- 1Department of Acupuncture and Moxibustion, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Gastroenterology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
The transient receptor potential vanilloid 1 (TRPV1) channel plays a dual role in peripheral neuropathic pain (NeuP) by acting as a “pain switch” through its sensitization and desensitization. Hyperalgesia, commonly resulting from tissue injury or inflammation, involves the sensitization of TRPV1 channels, which modulates sensory transmission from primary afferent nociceptors to spinal dorsal horn neurons. In chemotherapy-induced peripheral neuropathy (CIPN), TRPV1 is implicated in neuropathic pain mechanisms due to its interaction with ion channels, neurotransmitter signaling, and oxidative stress. Sensitization of TRPV1 in dorsal root ganglion neurons contributes to CIPN development, and inhibition of TRPV1 channels can reduce chemotherapy-induced mechanical hypersensitivity. In diabetic peripheral neuropathy (DPN), TRPV1 is involved in pain modulation through pathways including reactive oxygen species and cytokine production. TRPV1’s interaction with TRPA1 channels further influences chronic pain onset and progression. Therapeutically, capsaicin, a TRPV1 agonist, can induce analgesia through receptor desensitization, while TRPV1 antagonists and siRNA targeting TRPV1 show promise in preclinical studies. Cannabinoid modulation of TRPV1 provides another potential pathway for alleviating neuropathic pain. This review summarizes recent preclinical research on TRPV1 in association with peripheral NeuP.
1 Introduction
Neuropathic pain (NeuP) is caused by a lesion or disease affecting the peripheral or central somatosensory nervous system (Baron et al., 2010), as the International Association for the Study of Pain (IASP) defines (IASP, 1979). Depending on the lesion location, NeuP is classified into peripheral and central NeuP according the ICD-11 (Scholz et al., 2019). The prevalence of NeuP is as high as 7–10% of the general population, which is higher in certain specific populations (Torrance et al., 2006; Bouhassira et al., 2008; van Hecke et al., 2014). About 26% of patients with diabetes mellitus and 21% of patients with herpes zoster develop NeuP (van Hecke et al., 2014). The classic symptoms of NeuP involve positive symptoms such as spontaneous pain, hyperalgesia and allodynia, as well as negative symptoms such as decreased or loss of sensation (Scholz et al., 2019; Gilron et al., 2015). Meanwhile, NeuP is often accompanied by different temporal characteristics and pain properties (Gilron et al., 2015). NeuP is typically chronic and severe, impacting patients’ psychosocial and healthcare economic costs as well as their quality of life (Langley et al., 2013; Bates et al., 2019). The management of NeuP is extremely challenging for clinicians due to the refractory treatment (Deng et al., 2016). An epidemiologic survey showed that about 10–20% of patients are not correctly identified (Freynhagen and Bennett, 2009) and about 30–60% are not treated appropriately (Martinez et al., 2014), which may be related to insufficient information about the pathophysiologic mechanisms of the diseases (Moisset et al., 2020). Over the past decades, researchers have begun to investigate the cellular and molecular mechanisms involved in the pathogenesis of NeuP. Studies have revealed that significant mechanisms observed under the NeuP condition, include ectopic activity (Amir et al., 2005), peripheral sensitization (Kiguchi et al., 2014), central sensitization (Koltzenburg et al., 1994), impaired inhibitory regulation (Torsney and MacDermott, 2006), and microglia activation (Thacker et al., 2009).
Transient Receptor Potential (TRP) channels is a non-selective cation channels, consisting of a broad range of channels (Samanta et al., 2018), which could be categorized into TRPC, TRPV, TRPA, TRPM, TRPP, and TRPML (Venkatachalam et al., 2014). The TRP channels are implicated in the transduction of sensory information, including thermosensation (Damann et al., 2008), taste (Dhaka et al., 2006), hearing (Clapham, 2003), pain sensation (Clapham, 2003), etc. The abnormal function of TRP channels may lead to skin (Moran, 2018), airway (Marwaha et al., 2016), endocrine (Brandt et al., 2012) and gut (Cao et al., 2013). Furthermore, TRP channels are essential molecular components in acute inflammation and chronic pain conditions (Liao et al., 2013). Transient receptor potential channel vanilloid subtype 1 (TRPV1) channel is of the most studied targeting mechanisms in NeuP researches due to its widespread expression in neuronal cells and its critical role in pain perception and modulation (Moiseenkova-Bell et al., 2008; Dangi and Sharma, 2024). In this review, we provide a systematic overview of TRPV1, with a particular focus on their role and research progress in NeuP.
2 Structure and expression of TRPV1 channel
2.1 Molecular structure
TRPV1 was the first mammalian TRP channel whose structure was determined and cloned (Xu et al., 2007). The TRPV1 protein consists of four subunits, each containing six transmembrane structural domains (S1–S6) and two long intracellular N-terminal and C-terminal (Binder et al., 2011). Four independently folded S1–S4 structural domains surround to form the intervening pore loop region, which constitutes an ion-permeable channel with S5 and S6 (Valdes et al., 2011). Single-particle electron cryomicroscopy identified a fourfold symmetric structure of TRPV1, consisting of two regions, a large basket-like domain and a small compact domain, corresponding to the N-terminal, C-terminal region and transmembrane region, respectively (Caterina et al., 1997). Dual gating mechanism regulates the opening of TRPV1, where the upper gate is a selectivity filter formed by a funnel-shaped extracellular pore and the lower gate is located in the middle of the S6 helix and is involved in the dilation of a hydrophobic constriction (Xu et al., 2007). Some studies identified allelic variants of TRPV1 in specific populations (Sondermann, 2019), which may be associated with cold sensitivity (Caterina et al., 2000) and risk of developing knee osteoarthritis (Benítez-Angeles et al., 2020).
2.2 Expression patterns in tissues
TRPV1 is abundantly expressed in peripheral sensory neurons of the dorsal root ganglia (DRG), vagus and trigeminal ganglia (Arora et al., 2021). In addition, TRPV1 is also expressed in the intestinal mucosal epithelium, skin epidermis and immune cells, and others (Tominaga et al., 1998). As a pain and heat sensor for humans (Sanz-Salvador et al., 2012), it can be activated by a broad range of physical and chemical stimuli such as toxic heat (>43°C), divalent cations, low pH, inflammatory mediators, and animal toxins (Arora et al., 2021). Activation of the channel leads to a large Ca2+ and Na+ influx, generating neuronal depolarization and action potential discharges, which may also lead to calcium overload (Sanz-Salvador et al., 2012; Bujak et al., 2019). The activation of TRPV1 is enhanced when multiple stimuli are present simultaneously (Colloca et al., 2017). However, persistent stimulation reduces neuronal excitability, leading to a basic or complete insensitivity to subsequent stimuli, and thus specific desensitization (tachyphylaxis) occurs (Figure 1) (Bujak et al., 2019; Wang et al., 2022). To date, TRPV1 agonists (capsaicin, Resiniferatoxin), as well as antagonists (capsazepine, SB-705498, or NEO6860), have been used for the treatment of migraine, osteoarthritis, atopic dermatitis, and NeuP (Petitjean et al., 2015).
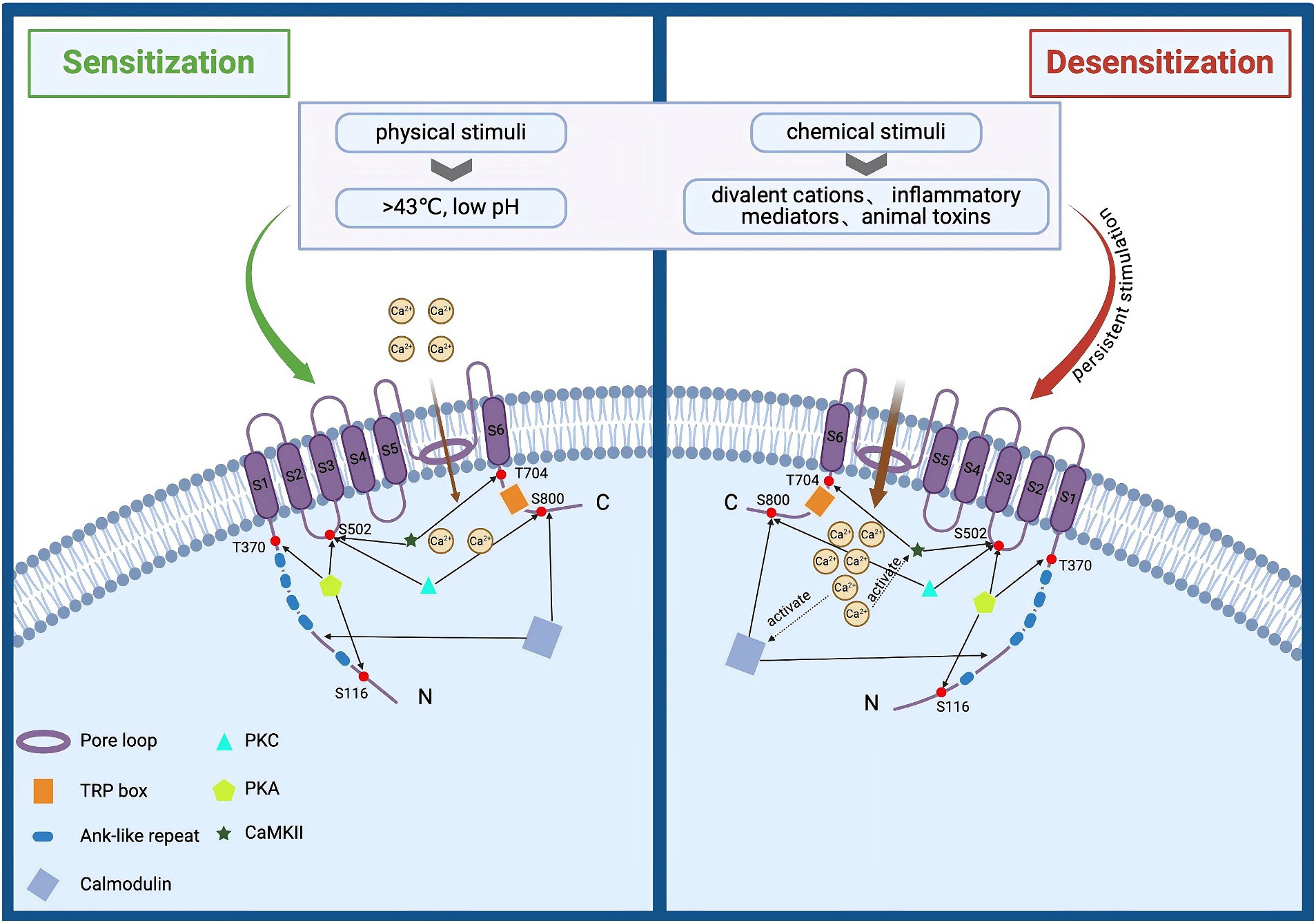
Figure 1. Structural and functional overview of TRPV1 activation and desensitization. TRPV1 is composed of three parts: intracellular N and C termini, six transmembrane domains (S1–S6), and a pore loop region formed between S5 and S6. It can be activated by various physical and chemical stimuli, such as noxious heat (>43°C), divalent cations, low pH, inflammatory mediators, and animal toxins. Activation of this channel leads to significant influxes of Ca2+ and Na+, causing neuronal depolarization and action potential discharge. Prolonged stimulation enhances TRPV1 activation, reducing neuronal excitability and resulting in near or complete insensitivity to subsequent stimuli, a phenomenon known as specific desensitization. Additionally, elevated intracellular calcium levels can activate the calcium-dependent protease calpain, which degrades cytoskeletal components within axons, leading to axonal structural damage and functional loss.
