- 1Department of Pain Management, Shandong Provincial Hospital, Shandong University, Jinan, China
- 2Department of Pain Management, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: Zoster-associated pain (ZAP) is notoriously difficult to treat. Pulsed radiofrequency (PRF) and short-term nerve electrical stimulation (st-NES) have been proven effective treatments for ZAP. However, it is still unclear which technique provides improved analgesia in ZAP. This study is based on a large-scale, long-term follow-up to evaluate the efficacy and safety between st-NES and PRF.
Materials and methods: All eligible ZAP patients treated with st-NES or PRF in our department were enrolled. Cohorts were divided into the st-NES group and the PRF group. A 1:1 ratio propensity score matching (PSM) was used to balance the baseline characteristics. The PS-matched cohort was adopted to investigate the efficacy and safety of the two treatments. The ordinal regression analysis was performed to determine the variables affecting the treatment effect of ZAP.
Results: A total of 226 patients were included after PSM. The numerical rating scale (NRS) scores in st-NES and PRF groups considerably reduced compared to baseline levels after treatment. The NRS scores in the st-NES group were obviously lower than those in the PRF group at discharge, 1, 3, 6, 12, and 24 months. During the follow-up period, the NRS reduction rate remained higher in the st-NES group than in the PRF group (P < 0.01). The dosage of medication, Pittsburgh Sleep Quality Index (PSQI) score, and the number of patients with aggravated pain after discharge in the st-NES group were significantly less than in the PRF group after treatment.
Conclusion: Short-term nerve electrical stimulation has been shown to be more advantageous than PRF for pain relief and quality of life improvement for ZAP patients.
Introduction
Zoster-associated pain (ZAP) is common and difficult to treat (Forbes et al., 2016). Evidence suggested that 15% to –45% of patients with shingles subsequently progress to postherpetic neuralgia (PHN), especially in those over 60 years of age (Gershon et al., 2015; Moshayedi et al., 2018; Salvetti et al., 2019; Zhou J. et al., 2021). The generally accepted classification of ZAP is as follows: (i) acute herpetic neuralgia (AHN) within 30 days of onset, (ii) subacute herpetic neuralgia (SHN), pain recorded between 30 and 90 days, (iii) postherpetic neuralgia (PHN), defined as pain lasting more than 90 days after the presentation (Whitley et al., 2010).
Currently, the treatment for ZAP is primarily based on symptom control (van Wijck et al., 2006; Johnson and Rice, 2014). Medications are the most fundamental and principal management for ZAP, with pregabalin and gabapentin being the most commonly used first-line therapeutic agents (Finnerup et al., 2021). However, complete pain relief cannot be achieved by taking medications, and 20–40% of patients do not respond to the drugs (Binder and Baron, 2016). Conservative treatments such as acupuncture and physical therapy combined with pharmacy have been reported to provide greater pain relief for ZAP (Fleckenstein et al., 2009; Zhou Q. et al., 2021). However, these therapeutic effects are still limited and lack quality supporting evidence. Invasive treatments, including nerve blocks and neuromodulation, provide alternatives for ZAP patients who are not satisfied with the results of drugs and conservative treatments (Makharita and Amr, 2020). It has been reported that local anesthetics and steroids injections could alleviate AHN (van Wijck et al., 2006), while the therapeutic effects of PHN were usually unsatisfactory and disappointing. Neuromodulations, including PRF and NES, have been rapidly developed in the management of chronic pain over the past 20 years (Liu et al., 2020; Knotkova et al., 2021). NES includes st-NES and permanent NES. Although permanent NES can achieve prolonged analgesia, its application is limited by the high costs and high incidence of complications (Kumar et al., 2006; Deer et al., 2014). Hence, st-NES have been increasingly used for intractable pain including ZAP. Recent reports have indicated that PRF and st-NES are effective treatments for ZAP (Johnson and Burchiel, 2004; Yanamoto and Murakawa, 2012; Dong et al., 2017; Kim et al., 2017; Li et al., 2018; Makharita et al., 2018; Huang et al., 2020). However, it is still unclear which technique provides improved analgesia in ZAP. Previously published studies comparing the efficacy of st-NES and PRF for ZAP are inconsistent (Liu et al., 2020; Song, 2021; Sheng et al., 2022).
Therefore, we compared st-NES with PRF in the effectiveness of treatment for ZAP through PSM and investigated the factors that influenced the therapeutic effects of ZAP. We have further explored the efficacy of these two treatments in comparison to each other in terms of disease course and location. NRS score and the NRS reduction rate were selected as the primary endpoints. The secondary endpoints included medication consumption, PSQI score, the aggravation of pain, and side effects.
Materials and methods
Study design
This retrospective study was approved by the Ethics Committee of the Shandong Provincial Hospital. Inclusion criteria were as follows: (1) the patient’s clinical data was integrity, (2) ZAP with a precise diagnosis, (3) pre-operative NRS ≥ 4, (4) resistance to pharmacological treatment or intolerable side effects of drugs, and (5) stimulation treatment for 7–10 days. Exclusion criteria were as follows: (1) companies with other chronic pain in the same site with ZAP, (2) invasive treatment for ZAP within 2 weeks before this admission, (3) stimulation treatment for less than 7 days, and (4) patients with lost follow-up.
Study population
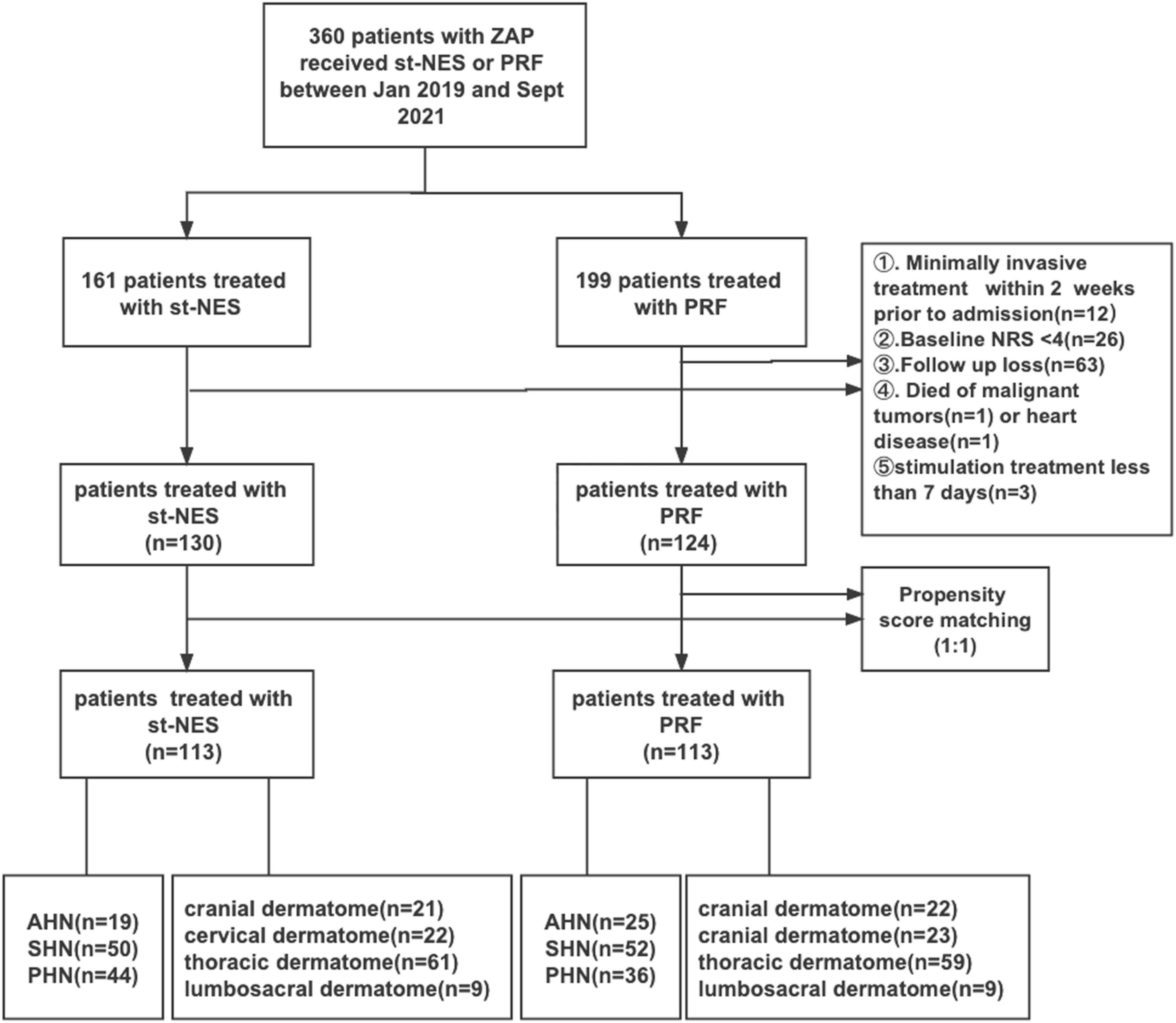
As shown in Figure 1, Between January 2019 and September 2021, 360 consecutive patients with ZAP were hospitalized in our department receiving PRF or st-NES. A total of 254 patients were ultimately enrolled in the current study (st-NES group, n = 130 and PRF group, n = 124). The following were excluded from the study: lost to follow-up (n = 63), accepting invasive treatment within 2 weeks before admission (n = 12), baseline NRS < 4 (n = 26), and died of malignant tumors (n = 1) or heart disease (n = 1), stimulation treatment for less than 7 days (n = 3). After PSM, 113 patients were included in each group. The PS-matched cohort was further divided into different subgroups according to disease duration (19 AHN patients, 50 SHN patients, and 44 PHN patients in the st-NES group and 25 AHN patients, 52 SHN patients, and 36 PHN patients in the PRF group) and sites (st-NES group: cranial dermatome n = 21, cervical dermatome n = 22, thoracic dermatome n = 61, lumbosacral dermatome n = 9. PRF group: cranial dermatome n = 22, cervical dermatome n = 23, thoracic dermatome n = 59, lumbosacral dermatome n = 9).
