- 1Department of Otolaryngology—Head and Neck Surgery, Stanford University School of Medicine, Stanford, CA, United States
- 2Department of Otolaryngology—Head and Neck Surgery, Massachusetts Eye and Ear and Harvard Medical School, Boston, MA, United States
- 3University of Minnesota Medical School, Minneapolis, MN, United States
The fibroblast growth factor 2 (FGF2) is a member of the FGF family which is involved in key biological processes including development, cellular proliferation, wound healing, and angiogenesis. Although the utility of the FGF family as therapeutic agents has attracted attention, and FGF2 has been studied in several clinical contexts, there remains an incomplete understanding of the molecular and clinical function of FGF2 in the auditory system. In this review, we highlight the role of FGF2 in inner ear development and hearing protection and present relevant clinical studies for tympanic membrane (TM) repair. We conclude by discussing the future implications of FGF2 as a potential therapeutic agent.
Introduction
Fibroblast growth factors (FGFs) comprise a large family of proteins which are involved in several biological functions including embryonic development, cell growth and differentiation, angiogenesis, and wound healing (Beenken and Mohammadi, 2009). Various experimental approaches including tissue ablation, transplantation, and development of knockout animals have revealed the important function of FGF signaling in auditory development and function. Several members of the FGF gene family and its receptors have been found to control cell proliferation and specification during the development of sensory progenitors (Huh et al., 2012, 2015; Mansour et al., 2013; Ono et al., 2014) and the inner ear sensory epithelia (Vendrell et al., 2000; Carnicero et al., 2004).
FGF2 binds and activates FGF receptors (FGFRs) primarily through the RAS-mitogen activated protein kinase (MAPK) pathway to regulate cellular function in the skin, blood vessels, tendons, ligaments, bone, and teeth (Beenken and Mohammadi, 2009; Yun et al., 2010; Park et al., 2017). FGF2 is present in human spermatozoa and exposure to recombinant FGF2 has been used to improve recovery of sperm motility (Garbarino Azúa et al., 2017). A role for FGF2 has been described in learning and memory (Graham and Richardson, 2011), neuropsychiatric disorders including anxiety (Perez et al., 2009; Eren-Koçak et al., 2011; Turner et al., 2012; Salmaso et al., 2016), and depression (Mallei et al., 2002; Maragnoli et al., 2004; Riva et al., 2005; Elsayed et al., 2012; Turner et al., 2012; Tang et al., 2017), stress-related disorders (Molteni et al., 2001a, b; Fumagalli et al., 2005; Xia et al., 2013), and schizophrenia (Klejbor et al., 2006; Terwisscha van Scheltinga et al., 2010). Increased levels of FGF2 have been shown to have positive effects in models of neurodegenerative diseases such as Parkinson’s disease (Claus et al., 2004; Timmer et al., 2007), Alzheimer’s disease (Cummings et al., 1993; Kiyota et al., 2011), multiple sclerosis (Ruffini et al., 2001), and traumatic brain injury (Sun et al., 2009; Thau-Zuchman et al., 2012). In contrast, it has been suggested that FGF2 may be a positive regulator for nicotine, amphetamine, cocaine, and alcohol use (Even-Chen and Barak, 2019). These early studies suggest that the role of FGF2 may vary based on the disease context. Nevertheless, there are currently no FDA-approved FGF2 therapies for any pathology.
Despite the discoveries indicating the growing importance of FGF2, there is a lack of complete understanding of FGF2’s role in the auditory system. In this review, we summarize the literature on FGF2 in the auditory system including its function in inner ear development, role in hearing protection, and application in clinical trials of tympanic membrane (TM) reconstruction.
FGF2 in Auditory Development and Maintenance
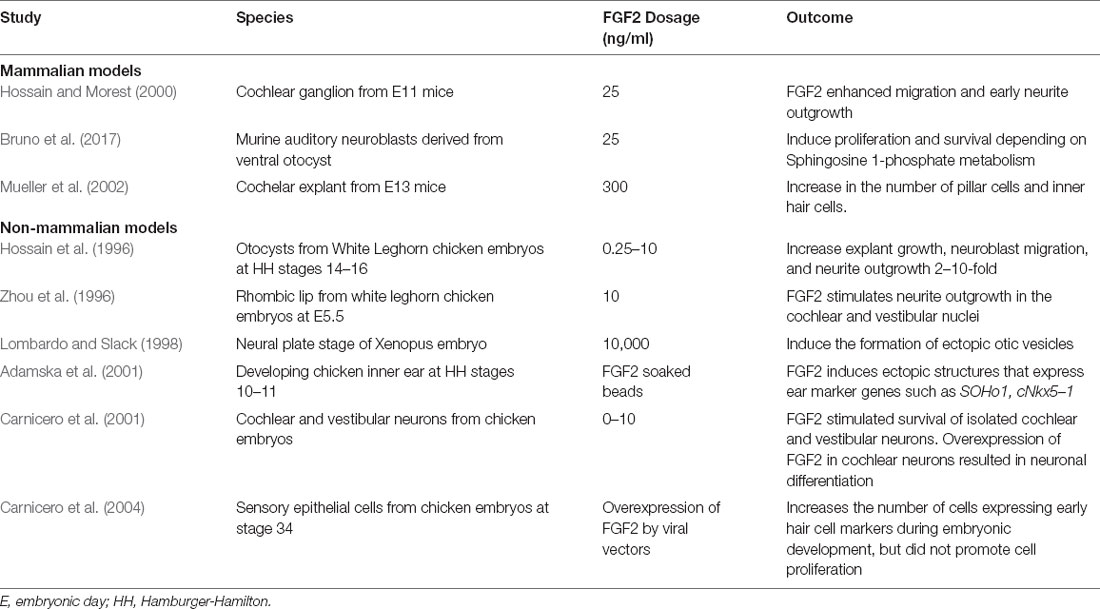
FGF2 has been proposed to fulfill many functions during vertebrate auditory development. The expression of FGF2 has been detected through messenger RNA (mRNA) and protein levels in the cochlea and vestibule throughout the life span of animal models (Figure 1). The precise locations of expression are described in the following paragraphs. Furthermore, several studies have examined the impact of exogenously added FGF2 on various developmental stage models (Table 1).
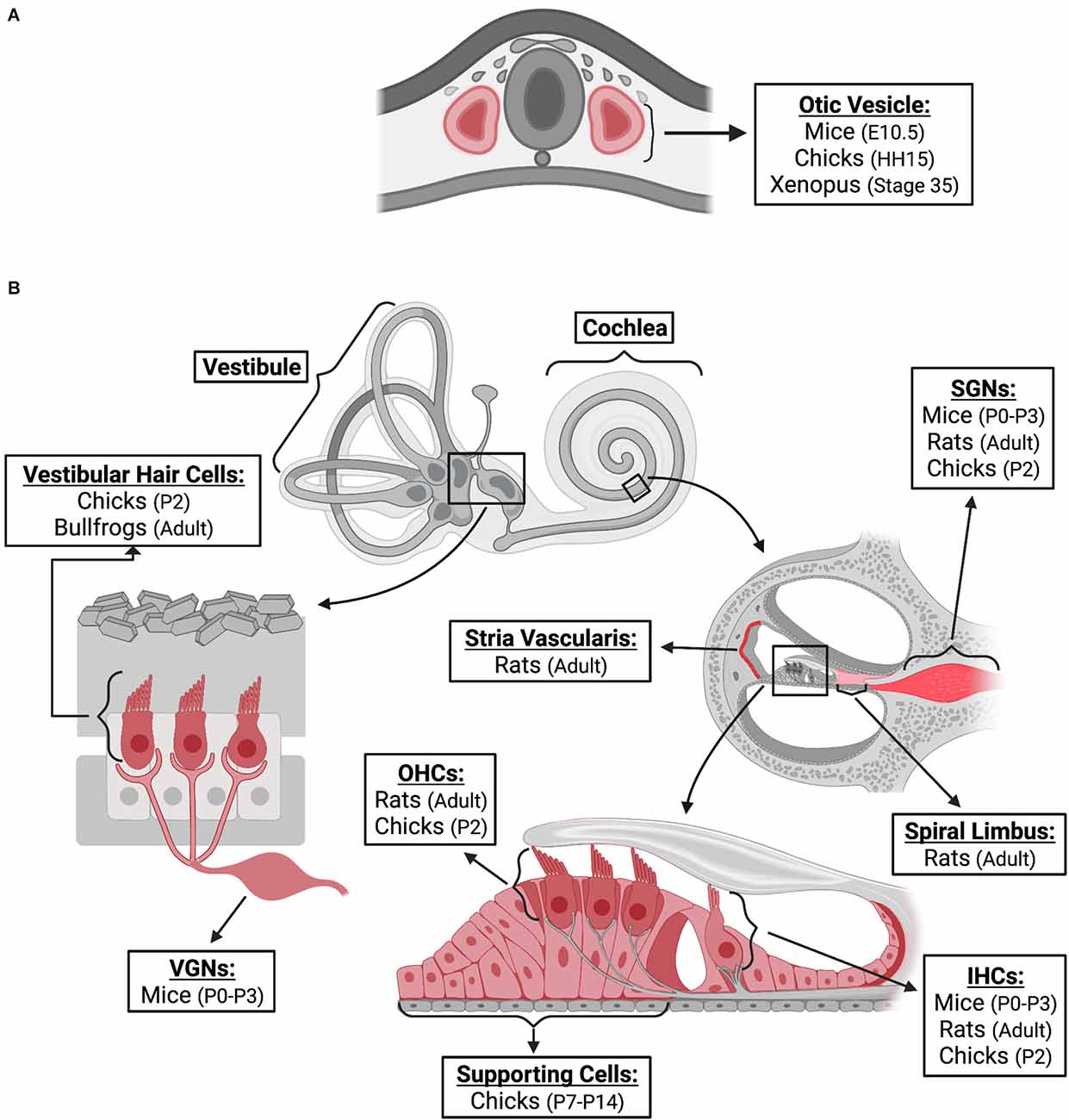
Figure 1. FGF2 expression in inner ear. FGF2 expression locations are indicated in red. The species, and age at which expression was confirmed, are provided for different locations of the inner ear during embryonic development (A) and post-natal (B) stages. Locations of expression are also provided for non-mammalian models; however, the depiction is based on similar structures of the mammalian inner ear. FGF2, fibroblast growth factor 2; E, embryonic; HH, hamburger-hamilton; P, post-natal; VGNs, vestibular ganglion neurons; SGNs, spiral ganglion neurons; OHCs, outer hair cells; IHCs, inner hair cells. Created with BioRender.com.
