- Department of Anesthesiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Purpose: To explore the potential therapeutic strategies of different types of neuropathic pain (NP) and to summarize the cutting-edge novel approaches for NP treatment based on the clinical trials registered on ClinicalTrials.gov.
Methods: The relevant clinical trials were searched using ClinicalTrials.gov Dec 08, 2022. NP is defined as a painful condition caused by neurological lesions or diseases. All data were obtained and reviewed by the investigators to confirm whether they were related to the current topic.
Results: A total of 914 trials were included in this study. They were divided into painful diabetic neuropathy (PDN), postherpetic neuralgia (PHN), sciatica (SC), peripheral nerve injury-related NP (PNI), trigeminal neuralgia (TN), chemotherapy-induced NP (CINP), general peripheral NP (GPNP) and spinal cord injury NP (SCI-NP). Potential novel therapeutic strategies, such as novel drug targets and physical means, were discussed for each type of NP.
Conclusion: NP treatment is mainly dominated by drug therapy, and physical means have become increasingly popular. It is worth noting that novel drug targets, new implications of conventional medicine, and novel physical means can serve as promising strategies for the treatment of NP. However, more attention needs to be paid to the challenges of translating research findings into clinical practice.
1. Introduction
Neuropathic pain (NP) is a significant contributor to the global burden of disease. Its prevalence varies between 6.9 and 10% in the general population, and is expected to increase by 2–3% annually, as the older population grows and the incidence of diabetes and cancer increases (Van Hecke et al., 2014). NP leads to a substantial economic burden on healthcare resources and societal costs. It is estimated that the direct medical costs of NP would exceed 635 billion per year in the United States (Gaskin and Richard, 2012). Most patients with NP complain of persistent or intermittent spontaneous pain (Finnerup et al., 2021). Lesions or diseases involving the somatosensory nervous system not only lead to a loss of function, but also increase pain sensitivity and spontaneous pain (Scholz et al., 2019). Moreover, NP patients are usually associated with severe anxiety, depression, sleep disturbances, and even suicide. However, the treatment of NP remains unsatisfied and challenging.
The current recommendations for the pharmacological treatment of NP were published by NP Special Interest group (NeuPSIG) of the International Association for the Study of Pain (IASP). Drugs with moderate-to-high quality of evidence and strong recommendation include tricyclic antidepressants (TCA), gabapentin, pregabalin, and serotonin noradrenaline reuptake inhibitors (SNRIs; duloxetine and venlafaxine), which are recommended as first-line therapies. The less recommended drugs include capsaicin 8% patches and lidocaine patches (Finnerup et al., 2015). Opioids and subcutaneous injections of botulinum-toxin type A for peripheral NP are weakly graded and recommended for third-line therapies (Finnerup et al., 2021). Opioids should be reserved for patients who do not respond to other treatment options and are at low risk of adverse reactions. Besides, the efficacy of analgesics (e.g., NSAIDs) in NP patients is still unclear (Scholz et al., 2019).
Although the guidelines recommend multiple therapeutic interventions, the first-line drugs provide unsatisfactory pain relief in the clinic (Finnerup et al., 2015). Gabapentin and pregabalin, which target voltage-dependent calcium channels α2δ1 subunit (Li et al., 2019), perform well for postherpetic neuralgia (PHN) and painful diabetic neuropathy, but not for sciatica and migraine (Finnerup et al., 2015). The common side effects of gabapentin and pregabalin include dizziness, drowsiness, euphoria, peripheral edema and weight gain (Finnerup et al., 2021). TCA and SNRIs inhibit presynaptic reuptake of serotonin and norepinephrine (Kremer et al., 2016). The common side effects of TCA include dry mouth, drowsiness and cardiovascular related effects, while SNRIs have side effects of nausea, insomnia and hyperhidrosis at an early stage (Kremer et al., 2016; Attal and Bouhassira, 2021). Lidocaine acts through a dependent voltage-gated blockade of sodium channels (Devor et al., 1992). The absorption of lidocaine into plasma leads to a potential risk of systemic adverse reactions. Capsaicin binds to TRPV1, and repeated or single application of high concentrations can cause reversible effects of intraepidermal nerve fiber density and pain receptor desensitization (Anand and Bley, 2011). The application of a capsaicin patch can lead to local skin redness or itching (Backonja et al., 2008). Hence, it is of great importance to develop novel therapeutic strategies for the clinical treatment of NP.
To achieve this goal, researchers are exploring new ways. With the rapid development of science and technology, scientists and clinicians explored deeper understandings of the mechanism of NP. Besides, several novel therapeutic strategies are invented and tested based on these mechanisms. For example, methadone is now being tested for the clinical management of NP (NCT05235191). Transcutaneous pulsed radiofrequency therapy is applied to test its effectiveness in the alleviation of PDN symptoms (NCT05480527). In this review, we searched the currently ongoing or completed clinical trials on NP treatment through ClinicalTrials.gov, which is the largest resource of the clinical research website of the US National Library of Medicine. This review aimed to explore new therapeutic strategies for different types of NP and to summarize the cutting-edge novel potential approaches for NP treatment.
2. Materials and methods
2.1. Data collection and processing
ClinicalTrials.gov was used to search the relevant clinical trials. The search was done on December 08, 2022 by ZL Du and J Z. NP is defined as a painful condition caused by neurological lesions or diseases. All trials were downloaded as a.tsv file, and then reviewed by WF Yu and XY Gu to confirm whether they were related to the current topic. Several diseases, such as complex regional pain syndrome type 1, low back pain without radicular pain, fibromyalgia and atypical facial pain, were not included because they did not meet the current definition of NP (Finnerup et al., 2016). The inclusion criteria of key trials were as follows: (1) completed within 2 years; (2) recruiting or not yet recruiting; (3) did not include drugs which were recommended as first-line therapy.
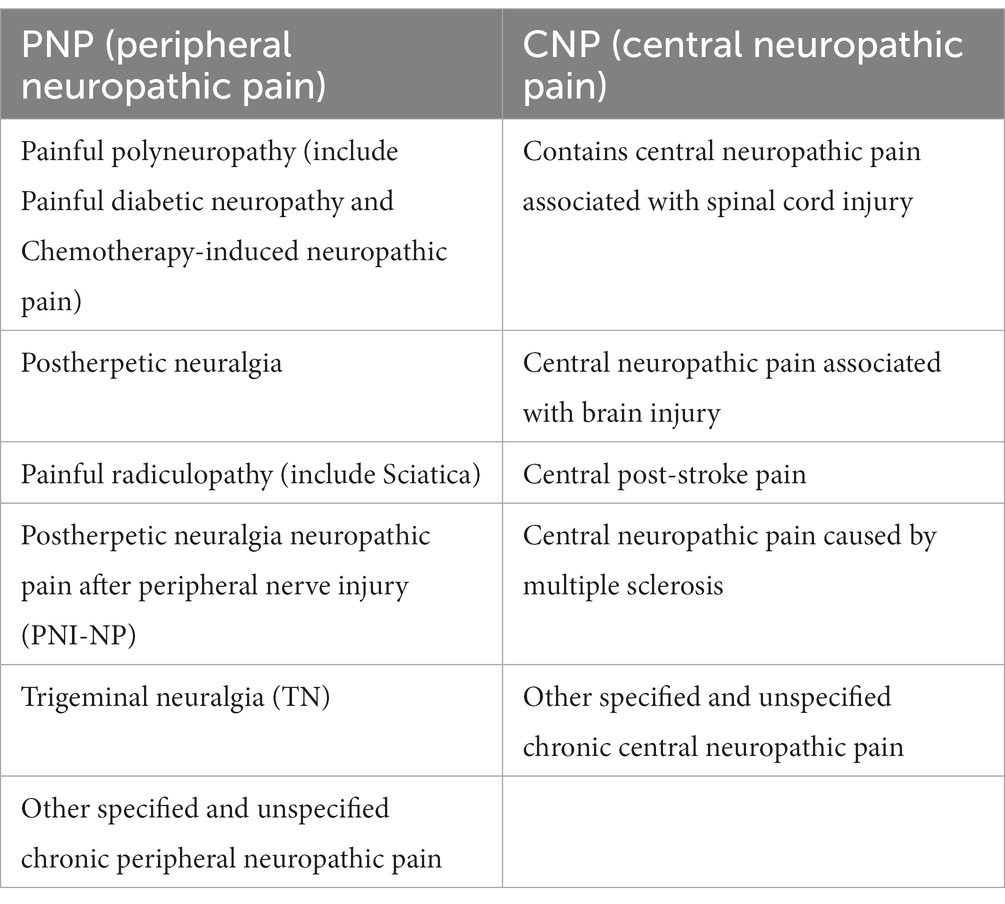
The classification of NP was defined as peripheral neuropathic pain (PNP) or central neuropathic pain (CNP) according to ICD-11 (Table 1). The trials related to NP treatment were further collected and summarized. The intervention was systemic or topical treatment for at least 3 weeks.