Surgical procedures
The procedure of st-NES was as follows: Short-term peripheral nerve stimulation (st-PNS) was applied in patients with ZAP in the cranial dermatome, short-term spinal cord stimulation (st-SCS) was applied in the cervical, thoracic or lumbosacral dermatome. In this study, st-PNS included supraorbital nerve and gasserian ganglion stimulation. X-ray guided exposure of the optimal puncture site and locating the target position of the electrode. The electrode was implanted through a puncture needle at a proper physiologic and anatomic position with local or general anesthesia. For supraorbital nerve stimulation, the supraorbital notch and supraorbital rim were located, slowly local infiltrate anesthesia with 1% lidocaine was performed from the medial edge of the contralateral brow arch to the superior edge of the affected brow arch to ensure that the supraorbital nerve was not blocked, then placed the puncture needle and reached the periosteal surface. The anterioposterior film showed that the puncture needle was located approximately 1 cm from the superior orbital rim of the affected side (Vaisman et al., 2012; Wan and Song, 2021). For gasserian ganglion stimulation, the oval foramen on the affected side was exposed as the puncture target, and the puncture needle was inserted in the established direction under general anesthesia. The direction and depth of the needle were adjusted under fluoroscopy. The electrode was placed after the needle tip passed through the oval fossa until the upper end of the electrode was shown to reach the junction between the sella turcica, the clivus, and the petrous part of the temporal bone on the lateral fluoroscopy. For spinal cord electrical stimulation, the puncture point was usually 1–2 segments below the target vertebral segment, and the electrode was located in the epidural space on lateral film (Taub et al., 1997). Then, Electrical stimulation was performed, and the electrode position and parameters (frequency, pulse width, and voltage) were adjusted to ensure adequate paresthesia caused by the stimulus could cover the painful area entirely. Then the position of the electrode was considered appropriate, and a fixation suture was applied to it (Song et al., 2014). The specific parameters were set according to the patient’s sensation, with frequencies mainly in the range of 60–100 Hz and without exceeding 300, pulse width usually between 90 and 300 us and no more than 1,000 us, and a voltage mainly varied from 0.5 to 5.5 V. In addition, the stimulus electrode and electric pulse generator were Medtronic (1*8 compact 3878-75, Medtronic, Minneapolis, MN, United States), and all patients were placed with a single electrode. The stimulation was continuous and performed for 7–10 days, and then the electrode was removed after the treatment. During the treatment, the parameters of the electrode were constantly adjusted to ensure that pain could be replaced entirely by pleasant paresthesia.
The targets of PRF in this study included the supraorbital nerve, the gasserian ganglion, and the dorsal root ganglion. The procedure of PRF is as follows: the anatomical area of the involved nerve was found under X-ray or ultrasound guidance and punctured with a radiofrequency needle with local anesthesia until the tip of the needle reached a satisfactory position. The supraorbital foramen was the puncture point for the supraorbital nerve PRF, the PRF needle was inserted perpendicular to the skin, and after the sense of falling, the PRF needle core was placed (Zhang et al., 2020). Similarly, the foramen ovale was the puncture target of the gasserian ganglion radiofrequency, and the puncture needle was inserted until it reached the foramen ovale. Then the tip of the needle was adjusted while inserting the needle core until the tip of the needle reached the clivus in the lateral fluoroscopy (Ding et al., 2019). For dorsal root ganglion PRF, the target vertebral segment was positioned as the puncture point and punctured under fluoroscopy or ultrasound until the needle tip was above or below the intervertebral foramen. After back drawing without blood or fluid, a stimulation test was performed under local anesthesia (Geurts et al., 2003). Sensory stimulation at a voltage of 0.2–0.6 V and a frequency of 50 Hz was implemented to ensure the needle tip position was closer to the surface of the ganglion or nerve. Subsequently, the parameters and their values were as follows: pulse width 15 or 20 ms, temperature 42°C, frequency 2 Hz, voltage 40–70 V, duration 600 s. Patients were usually treated with PRF 2–3 times during hospitalization, depending on the level of pain relief. If the pain was not relieved within 3 days after the treatment or the result was unsatisfactory, a further PRF treatment would be performed.
Measurements
The primary outcomes include the numerical rating scale (NRS) and NRS reduction rate. NRS reduction rate was calculated as another indicator to assess the pain relief level of the treatment (Excellent: NRS reduction ≥80%, Medium: 50% ≤ NRS reduction <80%, Poor: NRS reduction <50%) (Rigoard et al., 2019, 2021). NRS score and NRS reduction rate were evaluated at baseline (pre-operative), discharge (post-operative), 1, 3, 6, 12, and 24 months after discharge.
The Pittsburgh Sleep Quality Index (PSQI), analgesic consumption, and the aggravation of pain after discharge were used as secondary outcome indicators. The PSQI was recorded at baseline, discharge, and final follow-up. Analgesic consumption (pregabalin or gabapentin) was recorded at pre-operative, 1, 3, 6, 12, and 24 months after discharge. the aggravation of pain and adverse effects were assessed post-operatively.
Propensity score matching analysis
Propensity score matching (PSM) analysis was utilized to restrain confounding factors and settle possible patient selection bias. The PSM was based on age, side, duration of ZAP, and base NRS. Therefore, rigorous adjustment was implemented using nearest neighbor matching without replacement and the caliper width of 0.1 for significant differences in the underlying characteristics of PSM patients. After PSM, a P-value above 0.05 indicated a significant imbalance between groups.
Statistical analysis
All data were analyzed using IBM SPSS Statistics 26.0 (SPSS IBM Corporation, Chicago, IL, USA). Continuous data are expressed as the mean ± standard error of the mean (x ± SEM), and the enumeration data are presented as numbers and proportions. Comparisons between two groups were performed using the chi-square test, independent samples t-test, and Mann–Whitney U-test, and appropriate statistical methods were selected based on the type of variables and whether they conformed to a normal distribution. Univariate χ2 analysis was first performed in analyzing prognostic factors. Afterward, the factor with P < 0.1 in the results of the univariate analysis was used as an independent variable entered the Ordinal regression analysis. At the same time, the odds ratio (OR) and its 95% confidence interval (CI) were calculated. P < 0.05 was deemed statistically significant.
Results
General characteristics
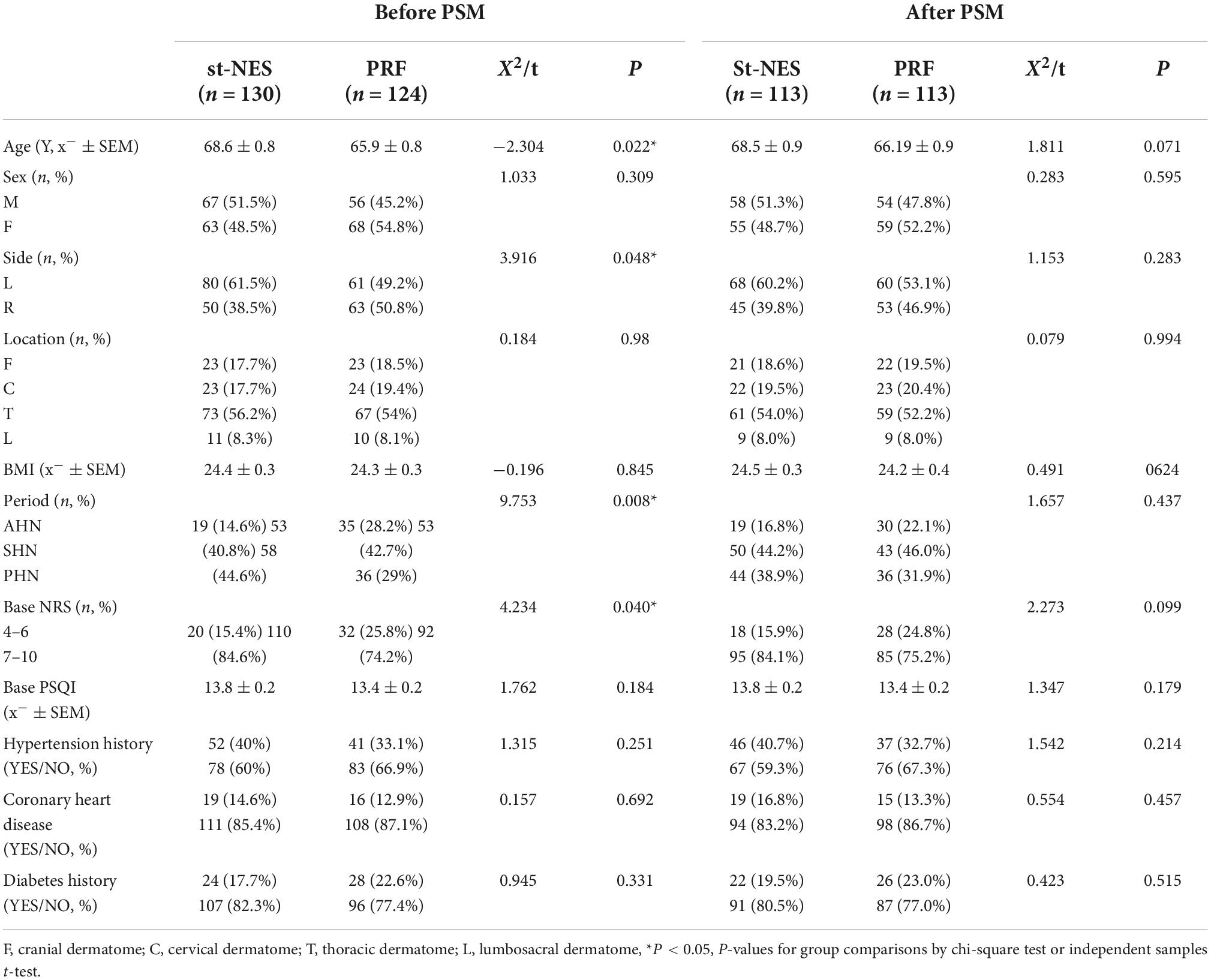
The general characteristics of the patients are summarized in Table 1. The differences in baseline information between the two groups were statistically significant before PSM (P = 0.008–0.048, Table 1). We matched the two groups according to a sample size of 1:1, and all basic characteristics increased to P > 0.05 between the groups after PSM.
