
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mol. Neurosci. , 15 February 2022
Sec. Molecular Signalling and Pathways
Volume 15 - 2022 | https://doi.org/10.3389/fnmol.2022.829204
This article is part of the Research Topic The Mechanism on Development and Regeneration of Inner Ear Hair Cells View all 11 articles
Hair cells are mechanosensitive cells in the inner ear, characterized by dozens to hundreds of actin-based stereocilia and one tubulin-based kinocilium on the apical surface of each cell. Two types of hair cells, namely cochlear hair cells and vestibular hair cells (VHCs), are responsible for the sensation of sound and balancing information, respectively. In each hair cell, the stereocilia are organized into rows of increasing heights with the mechano-electrical transduction (MET) channels localized at the tips of shorter-row stereocilia. A so-called “row 2 protein complex” also localizes at the tips of shorter-row mechanotransducing stereocilia, which plays important roles in the maintenance of mechanotransducing stereocilia. Recently, we and others identified BAIAP2L2 as a new component of row 2 complex. Baiap2l2 inactivation causes degeneration of the mechanotransducing stereocilia in cochlear hair cells, and leads to profound hearing loss in mice. In the present work, we examined the role of BAIAP2L2 in the VHC stereocilia. Confocal microscopy reveals that BAIAP2L2 immunoreactivity is localized at the tips of shorter-row stereocilia in VHCs. However, stereocilia development and maintenance are unaffected in Baiap2l2–/– VHCs. Meanwhile, MET function of VHCs as well as vestibular functions are also unaffected in Baiap2l2–/– mice. Further investigations show that the stereociliary tip localization of CAPZB2, another known row 2 complex component, is not affected in Baiap2l2–/– VHCs, consistent with the unaltered stereocilia morphology. Taken together, our present data show that BAIAP2L2 inactivation does not affect vestibular hair cell stereocilia.
Hair cells are the mechanosensory cells in the inner ear, responsible for converting the mechanical signals into electrical signals, a process referred to as mechano-electrical transduction (MET). Each hair cell harbors dozens to hundreds of actin-based stereocilia and one microtubule-based kinocilium at the apical surface, collectively named hair bundle (Flock and Cheung, 1977). The stereocilia play a pivotal role in MET, whereas the kinocilium is important for hair bundle development (Hudspeth and Jacobs, 1979; Jones et al., 2008).
There are two different types of hair cells in the mammalian inner ear, namely cochlear hair cells and vestibular hair cells (VHCs), which are responsible for the sensation of sound and balancing information, respectively. In each mammalian cochlear hair cell, stereocilia are organized into three rows of increasing heights, forming a characteristic staircase-like pattern (Tilney et al., 1980). Similarly, the stereocilia in mammalian VHCs form a staircase-like pattern with more rows of increasing heights (Krey and Barr-Gillespie, 2019). The MET channels are localized at the tips of shorter-row stereocilia, which are therefore referred to as mechanotransducing stereocilia (Beurg et al., 2009). In either type of hair cells, the development and maintenance of stereocilia is tightly regulated, and several proteins have been identified to play important roles in regulating stereocilia length (Barr-Gillespie, 2015; McGrath et al., 2017; Krey and Barr-Gillespie, 2019; Velez-Ortega and Frolenkov, 2019).
At the tips of the tallest-row stereocilia, there is a so-called “row 1 protein complex” that controls the identity and development of the tallest-row stereocilia (Tadenev et al., 2019; Krey et al., 2020). Meanwhile, at the tips of shorter-row mechanotransducing stereocilia resides a “row 2 protein complex”, which consists of MYO15A-L, EPS8L2, TWF2, and CAPZB2 (Peng et al., 2009; Rzadzinska et al., 2009; Furness et al., 2013; Fang et al., 2015; Avenarius et al., 2017). Evidences suggest that deficiency of row 2 complex components leads to degeneration of the mechanotransducing stereocilia (Furness et al., 2013; Fang et al., 2015). Recently, we and others identified BAI1-associated protein 2-like 2 (BAIAP2L2, also known as Pinkbar) as a new component of row 2 complex (Carlton et al., 2021; Yan et al., 2021). BAIAP2L2 inactivation causes degeneration of the mechanotransducing stereocilia in cochlear hair cells, and leads to profound hearing loss in mice (Carlton et al., 2021; Yan et al., 2021). Furthermore, the stereociliary tip localization of the known row 2 complex component, CAPZB2, is abolished in Baiap2l2 knockout mice, suggesting that BAIAP2L2 is indispensable for the formation of row 2 complex in cochlear hair cells (Yan et al., 2021).
In the present work, we further investigate the role of BAIAP2L2 in VHC stereocilia. Surprisingly, our results show that albeit localizing at the tips of shorter-row VHC stereocilia, BAIAP2L2 is not required for the development or maintenance of VHC stereocilia, which is in sharp contrast to the results observed in cochlear hair cells.
Animal experiments were approved by the Animal Ethics Committee of Shandong University School of Life Sciences (Permit Number: SYDWLL-2020-31) and performed accordingly. Baiap2l2 and Lhfpl5 knockout mice were established and maintained as previously reported (Xiong et al., 2012; Yan et al., 2021).