3. Results
3.1. Overview of the included clinical trials
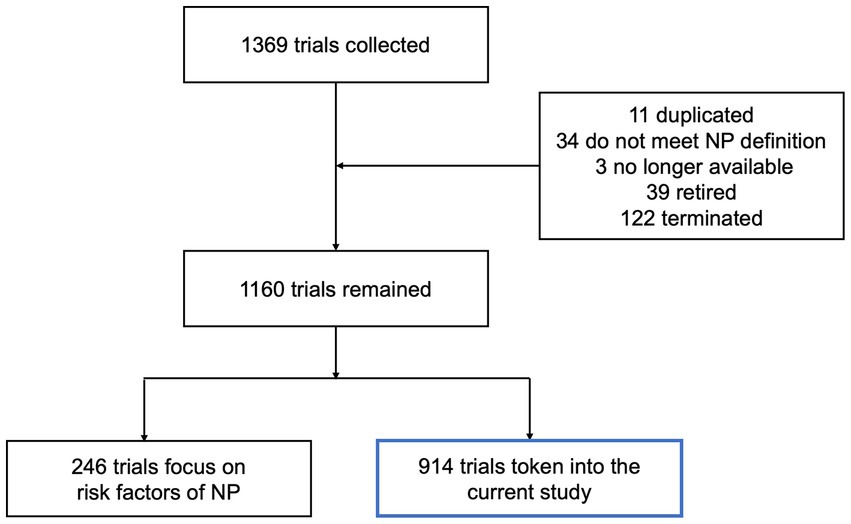
A total of 1,369 clinical trials related to NP were identified. After reviewing, there were 11 duplicated trials, 34 trials did not meet the NP definition, 3 were no longer available, 39 were retired, and 122 were terminated. Meanwhile, 246 trials focused on the risk factors, objective measures and economic burden of NP, as well as its effect on other functions. As a result, 914 trials were included in this study (Figure 1). Among them, 793 trials were focused on peripheral NP (PNP), including 132 painful diabetic neuropathy (PDN), 123 postherpetic neuralgia (PHN), 125 sciatica (SC), 76 peripheral nerve injury-related NP (PNI), 64 trigeminal neuralgia (TN), 25 chemotherapy-induced NP (CINP), 154 general PNP (GPNP) and others (including pudendal neuralgia, post-amputation NP, HIV-related NP). The remaining 121 trials were focused on central NP (CPN). Almost half of them (54 of 121) were concentrated on spinal cord injury NP (SCI-NP; Figure 2). The potential therapeutic strategies for NP are summarized as follows.
4. Peripheral neuropathic pain
4.1. Painful diabetic neuropathy
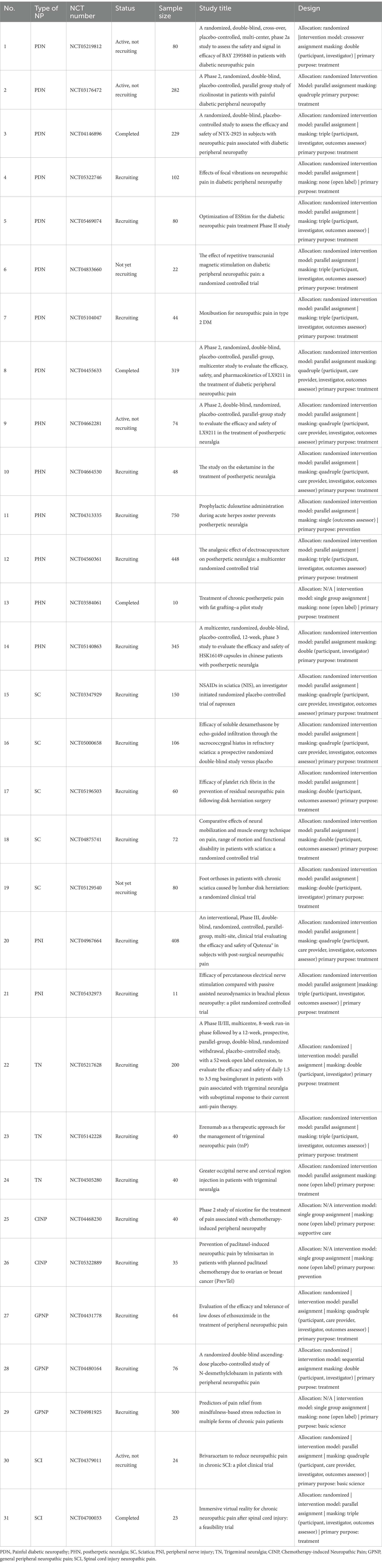
PDN is a frequent subtype of PNP, which is defined as “pain as a direct consequence of abnormalities in the peripheral somatosensory system of diabetes patients” (Jensen et al., 2011). PDN is one of the painful polyneuropathy. The prevalence of PDN ranged from 13 to 35% in patients with diabetes mellitus (Ponirakis et al., 2021). PDN is a frequent complication of long-term diabetes, and one of the leading causes of morbidity and disability (Rosenberger et al., 2020). Due to the improvement in living standards and increased prevalence of diabetes, PDN has attracted considerable attention, with the second most trials (132/914, 14.4%) until now. Pregabalin and duloxetine, together with tapentadol as a supplement, is the current first-line therapy for PDN according to the FDA recommendation (Rosenberger et al., 2020). Unwanted side effects are the main issue. The ongoing trials for PDN treatment are mainly focused on novel molecular targets and physical means.
4.1.1. Potential drugs
In NCT05219812, a new drug BAY 2395840 that blocks bradykinin receptor B1 (BDKRB1) is being tested (Table 2, Line 1). BDKRB1 was upregulated on sensory C-fibers, astrocytes and microglia in the spinal cord of streptozotocin (STZ)-induced diabetic rats, and peripheral blockade of BDKRB1 reversed tactile and cold allodynia at 3–6 h post-treatment in diabetic rats (Talbot et al., 2010). Inhibiting BDKRB1/RAS/MEK signaling pathway exhibited a better outcome in NP (Xie et al., 2021). The current trial by Bayer aimed to test the effectiveness and safety of BAY2395840 on participants who suffered from PDN.
NCT03176472 is a phase II trial of ricolinostat (ACY-1215), a histone deacetylase 6 (HDAC6) inhibitor developed by Regenacy Pharmaceuticals LLC for treating PDN (Table 2, Line 2). Mechanical allodynia, cognitive impairment, and depressive-like behavior caused by spared nerve ligation (SNL) model were attenuated by continuous intraperitoneal injection of ricolinostat (ACY-1215). Moreover, ACY-1215 administration could suppress SNL-induced neuroinflammatory responses in the ipsilateral spinal dorsal horn, hippocampus and prefrontal cortex (Chen et al., 2022). It is worth noting that cytoplasmic HDAC6 regulates the acetylation of various non-histone proteins in healthy neurons (LoPresti, 2020). Hence, the side effects of ricolinostat (ACY-1215) should not be ignored.
NCT04146896 has completed a novel small molecule called NYX-2925 that modulates the N-methyl-D-aspartate receptor (NMDAR) (Table 2, Line 3). Blocking NMDAR could affect the central pain processing, thereby reducing chronic pain and other associated symptoms (Houck et al., 2019). NYX-2925 modulates NMDARs in a highly specific and selective manner to normalize NMDAR-dependent plasticity, which is a possible mechanism for the long-duration effects observed in these pain models. Notably, NYX-2925 is varying from ketamine (an NMDAR antagonist), rather than fully turning the receptor “on” (agonist) or “off” (antagonist). It is more likely to normalize NMDAR function, thus enhancing communication between neural cells and avoiding the issues associated with overactivation (pain sensitivity) or excessive inhibition (loss of protective reflexes) of NMDAR that have plagued NMDAR-targeted drug development historically. Since no drugs targeting NMDAR have been applied clinically and NMDAR plays an important role in NP, NYX-2925 is expected to be a new direction for NP treatment.
4.1.2. Potential physical means
NCT05322746 is mainly concentrated on focal muscle vibration (FMV), a technique that applies a vibratory stimulus to a specific muscle or its tendon using a mechanical device (Table 2, Line 4). FMV is an innovative form of vibration with non-invasive intervention, which promotes neuroplasticity and durable motor recovery (Murillo et al., 2014). The vibration of a specific muscle can increase the motor-evoked potential, enhance the changes in corticospinal excitability, and produce involuntary contraction in the vibrated muscle (Chandrashekhar et al., 2021). FMV is expected to be a better approach than whole body vibration (WBV), which is effective for reducing PDN-associated pain over the two-and four-week intervals and has persistence in pain reduction beyond the day of treatment (Kessler et al., 2020). Chandrashekhar and colleagues’ pilot work demonstrated a significant improvement in average pain by wearable FMV applied to individuals with PDN (Chandrashekhar et al., 2021). The above preliminary results of wearable FMV are fascinating. Such type of FMV device is simple to wear and easy to use, which may change the management of PDN.
Other potential treatments for PDN include transcranial direct current stimulation (tDCS) (NCT05469074), repetitive transcranial magnetic stimulation (rTMS) (NCT 04833660), moxibustion (NCT05104047), LX9211 (NCT04455633, this drug will be explained below) (Table 2, Line 5–8) and so on. Drug therapy remains the main way to treat PDN, and physiotherapy is of great significance for PDN treatment. However, there is a lack of evidence on the use of combination therapies in combating PDN. The largest combination trial, COMBO-DN, compared the combination of pregabalin and duloxetine at moderate doses with high-dose monotherapy, and found neither approach to be statistically superior (Tesfaye et al., 2013).
4.2. Postherpetic neuralgia
PHN is defined as pain persisting for 3 months after the onset or healing from herpes zoster (HZ), a condition caused by Varizella-Zooster virus (VZV). The disease is caused by reactivation of a latent VZV infection in the sensory ganglia. Pain is the most prominent symptom in 90% of HZ patients (Zhang et al., 2022). The incidence of PHN continues to rise steadily, especially in older patients, possibly due to weakened immunity. PHN is considered as a chronic pain syndrome, which may be attributed to its persistence in a subset of patients over a long period of time (Pappagallo et al., 2000). The standard treatment is a combination of antivirals and pain medications. The commonly used painkillers include topical lidocaine patches, pregabalin, gabapentin and antidepressants (Cui et al., 2018). Although various treatments are available, patients, especially those with chronic PHN, are perpetually disappointed with the effectiveness of pain management and had to endure adverse drug reactions. Therefore, higher requirements for the treatment of PHN need to be put forward. The current trials working on PHN are summarized as follows.
