- 1Department of Medicine, Qingdao University, Qingdao, Shandong, China
- 2Department of Neurosurgery, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Introduction
Anxiety disorders are common chronic mental illnesses with complex causes, a high relapse rate, and a high prevalence of depressive symptoms (Zwanzger, 2016). Anxiety disorders are characterized by panic, nervousness, and irritability, as well as somatic and behavioral symptoms (Nechita et al., 2018). Anxiolytics are frequently used in conjunction with antidepressants in clinical practice to treat anxiety disorders. Despite the fact that the therapeutic effect is satisfactory, the drug combination causes uncontrollable side effects (Behlke et al., 2020). As a result, 273 million people worldwide suffer psychological and physical pain as a result of anxiety disorders (Whiteford et al., 2013). It was recently discovered that the midbrain dopamine system is a critical structure that regulates mood, motivation, reward, and salience. Furthermore, anxiety and depression are linked to the midbrain dopamine system (Mitsi and Zachariou, 2016). In 2013, Chaudhury et al. (2013) discovered that the ventral tegmental area (VTA) of the midbrain led to a sub-circulation of dopaminergic neurons in the nucleus accumbens (NAc) that rapidly modulated depression-related behavior. Similarly, VTA can be projected onto the basolateral amygdala (BLA), a critical part of the human brain that regulates anxiety processes (Felix-Ortiz et al., 2013). However, the neural circuitry mechanisms in anxiety disorders and anxious depressive states remain unknown. Furthermore, the relationship between VTA-BLA dopamine neurons and anxiety disorders requires additional research.
The decreasing activity of VTA project to BLA neurons induces the anxiety-like behavior
Because anxiety and depression frequently coexist, the line between these two disorders has always been blurred (Demyttenaere and Heirman, 2020). We learned from the World Health Organization's (WHO) International Classification of Diseases (ICD) that symptoms of anxiety and depression frequently overlap. Anxiety disorders can also cause depressed moods and loss of interest, and depression can cause anxiety. However, the two diseases have distinct typical symptoms (Fang et al., 2019; Williamson et al., 2021). Unconditioned panic attacks, hyperarousal, and compulsions can occur in patients with anxiety disorders. Depression patients exhibit core symptoms such as a lack of pleasure, negative psychology, and emotion. As the midbrain limbic dopamine system has been studied further in recent years, researchers have discovered that the amygdala receives DA neuronal projections from the VTA and is involved in the emergence of anxiety-like behavior (Nguyen et al., 2021). Under optogenetic conditions, VTA-BLA and CeA-VTA DA neurons successfully modulate anxiety-like behavior in mice (Jiang et al., 2021). Furthermore, depression-like behavior in mice is regulated by VTA-PFC, VTA-NAC, LDTg-VTA, and LC-VTA DA neurons (Saddoris et al., 2015; Isingrini et al., 2016; Fernandez et al., 2018; Huang et al., 2020). A VTA is a complex brain structure made up of 60% dopaminergic neurons (DA neurons), 35% GABAergic neurons (GABA neurons), and 5% glutamatergic neurons (Yamaguchi et al., 2015). It regulates the release of neurotransmitters and peptides, which controls reward consumption, learning, memory, and addictive behavior (Polter and Kauer, 2014; Morales and Margolis, 2017). The VTA also has close connections to various brain regions whose input and output projections constitute a complex network of behavioral relationships in the VTA (Figure 1).
