
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Neurosci. , 17 October 2017
Sec. Molecular Signalling and Pathways
Volume 10 - 2017 | https://doi.org/10.3389/fnmol.2017.00333
This article is part of the Research Topic Gap Junctions, Hemichannels and Pannexons: New Implications in Neuroplasticity and Neuroinflammation View all 19 articles
 Jennifer Ariazi1
Jennifer Ariazi1 Andrew Benowitz1
Andrew Benowitz1 Vern De Biasi1
Vern De Biasi1 Monique L. Den Boer2
Monique L. Den Boer2 Stephanie Cherqui3
Stephanie Cherqui3 Haifeng Cui1
Haifeng Cui1 Nathalie Douillet4
Nathalie Douillet4 Eliseo A. Eugenin5,6*
Eliseo A. Eugenin5,6* David Favre7
David Favre7 Spencer Goodman3
Spencer Goodman3 Karine Gousset8
Karine Gousset8 Dorit Hanein9
Dorit Hanein9 David I. Israel10
David I. Israel10 Shunsuke Kimura11
Shunsuke Kimura11 Robert B. Kirkpatrick1
Robert B. Kirkpatrick1 Nastaran Kuhn12
Nastaran Kuhn12 Claire Jeong13
Claire Jeong13 Emil Lou14
Emil Lou14 Robbie Mailliard15
Robbie Mailliard15 Stephen Maio13
Stephen Maio13 George Okafo4*
George Okafo4* Matthias Osswald16,17
Matthias Osswald16,17 Jennifer Pasquier18
Jennifer Pasquier18 Roel Polak2
Roel Polak2 Gabriele Pradel19
Gabriele Pradel19 Bob de Rooij2
Bob de Rooij2 Peter Schaeffer1
Peter Schaeffer1 Vytenis A. Skeberdis20
Vytenis A. Skeberdis20 Ian F. Smith21
Ian F. Smith21 Ahmad Tanveer22
Ahmad Tanveer22 Niels Volkmann9
Niels Volkmann9 Zhenhua Wu1
Zhenhua Wu1 Chiara Zurzolo23
Chiara Zurzolo23Cell-to-cell communication is essential for the organization, coordination, and development of cellular networks and multi-cellular systems. Intercellular communication is mediated by soluble factors (including growth factors, neurotransmitters, and cytokines/chemokines), gap junctions, exosomes and recently described tunneling nanotubes (TNTs). It is unknown whether a combination of these communication mechanisms such as TNTs and gap junctions may be important, but further research is required. TNTs are long cytoplasmic bridges that enable long-range, directed communication between connected cells. The proposed functions of TNTs are diverse and not well understood but have been shown to include the cell-to-cell transfer of vesicles, organelles, electrical stimuli and small molecules. However, the exact role of TNTs and gap junctions for intercellular communication and their impact on disease is still uncertain and thus, the subject of much debate. The combined data from numerous laboratories indicate that some TNT mediate a long-range gap junctional communication to coordinate metabolism and signaling, in relation to infectious, genetic, metabolic, cancer, and age-related diseases. This review aims to describe the current knowledge, challenges and future perspectives to characterize and explore this new intercellular communication system and to design TNT-based therapeutic strategies.
Cell-to-cell communication is essential to all biological processes. Tunneling nanotubes (TNTs), also named cytonemes and tumor microtubes, are a recently discovered form of the long-distance communication system between cells (Onfelt et al., 2004; Rustom et al., 2004; Gerdes et al., 2007). Consisting of long cytoplasmic, open-ended or connexin-containing protrusions that can connect cells, the proposed functions of these structures are diverse and have been shown to include the long-range exchange of organelles, vesicles, and small molecules between connected cells (Gerdes et al., 2007). Data from in vitro and ex vivo studies indicate that TNTs are minimally observed in uninfected cells (Eugenin et al., 2009a; Gerdes et al., 2013). In contrast, in vitro TNT formation and TNT-mediated intercellular communication are significantly higher in several pathologic forms of disease, including, virus infection, cancer, synucleinopathies (Parkinson's disease, Lewy bodies, and multiple system atrophy) as well as tauopathies, and prion-associated diseases (Gerdes and Carvalho, 2008; Eugenin et al., 2009a; Gousset et al., 2009; Abounit and Zurzolo, 2012; Wang and Gerdes, 2012; Gerdes et al., 2013; Austefjord et al., 2014; Abounit et al., 2015, 2016a,b; Desir et al., 2016; Tardivel et al., 2016). Several laboratories observed the presence of connexin and gap junction channels in TNTs, but the role of gap junctions (GJ) in these processes and these diseases is still under active investigation. These observations open the possibility of a long-range gap junctional communication mediated by the TNT processes.
