
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Neurosci., 04 January 2019
Sec. Pain Mechanisms and Modulators
Volume 11 - 2018 | https://doi.org/10.3389/fnmol.2018.00464
This article is part of the Research TopicMetabotropic Glutamate Receptors and Neurological/Psychiatric DisordersView all 12 articles
Pain is an essential protective mechanism meant to prevent tissue damages in organisms. On the other hand, chronic or persistent pain caused, for example, by inflammation or nerve injury is long lasting and responsible for long-term disability in patients. Therefore, chronic pain and its management represents a major public health problem. Hence, it is critical to better understand chronic pain molecular mechanisms to develop innovative and efficient drugs. Over the past decades, accumulating evidence has demonstrated a pivotal role of glutamate in pain sensation and transmission, supporting glutamate receptors as promising potential targets for pain relieving drug development. Glutamate is the most abundant excitatory neurotransmitter in the brain. Once released into the synapse, glutamate acts through ionotropic glutamate receptors (iGluRs), which are ligand-gated ion channels triggering fast excitatory neurotransmission, and metabotropic glutamate receptors (mGluRs), which are G protein-coupled receptors modulating synaptic transmission. Eight mGluRs subtypes have been identified and are divided into three classes based on their sequence similarities and their pharmacological and biochemical properties. Of note, all mGluR subtypes (except mGlu6 receptor) are expressed within the nociceptive pathways where they modulate pain transmission. This review will address the role of mGluRs in acute and persistent pain processing and emerging pharmacotherapies for pain management.
Acute pain is an important protective function, detecting harmful stimuli and preventing body damage. However, chronic pain persists for a long time after the initial affliction, losing its role as a warning signal and must be considered as a disease per se. Patients suffering from chronic pain not only experience exacerbated responses to both painful (hyperalgesia) and non-painful stimuli (allodynia) (Sandkühler, 2009) but also frequently express emotional and cognitive impairments often resulting in anxiety and depression (McWilliams et al., 2003; Moriarty et al., 2011; Bushnell et al., 2013).
Glutamate is the main excitatory neurotransmitter in the nervous system of adult mammals. Among the neurotransmitters involved in pain transmission from the periphery to the brain, glutamate has a leading role. Glutamate is also involved in central sensitization, which is associated with chronic pain. Glutamate action is mediated through ionotropic and metabotropic receptors. Ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels involved in the fast synaptic response to glutamate. Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors that are responsible for the slow neuromodulatory response to glutamate. Eight mGluRs have been identified so far. They are named mGlu1 to mGlu8 receptors by chronological order of discovery. Later, based on their sequence homology, signalization and pharmacology, they were subdivided in three groups. Group I mGluRs (mGlu1 and 5) are canonically coupled to Gαq/11 and lead to phospholipase C (PLC) activation that promotes neuronal excitability and are mostly expressed postsynaptically. In contrast, group II (mGlu2 and 3) and group III (mGlu4, 6, 7, and 8) mGluRs are predominantly coupled to Gαi/o triggering adenylate cyclase (AC) inhibition. Group II and III mGluRs also regulate neuronal excitability and synaptic transmission through Gβγ subunits, which notably inhibit voltage-sensitive calcium channels and activate potassium channels. Both group II and group III mGluRs are mainly localized on presynaptic terminals. Both iGluRs and mGluRs (except mGlu6 receptor) are expressed all along the pain neuraxis where they shape the transmission of pain information (Figure 1). They are also involved in the induction and the maintenance of central sensitization of the pain pathway (Latremoliere and Woolf, 2009). This phenomenon is associated with hyperexcitability of the glutamatergic system which leads to the development of the main sensory symptoms observed in persons suffering from chronic pain.
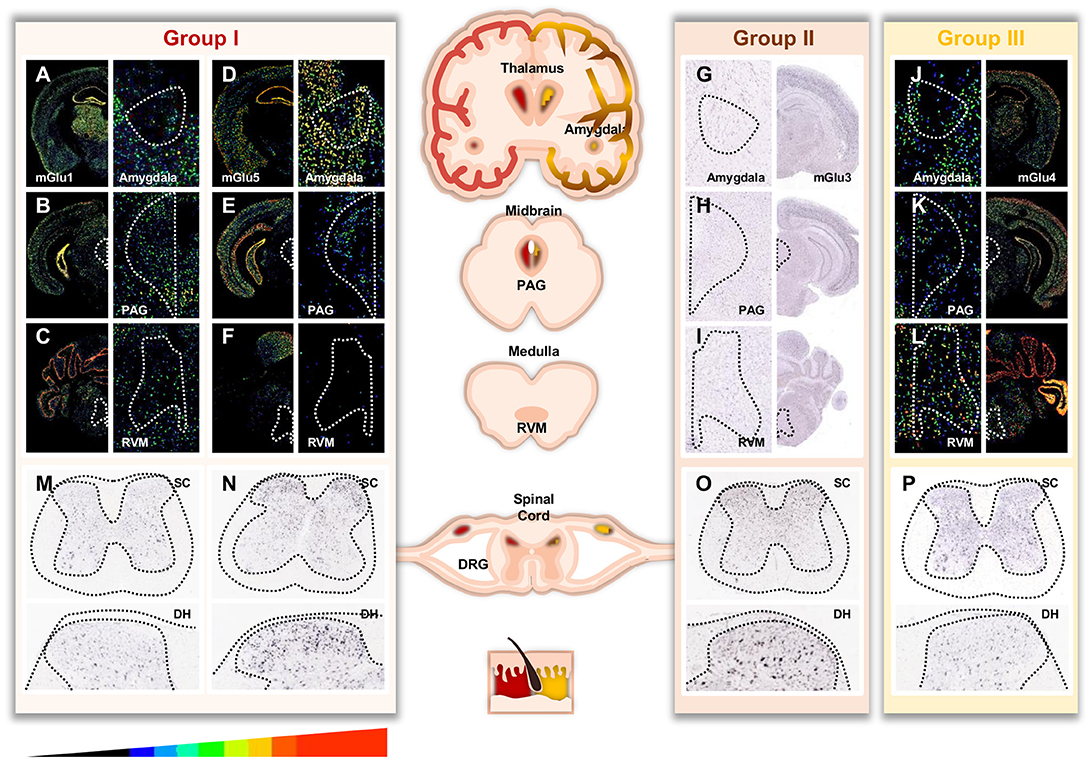
Figure 1. Distribution of mGluRs throughout important areas involved in pain. For (A-F, J-L) pictures, masks with pseudo colors were used to color scale the relative expression level of mGluR transcripts across sections (scale displayed at the bottom of the figure). For (G-I, M-P), no expression filter was applied to recolour the ISH pictures. Image credit: Allen Institute. Masked ISH images of mGlu1 (A) and mGlu5 (B) transcripts in mice coronal section, notably in Thalamus and Amygdala. CeA (central nucleus of the amygdala) is magnified in the right panels (white dotted line, drawn according to the Allen Brain Atlas). Distribution of mGlu1 (B,C) and mGlu5 (E,F) mRNA in mice midbrain and medulla sections involved in descending modulation of pain. Magnification of the periaqueductal gray (PAG) and rostro ventral medulla (RVM) areas are shown in the right panels (white dotted line, drawn according to the Allen Brain Atlas). ISH images of mGlu3 (G) transcript in mice coronal section, notably in Thalamus and Amygdala. CeA is magnified in the left panel (white dotted line). Distribution of mGlu3 (H,I) mRNA in mice midbrain and medulla. Magnification of the PAG and RVM nucleus are shown in the left panels (white dotted line). Masked ISH images of mGlu4 (J) transcript in mice coronal section, notably in Thalamus and Amygdala. CeA is magnified in the left panel (white dotted line). Distribution of mGlu4 (K,L) mRNA in mice midbrain and medulla. Magnification of the PAG and RVM nucleus are shown in the left panels (white dotted line). Images are available for mGlu1 receptor (GMR1 gene) at http://mouse.brain-map.org/experiment/show/79591723, for mGlu5 receptor (GRM5 gene) at http://mouse.brain-map.org/experiment/show/73512423, for mGlu3 receptor (GMR3 gene) at http://mouse.brain-map.org/experiment/show/539, and for mGlu4 receptor (GRM4 gene) at http://mouse.brain-map.org/experiment/show/71247631. Distribution of mGlu1 (M), mGlu5 (N), mGlu3 (O), mGlu4 (P) transcripts in mice spinal cord. Bottom panels are magnification of the dorsal horn. Images are available for mGlu1 at http://mousespinal.brain-map.org/imageseries/show.html?id=100036413, for mGlu5 receptor at http://mousespinal.brain-map.org/imageseries/show.html?id=100033614, for mGlu3 receptor at http://mousespinal.brain-map.org/imageseries/show.html?id=100039062 and for mGlu4 receptor at http://mousespinal.brain-map.org/imageseries/show.html?id=100018200.
Acting on the molecular mechanisms of glutamatergic transmission may, therefore, be a way of developing future analgesics counteracting chronic pain. However, even if iGluR selective antagonists have proven efficacious in releasing several pain states, drastically inhibiting glutamatergic transmission via iGluR blocking inevitably induces numerous side effects, notably hallucinations, ataxia and sedation (Bleakman et al., 2006). Therefore, the strategy of pharmacological modulation of mGluRs for the treatment of pain has been favored and significant effort has been devoted to better understanding the expression, the function and the role of these receptors in pain processing. The present review will focus on the role of mGluRs in acute and chronic pain at different levels–from the periphery to higher brain center involved in the perception and modulation of pain–and report the recent advances in the pharmacological strategy used to achieve mGluRs modulation.
Both orthosteric and allosteric ligands are available for pharmacological manipulation of mGluRs. Given their different binding sites, orthosteric ligands and allosteric modulators have specific pharmacological properties.
Orthosteric ligands are binding in the same pocket than the natural ligand (the orthosteric pocket). They are also referred to as competitive ligands. In mGluRs, the glutamate-binding pocket is located in the extracellular domain of the receptor. Due to the high degree of conservation of the glutamate-binding pocket among the mGluRs, the identification of subtype selective ligands is highly challenging. Therefore, many orthosteric ligands are selective for a specific group but do not discriminate between receptors within the group. The typical specific group I, II or III mGluRs agonists are S-3, 5-DHPG, LY379268 and L-AP4, respectively, and have been used in many preclinical studies. Recently, selective orthosteric ligands have been generated, LY2794193 for mGlu3 receptor (Monn et al., 2015, 2018) and LSP4-2022 for mGlu4 receptor (Goudet et al., 2012). They bind to residues of the orthosteric site and to specific residues and pockets surrounding the glutamate-binding pocket. LSP4-2022 has notably been used in several pain studies.
