- 1Department of Health School, Shanghai Normal University Tianhua College, Shanghai, China
- 2Department of Sport Rehabilitation, Shanghai University of Sport, Shanghai, China
- 3Rehabilitation Medicine Center, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 4School of Rehabilitation Medicine, Wenzhou Medical University, Wenzhou, Zhejiang, China
- 5Department of Rehabilitation Medicine, Shanghai University of Medicine and Health Sciences Affiliated Zhoupu Hospital, Shanghai, China
Fibromyalgia syndrome (FMS) is a recurrent pain condition that can be challenging to treat. Transcranial direct current stimulation (tDCS) has become a promising non-invasive therapeutic option in alleviating FMS pain, but the mechanisms underlying its effectiveness are not yet fully understood. In this article, we discuss the most current research investigating the analgesic effects of tDCS on FMS and discuss the potential mechanisms. TDCS may exert its analgesic effects by influencing neuronal activity in the brain, altering cortical excitability, changing regional cerebral blood flow, modulating neurotransmission and neuroinflammation, and inducing neuroplasticity. Overall, evidence points to tDCS as a potentially safe and efficient pain relief choice for FMS by multiple underlying mechanisms. This article provides a thorough overview of our ongoing knowledge regarding the mechanisms underlying tDCS and emphasizes the possibility of further studies to improve the clinical utility of tDCS as a pain management tool.
1 Introduction
Fibromyalgia syndrome (FMS) is a chronic disorder characterized by widespread musculoskeletal pain and tenderness in at least 11 areas for over 3 months (Galvez-Sánchez and Reyes Del Paso, 2020). Persistent musculoskeletal pain was linked to worse physical and cognitive function, burdening individuals and society (Xu et al., 2023; Zheng et al., 2023). Along with musculoskeletal pain, people with FMS often report fatigue, dyscognition, stiffness, sleep disturbances, mood issues, and hypervigilance, further reducing quality of life (Arnold et al., 2019; Gyorfi et al., 2022). FMS affects 2–4% of people worldwide (Häuser and Fitzcharles, 2018), with 7% of women aged 50–80 affected (White and Robinson, 2015). Despite improvements in FMS therapy, managing pain remains difficult for healthcare providers. Brain stimulation has been shown to alleviate FMS in recent clinical trials (Hou et al., 2016), giving hope for people with FMS.
Transcranial direct current stimulation (tDCS), a typical non-invasive brain stimulation technique, is being considered as an FMS treatment (Teixeira et al., 2022). It can change the polarity-dependent excitability of the cerebral cortex by delivering a low electrical current to specific brain areas via two electrodes on the scalp (Vicario et al., 2020). Anodal tDCS usually depolarizes and excites the neuronal membrane potential, while cathodal tDCS does the opposite (Sehm et al., 2013; Ho et al., 2016). Compared to other brain stimulation methods, TDCS is non-invasive, inexpensive, and safe (Mosayebi Samani et al., 2019).
Current research on tDCS for FMS is promising, and it has been recommended by the European Chapter of the International Federation of Clinical Neurophysiology as a possible effective treatment for FMS (Level B) (Lefaucheur et al., 2017). Despite multiple clinic studies (Caumo et al., 2022; Ramasawmy et al., 2022) and systematic reviews (Lloyd et al., 2020; Teixeira et al., 2022) showing that tDCS reduces FMS pain, a specific research gap remains. Initial results, like pain alleviation, are the focus of these investigations. The complicated pathophysiological changes in FMS pain and the underlying mechanisms by which tDCS relieves FMS pain are unknown. Thus, a deeper understanding of the mechanism of tDCS in FMS is required to enhance its validity and repeatability (van Boekholdt et al., 2021). Moreover, tDCS treatment parameters in FMS vary greatly across different studies. Some studies (Matias et al., 2022; Ramasawmy et al., 2022) recommend stimulating the left primary motor cortex (M1), while others (Forogh et al., 2021; Caumo et al., 2022) propose the dorsolateral prefrontal cortex (DLPFC) as more efficacious. Key treatment parameters like stimulation duration, intensity, and frequency are also inconsistently described and used. Lack of consistency makes clinical use of tDCS for FMS difficult. Thus, our work aims to (1) improve understanding of FMS’s complex pathophysiological changes and the mechanisms by which tDCS reduces pain; and (2) evaluate the effects and treatment parameters of tDCS on FMS.
2 Effect of tDCS on pain for fibromyalgia
Many studies have aimed to enhance the impact of tDCS stimulation on pain symptoms in fibromyalgia patients. However, the complexity and heterogeneity across these studies prompted us to conduct a scoping review following established guidelines, including the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-SR) Statement, to provide a concise and efficient summary of the existing literature (Peters et al., 2015; Tricco et al., 2018). Eligibility criteria were developed using the SPIDER approach (Cooke et al., 2012).
2.1 Specify sample
The review included patients diagnosed with fibromyalgia by local rheumatology associations or other formal institutions. Most studies excluded individuals receiving additional medication to prevent a potential impact on trial results. To ensure study homogeneity, female subjects were predominantly included, given the higher prevalence of fibromyalgia in women. Additionally, certain studies specified a minimum 6-month duration of chronic pain among participants to investigate its effects on individuals with prolonged pain experiences.
2.2 Phenomenon of interest
Most studies aimed to explore the impact of tDCS stimulation on pain, disability, and quality of life in fibromyalgia patients. The primary brain regions stimulated were M1 and DLPFC, typically with an intensity of 1–2 mA and a duration of 20 min. Randomized controlled trials commonly employed sham tDCS as controls, while only one study compared the effects of repetitive transcranial magnetic stimulation (rTMS) and tDCS on pain and quality of life in fibromyalgia patients (Forogh et al., 2021).
2.3 Design of the study
Most of the studies were randomized controlled trials, with five studies using a double-blind approach (Riberto et al., 2011; Villamar et al., 2013; Khedr et al., 2017; Caumo et al., 2022; Ramasawmy et al., 2022), two studies using a cross-over design (Valle et al., 2009; Villamar et al., 2013), and two studies exploring the long-term efficacy of tDCS (Cummiford et al., 2016; Silva et al., 2017).