3 Mechanisms of NeuP
The pathogenesis of the NeuP is of complexity and has not yet been fully elucidated (Finnerup et al., 2021). Most of the current potential pathogenic mechanisms center on neuronal cells, encompassing the excitability of primary sensory neurons and the imbalance between excitatory and inhibitory synaptic transmission within the central nervous system (CNS) (Nichols et al., 1999). NeuP is typically characterized by ongoing or intermittent spontaneous pain or mechanical allodynia (Willis and Congeshall, 1991). Spontaneous pain may be caused by ectopic activity of damaged nerve fibers, and evoked pain primarily involves peripheral and central sensitization (Inoue and Tsuda, 2018). The reasons for sensitization of nociceptors generally include alteration of ion channels, activation of immune cells, glial-derived mediators, and epigenetic regulation (Inoue and Tsuda, 2018). At the spinal cord level, the underlying synaptic plasticity is not fully clarified. It has been shown that projection neurons in layer I of the spinal dorsal horn form synaptic connections with nociceptor C as well as Aδ fibers. The nociceptive projection neurons in layer I are activated through a complex neural circuit consisting of excitatory and inhibitory interneurons, which then send out projection fibers to carry that stimulus information to the superior centers (Mika et al., 2013; Ji et al., 2019). Peripheral nerve impairment via plastic modification of neuronal synapses and networks leads to changes in the balance between synaptic excitation and inhibition in layer I projection neurons, which may be driven in part by changes in excitatory and inhibitory interneurons in layer II or layer III, that may be related to the development and maintenance of pain hypersensitivity responses (Hains and Waxman, 2006).
The mechanisms of central NeuP involve intricate interactions and maladaptive plasticity within spinal and brain circuits related to nociception and antinociception, along with neuronal hyperexcitability and neuro-immune interactions, contributing to the complexity of this condition (Rosner et al., 2023). Recently, microglia activation was suggested to be involved in central NeuP pathophysiology, leading to the dysregulation of the MED1/BDNF/TrkB signaling pathway within the CNS following thalamic hemorrhage, which in turn induces pain and depression (Infantino et al., 2022). There is also research suggesting that the activation of microglia leads to the reorganization of neural networks within sensory pathways, particularly in the thalamus and primary somatosensory cortex. Microglial depletion can effectively prevent and alleviate mechanical hyperalgesia and abnormal axonal regeneration caused by thalamic hemorrhage (Hiraga et al., 2020).
Glial cells make up about 70% of the total number of cells in the CNS and comprise a variety of cell types including oligodendrocytes, astrocytes, microglia and satellite cells (Wahlman et al., 2018). It has been suggested that microglia and astrocytes are the critical cells that contribute to the development of acute and chronic pain following peripheral and central nerve injury (Old et al., 2015; Ji and Xu, 2021). Microglia and astrocytes respond to peripheral input signals and release proinflammatory mediators (Ji et al., 2018), such as cytokines and chemokines, which can sensitize neurons through activation of their cognate receptors, thereby promoting central sensitization and producing allodynia, hyperalgesia and spontaneous pain (Gim et al., 2011). Microglia are the resident immune cells of CNS and can switch between different activation states in response to various stimuli, primarily classified into M1 (pro-inflammatory) and M2 (anti-inflammatory/repair) (Colton and Wilcock, 2010). Under pathological conditions, microglia often adopt the M1 phenotype, producing pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and releasing reactive oxygen species (ROS) and nitric oxide (NO), thereby exacerbating neuroinflammation and neuronal damage (Correale, 2014). For example, following spinal cord injury, microglia rapidly switch to the M1 state, aggravating tissue damage through the secretion of these inflammatory mediators (Kigerl et al., 2009). In contrast, in the presence of anti-inflammatory signals such as IL-4 and IL-10, microglia can polarize to the M2 phenotype (Tang and Le, 2016). M2 microglia are involved in tissue repair and the resolution of inflammation, producing anti-inflammatory cytokines, promoting the phagocytosis of debris, and supporting neuronal survival and regeneration. The M2 state is crucial during the recovery process following CNS injury (Jin and Yamashita, 2016). Microglia can switch between M1 and M2 states in response to changes in the local microenvironment. Activation of Toll-like receptor 4 (TLR4) drives microglia toward the M1 state, while engagement of anti-inflammatory receptors can induce the M2 phenotype (Zhang et al., 2022). In Neup, the persistent activation of M1 microglia is associated with chronic pain conditions. Pro-inflammatory cytokines released by M1 microglia sensitize pain pathways and maintain the pain state (DeLeo and Yezierski, 2001). Conversely, promoting the conversion to the M2 phenotype has been proposed as a therapeutic strategy to alleviate chronic pain (Song and Suk, 2017; Jin et al., 2020).
In addition, an increasing number of studies have explored the mechanisms of NeuP in terms of altered lipid metabolism of neurolemma, inflammatory cellular glucose metabolism, and glial cellular glucose metabolism in recent years. Studies have shown that nerve injury produces sphingosine-1-phosphate (S1P), and spinal dorsal horn pairs drive NeuP through selective activation of S1P receptor subtype 1 in astrocytes (Hori et al., 2021). Reprogramming of glucose metabolism in microglia promotes the shift of microglia to a pro-inflammatory phenotype as well as increased ROS production. Reprogramming of glucose metabolism in glial cells also contributes to hyperalgesia and allodynia in NeuP (Ramal-Sanchez et al., 2021).
4 Functions of TRPV1 in pain regulation
In recent years, TRPV1 ion channels have been increasingly reported to be involved in the regulation of a variety of physiopathological processes in living organisms (Katz et al., 2023; Petroianu et al., 2023; Schumacher, 2010), especially for its role as a crucial mechanism in the development of pain (Ji et al., 2003). TRPV1 receptors are highly expressed mainly on C and some Aδ nociception nerves (nociceptor), is a pivotal molecule in mediating both thermosensory and thermal pain sensitization formation (Arora et al., 2021). Injury leads to activation of TRP nociceptors in the periphery and action potentials are conducted along afferent sensory fibers to dorsal horn synapses. Subsequently, the signal crosses the spinal-thalamic lateral fasciculus, the thalamus, and the sensory cortex of the parietal lobe of the thalamocortex to localize the pain (Wang et al., 2004). Activation of TRPV1 in the periaqueductal gray promotes the release of glutamate, which activates antinociceptive neurons in the rostral ventromedial medulla, thereby modulating pain signal transmission and antinociceptive responses in the CNS (Starowicz et al., 2007).
TRPV1 plays a crucial role in neuroinflammatory responses by sensing stimuli such as high temperatures, acidic environments, and endogenous lipid molecules, leading to calcium influx (Tominaga et al., 1998; Kwon et al., 2021). This activation of sensory neurons results in the release of inflammatory mediators such as calcitonin gene-related peptide (CGRP) and substance P (Herbert and Holzer, 2002). These mediators further activate microglial and astrocytic cells within the CNS, leading to the release of additional pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6, which amplify the inflammatory response and increase pain sensitivity (Vergne-Salle and Bertin, 2021). Additionally, TRPV1 activation exacerbates the inflammatory response through neuro-immune interactions. The inflammatory mediators released by neurons act not only on glial cells but also affect immune cells such as T cells and macrophages (Li and Gupta, 2019). This leads to the aggregation and activation of these cells at the site of inflammation, releasing more inflammatory mediators and further intensifying the inflammation. Persistent TRPV1 activation may also impact the function of the endogenous opioid system, further influencing pain (Zygmunt et al., 1999).
Many endogenous inflammatory mediators (such as prostaglandin E2 and bradykinin, as well as nerve injury factors like nerve growth factor and TNF-α, etc.) have been shown to act directly or sensitize TRPV1 through secondary messengers and/or protein modifications (Premkumar and Ahern, 2000; Ji et al., 2023), leading to allodynia and hyperalgesia (Zhang et al., 2007). TRPV1 sensitization is facilitated by kinases such as protein kinase A (PKA), protein kinase C (PKC), and calcium/calmodulin-dependent protein kinase II (CaMKII) (Wang et al., 2004; Sinharoy et al., 2015). PKC is a prominent participant in pain signaling that can phosphorylate many substrate proteins to regulate the sensitivity of nociceptors (Rathee et al., 2002). PKC regulates the activity of TRPV1 channels mainly through two sites, S502 and S800, and phosphorylation of these two sites sensitizes and facilitates the opening of TRPV1 channels to enable calcium ions to flow into the cell (Numazaki et al., 2002). It was found that PKCε inhibitors completely blocked the enhancement of TRPV1 expression and provided a more significant functional relationship between PKCε and TRPV1 sensitization (Studer and McNaughton, 2010). c-AMP-dependent PKA phosphorylates the n-terminus of TRPV1 (Jung et al., 2004) and regulates channel sensitization directly through the S116, T144, T370, S502, and S800 sites (Sun et al., 2018; Ferreira et al., 2020). Elevated calcium levels in the cell can activate CaMKII, and active CaMKII can directly phosphorylate TRPV1 channels at specific sites Ser 502 and Thr 704 (Anand and Bley, 2011). Dysregulated lipid metabolism may also impact TRPV1 activation or sensitivity, leading to heightened pain signaling and increased pain perception in neuropathic conditions (Szolcsányi, 1993).
It is becoming evident that Botulinum neurotoxins (BoNTs) also regulate the expression and function of TRP channels, which may explain their analgesic effects (Go et al., 2021).
When BoNT-A enters the cell, synaptosomal-associated protein 25 kDa (SNAP25) is cleaved by the protease activity of BoNT-A(1′) (Dong et al., 2006), thereby inhibiting exocytosis. The failure of TRPV1 to translocate to the plasma membrane makes TRPV1 susceptible to ubiquitination and subsequent proteasomal degradation, leading to a decrease in TRPV1 levels, which mediates its antinociceptive effects (Shimizu et al., 2012). Additionally, estrogen and progesterone can influence pain perception by regulating the expression and function of the TRPV1 receptor (Chen et al., 2021; Ortíz-Rentería et al., 2018). Activation of Sig-1R can enhance the sensitization of TRPV1, leading to increased neuronal response to pain stimuli (Zheng and Trudeau, 2023). In females, mechanical pain from paclitaxel-induced CIPN is linked to the IL-23/IL-17A/TRPV1 axis (Luo et al., 2021), while male sensory neurons show greater paclitaxel-induced TRPM8 activity compared to females (Villalba-Riquelme et al., 2022). An increasing number of studies have highlighted the gender dimorphism in chronic pain (Cabañero et al., 2022).
5 Role of TRPV1 in mechanisms of NeuP
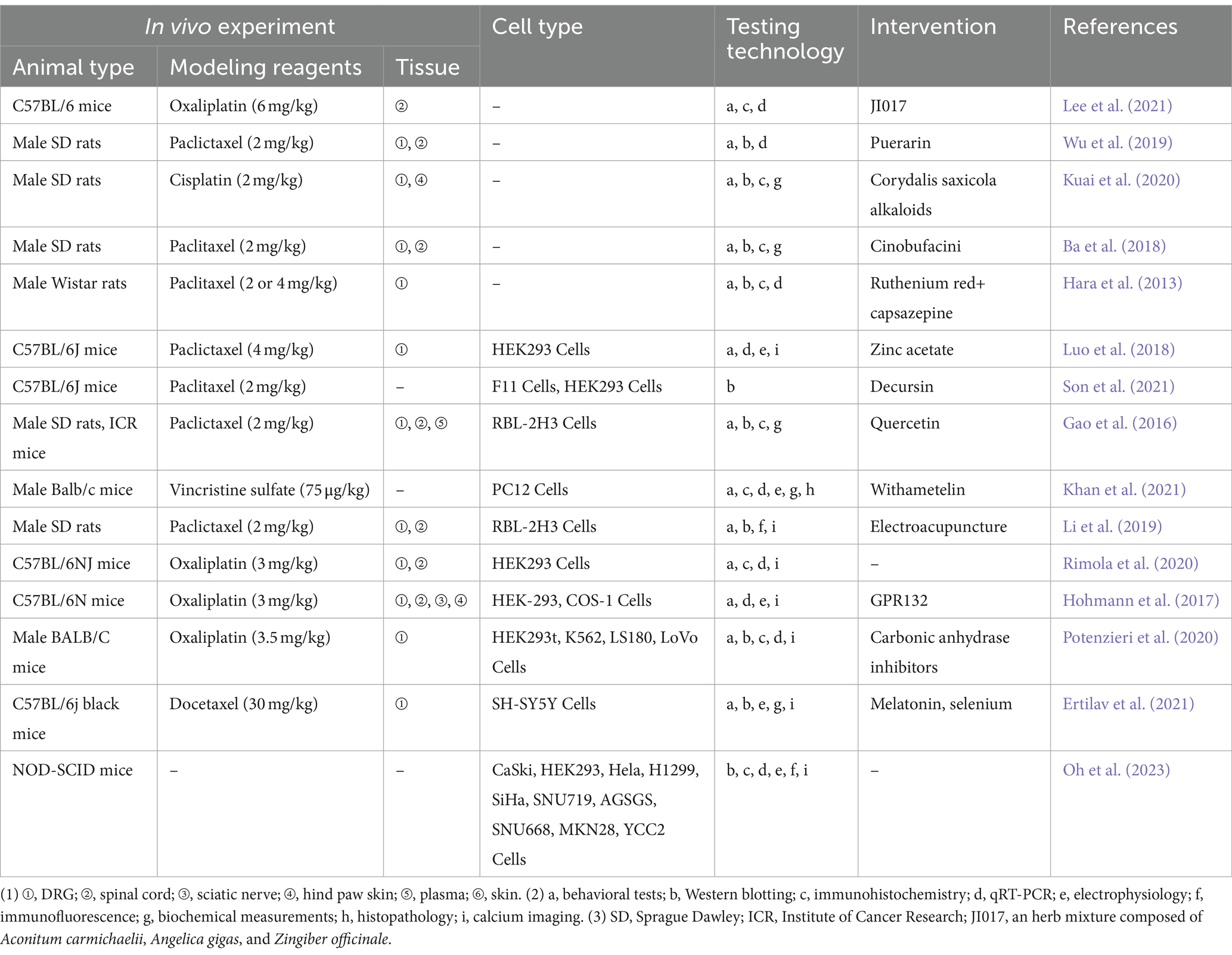
Hyperalgesia caused by tissue injury or inflammation is typically accompanied with sensitization to TRPV1 channel activity, which is important in the modulation of sensory transmission from primary afferent nociceptors to neurons in the spinal dorsal horn (Xu et al., 2022; Shim et al., 2019). Preclinical models used for peripheral neuropathic pain research commonly include chronic constriction injury (CCI) of the sciatic nerve, diabetic peripheral neuralgia (DPN), chemotherapy-induced neuropathic pain (CIPN), and etc. (Xu and Wang, 2024; Jaggi et al., 2011). The following section primarily focuses on studies based on these various rodent models of peripheral neuropathic pain.