In pathological conditions, TNT numbers can increase and facilitate the intercellular spread of infectious and toxic agents. To date, TNT formation has been observed in tissue culture in many different mammalian cell types (from epithelial to endothelial, mesenchymal and stem cells), immune cells (including B, T, NK cells, neutrophils, monocyte/macrophages and dendritic cells), neurons, glial cells and cancer cells, suggesting that their presence is more ubiquitous than initially thought (see review by Gerdes et al., 2007). In vivo, TNT-like protrusions called cytonemes have been observed in the imaginal disc development of Drosophila (Kornberg, 1999; Hsiung et al., 2005) and prior to fertilization of Plasmodium gametes in the midgut of the Anopheles malaria vector (Rupp et al., 2011). Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut (Rupp et al., 2011). Furthermore, TNT-like structures have been commonly observed between immune cells in lymph nodes (see review by Onfelt et al., 2004; Gerdes et al., 2007; Zaccard et al., 2016) and between dendritic cells in mouse cornea (Chinnery et al., 2008). Other examples of TNT-like structures observed in tissues have been reported in malignant tumors resected from human cancer patients (Pasquier et al., 2013; Ady et al., 2014; Antanaviciute et al., 2014; Thayanithy et al., 2014b), in leukemic cells obtained from bone marrow aspirates of pediatric patients (Polak et al., 2015) and in cardiac myocytes and non-myocyte cells in heart damage (Quinn et al., 2016). Moreover, an impressive in vivo demonstration of TNT-like structures (named tumor microtubes, TMs) has been reported in malignant gliomas, providing further support for a potentially important role for direct intercellular communication by TNT and GJ in tumor development and progression (Osswald et al., 2016). Interestingly, Dr. Gerdes's laboratory demonstrate that TNT between different cell types are electrically coupled by a mechanism involving gap junctions (Wang et al., 2010, 2012; Wang and Gerdes, 2012; Gerdes et al., 2013; Austefjord et al., 2014).
On September 22-23, 2016, academic leaders in the TNT field (see authors list) met in Collegeville, Pennsylvania, USA to discuss “Tunneling nanotubes (TNTs): Cell to Cell Social Networking in Disease.” In addition to the basic biology experts from Europe, Asia, and the United States, the meeting had extensive interest and attendance from researchers from the pharmaceutical industry, and the U.S. National Institutes of Health (NIH); this unique combination of basic and translational research expertise produced vigorous discussion and debate on several important aspects of this new field of the biology of intercellular communication including TNT and the role of GJ in health and disease. The focus was to clarify what defines TNT structures, what signals trigger their formation and accountability for their differential permeability and selectivity. Lastly, the potential use of TNTs to rescue cells from cell death or metabolic distress and as novel therapeutic approaches were considered. The conclusions drawn from the discussions are summarized in this review.
In the last 10 years, there have been many descriptions and observations of cellular protrusions connecting cells, which appear quite different from TNTs. Hence, it is critical to be able to distinguish TNTs from other types of cell projections. The similarity between TNT and GJ channels were highlighted (Rustom et al., 2004; Watkins and Salter, 2005). Some TNTs have been shown to possess GJ components (Wang et al., 2010; Wang and Gerdes, 2012). Data from Drs. Osswald and Eugenin showed that connexin-43 (Cx43) is present in the TNT-like structures under various contexts (between astrocytoma cells or between macrophages) and that inhibition of GJ channels does not prevent their formation but does interfere with normal communication between TNT connected cells. These data suggest that the two communication systems evolved to complement each other in coordinating cell-to-cell communication.
