- 1School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2International Collaborative Center on Big Science Plan for Purine Signaling, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Rudolf Boehm Institute for Pharmacology and Toxicology, University of Leipzig, Leipzig, Germany
P2X7 receptors are members of the ATP-gated cationic channel family with a preferential localization at the microglial cells, the resident macrophages of the brain. However, these receptors are also present at neuroglia (astrocytes, oligodendrocytes) although at a considerably lower density. They mediate necrosis/apoptosis by the release of pro-inflammatory cytokines/chemokines, reactive oxygen species (ROS) as well as the excitotoxic (glio)transmitters glutamate and ATP. Besides mediating cell damage i.e., superimposed upon chronic neurodegenerative processes in Alzheimer’s Disease, Parkinson’s Disease, multiple sclerosis, and amyotrophic lateral sclerosis, they may also participate in neuroglial signaling to neurons under conditions of high ATP concentrations during any other form of neuroinflammation/neurodegeneration. It is a pertinent open question whether P2X7Rs are localized on neurons, or whether only neuroglia/microglia possess this receptor-type causing indirect effects by releasing the above-mentioned signaling molecules. We suggest as based on molecular biology and functional evidence that neurons are devoid of P2X7Rs although the existence of neuronal P2X7Rs cannot be excluded with absolute certainty.
Introduction
As a last member of the ATP-gated cationic channel family P2X, the P2X7 receptor (R) was cloned and characterized by Surprenant et al. (1996). These authors already described several important properties of the receptor, such as stimulation by pathophysiologically large concentrations of extracellular ATP in the high micromolar range, a gradual increase of the receptor-current, when induced by repetitive or continuous activation, and a unique long cytoplasmic tail which was required for the lytic/necrotic actions of ATP. An increase of P2X7R current amplitude on long-lasting contact with ATP was thought for many years to be due to progressive dilation of the P2X7R channel (Virginio et al., 1999). Dilation of the receptor-channel itself or its association with the membrane channel pannexin-1 (Panx-1; Pelegrin and Surprenant, 2006) was supposed to allow the entry of large molecules (e.g., Yo-Pro-1) into the cell interior or the loss of important cell ingredients into the extracellular space leading to necrosis. However, more recently convincing evidence has been presented that these earlier hypotheses are probably misleading (Di Virgilio et al., 2018; Ugur and Ugur, 2019) and that the P2X7R channel permits the passage of large cationic molecules immediately from its initial activation, but at a much slower pace than that of the small cations Na+, K+, and Ca2+ (Harkat et al., 2017). Further, it has been proposed that the C-terminus of the P2X7R is needed for activation of the macropore and, in consequence for membrane permeabilization (Smart et al., 2003). However, it appears that the lack of a C-terminal tail does not fully abolish, but only slows down the formation of the macropore (Karasawa et al., 2017).
There has been a long-lasting controversy whether P2X7Rs are localized at neurons, or whether neuronal effects are indirect, due to the stimulation of astrocytic P2X7Rs, which on their behalf interact with neurons via releasing various signaling molecules (Illes et al., 2017; Miras-Portugal et al., 2017). This overview aims to discuss arguments supporting the idea that neuroactive P2X7Rs are probably localized at astrocytes/oligodendrocytes, and enlist novel molecular biology results suggesting that these receptors are missing at neurons. We did not intend to give a relatively complete survey of the relevant literature as presented in our recent review (Illes et al., 2017), but rather limited ourselves to the most relevant aspects of the neuroglia-mediated signaling to neurons.
Astrocyte-Neuron Interaction Via P2x7 Receptors
Astrocytic P2X7Rs
In the CNS, P2X7Rs occur at the highest density at microglia (Bhattacharya and Biber, 2016; Illes et al., 2020), but are present also at astrocytes as confirmed by mRNA and protein measurements (Fischer et al., 2009; Illes et al., 2012). Hippocampal (Kukley et al., 2001) as well as accumbal and cortical astrocytes of rats stained for P2X7R immunoreactivity (IR; Franke et al., 2012), although the latter ones only after mechanical or ischemic injury. Similarly, cerebral cortices of AD patients and an AD mouse model (APPPS1) expressed P2X7R-IR in astrocytes (Martin et al., 2019).
For some time, it was questioned whether the immunohistochemically identified astrocytic P2X7Rs have also functional significance (Jabs et al., 2007), but later unequivocal evidence was forwarded to confirm this assumption (Illes et al., 2012; Verkhratsky et al., 2012). ATP and the prototypic P2X7R agonist dibenzoyl-ATP (Bz-ATP) induced current responses in mixed astrocyte/neuron cultures prepared from the rodent neocortex (Duan et al., 2003; Nörenberg et al., 2010; Rubini et al., 2014) and in brain slices of the pre-frontal cortex (Oliveira et al., 2011). The measurement of the ATP-induced increase of intracellular Ca2+ ([Ca2+]i) also confirmed the existence of functional P2X7Rs at cultured astrocytes (Fumagalli et al., 2003; Fischer et al., 2009).
Astrocytic Signaling via P2X7R Activation
Astrocytes may communicate with neurons using several signaling molecules released by exocytotic and non-exocytotic pathways (Figure 1; Illes et al., 2019a). In the first line, the P2X7R pore is believed to be involved in the production and release of neuroinflammatory cytokines. The best characterized immune response associated with P2X7R stimulation is a leucine-rich repeat, pyrin domain containing 3 (NLRP3) inflammasome activation and the consequent interleukin-1β (IL-1β) secretion from macrophages/microglia (Di Virgilio et al., 2017; Illes et al., 2020). The maturation and release of IL-1β and IL-18 are mediated by NLRP3 stimulation, but activated microglia may secrete also further pro-inflammatory cytokines such as IL-6 and tumor necrosis factor-α (TNF-α) by other mechanisms (Shieh et al., 2014). Although the primary source of pro-inflammatory cytokines are microglial cells, astrocytes also appear to participate in this process and release IL-1β, IL-6, and TNF-α (Choi et al., 2014).
