- 1Beijing Hui-Long-Guan Hospital, Peking University, Beijing, China
- 2Key Laboratory of Psychosomatic Medicine, Inner Mongolia Medical University, Huhhot, China
- 3Department of Biomedical Engineering, College of Engineering, Peking University, Beijing, China
- 4Xinjiang Key Laboratory of Neurological Disorder Research, The Second Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 5Department of Anesthesiology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, China
- 6Department of Anesthesiology, The Affiliated Hospital of Inner Mongolia Medical University, Huhhot, China
- 7School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou, China
- 8Department of Psychiatry & Mind-Body Interface Laboratory (MBI-Lab), China Medical University Hospital, Taichung, Taiwan
- 9College of Medicine, China Medical University, Taichung, Taiwan
- 10An-Nan Hospital, China Medical University, Tainan, Taiwan
- 11The Affiliated Kangning Hospital, Wenzhou Medical University, Wenzhou, China
- 12School of Mental Health, Wenzhou Medical University, Wenzhou, China
Objectives: Cigarette smoking is associated with postoperative pain perception, which might be mediated by beta-endorphin and substance P. These effects on postoperative pain perception have never been investigated in human cerebrospinal fluid (CSF), which reflects biochemical alterations in the brain. Therefore, we investigated the associations among cigarette smoking, postoperative pain, and levels of beta-endorphin and substance P in human CSF.
Methods: We recruited 160 Chinese men (80 active smokers and 80 nonsmokers) who underwent lumbar puncture before anterior cruciate ligament reconstruction, and 5-ml CSF samples were collected. Pain visual analog scale (VAS) scores, post-anesthetic recovery duration (PARD), and smoking variables were obtained. CSF levels of beta-endorphin and substance P were measured.
Results: Compared to non-smokers, active smokers had significantly higher pain VAS (2.40 ± 0.67 vs. 1.70 ± 0.86, p < 0.001) and PARD scores (9.13 ± 2.11 vs. 7.27 ± 1.35, p = 0.001), lower CSF beta-endorphin (33.76 ± 1.77 vs. 35.66 ± 2.20, p = 0.001) and higher CSF substance P (2,124.46 ± 217.34 vs. 1,817.65 ± 302.14, p < 0.001) levels. Pain VAS scores correlated with PARD in active smokers (r = 0.443, p = 0.001).
Conclusions: Cigarette smoking is associated with increased postoperative pain intensity, shown by delayed pain perception, higher pain VAS scores, and lower beta-endorphin and higher substance P levels in the CSF of active smokers. The more extended postoperative pain perception is delayed, the more pain intensity increases.
Introduction
Approximately 75% of patients who undergo surgery experience acute postoperative pain, which can persist for up to 7 days after surgery (Gupta et al., 2010). Severe persistent pain affects 2–10% of adults (Lespasio et al., 2019), and it can eventually become chronic, even with medication (Chapman and Vierck, 2017).
Evidence suggests that chronic exposure to cigarette smoke may exaggerate pain symptoms (Carstens et al., 2001; Anderson et al., 2004; Shi et al., 2010). A recent study showed that smokers take more oral morphine than nonsmokers (Howard et al., 2019), and smoking intensity is positively associated with chronic opioid use after surgery (Pang et al., 2017). Clinical studies reported that smokers require more pain medication for postoperative pain than nonsmokers or past smokers (Chiang et al., 2016). Nevertheless, several studies generated conflicting findings to the effect that cigarette smoke demonstrates an acute pain-inhibitory effect in humans and animal models (Shi et al., 2010; Ditre et al., 2011).
The relationship between pain and cigarette smoke is possibly mediated by changes in the endogenous opioid system (Shi et al., 2010). Beta-endorphin is an endogenous ligand for the mu-subtype opioid receptor (Sprouse-Blum et al., 2010) and is 18–33 times more potent as an analgesic than morphine (Loh et al., 1976). Animal studies showed that acute and chronic treatment with nicotine decreases beta-endorphin content in the hypothalamus, altering beta-endorphin synthesis and release in the brain (Gudehithlu et al., 2012). Patients undergoing general anesthesia experienced a significant increase in beta-endorphin during surgery, which was relieved after the co-administration of fentanyl (Dubois et al., 1982).
Chronic cigarette smoking enhances the expression of tachykinin receptors in the endothelium, mediating the effects of substance P (Zakharchuk et al., 2017). Substance P is a pro-nociceptive neuropeptide (a tachykinin) that mediates pain transmission (Stein, 1995; Snijdelaar et al., 2000; Hokfelt et al., 2003). Inhibiting substance P relieves pain (Zieglgansberger, 2019). Studies in transgenic mice showed that mice lacking substance P do not respond to moderate or severe pain (Hokfelt et al., 2003). Substantial evidence suggested that substance P plays an essential role in eliciting pain sensation in both the central nervous system (CNS) and the peripheral nervous system (PNS; Jasmin et al., 2002; Chang et al., 2019); cigarette smoking was found to promote neurogenic substance P release (Xu and Xu, 2010). In contrast to the role of beta-endorphin, substance P enhances pain sensation in the PNS, and when beta-endorphin binds to opioid receptors followed by a decrease in substance P levels, an analgesic state may result (Butler et al., 2015). These findings suggest that beta-endorphin and substance P may play different roles in postoperative pain intensity.