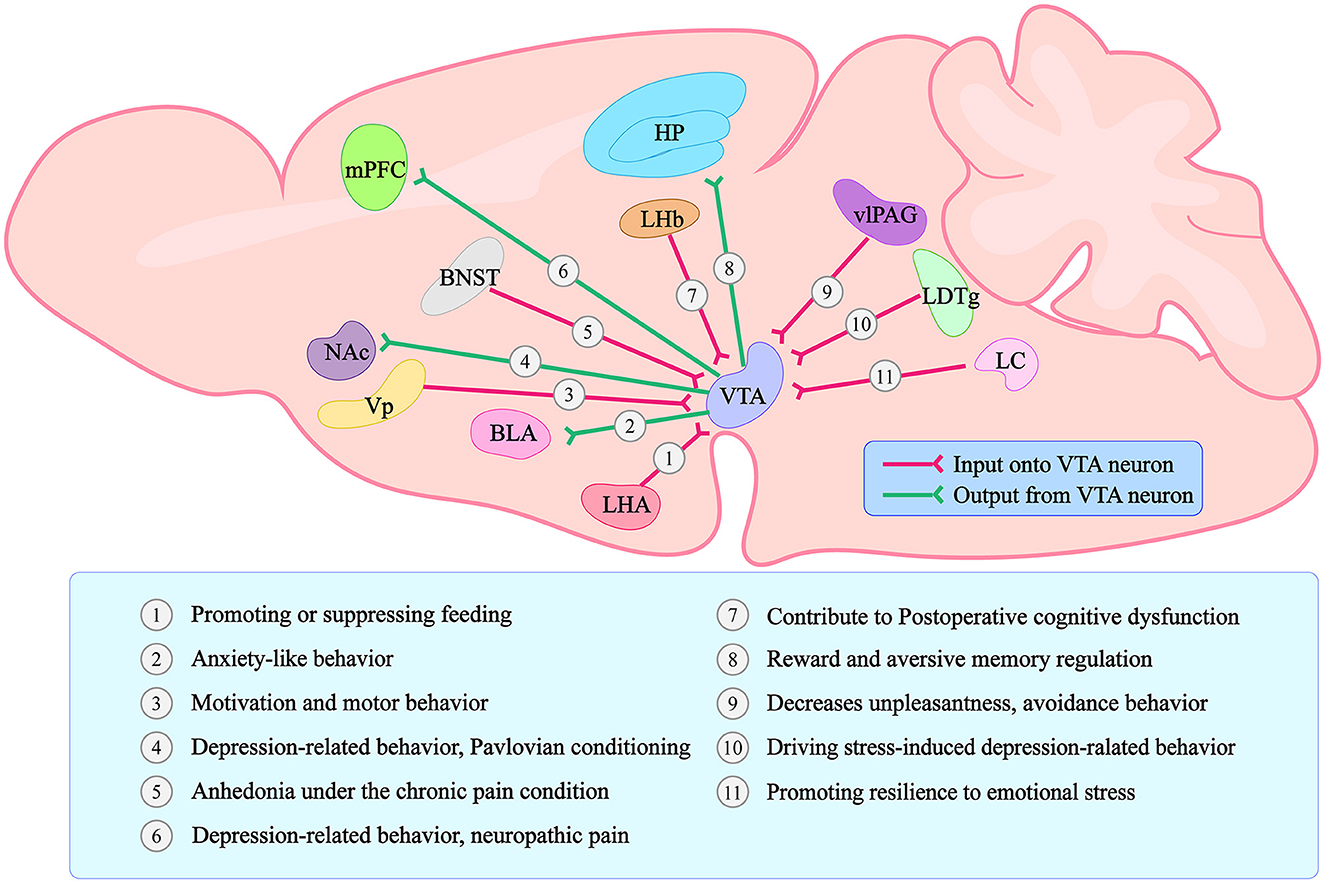
Figure 1. Graphical summary of the VTA connections with other brain regions and the main functions regarding each different projection. Forms and positions can only represent the general structure and position. The lines with arrows only represent a simplified schematic of the general projection pattern. VTA stands for Ventral Tegmental Area; LHA stands for lateral hypothalamic area; BLA stands for basolateral amygdala; Vp stands for ventral pallidum; NAc stands for Nucleus Accumbens; BNST stands for bed nucleus of the stria terminalis; mPFC stands for medial prefrontal Cortex; LHb stands for lateral habenula; HP stands for Hippocampus; vlPAG stands for ventrolateral periaqueductal gray; LDTg stands for laterodorsal tegmentum; LC stands for locus coeruleus.