Comparison of the efficacy of the two groups
Primary endpoints
Pain relief
The baseline average NRS score for the st-NES group was 8.1 ± 0.1, and for the PRF group, it was 7.8 ± 0.2, which were significantly reduced to 2.2 ± 0.3 and 3.7 ± 0.2 at discharge, respectively. Low pain scores were sustained at 1-month to 24-month follow-ups. NRS score in the st-NES group was reported to be significantly lower than in the PRF group at each time point after treatment (P < 0.01, Figure 2A). During the follow-up period, the NRS reduction rate remained higher in the st-NES group than in the PRF group (P < 0.01, Figure 2B). Additionally, 55–84% of patients achieved an NRS score ≤3 during follow-up in the st-NES group (Figure 2C), and it was 29–46% in the PRF group (Figure 2D).
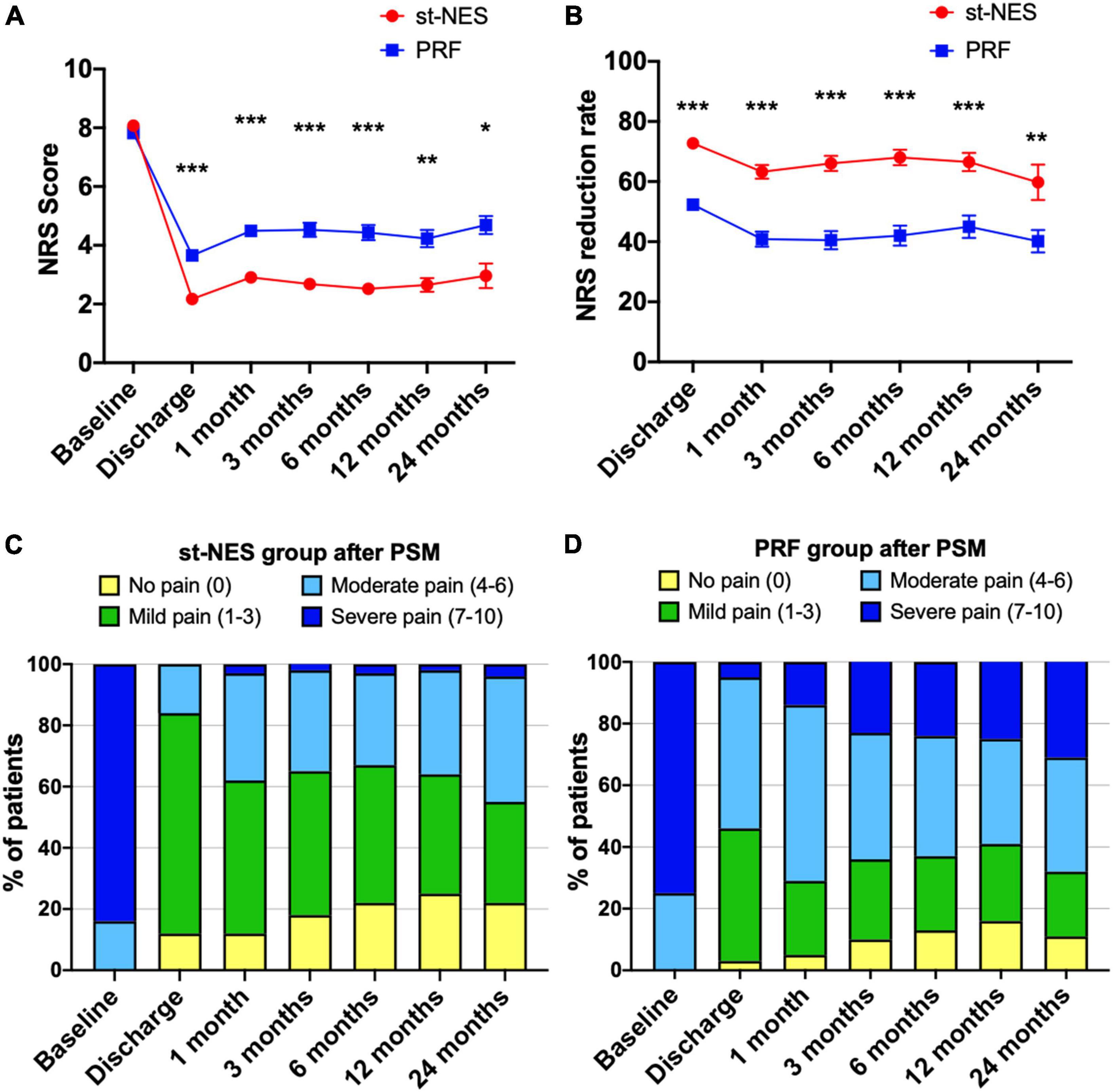
Figure 2. (A,B) Comparison of the efficacy of the two groups on pain relief after propensity score matching (PSM) by numerical rating scale (NRS) score or NRS reduction rate [n = 226, *P < 0.05, **P < 0.01***P < 0.001, indicate pulsed radiofrequency (PRF) group vs. short-term nerve electrical stimulation (st-NES) group, P-values for group comparison by Mann–Whitney U-test]. (C,D) Percentage of patients with different pain levels in the st-NES group or PRF group.
The percentage of patients with different outcomes of pain relief
A total of 91% of patients at discharge, 77, and 67% of patients at 6 and 24 months had excellent and medium outcomes in the st-NES group. In the PRF group, patients with excellent and medium outcomes at discharge, 6 and 24 months were 63, 50, and 42%, respectively (1–6 months P < 0.0001 Figures 3A–D, 12 and 24 months P = 0.002, P = 0.019, Figures 3E,F).
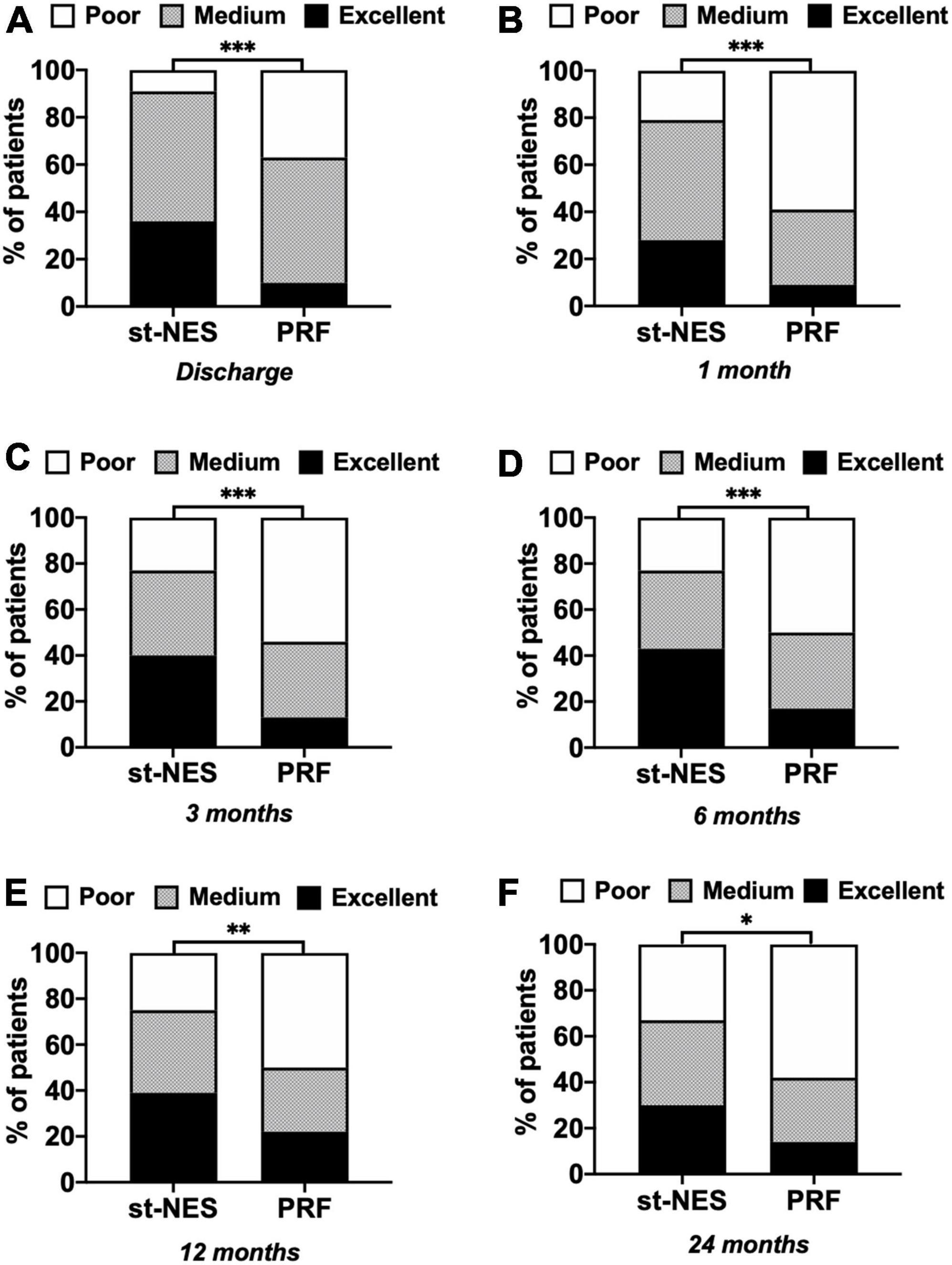
Figure 3. (A–F) The proportion of people with excellent, medium, and poor outcomes of numerical rating scale (NRS) reduction rate in short-term nerve electrical stimulation (st-NES) and pulsed radiofrequency (PRF) group at discharge, 1, 3, 6, 12, and 24 months follow-up (n = 226, *P < 0.05, **P < 0.01, ***P < 0.00, indicate PRF group vs. st-NES group, P-values for group comparison by chi-square test).