Neuropeptides like beta-endorphin and substance P cannot cross the blood-brain barrier (Riley et al., 2017), making the cerebrospinal fluid (CSF) an ideal medium to investigate biochemical alterations relating to pain in the human brain. This study used CSF to determine the association between pain intensity, beta-endorphin and substance P levels, and post-anesthetic recovery duration (PARD) in active smokers and explore the factors related to postoperative pain intensity.
Materials and Methods
Subjects
Because there are few female smokers in China, we, therefore, decided to exclude females from the study, and a total of 160 Chinese male subjects scheduled for anterior cruciate ligament single bundle reconstruction in the morning were recruited without surgery of meniscus repairing between September 2014 and January 2016. Of these, 80 were active smokers (the case group), and 80 were nonsmokers (the control group).
Sociodemographic data, including age, body mass index, and years of education, were collected. Clinical data, including substance abuse and dependence history, were obtained according to self-reporting and confirmed by the next of kin and family members. Exclusion criteria were as follows: (1) a family history of psychosis or neurological diseases, or CNS diseases determined using the Mini-International Neuropsychiatric Interview; and (2) systemic diseases based on the medical history and admitting diagnosis.
Subjects who had never smoked and had no history of substance abuse or dependence were assigned to the nonsmoker group. Active smokers were defined as those who had consumed half a pack of cigarettes (half pack = 10 cigarettes) or more per day for at least 1 year. Smokers that consumed fewer than 10 cigarettes per day were excluded from this study. Active smokers were further grouped into younger smokers (<40 years old) vs. older smokers (≥40 years old), according to the standard diagnostic manual of the American Psychiatric Association. Active smokers were divided into moderate smokers (n = 38, ≥10 and <20 cigarettes per day) and heavy smokers (n = 42, ≥20 cigarettes per day; the maximum was 40 cigarettes per day) according to the World Health Organization criteria. No subjects had a history of alcohol abuse or psychiatric disorders, according to the Diagnostic and Statistical Manual of Mental Disorders, fourth Edition. All subjects were unrelated.
The Institutional Review Board of the Inner Mongolian Medical University approved the study that was performed according to the Declaration of Helsinki. Written informed consent was obtained, and no financial compensation was provided to the subjects.
Assessments, Biological Sample Collection, and Laboratory Tests
Information was obtained from active smokers, including the age of smoking onset, years of cigarette smoking, the average daily number of cigarettes smoked, and the maximum daily number of cigarettes smoked.
The data of high-density lipoprotein, low-density lipoprotein, alanine aminotransferase test, cholesterol, triglyceride, gamma-glutamyl transferase, and aspartate aminotransferase came from routine tests to evaluate physical condition on admission. These peripheral metabolic marker levels were measured in the morning on the 2nd hospital day after an overnight fasting period using a biochemistry analyzer (HITACH 7600, Hitachi Co., Tokyo, Japan).
Before surgery, the Self-rating Anxiety Scale (SAS) and the 13-item Beck Depression Inventory (BDI) were used to evaluate self-reported preoperative anxiety and symptoms of depression. An original raw score cutoff of 40 for SAS was identified previously (Dunstan and Scott, 2020). The ranges of BDI score cutoff points for various categories of depression were as follows: 0–4 (no depression), 5–7 (mild depression), 8–15 (moderate depression), and 16–39 (severe depression; Pomerleau, 1992). The interval between assessment and lumbar puncture was less than 24 h.
Lumbar puncture is part of normal clinical practice for patients undergoing anterior cruciate ligament reconstructive surgery in China, making the CSF sample conveniently accessible and decreasing the likelihood of disease entities affecting the CSF sample. Preoperative smoking cessation is not required for this kind of operation. In this study, 3 ml 0.5% ropivacaine was administered as local anesthesia in the morning before surgery by a licensed anesthetist, and a 5-ml CSF sample was obtained for analysis via intrathecal collection before ropivacaine was conducted.
It takes 40–50 min to complete anterior cruciate ligament reconstruction. Analgesics were not used before surgery, including opioid therapy and other medications; preoperative pain intensity was not recorded because there were no subjective painful symptoms. The anesthetist recorded the time of surgery completion, and the duty nurse recorded the time to postoperative pain perception. The duration between the two points was defined as PARD. Analgesics were not used after surgery. At the time of pain perception, the pain visual analog scale (VAS) was used to assess acute postoperative pain perception for all subjects. The scores ranged from 0 (“no pain”) to 10 (“most severe pain ever”).
Each CSF sample was then distributed into 0.5 ml tubes and immediately frozen at −80°C for storage. The period from hospitalization to surgery was a maximum of two days. CSF analyses to quantify beta-endorphin (Catalog#: RK-022-14) and substance P (Catalog#: RK-061-05) levels were performed using commercial radioimmunoassay kits from Phoenix (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA). The detection ranges of radioimmunoassay kits are 10–1,280 pg/ml. The cross reactivities of the peptides for beta-endorphin and substance P are both 100%. Deionized water of enzyme inactivation was set as the negative control for the assays. The positive control was included in the kits. All experimental procedures followed the manufacturer’s instructions, and the data analyst was blinded to the clinical data.
Statistical Analysis
The normality of variance was performed using the Shapiro–Wilk test. The homogeneity of variance was performed using Levene’s test. Multi-collinearity was calculated using stepwise multiple regression among covariates estimated via tolerance and variance inflation factor (VIF) as the cutoff recommended thresholds for tolerance <0.1, and VIF >10 (Kim, 2019). Analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were used to compare differences of variables between groups. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Figures were created using GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA, USA). All the tests were two-sided, and the significance threshold was set at p < 0.05.