Allosteric modulators regulate the activity of a receptor by binding at a site distinct from the orthosteric site of endogenous ligands. In mGluRs, the binding site for most synthetic allosteric modulators which has been identified so far is located in the seven transmembrane domain. Interestingly, this pocket is less well conserved between the different receptors of the family, allowing the discovery of subtype selective ligands. Allosteric modulators may inhibit (negatively modulate) or potentiate (positively modulate) the activity of a co-binding orthosteric ligand at a target receptor and so can act as negative or positive allosteric modulators, respectively. Moreover, neutral allosteric ligands capable of inhibiting the action of either positive or negative allosteric modulators but devoid of activity by themselves have also been described (also referred to as silent allosteric modulators, SAM). Negative allosteric modulators (NAM) act as non-competitive antagonists and can have inverse agonist properties, meaning that they can inhibit the constitutive activity of the receptor. Interestingly, due to their non-competitive mode of action, the action of NAMs is less dependent on the concentration of endogenous ligands. Positive allosteric modulators (PAM) can enhance either the potency or the efficacy, or both, of orthosteric agonists. Therefore, in contrast to agonists that maintain the receptor active, pure PAMs potentiate the cellular response resulting from the action of the endogenous ligand. Some PAMs can also directly activate the receptor, referred to as agoPAMs, although such activity is usually partial.
The first described allosteric modulators of mGluRs were CPCCOEt, BAY36-7620 and MPEP, which display inverse agonist activity on mGlu1 and mGlu5 receptors (Litschig et al., 1999; Pagano et al., 2000; Carroll et al., 2001). Shortly after, a series of PAMs of mGlu1 receptors were described (Knoflach et al., 2001). To date, PAMs and NAMs have been described for most mGluRs [see (Lindsley et al., 2016) for a review] and have proven to be useful in exploring the function of mGluRs in pain.
Photopharmacology is a recent advance in the field of mGluRs. It is based on freely diffusible, light-operated ligands to control the function of the ligand on its target by light. Contrary to optogenetics, neither genetic modification of the targeted receptor nor exogenous expression are required, enabling the photocontrol of endogenous receptors. Two types of drugs have been developed for photopharmacology: photoactivable and photoswitchable ligands (Goudet et al., 2018). It allows the pharmacological manipulation of mGluRs with high spatial and temporal precision and holds great promise for exploring their physiological and pathological functions, notably in pain (Font et al., 2017; Gómez-Santacana et al., 2017; Zussy et al., 2018).
Since mGluRs are extensively expressed along the pain neuraxis (Figure 1), several preclinical studies have been performed to evaluate the impact of mGluRs ligands on pain following systemic administration (Tables 1–3). These preclinical studies outline the role of these different receptors on the regulation of pain. Additional studies have been performed to explore the role of these receptors at precise locations of the pain pathways and will be described in the following paragraphs.
Systemic administration of mGlu1 receptor antagonists are inefficient at altering normal pain threshold in naive animals (Maione et al., 1998; Sevostianova and Danysz, 2006). However, mGlu1 receptor inhibition relieves both mechanical and thermal hypersensitivity in various models of both inflammatory and neuropathic pain (Table 1) (Varty et al., 2005; El-Kouhen et al., 2006; Sevostianova and Danysz, 2006; Satow et al., 2008; Zhu et al., 2008). Similarly, systemic administration of mGlu5 receptor antagonists fails to modify basal thermal threshold (Sevostianova and Danysz, 2006), whereas it prevents mechanical and thermal hyperalgesia in a broad range of pain conditions from sub-chronic inflammatory pain to long lasting neuropathic pain (Table 1) (Walker et al., 2001a,b; Hudson et al., 2002; Zhu et al., 2004; Varty et al., 2005; Sevostianova and Danysz, 2006; Satow et al., 2008; Jacob et al., 2009; Montana et al., 2009; Zammataro et al., 2011). Of note, mGlu1 receptor inhibition induces motor and cognitive side effects at analgesic doses that could limit its use in clinical trials (El-Kouhen et al., 2006; Zhu et al., 2008). Consequently, mGlu5 receptor seems to be a better target to develop analgesic drugs. Although mGlu5 antagonists have been reported to induce tolerance and some locomotor deficits (Varty et al., 2005; Sevostianova and Danysz, 2006), it is interesting to point out that mGlu5 receptor antagonists reduce anxiety in naïve animals, a comorbidity often associated with chronic pain states (Varty et al., 2005).
Systematically administrated group II selective agonists have proven anti-hyperalgesic effects in both inflammatory and neuropathic pain without altering basal pain thresholds in healthy animals (Table 2) (Sharpe et al., 2002; Simmons et al., 2002; Satow et al., 2008; Johnson et al., 2017). Interestingly, selective group II mGluRs agonists have entered into clinical trials for the treatment of schizophrenia suggesting a safe profile of the drug in humans (Li et al., 2015; Muguruza et al., 2016).
Only a few studies have investigated the effect of systemic administration of group III selective compounds in pain perception (Table 3). Systemic delivery of mGlu4 receptor agonist alleviates mechanical hypersensitivity provoked by carrageenan-induced inflammation (Vilar et al., 2013). AMN082, an mGlu7 receptor PAM prevents hyperalgesia in inflammatory models (Dolan et al., 2009). The same compound injected systematically reduces mechanical allodynia and thermal hyperalgesia induced by chronic constriction injury to the sciatic nerve and potentiates the effect of morphine (Osikowicz et al., 2008). This drug also exhibits antidepressant-like and anxiolytic-like effects (Bradley et al., 2012). In addition to the mGlu7 receptor, other mechanisms can contribute to these effects since the AMN082 compound is rapidly metabolized in vivo into a monoamine transporter inhibitor (Sukoff Rizzo et al., 2011). Surprisingly, systemically administrated mGlu7 receptor negative allosteric modulators (NAMs) also have anti-hyperalgesic effects in neuropathic pain models (Palazzo et al., 2015). As detailed further in this review, pharmacological activation of mGlu7 receptors can lead to opposite effects depending on the administration site. Neuropathic pain induces variation in mGlu7 receptor expression that could imbalance the pronociceptive and antinociceptive role of mGlu7 receptor (Osikowicz et al., 2009; Palazzo et al., 2013, 2015).
Systemic delivery of a mGlu8 receptor agonist also decreases nociceptive responses in inflammatory and neuropathic models, which is inhibited by blocking group III mGluRs in the PAG (Marabese et al., 2007).
Sensory transmission initiates with the detection by primary afferents in the periphery of a broad range of stimuli such as mechanical, thermal or chemical stimuli. Primary afferents are specialized neurons translating information detected at the periphery into electrical signals which are conveyed through their cell bodies located in the dorsal root ganglia (DRG) to their projections into the dorsal horn of the spinal cord. Spinal neurons then project to higher centers in the brain which process the sensory information. After nerve injury or inflammation, a number of dysregulations occur in sensory neurons affecting activity, properties or gene expression, driving an increased sensitivity to both non-noxious and noxious stimuli with or without ectopic activities. Because the primary afferents are the first relay of nociceptive transmission and can trigger the chronicization of pain, they represent an interesting target for the development of analgesic drugs.
Early evidence of a glutamate role in nociceptive transmission at the periphery derived from the observation of thermal and mechanical hypersensitivity following subcutaneous injection of glutamate into naive rat hind paw (Carlton et al., 1995; Jackson et al., 1995), first believed to be only triggered by iGluR activation (Zhou et al., 1996). Furthermore, in rodents, glutamate concentration rises in inflamed tissue (Omote et al., 1998) and after sciatic nerve stimulation (deGroot et al., 2000). Elevated levels of glutamate have also been measured in synovial fluid from knee joints of arthritis patients highlighting the clinical relevance of glutamate modulation as a peripheral mediator of pain perception (McNearney et al., 2000). Since then, an increasing number of studies have reported the involvement of mGluRs at the periphery.
Recently, a single-cell transcriptome analysis has reported the expression of mGluR transcripts in mice DRG. Among the most expressed are mGlu7, mGlu3, mGlu4, mGlu8, and mGlu5 receptors (Usoskin et al., 2015). Transcriptome analysis provides evidence for the expression of mGluRs in cell bodies but whether these receptors are expressed at the peripheral terminal, the spinal projection endings, or both, must be further investigated. mGluRs expression has also been reported in trigeminal ganglia, notably mGlu1, mGlu2/3, and mGlu8 receptors (Boye Larsen et al., 2014).
Group I mGlu1 and mGlu5 receptors are expressed in nociceptive afferents (Bhave et al., 2001; Walker et al., 2001a,b). Together with iGluR, group I mGluRs are involved in capsaicin induced glutamate release, a process that could contribute to nociceptive responses evoked by the TRPV1 agonist (Jin et al., 2009). Intraplantar injection of group I agonists in rodents enhances thermal sensitivity and reciprocally, peripherally applied group I antagonist reduced hyperalgesia in animal models of inflammatory or neuropathic pain (Table 4) (Dogrul et al., 2000; Bhave et al., 2001; Walker et al., 2001a,b). Application of mGlu5 receptor antagonist at peripheral afferent endings also reduces visceral nociception (Table 5) (Lindström et al., 2008). More recently, the analgesic potential of peripheral mGlu5 receptor blockade has been highlighted using an mGlu5 selective photoactivable NAM. Photoactivable ligands, also called caged-ligands, are constituted of a ligand linked to a photo-labile protecting group that will be removed following illumination, enabling the precise control of the onset of drug activity at a specific location (Goudet et al., 2018). Following systemic injection of the inactive caged-mGlu5 NAM, analgesia in both phases of the formalin test can be induced by local illumination in the paw (Table 5) (Font et al., 2017).
Primary sensory neurons express mGlu2 and mGlu3 receptors in both peripheral terminals and dorsal horn projection (Carlton et al., 2001; Carlton and Hargett, 2007). In DRG, mGlu2/3 receptors are largely co-localized with TRPV1 channel (Carlton et al., 2009). Consistent with this co-expression, group II mGluR antagonists increase hyperalgesia evoked by capsaicin, a TRPV1 agonist, and this effect is blocked by group II mGluR agonists (Table 6) (Carlton et al., 2011). However, a recent report has demonstrated that mGlu2/3 receptors activation abolishes TRPV1 sensitization in mouse sensory neurons, but not in humans (Sheahan et al., 2018).