Mammalian Models
Endogenous FGF2 Expression
In mice, FGF2 protein was expressed as early as the embryonic day (E)-10.5 stage (when the otocyst is closed as a sac) of inner ear development in the otocyst epithelium and neuroepithelium (Frenz et al., 1994). Over the period E10.5–E15.5, FGF2 mRNA expression showed large increases of ~103 fold, and this expression was relatively evenly distributed throughout the mouse cochlea (lateral wall, the center of modiolus, sensory/neural area) except in the cartilage where it was low in the postnatal day (P)-0 mouse cochlea (Pickles, 2001). During the neonatal period of mice (P0-P3), FGF2 immunoreactivity was present in inner ear hair cells, spiral ganglion neurons (SGNs), vestibular ganglion neurons (VGNs), and the auditory brainstem (Després et al., 1991). In the adult stage of rats, FGF2 immunoreactivity was observed in the inner (IHCs) and outer hair cells (OHCs) of the organ of Corti, SGNs, spiral limbus, and stria vascularis (Silva et al., 2005). In the central auditory pathways, FGF2 immunoreactivity was found in the cytoplasm of the neurons of the cochlear nuclei, trapezoid body nuclei, medial geniculate nucleus, and inferior colliculus (Silva et al., 2005). These studies demonstrate that FGF2 can play an important role in embryonic and postnatal auditory development and may also influence the maintenance of adult auditory structure and function.
However, these observations conflict with findings that FGF2 mRNA was undetectable in the rat cochlea at any age (E 16—P > 60; Luo et al., 1993), and that FGF2-like protein was widely distributed in the auditory brainstem but not found in the adult rat cochlea (Després et al., 1991). It is possible that immunostaining of FGF2 observed in adult rat cochlea represented FGF1 immunoreactivity as FGF1 shares 55% homology with FGF2 and they have similar physiological actions and interact with the same receptors. However, this is less likely as the absorption of the FGF2 antibody by FGF1 did not modify the pattern of FGF2 immunoreactivity (Silva et al., 2005). Also, the specificity of the immunoreaction was demonstrated by the complete disappearance of immunoreactivity in the central and peripheral auditory pathways after absorption of FGF2 antibody by FGF2 protein (Silva et al., 2005). These contradicting results may reflect a loss of antigenicity due to decalcification which may have impaired proper localization of endogenous FGF2 mRNA and protein in the cochlea. Additionally, these discrepancies may be related to species or strain differences. For example, Silva et al. (2005) utilized Wistar rats whereas both Després et al. (1991) and Luo et al. (1993) utilized Sprague-Dawley rats. Furthermore, Silva et al. (2005) used male rats whereas gender was not specified in the other two studies. Only one study (Luo et al., 1993) explored the level of FGF2 expression in embryonic stage rats. Comparing these findings with embryonic studies done in mice likely introduces confounding factors related to species differences.
Impact of Exogenous FGF2
When FGF2 was added to the cochlear ganglion of E11 stage mice, it enhanced the migration and initial differentiation of cochlear ganglion neurons (Hossain and Morest, 2000). In addition, FGF2 was found to stimulate the proliferation of mouse auditory neuroblasts and protected these cells from apoptosis (Bruno et al., 2017). Interestingly treatment with FGF2 also led to a greater than two-fold increase in the number of pillar cells (located in the region between the single row of IHCs and the first row of OHCs) and to a small increase in the number of IHCs (Mueller et al., 2002).
Non-mammalian Models
Endogenous FGF2 Expression
During inner ear development in chicken, weak expression of FGF2 was observed in the otic placode, neural tube, and notochord at stage Hamburger-Hamilton (HH)-11 (40–45 h). Expression was increased during the formation of the otic vesicle at HH15 (50–55 h; Vendrell et al., 2000). FGF2 mRNA expression was detected in the otocyst at E5 and in the cochlea, cochlear nerve ganglion, cochlear hair cells, and vestibular hair cells at P2 (Pickles and van Heumen, 1997). FGF2 expression begins early, and thus, it is thought to regulate cell proliferation. At the young chick stage, FGF2 was localized in the nuclei of supporting cells throughout the entire length of the basilar papilla, but not hair cells (Lee and Cotanche, 1996). This suggests that supporting cells have the potential to proliferate via a signaling pathway involving FGF2 (Lee and Cotanche, 1996). In the pre-larval Xenopus embryo (stage 35), FGF2 protein was expressed in the otic vesicle (Song and Slack, 1994). In the adult stage of bullfrogs, vestibular hair cells expressed FGF2 and the receptors FGFR1 and FGFR2 while supporting cells did not express either molecule (Cristobal et al., 2002).
Impact of Exogenous FGF2
The addition of FGF2 has been found to promote explanted cell culture and neurite growth as well as increase neuroblast migration in otocysts extracted from chick embryos (Hossain et al., 1996). It has also been shown to stimulate neurite outgrowth in the cochlear and vestibular nuclei of chick embryos (Zhou et al., 1996). In addition, when applied via heparin beads at the neural plate stage in the Xenopus embryo, FGF2 was found to induce the formation of ectopic otic vesicles (Lombardo and Slack, 1998). Furthermore, when introduced in the developing chick inner ear, it induced ectopic structures that expressed ear marker genes and increased the size of the vestibulo-cochlear ganglion (Adamska et al., 2001). It was also found to stimulate survival of isolated chicken embryonic cochlear and vestibular neurons and to promote neuronal differentiation (Carnicero et al., 2001). In sensory epithelial cells from chicken embryos, the addition of FGF2 increased the number of cells expressing early hair cell markers but did not promote cell proliferation (Carnicero et al., 2004).
The current studies from vertebrate models lend support to the important role of FGF2 in auditory development. However, further investigation is required to better understand the precise spatial distribution and chronological sequence of FGF2 expression in ear development. Future studies must carefully consider the role of animal species, age, and environmental conditions in the experimental design.
FGF2 in Inner Ear Cell Survival, Proliferation, and Differentiation
The effect of FGF2 on post-development stage sensory epithelial and neural cells in vitro has been well characterized (Table 2). FGF2 was found to promote survival and neuritogenesis of adult rat auditory neurons (Lefebvre et al., 1991) and to significantly enhance the proliferation of rat utricular supporting cells (Zheng et al., 1997). Furthermore, FGF2 stimulated immortalized rat utricular epithelial cell proliferation and differentiation into cells expressing early hair cell markers (Zheng et al., 1998). FGF2 also increased P5 rat Schwann cell proliferation (Hansen et al., 2001). Following mechanical changes induced during isolation and dissociation procedures, FGF2 strongly promoted regeneration of murine SGNs via FGFR-3-IIIc receptor (Wei et al., 2007). In acoustic ganglia extracted from fully developed chicks, FGF2 was shown to enhance the survival of statoacoustic neurons (Warchol and Kaplan, 1999).
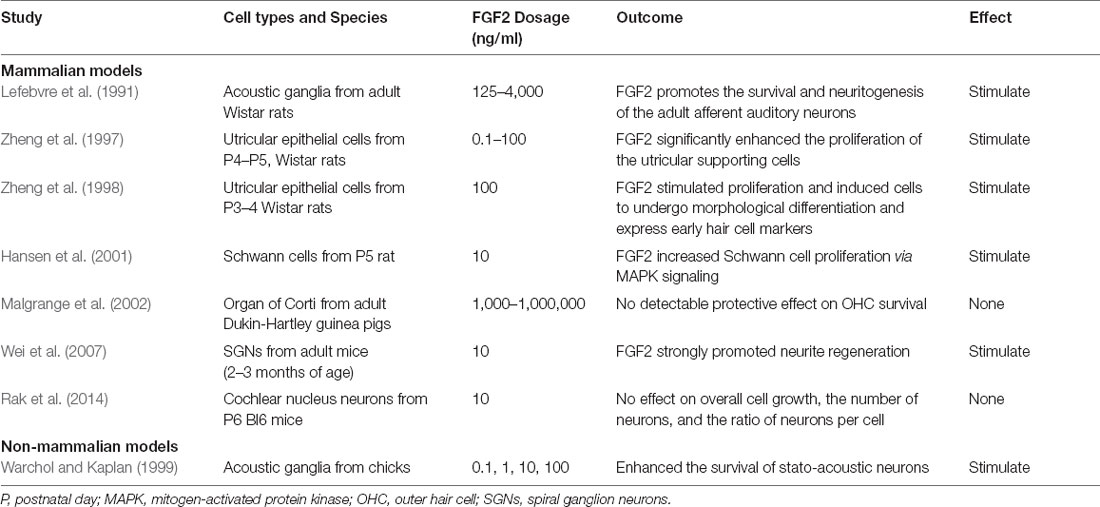
Table 2. In vitro studies of FGF2 in sensory and neural cell survival, proliferation, and differentiation.
Not all studies have shown the stimulatory effect of FGF2 in cell cultures. FGF2 had no detectable influence on the survival of guinea pig OHCs (Malgrange et al., 2002) or dissociated mouse cochlear nucleus neurons (Rak et al., 2014).