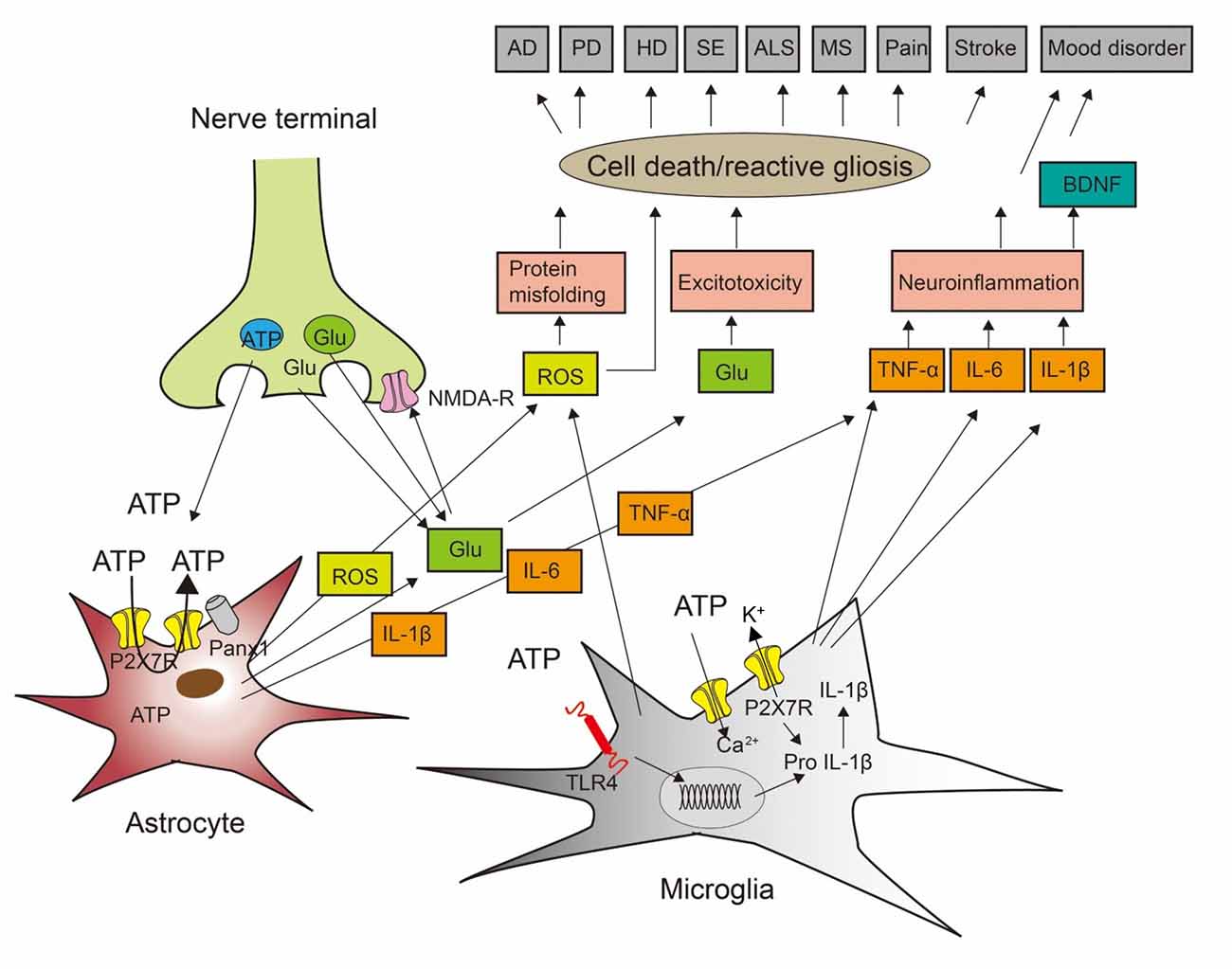
Figure 1. Cross-talk between neurons, astrocytes, and microglia to induce cell death during neurodegenerative and psychiatric illnesses. ATP and glutamate (Glu) are neurotransmitters released from nerve terminals but also gliotransmitters released/secreted from astrocytes and microglia. In consequence, ATP may be released by exocytotic mechanisms from neurons/astrocytes, but also through the P2X7 receptors (P2X7Rs) themselves or pannexin-1 (Panx-1) channels from astrocytes. P2X7Rs are nonselective cationic channels and thereby entry pathways for extracellular Ca2+ into the cell interior and exit pathways for the loss of K+. Stimulation of the toll-like receptor TLR4R by lipopolysaccharide (LPS) primes the generation of the inflammatory cytokine interleukin-1β (IL-1β) by the cleavage of pro-IL-1β. This process involves nucleotide-binding, leucine-rich repeat, pyrin domain containing 3 (NLRP3) inflammasome-mediated caspase-1 activation. Impoverishment in cytoplasmic K+ by P2X7R activation is a major stimulus for NLRP3 activation and therefore boosts IL-1β production and secretion. Presynaptic N-methyl-D-aspartate (NMDA) receptors facilitate the release of glutamate. Reactive oxygen species (ROS) are secreted from microglial cells. ROS induces protein misfolding, glutamate is in high concentrations an excitotoxin, and the inflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) cause neuroinflammation, leading also to the release of brain-derived neurotrophic factor (BDNF). AD, Alzheimer’s disease; PD, Parkinson’s disease; HD, Huntington’s disease; SE, status epilepticus; ALS, amyotrophic lateral sclerosis; MS, multiple sclerosis. Modified with permission from Illes et al. (2019b).
Reactive oxygen species (ROS) are another signaling molecule released by macrophages/microglia via P2X7R-induced activation of NADPH oxidase 2 (Apolloni et al., 2014; Bartlett et al., 2014). Astrocytes were also described to increase ROS production through NADPH oxidase, subsequently leading to IL-6 production (Munoz et al., 2020). The above-mentioned class of cytokines and chemokines, such as CCL2 or CCL5, as well as ROS, are neuroinflammatory molecules putting a further burden onto neurons already damaged by various neurodegenerative illnesses such as Alzheimer’s disease (AD; Illes et al., 2019b), Parkinson’s disease (PD; Carmo et al., 2014), amyotrophic lateral sclerosis (Apolloni et al., 2014), and multiple sclerosis (MS; Domercq and Matute, 2019). However, this damage is mostly due to a massive microglial outflow of cytokines/chemokines, while their astrocytic counterparts are likely to induce more moderate reactions such as depressive-like behavior in rodents and major depression in humans by interacting with cortico-limbic neuronal circuits (Koo and Duman, 2009; Maes et al., 2012).
Astrocytic Glutamate/GABA Stimulate Neighboring Neurons
In addition to these pro-inflammatory mediators the activation of astrocytic P2X7Rs was shown to release the (glio)transmitters glutamate (Duan et al., 2003; Bardoni et al., 2010), GABA (Wang et al., 2002; Wirkner et al., 2005), and ATP (Suadicani et al., 2006; Xiong et al., 2018). The potent, although non-selective P2X7R agonist Bz-ATP induced transient and tonic glutamate release, manifesting as transient (slow inward current; SIC) and tonic current, respectively in CA1 neurons of hippocampal slices (Fellin et al., 2006). The SICs were due to N-Methyl-D-Aspartate (NMDA) receptor activation (blocked by AP5), while the tonic current responses were mediated by P2X7Rs (blocked by oxidized ATP). In dorsal horn lamina II (substantia gelatinosa, SG) neurons of spinal cord slices, only SICs were recorded after Bz-ATP application (Bardoni et al., 2010). Both in the hippocampus and in the spinal cord, the source of glutamate exerting the respective electrophysiological responses were supposed to be astrocytes, which exhibited [Ca2+]i elevations accompanying the transmembrane currents.
In good correlation with the above findings, Bz-ATP evoked inward currents in CA1 and lamina II neurons and astrocytes kept in slice preparations; a low Ca2+/no Mg2+ (low X2+)-containing incubation medium was used in both cases to facilitate the P2X7R-mediated responses (Ficker et al., 2014; Gao et al., 2017). Whereas the antagonism of P2X7Rs strongly inhibited both the neuronal and astrocytic current responses, a combination of antagonists for AMPA, NMDA and GABAARs almost abolished the Bz-ATP-induced current amplitudes in the neurons only (Ficker et al., 2014). It was concluded that P2X7Rs are localized at astrocytes, the stimulation of which causes the release of glutamate (and probably GABA) stimulating their respective receptors at the neighboring neurons. Also, Bz-ATP potentiated the frequency of spontaneous postsynaptic currents in CA1 neurons (Khan et al., 2019). This effect on CA1 neurons was inhibited by the GABAAR antagonist gabazine or the astrocytic toxin fluorocitrate, suggesting stimulation of P2X7Rs at stratum radiatum astrocytes synapsing onto CA1 neurons. A stimulatory effect of astrocytic P2X7Rs executed via the release of glutamate onto noradrenergic locus coeruleus neurons was recorded as an increased frequency of the glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs) of these neurons (Khakpay et al., 2010).
Astrocytic ATP Stimulates Neighboring Neurons
Similar to glutamate/GABA also ATP itself may be released from astrocytes via P2X7R stimulation to modulate neuronal functions. As mentioned in the Introduction, Panx-1 channels are opened by activation of P2X7Rs (Pelegrin and Surprenant, 2006; Locovei et al., 2007), and both ATP release and dye uptake induced by this stimulus are blocked by Panx-1 knockdown strategies (Iglesias et al., 2008; Suadicani et al., 2012). In the rat hypothalamic paraventricular nucleus, an increase in the amplitude of miniature excitatory postsynaptic currents (mEPSCs) was observed in response to noradrenaline that released ATP from glial cells (Gordon et al., 2005). It was suggested that ATP acts at postsynaptic P2X7Rs localized at magnocellular neurosecretory cells to promote the insertion of AMPA receptors, resulting in the up-regulation of synaptic efficacy (i.e., mEPSC amplitude).
Whereas pannexin channels only function as release pathways from astrocytes, two opposed connexins form gap junctions between individual astrocytes connecting the cytoplasm of adjacent cells (Verkhratsky and Nedergaard, 2018; Theis and Giaume, 2012). Gap junctions serve as pathways for the exchange of small molecules of less than about 1 kDa, including ions, energy metabolites, neurotransmitters, and signaling molecules, that co-ordinate the metabolic and functional activities of connected cells (Pannasch and Rouach, 2013; Cheung et al., 2014). On the other hand, unopposed connexin hemichannels are exit pathways for small molecules from the intracellular space allowing e.g., the release of ATP in a similar manner as pannexins do. It is noteworthy, that although the excitotoxic glutamate/ATP were the primary gliotransmitters released from astrocytes, GABA may be also released under certain conditions to mediate inhibition of neuronal circuits.
