
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Neurosci. , 30 January 2023
Sec. Molecular Signalling and Pathways
Volume 16 - 2023 | https://doi.org/10.3389/fnmol.2023.1074703
This article is part of the Research Topic Myelin in White Matter Function and Related Diseases View all 7 articles
A correction has been applied to this article in:
Corrigendum: Network pharmacology integrated with experimental validation to explore the therapeutic role and potential mechanism of Epimedium for spinal cord injury
 Xuanhao Fu†
Xuanhao Fu† Boyuan Ma†
Boyuan Ma† Mengmeng Zhou†
Mengmeng Zhou† Yuelin Cheng
Yuelin Cheng Linyan Liu
Linyan Liu Shunli Kan
Shunli Kan Chengjiang Liu
Chengjiang Liu Xinyan Zhao
Xinyan Zhao Sa Feng
Sa Feng Haoqiang Zhu
Haoqiang Zhu Wei Hu
Wei Hu Zehua Jiang
Zehua Jiang Rusen Zhu*
Rusen Zhu*Objective: Epimedium (EPI) is a common Chinese herb with neuroprotective effects against a variety of central nervous system disorders, especially spinal cord injury (SCI). In this study, we performed network pharmacology and molecular docking analyses to reveal the mechanism underlying EPI treatment of SCI, then validated its efficacy using animal models.
Methods: The active ingredients and targets of EPI were screened by Traditional Chinese Medicine Systems Pharmacology (TCMSP) and their targets annotated on the UniProt platform. SCI-related targets were searched from OMIM, TTD, and GeneCards databases. We employed the STRING platform to construct a protein–protein interaction (PPI) network then visualized the results using Cytoscape (3.8.2) software. We also subjected key EPI targets to ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, then docked the main active ingredients with the key targets. Finally, we established an SCI rat model to evaluate efficacy of EPI in treating SCI and validate the effects of different biofunctional modules predicted by network pharmacology.
Results: A total of 133 EPI targets were associated with SCI. GO terms and KEGG pathway enrichment results showed that EPI’s effect in treating SCI was significantly associated with inflammatory response, oxidative stress and the PI3K/AKT signaling pathway. Molecular docking results indicated that EPI’s active ingredients have a high affinity for the key targets. Results from animal experiments revealed that EPI not only markedly improved Basso, Beattie, and Bresnahan scores in SCI rats, but also significantly improved p-PI3K/PI3K and p-AKT/AKT ratio. Moreover, EPI treatment not only mediated a significant decrease in malondialdehyde (MDA) but also increased both superoxide dismutase (SOD), and glutathione (GSH). However, this phenomenon was successfully reversed by LY294002, a PI3K inhibitor.
Conclusion: EPI improves behavioral performance in SCI rats through anti-oxidative stress, which may be mediated by activation of the PI3K/AKT signaling pathway.
Spinal cord injury (SCI) is a serious traumatic disease of the central nervous system, characterized by a high disability rate, resulting in abnormalities or loss of motor, sensory, and autonomic functions (Ahuja et al., 2017). SCI has a high annual incidence rate worldwide, about 40–80/1,000,000 people, a phenomenon that results in heavy economic burden on individuals, families, and society (Squair et al., 2016; Kumar et al., 2018; Alizadeh et al., 2019). Both primary and secondary injuries have been associated with SCI pathophysiology (Witiw and Fehlings, 2015). While primary injury refers to mechanical and direct injury to the spinal cord, secondary injury denotes several pathophysiological processes building on the primary injury, such as oxidative stress, inflammatory response, and apoptosis (Orr and Gensel, 2017; Zrzavy et al., 2021). Continuous and extensive cell death, coupled with tissue damage eventually cause permanent sensory and motor impairment (Liu et al., 2020). Although SCI currently lacks effective prevention and treatment modalities, numerous investigations have associated marked reduction of secondary injury with significant alleviation of disability rates in SCI patients (Song et al., 2021; Zhao et al., 2021). Therefore, promoting recovery of neurological function after SCI has great potential as a prevention and treatment modality for secondary injury.
Epimedium (EPI), a genus in family Berberidaceae with about 52 species, has been found to contain more than 260 compounds (Wu et al., 2003). In traditional Korean and Chinese clinics, EPI has been used for treatment of central nervous system-related diseases, such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, due to its neuroplasticity (Cho et al., 2017). In addition, EPI significantly restored motor function in rats after SCI, possibly by activating thePI3K/AKT signaling pathway (Li et al., 2019). On the other hand, Li demonstrated that EPI inhibited the mitochondrial apoptotic pathway to attenuate pro-inflammatory molecules and oxidative stress, which may be a key mechanism for enhanced motor recovery after SCI (Li et al., 2018). These evidences suggest that EPI is a very promising area of research in spinal cord repair and neurological recovery after SCI. To date, however, its necessitating pharmacological effects and underlying mechanisms of action in SCI remain unclear.
Network pharmacology, first proposed by Hopkins in the UK, is a technique used to systematically study the biological effects of complex drugs by integrating drug action with biological networks based on systems biology and multidirectional pharmacology (Hopkins, 2007). Numerous studies have shown that network pharmacology is a practical approach for systematic exploration of the relationship between herbal medicines and diseases (Wang et al., 2022; Zhou et al., 2022). In this study, we employed a compounds-targets network, based on pharmacology technique, in combination with molecular docking and animal experiments to reveal the effect of EPI and underlying mechanism of action in SCI. The overall flowchart of this study is shown in Figure 1.
Traditional Chinese Medicine Systems Pharmacology (TCMSP) is a comprehensive pharmacological database of traditional Chinese medicine, comprising 3,311 targets and 29,384 components of 499 Chinese herbal medicines included in The Chinese Pharmacopoeia (Ru et al., 2014). In the process of drug screening, oral bioavailability (OB) is one of the most important pharmacokinetic parameters in the ADME characteristics (i.e., absorption, distribution, metabolism, excretion) of a drug, and is also an important indicator of the relative amount of drug delivered to the systemic circulation when administered orally. Drug-like properties represent the similarity of a compound to known drugs, and compounds with a high drug-like index (DL) are more likely to become drugs. We screened out active ingredients that met the various criteria, namely drug-likeness (DL) ≥ 0.18 and oral bioavailability (OB) ≥ 30% (Tsaioun et al., 2016; Sheng et al., 2021). Additionally, we obtained information related to the active ingredients ‘target proteins, then added gene names to the target protein using UniProt (https://www.Uniprot.org/; UniProt Consortium, 2018).
We searched GeneCards (Stelzer et al., 2016), OMIM (Hamosh et al., 2005), and TTD (Wang et al., 2020) databases for SCI targets using “Spinal cord Injury” as a keyword (retrieved 17/11/2021), then processed the disease targets to obtain SCI-related targets. Finally, we analyzed the intersection between EPI-related and SCI-related targets using VennDiagram packages implemented in R software.
We employed the STRING database to build a PPI network, we uploaded the potential therapeutic targets of EPI for SCI to the STRING database and set the parameter to an interaction value >0.7 (Merico et al., 2010; Szklarczyk et al., 2017). We then used the CytoNCA plug-in in Cytoscape (3.8.2) software to obtain the key targets.
Next, we explored the biological processes and signaling pathways associated with EPI in treatment of SCI by performing GO and KEGG pathway enrichment analyses using the clusterProfiler package in R (Kanehisa et al., 2008; Gene Ontology, 2015). Statistical significance threshold of enrichment analysis was set at p ≤ 0.05.
The interrelationships between key targets and active ingredients of EPI were visualized using the Sankey diagram. We also generated 3D structures of the active compounds using Chem Office software. These 3D structures were then downloaded from the PDB database, then the proteins dehydrated and dephosphorylated using PyMOL software (Rose et al., 2018). Autodock Vina was used to assess molecular docking (Trott and Olson, 2010).
