- 1Department of Sport Rehabilitation, Shanghai University of Sport, Shanghai, China
- 2Department of Rehabilitation Medicine, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, China
- 3Shanghai Shangti Orthopaedic Hospital, Department of Rehabilitation Medicine, Shanghai, China
Complex regional pain syndrome characterized by severe pain and dysfunction seriously affects patients’ quality of life. Exercise therapy is gaining attention because it can effectively relieve pain and improve physical function. Based on the previous studies, this article summarized the effectiveness and underlying mechanisms of exercise interventions for complex regional pain syndrome, and described the gradual multistage exercise program. Exercises suitable for patients with complex regional pain syndrome mainly include graded motor imagery, mirror therapy, progressive stress loading training, and progressive aerobic training. In general, exercise training for patients with complex regional pain syndrome not only alleviates pain but also improves physical function and positive mental status. The underlying mechanisms of exercise interventions for complex regional pain syndrome include the remodeling of abnormal central and peripheral nervous system, the regulation of vasodilation and adrenaline levels, the release of endogenous opioids, and the increased anti-inflammatory cytokines. This article provided a clear explanation and summary of the research on exercise for complex regional pain syndrome. In the future, more high-quality studies with sufficient sample sizes may provide more exercise regimens and better evidence of efficacy.
1. Introduction
Complex regional pain syndrome (CRPS) is a chronic pain condition characterized by autonomic and inflammatory features and usually affects the distal limb (Bruehl, 2015; Smart et al., 2016; Goebel et al., 2019). The pathogenesis of this disorder is not fully understood, but it is usually triggered by a limb injury, such as trauma or surgery with or without specific nerve injuries. CRPS may develop after major trauma, minor injury, or surgery, and progress from self-limited and mild symptoms to chronic disease (Urits et al., 2018). Female and individuals with upper extremity injuries or suffered from a high-energy trauma are at a higher risk of developing CRPS (de Mos et al., 2007; Petersen et al., 2018). Patients with CRPS usually suffer from skin temperature changes allodynia, hyperalgesia, oedema, and impaired motor function (Petersen et al., 2018). In many instances, the development of CRPS is debilitating and severely reducing patients’ life quality, placing an enormous burden on their families (van Velzen et al., 2014). Although some symptoms of CRPS may get better spontaneously, aggressive treatment should not be delayed because progressive deterioration of symptoms are related to poor prognosis (Bean et al., 2014; Urits et al., 2018). Appropriate management may hasten the recovery of CRPS (Bean et al., 2016). The common treatment of CRPS is symptomatic including physical therapies, occupational therapies, psychological therapies, anti-inflammatories, neuropathic pain medications, and interventional procedures (Urits et al., 2018; Harden et al., 2022). Exercise therapy is an effective and affordable component of physical therapy in the management of CRPS (Smidt et al., 2005; McCormick et al., 2015). Previous studies have reported that exercise can reduce pain and edema volume and improve overall function in daily life activities for patients with CRPS (Sherry et al., 1999; McCormick et al., 2015; Sezgin Ozcan et al., 2019; Harden et al., 2022). Exercise can also reduce negative mood and improve patients’ general well-being. However, the potential therapeutic mechanism of exercise intervention for CRPS is lacking. This review summarizes the effectiveness of exercise on CRPS and comprehensively discusses the underlying mechanisms behind it to help researchers better understand the progress in this area.
2. Effect of exercise on CRPS
For patients with CRPS, starting exercise rehabilitation early provides the best probability of a good outcome and minimizes distress according to the practical guidelines (Goebel et al., 2019; Harden et al., 2022). The guideline for the management of CRPS from the European Pain Federation Working Group in 2019 recommended that patients with CRPS take appropriate, generally gentle, and graded exercises as soon as possible in the presence of pain and avoid immobilization of the CRPS limb (Goebel et al., 2019). Another guideline published in 2022 recommended that the principle of functional restoration for CRPS is based on a gradual and steady advancement: from activation of premotor and primary motor cortices to very gentle active movements, to stress loading and aerobic training, then to movements that comprise more active load bearing, and finally to vocational rehabilitation, thereby preparing to resume patients’ daily life and work (Harden et al., 2022).
Graded motor imagery consists of limb laterality recognition, motor imagery, and consecutive mirror therapy, which is designed specifically for patients with longstanding CRPS to shorten the prognostic course of CRPS (Moseley, 2004). Mirror therapy is among the most effective treatments to improve functional impairment for patients with acute CRPS (Cacchio et al., 2009a). Moseley (2006) reported that 6 weeks of graded motor imagery training significantly improved pain severity and functional impairment in patients with CRPS, and the effect was maintained at 6 months of follow-up. Mirror therapy and graded motor imagery can significantly relieve pain and improve motor control by helping the patients focus on the affected extremity, increase perceived ownership of that extremity, reduce kinesiophobia, and correct the mismatch between the motor and sensory systems (Cacchio et al., 2009a; Mccabe, 2011). Gentle active movements like initiating from active range of motion are performed to manage edema and conduct preliminary desensitization (Stanton-Hicks et al., 1998). A case reported that a female patient with CRPS-type 1 had pain and edema relief and function improvement after 20 days of range of motion exercise (Oh et al., 2019). Stress loading and aerobic training are also recommended. Although stress loading may initially increase symptoms in the affected extremities, pain and swelling usually decrease after several days (Harden et al., 2022). Watson and Carlson (1987) reported that 3 years of stress loading training for 41 CRPS patients remarkably improved pain and dysfunction, enhanced muscle strength, and greatly return to daily activities. Pain exposure physical therapy involves a progressive loading exercise program and management of pain avoidance behavior. Two studies found that 4 weeks to 3 months of pain exposure physical therapy for patients with CRPS-type 1 substantially reduced pain and functional limitations (van de Meent et al., 2011; Barnhoorn et al., 2015). Aerobic training contributes to manage edema, optimize range of motion, and improve circulation. A single-blind randomized controlled trial concluded that 4 weeks of aerobic exercise significantly reduced the signs and symptoms of CRPS-type 1 compared with conventional therapy (Topcuoglu et al., 2015). In addition, aquatic therapy is especially valuable for patients with CRPS (Harden et al., 2022). The gentle compressive force provided by hydrostatic pressure gentle around the extremity may reduce the widespread edema, dampen sympathetic nerve activity, and finally relieve pain (Yamazaki et al., 2000; Hall et al., 2008; Harden et al., 2022). Aquatic therapy is also beneficial to reduce extremity weight loading, and buoyancy may facilitate early recovery of functional activities (Saquetto et al., 2019; Harden et al., 2022). Sezgin Ozcan et al. (2019) reported that patients with CRPS-type 1 achieved better improvements on neuropathic pain and edema volume after performing active range of motion exercises in the water compared with the conventional rehabilitation program. Details of studies on exercise interventions for CRPS are presented in Table 1.