In cultured DRG neurons, group II mGluRs also negatively regulate TTX resistant sodium channels (Yang and Gereau, 2004). Local administration of group II agonist in the knee joint both prevents and reduces carrageenan-induced arthritis (Lee et al., 2013). Due to the lack of selective compounds that can discriminate between mGlu2 and mGlu3 receptors, the individual contribution of those two receptors to pain modulation has remained unclear for a long time. However, the generation of mGlu2 and mGlu3 receptor knockout mice allowed the precise investigation of the role of each subtype in nociception and revealed a predominant role of the mGlu2 over mGlu3 receptor (Zammataro et al., 2011).
In line with the pharmacological evidence, mGlu2 receptor overexpression in DRG induces analgesia in models of inflammatory and neuropathic pain (Chiechio et al., 2002, 2009). L-acetylcarnitine, a drug known to enhance mGlu2 receptor expression in DRG through epigenetic mechanisms induces a long-lasting analgesia in both inflammatory and neuropathic pain models (Notartomaso et al., 2017). Strikingly, N-acetyl-cysteine, a drug enhancing mGlu2 receptor expression in rodents, reduces nociceptive transmission in humans (Truini et al., 2015). Moreover, in a recent report using cultured DRG neurons from both mice and humans, PGE2 evoked neuron hyperexcitability was blocked by group II mGluR activation (Davidson et al., 2016). This data suggests that activation of group II mGluRs leads to an analgesic effect in rodents and humans, making group II mGluRs an interesting target for development of peripherally active drugs for the treatment of chronic pain.
Most group III mGluRs are expressed in the pain pathway, except the mGlu6 receptor which is expressed mainly in the retina (Vardi et al., 2000). The presence of mGlu4, mGlu7, and mGlu8 receptors have been detected in DRG and trigeminal ganglia (Li et al., 1996; Azkue et al., 2001; Carlton and Hargett, 2007). The mGlu8 receptor is expressed in DRG and peripheral terminals where it is widely co-expressed with TRPV1. Intraplantar injection of group III agonists significantly reduced capsaicin evoked pain behavior (Table 7; Govea et al., 2012). Similar to group II agonists, local administration in the knee joint of group III mGluRs agonist provokes analgesia in carrageenan-induced arthritic pain model (Lee et al., 2013). Specific contribution of each subtype to the antinociceptive effect of broad range group III mGluRs need to be further investigated.
The spinal cord (SC) is the first relay in the transmission of sensory information from the periphery to the brain. It is submitted to control from peripheral inputs, interneurons within the SC and both inhibitory and excitatory descending pathways from supraspinal regions. This network makes the SC an important site for the modulation of signals generated at the periphery. Any alteration in neurons from the SC network can imbalance spinal relay and lead to chronic pain conditions.
The dorsal horn (DH) of the SC which receives nociceptive inputs is organized into different laminae, from the superficial laminae I to the deep laminae V. Most nociceptive fibers (Aδ- and C-fibers) superficially innervate laminae I-III and, to a lesser extent, laminae V, whereas low-threshold Aβ-fibers mainly project into laminae III–VI. Early studies have demonstrated that glutamate is released from primary afferent neurons into the DH in response to both acute and persistent painful stimuli, highlighting a role of the glutamatergic system in nociceptive transmission (Sluka and Westlund, 1992; Sorkin et al., 1992).
According to a recent single-cell RNA sequencing study of sensory neurons in the mouse DH, all mGluRs except mGlu6, are expressed within the spinal cord, the highest expression levels being measured for mGlu5 and 7 receptors (Häring et al., 2018). This high throughput data is in line with previous histological and pharmacological studies detailed below, and draw further attention to the relevance of targeting glutamate synapses for pain modulation in the dorsal horn of the spinal cord.
Immunoreactive cell bodies for group I mGluRs are widely spread throughout the superficial laminae of DH (Jia et al., 1999; Tang and Sim, 1999; Hudson et al., 2002). Intrathecal administration of group I mGluR agonists provokes hyperalgesia whereas group I mGluR antagonists induces analgesia in inflammatory and neuropathic pain models (Table 4) (Fisher and Coderre, 1996, 1998; Young et al., 1997; Fisher et al., 1998). Intrathecal injection of mGlu5 antagonist also reverses paclitaxel-induced neuropathic pain (Table 5; Xie et al., 2017). DH neuron excitability is increased after activation of spinal group I mGluRs in part due to due to inhibition of a voltage gated potassium channel (Hu et al., 2007). In line with this pharmacological evidence, knockdown or antibody approaches targeting mGlu1 receptor have demonstrated an antinociceptive effect in various pain models (Fundytus et al., 1998, 2001; Noda et al., 2003). Interestingly, recent studies have reported enhanced mGlu5 expression at the nuclear membrane in DH neurons after nerve injury. Using permeable mGlu5 antagonists reaching the cytoplasm, the authors have demonstrated that blocking intracellular mGlu5 had a greater antinociceptive effect than by blocking cell membrane expressed mGlu5 (Vincent et al., 2016). Pre-treatment with an excitatory amino acid transporter (EAAT) inhibitor, which is meant to decrease intracellular glutamate levels, decreases pain-related behavior in an inflammatory pain model (Vincent et al., 2017).
Among group II mGluRs, mGlu3 receptor is the most expressed in the DH, and its transcript is restricted to laminae II (Valerio et al., 1997; Berthele et al., 1999; Jia et al., 1999). However, only mGlu2 receptor expression appears to be enhanced in the SC (and DRG neurons) after administration of L-acetylcarnitine and histone deacetylase inhibitors, two compounds with antinociceptive properties, suggesting a greater role of spinal mGlu2 receptors in pain modulation (Chiechio et al., 2002, 2009). This discrepancy could be explained by expression pattern differences. Indeed, mGlu2 receptor is mostly pre-synaptic, while mGlu3 receptor is both pre- and post-synaptic (Nicoletti et al., 2011). Moreover, mGlu2 is expressed in microglia while mGlu3 is expressed in both microglia and astrocytes (Spampinato et al., 2018).
Transcripts of two group III members, mGlu4 and mGlu7 receptors, are detected in the spinal cord (Valerio et al., 1997). The expression of mGlu4 receptor is restricted to inner laminae II of the DH receiving nociceptive Aδ- and C-fibers inputs whereas mGlu7 receptor is expressed in both laminae I and II (Valerio et al., 1997; Vilar et al., 2013). In addition, the mGlu4 receptor may be expressed in spinal neurons, since its expression can still be observed after rhizotomy of the afferent fibers (Vilar et al., 2013). Activation of spinal group III mGluRs depletes glutamate release from primary afferents in nerve-injured rats (Table 7; Zhang et al., 2009). Furthermore, intrathecal administration of the group III broad-spectrum agonist L-AP4 reduces capsaicin-induced hypersensitivity and neuropathic pain symptoms (Fisher et al., 2002; Chen and Pan, 2005; Soliman et al., 2005). Intrathecal administration of the mGlu4 receptor PAM or agonist inhibits both inflammatory and neuropathic pain without altering acute pain thresholds in naive animals (Table 8; Goudet et al., 2008; Wang et al., 2011; Vilar et al., 2013). Conversely, the antiallodynic action of an mGlu4 agonist in inflammatory pain can be blocked by a photoswitchable mGlu4 NAM (Rovira et al., 2016). Positive allosteric modulation of spinal mGlu7 alleviates mechanical allodynia and thermal hyperalgesia induced by either carrageenan or skin incisions (Dolan et al., 2009). However, intrathecally administrated mGlu7 PAM has failed to relieve neuropathic pain (Wang et al., 2011). Both studies used the mGlu7 PAM named AMN082 (Mitsukawa et al., 2005). As mentioned earlier in the text, in vivo, AMN082 is rapidly metabolized and one of its metabolite inhibits several monoamine transporters (Sukoff Rizzo et al., 2011). Therefore, in vivo actions of AMN082 should be interpreted with caution since it may have multiple mode of action.
Integration of the nociceptive signal in the brain translates into a complex pain experience (Hunt and Mantyh, 2001). Pain processing in the supraspinal nervous system involves both ascending and descending pathways. Briefly, two main ascending pathways have been identified. The first one, the spinoparabrachial pathway, originates from the superficial dorsal horn and projects to areas of the brain concerned with affect: the parabrachial area (PB), the ventral medial nucleus (VMN) or the amygdala. The second one, the spinothalamic pathway, starts from the deep DH and projects to the thalamus and other areas of the cortex concerned with discrimination and affect. Different brain areas are involved in pain integration and processing. They are referred to as the pain matrix, a concept first described by Ronald Melzack in the late eighties (Melzack, 1990). It comprises several regions such as the primary and secondary sensorimotor cortex, insula, anterior cingulate cortex, thalamus, striatum, brainstem and cerebellum (Garcia-Larrea and Peyron, 2013). Descending pathways also involve high brain centers such as amygdala, hypothalamus and VMH, and nucleus in the midbrain and the brainstem, respectively, periaqueductal gray (PAG) and rostral ventromedial medulla (RVM).
mGluRs are widely express in neurons, astrocytes, oligodendrocytes, and microglia throughout the brain areas involved in pain processing. Consequently, there is an increasing interest in understanding the contribution of supraspinal mGluRs to pain modulation and many groups have investigated their potential for alleviating pain.
Although it is clearly established that activation of group I mGluRs at both the periphery and the spinal cord promotes pain, group I activation at the supraspinal level can elicit both antinociceptive and pronociceptive effects depending on the region investigated (Tables 4, 5). For instance, when applied in the amygdala, group I agonist promotes nociception (Li and Neugebauer, 2004; Kolber et al., 2010; Ren and Neugebauer, 2010; Tappe-Theodor et al., 2011). Reciprocally, stereotaxic injection of mGlu1 and mGlu5 receptor antagonists in the amygdala inhibits pain-related responses in a model of arthritic pain (Han and Neugebauer, 2005). Similarly, intra basolateral amygdala administration of group I mGluRs agonist alleviates inflammatory pain, an effect at least in part due to inhibition of prefrontal cortex neurons activity (Luongo et al., 2013). When applied to the thalamus, mGlu1 PAM potentiated nociceptive responses of thalamic neurons (Salt et al., 2014). Conversely, when administrated in the PAG, a region involved in modulation of the descending pain pathway, activation of group I mGluRs decreases the nociceptive response, likely through the inhibition of the GABAergic transmission (Maione et al., 2000; Drew and Vaughan, 2004). Moreover, PAG expressed mGlu5 contribute to the antinociceptive effect provoked by RVM cannabinoid receptor activation (de Novellis et al., 2005).