Utricles and saccules were dissected out and fixed with 4% paraformaldehyde (PFA) in PBS for 20 min, followed by permeabilization and blocking with PBT1 (0.1% Triton X-100, 1% BSA, and 5% heat-inactivated goat serum in PBS, pH 7.3) for 40 min. Afterwards, the samples were incubated with primary antibody in PBT1 overnight at 4°C, followed by incubation with corresponding secondary antibody in PBT2 (0.1% Triton X-100 and 0.1% BSA in PBS) for 2 h. After incubation with TRITC-conjugated phalloidin (Sigma-Aldrich, Cat. No. P1951) in PBS for 30 min, the samples were mounted in PBS/glycerol (1:1) and imaged using a confocal microscope with a 1.4NA/63 × Kort M27 objective lens (LSM 900, Zeiss, Germany). The antibodies used in the present study are as follows: rabbit anti-BAIAP2L2 antibody (Sigma-Aldrich, Cat. No. HPA003043); rabbit anti-CAPZB2 antibody (Merck, Cat. No. AB6017); mouse anti-EPS8 antibody (BD Biosciences, Cat. No. 610143); Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Thermo Fisher Scientific, Cat. No. A21206); Alexa Fluor 488-conjugated donkey anti-mouse IgG (Thermo Fisher Scientific, Cat. No. A21202).
Mouse temporal bone was dissected out and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer overnight at 4°C. Then the utricle and saccule were taken out of the temporal bone and post-fixed with 1% osmium tetroxide in 0.1 M phosphate buffer at 4°C for 2 h. After dehydration in ethanol and critically point drying using a Leica EM CPD300 (Leica, Germany), samples were mounted and sputter coated with platinum (15 nm) using a Cressington 108 sputter coater (Cressington, United Kingdom). Images were taken using a Quanta250 field-emission scanning electron microscope (FEI, Netherlands).
The sensory epithelia of utricle and saccule were dissected out and incubated with 3 μM FM 1-43FX (Thermo Fisher, Cat. No. F35355) in PBS for 40 s, then fixed with 4% PFA at room temperature for 20 min. After mounting in PBS-glycerol (1:1), the samples were imaged using a confocal microscope with a 0.8NA/20 × Kort M27 objective lens using identical settings (LSM 700, Zeiss, Germany). The relative fluorescence intensity of individual hair cell was measured and analyzed using ImageJ software.
Vestibular function of mice was evaluated as described previously (Li et al., 2021). Circling stereotyped movement was counted to record compulsive circles around the animal’s hips. Swimming test was performed to observe swimming behavior ranging from normal swimming to drowning. Swimming test scores were defined as follows: 0, normal swimming; 1, irregular swimming; 2, immobile floating; and 3, underwater tumbling. For rotarod test, mice were placed on the rod apparatus (HB-600, Ruanlong, China) that was set to accelerate from 0 to 50 rpm over a 3-min period. Mice were trained for seven consecutive days and the time before dropping was recorded on day 4–7. Four trials were performed on each day and the second, third and fourth trials were measured and analyzed.
All experiments were performed at least three times independently. Data were shown as means ± standard error of mean (SEM). Student’s two-tailed unpaired t test was used to determine statistical significance when the results show normal distribution; otherwise, Mann-Whitney U test is used. P < 0.05 was considered statistically significant.
We first examined the localization of BAIAP2L2 in the VHC stereocilia by performing whole-mount immunostaining and confocal microscopy using a specific anti-BAIAP2L2 antibody. The stereociliary F-actin core was visualized by TRITC-conjugated phalloidin. The results reveal that BAIAP2L2 immunoreactivity is localized at the tips of utricular hair cell stereocilia at postnatal day 8 (P8) (Figure 1A and Supplementary Figure 1A, top panel). Noticeably, BAIAP2L2 immunoreactivity is more enriched at the tips of the shorter-row stereocilia (Figure 1A, top panel). Similar results were obtained in P30 utricular hair cells (Figure 1A and Supplementary Figure 1A, middle panel). No BAIAP2L2 immunoreactivity is detected in the homozygous Baiap2l2 knockout mice, confirming the specificity of the antibody (Figure 1A and Supplementary Figure 1A, bottom panel). Similar expression pattern was also observed in saccular hair cells (Figure 1B and Supplementary Figure 1B).