Results
Demographic and Clinical Characteristics
Using Levene’s test, the homogeneity of variance was performed for sociodemographic and clinical variables (all p > 0.05). Consequently, ANOVA was used in Table 1.
The SAS raw scores and the BDI scores were ≤40 and ≤15, respectively, for all subjects. Nonsmokers had more years of education (13.33 ± 2.58 vs. 11.91 ± 3.16 years, p = 0.003), while older age and higher BDI scores were seen in active smokers (31.45 ± 9.03 vs. 34.71 ± 10.69 and 2.67 ± 3.12 vs. 0.79 ± 1.17, both p < 0.05). No differences were identified in age, SAS score, or clinical characteristics between the groups (all p > 0.05; Table 1).
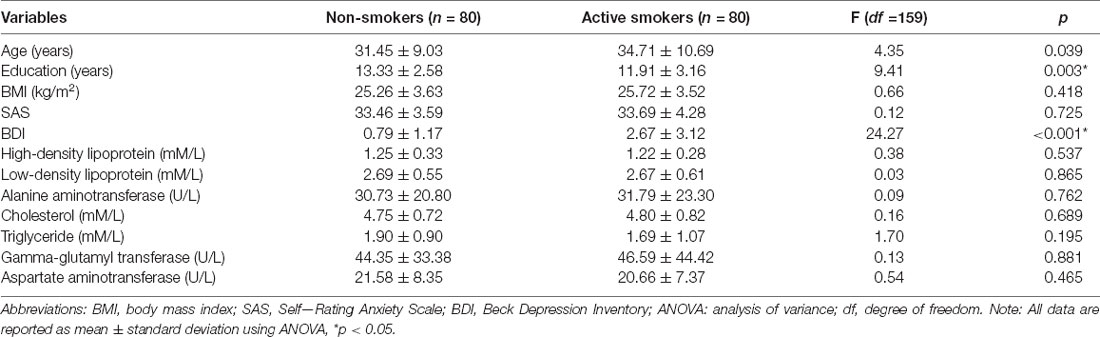
Table 1. Differences in demographic and clinical characteristics between nonsmokers and active smokers.
Pain Perception and CSF Biomarkers
The normality of the residuals from the beta-endorphin and substance P models was determined using the Shapiro–Wilk test (p > 0.05). The homoscedasticity of residuals of the variances was determined using Levene’s test. The residuals were equally distributed (all p > 0.05). Stepwise multiple regression analyses of four pain-related variables, year, education, and BDI, showed that no variable was removed from models (all tolerance >0.8 and VIF <2). Because there was collinearity of age with age at smoking onset and years of cigarette smoking, respectively (tolerance < 0.1 and VIF > 30), age was removed from models in active smokers. Therefore, analysis of covariance ANCOVA was used to compare differences in pain-related variables between groups in Tables 2, 3.
The pain VAS scores, PARD, and CSF levels of substance P in nonsmokers were remarkably lower than those in active smokers, while concentrations of beta-endorphin were significantly higher in nonsmokers when adjusting for years of education, BDI, and the other variables (all p < 0.05; Table 2, Figures 1, 2). ANCOVA was used for each variable, with years of education, BDI, smoking-related variables, and the other variables as covariates. There were no differences in pain VAS scores, PARD, or CSF levels of beta-endorphin and substance P between younger and elder smokers, moderate smokers, and heavy smokers, respectively (all p > 0.05; Table 3).

Figure 1. Significant differences in (A) pain visual analog scale (VAS) scores and (B) post-anesthetic recovery duration (PARD) between nonsmokers and active smokers (*p < 0.05).
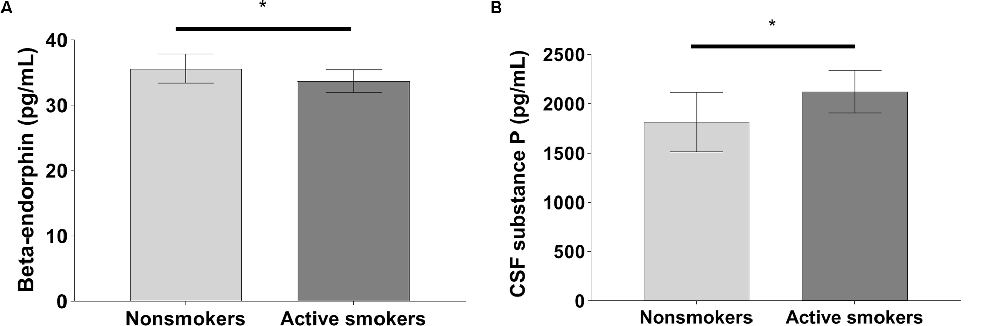
Figure 2. Significant differences in (A) cerebrospinal fluid (CSF) beta-endorphin levels, and (B) CSF substance P levels between nonsmokers and active smokers (*p < 0.05).
Correlation of CSF Biomarkers and Smoking Status With Pain
No correlation between CSF biomarkers and pain VAS scores or PARD was found in nonsmokers, adjusting for age, education, BDI, and other related variables (all p > 0.05). In active smokers, partial correlation analysis was performed between pain VAS scores and PARD (r = 0.443, p = 0.001, Figure 3), adjusting for years of education, BDI, and smoking-related variables.