Adult female Sprague–Dawley rats (10-week-old) were purchased from Beijing Huafukang Biotechnology Co (license no: SCXK (Jing) 2019-008). Prior to experimental treatments, all rats were acclimated for 7 days in a controlled environment under a 12-h light/dark cycle with free access to food and drink. All animal experiments were approved by the Experimental Animal Ethics Committee of Tianjin People’s Hospital. Rats were anesthetized by intraperitoneal injection (ip), using 1% sodium pentobarbital (50 mg/kg), with the appropriate anesthetic effect confirmed by the pain reflex in the tail. After anesthesia, the rats were fixed in prone position, and the dorsum routinely prepared and disinfected with skin. The rats were subjected to laminectomy, at the Tenth thoracic vertebra (T10) level by incising each subcutaneous layer sequentially to fully expose the T10 segment of the spinal cord, which was not subject to impact injury. Next, a 10-gram impactor device (diameter: 2 mm) was lowered into the spinal cord from a height of 5 cm, to generate moderate spinal cord contusion (Yacoub et al., 2014). After modeling, the skin and muscle were stitched and the skin disinfected again with an iodophor. Rats in the sham group were subjected to laminectomy in the absence of contusion. To prevent infection, the rats were given penicillin, administered intramuscularly at a dose of 16 × 104 U per animal once daily for 3 consecutive days from the date of surgery. After surgery, the bladder was massaged twice a day, in the morning and evening, before its activity was restored.
EPI was decocted twice (1 g/10 ml; 1.5 h each) in distilled water, then combined. The combined extracts were cooled to room temperature, and subsequently stored at −20°C in dry, sealed and contamination-free conditions until further use. LY294002 (154447-36-6), a PI3K inhibitor, was purchased from Aladdin, China and administered by intraperitoneal injection. After surgery, 40 rats were randomly divided into 4 groups and orally administered with EPI extract for 4 weeks. The treatments were as follows: sham group (sham surgery, 0.135 g/kg per day distilled water), SCI group (SCI, 0.135 g/kg per day distilled water), SCI + EPI group (SCI, 0.135 g/kg per day EPI), and SCI + EPI + LY group (SCI, 0.135 g/kg per day EPI, 1.2 mg/kg per day LY294002). Since oral administration is the main route of herbal drug delivery, we administered EPI orally to rats. The EPI dosage was adopted from the Chinese pharmacopeia clinical dose converted to body surface area (State Pharmacopoeia Commission of the PRC, 2015).
Researchers, who were completely blinded to the study, assessed hind limb function of the rats by observing their hip, knee, and ankle joint walking movements for 5 min at1, 7, 14, 21, and 28 days post-operation. The function of the hind limbs was evaluated according to the Basso, Beattie, and Bresnahan (BBB) scores, where 0 and 21 considered complete paralysis and normal gait, respectively.
Spinal cord tissues were obtained from the rats on the 28th day after operation. Firstly, ventricular perfusion with 0.9% physiological saline was performed under anesthesia, and 4% paraformaldehyde (PFA) injected to fix the tissue. Next, tissue from the center of the injury (1 cm) was obtained and immediately stored at-80°C to await western blot analysis. For H&E staining, spinal cord samples were fixed with 4% PFA, dehydrated in ethanol, dipped in wax, embedded in paraffin and cut into 6 μm sections using a microtome. The sections were then dewaxed and rehydrated, then stained with hematoxylin followed by eosin. The sections were permeabilized using xylene then visualized under a light microscope.
Spinal cord tissues, 1.0 cm around the center of the lesion, were collected from 3 individual rats and lysed in RIPA buffer (P0013B Beyotime, China) with a protease inhibitor cocktail (ST506 Beyotime, China). The tissue lysates were centrifuged at 12,000 rpm for 10 min at 4°C, and protein concentration determined using the double Bicinchoninic Acid (BCA) method (P0010, Beyotime, China). Equal concentrations of protein samples were separated on a 12% SDS polyacrylamide gel electrophoresis, then transferred to a polyvinylidene fluoride membrane. Next, the PVDF papers were blocked using skim milk for 1.5 h, then incubated overnight with the primary antibodies against GAPDH (1: 1000, Goodhere), phosphor-Akt (1: 3000, Proteintech Group), Akt (1: 2000, Affinity), phosphor-PI3K (1:2000, CST), and PI3K (1: 1000, Abcam) at 4°C. The membranes were washed 3 times using Tris-buffered saline Tween, then incubated with the secondary antibodies for 1 h at room temperature. Using ImageJ software, chemiluminescence was identified and measured.
Data were first tested for normality using the Shapiro–Wilk normality test, then normally-distributed data subjected to one-way analysis of variance (ANOVA). Both non-normally distributed data and those with non-uniform variance were subjected to the Kruskal Wallis analysis. Statistical significance was set at p < 0.05.
Using OB ≥ 30% and DL ≥ 0.18 as filtering conditions resulted in a total of 24 active EPI ingredients from the TCMSP database (Table 1). From these ingredients, we screened out 204 EPI-related targets using the TCMSP database and standardized by the UniProt database.
Combining the three databases, coupled with deleting of repeated targets resulted in 2456 SCI-related targets by GeneCards, OMIM, and TDD databases. Analysis of the intersection between 204 EPI-related and 2,456 and SCI-related targets, resulted in 133 potential targets of EPI for SCI (Figure 2).
Based on 133 potential targets and 24 active ingredients, the compounds-targets network diagram of EPI to treat SCI was plotted (Figure 3). Collectively, these results suggested that EPI may act synergistically through multiple components and multiple targets during treatment of SCI.
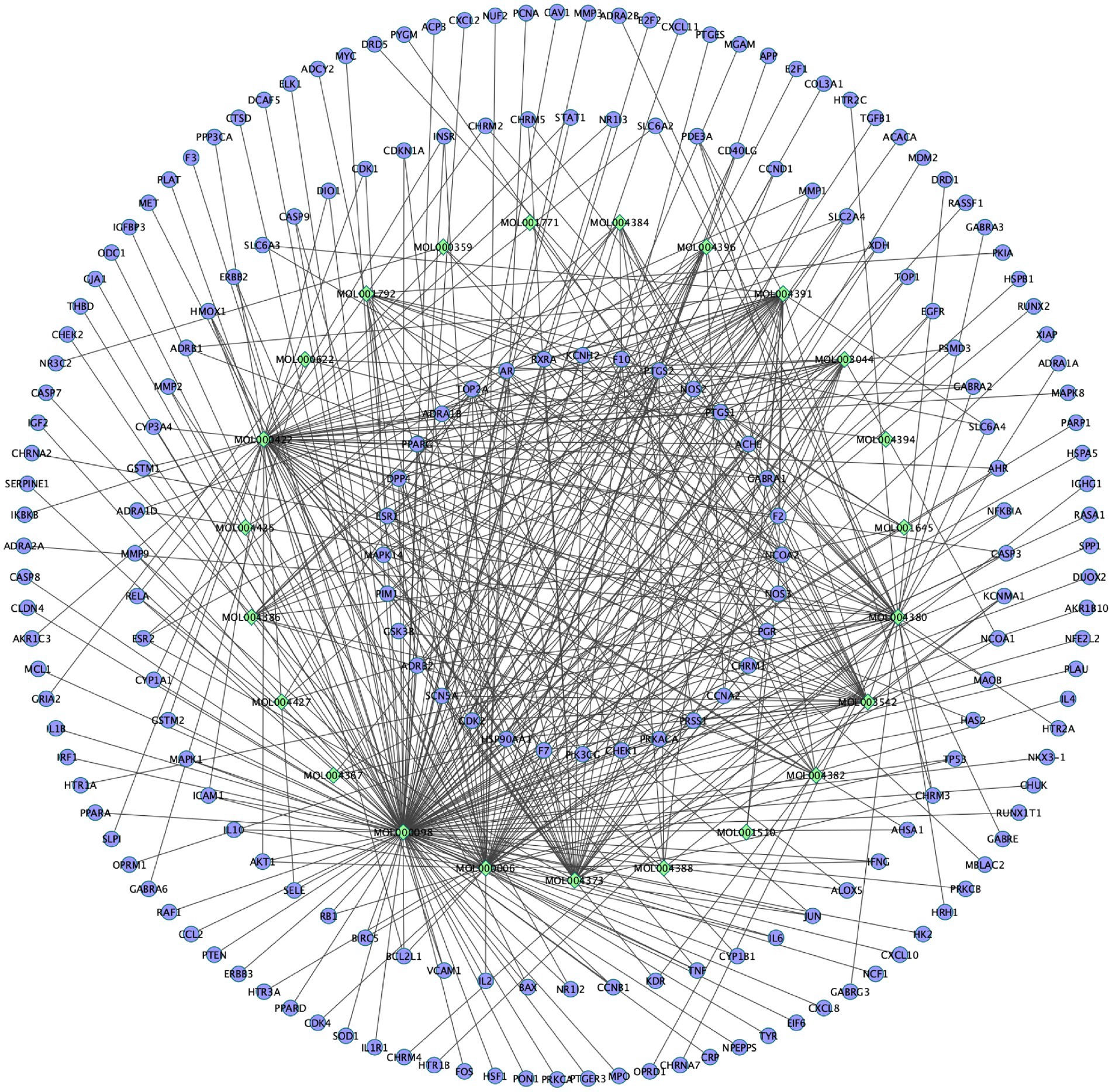
Figure 3. The compounds-targets network diagram of EPI to treat SCI. Rhombic nodes represent active ingredients in EPI, circular nodes represent 133 potential targets of EPI for SCI.