However, glutamate, GABA, ATP, and the glutamatergic co-agonist D-serine may be also outpoured from astrocytes by Ca2+-dependent, exocytotic/vesicular mechanisms (Lalo et al., 2014a, b). They were suggested to be released from astrocytic processes enwrapping the contact points (synapses) of the presynaptic neuronal elements with the dendritic specializations of the postsynaptic neuron. This tri-partite synapse is ideally suited to serve as an astrocyte-neuron communication interface (Araque et al., 1999; Halassa and Haydon, 2010).
Oligodendrocyte-Neuron Interaction Via P2x7 Receptors
Neurotoxic P2X7Rs at Oligodendrocytes
Although astrocytes in the CNS have attracted a lot of attention, oligodendrocytes outnumber them by almost a factor of two and are certainly of comparable importance for neuronal functions (Pelvig et al., 2008; Illes et al., 2019a). They constitute the myelin sheath of neuronal fiber tracts and thereby insulate individual axons permitting spatially separated and rapid conduction of action potentials. Ample evidence documents that oligodendrocytes and their precursors express P2X7Rs (Matute, 2008); Bz-ATP evoked an elevation in the cytosolic concentration Ca2+, and the cationic dye Yo-Pro-1 was taken up by oligodendrocytes in optic nerve glia (James and Butt, 2002; Matute, 2008; Butt, 2011). P2X7Rs in oligodendrocytes are highly permeable to Ca2+ and prolonged activation of these receptors is lethal to differentiated oligodendrocytes in culture and to mature oligodendrocytes in isolated optic nerves in vitro and in vivo (Matute, 2008). In this respect, ATP would act like the excitotoxic glutamate and damage the white matter of the CNS (Matute, 2011).
Primary cultures of oligodendrocytes derived from optic nerves of rats were subjected to in vitro ischemia achieved by replacing O2 with N2, external glucose with sucrose, and adding iodoacetate to the incubation medium to block glycolysis (Domercq et al., 2010). This treatment induced inward current, which could be reversed by the P2X7R antagonist Brilliant Blue G (BBG), the ATP degrading enzyme apyrase, and by blockers of pannexin hemichannels. It was suggested that ischemia releases ATP from cultured oligodendrocytes, which activates P2X7Rs at these very cells aggravating ischemic damage. Furthermore, sustained activation of P2X7Rs in vivo causes morphological lesions that are reminiscent of the major features of MS plaques, i.e., demyelination, oligodendrocyte death, and axonal damage (Matute et al., 2007). In an animal model of MS, experimental autoimmune encephalomyelitis, treatment with BBG ameliorated the neurological symptoms and restored normal axon conduction velocity in the optic nerve.
Possible Involvement of Oligodendrocytic P2X7Rs in Developmental Effects
As early as in 2006 it has been suggested that axonal firing stimulates different purinergic receptors at Schwann cells and oligodendrocyte progenitors regulating development and myelination (Fields, 2006). Moreover, P2X7Rs were found to evoke Ca2+ transients in growth cones of e.g., axons of hippocampal neurons kept in culture and thereby interfere with axonal growth (Diaz-Hernandez et al., 2008). Pharmacological inhibition of P2X7Rs or its silencing by shRNA interference induced longer and more branched axons, coupled with morphological changes in the growth cone. Signaling by this receptor was through adenylate cyclase which also coordinated its interaction with P2Y1 and P2Y13Rs which promoted axonal elongation (del Puerto et al., 2012). It is well established that adult neural progenitor cells (NPCs) located in the so-called neurogenic niches of the brain and being the common ancestors of newly generated neurons, astrocytes, and oligodendrocytes possess P2X7Rs (Tang and Illes, 2017; Leeson et al., 2019). Hence, we tentatively suggest that growth cones are developmentally immature cellular constituents that express NPC-like properties regulating axonal growth and branching.
The axon initial segment is the site where action potentials are triggered due to the high concentration of voltage-gated ion channels (Bender and Trussell, 2012). In dissociated cultured hippocampal neurons and hippocampal slice cultures, P2X7R activation decreased both sodium current amplitude and intrinsic neuronal excitability while P2X7R inhibition had the opposite effect (Del Puerto et al., 2015). Although not explicitly stated by the authors, we assume that P2X7Rs were situated at oligodendrocytes rather than the neurons themselves, implicating the signaling by oligodendrocyte cell products to neurons. Of course, the participation of astrocytes cannot be excluded either.
Oligodendrocyte Precursor Cells (NG2 Glia)
Besides astrocytes and oligodendrocytes, NG2 glial cells (expressing the proteoglycan NG2 in addition to the classic immunohistochemical markers for oligodendrocytes) emerged during the last decades as the third type of neuroglial cells in the CNS (Seifert and Steinhäuser, 2018; Bedner et al., 2020). These cells are also referred to as oligodendrocyte precursor cells (OPCs) because in the white matter they differentiate into myelinating oligodendrocytes (Nishiyama et al., 2016). However, in the gray matter, NG2 glia does not differentiate into oligodendrocytes but maintains its phenotype throughout postnatal life. These phenotypic characteristics include the expression of functional voltage-gated K+, Na+, and Ca2+ channels, without the ability to generate action potentials (Haberlandt et al., 2011). Rodent NG2 glia receives direct synaptic inputs from glutamatergic and GABAergic neurons, a feature that is unique among glial cells (Bergles et al., 2000). It is noteworthy that in the hippocampus, the number of NG2 cells amounts to about 20–25% of that of astrocytes (Degen et al., 2012).
We suggest that P2X7R possessing NG2 cells should be also considered as oligodendrocyte-like executors of neuronal effects. ATP/Bz-ATP increased [Ca2+]i in purified (less than 2% astrocytes and microglia), cultured NG2-immunopositive OPCs (Agresti et al., 2005). The Bz-ATP-induced raise in [Ca2+]i fully depended on extracellular Ca2+ and was blocked in presence of the P2X7R antagonistic oxidized ATP.
Thus, there is strong, direct evidence for the presence of neurotoxic P2X7Rs at oligodendrocytes and less convincing, circumstantial evidence for developmentally active P2X7Rs at the same cell type. However, at the moment it is difficult to judge whether astrocytic or oligodendrocytic (NG2 glia) P2X7Rs are more important for signaling to neurons (see below current molecular biology evidence for the presence of this receptor at oligodendrocytes but not astrocytes).
Neuronal P2X7 Receptors per se?
Numerous experimental reports are suggesting assumedly unequivocal evidence for neuronal P2X7Rs. However, we will prove below for a few prominent examples that this evidence allows also the conclusion that P2X7Rs are localized at astrocytes/oligodendrocytes which by their signaling molecules indirectly modify neuronal functions.
Electrophysiological Evidence in Cell Culture, Brain Slice, and Isolated Cell Preparations
In segments of the guinea-pig ileum, myenteric neurons were impaled by sharp microelectrodes or patch-clamp pipettes, and ATP or Bz-ATP was used to evoke inward currents (Hu et al., 2001; Valdez-Morales et al., 2011). These currents were abolished by the P2X7R antagonistic oxidized ATP and Brilliant Blue G. However, all these preparations contained an ample population of glial cells certainly expressing P2X7Rs.
More refined methods were also used to elucidate the question of neuronal P2X7Rs. Immature hilar neurons with attached glutamate releasing nerve terminals were mechanically isolated from rat hippocampal slices (Cho et al., 2010). Bz-ATP increased the frequency of sEPSCs, and this effect was blocked by Brilliant Blue G. In most of the hilar neurons tested, the Bz-ATP-induced increase in sEPSC frequency was blocked by tetrodotoxin or Cd2+, suggesting that the activation of P2X7Rs leads to a presynaptic depolarization.
In an ingenious methodological approach, mechanical deformation of isolated retinal ganglion cells led to the release of ATP through pannexin hemichannels which were supposed to act back directly at the neurons to auto-stimulate their P2X7Rs (Xia et al., 2012). P2X7R activation was measured as an A438079 (selective P2X7R antagonist)-sensitive cationic inward current. Ganglion cells were seeded onto elastic silicone material and were introduced into a cell stretch chamber; all figures of the paper show data generated on a mixed retinal cell preparation, meaning the additional presence of astrocytes/Müller cells. In some cases, the neurobasal medium (NBM) was used for culture to obtain purified neuronal preparations. This procedure was however found to cause the acquirement of astrocytic properties by cultured striatal neurons (Rubini et al., 2006). These neurons kept in NBM fired only a single low amplitude spike on injection of a depolarizing current pulse, while their counterparts that were grown in Dulbecco’s modified Eagle’s medium (DMEM) fired series of high amplitude action potentials. Also, neurons cultured in NBM responded to ATP with [Ca2+]i transients due to the release of Ca2+ from intracellular pools, otherwise a property of only astrocytes in DMEM grown cultures.