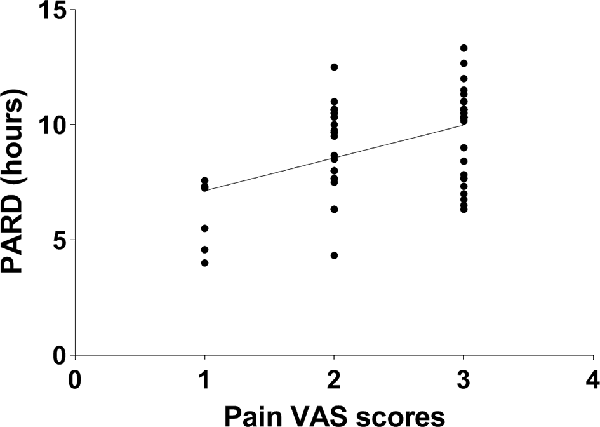
Figure 3. The pain visual analog scale (VAS) score was positively correlated with post-anesthetic recovery duration (PARD) in active smokers (r = 0.443, p = 0.001).
Partial correlation analysis was performed to test the correlation between pain-related and smoking-related variables in active smokers, adjusting for education, BDI, other pain-related and smoking-related variables as covariates, and there was no correlation (all p > 0.05; Table 4).
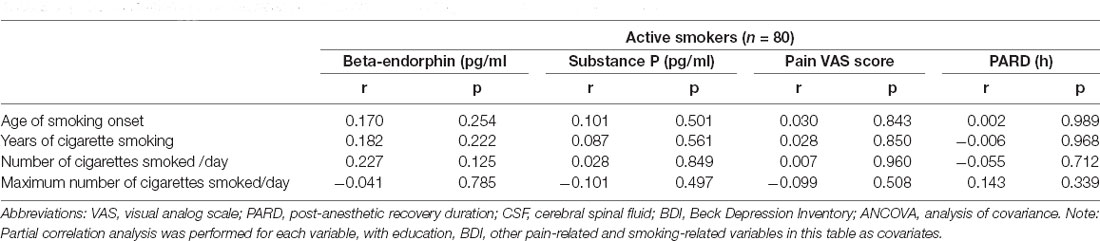
Table 4. The correlation of CSF between pain-related and smoking-related variables in active smokers.
Discussion
To the best of our knowledge, ours is the first study to use human CSF to calculate the association between cigarette smoking and postoperative pain. Our main finding is that cigarette smoking indeed increased postoperative pain intensity, with higher substance P and lower beta-endorphin levels in the CSF of active smokers than nonsmokers. Despite some conflicting results (Shi et al., 2010), several studies showed that cigarette smokers have more significant postoperative pain after total hip arthroplasty than a nonsmoking cohort (Etcheson et al., 2018). Furthermore, male active smokers require more morphine in the first 72 h after surgery than nonsmokers and past smokers (Chiang et al., 2016). Patients with smoking history were found to have increased analgesic intake compared to non-operative treatment (Forman et al., 2007). The administration of postoperative pain medication to coronary artery bypass graft patients showed that smokers require more pain medication than nonsmokers during the first 48 h after surgery (Creekmore et al., 2004). A prospective study demonstrated that smokers consume more opioids using patient-controlled analgesia during the first 24 h after surgery than nonsmokers (Steinmiller et al., 2012).
Beta-endorphin binds mu-opioid receptors at the presynaptic nerve terminals and inhibits the release of gamma-aminobutyric acid in the CNS, which inhibits the release of dopamine; the binding of beta-endorphin to these receptors leads to an overproduction of dopamine primarily associated with pleasure (Bressan and Crippa, 2005). Beta-endorphin relieves pain in adults (Stephan and Parsa, 2016) and fetuses (Lee et al., 2005). There are probably two functionally different beta-endorphin systems: one peripheral (release of beta-endorphin by the pituitary into the systemic circulation) and one central [synthesis in hypothalamic pro-opiomelanocortin (POMC) neurons]. Hypothalamic POMC neurons release beta-endorphin directly into the CSF of the third ventricle. The CSF serves as a transport medium for beta-endorphin to the distant brain and spinal sites. This is called “long-distance volume transmission” and is by no means unique for beta-endorphin. Hence, it makes physiological sense to speculate that low levels of CSF beta-endorphin would mirror a defective endogenous pain control system. Almay et al. reported that levels of unspecific “endorphins” were low in the CSF of patients with predominantly “neuralgic” pain, compared both to patients with what was labeled “psychogenic” pain and to healthy controls (Almay et al., 1978). Tonelli et al. (1988) found low CSF and beta-endorphin in patients scheduled for spinal cord stimulation, compared with historical controls. As a critical analgesic endogenous opioid (Bruehl et al., 2007), beta-endorphin is released from the pituitary gland (Veening et al., 2012) into the serum of humans (Bruehl et al., 2007) and mice (Rasmussen and Farr, 2003), following exposure to a painful stimulus. Previous studies have reported that patients undergoing general anesthesia experience a significant increase in beta-endorphin during surgery, inhibited by the co-administration of fentanyl (Cork et al., 1985). Similarly, patients who undergo dental surgery with local anesthesia have increased serum beta-endorphin levels during and after surgery. When fentanyl is co-administered, beta-endorphin levels in the serum are significantly reduced, and less pain is observed during the surgery (Hargreaves et al., 1983). All the studies mentioned above demonstrated the analgesic effect of beta-endorphin in the PNS.