Comparison in different disease duration
For AHN and SHN, the pain was significantly relieved with st-NES therapy than PRF therapy within 12 months, as seen by the lower NRS scores and higher NRS reduction rate (P = 0.0001–0.031 Figures 4A–D). No significant difference in NRS scores and NRS reduction rate was observed between the two groups after 12 months. For PHN, the average NRS score in the st-NES group was significantly reduced from 7.8 ± 0.2 to 2.5 ± 0.2 at discharge and remained between 3.3 ± 0.6 and 3.6 ± 0.3 from 1 to 24 months, that in the PRF group reduced from 7.9 ± 0.3 to 3.8 ± 0.3 and remained between 5.6 ± 0.3 and 6.1 ± 0.3 (P < 0.05, Figures 4E–F).
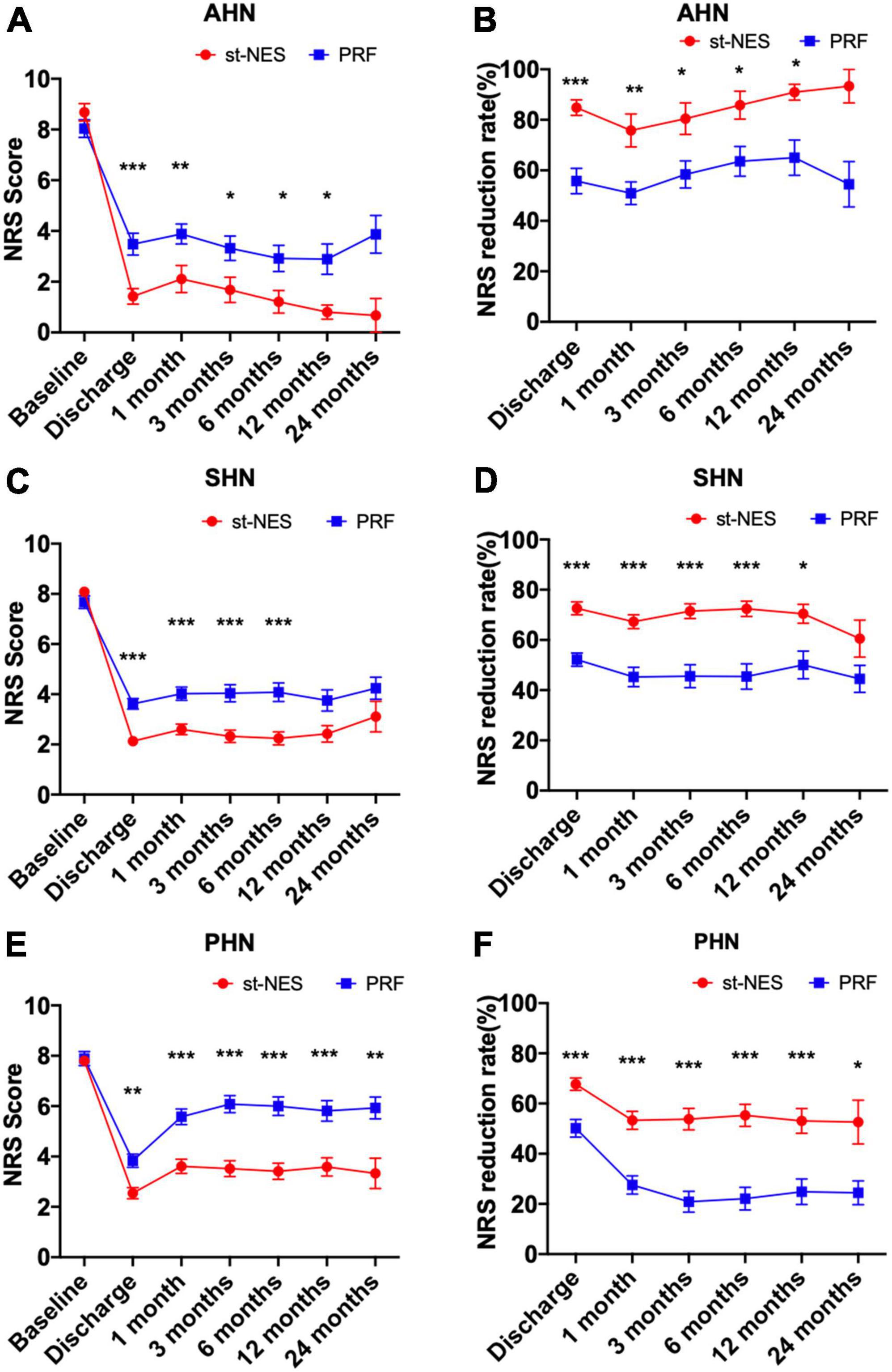
Figure 4. (A–F) Comparison of two treatment modalities for changes of numerical rating scale (NRS) score and NRS reduction rate at each time point during follow-up in acute herpetic neuralgia (AHN), subacute herpetic neuralgia (SHN), and postherpetic neuralgia (PHN), respectively [n = 226, *P < 0.05, **P < 0.01, ***P < 0.001, indicate pulsed radiofrequency (PRF) group vs. short-term nerve electrical stimulation (st-NES) group, P-values for group comparisons by Mann–Whitney U-test].
Comparison in different lesion sites
After treatments, NRS scores significantly declined in both groups at each time for ZAP occurring in the cranial, cervical, thoracic, and lumbosacral dermatome. However, in the cranial dermatome, NRS scores considerably decreased at discharge, 1, 3, and 6 months in the st-NES group compared with the PRF group (P ≤ 0.021, Figure 5A), there was no significant difference at 12 and 24 months. In the cervical dermatome and thoracic dermatome, compared with the NRS scores in the PRF group, the NRS scores in the st-NES group obviously declined at any follow-up interval (P ≤ 0.023, P ≤ 0.035, Figures 5B,C). Additionally, in the lumbosacral dermatome, there was no significant difference in NRS scores at each follow-up time in the two groups (P > 0.05, Figure 5D).
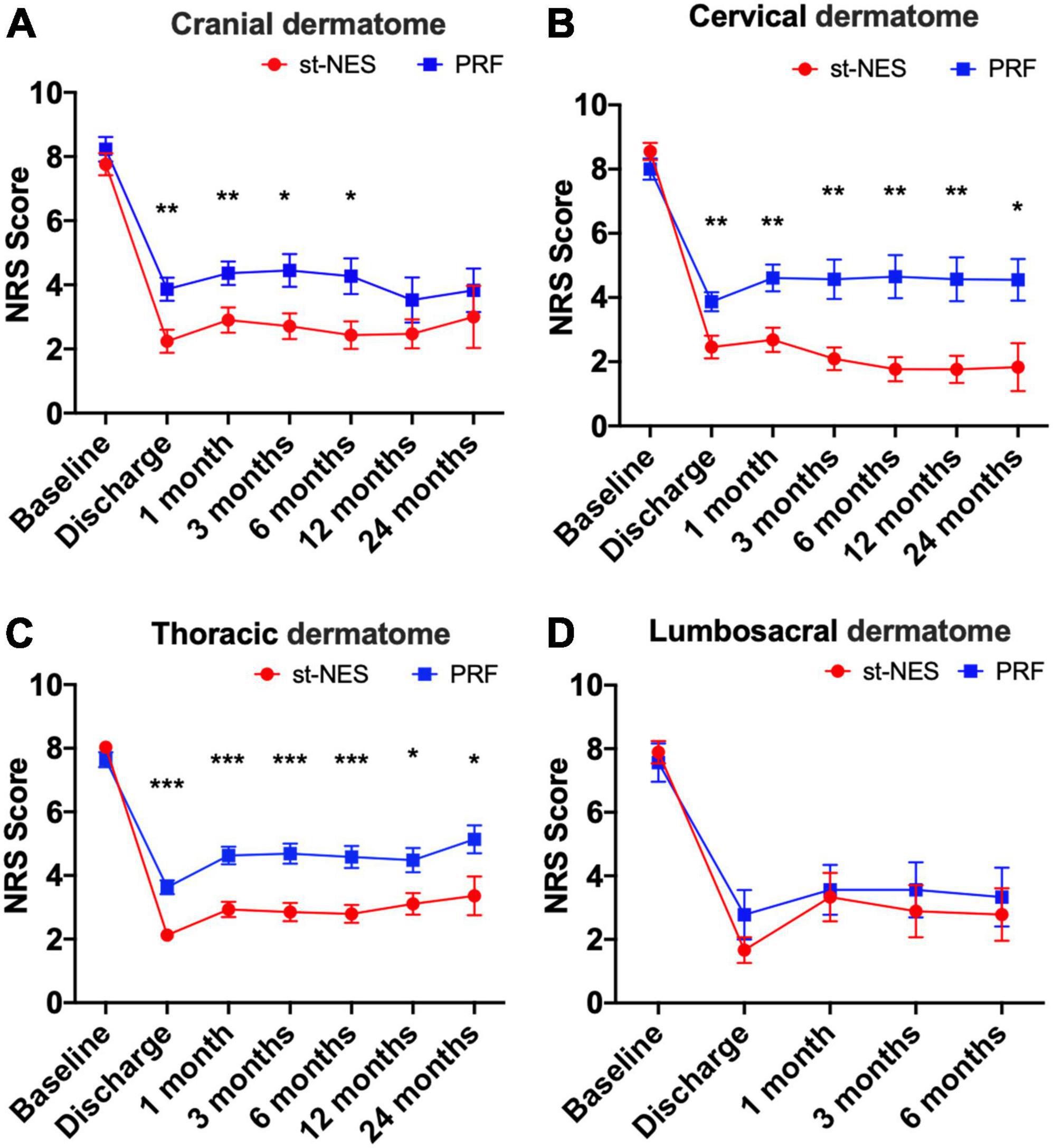
Figure 5. (A–D) Comparison of two groups treatment modalities at different sites [n = 226, *P < 0.05, **P < 0.01, ***P < 0.001, indicate pulsed radiofrequency (PRF) group vs. short-term nerve electrical stimulation (st-NES) group, P-values for group comparisons by Mann–Whitney U-test].