FGF2 as A Protective Or Restorative Agent in Auditory Trauma
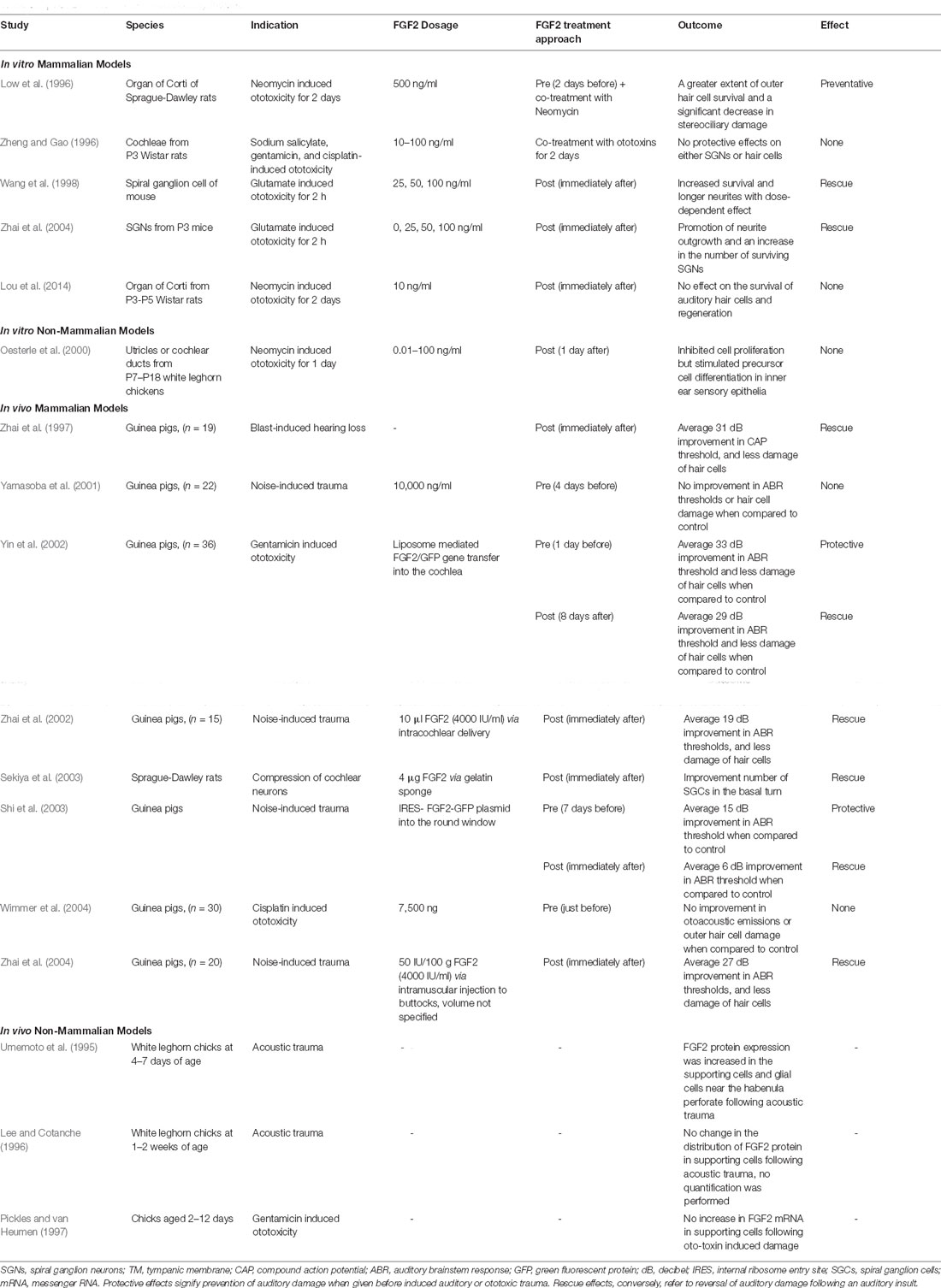
The therapeutic potential of FGF2 for induced auditory trauma has been explored in several pre-clinical models in both in vitro and in vivo experiments (Table 3). Evidence is mixed regarding the degree of effect conferred by FGF2 treatment; however, several studies have shown treatment efficacy. FGF2 has been shown to have both protective and rescue effects. Protective effects signify prevention of auditory damage when given before induced auditory or ototoxic trauma. Rescue effects, conversely, refer to a reversal of auditory damage following an auditory insult.
In vitro Studies
FGF2 was found to protect rat cochlear hair cells in the explanted organ of Corti from aminoglycoside injury when given as a pre-treatment or as a co-treatment with neomycin (Low et al., 1996). FGF2 also had a rescue effect on murine cochlear neurons from glutamate neurotoxicity by promoting neurite outgrowth and increasing the number of surviving SGNs when provided after glutamate application (Wang et al., 1998; Zhai et al., 2004). However, FGF2 was not always effective in ototoxic trauma. For example, FGF2 treatment didn’t affect either SGNs or hair cells when provided as a co- or post-treatment with ototoxins (Zheng and Gao, 1996; Lou X. et al., 2015). Interestingly, in utricles and cochlear ducts of chickens following neomycin-induced ototoxicity, FGF2 was found to inhibit cell proliferation which was not observed in mammals (Oesterle et al., 2000). Therefore, FGF2 may be involved in stimulating precursor cell differentiation as opposed to proliferation in the non-mammal inner ear epithelia.
In vivo Studies
In vivo pre-clinical studies of FGF2 have been performed with various delivery methods and animal models and have explored applications in auditory damage and hearing loss. Intramuscular or intracochlear delivery of FGF2 protein significantly improved the hearing threshold and reduced the loss of IHCs after noise exposure in guinea pigs (31, 27, and 19 mean dB improvement respectively when compared to control; Zhai et al., 1997, 2002, 2004). Application of FGF2 also ameliorated degeneration of cochlear nerve in rats after compression (Sekiya et al., 2003). Conversely, delivery of FGF2 prior to noise exposure or cisplatin-induced ototoxicity had no protective effect in guinea pigs (Yamasoba et al., 2001; Wimmer et al., 2004).
In addition, FGF2 gene therapy has also been investigated as a potential treatment modality. Administration of an FGF2 genetic construct via the internal ribosome entry site-FGF2-green fluorescent protein (IRES-FGF2-GFP) plasmid had a protective and rescue effect (15 and 6 mean dB improvement respectively when compared to control) following noise-induced trauma in guinea pigs (Shi et al., 2003). Additionally, liposome-mediated FGF2 gene transfer into the cochlea of guinea pigs had a similar protective and rescue (33 and 29 mean dB improvement respectively when compared to control) effect on hearing from gentamicin-induced ototoxicity (Yin et al., 2002).
Furthermore, endogenous FGF2 expression following acoustic trauma was studied in non-mammalian models. In contrast to mammalian models, non-mammalian vertebrates are capable of hair cell regeneration. Lee and Cotanche (1996) described an identical distribution pattern of FGF2 in supporting cells with exclusion from hair cells in both noise-exposed and control chicks, but they did not quantitatively evaluate expression levels. A separate study showed that FGF2 protein expression levels were increased in the supporting cell layer following noise-induced damage of the chick inner ear (Umemoto et al., 1995). Taken together, these results suggest that FGF2 may play a role in hair cell regeneration from supporting cells. Since regenerative processes are likely to recapitulate developmental processes, it is not surprising that regeneration appears to require the action of growth factors such as FGF2. One related study demonstrated no quantitative change in FGF2 mRNA expression in sensory epithelia after ototoxic damage when compared to untreated chicks (Pickles and van Heumen, 1997). It is important to note that this study did not specifically check localized expression in the supporting cells.
The current understanding of FGF2’s role in auditory trauma is still limited. Further investigation is required to determine the degree of FGF2’s protective or restorative effects in hearing as well as the mechanisms that may drive this function. In the meantime, FGF2 has been studied extensively in other auditory contexts, particularly in TM repair.
FGF2 for Tympanic Membrane Regeneration
The TM is a thin (~0.1 mm) layer of tissue which separates the external and middle ear. In the normal hearing process, the TM receives sound vibrations and transmits them to the auditory ossicles. The TM also serves as a barrier protecting the middle ear space from water, bacteria, or other foreign substances. Rupture of the TM can occur due to middle ear infections, barotrauma, loud sound exposure, or severe head trauma. While most TM perforations heal spontaneously within a few weeks, failure of healing can result in chronic perforation which can lead to hearing loss, infections, middle ear cholesteatoma, and other complications. Delayed healing may also necessitate surgical intervention for closure of the rupture. In recent years, alternative treatments for the repair of TM perforations have been explored including topically applied growth factors such as FGF2.
Proposed Role of FGF2 in Tympanic Membrane Healing
The precise mechanisms by which FGF2 functions in human TM repair remains unclear, however, studies performed in animal models have provided insight into its potential roles. TM healing proceeds through three stages: inflammatory, proliferative, and remodeling (Somers et al., 1997). The inflammatory stage, which occurs 48–72 h post-injury, is characterized by swelling and a local exudative reaction composed of interstitial fluid, lymph, and blood (Wang et al., 2004; Santa Maria et al., 2010). The proliferative stage occurs 3–4 days post-injury during which FGF2 facilitates migration and proliferation of keratinocytes at the perforation border (Ishibashi et al., 1998). Additionally, FGF2 has been found to intensify epithelial mitotic activity and mediate the connective tissue reaction in the middle epithelial layer (Ishibashi et al., 1998). Through this process, an epithelial bridge is formed over the area of injury (de Araújo et al., 2014). In the final stage of healing, the outer epithelial and inner mucosal layer thins, and fibroblasts in the middle layer become small and flattened (de Araújo et al., 2014). Levels of FGF2 are increased at the site of epithelial proliferation on day 3 post-injury with peak levels seen on day 5 post-injury (Werner et al., 1992; Ishibashi et al., 1998). When used therapeutically in rats (Vrabec et al., 1994), chinchillas (Kato and Jackler, 1996), and guinea pigs (Fina et al., 1991, 1993; Ozkaptan et al., 1997), animals treated with FGF2 had a shortened TM healing time and improved closure rate when compared with controls. The acceleration of closure time may be due in part to FGF2’s vasodilatory effects which stimulate increased local blood flow (Mondain and Ryan, 1994).