Morel et al. (2022) conducted experiments based on the preceding research to investigate the relationship between VTA-BLA neurons and anxiety disorders (Morel et al., 2022). The chronic social failure stress paradigm (CSDS) was used to first induce different phenotypes in C57BL/6J mice in this study. Following CSDS, mice of various phenotypes were subjected to depression-related social interaction test (SI), female urine sniffing test (FUST), and sucrose preference test (SP), as well as anxiety-related elevated plus maze (EPM) or open field test (OFT). They discovered that time spent in EPM open arms was related to time spent in the center of the open field using correlation data analysis. Time spent in EPM open arms, on the other hand, was not clearly related to social avoidance behavior. Female urine and sucrose preferences were also related to SI behavior but not to anxiety-like behavior test timing. It demonstrated that anxiety-like behavior was independent of depression-like behavior. The researchers then used a dual viral strategy to selectively label VTA projections to NAc and BLA neurons and counted the number of co-labeled neurons (only 2.7%). It was also shown that anxiety-like behavior was independent of depression-like behavior. Furthermore, VTA-BLA dopamine neurons in anxious mice had lower excitability, higher rheobase, Ih current, and sag amplitudes following whole-cell patch-clamp recordings and a video-tracking system synchronized with the fiber photometry system. All of these findings indicate that VTA-BLA dopamine neuronal activity is linked to anxiety-like behavior. Finally, Morel et al. (2022) used optogenetics to control the activity of VTA-BLA neurons selectively. The mice were found to have less time in the EPM open arm and the OFT center when subjected to sub-threshold social defeat stress (Sub.D) by using NpHR-optogenetic means to inhibit the activity of VTA-BLA neurons. In contrast, the mice increased their time in the EPM open arm and in the OFT center by using ChR2-optogenetic means to stimulate the activity of VTA-BLA neurons. As a result, Morel et al. discovered that VTA-BLA neurons regulate anxious behavior (Figure 2).
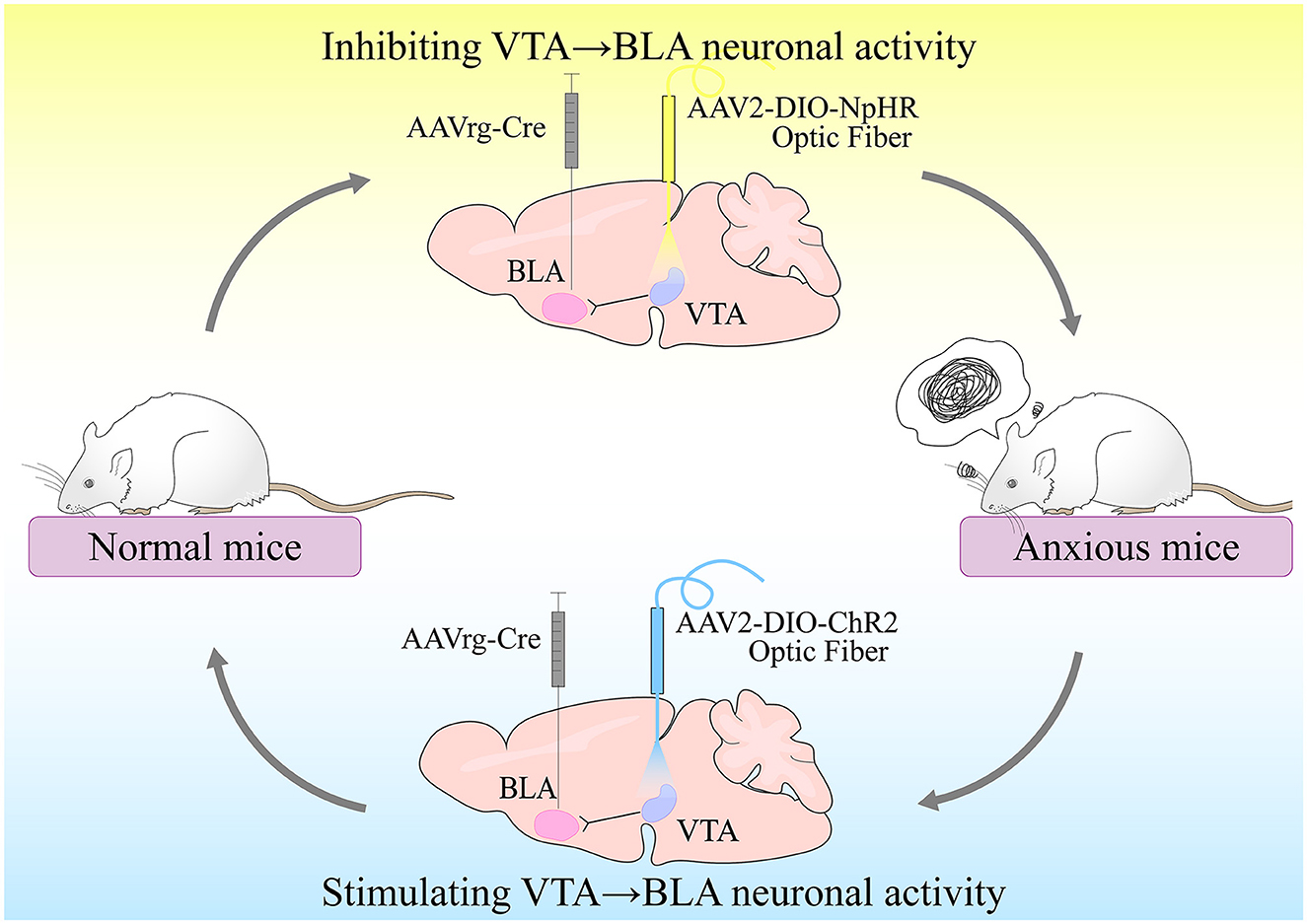
Figure 2. VTA-BLA neuronal activity controls anxiety-like behavior. Inhibition of VTA-BLA neuronal activity could produce anxiety-like behavior; activation of VTA-BLA neuronal activity could produce anxiolytic effects.