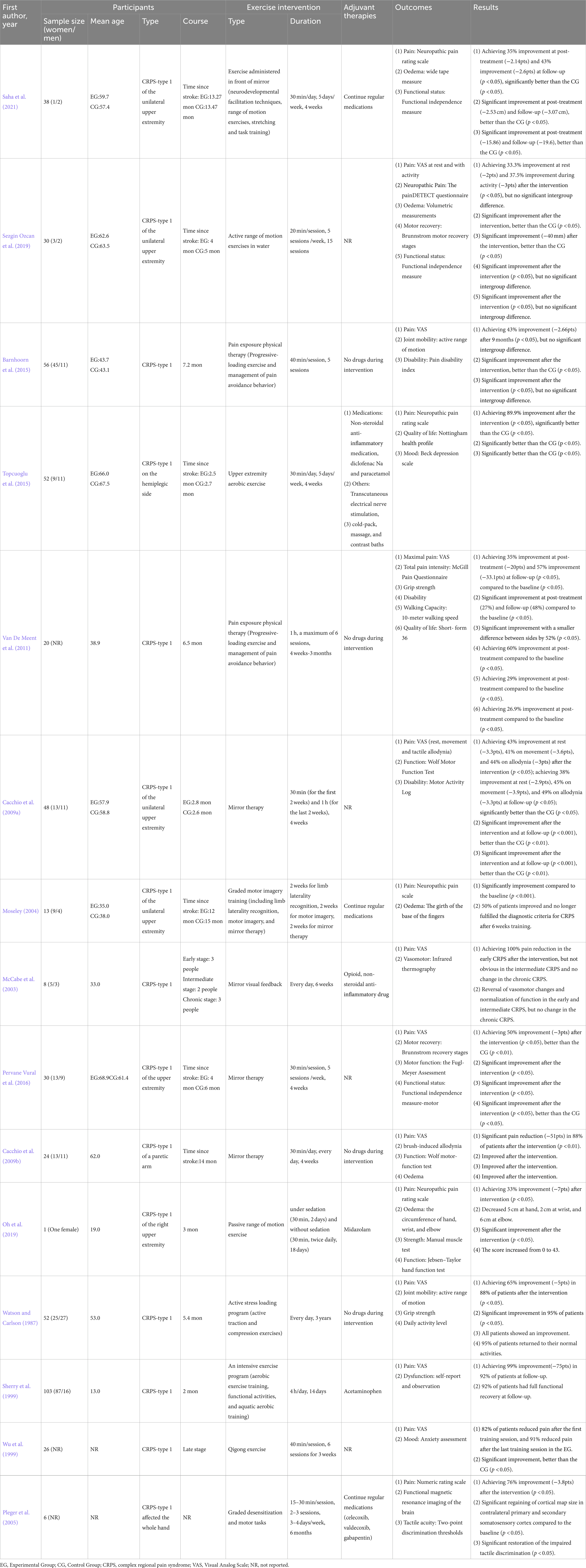
Table 1. General characteristics of clinical studies on exercise for complex regional pain syndrome.
3. Underlying therapeutic mechanisms of exercise on CRPS
Abnormal remodeling of the central nervous system is common in chronic pain, and CRPS is often secondary to damage of the nervous system, such as stroke (Baliki et al., 2011; Yang and Chang, 2019; Martins et al., 2022). When nervous system injury or abnormal nervous system function (central sensory signal amplification) occurs, the spinal cord and brain regions involved in pain processing can undergo great changes (Costigan et al., 2009; Coppieters et al., 2016). Patients with CRPS often present with extensive hypersensitivity to pain, decreased pain threshold, and increased duration of pain (Mendell, 2014; Birklein et al., 2015). Exercise can induce hypoalgesia (Rice et al., 2019; Wewege and Jones, 2021), and the analgesic effect of proximal motor site and distal nonmotor sites induced by aerobic exercise showed an overall effect (Zheng et al., 2021). Therefore, the analgesic effect of exercise on patients with CRPS may also involve a combination of various mechanisms (Figure 1).
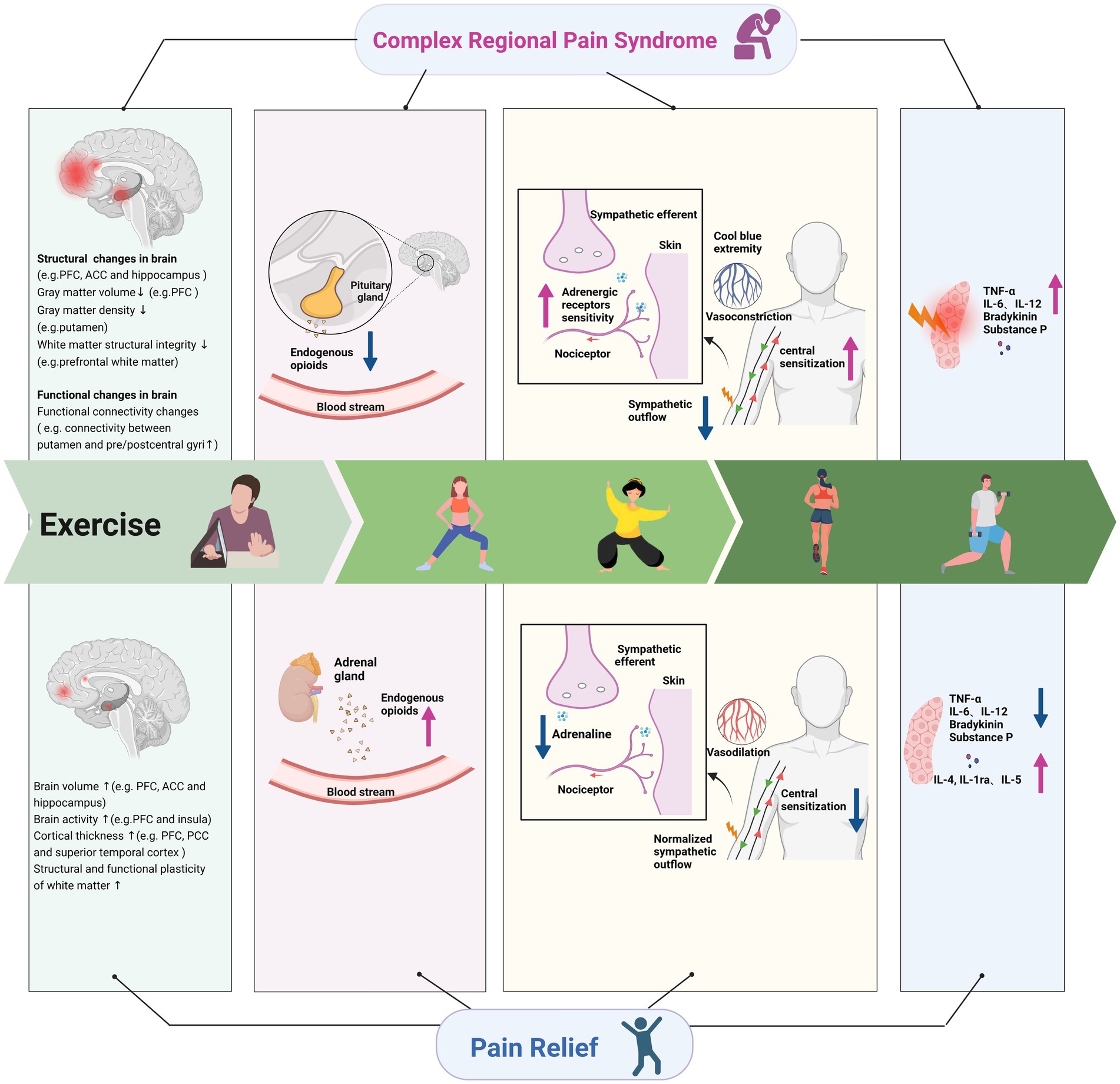
Figure 1. Underlying mechanisms of exercise on complex regional pain syndrome. ACC, anterior cingulate cortex; PFC, prefrontal cortex; PCC, posterior cingulate cortex; TNF, tumor necrosis factor; IL, interleukin; 5-HT, 5-hydroxytryptamine.