4.2.1. Potential drugs
In NCT04662281, a highly potent and selective adaptor-associated kinase 1 (AAK1) inhibitor, LX9211, is being tested (Table 2, Line 9). AAK1 belongs to the class of numb-associated family of protein kinases (NAKs), including BMP-2-inducible kinase (BIKE/BMP2K) and cyclin G-associated kinase (GAK). AAK1 is widely expressed in the brain, spinal cord, and dorsal root ganglia. LX-9211 demonstrated good efficacy in the STZ-induced diabetic PNP model and CCI model at oral doses of 0.3–1 mg/kg (Luo et al., 2022). The antinociceptive effects of AAK1 inhibitors were induced by the impaired endocytosis of the cell surface levels of μ2-containing GABAA channels. LX-9211 had completed phase I clinical trials and obtained a favorable safety and pharmacokinetics profile supportive of once-daily dosing. This compound received fast track designation by the FDA, and is currently in phase II trials for PDN (NCT04455633) and PHN (NCT04662281). More encouragingly, LX-9211 has significant and consistent benefits in the treatment of PDN, as reported by Lexicon pharmaceuticals Inc. at the 16th annual pain therapeutics summit.
NCT04664530 is a recruiting trial that works on esketamine, a well-known NMDA receptor inhibitor (Table 2, Line 10). Esketamine has both analgesic and antidepressant effects with few incidences of respiratory depression, delirium, hallucinations, nausea and vomiting (Riggs and Gould, 2021). Esketamine has an anti-depressant effect by increasing extracellular glutamate and mTORC1-dependent synapse formation (Shinohara et al., 2021). Anticonvulsants and antidepressants are clinically first-line drugs for the treatment of PHN, which are effective to treat PHN and alleviate patients’ depressive mood due to pain (Anosike et al., 2022). It should be taken into account that the long-term use of esketamine is addictive, limiting its application in treating chronic PHN. Esketamine is more effective against the acute-phase PHN (Riggs and Gould, 2021).
NCT04313335 is a prospective, randomized, open-label, endpoint-blinded study to investigate the preventive efficacy of the prophylactic use of duloxetine during acute herpes zoster (no complication, presents with vesicles within 72 h) on PHN in Beijing Tiantan Hospital (Table 2, Line 11). Duloxetine is a first-line drug for NP by inhibiting the reuptake of serotonin and norepinephrine (Marks et al., 2009). However, preventive intervention on PHN is still rare. A retrospective study revealed that the administration of gabapentin during the acute HZ period significantly decreased the incidence of PHN (Ghanavatian et al., 2019). Prophylactic medication is a novel choice and direction for relieving PHN.
4.2.2. Potential physical means
Electroacupuncture (EA) is a traditional therapy for pain relief via stimulating related acupuncture points (Heo et al., 2021). NCT04560361 was conducted by Nanjing University of Chinese Medicine to explore the effect of EA on PHN (Table 2, Line 12). Another trial NCT03584061 is a pilot study to investigate autologous fat grafting on chronic PHN (Table 2, Line 13). A clinical observation study reported that autologous fat grafting might relieve chronic pain resulting from HZ (Sollie et al., 2021).
In recent years, considerable attention has been paid to the treatment of PHN. Except for those mentioned above, some clinical trials demonstrated that the new calcium channel blocker HSK16149 (NCT05140863) might have a better therapeutic effect (Table 2, Line 14). Efforts to prevent VZV infection at source in different populations will be essential to improve the current poor outcomes associated with HZ infection (Zhang et al., 2022). Prophylactic medication is the current direction for the treatment of PHN, including HZ vaccines for elder people (Lang and Aspinall, 2021). Nevertheless, more drugs warrant further investigations in the near future.
4.3. Sciatica
SC is clinically diagnosed based on the symptoms of radiating pain in one leg with or without associated neurological deficits. SC can lead to severe discomfort and functional limitation with a prevalence of approximately 2–5% (Younes et al., 2006). Patients with SC usually reported aching and a sharp leg pain radiating below the knee and into the foot and toes, or coexisting with low back pain. The pain was described as a sudden or slow onset and varied in severity (Ropper et al., 2015). Inflammation or compression of the lumbosacral nerve roots (L4-S1) forming the sciatic nerve is accounted for SC (Valat et al., 2010). Disk herniation resulting from age-related degenerative changes is the most common cause (Konstantinou et al., 2015). Most patients improved over time with conservative treatment, including exercise, manual therapy and pain management (Jensen et al., 2019). Surgery was performed on serious or progressive neurologic deficits such as motor weakness or bladder dysfunction (Deyo and Mirza, 2016). However, pain medications had uncertain benefit for SC and might have adverse effects (Jensen et al., 2019), while surgery methods had unsatisfactory locating and injection issues. Novel potential treatments on SC are being tested in several clinical trials.
4.3.1. Potential drugs
In NCT03347929, a traditional non-selective NSAID, naproxen, is being tested, which has been approved for the treatment of inflammatory rheumatic conditions, osteoarthritis, primary dysmenorrhea, and musculoskeletal pain (Grøvle et al., 2022; Table 2, Line 15). However, the application of NSAIDs on SC remains a debate. Cochrane review of the existing trials found that NSAIDs were no more effective for pain reduction in SC patients compared with placebo or other drugs (Rasmussen-Barr et al., 2016). However, a survey indicated that 80% American physicians would recommend NSAIDs for the initial management of SC (Webster et al., 2005). The actual effect of NSAIDs on SC needs to be investigated further.
NCT05000658 is recruiting subjects for testing the efficacy of soluble dexamethasone in refractory SC (Table 2, Line 16). Dexamethasone is a synthetic corticosteroid with anti-inflammatory and immunosuppressive properties. National Institute for Health and Care Excellence (NICE) guidelines recommended the epidural injection of local anesthetic and steroid in the lumbar nerve root area for patients with acute and severe SC, where they would otherwise be considered for surgery (Jensen et al., 2019). The current trial is a phase III prospective randomized double-blind trial, which measures the efficacy of soluble dexamethasone by echo-guided infiltration through the sacro-coccygeal hiatus in intractable SC. This is an innovative operation, but its effectiveness may depend on the injection site.
In NCT05196503, the preventive effect of platelet-rich fibrin (PRF) on postsurgical NP after disk herniation surgery is being explored (Table 2, Line 17). PRF was isolated from venous blood without anticoagulant (Choukroun and Ghanaati, 2018). It is a natural fibrin matrix containing cytokines, platelet-derived growth factor (PDGF), bone morphogenetic protein (BMP), insulin-like growth factor-1 (IGF-1), transforming growth factor-β1 (TGF-β1), and vascular endothelial growth factor (VEGF) (Wu et al., 2016). PRF has increasingly been used in regenerative medicine and oral medicine due to its anti-inflammatory and analgesic properties. This effort may be a beneficial trial for preventing postsurgical NP.
4.3.2. Potential physical means
NCT04875741 demonstrates the comparative effects of Neural Mobilization (NM) and Muscle Energy Technique (MET) on SC (Table 2, Line 18). MET was developed by Fred Mitchell. It is a type of physical therapy, and is classified as a common conservative treatment for SC and other pathologies of the spine (Santos et al., 2022). MET is a form of manual or ‘hands-on’ therapy used by osteopathic physicians, chiropractors, and physical therapists. In this treatment type, the muscles of a patient are contracted by pushing against resistance provided by the therapist. The therapist then assists the patient in stretching, strengthening and relaxing those muscles. The goal of MET is to help restore normal muscle and joint mobility (Franke et al., 2015). A meta-analysis indicated that MET was unlikely to improve lumbar dysfunction, but might help reduce the intensity of low back pain, with moderate efficacy in subacute SC (Franke et al., 2015). NM is a movement-based treatment modality used in treating pathologies of the nervous system, which includes both slider and tensioner maneuvers (Plaza-Manzano et al., 2020). The underlying mechanisms of neural mobilization interventions include the restoration of homeostasis in and around the nerves and attenuation of intraneural edema through intraneural fluid dispersion in the nerve root and axon (Boudier-Revéret et al., 2017). Neurodynamic treatment demonstrated a significant reduction in leg pain intensity and improved function after 4 weeks of intervention in patients with chronic SC (Ferreira et al., 2016). The current trial provides strong evidence for the effectiveness of these two methods on SC.
In NCT05129540, whether the foot orthoses can alleviate SC is being evaluated (Table 2, Line 19). This is based on the idea that chronic SC may be related to abnormal stresses applied to the musculoskeletal system during the gait cycle due to foot alterations (Attal and Bouhassira, 2021). However, the trial is not yet recruiting participants.
It is helpful for patients to understand the natural course of SC to relieve symptoms such as pain (Jensen et al., 2019). Treatment of SC is still dominated by physical therapy and supplemented by surgery, but the effectiveness of these medications remains uncertain. Therefore, SC patients may require more effective treatments, including new physiotherapy approaches, advanced surgical methods and novel drug therapies.