5.1 Chemotherapy-induced NeuP
The pathological mechanisms of CIPN may be related to affecting the function of ion channels, signaling by neurotransmitters and neuromodulators, inflammatory mediators, transcription factors (Zajaczkowską et al., 2019), oxidative stress (Zhao et al., 2023), and mitochondrial dysfunction (Chiba et al., 2017). Moreover, structural brain abnormalities, such as axonopathy, small-fiber degeneration, demyelination, and atrophy, are often detected in the peripheral nerves of individuals with CIPN and rodent models of CIPN (Akhilesh et al., 2022). Platinum- and taxane-derived anticancer drugs, induced neurological damage models are widely applied. Spinal cord expression of TRPV1 receptors has been associated with NeuP induced by the aforementioned chemotherapeutic agents (Luo et al., 2018; Son et al., 2021). For instance, Paclitaxel (PTX) induced behavioral hypersensitivity by sensitizing TRPV1 in DRG neurons through TLR4 signaling (Li et al., 2024; Li et al., 2015). TRPV1 has a role in the development of CIPN, and spinal astrocytes and microglia are also engaged in the beginning and maintenance of CIPN (Lee et al., 2021). After the intrathecal injection of the oxaliplatin-treated satellite glial cells-secreted exosomes, mice developed mechanical hypersensitivity, with an increase in the percentage of reactive oxygen species-positive neurons and upregulation of acid-sensing ion channel 3 and TRPV1 expressions in DRG (Luo et al., 2018). TRPV1 is involved in the progression of mechanically allodynia/nociception and thermal hyperalgesia induced by chemotherapeutic agents such as paclitaxel and vincristine (Son et al., 2021). Inhibition of TRPV1 channels suppresses chemotherapeutic agent-induced mechanical hypersensitivity (Li et al., 2015; Chen and Chang, 2019; Oh et al., 2023). Zinc significantly decreased paclitaxel-induced NeuP in mice in a TRPV1-dependent manner (Li et al., 2015), and Decursin promotes the restoration of damaged neuronal networks and inhibits the pain transformation induced by a sudden increase in Ca2+ through the inhibition of TRPV1 (Chen and Chang, 2019). The overexpression of TRPV1 in DRG neurons and the pain reaction in paclitaxel-treated rats were significantly reduced by pharmacological blockade of TLR4, which indicates that TRPV1 expression and channel activity in CIPN are regulated by TLR4 (Guo et al., 2019). JI017 alleviates neuralgia by inhibiting TRPV1 expression and the activation of astrocytes in the superficial area of the spinal dorsal horn. However, JI017 only attenuated cold nociception while mechanical nociception remained unchanged, which may be related to its low CNS penetration rate (Oh et al., 2023). Resistance to chemotherapeutic agents and subsequent NeuP are the main factors affecting the course of chemotherapy in patients (Elafros et al., 2022). Chen et al. (2019) discovered that the development of cisplatin resistance is closely linked to the hyperactivation of the epidermal growth factor receptor (EGFR), driven by a transcriptional upregulation of TRPV1 through NANOG. Additionally, TRPV1 facilitates autophagy-mediated EGF secretion via Ca2+ influx, which in turn activates the EGFR-AKT signaling pathway, contributing to the acquisition of cisplatin resistance (Yagihashi et al., 2011). In addition, small interfering RNA (siRNA)-based therapeutics targeting TRPV1 has been verified in a number of experiments for the treatment of NeuP, including CIPN (Wang et al., 2012). Experimental studies related to the TRPV1 in the CIPN are presented in Table 1.
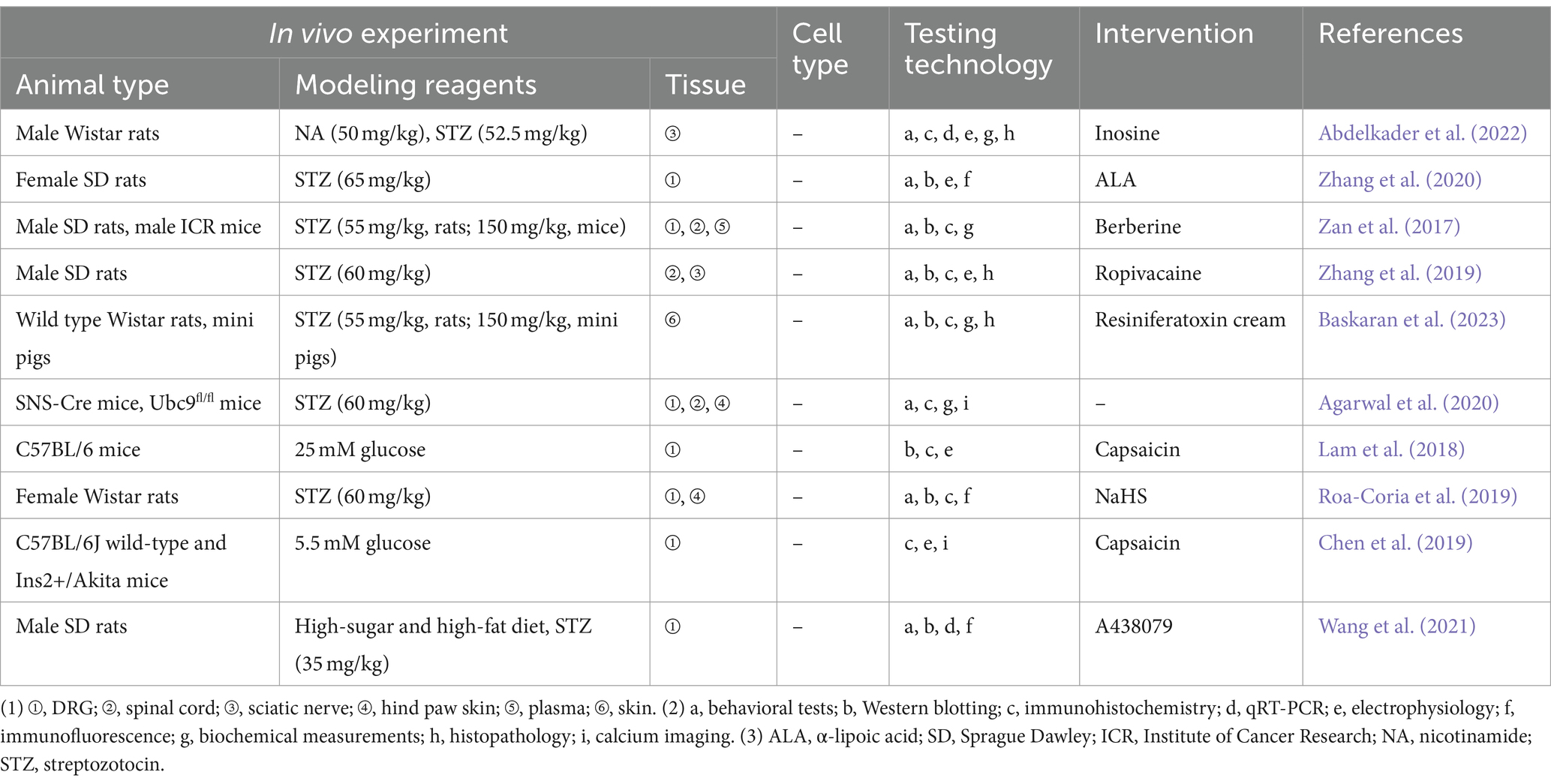
5.2 Diabetic peripheral neuralgia
Peripheral neuropathy is a common and characteristic complication of diabetes mellitus, causing numbness, tingling, burning pain in the skin, occasionally accompanied by hyperalgesia or allodynia (Mohammadi-Farani et al., 2014). Possible mechanisms of DPN include a vicious cycle involving the production of advanced glycation end products (AGEs), activation of PKC, amplification of the polyol pathway, and excessive release of ROS and cytokines (Carr and Frings, 2019). TRPV1 is linked to diabetes mellitus on multiple fronts, encompassing pancreatic function and insulin secretion, appetite regulation, and energy expenditure or thermogenesis (Zhang et al., 2019). Experimental studies related to the TRPV1 in the DPN are presented in Table 2. Hyperglycemia reduces the expression of cannabinoid receptor-1 (CB1) receptors and increases the expression of TRPV1 receptors in the PC12 cell line, leading to greater toxic effects from TRPV1 activation (Vincent et al., 2007). Enhanced expression of CGRP may promote injured peripheral nerve regeneration, and activated TRPV1 promotes calcium-dependent release of substance P and CGRP in peripheral nerve endings (Chen et al., 2019). Ropivacaine may exacerbate DPN nerve block by inhibiting TRPV1 expression in the dorsal horn, which in turn decreases CGRP release in the spinal cord (Lam et al., 2018). In vitro, receptor for advanced glycation end-products (RAGE) expression, signaling, and RAGE-induced ROS production contributed to apoptosis of DRG neurons exposed to high glucose conditions (Roa-Coria et al., 2019). In contrast, RAGE signaling-mediated TRPV1-associated aberrant responses (in terms of cytoplasmic signaling changes including Ca2+, PCK, and Src kinases) as well as ROS accumulation directly or indirectly results in TRPV1 function impairment, which are one of the contributing factors to DPN in the diabetic pathologic setting (Abdelkader et al., 2022; Zhang et al., 2020). Sensitization of peripheral TRPV1, TRPA1, and TRPC channels in non-peptidergic fibers by hydrogen sulfide synthesized by the cystathionine β-synthase enzyme, leading to hyperalgesia and loss of peripheral nerve fibers in a rat model of diabetes mellitus, was further validated by local peripheral injections of capsazepine, HC-030031, and SKF-96365 blockers (Agarwal et al., 2020). In addition, using 9-month-old Ins2+/Akita mice, Lam et al. (2018) found that capsaicin activation of TRPV1 in DRG neurons exhibited accelerated current decay, which may provide an explanation for the phenomenon of reduced pain in people with end-stage diabetic peripheral neuropathy in one way. Abdelkader et al. (2022) found that inosine alleviated pain through downregulation of PKC, TRPV1 expression, decreasing Substance P and Transforming growth factor beta in DPN rat model. α-lipoic acid (ALA) may alleviate NeuP in diabetes by regulating TRPV1 expression via affecting NF-κB (Wang Z. et al., 2020). SUMOylation is an important mechanism for protection against endogenous metabolic damage in DPN sensory neurons, and modulation of TRPV1 function through extra-sensory neuronal SUMOylation may yield novel strategies for treating and reversing DPN (Truini et al., 2015).