In an outstanding paper, authors used a selective photoactivable mGlu5 NAM enabling the precise spatiotemporal modulation of mGlu5 receptors to probe the involvement of thalamic mGlu5 receptors in pain processing. As expected, when injected systematically, the inactive caged compound has no effect on pain behavior of neuropathic animals. However, release of the active mGlu5 NAM by delivering light through implanted optical fibers in the ventrobasal thalamus, reduces neuropathic pain (Font et al., 2017).
An alternative photopharmacological strategy consists in using photoswitchable ligands that can be reversibly activated and inactivated by light (Goudet et al., 2018). This approach has been used to validate the role of amygdala-expressed mGlu5 in pain. A photoswitchable mGlu5 NAM has been injected locally in amygdala where it light-dependently reduced mechanical allodynia in a mice model of inflammatory pain (Gómez-Santacana et al., 2017), confirming previous preclinical studies (Han and Neugebauer, 2005).
Interestingly, global genetic disruption of mGlu5 in mice leads to increased basal mechanical withdrawal responses whereas conditional KO in the amygdala did not affect acute pain. However, both global and conditional KO prevent the establishment of mechanical hypersensitivity 180 min after formalin injection in the ipsi and contralateral paw (Kolber et al., 2010).
Accumulating evidence demonstrates that stimulation of group II mGluRs in supraspinal areas mediates analgesia (Table 6). Administration into the amygdala by microdialysis of group II agonist diminishes the response to noxious stimulation in an arthritis model of chronic pain (Li and Neugebauer, 2006). In the PAG, group II mGluR activation reinforces antinociceptive descending pathway (Maione et al., 2000). Local inhibition in the PAG or the RVM of the degradation of an endogenous peptide acting as an mGlu3 receptor agonist relieves pain in rat models of inflammatory and neuropathic pain (Yamada et al., 2012). However, studies have also reported a pronociceptive effect of CNS expressed group II mGluRs. For instance, blockage in the thalamus elicits antinociceptive effects, possibly via an inhibition of GABAergic inhibitory neurones (Neto and Castro-Lopes, 2000). Furthermore, microinjection of a group II agonist in the PAG induces pronociceptive effects by inhibiting descending pathway (Maione et al., 1998).
Broad range group III mGluR agonists were first used to elucidate the contribution of these receptors in pain processing in the CNS (Table 7). Early studies demonstrated that in the PAG a group III mGluR agonist facilitates pain related behavior (Maione et al., 1998, 2000), whereas in the amygdala group III agonist microinjection produces antinociceptive effects in an arthritis model (Li and Neugebauer, 2006). Development of more selective compounds for individual group III subtypes has allowed the more precise dissection of each members' contribution to nocifensive and affective pain responses within the CNS (Table 8). Of note, mGlu7 and mGlu8 have opposite effects in the PAG. Indeed, mGlu7 activation in PAG and amygdala is pronociceptive whereas mGlu8 activation is antinociceptive (Marabese et al., 2007; Palazzo et al., 2008). Similarly, in the nucleus tractus solitarius, mGlu7 activation has an antinociceptive effect on the cardiac-somatic reflex induced by pericardial capsaicin, while activation of mGlu8 receptors enhance cardiac nociception (Liu et al., 2012). Activation of mGlu7 in the nucleus accumbens by AMN082 has an antinociceptive effect and modulates relief learning (Kahl and Fendt, 2016). Blockade of mGlu7 in the PAG reduces the pain related behaviors in formalin and neuropathic pain models and differentially modulates RVM ON and OFF cell activity (Palazzo et al., 2013). Whereby, ON cells are neurons activated by noxious stimuli and inhibited by analgesics, and OFF cells are activated by analgesics and inhibited by painful stimuli (Palazzo et al., 2013).
Recently, dorsal striatum (DS) expressed mGlu7 receptors and their role in pain have been investigated. The DS is connected to the descending pain modulatory systems, including to the RVM. When locally administrated in the DS of sham animals, an mGlu7 PAM enhanced pain and simultaneously stimulates ON cells and inhibits OFF cells in the RVM. Whereas, in nerve-injured animals, the mGlu7 PAM has an anti-hyperalgesic effect in addition to increasing RVM OFF cell firing. This opposite effect of an mGluR7 PAM in acute or chronic pain conditions is assumed to be due to the recruitment of different pain pathways (Marabese et al., 2018). Interestingly, systemic administration of an mGluR7 PAM prevents the development of morphine tolerance (Gawel et al., 2018). A role of centrally expressed mGlu7 in epilepsy has also been reported (Sansig et al., 2001; Bertaso et al., 2008).
The first strong evidence of supraspinal mGlu4 involvement in pain processing is thanks to the recent development of an mGlu4 photoswitchable PAM allowing the time resolved control of endogenous receptors in freely behaving animals. Strikingly, dynamic modulation of mGlu4 receptor activation in the amygdala by the photoswitchable PAM reverses, in a light dependent manner, both inflammatory pain-related sensory and affective symptoms (Zussy et al., 2018). As compared to conventional compounds, this ligand enables precise temporal control of the mGlu4 receptor and, in contrast to optogenetics, allows endogenous receptor modulation, without the need of trangenesis. We expect that future development of photoswitchable ligands for other mGluRs will greatly improve our understanding of mGluRs in the pain neuraxis and co-morbidities associated with chronic pain conditions.
Beside neurons, mGluRs are also widely expressed in glial cells, noteworthy in microglia, astrocytes, and oligodendrocytes (for a recent review, see Spampinato et al., 2018). Astrocytes are the most abundant cell type in the brain, which are regulating neuronal function and remodeling synaptic structures. In addition to their physiological functions, astrocytes are involved in numerous diseases, such as chronic pain. Microglia act as resident macrophages, which function as sentinels of the CNS surveying potential damage. Following nerve injury, activated microglia surround the injured peripheral nerve terminals in the dorsal horn where they release different factors, such as brain-derived neurotrophic factor (BDNF), cytokines (TNFα, IL-1β, IL-6…) and glutamate, that will contribute to neuroinflammation, excitotoxicity and central sensitization. Numerous studies have shown that glial cells play a critical role in the development of neuropathic and inflammatory pain (Ji et al., 2013). For instance, microglia and astrocytes contribute to the central sensitization process that occurs in the setting of injury (Basbaum et al., 2009). Interestingly, all three groups of mGluRs are expressed in microglia and play a critical role in regulating microglial activity (Taylor et al., 2002, 2003; Byrnes et al., 2009; McMullan et al., 2012). In vitro, neuroinflammatory factors trigger an opposite regulation in the gene expression of the two predominant mGluR subtypes found in astrocytes and microglia, namely an upregulation of mGlu3 and a downregulation mGlu5 (Berger et al., 2012). Concerning group I mGluRs, activation of mGlu5 receptors inhibits microglial-associated inflammation and neurotoxicity (Byrnes et al., 2009), while little is known about mGlu1 receptors. Activation of group II mGluRs in vitro yields two opposite effects in cultured microglia, mGlu2 activation enhancing neurotoxicity whilst mGlu3 activation promotes neuroprotection (Taylor et al., 2002, 2005; Pinteaux-Jones et al., 2008). However, further studies are needed to understand the particular roles of these receptors, since activation of both mGlu2 and mGlu3 receptors have been reported to be neuroprotective in vivo (Fazio et al., 2018). Activation of group III mGluRs, notably mGlu4 receptors, reduces microglial reactivity (Taylor et al., 2003; Pinteaux-Jones et al., 2008; Ponnazhagan et al., 2016). Glial mGluRs modulate neuronal excitability and glutamate concentration in the synaptic and extrasynaptic regions (Pál, 2018). Of note, activation of group II and III, but not group I, attenuates export of glutamate from activated microglia through a cAMP-dependent mechanism (McMullan et al., 2012). Taken together, these results suggest that although less well studied than their neuronal counterparts, glial mGluRs may represent novel targets for the treatment of chronic pain.
The growing number of selective compounds for the different mGluRs has significantly improved our understanding of the specific role of each subtype in nociception. Numerous evidences tend to suggest these receptors are promising targets for the treatment of chronic pain. However, at doses proven to be analgesic, mGlu1 antagonists are associated with motor and cognitive impairment (El-Kouhen et al., 2006; Zhu et al., 2008). Similarly, deficits in motor coordination phenotype has also been observed in mGlu1 conditional knockouts in the cerebellum (Nakao et al., 2007). Although mGlu5 antagonists may have psychoactive properties (Swedberg et al., 2014), mGlu5 blockade seems to elicit less side effects than mGlu1, suggesting that targeting mGlu5 may be more promising for the development of new analgesics. Regarding group II agonists, which have proven antinociceptive effects, a major concern for the treatment of persistent pain is the development of tolerance after repeated systematic injections (Jones et al., 2005; Zammataro et al., 2011). Nevertheless, epigenetic upregulation of endogenous mGlu2 receptor expression could counteract the drawback of tolerance. Group III metabotropic receptors are of a particular interest in drug development because their targeting may also decrease affective and cognitive disorders associated with chronic pain such as anxiety, depression, or fear (Zussy et al., 2018).
Given the analgesic effects observed after targeting peripheral mGluRs, peripherally restricted molecules may have satisfying analgesic effectiveness while decreasing the central-associated side effects. Furthermore, the use of new pharmacological tools such as photoswitchable or caged ligands, which allow the spatiotemporal tuning of mGluRs, could reduce off-target effects related to the modulation of the glutamatergic system outside the pain neuraxis.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Ebba L. Lagerqvist for critical reading of the manuscript. This work was supported by a grant from the Agence Nationale de la Recherche (ANR-16-CE16-0010-01).