3.1. Improvement of sensitization of the central and peripheral nervous systems
CRPS pain and punctuate mechanical hyperalgesia can predict the cortical reorganization in the central nervous system (Maihöfner et al., 2003). Various studies have confirmed that exercise can regulate cortical reorganization (Carey et al., 2002; Pleger et al., 2005; Laible et al., 2012). The magnetoencephalography showed extensive reorganization of the primary somatosensory cortex contralateral to the affected side of CRPS, and pain reduction in CRPS correlated with recovery from cortical reorganization (Maihöfner et al., 2003, 2004). Pleger et al. (2005) concluded that one to six months of behavioral treatments consisting of graded sensorimotor retuning resulted in a sustained reduction in pain intensity for CRPS patients with intractable pain, which was accompanied by recovery of the impaired tactile discrimination and cortical map size in contralateral somatosensory cortexes. Brain functional magnetic resonance imaging in patients with CRPS revealed structural or functional changes in thalamus, hippocampus, amygdala, somatosensory cortex, primary motor cortex, prefrontal cortex (PFC), anterior cingulate cortex (ACC), insula cortex, and other brain areas (Bolwerk et al., 2013; Erpelding et al., 2016) involved in pain perception (Izquierdo-Alventosa et al., 2020). Bolwerk et al. (2013) found that the functional connectivity of sensorimotor cortex and intraparietal sulcus was more diffuse within other brain regions in CRPS patients. Physical exercise has been proved to induce structural plasticity in the human brain (Colcombe et al., 2006). Rogge et al. (2018) found that balance training for 12 weeks in healthy adults increased the cortical thickness in the superior frontal sulcus, the superior temporal cortex, the posterior cingulate cortex, the visual association cortices, and the precentral gyrus. Several studies revealed decreased gray matter volume in the PFC regions in CRPS patients compared with healthy subjects, and the atrophy in the PFC was correlated with the duration and intensity of CRPS pain (Geha et al., 2008; Barad et al., 2014; Lee et al., 2015). Exercise increased brain activities in the PFC and the anterior insula (Ellingson et al., 2016), and long-term brisk walking can increase the gray matter volume of PFC and ACC in healthy old people (Colcombe et al., 2006). The white matter integrity is widely affected in patients with CRPS (Geha et al., 2008; Hotta et al., 2017). The structural integrity of the prefrontal white matter in CRPS patients was lower than in healthy people due to the high degree of pain catastrophizing (Im et al., 2021). Mendez Colmenares et al. (2021) observed positive changes in the myelinating regions after 6 months of aerobic walking and dance intervention in healthy adults, thereby signifying that aerobic exercise training can induce the plasticity of white matter regions. Motor skill training activates neurons to release neurotransmitters, which promote the formation of mature myelinated oligodendrocytes (Gibson et al., 2014; Guo et al., 2020). In a mice model of chronic incomplete spinal cord injury, exercise induced oligodendrogenesis, increased axonal oligodendrocyte interactions, promoted white matter plasticity, and thus reduced hyperalgesia and neuropathic pain behavior (Faw et al., 2021).
CRPS pain is also related to gray matter hypertrophy in the left amygdala, left posterior hippocampus, and right hypothalamus (Barad et al., 2014). The two regions are generally associated with emotional intensity encoding and limbic reward processing (Rolls, 2015; Corbett et al., 2020). Aerobic training was showed to increase hippocampal volume in young healthy adults and old people without dementia (Erickson et al., 2011; Rogge et al., 2018). There was a positive correlation between increasing fitness levels and changes in the hippocampal perfusion after 3 months of intervention (Maass et al., 2016). Another study reported that mind–body exercise increased gray matter volume in the right hippocampus and in the bilateral ACC in people with mild cognitive impairment (Tao et al., 2019). Patients with CRPS were accompanied with bilateral decreases in gray matter density in the putamen and functional connectivity changes among the putamen, cerebellum and pre/postcentral gyri (Azqueta-Gavaldon et al., 2020). These abnormalities affected pain processing and implicated movement disorders. Nagamatsu et al. (2016) reported that healthy individuals with the greatest improvement in mobility had the greatest left putamen volume retention after 12 months of training.
3.2. Regulation of vasodilation and adrenaline levels
CRPS-type 1, known as reflex sympathetic dystrophy, is a sympathetically mediated peripheral pain condition. After the injury occurred, nociceptive fibers in the injured area initiate to express adrenergic receptors. Reduced sympathetic outflow following peripheral nerve injury leads to compensatory up-regulation of local adrenergic receptor sensitivity in the affected limb (Raja et al., 1992; Bruehl, 2010). This up-regulation may lead to exaggerated catecholamine responsiveness, which leads to excessive vasoconstriction, thereby causing the classic symptoms of cool blue extremity in chronic CRPS. Topcuoglu et al. (2015) suggested that an endothelium-related vasodilation mechanism caused by exercise could alleviate the situation. Mortensen et al. (Mortensen et al., 2014) found that 8 weeks of exercise training reduced the vasoconstrictor response to sympathetic nerve activity and improve the ability to override sympathetic vasoconstrictor activity. Another study indicated that physical training in patients with chronic heart failure restored endothelial dysfunction by enhanced endothelial release of nitric oxide to coordinate tissue perfusion (Hornig et al., 1996). In addition, catecholamine-induced nociceptive firing may promote central sensitization by maintaining elevated peripheral nociceptive input (Gracely et al., 1992). Central sensitization causes an increase in pain and catecholamine release that further causes a vicious cycle (Gracely et al., 1992). Therefore, a decrease in epinephrine levels caused by exercise is possibly beneficial (Kiilavuori et al., 1999). Animal experiments showed a decrease in vascular sensitivity after immediate exercise and long-term exercise training (Izawa et al., 1996), and exercise normalized sympathetic outflow by central antioxidant mechanisms (Gao et al., 2007). Negative emotions are also associated with increased catecholamine release (Charney et al., 1990; Light et al., 1998; Bruehl, 2010). Severe depressive symptoms were related to an elevated level of plasma epinephrine (Harden et al., 2004). Exercise may indirectly regulate the release of catecholamines by improving mood, and finally achieve pain relief in CRPS.