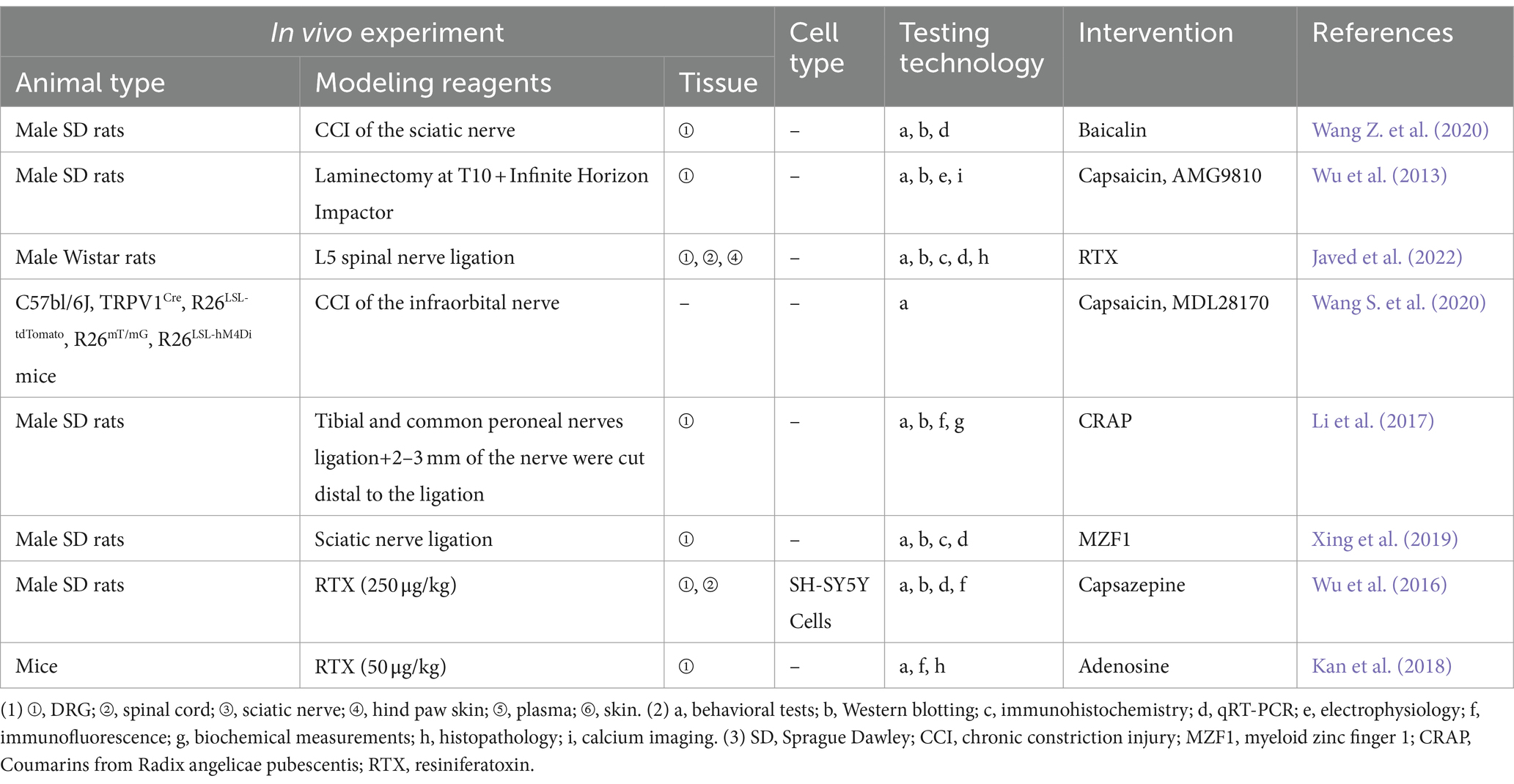
5.3 Other NeuP
The most common way for creating neuropathy in animals is to cause entire or partial traumatic nerve injury via ligation, transection, or compression (Chen and Pan, 2005). The key protein phospho-regulating effectors that promote nociceptive sensitization are mitogen-activated protein kinases (MAPK), and additional findings showed that baicalin inhibits TRPV1 up-regulation and extracellular signal-regulated kinase phosphorylation in CCI of the sciatic nerve rats’ DRG (Pan et al., 2003). PHN is common in the elderly and immunocompromised patients (Wu et al., 2016). The resiniferatoxin (RTX)-induced PHN model is a commonly used method of PHN modeling, which depletes TRPV1-expressing primary sensory neurons, causing severe degeneration of C-fiber afferent terminals as well as aberrant sprouting of myelinated afferent fibers in the II layer of the spinal dorsal horn (Zhang et al., 2021), which in turn exhibits the distinctive clinical features of PHN, i.e., thermosensory impairments and mechanical allodynia (Story et al., 2003). Wu et al. (2016) proposed that RTX may stimulate the TRPV1 receptor and its downstream signaling molecules to enhance the expression of netrin-1, and the increased expression of netrin-1 further activates repulsive receptor of netrin-1 (UNC5H2) and deleted in colorectal (DCC) at the central terminus of the remaining myelinated neurons in the DRG to promote myelinated fibers to sprout to the noxious neurons located in the superficial dorsal horn. Zhang et al. (2021) found that RTX treatment increased excitatory glutamatergic input from myelinated afferent nerves to the spinal dorsal horn through α2δ-1-dependent enhancement of N-methyl-D-aspartate receptor (NMDAR) activity, thereby causing mechanical allodynia, which further enriched the study of synaptic plasticity in PHN. Experimental studies related to the TRPV1 in the other NeuP are presented in Table 3.
6 Association between TRPA1 and TRPV1
There are evidences that TRPA1 and TRPV1 mutually regulate pain signal transduction (Weng et al., 2015; Spahn et al., 2014). TRPA1 is localized to a subset of TRPV1-positive sensory neurons, being present in 30–50% of these neurons. It is rarely detected in neurons that lack TRPV1 expression (Fischer et al., 2014; Shields et al., 2010). In cells co-expressing TRPA1 and TRPV1, these two TRP channels appear to form a complex or a heterogeneous channel at the cell membrane, thereby influencing the function of each other (Marwaha et al., 2016; Billeter et al., 2014). Shields et al. (2010) utilized selective elimination of the central terminus of TRPV1-expressing nociceptor in wild-type C57Bl/6 mice by intrathecal injection of capsaicin and found that the nociceptive reaction induced by the TRPA1-selective agonist mustard oil was also eliminated. The co-expression of TRPA1 and TRPV1 in nociceptive fibers is crucial for the initiation and progression of chronic pain (Akopian et al., 2007). Structurally, TRPA1 and TRPV1 share similar transmembrane domains. However, TRPA1 differs by having an additional pore helix lining the extracellular side of the ion permeation pathway, resulting in two pore helices per subunit (Mihara and Shibamoto, 2015). Studies of IMustard Oil (MO) rapid sensitization in Chinese hamster ovary cells expressing TRPA1 or TRPA1/TRPV1 showed that IMO experienced greater rapid sensitization in the absence of TRPV1. One possible explanation is that TRPV1 stabilizes the membrane surface expression of TRPA1 (Fernandes et al., 2012). Activation of TRPA1 did not sensitize TRPV1 without the involvement of calcium ions, suggesting that co-expression occurs in a calcium-dependent way. TRPA1 activation leads to enhanced accumulation of cAMP and subsequent stimulation of PKA subunit release, which in turn leads to phosphorylation and sensitization of TRPV1 (Wu et al., 2019). Functional crossover desensitization has also been reported between typical agonists of TRPA1 (allyl isothiocyanate, mustard) and TRPV1 (capsaicin) (Kuai et al., 2020). In addition, it was shown that TRPA1 and TRPV1 can form complexes in cell membranes that affect the properties of each other (Marwaha et al., 2016). The TRPA1 and TRPV1 channels are therefore described as “partners in crime” (Ba et al., 2018).
7 Basic drug targets
7.1 TRPV1 agonists
Capsaicin, a potent agonist of the TRPV1 channel, was extracted from the capsicum genus of spices (Abrams et al., 2021). Capsaicin has emerged as a useful tool in the research on pain pathways (Turnbull, 1850) and is currently approved for the treatment of PHN, HIV-associated neuropathy and DPN (Jancso and Jancso, 1949; Szolcsányi, 2005). High concentrations of capsaicin reversibly deactivate TRPV1 receptors, which leads to an analgesic effect (Blair, 2018). It has long been recognized that the initial application of capsaicin is painful and, paradoxically, repeated applications produce local analgesic effects (Touska et al., 2011; Tian et al., 2019). This is a desensitization response induced by prolonged gating of TRPV1 cation channels (Arora et al., 2021) that is closely associated with the duration of capsaicin exposure and the external calcium concentration, and which can be considered as a protective mechanism for neurons against calcium overload during repeated TRPV1 stimulation (Iftinca et al., 2021). Calcium influx following TRPV1 activation leads to channel desensitization. Acute desensitization refers to a rapid decline in the evoked inward current, while tachyphylaxis describes the reduction in current during repeated stimulation (Koplas et al., 1997). Compared to the short-term dysfunction induced by low doses of capsaicin, high doses of capsaicin often elicit dysfunction that lasts for months, which may be related to the structural ablation of TRPV1+ nerve endings (Campbell et al., 2021). Capsaicin induces calcium influx through TRPV1 channels, leading to the activation of the calcium-dependent protease calpain. Calpain then begins to degrade cytoskeletal components within the axon, resulting in structural damage and loss of function in the axon. Studies have shown that capsaicin-induced TRPV1+ sensory axon ablation is also associated with mitochondrial dysfunction. Inhibiting calcium influx or calpain activity can significantly reduce capsaicin-induced TRPV1+ axon ablation (Wang et al., 2017). Calcineurin, also known as protein phosphatase 2B, is a Ca2+-Calmodulin (CaM) phosphatase that has been shown to dephosphorylate the channel, thereby promoting its desensitization (Mohapatra and Nau, 2005). Ca2+ influx activates phospholipase C (PLC), leading to the depletion of the agonists Phosphatidylinositol 4,5-bisphosphate (PIP2) and Phosphatidylinositol 4-phosphate (PIP). This reduction in PIP2 and PIP levels limits the channel’ s activity, resulting in its desensitization (Lukacs et al., 2007; Lukacs et al., 2013). These findings not only enhance our understanding of the mechanisms behind capsaicin-induced analgesia but also provide a theoretical foundation for improving the use of capsaicin in pain treatment.
7.2 TRPV1 antagonists and TRPV1-targeted siRNA
TRPV1 antagonists work by blocking the TRPV1 receptor, preventing calcium influx, and thereby inhibiting the transmission of pain signals. However, the preclinical development of TRPV1 antagonists faces challenges, including potential side effects such as thermoregulation abnormalities (Szelenyi et al., 2004). Thereby, the aim of developing TRPV1 antagonists for pain treatment is to create medications that specifically inhibit the activation of TRPV1 channels by pain-inducing agents, without affecting their activation by thermal stimuli (Chahl, 2024). Subsequently, alternative strategies emerged to target the expression of the TRPV1 channel using genome-editing tools. In a preclinical study, mice treated with TRPV1-targeted siRNA showed a phenotype similar to that of TRPV1 knockout mice (Christoph et al., 2008). Research has shown that paratracheal delivery of TRPV1 siRNA suppresses TRPV1 upregulation in the DRG and spinal cord, effectively eliminating CFA-induced inflammation and chemotherapy-induced thermal hyperalgesia and mechanical allodynia (Kasama et al., 2007). TRPV1 antagonists, including TRPV1 siRNA, have potential roles in the treatment of neuropathic pain (Akhilesh et al., 2022).
