
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Neurosci., 19 August 2021
Sec. Molecular Signalling and Pathways
Volume 14 - 2021 | https://doi.org/10.3389/fnmol.2021.710303
This article is part of the Research TopicCell-Cell Interactions Controlling Neuronal Functionality in Health and DiseaseView all 12 articles
In the last decades, the effects of sedentary lifestyles have emerged as a critical aspect of modern society. Interestingly, recent evidence demonstrated that physical exercise plays an important role not only in maintaining peripheral health but also in the regulation of central nervous system function. Many studies have shown that physical exercise promotes the release of molecules, involved in neuronal survival, differentiation, plasticity and neurogenesis, from several peripheral organs. Thus, aerobic exercise has emerged as an intriguing tool that, on one hand, could serve as a therapeutic protocol for diseases of the nervous system, and on the other hand, could help to unravel potential molecular targets for pharmacological approaches. In the present review, we will summarize the cellular interactions that mediate the effects of physical exercise on brain health, starting from the factors released in myocytes during muscle contraction to the cellular pathways that regulate higher cognitive functions, in both health and disease.
Among the number of different activities that shape lifestyle, physical exercise is known to elicit various beneficial consequences on health.
At systemic level, it has been shown to exert a positive outcome in different systems: it prevents cardiovascular diseases and osteoporosis, improves glucose metabolism, and decreases the risk of developing cancer (Warburton et al., 2006). Moreover, the remarkable impact of voluntary physical activity on glucose metabolism exerts relevant beneficial effects in the insulin-resistant state (James et al., 1984) and in preventing type 2 diabetes in high-risk individuals (Pan et al., 1997; Kelley and Goodpaster, 1999).
Furthermore, physical exercise induces a wide range of beneficial effects on brain health at different levels: regardless of the specific type of aerobic activity, it reduces anxiety, depression (Sharma et al., 2006), and negative mood (Callaghan, 2004). At the cognitive level, a relationship between aerobic capacity, hippocampal plasticity and memory is widely recognised and accepted (Duzel et al., 2016), and physical exercise improves and maintains cognition in older individuals (Duzel et al., 2016). Studies conducted in animal models have shown that running modulates neurotransmitters, neurotrophin level, neuronal morphology and adult neurogenesis (Voss et al., 2013). Interestingly, running has also been shown to reinstate juvenile-like plasticity in the visual cortex of adult rats through a reduction of the intracortical inhibitory tone (Baroncelli et al., 2012). Very recently, this approach has been successfully applied to restore plasticity also in adult human subjects (Lunghi and Sale, 2015).
It is worth noting that while moderate physical activity is considered a safe practice – World Health Organization suggests 150–300 min of weekly moderate aerobic exercise for adults [World Health Organization (WHO), 2020], the effects of higher intensities on brain physiology are still controversial. Some studies reported that high-dose exercise induces higher increase of neurotrophins than moderate exercise (Marquez et al., 2015); on the other hand, it has also been reported that the exaggerated oxidative stress exerted by exhausting exercise could impair cognitive functionalities in mice (Rosa et al., 2007). In order to avoid possible misunderstandings, all the works here reported deal about moderate aerobic exercise.
Despite the ever-increasing number of studies investigating the effects of physical activity on the brain, the peripheral mechanisms that drive these beneficial events remain unclear. In particular, very little is known about the physiological processes involved in the translation of general muscle activation into the enhancement of brain molecular pathways involved in neural plasticity.
Given the complexity of the cellular and biochemical framework that underlies physical exercise’s output on brain physiology, a reductionistic approach to this issue is not possible and the present work is not aimed to suggest the pivotal role of a particular pathway or cellular network over others; it is rather conceived as a map that untangles some of the numerous ropes that span from muscular activation to brain health. Thus, this review focuses on the cellular interactions that regulate brain physiology in response to physical exercise in health and disease.
The journey from muscle contractions to neuronal modulation is assembled by numerous routes: the molecules released by peripheral organs are regulated through the somatotropic axis with feedback and feedforward mechanisms, that eventually contribute to environment perception and cognitive functionalities.
In response to physical exercise, several peripheral organs release a plethora of molecular factors in the bloodstream. Once in the circulatory system, some of these factors can cross the blood-brain barrier (BBB) and enter the brain where they affect the neuronal activity of CNS cells.
Skeletal muscle is one of the major sources of these exercise-induced circulating factors; during prolonged muscle contraction, myocytes produce, and secrete small proteins called myokines that act on various other organs including the heart, liver, pancreas, and the brain (Schnyder and Handschin, 2015). By definition, myokines are cytokines released by skeletal muscles, exerting both an autocrine control on muscle metabolism and a paracrine or endocrine regulation on other body parts (Pedersen et al., 2003). Among them, irisin, a recently discovered myokine (Boström et al., 2012), is getting increasing attention. In skeletal muscle, prolonged physical exercise activates the peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) through the 5′ AMP-activated protein kinase (AMPK). In turn, PGC-1α increases the blood concentration of irisin controlling the expression of fibronectin type III domain-containing protein 5 (FNDC5), the transmembrane protein from which irisin is cleaved. In the brain, this myokine exerts an antidepressant-like effect (Wang and Pan, 2016; Siteneski et al., 2018) and confers neuroprotection in animal models (Xia et al., 2017; Noda et al., 2018; Lourenco et al., 2019). Similarly, physical exercise increases the level of cathepsin B that has anti-amyloidogenic and neuroprotective functions (Mueller-Steiner et al., 2006) and whose expression is required for functional spatial memory, proper mood-related behavior, and adult neurogenesis (Moon et al., 2016).
Myocytes are not the only peripheral cell type modulating neuronal activity during locomotion; indeed, hepatocytes produce and secrete molecular factors in response to vigorous exercise as well. The insulin-like growth factor 1 is the earliest identified peripheral factor that mediates the interaction between peripheral and brain cells following physical activity (Carro et al., 2000). IGF-1 belongs to the insulin superfamily, a large group of peptides sharing a highly conserved structural motif consisting of two peptide chains linked by three disulfide bonds (Shabanpoor et al., 2009). Insulin and insulin-like growth factors (IGF-1 and IGF-2) are chiefly known for their role in controlling glucose metabolism peripherally, but they also have various functions in the CNS. In the developing brain, IGF-1 is locally expressed by all cell types; this peptide is indeed necessary for cell proliferation, survival, and differentiation as well as for proper axon guidance and synaptogenesis during CNS formation (Beck et al., 1995; O’Kusky et al., 2000; Cheng et al., 2001; Hodge et al., 2007; Hurtado-Chong et al., 2009; Croci et al., 2011; Fernandez and Torres-Alemán, 2012; for a review). However, the local synthesis of this peptide dramatically drops postnatally and, though IGF-1 is produced by all adult tissues, hepatocytes are the main source of this trophic factor in adulthood (Bartlett et al., 1991; García-Segura et al., 1991; Bondy et al., 1992).
The hepatic expression of IGF-1 is regulated by the growth hormone (GH) synthesized by the somatotropic cells in the pituitary gland (Hartman et al., 1993). Notably, the serum concentration of GH is increased in running trained subjects (Sutton and Lazarus, 1976; Hartman et al., 1993; Kraemer et al., 1993). When released into the serum, IGF-1 is bound by the IGF-binding proteins (IGFBPs), a group of six proteins that modulate the bioavailability of circulating IGF-1 (Allard and Duan, 2018) and transport it to the brain.
Stimulating the release of peripheral factors, physical exercise has a profound influence on both the developing and the adult CNS. To date, the mechanisms by which these messengers modify neuronal activity are incompletely understood; however, accumulating evidence suggests that they may ultimately promote epigenetic changes on the promoters of Brain-derived neurotrophic factor (BDNF) (Gomez-Pinilla et al., 2011), which is a master regulator of neural function (Kowiański et al., 2018). Consistent with this, a recent study (Sleiman et al., 2016) proved that β-hydroxybutyrate, one of the factors secreted by the liver during physical exercise, can increase the expression of this neurotrophin inactivating the histone deacetylases (HDACs), which are a class of enzymes known to decrease BDNF synthesis (Koppel and Timmusk, 2013).
The neural microenvironment is isolated from plasma by several selective membranes, including the BBB and the blood-cerebrospinal fluid (CSF) barrier (BCSFB). BBB supplies the brain with essential nutrients and mediates the efflux of many waste products (Begley and Brightman, 2003). It plays an important role in the homeostatic regulation of the brain microenvironment necessary for the stable and coordinated activity of neurons (Cserr and Bundgaard, 1984), limits small molecule permeation, regulates large molecular traffic, and also separates peripheral and central neurotransmitters pools (Abbott, 2004). BBB is formed by brain endothelial cells lining the cerebral microvasculature, but neurons, astrocytes, pericytes, extracellular matrix and microvessels are organized into well-structured neurovascular units (Hawkins and Davis, 2005), which are involved in the regulation of cerebral blood flow (Iadecola, 2004). However, the main structures responsible for the barrier properties of the BBB are tight junctions (TJs) (Reese and Karnovsky, 1967), that seal the intercellular cleft between one cell and another.
Blood-brain barrier is not the only barrier dividing the CNS from the periphery, a second interface is formed by the epithelial cells of the choroid plexus (CP) (Wolburg and Paulus, 2010). With the aim of separating blood and CSF compartments, the epithelial cells of the CP and the tanycytes of circumventricular organs (CVO) constitute the BCSFB (Segal, 2000). Similar to BBB, TJs between the CP epithelial cells inhibit paracellular diffusion of hydrophilic substances (Engelhardt and Sorokin, 2009). Compared to BBB, this barrier is leakier (Wolburg and Lippoldt, 2002), but this does not reflect an increase in the bioavailability of substances in the deep brain parenchyma (Spencer et al., 2020). Besides their barrier function, CP epithelial cells are involved in the supply and distribution of peptides into the brain, in the removal of toxic metabolites, in the excretion of xenobiotics (Engelhardt and Sorokin, 2009; Weiss et al., 2009), and in the production of CSF through free access to the blood compartment of the leaky blood vessels (Brown et al., 2004).
TJs act as a “physical barrier” (Abbott et al., 2006, 2010) that precludes the entry into the CNS of a whole series of molecules necessary for its functioning. Only small gaseous molecules, such as O2 and CO2, and a wide range of lipid-soluble molecules can passively diffuse across the lipid membranes of the epithelial cells (Liu et al., 2004). Most polar molecules cannot diffuse through the BBB thus, all endothelial cells express a large number of solute carrier (SLC) proteins in the membrane (Zhang et al., 2002) that mediate the influx and efflux of these molecules. Carrier-mediated transport (CMT) allows the entry of essential nutrients (i.e., glucose) into the brain and the elimination of metabolic waste.
Large molecules such as peptides and proteins also require a transport mechanism to cross the BBB: they can pass through the endothelial cell membranes via the specific receptor-mediated transcytosis (RMT), or by the less specific adsorptive-mediated transcytosis (AMT) (Pardridge, 2003; Wolka et al., 2003; Abbott et al., 2006). In particular, IGF-1 and IGF-2, undergo RMT across the BBB via separate type 1 and type 2 IGF receptors (IGF1R and IGF2R) (Pardridge, 2007). In the circulatory system, the great majority of serum IGF-1 forms a 150 kDa complex along with the acid-labile subunit (ALS) and the IGFBP3 (Delafontaine, 1995), i.e., the IGFBP with the highest affinity to IGF-1. Blood released IGF-1 can then enter the CNS via two distinct gateways: it can enter the CSF through the CP or the brain parenchyma through the BBB (Carro et al., 2005; Nishijima et al., 2010). These two structures are indeed well suited to transport serum IGF-1: the CP epithelium, the end-feet glial cells and the brain vessels in the BBB express high amounts of IGF-1Rs (García-Segura et al., 1991; Marks et al., 1991). The CSF concentration of IGF-1 linearly depends on the systemic level of this trophic factor; an observation suggesting that IGF-1 is constitutively transported into the CSF through the CP (Carro et al., 2000, 2005). This direct passage of IGF-1 into the CSF occurs through a mechanism involving the multiligand receptor megalin expressed in the CP epithelium (Carro et al., 2005). In parallel, serum IGF-1 is carried in the brain parenchyma via an activity-dependent mechanism; the entrance of IGF-1 through the BBB is guided by a process initiated by glutamate release at active brain regions (Nishijima et al., 2010). The release of glutamate by active synapses leads to Ca2+ influx in astrocytes with the subsequent release of prostaglandin E2, arachidonic acid derivatives, NO and ATP. These diffusible mediators induce local vasodilation increasing the BBB permeability to serum IGF-1, oxygen, and glucose. Furthermore, several of these same mediators activate the matrix metalloproteinase 9 (MMP9) that cleaving IGFBP3 releases bioavailable IGF-1. The MMP9 activity, therefore, raises the amount of serum IGF-1 able to bind the IGF-1Rs, which are locally expressed by the BBB (Nishijima et al., 2010). Crossed the BBB, IGF-1 can directly interact with active neurons or can be indirectly transported to active neurons by the end-feet glial cells. Upon entry in the adult brain, IGF-1 controls cellular homeostasis, modulates synaptic plasticity, promotes adult neurogenesis, and counteracts neurodegeneration (Fernandez and Torres-Alemán, 2012).