Clinical Studies of FGF2 for Tympanic Membrane Regeneration
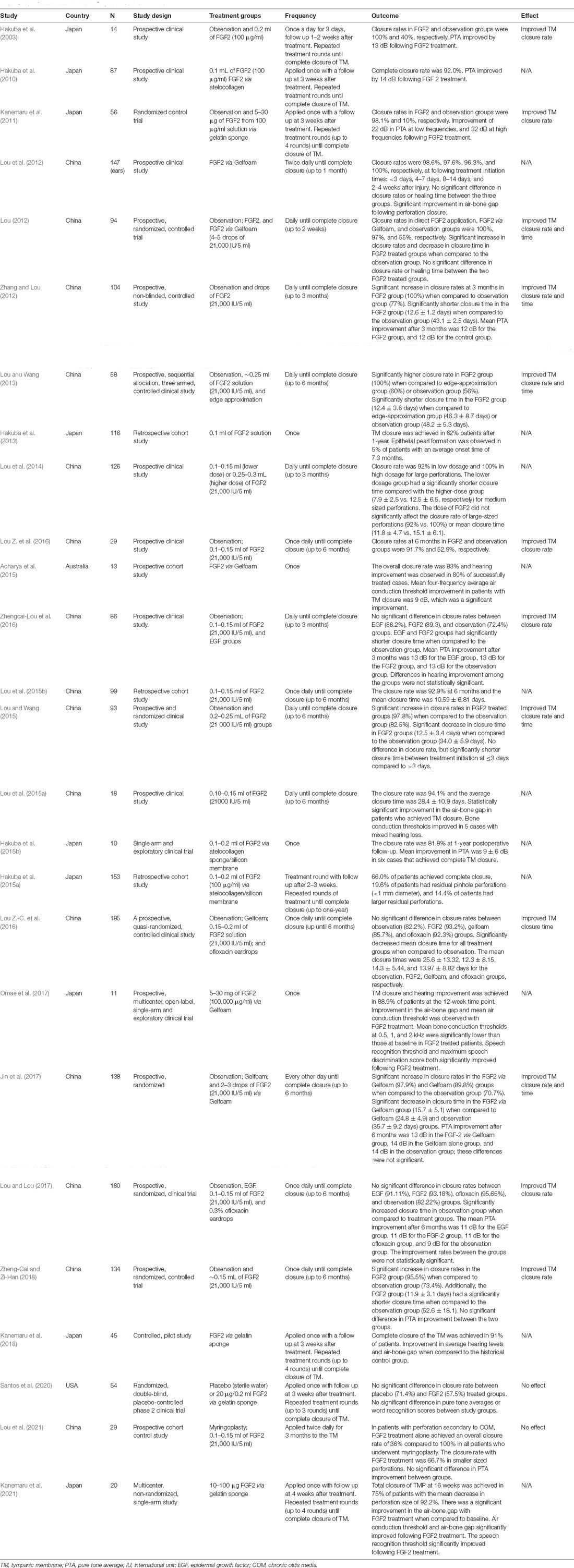
Building upon promising pre-clinical findings, several studies have been performed exploring the therapeutic potential of FGF2 for TM regeneration in patients (Table 4). Twelve of the identified studies did not have a control group for comparison (Klejbor et al., 2006; Hakuba et al., 2010, 2013, 2015a,b; Lou et al., 2014, 2015a,b; Lou X. et al., 2015; Acharya et al., 2015; Omae et al., 2017; Kanemaru et al., 2018; Kanemaru et al., 2021). The closure rate of TM perforations in these studies was reported between 62 and 100%.
The remaining 14 studies compared patients treated with FGF2 to either those who had no intervention, placebo, or an alternative treatment (e.g., Gelfoam, ofloxacin eardrops, and myringoplasty). Many of these studies reported improved closure rates (Hakuba et al., 2003; Kanemaru et al., 2011; Lou Z. et al., 2016), shortened closure times (Lou Z.-C. et al., 2016; Zhengcai-Lou et al., 2016; Lou and Lou, 2017; Zheng-Cai and Zi-Han, 2018), or both (Lou, 2012; Zhang and Lou, 2012; Lou and Wang, 2013, 2015; Jin et al., 2017) with FGF2 treatment when compared to observation. However, in a Phase II trial of 54 patients, no significant difference in TM closure rates or hearing improvement were observed when comparing FGF2 treatment with placebo (Santos et al., 2020). Treatment with FGF2 compared with Gelfoam alone significantly shortened time to closure but had no significant difference in closure rate (Lou Z.-C. et al., 2016). In a different study, a comparison between Gelfoam alone and Gelfoam with FGF2 showed that co-application with FGF2 significantly improved closure rates and decreased closure time (Jin et al., 2017). No significant differences in closure rates or time to closure were observed between patients treated with FGF2 and ofloxacin drops (Lou Z.-C. et al., 2016; Zheng-Cai and Zi-Han, 2018). When compared with traditional myringoplasty in patients with TM perforation secondary to chronic otitis media (COM), FGF2 treatment had a much lower overall closure rate (36 and 100%, respectively; Lou et al., 2021). However, when grouped by size, FGF2 treatment had a higher closure rate in smaller perforations when compared to medium-sized perforations in patients with COM (66.7% vs. 0%, respectively; Lou et al., 2021).
Consideration of FGF2 Activity in Tumors Related to Hearing Loss
FGF2 has been found to have tumor-promoting effects which is not unexpected given its classification as a growth factor. Conversely, studies have also shown that FGF2 may suppress tumor growth. In the auditory system, sporadic vestibular schwannomas (VS) are the most common tumors of the cerebellopontine angle, and often present with sensorineural hearing loss (SNHL; Mahaley et al., 1990). It was previously assumed that SNHL due to VS was mediated solely by tumor compression of the cochlear nerve, however, it is now recognized that VS-secreted factors potentiate damage to the auditory system (Dilwali et al., 2013, 2015; Wu et al., 2021). FGF2 has been identified as a VS-secreted factor that may serve a protective role against SNHL (Dilwali et al., 2013). Specifically, VS-secreted FGF2 levels had a negative correlation with the degree of SNHL (Dilwali et al., 2013, 2015) because they were positively correlated with word recognition scores, and negatively correlated with pure tone averages (Dilwali et al., 2015). These studies suggest that FGF2 may have protective effects on hearing. However, the dichotomous role of FGF2 in tumors requires further investigation.
Discussion
Knowledge of the role of FGF2 in auditory development and interest in its potential as a therapeutic agent has advanced considerably in the past decades. In this review, we comprehensively summarized: (1) the expression pattern and activity of FGF2 during inner ear development and maintenance in vertebrate models; (2) the effect of FGF2 on proliferation and differentiation of inner ear cells in vitro; (3) the role of FGF2 as a preventive and curative agent in auditory damage; (4) regenerative function of FGF2 in TM; and (5) FGF2 activity in tumors related to hearing loss. These studies highlight the potential of FGF2 based therapies for hearing disorders. However, there are many unanswered questions prior to verifying FGF2 as a useful therapeutic for hearing loss. Therefore, future studies need to be conducted to fill these gaps in knowledge which are outlined below.
Limitations of Preclinical Studies
Mammalian Studies
Both in vitro and in vivo experiments of induced auditory trauma with mammalian animal models (Table 3) have yielded variable results, calling for additional studies to define optimal treatment parameters prior to transitioning to clinical studies. Current in vitro evidence suggests that the stimulatory effect of FGF2 is concentration-dependent. The survival rate and length of mouse neurites were found to be directly correlated with the added concentration of FGF2 (Zhai et al., 2004). Treatment with a high concentration of FGF2 (500 ng/ml) had a stimulatory effect (Low et al., 1996) that was not seen with low concentration (10 ng/ml; Lou et al., 2014).
In existing in vivo studies, the degree of improvement with FGF2 varied between studies, partially owing to differences in the type of auditory insult (e.g., blast, noise, ototoxin, and compression). For studies that used noise-induced trauma, parameters such as the intensity (115–172 dB) and duration of exposure (4–5 h) varied significantly. Therefore, direct comparison even in these studies with the same mechanism of auditory trauma was challenging as more than one parameter was different. Two studies used the same noise parameters and differed only in route of FGF2 delivery (Zhai et al., 2002, 2004). In Zhai et al. (2002), FGF2 was delivered directly to the cochlea. In contrast, Zhai et al. (2004) delivered FGF2 at the same concentration (volume not specified) via intramuscular injection in the buttocks. The mean improvement in the ABR threshold was 27 dB for intramuscular delivery compared to 19 dB for intracochlear delivery (Zhai et al., 2002, 2004). Since the volume of drug was not specified in Zhai et al. (2004), it is difficult to conclude whether route or dosing led to the different outcomes. However, there was a uniform improvement of hearing threshold in all studies when FGF2 was given after an auditory insult (Zhai et al., 1997, 2002, 2004; Yin et al., 2002; Shi et al., 2003). This supports a rescue role for FGF2.
In contrast, in two studies in which FGF2 was given prior to noise-induced or ototoxic damage, there was no effect (Yamasoba et al., 2001; Wimmer et al., 2004). Considering the short biological half-life of free form FGF2 (less than 1 h; Edelman et al., 1993), its utility as a protective agent may be limited. In Wimmer et al. (2004), ototoxic damage was induced over a period of 5 days, and in Yamasoba et al. (2001), noise-induced trauma was started 4 days after FGF2 delivery. It may be that these studies missed the treatment window for FGF2. The lack of consistency in auditory trauma parameters and FGF2 treatment between current pre-clinical studies remains a limitation. Nonetheless, the consistent findings when given after auditory damage support further development as a rescue agent.
Interestingly, when provided as a genetic construct, FGF2 treatment was found to have both protective and rescue effects (Yin et al., 2002; Shi et al., 2003). Gene therapy using an FGF2 genetic construct can be tailored for precise FGF2 dosing and targeting. In the case of auditory trauma, such a genetic approach is expected to provide long-term and stable expression of FGF2 in specific target cells such as hair cells or SGNs. This long-lasting expression may underly the added protective effect seen with this approach.
Non-mammalian Studies
Studies performed in non-mammalian models have provided insight into FGF2 expression levels and distribution following induced auditory trauma. However, studies have not been consistent in their findings. It has been suggested that these inconsistencies may be a result of variable durations following induced auditory or ototoxic damage at which FGF2 levels were analyzed. For example, Umemoto et al. (1995) examined protein expression 1 day after noise exposure. Lee and Cotanche (1996) and Pickles and van Heumen (1997) measured mRNA and protein levels 2 days following noise or ototoxic damage. Additionally, the method by which auditory damage was induced varied between studies. Given that ototoxic damage works over a longer time period than acoustic damage, it may be that even if there were changes in FGF2, this would not be reflected in mRNA levels. Additionally, studies demonstrating no quantitative changes of FGF2 did note a redistribution of FGFR1, a high-affinity receptor for FGF2, from hair cells to supporting cells following the damage. Therefore, it may be that FGF2 protein was locally increased to promote regeneration of avian hair cells via stimulation of FGFR1. Another hypothesis is that FGF2 may be involved in stimulating precursor cell differentiation in inner ear epithelia since stimulation of postnatal avian inner ear epithelia by FGF2 did not lead to a direct increase in cell proliferation, but rather blocked mitogenesis (Oesterle et al., 2000).
Future studies can help clarify whether differences between the regenerative capacities of the avian and mammalian inner ear reflect, at least in part, variations in cellular patterns and timing of FGF2 expression. Similar experiments are needed in mammalian models to define FGF2 expression levels at different time points following auditory trauma, as these experiments may provide insights into a possible therapeutic window to repair damaged inner ear cells.