4.4. Peripheral nerve injury-related neuropathic pain
PNI-NP is a heterogeneous group of NP conditions caused by a peripheral nerve lesion, e.g., during surgery or trauma (Finnerup et al., 2021). There was a significant correlation between the risk of nerve damage during surgery and the risk of developing chronic NP (Haroutiunian et al., 2013), while the severity of injury and the type (transecting, stretching, crushing) of nerve damage were not significantly associated with the development of NP (Andersen et al., 2014). However, it remains unclear that most patients with nerve damage would develop pain while others did not. The percentage of those patients who experienced pain ranged from 67 to 95% (Teixeira et al., 2015). PNI-NP treatment is subjected to the standards of NP therapy and has special features. A follow-up with an experienced psychotherapist is strongly recommended (Mathews, 2014). Drug treatment, such as tricyclic antidepressants and calcium channel ligands (Teixeira et al., 2015), should be initiated early, which can be either aggressive or progressive. Surgical treatment is another option. Decompression, reconstruction, ablation and modulation are the four basic options for the surgical management of PNI-NP. The most optimal methods and means have been investigated by several researchers in order to better mitigate PNI.
4.4.1. Potential drugs
NCT04967664 was performed to confirm the efficacy and safety of the repeated topical application of Qutenza (capsaicin 8% topical system) versus low-dose capsaicin control (capsaicin 0.04% topical system) in subjects with moderate-to-severe postsurgical NP (Table 2, Line 20). Capsaicin 8% patches are recommended as a second-line therapy for NP, which can be applied for 30–60 min and repeated every 3 months (Finnerup et al., 2021). The possible mechanism underlying this analgesic effect is that capsaicin binds to TRPV1, which is important for pain signal transduction, and both single and repeated application of high capsaicin doses can lead to the desensitization and dysfunction of TRPV1. The most commonly reported adverse events are application site reactions, including dryness, erythema, oedema, pain, papules and pruritus. The analgesic effect of 8% capsaicin patch was better than that of 0.04% capsaicin patch in PHN patients, but the adverse event rates were higher (Backonja et al., 2008). However, it remains unclear how the capsaicin 8% topical system works with PNI-NP.
4.4.2. Potential physical means
In NCT05432973, the efficacy of transcutaneous electrical nerve stimulation (TENS) versus passive-assisted neurodynamics for pain relief in brachial plexus neuropathy is compared (Table 2, Line 21). TENS intervention was defined as pulsed electrical currents generated by a ‘standard TENS device’ administered across the intact surface of the skin using surface electrodes at the site of pain or over nerve bundles proximal (or near) to the site of pain, with the intention of stimulating peripheral nerves to alleviate pain (Johnson, 2021). TENS is a neuromodulation therapy, which has been widely used for symptomatic relief of pain. Physiological evidence suggests that TENS could inhibit the activity and excitability of central nociceptive transmission neurons (Johnson et al., 2022). A meta-TENS study provides moderate-certainty evidence that pain intensity is lower during or immediately after TENS compared with placebo, and without any serious adverse events (Johnson et al., 2022). Hence, TENS is a potential alternative for the treatment of PNI-NP.
The first-line therapy for PNI-NP is etiology treatment. Repair of peripheral nerve injury should be considered, such as reconnecting the damaged nerve through surgery, as well as medication or physical therapy to promote nerve repair. A variety of drugs have been used to relieve PNI-NP, while the nerve damage is hard to repair. The above trial might provide us with more possibility of providing adjuvant treatment on PNI-NP.
4.5. Trigeminal neuralgia
TN is the most common form of craniofacial NP disorder characterized by spontaneous and elicited paroxysms of electric shock–like or stabbing pain affecting one side of the face. Triggered paroxysmal pain is specific to TN, and is reported in 91–99% of NP patients (Di Stefano et al., 2018), indicating that this feature may be pathognomonic of TN. Intracranial vascular compression of the trigeminal nerve root is the main cause of TN (Cruccu et al., 2020). Carbamazepine and oxcarbazepine are recommended as first-line therapies for TN (Cruccu et al., 2008). Surgery is performed only if the standard doses of medications are not sufficient to control the symptoms or the side effects prevent their continuous use (Cruccu et al., 2020). Nonetheless, the first-line drugs have serious side effects, including dizziness, diplopia, ataxia and elevated transaminase levels, resulting in 23% of patients discontinuing medications (Cruccu et al., 2014). Thus, it is necessary to develop new treatment modalities for TN.
4.5.1. Potential drugs
NCT05217628 is a phase II/III trial to test the efficacy and safety of basimglurant (Table 2, Line 22). Basimglurant is a potent, selective, and safe metabotropic glutamate subtype 5 (mGlu5)-negative allosteric modulator with good oral bioavailability and long half-life supportive of once-daily administration in humans (Quiroz et al., 2016). mGluR5 is vital for the release of neurotransmitters and regulating the postsynaptic response of neurotransmitters in the central and peripheral nervous systems. mGlu5 at synaptic and extrasynaptic locations can enhance the activity of NMDA receptors with increasing NMDA currents. Inhibiting the downstream effects of mGlu5 receptor could down-regulate the function of NMDA (Tebano et al., 2005). Basimglurant might achieve positive results for the treatment of TN, except for its previous application on major depression.
In NCT05142228, the TN pain-related outcomes after injection of aimovig under the skin are being evaluated (Table 2, Line 23). Aimovig (erenumab) is the first antibody therapy targeting the calcitonin gene-related peptide (CGRP) receptor with FDA approval for migraine prevention with well tolerated and good safety profile (Wang et al., 2021). CGRP plays a pivotal role in the formation and maintenance of NP (Xie et al., 2017; Wang et al., 2021). This trial is an expanded application of aimovig (erenumab) on TN.
4.5.2. Potential physical means
NCT04505280 was conducted to investigate the efficacy of greater occipital nerve block and cervical injections with lidocaine (Table 2, Line 24). The pharmacological block of the greater occipital nerve has been proven to be effective in numerous headache and facial pain syndromes (Hoffmann et al., 2021). Nociceptive signals induced by stimulation of peripheral trigeminal afferents are transmitted along the trigeminal nerve passing the trigeminal ganglion and enter the brainstem into the trigeminocervical complex (TCC) (Goadsby et al., 2017). Nociceptive information originating in the occipital region of the head is transmitted through the occipital nerve into the TCC (Angus-Leppan et al., 1997). Previous animal studies suggest that neuronal signals originating from trigeminal and occipital neurons converge in the TCC, and within the TCC, around two-thirds of neurons receive afferent input from trigeminal and occipital neurons (Angus-Leppan et al., 1997). An occipital nerve block may inhibit neurons in TCC and relieve TN.
4.6. Chemotherapy-induced neuropathic pain
CINP in cancer patients is usually caused by neurotoxic chemotherapeutic agents that lead to peripheral neuropathies (Staff et al., 2017). CINP is one of the painful polyneuropathies, and is a severe adverse reaction that affects up to 80% of patients during cancer treatment (Sisignano et al., 2016). To date, there are no FDA-approved drugs for preventing or treating CINP, with emerging debate on its underlying mechanisms and therapeutic targets (Bae et al., 2021).
NCT04468230 was performed to assess the efficacy of short-term nicotine transdermal patch administration for the treatment of CINP in cancer stable or remission patients (Table 2, Line 25). Nicotine, the main stimulant in cigarette smoke, was found to have antinociceptive effects in animal and clinical studies (Rowley et al., 2008). A nicotine patch allows less variability in plasma nicotine with a slow steady level delivered through the skin over time. A systematic review and meta-analysis indicated that nicotine patches had an opioid-sparing effect in non-smokers who underwent surgery after general anesthesia, but did not significantly reduce the pain scores (Mishriky and Habib, 2014). Landim et al. found that nicotine patch was effective at controlling pain following third molar surgery (Landim et al., 2020). Nevetheless, the current clinical trial is a meaningful attempt under the condition of local anesthesia, rather than that of general anesthesia.
In NCT05322889, the effectiveness of telmisartan, an angiotensin AT1 receptor blocker, in CINP patients is being tested (Table 2, Line 26). Treatment with telmisartan in mice could lead to a strong reduction of CINP. Interestingly, its analgesic effect was associated with the inhibition of cytochrome P450 oxidase 2 J (CYP2J) rather than angiotensin AT1 receptor (Sisignano et al., 2016). Telmisartan has been on the market for a long time and has a good risk profile, low occurrence of side effects, and is generally well tolerated in patients. Hence, telmisartan may be a promising pharmacological treatment option for CINP patients.
4.7. Others
Numerous clinical trials are not focused on a particular type of PNP, thus, we classified them as GPNP.
NCT04431778 is a phase II trial that assesses the efficacy and tolerance of low ethosuximide (ETX) doses in GPNP patients (Table 2, Line 27). ETX is a low voltage-activated (LVA) calcium channel blocker, which completely removes painful neuropathic symptoms induced by sciatic and spinal nerve ligation in mice (Dogrul et al., 2003; Kerckhove et al., 2019). However, a proof-of-concept, randomized, double-blind and controlled trial (NCT02100046) showed that although ETX had an analgesic effect on NP in vivo, it was not effective for NP patients at a certain dose (30 ml (1,500 mg) per day) (Kerckhove et al., 2018). The current trial aims at testing the effect of low ETX doses on NP patients, which can further strengthen the clinical application of LVA calcium channel blockers.
In NCT04480164, the safety and efficacy of N-desmethylclobazam (NDMC) in GPNP patients are being evaluated (Table 2, Line 28). NDMC is the main metabolite of clobazam (CBZ), and may act as a GABAA receptor inhibitor (Ralvenius et al., 2016). CBZ is a family of benzodiazepine (BDZ), which has a sedative, anti-convulsive, analgesic and antianxiety effect. Previous studies demonstrated that classical BDZ activated α1-subtype GABAA receptors (Ralvenius et al., 2015) and α2-subtype GABAA receptors (Paul et al., 2014), leading to severe side effects (e.g., sedation, addiction and motor impairment). Moreover, the data from transgenic mice also supported that the beneficial effects of antipain could be induced by selective allosteric regulation of GABAA receptors without any cognitive and calming effects (Knabl et al., 2008). NMDC has a high selectivity profile with an obvious preference for GABAA α2-subtypes receptors rather than α1 receptors, which is mainly responsible for its sedative effects (Ralvenius et al., 2016). Since NDMC naturally occurs as the metabolite of CBZ, together with the safety profile known from its parent drug CBZ, it is very unlikely that NDMC has unexpected adverse reactions. Thus, GABA is not a common target for NP treatment, and its possible interaction with NDMC is worthy of further investigation.