A great number of CNS pathologies (including brain injury, stroke, multiple sclerosis, epilepsy, Parkinson’s disease, and Alzheimer’s disease) cause TJ dysfunctions (Förster, 2008) that, increasing BBB permeability, leading to the entry of neurotoxic substances into the brain. Lifestyle and bad eating habits also influence the functioning and integrity of the BBB. Metabolic syndrome, insulin resistance, type 2 diabetes, arterial hypertension, dyslipidaemia, and obesity cause low-grade inflammation due to increased blood levels of pro-inflammatory cytokines including Interleukin-1β,-6 (IL-1β,-6) and tumour necrosis factor-α (TNF-α) and the CNS response to inflammation could lead to endothelial cell damage which increases BBB permeability (Kim et al., 2012). It has been shown that moderate physical exercise can maintain endothelial health (D’Alessio, 2004; Middlebrooke et al., 2005). Furthermore, exercise induces a reduction in C-reactive protein (CRP), IL-1, IL-6, interferon-γ (INF-γ) levels in coronary artery disease patients (Goldhammer et al., 2005), demonstrating its anti-inflammatory effects. A reduction of TNF-α and IL-6 blood levels has been observed also in a group of healthy elderly women after 14 weeks of exercise (Chupel et al., 2018). Diabetes mellitus and obesity are frequently associated with oxidative stress (Houstis et al., 2006) that refers to elevated intracellular levels of reactive oxygen species (Schieber and Chandel, 2014) which produce a loss in TJs integrity (Baeten and Akassoglou, 2011) causing a direct damage on BBB. In animal model of obese type 2 diabetes regular and moderate physical exercise reduce oxidative stress (Teixeira-Lemos et al., 2011). Therefore, counteracting obesity, physical exercise indirectly protects the BBB. In short, physical exercise seems to be an important therapeutic resource that protects and repairs the BBB which in turn maintains homeostasis in the cerebral microenvironment.
The impact exerted on the CNS by exercise has been extensively studied in the hippocampus, a brain region embedded in the medial temporal lobe with a major role in learning and memory (Bird and Burgess, 2008).
In human subjects, moderate-intensity physical activity was correlated to increased hippocampal size, hippocampal blood flow, and memory (Erickson et al., 2011; Steventon et al., 2020). Likewise, exercise has been shown to improve spatial navigation (Ang et al., 2006; Aguiar et al., 2011), object recognition (Bechara and Kelly, 2013) and contextual fear memory (Greenwood et al., 2009) in rodents. These memory improvements appear to be mediated by the genesis of new CNS cells in the subgranular zone (SGZ), a stem-cell niche that lies between the hilus and the granule cell layer in the hippocampal dentate gyrus (Gonçalves et al., 2016). Physical exercise indeed promotes adult neurogenesis and newborn cell survival (Van Praag et al., 1999a,b) through a mechanism that could be mediated by BDNF expression (Liu and Nusslock, 2018). In fact, growing evidence supports the notion that BDNF is required for proper neuronal differentiation and survival in adulthood (Lee et al., 2002; Scharfman et al., 2005; Chan et al., 2008; Waterhouse et al., 2012). Moreover, this enhancement of neurogenesis is an exclusive process to the SGZ; physical exercise influences the production of new cells in the hippocampal formation but not in the subventricular zone, which is one of the two major stem-cell niches in the adult brain along with the SGZ (Brown et al., 2003).
Hippocampal neurogenesis is intimately connected with angiogenesis. Newborn cells cluster in striking proximity to blood vessels (Palmer et al., 2000) that are integral constituents of stem-cell niches since the vasculature serves not only as a conduit for nutrients, but also conveys signaling molecules that modulate stem-cell differentiation (Licht and Keshet, 2015). Physical exercise shapes the neurovascular interface augmenting the vascular density in the granular layer of the dentate gyrus. Vascular endothelial growth factor (VEGF) originating from outside the BBB represents an essential player in this angiogenic response to exercise. Following acute muscle contractions, skeletal myofibers release VEGF in circulation (Fabel et al., 2003; Rich et al., 2017). Once in the cortex, VEGF stimulates both the proliferation of neural cell precursors and the perfusion of blood capillaries in the SGZ (Jin et al., 2002; Fabel et al., 2003; van Praag et al., 2005; Ding et al., 2006; Clark et al., 2009; Rich et al., 2017). In agreement with this hypothesis, the peripheral injection of a VEGF antagonist abolishes exercise-induced SGZ neurogenesis but is ineffective in suppressing baseline neurogenesis (Fabel et al., 2003). However, according to recent lines of research, the local VEGF production by CNS resident cells might itself instruct the SGZ neurovascular interface. During physical exercise, hypoxic conditions favour the pyruvate conversion into L-lactate in skeletal muscles. This metabolite is then secreted in the bloodstream and transported in the CNS through the monocarboxylate carriers (Proia et al., 2016). In the dentate gyrus, L-lactate increases local VEGF release activating the lactate receptor – hydroxycarboxylic acid receptor 1 – (HCAR1) expressed by the perivascular pial and pericyte-like cells (Morland et al., 2017).
By promoting the genesis of granular cells, physical exercise modulates synaptic plasticity in the hippocampus. Adult newborn cells acquire the properties of completely mature granule cells and are functionally integrated into the hippocampal network by 4 weeks (Van Praag et al., 2002). During this process, newborn cells exhibit a narrow time window of enhanced plasticity that depends on the transient expression of the NR2B subunits of N-methyl-D-aspartate receptors (NMDAR) early (4–6 weeks) post-mitosis (Snyder et al., 2001; Ge et al., 2007). Long-term potentiation (LTP) is consistently enhanced in hippocampal slices of running mice as compared to sedentary animals. This increase in synaptic plasticity is tightly connected with running-induce SGZ neurogenesis; changes in LTP properties can be detected only in the dentate gyrus, where new granule cells born, and not in other hippocampal regions (Van Praag et al., 1999b; Farmer et al., 2004). Furthermore, adult-born cells modify not only the functional properties of the dentate gyrus but also rewire the hippocampal circuitry. Indeed, physical exercise reshapes the local innervation to newborn neurons and increases the distal projections coming to these cells from subcortical and cortical regions (Vivar et al., 2016; Sah et al., 2017).
Cortical areas are the headquarters of higher cognitive functions that allow us to respond adaptively to a constantly changing environment, for this reason these areas are highly influenced by physical activity, which represents a way to interact with the environment. Exercise increases cortical thickness in older individuals (Colcombe et al., 2006) and modulates neuronal protein expression via epigenetic regulation (Abel and Rissman, 2013; Kashimoto et al., 2016). Interestingly, exercise protects microglial cells from age-dependent loss of functionality, a fact that, in turn, improves cognitive functions in elderly subjects (Mela et al., 2020).
It is worth noticing that, locomotion is also able to directly modulate specific cortical areas: from an evolutionary point of view, the integration of motor coordination and sensory information -visual, auditory, and tactile (Lee et al., 2013; Dipoppa et al., 2018; Ayaz et al., 2019; Yavorska and Wehr, 2021) – is a process that is necessary for fundamental skills required for surviving such as foraging and threats escape.
The visual cortex represents, since the 1960s, the prime model to study the complex interaction between external cues – such as physical exercise – and cellular reorganization of neural circuits. The proper maturation of the visual system is a phenomenon strictly dictated by the mutual interaction between genetic programs and plasticity processes driven by environmental experience. Postnatal exposure to an enriched environment, a condition of enhanced physical exercise and sensory stimulation, conspicuously accelerates visual system development (Sale et al., 2004). A number of data now support the hypothesis that this acceleration is partly elicited by the increased amount of serum IGF-1 in enriched animals, which experience high levels of motor stimulation. Such hypothesis is supported by experiments showing that the exogenous supply of JB1 (an IGF-1R antagonist) or anti-IGF-I antiserum completely prevents this acceleration (Ciucci et al., 2007; Landi et al., 2009). The faster maturation of the visual system might be caused by the IGF-1 interaction with different pathways. Indeed, the increased presence of IGF-1 enhances BDNF expression, stimulates retinal development, promotes a precocious maturation of the GABAergic interneurons and a precocious decrease in the NKCC/KCC2 ratio (Sale et al., 2007; Baroncelli et al., 2017). Interfering with all these pathways, IGF-1 can eventually prompt the premature visual system development observed at behavioral, electrophysiological, and molecular level.
Number of studies are needed to evaluate whether other exercise-related molecules extend their action to the visual system. Nevertheless, a number of researches provided evidence that physical exercise acts on visual neurons promoting the release of different neuromodulators in the primary visual cortex; in particular, locomotion increases the amount of serotonin and acetylcholine (Jacobs and Fornal, 1999). In the CNS, serotonin is almost exclusively released by the serotoninergic neurons located in the raphe nuclei, which are a cluster of nuclei in the brain stem (Hornung, 2003). Neurotransmission of serotonin is involved in structural and functional remodeling of visual cortical circuits and is considered one of the non-visual neurochemical bases of attention, arousal, and motivation (Gu, 2007). Serotoninergic neurons produce serotonin through a process that is limited by the plasma level of tryptophan, a large neutral amino acid from which serotonin is synthesized (Russo et al., 2007; Höglund et al., 2019). Tryptophan is transported through the BBB via a transporter carrier shared by all the large neutral amino acids; therefore, tryptophan’s entry in the CNS does not depend on its plasma concentration per se, but on its plasma concentration with respect to other large neutral amino acids since they all compete for the same transporter (Fernstrom, 2005; Markus, 2008; Höglund et al., 2019). During physical exercise, the branched-chain amino acids – a subset of the large neutral amino acids – are transported into the muscles (Patrick and Ames, 2015). Consequently, physical exercise increases tryptophan transport into the brain decreasing the competition for the BBB transporter.
On the other hand, the effect of acetylcholine in shaping the circuitry of the visual cortex has been extensively studied in the last few years (Sugihara et al., 2016). The primary visual cortex receives direct input from the nucleus of the diagonal band of Broca, a cholinergic centre in the basal forebrain (Fu et al., 2014), specifically from the mesencephalic locomotor region (MLR), a midbrain region associated with the ascending reticular activating system described by Moruzzi and Magoun (1949). During locomotion, the enhanced activity of the MLR increases visual cortical responsiveness via a mechanism involving the vasoactive intestinal peptide (VIP) and the somatostatin (SST) positive cells, which are two of the major classes of inhibitory interneurons. To dissect this cellular mechanism, Fu et al. (2014) imaged calcium responses of these interneurons in awake mice free to run on a treadmill. They found that neural activity of VIP neurons is significantly increased during locomotion even in the absence of visual stimulation; SST neurons were instead inhibited by locomotion. These observations suggest the involvement of a circuit in which VIP neurons trigger the inhibition of SST neurons. Therefore, the activation of VIP cells boosts cortical excitation releasing pyramidal cells from the inhibition exerted by SST neurons, a mechanism termed disinhibition. VIP interneurons are activated by the basal forebrain through nicotinic acetylcholine receptors (nAChRs) (Alitto and Dan, 2012). Consistently, local infusion of nAChRs antagonists greatly reduces the response of VIP cells to locomotion, pinpointing acetylcholine as a crucial mediator of physical exercise in the primary visual cortex (Fu et al., 2014).
Physical exercise has a marked influence on several physiological processes; indeed, general myocyte contraction can, indirectly, control adult neurogenesis, cortical excitability, and the release of different neuromodulators such as BDNF. Given its multifactorial action, exercise, and its downstream cellular pathways, could represent potential targets for preventing or reversing the symptoms of those pathologies in which neuroprotection, plasticity and transmission are defective (Figure 1). In the following section, we describe amblyopia, Down syndrome, neurodegenerative diseases, and glioma as explicative cases.