Optimization of FGF2 Treatment for Clinical Studies
While clinical studies have revealed the therapeutic potential of FGF2 in TM repair, there remains uncertainty around the ideal dose, timing, duration, method of delivery, and patient selection for enhanced therapeutic effect.
One study examined two different doses of FGF2 for healing of medium (1/8 to 1/4 of TM) and large (greater than 1/4 of TM) perforations (Lou et al., 2014). They found that the lower dose (0.1–0.15 ml of 21,000 IU/5 ml recombinant bovine FGF2 solution) had a significantly shorter closure time for medium-sized perforations when compared to the higher dose (0.25–0.3 ml FGF2 solution). The FGF2 dose had no significant impact on medium-sized perforation closure rate, or large-sized closure rate and repair time. It may be that continuous application of a higher dose of FGF2 inhibits collagen synthesis in the fibrous layer, thereby prolonging time to closure (Ryan and Baird, 1993; Lou, 2012; Lou et al., 2014). However, several factors such as age, gender, duration of injury, and cause of injury may also impact healing time and rates.
With regards to the timing of FGF2 administration, Lou and Wang (2015) have shown that mean closure time was significantly shortened when FGF2 was applied 3 days after injury, which corresponds to the proliferative stage of healing. Two additional studies have compared initiation of FGF2 treatment at specified times of 3, 4–7, 8–14, and >15 days following injury (Lou et al., 2012, 2015b). There was no statistically significant difference in closure rate or healing time between these groups, although those treated in the 8–14-day window after injury had the shortest time to closure (Lou et al., 2012, 2015b). Even though these studies indicate that the best commencement time of application may be after the inflammatory stage of wound healing, further research is needed to clarify the best time to apply FGF2 for TM perforation.
No consensus has been reached on the preferred duration of FGF2 treatment. Many studies have administered FGF2 daily until the TM perforation is completely healed (Lou et al., 2012, 2014, 2015a,b, 2021; Lou, 2012; Zhang and Lou, 2012; Lou and Wang, 2013, 2015; Lou Z. et al., 2016; Lou Z.-C. et al., 2016; Zhengcai-Lou et al., 2016; Lou and Lou, 2017; Zheng-Cai and Zi-Han, 2018). However, this approach can be inconvenient for patients with prolonged healing times. Furthermore, prolonged treatment may also trigger otorrhea or cause excess moisture buildup which can impair healing and damage surrounding tissue (Okan et al., 2007). Previous studies with other growth factors have also found that large doses or long-term application can result in reperforation of the eardrum or formation of middle ear cholesteatoma (Hennessey et al., 1991; Dvorak et al., 1995). An alternative approach has been to administer FGF2 every other day and this has been found to reduce otorrhea while allowing FGF2 to exert a continual effect (Jin et al., 2017).
Various routes of FGF2 delivery have been studied including direct application or administration via biomaterials. It has been hypothesized that biomaterials could serve as scaffolds for epithelial migration and allow for sustained release of FGF2. Furthermore, biomaterial patches may even serve as protection from infection during the TM healing process (Kanemaru et al., 2011). Several studies administered FGF2 with either atelocollagen or Gelfoam with beneficial effects on TM healing (Hakuba et al., 2010, 2013, 2015a,b; Kanemaru et al., 2011, 2018, 2021; Lou et al., 2012; Lou, 2012; Acharya et al., 2015; Jin et al., 2017; Omae et al., 2017). However, when FGF2 administered directly was compared to FGF2 provided via Gelfoam, no significant difference was observed in closure rate or healing time (Lou, 2012). The benefits of FGF2 via biological material patching need to be further validated in studies involving proper control groups.
The conflicting results of FGF2 therapeutic efficacy from recent studies (Santos et al., 2020; Lou et al., 2021) may be related to differences in patient selection criteria. For example, Santos et al. (2020) included patients with prior surgically repaired ears. Previous studies have suggested that surgical tympanoplasty can damage progenitor cells at the umbo or annulus of the TM which can inhibit healing (Kanemaru et al., 2011; Hakuba et al., 2013). Even so, Santos et al. (2020) did not see a difference in response to FGF2 when comparing surgical and non-surgical ears in their study group. Furthermore, Lou et al. (2021) found that the FGF2 application for TM perforation due to COM was not effective. This suggests that FGF2 may have an improved effect in TM perforation secondary to traumatic perforation as opposed to other causes. In contrast, Kanemaru et al. (2018) found FGF2 treatment to be beneficial even in patients with TM perforation who had cholesteatomas, tumors, and severe calcification. While other treatment variables were not constant between these studies, they do highlight that FGF2 treatment may have improved efficacy in a more restrictive patient subgroup.
There remain large gaps in understanding of the optimal treatment regimen of FGF2 for TM repair. However, current evidence suggests that treatment initiation 3 days following TM injury, and an every other day dosing strategy might be a promising starting point. Furthermore, restriction of patient populations to those with traumatic perforations in non-surgically repaired ears may show the most benefit. Early evidence does not support a role for biomaterials. Future studies using well-defined patient populations with a longer period of follow-up will be useful in determining these treatment parameters.
Implications in Hearing Loss
The link between middle- and inner-ear studies of FGF2 has not been explored in depth. Early evidence suggests potential FGF2 penetration into and activity within the inner ear even when applied to the middle ear. Damage to the TM is often reflected on an audiogram by an increase in the air-conduction threshold with an associated gap between air and bone conduction (air-bone gap). Repair of the TM is expected to improve the air conduction threshold, and thereby close the air-bone gap. Nearly all the clinical studies of FGF2 for TM repair reported improvements in PTA air conduction and air-bone gap in patients who achieved TM closure. Two studies, however, also reported improvements in bone conduction thresholds, which they attributed to improved sound transmission due to TM repair (Lou et al., 2015a; Omae et al., 2017). While improvements in bone conduction thresholds have been reported after addressing the cause of conductive hearing loss (Vijayendra and Parikh, 2011), bone conduction thresholds are typically thought to reflect inner ear processes. Therefore, the clinically improved bone conduction thresholds may, at least partly, be a result of FGF2 activity in the inner ear. This is not implausible given the pre-clinical studies outlined previously which support a rescue and proliferative effect of FGF2. Although FGF2 was applied to the middle ear in clinical studies of TM repair, some amount may have penetrated the inner ear, e.g., via the round window membrane, allowing for localized activity. This potential therapeutic effect in hearing is in line with previous findings of elevated levels of vestibular schwannoma-secreted FGF2 being associated with better hearing (Dilwali et al., 2013, 2015).
Hearing loss is the most common sensory deficit worldwide, and the burden of disability is projected to increase in the coming years (Sheffield and Smith, 2019). There are no approved pharmacological therapies for hearing loss. Therefore, the search for novel treatments for hearing loss is an important endeavor. Overall, the existing evidence reveals a therapeutic role of FGF2 in auditory disorders, especially in TM regeneration. Early studies suggest the benefit of FGF2 for TM repair in specific patient populations. However, additional focus must be placed on optimizing treatment strategy and defining therapeutic window. The relationship between FGF2 and sensorineural hearing is still unclear. Existing pre-clinical evidence suggests a therapeutic effect conferred by FGF2 treatment, but the mechanism of this effect is largely unknown. Future studies investigating the link between FGF2 and sensorineural hearing are needed to fill current gaps in knowledge and translate them to clinical studies. These studies may help accelerate the discovery of novel treatment approaches for a patient population that has few non-surgical options.