Adwanikar, H., Karim, F., and Gereau, R. W. (2004). Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain 111, 125–135. doi: 10.1016/j.pain.2004.06.009
Ahn, D. K., Kim, K. H., Jung, C. Y., Choi, H. S., Lim, E. J., Youn, D. H., et al. (2005). Role of peripheral group I and II metabotropic glutamate receptors in IL-1β-induced mechanical allodynia in the orofacial area of conscious rats. PAIN 118:53–60. doi: 10.1016/j.pain.2005.07.017
Azkue, J. J., Murga, M., Fernández-Capetillo, O., Mateos, J. M., Elezgarai, I., Benítez, R., et al. (2001). Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J. Comp. Neurol. 430, 448–457. doi: 10.1002/1096-9861(20010219)430:4 < 448::AID-CNE1042>3.0.CO;2-O
Basbaum, A. I., Bautista, D. M., Scherrer, G., and Julius, D. (2009). Cellular and molecular mechanisms of pain. Cell 139, 267–284. doi: 10.1016/j.cell.2009.09.028
Berger, J. V., Dumont, A. O., Focant, M. C., Vergouts, M., Sternotte, A., Calas, A.-G., et al. (2012). Opposite regulation of metabotropic glutamate receptor 3 and metabotropic glutamate receptor 5 by inflammatory stimuli in cultured microglia and astrocytes. Neuroscience 205, 29–38. doi: 10.1016/j.neuroscience.2011.12.044
Bertaso, F., Zhang, C., Scheschonka, A., De, F. B., Fontanaud, P., Marin, P., et al. (2008). PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat. Neurosci. 11, 940–948. doi: 10.1038/nn.2142
Berthele, A., Boxall, S. J., Urban, A., Anneser, J. M. H., Zieglgänsberger, W., Urban, L., et al. (1999). Distribution and developmental changes in metabotropic glutamate receptor messenger RNA expression in the rat lumbar spinal cord. Dev. Brain Res. 112, 39–53. doi: 10.1016/S0165-3806(98)00156-4
Bhave, G., Karim, F., Carlton, S. M., and Iv, R. W. G. (2001). Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat. Neurosci. 4, 417–423. doi: 10.1038/86075
Bleakman, D., Alt, A., and Nisenbaum, E. S. (2006). Glutamate receptors and pain. Semin. Cell Dev. Biol. 17, 592–604. doi: 10.1016/j.semcdb.2006.10.008
Boye Larsen, D., Ingemann Kristensen, G., Panchalingam, V., Laursen, J. C., Nørgaard Poulsen, J., Skallerup Andersen, M., et al. (2014). Investigating the expression of metabotropic glutamate receptors in trigeminal ganglion neurons and satellite glial cells: implications for craniofacial pain. J. Recept. Signal Transduct. Res. 34, 261–269. doi: 10.3109/10799893.2014.885049
Bradley, S. R., Uslaner, J. M., Flick, R. B., Lee, A., Groover, K. M., and Hutson, P. H. (2012). The mGluR7 allosteric agonist AMN082 produces antidepressant-like effects by modulating glutamatergic signaling. Pharmacol. Biochem. Behav. 101, 35–40. doi: 10.1016/j.pbb.2011.11.006
Bushnell, M. C., Ceko, M., and Low, L.A. (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 14, 502–511. doi: 10.1038/nrn3516
Byrnes, K. R., Stoica, B., Loane, D. J., Riccio, A., Davis, M. I., and Faden, A. I. (2009). Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 57, 550–560. doi: 10.1002/glia.20783
Carlton, S. M., Du, J., and Zhou, S. (2009). Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain Res. 1248, 86–95. doi: 10.1016/j.brainres.2008.10.066
Carlton, S. M., and Hargett, G. L. (2007). Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J. Comp. Neurol. 501, 780–789. doi: 10.1002/cne.21285
Carlton, S. M., Hargett, G. L., and Coggeshall, R. E. (1995). Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci. Lett. 197, 25–28. doi: 10.1016/0304-3940(95)11889-5
Carlton, S. M., Hargett, G. L., and Coggeshall, R. E. (2001). Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience 105, 957–969. doi: 10.1016/S0306-4522(01)00238-X
Carlton, S. M., Zhou, S., Govea, R., and Du, J. (2011). Group II/III metabotropic glutamate receptors exert endogenous activity-dependent modulation of TRPV1 receptors on peripheral nociceptors. J. Neurosci. Off. J. Soc. Neurosci. 31, 12727–12737. doi: 10.1523/JNEUROSCI.6558-10.2011
Carroll, F. Y., Stolle, A., Beart, P. M., Voerste, A., Brabet, I., Mauler, F., et al. (2001). BAY36-7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol. Pharmacol. 59, 965–973. doi: 10.1124/mol.59.5.965
Chen, S.-R., and Pan, H.-L. (2005). Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neurons in normal and nerve-injured Rats. J. Pharmacol. Exp. Ther. 312, 120–126. doi: 10.1124/jpet.104.073817
Chiechio, S., Caricasole, A., Barletta, E., Storto, M., Catania, M. V., Copani, A., et al. (2002). l-acetylcarnitine induces analgesia by selectively up-regulating mGlu2 metabotropic glutamate receptors. Mol. Pharmacol. 61, 989–996. doi: 10.1124/mol.61.5.989
Chiechio, S., Zammataro, M., Morales, M. E., Busceti, C. L., Drago, F., Gereau, R. W., et al. (2009). Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol. Pharmacol. 75, 1014–1020. doi: 10.1124/mol.108.054346
Chung, G., Kim, C. Y., Yun, Y.-C., Yoon, S. H., Kim, M.-H., Kim, Y. K., et al. (2017). Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats. Sci. Rep. 7:9743. doi: 10.1038/s41598-017-09991-8
Chung, M.-K., Lee, J., Joseph, J., Saloman, J., and Ro, J. Y. (2015). Peripheral group I metabotropic glutamate receptor activation leads to muscle mechanical hyperalgesia through TRPV1 phosphorylation in the rat. J. Pain 16, 67–76. doi: 10.1016/j.jpain.2014.10.008
Davidson, S., Golden, J. P., Copits, B. A., Ray, P. R., Vogt, S. K., Valtcheva, M. V., et al. (2016). Group II mGluRs suppress hyperexcitability in mouse and human nociceptors. Pain 157, 2081–2088. doi: 10.1097/j.pain.0000000000000621
de Novellis, V., Mariani, L., Palazzo, E., Vita, D., Marabese, I., Scafuro, M., et al. (2005). Periaqueductal grey CB1 cannabinoid and metabotropic glutamate subtype 5 receptors modulate changes in rostral ventromedial medulla neuronal activities induced by subcutaneous formalin in the rat. Neuroscience 134, 269–281. doi: 10.1016/j.neuroscience.2005.03.014
deGroot, J., Zhou, S., and Carlton, S. M. (2000). Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport 11, 497–502. doi: 10.1097/00001756-200002280-00014
Dogrul, A., Ossipov, M. H., Lai, J., Malan, T. P., and Porreca, F. (2000). Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci. Lett. 292, 115–118. doi: 10.1016/S0304-3940(00)01458-0
Dolan, S., Gunn, M. D., Biddlestone, L., and Nolan, A. M. (2009). The selective metabotropic glutamate receptor 7 allosteric agonist AMN082 inhibits inflammatory pain-induced and incision-induced hypersensitivity in rat. Behav. Pharmacol. 20, 596–604. doi: 10.1097/FBP.0b013e32832ec5d1
Dolan, S., and Nolan, A. M. (2000). Behavioural evidence supporting a differential role for group I and II metabotropic glutamate receptors in spinal nociceptive transmission. Neuropharmacology 39, 1132–1138. doi: 10.1016/S0028-3908(99)00200-2
Drew, G. M., and Vaughan, C. W. (2004). Multiple metabotropic glutamate receptor subtypes modulate GABAergic neurotransmission in rat periaqueductal grey neurons in vitro. Neuropharmacology 46, 927–934. doi: 10.1016/j.neuropharm.2004.01.015
Du, J., Zhou, S., and Carlton, S. M. (2008). Group II metabotropic glutamate receptor activation attenuates peripheral sensitization in inflammatory states., Group II metabotropic glutamate receptor activation attenuates peripheral sensitization in inflammatory states. Neurosci. Neurosci. 154, 754–766. doi: 10.1016/j.neuroscience.2008.03.084
El-Kouhen, O., Lehto, S. G., Pan, J. B., Chang, R., Baker, S. J., Zhong, C., et al. (2006). Blockade of mGluR1 receptor results in analgesia and disruption of motor and cognitive performances: effects of A-841720, a novel non-competitive mGluR1 receptor antagonist. Br. J. Pharmacol. 149, 761–774. doi: 10.1038/sj.bjp.0706877
Fazio, F., Ulivieri, M., Volpi, C., Gargaro, M., and Fallarino, F. (2018). Targeting metabotropic glutamate receptors for the treatment of neuroinflammation. Curr. Opin. Pharmacol. 38, 16–23. doi: 10.1016/j.coph.2018.01.010
Fisher, K., and Coderre, T. J. (1996). Comparison of nociceptive effects produced by intrathecal administration of mGluR agonists. Neuroreport 7, 2743–2747. doi: 10.1097/00001756-199611040-00067
Fisher, K., and Coderre, T. J. (1998). Hyperalgesia and allodynia induced by intrathecal (RS)-dihydroxyphenylglycine in rats. Neuroreport 9, 1169–1172. doi: 10.1097/00001756-199804200-00038
Fisher, K., Fundytus, M. E., Cahill, C. M., and Coderre, T. J. (1998). Intrathecal administration of the mGluR compound, (S)-4CPG, attenuates hyperalgesia and allodynia associated with sciatic nerve constriction injury in rats. Pain 77, 59–66. doi: 10.1016/S0304-3959(98)00082-7
Fisher, K., Lefebvre, C., and Coderre, T. J. (2002). Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol. Biochem. Behav. 73, 411–418. doi: 10.1016/S0091-3057(02)00832-8
Font, J., López-Cano, M., Notartomaso, S., Scarselli, P., Di Pietro, P., Bresolí-Obach, R., et al. (2017). Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator. Elife 6:e23545. doi: 10.7554/eLife.23545
Fundytus, M. E., Fisher, K., Dray, A., Henry, J. L., and Coderre, T. J. (1998). In vivo antinociceptive activity of anti-rat mGluR1 and mGluR5 antibodies in rats. Neuroreport 9, 731–735. doi: 10.1097/00001756-199803090-00031
Fundytus, M. E., Yashpal, K., Chabot, J. G., Osborne, M. G., Lefebvre, C. D., Dray, A., et al. (2001). Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br. J. Pharmacol. 132, 354–367. doi: 10.1038/sj.bjp.0703810
Garcia-Larrea, L., and Peyron, R. (2013). Pain matrices and neuropathic pain matrices: a review. Pain 154(Suppl. 1), S29–S43. doi: 10.1016/j.pain.2013.09.001
Gawel, K., Jenda-Wojtanowska, M., Gibula-Bruzda, E., Kedzierska, E., Filarowska, J., Marszalek-Grabska, M., et al. (2018). The influence of AMN082, metabotropic glutamate receptor 7 (mGlu7) allosteric agonist on the acute and chronic antinociceptive effects of morphine in the tail-immersion test in mice: Comparison with mGlu5 and mGlu2/3 ligands. Physiol. Behav. 185, 112–120. doi: 10.1016/j.physbeh.2017.12.035