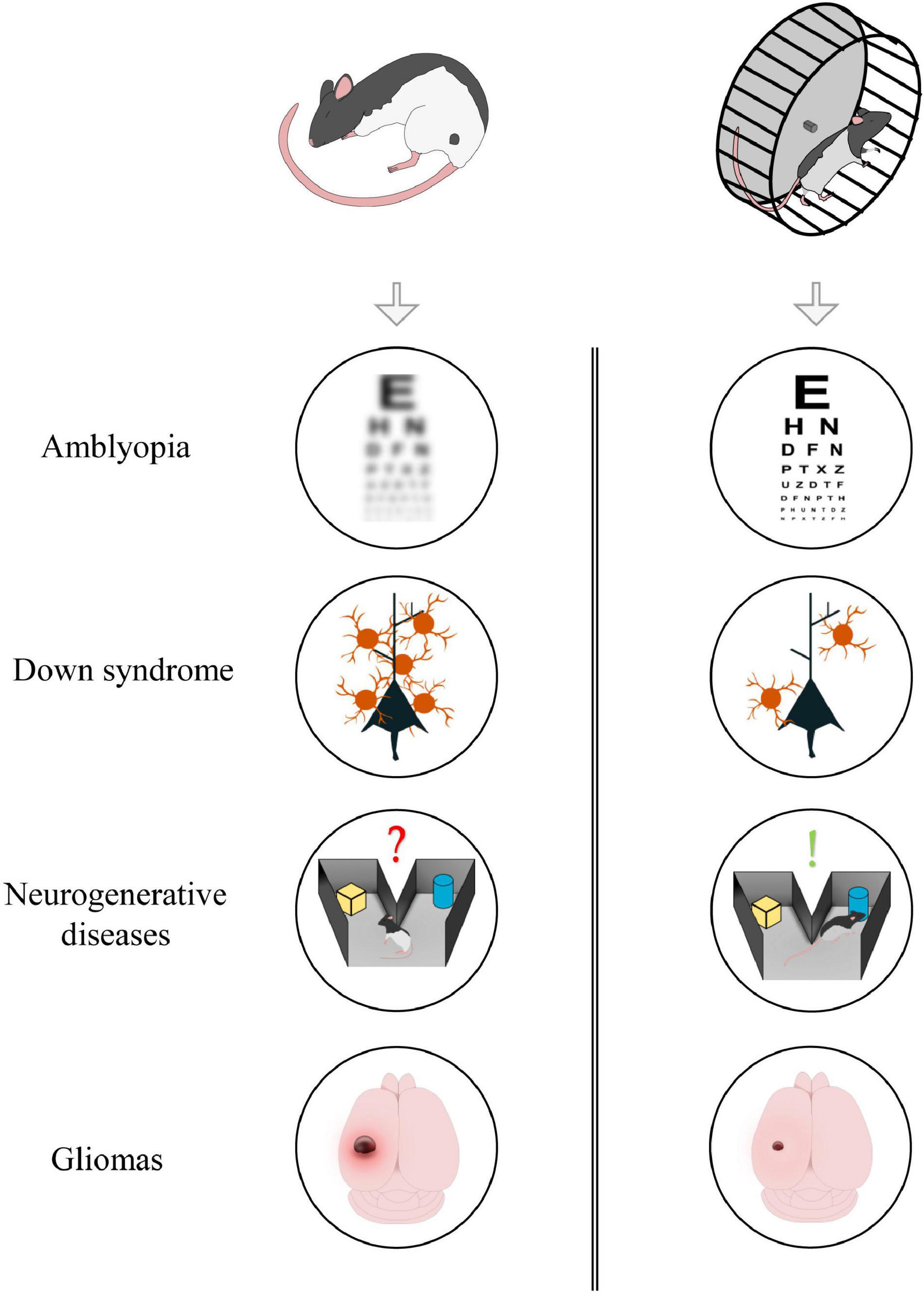
Figure 1. Aerobic exercise could exert beneficial effects in different forms of neuropathology. From top to bottom: exercise induces visual recovery in adult amblyopic subjects; it reduces GABAergic innervation in animal model of Down Syndrome; it improves memory formation and consolidation in neurodegenerative diseases; it contributes in reducing tumoral mass in glioma-bearing mice.
Amblyopia, with an incidence of 1–5%, is the prevalent monocular impairment in the world human population (Holmes and Clarke, 2006). The central physiological mechanism in amblyopia is considered inter-ocular suppression, which is the empowering of the inputs coming from the apparently healthy eye at the expense of those coming from the amblyopic eye (Antonini et al., 1999). This suppression is evident at the level of the primary visual cortex and appears to be mediated by the inhibitory circuitry (Sengpiel et al., 2005).
Amblyopia, if not promptly treated during childhood, leads to severe visual impairments including loss of visual acuity and defective stereopsis. Occlusion therapy, i.e., the temporary exclusion of the healthy eye from visual activity by means of an eye patch, completely reverses amblyopia when performed during the critical period for binocular vision (Loudon and Simonsz, 2005). Nevertheless, amblyopia is an almost untreatable disease in adulthood; the occlusion therapy is indeed completely ineffective in adult subjects due to the dramatic decline in cortical plasticity caused by the maturation of different plasticity-limiting factors as the GABAergic and the cholinergic systems (Fagiolini and Hensch, 2000; Morishita et al., 2010).
Amblyopia can be artificially induced in animal models depriving one eye of visual stimuli through a long-term lid suture. Several studies have proved physical exercise as an effective strategy to treat amblyopia in adult rodents. Employing adult amblyopic rats, it has been recently shown that voluntary physical exercise under binocular conditions promotes a full and long-lasting recovery of both visual acuity and depth perception (Sansevero et al., 2020). Moreover, physical exercise can also promote amblyopia recovery in humans; indeed, it has been shown that a short-term occlusion of the amblyopic eye coupled with moderate physical activity improve visual acuity and stereo-sensitivity in adult patients (Lunghi et al., 2019). Notably, the exercise-induced factors may be directly involved in promoting the beneficial effects exerted by physical exercise; pharmacological interventions enhancing IGF-1, serotonin or serotonin transporters can counteract visual impairments in adult amblyopic rodents even in the absence of intense exercise (Vetencourt et al., 2008; Maya Vetencourt et al., 2011; Maya-Vetencourt et al., 2012). However, a thorough analysis is still needed to understand whether these molecular factors promote amblyopia recovery increasing BDNF expression. BDNF itself, indeed, can be successful in eliciting visual function recovery in adult animals (Sansevero et al., 2019).
It is worth noting that short-distance cellular interactions also play a crucial role in promoting amblyopia recovery in physical active animals. Recent studies have indeed demonstrated that activation of the VIP-SST disinhibitory circuit promoted, during locomotion, by cholinergic afferents originating from the mesencephalic locomotor region, induces a complete recovery of responsiveness in both excitatory and inhibitory neurons in the primary visual cortex (Kaneko and Stryker, 2014; Sansevero et al., 2020). In this scenario, physical exercise promotes amblyopia recovery releasing the excitatory neurons from excessive levels of inhibition exerted by SST interneurons.
Based on the above-reported studies, a complex and interactive model could be postulated in which both long- and short-range interactions contribute to visual function recovery in running amblyopic animals; with long-distance cellular interaction controlling the expression of pro-plastic genes – like bdnf – and short-distance cellular interaction increasing cortical responsiveness through the release of pyramidal neurons from the local inhibition.
Down syndrome (DS), a developmental disorder elicited by the partial or total triplication of the chromosome 21 (Hsa21), is the most common genetic cause of intellectual disability in humans, with an incidence ranging from 1 in 700 to 1 in 1000 live births (Roizen and Patterson, 2003). People with DS exhibit major cognitive deficits in learning and memory along with a number of moderate to severe impairments in motor function, language, and sensory processing (Contestabile et al., 2010; Dierssen, 2012).
The last two decades have brought the development of several mouse models with DS-related features (Gupta et al., 2016; Herault et al., 2017). To date, Ts65Dn is one of the most commonly used and best-studied model of DS. Ts65Dn is characterized by the triplication of the distal segment of Mm16, the mouse chromosome harbouring the largest syntenic region of homology to Hsa21 (Davisson et al., 1990; Duchon et al., 2011). Ts65Dn mice recapitulate the main hallmarks of DS phenotypes. Ts65Dn indeed displays impairments in learning and memory (Hunter et al., 2003), motor dysfunction (Costa et al., 1999), visual deficits (Scott-McKean et al., 2010) and several anatomical alterations including craniofacial dysmorphology (Richtsmeier et al., 2002), hippocampal hypocellularity (Guidi et al., 2008), and impaired neurogenesis (Lorenzi and Reeves, 2006). The Ts65Dn phenotype correlates with major functional deficits in synaptic plasticity, particularly in the hippocampus where the possibility to induce LTP is drastically compromised (Siarey et al., 1997, 1999). This LTP failure is largely determined by alterations in the GABAergic system (Kleschevnikov et al., 2004; Costa and Grybko, 2005). Indeed, marked morphological and functional changes have been detected in the GABAergic circuitries of both the cerebral cortex and the hippocampus of Ts65Dn mice (Belichenko et al., 2009; Chakrabarti et al., 2010; Best et al., 2012; Martin et al., 2020). Therefore, this large body of evidence has now led to the notion that the excessive level of cortical inhibition is the major functional impairment in this DS model (Best et al., 2007). Accordingly, pharmacological interventions targeting the GABAergic system can completely reverse the defects displayed by Ts65Dn mice (Fernandez et al., 2007), confirming the prime role of overinhibition in DS pathogenesis (Baroncelli et al., 2011; Contestabile et al., 2017).
Physical exercise emerged as an appealing strategy to ameliorate Ts65Dn mice conditions, notably for its role in restoring the proper excitatory/inhibitory balance in the CNS (Sale et al., 2014); an effect largely ascribed to the activation of an interaction network between specific GABAergic cell subtypes (Stryker, 2014). Complex sensory-motor stimulation, including a high level of voluntary physical exercise, can indeed restore spatial navigation, hippocampal plasticity, and brain development in Ts65Dn promoting a reduction in the GABA transmission paralleled by an increase in BDNF expression (Baroncelli et al., 2011; Begenisic et al., 2015). Strikingly, the overexpression of this neurotrophic factor has been extensively associated with reduced inhibition in the adult brain (Sale et al., 2010; Baroncelli et al., 2011). Recent studies have also shown that physical exercise per se might exert a positive effect on the DS phenotype promoting recovery of learning and cognitive performances in Ts65Dn mice (Llorens-Martín et al., 2010; Parrini et al., 2017). In human subjects with DS, exercise has been shown to improve physical fitness (Rimmer et al., 2004; Dodd and Shields, 2005; Mendonca et al., 2011). Nonetheless, little is known about the effects of physical exercise on cognitive functions. Recent studies, however, seem to suggest that exercise might also improve executive functions in DS individuals (Pape et al., 2021). Likewise, the exercise-related factors might represent promising targets for therapeutic application to DS since they could be particularly effective in reducing overinhibition by increasing the neuronal expression of BDNF. Interestingly, a recent work showed that either physical exercise or a BDNF-mimetic pharmacological intervention can rescue cognitive functions and synaptic plasticity in Ts65Dn mice (Parrini et al., 2017). Moreover, it is noteworthy to point out that pharmacological strategies based on exercise-related factors can circumvent the major throwback of BDNF administration, i.e., the impossibility for this neurotrophic factor to efficiently cross the blood-brain barrier when delivered peripherally (Nagahara and Tuszynski, 2011).
Neurodegenerative diseases (NDs) are a heterogeneous group of pathologies resulting from the progressive loss of selective neuronal types in specific CNS regions. Major NDs include Alzheimer’s disease (AD), Parkinson’s disease (PD), and dementia with Lewy bodies (DLB) (Erkkinen et al., 2018). Ageing is nowadays considered the primary risk factor for neurodegeneration; most NDs occur after the fourth decade of life and their prevalence increases with increasing age (Wyss-Coray, 2016; Hou et al., 2019).
Although the clinical symptoms are diverse depending on the core population of neurons involved in the neurodegenerative process, NDs can be broadly classified as: (1) diseases causing cognitive decline, dementia, and alterations in higher cortical functions; (2) diseases characterized by motor dysfunctions including hyperkinetic and hypokinetic movements (Kovacs, 2018). However, in spite of their heterogeneity in clinical symptoms, numerous NDs share common pathological features: deposition of misfolded proteins, oxidative stress, apoptosis, and neuroinflammation (Dugger and Dickson, 2017; Marsh, 2019).