A related issue is whether all TNTs are open-ended and what is the mechanism of their formation. Some reports described the intercellular exchange of Ca2+ signals between distant cells are mediated via TNTs (Watkins and Salter, 2005; Hase et al., 2009; Wang et al., 2010, 2012; He et al., 2011; Smith et al., 2011; Wittig et al., 2012; Al Heialy et al., 2015; Osswald et al., 2015) suggesting some form of membrane/cytosolic continuity along these structures or active GJ channels are present at the end of the process (Wang et al., 2010, 2012; Wang and Gerdes, 2012; Gerdes et al., 2013; Austefjord et al., 2014). The mechanisms involved in this process of intercellular Ca2+ wave propagation are not well understood, but GJ are thought to be intimately involved (Wang et al., 2010, 2012; Wittig et al., 2012; Lock et al., 2016). Further, the observation in lymphocytes that TNTs are not permeable to Ca2+ highlight the diverse phenotype in their physiological properties (Davis and Sowinski, 2008; Sowinski et al., 2008). Characterization of TNTs in untreated cells in culture indicates that TNTs are uniformly F-actin positive and have low expression of tubulin (Onfelt et al., 2006; Rupp et al., 2011; Gousset et al., 2013; Thayanithy et al., 2014a; Astanina et al., 2015; Polak et al., 2015) suggesting that actin regulators and actin-driven motors might be implicated in the formation and/or function of TNTs. In PC12 cells, the immunocytochemical analysis demonstrates that synaptophysin, a marker of synaptic vesicles, as well as Myosin-X (Myo10) and Va (MyoVa), both actin-based motor proteins, were present inside TNTs (Rustom et al., 2004). These data were confirmed in other cell types (Gousset et al., 2013; Schiller et al., 2013; Reichert et al., 2016; Tardivel et al., 2016), and M-Sec through Ral-mediated actin remodeling was shown to be involved in TNT formation as reported by Dr. Kimura (Hase et al., 2009; Ohno et al., 2010). Furthermore, recent data indicated that TNT mediates a long-range transmission of IP3 by a gap junction-dependent mechanism (Lock et al., 2016). Nonetheless, it is still entirely unknown which proteins are involved in the formation, stability, and transport associated with TNTs and is very likely that different mechanisms will participate in the formation of these structures and are prevalent in different cell types.
Filamentous Actin (F-Actin), M-Sec, myosin Va, and X, as well as Cx43, are well-known components of TNTs, and the blocking any of these components reduces or prevents communication. Preliminary data from Dr. Den Boer showed that various types of actin inhibitors, but not tubulin inhibitors, will reduce the level of TNT signaling in leukemia. Novel data from Dr. Zurzolo showed that TNTs and filopodial extensions (which look very similar in confocal microscopy) have different requirements and rely on different actin regulators (Abounit et al., 2015). This is consistent with the previous observation made from the same group (Gousset et al., 2013).
Several groups have demonstrated that HIV-infected cells (e.g., those containing proteins or infected with HIV) can send TNTs to neighboring uninfected or healthy cells, resulting in the spread of infection or aggregation of toxic viral proteins. Dr. Gousset indicated that the transfer of the HIV-1 Nef accessory protein is mediated via TNTs between a macrophage cell line and T cells. Using this Nef model system, it was shown that Nef transfer occurred through a Myo10-dependent mechanism. Similarly, diseased cells lacking functional lysosomes have also been shown to induce TNT formation from nearby healthy cells to facilitate lysosome delivery into diseases cells (Abounit et al., 2015, 2016a). Interestingly, lysosomal dysfunction occurs in neurodegenerative disease. Dr. Zurzolo's group recently showed that lysosomes could be transferred through TNTs to mediate the intercellular spreading of misfolded alpha-synuclein in a neuronal cell model of Parkinson's disease (Abounit et al., 2015, 2016b). Lysosomal cross-correction via TNTs was also shown in the context of a lysosomal storage disorder after hematopoietic stem cell transplantation resulting in long-term tissue preservation (Yasuda et al., 2011; Astanina et al., 2015; Naphade et al., 2015; Abounit et al., 2016a). Similar TNT transfer mechanisms have been observed for mitochondria in different diseases (Han et al., 2016; Jackson et al., 2016; Jiang et al., 2016; Reichert et al., 2016; Sinclair et al., 2016; Wang et al., 2016; Zhang et al., 2016).