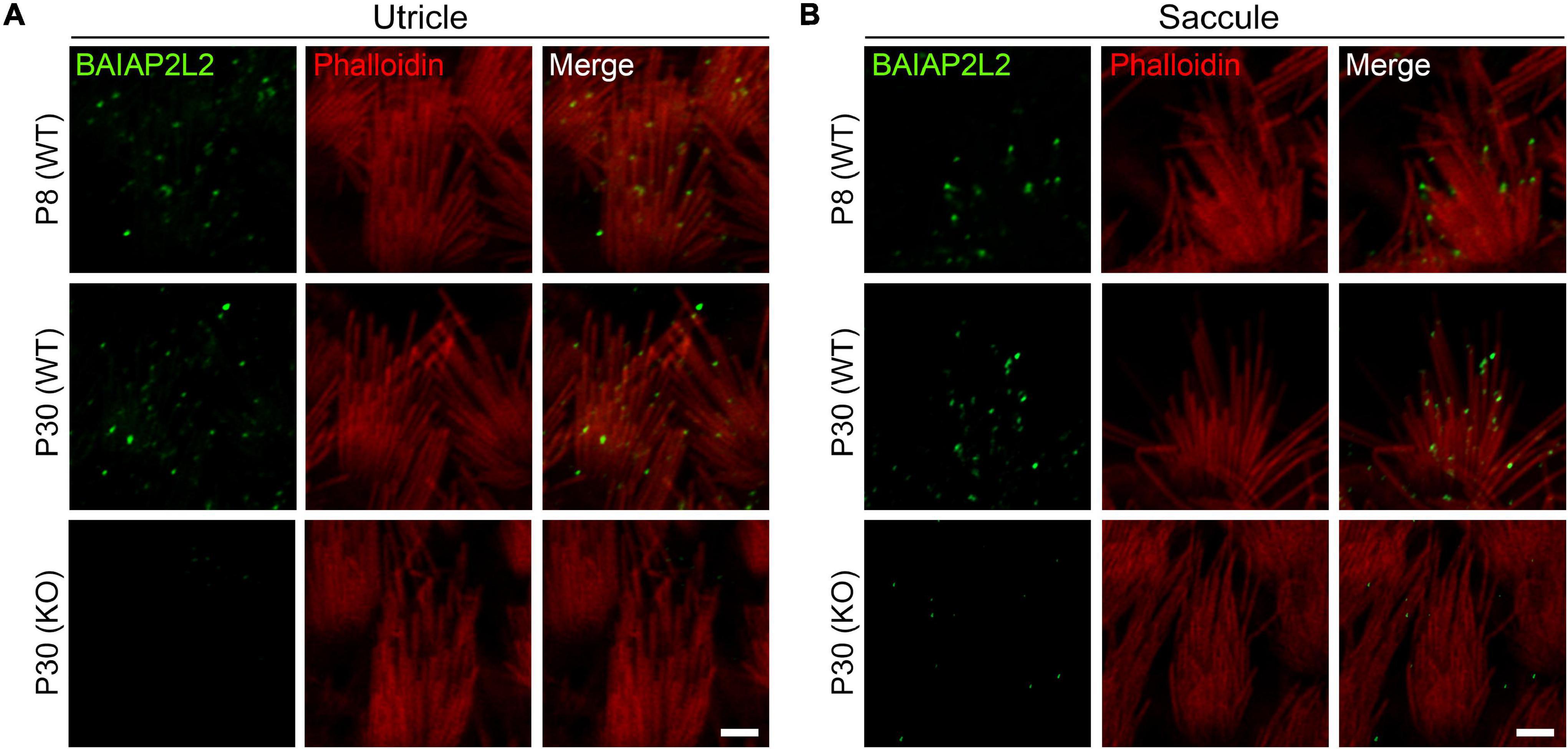
Figure 1. BAIAP2L2 is localized at the tips of shorter-row stereocilia in VHCs. Whole-mount immunostaining using a specific anti-BAIAP2L2 antibody (green) was performed to examine the localization of BAIAP2L2 in the stereocilia of utricular (A) and saccular (B) hair cells. Steoreociliary F-actin core was visualized using TRITC-conjugated phalloidin (red). The genotypes and ages of mice are indicated. Scale bar, 2 μm.
Phalloidin staining reveals largely unaffected stereocilia morphology in Baiap2l2–/– VHCs (Figures 1A,B). Scanning electron microscopy (SEM) was then employed to further examine the morphology of VHC stereocilia in Baiap2l2 knockout mice. When examined at P8, the morphology of both utricular and saccular VHC stereocilia in Baiap2l2–/– mice is indistinguishable from that in control mice (Figures 2A,B). Similar results were observed in Baiap2l2–/– mice at P30 (Figures 2C,D). Taken together, our present data suggest that loss of BAIAP2L2 does not affect the development or maintenance of VHC stereocilia.
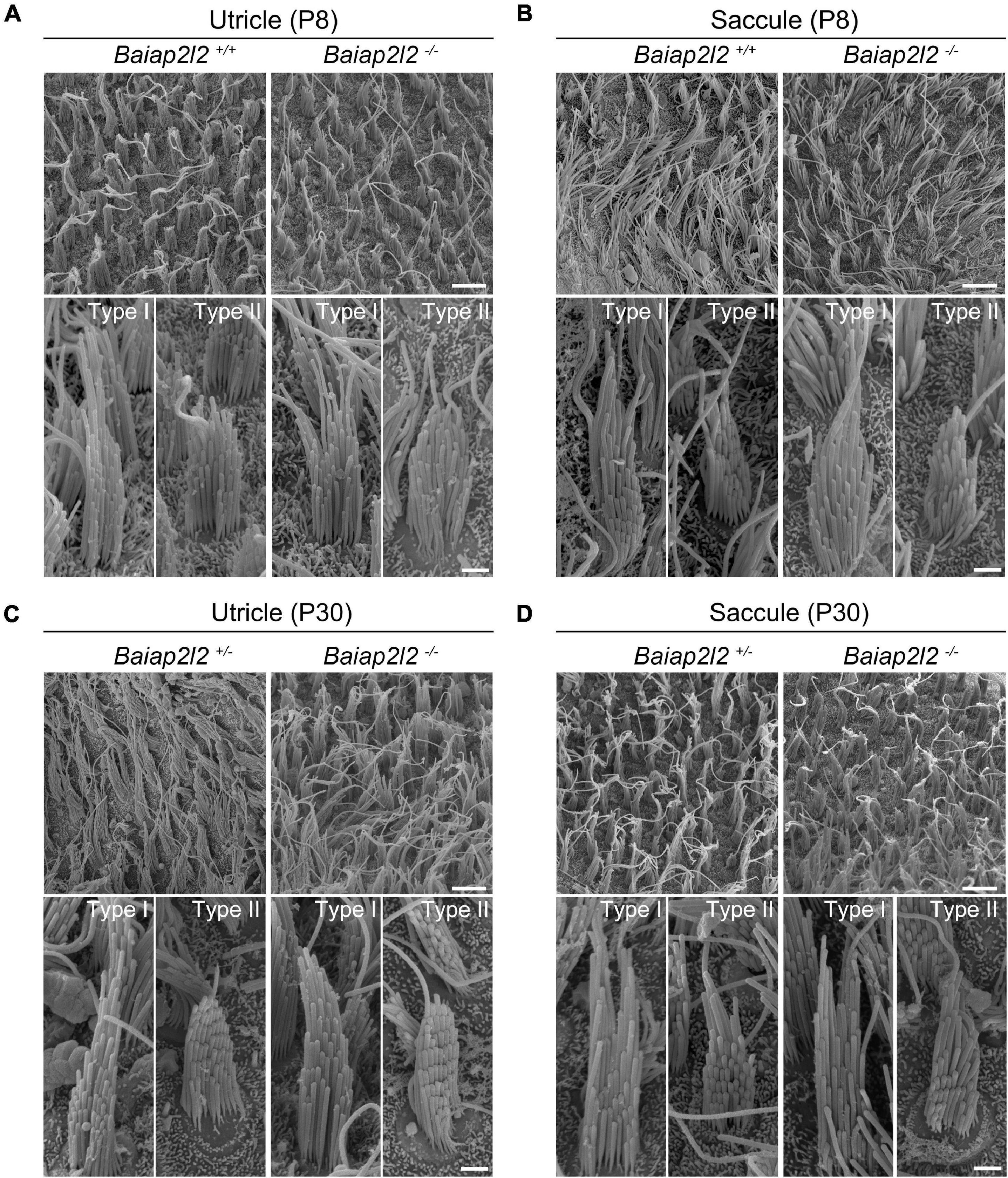
Figure 2. Stereocilia morphology is unaffected in VHCs of Baiap2l2 knockout mice. SEM was performed to examine the stereocilia morphology of P8 utricle (A), P8 saccule (B), P30 utricle (C), and P30 saccule (D) in mice of different genotypes as indicated. In each panel, low-magnification images and high magnification images of type I and II VHCs are shown at the top and bottom, respectively. Scale bar, 5 μm (in low-magnification images) and 1 μm (in high-magnification images).