3.3. Release of endogenous opioids
Endogenous opioids, such as endorphin and enkephalin, may play a vital role in exercise-induced reduction of CRPS pain (Misra et al., 2017). A study suggested that failed opioid modulation in the regional sympathetic ganglia may trigger or contribute to CRPS-type 1 accompanied by intense pain (Hannington-Kiff, 1991). One explanation for this mechanism is the characteristic of CRPS that complicates minor injuries, indicating that minor injury fails to start or maintain adequate opioid modulation in the regional sympathetic ganglia. The alternative explanation is the developing tolerance to regionally increase opioid activity (Hannington-Kiff, 1991). A study showed that immunoreactive β-endorphin levels in peripheral blood mononuclear cells were significantly lower in the CRPS patients (Takahashi et al., 2000). These findings suggest that regional increases in endogenous opioids might be effective. Adequate exercise intensity and duration were demonstrated to increase circulating β-endorphin and enkephalin levels (Goldfarb et al., 1990). Physical therapy combined with active use of the affected limb may offer a conducive way to sustain regional opioid modulation and safeguard the limb from CRPS-type 1 (Hannington-Kiff, 1991). The results of Pierce et al. showed a remarkable increase in β-endorphin levels after 45 min of high-intensity aerobic exercise (Pierce et al., 1993). Plasma beta-endorphin concentration was elevated after light load blood flow restriction resistance exercise (Hughes and Patterson, 2020). Another study, which included 59 healthy women, found a significant increase in plasma proenkephalin peptide F with acute exercise, and this effect was greater in the combination of strength and endurance training group (DuPont et al., 2017). Furthermore, in a mice model of CRPS-type 1, Martins et al. found that swimming exercise decreased allodynia, and the antiallodynic effect induced by exercise was reversed by the pretreatment with a nonselective opioid receptor antagonist (naloxone), confirming the role of the endogenous opioid in the antiallodynic effect of exercise (Martins et al., 2013). Chromaffin cells of the adrenal medulla are thought to be rich source of endogenous opioids. Results from a study showed that the bilateral adrenalectomy in mice suppressed the analgesia effects of high intensity swimming exercise, suggesting that endogenous opioids released by adrenal glands may conduce to the analgesia effect induced by exercise (Mazzardo-Martins et al., 2010).
3.4. Increased levels of anti-inflammatory cytokines
Persistent inflammatory activities in patients with CRPS lead to visible signs, such as edema, severe pain, temperature increase, and hyperalgesia. Pain catastrophizing in patients with CRPS is related to elevated pro-inflammatory cytokine activity in reaction to painful stimuli (Edwards et al., 2008). Cytokines cause pain and hyperalgesia through the sensitization of nociceptors and release abundant neuropeptides (Birklein et al., 2015). In the samples of CRPS patients’ serum and cerebrospinal fluid, increased levels of proinflammatory cytokines (interleukin–6, interleukin-12, tumor necrosis factor alpha receptors), decreased levels of anti-inflammatory cytokine, and increased levels of neuropeptides (bradykinin, calcitonin substance P and gene-related peptide) were found (Marinus et al., 2011). Exercise may participate in inhibiting regulated neuropeptide signaling and inflammatory mediator expression and reversing nociceptive sensitization. A study in the mouse model of CRPS found that 4 weeks of running wheel exercise can reverse the upregulation of neuropeptide and inflammatory mediator expression (Shi et al., 2018). However, the pain behaviors recurred when exercise stopped for 2 weeks, this nociceptive sensitization was related to increased neuropeptide levels, interleukin-6, and nerve growth factor expression (Shi et al., 2018). Evidence from animal and clinical studies showed that exercise can increase anti-inflammatory cytokines and reduce the levels of pro-inflammatory cytokines (Kohut et al., 2006; Tsai et al., 2017; Paolucci et al., 2018). Six weeks of high-intensity interval training decreased the level of tumor necrosis factor alpha in healthy adults (Paolucci et al., 2018). Two weeks of treadmill exercise improved neuropathic pain (mechanical hyperalgesia and avoidance behavior) in mice. Meanwhile treadmill exercise increased the levels of M2 macrophages which secretes anti-inflammatory cytokines, decreased the levels of M1 macrophages which secretes proinflammatory cytokines, and increased anti-inflammatory cytokine concentrations (interleukin-4, interleukin-1ra, and interleukin-5; Bobinski et al., 2018). Therefore, exercise modulates the immune system to promote healing and analgesia (Sluka et al., 2018; Simpson et al., 2021).
4. Conclusion
The exercise rehabilitation for patients with CRPS is important and beneficial. As a complementary treatment, exercise including graded motor imagery, progressive stress loading training, and aerobic training can improve patients’ pain and disabilities. This article briefly summarized the effectiveness of different types of exercise on CRPS and indicated the underlying mechanisms of the alleviating effect of exercise on CRPS, including the remodeling of abnormal brain structure and brain function, the reduction in peripheral sensitization, the regulation of vasodilation and adrenaline levels, the release of endogenous opioids, and the increased anti-inflammatory cytokines. Therefore, exercise is a feasible and effective treatment for patients with CRPS to improve pain intensity, physical function, and mental health. In addition, the characteristics of capricious symptom, difficult diagnosis, and refractory of CRPS indicate the need for the matched graded multi-professional care. Some complementary treatments such as medications, transcutaneous electrical nerve stimulation (Sutbeyaz et al., 2005; Anandkumar and Manivasagam, 2014; Bilgili et al., 2016), acupuncture (Peng et al., 2018), and manual lymphatic drainage (Duman et al., 2009), have also shown great effect to improve pain and oedema in combination with exercise therapy (Melf-Marzi et al., 2022). Given the comprehensive search, we found a lack of sufficient high- quality clinical studies about CRPS to carry out deeper analyses, which is one of the limitations of this study. Besides, direct evidence of neurophysiological changes in CRPS patients is also unclear. Thus, more clinical studies with larger sample size and detailed protocol in the future could provide more valuable insights. The parameters of different exercise therapy for different stages of CRPS and the integration of exercise therapy and other methods still need further exploration.
Author contributions
X-QW and XS conceived the review. T-SL and RW searched the literature to identify eligible studies and drafted the manuscript and extracted related information. T-SL made the figure and table. All authors contributed to the revision of manuscript, and they have read and approved the final version of the manuscript and the order of presentation of the authors.