2.4 Evaluation
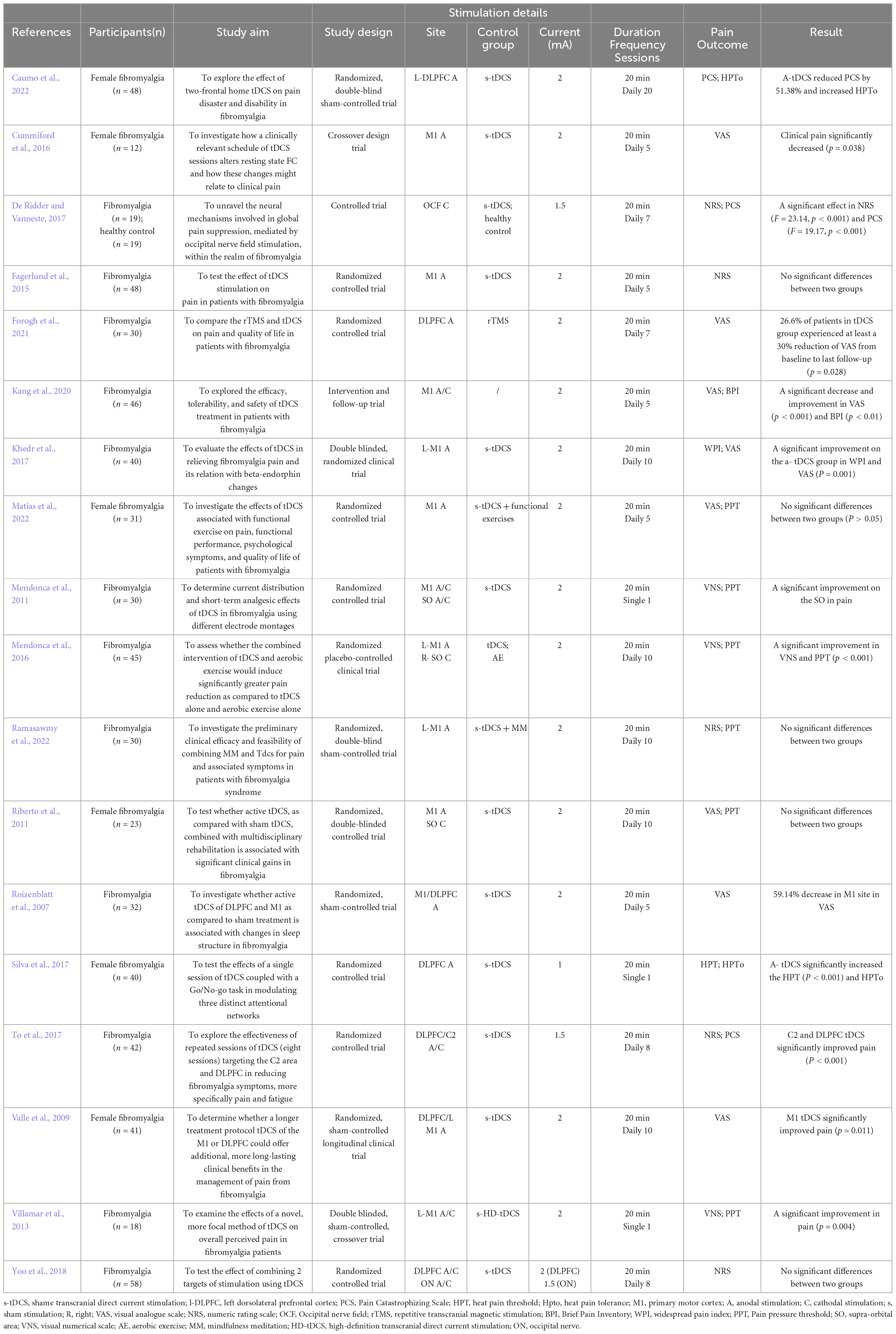
This study primarily investigated the impact of tDCS stimulation on pain relief in fibromyalgia patients, focusing on pain outcome measures. Visual Analogue Scale (VAS), Numeric Rating Scale (NRS), and Pain Pressure Threshold (PPT) were chosen as the primary pain indicators. Most studies observed that applying 2 mA anodic tDCS to the M1 and DLPFC regions effectively alleviated pain (Roizenblatt et al., 2007; Valle et al., 2009; Villamar et al., 2013; Cummiford et al., 2016; Khedr et al., 2017; Silva et al., 2017; Kang et al., 2020; Forogh et al., 2021; Caumo et al., 2022).
2.5 Research type
The majority of literature in this study adopts quantitative research methods, primarily comparing the impacts of genuine and sham tDCS stimulation on pain and other functions in patients. While some studies reported no significant changes in pain with sham tDCS, one interesting finding contradicted this trend, suggesting that sham tDCS could exhibit similar analgesic effects, possibly linked to the placebo analgesic effect (Caumo et al., 2022).
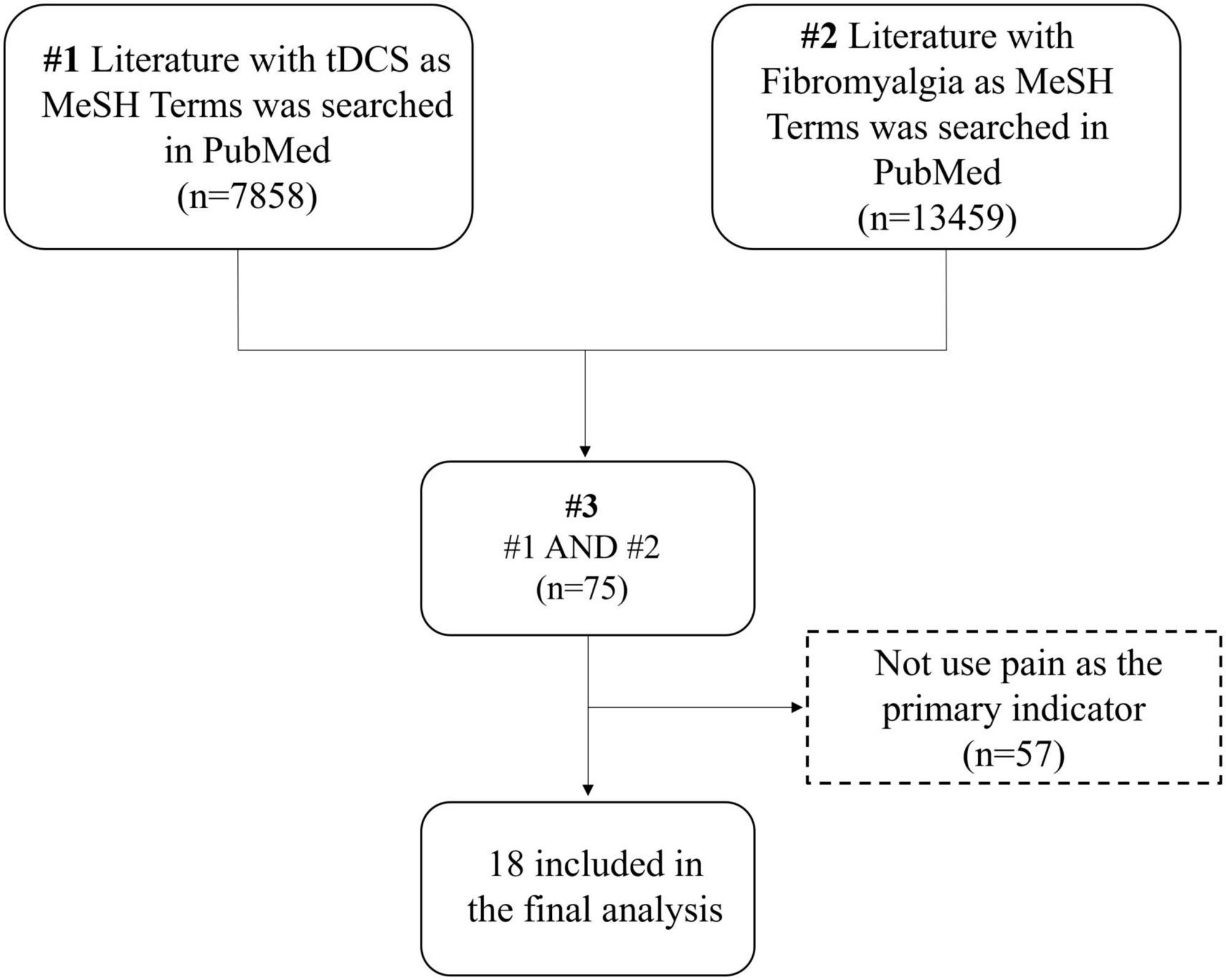
“Transcranial Direct Current Stimulation” and “Fibromyalgia” served as MeSH Terms. On November 7, 2022, 75 pertinent studies were retrieved from the PubMed database, underwent manual screening, and ultimately, 18 relevant studies were included (Figure 1). The detailed search strategy is available in the Supplementary material. Research indicates that 10 sessions of anodal tDCS in the M1 region can decrease pain levels in fibromyalgia patients. The development of this condition may be linked to alterations in serum endorphin levels (Khedr et al., 2017). Whether applied singly or periodically, tDCS mitigates pain perception, and stimulating the DLPFC region proves beneficial for relieving fatigue (To et al., 2017). A single 2 mA, 20-min session of tDCS stimulation in the M1 and Supra-orbital area (SO) can yield positive clinical effects (To et al., 2017). Moreover, limited research has addressed enhancing functional connectivity in pain-related brain regions through tDCS. Future studies should employ multiple imaging techniques to observe changes in the brain mechanisms of tDCS analgesia (Caumo et al., 2022). Table 1 provides details on tDCS stimulus parameters and the results of the included studies.