The intracellular and extracellular signals involved in the formation, permeability, and directionality of these TNTs are unknown. Interestingly, experiments using different tumor cell lines, primary astrocytes, acute leukemia cells, T cells, and macrophages demonstrate that the formation, communication, transfer of metabolites and the collapse of the TNTs are extremely fast (30–60 s) and can reach distances up to 300 μm. To further understand the properties of TNTs and GJ either the identification of novel proteins and lipids capable of supporting these mechanisms or identification of new TNT-related functions of existing proteins are required. The main conclusion was that several types of TNTs are present in multiple cell types and tissues. Further research is required to identify potential biomarkers of TNT formation for different cell types is therefore warranted. Moreover, an agreed definition of a TNT has been the subject of much debate and consensus amongst TNT scientists is a tubular membrane connection between non-adjacent cells that allow direct intercellular communication, not necessarily gap junction-mediated. They contain F-actin, are open-ended and have a variable diameter from 50 to 800 nm. Although different types of tubular, membranous connections have been observed to form between distant cells, the term “TNT-like structure” can be ascribed to these cellular structures, provided that they fulfill the essential requirement of allowing intercellular exchanges of any material, (e.g., vesicular, particulate, ionic, molecular, organismic) between the connected cells (see Figure 1). To identify TNT-associated structures, there is a need for new or improved super-resolution and electron microscopy methods that can structurally characterize this new intercellular communication system in more detail. It will also be important to describe TNTs in different cell types and situations, where expression of one TNT type may predominate. Also, more data using live imaging systems are needed to describe the mechanism of transfer.
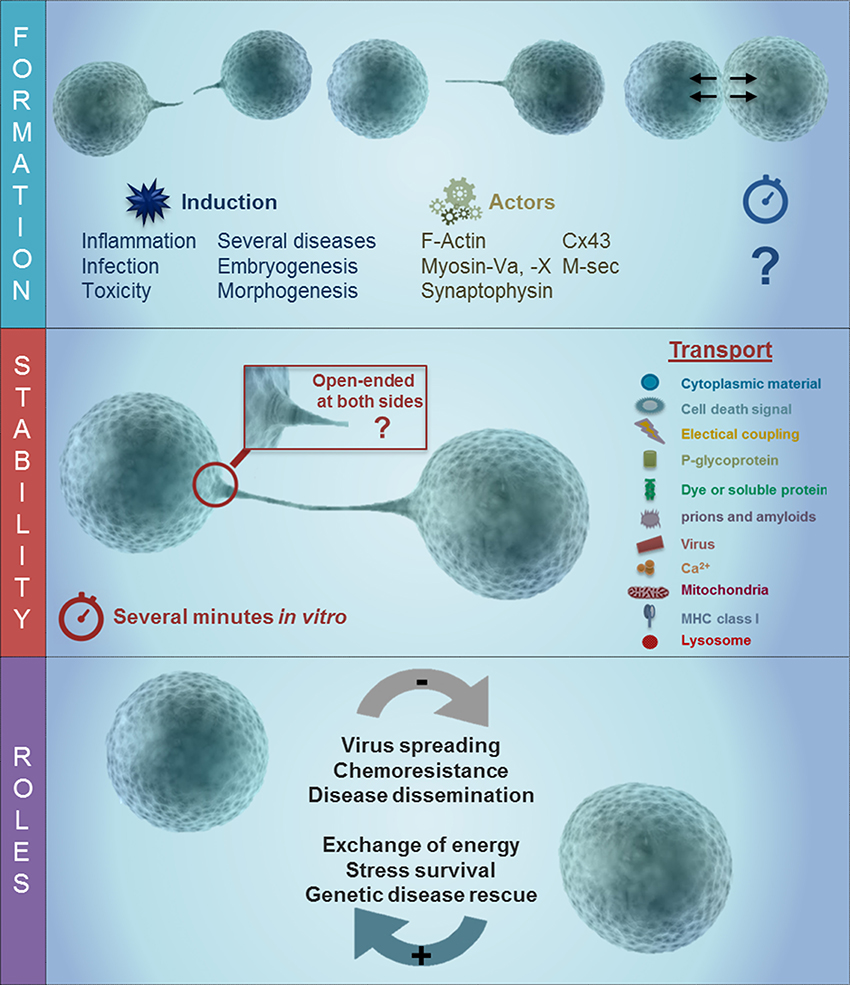
Figure 1. Schematic of TNT formation and the potential role of gap junction channels during long rage communication. As described in the text, TNT have at least 3 different stages, including formation, stabilization, and the transport of the cargo. The last one is associated with several different roles in disease including viral spreading, chemoresistance, and disease dissemination as well as an energy associated survival, genetic disease rescue and stress survival. TNT formation is triggered by inflammation, infection, toxicity, in several disease, and embryogenesis/morphogenesis. Some of the proteins involved in the formation of TNT are actin, Myosin Va and X, synaptophysin, Cx43, and M-sec. Following the formation of the TNT process, there are at least 2 different types of tubes, a synaptic and open-ended process. The formation of these long rage TNT enable the connected cells to share multiple proteins and lipids.