The unaffected stereocilia morphology suggests that MET function might be normal in Baiap2l2–/– VHCs. We then examined the MET function of Baiap2l2–/– VHCs by performing FM 1-43FX uptake experiment. FM 1-43FX is a fixable fluorescent dye that could enter hair cells through MET channels when applied briefly, therefore is often used as an indicator of hair cell MET function (Gale et al., 2001; Meyers et al., 2003). The results show that FM 1-43FX dye uptake of utricular VHCs in Baiap2l2–/– mice at P8 is comparable to that in control mice (Supplementary Figures 2A,B). Similar results were obtained in P8 saccular VHCs (Supplementary Figures 2C,D). We then examined the MET function of adult VHCs at P30 by performing FM 1-43FX uptake experiment, which also did not reveal any difference between Baiap2l2–/– and control mice (Figures 3A–D). Therefore, our present data suggest that the MET function of VHCs is not affected by loss of BAIAP2L2.
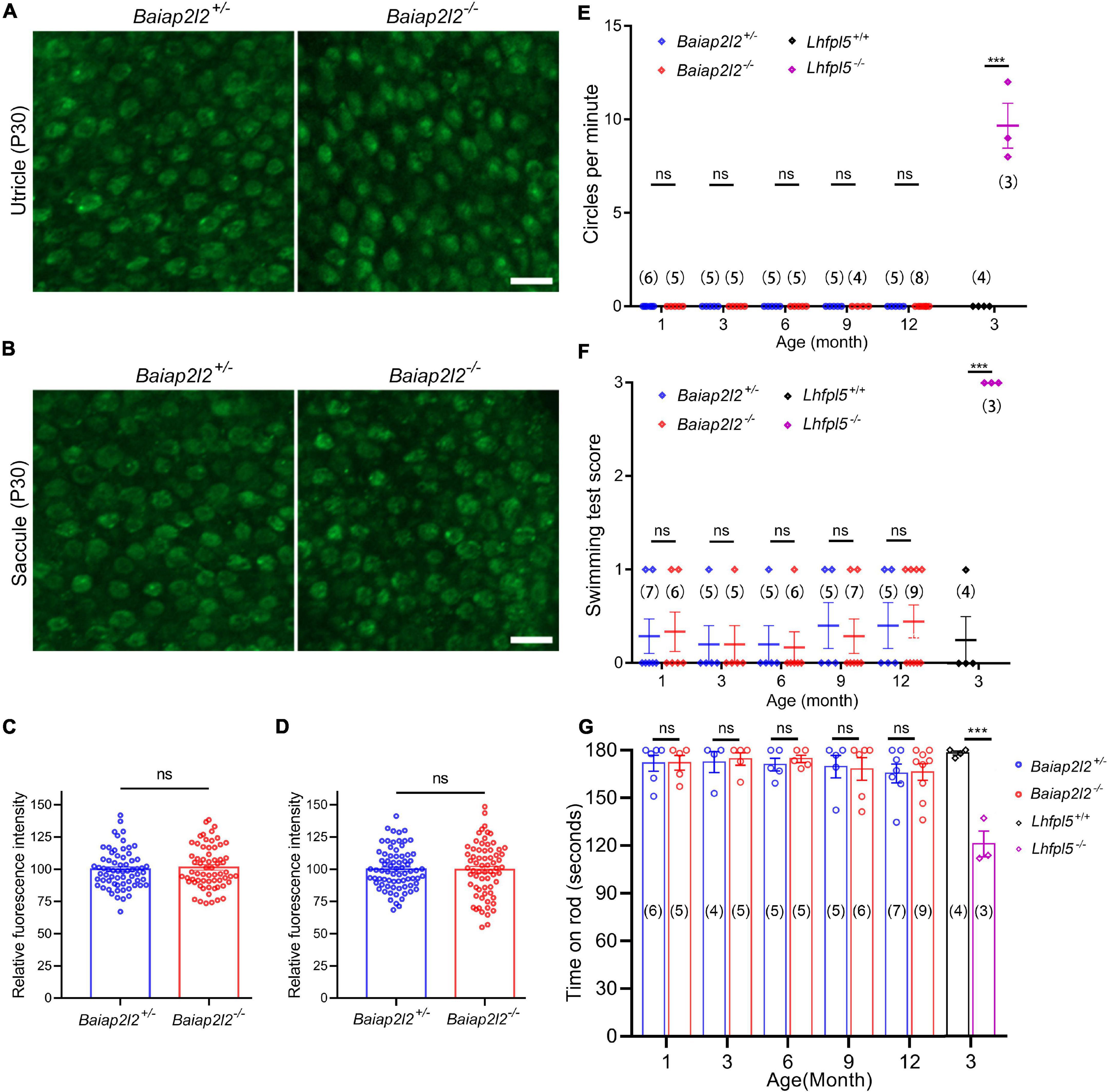
Figure 3. Vestibular function is unaffected in Baiap2l2 knockout mice. (A,B) FM1-43FX uptake by P30 utricular (A) and saccular (B) hair cells from Baiap2l2+/– or Baiap2l2–/– mice was examined using confocal microscope. Scale bars, 10 μm. (C,D) FM1-43FX uptake was quantified according to the results from (A,B), respectively. (E–G) The vestibular function of Baiap2l2+/– or Baiap2l2–/– mice of different ages was evaluated by examining circling stereotyped movement (E), swimming test (F), and rotarod test (G). Lhfpl5–/– mice were included as positive control. Numbers of animals in each group are indicated in brackets. ns, not significant; ***p < 0.001.