3 Mechanisms of tDCS for fibromyalgia syndrome
Despite the unknown pathophysiology of FMS, pain is connected to central sensitization (Rehm et al., 2021), a heightened sensitivity of the nervous system that overreacts to stimuli. This process is essential in FMS, causing widespread pain and other sensory-related symptoms. Abnormal brain neural networks and excitability, neuroinflammatory processes, neurotransmitter imbalances, abnormal cerebral blood flow, and disrupted neuroplasticity in the pain-processing region may be involved in this process and contribute to FMS pain (Gyorfi et al., 2022). Although tDCS has shown promise in reducing FMS pain, its specific mechanism is unknown, and no biomarkers are available to predict a patient’s response. The consensus is that tDCS depolarizes or hyperpolarizes neuronal membrane potential, affecting neural excitability (Lefaucheur and Wendling, 2019). This knowledge allows for further study of its mechanics. Multiple systems may be involved in how tDCS reduces FMS pain. Possible mechanisms are the promotion of cortical excitability recovery as well as effects on neuroinflammation, neurotransmission, regional cerebral blood flow (rCBF), and neuroplasticity. The mechanism of tDCS on FMS pain is depicted in Figure 2.
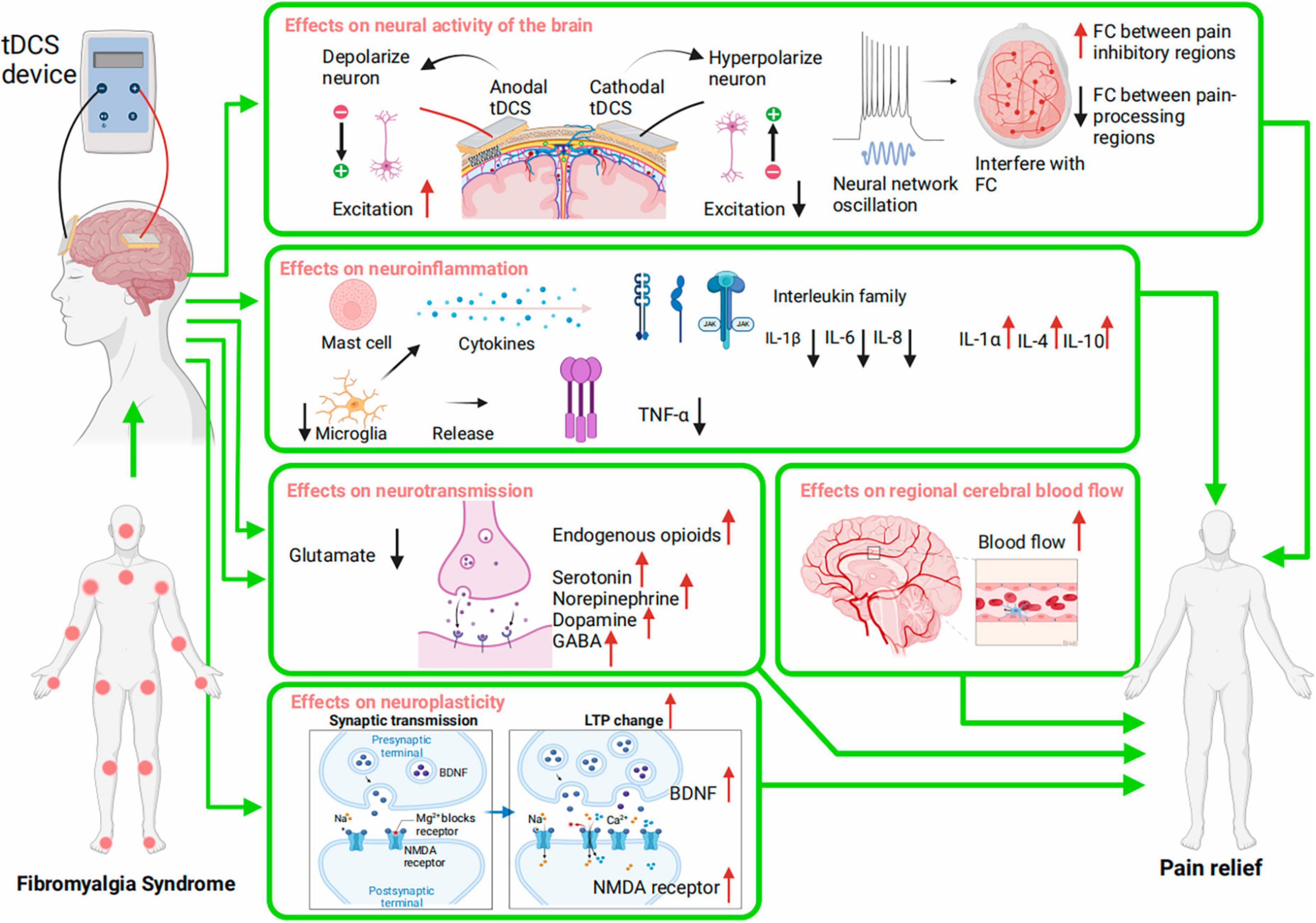
Figure 2. Potential Mechanisms of tDCS for pain in Fibromyalgia Syndrome (FMS). The mechanisms include regulation of neural activity; modulation of neuroinflammation; regulation of neurotransmission; regulation of regional cerebral blood flow; modulation of neuroplasticity. FC, function connectivity; TNF, tumor necrosis factor; IL, interleukin; GABA, gamma-aminobutyric acid; LTP, long-term potentiation; BDNF, brain-derived neurotrophic factor; NMDA receptors, N-methyl-D-aspartate receptors.
3.1 Effects on neural activities of the brain
With aberrant central nervous system excitability (Thabit et al., 2021), pain-processing brain regions in FMS sufferers are more hypersensitive to pressure stimuli than healthy people (Truini et al., 2015).
An abnormal mix of enhanced and reduced Functional Connectivity (FC) patterns across the pain matrix was found in individuals with FMS (Cifre et al., 2012), indicating the neural networks involved in pain perception and processing are functioning abnormally.
Anodal tDCS increases while cathodal decreases neural excitability in targeted areas (Pellicciari et al., 2013). A study (Auvichayapat et al., 2018) found that anodal tDCS on M1 increased neuronal activity in that area and decreased neuropathic SCI pain, implying that pain relief occurs by increasing M1 excitability, which is related to pain process (Zortea et al., 2019). Another study discovered that anodal tDCS targeting DLPFC increased DLPFC neural excitability, emotion, and pain relief in participants (Maeoka et al., 2012). Since the DLPFC regulates emotional pain perception, the change may affect how people perceive painful sensations. These synchronous changes suggest tDCS may be helpful to regulate abnormal pain processing and perception in FMS.
Patients with FMS displayed decreased FC between key pain-modulating regions (Jensen et al., 2012) and altered FC among pain process regions and sensorimotor areas (Flodin et al., 2014) relative to healthy individuals. Pain intensity correlates with FC sensory integration disturbances (Pujol et al., 2014). Polanía et al. (2012) found that anodal tDCS on M1 improved the FC between the left thalamus and ipsilateral M1 in healthy persons. Cummiford et al. (2016) found that anodal tDCS on the left M1 and cathodal tDCS on the right supraorbital cortex in FMS sufferers reduced FC between the left ventral posterolateral thalamus seed and left inferior parietal lobule, and FC between periaqueductal gray seed and posterior cingulate, followed by decreased pain. TDCS appears able to modulate disrupted FC in FMS, which may underlie its pain-relieving effects in these patients.
Moreover, tDCS can alter oscillatory activity of brain at a network level (Donaldson et al., 2019). These oscillations integrate and separate brain areas involved in sensory-painful perception and processing (Kim and Davis, 2021). Research found enhanced oscillations in the anterior cingulate and prefrontal cortex of FMS patients correlated with increased pain, fatigue, and stress during rest (Fallon et al., 2018). Another study demonstrated that a high-definition tDCS modulated oscillations and reduced FMS pain (Castillo-Saavedra et al., 2016). These alterations suggest that tDCS may reduce pain by modulating abnormal neural oscillations in FMS.
In all, tDCS may relieve FMS pain by modulating cortical excitability, FC, and neural oscillations.