Another important question under consideration is the timing and location of TNT formation. Several reports indicated that viruses, such as herpes (La Boissiere et al., 2004; Sherer et al., 2007), influenza (Kumar et al., 2017), and pseudorabies viruses (Favoreel et al., 2005), can be transmitted through long extensions without contact with the extracellular environment, suggesting that viruses may have evolved to use TNTs to spread efficiently between connected cells (Figure 1). The signals that guide the formation of TNTs are not entirely known. However, a re-examination of older reports through the prism of the current knowledge of TNTs indicates that there were published descriptions of increased formation of TNT-like structures in inflammatory conditions. In particular, TNT-like structures have been observed under the following pathological conditions in vitro: cell infected with Listeria monocytogenes and Mycobacterium Bovis (Dramsi and Cossart, 1998; Wehland and Carl, 1998; Onfelt et al., 2006), in astrocytes treated with H2O2 (Zhu et al., 2005), microglia activated with PMA and calcium ionophore (Martinez et al., 2002), monocyte/macrophages treated with LPS plus IFN-γ (Eugenin et al., 2003), lymphocytes and human macrophages infected with HIV (Sowinski et al., 2008; Eugenin et al., 2009b), mouse neuronal CAD cells and primary neurons and astrocytes infected with exogenous PrP (Gousset et al., 2009, 2013), and more recently neurons treated with pathogenic amyloid aggregates (Costanzo et al., 2013; Abounit et al., 2016a,b). It is therefore not surprising that TNT-like structures have also been identified in normal hematopoietic (CD34+) progenitor cells and lymphoid leukemia cells and that interference with TNT signaling in the hematopoietic context results in altered secretion of cytokines (Polak et al., 2015). Interestingly, most of these treatments are also associated with the formation and functional gap junctional communication, especially in immune cells.
Dr. Zurzolo's group proposed that diseases associated with the spread of the misfolded aggregated proteins within the CNS (like a prion, Alzheimer, Parkinson, and Huntington disease) might involve TNT-mediated spreading (Abounit and Zurzolo, 2012; Delage and Zurzolo, 2013; Abounit et al., 2015, 2016a,b; Delage et al., 2016). They demonstrated that prion protein, PolyQ Huntingtin, fibrillar tau and alpha-synuclein transfer between neurons in culture using TNTs as the predominant mechanism of dissemination (Abounit et al., 2016a,b). Together with the postulated role of TNTs in HIV spreading within the central nervous system (Eugenin et al., 2009a,b; Abounit et al., 2016a), this suggests that multiple diseases can use TNTs to spread toxicity and infection, identifying TNTs as an exciting new potential therapeutic target. Indeed, inhibition of TNTs may block or reduce the amplification of several diseases including HIV, Parkinson's disease, Lewy bodies, and multiple system atrophy as well as tauopathies (Gousset et al., 2009, 2013; Abounit and Zurzolo, 2012; Costanzo et al., 2013; Abounit et al., 2016a,b). Dr. Zurzolo presented data showing that misfolded aggregated tau leads to an increase in TNT formation in culture, but the role of gap junction channels in these tubes was not examined (Abounit et al., 2016b). In agreement with Dr. Zurzolo's findings, several groups have identified TNT like structures in tau related pathologies and their potential role in disease by facilitating electrical coupling and calcium signaling between distant cells (Gerdes et al., 2007; Wang et al., 2012; Wittig et al., 2012; Tardivel et al., 2016), supporting further a potential role of gap junction channels in TNT biology.