Next, we used the STRING program to construct a PPI network of the 133 potential EPI targets for SCI (Figure 4A). The resulting network contained 126 nodes and 1968 edges. The top 10 genes in the Degree algorithm were AKT1, TP53, JUN, TNF, CASP3, EGFR, IL6, HSP90AA1, MAPK1, and MYC, which can affect a wider range of proteins (Figures 4B,C).
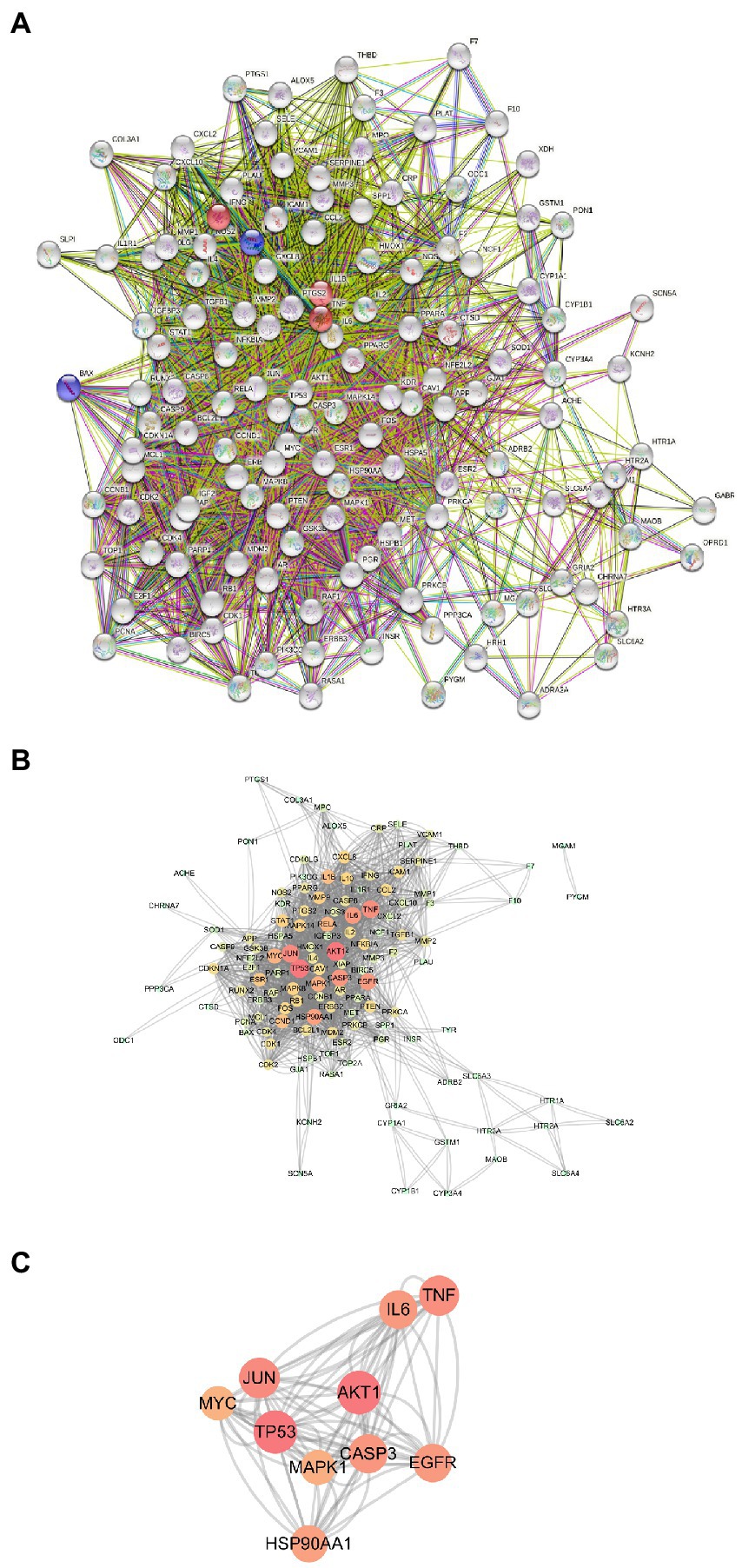
Figure 4. PPI Network Analysis. (A) PPI network of 133 potential targets of EPI for SCI. (B) PPI network constructed with Cytoscape software (The darker the node color, the higher the number of connected proteins). (C) The key targets of EPI for SCI obtained.
To elucidate the potential mechanism underlying EPI’s action in SCI, we used the clusterProfiler package to perform GO and KEGG pathway enrichment analyses on the 133 potential EPI targets. The top 10 GO items and enriched KEGG pathways are shown in Figures 5A,B while detailed information is shown in Tables 2 and 3. In addition, KEGG analysis indicated that the PI3K/AKT signaling pathway was enriched with the most genes (Figure 5C). These results suggested that EPI may be regulating SCI repair by modulating oxidative stress, inflammatory response, neuron death, and PI3K/AKT signaling pathways.