3.2 Effects on neuroinflammation
Neuroinflammation refers to inflammatory processes within the central nervous system that are known to exacerbate pain sensations in FMS (Mendieta et al., 2016). An imbalance between pro- and anti-inflammatory cytokines in cerebrospinal fluid (CSF) is common in FMS. Studies showed increased pro-inflammatory chemokines/cytokines interleukin 1 (IL-1), IL-6, IL-8, and TNF-α, and decreased anti-inflammatory cytokines IL-4 and IL-10 in the CSF of FMS sufferers compared to healthy individuals (Ross et al., 2010; Mendieta et al., 2016). Moreover, microglia and mast cells (MCs) are engaged in FMS, activated to secrete more pro-inflammatory Cytokines (Theoharides et al., 2019). Pro-inflammatory cytokine dysregulation aggravates low-grade inflammation in CNS, activates or even sensitizes nociceptors, causes pain sensitization, and triggers hyperalgesia (Siracusa et al., 2021).
Transcranial direct current stimulation may reduce FMS pain by modulating neuroinflammation, possibly achieved by stimulating brain immune cells, such as MCs and glial cells, to regulate pro-inflammatory cytokines release. Research showed tDCS can reduce the activation of microglia (Walter et al., 2022), a type of essential glial cell in the neuroinflammatory process, thus decreasing the synthesis of TNF and other inflammatory mediators (Guo et al., 2020).
Animal models show that tDCS can change neuroinflammatory mediators. IL-1β (Lopes et al., 2020; Regner et al., 2020) and IL-6 (Guo et al., 2020) were reduced in the CNS structure of animals following tDCS stimulation, while IL-1α (Santos et al., 2020), IL-10 (Santos et al., 2020), and IL-4 (Lopes et al., 2019) were increased. Moreover, animals in these experiments showed analgesic response after tDCS stimulation, which provided a window into the pain relief caused by neuroinflammatory modulation. Human studies also confirmed the analgesic effect of tDCS caused by the regulation of neuroinflammation. A sham-controlled study found plasma IL-8 reduced significantly among bipolar disorder sufferers after using tDCS (Goerigk et al., 2021). In other studies, depressed individuals had a non-significant decrease in plasma IL-6 and TNF-α compared to the sham group after tDCS activation (Brunoni et al., 2014, 2018). These findings suggest tDCS may relieve FMS pain by modulating neuroinflammation through balancing pro- and anti-inflammatory cytokines. Further tDCS studies in FMS patients with a focus on cytokines are needed to confirm the consistency of the changed cytokine and analgesic response, verifying its ability to influence neuroinflammation for pain relief.
3.3 Effects on neurotransmission
Pain in FMS may be associated with an impairment of excitatory and inhibitory neurotransmission (Harris, 2010). Abnormal levels of neurotransmitters were found in the CSF and brain of FMS patients, such as glutamate and substance P, serotonin (5-HT), noradrenaline, dopamine, and gamma-aminobutyric acid (GABA) (Clauw et al., 2011). Changed neurotransmitter levels increased pro-nociceptive transmission and reduced anti-nociceptive transmission. Changed endogenous cerebral opioid activation is another anomaly in FMS (Schrepf et al., 2016).
Transcranial direct current stimulation shows promise for reducing FMS pain by regulating neurotransmitters implicated in its complex pathophysiology. Increased levels of glutamate (excitatory) and reduced levels of GABA (inhibitory) contribute to FMS hyperalgesia (Harris, 2010; Pomares et al., 2020). Studies (Zhao et al., 2020; Lengu et al., 2021) show that tDCS can modulate cortical levels of GABA and glutamate, impacting neuronal signaling. Bifrontal tDCS (anode over left DLPFC and cathode over right DLPFC with a current of 2 mA) increased dopamine in the ventral striatum in healthy participants (Fonteneau et al., 2018). Research found that tDCS with an anode on the left and a cathode on the right DLPFC in healthy subjects enhanced left striatal GABA, correlated with increased right striatal dopamine, and decreased GABA in the left DLPFC (Bunai et al., 2021). Additional research shows tDCS can also affect serotonin (Brunoni et al., 2013) and noradrenaline (Mishima et al., 2019) release. Changes in transmitters induced by tDCS may activate pain inhibitory pathways to cause pain relief in FMS.
Deficiencies in an endogenous pain management system may induce widespread pain in FMS (Schrepf et al., 2016). The intrinsic pain-regulating system modulates spinal cord pain signals via the descending brainstem-to-spinal cord pathway. This system appears to be strengthened by TDCS to reduce pain signaling and thus relieve pain (DosSantos et al., 2018). Research also linked pain relief to increased beta-endorphin levels (Chaudhry and Gossman, 2021). A review found that tDCS enhances dysfunctional neuronal circuitries involved in the pain-descending inhibitory system associated with opioids, thereby reducing chronic non-cancer-related pain (Zortea et al., 2019). DosSantos et al. (2012) discovered that tDCS over M1 boosted the endogenous-opioid release and the experimental cold pain threshold in a subject with trigeminal neuropathic pain. Another clinical trial (Khedr et al., 2017) indicated that left M1 tDCS reduced pain, improved mood, and boosted β-endorphin levels in FMS sufferers. To summarize, tDCS affects glutamate, serotonin, noradrenaline, dopamine, GABA, and endogenous brain opioids. These modulations may explain tDCS’ analgesic impact.
3.4 Effects on regional cerebral blood flow
People with FMS suffer abnormal rCBF and metabolism in pain-related regions, which may contribute to pain severity. Patients with FMS have lower CBF than controls in different brain regions, including the thalamus, caudate nucleus, pontine tegmentum, and basal ganglia (Kwiatek et al., 2000; Schmidt-Wilcke et al., 2007; Shokouhi et al., 2016). Given that these areas play a crucial role in processing and regulating pain, the reduction in CBF may be a major consideration in the heightened sensitivity to pain and chronic discomfort suffered by patients with FMS. Alterations in metabolism were also found in different regions of the brain in individuals with FMS (Guedj et al., 2008), which were related to how well the disorder would progress (Usui et al., 2017).
A study (Zheng et al., 2011) showed that anodal tDCS significantly raised rCBF (17.1%) during stimulation, which returned to baseline afterward, while cathodal tDCS caused a smaller rCBF increase in participants. La Rocca et al. (2022) found TDCS stimulation on M1 restored basic cortical hypometabolism in patients with FMS. Jales Junior et al. (2015) found that tDCS significantly increased rCBF in basal ganglia, and this alteration correlates with reduced pain in patients with FMS. These regions are critical to pain processing. Negative rCBF and cortical hypometabolism can affect neuronal function and pain processing. These studies collectively suggest that tDCS modulates rCBF and hypometabolism, which may normalize the dysfunctional neural circuits involved in pain perception, thereby reducing the pain experienced by FMS patients.
3.5 Effects on neuroplasticity
Transcranial direct current stimulation may reduce FMS pain by altering the brain’s pain response by inducing plasticity. Neuroplastic changes, including long-term potentiation (LTP) and long-term depression (LTD) (Kourosh-Arami et al., 2021), refer to the ability of the brain to reshape itself by generating new neural connections. Because of this adaptability, FMS causes an overactive brain pain processing system and generalized widespread pain (Gerra et al., 2021; Jayakar et al., 2021; Mezhov et al., 2021). Neuroplastic changes are associated with brain-derived neurotrophic factor (BDNF), which affects neuronal growth and synaptic connectivity. Research indicates that the BDNF levels in participants with FMS were lower than those in healthy controls (Iannuccelli et al., 2022).
Evidence shows that tDCS can cause cerebral excitability alterations that can persist longer than the stimulation period (Farnad et al., 2021; Santos et al., 2021), offering compelling insights into its potential impact on neuroplasticity. Further substantiating this view are animal experiments, which have demonstrated that tDCS enhanced LTP, reduced LTD, and increased BDNF concentration in some areas in the brain of rats (Kronberg et al., 2017; Yu et al., 2019). Another investigation suggested that tDCS can decrease BDNF levels and decrease pain in people with knee pain, and it supported an association between change in BDNF and change in clinical pain (Suchting et al., 2021).