The study challenged the previously held belief that anxiety and depression share neural circuits and investigated the link between anxiety-like behavior and the midbrain dopamine system, which not only improved understanding of the midbrain dopamine system but also provided practical experimental thinking to investigate neural circuits. The experimental procedure of this study is impressive, but we still wish to discuss some details. First, NAc could not only receive neural projections from BLA, but it could also induce changes in anxiety behavior in previous studies (Zhang et al., 2020; Huang et al., 2021; Khastkhodaei et al., 2021). Sun et al. discovered that the VTA-BLA-NAc neural circuit regulates reward effects and motivated behavior (Sun et al., 2021). So, whether the presence of 2.7% of co-labeled neurons could indicate that anxiety-like behavior was not completely independent of depression-like behavior. Second, the VTA is a heterogeneous structure in which GABAergic and glutamatergic neurons modulate DA neurons (Gordon-Fennell and Stuber, 2021; Miranda-Barrientos et al., 2021). GABA neurons in the VP, for example, can be projected into VTA, which acts on DA neurons and modulates motivation; glutamatergic neurons in the LHb can also be projected into VTA, which inhibits DA neurons and modulates reward (Omelchenko et al., 2009; Hjelmstad et al., 2013). Whether the authors' electrophysiological experiments require them to differentiate DA neurons and selectively inhibit peripheral neurons. Finally, the authors did not explore the role of VTA-NAc dopaminergic neurons in anxiety (Nguyen et al., 2021). Whether adding control experiments can highlight the specific regulation of anxiety by VTA-BLA and make the experiment more complete. Whether the use of separate retrograde tracers in the same slice to record different dopaminergic neurons in VTA-NAC and VTA-BLA, would allow for more standardization of the experimental procedure.
Anxiety disorders have previously been linked to differential methylation of specific genes (MAOA, CRHR1, OXTR) (Schartner et al., 2017), and functional magnetic resonance imaging has revealed that the temporal and prefrontal regions of the brain respond differently in patients with anxiety disorders (Marin et al., 2020). In the treatment of anxiety disorders, the use of selective serotonin reuptake inhibitors (SSRI), selective serotonin norepinephrine reuptake inhibitors (SNRI), buspirone, benzodiazepines, and other drugs, in conjunction with psychotherapy that can enhance the effect of antidepressants (Strawn et al., 2018), has yielded positive results. However, the use of anti-anxiety medications causes many side effects in patients with anxiety disorders, such as allergies, headaches, gastrointestinal disorders, and so on (Balon and Starcevic, 2020; Panayotis et al., 2021). Furthermore, when selecting a drug, clinicians must consider a number of factors, including the patient's age, comorbidities, and tolerability (Katzman et al., 2014). Thanks to the efforts of Carole Morel and others, we have turned our perspective to the neuroscience research based on VTA-BLA, which will be the crucial part for the development of new anti-anxiety drugs. Anxiety is a common and non-negligible psychiatric symptom of the world's two most common neurodegenerative diseases, Alzheimer's disease (AD) and Parkinson's disease (PD) (Schrag and Taddei, 2017; Mendez, 2021). As a result, this research provides new hope for improving the quality of life of people suffering from neurodegenerative diseases.