Gómez-Santacana, X., Pittolo, S., Rovira, X., Lopez, M., Zussy, C., Dalton, J. A. R., et al. (2017). Illuminating phenylazopyridines to photoswitch metabotropic glutamate receptors: from the flask to the animals. ACS Cent. Sci. 3, 81–91. doi: 10.1021/acscentsci.6b00353
Goudet, C., Chapuy, E., Alloui, A., Acher, F., Pin, J.-P., and Eschalier, A. (2008). Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain 137, 112–124. doi: 10.1016/j.pain.2007.08.020
Goudet, C., Rovira, X., and Llebaria, A. (2018). Shedding light on metabotropic glutamate receptors using optogenetics and photopharmacology. Curr. Opin. Pharmacol. 38, 8–15. doi: 10.1016/j.coph.2018.01.007
Goudet, C., Vilar, B., Courtiol, T., Deltheil, T., Bessiron, T., Brabet, I., et al. (2012). A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 26, 1682–1693. doi: 10.1096/fj.11-195941
Govea, R. M., Zhou, S., and Carlton, S. M. (2012). Group III metabotropic glutamate receptors and transient receptor potential vanilloid 1 co-localize and interact on nociceptors. Neuroscience 217, 130–139. doi: 10.1016/j.neuroscience.2012.05.014
Hama, A. T. (2003). Acute activation of the spinal cord metabotropic glutamate subtype-5 receptor leads to cold hypersensitivity in the rat. Neuropharmacology 44, 423–430. doi: 10.1016/S0028-3908(03)00026-1
Han, J. S., and Neugebauer, V. (2005). mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 113, 211–222. doi: 10.1016/j.pain.2004.10.022
Häring, M., Zeisel, A., Hochgerner, H., Rinwa, P., Jakobsson, J. E. T., Lönnerberg, P., et al. (2018). Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 21, 869–880. doi: 10.1038/s41593-018-0141-1
Hu, H.-J., Alter, B. J., Carrasquillo, Y., Qiu, C.-S., and Gereau, R. W. (2007). Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J. Neurosci. Off. J. Soc. Neurosci. 27, 13181–13191. doi: 10.1523/JNEUROSCI.0269-07.2007
Hudson, L. J., Bevan, S., McNair, K., Gentry, C., Fox, A., Kuhn, R., et al. (2002). Metabotropic glutamate receptor 5 upregulation in a-fibers after spinal nerve injury: 2-methyl-6-(Phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J. Neurosci. 22, 2660–2668. doi: 10.1523/JNEUROSCI.22-07-02660.2002
Hunt, S. P., and Mantyh, P. W. (2001). The molecular dynamics of pain control. Nat. Rev. Neurosci. 2, 83–91. doi: 10.1038/35053509
Jackson, D. L., Graff, C. B., Richardson, J. D., and Hargreaves, K. M. (1995). Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur. J. Pharmacol. 284, 321–325. doi: 10.1016/0014-2999(95)00449-U
Jacob, W., Gravius, A., Pietraszek, M., Nagel, J., Belozertseva, I., Shekunova, E., et al. (2009). The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology 57, 97–108. doi: 10.1016/j.neuropharm.2009.04.011
Ji, R.-R., Berta, T., and Nedergaard, M. (2013). Glia and pain: is chronic pain a gliopathy? Pain 154(Suppl. 1), S10–S28. doi: 10.1016/j.pain.2013.06.022
Jia, H., Rustioni, A., and Valtschanoff, J. G. (1999). Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J. Comp. Neurol. 410, 627–642. doi: 10.1002/(SICI)1096-9861(19990809)410:4<627::AID-CNE9>3.0.CO;2-8
Jin, Y.-H., Yamaki, F., Takemura, M., Koike, Y., Furuyama, A., and Yonehara, N. (2009). Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J. Pharmacol. Sci. 109, 233–241. doi: 10.1254/jphs.08262FP
Johnson, M. P., Muhlhauser, M. A., Nisenbaum, E. S., Simmons, R. M. A., Forster, B. M., Knopp, K. L., et al. (2017). Broad spectrum efficacy with LY2969822, an oral prodrug of metabotropic glutamate 2/3 receptor agonist LY2934747, in rodent pain models. Br. J. Pharmacol. 174, 822–835. doi: 10.1111/bph.13740
Jones, C. K., Eberle, E. L., Peters, S. C., Monn, J. A., and Shannon, H. E. (2005). Analgesic effects of the selective group II (mGlu2/3) metabotropic glutamate receptor agonists LY379268 and LY389795 in persistent and inflammatory pain models after acute and repeated dosing. Neuropharmacology 49 (Suppl. 1), 206–218. doi: 10.1016/j.neuropharm.2005.05.008
Kahl, E., and Fendt, M. (2016). Metabotropic glutamate receptors 7 within the nucleus accumbens are involved in relief learning in rats. Curr. Neuropharmacol. 14, 405–412. doi: 10.2174/1570159X13666150425002017
Karim, F., Wang, C.-C., and Gereau, R. W. (2001). Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J. Neurosci. 21, 3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001
Knoflach, F., Mutel, V., Jolidon, S., Kew, J. N., Malherbe, P., Vieira, E., et al. (2001). Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc. Natl. Acad. Sci. U.S.A. 98, 13402–13407. doi: 10.1073/pnas.231358298
Kolber, B. J., Montana, M. C., Carrasquillo, Y., Xu, J., Heinemann, S. F., Muglia, L. J., et al. (2010). Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J. Neurosci. Off. J. Soc. Neurosci. 30, 8203–8213. doi: 10.1523/JNEUROSCI.1216-10.2010
Latremoliere, A., and Woolf, C. J. (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain Off. J. Am. Pain Soc. 10, 895–926. doi: 10.1016/j.jpain.2009.06.012
Lee, J.-S., and Ro, J. Y. (2007). Peripheral metabotropic glutamate receptor 5 mediates mechanical hypersensitivity in craniofacial muscle via protein kinase C dependent mechanisms. Neuroscience 146, 375–383. doi: 10.1016/j.neuroscience.2007.01.015
Lee, K. S., Park, E. H., Cho, H., Kim, Y. I., and Han, H. C. (2013). Peripheral group II and III metabotropic glutamate receptors in the knee joint attenuate carrageenan-induced nociceptive behavior in rats. Neurosci. Lett. 542, 21–25. doi: 10.1016/j.neulet.2013.03.006
Li, J. L., Ohishi, H., Kaneko, T., Shigemoto, R., Neki, A., Nakanishi, S., et al. (1996). Immunohistochemical localization of a metabotropic glutamate receptor, mGluR7, in ganglion neurons of the rat; with special reference to the presence in glutamatergic ganglion neurons. Neurosci. Lett. 204, 9–12. doi: 10.1016/0304-3940(95)12299-0
Li, M.-L., Hu, X.-Q., Li, F., and Gao, W.-J. (2015). Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: still promising or a dead end? Prog. Neuropsychopharmacol. Biol. Psychiatry 60, 66–76. doi: 10.1016/j.pnpbp.2015.02.012
Li, W., and Neugebauer, V. (2004). Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J. Neurophysiol. 91, 13–24. doi: 10.1152/jn.00485.2003
Li, W., and Neugebauer, V. (2006). Differential changes of group II and group III mGluR function in central amygdala neurons in a model of arthritic pain. J. Neurophysiol. 96, 1803–1815. doi: 10.1152/jn.00495.2006
Li, Z., Ji, G., and Neugebauer, V. (2011). Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J. Neurosci. Off. J. Soc. Neurosci. 31, 1114–1127. doi: 10.1523/JNEUROSCI.5387-10.2011
Lindsley, C. W., Emmitte, K. A., Hopkins, C. R., Bridges, T. M., Gregory, K. J., Niswender, C. M., et al. (2016). Practical strategies and concepts in GPCR allosteric modulator discovery: recent advances with metabotropic glutamate receptors. Chem. Rev. 116, 6707–6741. doi: 10.1021/acs.chemrev.5b00656
Lindström, E., Brusberg, M., Hughes, P. A., Martin, C. M., Brierley, S. M., Phillis, B. D., et al. (2008). Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain 137, 295–305. doi: 10.1016/j.pain.2007.09.008
Litschig, S., Gasparini, F., Rueegg, D., Stoehr, N., Flor, P. J., Vranesic, I., et al. (1999). CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol. Pharmacol. 55, 453–461.
Liu, X. H., Han, M., Zhu, J. X., Sun, N., Tang, J. S., Huo, F. Q., et al. (2012). Metabotropic glutamate subtype 7 and 8 receptors oppositely modulate cardiac nociception in the rat nucleus tractus solitarius. Neuroscience 220, 322–329. doi: 10.1016/j.neuroscience.2012.05.024
Lorrain, D. S., Correa, L., Anderson, J., and Varney, M. (2002). Activation of spinal group I metabotropic glutamate receptors in rats evokes local glutamate release and spontaneous nociceptive behaviors: effects of 2-methyl-6-(phenylethynyl)-pyridine pretreatment. Neurosci. Lett. 327, 198–202. doi: 10.1016/S0304-3940(02)00393-2
Luongo, L., de Novellis, V., Gatta, L., Palazzo, E., Vita, D., Guida, F., et al. (2013). Role of metabotropic glutamate receptor 1 in the basolateral amygdala-driven prefrontal cortical deactivation in inflammatory pain in the rat. Neuropharmacology 66, 317–329. doi: 10.1016/j.neuropharm.2012.05.047
Maione, S., Marabese, I., Leyva, J., Palazzo, E., de Novellis, V., and Rossi, F. (1998). Characterisation of mGluRs which modulate nociception in the PAG of the mouse. Neuropharmacology 37, 1475–1483. doi: 10.1016/S0028-3908(98)00126-9
Maione, S., Oliva, P., Marabese, I., Palazzo, E., Rossi, F., Berrino, L., et al. (2000). Periaqueductal gray matter metabotropic glutamate receptors modulate formalin-induced nociception. Pain 85, 183–189. doi: 10.1016/S0304-3959(99)00269-9
Marabese, I., Boccella, S., Iannotta, M., Luongo, L., de Novellis, V., Guida, F., et al. (2018). Metabotropic glutamate receptor subtype 7 in the dorsal striatum oppositely modulates pain in sham and neuropathic rats. Neuropharmacology 135, 86–99. doi: 10.1016/j.neuropharm.2018.03.003
Marabese, I., de Novellis, V., Palazzo, E., Scafuro, M. A., Vita, D., Rossi, F., et al. (2007). Effects of (S)-3,4-DCPG, an mGlu8 receptor agonist, on inflammatory and neuropathic pain in mice. Neuropharmacology 52, 253–262. doi: 10.1016/j.neuropharm.2006.04.006
McMullan, S. M., Phanavanh, B., Guo Li, G., and Barger, S. W. (2012). Metabotropic glutamate receptors inhibit microglial glutamate release. ASN Neuro 4:e00094. doi: 10.1042/AN20120044
McNearney, T., Speegle, D., Lawand, N., Lisse, J., and Westlund, K. N. (2000). Excitatory amino acid profiles of synovial fluid from patients with arthritis. J. Rheumatol. 27, 739–745.