Transcranial direct current stimulation can induce neuroplasticity in a manner dependent on N-methyl-D-aspartate receptors (NMDARs) (Liebetanz et al., 2002; Nitsche et al., 2003), which can regulate signaling pathways by allowing positively charged ions, such as calcium, to enter the cell and strengthen the synapse. Research also discovered that tDCS increased the amount of NMDA receptors and subsequently enhanced pain-related responses in animals (Li et al., 2022a,b). This suggests tDCS enhances NMDAR-mediated synaptic plasticity by increasing neuronal membrane NMDAR density, heightening synaptic responsiveness crucial to modulating pain.
Accordingly, tDCS can trigger long-term neuroplastic changes in the brain. These changes are crucial in FMS, as they can lead to a reorganization of the pain processing pathways in the brain. Alterations in plasticity-related pathways may be accomplished by inducing LTP and upregulating BDNF or NMDARs.
4 Conclusion
Overall, we found that tDCS may reduce FMS pain by altering neuronal activity, regulating neuroinflammation and neurotransmission, accelerating rCBF, and inducing neuroplasticity. Deeper exploration, such as molecular studies, is needed to fill the ongoing gaps between the complex pathophysiological factors underlying FMS pain and the specific molecular changes by which tDCS reduces FMS pain., thus optimizing the efficacy of tDCS in FMS pain management. M1 and DLPFC areas in FMS sufferers are typically stimulated with 1–2 mA of tDCS for 20 min. Research on tDCS in FMS often delivers inconsistent outcomes because of different treatment protocols. This variability challenges synthesizing evidence and limits research results to broader patient populations, which underscores the need for standardized protocols to increase the comparability and generalizability of tDCS results in FMS.
While our research highlights the potential of tDCS in FMS pain relief, we need to admit that our limitations for possible biased sampling cannot be ruled out without a robust systematic literature assessment. Further research utilizing rigorous quality evaluation approaches is needed to enhance confidence in synthesizing findings. Also, this work primarily addressed immediate outcomes like pain reduction rather than long-term efficacy and impact on other symptoms. Pain location and perception vary among FMS sufferers, and they may have multi-faceted impairments beyond pain alone. It is therefore imperative that future research employs longitudinal study designs to evaluate the sustained effects of tDCS on pain symptoms and the broader spectrum of FMS manifestations. Future studies should also focus on identifying biomarkers to predict individual responses to tDCS, enhancing the treatment’s efficacy and personalization.
Author contributions
X-QW: Conceptualization, Supervision, Writing—review and editing. SW: Methodology, Resources, Writing—original draft, Writing—review and editing. S-HD: Methodology, Resources, Writing—original draft, Writing—review and editing. J-YL: Conceptualization, Supervision, Formal analysis, Validation, Investigation, Resources, Visualization, Writing—review and editing
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81871844); Joint Research Project on Health and Family Planning of Pudong New Area Health Commission (PW2021D-07); and Shanghai University of Medicine and Health Sciences Clinical Research Center (22MC2022002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2024.1269636/full#supplementary-material
References
Arnold, L. M., Bennett, R. M., Crofford, L. J., Dean, L. E., Clauw, D. J., Goldenberg, D. L., et al. (2019). AAPT diagnostic criteria for fibromyalgia. J. Pain 20, 611–628. doi: 10.1016/j.jpain.2018.10.008
Auvichayapat, P., Keeratitanont, K., Janyachareon, T., and Auvichayapat, N. (2018). The effects of transcranial direct current stimulation on metabolite changes at the anterior cingulate cortex in neuropathic pain: a pilot study. J. Pain Res. 11, 2301–2309. doi: 10.2147/JPR.S172920
Brunoni, A. R., Kemp, A. H., Shiozawa, P., Cordeiro, Q., Valiengo, L. C., Goulart, A. C., et al. (2013). Impact of 5-HTTLPR and BDNF polymorphisms on response to sertraline versus transcranial direct current stimulation: implications for the serotonergic system. Eur. Neuropsychopharmacol. 23, 1530–1540. doi: 10.1016/j.euroneuro.2013.03.009
Brunoni, A. R., Machado-Vieira, R., Zarate, C. A., Valiengo, L., Vieira, E. L., Benseñor, I. M., et al. (2014). Cytokines plasma levels during antidepressant treatment with sertraline and transcranial direct current stimulation (tDCS): results from a factorial, randomized, controlled trial. Psychopharmacology 231, 1315–1323. doi: 10.1007/s00213-013-3322-3
Brunoni, A. R., Padberg, F., Vieira, E. L. M., Teixeira, A. L., Carvalho, A. F., Lotufo, P. A., et al. (2018). Plasma biomarkers in a placebo-controlled trial comparing tDCS and escitalopram efficacy in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 86, 211–217. doi: 10.1016/j.pnpbp.2018.06.003
Bunai, T., Hirosawa, T., Kikuchi, M., Fukai, M., Yokokura, M., Ito, S., et al. (2021). tDCS-induced modulation of GABA concentration and dopamine release in the human brain: a combination study of magnetic resonance spectroscopy and positron emission tomography. Brain Stimul. 14, 154–160. doi: 10.1016/j.brs.2020.12.010
Castillo-Saavedra, L., Gebodh, N., Bikson, M., Diaz-Cruz, C., Brandao, R., Coutinho, L., et al. (2016). Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J. Pain 17, 14–26. doi: 10.1016/j.jpain.2015.09.009
Caumo, W., Alves, R. L., Vicuña, P., Alves, C. F. D. S., Ramalho, L., Sanches, P. R. S., et al. (2022). Impact of bifrontal home-based transcranial direct current stimulation in pain catastrophizing and disability due to pain in fibromyalgia: a randomized, double-blind sham-controlled study. J. Pain 23, 641–656. doi: 10.1016/j.jpain.2021.11.002
Chaudhry, S. R., and Gossman, W. (2021). Biochemistry, endorphin. Treasure Island, FL: StatPearls Publishing.