Presynaptic P2X7Rs
It has repeatedly been reported that when hippocampal brain slices or cultured cerebrocortical cells were pre-incubated with [3H]glutamate or [3H]GABA, ATP/Bz-ATP caused a release of the transmitters previously incorporated into their storage pools. All effects were blocked by conventional P2X7R antagonistic drugs (Sperlagh et al., 2002; Wirkner et al., 2005) or by using P2X7R knockout (KO) mice (Papp et al., 2004).
More convincing evidence for the existence of presynaptic P2X7Rs was supplied, when pinched-off nerve terminals (synaptosomes) were prepared from various parts of the rodent and human brain (mouse cerebellum, Sanchez-Nogueiro et al., 2005; rat caudal brainstem, D’Amico et al., 2010; Curro et al., 2020; neocortex of epileptic humans, Barros-Barbosa et al., 2018). Once again, after loading of the transmitter pools with radioactively labeled excitatory amino acids, subsequent application of ATP/Bz-ATP led to the release of tritium considered to be a measure of transmitter release.
P2X7R stimulation can initiate the influx of Ca2+ through the membrane of mice midbrain synaptosomes (Marin-Garcia et al., 2008). This was documented by microfluorimetric measurements of [Ca2+]i transients induced by ATP/Bz-ATP and the blockade of this effect by Brilliant Blue G.
All these results were considered to be convincing evidence for the exclusive neuronal location of release-regulating P2X7Rs. However, it was not taken into account that the crude synaptosomal preparations used are not really homogenous and contain also glial material, microsomes, and mitochondria (Henn et al., 1976; Olde and Johansson, 1989).
Retinal Ganglion Cells
P2X7R-mRNA and protein have been identified in the rat retina at different postnatal developmental stages (Brändle et al., 1998). In the human retina, P2X7Rs are present in Müller cells, where they function as targets of ATP, coupled with Ca2+-mediated signaling pathways (Pannicke et al., 2000). In preparations obtained from patients suffering from proliferative vitreoretinopathy, stimulation by Bz-ATP of human Müller cells induced larger membrane currents than in preparations from healthy patients (Bringmann et al., 2001).
Genetic association studies have implicated purinergic receptors in the development of age-related macular degeneration (Gu et al., 2013). It was suggested that P2X7Rs identified by their immunoreactivity at retinal ganglion cells may be the reason for the observed apoptotic reactions (Franke et al., 2005). A similar approach was used to explain the deleterious molecular changes initiated by P2X7R activation in the mice glaucomatous retina (Perez de Lara et al., 2019). However, the allegedly specific increase in P2X7R-IR and function of neurons is tightly accompanied by similar changes in retinal glial cells probably being the primary targets of large extracellular ATP concentrations.
Stress-Induced Increase in P2X7Rs
It has repeatedly been suggested that neuronal P2X7Rs may be absent or down-regulated at resting conditions but become expressed on stressful conditions such as temporal lobe epilepsy (Engel et al., 2012; Jimenez-Pacheco et al., 2016), ischemic damage to the brain (Franke et al., 2004), neuropathic pain (Andó and Sperlagh, 2013; Zhang et al., 2019), and neurodegenerative diseases (AD; Diaz-Hernandez et al., 2012; Rodrigues et al., 2015; Huntington’s disease and PD, Diaz-Hernandez et al., 2009; Glaser et al., 2020; MS, Sharp et al., 2008; Grygorowicz et al., 2010). Without any doubt these injurious conditions activate microglial cells as well as astrocytes/oligodendrocytes and facilitate the release of neurodamaging signaling molecules (cytokines, chemokines, reactive oxygen and nitrogen species, glutamate/ATP), possibly facilitating neuronal functions. However, the more marked and most likely spurious immunohistochemical staining of P2X7Rs at neurons is by no way an indication of their existence as functional receptors.
Developmental Models for P2X7Rs
Adult NPCs of the subgranular zone in the mouse hippocampus contain functional P2X7Rs, while in the mature granule cells, the developmental successors of the NPCs, P2X7Rs are missing (Rozmer et al., 2017). Undifferentiated Neuro-2a neuroblastoma cells possess functional P2X7Rs as proven by [Ca2+]i transients and inward current responses caused by Bz-ATP application (Gomez-Villafuertes et al., 2009). Neuronal differentiation of these cells by retinoic acid treatment was associated with decreases in the expression and function of P2X7Rs (Wu et al., 2009). Inhibition of P2X7Rs by pharmacological antagonists or shRNA interference resulted in increased neurogenesis, whereas P2X7R overexpression significantly reduced the formation of neurites (Gomez-Villafuertes et al., 2009; Wu et al., 2009). These data as a whole suggest that during neuronal differentiation of neuroglia-like cells the P2X7R function successively decreases.
In the above paragraphs, we enumerated a few experimental findings purportedly supporting the existence of neuronal P2X7Rs. However, because of the obvious difficulties in obtaining completely glia-free neuronal preparations, considerable doubts remain whether P2X7Rs are situated at the neurons themselves. We have to concede that our criticism does not exclude the possibility of neuronal P2X7Rs but together with the molecular biology evidence summarized in the next section, strongly argues for the absence of this receptor type at neurons.
Knockout and Transgenic P2x7 Receptor Mice Models
Knockout Strategies
The above results acquire significance because of a hypothesis forwarded recently on the non-neuronal localization of P2X7Rs (Rubini et al., 2014; Illes et al., 2017; Khan et al., 2019). It was suggested that glial, especially astrocytic P2X7Rs are indirect causes of neuronal effects. Initial doubts concerning the existence of neuronal P2X7Rs were raised by the observation that polyclonal antibodies binding to this receptor-type stained not only hippocampal structures of wild-type mice but also those of two separate P2X7R KO strains generated by the pharmaceutical companies Pfizer or Glaxo Smith Kline (GSK; Sim et al., 2004). This apparent riddle was resolved some time afterward, by demonstrating that none of the two KO strategies eliminated all immunologically and functionally active splice variants of the genuine receptor P2X7A (Bartlett et al., 2014; Sperlagh and Illes, 2014). In the GSK mouse, a widely expressed and strongly functional P2X7k splice variant escaped inactivation (Nicke et al., 2009). In the Pfizer mouse, C-terminally truncated variants of P2X7A (ΔC) were not depleted; when expressed in HEK293 cells, this variant inefficiently trafficked to the cell surface and agonist-evoked currents were small (Masin et al., 2012). Co-expressed with P2X7A, ΔC-P2X7 acted in a dominant-negative fashion to depress current responses.
More recently, a humanized P2rx7 allele generated by Metzger et al. (2017) was accessible to spatially and temporally controlled Cre-recombinase-mediated inactivation. In contrast to the “classic” KO mice, none of the described P2rx7 splice variants evaded this null allele. By selective disruption and assessment of P2rx7 expression in different brain regions and cell types it was found to exist in astrocytes, oligodendrocytes, and microglia, as repeatedly shown by P2X7-protein measurements (see previous sections), but only in a single neuronal structure of the brain, the glutamatergic pyramidal neurons of the hippocampal CA3 region. It is still unproven that this very circumscriptive expression of mRNA has an appropriate protein readout.
A powerful technique to investigate the cell-type-specific distribution of P2rx7 was for a couple of years the bacterial artificial chromosome (BAC)-transgenic reporter mouse model Tg(P2rx7-EGFP) FY174 Gsat1, a sequence encoding soluble enhanced green fluorescent protein (sEGFP) under the control of the P2rx7 promoter. Experimental results obtained on this mouse model were considered to strongly support the existence of neuronal P2X7Rs in the brain (Garcia-Huerta et al., 2012; Miras-Portugal et al., 2017; Martinez-Frailes et al., 2019). Specifically, status epilepticus induced by kainic acid infusion into the nucleus amygdala demonstrated the neuronal expression and up-regulation of P2X7R-mRNA in the hippocampus (Engel et al., 2012; Jimenez-Pacheco et al., 2016).