We then moved on to examine the vestibular function of Baiap2l2–/– mice. Mutation in Lhfpl5 gene, which encodes for MET component LHFPL5, leads to deafness and balance dysfunction (Longo-Guess et al., 2005). Therefore, Lhfpl5–/– mice were included in the present experiments as positive control. Consistent with the previous report, Lhfpl5–/– mice show typical circling stereotyped movement, suggesting of balance dysfunction (Figure 3E). However, Baiap2l2–/– mice at ages of up to 12 months do not show any circling stereotyped movement (Figure 3E).
The vestibular function of Baiap2l2–/– mice was further evaluated by swimming test and rotarod test. In both tests, Baiap2l2–/– mice at ages of up to 12 months perform indistinguishably from control Baiap2l2+/– mice (Figures 3F,G). In contrast, Lhfpl5–/– mice show abnormal swimming behavior and are easier to fall off the rotarod (Figures 3F,G). Taken together, our present data suggest that the vestibular function is not affected by loss of BAIAP2L2.
To explore the possible reason why loss of BAIAP2L2 does not affect VHC stereocilia or vestibular function, we tried to examine the stereociliary localization of other row 2 complex components in VHCs by performing whole-mount immunostaining and confocal microscopy. Here, we focused on CAPZB2 since its stereociliary tip localization in cochlear hair cells has been shown to depend on BAIAP2L2 (Yan et al., 2021). The results show that in the utricle of P30 control mice, CAPZB2 immunoreactivity is localized at the tips of shorter-row stereocilia as reported previously (Avenarius et al., 2017; Figure 4A, top panel). Interestingly, CAPZB2 immunoreactivity is unaffected in the utricle of Baiap2l2–/– mice (Figure 4A, bottom panel), which is consistent with the normal development and maintenance of VHC stereocilia in Baiap2l2–/– mice. Similar results were observed in the saccule (Figure 4B).