Cifre, I., Sitges, C., Fraiman, D., Muñoz, M. Á, Balenzuela, P., González-Roldán, A., et al. (2012). Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom. Med. 74, 55–62. doi: 10.1097/PSY.0b013e3182408f04
Clauw, D. J., Arnold, L. M., McCarberg, B. H., and FibroCollaborative (2011). The science of fibromyalgia. Mayo Clin. Proc. 86, 907–911. doi: 10.4065/mcp.2011.0206
Cooke, A., Smith, D., and Booth, A. (2012). Beyond PICO: the Spider tool for qualitative evidence synthesis. Qual. Health Res. 22, 1435–1443. doi: 10.1177/1049732312452938
Cummiford, C. M., Nascimento, T. D., Foerster, B. R., Clauw, D. J., Zubieta, J. K., Harris, R. E., et al. (2016). Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res. Ther. 18:40. doi: 10.1186/s13075-016-0934-0
De Ridder, D., and Vanneste, S. (2017). Occipital nerve field transcranial direct current stimulation normalizes imbalance between pain detecting and pain inhibitory pathways in fibromyalgia. Neurotherapeutics 14, 484–501. doi: 10.1007/s13311-016-0493-8
Donaldson, P. H., Kirkovski, M., Yang, J. S., Bekkali, S., and Enticott, P. G. (2019). High-definition tDCS to the right temporoparietal junction modulates slow-wave resting state power and coherence in healthy adults. J. Neurophysiol. 122, 1735–1744. doi: 10.1152/jn.00338.2019
DosSantos, M. F., Love, T. M., Martikainen, I. K., Nascimento, T. D., Fregni, F., Cummiford, C., et al. (2012). Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front. Psychiatry 3:93. doi: 10.3389/fpsyt.2012.00093
DosSantos, M. F., Oliveira, A. T., Ferreira, N. R., Carvalho, A. C. P., and Rosado de Castro, P. H. (2018). The contribution of endogenous modulatory systems to TMS- and tDCS-induced analgesia: evidence from PET studies. Pain Res. Manag. 2018:2368386. doi: 10.1155/2018/2368386
Fagerlund, A. J., Hansen, O. A., and Aslaksen, P. M. (2015). Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain 156, 62–71. doi: 10.1016/j.pain.0000000000000006
Fallon, N., Chiu, Y., Nurmikko, T., and Stancak, A. (2018). Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. Eur. J. Pain 22, 49–57. doi: 10.1002/ejp.1076
Farnad, L., Ghasemian-Shirvan, E., Mosayebi-Samani, M., Kuo, M. F., and Nitsche, M. A. (2021). Exploring and optimizing the neuroplastic effects of anodal transcranial direct current stimulation over the primary motor cortex of older humans. Brain Stimul. 14, 622–634. doi: 10.1016/j.brs.2021.03.013
Flodin, P., Martinsen, S., Löfgren, M., Bileviciute-Ljungar, I., Kosek, E., and Fransson, P. (2014). Fibromyalgia is associated with decreased connectivity between pain-and sensorimotor brain areas. Brain Connect. 4, 587–594. doi: 10.1089/brain.2014.0274
Fonteneau, C., Redoute, J., Haesebaert, F., Le Bars, D., Costes, N., Suaud-Chagny, M. F., et al. (2018). Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb. Cortex 28, 2636–2646. doi: 10.1093/cercor/bhy093
Forogh, B., Haqiqatshenas, H., Ahadi, T., Ebadi, S., Alishahi, V., and Sajadi, S. (2021). Repetitive transcranial magnetic stimulation (rTMS) versus transcranial direct current stimulation (tDCS) in the management of patients with fibromyalgia: a randomized controlled trial. Neurophysiol. Clin. 51, 339–347. doi: 10.1016/j.neucli.2021.03.002
Galvez-Sánchez, C. M., and Reyes Del Paso, G. A. (2020). Diagnostic criteria for fibromyalgia: critical review and future perspectives. J. Clin. Med. 9:1219. doi: 10.3390/jcm9041219
Gerra, M. C., Carnevali, D., Ossola, P., González-Villar, A., Pedersen, I. S., Triñanes, Y., et al. (2021). DNA methylation changes in fibromyalgia suggest the role of the immune-inflammatory response and central sensitization. J. Clin. Med. 10:4992. doi: 10.3390/jcm10214992
Goerigk, S., Cretaz, E., Sampaio-Junior, B., Vieira, É. L. M., Gattaz, W., Klein, I., et al. (2021). Effects of tDCS on neuroplasticity and inflammatory biomarkers in bipolar depression: results from a sham-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 105:110119. doi: 10.1016/j.pnpbp.2020.110119
Guedj, E., Cammilleri, S., Niboyet, J., Dupont, P., Vidal, E., Dropinski, J. P., et al. (2008). Clinical correlate of brain SPECT perfusion abnormalities in fibromyalgia. J. Nucl. Med. 49, 1798–1803. doi: 10.2967/jnumed.108.053264
Guo, T., Fang, J., Tong, Z. Y., He, S., and Luo, Y. (2020). Transcranial direct current stimulation ameliorates cognitive impairment via modulating oxidative stress, inflammation, and autophagy in a rat model of vascular dementia. Front. Neurosci. 14:28. doi: 10.3389/fnins.2020.00028
Gyorfi, M., Rupp, A., and Abd-Elsayed, A. (2022). Fibromyalgia pathophysiology. Biomedicines 10:3070. doi: 10.3390/biomedicines10123070
Harris, R. E. (2010). Elevated excitatory neurotransmitter levels in the fibromyalgia brain. Arthritis Res. Ther. 12:141. doi: 10.1186/ar3136
Häuser, W., and Fitzcharles, M. A. (2018). Facts and myths pertaining to fibromyalgia. Dialogues Clin. Neurosci. 20, 53–62. doi: 10.31887/DCNS.2018.20.1/whauser
Ho, K. A., Taylor, J. L., Chew, T., Gálvez, V., Alonzo, A., Bai, S., et al. (2016). The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimul. 9, 1–7. doi: 10.1016/j.brs.2015.08.003
Hou, W. H., Wang, T. Y., and Kang, J. H. (2016). The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology 55, 1507–1517. doi: 10.1093/rheumatology/kew205
Iannuccelli, C., Lucchino, B., Gioia, C., Dolcini, G., Rabasco, J., Venditto, T., et al. (2022). Gender influence on clinical manifestations, depressive symptoms and brain-derived neurotrophic factor (BDNF) serum levels in patients affected by fibromyalgia. Clin. Rheumatol. 41, 2171–2178. doi: 10.1007/s10067-022-06133-y
Jales Junior, L. H., Costa, D. L., Jales Neto, L. H., Ribeiro, J. P. M., Freitas, W. J. Sd. N., and Teixeira, M. J. (2015). Transcranial direct current stimulation in fibromyalgia: effects on pain and quality of life evaluated clinically and by brain perfusion scintigraphy. Rev. Dor 16, 37–42. doi: 10.5935/1806-0013.20150008
Jayakar, S., Shim, J., Jo, S., Bean, B. P., Singeç, I., and Woolf, C. J. (2021). Developing nociceptor-selective treatments for acute and chronic pain. Sci. Transl. Med. 13:eabj9837. doi: 10.1126/scitranslmed.abj9837
Jensen, K. B., Loitoile, R., Kosek, E., Petzke, F., Carville, S., Fransson, P., et al. (2012). Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain 8:32. doi: 10.1186/1744-8069-8-32
Kang, J. H., Choi, S. E., Park, D. J., Xu, H., Lee, J. K., and Lee, S. S. (2020). Effects of add-on transcranial direct current stimulation on pain in Korean patients with fibromyalgia. Sci. Rep. 10:12114. doi: 10.1038/s41598-020-69131-7
Khedr, E. M., Omran, E. A. H., Ismail, N. M., El-Hammady, D. H., Goma, S. H., Kotb, H., et al. (2017). Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul. 10, 893–901. doi: 10.1016/j.brs.2017.06.006
Kim, J. A., and Davis, K. D. (2021). Neural oscillations: understanding a neural code of pain. Neuroscientist 27, 544–570. doi: 10.1177/1073858420958629
Kourosh-Arami, M., Hosseini, N., and Komaki, A. (2021). Brain is modulated by neuronal plasticity during postnatal development. J. Physiol. Sci. 71:34.
Kronberg, G., Bridi, M., Abel, T., Bikson, M., and Parra, L. C. (2017). Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. 10, 51–58. doi: 10.1016/j.brs.2016.10.001
Kwiatek, R., Barnden, L., Tedman, R., Jarrett, R., Chew, J., Rowe, C., et al. (2000). Regional cerebral blood flow in fibromyalgia: single-photon–emission computed tomography evidence of reduction in the pontine tegmentum and thalami. Arthritis Rheum. 43, 2823–2833.