In search of candidate molecular and cellular mechanisms underlying neurodegeneration, different mouse strains have been generated to model NDs exploiting either genetically based or pharmacologic-based strategies (Dawson et al., 2018; Fisher and Bannerman, 2019). Accumulating evidence shows that physical exercise and exercise-related factors can confer neuroprotection in mouse models of NDs. In recent years, physical exercise emerged as a promising non-invasive strategy to ameliorate neuronal loss, memory impairments, oxidative stress, neuroinflammation, and motor dysfunctions (Souza et al., 2013; Hüttenrauch et al., 2016; Do et al., 2018; Klemann et al., 2018; Palasz et al., 2019). Remarkably, exercise seems to reverse the genetic pattern of inflammation and apoptotic markers set in motion by the progression of NDs (Hüttenrauch et al., 2016; Do et al., 2018) probably through epigenetic mechanisms (Ferioli et al., 2019). A large number of studies has also analyzed the neuroprotective action of specific exercise-released factors. Lourenco et al. (2019) recently reported that irisin levels are reduced in AD mice. Moreover, these authors showed that the peripheral overexpression of irisin attenuates synaptic and memory impairments and that the blockade of either peripheral or brain irisin conversely hampers the neuroprotective effects of physical exercise in AD model. The effect of another myokine, cathepsin B, on AD is instead controversial. According to some studies, cathepsin B can exert neuroprotective actions and promote learning and memory in AD mice (Mueller-Steiner et al., 2006; Embury et al., 2017); on the contrary, other studies have associated this myokine with AD onset and amyloid plaque accumulation (Cataldo and Nixon, 1990; Hook et al., 2005). In addition to myokines, IGF-1 and β-hydroxybutyrate can mitigate the phenotype of various mouse models of NDs (Carro et al., 2006; Lim et al., 2011). IGF-1 can serve as a protective agent against neuroinflammation modulating the activation of CNS resident cells. This growth factor indeed attenuates the inflammatory response of astrocytes (Bellini et al., 2011), decreases the expression of pro-inflammatory cytokine (Park et al., 2011), and it might also repress neurotoxic microglia (Grinberg et al., 2013; Rodriguez-Perez et al., 2016).
The results obtained in mouse models of NDs encourage stronger efforts in the application of physical exercise to human patients; in particular, exercise-related factors may be promising pharmacological targets to counteract, or at least to prevent, the progression of NDs.
Gliomas (GLs) are primary brain tumours that arise from glial or precursor cells and represent approximately 25.1% of all CNS tumours and 80.8% of malignant tumours. Although the 5-year survival rate for non-malignant primary brain tumours is 91.7%, for malignant tumours this rate drops to 36% and the worst prognosis is for patients diagnosed with glioblastoma (the most malignant GLs) whose 5-year survival rate is 7.2% (Ostrom et al., 2020).
Researchers are paying more and more attention to the glioma microenvironment and to the crosstalk between glioma and peritumoral cells. Glioma cells secrete an aberrant quantity of the excitatory neurotransmitter glutamate, which leads peritumoral neurons to an excitotoxic death via hyperactivation of NMDAR and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPAR) in peritumoral neurons that in turn can make it easier for glioma to invade nearby CNS parenchyma (Ye and Sontheimer, 1999). Concurrently, the autocrine activation of NMDAR and AMPAR promotes glioma invasion (Lyons et al., 2007; Piao et al., 2009; Nandakumar et al., 2019). Another line of research explores the ability of glioma cells to create contact with neurons forming the neuron-glioma synapses (NGS). Under normal conditions, there are synaptic contacts between neurons and normal oligodendroglial precursor cells (OPCs) (Bergles and Jahr, 2000). Recently, Venkatesh et al. showed that the characteristics of NGS are similar to synapses formed between neurons and normal OPCs (Venkatesh et al., 2019). NGS, whose activity is mediated by AMPA receptors, promotes glioma progression (Venkatesh et al., 2019); indeed, AMPA antagonism reduces the glioma cell proliferation (Venkataramani et al., 2019). Furthermore, the increase in glutamate release stimulates peritumoral neurons to release BDNF and Neuroligin 3 (NLGN3) that in turn stimulate the glioma cells to proliferation and infiltration and sustained NGS formation (Venkatesh et al., 2015, 2017, 2019; Venkataramani et al., 2019).
Physical exercise might represent a safe, easy, and side-effect-free treatment for GLs. Tantillo et al. (2020) have recently examined the effect of physical exercise in mice injected with tumour cells (GL261) into the primary motor cortex. Compared to the sedentary group, the trained group showed a reduction in glioma cell proliferation and a slowdown in the emergence of motor deficits, while no difference in tumour volume emerged between the two groups (Tantillo et al., 2020). Consistent with this evidence, other studies have shown that physical exercise suppresses tumour growth and can decrease the risk of a number of cancers such as those of the colon, breast and endometrium (Booth et al., 2012). Hence, physical exercise could be used as a protective factor as well as an adjuvant treatment. Indeed, it has been shown that physical exercise, performed during the temozolomide treatment, prolongs the survival of glioma-mice (Lemke et al., 2016). Consistently, physical exercise can also ameliorate the quality of life in human patients (Cordier et al., 2019).
Exercise increases IGF-1 levels both in the periphery (Carro et al., 2001) and the brain (Carro et al., 2000) and increases the BDNF expression (Vaynman and Gomez-Pinilla, 2005) in specific brain areas. These exercise-related factors may be responsible for reducing or slowing down the progression of glioma modifying the glioma microenvironment (Garofalo et al., 2015, 2017). BDNF stimulates the production of Interleukin-15 (IL-15) in the brain of glioma-mice, and IL-15, in turn, stimulates natural kill (NK) cells to produce Interferon-gamma (IFN-γ) that affects the phenotype of myeloid cells, promoting the transition to an anti-tumour state (Garofalo et al., 2017). Aerobic respiration, which occurs in the mitochondria of eukaryotic cells, generates energy and, as a result of this oxidative metabolism, several reactive species are produced [among which reactive oxygen species (ROS) is the most abundant]. But, when the physiological balance between production and elimination of reactive species is broken, oxidative stress is generated. Oxidative stress is related to a wide variety of human diseases, including cancer (Sosa et al., 2013). Physical exercise promotes brain function, increases the resistance against oxidative stress and facilitates recovery from oxidative stress (Radak et al., 2013; Accattato et al., 2017).
Further studies are needed to better understand the molecular mechanisms and the cellular interactions underlying the efficacy of physical exercise as a therapy for GLs. Nevertheless, the obtained results suggest that physical exercise might be a valid and effective strategy, not only in slowing down the progression of glioma but also in improving the life quality of GLs patients.
We analyzed the impact that physical exercise exerts on brain activity (Figure 2). The work reviewed in this article has shown that physical exercise and brain health are tightly related; indeed, the interplay between brain and muscle activation begins with long-range communications: motoneurons activate myocytes that, in turn, release a large number of factors in the circulation that may reach the brain and regulate the somatotropic axis. On the other hand, in different brain regions, exercise promotes the activation of specific short-range cellular interaction that mediates disparate processes, encompassing from sensory integration to plastic and metabolic changes.
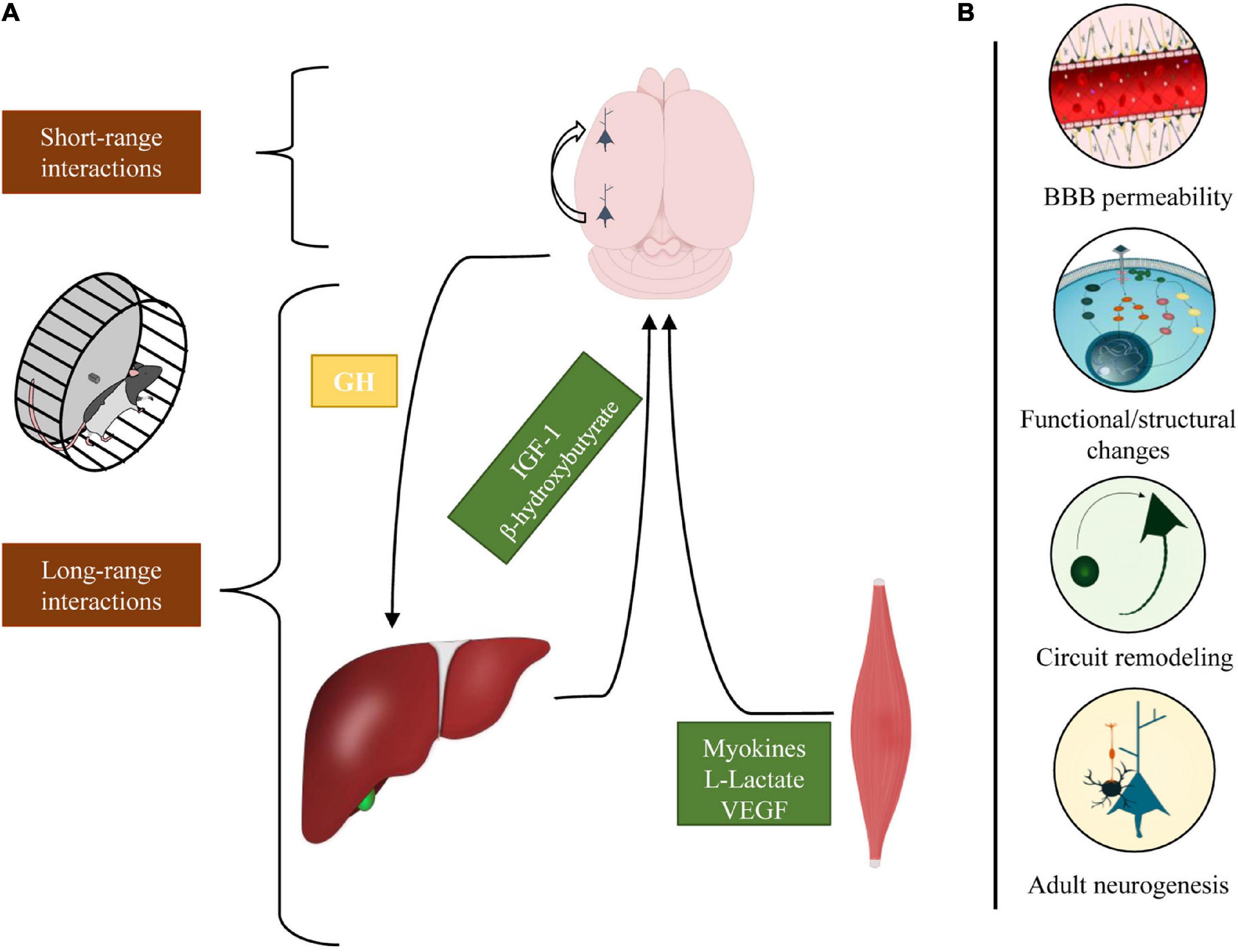
Figure 2. Schematic representation of the different cellular interactions engaged by physical exercise. (A) In response to physical exercise, multiple peripheral organs release molecular factors that modulate different neurophysiological processes. The brain, in turn, regulates the inflow of these factors through the somatotropic axis (long-range interactions). Concomitantly, exercise activates intra-cortical neuronal circuits that enhance perceptual and cognitive functions (short-range interactions). (B) Physical exercise exerts various effects on the brain modulating BBB permeability, eliciting functional/structural changes, stimulating neurogenesis, and shaping cortical circuits.
The first consideration, that should emerge from the present work, is that a complete comprehension of the mechanisms that act during exercise on the brain and vice-versa is far from being reached. We marked some boundaries of this tangled network, and we hope they could represent a helpful starting point for future research. As a consequence of that, from a research point of view, the application of multiple experimental approaches but also the commitment of scientists with different formations and perspectives is necessary to develop a field that engages many aspects of general physiology.
Finally, it is important to mention the enormous impact that lifestyle exerts on physiological and pathological brain processes. Effective treatments for most neurodegenerative and neurodevelopmental disorders are still lacking, a fact that entails a huge economic impact on healthcare systems and tremendous consequences on the life quality of families that face such conditions. Given this situation, maintaining an active life, both intellectually and physically, remains one of the few compelling strategies to prevent cognitive decline in the elderly.
GS and AC conceived the work and wrote the manuscript. ID wrote the manuscript. All authors contributed to the article and approved the submitted version.