Discussion
The co-morbidity of anxiety and depression presents a clinical treatment and diagnostic challenge that has plagued countless patients and physicians (Choi et al., 2020). By demonstrating that VTA-BLA induces anxiety-like behavior via optogenetics, this study provided new insights into anxiety and depression and inspired the development of novel anxiolytic drugs. It also allowed us to complement further the neurobehavioral network associated with the VTA. In 2021, Nguyen et al. discovered that activation of VTA-amygdala DA neurons blocked the anxiety effects of nicotine (Nguyen et al., 2021); however, Jiang et al. (2021) discovered that activating corticotrophin-releasing hormone-mediated CeA-VTA terminals increased opioid withdrawal-induced anxiety and inhibiting CeA-VTA decreased anxiety. That is the inverse direction of the VTA-BLA regulation of anxiety. Because BLA and CeA are two critical functional regions in the amygdala, we wondered whether the anxiety-related VTA-BLA-CeA closed neural loop exists and what kind of connection exists between BLA and CeA.
Anxiety is a non-motor symptom that is common in AD and PD. Krashia et al. (2022) have recently proposed the VTA dopamine system as a therapeutic target for neuropsychiatric symptoms of AD. And PD is caused by the selective deletion of dopaminergic neurons in the dense part of the substantia nigra (SNc) (Tang et al., 2020; Zhang et al., 2021). Interestingly, Suzuki et al., found that a reduction in the number of VTA DA neurons was observed after unilateral injection of 6-hydroxydopamine (6-OHDA) in the midbrain of mice, and Alvarsson et al. (2016) found that overexpression of α-synuclein within the VTA after 3 weeks of adenovirus injection could lead to motor disability (Suzuki et al., 2010). It made us wonder whether VTA neural circuits are associated with the deficiency of SNc dopaminergic neurons and whether VTA could also be a new therapeutic target for PD.
Author contributions
JZ, PS, and HL conceived the article. JZ and HL wrote the first draft and reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32000795), Shandong Province Natural Science Foundation (ZR2020QC095), China Postdoctoral Science Foundation (2017M622129), and Special Fund for Youth of Applied Foundational Research Program of Qingdao (No. 19-6-2-43-cg).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alvarsson, A., Caudal, D., Björklund, A., and Svenningsson, P. (2016). Emotional memory impairments induced by AAV-mediated overexpression of human α-synuclein in dopaminergic neurons of the ventral tegmental area. Behav. Brain Res. 296, 129–133. doi: 10.1016/j.bbr.08034
Balon, R., and Starcevic, V. (2020). Role of benzodiazepines in anxiety disorders. Adv. Exp. Med. Biol. 1191, 20. doi: 10.1007./978-981-32-9705-0_20
Behlke, L. M., Lenze, E. J., and Carney, R. M. (2020). The cardiovascular effects of newer antidepressants in older adults and those with or at high risk for cardiovascular diseases. CNS Drugs 34, 1133–1147. doi: 10.1007./s40263-020-00763-z
Chaudhury, D., Walsh, J. J., Friedman, A. K., Juarez, B., and Kuet, S. M. (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 7433. doi: 10.1038./nature11713
Choi, K. W., Kim, Y. K., and Jeon, H. J. (2020). Comorbid anxiety and depression: clinical and conceptual consideration and transdiagnostic treatment. Adv. Exp. Med. Biol. 1191, 219–235. doi: 10.1007./978-981-32-9705-0_14
Demyttenaere, K., and Heirman, E. (2020). The blurred line between anxiety and depression: hesitations on comorbidity, thresholds and hierarchy. Int. Rev. Psychiatry. 32, 455–465. doi: 10.1080./09540261.2020.1764509
Fang, H., Tu, S., Sheng, J., and Shao, A. (2019). Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 23, 2324–2332. doi: 10.1111./jcmm.14170
Felix-Ortiz, A. C., Beyeler, A., Seo, C., Leppla, C. A., and Wildeset, C. P. (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 79, 658–664. doi: 10.1016/j.neuron.06016