Transgenic Strategies
However, later on, the Tg(RP24-114E20P2X7451P-StrepHis-EGFP)Ani reporter mouse was generated (Kaczmarek-Hajek et al., 2018). Extensive characterization of these transgenic mice revealed dominant P2X7-EGFP protein expression in microglia, Bergmann glia, and oligodendrocytes, but not in neurons. These findings were confirmed by microglia- and oligodendrocyte-specific P2X7 deletion and a novel P2X7-specific nanobody. By using this model mouse, status epilepticus induced by intra-amygdala injection of kainic acid confirmed co-localization of P2X7-EGFP with cell-type-specific markers in oligodendrocytes, but not in neurons or astrocytes (Morgan et al., 2020).
Although in both P2X7 reporter models the EGFP constructs are expected to be expressed under the control of the P2rx7 promoter, there were substantial differences in their cell-type-specific expression, and serious concerns were raised regarding the reliability of the reporter expression in the sEGFP mouse (Ramirez-Fernandez et al., 2020). For example, the sEGFP model overexpresses a P2X4 passenger gene, and sEGFP shows clear neuronal localization, but appears to be absent in microglia.
Conclusions
In conclusion, molecular biology and functional data suggest that P2X7Rs are probably missing in neurons of the CNS, while their presence in neuroglial cells (astrocytes, oligodendrocytes) can be taken for granted. Thus, P2X7R-mediated effects in neurons are suggested to be indirect, due to neuroglia-neuron interaction by various signaling molecules. Nonetheless, at the present stage, the existence of neuronal P2X7Rs cannot be negated with absolute certainty.
Author Contributions
PI drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants of the National Key R&D Program of China (2019YFC1709101) and the Project First-Class Disciplines Development of Chengdu University of Traditional Chinese Medicine (CZYHW1901) awarded to build up the “International Collaborative Centre on Big Science Plan for Purine Signalling,” and the Science and Technology Program of Sichuan Province, China (2019YFH0108). The stay and work of PI in Chengdu was supported by a grant from the State Administration of Foreign Experts Affairs (G20190236012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article is dedicated to the memory of the late Professor Geoffrey Burnstock.
Footnotes
References
Agresti, C., Meomartini, M. E., Amadio, S., Ambrosini, E., Serafini, B., Franchini, L., et al. (2005). Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50, 132–144. doi: 10.1002/glia.20160
Andó, R. D., and Sperlagh, B. (2013). The role of glutamate release mediated by extrasynaptic P2X7 receptors in animal models of neuropathic pain. Brain Res. Bull. 93, 80–85. doi: 10.1016/j.brainresbull.2012.09.016
Apolloni, S., Amadio, S., Parisi, C., Matteucci, A., Potenza, R. L., Armida, M., et al. (2014). Spinhennal cord pathology is ameliorated by P2X7 antagonism in a SOD1-mutant mouse model of amyotrophic lateral sclerosis. Dis. Model. Mech. 7, 1101–1109. doi: 10.1242/dmm.017038
Araque, A., Parpura, V., Sanzgiri, R. P., and Haydon, P. G. (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. doi: 10.1016/s0166-2236(98)01349-6
Bardoni, R., Ghirri, A., Zonta, M., Betelli, C., Vitale, G., Ruggieri, V., et al. (2010). Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J. Physiol. 588, 831–846. doi: 10.1113/jphysiol.2009.180570
Barros-Barbosa, A. R., Oliveira, A., Lobo, M. G., Cordeiro, J. M., and Correia-de-Sa, P. (2018). Under stressful conditions activation of the ionotropic P2X7 receptor differentially regulates GABA and glutamate release from nerve terminals of the rat cerebral cortex. Neurochem. Int. 112, 81–95. doi: 10.1016/j.neuint.2017.11.005
Bartlett, R., Stokes, L., and Sluyter, R. (2014). The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 66, 638–675. doi: 10.1124/pr.113.008003
Bedner, P., Jabs, R., and Steinhäuser, C. (2020). Properties of human astrocytes and NG2 glia. Glia 68, 756–767. doi: 10.1002/glia.23725
Bender, K. J., and Trussell, L. O. (2012). The physiology of the axon initial segment. Annu. Rev. Neurosci. 35, 249–265. doi: 10.1146/annurev-neuro-062111-150339
Bergles, D. E., Roberts, J. D., Somogyi, P., and Jahr, C. E. (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. doi: 10.1038/35012083
Bhattacharya, A., and Biber, K. (2016). The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 64, 1772–1787. doi: 10.1002/glia.23001
Brändle, U., Kohler, K., and Wheeler-Schilling, T. H. (1998). Expression of the P2X7-receptor subunit in neurons of the rat retina. Brain Res. Mol. Brain Res. 62, 106–109. doi: 10.1016/s0169-328x(98)00254-x
Bringmann, A., Pannicke, T., Moll, V., Milenkovic, I., Faude, F., Enzmann, V., et al. (2001). Upregulation of P2X7 receptor currents in Müller glial cells during proliferative vitreoretinopathy. Invest Ophthalmol. Vis. Sci. 42, 860–867.
Butt, A. M. (2011). ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin. Cell Dev. Biol. 22, 205–213. doi: 10.1016/j.semcdb.2011.02.023
Carmo, M. R., Menezes, A. P., Nunes, A. C., Pliassova, A., Rolo, A. P., Palmeira, C. M., et al. (2014). The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 81, 142–152. doi: 10.1016/j.neuropharm.2014.01.045
Cheung, G., Chever, O., and Rouach, N. (2014). Connexons and pannexons: newcomers in neurophysiology. Front. Cell Neurosci. 8:348. doi: 10.3389/fncel.2014.00348
Cho, J. H., Choi, I. S., and Jang, I. S. (2010). P2X7 receptors enhance glutamate release in hippocampal hilar neurons. Neuroreport 21, 865–870. doi: 10.1097/WNR.0b013e32833d9142
Choi, S. S., Lee, H. J., Lim, I., Satoh, J., and Kim, S. U. (2014). Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One 9:e92325. doi: 10.1371/journal.pone.0092325
Curro, D., Navarra, P., Samengo, I., and Martire, M. (2020). P2X7 receptors exert a permissive effect on the activation of presynaptic AMPA receptors in rat trigeminal caudal nucleus glutamatergic nerve terminals. J. Headache Pain 21:83. doi: 10.1186/s10194-020-01153-y
D’Amico, M., Samengo, I., Navarra, P., Taglialatela, M., and Martire, M. (2010). AMPA- and P2X7-receptor-mediated facilitation of [3H]d-aspartate release from nerve terminals isolated from the rat caudal brainstem. Neurochem. Int. 57, 623–628. doi: 10.1016/j.neuint.2010.07.009
Degen, J., Dublin, P., Zhang, J., Dobrowolski, R., Jokwitz, M., Karram, K., et al. (2012). Dual reporter approaches for identification of Cre efficacy and astrocyte heterogeneity. FASEB J. 26, 4576–4583. doi: 10.1096/fj.12-207183
del Puerto, A., Diaz-Hernandez, J. I., Tapia, M., Gomez-Villafuertes, R., Benitez, M. J., Zhang, J., et al. (2012). Adenylate cyclase 5 coordinates the action of ADP, P2Y1, P2Y13 and ATP-gated P2X7 receptors on axonal elongation. J. Cell Sci. 125, 176–188. doi: 10.1242/jcs.091736
Del Puerto, A., Fronzaroli-Molinieres, L., Perez-Alvarez, M. J., Giraud, P., Carlier, E., Wandosell, F., et al. (2015). ATP-P2X7 Receptor Modulates Axon Initial Segment Composition and Function in Physiological Conditions and Brain Injury. Cereb. Cortex 25, 2282–2294. doi: 10.1093/cercor/bhu035
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L., and Falzoni, S. (2017). The P2X7 Receptor in Infection and Inflammation. Immunity 47, 15–31. doi: 10.1016/j.immuni.2017.06.020
Di Virgilio, F., Schmalzing, G., and Markwardt, F. (2018). The Elusive P2X7 Macropore. Trends Cell Biol. 28, 392–404. doi: 10.1016/j.tcb.2018.01.005
Diaz-Hernandez, J. I., Gomez-Villafuertes, R., Leon-Otegui, M., Hontecillas-Prieto, L., del Puerto, A., Trejo, J. L., et al. (2012). In vivo P2X7 inhibition reduces amyloid plaques in alzheimer’s disease through GSK3β and secretases. Neurobiol. Aging 33, 1816–1828. doi: 10.1016/j.neurobiolaging.2011.09.040
Diaz-Hernandez, M., del Puerto, A., Diaz-Hernandez, J. I., Diez-Zaera, M., Lucas, J. J., Garrido, J. J., et al. (2008). Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J. Cell Sci. 121, 3717–3728. doi: 10.1242/jcs.034082
Diaz-Hernandez, M., Diez-Zaera, M., Sanchez-Nogueiro, J., Gomez-Willafuertes, R., Canals, J. M., Alberch, J., et al. (2009). Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 23, 1893–1906. doi: 10.1096/fj.08-122275
Domercq, M., and Matute, C. (2019). Targeting P2X4 and P2X7 receptors in multiple sclerosis. Curr. Opin. Pharmacol. 47, 119–125. doi: 10.1016/j.coph.2019.03.010
Domercq, M., Perez-Samartin, A., Aparicio, D., Alberdi, E., Pampliega, O., and Matute, C. (2010). P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 58, 730–740. doi: 10.1002/glia.20958
Duan, S., Anderson, C. M., Keung, E. C., Chen, Y., Chen, Y., and Swanson, R. A. (2003). P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003
Engel, T., Gomez-Villafuertes, R., Tanaka, K., Mesuret, G., Sanz-Rodriguez, A., Garcia-Huerta, P., et al. (2012). Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 26, 1616–1628. doi: 10.1096/fj.11-196089
Fellin, T., Pozzan, T., and Carmignoto, G. (2006). Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J. Biol. Chem. 281, 4274–4284. doi: 10.1074/jbc.M510679200
Ficker, C., Rozmer, K., Kato, E., Ando, R. D., Schumann, L., Krügel, U., et al. (2014). Astrocyte-neuron interaction in the substantia gelatinosa of the spinal cord dorsal horn via P2X7 receptor-mediated release of glutamate and reactive oxygen species. Glia 62, 1671–1686. doi: 10.1002/glia.22707
Fields, R. D. (2006). Nerve impulses regulate myelination through purinergic signalling. Novartis Found. Symp. 276, 148–158.