GS was supported by Fondazione Umberto Veronesi.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbott, N. J. (2004). Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 45, 545–552. doi: 10.1016/j.neuint.2003.11.006
Abbott, N. J., Patabendige, A. A. K., Dolman, D. E. M., Yusof, S. R., and Begley, D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25. doi: 10.1016/j.nbd.2009.07.030
Abbott, N. J., Rönnbäck, L., and Hansson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. doi: 10.1038/nrn1824
Abel, J. L. B., and Rissman, E. F. (2013). Running-induced epigenetic and gene expression changes in the adolescent brain. Int. J. Dev. Neurosci. 31, 382–390. doi: 10.1016/j.ijdevneu.2012.11.002
Accattato, F., Greco, M., Pullano, S. A., Caré, I., Fiorillo, A. S., Pujia, A., et al. (2017). Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS One 12:1–13. doi: 10.1371/journal.pone.0178900
Aguiar, A. S., Castro, A. A., Moreira, E. L., Glaser, V., Santos, A. R. S., Tasca, C. I., et al. (2011). Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 132, 560–567. doi: 10.1016/j.mad.2011.09.005
Alitto, H. J., and Dan, Y. (2012). Cell-type-specific modulation of neocortical activity by basal forebrain input. Front. Syst. Neurosci. 6:79. doi: 10.3389/fnsys.2012.00079
Allard, J. B., and Duan, C. (2018). IGF-binding proteins: why do they exist and why are there so many? Front. Endocrinol. 9:117. doi: 10.3389/fendo.2018.00117
Ang, E. T., Dawe, G. S., Wong, P. T. H., Moochhala, S., and Ng, Y. K. (2006). Alterations in spatial learning and memory after forced exercise. Brain Res. 1113, 186–193. doi: 10.1016/j.brainres.2006.07.023
Antonini, A., Fagiolini, M., and Stryker, M. P. (1999). Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19, 4388–4406.
Ayaz, A., Stäuble, A., Hamada, M., Wulf, M. A., Saleem, A. B., and Helmchen, F. (2019). Layer-specific integration of locomotion and sensory information in mouse barrel cortex. Nat. Commun. 10, 1–14. doi: 10.1038/s41467-019-10564-8
Baeten, K. M., and Akassoglou, K. (2011). Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 71, 1018–1039. doi: 10.1002/dneu.20954
Baroncelli, L., Bonaccorsi, J., Milanese, M., Bonifacino, T., Giribaldi, F., Manno, I., et al. (2012). Enriched experience and recovery from amblyopia in adult rats: impact of motor, social and sensory components. Neuropharmacology 62, 2387–2396. doi: 10.1016/j.neuropharm.2012.02.010
Baroncelli, L., Braschi, C., Spolidoro, M., Begenisic, T., Maffei, L., and Sale, A. (2011). Brain Plasticity and Disease: a Matter of Inhibition. Neural. Plast. 2011, 1–11. doi: 10.1155/2011/286073
Baroncelli, L., Cenni, M. C., Melani, R., Deidda, G., Landi, S., Narducci, R., et al. (2017). Early IGF-1 primes visual cortex maturation and accelerates developmental switch between NKCC1 and KCC2 chloride transporters in enriched animals. Neuropharmacology 113, 167–177. doi: 10.1016/j.neuropharm.2016.02.034
Bartlett, W. P., Li, X. S., Williams, M., and Benkovic, S. (1991). Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev. Biol. 147, 239–250. doi: 10.1016/S0012-1606(05)80021-1
Bechara, R. G., and Kelly, ÁM. (2013). Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav. Brain Res. 245, 96–100. doi: 10.1016/j.bbr.2013.02.018
Beck, K. D., Powell-Braxtont, L., Widmer, H. R., Valverde, J., and Hefti, F. (1995). Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14, 717–730. doi: 10.1016/0896-6273(95)90216-3
Begenisic, T., Sansevero, G., Baroncelli, L., Cioni, G., and Sale, A. (2015). Early environmental therapy rescues brain development in a mouse model of Down syndrome. Neurobiol. Dis. 82, 409–419. doi: 10.1016/j.nbd.2015.07.014
Begley, D. J., and Brightman, M. W. (2003). Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. 37, 13–25. doi: 10.1007/978-3-0348-8049-7_2
Belichenko, P. V., Kleschevnikov, A. M., Masliah, E., Wu, C., Takimoto-Kimura, R., Salehi, A., et al. (2009). Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of down syndrome. J. Comp. Neurol. 512, 453–466. doi: 10.1002/cne.21895
Bellini, M. J., Hereñú, C. B., Goya, R. G., and Garcia-Segura, L. M. (2011). Insulin-like growth factor-I gene delivery to astrocytes reduces their inflammatory response to lipopolysaccharide. J. Neuroinflamm. 8:21. doi: 10.1186/1742-2094-8-21
Best, T. K., Cramer, N. P., Chakrabarti, L., Haydar, T. F., and Galdzicki, Z. (2012). Dysfunctional hippocampal inhibition in the Ts65Dn mouse model of Down syndrome. Exp. Neurol. 233, 749–757. doi: 10.1016/j.expneurol.2011.11.033
Best, T. K., Siarey, R. J., and Galdzicki, Z. (2007). Ts65Dn, a mouse model of down syndrome, exhibits increased GABA B-induced potassium current. J. Neurophysiol. 97, 892–900. doi: 10.1152/jn.00626.2006
Bird, C. M., and Burgess, N. (2008). The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 9, 182–94. doi: 10.1038/nrn2335
Bondy, C. A., Bach, M. A., and Lee, W. H. (1992). Mapping of brain insulin and insulin-like growth factor receptor gene expression by in situ hybridization. Neuroprotocols 1, 240–249. doi: 10.1016/1058-6741(92)90034-U
Booth, F. W., Roberts, C. K., and Laye, M. J. (2012). Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2, 1143–1211. doi: 10.1002/cphy.c110025
Boström, P., Wu, J., Jedrychowski, M. P., Korde, A., Ye, L., Lo, J. C., et al. (2012). A PGC1a dependent myokine that derives browning of white fat and thermogenesis. Nature 481, 463–468. doi: 10.1038/nature10777.A
Brown, J., Cooper-Kuhn, C. M., Kempermann, G., Van Praag, H., Winkler, J., Gage, F. H., et al. (2003). Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x
Brown, P. D., Davies, S. L., Speake, T., and Millar, I. D. (2004). Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129, 955–968. doi: 10.1016/j.neuroscience.2004.07.003
Callaghan, P. (2004). Exercise: a neglected intervention in mental health care? J. Psychiatr. Ment. Health Nurs. 11, 476–83. doi: 10.1111/j.1365-2850.2004.00751.x
Carro, E., Nuñez, A., Busiguina, S., and Torres-Aleman, I. (2000). Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20, 2926–2933.
Carro, E., Spuch, C., Trejo, J. L., Antequera, D., and Torres-Aleman, I. (2005). Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J. Neurosci. 25, 10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005
Carro, E., Trejo, J. L., Busiguina, S., and Torres-Aleman, I. (2001). Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 21, 5678–5684.
Carro, E., Trejo, J. L., Gerber, A., Loetscher, H., Torrado, J., Metzger, F., et al. (2006). Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol. Aging 27, 1250–1257. doi: 10.1016/j.neurobiolaging.2005.06.015
Cataldo, A. M., and Nixon, R. A. (1990). Enzymatically active lysomal proteases are associated with amyloid deposits in Alzheimer brain. Proc. Natl. Acad. Sci. U. S. A. 87, 3861–3865. doi: 10.1073/pnas.87.10.3861
Chakrabarti, L., Best, T. K., Cramer, N. P., Carney, R. S. E., Isaac, J. T. R., Galdzicki, Z., et al. (2010). Olig1 and Olig2 triplication causes developmental brain defects in Down syndrome. Nat. Neurosci. 13, 927–934. doi: 10.1038/nn.2600
Chan, J. P., Cordeira, J., Calderon, G. A., Iyer, L. K., and Rios, M. (2008). Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol. Cell. Neurosci. 39, 372–383. doi: 10.1016/j.mcn.2008.07.017
Cheng, C. M., Cohen, M., Tseng, V., and Bondy, C. A. (2001). Endogenous IGF1 enhances cell survival in the postnatal dentate gyrus. J. Neurosci. Res. 64, 341–347. doi: 10.1002/jnr.1084
Chupel, M. U., Minuzzi, L. G., Furtado, G., Santos, M. L., Hogervorst, E., Filaire, E., et al. (2018). Exercise and taurine in inflammation, cognition, and peripheral markers of blood-brain barrier integrity in older women. Appl. Physiol. Nutr. Metab. 43, 733–741. doi: 10.1139/apnm-2017-0775
Ciucci, F., Putignano, E., Baroncelli, L., Landi, S., Berardi, N., and Maffei, L. (2007). Insulin-Like Growth Factor 1 (IGF-1) Mediates the Effects of Enriched Environment (EE) on Visual Cortical Development. PLoS One 2:e475. doi: 10.1371/journal.pone.0000475
Clark, P. J., Brzezinska, W. J., Puchalski, E. K., Krone, D. A., and Rhodes, J. S. (2009). Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus 19, 937–950. doi: 10.1002/hipo.20543
Colcombe, S. J., Erickson, K. I., Scalf, P. E., Kim, J. S., Prakash, R., McAuley, E., et al. (2006). Aerobic exercise training increases brain volume in aging humans. J. Gerontol A Biol. Sci. Med. Sci. 61, 1166–1170. doi: 10.1093/gerona/61.11.1166
Contestabile, A., Benfenati, F., and Gasparini, L. (2010). Communication breaks-Down: from neurodevelopment defects to cognitive disabilities in Down syndrome. Prog. Neurobiol. 91, 1–22. doi: 10.1016/j.pneurobio.2010.01.003
Contestabile, A., Magara, S., and Cancedda, L. (2017). The GABAergic hypothesis for cognitive disabilities in down syndrome. Front. Cell. Neurosci. 11:54. doi: 10.3389/fncel.2017.00054
Cordier, D., Gerber, M., and Brand, S. (2019). Effects of two types of exercise training on psychological well - being, sleep, quality of life and physical fitness in patients with high - grade glioma (WHO III and IV): study protocol for a randomized controlled trial. Cancer Commun. 39:46. doi: 10.1186/s40880-019-0390-8
Costa, A. C. S., and Grybko, M. J. (2005). Deficits in hippocampal CA1 LTP induced by TBS but not HFS in the Ts65Dn mouse: a model of Down syndrome. Neurosci. Lett. 382, 317–322. doi: 10.1016/j.neulet.2005.03.031
Costa, A. C. S., Walsh, K., and Davisson, M. T. (1999). Motor dysfunction in a mouse model for Down syndrome. Physiol. Behav. 68, 211–220. doi: 10.1016/S0031-9384(99)00178-X
Croci, L., Barili, V., Chia, D., Massimino, L., Van Vugt, R., Masserdotti, G., et al. (2011). Local insulin-like growth factor i expression is essential for Purkinje neuron survival at birth. Cell Death Differ. 18, 48–59. doi: 10.1038/cdd.2010.78
Cserr, H. F., and Bundgaard, M. (1984). Blood-brain interfaces in vertebrates: a comparative approach. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 246, R277–88. doi: 10.1152/ajpregu.1984.246.3.r277
D’Alessio, P. (2004). Aging and the endothelium. Exp. Gerontol. 39, 165–171. doi: 10.1016/j.exger.2003.10.025
Davisson, M. T., Schmidt, C., and Akeson, E. C. (1990). Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog. Clin. Biol. Res. 360, 263–280.