In addition, the operation method of peripheral nerve stimulation and pulsed radiofrequency therapy for herpetic neuralgia in the cranial dermatome will affect the curative effect. We divided the target nerves of peripheral nerve modulation into the supraorbital nerve region and the gasserian ganglion region and compared the efficacy of peripheral nerve electrical stimulation and radiofrequency in the same regions. As shown in Supplementary Figure 1, the NRS scores for supraorbital nerve electrical stimulation were significantly lower than supraorbital nerve radiofrequency within 6 months after discharge (Supplementary Figure 1A), while the efficacy of both was comparable after 6 months. The effects of gasserian ganglion stimulation and gasserian ganglion radiofrequency are always comparable (Supplementary Figure 1B).
Secondary endpoints
Medication consumption
Compared to pre-operative dosages, the average dosages of pregabalin and gabapentin significantly decreased after treatment in the two groups post-operatively (P < 0.05, Figures 6A,B). There was no significant difference in the dosages of pregabalin at any time interval in the two groups, and the dosages of gabapentin obviously declined at 1- and 3-month in the st-NES group compared with the PRF group follow-up (P ≤ 0.033, Figure 6B). During the follow-up period, 54.0% of patients had stopped taking any analgesic within 12 months in the st-NES group and 69.0% in the PRF group (P = 0.067, Figures 6C,D).
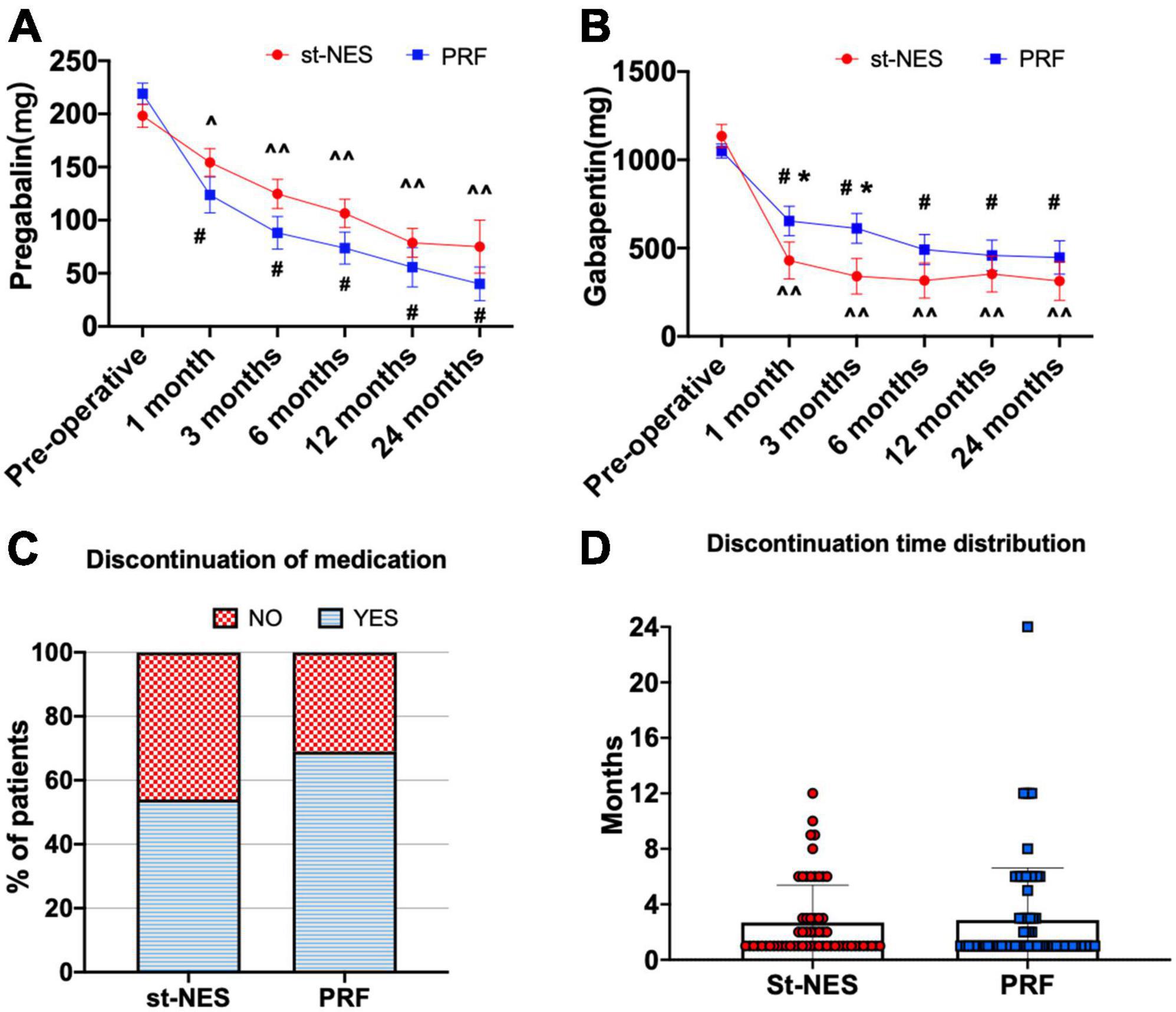
Figure 6. (A,B) The dosage of pregabalin and gabapentin before and after treatment [∧P < 0.05 and ∧∧P < 0.0001 indicate pre-operative vs. post-operative dosage in the short-term nerve electrical stimulation (st-NES) group, #P < 0.0001 indicate pre-operative vs. post-operative dosage in the pulsed radiofrequency (PRF) group, *P < 0.05 indicate PRF group vs. st-NES group, P-values for inter- and intra-group comparisons by Mann–Whitney U-test]. (C) Proportion of patients who discontinued medication during the follow-up. (D) Distribution of discontinuation times and number of patients.
Pittsburgh Sleep Quality Index and the aggravation of pain after discharge
As shown in Figure 7A, the average PSQI scores in the two groups declined at discharge, which further declined at the end of follow-up. Compared to baseline, the average scores in the st-NES group decreased by 5.5 points at discharge and 7.0 points at the end of follow-up, which decreased more than those in the PRF group (P ≤ 0.001, Figure 7A). The number of people with pain worse after discharge than at discharge was significantly higher in the PRF group than in the st-NES group (P < 0.0001, Figure 7B). A total of 45.1% of patients in the PRF group and 17.7% in the st-NES group experienced an aggravation of pain within 6 months after discharge (P < 0.0001, Figure 7B).