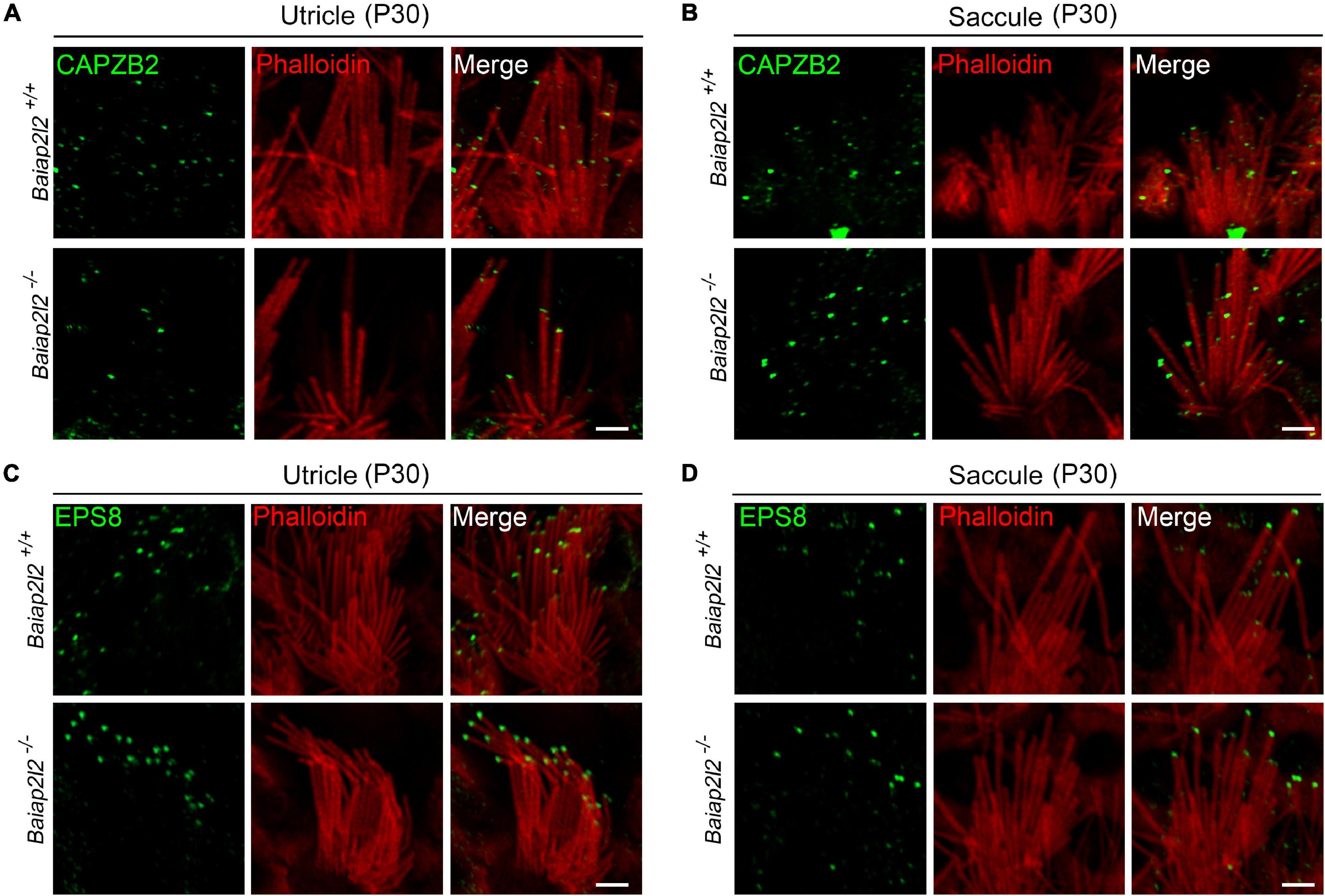
Figure 4. Stereociliary tip localization of CAPZB2 and EPS8 is unaffected in VHCs of Baiap2l2 knockout mice. Whole-mount immunostaining and confocal microscopy were performed to examine the localization of CAPZB2 in utricular (A) and saccular (B) hair cells of P30 Baiap2l2+/+ or Baiap2l2–/– mice. Similar experiments were performed to examine the localization of EPS8 in utricular (C) and saccular (D) hair cells of P30 Baiap2l2+/+ or Baiap2l2–/– mice. TRITC-conjugated phalloidin was used to visualize stereociliary F-actin core. Scale bars, 2 μm.
EPS8 is a row 1 complex component and is responsible for the stereociliary tip localization of BAIAP2L2 in cochlear hair cells (Carlton et al., 2021). Our results show that EPS8 immunoreactivity is localized at the tips of the taller-row stereocilia in the utricle or saccule of both Baiap2l2+/+ and Baiap2l2–/– mice (Figures 4C,D). Taken together, the present data show that row 1 and row 2 complex are largely unaffected in the VHC stereocilia of Baiap2l2–/– mice.
BAIAP2L2 is a recently identified row 2 complex component that localizes at the tips of shorter-row mechanotransducing stereocilia in cochlear hair cells (Carlton et al., 2021; Yan et al., 2021). BAIAP2L2 inactivation results in mechanotransducing stereocilia degeneration in cochlear hair cells, and leads to profound hearing loss (Carlton et al., 2021; Yan et al., 2021). In the present work, we show that BAIAP2L2 is also localized at the tips of shorter-row stereocilia in VHCs. Unexpectedly, our data reveal that BAIAP2L2 inactivation does not affect the development/maintenance of VHC stereocilia as well as vestibular function.
There are evidences suggesting that deficiency of row 2 complex components might affect cochlear and vestibular hair cells differently. For example, EPS8L2 or MYO15A-L inactivation results in degeneration of the mechanotransducing stereocilia in cochlear hair cells, but does not significantly affect vestibular function and/or VHC stereocilia morphology (Furness et al., 2013; Fang et al., 2015). ESP8L2 localizes at the tips of most VHC stereocilia including the taller ones (Furness et al., 2013; Avenarius et al., 2017). Meanwhile, row 1 complex component EPS8 is mostly enriched at the tips of taller-row VHC stereocilia, raising the possibility that row 1 and 2 complex components might work cooperatively in VHC stereocilia and compensate for the loss of each other (Furness et al., 2013; Avenarius et al., 2017). Our present results suggest that similar functional compensation might also happen in Baiap2l2 knockout mice, which awaits further investigations.