Dawson, T. M., Golde, T. E., and Lagier-Tourenne, C. (2018). Animal models of neurodegenerative diseases. Nat. Neurosci. 21, 1370–1379. doi: 10.1038/s41593-018-0236-8
Delafontaine, P. (1995). Insulin-like growth factor I and its binding proteins in the cardiovascular system. Cardiovasc. Res. 30, 825–834. doi: 10.1016/S0008-6363(95)00163-8
Dierssen, M. (2012). Down syndrome: the brain in trisomic mode. Nat. Rev. Neurosci. 13, 844–858. doi: 10.1038/nrn3314
Ding, Y.-H., Li, J., Zhou, Y., Rafols, J., Clark, J., and Ding, Y. (2006). Cerebral Angiogenesis and Expression of Angiogenic Factors in Aging Rats after Exercise. Curr. Neurovasc. Res. 3, 15–23. doi: 10.2174/156720206775541787
Dipoppa, M., Ranson, A., Krumin, M., Pachitariu, M., Carandini, M., Harris, K. D., et al. (2018). Vision and Locomotion Shape the Interactions between Neuron Types in Mouse Visual Cortex Article Vision and Locomotion Shape the Interactions between Neuron Types in Mouse Visual Cortex. Neuron 98, 602–615.e8. doi: 10.1016/j.neuron.2018.03.037
Do, K., Laing, B. T., Landry, T., Bunner, W., Mersaud, N., Matsubara, T., et al. (2018). The effects of exercise on hypothalamic neurodegeneration of Alzheimer’s disease mouse model. PLoS One 13:e0190205. doi: 10.1371/journal.pone.0190205
Dodd, K. J., and Shields, N. (2005). A systematic review of the outcomes of cardiovascular exercise programs for people with Down syndrome. Arch. Phys. Med. Rehabil. 86, 2051–2058. doi: 10.1016/j.apmr.2005.06.003
Duchon, A., Raveau, M., Chevalier, C., Nalesso, V., Sharp, A. J., and Herault, Y. (2011). Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: relevance for modeling down syndrome. Mamm. Genome 22, 674–684. doi: 10.1007/s00335-011-9356-0
Dugger, B. N., and Dickson, D. W. (2017). Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 9:a028035. doi: 10.1101/cshperspect.a028035
Duzel, E., van Praag, H., and Sendtner, M. (2016). Can physical exercise in old age improve memory and hippocampal function? Brain 139, 662–673. doi: 10.1093/brain/awv407
Embury, C. M., Dyavarshetty, B., Lu, Y., Wiederin, J. L., Ciborowski, P., Gendelman, H. E., et al. (2017). Cathepsin B Improves ß-Amyloidosis and Learning and Memory in Models of Alzheimer’s Disease. J. Neuroimmune Pharmacol. 12, 340–352. doi: 10.1007/s11481-016-9721-6
Engelhardt, B., and Sorokin, L. (2009). The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 31, 497–511. doi: 10.1007/s00281-009-0177-0
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Erkkinen, M. G., Kim, M. O., and Geschwind, M. D. (2018). Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 10:a033118. doi: 10.1101/cshperspect.a033118
Fabel, K., Fabel, K., Tam, B., Kaufer, D., Baiker, A., Simmons, N., et al. (2003). VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x
Fagiolini, M., and Hensch, T. K. (2000). Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404, 183–186. doi: 10.1038/35004582
Farmer, J., Zhao, X., van Praag, H., Wodtke, K., Gage, F. H., and Christie, B. R. (2004). Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124, 71–79. doi: 10.1016/j.neuroscience.2003.09.029
Ferioli, M., Zauli, G., Maiorano, P., Milani, D., Mirandola, P., and Neri, L. M. (2019). Role of physical exercise in the regulation of epigenetic mechanisms in inflammation, cancer, neurodegenerative diseases, and aging process. J. Cell. Physiol. 234. doi: 10.1002/jcp.28304 Epub online ahead of print.
Fernandez, A. M., and Torres-Alemán, I. (2012). The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 13, 225–239. doi: 10.1038/nrn3209
Fernandez, F., Morishita, W., Zuniga, E., Nguyen, J., Blank, M., Malenka, R. C., et al. (2007). Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat. Neurosci. 10, 411–413. doi: 10.1038/nn1860
Fernstrom, J. D. (2005). Branched-chain amino acids and brain function. J. Nutr. 135, 1539S–1546S. doi: 10.1093/jn/135.6.1539s
Fisher, E. M. C., and Bannerman, D. M. (2019). Mouse models of neurodegeneration: know your question, know your mouse. Sci. Transl. Med. 11:eaaq1818. doi: 10.1126/scitranslmed.aaq1818
Förster, C. (2008). Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 130, 55–70. doi: 10.1007/s00418-008-0424-9
Fu, Y., Tucciarone, J. M., Espinosa, J. S., Sheng, N., Darcy, D. P., Nicoll, R. A., et al. (2014). A Cortical Circuit for Gain Control by Behavioral State. Cell 156, 1139–1152. doi: 10.1016/j.cell.2014.01.050
García-Segura, L. M., Pérez, J., Pons, S., Rejas, M. T., and Torres-Alemán, I. (1991). Localization of insulin-like growth factor I (IGF-I)-like immunoreactivity in the developing and adult rat brain. Brain Res. 560, 167–174. doi: 10.1016/0006-8993(91)91228-S
Garofalo, S., D’Alessandro, G., Chece, G., Brau, F., Maggi, L., Rosa, A., et al. (2015). Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat. Commun. 6:6623. doi: 10.1038/ncomms7623
Garofalo, S., Porzia, A., Mainiero, F., Angelantonio, S., Di, Cortese, B., et al. (2017). Environmental stimuli shape microglial plasticity in glioma. Elife 6:e33415.
Ge, S., Yang, C. H., Hsu, K. S., Ming, G. L., and Song, H. (2007). A Critical Period for Enhanced Synaptic Plasticity in Newly Generated Neurons of the Adult Brain. Neuron 54, 559–566. doi: 10.1016/j.neuron.2007.05.002
Goldhammer, E., Tanchilevitch, A., Maor, I., Beniamini, Y., Rosenschein, U., and Sagiv, M. (2005). Exercise training modulates cytokines activity in coronary heart disease patients. Int. J. Cardiol. 100, 93–99. doi: 10.1016/j.ijcard.2004.08.073
Gomez-Pinilla, F., Zhuang, Y., Feng, J., Ying, Z., and Fan, G. (2011). Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 33, 383–390. doi: 10.1111/j.1460-9568.2010.07508.x
Gonçalves, J. T., Schafer, S. T., and Gage, F. H. (2016). Adult Neurogenesis in the Hippocampus: from Stem Cells to Behavior. Cell 167, 897–914. doi: 10.1016/j.cell.2016.10.021
Greenwood, B. N., Strong, P. V., Foley, T. E., and Fleshner, M. (2009). A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus 19, 988–1001. doi: 10.1002/hipo.20534
Grinberg, Y. Y., Dibbern, M. E., Levasseur, V. A., and Kraig, R. P. (2013). Insulin-like growth factor-1 abrogates microglial oxidative stress and TNF-α responses to spreading depression. J. Neurochem. 126, 662–672. doi: 10.1111/jnc.12267
Gu, Q. (2007). Serotonin Involvement in plAsticity of the Visual Cortex, in: monoaminergic Modulation of Cortical Excitability. US: Springer. 113–124. doi: 10.1007/978-0-387-72256-6_7
Guidi, S., Bonasoni, P., Ceccarelli, C., Santini, D., Gualtieri, F., Ciani, E., et al. (2008). Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol. 18, 180–197. doi: 10.1111/j.1750-3639.2007.00113.x
Gupta, M., Dhanasekaran, A. R., and Gardiner, K. J. (2016). Mouse models of Down syndrome: gene content and consequences. Mamm. Genome 27, 538–555. doi: 10.1007/s00335-016-9661-8
Hartman, M. L., Veldhuis, J. D., and Thorner, M. O. (1993). Normal control of growth hormone secretion. Horm. Res. 40 37–47. doi: 10.1159/000183766
Hawkins, B. T., and Davis, T. P. (2005). The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57, 173–185. doi: 10.1124/pr.57.2.4
Herault, Y., Delabar, J. M., Fisher, E. M. C., Tybulewicz, V. L. J., Yu, E., and Brault, V. (2017). Rodent models in Down syndrome research: impact and future opportunities. DMM Dis. Model. Mech. 10, 1165–1186. doi: 10.1242/dmm.029728
Hodge, R. D., D’Ercole, A. J., and O’Kusky, J. R. (2007). Insulin-like growth factor-I (IGF-I) inhibits neuronal apoptosis in the developing cerebral cortex in vivo. Int. J. Dev. Neurosci. 25, 233–241. doi: 10.1016/j.ijdevneu.2007.03.004
Höglund, E., Øverli, Ø, and Winberg, S. (2019). Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front. Endocrinol. 10:158. doi: 10.3389/fendo.2019.00158
Holmes, J. M., and Clarke, M. P. (2006). Amblyopia. Lancet 367, 1343–1351. doi: 10.1016/S0140-6736(06)68581-4
Hook, V., Toneff, T., Bogyo, M., Greenbaum, D., Medzihradszky, K. F., Neveu, J., et al. (2005). Inhibition of cathepsin B reduces β-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate β-secretase of Alzheimer’s disease. Biol. Chem. 386, 931–940. doi: 10.1515/BC.2005.108
Hornung, J. P. (2003). The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 26, 331–343. doi: 10.1016/j.jchemneu.2003.10.002
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. doi: 10.1038/s41582-019-0244-7
Houstis, N., Rosen, E. D., and Lander, E. S. (2006). Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948. doi: 10.1038/nature04634
Hunter, C. L., Bimonte, H. A., and Granholm, A. C. E. (2003). Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav. Brain Res. 138, 121–131. doi: 10.1016/S0166-4328(02)00275-9
Hurtado-Chong, A., Yusta-Boyo, M. J., Vergaño-Vera, E., Bulfone, A., De Pablo, F., and Vicario-Abejón, C. (2009). IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur. J. Neurosci. 30, 742–755. doi: 10.1111/j.1460-9568.2009.06870.x
Hüttenrauch, M., Brauß, A., Kurdakova, A., Borgers, H., Klinker, F., Liebetanz, D., et al. (2016). Physical activity delays hippocampal neurodegeneration and rescues memory deficits in an Alzheimer disease mouse model. Transl. Psychiatry 6:e800. doi: 10.1038/tp.2016.65
Iadecola, C. (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 5, 347–360. doi: 10.1038/nrn1387
Jacobs, B. L., and Fornal, C. A. (1999). Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology 21, 9S–15S. doi: 10.1016/S0893-133X(99)00012-3
James, D. E., Kraegen, E. W., and Chisholm, D. J. (1984). Effect of exercise training on whole-body insulin sensitivity and responsiveness. J. Appl. Physiol. 56, 1217–1222. doi: 10.1152/jappl.1984.56.5.1217
Jin, K., Zhu, Y., Sun, Y., Mao, X. O., Xie, L., and Greenberg, D. A. (2002). Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 99, 11946–11950. doi: 10.1073/pnas.182296499
Kaneko, M., and Stryker, M. P. (2014). Sensory experience during locomotion promotes recovery of function in adult visual cortex. Elife 2014, 1–16. doi: 10.7554/eLife.02798.001
Kashimoto, R. K., Toffoli, L. V., Manfredo, M. H. F., Volpini, V. L., Martins-Pinge, M. C., Pelosi, G. G., et al. (2016). Physical exercise affects the epigenetic programming of rat brain and modulates the adaptive response evoked by repeated restraint stress. Behav. Brain Res. 296, 286–289. doi: 10.1016/j.bbr.2015.08.038
Kelley, D. E., and Goodpaster, B. H. (1999). Effects of physical activity on insulin action and glucose tolerance in obesity. Med. Sci. Sports Exerc. 31, S619–S623.