Funding
The study was supported by the Science and Technology Commission of Shanghai Municipality [grant numbers 19080503100 and 21S31902400], the Fok Ying-Tong Education Foundation of China [grant number 161092], the Talent Development Fund of Shanghai Municipal [grant number 2021081], the Shanghai Clinical Research Center for Rehabilitation Medicine [grant number 21MC1930200], and the Shanghai Key Lab of Human Performance (Shanghai University of Sport) [grant number 11DZ2261100], as well as by the Shanghai Frontiers Science Research Base of Exercise and Metabolic Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anandkumar, S., and Manivasagam, M. (2014). Multimodal physical therapy management of a 48-year-old female with post-stroke complex regional pain syndrome. Physiother. Theory Pract. 30, 38–48. doi: 10.3109/09593985.2013.814186
Azqueta-Gavaldon, M., Youssef, A. M., Storz, C., Lemme, J., Schulte-Göcking, H., Becerra, L., et al. (2020). Implications of the putamen in pain and motor deficits in complex regional pain syndrome. Pain 161, 595–608. doi: 10.1097/j.pain.0000000000001745
Baliki, M. N., Schnitzer, T. J., Bauer, W. R., and Apkarian, A. V. (2011). Brain morphological signatures for chronic pain. PLoS One 6:e26010. doi: 10.1371/journal.pone.0026010
Barad, M. J., Ueno, T., Younger, J., Chatterjee, N., and Mackey, S. (2014). Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J. Pain 15, 197–203. doi: 10.1016/j.jpain.2013.10.011
Barnhoorn, K. J., Van De Meent, H., Van Dongen, R. T., Klomp, F. P., Groenewoud, H., Samwel, H., et al. (2015). Pain exposure physical therapy (PEPT) compared to conventional treatment in complex regional pain syndrome type 1: a randomised controlled trial. BMJ Open 5:e008283. doi: 10.1136/bmjopen-2015-008283
Bean, D. J., Johnson, M. H., Heiss-Dunlop, W., and Kydd, R. R. (2016). Extent of recovery in the first 12 months of complex regional pain syndrome type-1: a prospective study. Eur. J. Pain 20, 884–894. doi: 10.1002/ejp.813
Bean, D. J., Johnson, M. H., and Kydd, R. R. (2014). The outcome of complex regional pain syndrome type 1: a systematic review. J. Pain 15, 677–690. doi: 10.1016/j.jpain.2014.01.500
Bilgili, A., Çakır, T., Doğan, Ş., Erçalık, K., Filiz, T., and Toraman, F. (2016). The effectiveness of transcutaneous electrical nerve stimulation in the management of patients with complex regional pain syndrome: a randomized, double-blinded, placebo-controlled prospective study. J. Back Musculoskelet. Rehabil. 29, 661–671. doi: 10.3233/BMR-160667
Birklein, F., Oneill, D., and Schlereth, T. (2015). Complex regional pain syndrome: an optimistic perspective. Neurology 84, 89–96. doi: 10.1212/wnl.0000000000001095
Bobinski, F., Teixeira, J. M., Sluka, K. A., and Santos, A. R. S. (2018). Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 159, 437–450. doi: 10.1097/j.pain.0000000000001109
Bolwerk, A., Seifert, F., and Maihöfner, C. (2013). Altered resting-state functional connectivity in complex regional pain syndrome. J. Pain 14, 1107–1115.e8. doi: 10.1016/j.jpain.2013.04.007
Bruehl, S. (2010). An update on the pathophysiology of complex regional pain syndrome. Anesthesiology 113, 713–725. doi: 10.1097/ALN.0b013e3181e3db38
Cacchio, A., De Blasis, E., De Blasis, V., Santilli, V., and Spacca, G. (2009a). Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil. Neural Repair 23, 792–799. doi: 10.1177/1545968309335977
Cacchio, A., De Blasis, E., Necozione, S., Di Orio, F., and Santilli, V. (2009b). Mirror therapy for chronic complex regional pain syndrome type 1 and stroke. N. Engl. J. Med. 361, 634–636. doi: 10.1056/NEJMc0902799
Carey, J. R., Kimberley, T. J., Lewis, S. M., Auerbach, E. J., Dorsey, L., Rundquist, P., et al. (2002). Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain 125, 773–788. doi: 10.1093/brain/awf091
Charney, D. S., Woods, S. W., Nagy, L. M., Southwick, S. M., Krystal, J. H., and Heninger, G. R. (1990). Noradrenergic function in panic disorder. J. Clin. Psychiatry 51 Suppl A, 5–11.
Colcombe, S. J., Erickson, K. I., Scalf, P. E., Kim, J. S., Prakash, R., Mcauley, E., et al. (2006). Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1166–1170. doi: 10.1093/gerona/61.11.1166
Coppieters, I., Meeus, M., Kregel, J., Caeyenberghs, K., De Pauw, R., Goubert, D., et al. (2016). Relations between brain alterations and clinical pain measures in chronic musculoskeletal pain: a systematic review. J. Pain 17, 949–962. doi: 10.1016/j.jpain.2016.04.005
Corbett, B., Rajah, M. N., and Duarte, A. (2020). Preparing for the worst: evidence that older adults proactively downregulate negative affect. Cereb. Cortex 30, 1291–1306. doi: 10.1093/cercor/bhz166
Costigan, M., Scholz, J., and Woolf, C. J. (2009). Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 32, 1–32. doi: 10.1146/annurev.neuro.051508.135531
De Mos, M., De Bruijn, A. G., Huygen, F. J., Dieleman, J. P., Stricker, B. H., and Sturkenboom, M. C. (2007). The incidence of complex regional pain syndrome: a population-based study. Pain 129, 12–20. doi: 10.1016/j.pain.2006.09.008
Duman, I., Ozdemir, A., Tan, A. K., and Dincer, K. (2009). The efficacy of manual lymphatic drainage therapy in the management of limb edema secondary to reflex sympathetic dystrophy. Rheumatol. Int. 29, 759–763. doi: 10.1007/s00296-008-0767-5
Dupont, W. H., Kraemer, W. J., Nindl, B. C., Lee, E. C., Fragala, M. S., Hatfield, D. L., et al. (2017). The effects of different exercise training modalities on plasma proenkephalin peptide F in women. Peptides 91, 26–32. doi: 10.1016/j.peptides.2017.02.006
Edwards, R. R., Kronfli, T., Haythornthwaite, J. A., Smith, M. T., Mcguire, L., and Page, G. G. (2008). Association of catastrophizing with interleukin-6 responses to acute pain. Pain 140, 135–144. doi: 10.1016/j.pain.2008.07.024
Ellingson, L. D., Stegner, A. J., Schwabacher, I. J., Koltyn, K. F., and Cook, D. B. (2016). Exercise strengthens central nervous system modulation of pain in fibromyalgia. Brain Sci. 6:8. doi: 10.3390/brainsci6010008
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Erpelding, N., Simons, L., Lebel, A., Serrano, P., Pielech, M., Prabhu, S., et al. (2016). Rapid treatment-induced brain changes in pediatric CRPS. Brain Struct. Funct. 221, 1095–1111. doi: 10.1007/s00429-014-0957-8
Faw, T. D., Lakhani, B., Schmalbrock, P., Knopp, M. V., Lohse, K. R., Kramer, J. L. K., et al. (2021). Eccentric rehabilitation induces white matter plasticity and sensorimotor recovery in chronic spinal cord injury. Exp. Neurol. 346:113853. doi: 10.1016/j.expneurol.2021.113853
Gao, L., Wang, W., Liu, D., and Zucker, I. H. (2007). Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115, 3095–3102. doi: 10.1161/circulationaha.106.677989
Geha, P. Y., Baliki, M. N., Harden, R. N., Bauer, W. R., Parrish, T. B., and Apkarian, A. V. (2008). The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 60, 570–581. doi: 10.1016/j.neuron.2008.08.022
Gibson, E. M., Purger, D., Mount, C. W., Goldstein, A. K., Lin, G. L., Wood, L. S., et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304. doi: 10.1126/science.1252304
Goebel, A., Barker, C., Birklein, F., Brunner, F., Casale, R., Eccleston, C., et al. (2019). Standards for the diagnosis and management of complex regional pain syndrome: results of a European pain federation task force. Eur. J. Pain 23, 641–651. doi: 10.1002/ejp.1362
Goldfarb, A. H., Hatfield, B. D., Armstrong, D., and Potts, J. (1990). Plasma beta-endorphin concentration: response to intensity and duration of exercise. Med. Sci. Sports Exerc. 22, 241–244.