La Rocca, M., Clemente, L., Gentile, E., Ricci, K., Delussi, M., and de Tommaso, M. (2022). Effect of single session of anodal M1 transcranial direct current stimulation—TDCS—On cortical hemodynamic activity: a pilot study in fibromyalgia. Brain Sci. 12:1569. doi: 10.3390/brainsci12111569
Lefaucheur, J. P., and Wendling, F. (2019). Mechanisms of action of tDCS: a brief and practical overview. Neurophysiol. Clin. 49, 269–275. doi: 10.1016/j.neucli.2019.07.013
Lefaucheur, J. P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92. doi: 10.1016/j.clinph.2016.10.087
Lengu, K., Ryan, S., Peltier, S. J., Tyszkowski, T., Kairys, A., Giordani, B., et al. (2021). Effects of high definition-transcranial direct current stimulation on local GABA and glutamate levels among older adults with and without mild cognitive impairment: an exploratory study. J. Alzheimers Dis. 84, 1091–1102. doi: 10.3233/JAD-201091
Li, X., Ye, Y., Wang, L., Zhou, W., Chu, X., and Li, T. (2022a). Botulinum toxin type A combined with transcranial direct current stimulation reverses the chronic pain induced by osteoarthritis in rats. Toxicon 212, 42–48. doi: 10.1016/j.toxicon.2022.04.005
Li, X., Zhou, W., Wang, L., Ye, Y., and Li, T. (2022b). Transcranial direct current stimulation alleviates the chronic pain of osteoarthritis by modulating NMDA receptors in midbrain periaqueductal gray in rats. J. Pain Res. 15, 203–214. doi: 10.2147/JPR.S333454
Liebetanz, D., Nitsche, M. A., Tergau, F., and Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125, 2238–2247. doi: 10.1093/brain/awf238
Lloyd, D. M., Wittkopf, P. G., Arendsen, L. J., and Jones, A. K. P. (2020). Is transcranial direct current stimulation (tDCS) effective for the treatment of pain in fibromyalgia? A systematic review and meta-analysis. J. Pain 21, 1085–1100. doi: 10.1016/j.jpain.2020.01.003
Lopes, B. C., Cioato, S. G., Medeiros, H., Souza, V., Oliveira, C. L., Medeiros, L. F., et al. (2019). “Forced-exercise and transcranial direct current stimulation (tDCS) provide antinociceptive effects and modulate inflammatory and neurotrophic parameters in the spinal cord in a chronic pain model: long-term effects,” in Proceedings of the XLII Reunião Anual da SBNeC, Campos do Jordão.
Lopes, B. C., Medeiros, L. F., Silva de Souza, V., Cioato, S. G., Medeiros, H. R., Regner, G. G., et al. (2020). Transcranial direct current stimulation combined with exercise modulates the inflammatory profile and hyperalgesic response in rats subjected to a neuropathic pain model: long-term effects. Brain Stimul. 13, 774–782. doi: 10.1016/j.brs.2020.02.025
Maeoka, H., Matsuo, A., Hiyamizu, M., Morioka, S., and Ando, H. (2012). Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci. Lett. 512, 12–16. doi: 10.1016/j.neulet.2012.01.037
Matias, M. G. L., Germano Maciel, D., França, I. M., Cerqueira, M. S., Silva, T. C. L. A., Okano, A. H., et al. (2022). Transcranial direct current stimulation associated with functional exercise program for treating fibromyalgia: a randomized controlled trial. Arch. Phys. Med. Rehabil. 103, 245–254. doi: 10.1016/j.apmr.2021.06.029
Mendieta, D., De la Cruz-Aguilera, D. L., Barrera-Villalpando, M. I., Becerril-Villanueva, E., Arreola, R., Hernández-Ferreira, E., et al. (2016). IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J. Neuroimmunol. 290, 22–25. doi: 10.1016/j.jneuroim.2015.11.011
Mendonca, M. E., Santana, M. B., Baptista, A. F., Datta, A., Bikson, M., Fregni, F., et al. (2011). Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J. Pain 12, 610–617. doi: 10.1016/j.jpain.2010.12.015
Mendonca, M. E., Simis, M., Grecco, L. C., Battistella, L. R., Baptista, A. F., and Fregni, F. (2016). Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front. Hum. Neurosci. 10:68. doi: 10.3389/fnhum.2016.00068
Mezhov, V., Guymer, E., and Littlejohn, G. (2021). Central sensitivity and fibromyalgia. Internal Med. J. 51, 1990–1998. doi: 10.1111/imj.15430
Mishima, T., Nagai, T., Yahagi, K., Akther, S., Oe, Y., Monai, H., et al. (2019). Transcranial direct current stimulation (tDCS) induces adrenergic receptor-dependent microglial morphological changes in mice. eNeuro 6:ENEURO.0204-19.2019. doi: 10.1523/ENEURO.0204-19.2019
Mosayebi Samani, M. M., Agboada, D., Jamil, A., Kuo, M. F., and Nitsche, M. A. (2019). Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 119, 350–361. doi: 10.1016/j.cortex.2019.04.016
Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., Lang, N., et al. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 553, 293–301. doi: 10.1113/jphysiol.2003.049916
Pellicciari, M. C., Brignani, D., and Miniussi, C. (2013). Excitability modulation of the motor system induced by transcranial direct current stimulation: a multimodal approach. Neuroimage 83, 569–580. doi: 10.1016/j.neuroimage.2013.06.076
Peters, M. D., Godfrey, C. M., Khalil, H., McInerney, P., Parker, D., and Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 13, 141–146. doi: 10.1097/XEB.0000000000000050
Polanía, R., Paulus, W., and Nitsche, M. A. (2012). Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 33, 2499–2508. doi: 10.1002/hbm.21380
Pomares, F. B., Roy, S., Funck, T., Feier, N. A., Thiel, A., Fitzcharles, M. A., et al. (2020). Upregulation of cortical GABAA receptor concentration in fibromyalgia. Pain 161, 74–82. doi: 10.1097/j.pain.0000000000001707
Pujol, J., Macià, D., Garcia-Fontanals, A., Blanco-Hinojo, L., López-Solà, M., Garcia-Blanco, S., et al. (2014). The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain 155, 1492–1503. doi: 10.1016/j.pain.2014.04.028
Ramasawmy, P., Khalid, S., Petzke, F., and Antal, A. (2022). Pain reduction in fibromyalgia syndrome through pairing transcranial direct current stimulation and mindfulness meditation: a randomized, double-blinded, sham-controlled pilot clinical trial. Front. Med. 9:908133. doi: 10.3389/fmed.2022.908133
Regner, G. G., Torres, I. L. S., de Oliveira, C., Pflüger, P., da Silva, L. S., Scarabelot, V. L., et al. (2020). Transcranial direct current stimulation (tDCS) affects neuroinflammation parameters and behavioral seizure activity in pentylenetetrazole-induced kindling in rats. Neurosci. Lett. 735:135162. doi: 10.1016/j.neulet.2020.135162
Rehm, S., Sachau, J., Hellriegel, J., Forstenpointner, J., Børsting Jacobsen, H., Harten, P., et al. (2021). Pain matters for central sensitization: sensory and psychological parameters in patients with fibromyalgia syndrome. Pain Rep. 6:e901. doi: 10.1097/PR9.0000000000000901
Riberto, M., Marcon Alfieri, F., Monteiro de Benedetto Pacheco, K., Dini Leite, V., Nemoto Kaihami, H., Fregni, F., et al. (2011). Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol. J. 5, 45–50. doi: 10.2174/1874312901105010045
Roizenblatt, S., Fregni, F., Gimenez, R., Wetzel, T., Rigonatti, S. P., Tufik, S., et al. (2007). Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled study. Pain Pract. 7, 297–306. doi: 10.1111/j.1533-2500.2007.00152.x
Ross, R. L., Jones, K. D., Bennett, R. M., Ward, R. L., Druker, B. J., and Wood, L. J. (2010). Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol. J. 3, 9–18. doi: 10.2174/1874226201003010009
Santos, D. S., Lopes, B. C., Medeiros, L. F., Assumpção, J. A. F., de Souza, A., Salvi, A. A., et al. (2020). Transcranial direct current stimulation (tDCS) induces analgesia in rats with neuropathic pain and alcohol abstinence. Neurochem. Res. 45, 2653–2663. doi: 10.1007/s11064-020-03116-w
Santos, D. S., Medeiros, L. F., Stein, D. J., De Macedo, I. C., Da Silva Rios, D. E., De Oliveira, C., et al. (2021). Bimodal transcranial direct current stimulation reduces alcohol consumption and induces long-term neurochemical changes in rats with neuropathic pain. Neurosci. Lett. 759:136014. doi: 10.1016/j.neulet.2021.136014
Schmidt-Wilcke, T., Luerding, R., Weigand, T., Jürgens, T., Schuierer, G., Leinisch, E., et al. (2007). Striatal grey matter increase in patients suffering from fibromyalgia–a voxel-based morphometry study. Pain 132(Suppl. 1), S109–S116. doi: 10.1016/j.pain.2007.05.010
Schrepf, A., Harper, D. E., Harte, S. E., Wang, H., Ichesco, E., Hampson, J. P., et al. (2016). Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain 157, 2217–2225. doi: 10.1097/j.pain.0000000000000633
Sehm, B., Kipping, J., Schäfer, A., Villringer, A., and Ragert, P. (2013). A comparison between uni- and bilateral tDCS effects on functional connectivity of the human motor cortex. Front. Hum. Neurosci. 7:183. doi: 10.3389/fnhum.2013.00183
Shokouhi, M., Davis, K. D., Moulin, D. E., Morley-Forster, P., Nielson, W. R., Bureau, Y., et al. (2016). Basal ganglia perfusion in fibromyalgia is related to pain disability and disease impact: an Arterial Spin Labeling Study. Clin. J. Pain 32, 495–505. doi: 10.1097/AJP.0000000000000295
Silva, A. F., Zortea, M., Carvalho, S., Leite, J., Torres, I. L., Fregni, F., et al. (2017). Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci. Rep. 7:135. doi: 10.1038/s41598-017-00185-w
Siracusa, R., Paola, R. D., Cuzzocrea, S., and Impellizzeri, D. (2021). Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int. J. Mol. Sci. 22:3891. doi: 10.3390/ijms22083891
Suchting, R., Teixeira, A. L., Ahn, B., Colpo, G. D., Park, J., and Ahn, H. (2021). Changes in brain-derived neurotrophic factor from active and sham transcranial direct current stimulation in older adults with knee osteoarthritis. Clin. J. Pain 37, 898–903. doi: 10.1097/AJP.0000000000000987
Teixeira, P. E., Pacheco-Barrios, K., Branco, L. C., de Melo, P. S., Marduy, A., Caumo, W., et al. (2022). The analgesic effect of transcranial direct current stimulation in fibromyalgia: a systematic review, meta-analysis, and meta-regression of potential influencers of clinical effect. Neuromodulation 26, 715–727.