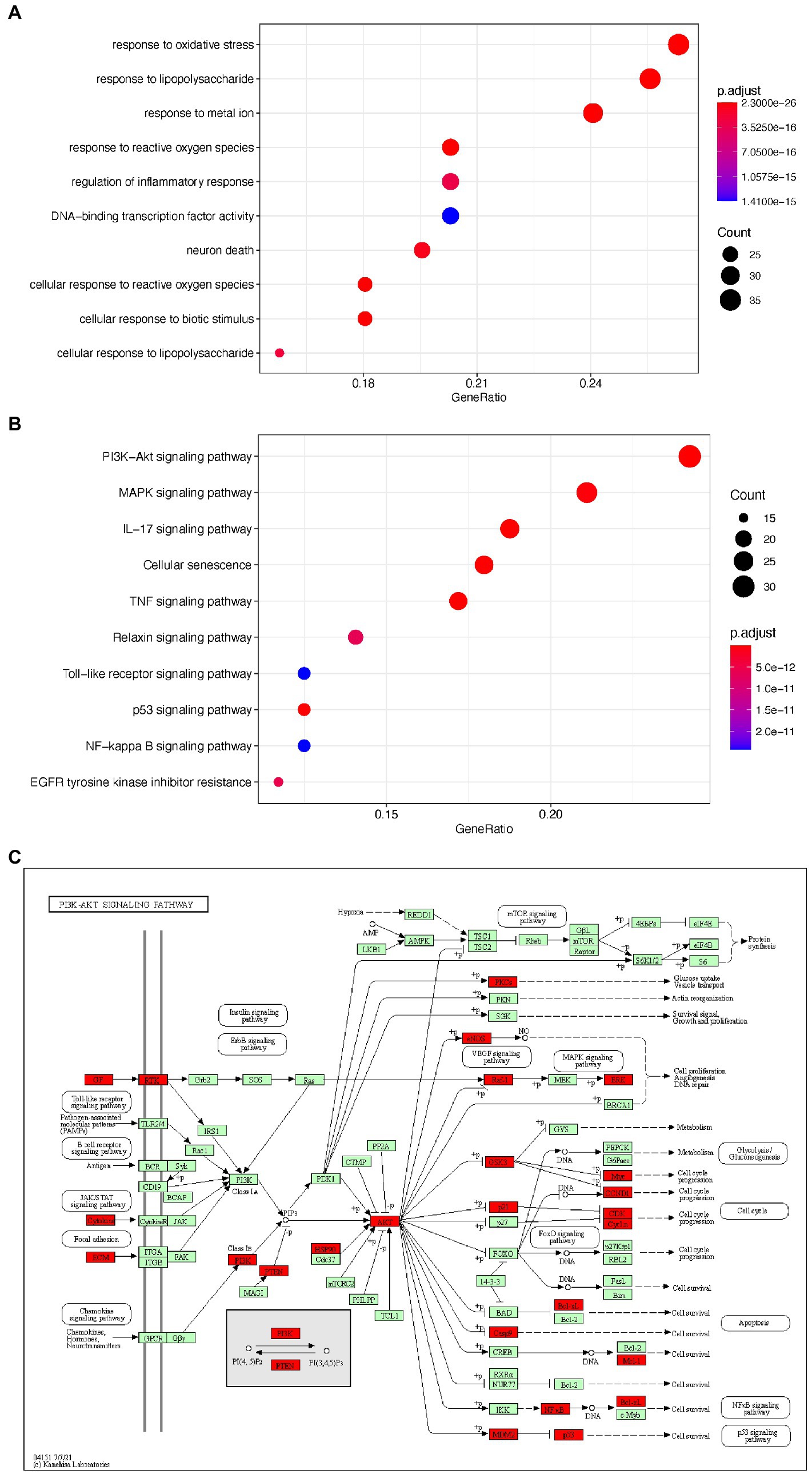
Figure 5. The GO and KEGG enrichment analysis of the potential targets of EPI for SCI. (A) the top 10 enriched GO terms. (B) The top 10 enriched KEGG signaling pathways. (C) PI3K/AKT signaling pathway map (Green and red are all genes on the PI3K signaling pathway, and red represents the targets of EPI for SCI). PI3K/AKT signaling pathway map is mined here from https://www.kegg.jp/kegg/mapper/.
To validate results of the network pharmacology, we used molecular docking to evaluate the interaction between active ingredients and key targets. A resulting Sankey diagram revealed that the 10 key targets were related to active EPI ingredients. Particularly, quercetin was associated with 10 targets, luteolin corresponded to 9 targets, kaempferol corresponded to 5 targets, while other active ingredients corresponded to 1 target each (Figure 6A). Molecular docking scores suggested that most of active ingredients bind core target proteins with binding energies below-5.0, indicating that the target specifically binds the compound (Figure 6B). The active ingredients and targets with the highest scoring free binding energy are shown in corresponding molecular docking diagrams (Figures 6C–L). Collectively, these results revealed that active ingredients have a strong affinity for the identified targets.
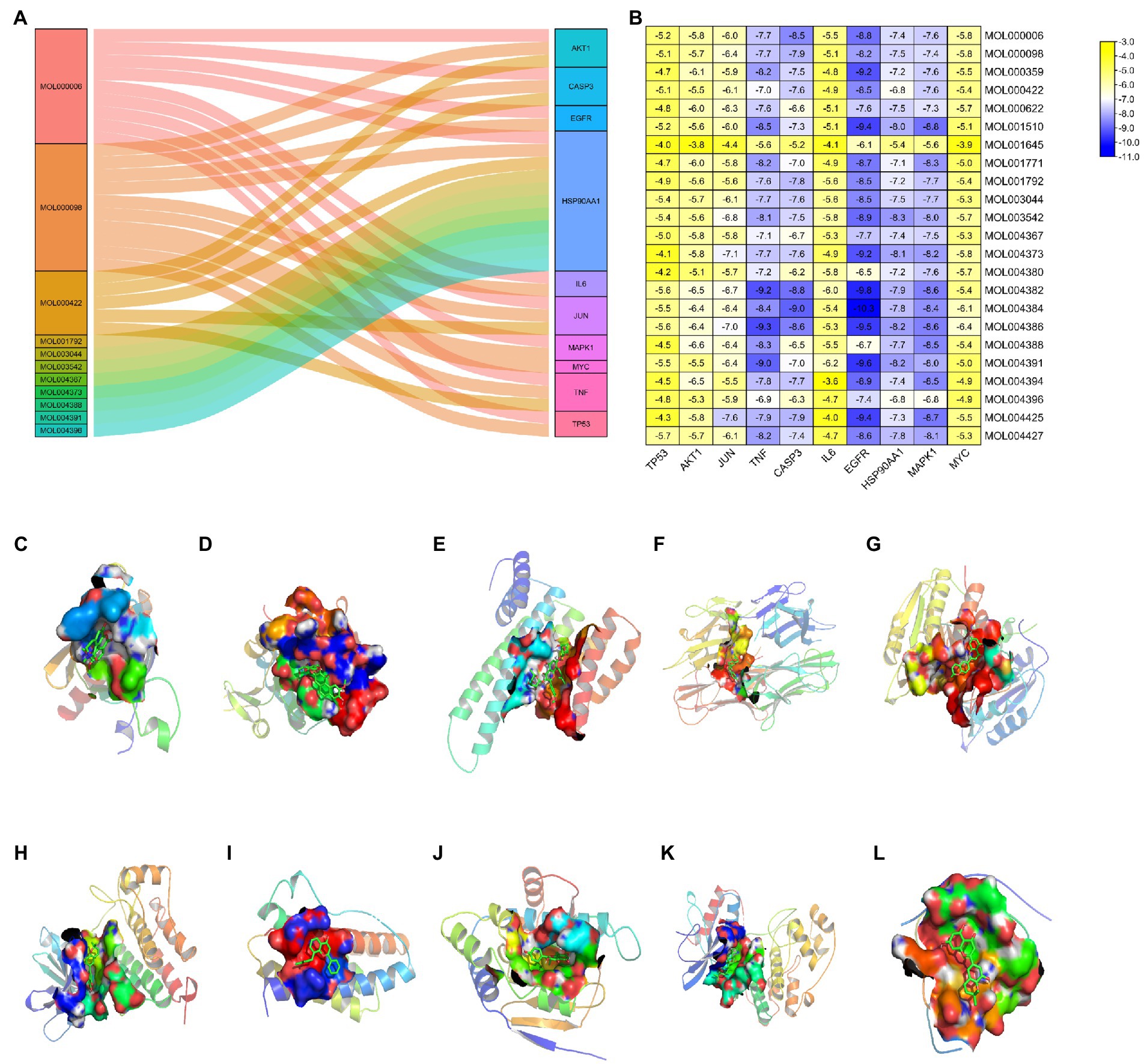
Figure 6. Molecular docking between active ingredients and key targets. (A) Sankey diagram of “compounds-targets.” (B) Heatmap of the scores of molecular docking in this study. The darker the color, the better the binding of active ingredient to the key target. (C) MOL004388 to AKT1. (D) MOL004427 to TP53. (E) MOL004425 to JUN. (F) MOL004386 to TNF. (G) MOL004384 to CASP3. (H) MOL004384 to EGFR. (I) MOL004391 to IL6. (J) MOL003542 to HSP90AA1. (K) MOL001510 to MAPK1. (L) MOL000006 to MYC.