Gracely, R. H., Lynch, S. A., and Bennett, G. J. (1992). Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain 51, 175–194. doi: 10.1016/0304-3959(92)90259-e
Guo, L. Y., Lozinski, B., and Yong, V. W. (2020). Exercise in multiple sclerosis and its models: focus on the central nervous system outcomes. J. Neurosci. Res. 98, 509–523. doi: 10.1002/jnr.24524
Hall, J., Swinkels, A., Briddon, J., and Mccabe, C. S. (2008). Does aquatic exercise relieve pain in adults with neurologic or musculoskeletal disease? A systematic review and meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 89, 873–883. doi: 10.1016/j.apmr.2007.09.054
Hannington-Kiff, J. G. (1991). Does failed natural opioid modulation in regional sympathetic ganglia cause reflex sympathetic dystrophy? Lancet 338, 1125–1127. doi: 10.1016/0140-6736(91)91974-y
Harden, R. N., Mccabe, C. S., Goebel, A., Massey, M., Suvar, T., Grieve, S., et al. (2022). Complex regional pain syndrome: practical diagnostic and treatment guidelines. Pain Med. 23, S1–s53. doi: 10.1093/pm/pnac046
Harden, R. N., Rudin, N. J., Bruehl, S., Kee, W., Parikh, D. K., Kooch, J., et al. (2004). Increased systemic catecholamines in complex regional pain syndrome and relationship to psychological factors: a pilot study. Anesth. Analg. 99, 1478–1485. doi: 10.1213/01.Ane.0000132549.25154
Hornig, B., Maier, V., and Drexler, H. (1996). Physical training improves endothelial function in patients with chronic heart failure. Circulation 93, 210–214. doi: 10.1161/01.cir.93.2.210
Hotta, J., Zhou, G., Harno, H., Forss, N., and Hari, R. (2017). Complex regional pain syndrome: the matter of white matter? Brain Behav. 7:e00647. doi: 10.1002/brb3.647
Hughes, L., and Patterson, S. D. (2020). The effect of blood flow restriction exercise on exercise-induced hypoalgesia and endogenous opioid and endocannabinoid mechanisms of pain modulation. J. Appl. Physiol. 128, 914–924. doi: 10.1152/japplphysiol.00768.2019
Im, J. J., Kim, J., Jeong, H., Oh, J. K., Lee, S., Lyoo, I. K., et al. (2021). Prefrontal White matter abnormalities associated with pain catastrophizing in patients with complex regional pain syndrome. Arch. Phys. Med. Rehabil. 102, 216–224. doi: 10.1016/j.apmr.2020.07.006
Izawa, T., Morikawa, M., Mizuta, T., Nagasawa, J., Kizaki, T., Oh-Ishi, S., et al. (1996). Decreased vascular sensitivity after acute exercise and chronic exercise training in rat thoracic aorta. Res. Commun. Mol. Pathol. Pharmacol. 93, 331–342.
Izquierdo-Alventosa, R., Inglés, M., Cortés-Amador, S., Gimeno-Mallench, L., Chirivella-Garrido, J., Kropotov, J., et al. (2020). Low-intensity physical exercise improves pain catastrophizing and other psychological and physical aspects in women with fibromyalgia: a randomized controlled trial. Int. J. Environ. Res. Public Health 17:3634. doi: 10.3390/ijerph17103634
Kiilavuori, K., Näveri, H., Leinonen, H., and Härkönen, M. (1999). The effect of physical training on hormonal status and exertional hormonal response in patients with chronic congestive heart failure. Eur. Heart J. 20, 456–464. doi: 10.1053/euhj.1998.1277
Kohut, M. L., Mccann, D. A., Russell, D. W., Konopka, D. N., Cunnick, J. E., Franke, W. D., et al. (2006). Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 20, 201–209. doi: 10.1016/j.bbi.2005.12.002
Laible, M., Grieshammer, S., Seidel, G., Rijntjes, M., Weiller, C., and Hamzei, F. (2012). Association of activity changes in the primary sensory cortex with successful motor rehabilitation of the hand following stroke. Neurorehabil. Neural Repair 26, 881–888. doi: 10.1177/1545968312437939
Lee, D. H., Lee, K. J., Cho, K. I., Noh, E. C., Jang, J. H., Kim, Y. C., et al. (2015). Brain alterations and neurocognitive dysfunction in patients with complex regional pain syndrome. J. Pain 16, 580–586. doi: 10.1016/j.jpain.2015.03.006
Light, K. C., Kothandapani, R. V., and Allen, M. T. (1998). Enhanced cardiovascular and catecholamine responses in women with depressive symptoms. Int. J. Psychophysiol. 28, 157–166. doi: 10.1016/s0167-8760(97)00093-7
Maass, A., Düzel, S., Brigadski, T., Goerke, M., Becke, A., Sobieray, U., et al. (2016). Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 131, 142–154. doi: 10.1016/j.neuroimage.2015.10.084
Maihöfner, C., Handwerker, H. O., Neundörfer, B., and Birklein, F. (2003). Patterns of cortical reorganization in complex regional pain syndrome. Neurology 61, 1707–1715. doi: 10.1212/01.wnl.0000098939.02752.8e
Maihöfner, C., Handwerker, H. O., Neundörfer, B., and Birklein, F. (2004). Cortical reorganization during recovery from complex regional pain syndrome. Neurology 63, 693–701. doi: 10.1212/01.wnl.0000134661.46658.b0
Marinus, J., Moseley, G. L., Birklein, F., Baron, R., Maihöfner, C., Kingery, W. S., et al. (2011). Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 10, 637–648. doi: 10.1016/s1474-4422(11)70106-5
Martins, D., Dipasquale, O., Veronese, M., Turkheimer, F., Loggia, M. L., Mcmahon, S., et al. (2022). Transcriptional and cellular signatures of cortical morphometric remodelling in chronic pain. Pain 163, e759–e773. doi: 10.1097/j.pain.0000000000002480
Martins, D. F., Mazzardo-Martins, L., Soldi, F., Stramosk, J., Piovezan, A. P., and Santos, A. R. (2013). High-intensity swimming exercise reduces neuropathic pain in an animal model of complex regional pain syndrome type I: evidence for a role of the adenosinergic system. Neuroscience 234, 69–76. doi: 10.1016/j.neuroscience.2012.12.042