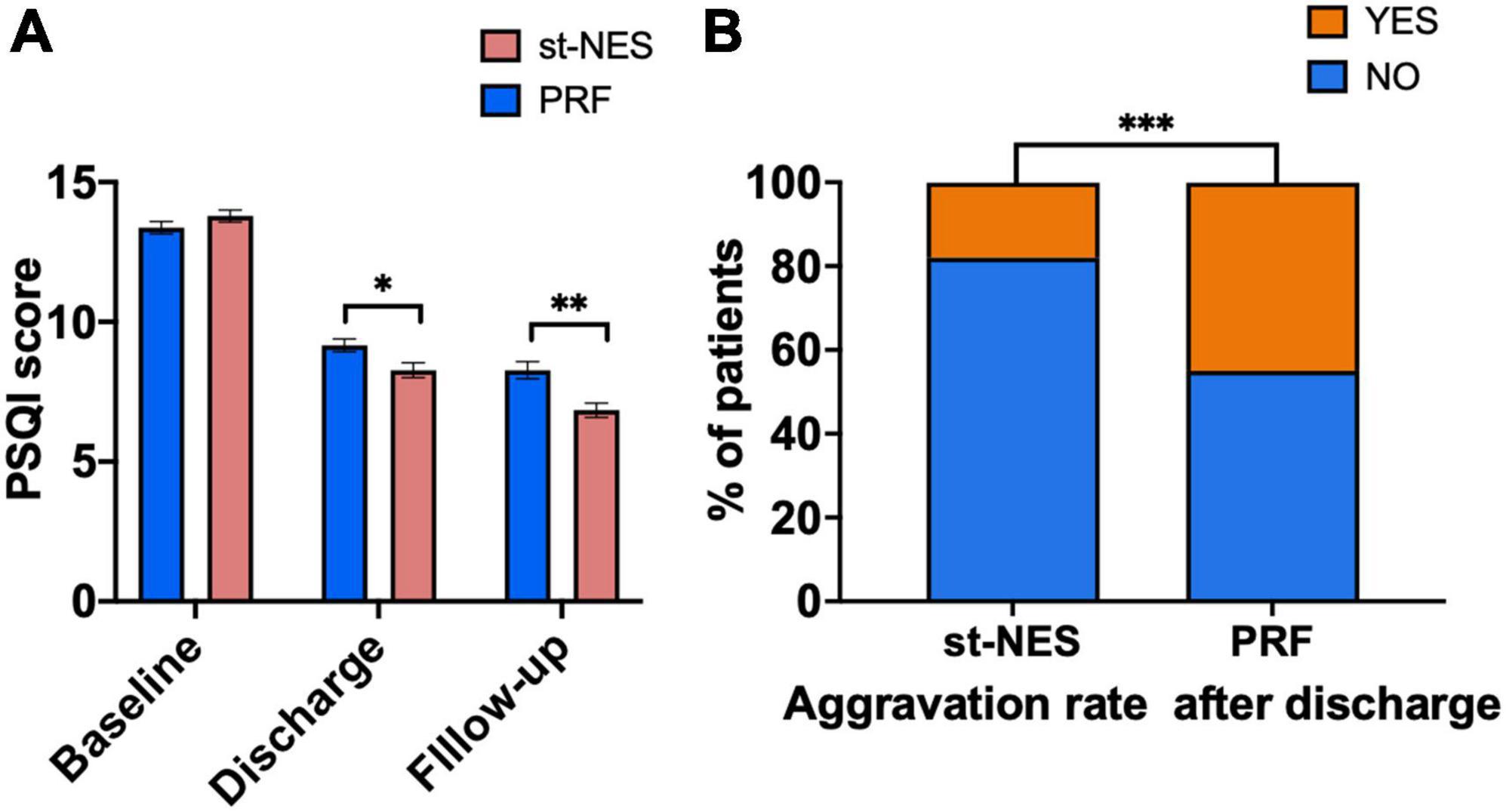
Figure 7. (A) Pittsburgh Sleep Quality Index (PSQI) scores decreased at discharge and final follow-up period. (B) Aggravation rates of the two groups (n = 226, *P < 0.05, **P < 0.01, ***P < 0.001, P-values for group comparison by Mann–Whitney U-test).
Side effects
One patient developed leakage of cerebrospinal fluid, and two patients developed a local infection of the puncture sites in the st-NES group. These complications resulted in electrodes being removed prematurely for less than 10 days and disappearing after rehydration and administration of antibiotics. No other serious adverse events (including prolonged bleeding, hematoma formation, spinal cord injury, etc.) were found in the two groups.
Influential factors
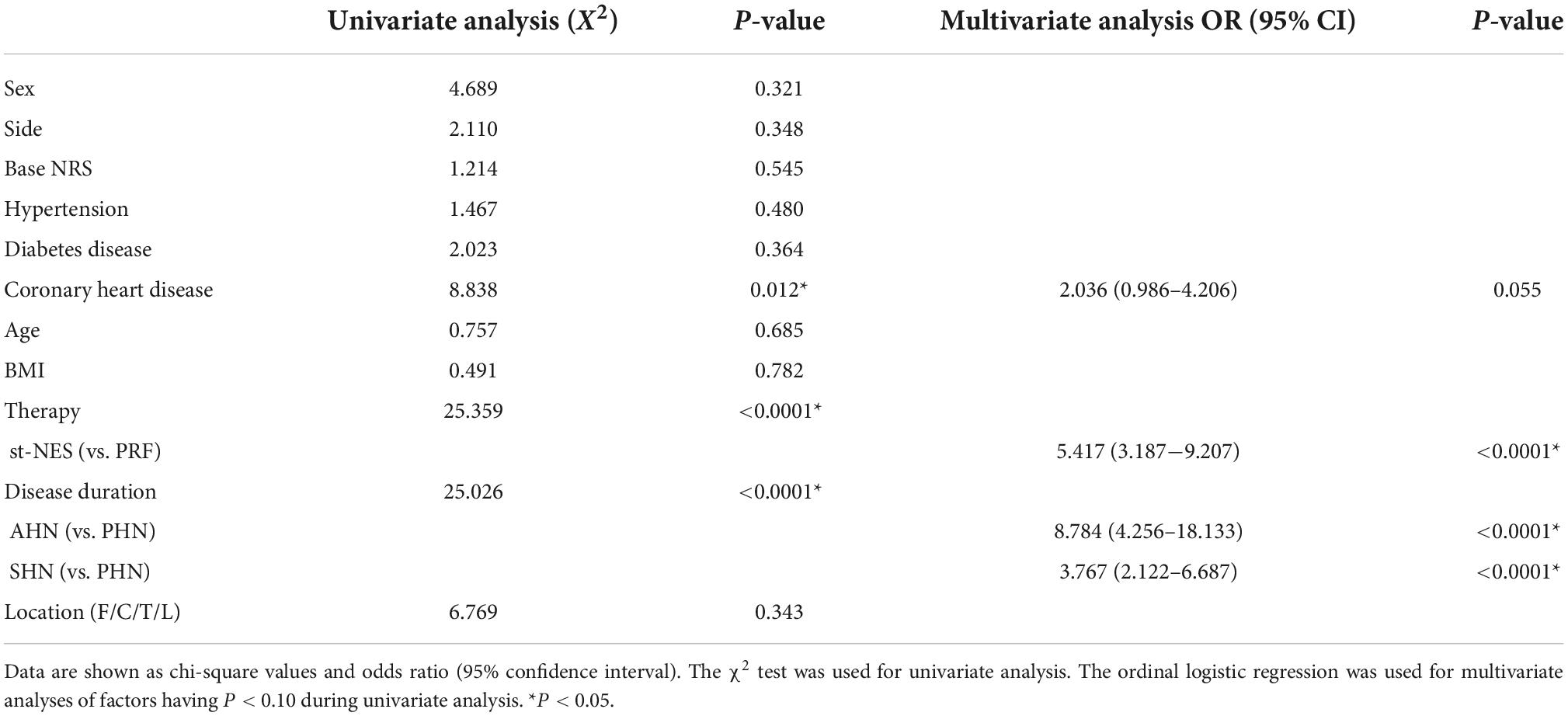
The cohort before PSM was used for risk factors analysis. Coronary heart disease, therapies, and disease duration may affect the effectiveness of ZAP treatment according to the results of the univariate analysis (P = 0.012, P < 0.0001, P < 0.0001, Table 2). Multivariable analysis showed that the therapeutic effect in the st-NES group was more significant than those in the PRF group (OR:5.417, 95% CI: 3.187–9.207, P < 0.0001, Table 2), and patients with shorter duration of disease were prone to achieve more excellent therapeutic results (AHN vs. PHN OR:8.784, 95% CI: 4.256–18.133, P < 0.0001, SHN vs. PHN OR:3.767, 95% CI: 4.256–18.133, P < 0.0001, Table 2).
Discussion
Renovative varicella-zoster virus (VZV) leads to extensive necrosis of skin and nerve cells and triggers abnormal action of neurons resulting in ZAP (Wall and Gutnick, 1974; Devor, 1991; Head et al., 1997). Currently, a proportion of patients still turned PHN after performing interventions in the acute and subacute phases. PHN is the most common complication of herpes zoster and has produced moderate to severe pain for years, severely affecting the quality of life of patients and their families (Johnson, 2010). PRF and NES offer alternatives when conservative treatment and medication for intractable and refractory pain are not effective (Kemler et al., 2000; Eyigor et al., 2010; Assaf et al., 2016).
At present, PRF and st-NES, including st-SCS and st-PNS, were reported to be safe and effective for ZAP (Yanamoto and Murakawa, 2012; Ke et al., 2013; Dong et al., 2017; Wu et al., 2020; Wan and Song, 2021). However, there are relatively few studies on st-NES for treating ZAP compared to PRF, and the comparative efficacy between st-NES and PRF for ZAP is unclear. The present study was designed to determine the effect of st-NES and PRF in treating ZAP. We found that both PRF and st-NES could induce pain alleviation, and pain relief reported in patients treated with st-NES is higher than in those treated with PRF at any time interval. A previous study enrolled 91 AHN and SHN patients, followed up to 6 months, then observed that st-SCS achieved better pain relief than PRF for ZAP at 1-and 6-month follow-ups (Song, 2021). In addition, a recent study with a sample size of 70 PHN patients and a follow-up period of 12 months demonstrated that the VAS scores in the st-SCS group were reported notably lower than those in the PRF group at 3, 6, and 12 months after treatment (Sheng et al., 2022). Our findings are consistent with those studies. Furthermore, we observed that at 24 months follow-up, the st-NES group still maintained a lower NRS score and higher NRS reduction rate.