7.3 Cannabinoid modulation
As an integral part of the extended endocannabinoid system (De Petrocellis et al., 2017), TRPV1 interacts with endocannabinoids through complex molecular mechanisms, thereby regulating the pathophysiological processes of neuropathic pain (Starowicz and Przewlocka, 2012). Firstly, TRPV1 can directly interact with endocannabinoids. For example, Anandamide (AEA) is not only a partial agonist of CB1 receptors but also an agonist of TRPV1. When AEA binds to TRPV1, it leads to the opening of TRPV1 channel, causing Ca2+ influx, which subsequently induces depolarization and the generation of action potentials in sensory neurons (Fenwick et al., 2017). Secondly, the endocannabinoid system can influence the occurrence and development of neuropathic pain by regulating TRPV1 expression and function. Studies have found that activation of CB1 receptors can inhibit TRPV1 expression and function. For example, treatment with CB1 receptor agonists can reduce TRPV1 expression in sensory neurons, thereby alleviating pain (McDowell et al., 2013). This mechanism may be achieved by lowering intracellular cAMP levels and inhibiting PKA activity, which in turn reduces the transcription and translation of the TRPV1 gene (Vetter et al., 2006). Additionally, TRPV1 may be involved in the degradation process of endocannabinoids. The degradation of endocannabinoids primarily relies on the enzyme fatty acid amide hydrolase (FAAH) (Vandevoorde and Lambert, 2005). For instance, research indicates that increasing doses of a locally injected FAAH inhibitor elevate spinal AEA levels, which in turn produce anti-hyperalgesic and anti-allodynic effects. These effects are achieved through mechanisms that progressively involve the desensitization of TRPV1 channels (Starowicz et al., 2013).
8 Conclusion and perspectives
TRPV1 plays a dual role in peripheral NeuP, acting as a “switch” for pain through its sensitization and desensitization processes. In CIPN and DPN, the sensitization of TRPV1 channels is a key mechanism. Inhibiting TRPV1 channels can significantly reduce mechanical hypersensitivity and pain. Clinically, capsaicin, a TRPV1 agonist, alleviates pain by inducing receptor desensitization, while TRPV1 antagonists and siRNA targeting TRPV1 show promise in preclinical studies. Cannabinoid modulation of TRPV1 offers another potential pathway for alleviating neuropathic pain. Future research should focus on the immunomodulation and metabolic functions of the TRPV1 receptor, as well as the application of novel gene editing and RNA interference technologies, with the aim of developing more effective pain treatment strategies.
Author contributions
NG: Conceptualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Writing – original draft, Writing – review & editing. WW: Conceptualization, Methodology, Software, Validation, Writing – review & editing. ZL: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. YG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by High Level Chinese Medical Hospital Promotion Project (Grant no. HLCMHPP2023089) and Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (Grant no. CI2021A02306). The funding agency had no role in the design or conduct of the study.
Acknowledgments
The authors appreciate the publications included in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelkader, N. F., Ibrahim, S. M., Moustafa, P. E., and Elbaset, M. A. (2022). Inosine mitigated diabetic peripheral neuropathy via modulating GLO1/AGEs/RAGE/NF-κB/Nrf2 and TGF-β/PKC/TRPV1 signaling pathways. Biomed. Pharmacother. 145:112395. doi: 10.1016/j.biopha.2021.112395
Abrams, R. M. C., Pedowitz, E. J., and Simpson, D. M. (2021). A critical review of the capsaicin 8% patch for the treatment of neuropathic pain associated with diabetic peripheral neuropathy of the feet in adults. Expert. Rev. Neurother. 21, 259–266. doi: 10.1080/14737175.2021.1874920
Agarwal, N., Taberner, F. J., Rangel Rojas, D., Moroni, M., Omberbasic, D., Njoo, C., et al. (2020). SUMOylation of enzymes and ion channels in sensory neurons protects against metabolic dysfunction, neuropathy, and sensory loss in diabetes. Neuron 107, 1141–1159.e7. doi: 10.1016/j.neuron.2020.06.037
Akhilesh,, Uniyal, A., Gadepalli, A., Tiwari, V., Allani, M., Chouhan, D., et al. (2022). Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain. Life Sci. 288:120187. doi: 10.1016/j.lfs.2021.120187
Akopian, A. N., Ruparel, N. B., Jeske, N. A., and Hargreaves, K. M. (2007). Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J. Physiol. 583, 175–193. doi: 10.1113/jphysiol.2007.133231
Amir, R., Kocsis, J. D., and Devor, M. (2005). Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons. J. Neurosci. 25, 2576–2585. doi: 10.1523/JNEUROSCI.4118-04.2005
Anand, P., and Bley, K. (2011). Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 107, 490–502. doi: 10.1093/bja/aer260
Arora, V., Campbell, J. N., and Chung, M. K. (2021). Fight fire with fire: neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 220:107743. doi: 10.1016/j.pharmthera.2020.107743
Ba, X., Wang, J., Zhou, S., Luo, X., Peng, Y., Yang, S., et al. (2018). Cinobufacini protects against paclitaxel-induced peripheral neuropathic pain and suppresses TRPV1 up-regulation and spinal astrocyte activation in rats. Biomed. Pharmacother. 108, 76–84. doi: 10.1016/j.biopha.2018.09.018
Baron, R., Binder, A., and Wasner, G. (2010). Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 9, 807–819. doi: 10.1016/S1474-4422(10)70143-5
Baskaran, P., Mohandass, A., Gustafson, N., Bennis, J., Louis, S., Alexander, B., et al. (2023). Evaluation of a polymer-coated nanoparticle cream formulation of resiniferatoxin for the treatment of painful diabetic peripheral neuropathy. Pain 164, 782–790. doi: 10.1097/j.pain.0000000000002765
Bates, D., Schultheis, B. C., Hanes, M. C., Jolly, S. M., Chakravarthy, K. V., Deer, T. R., et al. (2019). A comprehensive algorithm for management of neuropathic pain. Pain Med. 20, S2–S12. doi: 10.1093/pm/pnz075
Benítez-Angeles, M., Morales-Lázaro, S. L., Juárez-González, E., and Rosenbaum, T. (2020). TRPV1: structure, endogenous agonists, and mechanisms. Int. J. Mol. Sci. 21:3421. doi: 10.3390/ijms21103421
Billeter, A. T., Hellmann, J. L., Bhatnagar, A., and Polk, H. C. (2014). Transient receptor potential ion channels: powerful regulators of cell function. Ann. Surg. 259, 229–235. doi: 10.1097/SLA.0b013e3182a6359c
Binder, A., May, D., Baron, R., Maier, C., Tölle, T. R., Treede, R. D., et al. (2011). Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One 6:e17387. doi: 10.1371/journal.pone.0017387
Blair, H. A. (2018). Capsaicin 8% dermal patch: a review in peripheral neuropathic pain. Drugs 78, 1489–1500. doi: 10.1007/s40265-018-0982-7
Bouhassira, D., Lantéri-Minet, M., Attal, N., Laurent, B., and Touboul, C. (2008). Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136, 380–387. doi: 10.1016/j.pain.2007.08.013
Brandt, M. R., Beyer, C. E., and Stahl, S. M. (2012). TRPV1 antagonists and chronic pain: beyond thermal perception. Pharmaceuticals 5, 114–132. doi: 10.3390/ph5020114
Bujak, J. K., Kosmala, D., Szopa, I. M., Majchrzak, K., and Bednarczyk, P. (2019). Inflammation, cancer and immunity-implication of TRPV1 channel. Front. Oncol. 9:1087. doi: 10.3389/fonc.2019.01087
Cabañero, D., Villalba-Riquelme, E., Fernández-Ballester, G., Fernández-Carvajal, A., and Ferrer-Montiel, A. (2022). ThermoTRP channels in pain sexual dimorphism: new insights for drug intervention. Pharmacol. Ther. 240:108297. doi: 10.1016/j.pharmthera.2022.108297
Campbell, J. N., Stevens, R., Hanson, P., Connolly, J., Meske, D. S., Chung, M. K., et al. (2021). Injectable capsaicin for the Management of Pain due to osteoarthritis. Molecules 26:778. doi: 10.3390/molecules26040778
Cao, E., Liao, M., Cheng, Y., and Julius, D. (2013). TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118. doi: 10.1038/nature12823
Carr, R., and Frings, S. (2019). Neuropeptides in sensory signal processing. Cell Tissue Res. 375, 217–225. doi: 10.1007/s00441-018-2946-3
Caterina, M. J., Leffler, A., Malmberg, A. B., Martin, W. J., Trafton, J., Petersen-Zeitz, K. R., et al. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313. doi: 10.1126/science.288.5464.306
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. doi: 10.1038/39807
Chahl, L. A. (2024). TRPV1 channels in the central nervous system as drug targets. Pharmaceuticals 17:756. doi: 10.3390/ph17060756
Chen, S. H., and Chang, J. Y. (2019). New insights into mechanisms of cisplatin resistance: from tumor cell to microenvironment. Int. J. Mol. Sci. 20:4136. doi: 10.3390/ijms20174136
Chen, X., Duan, Y., Riley, A. M., Welch, M. A., White, F. A., Grant, M. B., et al. (2019). Long-term diabetic microenvironment augments the decay rate of capsaicin-induced currents in mouse dorsal root ganglion neurons. Molecules 24:775. doi: 10.3390/molecules24040775
Chen, S. R., and Pan, H. L. (2005). Effect of systemic and intrathecal gabapentin on allodynia in a new rat model of postherpetic neuralgia. Brain Res. 1042, 108–113. doi: 10.1016/j.brainres.2005.02.024
Chen, Q., Zhang, W., Sadana, N., and Chen, X. (2021). Estrogen receptors in pain modulation: cellular signaling. Biol. Sex Differ. 12, 1–10. doi: 10.1186/s13293-021-00364-5
Chiba, T., Oka, Y., Sashida, H., Kanbe, T., Abe, K., Utsunomiya, I., et al. (2017). Vincristine-induced peripheral neuropathic pain and expression of transient receptor potential vanilloid 1 in rat. J. Pharmacol. Sci. 133, 254–260. doi: 10.1016/j.jphs.2017.03.004
Christoph, T., Bahrenberg, G., De Vry, J., Englberger, W., Erdmann, V. A., Frech, M., et al. (2008). Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol. Cell. Neurosci. 37, 579–589. doi: 10.1016/j.mcn.2007.12.006
Clapham, D. E. (2003). TRP channels as cellular sensors. Nature 426, 517–524. doi: 10.1038/nature02196
Colloca, L., Ludman, T., Bouhassira, D., Baron, R., Dickenson, A. H., Yarnitsky, D., et al. (2017). Neuropathic pain. Nat. Rev. Dis. Primers 3:17002. doi: 10.1038/nrdp.2017.2
Colton, C., and Wilcock, D. M. (2010). Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets 9, 174–191. doi: 10.2174/187152710791012053
Correale, J. (2014). The role of microglial activation in disease progression. Mult. Scler. 20, 1288–1295. doi: 10.1177/1352458514533230
Damann, N., Voets, T., and Nilius, B. (2008). TRPs in our senses. Curr. Biol. 18, R880–R889. doi: 10.1016/j.cub.2008.07.063
Dangi, A., and Sharma, S. S. (2024). Pharmacological agents targeting transient receptor potential (TRP) channels in neuropathic pain: preclinical and clinical status. Eur. J. Pharmacol. 980:176845. doi: 10.1016/j.ejphar.2024.176845
De Petrocellis, L., Nabissi, M., Santorini, G., and Ligristi, A. (2017). Actions and regulation of ionotropic cannabinoid receptors. Adv. Pharmacol. 80, 249–289. doi: 10.1016/bs.apha.2017.04.001
DeLeo, J. A., and Yezierski, R. P. (2001). The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90, 1–6. doi: 10.1016/s0304-3959(00)00490-5
Deng, Y., Luo, L., Hu, Y., Fang, K., and Liu, J. (2016). Clinical practice guidelines for the management of neuropathic pain: a systematic review. BMC Anesthesiol. 16:12. doi: 10.1186/s12871-015-0150-5
Dhaka, A., Viswanath, V., and Patapoutian, A. (2006). Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135–161. doi: 10.1146/annurev.neuro.29.051605.112958
Dong, M., Yeh, F., Tepp, W. H., Dean, C., Johnson, E. A., Janz, R., et al. (2006). SV2 is the protein receptor for botulinum neurotoxin a. Science 312, 592–596. doi: 10.1126/science.1123654
Elafros, M. A., Andersen, H., Bennett, D. L., Savelieff, M. G., Viswanathan, V., Callaghan, B. C., et al. (2022). Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 21, 922–936. doi: 10.1016/S1474-4422(22)00188-0
Ertilav, K., Nazıroğlu, M., Ataizi, Z. S., and Yıldızhan, K. (2021). Melatonin and selenium suppress docetaxel-induced TRPV1 activation, neuropathic pain and oxidative neurotoxicity in mice. Biol. Trace Elem. Res. 199, 1469–1487. doi: 10.1007/s12011-020-02250-4
Fenwick, A. J., Fowler, D. K., Wu, S. W., Shaffer, F. J., Lindberg, J. E. M., Kinch, D. C., et al. (2017). Direct anandamide activation of TRPV1 produces divergent calcium and current responses. Front. Mol. Neurosci. 10:200. doi: 10.3389/fnmol.2017.00200