Substance P is one of the main transmitters of nociceptive pain (Li et al., 2012). Substantial evidence suggests that substance P plays an essential role in eliciting pain sensation in both the CNS and PNS. In the CNS, substance P results in central sensitization by activating excitatory post-synaptic potential (De Koninck and Henry, 1991). In people with fibromyalgia, a chronic pain disorder, there are elevated substance P levels in CSF (Chang et al., 2019). Chemical ablation of neurons expressing substance P receptors in animals (Mantyh et al., 1997) and genetic disruption of the encoding gene of substance P or its receptor (Cao et al., 1998) reduced pain responses. A study demonstrated that 2 h after 2.5% formalin injection substance, P-expression in the distal CSF-contacting neurons was upregulated significantly, which may be considered essential for nociceptive processing (Lu et al., 2009). Together, these studies support the notion that substance P is an important signal molecule in pain transmission.
The evidence suggests higher substance P and lower beta-endorphin levels increase the intensity of postoperative pain, consistent with our results, as demonstrated by higher pain VAS scores after surgery. Studies reported that chronic exposure to nicotine and cigarette smoking reduces pain inhibition, agreeing with our findings (Carstens et al., 2001; Anderson et al., 2004; Simons et al., 2005). More importantly, ropivacaine is metabolized extensively by cytochrome P450 (CYP) enzymes (Arlander et al., 1998), and cigarette smoking increases the CYP-mediated metabolism of ropivacaine (Jokinen et al., 2001). CSF samples in our study were obtained before intrathecal injection of ropivacaine, which strongly indicates that smoking is more likely to enhance postoperative pain independently of ropivacaine.
Our secondary findings suggest delayed pain perception in active smokers and that pain VAS score positively correlated with PARD. First, cigarette smoking accelerates the metabolism of ropivacaine in the liver (Jokinen et al., 2001). Second, chronic cigarette smoking enhances tachykinin synthesis, stimulates neurogenic substance P release (Kwong et al., 2001; Xu and Xu, 2010), and accelerates the thinning of the brain’s cortex (Karama et al., 2015), which is one factor resulting in peripheral neuropathy (Richardson et al., 2009), such as axon loss and demyelination. Demyelinating neuropathy is characterized by a reduction in conduction velocity (Chung et al., 2014), which is also found in the sensory nerve (Walsh et al., 2015; Palve and Palve, 2018). Thus, this may be one reason for the longer duration in active smokers. Taken together, these findings suggest that cigarette smoking postpones postoperative pain. The evidence from these studies supports the notion that higher CSF substance P levels are found in active smokers with degenerating nerve conduction, further explaining why active smokers experience a higher pain intensity after a longer PARD. The positive correlation of pain VAS scores with PARD indicates that the longer the postoperative pain perception was delayed, the greater the pain intensity was. Since subjects were not required to stop smoking before or after surgery, withdrawal responses were not considered in the present study.
A study reported that the use of ropivacaine may reduce noxious input during surgery (Papaziogas et al., 2001); CSF samples were obtained prior to spinal anesthesia in the present study, which indicate the levels of beta-endorphin and substance P were the baseline levels and did not change due to surgery or ropivacaine. Thus, the CSF beta-endorphin and substance P levels can relate to postoperative pain in the present study. Moreover, active smokers who consumed less than half a pack of cigarettes (<10 cigarettes) per day, or had smoked for less than 1 year, were not included in our study, which might be another reason that no correlation was observed.
There are limitations to this study. First, we did not explore the immediate effect of cigarette smoking on pain perception because our subjects were all chronic cigarette smokers. Second, all subjects underwent surgery for anterior cruciate ligament injuries, which may not represent other types of postoperative pain. Thirdly, we did not record pack-year, since it was difficult to remember the amount for the subjects, though the common clinical quantification index of smoking exposure is pack-year. However, we collected smoking habit variables including the age of smoking onset, years of cigarette smoking, the average daily number of cigarettes smoked, and maximum daily number of cigarettes smoked instead. Finally, ropivacaine is metabolized extensively in the liver, predominantly by CYP, and its polymorphisms may play a role in the differences in ropivacaine metabolism. However, we did not detect the genetic polymorphisms of CYP in all subjects in the present study, which may be a slight confounder of our results, and genotyping these polymorphisms will be the subject of our future study.
Conclusions
The relationship between cigarette smoking and postoperative pain is complex. Chronic cigarette smoking is associated with higher intensity postoperative pain, as shown by lower beta-endorphin and higher substance P levels in the CSF. The delay of postoperative pain perception correlates with greater pain intensity. These findings suggest that more analgesics should be considered for active smokers after surgery.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Fan Wang, RmFuV2FuZ0Biam11LmVkdS5jbg==.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Ethics Committee of Inner Mongolia Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YL and K-PS provided the idea for the study. FW, K-PS, and YL contributed to the study design. FW and HL led the drafting of the manuscript and the statistical analyses. QM, YK, SY, LS, and H-CC collected the clinical data. H-CC helped edit the manuscript. All authors approved the final manuscript for submission.