McWilliams, L. A., Cox, B. J., and Enns, M. W. (2003). Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain 106, 127–133. doi: 10.1016/S0304-3959(03)00301-4
Melzack, R. (1990). Phantom limbs and the concept of a neuromatrix. Trends Neurosci. 13, 88–92. doi: 10.1016/0166-2236(90)90179-E
Mitsukawa, K., Yamamoto, R., Ofner, S., Nozulak, J., Pescott, O., Lukic, S., et al. (2005). A selective metabotropic glutamate receptor 7 agonist: Activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc. Natl. Acad. Sci. U.S.A. 102, 18712–18717. doi: 10.1073/pnas.0508063102
Monn, J. A., Henry, S. S., Massey, S. M., Clawson, D. K., Chen, Q., Diseroad, B. A., et al. (2018). Synthesis and pharmacological characterization of C4β-amide-substituted 2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylates. Identification of (1 S,2 S,4 S,5 R,6 S)-2-amino-4-[(3-methoxybenzoyl)amino]bicyclo[3.1.0]hexane-2,6-dicarboxylic Acid (LY2794193), a highly potent and selective mGlu3 receptor agonist. J. Med. Chem. 61, 2303–2328. doi: 10.1021/acs.jmedchem.7b01481
Monn, J. A., Prieto, L., Taboada, L., Pedregal, C., Hao, J., Reinhard, M. R., et al. (2015). Synthesis and pharmacological characterization of C4-disubstituted analogs of 1S,2S,5R,6S-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylate: identification of a potent, selective metabotropic glutamate receptor agonist and determination of agonist-bound human mGlu2 and mGlu3 amino terminal domain structures. J. Med. Chem. 58, 1776–1794. doi: 10.1021/jm501612y
Montana, M. C., Cavallone, L. F., Stubbert, K. K., Stefanescu, A. D., Kharasch, E. D., and Gereau, R. W. (2009). The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity compared with the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J. Pharmacol. Exp. Ther. 330, 834–843. doi: 10.1124/jpet.109.154138
Montana, M. C., Conrardy, B. A., Cavallone, L. F., Kolber, B. J., Rao, L. K., Greco, S. C., et al. (2011). Metabotropic glutamate receptor 5 antagonism with fenobam: examination of analgesic tolerance and side effect profile in mice. Anesthesiology 115, 1239–1250. doi: 10.1097/ALN.0b013e318238c051
Moriarty, O., McGuire, B. E., and Finn, D. P. (2011). The effect of pain on cognitive function: a review of clinical and preclinical research. Prog. Neurobiol. 93, 385–404. doi: 10.1016/j.pneurobio.2011.01.002
Muguruza, C., Meana, J. J., and Callado, L. F. (2016). Group II metabotropic glutamate receptors as targets for novel antipsychotic drugs. Front. Pharmacol. 7:130. doi: 10.3389/fphar.2016.00130
Nakao, H., Nakao, K., Kano, M., and Aiba, A. (2007). Metabotropic glutamate receptor subtype-1 is essential for motor coordination in the adult cerebellum. Neurosci. Res. 57, 538–543. doi: 10.1016/j.neures.2006.12.014
Neto, F. L., and Castro-Lopes, J. M. (2000). Antinociceptive effect of a group II metabotropic glutamate receptor antagonist in the thalamus of monoarthritic rats. Neurosci. Lett. 296, 25–28. doi: 10.1016/S0304-3940(00)01613-X
Nicoletti, F., Bockaert, J., Collingridge, G. L., Conn, P. J., Ferraguti, F., Schoepp, D. D., et al. (2011). Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041. doi: 10.1016/j.neuropharm.2010.10.022
Noda, K., Anzai, T., Ogata, M., Akita, H., Ogura, T., and Saji, M. (2003). Antisense knockdown of spinal-mGluR1 reduces the sustained phase of formalin-induced nociceptive responses. Brain Res. 987, 194–200. doi: 10.1016/S0006-8993(03)03330-4
Notartomaso, S., Mascio, G., Bernabucci, M., Zappulla, C., Scarselli, P., Cannella, M., et al. (2017). Analgesia induced by the epigenetic drug, L-acetylcarnitine, outlasts the end of treatment in mouse models of chronic inflammatory and neuropathic pain. Mol. Pain 13:1744806917697009. doi: 10.1177/1744806917697009
Omote, K., Kawamata, T., Kawamata, M., and Namiki, A. (1998). Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 787, 161–164. doi: 10.1016/S0006-8993(97)01568-0
Osikowicz, M., Mika, J., Makuch, W., and Przewlocka, B. (2008). Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain 139, 117–126. doi: 10.1016/j.pain.2008.03.017
Osikowicz, M., Skup, M., Mika, J., Makuch, W., Czarkowska-Bauch, J., and Przewlocka, B. (2009). Glial inhibitors influence the mRNA and protein levels of mGlu2/3, 5 and 7 receptors and potentiate the analgesic effects of their ligands in a mouse model of neuropathic pain. Pain 147, 175–186. doi: 10.1016/j.pain.2009.09.002
Pagano, A., Ruegg, D., Litschig, S., Stoehr, N., Stierlin, C., Heinrich, M., et al. (2000). The non-competitive antagonists 2-methyl-6-(phenylethynyl)pyridine and 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester interact with overlapping binding pockets in the transmembrane region of group I metabotropic glutamate receptors. J. Biol. Chem. 275, 33750–33758. doi: 10.1074/jbc.M006230200
Pál, B. (2018). Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell. Mol. Life Sci. CMLS. 75:2917. doi: 10.1007/s00018-018-2837-5
Palazzo, E., Fu, Y., Ji, G., Maione, S., and Neugebauer, V. (2008). Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology 55, 537–545. doi: 10.1016/j.neuropharm.2008.05.007
Palazzo, E., Marabese, I., Luongo, L., Boccella, S., Bellini, G., Giordano, M. E., et al. (2013). Effects of a metabotropic glutamate receptor subtype 7 negative allosteric modulator in the periaqueductal grey on pain responses and rostral ventromedial medulla cell activity in rat. Mol. Pain 9:44. doi: 10.1186/1744-8069-9-44
Palazzo, E., Romano, R., Luongo, L., Boccella, S., De Gregorio, D., Giordano, M. E., et al. (2015). MMPIP, an mGluR7-selective negative allosteric modulator, alleviates pain and normalizes affective and cognitive behavior in neuropathic mice. Pain 156, 1060–1073. doi: 10.1097/j.pain.0000000000000150
Pinteaux-Jones, F., Sevastou, I. G., Fry, V. A. H., Heales, S., Baker, D., and Pocock, J. M. (2008). Myelin-induced microglial neurotoxicity can be controlled by microglial metabotropic glutamate receptors. J. Neurochem. 106, 442–454. doi: 10.1111/j.1471-4159.2008.05426.x
Ponnazhagan, R., Harms, A. S., Thome, A. D., Jurkuvenaite, A., Gogliotti, R., Niswender, C. M., et al. (2016). The Metabotropic glutamate receptor 4 positive allosteric modulator ADX88178 inhibits inflammatory responses in primary microglia. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 11, 231–237. doi: 10.1007/s11481-016-9655-z
Ren, W., and Neugebauer, V. (2010). Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Mol. Pain 6:93. doi: 10.1186/1744-8069-6-93
Rossi, F., Marabese, I., De Chiaro, M., Boccella, S., Luongo, L., Guida, F., et al. (2013). Dorsal striatum metabotropic glutamate receptor 8 affects nocifensive responses and rostral ventromedial medulla cell activity in neuropathic pain conditions. J. Neurophysiol. 111, 2196–2209. doi: 10.1152/jn.00212.2013
Rovira, X., Trapero, A., Pittolo, S., Zussy, C., Faucherre, A., Jopling, C., et al. (2016). OptoGluNAM4.1, a photoswitchable allosteric antagonist for real-time control of mGlu4 receptor activity. Cell Chem. Biol. 23, 929–934. doi: 10.1016/j.chembiol.2016.06.013
Salt, T. E., Jones, H. E., Copeland, C. S., and Sillito, A. M. (2014). Function of mGlu1 receptors in the modulation of nociceptive processing in the thalamus. Neuropharmacology 79, 405–411. doi: 10.1016/j.neuropharm.2013.12.016
Sandkühler, J. (2009). Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758. doi: 10.1152/physrev.00025.2008
Sansig, G., Bushell, T. J., Clarke, V. R., Rozov, A., Burnashev, N., Portet, C., et al. (2001). Increased seizure susceptibility in mice lacking metabotropic glutamate receptor 7. J. Neurosci. Off. J. Soc. Neurosci. 21, 8734–8745. doi: 10.1523/JNEUROSCI.21-22-08734.2001
Satow, A., Maehara, S., Ise, S., Hikichi, H., Fukushima, M., Suzuki, G., et al. (2008). Pharmacological effects of the metabotropic glutamate receptor 1 antagonist compared with those of the metabotropic glutamate receptor 5 antagonist and metabotropic glutamate receptor 2/3 agonist in rodents: detailed investigations with a selective allosteric metabotropic glutamate receptor 1 antagonist, FTIDC [4-[1-(2-fluoropyridine-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methyl-3,6-dihydropyridine-1(2H)-carboxamide]. J. Pharmacol. Exp. Ther. 326, 577–586. doi: 10.1124/jpet.108.138107
Sevostianova, N., and Danysz, W. (2006). Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology 51, 623–630. doi: 10.1016/j.neuropharm.2006.05.004
Sharpe, E. F., Kingston, A. E., Lodge, D., Monn, J. A., and Headley, P. M. (2002). Systemic pre-treatment with a group II mGlu agonist, LY379268, reduces hyperalgesia in vivo. Br. J. Pharmacol. 135, 1255–1262. doi: 10.1038/sj.bjp.0704583
Sheahan, T. D., Valtcheva, M. V., McIlvried, L. A., Pullen, M. Y., Baranger, D. A. A., and Gereau, R. W. (2018). Metabotropic glutamate receptor 2/3 (mGluR2/3) activation suppresses TRPV1 sensitization in mouse, but not human, sensory neurons. eNeuro 5:ENEURO.0412-17.2018. doi: 10.1523/ENEURO.0412-17.2018
Simmons, R. M. A., Webster, A. A., Kalra, A. B., and Iyengar, S. (2002). Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol. Biochem. Behav. 73, 419–427. doi: 10.1016/S0091-3057(02)00849-3
Sluka, K. A., and Westlund, K. N. (1992). An experimental arthritis in rats: Dorsal horn aspartate and glutamate increases. Neurosci. Lett. 145, 141–144. doi: 10.1016/0304-3940(92)90006-S
Soliman, A. C., Yu, J. S. C., and Coderre, T. J. (2005). mGlu and NMDA receptor contributions to capsaicin-induced thermal and mechanical hypersensitivity. Neuropharmacology 48, 325–332. doi: 10.1016/j.neuropharm.2004.10.014
Sorkin, L. S., Westlund, K. N., Sluka, K. A., Dougherty, P. M., and Willis, W. D. (1992). Neural changes in acute arthritis in monkeys. IV. Time-course of amino acid release into the lumbar dorsal horn. Brain Res. Rev. 17, 39–50. doi: 10.1016/0165-0173(92)90005-7
Spampinato, S. F., Copani, A., Nicoletti, F., Sortino, M. A., and Caraci, F. (2018). Metabotropic glutamate receptors in glial cells: a new potential target for neuroprotection? Front. Mol. Neurosci. 11:414. doi: 10.3389/fnmol.2018.00414
Sukoff Rizzo, S. J., Leonard, S. K., Gilbert, A., Dollings, P., Smith, D. L., Zhang, M.-Y., et al. (2011). The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise? J. Pharmacol. Exp. Ther. 338, 345–352. doi: 10.1124/jpet.110.177378
Swedberg, M. D. B., Ellgren, M., and Raboisson, P. (2014). mGluR5 antagonist-induced psychoactive properties: MTEP drug discrimination, a pharmacologically selective non-NMDA effect with apparent lack of reinforcing properties. J. Pharmacol. Exp. Ther. 349, 155–164. doi: 10.1124/jpet.113.211185
Tang, F. R., and Sim, M. K. (1999). Pre- and/or post-synaptic localisation of metabotropic glutamate receptor 1alpha (mGluR1alpha) and 2/3 (mGluR2/3) in the rat spinal cord. Neurosci. Res. 34, 73–78. doi: 10.1016/S0168-0102(99)00035-8
Tappe-Theodor, A., Fu, Y., Kuner, R., and Neugebauer, V. (2011). Homer1a signaling in the amygdala counteracts pain-related synaptic plasticity, mGluR1 function and pain behaviors. Mol. Pain 7:38. doi: 10.1186/1744-8069-7-38
Taylor, D. L., Diemel, L. T., Cuzner, M. L., and Pocock, J. M. (2002). Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromogranin A, a peptide up-regulated in Alzheimer's disease. J. Neurochem. 82, 1179–1191.