Mazzardo-Martins, L., Martins, D. F., Marcon, R., Dos Santos, U. D., Speckhann, B., Gadotti, V. M., et al. (2010). High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. J. Pain 11, 1384–1393. doi: 10.1016/j.jpain.2010.03.015
Mccabe, C. (2011). Mirror visual feedback therapy. A practical approach. J. Hand Ther. 24, 170-8–170-179. doi: 10.1016/j.jht.2010.08.003
Mccabe, C. S., Haigh, R. C., Ring, E. F., Halligan, P. W., Wall, P. D., and Blake, D. R. (2003). A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology (Oxford) 42, 97–101. doi: 10.1093/rheumatology/keg041
Mccormick, Z. L., Gagnon, C. M., Caldwell, M., Patel, J., Kornfeld, S., Atchison, J., et al. (2015). Short-term functional, emotional, and pain outcomes of patients with complex regional pain syndrome treated in a comprehensive interdisciplinary pain management program. Pain Med. 16, 2357–2367. doi: 10.1111/pme.12817
Melf-Marzi, A., Böhringer, B., Wiehle, M., and Hausteiner-Wiehle, C. (2022). Modern principles of diagnosis and treatment in complex regional pain syndrome. Dtsch. Arztebl. Int. 119, 879–886. doi: 10.3238/arztebl.m2022.0358
Mendell, L. M. (2014). Constructing and deconstructing the gate theory of pain. Pain 155, 210–216. doi: 10.1016/j.pain.2013.12.010
Mendez Colmenares, A., Voss, M. W., Fanning, J., Salerno, E. A., Gothe, N. P., Thomas, M. L., et al. (2021). White matter plasticity in healthy older adults: the effects of aerobic exercise. NeuroImage 239:118305. doi: 10.1016/j.neuroimage.2021.118305
Misra, U. K., Kalita, J., Tripathi, G., and Bhoi, S. K. (2017). Role of β endorphin in pain relief following high rate repetitive transcranial magnetic stimulation in migraine. Brain Stimul. 10, 618–623. doi: 10.1016/j.brs.2017.02.006
Mortensen, S. P., Nyberg, M., Gliemann, L., Thaning, P., Saltin, B., and Hellsten, Y. (2014). Exercise training modulates functional sympatholysis and α-adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J. Physiol. 592, 3063–3073. doi: 10.1113/jphysiol.2014.273722
Moseley, G. L. (2004). Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain 108, 192–198. doi: 10.1016/j.pain.2004.01.006
Moseley, G. L. (2006). Graded motor imagery for pathologic pain: a randomized controlled trial. Neurology 67, 2129–2134. doi: 10.1212/01.wnl.0000249112.56935.32
Nagamatsu, L. S., Weinstein, A. M., Erickson, K. I., Fanning, J., Awick, E. A., Kramer, A. F., et al. (2016). Exercise mode moderates the relationship between mobility and basal ganglia volume in healthy older adults. J. Am. Geriatr. Soc. 64, 102–108. doi: 10.1111/jgs.13882
Oh, H. M., Kim, C. H., and Kim, A. R. (2019). Dramatic effect in passive ROM exercise under sedation in a patient with intractable complex regional pain syndrome (type I): a case report. Medicine (Baltimore) 98:e14990. doi: 10.1097/md.0000000000014990
Paolucci, E. M., Loukov, D., Bowdish, D. M. E., and Heisz, J. J. (2018). Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 133, 79–84. doi: 10.1016/j.biopsycho.2018.01.015
Peng, L., Zhang, C., Zhou, L., Zuo, H. X., He, X. K., and Niu, Y. M. (2018). Traditional manual acupuncture combined with rehabilitation therapy for shoulder hand syndrome after stroke within the Chinese healthcare system: a systematic review and meta-analysis. Clin. Rehabil. 32, 429–439. doi: 10.1177/0269215517729528
Pervane Vural, S., Nakipoglu Yuzer, G. F., Sezgin Ozcan, D., Demir Ozbudak, S., and Ozgirgin, N. (2016). Effects of Mirror therapy in stroke patients with complex regional pain syndrome type 1: a randomized controlled study. Arch. Phys. Med. Rehabil. 97, 575–581. doi: 10.1016/j.apmr.2015.12.008
Petersen, P. B., Mikkelsen, K. L., Lauritzen, J. B., and Krogsgaard, M. R. (2018). Risk factors for Post-treatment complex regional pain syndrome (CRPS): an analysis of 647 cases of CRPS from the Danish patient compensation association. Pain Pract. 18, 341–349. doi: 10.1111/papr.12610
Pierce, E. F., Eastman, N. W., Tripathi, H. L., Olson, K. G., and Dewey, W. L. (1993). Beta-endorphin response to endurance exercise: relationship to exercise dependence. Percept. Mot. Skills 77, 767–770. doi: 10.2466/pms.1993.77.3.767
Pleger, B., Tegenthoff, M., Ragert, P., Förster, A. F., Dinse, H. R., Schwenkreis, P., et al. (2005). Sensorimotor retuning [corrected] in complex regional pain syndrome parallels pain reduction. Ann. Neurol. 57, 425–429. doi: 10.1002/ana.20394
Raja, S. N., Davis, K. D., and Campbell, J. N. (1992). The adrenergic pharmacology of sympathetically-maintained pain. J. Reconstr. Microsurg. 8, 63–69. doi: 10.1055/s-2007-1006686
Rice, D., Nijs, J., Kosek, E., Wideman, T., Hasenbring, M. I., Koltyn, K., et al. (2019). Exercise-induced Hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J. Pain 20, 1249–1266. doi: 10.1016/j.jpain.2019.03.005
Rogge, A. K., Röder, B., Zech, A., and Hötting, K. (2018). Exercise-induced neuroplasticity: balance training increases cortical thickness in visual and vestibular cortical regions. NeuroImage 179, 471–479. doi: 10.1016/j.neuroimage.2018.06.065
Rolls, E. T. (2015). Limbic systems for emotion and for memory, but no single limbic system. Cortex 62, 119–157. doi: 10.1016/j.cortex.2013.12.005
Saha, S., Sur, M., Ray Chaudhuri, G., and Agarwal, S. (2021). Effects of mirror therapy on oedema, pain and functional activities in patients with poststroke shoulder-hand syndrome: a randomized controlled trial. Physiother. Res. Int. 26:e1902. doi: 10.1002/pri.1902
Saquetto, M. B., Da Silva, C. M., Martinez, B. P., Sena, C. D. C., Pontes, S. S., Da Paixão, M. T. C., et al. (2019). Water-based exercise on functioning and quality of life in Poststroke persons: a systematic review and Meta-analysis. J. Stroke Cerebrovasc. Dis. 28:104341. doi: 10.1016/j.jstrokecerebrovasdis.2019.104341