Apart from pain relief, medication consumption, PSQI score, and the aggravation of pain relevant to the quality of life were assessed in our study. We only counted the dosage of pregabalin and gabapentin due to irregular use of opioids, antidepressants, and other medications in patients. Consequently, a significant reduction in the dosage of these two drugs after surgery could be founded in the present study. Moreover, more than 50% of patients had stopped taking analgesic medication in both groups during the follow-up period. Most patients stopped taking medication gradually as the pain could be maintained at a lower level without a tendency to rebound. Lack of continuous sound sleep is widespread concern for patients with ZAP. In this study, PSQI scores decreased in the two groups, which observed in the st-NES group apparently declined compared to the PRF group. Additionally, patients treated with PRF were prone to have worse pain after discharge than at discharge compared to patients treated with st-NES. The results from secondary-endpoint assessments in the two groups further supported the NRS and NRS reduction rate analysis. Therefore, the superior long- and short-term efficacy of st-NES over PRF was derived from this observational study. This result may be explained by the fact that st-NES is continuous for 7–10 days while PRF lasts for 10 min once a time. The difference in the mechanism of these two treatment modalities may also be the cause. Prior studies reported that PRF could alternate the expression of inflammatory cytokines to induce analgesia, such as IL-6, IL-17, IFN-γ, TNFα, and IGF-2 (Das et al., 2018; Sam et al., 2021). Expression of the neurotransmitter GABA and the inhibitory GABAergic interneurons in the dorsal horn of the spinal cord and ganglion have been identified in a rat model as being involved in the mechanism of NES-mediated analgesia (Cui et al., 1997; Daniele and MacDermott, 2009; Takeda et al., 2013; Sun et al., 2018; Meuwissen et al., 2020). In addition, several reports have mentioned that electrical stimulation changes the electrical state of individual neurons, causing neurotransmitter activity, altering neuronal circuits, and leading to changes in pain and function (Gilmore et al., 2019; Sivanesan et al., 2019; Knotkova et al., 2021). Hence, the altered individual neurons and the neurotransmitters can still relieve pain by reducing the excitability of sensory neurons after the electrodes are removed.
Moreover, we further observed the comparative effects of the two treatments in different disease duration and sites, respectively. For AHN and SHN, the long-term efficacy observed in the st-NES group was comparable with the PRF group. However, st-NES was more effective than PRF for PHN in pain relief. A previous study reported no significant difference between the st-SCS and PRF within 24 weeks post-operatively in patients with SHN and PHN (Liu et al., 2020). The differences observed between us and that study may be due to differences in sample size and follow-up time. In the cervical and thoracic dermatome, the long and short-term efficacy of st-NES was greater than PRF. The statistical analysis did not include the NRS scores at 12 and 24 months in the lumbosacral dermatome because of the small sample size.
The influential factors associated with the therapeutic outcome of ZAP were evaluated. Ultimately, treatments and disease duration were influential factors in the efficacy of ZAP, which means that patients with st-NES experienced greater effectiveness than PRF, and the longer the course of the disease, the worse the outcome of the patients. This result differed from a previous study, which showed no effect of disease duration on the efficacy of ZAP (Liu et al., 2020). This inconsistency may result from the different disease duration of included patients between the two studies.
This study is real-world-based, where patients were admitted to the hospital for further treatment only when medication was ineffective or the side effects were intolerable. We have introduced PRF and st-NES to the patients in detail, and they chose the specific method. Therefore, each patient as their own control could indicate that st-NES and PRF were more effective than oral medications. However, several limitations of this study should be addressed in future research. First, this is a retrospective analysis, lacking strict randomized control. Thus we used propensity score matching methods to avoid the impacts of other variables on endpoints, and the obtained results were reviewed by various statistical methods to ensure the reliability of the data. Second, patients enrolled in one pain management center despite the large sample size. Future multiple centers studies are warranted to validate our findings.
Conclusion
This study showed that st-NES and PRF are effective and safe in treating ZAP. St-NES provides better pain relief and sleeps improvement than PRF for ZAP patients. We further found that st-NES is more effective than PRF within 12 months in AHN, SHN patients, and patients with ZAP in the cranial dermatome, but the efficacy of st-NES and PRF is comparable after 12 months. There is no significant difference in the efficacy of the two treatment modalities for ZAP in the lumbosacral dermatome. In addition, the prognosis of ZAP is related to the type of treatment and duration of the disease, with no correlation to gender, age, underlying diseases, BMI, location, or side of the disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LL contributed to writing of the manuscript. LL and W-JZ contributed to statistical analysis. LL, W-JZ, and TS contributed to study design. LL, W-JZ, S-XX, W-SG, R-RY, X-HJ, and S-YL contributed to collecting data. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2022.1069058/full#supplementary-material
References
Assaf, A. T., Hillerup, S., Rostgaard, J., Puche, M., Blessmann, M., Kohlmeier, C., et al. (2016). Technical and surgical aspects of the sphenopalatine ganglion (SPG) microstimulator insertion procedure. Int. J. Oral. Maxillofac. Surg. 45, 245–254. doi: 10.1016/j.ijom.2015.09.023
Binder, A., and Baron, R. (2016). The pharmacological therapy of chronic neuropathic pain. Dtsch. Arztebl. Int. 113, 616–625. doi: 10.3238/arztebl.2016.0616
Cui, J. G., O’Connor, W. T., Ungerstedt, U., Linderoth, B., and Meyerson, B. A. (1997). Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain 73, 87–95. doi: 10.1016/s0304-3959(97)00077-8
Daniele, C. A., and MacDermott, A. B. (2009). Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J. Neurosci. 29, 686–695. doi: 10.1523/JNEUROSCI.5120-08.2009
Das, B., Conroy, M., Moore, D., Lysaght, J., and McCrory, C. (2018). Human dorsal. root ganglion pulsed radiofrequency treatment modulates cerebrospinal fluid lymphocytes and neuroinflammatory markers in chronic radicular pain. Brain Behav. Immun. 70, 157–165. doi: 10.1016/j.bbi.2018.02.010
Deer, T. R., Mekhail, N., Provenzano, D., Pope, J., Krames, E., Thomson, S., et al. (2014). The appropriate use of neurostimulation: Avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation 17, 571–598. doi: 10.1111/ner.12206
Devor, M. (1991). Neuropathic pain and injured nerve: Peripheral mechanisms. Br. Med. Bull. 47, 619–630. doi: 10.1093/oxfordjournals.bmb.a072496
Ding, Y., Hong, T., Li, H., Yao, P., and Zhao, G. (2019). Efficacy of CT guided pulsed radiofrequency treatment for trigeminal postherpetic neuralgia. Front. Neurosci. 13:708. doi: 10.3389/fnins.2019.00708
Dong, D.-S., Yu, X., Wan, C.-F., Liu, Y., Zhao, L., Xi, Q., et al. (2017). Efficacy of. short-term spinal cord stimulation in acute/subacute zoster-related pain: A retrospective study. Pain Physician 20:E633–E645.
Eyigor, C., Eyigor, S., Korkmaz, O. K., and Uyar, M. (2010). Intra-articular corticosteroid injections versus pulsed radiofrequency in painful shoulder: A prospective, randomized, single-blinded study. Clin. J. Pain 26, 386–392. doi: 10.1097/AJP.0b013e3181cf5981
Finnerup, N. B., Kuner, R., and Jensen, T. S. (2021). Neuropathic pain: From. mechanisms to treatment. Physiol. Rev. 101, 259–301. doi: 10.1152/physrev.00045.2019
Fleckenstein, J., Kramer, S., Hoffrogge, P., Thoma, S., Lang, P. M., Lehmeyer, L., et al. (2009). Acupuncture in acute herpes zoster pain therapy (ACUZoster) - design and protocol of a randomised controlled trial. BMC Complement Altern. Med. 9:31. doi: 10.1186/1472-6882-9-31
Forbes, H. J., Thomas, S. L., Smeeth, L., Clayton, T., Farmer, R., Bhaskaran, K., et al. (2016). A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain 157, 30–54. doi: 10.1097/j.pain.0000000000000307
Gershon, A. A., Breuer, J., Cohen, J. I., Cohrs, R. J., Gershon, M. D., Gilden, D., et al. (2015). Varicella zoster virus infection. Nat. Rev. Dis. Primers 1:15016. doi: 10.1038/nrdp.2015.16
Geurts, J. W. M., Knape, J. T. A., and Groen, G. J. (2003). Radiofrequency lesioning. of dorsal root ganglia for chronic lumbosacral radicular pain: A randomised, double-blind, controlled trial. Lancet 361:6. doi: 10.1016/s0140-6736(03)12115-0
Gilmore, C., Ilfeld, B., Rosenow, J., Li, S., Desai, M., Hunter, C., et al. (2019). Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial. Reg. Anesth. Pain Med. 44, 637–645. doi: 10.1136/rapm-2018-100109
Head, H., Campbell, A. W., and Kennedy, P. G. (1997). The pathology of herpes. zoster and its bearing on sensory localisation. Rev. Med. Virol. 7, 131–143. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7
Huang, J., Yang, S., Yang, J., Sun, W., Jiang, C., Zhou, J., et al. (2020). Early treatment with temporary spinal cord stimulation effectively prevents development of postherpetic neuralgia. Pain Physician 23:E219–E230.
Johnson, M. D., and Burchiel, K. J. (2004). Peripheral stimulation for treatment of. trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: A pilot study. Neurosurgery 55, 135–141.
Johnson, R. W. (2010). Herpes zoster and postherpetic neuralgia. Expert Rev. Vaccines 9, 21–26. doi: 10.1586/erv.10.30
Johnson, R. W., and Rice, A. S. C. (2014). Postherpetic neuralgia. N. Engl. J. Med. 371, 1526–1533. doi: 10.1056/NEJMcp1403062
Ke, M., Yinghui, F., Yi, J., Xeuhua, H., Xiaoming, L., Zhijun, C., et al. (2013). Efficacy. of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: A randomized, double-blinded, controlled trial. Pain Physician 16, 15–25.
Kemler, M. A., Barendse, G. A., van Kleef, M., de Vet, H. C., Rijks, C. P., Furnée, C. A., et al. (2000). Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N. Engl. J. Med. 343, 618–624. doi: 10.1056/NEJM200008313430904
Kim, K., Jo, D., and Kim, E. (2017). Pulsed radiofrequency to the dorsal root. ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician 20:E411–E418.