Fernandez, S. P., Broussot, L., Marti, F., Contesse, T., and Mouskaet, X. (2018). Mesopontine cholinergic inputs to midbrain dopamine neurons drive stress-induced depressive-like behaviors. Nat. Commun. 9, 4449. doi: 10.1038./s41467-018-06809-7
Gordon-Fennell, A., and Stuber, G. D. (2021). Illuminating subcortical GABAergic and glutamatergic circuits for reward and aversion. Neuropharmacology. 198, 108725. doi: 10.1016./j.neuropharm.2021.108725
Hjelmstad, G. O., Xia, Y., Margolis, E. B., and Fields, H. L. (2013). Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J. Neurosci. 33, 6454–6459. doi: 10.1523./jneurosci.0178-13.2013
Huang, L., Chen, Y., Jin, S., Lin, L., and Duanet, S. (2021). Organizational principles of amygdalar input-output neuronal circuits. Mol. Psychiatry. 26, 7118–7129. doi: 10.1038./s41380-021-01262-3
Huang, S., Zhang, Z., Gambeta, E., Xu, S. C., and Thomaset, C. (2020). Dopamine inputs from the ventral tegmental area into the medial prefrontal cortex modulate neuropathic pain-associated behaviors in mice. Cell Rep. 31, 107812. doi: 10.1016./j.celrep.2020.107812
Isingrini, E., Perret, L., Rainer, Q., Amilhon, B., and Gumaet, E. (2016). Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci. 19, 560–563. doi: 10.1038./nn.4245
Jiang, C., Yang, X., He, G., Wang, F., and Wanget, Z. (2021). CRH (CeA → VTA) inputs inhibit the positive ensembles to induce negative effect of opiate withdrawal. Mol. Psychiatry. 26, 6170–6186. doi: 10.1038./s41380-021-01321-9
Katzman, M. A., Bleau, P., Blier, P., Chokka, P., and Kjernistedet, K. (2014). Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress, and obsessive-compulsive disorders. BMC Psychiatry. 14(Suppl 1), 1–83. doi: 10.1186./1471-244x-14-s1-s1
Khastkhodaei, Z., Muthuraman, M., Yang, J. W., Groppa, S., and Luhmann, H. J. (2021). Functional and directed connectivity of the cortico-limbic network in mice in vivo. Brain Struct. Funct. 226, 685–700. doi: 10.1007/s00429-020-02202-7
Krashia, P., Spoleti, E., and D'Amelio, M. (2022). The VTA dopaminergic system as diagnostic and therapeutical target for Alzheimer's disease. Front Psychiatry 13, 685–700. doi: 10.3389./fpsyt.2022.1039725
Marin, M. F., Hammoud, M. Z., Klumpp, H., Simon, N. M., and Milad, M. R. (2020). Multimodal categorical and dimensional approaches to understanding threat conditioning and its extinction in individuals with anxiety disorders. JAMA Psychiatry 77, 618–627. doi: 10.1001./jamapsychiatry.2019.4833
Mendez, M. F. (2021). The relationship between anxiety and Alzheimer's disease. J Alzheimers Dis Rep. 5, 171–177. doi: 10.3233./adr-210294
Miranda-Barrientos, J., Chambers, I., Mongia, S., Liu, B., and Wanget, H. L. (2021). Ventral tegmental area GABA, glutamate, and glutamate-GABA neurons are heterogeneous in their electrophysiological and pharmacological properties. Eur. J. Neurosci. 54, 4061–4084. doi: 10.1111./ejn.15156
Mitsi, V., and Zachariou, V. (2016). Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience 338, 81–92. doi: 10.1016/j.neuroscience.05017
Morales, M., and Margolis, E. B. (2017). Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85. doi: 10.1038./nrn.2016.165
Morel, C., Montgomery, S. E., Li, L., Durand-de Cuttoli, R., Teichman, E. M., Juarez, B., et al. (2022). Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors. Nat. Commun. 13, 1532. doi: 10.1038./s41467-022-29155-1
Nechita, D., Nechita, F., and Motorga, R. (2018). A review of the influence the anxiety exerts on human life. Rom. J. Morphol. Embryol. 59, 1045–1051. Available online at: https://rjme.ro/RJME/resources/files/59041810451051.pdf
Nguyen, C., Mondoloni, S., Le Borgne, T., Centeno, I., and Comeet, M. (2021). Nicotine inhibits the VTA-to-amygdala dopamine pathway to promote anxiety. Neuron. 109, 2604–2615. doi: 10.1016/j.neuron.0013