In NCT04981925, the predictors of pain relief for mindfulness-based stress reduction (MBSR) are identified (Table 2, Line 29). MBSR is one of the mindfulness-based interventions, and individuals primarily have a non-judgmental attitude toward all experiences (Majeed et al., 2018). MBSR exhibited beneficial effects on the physical and mental health of patients with chronic pain compared to cognitive behavioral therapy (CBT) (Veehof et al., 2011). MBSR treatment had a greater improvement at 26 weeks in low back pain compared with usual care (NCT01467843) (Cherkin et al., 2016). Additionally, MBSR is effective against pain reduction, as well as improvements in physical function, mood and sleep disturbance in patients with chronic pain (Burns et al., 2022). MBSR is a potential therapeutic modality for chronic NP without any side effects. The current trial (NCT04981925) is helpful for the identification of potential molecular/physical targets in NP patients.
5. Central neuropathic pain
5.1. Spinal cord injury-related neuropathic pain
SCI-NP is defined as pain associated with a lesion or disease of the somatosensory nervous system, and remains difficult to treat (Finnerup et al., 2016). SCI-NP is typically experienced at or below the zone of injury, and is classified as sharp, burning or electric (Bryce et al., 2012). Principles of SCI-NP treatments include self-management, psychological interventions, pharmacological treatment, neuromodulation, and personalized medicine (Attal, 2021). SCI-NP is minimally responsive to the existing pharmacological interventions (Boldt et al., 2014), which may have serious adverse events. The intractable nature of SCI-NP is a strong impetus to explore alternative therapeutic approaches.
In NCT04379011, whether brivaracetam can reduce SCI-NP is being explored (Table 2, Line 30). Brivaracetam (an antiepileptic drug) is a synaptic vesicle protein 2 (SVP2) ligand, which displays similar selectivity, but higher affinity than its parent compound levetiracetam (Kenda et al., 2004). Tsymbalyuk et al. reported that brivaracetam significantly reduced microglial activation, TNF expression, and leukocyte infiltration into the dorsal horn, in conjunction with reduced NP behaviors in a murine sciatic nerve injury model (Tsymbalyuk et al., 2019). In real-world clinical practice, brivaracetam is sometimes used to relieve TN, either as monotherapy or in combination (Pergolizzi et al., 2022). Since brivaracetam is a ligand of SVP2, and the latter is a potential target of NP (Kenda et al., 2004), it can serve as a mechanism-based pharmacological intervention for SCI-NP.
NCT04700033 showed that immersive interactive virtual walking could reduce NP, leading to a significant decline in depressive symptomatology among patients with SCI (Trost et al., 2022; Table 2, Line 31). Immersive virtual reality (IVR) provides a completely immersive experience for participants and gives users the feeling of being in a virtual world. However, the mechanism of action is still unclear. The study’s sample size was small, and participants were not randomly assigned to study conditions, thus introducing a potential bias. IVR acts as a new type of wearable device, and may be a promising treatment for SCI-NP.
SCI-NP has lifelong impacts on health and well-being. Therefore, improving the management of this complicated pain condition is an important goal. The translation of knowledge between basic and clinical research, as well as the clinical management of NP after SCI can be realized by uncovering the reliable and valid clinical NP phenotypes (e.g., pain type, pain symptoms, sensory function and dysfunction, and psychosocial factors), translatable biomarkers, and therapeutic targets (Widerstrom-Noga, 2017).
6. Discussion
In this review, we briefly summarize the existing clinical trials on the treatment of NP to enrich the knowledge of potential novel therapeutic strategies, including drug targets, new implications of conventional medicine and physical means. NP treatment is mainly dominated by drug therapy, and physical means have become increasingly popular. These potential strategies offer new hope for NP treatment and encourage us to uncover the mechanisms of NP heterogeneity.
Drug therapy remains the dominant mode of NP therapy. With the development of basic science and technology, there have been increasing numbers of novel molecular targets and molecular compounds of NP being discovered and synthesized, respectively. The AAK1 inhibitor LX9211, which is effective against NP relief in rats, has great potential for PHN treatment (NCT04662281). More importantly, uncovering the mechanisms of NP helps the clinicians to explore the new implications of conventional drugs for pain relief. For example, NDMC is the first drug used to treat NP by targeting GABAARs (NCT04480164). Erenumab might treat TN like its application on migraine since they share the same CRGP pathway (NCT05142228). Optimizing the dosage and duration of conventional drugs might also work well. The current trials have compared the effects of different concentrations of capsaicin patches on various types of NP (NCT04967664), or prophylactic use of duloxetine to reduce PHN (NCT04313335). With a deeper understanding of the mechanisms of NP, we believe that there is great potential for the application of new medicines in the clinical setting.
The inevitable problems with medication are adverse events and possible analgesic effects on different types of NP. Adverse reactions of gabapentin and pregabalin include dizziness, drowsiness, confusion and fatigue, and they are ineffective for SC. Local injection of botulinum toxin A has a beneficial effect on PNI, but a detrimental effect on other types of NP. A large-scale study found that the combination of nortriptyline with morphine at moderate doses was more effective than monotherapy at higher doses for the treatment of PNP (Gilron et al., 2015). The combination of different drugs may reduce potential side effects without diminishing the analgesic effect. Several clinical trials are under way on the combinations of different drugs, which may be a future direction to eliminate or reduce the side effects to a bearable level.
In addition to drug therapies, physical means are getting popular. Patients who enhance their strength and endurance through physical therapy for general health often report a reduction in pain compared with before treatment (Mathews, 2014). Neuromodulation therapy is one of the successful means for NP by promoting neuroplasticity (Knotkova et al., 2021), which includes deep brain and motor cortex stimulation, peripheral nerve stimulation, and the non-invasive treatments of repetitive transcranial magnetic stimulation, transcranial direct current stimulation and TENS. A meta-analysis revealed the analgesic effect of noninvasive brain stimulation (NIBS) on patients with NP, and recommended its clinical application (Gao et al., 2022). Another systematic review and meta-analysis proved the analgesic effect of rTMS on NP and stimulus sites. The combination of stimulus frequency and site as well as the location of pain are critical modulators of the rTMS effect (Jiang et al., 2022). However, the placebo effect of neuromodulation could not be neglected (Knotkova et al., 2021).
With the advancement of technology, several novel physical approaches are shown to be effective in preventing NP, including VR and Sana Pain Reliever (Sana PR). VR could improve patients’ mental state and reduce patients’ anxiety (Trost et al., 2022). The movement of SCI-NP patients could be simulated with the aid of VR. Sana PR is another device to provide Audio Visual Stimulation (AVS), which enhances performance stability and symptom management. These novel devices might help clinicians to have multiple choices in treating NP patients individually.
The current study has some limitations. Firstly, we only searched clinical trials on ClinicalTrials.gov and might miss out potential trials in other databases. Secondly, we did not consider trials completed over 5 years as the inclusion criteria. Thirdly, the current search was done on December 8, 2022 to avoid frequent updates of the database. Practitioner could update the current knowledge on ClinicalTrials.gov with the search term “Neuropathic Pain” in the “Condition or disease” dialog. Those who are interested in a specific pain sub-type or treatment type could apply an advanced search with the search terms of a specific pain sub-type in “Other terms” dialog or choose the “Study type” (Interventional or Observational) below. Fourthly, less attention has been paid to multimodal pain management programs, drug combination therapy, dietary therapy and psychological intervention. Several of them proved their effectiveness for chronic pain. For example, dietary supplementation of gingerols–and shogaols-enriched ginger root extract could attenuate pain-associated behaviors (Shen et al., 2022). Omega-3 fatty acids may be of great benefit in the management of NP patients (Ko et al., 2010). It is worth noting that the combined approaches have become increasingly important in treating complex NP. We paid less attention to those combination treatment, as most of the trials were related to gabapentin or pregabalin, the first-line therapies for patients with NP. Additionally, only a few trials (51/914, 5.6%) reported on the combined therapy on NP. In the future, it is important to consider conducting basic studies to uncover the potential mechanisms of combined therapy, as well as mechanism-based clinical studies. Fifthly, the reasons for retired or terminated trials might provide valuable information for further guidance on NP treatment. However, this information could not be found directly through ClinicalTrials.gov. Trial design, small sample size, failure to achieve the endpoint of outcome, and potential toxic effects in both animal and human experiments could be the reasons behind a retired or terminated trial. Thus, is imperative to consider the potential biases in the study design when interpreting the results. Finally, CNP was seldom discussed and some rare types of NP were not covered, such as occipital neuralgia, pudendal neuralgia and NP in Parkinson’s disease. Despite these limitations, the current review provides researchers and clinicians with new hopes for the treatment of NP and encourages us to invent more therapeutic approaches.
7. Conclusion
NP remains difficult to treat and represents a huge unmet medical need. NP treatment is mainly dominated by drug therapy, and physical means have become increasingly popular. It should be highlighted that novel drug targets, new implications of conventional medicine, and novel physical means are the promising strategies for NP treatment. Nevertheless, more attention needs to be paid to the challenges of translating research findings into clinical practice.