Funding
This work was supported by the following grants: The 10th Inner Mongolia Autonomous Region “Prairie excellence” Project (FW), The Technology Support Project of Xinjiang (2017E0267, FW), Natural Science Foundation of Xinjiang Uyghur Autonomous Region (2018D01C228, FW, and 2018D01C239, HL), Tianshan Youth Project—Outstanding Youth Science and Technology Talents of Xinjiang (2017Q007, FW), Beijing Natural Science Foundation (7152074, FW), the Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences (YL), MOST (106-2314-B-039-027-MY3, 108-2320-B-039-048, and 108-2314-B-039-016, K-PS) from the Ministry of Science and Technology, Taiwan; NHRI-EX108-10528NI (K-PS) from the National Health Research Institutes, Taiwan; MYRG2018-00242-ICMS (K-PS) from University of Macau, China; and CRS-108-048, CMU108-SR-106, DMR-108-216, CMRC-CMA-3, DMR-109-102, DMR-109-244, DMR-HHC-109-11, DMR-HCC-109-12, and the Chinese Medicine Research Center (K-PS) from the China Medical University, Taichung, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the teachers and students for their involvement in this study. We also thank all the participants in our study for their time and cooperation.
References
Almay, B., Johansson, F., Von Knorring, L., Terenius, L., and Wahlstrom, A. (1978). Endorphins in chronic pain. I. Differences in CSF endorphin levels between organic and psychogenic pain syndromes. Pain 5, 153–162. doi: 10.1016/0304-3959(78)90037-4
Anderson, K. L., Pinkerton, K. E., Uyeminami, D., Simons, C. T., Carstens, M. I., and Carstens, E. (2004). Antinociception induced by chronic exposure of rats to cigarette smoke. Neurosci. Lett. 366, 86–91. doi: 10.1016/j.neulet.2004.05.020
Arlander, E., Ekström, G., Alm, C., Carrillo, J., Bielenstein, M., Böttiger, Y., et al. (1998). Metabolism of ropivacaine in humans is mediated by CYP1A2 and to a minor extent by CYP3A4: an interaction study with fluvoxamine and ketoconazole as in vivo inhibitors. Clini. Pharmacol. Ther. 64, 484–491. doi: 10.1016/S0009-9236(98)90131-X
Bressan, R., and Crippa, J. (2005). The role of dopamine in reward and pleasure behaviour – review of data from preclinical research. Acta Psychiatr. Scand. Suppl. 427, 14–21. doi: 10.1111/j.1600-0447.2005.00540.x
Bruehl, S., Chung, O. Y., Burns, J. W., and Diedrich, L. (2007). Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain 130, 208–215. doi: 10.1016/j.pain.2006.11.013
Butler, M. G., Nelson, T. A., Driscoll, D. J., and Manzardo, A. M. (2015). Evaluation of plasma substance P and beta-endorphin levels in children with prader-willi syndrome. J. Rare Disord. 3.
Cao, Y. Q., Mantyh, P. W., Carlson, E. J., Gillespie, A. M., Epstein, C. J., and Basbaum, A. I. (1998). Primary afferent tachykinins are required to experience moderate to intense pain. Nature 392, 390–394. doi: 10.1038/32897
Carstens, E., Anderson, K. A., Simons, C. T., Carstens, M. I., and Jinks, S. L. (2001). Analgesia induced by chronic nicotine infusion in rats: differences by gender and pain test. Psychopharmacology (Berl) 157, 40–45. doi: 10.1007/s002130100770
Chang, C. T., Jiang, B. Y., and Chen, C. C. (2019). Ion channels involved in substance P-mediated nociception and antinociception. Int. J. Mol. Sci. 20:1596. doi: 10.3390/ijms20071596
Chapman, C. R., and Vierck, C. J. (2017). The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J. Pain 18, 359.e1–359.e38. doi: 10.1016/j.jpain.2016.11.004
Chiang, H. L., Chia, Y. Y., Lin, H. S., and Chen, C. H. (2016). The implications of tobacco smoking on acute postoperative pain: a prospective observational study. Pain Res. Manag. 2016:9432493. doi: 10.1155/2016/9432493
Chung, T., Prasad, K., and Lloyd, T. E. (2014). Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin. N. Am. 24, 49–65. doi: 10.1016/j.nic.2013.03.023
Cork, R. C., Hameroff, S. R., and Weiss, J. L. (1985). Effects of halothane and fentanyl anesthesia on plasma beta-endorphin immunoreactivity during cardiac surgery. Anesth. Analg. 64, 677–680.
Creekmore, F. M., Lugo, R. A., and Weiland, K. J. (2004). Postoperative opiate analgesia requirements of smokers and nonsmokers. Ann. Pharmacother. 38, 949–953. doi: 10.1345/aph.1D580
De Koninck, Y., and Henry, J. L. (1991). Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc. Natl. Acad. Sci. U S A 88, 11344–11348. doi: 10.1073/pnas.88.24.11344
Ditre, J., Brandon, T., Zale, E., and Meagher, M. (2011). Pain, nicotine and smoking: research findings and mechanistic considerations. Psychol. Bull. 137, 1065–1093. doi: 10.1037/a0025544
Dubois, M., Pickar, D., Cohen, M., Gadde, P., Macnamara, T. E., and Bunney, W. E. (1982). Effects of fentanyl on the response of plasma beta-endorphin immunoreactivity to surgery. Anesthesiology 57, 468–472. doi: 10.1097/00000542-198212000-00006
Dunstan, D. A., and Scott, N. (2020). Norms for zung’s self-rating anxiety scale. BMC Psychiatry 20:90. doi: 10.1186/s12888-019-2427-6
Etcheson, J. I., Gwam, C. U., George, N. E., Walia, N., Jerjian, C., Han, G. R., et al. (2018). Opiate pain medication consumption in cigarette smokers following total hip arthroplasty. Joints 6, 157–160. doi: 10.1055/s-0038-1673405
Forman, J. P., Rimm, E. B., and Curhan, G. C. (2007). Frequency of analgesic use and risk of hypertension among men. Arch. Intern. Med. 167, 394–399. doi: 10.1001/archinte.167.4.394
Gudehithlu, K., Duchemin, A., Tejwani, G., Neff, N., and Hadjiconstantinou, M. (2012). Nicotine-induced changes of brain β-endorphin. Neuropeptides 46, 125–131. doi: 10.1016/j.npep.2012.03.001
Gupta, A., Kaur, K., Sharma, S., Goyal, S., Arora, S., and Murthy, R. S. (2010). Clinical aspects of acute post-operative pain management & its assessment. J. Adv. Pharm. Technol. Res. 1, 97–108.