One of the candidates responsible for this possible compensation is its homolog BAIAP2L1 (also known as IRTKS), whose expression has been detected in the hair cells through transcriptome studies (umgear.org). In the gut, BAIAP2L1 could localize EPS8 to the developing microvilli of the brush border and regulate their growth (Postema et al., 2018). Examination of the precise localization of BAIAP2L1 in the stereocilia of cochlear and vestibular hair cells, and analysis of Baiap2l1 knockout mice and Baiap2l1/Baiap2l2 double knockout mice will help to address this question.
Similarly to BAIAP2L2, CAPZB2 and TWF2 are more enriched at the tips of shorter-row VHC stereocilia (Avenarius et al., 2017). CAPZB2 is a capping protein that binds to the barbed ends of F-actin and prevents both actin polymerization and depolymerization (Caldwell et al., 1989). CAPZB2 functions as a heterodimer formed together with CAPZA1/2, both of which are detected at the tips of stereocilia (Shin et al., 2013; Avenarius et al., 2017). In sharp contrast to BAIAP2L2/EPS8L2/MYO15A-L, CAPZB2 inactivation leads to VHC stereocilia deficits as well as compromised vestibular function (Avenarius et al., 2017). Capzb2-deficient VHC stereocilia are normal when examined at P2, but become severely disrupted at P7-P9 in some VHCs (Avenarius et al., 2017). A common phenotype in Capzb2-deficient VHCs at P7-P9 is missing of the shortest stereocilia, with the intermediate or highest stereocilia largely unaffected (Avenarius et al., 2017). Consistent with the important role of CAPZB2 in VHC stereocilia, the stereociliary tip localization of CAPZB2 is unaffected in Baiap2l2-deficient VHCs. In contrast, in the cochlear hair cells, the stereociliary tip localization of CAPZB2 is dependent on functional BAIAP2L2 (Yan et al., 2021). The different dependency of CAPZB2 localization on BAIAI2L2 in cochlear and vestibular hair cells might explain the different auditory and balancing phenotypes in Baiap2l2 knockout mice. Detailed examination of the localization of row 2 proteins in VHC stereocilia using super-resolution microscopy might help to learn more about the underlying mechanism (Liu et al., 2019; Qi et al., 2019, 2020).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Animal Ethics Committee of Shandong University School of Life Sciences.
WZ and ZX: study concept and design. KY, CQ, YW, and WZ: acquisition of data. KY, CQ, YW, WZ, and ZX: analysis and interpretation of data. KY, WZ, and ZX: drafting the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (82071051 and 81771001), Shandong Provincial Natural Science Foundation (ZR2020ZD39), and Start-up funds from Shandong University (2019GN115).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Wei Xiong (Tsinghua University) for Lhfpl5 knockout mice. We also thank Sen Wang, Xiaomin Zhao, and Haiyan Yu from the core facilities for Life and Environmental Sciences, Shandong University for the technical support in SEM and confocal microscopy.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2022.829204/full#supplementary-material
Avenarius, M. R., Krey, J. F., Dumont, R. A., Morgan, C. P., Benson, C. B., Vijayakumar, S., et al. (2017). Heterodimeric capping protein is required for stereocilia length and width regulation. J. Cell Biol. 216, 3861–3881. doi: 10.1083/jcb.201704171
Barr-Gillespie, P. G. (2015). Assembly of hair bundles, an amazing problem for cell biology. Mol. Biol. Cell 26, 2727–2732. doi: 10.1091/mbc.E14-04-0940
Beurg, M., Fettiplace, R., Nam, J. H., and Ricci, A. J. (2009). Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat. Neurosci. 12, 553–558. doi: 10.1038/nn.2295
Caldwell, J. E., Heiss, S. G., Mermall, V., and Cooper, J. A. (1989). Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry 28, 8506–8514. doi: 10.1021/bi00447a036
Carlton, A. J., Halford, J., Underhill, A., Jeng, J. Y., Avenarius, M. R., Gilbert, M. L., et al. (2021). Loss of Baiap2l2 destabilizes the transducing stereocilia of cochlear hair cells and leads to deafness. J. Physiol. 599, 1173–1198. doi: 10.1113/JP280670
Fang, Q., Indzhykulian, A. A., Mustapha, M., Riordan, G. P., Dolan, D. F., Friedman, T. B., et al. (2015). The 133-kDa N-terminal domain enables myosin 15 to maintain mechanotransducing stereocilia and is essential for hearing. Elife 4:e08627. doi: 10.7554/eLife.08627
Flock, A., and Cheung, H. C. (1977). Actin filaments in sensory hairs of inner ear receptor cells. J. Cell Biol. 75, 339–343. doi: 10.1083/jcb.75.2.339
Furness, D. N., Johnson, S. L., Manor, U., Ruttiger, L., Tocchetti, A., Offenhauser, N., et al. (2013). Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2. Proc. Natl. Acad. Sci. U. S. A. 110, 13898–13903. doi: 10.1073/pnas.1304644110