Kim, S. Y., Buckwalter, M., Soreq, H., Vezzani, A., and Kaufer, D. (2012). Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia 53, 37–44. doi: 10.1111/j.1528-1167.2012.03701.x
Klemann, C. J. H. M., Xicoy, H., Poelmans, G., Bloem, B. R., Martens, G. J. M., and Visser, J. E. (2018). Physical Exercise Modulates L-DOPA-Regulated Molecular Pathways in the MPTP Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 55, 5639–5657. doi: 10.1007/s12035-017-0775-0
Kleschevnikov, A. M., Belichenko, P. V., Villar, A. J., Epstein, C. J., Malenka, R. C., and Mobley, W. C. (2004). Hippocampal Long-Term Potentiation Suppressed by Increased Inhibition in the Ts65Dn Mouse, a Genetic Model of Down Syndrome. J. Neurosci. 24, 8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004
Koppel, I., and Timmusk, T. (2013). Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology 75, 106–115. doi: 10.1016/j.neuropharm.2013.07.015
Kovacs, G. G. (2018). Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 145, 301–307. doi: 10.1016/B978-0-12-802395-2.00021-3
Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., and Moryś, J. (2018). BDNF: a Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 38, 579–593. doi: 10.1007/s10571-017-0510-4
Kraemer, R. R., Blair, M. S., McCaferty, R., and Castracane, V. D. (1993). Running-induced alterations in growth hormone, prolactin, triiodothyronine, and thyroxine concentrations in trained and untrained men and women. Res. Q. Exerc. Sport 64, 64–73. doi: 10.1080/02701367.1993.10608780
Landi, S., Ciucci, F., Maffei, L., Berardi, N., and Cenni, M. C. (2009). Setting the pace for retinal development: environmental enrichment acts through insulin-like growth factor 1 and brain-derived neurotrophic factor. J. Neurosci. 29, 10809–10819. doi: 10.1523/JNEUROSCI.1857-09.2009
Lee, J., Duan, W., and Mattson, M. P. (2002). Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 82, 1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x
Lee, S., Kruglikov, I., Huang, Z. J., Fishell, G., and Rudy, B. (2013). A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 16, 1662–1670. doi: 10.1038/nn.3544
Lemke, D., Pledl, H. W., Zorn, M., Jugold, M., Green, E., Blaes, J., et al. (2016). Slowing down glioblastoma progression in mice by running or the anti-malarial drug dihydroartemisinin? Induction of oxidative stress in murine glioblastoma therapy. Oncotarget 7, 56713–56725. doi: 10.18632/oncotarget.10723
Licht, T., and Keshet, E. (2015). The vascular niche in adult neurogenesis. Mech. Dev. 138, 56–62. doi: 10.1016/j.mod.2015.06.001
Lim, S., Chesser, A. S., Grima, J. C., Rappold, P. M., Blum, D., Przedborski, S., et al. (2011). D-β-hydroxybutyrate is protective in mouse models of Huntington’s disease. PLoS One 6:e24620. doi: 10.1371/journal.pone.0024620
Liu, P. Z., and Nusslock, R. (2018). Exercise-mediated neurogenesis in the hippocampus via BDNF. Front. Neurosci. 12:52. doi: 10.3389/fnins.2018.00052
Liu, X., Tu, M., Kelly, R. S., Chen, C., and Smith, B. J. (2004). Development of a computational approach to predict blood-brain barrier permeability. Drug Metab. Dispos. 32, 132–139. doi: 10.1124/dmd.32.1.132
Llorens-Martín, M. V., Rueda, N., Tejeda, G. S., Flórez, J., Trejo, J. L., and Martínez-Cué, C. (2010). Effects of voluntary physical exercise on adult hippocampal neurogenesis and behavior of Ts65Dn mice, a model of Down syndrome. Neuroscience 171, 1228–1240. doi: 10.1016/j.neuroscience.2010.09.043
Lorenzi, H. A., and Reeves, R. H. (2006). Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 1104, 153–159. doi: 10.1016/j.brainres.2006.05.022
Loudon, S. E., and Simonsz, H. J. (2005). The History of the Treatment of Amblyopia. Strabismus 13, 93–106. doi: 10.1080/09273970590949818
Lourenco, M. V., Frozza, R. L., de Freitas, G. B., Zhang, H., Kincheski, G. C., Ribeiro, F. C., et al. (2019). Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175. doi: 10.1038/s41591-018-0275-4
Lunghi, C., and Sale, A. (2015). A cycling lane for brain rewiring. Curr. Biol. 25, R1122–R1123. doi: 10.1016/j.cub.2015.10.026
Lunghi, C., Sframeli, A. T., Lepri, A., Lepri, M., Lisi, D., Sale, A., et al. (2019). A new counterintuitive training for adult amblyopia. Ann. Clin. Transl. Neurol. 6, 274–284. doi: 10.1002/acn3.698
Lyons, S. A., Chung, W. J., Weaver, A. K., Ogunrinu, T., and Sontheimer, H. (2007). Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 67, 9463–9471. doi: 10.1158/0008-5472.CAN-07-2034
Marks, J. L., Porte, D., and Baskin, D. G. (1991). Localization of type I insulin-like growth factor receptor messenger RNA in the adult rat brain by in situ hybridization. Mol. Endocrinol. 5, 1158–1168. doi: 10.1210/mend-5-8-1158
Markus, C. R. (2008). Dietary amino acids and brain serotonin function; Implications for stress-related affective changes. NeuroMolecular Med. 10, 247–58. doi: 10.1007/s12017-008-8039-9
Marquez, C. M. S., Vanaudenaerde, B., Troosters, T., and Wenderoth, N. (2015). High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. 119, 1363–73. doi: 10.1152/japplphysiol.00126.2015
Marsh, A. P. (2019). Molecular mechanisms of proteinopathies across neurodegenerative disease: a review. Neurol. Res. Pract. 1:35. doi: 10.1186/s42466-019-0039-8
Martin, J. Z. D. S., Donato, C., Peixoto, J., Aguirre, A., Choudhary, V., De Stasi, A. M., et al. (2020). Alterations of specific cortical GABAergic circuits underlie abnormal network activity in a mouse model of down syndrome. Elife 9, 1–54. doi: 10.7554/ELIFE.58731
Maya Vetencourt, J. F., Tiraboschi, E., Spolidoro, M., Castrén, E., and Maffei, L. (2011). Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur. J. Neurosci. 33, 49–57. doi: 10.1111/j.1460-9568.2010.07488.x
Maya-Vetencourt, J. F., Baroncelli, L., Viegi, A., Tiraboschi, E., Castren, E., Cattaneo, A., et al. (2012). IGF-1 Restores Visual Cortex Plasticity in Adult Life by Reducing Local GABA Levels. Neural. Plast. 2012, 1–10. doi: 10.1155/2012/250421
Mela, V., Mota, B. C., Milner, M., McGinley, A., Mills, K. H. G., Kelly, ÁM., et al. (2020). Exercise-induced re-programming of age-related metabolic changes in microglia is accompanied by a reduction in senescent cells. Brain. Behav. Immun. 87, 413–428. doi: 10.1016/j.bbi.2020.01.012
Mendonca, G. V., Pereira, F. D., and Fernhall, B. (2011). Effects of combined aerobic and resistance exercise training in adults with and without down syndrome. Arch. Phys. Med. Rehabil. 92, 37–45. doi: 10.1016/j.apmr.2010.09.015
Middlebrooke, A. R., Armstrong, N., Welsman, J. R., Shore, A. C., Clark, P., and MacLeod, K. M. (2005). Does aerobic fitness influence microvascular function in healthy adults at risk of developing Type 2 diabetes? Diabet. Med. 22, 483–489. doi: 10.1111/j.1464-5491.2005.01455.x
Moon, H. Y., Becke, A., Berron, D., Becker, B., Sah, N., Benoni, G., et al. (2016). Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 24, 332–340. doi: 10.1016/j.cmet.2016.05.025
Morishita, H., Miwa, J. M., Heintz, N., and Hensch, T. K. (2010). Lynx1, a Cholinergic Brake. Limits Plasticity in Adult Visual Cortex. Science 330, 1238–1240. doi: 10.1126/science.1195320
Morland, C., Andersson, K. A., Haugen, ØP., Hadzic, A., Kleppa, L., Gille, A., et al. (2017). Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8, 1–9. doi: 10.1038/ncomms15557
Moruzzi, G., and Magoun, H. W. (1949). Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1, 455–473.
Mueller-Steiner, S., Zhou, Y., Arai, H., Roberson, E. D., Sun, B., Chen, J., et al. (2006). Antiamyloidogenic and Neuroprotective Functions of Cathepsin B: implications for Alzheimer’s Disease. Neuron 51, 703–714. doi: 10.1016/j.neuron.2006.07.027
Nagahara, A. H., and Tuszynski, M. H. (2011). Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 10, 209–219. doi: 10.1038/nrd3366
Nandakumar, D. N., Ramaswamy, P., Prasad, C., Srinivas, D., and Goswami, K. (2019). Glioblastoma invasion and NMDA receptors: a novel prospect. Physiol. Int. 106, 250–260. doi: 10.1556/2060.106.2019.22
Nishijima, T., Piriz, J., Duflot, S., Fernandez, A. M., Gaitan, G., Gomez-Pinedo, U., et al. (2010). Neuronal Activity Drives Localized Blood-Brain-Barrier Transport of Serum Insulin-like Growth Factor-I into the CNS. Neuron 67, 834–846. doi: 10.1016/j.neuron.2010.08.007
Noda, Y., Kuzuya, A., Tanigawa, K., Araki, M., Kawai, R., Ma, B., et al. (2018). Fibronectin type III domain-containing protein 5 interacts with APP and decreases amyloid β production in Alzheimer’s disease 11 Medical and Health Sciences 1109 Neurosciences Tim Bliss. Mol. Brain 11:61. doi: 10.1186/s13041-018-0401-8
O’Kusky, J. R., Ye, P., and D’Ercole, A. J. (2000). Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J. Neurosci. 20, 8435–8442. doi: 10.1523/jneurosci.20-22-08435.2000
Ostrom, Q. T., Patil, N., Cioffi, G., Waite, K., Kruchko, C., and Barnholtz-Sloan, J. S. (2020). CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro. Oncol. 22, iv1–iv96. doi: 10.1093/neuonc/noaa200
Palasz, E., Niewiadomski, W., Gasiorowska, A., Wysocka, A., Stepniewska, A., and Niewiadomska, G. (2019). Exercise-Induced Neuroprotection and Recovery of Motor Function in Animal Models of Parkinson’s Disease. Front. Neurol. 10:1143. doi: 10.3389/fneur.2019.01143
Palmer, T. D., Willhoite, A. R., and Gage, F. H. (2000). Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479–494. doi: 10.1002/1096-9861(20001002)425:4<479::AID-CNE2>3.0.CO;2-3
Pan, X.-R., Li, G.-W., Hu, Y.-H., Wang, J.-X., Yang, W.-Y., An, Z.-X., et al. (1997). Effects of Diet and Exercise in Preventing NIDDM in People With Impaired Glucose Tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 20, 537–544. doi: 10.2337/DIACARE.20.4.537
Pape, S. E., Baksh, R. A., Startin, C., Hamburg, S., Hithersay, R., and Strydom, A. (2021). The Association between Physical Activity and CAMDEX-DS Changes Prior to the Onset of Alzheimer’s Disease in Down Syndrome. J. Clin. Med. 10:1882. doi: 10.3390/jcm10091882
Pardridge, W. M. (2003). Blood-brain barrier drug targeting: the future of brain drug development. Mol. Interv. 3, 90–105. doi: 10.1124/mi.3.2.90
Pardridge, W. M. (2007). Blood-brain barrier delivery. Drug Discov. Today 12, 54–61. doi: 10.1016/j.drudis.2006.10.013
Park, S. E., Dantzer, R., Kelley, K. W., and McCusker, R. H. (2011). Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflamm. 8:12. doi: 10.1186/1742-2094-8-12
Parrini, M., Ghezzi, D., Deidda, G., Medrihan, L., Castroflorio, E., Alberti, M., et al. (2017). Aerobic exercise and a BDNF-mimetic therapy rescue learning and memory in a mouse model of Down syndrome. Sci. Rep. 7:16825. doi: 10.1038/s41598-017-17201-8
Patrick, R. P., and Ames, B. N. (2015). Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 29, 2207–22. doi: 10.1096/fj.14-268342
Pedersen, B. K., Steensberg, A., Fischer, C., Keller, C., Keller, P., Plomgaard, P., et al. (2003). Searching for the exercise factor: is IL-6 a candidate?. J. Muscle Res. Cell. Motil. 24, 113–119. doi: 10.1023/A:1026070911202
Piao, Y., Lu, L., and De Groot, J. (2009). AMPA receptors promote perivascular glioma invasion via β1 integrin-dependent adhesion to the extracellular matrix. Neuro. Oncol. 11, 260–273. doi: 10.1215/15228517-2008-094
Proia, P., di Liegro, C. M., Schiera, G., Fricano, A., and Di Liegro, I. (2016). Lactate as a metabolite and a regulator in the central nervous system. Int. J. Mol. Sci. 17:1450. doi: 10.3390/ijms17091450
Radak, Z., Marton, O., Nagy, E., Koltai, E., and Goto, S. (2013). The complex role of physical exercise and reactive oxygen species on brain. J. Sport Heal. Sci. 2, 87–93. doi: 10.1016/j.jshs.2013.04.001
Reese, T. S., and Karnovsky, M. J. (1967). Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34, 207–217. doi: 10.1083/jcb.34.1.207
Rich, B., Scadeng, M., Yamaguchi, M., Wagner, P. D., and Breen, E. C. (2017). Skeletal myofiber vascular endothelial growth factor is required for the exercise training-induced increase in dentate gyrus neuronal precursor cells. J. Physiol. 595, 5931–5943. doi: 10.1113/JP273994