Fischer, W., Appelt, K., Grohmann, M., Franke, H., Nörenberg, W., and Illes, P. (2009). Increase of intracellular Ca2+ by P2X and P2Y receptor-subtypes in cultured cortical astroglia of the rat. Neuroscience 160, 767–783. doi: 10.1016/j.neuroscience.2009.02.026
Franke, H., Günther, A., Grosche, J., Schmidt, R., Rossner, S., Reinhardt, R., et al. (2004). P2X7 receptor expression after ischemia in the cerebral cortex of rats. J. Neuropathol. Exp. Neurol. 63, 686–699. doi: 10.1093/jnen/63.7.686
Franke, H., Klimke, K., Brinckmann, U., Grosche, J., Francke, M., Sperlagh, B., et al. (2005). P2X7 receptor-mRNA and -protein in the mouse retina; changes during retinal degeneration in BALBCrds mice. Neurochem. Int. 47, 235–242. doi: 10.1016/j.neuint.2005.04.022
Franke, H., Verkhratsky, A., Burnstock, G., and Illes, P. (2012). Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 8, 629–657. doi: 10.1007/s11302-012-9300-0
Fumagalli, M., Brambilla, R., D’Ambrosi, N., Volonte, C., Matteoli, M., Verderio, C., et al. (2003). Nucleotide-mediated calcium signaling in rat cortical astrocytes, Role of P2X and P2Y receptors. Glia 43, 218–230. doi: 10.1002/glia.10248
Gao, P., Ding, X., Khan, T. M., Rong, W., Franke, H., and Illes, P. (2017). P2X7 receptor-sensitivity of astrocytes and neurons in the substantia gelatinosa of organotypic spinal cord slices of the mouse depends on the length of the culture period. Neuroscience 349, 195–207. doi: 10.1016/j.neuroscience.2017.02.030
Garcia-Huerta, P., Diaz-Hernandez, M., Delicado, E. G., Pimentel-Santillana, M., Miras-Portugal, M. T., and Gomez-Villafuertes, R. (2012). The specificity protein factor Sp1 mediates transcriptional regulation of P2X7 receptors in the nervous system. J. Biol. Chem. 287, 44628–44644. doi: 10.1074/jbc.M112.390971
Glaser, T., Andrejew, R., Oliveira-Giacomelli, A., Ribeiro, D. E., Bonfim, M. L., Ye, Q., et al. (2020). Purinergic Receptors in Basal Ganglia Diseases: Shared Molecular Mechanisms between Huntington’s and Parkinson’s Disease. Neurosci. Bull. 36, 1299–1314. doi: 10.1007/s12264-020-00582-8
Gomez-Villafuertes, R., del Puerto, A., Diaz-Hernandez, M., Bustillo, D., Diaz-Hernandez, J. I., Huerta, P. G., et al. (2009). Ca2+/calmodulin-dependent kinase II signalling cascade mediates P2X7 receptor-dependent inhibition of neuritogenesis in neuroblastoma cells. FEBS J. 276, 5307–5325. doi: 10.1111/j.1742-4658.2009.07228.x
Gordon, G. R., Baimoukhametova, D. V., Hewitt, S. A., Rajapaksha, W. R., Fisher, T. E., and Bains, J. S. (2005). Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat. Neurosci. 8, 1078–1086. doi: 10.1038/nn1498
Grygorowicz, T., Struzynska, L., Sulkowski, G., Chalimoniuk, M., and Sulejczak, D. (2010). Temporal expression of P2X7 purinergic receptor during the course of experimental autoimmune encephalomyelitis. Neurochem. Int. 57, 823–829. doi: 10.1016/j.neuint.2010.08.021
Gu, B. J., Baird, P. N., Vessey, K. A., Skarratt, K. K., Fletcher, E. L., Fuller, S. J., et al. (2013). A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age-related macular degeneration. FASEB J. 27, 1479–1487. doi: 10.1096/fj.12-215368
Haberlandt, C., Derouiche, A., Wyczynski, A., Haseleu, J., Pohle, J., Karram, K., et al. (2011). Gray matter NG2 cells display multiple Ca2+-signaling pathways and highly motile processes. PLoS One 6:e17575. doi: 10.1371/journal.pone.0017575
Halassa, M. M., and Haydon, P. G. (2010). Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 72, 335–355. doi: 10.1146/annurev-physiol-021909-135843
Harkat, M., Peverini, L., Cerdan, A. H., Dunning, K., Beudez, J., Martz, A., et al. (2017). On the permeation of large organic cations through the pore of ATP-gated P2X receptors. Proc. Natl. Acad. Sci. U S A 114, E3786–E3795. doi: 10.1073/pnas.1701379114
Henn, F. A., Anderson, D. J., and Rustad, D. G. (1976). Glial contamination of synaptosomal fractions. Brain Res. 101, 341–344. doi: 10.1016/0006-8993(76)90274-2
Hu, H. Z., Gao, N., Lin, Z., Gao, C., Liu, S., Ren, J., et al. (2001). P2X7 receptors in the enteric nervous system of guinea-pig small intestine. J. Comp. Neurol. 440, 299–310. doi: 10.1002/cne.1387
Iglesias, R., Locovei, S., Roque, A., Alberto, A. P., Dahl, G., Spray, D. C., et al. (2008). P2X7 receptor-pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 295, C752–C760. doi: 10.1152/ajpcell.00228.2008
Illes, P., Burnstock, G., and Tang, Y. (2019a). Astroglia-Derived ATP Modulates CNS Neuronal Circuits. Trends Neurosci. 42, 885–898. doi: 10.1016/j.tins.2019.09.006
Illes, P., Khan, T. M., and Rubini, P. (2017). Neuronal P2X7 Receptors Revisited: Do They Really Exist? J. Neurosci. 37, 7049–7062. doi: 10.1523/JNEUROSCI.3103-16.2017
Illes, P., Rubini, P., Huang, L., and Tang, Y. (2019b). The P2X7 receptor: a new therapeutic target in Alzheimer’s disease. Expert Opin. Ther. Targets 23, 165–176. doi: 10.1080/14728222.2019.1575811
Illes, P., Rubini, P., Ulrich, H., Zhao, Y., and Tang, Y. (2020). Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 9:1108. doi: 10.3390/cells9051108
Illes, P., Verkhratsky, A., Burnstock, G., and Franke, H. (2012). P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neuroscientist 18, 422–438. doi: 10.1177/1073858411418524
Jabs, R., Matthias, K., Grote, A., Grauer, M., Seifert, G., and Steinhäuser, C. (2007). Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia 55, 1648–1655. doi: 10.1002/glia.20580
James, G., and Butt, A. M. (2002). P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur. J. Pharmacol. 447, 247–260. doi: 10.1016/s0014-2999(02)01756-9
Jimenez-Pacheco, A., Diaz-Hernandez, M., Arribas-Blazquez, M., Sanz-Rodriguez, A., Olivos-Ore, L. A., Artalejo, A. R., et al. (2016). Transient p2X7 receptor antagonism produces lasting reductions in spontaneous seizures and gliosis in experimental temporal lobe epilepsy. J. Neurosci. 36, 5920–5932. doi: 10.1523/JNEUROSCI.4009-15.2016
Kaczmarek-Hajek, K., Zhang, J., Kopp, R., Grosche, A., Rissiek, B., Saul, A., et al. (2018). Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife 7:e36217. doi: 10.7554/eLife.36217
Karasawa, A., Michalski, K., Mikhelzon, P., and Kawate, T. (2017). The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. Elife 6:e31186. doi: 10.7554/eLife.31186
Khakpay, R., Polster, D., Köles, L., Skorinkin, A., Szabo, B., Wirkner, K., et al. (2010). Potentiation of the glutamatergic synaptic input to rat locus coeruleus neurons by P2X7 receptors. Purinergic Signal 6, 349–359. doi: 10.1007/s11302-010-9198-3
Khan, M. T., Deussing, J., Tang, Y., and Illes, P. (2019). Astrocytic rather than neuronal P2X7 receptors modulate the function of the tri-synaptic network in the rodent hippocampus. Brain Res. Bull. 151, 164–173. doi: 10.1016/j.brainresbull.2018.07.016
Koo, J. W., and Duman, R. S. (2009). Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr. Opin. Investig. Drugs 10, 664–671.