Author contributions
ZD and JZ collected and analyzed the data. ZD, JZ, and XH reviewed and confirmed the data analysis. ZD, JZ, WY, and XG wrote the manuscript. WY and XG designed the project and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Technological Innovation 2030-Major Projects of “Brain Science and Brain-like Research” (2022ZD0206200) to XG, Shanghai Rising-Star Program (21QC1400300) to XG, the promoting project of Renji Hospital (RJTJ23-RC-013) to XG, the Medical Engineering Cross Research Foundation of Shanghai Jiao Tong University (YG2022QN035) to XH, National Natural Science Foundation of China (Nos. 81701092, 81971223, and 32030043) to XG and WY, and Shanghai Engineering Research Center of Peri-operative Organ Support and Function Preservation (20DZ2254200) to WY.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anand, P., and Bley, K. (2011). Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 107, 490–502. doi: 10.1093/bja/aer260
Andersen, K. G., Aasvang, E. K., Kroman, N., and Kehlet, H. (2014). Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta Anaesthesiol. Scand. 58, 1240–1248. doi: 10.1111/aas.12393
Angus-Leppan, H., Lambert, G. A., and Michalicek, J. (1997). Convergence of occipital nerve and superior sagittal sinus input in the cervical spinal cord of the cat. Cephalalgia 17, 625–630. doi: 10.1046/j.1468-2982.1997.1706625.x
Anosike, U. G., Ouko, I., Mwaura, A. W., Ongidi, I., and Mbonu, C. C. (2022). Phenotypes and genotypes in Postherpetic neuralgia drug therapy: a narrative mini-review. Clin. J. Pain 38, 536–540. doi: 10.1097/AJP.0000000000001045
Attal, N. (2021). Spinal cord injury pain. Rev. Neurol. (Paris) 177, 606–612. doi: 10.1016/j.neurol.2020.07.003
Attal, N., and Bouhassira, D. (2021). Advances in the treatment of neuropathic pain. Curr. Opin. Neurol. 34, 631–637. doi: 10.1097/WCO.0000000000000980
Backonja, M., Wallace, M. S., Blonsky, E. R., Cutler, B. J., Malan, P., Rauck, R., et al. (2008). NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 7, 1106–1112. doi: 10.1016/S1474-4422(08)70228-X
Bae, E. H., Greenwald, M. K., and Schwartz, A. G. (2021). Chemotherapy-induced peripheral neuropathy: mechanisms and therapeutic avenues. Neurotherapeutics 18, 2384–2396. doi: 10.1007/s13311-021-01142-2
Boldt, I., Eriks-Hoogland, I., Brinkhof, M. W. G., de Bie, R., Joggi, D., and von Elm, E. (2014). Non-pharmacological interventions for chronic pain in people with spinal cord injury. Cochrane Database Syst. Rev. 11:CD009177. doi: 10.1002/14651858.CD009177.pub2
Boudier-Revéret, M., Gilbert, K. K., Allégue, D. R., Moussadyk, M., Brismée, J. M., Sizer, P. S. Jr., et al. (2017). Effect of neurodynamic mobilization on fluid dispersion in median nerve at the level of the carpal tunnel: a cadaveric study. Musculoskelet. Sci. Pract. 31, 45–51. doi: 10.1016/j.msksp.2017.07.004
Bryce, T. N., Biering-Sørensen, F., Finnerup, N. B., Cardenas, D. D., Defrin, R., Lundeberg, T., et al. (2012). International spinal cord injury pain classification: part I. background and description. March 6-7, 2009. Spinal Cord 50, 413–417. doi: 10.1038/sc.2011.156
Burns, J. W., Jensen, M. P., Thorn, B., Lillis, T. A., Carmody, J., Newman, A. K., et al. (2022). Cognitive therapy, mindfulness-based stress reduction, and behavior therapy for the treatment of chronic pain: randomized controlled trial. Pain 163, 376–389. doi: 10.1097/j.pain.0000000000002357
Chandrashekhar, R., Wang, H., Dionne, C., James, S., and Burzycki, J. (2021). Wearable focal muscle vibration on pain, balance, mobility, and sensation in individuals with diabetic peripheral neuropathy: a pilot study. Int. J. Environ. Res. Public Health 18:2415. doi: 10.3390/ijerph18052415
Chen, C., Liu, A., Lu, Q., Luo, L., Li, J., Ke, J., et al. (2022). HDAC6 inhibitor ACY-1215 improves neuropathic pain and its comorbidities in rats of peripheral nerve injury by regulating neuroinflammation. Chem. Biol. Interact. 353:109803. doi: 10.1016/j.cbi.2022.109803
Cherkin, D. C., Sherman, K. J., Balderson, B. H., Cook, A. J., Anderson, M. L., Hawkes, R. J., et al. (2016). Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on Back pain and functional limitations in adults with chronic Low Back pain: a randomized clinical trial. JAMA 315, 1240–1249. doi: 10.1001/jama.2016.2323
Choukroun, J., and Ghanaati, S. (2018). Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients' own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg. 44, 87–95. doi: 10.1007/s00068-017-0767-9
Cruccu, G., Di Stefano, G., and Truini, A. (2020). Trigeminal Neuralgia. N. Engl. J. Med. 383, 754–762. doi: 10.1056/NEJMra1914484
Cruccu, G., Gronseth, G., Alksne, J., Argoff, C., Brainin, M., Burchiel, K., et al. (2008). AAN-EFNS guidelines on trigeminal neuralgia management. Eur. J. Neurol. 15, 1013–1028. doi: 10.1111/j.1468-1331.2008.02185.x
Cruccu, G., Pennisi, E. M., Antonini, G., Biasiotta, A., di Stefano, G., la Cesa, S., et al. (2014). Trigeminal isolated sensory neuropathy (TISN) and FOSMN syndrome: despite a dissimilar disease course do they share common pathophysiological mechanisms? BMC Neurol. 14:248. doi: 10.1186/s12883-014-0248-2
Cui, J. Z., Zhang, J. W., Yan, F., Yang, X. N., Wang, X. L., Zhao, Z. B., et al. (2018). Effect of single intra-cutaneous injection for acute thoracic herpes zoster and incidence of Postherpetic neuralgia. Pain Manag. Nurs. 19, 186–194. doi: 10.1016/j.pmn.2017.09.002
Devor, M., Wall, P. D., and Catalan, N. (1992). Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain 48, 261–268. doi: 10.1016/0304-3959(92)90067-L
Deyo, R. A., and Mirza, S. K. (2016). CLINICAL PRACTICE. Herniated lumbar intervertebral disk. N. Engl. J. Med. 374, 1763–1772. doi: 10.1056/NEJMcp1512658
Di Stefano, G., Maarbjerg, S., Nurmikko, T., Truini, A., and Cruccu, G. (2018). Triggering trigeminal neuralgia. Cephalalgia 38, 1049–1056. doi: 10.1177/0333102417721677
Dogrul, A., Gardell, L. R., Ossipov, M. H., Tulunay, C. F., Lai, J., and Porreca, F. (2003). Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain 105, 159–168. doi: 10.1016/S0304-3959(03)00177-5
Ferreira, G., Stieven, F., Araujo, F., Wiebusch, M., Rosa, C., Plentz, R., et al. (2016). Neurodynamic treatment did not improve pain and disability at two weeks in patients with chronic nerve-related leg pain: a randomised trial. J. Physiother. 62, 197–202. doi: 10.1016/j.jphys.2016.08.007
Finnerup, N. B., Attal, N., Haroutounian, S., McNicol, E., Baron, R., Dworkin, R. H., et al. (2015). Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14, 162–173. doi: 10.1016/S1474-4422(14)70251-0
Finnerup, N. B., Haroutounian, S., Kamerman, P., Baron, R., Bennett, D. L. H., Bouhassira, D., et al. (2016). Neuropathic pain: an updated grading system for research and clinical practice. Pain 157, 1599–1606. doi: 10.1097/j.pain.0000000000000492
Finnerup, N. B., Kuner, R., and Jensen, T. S. (2021). Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 101, 259–301. doi: 10.1152/physrev.00045.2019
Franke, H., Fryer, G., Ostelo, R. W. J. G., and Kamper, S. J., Cochrane Back and Neck Group (2015). Muscle energy technique for non-specific low-back pain. Cochrane Database Syst. Rev. 2:CD009852. doi: 10.1002/14651858.CD009852.pub2
Gao, C., Zhu, Q., Gao, Z., Zhao, J., Jia, M., and Li, T. (2022). Can noninvasive brain stimulation improve pain and depressive symptoms in patients with neuropathic pain? A systematic review and meta-analysis. J. Pain Symptom Manag. 64, e203–e215. doi: 10.1016/j.jpainsymman.2022.05.002