Fernandes, E. S., Fernandes, M. A., and Keeble, J. E. (2012). The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br. J. Pharmacol. 166, 510–521. doi: 10.1111/j.1476-5381.2012.01851.x
Ferreira, L. G. B., Faria, J. V., Dos Santos, J. P. S., et al. (2020). Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 887:173356. doi: 10.1016/j.ejphar.2020.173356
Finnerup, N. B., Kuner, R., and Jensen, T. S. (2021). Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 101, 259–301. doi: 10.1152/physrev.00045.2019
Fischer, M. J. M., Balasuriya, D., Jeggle, P., Goetze, T. A., McNaughton, P. A., Reeh, P. W., et al. (2014). Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch Eur J Physiol. 466, 2229–2241. doi: 10.1007/s00424-014-1497-z
Freynhagen, R., and Bennett, M. I. (2009). Diagnosis and management of neuropathic pain. BMJ 339:b3002. doi: 10.1136/bmj.b3002
Gao, W., Zan, Y., Wang, Z. J., Hu, X. Y., and Huang, F. (2016). Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol. Sin. 37, 1166–1177. doi: 10.1038/aps.2016.58
Gilron, I., Baron, R., and Jensen, T. (2015). Neuropathic pain: principles of diagnosis and treatment. Mayo Clin. Proc. 90, 532–545. doi: 10.1016/j.mayocp.2015.01.018
Gim, G. T., Lee, J. H., Park, E., Sung, Y. H., Kim, C. J., Hwang, W. W., et al. (2011). Electroacupuncture attenuates mechanical and warm allodynia through suppression of spinal glial activation in a rat model of neuropathic pain. Brain Res. Bull. 86, 403–411. doi: 10.1016/j.brainresbull.2011.09.010
Go, E. J., Ji, J., Kim, Y. H., Berta, T., and Park, C. K. (2021). Transient receptor potential channels and botulinum neurotoxins in chronic pain. Front. Mol. Neurosci. 14:772719. doi: 10.3389/fnmol.2021.772719
Guo, S. H., Lin, J. P., Huang, L. E., Yang, Y., Chen, C. Q., Li, N. N., et al. (2019). Silencing of spinal Trpv1 attenuates neuropathic pain in rats by inhibiting CAMKII expression and ERK2 phosphorylation. Sci. Rep. 9:2769. doi: 10.1038/s41598-019-39184-4
Hains, B. C., and Waxman, S. G. (2006). Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 26, 4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006
Hara, T., Chiba, T., Abe, K., Makabe, A., Ikeno, S., Kawakami, K., et al. (2013). Effect of paclitaxel on transient receptor potential vanilloid 1 in rat dorsal root ganglion. Pain 154, 882–889. doi: 10.1016/j.pain.2013.02.023
Herbert, M. K., and Holzer, P. (2002). Die Neurogene Entzündung – I. Grundlegende Mechanismen, Physiologie und Pharmakologie – [neurogenic inflammation. I. Basic mechanisms, physiology and pharmacology]. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 37, 314–325. doi: 10.1055/s-2002-32233
Hiraga, S. I., Itokazu, T., Hoshiko, M., Takaya, H., Nishibe, M., and Yamashita, T. (2020). Microglial depletion under thalamic hemorrhage ameliorates mechanical allodynia and suppresses aberrant axonal sprouting. JCI Insight 5:e131801. doi: 10.1172/jci.insight.131801
Hohmann, S. W., Angioni, C., Tunaru, S., Lee, S., Woolf, C. J., Offermanns, S., et al. (2017). The G2A receptor (GPR132) contributes to oxaliplatin-induced mechanical pain hypersensitivity. Sci. Rep. 7:446. doi: 10.1038/s41598-017-00591-0
Hori, Y., Temma, T., Wooten, C., Sobowale, C., Chan, C., Swid, M., et al. (2021). Cardiac afferent signaling partially underlies premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 18, 1586–1595. doi: 10.1016/j.hrthm.2021.04.004
IASP . (1979). IASP taxonomy. Available at: https://www.iasp-pain.org/terminology?navItemNumber=576.2019 (Accessed November 26, 2023).
Iftinca, M., Defaye, M., and Altier, C. (2021). TRPV1-targeted drugs in development for human pain conditions. Drugs 81, 7–27. doi: 10.1007/s40265-020-01429-2
Infantino, R., Schiano, C., Luongo, L., Paino, S., Mansueto, G., Boccella, S., et al. (2022). MED1/BDNF/TrkB pathway is involved in thalamic hemorrhage-induced pain and depression by regulating microglia. Neurobiol. Dis. 164:105611. doi: 10.1016/j.nbd.2022.105611
Inoue, K., and Tsuda, M. (2018). Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 19, 138–152. doi: 10.1038/nrn.2018.2
Jaggi, A. S., Jain, V., and Singh, N. (2011). Animal models of neuropathic pain. Fundam. Clin. Pharmacol. 25, 1–28. doi: 10.1111/j.1472-8206.2009.00801.x
Javed, H., Johnson, A. M., Challagandla, A. K., Emerald, B. S., and Shehab, S. (2022). Cutaneous injection of Resiniferatoxin completely alleviates and prevents nerve-injury-induced neuropathic pain. Cells 11:4049. doi: 10.3390/cells11244049
Ji, R. R., Donnelly, C. R., and Nedergaard, M. (2019). Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 20, 667–685. doi: 10.1038/s41583-019-0218-1
Ji, J., Huh, Y., and Ji, R. R. (2023). Inflammatory mediators, nociceptors, and their interactions in pain[M]//Neuroimmune interactions in pain: mechanisms and therapeutics. Cham: Springer International Publishing, 87–119.
Ji, R. R., Kohno, T., Moore, K. A., and Woolf, C. J. (2003). Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705. doi: 10.1016/j.tins.2003.09.017
Ji, R. R., Nackley, A., Huh, Y., Terrando, N., and Maixner, W. (2018). Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129, 343–366. doi: 10.1097/ALN.0000000000002130
Ji, A., and Xu, J. (2021). Neuropathic pain: biomolecular intervention and imaging via targeting microglia activation. Biomol. Ther. 11:1343. doi: 10.3390/biom11091343
Jin, J., Guo, J., Cai, H., Zhao, C., Wang, H., Liu, Z., et al. (2020). M2-like microglia polarization attenuates neuropathic pain associated with Alzheimer's disease. J. Alzheimers Dis. 76, 1255–1265. doi: 10.3233/JAD-200099
Jin, X., and Yamashita, T. (2016). Microglia in central nervous system repair after injury. J. Biochem. 159, 491–496. doi: 10.1093/jb/mvw009
Jung, J., Shin, J. S., Lee, S. Y., Hwang, S. W., Koo, J., Cho, H., et al. (2004). Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 279, 7048–7054. doi: 10.1074/jbc.M311448200
Kan, H. W., Chang, C. H., Lin, C. L., Lee, Y. C., Hsieh, S. T., and Hsieh, Y. L. (2018). Downregulation of adenosine and adenosine A1 receptor contributes to neuropathic pain in resiniferatoxin neuropathy. Pain 159, 1580–1591. doi: 10.1097/j.pain.0000000000001246
Kasama, S., Kawakubo, M., Suzuki, T., Nishizawa, T., Ishida, A., and Nakayama, J. (2007). RNA interference-mediated knock-down of transient receptor potential vanilloid 1 prevents forepaw inflammatory hyperalgesia in rat. Eur. J. Neurosci. 25, 2956–2963. doi: 10.1111/j.1460-9568.2007.05584.x
Katz, B., Zaguri, R., Edvardson, S., Maayan, C., Elpeleg, O., Lev, S., et al. (2023). Nociception and pain in humans lacking a functional TRPV1 channel. J. Clin. Invest. 133:e153558. doi: 10.1172/JCI153558
Khan, A., Shal, B., Khan, A. U., Ullah, R., Baig, M. W., Ul Haq, I., et al. (2021). Suppression of TRPV1/TRPM8/P2Y nociceptors by Withametelin via downregulating MAPK Signaling in mouse model of vincristine-induced neuropathic pain. Int. J. Mol. Sci. 22:6084. doi: 10.3390/ijms22116084
Kigerl, K. A., Gensel, J. C., Ankeny, D. P., Alexander, J. K., Donnelly, D. J., and Popovich, P. G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009
Kiguchi, N., Kobayashi, Y., Kadowaki, Y., Fukazawa, Y., Saika, F., and Kishioka, S. (2014). Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. J. Neurochem. 129, 169–178. doi: 10.1111/jnc.12614
Koltzenburg, M., Torebjörk, H. E., and Wahren, L. K. (1994). Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain 117, 579–591. doi: 10.1093/brain/117.3.579
Koplas, P. A., Rosenberg, R. L., and Oxford, G. S. (1997). The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 17, 3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997
Kuai, C. P., Ju, L. J., Hu, P. P., and Huang, F. (2020). Corydalis saxicola alkaloids attenuate cisplatin-induced neuropathic pain by reducing loss of IENF and blocking TRPV1 activation. Am. J. Chin. Med. 48, 407–428. doi: 10.1142/S0192415X20500214
Kwon, D. H., Zhang, F., Suo, Y., Bouvette, J., Borgnia, M. J., and Lee, S. Y. (2021). Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat. Struct. Mol. Biol. 28, 554–563. doi: 10.1038/s41594-021-00616-3
Lam, D., Momeni, Z., Theaker, M., Jagadeeshan, S., Yamamoto, Y., Ianowski, J. P., et al. (2018). RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS One 13:e0193312. doi: 10.1371/journal.pone.0193312
Langley, P. C., Van Litsenburg, C., Cappelleri, J. C., and Carroll, D. (2013). The burden associated with neuropathic pain in Western Europe. J. Med. Econ. 16, 85–95. doi: 10.3111/13696998.2012.729548
Lee, J. H., Ji, H., Ko, S. G., and Kim, W. (2021). JI017 attenuates Oxaliplatin-induced cold allodynia via spinal TRPV1 and astrocytes inhibition in mice. Int. J. Mol. Sci. 22:8811. doi: 10.3390/ijms22168811
Li, Y., Adamek, P., Zhang, H., Tatsui, C. E., Rhines, L. D., Mrozkova, P., et al. (2015). The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J. Neurosci. 35, 13487–13500. doi: 10.1523/JNEUROSCI.1956-15.2015
Li, Y. R., and Gupta, P. (2019). Immune aspects of the bi-directional neuroimmune facilitator TRPV1. Mol. Biol. Rep. 46, 1499–1510. doi: 10.1007/s11033-018-4560-6
Li, Y., Yin, C., Li, X., Liu, B., Wang, J., Zheng, X., et al. (2019). Electroacupuncture alleviates paclitaxel-induced peripheral neuropathic pain in rats via suppressing TLR4 Signaling and TRPV1 upregulation in sensory neurons. Int. J. Mol. Sci. 20:5917. doi: 10.3390/ijms20235917
Li, W. W., Zhao, Y., Liu, H. C., Liu, J., Chan, S. O., Zhong, Y. F., et al. (2024). Roles of thermosensitive transient receptor channels TRPV1 and TRPM8 in paclitaxel-induced peripheral neuropathic pain. Int. J. Mol. Sci. 25:5813. doi: 10.3390/ijms25115813
Li, R., Zhao, C., Yao, M., Song, Y., Wu, Y., and Wen, A. (2017). Analgesic effect of coumarins from Radix angelicae pubescentis is mediated by inflammatory factors and TRPV1 in a spared nerve injury model of neuropathic pain. J. Ethnopharmacol. 195, 81–88. doi: 10.1016/j.jep.2016.11.046
Liao, M., Cao, E., Julius, D., and Cheng, Y. (2013). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. doi: 10.1038/nature12822
Lukacs, V., Thyagarajan, B., Varnai, P., Balla, A., Balla, T., and Rohacs, T. (2007). Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 27, 7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007
Lukacs, V., Yudin, Y., Hammond, G. R., Sharma, E., Fukami, K., and Rohacs, T. (2013). Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J. Neurosci. 33, 11451–11463. doi: 10.1523/JNEUROSCI.5637-12.2013
Luo, J., Bavencoffe, A., Yang, P., Feng, J., Yin, S., Qian, A., et al. (2018). Zinc inhibits TRPV1 to alleviate chemotherapy-induced neuropathic pain. J. Neurosci. 38, 474–483. doi: 10.1523/JNEUROSCI.1816-17.2017
Luo, X., Chen, O., Wang, Z., Bang, S., Ji, J., Lee, S. H., et al. (2021). IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 109, 2691–2706.e5. doi: 10.1016/j.neuron.2021.06.015
Martinez, V., Attal, N., Vanzo, B., Vicaut, E., Gautier, J. M., Bouhassira, D., et al. (2014). Adherence of French GPs to chronic neuropathic pain clinical guidelines: results of a cross-sectional, randomized, "e" case-vignette survey. PLoS One 9:e93855. doi: 10.1371/journal.pone.0093855
Marwaha, L., Bansal, Y., Singh, R., Saroj, P., Bhandari, R., and Kuhad, A. (2016). TRP channels: potential drug target for neuropathic pain. Inflammopharmacology 24, 305–317. doi: 10.1007/s10787-016-0288-x
McDowell, T. S., Wang, Z. Y., Singh, R., and Bjorling, D. (2013). CB1 cannabinoid receptor agonist prevents NGF-induced sensitization of TRPV1 in sensory neurons. Neurosci. Lett. 551, 34–38. doi: 10.1016/j.neulet.2013.06.066
Mihara, S., and Shibamoto, T. (2015). The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin. Immunol. 11:11. doi: 10.1186/s13223-015-0074-0
Mika, J., Zychowska, M., Popiolek-Barczyk, K., Rojewska, E., and Przewlocka, B. (2013). Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 716, 106–119. doi: 10.1016/j.ejphar.2013.01.072
Mohammadi-Farani, A., Ghazi-Khansari, M., and Sahebgharani, M. (2014). Glucose concentration in culture medium affects mRNA expression of TRPV1 and CB1 receptors and changes capsaicin toxicity in PC12 cells. Iran. J. Basic Med. Sci. 17, 673–378.