Kukley, M., Barden, J. A., Steinhäuser, C., and Jabs, R. (2001). Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia 36, 11–21. doi: 10.1002/glia.1091
Lalo, U., Palygin, O., Rasooli-Nejad, S., Andrew, J., Haydon, P. G., and Pankratov, Y. (2014b). Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS. Biol. 12:e1001747. doi: 10.1371/journal.pbio.1001747
Lalo, U., Rasooli-Nejad, S., and Pankratov, Y. (2014a). Exocytosis of gliotransmitters from cortical astrocytes, implications for synaptic plasticity and aging. Biochem. Soc. Trans. 42, 1275–1281. doi: 10.1042/BST20140163
Leeson, H. C., Chan-Ling, T., Lovelace, M. D., Brownlie, J. C., Gu, B. J., and Weible, M. W. (2019). P2X7 receptor signaling during adult hippocampal neurogenesis. Neural Regen. Res. 14, 1684–1694. doi: 10.4103/1673-5374.257510
Locovei, S., Scemes, E., Qiu, F., Spray, D. C., and Dahl, G. (2007). Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 581, 483–488. doi: 10.1016/j.febslet.2006.12.056
Maes, M., Song, C., and Yirmiya, R. (2012). Targeting IL-1 in depression. Expert Opin. Ther. Targets 16, 1097–1112. doi: 10.1517/14728222.2012.718331
Marin-Garcia, P., Sanchez-Nogueiro, J., Gomez-Villafuertes, R., Leon, D., and Miras-Portugal, M. T. (2008). Synaptic terminals from mice midbrain exhibit functional P2X7 receptor. Neuroscience 151, 361–373. doi: 10.1016/j.neuroscience.2007.10.038
Martin, E., Amar, M., Dalle, C., Youssef, I., Boucher, C., Le, D. C., et al. (2019). New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol. Psychiatry 24, 108–125. doi: 10.1038/s41380-018-0108-3
Martinez-Frailes, C., Di Lauro, C., Bianchi, C., de Diego-Garcia, L., Sebastian-Serrano, A., Bosca, L., et al. (2019). Amyloid Peptide Induced Neuroinflammation Increases the P2X7 Receptor Expression in Microglial Cells, Impacting on Its Functionality. Front. Cell. Neurosci. 13:143. doi: 10.3389/fncel.2019.00143
Masin, M., Young, C., Lim, K., Barnes, S. J., Xu, X. J., Marschall, V., et al. (2012). Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor, re-evaluation of P2X7 knockouts. Br. J. Pharmacol. 165, 978–993. doi: 10.1111/j.1476-5381.2011.01624.x
Matute, C. (2008). P2X7 receptors in oligodendrocytes: a novel target for neuroprotection. Mol. Neurobiol. 38, 123–128. doi: 10.1007/s12035-008-8028-x
Matute, C. (2011). Glutamate and ATP signalling in white matter pathology. J. Anat. 219, 53–64. doi: 10.1111/j.1469-7580.2010.01339.x
Matute, C., Torre, I., Perez-Cerda, F., Perez-Samartin, A., Alberdi, E., Etxebarria, E., et al. (2007). P2X7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 27, 9525–9533. doi: 10.1523/JNEUROSCI.0579-07.2007
Metzger, M. W., Walser, S. M., Aprile-Garcia, F., Dedic, N., Chen, A., Holsboer, F., et al. (2017). Genetically dissecting P2rx7 expression within the central nervous system using conditional humanized mice. Purinergic Signal 13, 153–170. doi: 10.1007/s11302-016-9546-z
Miras-Portugal, M. T., Sebastian-Serrano, A., de Diego, G. L., and Diaz-Hernandez, M. (2017). Neuronal p2X7 receptor, involvement in neuronal physiology and pathology. J. Neurosci. 37, 7063–7072. doi: 10.1523/JNEUROSCI.3104-16.2017
Morgan, J., Alves, M., Conte, G., Menendez-Mendez, A., de Diego-Garcia, L., de Leo, G., et al. (2020). Characterization of the expression of the ATP-gated P2X7 receptor following status epilepticus and during epilepsy using a P2X7-EGFP reporter mouse. Neurosci. Bull. 36, 1242–1258. doi: 10.1007/s12264-020-00573-9
Munoz, F. M., Patel, P. A., Gao, X., Mei, Y., Xia, J., Gilels, S., et al. (2020). Reactive oxygen species play a role in P2X7 receptor-mediated IL-6 production in spinal astrocytes. Purinergic Signal 16, 97–107. doi: 10.1007/s11302-020-09691-5
Nicke, A., Kuan, Y. H., Masin, M., Rettinger, J., Marquez-Klaka, B., Bender, O., et al. (2009). A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J. Biol. Chem. 284, 25813–25822. doi: 10.1074/jbc.M109.033134
Nishiyama, A., Boshans, L., Goncalves, C. M., Wegrzyn, J., and Patel, K. D. (2016). Lineage, fate and fate potential of NG2-glia. Brain Res. 1638, 116–128. doi: 10.1016/j.brainres.2015.08.013
Nörenberg, W., Schunk, J., Fischer, W., Sobottka, H., Riedel, T., Oliveira, J. F., et al. (2010). Electrophysiological classification of P2X7 receptors in rat cultured neocortical astroglia. Br. J. Pharmacol. 160, 1941–1952. doi: 10.13287/j.1001-9332.202101.027
Olde, B., and Johansson, G. (1989). Heterogeneity of a crude synaptosomal preparation, studied by affinity partitioning using hexaethonium-poly(ethylene glycol). Mol. Cell Biochem. 87, 153–160.