Thabit, M. N., Ezat, A., Ismael, M. A., and Hadad, S. (2021). Altered spinal excitability in patients with primary fibromyalgia: a case-control study. J. Clin. Neurol. 17, 121–127. doi: 10.3988/jcn.2021.17.1.121
Theoharides, T. C., Tsilioni, I., and Bawazeer, M. (2019). Mast cells, neuroinflammation and pain in fibromyalgia syndrome. Front. Cell. Neurosci. 13:353. doi: 10.3389/fncel.2019.00353
To, W. T., James, E., Ost, J., Hart, J., De Ridder, D., and Vanneste, S. (2017). Differential effects of bifrontal and occipital nerve stimulation on pain and fatigue using transcranial direct current stimulation in fibromyalgia patients. J. Neural Transm. 124, 799–808. doi: 10.1007/s00702-017-1714-y
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Internal Med. 169, 467–473. doi: 10.7326/M18-0850
Truini, A., Gerardi, M. C., Di Stefano, G., La Cesa, S., Iannuccelli, C., Pepe, A., et al. (2015). Hyperexcitability in pain matrices in patients with fibromyalgia. Clin. Exp. Rheumatol. 33(1 Suppl. 88), S68–S72.
Usui, C., Soma, T., Hatta, K., Aratani, S., Fujita, H., Nishioka, K., et al. (2017). A study of brain metabolism in fibromyalgia by positron emission tomography. Prog. Neuropsychopharmacol. Biol. Psychiatry 75, 120–127. doi: 10.1016/j.pnpbp.2017.01.012
Valle, A., Roizenblatt, S., Botte, S., Zaghi, S., Riberto, M., Tufik, S., et al. (2009). Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J. Pain Manag. 2, 353–361.
van Boekholdt, L., Kerstens, S., Khatoun, A., Asamoah, B., and Mc Laughlin, M. (2021). tDCS peripheral nerve stimulation: a neglected mode of action? Mol. Psychiatry 26, 456–461. doi: 10.1038/s41380-020-00962-6
Vicario, C. M., Salehinejad, M. A., Avenanti, A., and Nitsche, M. A. (2020). “Transcranial direct current stimulation (tDCS) in anxiety disorders,” in Non invasive brain stimulation in psychiatry and clinical neurosciences, eds B. Dell’Osso and G. Di Lorenzo (Cham: Springer), 301–317.
Villamar, M. F., Wivatvongvana, P., Patumanond, J., Bikson, M., Truong, D. Q., Datta, A., et al. (2013). Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J. Pain 14, 371–383. doi: 10.1016/j.jpain.2012.12.007
Walter, H. L., Pikhovych, A., Endepols, H., Rotthues, S., Bärmann, J., Backes, H., et al. (2022). Transcranial-direct-current-stimulation accelerates motor recovery after cortical infarction in mice: the interplay of structural cellular responses and functional recovery. Neurorehabil. Neural Repair 36, 701–714. doi: 10.1177/15459683221124116
White, H. D., and Robinson, T. D. (2015). A novel use for testosterone to treat central sensitization of chronic pain in fibromyalgia patients. Int. Immunopharmacol. 27, 244–248. doi: 10.1016/j.intimp.2015.05.020
Xu, H. R., Zhang, Y. H., Ngo, T. L., Yang, Q. H., Du, S. H., and Wang, X. Q. (2023). Association between smoking and incident back pain: a prospective cohort study with 438 510 participants. J. Glob. Health 13:04152. doi: 10.7189/jogh.13.04152
Yoo, H. B., Ost, J., Joos, W., Van Havenbergh, T., De Ridder, D., and Vanneste, S. (2018). Adding prefrontal transcranial direct current stimulation before occipital nerve stimulation in fibromyalgia. Clin. J. Pain 34, 421–427. doi: 10.1097/AJP.0000000000000552
Yu, T. H., Wu, Y. J., Chien, M. E., and Hsu, K. S. (2019). Transcranial direct current stimulation induces hippocampal metaplasticity mediated by brain-derived neurotrophic factor. Neuropharmacology 144, 358–367. doi: 10.1016/j.neuropharm.2018.11.012
Zhao, X., Ding, J., Pan, H., Zhang, S., Pan, D., Yu, H., et al. (2020). Anodal and cathodal tDCS modulate neural activity and selectively affect GABA and glutamate syntheses in the visual cortex of cats. J. Physiol. 598, 3727–3745. doi: 10.1113/JP279340
Zheng, X., Alsop, D. C., and Schlaug, G. (2011). Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage 58, 26–33. doi: 10.1016/j.neuroimage.2011.06.018
Zheng, Y. N., Liu, H., Chen, P. J., and Wang, X. Q. (2023). Association of persistent musculoskeletal pain with dementia risk score in adults aged 45 years or older: the China health and retirement longitudinal study. Brain Behav. Immun. 116, 185–192. doi: 10.1016/j.bbi.2023.12.015
Zortea, M., Ramalho, L., Alves, R. L., Alves, C. F. D. S., Braulio, G., Torres, I. L. D. S., et al. (2019). Transcranial direct current stimulation to improve the dysfunction of descending pain modulatory system related to opioids in chronic non-cancer pain: an integrative review of neurobiology and meta-analysis. Front. Neurosci. 13:1218. doi: 10.3389/fnins.2019.01218
Keywords: chronic pain, mechanisms, brain modulation, tDCS, fibromyalgia syndrome
Citation: Wang S, Du S-H, Wang X-Q and Lu J-Y (2024) Mechanisms of transcranial direct current stimulation (tDCS) for pain in patients with fibromyalgia syndrome. Front. Mol. Neurosci. 17:1269636. doi: 10.3389/fnmol.2024.1269636
Received: 16 August 2023; Accepted: 10 January 2024;
Published: 30 January 2024.
Edited by:
Victor Ramírez-Amaya, Medical Research Institute Mercedes and Martín Ferreyra (INIMEC), ArgentinaCopyright © 2024 Wang, Du, Wang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Qiang Wang, d2FuZ3h1ZXFpYW5nQHdtdS5lZHUuY24=; Jun-Yan Lu, aHVhcnMwM0AxNjMuY29t
†These authors have contributed equally to this work
 Shan Wang1†
Shan Wang1† Xue-Qiang Wang
Xue-Qiang Wang