Richtsmeier, J. T., Zumwalt, A., Carlson, E. J., Epstein, C. J., and Reeves, R. H. (2002). Craniofacial phenotypes in segmentally trisomic mouse models for Down syndrome. Am. J. Med. Genet. 107, 317–324. doi: 10.1002/ajmg.10175
Rimmer, J. H., Heller, T., Wang, E., and Valerio, I. (2004). Improvements in Physical Fitness in Adults With Down Syndrome. Am J Ment Retard. 109, 165–174. doi: 10.1352/0895-80172004109<165
Rodriguez-Perez, A. I., Borrajo, A., Diaz-Ruiz, C., Garrido-Gil, P., and Labandeira-Garcia, J. L. (2016). Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: role in neuroinflammation and aging. Oncotarget 7, 30049–30067. doi: 10.18632/oncotarget.9174
Roizen, N. J., and Patterson, D. (2003). Down’s syndrome. Lancet 361, 1281–1289. doi: 10.1016/S0140-6736(03)12987-X
Rosa, E. F., Takahashi, S., Aboulafia, J., Nouailhetas, V. L. A., and Oliveira, M. G. M. (2007). Oxidative stress induced by intense and exhaustive exercise impairs murine cognitive function. J. Neurophysiol. 98, 1820–6. doi: 10.1152/jn.01158.2006
Russo, S., Kema, I., Bosker, F., Haavik, J., and Korf, J. (2007). Tryptophan as an evolutionarily conserved signal to brain serotonin: molecular evidence and psychiatric implications. World J. Biol. Psychiatry 10, 1–11. doi: 10.1080/15622970701513764
Sah, N., Peterson, B. D., Lubejko, S. T., Vivar, C., and Van Praag, H. (2017). Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci. Rep. 7:10903. doi: 10.1038/s41598-017-11268-z
Sale, A., Berardi, N., and Maffei, L. (2014). Environment and Brain Plasticity: towards an Endogenous Pharmacotherapy. Physiol. Rev. 94, 189–234. doi: 10.1152/physrev.00036.2012
Sale, A., Berardi, N., Spolidoro, M., Baroncelli, L., and Maffei, L. (2010). GABAergic inhibition in visual cortical plasticity. Front. Cell. Neurosci. 4:10. doi: 10.3389/fncel.2010.00010
Sale, A., Cenni, M. C., Clussi, F., Putignano, E., Chierzi, S., and Maffei, L. (2007). Maternal enrichment during pregnancy accelerates retinal development of the fetus. PLoS One 2:e1160. doi: 10.1371/journal.pone.0001160
Sale, A., Putignano, E., Cancedda, L., Landi, S., Cirulli, F., Berardi, N., et al. (2004). Enriched environment and acceleration of visual system development. Neuropharmacology 47, 649–660. doi: 10.1016/j.neuropharm.2004.07.008
Sansevero, G., Baroncelli, L., Scali, M., and Sale, A. (2019). Intranasal BDNF administration promotes visual function recovery in adult amblyopic rats. Neuropharmacology 145, 114–122. doi: 10.1016/j.neuropharm.2018.02.006
Sansevero, G., Torelli, C., Mazziotti, R., Consorti, A., Pizzorusso, T., Berardi, N., et al. (2020). Running towards amblyopia recovery. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-69630-7
Scharfman, H., Goodman, J., Macleod, A., Phani, S., Antonelli, C., and Croll, S. (2005). Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 192, 348–356. doi: 10.1016/j.expneurol.2004.11.016
Schieber, M., and Chandel, N. S. (2014). ROS function in redox signaling. Curr. Biol. 24, 453–462. doi: 10.1016/j.cub.2014.03.034.ROS
Schnyder, S., and Handschin, C. (2015). Skeletal muscle as an endocrine organ: pGC-1α, myokines and exercise. Bone 80, 115–125. doi: 10.1016/j.bone.2015.02.008
Scott-McKean, J. J., Chang, B., Hurd, R. E., Nusinowitz, S., Schmidt, C., Davisson, M. T., et al. (2010). The mouse model of Down syndrome Ts65Dn presents visual deficits as assessed by pattern visual evoked potentials. Invest. Ophthalmol. Vis. Sci. 51, 3300–3308. doi: 10.1167/iovs.09-4465
Segal, M. B. (2000). The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell. Mol. Neurobiol. 20, 183–196. doi: 10.1023/A:1007045605751
Sengpiel, F., Jirmann, K.-U., Vorobyov, V., and Eysel, U. T. (2005). Strabismic Suppression Is Mediated by Inhibitory Interactions in the Primary Visual Cortex. Cereb. Cortex 16, 1750–1758. doi: 10.1093/cercor/bhj110
Shabanpoor, F., Separovic, F., and Wade, J. D. (2009). Chapter 1 The Human Insulin Superfamily of Polypeptide Hormones. Vitam. Horm. 80, 1–31. doi: 10.1016/S0083-6729(08)00601-8
Sharma, A., Madaan, V., and Petty, F. D. (2006). Exercise for mental health. Prim. Care Companion J. Clin. Psychiatry. 8:106. doi: 10.4088/pcc.v08n0208a
Siarey, R. J., Carlson, E. J., Epstein, C. J., Balbo, A., Rapoport, S. I., and Galdzicki, Z. (1999). Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology 38, 1917–1920.
Siarey, R. J., Stoll, J., Rapoport, S. I., and Galdzicki, Z. (1997). Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down Syndrome. Neuropharmacology 36, 1549–1554. doi: 10.1016/S0028-3908(97)00157-3
Siteneski, A., Cunha, M. P., Lieberknecht, V., Pazini, F. L., Gruhn, K., Brocardo, P. S., et al. (2018). Central irisin administration affords antidepressant-like effect and modulates neuroplasticity-related genes in the hippocampus and prefrontal cortex of mice. Prog. Neuro Psychopharmacol. Biol. Psychiatry 84, 294–303. doi: 10.1016/j.pnpbp.2018.03.004
Sleiman, S. F., Henry, J., Al-Haddad, R., El Hayek, L., Abou Haidar, E., Stringer, T., et al. (2016). Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 5, 1–21. doi: 10.7554/eLife.15092
Snyder, J. S., Kee, N., and Wojtowicz, J. M. (2001). Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 85, 2423–2431. doi: 10.1152/jn.2001.85.6.2423
Sosa, V., Moliné, T., Somoza, R., Paciucci, R., Kondoh, H., and LLeonart, M. E. (2013). Oxidative stress and cancer: an overview. Ageing Res. Rev. 12, 376–390. doi: 10.1016/j.arr.2012.10.004
Souza, L. C., Filho, C. B., Goes, A. T. R., Fabbro, L., Del, De Gomes, M. G., Savegnago, L., et al. (2013). Neuroprotective effect of physical exercise in a mouse model of alzheimer’s disease induced by β-Amyloid1-40 peptide. Neurotox. Res. 24, 148–163. doi: 10.1007/s12640-012-9373-0
Spencer, A. P., Torrado, M., Custódio, B., Silva-Reis, S. C., Santos, S. D., Leiro, V., et al. (2020). Breaking barriers: bioinspired strategies for targeted neuronal delivery to the central nervous system. Pharmaceutics 12:192. doi: 10.3390/pharmaceutics12020192
Steventon, J. J., Foster, C., Furby, H., Helme, D., Wise, R. G., and Murphy, K. (2020). Hippocampal Blood Flow Is Increased after 20 min of Moderate-Intensity Exercise. Cereb. Cortex 30, 525–533. doi: 10.1093/cercor/bhz104
Stryker, M. P. (2014). A neural circuit that controls cortical state, plasticity, and the gain of sensory responses in mouse. Cold Spring Harb. Symp. Quant. Biol. 79, 1–9. doi: 10.1101/sqb.2014.79.024927
Sugihara, H., Chen, N., and Sur, M. (2016). Cell-specific modulation of plasticity and cortical state by cholinergic inputs to the visual cortex. J. Physiol. Paris 110, 37–43. doi: 10.1016/j.jphysparis.2016.11.004
Sutton, J., and Lazarus, L. (1976). Growth hormone in exercise: comparison of physiological and pharmacological stimuli. J. Appl. Physiol. 41, 523–527. doi: 10.1152/jappl.1976.41.4.523
Tantillo, E., Colistra, A., Baroncelli, L., Costa, M., Caleo, M., and Vannini, E. (2020). Voluntary physical exercise reduces motor dysfunction and hampers tumor cell proliferation in a mouse model of glioma. Int. J. Environ. Res. Public Health 17, 1–12. doi: 10.3390/ijerph17165667
Teixeira-Lemos, E., Nunes, S., Teixeira, F., and Reis, F. (2011). Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc. Diabetol. 10, 1–15. doi: 10.1186/1475-2840-10-12
Van Praag, H., Christie, B. R., Sejnowski, T. J., and Gage, F. H. (1999a). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 13427–13431. doi: 10.1073/pnas.96.23.13427
Van Praag, H., Kempermann, G., and Gage, F. H. (1999b). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270. doi: 10.1038/6368
Van Praag, H., Schinder, A. F., Christle, B. R., Toni, N., Palmer, T. D., and Gage, F. H. (2002). Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034. doi: 10.1038/4151030a
van Praag, H., Shubert, T., Zhao, C., and Gage, F. H. (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005
Vaynman, S., and Gomez-Pinilla, F. (2005). License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil. Neural Repair. 19, 283–295. doi: 10.1177/1545968305280753
Venkataramani, V., Tanev, D. I., Strahle, C., Studier-Fischer, A., Fankhauser, L., Kessler, T., et al. (2019). Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538. doi: 10.1038/s41586-019-1564-x
Venkatesh, H. S., Johung, T. B., Caretti, V., Noll, A., Tang, Y., Nagaraja, S., et al. (2015). Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816. doi: 10.1016/j.cell.2015.04.012
Venkatesh, H. S., Morishita, W., Geraghty, A. C., Silverbush, D., Gillespie, S. M., Arzt, M., et al. (2019). Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545. doi: 10.1038/s41586-019-1563-y
Venkatesh, H. S., Tam, L. T., Woo, P. J., Lennon, J., Nagaraja, S., Gillespie, S. M., et al. (2017). Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537. doi: 10.1038/nature24014
Vetencourt, J. F. M., Sale, A., Viegi, A., Baroncelli, L., De Pasquale, R., O’Leary, O. F., et al. (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320, 385–388. doi: 10.1126/science.1150516
Vivar, C., Peterson, B. D., and van Praag, H. (2016). Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage 131, 29–41. doi: 10.1016/j.neuroimage.2015.11.031
Voss, M. W., Vivar, C., Kramer, A. F., and van Praag, H. (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 17, 525–544. doi: 10.1016/j.tics.2013.08.001
Wang, S., and Pan, J. (2016). Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. Biophys. Res. Commun. 474, 22–28. doi: 10.1016/j.bbrc.2016.04.047
Warburton, D. E. R., Nicol, C. W., and Bredin, S. S. D. (2006). Health benefits of physical activity: the evidence. CMAJ 174, 801–809. doi: 10.1503/cmaj.051351
Waterhouse, E. G., An, J. J., Orefice, L. L., Baydyuk, M., Liao, G. Y., Zheng, K., et al. (2012). BDNF promotes differentiation and maturation of adult-born neurons through GABArgic transmission. J. Neurosci. 32, 14318–14330. doi: 10.1523/JNEUROSCI.0709-12.2012
Weiss, N., Miller, F., Cazaubon, S., and Couraud, P. O. (2009). The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta Biomembr. 1788, 842–857. doi: 10.1016/j.bbamem.2008.10.022
Wolburg, H., and Lippoldt, A. (2002). Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul. Pharmacol. 38, 323–337. doi: 10.1016/S1537-1891(02)00200-8
Wolburg, H., and Paulus, W. (2010). Choroid plexus: biology and pathology. Acta Neuropathol. 119, 75–88. doi: 10.1007/s00401-009-0627-8
Wolka, A. M., Huber, J. D., and Davis, T. P. (2003). Pain and the blood-brain barrier: obstacles to drug delivery. Adv. Drug Deliv. Rev. 55, 987–1006. doi: 10.1016/S0169-409X(03)00100-5
World Health Organization (WHO) (2020). Physical activity. Available online at: URL https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed July 21, 2021).
Wyss-Coray, T. (2016). Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186. doi: 10.1038/nature20411
Xia, D. Y., Huang, X., Bi, C. F., Mao, L. L., Peng, L. J., and Qian, H. R. (2017). PGC-1α or FNDC5 is involved in modulating the effects of Aβ1-42 oligomers on suppressing the expression of BDNF, a beneficial factor for inhibiting neuronal apoptosis, Aβ deposition and cognitive decline of APP/PS1 Tg mice. Front. Aging Neurosci. 9:65. doi: 10.3389/fnagi.2017.00065
Yavorska, I., and Wehr, M. (2021). Effects of Locomotion in Auditory Cortex Are Not Mediated by the VIP Network. Front. Neural Circuits 15:24. doi: 10.3389/fncir.2021.618881
Ye, Z. C., and Sontheimer, H. (1999). Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 59, 4383–4391.
Keywords: physical exercise, brain physiology, brain pathology, myocytes, neurons, microglia, neurodegeneration, neurodevelopmental disorders
Citation: Consorti A, Di Marco I and Sansevero G (2021) Physical Exercise Modulates Brain Physiology Through a Network of Long- and Short-Range Cellular Interactions. Front. Mol. Neurosci. 14:710303. doi: 10.3389/fnmol.2021.710303
Received: 15 May 2021; Accepted: 23 July 2021;
Published: 19 August 2021.
Edited by:
Rosa Chiara Paolicelli, University of Lausanne, SwitzerlandReviewed by:
Aine Kelly, Trinity College Dublin, IrelandCopyright © 2021 Consorti, Di Marco and Sansevero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriele Sansevero, Z2FicmllbGUuc2Fuc2V2ZXJvQGluLmNuci5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.