Mohapatra, D. P., and Nau, C. (2005). Regulation of Ca2+−dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 280, 13424–13432. doi: 10.1074/jbc.M410917200
Moiseenkova-Bell, V. Y., Stanciu, L. A., Serysheva, I. I., Tobe, B. J., and Wensel, T. G. (2008). Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 105, 7451–7455. doi: 10.1073/pnas.0711835105
Moisset, X., Bouhassira, D., Avez Couturier, J., Alchaar, H., Conradi, S., Delmotte, M. H., et al. (2020). Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev. Neurol. 176, 325–352. doi: 10.1016/j.neurol.2020.01.361
Moran, M. M. (2018). TRP channels as potential drug targets. Annu. Rev. Pharmacol. Toxicol. 58, 309–330. doi: 10.1146/annurev-pharmtox-010617-052832
Nichols, M. L., Allen, B. J., Rogers, S. D., Ghilardi, J. R., Honore, P., Luger, N. M., et al. (1999). Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science 286, 1558–1561. doi: 10.1126/science.286.5444.1558
Numazaki, M., Tominaga, T., Toyooka, H., and Tominaga, M. (2002). Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J. Biol. Chem. 277, 13375–13378. doi: 10.1074/jbc.C200104200
Oh, S. J., Lim, J. Y., Son, M. K., Ahn, J. H., Song, K. H., Lee, H. J., et al. (2023). TRPV1 inhibition overcomes cisplatin resistance by blocking autophagy-mediated hyperactivation of EGFR signaling pathway. Nat. Commun. 14:2691. doi: 10.1038/s41467-023-38318-7
Old, E. A., Clark, A. K., and Malcangio, M. (2015). The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb. Exp. Pharmacol. 227, 145–170. doi: 10.1007/978-3-662-46450-2_8
Ortíz-Rentería, M., Juárez-Contreras, R., González-Ramírez, R., et al. (2018). TRPV1 channels and the progesterone receptor sig-1R interact to regulate pain. Proc. Natl. Acad. Sci. 115, E1657–E1666. doi: 10.1073/pnas.1715972115
Pan, H. L., Khan, G. M., Alloway, K. D., and Chen, S. R. (2003). Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J. Neurosci. 23, 2911–2919. doi: 10.1523/JNEUROSCI.23-07-02911.2003
Petitjean, H., Pawlowski, S. A., Fraine, S. L., Sharif, B., Hamad, D., Fatima, T., et al. (2015). Dorsal horn Parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep. 13, 1246–1257. doi: 10.1016/j.celrep.2015.09.080
Petroianu, G. A., Aloum, L., and Adem, A. (2023). Neuropathic pain: mechanisms and therapeutic strategies. Front. Cell Dev. Biol. 11:1072629. doi: 10.3389/fcell.2023.1072629
Potenzieri, A., Riva, B., Rigolio, R., Chiorazzi, A., Pozzi, E., Ballarini, E., et al. (2020). Oxaliplatin-induced neuropathy occurs through impairment of haemoglobin proton buffering and is reversed by carbonic anhydrase inhibitors. Pain 161, 405–415. doi: 10.1097/j.pain.0000000000001722
Premkumar, L. S., and Ahern, G. P. (2000). Induction of vanilloid receptor channel activity by protein kinase C. Nature 408, 985–990. doi: 10.1038/35050121
Ramal-Sanchez, M., Bernabò, N., Valbonetti, L., Cimini, C., Taraschi, A., Capacchietti, G., et al. (2021). Role and modulation of TRPV1 in mammalian spermatozoa: an updated review. Int. J. Mol. Sci. 22:4306. doi: 10.3390/ijms22094306
Rathee, P. K., Distler, C., Obreja, O., Neuhuber, W., Wang, G. K., Wang, S. Y., et al. (2002). PKA/AKAP/VR-1 module: a common link of Gs-mediated signaling to thermal hyperalgesia. J. Neurosci. 22, 4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002
Rimola, V., Hahnefeld, L., Zhao, J., Jiang, C., Angioni, C., Schreiber, Y., et al. (2020). Lysophospholipids contribute to Oxaliplatin-induced acute peripheral pain. J. Neurosci. 40, 9519–9532. doi: 10.1523/JNEUROSCI.1223-20.2020
Roa-Coria, J. E., Pineda-Farias, J. B., Barragán-Iglesias, P., Quiñonez-Bastidas, G. N., Zúñiga-Romero, Á., Huerta-Cruz, J. C., et al. (2019). Possible involvement of peripheral TRP channels in the hydrogen sulfide-induced hyperalgesia in diabetic rats. BMC Neurosci. 20:1. doi: 10.1186/s12868-018-0483-3
Rosner, J., de Andrade, D. C., Davis, K. D., Gustin, S. M., Kramer, J. L. K., Seal, R. P., et al. (2023). Central neuropathic pain. Nat. Rev. Dis. Primers 9:73. doi: 10.1038/s41572-023-00484-9
Samanta, A., Hughes, T. E. T., and Moiseenkova-Bell, V. Y. (2018). Transient receptor potential (TRP) channels. Subcell. Biochem. 87, 141–165. doi: 10.1007/978-981-10-7757-9_6
Sanz-Salvador, L., Andrés-Borderia, A., Ferrer-Montiel, A., and Planells-Cases, R. (2012). Agonist-and Ca2+−dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J. Biol. Chem. 287, 19462–19471. doi: 10.1074/jbc.M111.289751
Scholz, J., Finnerup, N. B., Attal, N., Aziz, Q., Baron, R., Bennett, M. I., et al. (2019). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160, 53–59. doi: 10.1097/j.pain.0000000000001365
Schumacher, M. A. (2010). Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 10, 185–200. doi: 10.1111/j.1533-2500.2010.00358.x
Shields, S. D., Cavanaugh, D. J., Lee, H., Anderson, D. J., and Basbaum, A. I. (2010). Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors. Pain 151, 422–429. doi: 10.1016/j.pain.2010.08.001
Shim, H. S., Bae, C., Wang, J., Lee, K. H., Hankerd, K. M., Kim, H. K., et al. (2019). Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain 15:1744806919840098. doi: 10.1177/1744806919840098
Shimizu, T., Shibata, M., Toriumi, H., Iwashita, T., Funakubo, M., Sato, H., et al. (2012). Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-a. Neurobiol. Dis. 48, 367–378. doi: 10.1016/j.nbd.2012.07.010
Sinharoy, P., Zhang, H., Sinha, S., Prudner, B. C., Bratz, I. N., and Damron, D. S. (2015). Propofol restores TRPV1 sensitivity via a TRPA1-, nitric oxide synthase-dependent activation of PKCε. Pharmacol. Res. Perspect. 3:e00153. doi: 10.1002/prp2.153
Son, D. B., Choi, W., Kim, M., Go, E. J., Jeong, D., Park, C. K., et al. (2021). Decursin alleviates mechanical allodynia in a paclitaxel-induced neuropathic pain mouse model. Cells 10:547. doi: 10.3390/cells10030547
Sondermann, J. R. (2019). Identification and characterization of protein complexes involved in different pain states in vertebrates, Ph.D. thesis Max Planck Institute of the Georg-August University School of Science
Song, G. J., and Suk, K. (2017). Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front. Aging Neurosci. 9:139. doi: 10.3389/fnagi.2017.00139
Spahn, V., Stein, C., and Zöllner, C. (2014). Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol. Pharmacol. 85, 335–344. doi: 10.1124/mol.113.088997
Starowicz, K., Maione, S., Cristino, L., Palazzo, E., Marabese, I., Rossi, F., et al. (2007). Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J. Neurosci. 27, 13739–13749. doi: 10.1523/JNEUROSCI.3258-07.2007
Starowicz, K., Makuch, W., Korostynski, M., Malek, N., Slezak, M., Zychowska, M., et al. (2013). Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS One 8:e60040. doi: 10.1371/journal.pone.0060040
Starowicz, K., and Przewlocka, B. (2012). Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 367, 3286–3299. doi: 10.1098/rstb.2011.0392
Story, G. M., Peier, A. M., Reeve, A. J., Eid, S. R., Mosbacher, J., Hricik, T. R., et al. (2003). ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. doi: 10.1016/s0092-8674(03)00158-2
Studer, M., and McNaughton, P. A. (2010). Modulation of single-channel properties of TRPV1 by phosphorylation. J. Physiol. 588, 3743–3756. doi: 10.1113/jphysiol.2010.190611
Sun, L., Li, H., Tai, L. W., Gu, P., and Cheung, C. W. (2018). Adiponectin regulates thermal nociception in a mouse model of neuropathic pain. Br. J. Anaesth. 120, 1356–1367. doi: 10.1016/j.bja.2018.01.016
Szelenyi, Z., Hummel, Z., Szolcsanyi, J., and Davis, J. B. (2004). Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur. J. Neurosci. 19, 1421–1424. doi: 10.1111/j.1460-9568.2004.03221.x
Szolcsányi, J. (1993). “Actions of capsaicin on sensory receptors” in Capsaicin in the study of pain. ed. J. N. Wood (London, UK: Academic Press), 1–33.
Szolcsányi, J. (2005). “Hot peppers, pain and analgesics” in Turning up the heat on pain: TRPV1 receptors in pain and inflammation. eds. A. B. Malmberg and K. R. Bley (Switzerland: Birkhäuser Verlag Basel), 3–22.