Hargreaves, K. M., Dionne, R. A., and Mueller, G. P. (1983). Plasma beta-endorphin-like immunoreactivity, pain and anxiety following administration of placebo in oral surgery patients. J. Dent. Res. 62, 1170–1173. doi: 10.1177/00220345830620111601
Hokfelt, T., Bartfai, T., and Bloom, F. (2003). Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2, 463–472. doi: 10.1016/s1474-4422(03)00482-4
Howard, R., Fry, B., Gunaseelan, V., Lee, J., Waljee, J., Brummett, C., et al. (2019). Association of opioid prescribing with opioid consumption after surgery in michigan. JAMA Surg. 154:e184234. doi: 10.1001/jamasurg.2018.4234
Jasmin, L., Tien, D., Weinshenker, D., Palmiter, R., Green, P., Janni, G., et al. (2002). The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc. Natl. Acad. Sci. U S A 99, 1029–1034. doi: 10.1073/pnas.012598599
Jokinen, M. J., Olkkola, K. T., Ahonen, J., and Neuvonen, P. J. (2001). Effect of rifampin and tobacco smoking on the pharmacokinetics of ropivacaine. Clin. Pharmacol. Ther. 70, 344–350. doi: 10.1016/S0009-9236(01)29243-1
Karama, S., Ducharme, S., Corley, J., Chouinard-Decorte, F., Starr, J. M., Wardlaw, J. M., et al. (2015). Cigarette smoking and thinning of the brain’s cortex. Mol. Psychiatry 20, 778–785. doi: 10.1038/mp.2014.187
Kim, J. H. (2019). Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 72, 558–569. doi: 10.4097/kja.19087
Kwong, K., Wu, Z. X., Kashon, M. L., Krajnak, K. M., Wise, P. M., and Lee, L. Y. (2001). Chronic smoking enhances tachykinin synthesis and airway responsiveness in guinea pigs. Am. J. Respir. Cell. Mol. Biol. 25, 299–305. doi: 10.1165/ajrcmb.25.3.4557
Lee, S. J., Ralston, H. J., Drey, E. A., Partridge, J. C., and Rosen, M. A. (2005). Fetal pain: a systematic multidisciplinary review of the evidence. JAMA 294, 947–954. doi: 10.1001/jama.294.8.947
Lespasio, M. J., Guarino, A. J., Sodhi, N., and Mont, M. A. (2019). Pain management associated with total joint arthroplasty: a primer. Perm. J. 23, 18–169. doi: 10.7812/TPP/18-169
Li, W. W., Guo, T. Z., Liang, D. Y., Sun, Y., Kingery, W. S., and Clark, J. D. (2012). Substance P signaling controls mast cell activation, degranulation and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 116, 882–895. doi: 10.1097/ALN.0b013e31824bb303
Loh, H. H., Tseng, L. F., Wei, E., and Li, C. H. (1976). beta-endorphin is a potent analgesic agent. Proc. Natl. Acad. Sci. U S A 73, 2895–2898. doi: 10.1073/pnas.73.8.2895
Lu, X., Geng, X., Zhang, L., Zeng, Y., Dong, H., and Yu, H. (2009). Substance P expression in the distal cerebrospinal fluid-contacting neurons and spinal trigeminal nucleus in formalin-induced the orofacial inflammatory pain in rats. Brain Res. Bull. 78, 139–144. doi: 10.1016/j.brainresbull.2008.11.011
Mantyh, P. W., Rogers, S. D., Honore, P., Allen, B. J., Ghilardi, J. R., Li, J., et al. (1997). Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278, 275–279. doi: 10.1126/science.278.5336.275
Palve, S. S., and Palve, S. B. (2018). Impact of aging on nerve conduction velocities and late responses in healthy individuals. J. Neurosci. Rural Pract. 9, 112–116. doi: 10.4103/jnrp.jnrp_323_17
Pang, J., Tringale, K. R., Tapia, V. J., Moss, W. J., May, M. E., Furnish, T., et al. (2017). Chronic opioid use following surgery for oral cavity cancer. JAMA Otolaryngol. Head Neck Surg. 143, 1187–1194. doi: 10.1001/jamaoto.2017.0582
Papaziogas, B., Argiriadou, H., Papagiannopoulou, P., Pavlidis, T., Georgiou, M., Sfyra, E., et al. (2001). Preincisional intravenous low-dose ketamine and local infiltration with ropivacaine reduces postoperative pain after laparoscopic cholecystectomy. Surg. Endosc. 15, 1030–1033. doi: 10.1007/s004640090124
Pomerleau, O. F. (1992). Nicotine and the central nervous system: biobehavioral effects of cigarette smoking. Am. J. Med. 93, 2S–7S. doi: 10.1016/0002-9343(92)90619-m
Rasmussen, N. A., and Farr, L. A. (2003). Effects of morphine and time of day on pain and beta-endorphin. Biol. Res. Nurs. 5, 105–116. doi: 10.1177/1099800403257166