To assess whether EPI promotes recovery of motor function in SCI rats, we first established an SCI rat model then analyzed the resulting BBB scores to understand behavioral performance. Rats in the sham group exhibited a BBB motor score of 21 at each postoperative time point (1, 7, 14, and 28 days), with no evidence of hindlimb motor dysfunction (Figure 7A). Conversely, rats in SCI, SCI + EPI, and SCI + EPI + LY groups exhibited severe motor dysfunction at 1 day post-operation, as evidenced by a score close to 0. It continued to rise during the experimental period but remained significantly lower in the SCI group, EPI, and EPI + LY group than in the sham group at all time points. Rats in the SCI + EPI group exhibited significantly higher BBB scores at 14, 21, and 28 d after SCI, relative to their SCI counterparts, while those in the SCI + EPI had a significantly higher behavior compared to those in the SCI + EPI + LY group at 21 and 28 d after SCI. There were no significant differences between rats in the SCI and SCI + EPI + LY groups at individual time points. We further analyzed the effect of EPI on spinal cord histopathology using H&E staining and found that rats in the SCI group exhibited significantly higher congestion, structural disorders, and structural damage than those in the sham group (Figure 7B). Notably, EPI treatment significantly improved the above-mentioned protective effect pathological symptoms, consistent with results of motor assessment. Overall, these results indicated that EPI not only reduced the degree of edema and cavitation of spinal cord tissue, but also improved motor function in the hind limbs of rats after SCI.
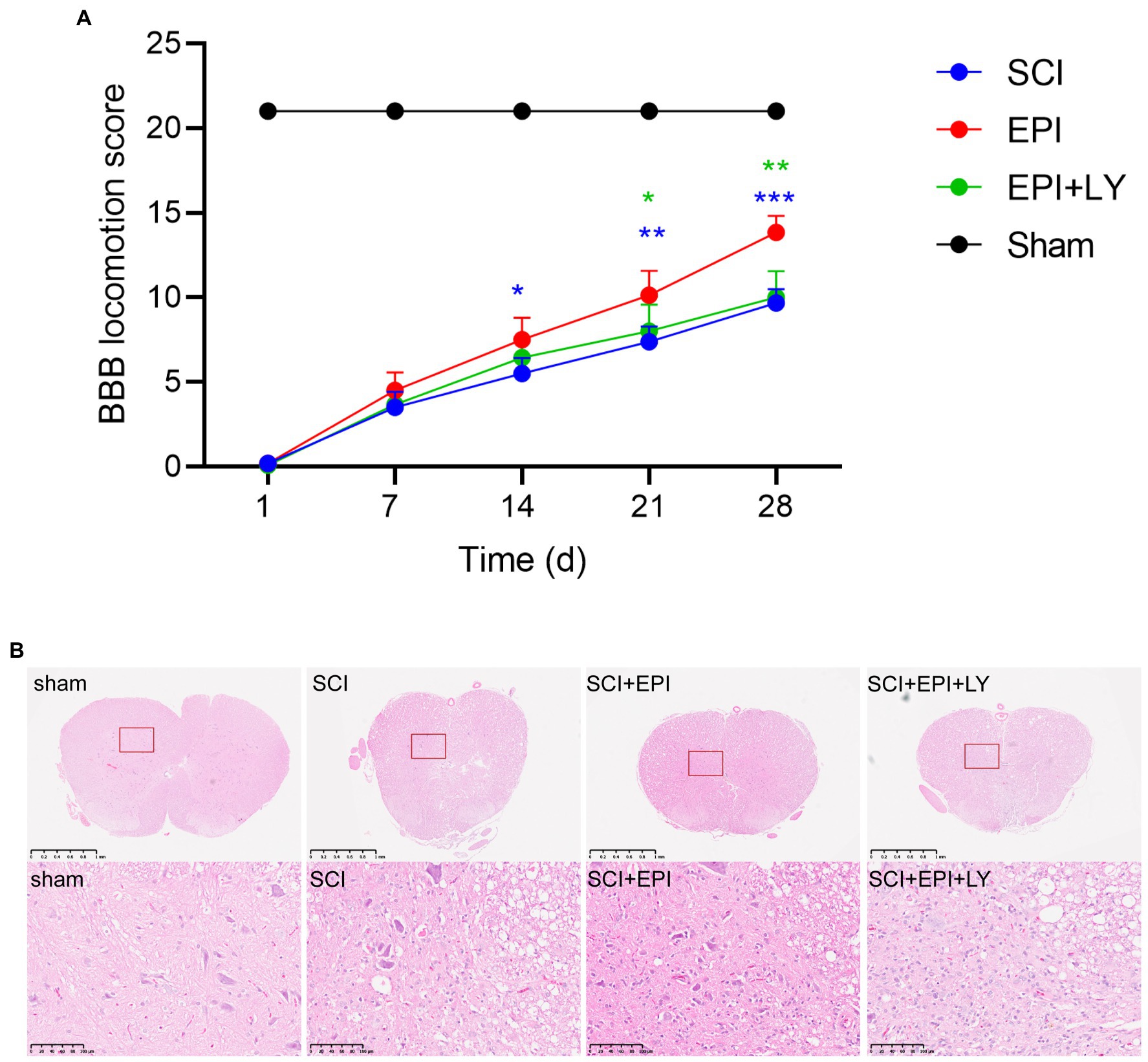
Figure 7. EPI enhances behavioral performance and tissue repair in SCI rats (N = 3 per group). (A) Use BBB score to evaluate the recovery of motor function in sham group, SCI group, SCI + EPI group, and SCI + EPI + LY group, respectively, in 3, 7, 14, 21, 28 days (N = 10 per group). (B) HE-stained transverse sections of spinal cords from sham group, SCI group, SCI + EPI group, and SCI + EPI + LY group (N = 3 per group). * means p < 0.05, ** means p < 0.01, and *** means p < 0.001.
Network pharmacology analysis results revealed that EPI treats SCI by modulating the PI3K/Akt signaling pathway. To verify the importance of the PI3K/Akt signaling pathway in SCI treatment, we analyzed expression levels of p-PI3K, PI3K, p-Akt, and Akt proteins in the spinal cord tissue of rats after EPI treatment. We also used LY294002, a specific PI3K inhibitor, to inhibit PI3K/Akt signaling pathway to further validate the importance of EPI for SCI recovery. Western blots revealed that the p-PI3K/PI3K ratio was significantly higher in the spinal cord of SCI rats relative to those in the sham group, whereas the p-AKT/AKT ratio was not significantly different between the groups (Figures 8A–C). p-PI3K/PI3K and p-AKT/AKT ratio significantly increased after EPI treatment, compared with the SCI group, and these increases were significantly inhibited by the LY294002 treatment. Overall, these results indicated that EPI activated the PI3K/AKT signaling pathway in SCI rats, which was consistent with results of network pharmacology analysis.