Gale, J. E., Marcotti, W., Kennedy, H. J., Kros, C. J., and Richardson, G. P. (2001). FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 21, 7013–7025. doi: 10.1523/Jneurosci.21-18-07013.2001
Hudspeth, A. J., and Jacobs, R. (1979). Stereocilia mediate transduction in vertebrate hair cells. Proc. Natl. Acad. Sci. U. S. A. 76, 1506–1509. doi: 10.1073/pnas.76.3.1506
Jones, C., Roper, V. C., Foucher, I., Qian, D., Banizs, B., Petit, C., et al. (2008). Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 40, 69–77. doi: 10.1038/ng.2007.54
Krey, J. F., and Barr-Gillespie, P. G. (2019). Molecular composition of vestibular hair bundles. Cold Spring Harb. Perspect. Med. 9:a033209. doi: 10.1101/cshperspect.a033209
Krey, J. F., Chatterjee, P., Dumont, R. A., O’Sullivan, M., Choi, D., Bird, J. E., et al. (2020). Mechanotransduction-dependent control of stereocilia dimensions and row identity in inner hair cells. Curr. Biol. 30, 442–454. doi: 10.1016/j.cub.2019.11.076
Li, N., Xi, Y., Du, H., Zhou, H., and Xu, Z. (2021). Annexin A4 is dispensable for hair cell development and function. Front. Cell Dev. Biol. 9:680155. doi: 10.3389/fcell.2021.680155
Liu, Y., Qi, J., Chen, X., Tang, M., Chu, C., Zhu, W., et al. (2019). Critical role of spectrin in hearing development and deafness. Sci. Adv. 5:eaav7803. doi: 10.1126/sciadv.aav7803
Longo-Guess, C. M., Gagnon, L. H., Cook, S. A., Wu, J., Zheng, Q. Y., and Johnson, K. R. (2005). A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc. Natl. Acad. Sci. U. S. A. 102, 7894–7899. doi: 10.1073/pnas.0500760102
McGrath, J., Roy, P., and Perrin, B. J. (2017). Stereocilia morphogenesis and maintenance through regulation of actin stability. Semin. Cell Dev. Biol. 65, 88–95. doi: 10.1016/j.semcdb.2016.08.017
Meyers, J. R., MacDonald, R. B., Duggan, A., Lenzi, D., Standaert, D. G., Corwin, J. T., et al. (2003). Lighting up the senses: fM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 23, 4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003
Peng, A. W., Belyantseva, I. A., Hsu, P. D., Friedman, T. B., and Heller, S. (2009). Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J. Neurosci. 29, 15083–15088. doi: 10.1523/JNEUROSCI.2782-09.2009
Postema, M. M., Grega-Larson, N. E., Neininger, A. C., and Tyska, M. J. (2018). IRTKS (BAIAP2L1) elongates epithelial microvilli using EPS8-dependent and independent mechanisms. Curr. Biol. 28, 2876–2888. doi: 10.1016/j.cub.2018.07.022
Qi, J., Liu, Y., Chu, C., Chen, X., Zhu, W., Shu, Y., et al. (2019). A cytoskeleton structure revealed by super-resolution fluorescence imaging in inner ear hair cells. Cell. Discov. 5:12. doi: 10.1038/s41421-018-0076-4
Qi, J., Zhang, L., Tan, F., Liu, Y., Chu, C., Zhu, W., et al. (2020). Espin distribution as revealed by super-resolution microscopy of stereocilia. Am. J. Transl. Res. 12, 130–141.
Rzadzinska, A. K., Nevalainen, E. M., Prosser, H. M., Lappalainen, P., and Steel, K. P. (2009). MyosinVIIa interacts with Twinfilin-2 at the tips of mechanosensory stereocilia in the inner ear. PLoS One 4:e7097. doi: 10.1371/journal.pone.0007097
Shin, J. B., Krey, J. F., Hassan, A., Metlagel, Z., Tauscher, A. N., Pagana, J. M., et al. (2013). Molecular architecture of the chick vestibular hair bundle. Nat. Neurosci. 16, 365–374. doi: 10.1038/nn.3312
Tadenev, A. L. D., Akturk, A., Devanney, N., Mathur, P. D., Clark, A. M., Yang, J., et al. (2019). GPSM2-GNAI specifies the tallest stereocilia and defines hair bundle row identity. Curr. Biol. 92:e924. doi: 10.1016/j.cub.2019.01.051
Tilney, L. G., Derosier, D. J., and Mulroy, M. J. (1980). The organization of actin filaments in the stereocilia of cochlear hair cells. J. Cell Biol. 86, 244–259. doi: 10.1083/jcb.86.1.244
Velez-Ortega, A. C., and Frolenkov, G. I. (2019). Building and repairing the stereocilia cytoskeleton in mammalian auditory hair cells. Hear. Res. 376, 47–57. doi: 10.1016/j.heares.2018.12.012
Xiong, W., Grillet, N., Elledge, H. M., Wagner, T. F. J., Zhao, B., Johnson, K. R., et al. (2012). TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151, 1283–1295. doi: 10.1016/j.cell.2012.10.041
Keywords: inner ear, vestibular hair cells, stereocilia, BAIAP2L2, CAPZB2
Citation: Yan K, Qu C, Wang Y, Zong W and Xu Z (2022) BAIAP2L2 Inactivation Does Not Affect Stereocilia Development or Maintenance in Vestibular Hair Cells. Front. Mol. Neurosci. 15:829204. doi: 10.3389/fnmol.2022.829204
Received: 05 December 2021; Accepted: 26 January 2022;
Published: 15 February 2022.
Edited by:
Dongdong Ren, Fudan University, ChinaReviewed by:
Jeremy Duncan, Western Michigan University, United StatesCopyright © 2022 Yan, Qu, Wang, Zong and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Zong, d2Vuem9uZ0BzZHUuZWR1LmNu; Zhigang Xu, eHV6Z0BzZHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.