Gaskin, D. J., and Richard, P. (2012). The economic costs of pain in the United States. J. Pain 13, 715–724. doi: 10.1016/j.jpain.2012.03.009
Ghanavatian, S., Wie, C. S., Low, R. S., Zhang, N., Montoya, J. M., Dhaliwal, G. S., et al. (2019). Premedication with gabapentin significantly reduces the risk of Postherpetic neuralgia in patients with neuropathy. Mayo Clin. Proc. 94, 484–489. doi: 10.1016/j.mayocp.2018.11.004
Gilron, I., Tu, D., Holden, R. R., Jackson, A. C., and DuMerton-Shore, D. (2015). Combination of morphine with nortriptyline for neuropathic pain. Pain 156, 1440–1448. doi: 10.1097/j.pain.0000000000000149
Goadsby, P. J., Holland, P. R., Martins-Oliveira, M., Hoffmann, J., Schankin, C., and Akerman, S. (2017). Pathophysiology of migraine: a disorder of sensory processing. Physiol. Rev. 97, 553–622. doi: 10.1152/physrev.00034.2015
Grøvle, L., Hasvik, E., Holst, R., and Haugen, A. J. (2022). NSAIDs in sciatica (NIS): study protocol for an investigator-initiated multicentre, randomized placebo-controlled trial of naproxen in patients with sciatica. Trials 23:493. doi: 10.1186/s13063-022-06441-3
Haroutiunian, S., Nikolajsen, L., Finnerup, N. B., and Jensen, T. S. (2013). The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 154, 95–102. doi: 10.1016/j.pain.2012.09.010
Heo, I., Shin, B. C., Cho, J. H., Ha, I. H., Hwang, E. H., Lee, J. H., et al. (2021). Multicentre randomised controlled clinical trial of electroacupuncture with usual care for patients with non-acute pain after back surgery. Br. J. Anaesth. 126, 692–699. doi: 10.1016/j.bja.2020.10.038
Hoffmann, J., Mehnert, J., Koo, E. M., and May, A. (2021). Greater occipital nerve block modulates nociceptive signals within the trigeminocervical complex. J. Neurol. Neurosurg. Psychiatry 92, 1335–1340. doi: 10.1136/jnnp-2021-326433
Houck, D. R., Sindelar, L., Sanabria, C. R., Stanworth, S. H., Krueger, M., Suh, M., et al. (2019). NYX-2925, a novel N-methyl-D-aspartate receptor modulator: a First-in-human, randomized, double-blind study of safety and pharmacokinetics in adults. Clin. Transl. Sci. 12, 164–171. doi: 10.1111/cts.12584
Jensen, T. S., Baron, R., Haanpää, M., Kalso, E., Loeser, J. D., Rice, A. S. C., et al. (2011). A new definition of neuropathic pain. Pain 152, 2204–2205. doi: 10.1016/j.pain.2011.06.017
Jensen, R. K., Kongsted, A., Kjaer, P., and Koes, B. (2019). Diagnosis and treatment of sciatica. BMJ 367:l6273. doi: 10.1136/bmj.l6273
Jiang, X., Yan, W., Wan, R., Lin, Y., Zhu, X., Song, G., et al. (2022). Effects of repetitive transcranial magnetic stimulation on neuropathic pain: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 132, 130–141. doi: 10.1016/j.neubiorev.2021.11.037
Johnson, M. I. (2021). Resolving long-standing uncertainty about the clinical efficacy of transcutaneous electrical nerve stimulation (TENS) to relieve pain: A comprehensive review of factors influencing outcome. Medicina (Kaunas) 57:378. doi: 10.3390/medicina57040378
Johnson, M. I., Paley, C. A., Jones, G., Mulvey, M. R., and Wittkopf, P. G. (2022). Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open 12:e051073. doi: 10.1136/bmjopen-2021-051073
Kenda, B. M., Matagne, A. C., Talaga, P. E., Pasau, P. M., Differding, E., Lallemand, B. I., et al. (2004). Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J. Med. Chem. 47, 530–549. doi: 10.1021/jm030913e
Kerckhove, N., Boudieu, L., Ourties, G., Bourdier, J., Daulhac, L., Eschalier, A., et al. (2019). Ethosuximide improves chronic pain-induced anxiety–and depression-like behaviors. Eur. Neuropsychopharmacol. 29, 1419–1432. doi: 10.1016/j.euroneuro.2019.10.012
Kerckhove, N., Pereira, B., Soriot-Thomas, S., Alchaar, H., Deleens, R., Hieng, V. S., et al. (2018). Efficacy and safety of a T-type calcium channel blocker in patients with neuropathic pain: a proof-of-concept, randomized, double-blind and controlled trial. Eur. J. Pain 22, 1321–1330. doi: 10.1002/ejp.1221
Kessler, N. J., Lockard, M. M., and Fischer, J. (2020). Whole body vibration improves symptoms of diabetic peripheral neuropathy. J. Bodyw. Mov. Ther. 24, 1–3. doi: 10.1016/j.jbmt.2020.01.004
Knabl, J., Witschi, R., Hösl, K., Reinold, H., Zeilhofer, U. B., Ahmadi, S., et al. (2008). Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451, 330–334. doi: 10.1038/nature06493
Knotkova, H., Hamani, C., Sivanesan, E., le Beuffe, M. F. E., Moon, J. Y., Cohen, S. P., et al. (2021). Neuromodulation for chronic pain. Lancet 397, 2111–2124. doi: 10.1016/S0140-6736(21)00794-7
Ko, G. D., Nowacki, N. B., Arseneau, L., Eitel, M., and Hum, A. (2010). Omega-3 fatty acids for neuropathic pain: case series. Clin. J. Pain 26, 168–172. doi: 10.1097/AJP.0b013e3181bb8533
Konstantinou, K., Dunn, K. M., Ogollah, R., Vogel, S., and Hay, E. M. (2015). Characteristics of patients with low back and leg pain seeking treatment in primary care: baseline results from the ATLAS cohort study. BMC Musculoskelet. Disord. 16:332. doi: 10.1186/s12891-015-0787-8
Kremer, M., Salvat, E., Muller, A., Yalcin, I., and Barrot, M. (2016). Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience 338, 183–206. doi: 10.1016/j.neuroscience.2016.06.057
Landim, F. S., Laureano Filho, J. R., Nascimento, J., and do Egito Vasconcelos, B. C. (2020). Effectiveness of nicotine patch for the control of pain, oedema, and trismus following third molar surgery: a randomized clinical trial. Int. J. Oral Maxillofac. Surg. 49, 1508–1517. doi: 10.1016/j.ijom.2019.08.013
Lang, P. O., and Aspinall, R. (2021). Vaccination for quality of life: herpes-zoster vaccines. Aging Clin. Exp. Res. 33, 1113–1122. doi: 10.1007/s40520-019-01374-5
Li, Q., Lu, J., Zhou, X., Chen, X., Su, D., Gu, X., et al. (2019). High-voltage-activated Calcium Channel in the afferent pain pathway: an important target of pain therapies. Neurosci. Bull. 35, 1073–1084. doi: 10.1007/s12264-019-00378-5
LoPresti, P. (2020). HDAC6 in diseases of cognition and of neurons. Cells 10:12. doi: 10.3390/cells10010012
Luo, G., Chen, L., Kostich, W. A., Hamman, B., Allen, J., Easton, A., et al. (2022). Discovery of (S)-1-((2′,6-Bis(difluoromethyl)-[2,4′-bipyridin]-5-yl)oxy)-2,4-dimethylpentan-2-amine (BMS-986176/LX-9211): a highly selective, CNS penetrable, and orally active adaptor Protein-2 associated kinase 1 inhibitor in clinical trials for the treatment of neuropathic pain. J. Med. Chem. 65, 4457–4480. doi: 10.1021/acs.jmedchem.1c02131
Majeed, M. H., Ali, A. A., and Sudak, D. M. (2018). Mindfulness-based interventions for chronic pain: evidence and applications. Asian J. Psychiatr. 32, 79–83. doi: 10.1016/j.ajp.2017.11.025
Marks, D. M., Shah, M., Patkar, A., Masand, P., Park, G. Y., and Pae, C. U. (2009). Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr. Neuropharmacol. 7, 331–336. doi: 10.2174/157015909790031201
Mathews, M. (2014). Multimodal treatment of pain. Neurosurg. Clin. N. Am. 25, 803–808. doi: 10.1016/j.nec.2014.07.005
Mishriky, B. M., and Habib, A. S. (2014). Nicotine for postoperative analgesia: a systematic review and meta-analysis. Anesth. Analg. 119, 268–275. doi: 10.1213/ANE.0b013e3182a8fa7b
Murillo, N., Valls-Sole, J., Vidal, J., Opisso, E., Medina, J., and Kumru, H. (2014). Focal vibration in neurorehabilitation. Eur. J. Phys. Rehabil. Med. 50, 231–242.