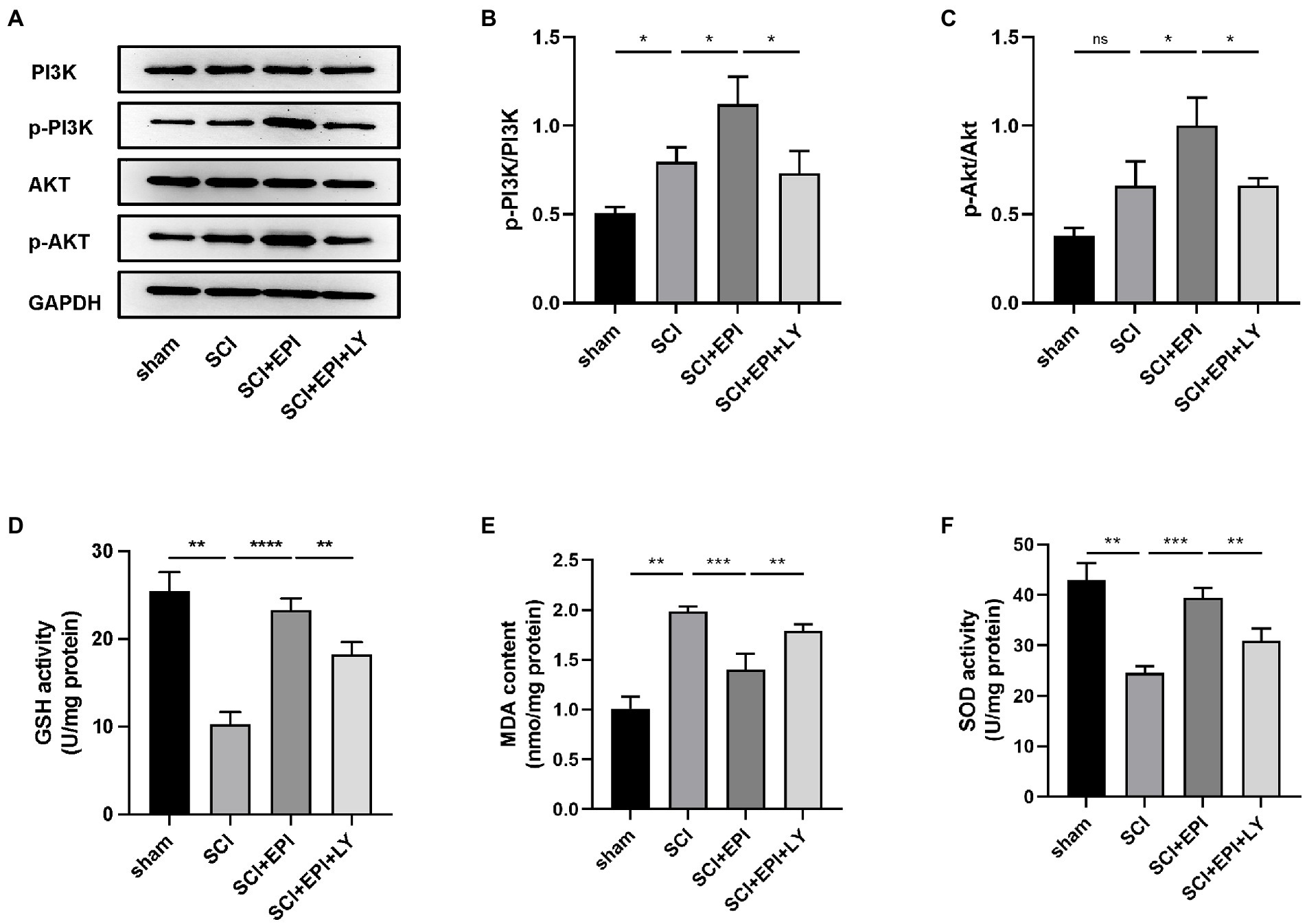
Figure 8. Investigation of the PI3K/AKT signaling pathway in the antioxidant effect of EPI (N = 3 per group). (A) The expressions of PI3K, p-PI3K, AKT, and p-AKT were detected by Western blot assays. (B,C) Quantitative analysis of western blot assays. (D–F) The concentration of GSH, MDA, and SOD in sham group, SCI group, SCI + EPI group, and SCI + EPI + LY. * means p < 0.05, ** means p < 0.01, and *** means p < 0.001.
Next, we investigated whether EPI affects the ability to resist oxidative stress through PI3K/AKT pathway by analyzing the effect of EPI on spinal cord tissue in SCI rats. Experimental results revealed that GSH and SOD activities were significantly lower while MDA activity was significantly higher in spinal cord tissue of SCI rats, relative to those in the sham group, consistent with previous results by Li et al. (2022; Figures 8E,F). Further, rats in the SCI + EPI group had significantly higher GSH activities than their SCI counterparts, whereas those in the SCI + EPI + LY group had lower activities than their SCI + EPI counterparts (Figure 8D). Rats in the SCI + EPI group exhibited significantly lower MDA levels than those in the SCI group while those in the SCI + EPI + LY group were higher than in the SCI + EPI group (Figure 8E). However, SOD expression was downregulated in the SCI + EPI group than in the SCI + EPI group, and higher than that in the SCI + EPI + LY group (Figure 8F). These results indicated that LY294002 affected the ability of EPI to reduce oxidative stress by supplementing the role of EPI in activating PI3K/AKT signaling pathway. Collectively, these results indicated that EPI alleviates oxidative stress in the spinal cord tissue of SCI rats by activating the PI3K/AKT signaling pathway.
SCI, a major traumatic disorder of the central nervous system that can lead to permanent motor and sensory impairment, has been shown to affect approximately 930,000 people worldwide each year (Disease et al., 2018). In recent years, several studies have revealed the active ingredients of EPI and demonstrated its neuroprotective effects in SCI (Tohda and Nagata, 2012; Jia et al., 2019). Traditional research on drugs for SCI is limited to a one-drug, one-target model, but SCI is a multi-target and multifactorial disease and it is difficult to achieve ideal therapeutic results based on a single target alone. By collecting drug and disease information from public databases, network pharmacology explains the molecular mechanism of the active ingredients of EPI in the treatment of SCI from multi-component, multi-target, and multi-pathway. Thus, we employed this new approach, coupled with animal experiments, to investigate the protective effect and mechanism underlying EPI action in the treatment of SCI. Analysis of biological activities, core therapeutic targets, and potential mechanisms underlying EPI’s action in SCI revealed 24 active ingredients and 133 potential therapeutic targets. In addition, PPI network analysis revealed 10 key EPI targets, namely Akt1, Tp53, Jun, Tnf, Casp3, Egfr, Il6, Hsp90aa1, Mapk1, and Myc, which were closely related to SCI development. These proteins are regarded as core proteins that may play an essential role in the therapeutic effect of EPI on SCI. TNF activity increases, participating in the inflammatory and injury cascade response, exacerbating ischemia and hypoxia in the injury area, and inducing axonal damage and degeneration after SCI (Yan et al., 2001; Pineau and Lacroix, 2007). Recent studies have shown that conditional elimination of TNF in macrophages and neutrophils improves recovery of motor function and reduces the area of injury in mice with SCI (Ellman et al., 2020). Inflammatory mediators such as TNF-α and IL6 mediate the recruitment of inflammatory cells to the injury site. Targeting these cytokines may be a potential strategy to reduce secondary injury in SCI (Esposito and Cuzzocrea, 2011; Kim et al., 2021). Molecular docking results revealed that luteolin and Yinyanghuo E had high affinity and were bound to IL6 and TNF, respectively. These results affirmed the role played by IL6 and TNF in EPI for SCI.