Oliveira, J. F., Riedel, T., Leichsenring, A., Heine, C., Franke, H., Krügel, U., et al. (2011). Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb. Cortex 21, 806–820. doi: 10.1093/cercor/bhq154
Pannasch, U., and Rouach, N. (2013). Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 36, 405–417. doi: 10.1016/j.tins.2013.04.004
Pannicke, T., Fischer, W., Biedermann, B., Schädlich, H., Grosche, J., Faude, F., et al. (2000). P2X7 receptors in Müller glial cells from the human retina. J. Neurosci. 20, 5965–5972. doi: 10.1523/jneurosci.20-16-05965.2000
Papp, L., Vizi, E. S., and Sperlagh, B. (2004). Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor−/− mice. Neuroreport 15, 2387–2391. doi: 10.1097/00001756-200410250-00017
Pelegrin, P., and Surprenant, A. (2006). Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082. doi: 10.1038/sj.emboj.7601378
Pelvig, D. P., Pakkenberg, H., Stark, A. K., and Pakkenberg, B. (2008). Neocortical glial cell numbers in human brains. Neurobiol. Aging 29, 1754–1762. doi: 10.1016/neurobiolaging.2007.04.013
Perez de Lara, M., Aviles-Trigueros, M., Guzman-Aranguez, A., Valiente-Soriano, F. J., de la Villa, P., Vidal-Sanz, M., et al. (2019). Potential role of P2X7 receptor in neurodegenerative processes in a murine model of glaucoma. Brain Res. Bull. 150, 61–74. doi: 10.1016/j.brainresbull.2019.05.006
Ramirez-Fernandez, A., Urbina-Trevino, L., Conte, G., Alves, M., Rissiek, B., Durner, A., et al. (2020). Deviant reporter expression and P2X4 passenger gene overexpression in the soluble EGFP BAC transgenic P2X7 reporter mouse model. Sci. Rep. 10:19876. doi: 10.1038/s41598-020-76428-0
Rodrigues, R.J., Tome, A.R., and Cunha, R.A. (2015). ATP as a multi-target danger signal in the brain. Front. Neurosci. 9:148. doi: 10.3389/fnins.2015.00148
Rozmer, K., Gao, P., Araujo, M. G. L., Khan, M. T., Liu, J., Rong, W., et al. (2017). Pilocarpine-induced status epilepticus increases the sensitivity of P2X7 and P2Y1 receptors to nucleotides at neural progenitor cells of the juvenile rodent hippocampus. Cereb. Cortex 27, 3568–3585. doi: 10.1093/cercor/bhw178
Rubini, P., Pagel, G., Mehri, S., Marquardt, P., Riedel, T., and Illes, P. (2014). Functional P2X7 receptors at cultured hippocampal astrocytes but not neurons. Naunyn Schmiedebergs Arch. Pharmacol. 387, 943–954. doi: 10.1007/s00210-014-1005-1
Rubini, P., Pinkwart, C., Franke, H., Gerevich, Z., Nörenberg, W., and Illes, P. (2006). Regulation of intracellular Ca2+ by P2Y1 receptors may depend on the developmental stage of cultured rat striatal neurons. J. Cell. Physiol. 209, 81–93. doi: 10.1002/jcp.20705
Sanchez-Nogueiro, J., Marin-Garcia, P., and Miras-Portugal, M. T. (2005). Characterization of a functional P2X7-like receptor in cerebellar granule neurons from P2X7 knockout mice. FEBS Lett. 579, 3783–3788. doi: 10.1016/j.febslet.2005.05.073
Seifert, G., and Steinhäuser, C. (2018). Heterogeneity and function of hippocampal macroglia. Cell Tissue Res. 373, 653–670. doi: 10.1007/s00441-017-2746-1
Sharp, A. J., Polak, P. E., Simonini, V., Lin, S. X., Richardson, J. C., Bongarzone, E. R., et al. (2008). P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J. Neuroinflammation 5:33. doi: 10.1186/1742-2094-5-33
Shieh, C. H., Heinrich, A., Serchov, T., van Calker, D., and Biber, K. (2014). P2X7-dependent, but differentially regulated release of IL-6, CCL2 and TNF-α in cultured mouse microglia. Glia 62, 592–607. doi: 10.1002/glia.22628
Sim, J. A., Young, M. T., Sung, H. Y., North, R. A., and Surprenant, A. (2004). Reanalysis of P2X7 receptor expression in rodent brain. J. Neurosci. 24, 6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004
Smart, M. L., Gu, B., Panchal, R. G., Wiley, J., Cromer, B., Williams, D. A., et al. (2003). P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J. Biol. Chem. 278, 8853–8860. doi: 10.1074/jbc.M211094200
Sperlagh, B., and Illes, P. (2014). P2X7 receptor, an emerging target in central nervous system diseases. Trends Pharmacol. Sci. 35, 537–547. doi: 10.1016/j.tips.2014.08.002
Sperlagh, B., Köfalvi, A., Deuchars, J., Atkinson, L., Milligan, C. J., Buckley, N. J., et al. (2002). Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem. 81, 1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x
Suadicani, S. O., Brosnan, C. F., and Scemes, E. (2006). P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006
Suadicani, S. O., Iglesias, R., Wang, J., Dahl, G., Spray, D. C., and Scemes, E. (2012). ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia 60, 1106–1116. doi: 10.1002/glia.22338
Surprenant, A., Rassendren, F., Kawashima, E., North, R. A., and Buell, G. (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738. doi: 10.1126/science.272.5262.735
Tang, Y., and Illes, P. (2017). Regulation of adult neural progenitor cell functions by purinergic signaling. Glia 65, 213–230. doi: 10.1002/glia.23056
Theis, M., and Giaume, C. (2012). Connexin-based intercellular communication and astrocyte heterogeneity. Brain Res. 1487, 88–98. doi: 10.1016/j.brainres.2012.06.045
Ugur, M., and Ugur, ÖO. (2019). A Mechanism-Based Approach to P2X7 Receptor Action. Mol. Pharmacol. 95, 442–450. doi: 10.1124/mol.118.115022
Valdez-Morales, E., Guerrero-Alba, R., Linan-Rico, A., Espinosa-Luna, R., Zarazua-Guzman, S., Miranda-Morales, M., et al. (2011). P2X7 receptors contribute to the currents induced by ATP in guinea pig intestinal myenteric neurons. Eur. J. Pharmacol. 668, 366–372. doi: 10.1016/j.ejphar.2011.07.019
Verkhratsky, A., and Nedergaard, M. (2018). Physiology of astroglia. Physiol. Rev. 98, 239–389. doi: 10.1152/physrev.00042.2016
Verkhratsky, A., Pankratov, Y., Lalo, U., and Nedergaard, M. (2012). P2X receptors in neuroglia. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1:10. doi: 10.1002/wmts.12
Virginio, C., MacKenzie, A., Rassendren, F. A., North, R. A., and Surprenant, A. (1999). Pore dilation of neuronal P2X receptor channels. Nat. Neurosci. 2, 315–321. doi: 10.1038/7225
Wang, C. M., Chang, Y. Y., Kuo, J. S., and Sun, S. H. (2002). Activation of P2X7 receptors induced [3H]GABA release from the RBA-2 type-2 astrocyte cell line through a Cl−/HCO3−-dependent mechanism. Glia 37, 8–18. doi: 10.1002/glia.10004
Wirkner, K., Köfalvi, A., Fischer, W., Günther, A., Franke, H., Gröger-Arndt, H., et al. (2005). Supersensitivity of P2X receptors in cerebrocortical cell cultures after in vitro ischemia. J. Neurochem. 95, 1421–1437. doi: 10.1111/j.1471-4159.2005.03465.x
Wu, P. Y., Lin, Y. C., Chang, C. L., Lu, H. T., Chin, C. H., Hsu, T. T., et al. (2009). Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro-2a neuroblastoma cells. Cell Signal. 21, 881–891. doi: 10.1016/j.cellsig.2009.01.036
Xia, J., Lim, J. C., Lu, W., Beckel, J. M., Macarak, E. J., Laties, A. M., et al. (2012). Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J. Physiol. 590, 2285–2304. doi: 10.1113/jphysiol.2012.227983
Xiong, Y., Sun, S., Teng, S., Jin, M., and Zhou, Z. (2018). Ca2+-dependent and Ca2+-independent ATP release in astrocytes. Front. Mol. Neurosci. 11:224. doi: 10.3389/fnmol.2018.00224
Keywords: P2X7 receptors, astrocytes, oligodendrocytes, neurons, signaling molecules
Citation: Zhao Y-F, Tang Y and Illes P (2021) Astrocytic and Oligodendrocytic P2X7 Receptors Determine Neuronal Functions in the CNS. Front. Mol. Neurosci. 14:641570. doi: 10.3389/fnmol.2021.641570
Received: 14 December 2020; Accepted: 19 January 2021;
Published: 09 February 2021.
Edited by:
David Blum, Inserm U1172 Centre de Recherche Jean Pierre Aubert, FranceReviewed by:
Cecile Delarasse, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceRodrigo A. Cunha, University of Coimbra, Portugal
Copyright © 2021 Zhao, Tang and Illes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Illes, peter.illes@medizin.uni-leipzig.de
 Ya-Fei Zhao
Ya-Fei Zhao Yong Tang
Yong Tang Peter Illes
Peter Illes