Pappagallo, M., Oaklander, A. L., Quatrano-Piacentini, A. L., Clark, M. R., and Raja, S. N. (2000). Heterogenous patterns of sensory dysfunction in postherpetic neuralgia suggest multiple pathophysiologic mechanisms. Anesthesiology 92, 691–698. doi: 10.1097/00000542-200003000-00013
Paul, J., Yévenes, G. E., Benke, D., Lio, A. D., Ralvenius, W. T., Witschi, R., et al. (2014). Antihyperalgesia by alpha2-GABAA receptors occurs via a genuine spinal action and does not involve supraspinal sites. Neuropsychopharmacology 39, 477–487. doi: 10.1038/npp.2013.221
Pergolizzi, J. V., Gharibo, C., Magnusson, P., Breve, F., LeQuang, J. A., and Varrassi, G. (2022). Pharmacotherapeutic management of trigeminal neuropathic pain: an update. Expert. Opin. Pharmacother. 23, 1155–1164. doi: 10.1080/14656566.2022.2087507
Plaza-Manzano, G., Cancela-Cilleruelo, I., Fernández-de-las-Peñas, C., Cleland, J. A., Arias-Buría, J. L., Thoomes-de-Graaf, M., et al. (2020). Effects of adding a Neurodynamic mobilization to motor control training in patients with lumbar radiculopathy due to disc herniation: a randomized clinical trial. Am. J. Phys. Med. Rehabil. 99, 124–132. doi: 10.1097/PHM.0000000000001295
Ponirakis, G., Elhadd, T., Chinnaiyan, S., Hamza, A. H., Sheik, S., Kalathingal, M. A., et al. (2021). Prevalence and risk factors for diabetic neuropathy and painful diabetic neuropathy in primary and secondary healthcare in Qatar. J. Diabetes Investig. 12, 592–600. doi: 10.1111/jdi.13388
Quiroz, J. A., Tamburri, P., Deptula, D., Banken, L., Beyer, U., Rabbia, M., et al. (2016). Efficacy and safety of Basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA Psychiat. 73, 675–684. doi: 10.1001/jamapsychiatry.2016.0838
Ralvenius, W. T., Acuña, M. A., Benke, D., Matthey, A., Daali, Y., Rudolph, U., et al. (2016). The clobazam metabolite N-desmethyl clobazam is an alpha2 preferring benzodiazepine with an improved therapeutic window for antihyperalgesia. Neuropharmacology 109, 366–375. doi: 10.1016/j.neuropharm.2016.07.004
Ralvenius, W. T., Benke, D., Acuña, M. A., Rudolph, U., and Zeilhofer, H. U. (2015). Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat. Commun. 6:6803. doi: 10.1038/ncomms7803
Rasmussen-Barr, E., Held, U., Grooten, W. J. A., Roelofs, P. D. D. M., Koes, B. W., van Tulder, M. W., et al. (2016). Non-steroidal anti-inflammatory drugs for sciatica. Cochrane Database Syst. Rev. 2017:CD012382. doi: 10.1002/14651858.CD012382
Riggs, L. M., and Gould, T. D. (2021). Ketamine and the future of rapid-acting antidepressants. Annu. Rev. Clin. Psychol. 17, 207–231. doi: 10.1146/annurev-clinpsy-072120-014126
Ropper, A. H., Longo, D. L., and Zafonte, R. D. (2015). Sciatica. N. Engl. J. Med. 372, 1240–1248. doi: 10.1056/NEJMra1410151
Rosenberger, D. C., Blechschmidt, V., Timmerman, H., Wolff, A., and Treede, R. D. (2020). Challenges of neuropathic pain: focus on diabetic neuropathy. J. Neural Transm. (Vienna) 127, 589–624. doi: 10.1007/s00702-020-02145-7
Rowley, T. J., Payappilly, J., Lu, J., and Flood, P. (2008). The antinociceptive response to nicotinic agonists in a mouse model of postoperative pain. Anesth. Analg. 107, 1052–1057. doi: 10.1213/ane.0b013e318165e0c0
Santos, G. K., de Oliveira, R. G., de Oliveira, L. C., de Oliveira, C. F. C., Andraus, R. A., Ngomo, S., et al. (2022). Effectiveness of muscle energy technique in patients with nonspecific low back pain: a systematic review with meta-analysis. Eur. J. Phys. Rehabil. Med. 58, 827–837. doi: 10.23736/S1973-9087.22.07424-X
Scholz, J., Finnerup, N. B., Attal, N., Aziz, Q., Baron, R., Bennett, M. I., et al. (2019). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160, 53–59. doi: 10.1097/j.pain.0000000000001365
Shen, C. L., Wang, R., Ji, G., Elmassry, M. M., Zabet-Moghaddam, M., Vellers, H., et al. (2022). Dietary supplementation of gingerols–and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 100:108904. doi: 10.1016/j.jnutbio.2021.108904
Shinohara, R., Aghajanian, G. K., and Abdallah, C. G. (2021). Neurobiology of the rapid-acting antidepressant effects of ketamine: impact and opportunities. Biol. Psychiatry 90, 85–95. doi: 10.1016/j.biopsych.2020.12.006
Sisignano, M., Angioni, C., Park, C. K., Meyer dos Santos, S., Jordan, H., Kuzikov, M., et al. (2016). Targeting CYP2J to reduce paclitaxel-induced peripheral neuropathic pain. Proc. Natl. Acad. Sci. U. S. A. 113, 12544–12549. doi: 10.1073/pnas.1613246113
Sollie, M., Thomsen, J. B., and Sorensen, J. A. (2021). Autologous fat grafting seems to alleviate postherpetic neuralgia–a feasibility study investigating patient-reported levels of pain. J. Plast. Reconstr. Aesthet. Surg. 74, 350–356. doi: 10.1016/j.bjps.2020.08.038
Staff, N. P., Grisold, A., Grisold, W., and Windebank, A. J. (2017). Chemotherapy-induced peripheral neuropathy: a current review. Ann. Neurol. 81, 772–781. doi: 10.1002/ana.24951
Talbot, S., Chahmi, E., Dias, J. P., and Couture, R. (2010). Key role for spinal dorsal horn microglial kinin B1 receptor in early diabetic pain neuropathy. J. Neuroinflammation 7:36. doi: 10.1186/1742-2094-7-36
Tebano, M. T., Martire, A., Rebola, N., Pepponi, R., Domenici, M. R., Gro, M. C., et al. (2005). Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J. Neurochem. 95, 1188–1200. doi: 10.1111/j.1471-4159.2005.03455.x
Teixeira, M. J., da Paz, M. G., Bina, M. T., Santos, S. N., Raicher, I., Galhardoni, R., et al. (2015). Neuropathic pain after brachial plexus avulsion-central and peripheral mechanisms. BMC Neurol. 15:73. doi: 10.1186/s12883-015-0329-x
Tesfaye, S., Wilhelm, S., Lledo, A., Schacht, A., Tölle, T., Bouhassira, D., et al. (2013). Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”–a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 154, 2616–2625. doi: 10.1016/j.pain.2013.05.043
Trost, Z., Anam, M., Seward, J., Shum, C., Rumble, D., Sturgeon, J., et al. (2022). Immersive interactive virtual walking reduces neuropathic pain in spinal cord injury: findings from a preliminary investigation of feasibility and clinical efficacy. Pain 163, 350–361. doi: 10.1097/j.pain.0000000000002348
Tsymbalyuk, S., Smith, M., Gore, C., Tsymbalyuk, O., Ivanova, S., Sansur, C., et al. (2019). Brivaracetam attenuates pain behaviors in a murine model of neuropathic pain. Mol. Pain 15:6503. doi: 10.1177/1744806919886503
Valat, J.-P., Genevay, S., Marty, M., Rozenberg, S., and Koes, B. (2010). Sciatica. Best Pract. Res. Clin. Rheumatol. 24, 241–252. doi: 10.1016/j.berh.2009.11.005
Van Hecke, O., Austin, S. K., Khan, R. A., Smith, B. H., and Torrance, N. (2014). Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155, 654–662. doi: 10.1016/j.pain.2013.11.013
Veehof, M. M., Oskam, M. J., Schreurs, K. M. G., and Bohlmeijer, E. T. (2011). Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 152, 533–542. doi: 10.1016/j.pain.2010.11.002
Wang, L. L., Wang, H. B., Fu, F. H., and Yu, L. C. (2021). Role of calcitonin gene-related peptide in pain regulation in the parabrachial nucleus of naive rats and rats with neuropathic pain. Toxicol. Appl. Pharmacol. 414:115428. doi: 10.1016/j.taap.2021.115428
Webster, B. S., Courtney, T. K., Huang, Y. H., Matz, S., and Christiani, D. C. (2005). Physicians' initial management of acute low back pain versus evidence-based guidelines. Influence of sciatica. J. Gen. Intern. Med. 20, 1132–1135. doi: 10.1111/j.1525-1497.2005.0230.x
Widerstrom-Noga, E. (2017). Neuropathic pain and spinal cord injury: phenotypes and pharmacological management. Drugs 77, 967–984. doi: 10.1007/s40265-017-0747-8
Wu, M., Chen, G., and Li, Y. P. (2016). TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 4:16009. doi: 10.1038/boneres.2016.9
Xie, H., Lu, F., Liu, W., Wang, E., Wang, L., and Zhong, M. (2021). Remimazolam alleviates neuropathic pain via regulating bradykinin receptor B1 and autophagy. J. Pharm. Pharmacol. 73, 1643–1651. doi: 10.1093/jpp/rgab080
Xie, X., Pascual, C., Lieu, C., Oh, S., Wang, J., Zou, B., et al. (2017). Analgesic microneedle patch for neuropathic pain therapy. ACS Nano 11, 395–406. doi: 10.1021/acsnano.6b06104
Younes, M., Béjia, I., Aguir, Z., Letaief, M., Hassen-Zrour, S., Touzi, M., et al. (2006). Prevalence and risk factors of disk-related sciatica in an urban population in Tunisia. Joint Bone Spine 73, 538–542. doi: 10.1016/j.jbspin.2005.10.022
Zhang, Y., Gao, Y. L., and Zhang, L. H. (2022). Methylprednisolone and local anesthetic for long-term postherpetic neuralgia: a meta-analysis. J. Dermatolog. Treat. 33, 2723–2729. doi: 10.1080/09546634.2022.2067820
Keywords: neuropathic pain, clinical trial, drug targets, physical means, therapeutic strategies
Citation: Du Z, Zhang J, Han X, Yu W and Gu X (2023) Potential novel therapeutic strategies for neuropathic pain. Front. Mol. Neurosci. 16:1138798. doi: 10.3389/fnmol.2023.1138798
Edited by:
Yan Lu, Fourth Military Medical University, ChinaReviewed by:
Fabien Marchand, INSERM U1107 Douleur et Biophysique Neurosensorielle (Neuro-Dol), FranceJacqueline Sagen, University of Miami, United States
Copyright © 2023 Du, Zhang, Han, Yu and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Yu, eXdmODA4QHllYWgubmV0; Xiyao Gu, Z3h5a2V2aW5AMTI2LmNvbQ==
†These authors have contributed equally this work
 Zelu Du
Zelu Du Jian Zhang
Jian Zhang Xu Han
Xu Han Weifeng Yu
Weifeng Yu Xiyao Gu
Xiyao Gu