Richardson, J. K., Ho, S., Wolf, J., and Spiegelberg, T. (2009). The nature of the relationship between smoking and ulnar neuropathy at the elbow. Am. J. Phys. Med. Rehabil. 88, 711–718. doi: 10.1097/PHM.0b013e3181b333e6
Riley, J. L. 3rd, Cruz-Almeida, Y., Dasilva Ribeiro, M. C., Simon, C. B., Eckert, N. R., Aguirre, M., et al. (2017). Age differences in the time course and magnitude of changes in circulating neuropeptides after pain evocation in humans. J. Pain 18, 1078–1086. doi: 10.1016/j.jpain.2017.04.006
Shi, Y., Weingarten, T., Mantilla, C., Hooten, W., and Warner, D. (2010). Smoking and pain: pathophysiology and clinical implications. Anesthesiology 113, 977–992. doi: 10.1097/ALN.0b013e3181ebdaf9
Simons, C. T., Cuellar, J. M., Moore, J. A., Pinkerton, K. E., Uyeminami, D., Carstens, M. I., et al. (2005). Nicotinic receptor involvement in antinociception induced by exposure to cigarette smoke. Neurosci. Lett. 389, 71–76. doi: 10.1016/j.neulet.2005.07.025
Snijdelaar, D. G., Dirksen, R., Slappendel, R., and Crul, B. J. (2000). Substance P. Eur. J. Pain 4, 121–135. doi: 10.1053/eujp.2000.0171
Sprouse-Blum, A., Smith, G., Sugai, D., and Parsa, F. (2010). Understanding endorphins and their importance in pain management. Hawaii Med. J. 69, 70–71.
Stein, C. (1995). The control of pain in peripheral tissue by opioids. N. Engl. J. Med. 332, 1685–1690. doi: 10.1056/NEJM199506223322506
Steinmiller, C., Diederichs, C., Roehrs, T., Hyde-Nolan, M., Roth, T., and Greenwald, M. (2012). Postsurgical patient-controlled opioid self-administration is greater in hospitalized abstinent smokers than nonsmokers. J. Opioid Manag. 8, 227–235. doi: 10.5055/jom.2012.0120
Stephan, B., and Parsa, F. (2016). Avoiding opioids and their harmful side effects in the postoperative patient: exogenous opioids, endogenous endorphins, wellness, mood and their relation to postoperative pain. Hawaii J. Med. Public Health 75, 63–67.
Tonelli, L., Setti, T., Falasca, A., Martignoni, E., Torcia, E., Calcaterra, F. M., et al. (1988). Investigation on cerebrospinal fluid opioids and neurotransmitters related to spinal cord stimulation. Appl. Neurophysiol. 51, 324–332. doi: 10.1159/000099977
Veening, J. G., Gerrits, P. O., and Barendregt, H. P. (2012). Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS 9:16. doi: 10.1186/2045-8118-9-16
Walsh, M., Sloane, L., Fischer, K., Austad, S., Richardson, A., and Van Remmen, H. (2015). Use of nerve conduction velocity to assess peripheral nerve health in aging mice. J. Gerontol. A. Biol. Sci. Med. Sci. 70, 1312–1319. doi: 10.1093/gerona/glu208
Xu, J., and Xu, F. (2010). Role of neurogenic substance P in overexpression of alveolar macrophages’ neurokinin 1 receptor in mice exposed to cigarette smoke. Exp. Lung Res. 36, 243–254. doi: 10.3109/01902140903398275
Zakharchuk, N. V., Chertok, V. M., Nevzorova, V. A., and Gonchar, E. Y. (2017). Effects of chronic tobacco smoking on the distribution of tachykinin receptors in rat pial arteries. Bull. Exp. Biol. Med. 163, 313–316. doi: 10.1007/s10517-017-3792-0
Keywords: postoperative pain, cigarette smoking, cerebrospinal fluid, beta-endorphin, substance P
Citation: Wang F, Li H, Mu Q, Shan L, Kang Y, Yang S, Chang H-C, Su K-P and Liu Y (2022) Association of Acute Postoperative Pain and Cigarette Smoking With Cerebrospinal Fluid Levels of Beta-Endorphin and Substance P. Front. Mol. Neurosci. 14:755799. doi: 10.3389/fnmol.2021.755799
Received: 09 August 2021; Accepted: 16 December 2021;
Published: 10 January 2022.
Edited by:
Ildikó Rácz, University Hospital Bonn, GermanyReviewed by:
Chihiro Nozaki, Waseda University, JapanIbrahim Tuncay, Bezmiâlem Vakdotlessif Üniversitesi, Turkey
Copyright © 2022 Wang, Li, Mu, Shan, Kang, Yang, Chang, Su and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanlong Liu, YmVuamFtaW5seWxAd211LmVkdS5jbg==; Kuan-Pin Su, Y29ib2xzdUBnbWFpbC5jb20=
† These authors have contributed equally to this work
 Fan Wang
Fan Wang Hui Li3,4†
Hui Li3,4† Qingshuang Mu
Qingshuang Mu Shizhuo Yang
Shizhuo Yang Kuan-Pin Su
Kuan-Pin Su Yanlong Liu
Yanlong Liu