Author Contributions
MJ and VS wrote the manuscript. KB prepared all the tables. VS prepared the figure. KS conceived, designed, and supervised the manuscript writing and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Institute on Deafness and Other Communication Disorders (NIDCD) grant R01DC015824 and the Remondi foundation (KS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya, A. N., Coates, H., Tavora-Vièira, D., and Rajan, G. P. (2015). A pilot study investigating basic fibroblast growth factor for the repair of chronic tympanic membrane perforations in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 79, 332–335. doi: 10.1016/j.ijporl.2014.12.014
Adamska, M., Herbrand, H., Adamski, M., Krüger, M., Braun, T., and Bober, E. (2001). FGFs control the patterning of the inner ear but are not able to induce the full ear program. Mech. Dev. 109, 303–313. doi: 10.1016/s0925-4773(01)00550-0
Beenken, A., and Mohammadi, M. (2009). The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253. doi: 10.1038/nrd2792
Bruno, M., Rizzo, I. M., Romero-Guevara, R., Bernacchioni, C., Cencetti, F., Donati, C., et al. (2017). Sphingosine 1-phosphate signaling axis mediates fibroblast growth factor 2-induced proliferation and survival of murine auditory neuroblasts. Biochim. Biophys. Acta Mol. Cell Res. 1864, 814–824. doi: 10.1016/j.bbamcr.2017.02.004
Carnicero, E., Garrido, J. J., Alonso, M. T., and Schimmang, T. (2001). Roles of fibroblast growth factor 2 during innervation of the avian inner ear. J. Neurochem. 77, 786–795. doi: 10.1046/j.1471-4159.2001.00283.x
Carnicero, E., Zelarayan, L. C., Rüttiger, L., Knipper, M., Alvarez, Y., Alonso, M. T., et al. (2004). Differential roles of fibroblast growth factor-2 during development and maintenance of auditory sensory epithelia. J. Neurosci. Res. 77, 787–797. doi: 10.1002/jnr.20222
Claus, P., Werner, S., Timmer, M., and Grothe, C. (2004). Expression of the fibroblast growth factor-2 isoforms and the FGF receptor 1-4 transcripts in the rat model system of Parkinson’s disease. Neurosci. Lett. 360, 117–120. doi: 10.1016/j.neulet.2004.01.046
Cristobal, R., Popper, P., Lopez, I., Micevych, P., De Vellis, J., and Honrubia, V. (2002). in vivo and in vitro localization of brain-derived neurotrophic factor, fibroblast growth factor-2 and their receptors in the bullfrog vestibular end organs. Brain Res. Mol. Brain Res. 102, 83–99. doi: 10.1016/s0169-328x(02)00202-4
Cummings, B. J., Su, J. H., and Cotman, C. W. (1993). Neuritic involvement within bFGF immunopositive plaques of Alzheimer’s disease. Exp. Neurol. 124, 315–325. doi: 10.1006/exnr.1993.1202
de Araújo, M. M., Murashima, A. A. B., Alves, V. M., Jamur, M. C., and Hyppolito, M. A. (2014). Spontaneous healing of the tympanic membrane after traumatic perforation in rats. Braz. J. Otorhinolaryngol. 80, 330–338. doi: 10.1080/21645515.2021.1932212
Després, G., Jalenques, I., and Romand, R. (1991). Basic FGF-like protein in the lower stato-acoustic system of the neonatal and adult rat. Neuroreport 2, 639–642. doi: 10.1097/00001756-199111000-00001
Dilwali, S., Landegger, L. D., Soares, V. Y. R., Deschler, D. G., and Stankovic, K. M. (2015). Secreted factors from human vestibular schwannomas can cause cochlear damage. Sci. Rep. 5:18599. doi: 10.1038/srep18599
Dilwali, S., Lysaght, A., Roberts, D., Barker, F. G., McKenna, M. J., and Stankovic, K. M. (2013). Sporadic vestibular schwannomas associated with good hearing secrete higher levels of fibroblast growth factor 2 than those associated with poor hearing irrespective of tumor size. Otol. Neurotol. 34, 748–754. doi: 10.1097/MAO.0b013e31828048ec
Dvorak, D. W., Abbas, G., Ali, T., Stevenson, S., and Welling, D. B. (1995). Repair of chronic tympanic membrane perforations with long-term epidermal growth factor. Laryngoscope 105, 1300–1304. doi: 10.1288/00005537-199512000-00007
Edelman, E. R., Nugent, M. A., and Karnovsky, M. J. (1993). Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc. Natl. Acad. Sci. U S A 90, 1513–1517. doi: 10.1073/pnas.90.4.1513
Elsayed, M., Banasr, M., Duric, V., Fournier, N. M., Licznerski, P., and Duman, R. S. (2012). Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol. Psychiatry 72, 258–265. doi: 10.1016/j.biopsych.2012.03.003
Eren-Koçak, E., Turner, C. A., Watson, S. J., and Akil, H. (2011). Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol. Psychiatry 69, 534–540. doi: 10.1016/j.biopsych.2010.11.020
Even-Chen, O., and Barak, S. (2019). The role of fibroblast growth factor 2 in drug addiction. Eur. J. Neurosci. 50, 2552–2561. doi: 10.1111/ejn.14133
Fina, M., Baird, A., and Ryan, A. (1993). Direct application of basic fibroblast growth factor improves tympanic membrane perforation healing. Laryngoscope 103, 804–809. doi: 10.1288/00005537-199307000-00015
Fina, M., Bresnick, S., Baird, A., and Ryan, A. (1991). Improved healing of tympanic membrane perforations with basic fibroblast growth factor. Growth Factors 5, 265–272. doi: 10.3109/08977199109000290
Frenz, D. A., Liu, W., Williams, J. D., Hatcher, V., Galinovic-Schwartz, V., Flanders, K. C., et al. (1994). Induction of chondrogenesis: requirement for synergistic interaction of basic fibroblast growth factor and transforming growth factor-beta. Development 120, 415–424. doi: 10.1242/dev.120.2.415
Fumagalli, F., Bedogni, F., Slotkin, T. A., Racagni, G., and Riva, M. A. (2005). Prenatal stress elicits regionally selective changes in basal FGF-2 gene expression in adulthood and alters the adult response to acute or chronic stress. Neurobiol. Dis. 20, 731–737. doi: 10.1016/j.nbd.2005.05.005
Garbarino Azúa, D. J., Saucedo, L., Giordana, S., Magri, M. L., Buffone, M. G., Neuspiller, F., et al. (2017). Fibroblast growth factor 2 (FGF2) is present in human spermatozoa and is related with sperm motility. The use of recombinant FGF2 to improve motile sperm recovery. Andrology 5, 990–998. doi: 10.1111/andr.12398
Graham, B. M., and Richardson, R. (2011). Memory of fearful events: the role of fibroblast growth factor-2 in fear acquisition and extinction. Neuroscience 189, 156–169. doi: 10.1016/j.neuroscience.2011.05.041
Hakuba, N., Hato, N., Okada, M., Mise, K., and Gyo, K. (2015a). Preoperative factors affecting tympanic membrane regeneration therapy using an atelocollagen and basic fibroblast growth factor. JAMA Otolaryngol. Head Neck Surg. 141, 60–66. doi: 10.1001/jamaoto.2014.2613
Hakuba, N., Hato, N., Omotehara, Y., Okada, M., and Gyo, K. (2013). Epithelial pearl formation following tympanic membrane regeneration therapy using an atelocollagen/silicone membrane and basic fibroblast growth factor: our experience from a retrospective study of one hundred sixteen patients. Clin. Otolaryngol. 38, 394–397. doi: 10.1111/coa.12164
Hakuba, N., Ikemune, K., Okada, M., and Hato, N. (2015b). Use of ambulatory anesthesia with manually assisted ventilation for tympanic membrane regeneration therapy in children. Am. J. Otolaryngol. 36, 153–157. doi: 10.1016/j.amjoto.2014.10.012
Hakuba, N., Iwanaga, M., Tanaka, S., Hiratsuka, Y., Kumabe, Y., Konishi, M., et al. (2010). Basic fibroblast growth factor combined with atelocollagen for closing chronic tympanic membrane perforations in 87 patients. Otol. Neurotol. 31, 118–121. doi: 10.1097/MAO.0b013e3181c34f01
Hakuba, N., Taniguchi, M., Shimizu, Y., Sugimoto, A., Shinomori, Y., and Gyo, K. (2003). A new method for closing tympanic membrane perforations using basic fibroblast growth factor. Laryngoscope 113, 1352–1355. doi: 10.1097/00005537-200308000-00016
Hansen, M. R., Vijapurkar, U., Koland, J. G., and Green, S. H. (2001). Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear. Res. 161, 87–98. doi: 10.1016/s0378-5955(01)00360-4
Hennessey, P. J., Nirgiotis, J. G., Shinn, M. N., and Andrassy, R. J. (1991). Continuous EGF application impairs long-term collagen accumulation during wound healing in rats. J. Pediatr. Surg. 26, 362–365. doi: 10.1016/0022-3468(91)90980-8
Hossain, W. A., and Morest, D. K. (2000). Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: immunohistochemistry and antibody perturbation. J. Neurosci. Res. 62, 40–55. doi: 10.1002/1097-4547(20001001)62:1<40::AID-JNR5>3.0.CO;2-L
Hossain, W. A., Zhou, X., Rutledge, A., Baier, C., and Morest, D. K. (1996). Basic fibroblast growth factor affects neuronal migration and differentiation in normotypic cell cultures from the cochleovestibular ganglion of the chick embryo. Exp. Neurol. 138, 121–143. doi: 10.1006/exnr.1996.0052
Huh, S.-H., Jones, J., Warchol, M. E., and Ornitz, D. M. (2012). Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biol. 10:e1001231. doi: 10.1371/journal.pbio.1001231
Huh, S.-H., Warchol, M. E., and Ornitz, D. M. (2015). Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling. eLife 4:e05921. doi: 10.7554/eLife.05921
Ishibashi, T., Shinogami, M., Ishimoto, S. I., Yoshida, K., and Kaga, K. (1998). Induction of KGF, basic FGF and TGFalpha mRNA expression during healing of experimental TM perforations. Acta Otolaryngol. 118, 701–704. doi: 10.1080/00016489850183214
Jin, Z.-H., Dong, Y.-H., and Lou, Z.-H. (2017). The effects of fibroblast growth factor-2 delivered via a Gelfoam patch on the regeneration of myringosclerotic traumatic eardrum perforations lying close to the malleus. Am. J. Otolaryngol. 38, 582–587. doi: 10.1016/j.amjoto.2017.06.005
Kanemaru, S.-I., Kanai, R., Omori, K., Yamamoto, N., Okano, T., Kishimoto, I., et al. (2021). Multicenter phase III trial of regenerative treatment for chronic tympanic membrane perforation. Auris Nasus Larynx 48, 1054–1060. doi: 10.1016/j.anl.2021.02.007
Kanemaru, S.-I., Kanai, R., Yoshida, M., Kitada, Y., Omae, K., and Hirano, S. (2018). Application of regenerative treatment for tympanic membrane perforation with cholesteatoma, tumor, or severe calcification. Otol. Neurotol. 39, 438–444. doi: 10.1097/MAO.0000000000001701
Kanemaru, S.-I., Umeda, H., Kitani, Y., Nakamura, T., Hirano, S., and Ito, J. (2011). Regenerative treatment for tympanic membrane perforation. Otol. Neurotol. 32, 1218–1223. doi: 10.1097/MAO.0b013e31822e0e53
Kato, M., and Jackler, R. K. (1996). Repair of chronic tympanic membrane perforations with fibroblast growth factor. Otolaryngol. Head Neck Surg. 115, 538–547. doi: 10.1016/s0194-5998(96)70008-6
Kiyota, T., Ingraham, K. L., Jacobsen, M. T., Xiong, H., and Ikezu, T. (2011). FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer’s disease and has therapeutic implications for neurocognitive disorders. Proc. Natl. Acad. Sci. U S A 108, E1339–1348. doi: 10.1073/pnas.1102349108