Knotkova, H., Hamani, C., Sivanesan, E., Le Beuffe, M. F. E., Moon, J. Y., Cohen, S. P., et al. (2021). Neuromodulation for chronic pain. Lancet 397, 2111–2124. doi: 10.1016/S0140-6736(21)00794-7
Kumar, K., Wilson, J. R., Taylor, R. S., and Gupta, S. (2006). Complications of spinal. cord stimulation, suggestions to improve outcome, and financial impact. J. Neurosurg. Spine 5, 191–203. doi: 10.3171/spi.2006.5.3.191
Li, D., Sun, G., Sun, H., Wang, Y., Wang, Z., and Yang, J. (2018). Combined therapy of pulsed radiofrequency and nerve block in postherpetic neuralgia patients: A randomized clinical trial. PeerJ 6:e4852. doi: 10.7717/peerj.4852
Liu, B., Yang, Y., Zhang, Z., Wang, H., Fan, B., and Sima, L. (2020). Clinical study of spinal cord stimulation and pulsed radiofrequency for management of herpes zoster-related pain persisting beyond acute phase in elderly patients. Pain Physician 8, 263–270.
Makharita, M. Y., and Amr, Y. M. (2020). Effect of repeated paravertebral injections. with local anesthetics and steroids on prevention of post-herpetic neuralgia. Pain Physician 23, 565–572.
Makharita, M. Y., El Bendary, H. M., Sonbul, Z. M., Ahmed, S. E. S., and Latif, M. A. (2018). Ultrasound-guided pulsed radiofrequency in the management of thoracic postherpetic neuralgia: A randomized, double-blinded, controlled trial. Clin. J. Pain 34, 1017–1024. doi: 10.1097/AJP.0000000000000629
Meuwissen, K. P. V., de Vries, L. E., Gu, J. W., Zhang, T. C., and Joosten, E. A. J. (2020). Burst and tonic spinal cord stimulation both activate spinal GABAergic mechanisms to attenuate pain in a rat model of chronic neuropathic pain. Pain Pract. 20, 75–87. doi: 10.1111/papr.12831
Moshayedi, P., Thomas, D., Rinaldo, C. R., Moossy, J. J., Maroon, J. C., Murdoch, G. H., et al. (2018). Subacute histopathological features in a case of varicella zoster virus myelitis and post-herpetic neuralgia. Spinal Cord Ser. Cases 4:33. doi: 10.1038/s41394-018-0068-5
Rigoard, P., Basu, S., Desai, M., Taylor, R., Annemans, L., Tan, Y., et al. (2019). Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: A multicenter randomized controlled trial. Pain 160, 1410–1420. doi: 10.1097/j.pain.0000000000001510
Rigoard, P., Roulaud, M., Goudman, L., Adjali, N., Ounajim, A., Voirin, J., et al. (2021). Comparison of spinal cord stimulation vs. dorsal root ganglion stimulation vs. association of both in patients with refractory chronic back and/or lower limb neuropathic pain: An international, prospective, randomized, double-blinded, crossover trial (BOOST-DRG Study). Medicina 58:7. doi: 10.3390/medicina58010007
Salvetti, A., Ferrari, V., Garofalo, R., Gazzaniga, P., Guerroni, A., Metrucci, A., et al. (2019). Incidence of herpes zoster and postherpetic neuralgia in Italian adults aged ≥50 years: A prospective study. Prev. Med. Rep. 14:100882. doi: 10.1016/j.pmedr.2019.100882
Sam, J., Catapano, M., Sahni, S., Ma, F., Abd-Elsayed, A., and Visnjevac, O. (2021). Pulsed radiofrequency in interventional pain management: Cellular and molecular mechanisms of action - an update and review. Pain Physician 24, 525–532.
Sheng, L., Liu, Z., Zhou, W., Li, X., Wang, X., and Gong, Q. (2022). Short-Term spinal. cord stimulation or pulsed radiofrequency for elderly patients with postherpetic neuralgia: A prospective randomized controlled trial. Neural Plast. 2022:7055697. doi: 10.1155/2022/7055697
Sivanesan, E., Stephens, K. E., Huang, Q., Chen, Z., Ford, N. C., Duan, W., et al. (2019). Spinal cord stimulation prevents paclitaxel-induced mechanical and cold hypersensitivity and modulates spinal gene expression in rats. Pain Rep. 9:e785. doi: 10.1097/PR9.0000000000000785
Song, J. J., Popescu, A., and Bell, R. L. (2014). Present and potential use of spinal cord stimulation to control chronic pain. Pain Physician 17, 235–246.
Song, T. (2021). Efficacy of pulsed radiofrequency or short-termspinal cord. stimulation for acute/subacutezoster-related pain: A randomized, double-blinded, controlled trial. Pain Phys. 24, 215–222. doi: 10.36076/ppj.2021/24/215
Sun, K.-F., Feng, W.-W., Liu, Y.-P., Dong, Y.-B., Gao, L., and Yang, H.-L. (2018). Electrical peripheral nerve stimulation relieves bone cancer pain by inducing arc protein expression in the spinal cord dorsal horn. J. Pain Res. 11, 599–609. doi: 10.2147/JPR.S149470
Takeda, M., Ikeda, M., Takahashi, M., Kanazawa, T., Nasu, M., and Matsumoto, S. (2013). Suppression of ATP-induced excitability in rat small-diameter trigeminal ganglion neurons by activation of GABAB receptor. Brain Res. Bull. 98, 155–162. doi: 10.1016/j.brainresbull.2013.08.005
Taub, E., Munz, M., and Tasker, R. R. (1997). Chronic electrical stimulation of the. gasserian ganglion for the relief of pain in a series of 34 patients. J. Neurosurg. 86, 197–202. doi: 10.3171/jns.1997.86.2.0197
Vaisman, J., Markley, H., Ordia, J., and Deer, T. (2012). The treatment of medically. intractable trigeminal autonomic cephalalgia with supraorbital/supratrochlear stimulation: A retrospective case series. Neuromodulation 15, 374–380. doi: 10.1111/j.1525-1403.2012.00455.x
van Wijck, A. J. M., Opstelten, W., Moons, K. G. M., van Essen, G. A., Stolker, R. J., Kalkman, C. J., et al. (2006). The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: A randomised controlled trial. Lancet 367, 219–224. doi: 10.1016/S0140-6736(06)68032-X
Wall, P. D., and Gutnick, M. (1974). Properties of afferent nerve impulses originating from a neuroma. Nature 248, 740–743. doi: 10.1038/248740a0
Wan, C.-F., and Song, T. (2021). Short-Term peripheral nerve stimulation relieve pain for elder herpes zoster ophthalmicus patients: A retrospective study. Neuromodulation 24, 1121–1126. doi: 10.1111/ner.13288
Whitley, R. J., Volpi, A., McKendrick, M., Wijck, A. V., and Oaklander, A. L. (2010). Management of herpes zoster and post-herpetic neuralgia now and in the future. J. Clin. Virol. 48:S20–S28. doi: 10.1016/S1386-6532(10)70005-6
Wu, C.-Y., Lin, H.-C., Chen, S.-F., Chang, W.-P., Wang, C.-H., Tsai, J.-C., et al. (2020). Efficacy of pulsed radiofrequency in herpetic neuralgia: A meta-analysis of randomized controlled trials. Clin. J. Pain 36, 887–895. doi: 10.1097/AJP.0000000000000867
Yanamoto, F., and Murakawa, K. (2012). The effects of temporary spinal cord. stimulation (or Spinal Nerve Root Stimulation) on the management of early postherpetic neuralgia from one to six months of its onset. Neuromodulation 15, 151–154. doi: 10.1111/j.1525-1403.2012.00438.x
Zhang, H., Ni, H., Liu, S., and Xie, K. (2020). Supraorbital nerve radiofrequency for. severe neuralgia caused by herpes zoster ophthalmicus. Pain Res. Manag. 2020:3191782. doi: 10.1155/2020/3191782
Zhou, J., Sun, W., Liu, Y., Yang, S., Wu, S., Wang, S., et al. (2021a). Clinical characteristics, treatment effectiveness, and predictors of response to pharmacotherapeutic interventions among patients with herpetic-related neuralgia: A retrospective analysis. Pain Ther. 10, 1511–1522. doi: 10.1007/s40122-021-00303-7
Keywords: acute herpetic neuralgia, postherpetic neuralgia, pulsed radiofrequency, subacute herpetic neuralgia, zoster-associated pain, short-term nerve electrical stimulation
Citation: Liu L, Zhang W-j, Xu S-x, Guo W-s, Yan R-r, Jiang X-h, Li S-y and Sun T (2022) Propensity score matching comparing short-term nerve electrical stimulation to pulsed radiofrequency for herpes zoster-associated pain: A retrospective study. Front. Mol. Neurosci. 15:1069058. doi: 10.3389/fnmol.2022.1069058
Received: 13 October 2022; Accepted: 24 October 2022;
Published: 28 November 2022.
Edited by:
Yan Lu, Fourth Military Medical University, ChinaReviewed by:
Jinfeng Liu, Harbin Medical University, ChinaLizu Xiao, Shenzhen Sixth Hospital of Guangdong Medical University, China
Cheng-fu Wan, China Medical University, China
Copyright © 2022 Liu, Zhang, Xu, Guo, Yan, Jiang, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Sun, c3VudGFvc2RwaEAxNjMuY29t
†These authors share first authorship
 Liu Liu1†
Liu Liu1† Shu-xiang Xu
Shu-xiang Xu Xiao-han Jiang
Xiao-han Jiang Tao Sun
Tao Sun