Tang, Y., and Le, W. (2016). Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194. doi: 10.1007/s12035-014-9070-5
Thacker, M. A., Clark, A. K., Bishop, T., Grist, J., Yip, P. K., Moon, L. D., et al. (2009). CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur. J. Pain 13, 263–272. doi: 10.1016/j.ejpain.2008.04.017
Tian, Q., Hu, J., Xie, C., Mei, K., Pham, C., Mo, X., et al. (2019). Recovery from tachyphylaxis of TRPV1 coincides with recycling to the surface membrane. Proc. Natl. Acad. Sci. USA 116, 5170–5175. doi: 10.1073/pnas.1819635116
Tominaga, M., Caterina, M. J., Malmberg, A. B., Rosen, T. A., Gilbert, H., Skinner, K., et al. (1998). The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543. doi: 10.1016/s0896-6273(00)80564-4
Torrance, N., Smith, B. H., Bennett, M. I., and Lee, A. J. (2006). The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J. Pain 7, 281–289. doi: 10.1016/j.jpain.2005.11.008
Torsney, C., and MacDermott, A. B. (2006). Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J. Neurosci. 26, 1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006
Touska, F., Marsakova, L., Teisinger, J., and Vlachova, V. (2011). A “cute” desensitization of TRPV1. Curr. Pharm. Biotechnol. 12, 122–129. doi: 10.2174/138920111793937826
Truini, A., Haanpaa, M., Provitera, V., Biasiotta, A., Stancanelli, A., Caporaso, G., et al. (2015). Differential myelinated and unmyelinated sensory and autonomic skin nerve fiber involvement in patients with ophthalmic postherpetic neuralgia. Front. Neuroanat. 9:105. doi: 10.3389/fnana.2015.00105
Turnbull, A. (1850). Tincture of capsaicin as a remedy for chilblains and toothache, vol. 1: Dublin Free Press, 95–96.
Valdes, A. M., De Wilde, G., Doherty, S. A., Lories, R. J., Vaughn, F. L., Laslett, L. L., et al. (2011). The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann. Rheum. Dis. 70, 1556–1561. doi: 10.1136/ard.2010.148122
van Hecke, O., Austin, S. K., Khan, R. A., Smith, B. H., and Torrance, N. (2014). Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155, 654–662. doi: 10.1016/j.pain.2013.11.013
Vandevoorde, S., and Lambert, D. M. (2005). Focus on the three key enzymes hydrolysing endocannabinoids as new drug targets. Curr. Pharm. Des. 11, 2647–2668. doi: 10.2174/1381612054546914
Venkatachalam, K., Luo, J., and Montell, C. (2014). Evolutionarily conserved, multitasking TRP channels: lessons from worms and flies. Handb. Exp. Pharmacol. 223, 937–962. doi: 10.1007/978-3-319-05161-1_9
Vergne-Salle, P., and Bertin, P. (2021). Chronic pain and neuroinflammation. Joint Bone Spine 88:105222. doi: 10.1016/j.jbspin.2021.105222
Vetter, I., Wyse, B. D., Monteith, G. R., Roberts-Thomson, S. J., and Cabot, P. J. (2006). The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase a pathway. Mol. Pain 2:22. doi: 10.1186/1744-8069-2-22
Villalba-Riquelme, E., de la Torre-Martínez, R., Fernández-Carvajal, A., and Ferrer-Montiel, A. (2022). Paclitaxel in vitro reversibly sensitizes the excitability of IB4(−) and IB4(+) sensory neurons from male and female rats. Br. J. Pharmacol. 179, 3693–3710. doi: 10.1111/bph.15809
Vincent, A. M., Perrone, L., Sullivan, K. A., Backus, C., Sastry, A. M., Lastoskie, C., et al. (2007). Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 148, 548–558. doi: 10.1210/en.2006-0073
Wahlman, C., Doyle, T. M., Little, J. W., Luongo, L., Janes, K., Chen, Z., et al. (2018). Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain 159, 1025–1034. doi: 10.1097/j.pain.0000000000001177
Wang, S., Bian, C., Yang, J., Arora, V., Gao, Y., Wei, F., et al. (2020). Ablation of TRPV1+ afferent terminals by capsaicin mediates long-lasting analgesia for trigeminal neuropathic pain. eNeuro 7, ENEURO.0118–ENEU20.2020. doi: 10.1523/ENEURO.0118-20.2020
Wang, Y., Kedei, N., Wang, M., Wang, Q. J., Huppler, A. R., Toth, A., et al. (2004). Interaction between protein kinase Cmu and the vanilloid receptor type 1. J. Biol. Chem. 279, 53674–53682. doi: 10.1074/jbc.M410331200
Wang, Q., Li, H. Y., Ling, Z. M., Chen, G., and Wei, Z. Y. (2022). Inhibition of Schwann cell pannexin 1 attenuates neuropathic pain through the suppression of inflammatory responses. J. Neuroinflammation 19:244. doi: 10.1186/s12974-022-02603-x
Wang, Z., Ling, D., Wu, C., Han, J., and Zhao, Y. (2020). Baicalin prevents the up-regulation of TRPV1 in dorsal root ganglion and attenuates chronic neuropathic pain. Vet Med Sci. 6, 1034–1040. doi: 10.1002/vms3.318
Wang, A., Shi, X., Yu, R., Qiao, B., Yang, R., and Xu, C. (2021). The P2X7 receptor is involved in diabetic neuropathic pain hypersensitivity mediated by TRPV1 in the rat dorsal root ganglion. Front. Mol. Neurosci. 14:663649. doi: 10.3389/fnmol.2021.663649
Wang, S., Wang, S., Asgar, J., Joseph, J., Ro, J. Y., Wei, F., et al. (2017). Ca2+ and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J. Biol. Chem. 292, 8291–8303. doi: 10.1074/jbc.M117.778290
Wang, P., Yan, Z., Zhong, J., Chen, J., Ni, Y., Li, L., et al. (2012). Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 61, 2155–2165. doi: 10.2337/db11-1503
Weng, H. J., Patel, K. N., Jeske, N. A., Bierbower, S. M., Zou, W., Tiwari, V., et al. (2015). Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron 85, 833–846. doi: 10.1016/j.neuron.2014.12.065
Willis, W. D., and Congeshall, R. E. (1991). “Function organization of dorsal horn interneurons” in Sensory mechanisms of the spinal cord. Ed. W. D. Willis Jr. (NewYork: Plenum), 153–215.
Wu, Y., Chen, J., and Wang, R. (2019). Puerarin suppresses TRPV1, calcitonin gene-related peptide and substance P to prevent paclitaxel-induced peripheral neuropathic pain in rats. Neuroreport 30, 288–294. doi: 10.1097/WNR.0000000000001199
Wu, Z., Yang, Q., Crook, R. J., O'Neil, R. G., and Walters, E. T. (2013). TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain 154, 2130–2141. doi: 10.1016/j.pain.2013.06.040
Wu, C. H., Yuan, X. C., Gao, F., Li, H. P., Cao, J., Liu, Y. S., et al. (2016). Netrin-1 contributes to myelinated afferent Fiber sprouting and neuropathic pain. Mol. Neurobiol. 53, 5640–5651. doi: 10.1007/s12035-015-9482-x
Xing, F., Gu, H., Niu, Q., Fan, X., Wang, Z., Yuan, J., et al. (2019). MZF1 in the dorsal root ganglia contributes to the development and maintenance of neuropathic pain via regulation of TRPV1. Neural Plast. 2019, 2782417–2782413. doi: 10.1155/2019/2782417
Xu, Y., Jiang, Z., and Chen, X. (2022). Mechanisms underlying paclitaxel-induced neuropathic pain: channels, inflammation and immune regulations. Eur. J. Pharmacol. 933:175288. doi: 10.1016/j.ejphar.2022.175288
Xu, H., Tian, W., Fu, Y., Oyama, T. T., Anderson, S., and Cohen, D. M. (2007). Functional effects of nonsynonymous polymorphisms in the human TRPV1 gene. Am. J. Physiol. Renal Physiol. 293, F1865–F1876. doi: 10.1152/ajprenal.00347.2007
Xu, S., and Wang, Y. (2024). Transient receptor potential channels: multiple modulators of peripheral neuropathic pain in several rodent models. Neurochem. Res. 49, 872–886. doi: 10.1007/s11064-023-04087-4
Yagihashi, S., Mizukami, H., and Sugimoto, K. (2011). Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig. 2, 18–32. doi: 10.1111/j.2040-1124.2010.00070.x
Zajaczkowską, R., Kocot-Kępska, M., Leppert, W., Wrzosek, A., Mika, J., and Wordliczek, J. (2019). Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 20:1451. doi: 10.3390/ijms20061451
Zan, Y., Kuai, C. X., Qiu, Z. X., and Huang, F. (2017). Berberine ameliorates diabetic neuropathy: TRPV1 modulation by PKC pathway. Am. J. Chin. Med. 45, 1709–1723. doi: 10.1142/S0192415X17500926
Zhang, H., Cang, C. L., Kawasaki, Y., Liang, L. L., Zhang, Y. Q., Ji, R. R., et al. (2007). Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J. Neurosci. 27, 12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007
Zhang, G. F., Chen, S. R., Jin, D., Huang, Y., Chen, H., and Pan, H. L. (2021). α2δ-1 upregulation in primary sensory neurons promotes NMDA receptor-mediated glutamatergic input in Resiniferatoxin-induced neuropathy. J. Neurosci. 41, 5963–5978. doi: 10.1523/JNEUROSCI.0303-21.2021
Zhang, N., Wei, H., Wu, W., Lin, P., Chen, Y., Liu, Z., et al. (2019). Effect of ropivacaine on peripheral neuropathy in streptozocin diabetes-induced rats through TRPV1-CGRP pathway. Biosci. Rep. 39:BSR20190817. doi: 10.1042/BSR20190817
Zhang, L., Zhang, X., Liu, Y., Wang, S., and Jia, G. (2022). Vagus nerve stimulation promotes the M1-to-M2 transition via inhibition of TLR4/NF-κB in microglial to rescue the reperfusion injury. J. Stroke Cerebrovasc. Dis. 31:106596. doi: 10.1016/j.jstrokecerebrovasdis.2022.106596
Zhang, B. Y., Zhang, Y. L., Sun, Q., Zhang, P. A., Wang, X. X., Xu, G. Y., et al. (2020). Alpha-lipoic acid downregulates TRPV1 receptor via NF-κB and attenuates neuropathic pain in rats with diabetes. CNS Neurosci. Ther. 26, 762–772. doi: 10.1111/cns.13303
Zhao, L., Liu, S., Zhang, X., Yang, J., Mao, M., Zhang, S., et al. (2023). Satellite glial cell-secreted exosomes after in-vitro oxaliplatin treatment presents a pro-nociceptive effect for dorsal root ganglion neurons and induce mechanical hypersensitivity in naïve mice. Mol. Cell. Neurosci. 126:103881. doi: 10.1016/j.mcn.2023.103881
Zheng, J., and Trudeau, M. C. (2023). Textbook of ion channels volume II: properties, function, and pharmacology of the superfamilies. Boca Raton, FL: CRC Press. 369–386.
Zygmunt, P. M., Petersson, J., Andersson, D. A., Chuang, H., Sørgård, M., Di Marzo, V., et al. (1999). Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457. doi: 10.1038/22761
Glossary
Keywords: TRPV1, peripheral neuropathic pain, molecular mechanisms, sensitization, desensitization
Citation: Gao N, Li M, Wang W, Liu Z and Guo Y (2024) The dual role of TRPV1 in peripheral neuropathic pain: pain switches caused by its sensitization or desensitization. Front. Mol. Neurosci. 17:1400118. doi: 10.3389/fnmol.2024.1400118
Edited by:
Xin Zhang, Duke University, United StatesReviewed by:
Rosmara Infantino, University of Galway, IrelandAntonio Ferrer-Montiel, Miguel Hernández University of Elche, Spain
Copyright © 2024 Gao, Li, Wang, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Liu, ZG9jdG9ybGl1emhlbkAxMjYuY29tcw==; Yufeng Guo, aW5lZmlyZW5lZWRsZUdZRkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Ning Gao
Ning Gao Meng Li
Meng Li Weiming Wang
Weiming Wang Zhen Liu
Zhen Liu Yufeng Guo1*
Yufeng Guo1*