Klejbor, I., Myers, J. M., Hausknecht, K., Corso, T. D., Gambino, A. S., Morys, J., et al. (2006). Fibroblast growth factor receptor signaling affects development and function of dopamine neurons - inhibition results in a schizophrenia-like syndrome in transgenic mice. J. Neurochem. 97, 1243–1258. doi: 10.1111/j.1471-4159.2006.03754.x
Lee, K. H., and Cotanche, D. A. (1996). Potential role of bFGF and retinoic acid in the regeneration of chicken cochlear hair cells. Hear. Res. 94, 1–13. doi: 10.1016/0378-5955(95)00220-0
Lefebvre, P. P., Van de Water, T. R., Weber, T., Rogister, B., and Moonen, G. (1991). Growth factor interactions in cultures of dissociated adult acoustic ganglia: neuronotrophic effects. Brain Res. 567, 306–312. doi: 10.1016/0006-8993(91)90809-a
Lombardo, A., and Slack, J. M. (1998). Postgastrulation effects of fibroblast growth factor on Xenopus development. Dev. Dyn. 212, 75–85. doi: 10.1002/(SICI)1097-0177(199805)212:1<L75::AID-AJA7>3.0.CO;2-#
Lou, Z. (2012). Healing large traumatic eardrum perforations in humans using fibroblast growth factor applied directly or via gelfoam. Otol. Neurotol. 33, 1553–1557. doi: 10.1021/acsbiomaterials.1c01190
Lou, Z., Huang, P., Yang, J., Xiao, J., and Chang, J. (2016). Direct application of bFGF without edge trimming on human subacute tympanic membrane perforation. Am. J. Otolaryngol. 37, 156–161. doi: 10.1016/j.amjoto.2015.11.004
Lou, Z., and Lou, Z. (2017). A comparative study to evaluate the efficacy of EGF, FGF-2 and 0.3% (w/v) ofloxacin drops on eardrum regeneration. Medicine (Baltimore) 96:e7654. doi: 10.1097/MD.0000000000007654
Lou, Z., Lou, Z., Jin, K., Sun, J., and Chen, Z. (2021). Topical application of bFGF alone for the regeneration of chronic tympanic membrane perforations: a preliminary case series. Stem Cells Int. 2021:5583046. doi: 10.1155/2021/5583046
Lou, Z.-C., Lou, Z.-H., Liu, Y.-C., and Chang, J. (2016). Healing human moderate and large traumatic tympanic membrane perforations using basic fibroblast growth factor, 0.3% ofloxacin eardrops and gelfoam patching. Otol. Neurotol. 37, 735–741. doi: 10.1097/MAO.0000000000001080
Lou, Z., Lou, Z., Tang, Y., and Xiao, J. (2015a). Utility of basic fibroblast growth factor in the repair of blast-induced total or near-total tympanic membrane perforations: a pilot study. Am. J. Otolaryngol. 36, 794–797. doi: 10.1016/j.amjoto.2015.08.007
Lou, Z., Yang, J., Tang, Y., and Xiao, J. (2015b). Risk factors affecting human traumatic tympanic membrane perforation regeneration therapy using fibroblast growth factor-2. Growth Factors 33, 410–418. doi: 10.3109/08977194.2015.1122003
Lou, Z., Tang, Y., and Wu, X. (2012). Analysis of the effectiveness of basic fibroblast growth factor treatment on traumatic perforation of the tympanic membrane at different time points. Am. J. Otolaryngol. 33, 244–249. doi: 10.1016/j.amjoto.2011.07.006
Lou, Z., and Wang, Y. (2015). Evaluation of the optimum time for direct application of fibroblast growth factor to human traumatic tympanic membrane perforations. Growth Factors 33, 65–70. doi: 10.3109/08977194.2014.980905
Lou, Z.-C., and Wang, Y.-B.-Z. (2013). Healing outcomes of large (>50%) traumatic membrane perforations with inverted edges following no intervention, edge approximation and fibroblast growth factor application; a sequential allocation, three-armed trial. Clin. Otolaryngol. 38, 289–296. doi: 10.1111/coa.12135
Lou, Z., Wang, Y., and Yu, G. (2014). Effects of basic fibroblast growth factor dose on traumatic tympanic membrane perforation. Growth Factors 32, 150–154. doi: 10.3109/08977194.2014.952411
Lou, X., Yuan, H., Xie, J., Wang, X., Yang, L., and Zhang, Y. (2015). Growth factors have a protective effect on neomycin-induced hair cell loss. Cell Biol. Int. 39, 65–73. doi: 10.1002/cbin.10347
Low, W., Dazert, S., Baird, A., and Ryan, A. F. (1996). Basic fibroblast growth factor (FGF-2) protects rat cochlear hair cells in organotypical culture from aminoglycoside injury. J. Cell. Physiol. 167, 443–450. doi: 10.1002/(SICI)1097-4652(199606)167:3<443::AID-JCP8>3.0.CO;2-P
Luo, L., Koutnouyan, H., Baird, A., and Ryan, A. F. (1993). Acidic and basic FGF mRNA expression in the adult and developing rat cochlea. Hear. Res. 69, 182–193. doi: 10.1016/0378-5955(93)90106-b
Mahaley, M. S., Mettlin, C., Natarajan, N., Laws, E. R., and Peace, B. B. (1990). Analysis of patterns of care of brain tumor patients in the United States: a study of the Brain Tumor section of the AANS and the CNS and the commission on cancer of the ACS. Clin. Neurosurg. 36, 347–352.
Malgrange, B., Rigo, J.-M., Coucke, P., Thiry, M., Hans, G., Nguyen, L., et al. (2002). Identification of factors that maintain mammalian outer hair cells in adult organ of corti explants. Hear. Res. 170, 48–58. doi: 10.1016/s0378-5955(02)00451-3
Mallei, A., Shi, B., and Mocchetti, I. (2002). Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol. Pharmacol. 61, 1017–1024. doi: 10.1124/mol.61.5.1017
Mansour, S. L., Li, C., and Urness, L. D. (2013). Genetic rescue of Muenke syndrome model hearing loss reveals prolonged FGF-dependent plasticity in cochlear supporting cell fates. Genes Dev. 27, 2320–2331. doi: 10.1101/gad.228957.113
Maragnoli, M. E., Fumagalli, F., Gennarelli, M., Racagni, G., and Riva, M. A. (2004). Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biol. Psychiatry 55, 1095–1102. doi: 10.1016/j.biopsych.2004.02.003
Molteni, R., Fumagalli, F., Magnaghi, V., Roceri, M., Gennarelli, M., Racagni, G., et al. (2001a). Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res. Brain Res. Rev. 37, 249–258. doi: 10.1016/s0165-0173(01)00128-x
Molteni, R., Lipska, B. K., Weinberger, D. R., Racagni, G., and Riva, M. A. (2001b). Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol. Psychiatry 6, 285–292. doi: 10.1038/sj.mp.4000865
Mondain, M., and Ryan, A. (1994). Effect of basic fibroblast growth factor on normal tympanic membrane. Am. J. Otolaryngol. 15, 344–350. doi: 10.1016/0196-0709(94)90133-3
Mueller, K. L., Jacques, B. E., and Kelley, M. W. (2002). Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J. Neurosci. 22, 9368–9377. doi: 10.1523/JNEUROSCI.22-21-09368.2002
Oesterle, E. C., Bhave, S. A., and Coltrera, M. D. (2000). Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. J. Comp. Neurol. 424, 307–326. doi: 10.1002/1096-9861(20000821)424:2<307::aid-cne9>3.0.co;2-m
Okan, D., Woo, K., Ayello, E. A., and Sibbald, G. (2007). The role of moisture balance in wound healing. Adv. Skin Wound Care 20, 39–53. doi: 10.1097/00129334-200701000-00013
Omae, K., Kanemaru, S.-I., Nakatani, E., Kaneda, H., Nishimura, T., Tona, R., et al. (2017). Regenerative treatment for tympanic membrane perforation using gelatin sponge with basic fibroblast growth factor. Auris Nasus Larynx 44, 664–671. doi: 10.1016/j.anl.2016.12.005
Ono, K., Kita, T., Sato, S., O’Neill, P., Mak, S.-S., Paschaki, M., et al. (2014). FGFR1-Frs2/3 signalling maintains sensory progenitors during inner ear hair cell formation. PLoS Genet. 10:e1004118. doi: 10.1371/journal.pgen.1004118
Ozkaptan, Y., Gerek, M., Simek, S., and Deveci, S. (1997). Effects of fibroblast growth factor on the healing process of tympanic membrane perforations in an animal model. Eur. Arch. Otorhinolaryngol. 254, S2–5. doi: 10.1007/BF02439709
Park, J. W., Hwang, S. R., and Yoon, I.-S. (2017). Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 22:1259. doi: 10.3390/molecules22081259
Perez, J. A., Clinton, S. M., Turner, C. A., Watson, S. J., and Akil, H. (2009). A new role for FGF2 as an endogenous inhibitor of anxiety. J. Neurosci. 29, 6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009
Pickles, J. O. (2001). The expression of fibroblast growth factors and their receptors in the embryonic and neonatal mouse inner ear. Hear. Res. 155, 54–62. doi: 10.1016/s0378-5955(01)00247-7
Pickles, J. O., and van Heumen, W. R. (1997). The expression of messenger RNAs coding for growth factors, their receptors and eph-class receptor tyrosine kinases in normal and ototoxically damaged chick cochleae. Dev. Neurosci. 19, 476–487. doi: 10.1159/000111245
Rak, K., Völker, J., Frenz, S., Scherzad, A., Schendzielorz, P., Radeloff, A., et al. (2014). Effects of the neurotrophic factors BDNF, NT-3 and FGF2 on dissociated neurons of the cochlear nucleus. Neuroreport 25, 960–964. doi: 10.1097/WNR.0000000000000220
Riva, M. A., Molteni, R., Bedogni, F., Racagni, G., and Fumagalli, F. (2005). Emerging role of the FGF system in psychiatric disorders. Trends Pharmacol. Sci. 26, 228–231. doi: 10.1016/j.tips.2005.03.001
Ruffini, F., Furlan, R., Poliani, P. L., Brambilla, E., Marconi, P. C., Bergami, A., et al. (2001). Fibroblast growth factor-II gene therapy reverts the clinical course and the pathological signs of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther. 8, 1207–1213. doi: 10.1038/sj.gt.3301523
Ryan, A. F., and Baird, A. (1993). Growth factors during proliferation of the middle ear mucosa. Acta Otolaryngol. 113, 68–74. doi: 10.3109/00016489309135769
Salmaso, N., Stevens, H. E., McNeill, J., ElSayed, M., Ren, Q., Maragnoli, M. E., et al. (2016). Fibroblast growth factor 2 modulates hypothalamic pituitary axis activity and anxiety behavior through glucocorticoid receptors. Biol. Psychiatry 80, 479–489. doi: 10.1016/j.biopsych.2016.02.026
Santa Maria, P. L., Redmond, S. L., Atlas, M. D., and Ghassemifar, R. (2010). Histology of the healing tympanic membrane following perforation in rats. Laryngoscope 120, 2061–2070. doi: 10.1002/lary.20998
Santos, F., Shu, E., Lee, D. J., Jung, D. H., Quesnel, A. M., Stankovic, K. M., et al. (2020). Topical fibroblast growth factor-2 for treatment of chronic tympanic membrane perforations. Laryngoscope Investig. Otolaryngol. 5, 657–664. doi: 10.1002/lio2.395
Sekiya, T., Shimamura, N., Yagihashi, A., and Suzuki, S. (2003). Effect of topically applied basic fibroblast growth factor on injured cochlear nerve. Neurosurgery 52, 900–907. doi: 10.1227/01.neu.0000053509.98561.16
Sheffield, A. M., and Smith, R. J. H. (2019). The Epidemiology of Deafness. Cold Spring Harb. Perspect. Med. 9:a033258. doi: 10.1101/cshperspect.a033258
Shi, L., Dong, M. M., Shi, W. J., Hu, Y. Y., Guo, W., Zhai, S. Q., et al. (2003). [Construction of bicistronic eukaryotic vector containing basic fibroblast growth factor and study of their functions in gene therapy for hearing impairment]. Zhonghua Er. Bi. Yan Hou Ke Za Zhi 38, 21–23.