Omelchenko, N., Bell, R., and Sesack, S. R. (2009). Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur. J. Neurosci. 30, 6294. doi: 10.1111./j.1460-9568.2009.06924.x
Panayotis, N., Freund, P. A., Marvaldi, L., Shalit, T., and Brandiset, A. (2021). β-sitosterol reduces anxiety and synergizes with established anxiolytic drugs in mice. Cell Rep Med. 2, 100281. doi: 10.1016./j.xcrm.2021.100281
Polter, A. M., and Kauer, J. A. (2014). Stress and VTA synapses: implications for addiction and depression. Eur. J. Neurosci. 39, 1179–1188. doi: 10.1111./ejn.12490
Saddoris, M. P., Cacciapaglia, F., Wightman, R. M., and Carelli, R. M. (2015). Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J Neurosci. 35, 11572–11582. doi: 10.1523./jneurosci.2344-15.2015
Schartner, C., Ziegler, C., Schiele, M. A., Kollert, L., and Weberet, H. (2017). CRHR1 promoter hypomethylation: an epigenetic readout of panic disorder? Eur. Neuropsychopharmacol. 27, 360–371. doi: 10.1016/j.euroneuro.01005
Schrag, A., and Taddei, R. N. (2017). Depression and anxiety in Parkinson's disease. Int. Rev. Neurobiol. 133, 623–655. doi: 10.1016/bs.irn.05024
Strawn, J. R., Geracioti, L., Rajdev, N., Clemenza, K., and Levine, A. (2018). Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin. Pharmacother. 19, 1057–1070. doi: 10.1080./14656566.2018.1491966
Sun, L., You, J., Sun, F., Cui, M., and Wanget, J. (2021). Reactivating a positive feedback loop VTA-BLA-NAc circuit associated with positive experience ameliorates the attenuated reward sensitivity induced by chronic stress. Neurobiol Stress. 15, 100370. doi: 10.1016./j.ynstr.2021.100370
Suzuki, K., Okada, K., Wakuda, T., Shinmura, C., and Kamenoet, Y. (2010). Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine. PLoS ONE 5, 9260. doi: 10.1371./journal.pone.0009260
Tang, J., Lu, L., Wang, Q., Liu, H., and Xueet, W. (2020). Crocin reverses depression-like behavior in parkinson disease mice via VTA-mPFC pathway. Mol. Neurobiol. 57, 3158–3170. doi: 10.1007./s12035-020-01941-2
Whiteford, H. A., Degenhardt, L., Rehm, J., Baxter, A. J., Ferrari, A. J., Erskine, H. E., et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 382, 9904. doi: 10.1016./s0140-6736(13)61611-6
Williamson, J. B., Jaffee, M. S., and Jorge, R. E. (2021). Post-traumatic stress disorder and anxiety-related conditions. Continuum 27, 1738–1763. doi: 10.1212./con.0000000000001054
Yamaguchi, T., Qi, J., Wang, H. L., Zhang, S., and Morales, M. (2015). Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur. J. Neurosci. 41, 760–772. doi: 10.1111./ejn.12818
Zhang, J., Liu, H., and Jiang, H. (2021). Commentary: dopamine-dependent early synaptic and motor dysfunctions induced by α-synuclein in the nigrostriatal circuit. Front. Aging Neurosci. 13, 3477–3491. doi: 10.3389./fnagi.2021.790224
Zhang, X. Y., Peng, S. Y., Shen, L. P., Zhuang, Q. X., and Liet, B. (2020). Targeting presynaptic H3 heteroreceptor in nucleus accumbens to improve anxiety and obsessive-compulsive-like behaviors. Proc. Natl. Acad. Sci. USA. 117, 32155–32164. doi: 10.1073./pnas.2008456117
Keywords: anxiety disorders, midbrain, ventral tegmental area, basolateral amygdala, anxiolytic
Citation: Zhao J, Sun P and Liu H (2023) Commentary: Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors. Front. Mol. Neurosci. 16:1117121. doi: 10.3389/fnmol.2023.1117121
Received: 06 December 2022; Accepted: 27 January 2023;
Published: 08 February 2023.
Edited by:
Xiao-Dong Wang, Zhejiang University, ChinaReviewed by:
Fabio Marti, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceLei Xiao, Fudan University, China
Copyright © 2023 Zhao, Sun and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heng Liu,  bGl1aGVuZzUxNkAxNjMuY29t;
bGl1aGVuZzUxNkAxNjMuY29t;  aGVuZ2xpdUBxZHUuZWR1LmNu
aGVuZ2xpdUBxZHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Jihu Zhao
Jihu Zhao Peng Sun2†
Peng Sun2† Heng Liu
Heng Liu