Sezgin Ozcan, D., Tatli, H. U., Polat, C. S., Oken, O., and Koseoglu, B. F. (2019). The effectiveness of Fluidotherapy in Poststroke complex regional pain syndrome: a randomized controlled study. J. Stroke Cerebrovasc. Dis. 28, 1578–1585. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.002
Sherry, D. D., Wallace, C. A., Kelley, C., Kidder, M., and Sapp, L. (1999). Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin. J. Pain 15, 218–223. doi: 10.1097/00002508-199909000-00009
Shi, X., Guo, T. Z., Li, W., Sahbaie, P., Rice, K. C., Sulima, A., et al. (2018). Exercise reverses nociceptive sensitization, upregulated neuropeptide signaling, inflammatory changes, anxiety, and memory impairment in a mouse tibia fracture model. Anesthesiology 129, 557–575. doi: 10.1097/aln.0000000000002332
Simpson, R. J., Boßlau, T. K., Weyh, C., Niemiro, G. M., Batatinha, H., Smith, K. A., et al. (2021). Exercise and adrenergic regulation of immunity. Brain Behav. Immun. 97, 303–318. doi: 10.1016/j.bbi.2021.07.010
Sluka, K. A., Frey-Law, L., and Hoeger Bement, M. (2018). Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 159, S91–s97. doi: 10.1097/j.pain.0000000000001235
Smart, K. M., Wand, B. M., and O'connell, N. E. (2016). Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst. Rev. 2016:Cd010853. doi: 10.1002/14651858.CD010853.pub2
Smidt, N., De Vet, H. C., Bouter, L. M., Dekker, J., Arendzen, J. H., De Bie, R. A., et al. (2005). Effectiveness of exercise therapy: a best-evidence summary of systematic reviews. Aust. J. Physiother. 51, 71–85. doi: 10.1016/s0004-9514(05)70036-2
Stanton-Hicks, M., Baron, R., Boas, R., Gordh, T., Harden, N., Hendler, N., et al. (1998). Complex regional pain syndromes: guidelines for therapy. Clin. J. Pain 14, 155–166. doi: 10.1097/00002508-199806000-00012
Sutbeyaz, S. T., Koseoglu, B. F., and Yeşiltepe, E. (2005). Simultaneous upper and lower extremity complex regional pain syndrome type I in tetraplegia. Spinal Cord 43, 568–572. doi: 10.1038/sj.sc.3101742
Takahashi, M., Yoshida, A., Yamanaka, H., Furuyama, Y., Horinouchi, T., Kato, M., et al. (2000). Lower β-endorphin content of peripheral blood mononuclear cells in patients with complex regional pain syndrome. J. Back Musculoskelet. Rehabil. 15, 31–36. doi: 10.3233/bmr-2000-15104
Tao, J., Liu, J., Chen, X., Xia, R., Li, M., Huang, M., et al. (2019). Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. Neuroimage Clin. 23:101834. doi: 10.1016/j.nicl.2019.101834
Topcuoglu, A., Gokkaya, N. K., Ucan, H., and Karakuş, D. (2015). The effect of upper-extremity aerobic exercise on complex regional pain syndrome type I: a randomized controlled study on subacute stroke. Top. Stroke Rehabil. 22, 253–261. doi: 10.1179/1074935714z.0000000025
Tsai, K. L., Huang, P. C., Wang, L. K., Hung, C. H., and Chen, Y. W. (2017). Incline treadmill exercise suppresses pain hypersensitivity associated with the modulation of pro-inflammatory cytokines and anti-inflammatory cytokine in rats with peripheral nerve injury. Neurosci. Lett. 643, 27–31. doi: 10.1016/j.neulet.2017.02.021
Urits, I., Shen, A. H., Jones, M. R., Viswanath, O., and Kaye, A. D. (2018). Complex regional pain syndrome, current concepts and treatment options. Curr. Pain Headache Rep. 22:10. doi: 10.1007/s11916-018-0667-7
Van De Meent, H., Oerlemans, M., Bruggeman, A., Klomp, F., Van Dongen, R., Oostendorp, R., et al. (2011). Safety of pain exposure physical therapy in patients with complex regional pain syndrome type 1. Pain 152, 1431–1438. doi: 10.1373/clinchem.2005.062117
Van Velzen, G. A. J., Perez, R., Van Gestel, M. A., Huygen, F., Van Kleef, M., Van Eijs, F., et al. (2014). Health-related quality of life in 975 patients with complex regional pain syndrome type 1. Pain 155, 629–634. doi: 10.1016/j.pain.2013.12.017
Watson, H. K., and Carlson, L. (1987). Treatment of reflex sympathetic dystrophy of the hand with an active stress loading program. J. Hand Surg. Am. 12, 779–785. doi: 10.1016/s0363-5023(87)80069-2
Wewege, M. A., and Jones, M. D. (2021). Exercise-induced Hypoalgesia in healthy individuals and people with chronic musculoskeletal pain: a systematic review and meta-analysis. J. Pain 22, 21–31. doi: 10.1016/j.jpain.2020.04.003
Wu, W. H., Bandilla, E., Ciccone, D. S., Yang, J., Cheng, S. C., Carner, N., et al. (1999). Effects of qigong on late-stage complex regional pain syndrome. Altern. Ther. Health Med. 5, 45–54.
Yamazaki, F., Endo, Y., Torii, R., Sagawa, S., and Shiraki, K. (2000). Continuous monitoring of change in hemodilution during water immersion in humans: effect of water temperature. Aviat. Space Environ. Med. 71, 632–639.
Yang, S., and Chang, M. C. (2019). Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int. J. Mol. Sci. 20:3130. doi: 10.3390/ijms20133130
Keywords: complex regional pain syndrome, exercise, pain, analgesic effect, analgesic mechanisms
Citation: Li T-S, Wang R, Su X and Wang X-Q (2023) Effect and mechanisms of exercise for complex regional pain syndrome. Front. Mol. Neurosci. 16:1167166. doi: 10.3389/fnmol.2023.1167166
Edited by:
Lingxiao Deng, Indiana University, United StatesReviewed by:
Jacqueline Sagen, University of Miami, United StatesCopyright © 2023 Li, Wang, Su and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Qiang Wang, d2FuZ3h1ZXFpYW5nQHN1cy5lZHUuY24=; Xuan Su, ODUxNjI1NTc1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Tian-Shu Li
Tian-Shu Li Rui Wang
Rui Wang Xuan Su2*
Xuan Su2* Xue-Qiang Wang
Xue-Qiang Wang