Silva, V. A., Gomide, V. C., and Chadi, G. (2005). Fibroblast growth factor-2 immunoreactivity is present in the central and peripheral auditory pathways of adult rats. J. Morphol. 265, 141–151. doi: 10.1002/jmor.10345
Somers, T. h., Houben, V., Goovaerts, G., Govaerts, P. J., and Offeciers, F. E. (1997). Histology of the perforated tympanic membrane and its muco-epithelial junction. Clin. Otolaryngol. Allied Sci. 22, 162–166. doi: 10.1046/j.1365-2273.1997.00006.x
Song, J., and Slack, J. M. (1994). Spatial and temporal expression of basic fibroblast growth factor (FGF-2) mRNA and protein in early xenopus development. Mech. Dev. 48, 141–151. doi: 10.1016/0925-4773(94)90055-8
Sun, D., Bullock, M. R., McGinn, M. J., Zhou, Z., Altememi, N., Hagood, S., et al. (2009). Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 216, 56–65. doi: 10.1016/j.expneurol.2008.11.011
Tang, M.-M., Lin, W.-J., Zhang, J.-T., Zhao, Y.-W., and Li, Y.-C. (2017). Exogenous FGF2 reverses depressive-like behaviors and restores the suppressed FGF2-ERK1/2 signaling and the impaired hippocampal neurogenesis induced by neuroinflammation. Brain Behav. Immun. 66, 322–331. doi: 10.1016/j.bbi.2017.05.013
Terwisscha van Scheltinga, A. F., Bakker, S. C., and Kahn, R. S. (2010). Fibroblast growth factors in schizophrenia. Schizophr. Bull. 36, 1157–1166. doi: 10.1093/schbul/sbp033
Thau-Zuchman, O., Shohami, E., Alexandrovich, A. G., and Leker, R. R. (2012). Combination of vascular endothelial and fibroblast growth factor 2 for induction of neurogenesis and angiogenesis after traumatic brain injury. J. Mol. Neurosci. 47, 166–172. doi: 10.1007/s12031-012-9706-8
Timmer, M., Cesnulevicius, K., Winkler, C., Kolb, J., Lipokatic-Takacs, E., Jungnickel, J., et al. (2007). Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J. Neurosci. 27, 459–471. doi: 10.1523/JNEUROSCI.4493-06.2007
Turner, C. A., Watson, S. J., and Akil, H. (2012). The fibroblast growth factor family: neuromodulation of affective behavior. Neuron 76, 160–174. doi: 10.1016/j.neuron.2012.08.037
Umemoto, M., Sakagami, M., Fukazawa, K., Ashida, K., Kubo, T., Senda, T., et al. (1995). Hair cell regeneration in the chick inner ear following acoustic trauma: ultrastructural and immunohistochemical studies. Cell Tissue Res. 281, 435–443. doi: 10.1007/BF00417861
Vendrell, V., Carnicero, E., Giraldez, F., Alonso, M. T., and Schimmang, T. (2000). Induction of inner ear fate by FGF3. Development 127, 2011–2019. doi: 10.1242/dev.127.10.2011
Vijayendra, H., and Parikh, B. (2011). Bone conduction improvement after surgery for conductive hearing loss. Indian J. Otolaryngol. Head Neck Surg. 63, 201–204. doi: 10.1007/s12070-011-0130-0
Vrabec, J. T., Schwaber, M. K., Davidson, J. M., and Clymer, M. A. (1994). Evaluation of basic fibroblast growth factor in tympanic membrane repair. Laryngoscope 104, 1059–1064. doi: 10.1288/00005537-199409000-00002
Wang, D., Han, D., and Yang, W. (1998). [Effect of basic fibroblast growth factor on cultured spiral ganglion cells following glutamate treatment]. Zhonghua Er. Bi. Yan Hou Ke Za Zhi 33, 165–168.
Wang, W.-Q., Wang, Z.-M., and Chi, F.-L. (2004). Spontaneous healing of various tympanic membrane perforations in the rat. Acta Otolaryngol. 124, 1141–1144. doi: 10.1080/00016480410022921
Warchol, M. E., and Kaplan, B. A. (1999). Macrophage secretory products influence the survival of statoacoustic neurons. Neuroreport 10, 665–668. doi: 10.1097/00001756-199903170-00001
Wei, D., Jin, Z., Järlebark, L., Scarfone, E., and Ulfendahl, M. (2007). Survival, synaptogenesis and regeneration of adult mouse spiral ganglion neurons in vitro. Dev. Neurobiol. 67, 108–122. doi: 10.1002/dneu.20336
Werner, S., Peters, K. G., Longaker, M. T., Fuller-Pace, F., Banda, M. J., and Williams, L. T. (1992). Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc. Natl. Acad. Sci. U S A 89, 6896–6900. doi: 10.1073/pnas.89.15.6896
Wimmer, C., Mees, K., Stumpf, P., Welsch, U., Reichel, O., and Suckfüll, M. (2004). Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otol. Neurotol. 25, 33–40. doi: 10.1097/00129492-200401000-00007
Wu, L., Vasilijic, S., Sun, Y., Chen, J., Landegger, L. D., Zhang, Y., et al. (2021). Losartan prevents tumor-induced hearing loss and augments radiation efficacy in NF2 schwannoma rodent models. Sci. Transl. Med. 13:eabd4816. doi: 10.1126/scitranslmed.abd4816
Xia, L., Zhai, M., Wang, L., Miao, D., Zhu, X., and Wang, W. (2013). FGF2 blocks PTSD symptoms via an astrocyte-based mechanism. Behav. Brain Res. 256, 472–480. doi: 10.1016/j.bbr.2013.08.048
Yamasoba, T., Altschuler, R. A., Raphael, Y., Miller, A. L., Shoji, F., and Miller, J. M. (2001). Absence of hair cell protection by exogenous FGF-1 and FGF-2 delivered to guinea pig cochlea in vivo. Noise Health 3, 65–78.
Yin, J., Zhai, S., Guo, W., Hu, Y., and Shi, L. (2002). [Protective and rescue effects of transgenic bFGF/GFP expression mediated by cationic liposome on gentamicin-induced guinea pig cochlear toxicity]. Zhonghua Yi Xue Za Zhi 82, 1192–1194.
Yun, Y.-R., Won, J. E., Jeon, E., Lee, S., Kang, W., Jo, H., et al. (2010). Fibroblast growth factors: biology, function and application for tissue regeneration. J. Tissue Eng. 2010:218142. doi: 10.4061/2010/218142
Zhai, S., Cheng, J., and Wang, J. (1997). [Treatment effects of fibroblast growth factors on blast-induced hearing loss]. Zhonghua Er Bi Yan Hou Ke Za Zhi 32, 354–356.
Zhai, S.-Q., Cheng, J.-C., Wang, J.-L., Yang, W.-Y., Gu, R., and Jiang, S.-C. (2002). Protective effect of basic fibroblast growth factor on auditory hair cells after noise exposure. Acta Otolaryngol. 122, 370–373. doi: 10.1080/00016480260000030
Zhai, S.-Q., Wang, D.-J., Wang, J.-L., Han, D.-Y., and Yang, W.-Y. (2004). Basic fibroblast growth factor protects auditory neurons and hair cells from glutamate neurotoxicity and noise exposure. Acta Otolaryngol. 124, 124–129. doi: 10.1080/00016480310015939
Zhang, Q., and Lou, Z. (2012). Impact of basic fibroblast growth factor on healing of tympanic membrane perforations due to direct penetrating trauma: a prospective non-blinded/controlled study. Clin. Otolaryngol. 37, 446–451. doi: 10.1111/coa.12017
Zheng, J. L., and Gao, W. Q. (1996). Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur. J. Neurosci. 8, 1897–1905. doi: 10.1111/j.1460-9568.1996.tb01333.x
Zheng, J. L., Helbig, C., and Gao, W. Q. (1997). Induction of cell proliferation by fibroblast and insulin-like growth factors in pure rat inner ear epithelial cell cultures. J. Neurosci. 17, 216–226. doi: 10.1523/JNEUROSCI.17-01-00216.1997
Zheng, J. L., Lewis, A. K., and Gao, W. Q. (1998). Establishment of conditionally immortalized rat utricular epithelial cell lines using a retrovirus-mediated gene transfer technique. Hear. Res. 117, 13–23. doi: 10.1016/s0378-5955(97)00205-0
Zheng-Cai, L., and Zi-Han, L. (2018). The short- and long-term adverse effects of FGF-2 on tympanic membrane perforations. Acta Otorhinolaryngol. Ital. 38, 264–272. doi: 10.14639/0392-100X-1480
Zhengcai-Lou, Zihan-Lou, and Yongmei-Tang (2016). Comparative study on the effects of EGF and bFGF on the healing of human large traumatic perforations of the tympanic membrane. Laryngoscope 126, E23–28. doi: 10.1002/lary.25715
Keywords: FGF2, hearing loss, tympanic membrane repair, auditory development, vestibular schwannoma
Citation: Jeong M, Bojkovic K, Sagi V and Stankovic KM (2021) Molecular and Clinical Significance of Fibroblast Growth Factor 2 in Development and Regeneration of the Auditory System. Front. Mol. Neurosci. 14:757441. doi: 10.3389/fnmol.2021.757441
Received: 12 August 2021; Accepted: 18 November 2021;
Published: 23 December 2021.
Edited by:
Laura Astolfi, University of Padua, ItalyReviewed by:
Luis Lassaletta, Madrid Health Service, SpainVerena Scheper, Hannover Medical School, Germany
Huib Versnel, University Medical Center Utrecht, Netherlands
Copyright © 2021 Jeong, Bojkovic, Sagi and Stankovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantina M. Stankovic, a3N0YW5rb3ZpY0BzdGFuZm9yZC5lZHU=
 Minjin Jeong
Minjin Jeong Katarina Bojkovic
Katarina Bojkovic Varun Sagi
Varun Sagi Konstantina M. Stankovic
Konstantina M. Stankovic