In the present study, GO terms indicated that EPI could treat SCI by modulating oxidative stress, inflammatory response, and neuron death, as well as other biological functional modules. The targets were significantly enriched in PI3K/Akt, MAPK, and IL-17 signaling pathway signaling pathways, which are closely related to the SCI signaling pathway. Among them, the PI3K-AKT signaling pathway appears to be the most enriched in SCI-related signaling pathways and seems to be the most important pathway for the treatment of SCI. Previous studies have shown that the PI3K/AKT signaling pathway plays important roles in secondary SCI, such as anti-inflammatory, anti-apoptotic, and regulating glial scar formation (Dong et al., 2014; Chen et al., 2016; Li et al., 2020). In addition, Wang showed that activation of the PI3K/Akt signaling pathway could inhibit excessive oxidative stress (Wang et al., 2020). These reports were consistent with our prediction which indicated that the PI3K/AKT signaling pathway is not only essential for EPI treatment of SCI but may also be regulating the observed antioxidant activity.
Phosphatidylinositol 3 kinases (PI3Ks), which belong to the lipid kinase family, are activated by various stimuli to produce phosphatidylinositol-3-phosphate (PIP3) which subsequently directly activates conversion of threonine proteins (AKT) into phosphorylated proteins (p-AKT) by stimulating serine-dependent activase (PDK; Heras-Sandoval et al., 2014). Notably, PI3K is required for the activation of Akt, a member of the serine/threonine protein activase family (Heras-Sandoval et al., 2014). In the present study, we administered the EPI to rats and found that EPI not only improved motor function, and reduced tissue damage, but also significantly improved p-PI3K/PI3K and p-AKT/AKT ratio after SCI. In addition, LY294002 decreased the ability of EPI to improve motor and tissue damage and reduced p-PI3K/PI3K and p-AKT/AKT ratio. Collectively, these findings suggested that EPI exerts a protective effect after SCI through the PI3K/Akt signaling pathway.
Previous studies have shown that oxidative stress markedly exacerbates both SCI and diabetic neuropathy (Fernyhough and McGavock, 2014; Zrzavy et al., 2021). Moreover, oxidative stress has not only been reportedly linked to the pathogenesis of SCI, but also to levels of oxidative markers such as SOD, GSH, and MDA in the spinal cord tissue (Li et al., 2022). Results of the present study showed that treatment with EPI extracts significantly increased SOD and GSH and decreased MDA, demonstrating that EPI may exert SCI protective effects through antioxidant effects. Furthermore, PI3K inhibitors partially reversed the antioxidant capacity of EPI extracts, suggesting that the PI3K/AKT signaling pathway may be a potential underlying mechanism EPI against oxidative stress after SCI.
This study had some limitations. Firstly, the data obtained from the online databases were based on researched and anticipated material, therefore unverified and undocumented active ingredients or genes might not be considered our study. Secondly, in addition to the biological modules validated in this experiment, EPI may also exert protective effects through other pathways. Further research explorations are needed to validate each biofunctional module. Thirdly, the basis of EPI’s treatment of SCI is that it contains a large number of compounds, and we will conduct more experiments to explore the role of key compounds in EPI.
In summary, we employed a combination of network pharmacology and animal experiments to effectively reveal the biological-molecular mechanisms underlying EPI’s action in treatment of SCI. Particularly, our results indicated that EPI ameliorates SCI in rats through antioxidant effects, which may be related to the activation of the PI3K/AKT signaling pathway. These findings affirm EPI’s significance as a potential therapy for treatment of SCI.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Experimental Animal Ethics Committee of Tianjin People’s Hospital.
XF, BM, MZ, and RZ designed the experiments and analyzed data. YC, LL, SK, CL, and ZJ performed the experiments. XZ, SF, HZ, and WH wrote the paper. All authors contributed to the article and approved the submitted version.
This study was supported by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-064B), Foundation of Tianjin Union Medical Center (2019JZPY01), and Tianjin Health Commission Science and Technology Project (ZC20225 and KJ20061).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahuja, C. S., Wilson, J. R., Nori, S., Kotter, M. R. N., Druschel, C., Curt, A., et al. (2017). Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3:17018. doi: 10.1038/nrdp.2017.18
Alizadeh, A., Dyck, S. M., and Karimi-Abdolrezaee, S. (2019). Traumatic spinal cord Injury: an overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 10:282. doi: 10.3389/fneur.2019.00282
Chen, C. H., Sung, C. S., Huang, S. Y., Feng, C. W., Hung, H. C., Yang, S. N., et al. (2016). The role of the PI3K/Akt/mTOR pathway in glial scar formation following spinal cord injury. Exp. Neurol. 278, 27–41. doi: 10.1016/j.expneurol.2016.01.023
Cho, J. H., Jung, J. Y., Lee, B. J., Lee, K., Park, J. W., and Bu, Y. (2017). Epimedii Herba: a promising herbal medicine for neuroplasticity. Phytother. Res. 31, 838–848. doi: 10.1002/ptr.5807
Disease, G. B. D., Injury, I., and Prevalence, C. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. doi: 10.1016/S0140-6736(18)32279-7
Dong, H., Zhang, X., Dai, X., Lu, S., Gui, B., Jin, W., et al. (2014). Lithium ameliorates lipopolysaccharide-induced microglial activation via inhibition of toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1 pathway. J. Neuroinflammation 11:140. doi: 10.1186/s12974-014-0140-4
Ellman, D. G., Lund, M. C., Nissen, M., Nielsen, P. S., Sørensen, C., Lester, E. B., et al. (2020). Conditional ablation of myeloid TNF improves functional outcome and decreases lesion size after spinal cord Injury in mice. Cells 9:2407. doi: 10.3390/cells9112407
Esposito, E., and Cuzzocrea, S. (2011). Anti-TNF therapy in the injured spinal cord. Trends Pharmacol. Sci. 32, 107–115. doi: 10.1016/j.tips.2010.11.009
Fernyhough, P., and McGavock, J. (2014). Mechanisms of disease: mitochondrial dysfunction in sensory neuropathy and other complications in diabetes. Handb. Clin. Neurol. 126, 353–377. doi: 10.1016/B978-0-444-53480-4.00027-8
Gene Ontology, C. (2015). Gene Ontology consortium: going forward. Nucleic Acids Res. 43, D1049–D1056. doi: 10.1093/nar/gku1179
Hamosh, A., Scott, A. F., Amberger, J. S., Bocchini, C. A., and McKusick, V. A. (2005). Online mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 33, D514–D517.
Heras-Sandoval, D., Perez-Rojas, J. M., Hernandez-Damian, J., and Pedraza-Chaverri, J. (2014). The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 26, 2694–2701. doi: 10.1016/j.cellsig.2014.08.019
Hopkins, A. L. (2007). Network pharmacology. Nat. Biotechnol. 25, 1110–1111. doi: 10.1038/nbt1007-1110
Jia, G., Zhang, Y., Li, W., and Dai, H. (2019). Neuroprotective role of icariin in experimental spinal cord injury via its antioxidant, antineuroinflammatory and antiapoptotic properties. Mol. Med. Rep. 20, 3433–3439. doi: 10.3892/mmr.2019.10537
Kanehisa, M., Araki, M., Goto, S., Hattori, M., Hirakawa, M., Itoh, M., et al. (2008). KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484.