Taylor, D. L., Diemel, L. T., and Pocock, J. M. (2003). Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J. Neurosci. 23, 2150–2160. doi: 10.1523/JNEUROSCI.23-06-02150.2003
Taylor, D. L., Jones, F., Kubota, E. S. F. C. S., and Pocock, J. M. (2005). Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. Off. J. Soc. Neurosci. 25, 2952–2964. doi: 10.1523/JNEUROSCI.4456-04.2005
Truini, A., Piroso, S., Pasquale, E., Notartomaso, S., Di Stefano, G., Lattanzi, R., et al. (2015). N-acetyl-cysteine, a drug that enhances the endogenous activation of group-II metabotropic glutamate receptors, inhibits nociceptive transmission in humans. Mol. Pain 11:14. doi: 10.1186/s12990-015-0009-2
Usoskin, D., Furlan, A., Islam, S., Abdo, H., Lönnerberg, P., Lou, D., et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153. doi: 10.1038/nn.3881
Valerio, A., Paterlini, M., Boifava, M., Memo, M., and Spano, P. (1997). Metabotropic glutamate receptor mRNA expression in rat spinal cord. NeuroReport 8, 2695–2699. doi: 10.1097/00001756-199708180-00012
Vardi, N., Duvoisin, R., Wu, G., and Sterling, P. (2000). Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J. Comp. Neurol. 423, 402–412. doi: 10.1002/1096-9861(20000731)423:3 < 402::AID-CNE4>3.0.CO;2-E
Varty, G. B., Grilli, M., Forlani, A., Fredduzzi, S., Grzelak, M. E., Guthrie, D. H., et al. (2005). The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology 179, 207–217. doi: 10.1007/s00213-005-2143-4
Vilar, B., Busserolles, J., Ling, B., Laffray, S., Ulmann, L., Malhaire, F., et al. (2013). Alleviating pain hypersensitivity through activation of type 4 metabotropic glutamate receptor. J. Neurosci. Off. J. Soc. Neurosci. 33, 18951–18965. doi: 10.1523/JNEUROSCI.1221-13.2013
Vincent, K., Cornea, V. M., Jong, Y.-J. I., Laferrière, A., Kumar, N., Mickeviciute, A., et al. (2016). Intracellular mGluR5 plays a critical role in neuropathic pain. Nat. Commun. 7:10604. doi: 10.1038/ncomms10604
Vincent, K., Wang, S. F., Laferrière, A., Kumar, N., and Coderre, T. J. (2017). Spinal intracellular metabotropic glutamate receptor 5 (mGluR5) contributes to pain and c-fos expression in a rat model of inflammatory pain. Pain 158, 705–716. doi: 10.1097/j.pain.0000000000000823
Walker, K., Bowes, M., Panesar, M., Davis, A., Gentry, C., Kesingland, A., et al. (2001a). Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function: I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology 40, 1–9. doi: 10.1016/S0028-3908(00)00113-1
Walker, K., Reeve, A., Bowes, M., Winter, J., Wotherspoon, G., Davis, A., et al. (2001b). mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology 40, 10–19. doi: 10.1016/S0028-3908(00)00114-3
Wang, H., Jiang, W., Yang, R., and Li, Y. (2011). Spinal metabotropic glutamate receptor 4 is involved in neuropathic pain. Neuroreport 22, 244–248. doi: 10.1097/WNR.0b013e3283453843
Xie, J.-D., Chen, S.-R., and Pan, H.-L. (2017). Presynaptic mGluR5 receptor controls glutamatergic input through protein kinase C-NMDA receptors in paclitaxel-induced neuropathic pain. J. Biol. Chem. 292, 20644–20654. doi: 10.1074/jbc.M117.818476
Yamada, T., Zuo, D., Yamamoto, T., Olszewski, R. T., Bzdega, T., Moffett, J. R., et al. (2012). NAAG peptidase inhibition in the periaqueductal gray and rostral ventromedial medulla reduces flinching in the formalin model of inflammation. Mol. Pain 8:67. doi: 10.1186/1744-8069-8-67
Yang, D., and Gereau, R. W. (2003). Peripheral group II metabotropic glutamate receptors mediate endogenous anti-allodynia in inflammation. Pain 106, 411–417. doi: 10.1016/j.pain.2003.08.011
Yang, D., and Gereau, R. W. (2004). Group II metabotropic glutamate receptors inhibit cAMP-dependent protein kinase-mediated enhancemednt of tetrodotoxin-resistant sodium currents in mouse dorsal root ganglion neurons. Neurosci. Lett. 357, 159–162. doi: 10.1016/j.neulet.2003.11.074
Young, M. R., Fleetwood-Walker, S. M., Dickinson, T., Blackburn-Munro, G., Sparrow, H., Birch, P. J., et al. (1997). Behavioural and electrophysiological evidence supporting a role for group I metabotropic glutamate receptors in the mediation of nociceptive inputs to the rat spinal cord. Brain Res. 777, 161–169.
Zammataro, M., Chiechio, S., Montana, M. C., Traficante, A., Copani, A., Nicoletti, F., et al. (2011). mGlu2 metabotropic glutamate receptors restrain inflammatory pain and mediate the analgesic activity of dual mGlu2/mGlu3 receptor agonists. Mol. Pain 7:6. doi: 10.1186/1744-8069-7-6
Zhang, H.-M., Chen, S.-R., and Pan, H.-L. (2009). Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience 158, 875–884. doi: 10.1016/j.neuroscience.2008.10.042
Zhou, H.-Y., Chen, S.-R., Chen, H., and Pan, H.-L. (2011). Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J. Pharmacol. Exp. Ther. 336, 254–264. doi: 10.1124/jpet.110.173112
Zhou, S., Bonasera, L., and Carlton, S. M. (1996). Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport 7, 895–900. doi: 10.1097/00001756-199603220-00012
Zhu, C. Z., Baker, S., EI-Kouhen, O., Lehto, S. G., Hollingsworth, P. R., Gauvin, D. M., et al. (2008). Analgesic activity of metabotropic glutamate receptor 1 antagonists on spontaneous post-operative pain in rats. Eur. J. Pharmacol. 580, 314–321. doi: 10.1016/j.ejphar.2007.09.047
Zhu, C. Z., Hsieh, G., Ei-Kouhen, O., Wilson, S. G., Mikusa, J. P., Hollingsworth, P. R., et al. (2005). Role of central and peripheral mGluR5 receptors in post-operative pain in rats. Pain 114, 195–202. doi: 10.1016/j.pain.2004.12.016
Zhu, C. Z., Wilson, S. G., Mikusa, J. P., Wismer, C. T., Gauvin, D. M., Lynch, J. J., et al. (2004). Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. Eur. J. Pharmacol. 506, 107–118. doi: 10.1016/j.ejphar.2004.11.005
Keywords: pain, GPCR (G-protein-coupled receptors), receptor, glutamate (Glu), neurotransmitter, chronic pain, pharmacology, neuromodulation
Citation: Pereira V and Goudet C (2019) Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors. Front. Mol. Neurosci. 11:464. doi: 10.3389/fnmol.2018.00464
Received: 11 September 2018; Accepted: 30 November 2018;
Published: 04 January 2019.
Edited by:
Enza Palazzo, Università degli Studi della Campania “Luigi Vanvitelli” Naples, ItalyReviewed by:
Katarzyna Starowicz, Institute of Pharmacology (PAN), PolandCopyright © 2019 Pereira and Goudet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cyril Goudet, Y3lyaWwuZ291ZGV0QGlnZi5jbnJzLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.