Kim, J., Joshi, H. P., Sheen, S. H., Kim, K. T., Kyung, J. W., Choi, H., et al. (2021). Resolvin D3 promotes inflammatory resolution, neuroprotection, and functional recovery after spinal cord injury. Mol. Neurobiol. 58, 424–438. doi: 10.1007/s12035-020-02118-7
Kumar, R., Lim, J., Mekary, R. A., Rattani, A., Dewan, M. C., Sharif, S. Y., et al. (2018). Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 113, e345–e363. doi: 10.1016/j.wneu.2018.02.033
Li, H., Kong, R., Wan, B., Yang, L., Zhang, S., Cao, X., et al. (2020). Initiation of PI3K/AKT pathway by IGF-1 decreases spinal cord injury-induced endothelial apoptosis and microvascular damage. Life Sci. 263:118572. doi: 10.1016/j.lfs.2020.118572
Li, H., Zhang, X., Qi, X., Zhu, X., and Cheng, L. (2019). Icariin inhibits endoplasmic reticulum stress-induced neuronal apoptosis after spinal cord injury through modulating the PI3K/AKT signaling pathway. Int. J. Biol. Sci. 15, 277–286. doi: 10.7150/ijbs.30348
Li, H., Zhang, X., Zhu, X., Qi, X., Lin, K., and Cheng, L. (2018). The effects of icariin on enhancing motor recovery through attenuating pro-inflammatory factors and oxidative stress via mitochondrial apoptotic pathway in the mice model of spinal cord Injury. Front. Physiol. 9:1617. doi: 10.3389/fphys.2018.01617
Li, Y., Zou, Z., An, J., Wu, Q., Tong, L., Mei, X., et al. (2022). Chitosan-modified hollow manganese dioxide nanoparticles loaded with resveratrol for the treatment of spinal cord injury. Drug Deliv. 29, 2498–2512. doi: 10.1080/10717544.2022.2104957
Liu, Z., Yao, X., Jiang, W., Li, W., Zhu, S., Liao, C., et al. (2020). Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-kappaB signaling pathway and pyroptosis after secondary spinal cord injury. J. Neuroinflammation 17:90. doi: 10.1186/s12974-020-01751-2
Merico, D., Isserlin, R., Stueker, O., Emili, A., and Bader, G. D. (2010). Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One 5:e13984. doi: 10.1371/journal.pone.0013984
Orr, M. B., and Gensel, J. C. (2017). Interactions of primary insult biomechanics and secondary cascades in spinal cord injury: implications for therapy. Neural Regen. Res. 12, 1618–1619. doi: 10.4103/1673-5374.217332
Pineau, I., and Lacroix, S. (2007). Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 500, 267–285. doi: 10.1002/cne.21149
Rose, A. S., Bradley, A. R., Valasatava, Y., Duarte, J. M., Prlić, A., and Rose, P. W. (2018). NGL viewer: web-based molecular graphics for large complexes. Bioinformatics 34, 3755–3758. doi: 10.1093/bioinformatics/bty419
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6:13. doi: 10.1186/1758-2946-6-13
Sheng, S., Yang, Z. X., Xu, F. Q., and Huang, Y. (2021). Network pharmacology-based exploration of synergistic mechanism of Guanxin II formula (II) for coronary heart disease. Chin. J. Integr. Med. 27, 106–114. doi: 10.1007/s11655-020-3199-z
Song, W. Y., Ding, H., Dunn, T., Gao, J. L., Labastida, J. A., Schlagal, C., et al. (2021). Low-dose metformin treatment in the subacute phase improves the locomotor function of a mouse model of spinal cord injury. Neural Regen. Res. 16, 2234–2242. doi: 10.4103/1673-5374.310695
Squair, J. W., White, B. A., Bravo, G. I., Martin Ginis, K. A., and Krassioukov, A. V. (2016). The economic burden of autonomic dysreflexia during hospitalization for individuals with spinal cord Injury. J. Neurotrauma 33, 1422–1427. doi: 10.1089/neu.2015.4370
State Pharmacopoeia Commission of the PRC Pharmacopoeia of the People’s republic of China. China Medical Science Press, Beijing 1, 332 (2015).
Stelzer, G., Rosen, N., Plaschkes, I., Zimmerman, S., Twik, M., Fishilevich, S., et al. (2016). The GeneCards suite: from Gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinformatics 54, 1.30.31–1.30.33. doi: 10.1002/cpbi.5
Szklarczyk, D., Morris, J. H., Cook, H., Kuhn, M., Wyder, S., Simonovic, M., et al. (2017). The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 45, D362–d368. doi: 10.1093/nar/gkw937
Tohda, C., and Nagata, A. (2012). Epimedium koreanum extract and its constituent icariin improve motor dysfunction in spinal cord injury. Evid. Based Complement. Alternat. Med. 2012:731208
Trott, O., and Olson, A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461. doi: 10.1002/jcc.21334
Tsaioun, K., Blaauboer, B. J., and Hartung, T. (2016). Evidence-based absorption, distribution, metabolism, excretion (ADME) and its interplay with alternative toxicity methods. ALTEX 33, 343–358. doi: 10.14573/altex.1610101
UniProt Consortium (2018). UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46:2699. doi: 10.1093/nar/gky092
Wang, M., Cui, B., Gong, M., Liu, Q., Zhuo, X., Lv, J., et al. (2022). Arctium lappa leaves based on network pharmacology and experimental validation attenuate atherosclerosis by targeting the AMPK-mediated PPARG/LXRalpha pathway. Biomed. Pharmacother. 153:113503. doi: 10.1016/j.biopha.2022.113503
Wang, Y., Zhang, S., Li, F., Zhou, Y., Zhang, Y., Wang, Z., et al. (2020). Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 48, D1031–D1041. doi: 10.1093/nar/gkz981
Wang, H., Zheng, Z., Han, W., Yuan, Y., Li, Y., Zhou, K., et al. (2020). Metformin promotes axon regeneration after spinal cord injury through inhibiting oxidative stress and stabilizing microtubule. Oxidative Med. Cell. Longev. 2020:9741369. doi: 10.1155/2020/9741369
Witiw, C. D., and Fehlings, M. G. (2015). Acute spinal cord Injury. J. Spinal Disord. Tech. 28, 202–210. doi: 10.1097/BSD.0000000000000287
Wu, H., Lien, E. J., and Lien, L. L. (2003). Chemical and pharmacological investigations of Epimedium species: a survey. Prog. Drug Res. 60, 1–57.
Yacoub, A., Hajec, M. C., Stanger, R., Wan, W., Young, H., and Mathern, B. E. (2014). Neuroprotective effects of perflurocarbon (oxycyte) after contusive spinal cord injury. J. Neurotrauma 31, 256–267. doi: 10.1089/neu.2013.3037
Yan, P., Li, Q., Kim, G. M., Xu, J., Hsu, C. Y., and Xu, X. M. (2001). Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J. Neurotrauma 18, 563–568. doi: 10.1089/089771501300227369
Zhao, H., Mei, X., Yang, D., and Tu, G. (2021). Resveratrol inhibits inflammation after spinal cord injury via SIRT-1/NF-kappaB signaling pathway. Neurosci. Lett. 762:136151. doi: 10.1016/j.neulet.2021.136151
Zhou, W., Zhang, H., Wang, X., Kang, J., Guo, W., Zhou, L., et al. (2022). Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine 95:153837. doi: 10.1016/j.phymed.2021.153837
Keywords: spinal cord injury, Epimedium, network pharmacology, molecular docking, pharmacological mechanism
Citation: Fu X, Ma B, Zhou M, Cheng Y, Liu L, Kan S, Liu C, Zhao X, Feng S, Zhu H, Hu W, Jiang Z and Zhu R (2023) Network pharmacology integrated with experimental validation to explore the therapeutic role and potential mechanism of Epimedium for spinal cord injury. Front. Mol. Neurosci. 16:1074703. doi: 10.3389/fnmol.2023.1074703
Received: 19 October 2022; Accepted: 09 January 2023;
Published: 30 January 2023.
Edited by:
Yun Li, University of Wyoming, United StatesReviewed by:
Jiaman Dai, University of Wyoming, United StatesCopyright © 2023 Fu, Ma, Zhou, Cheng, Liu, Kan, Liu, Zhao, Feng, Zhu, Hu, Jiang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rusen Zhu, ✉ enJzc3BpbmVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.