Another important role of TNTs in disease may be linked to modulation of the tumor microenvironment. Data from Dr. Den Boer showed that TNTs are actively formed between leukemic cells and bone marrow-derived mesenchymal stromal cells. This interaction is beneficial to the viability of leukemic cells and induces chemo-resistance, which can be abrogated by disrupting the TNTs (Polak et al., 2015). Only recently, Drs. Winkler and Osswald demonstrated that TNT-like structures are essential in the pathogenesis of astrocytomas including the participation of connexin containing channels (Osswald et al., 2015, 2016; Winkler, 2016; Jung et al., 2017; Weil et al., 2017).
As indicated above, the exact role of TNTs and GJ channels is unclear. However, there is evidence that a specific type of TNT-like structures (called cytonemes) have been observed during developmental stages of several organisms like Drosophila and have been postulated to play a role in embryonic development, differentiation, and morphogenesis (Ramirez-Weber and Kornberg, 1999; Roy et al., 2011, 2014; Rojas-Rios et al., 2012; Bilioni et al., 2013; Bischoff et al., 2013; Kornberg, 2014; Kornberg and Roy, 2014; Huang and Kornberg, 2016; Karlikow et al., 2016). Further, TNT-like structures were found in the unicellular malaria parasites during gametogenesis, which takes place in the midgut of the Anopheles mosquito and proposed to be important for the initial contact between mating partners (Rupp et al., 2011). Although the role of TNTs in normal cells was not specifically addressed, there is a large body of data supporting the presence and the need of TNT-like communication during development and immune cell activation (Kornberg, 1999; Ramirez-Weber and Kornberg, 1999; Roy et al., 2011, 2014; Bilioni et al., 2013; Bischoff et al., 2013; Briscoe and Vincent, 2013; Polak et al., 2015; Huang and Kornberg, 2016; Karlikow et al., 2016). A recent report from the Mailliard group describes the induction and regulation of TNTs in dendritic cells as a normal component of their function as mediators of adaptive immunity (Zaccard et al., 2015). In this study, dendritic cells matured under type-1 pro-inflammatory conditions acquired a unique program to rapidly form intercellular networks of tunneling nanotube-like structures upon subsequent antigen-driven interaction with CD4+ T-helper (TH) cells. This immune process, which they termed dendritic cell “reticulation,” is induced by the TH cell-derived factor CD40L, and serves to facilitate the functional intercellular transfer of antigens and endosomal vesicles (Zaccard et al., 2015). Interestingly, this process is differentially regulated by the opposing activity of the respective TH1- and TH2-associated cytokines IFN-γ and IL-4. Importantly, they also describe how the induction and regulation of TNT networks in dendritic cells can be exploited by pathogens such as HIV to facilitate cell-to-cell spread (Mailliard et al., 2013; Zaccard et al., 2015). Similar results of antigen sharing has been described in the context of GJ communication (Neijssen et al., 2005; Matsue et al., 2006; Corvalan et al., 2007; Handel et al., 2007; Mendoza-Naranjo et al., 2007; Pang et al., 2009). Thus, it may be that a similar mechanism of amplification of the immune response can be mediated either by TNT's or by gap junctions.
Under inflammatory or pathological conditions in the context of a genetic lysosomal storage disorder, cystinosis, TNTs also serve as a delivery system to transfer “healthy” lysosomes. Indeed, following the systemic transplantation of wild-type hematopoietic stem and progenitor cells (HSPCs) in the mouse model of cystinosis, Ctns−/− mice, HSPCs differentiate into macrophages and generate TNTs that transfer cystinosis-bearing lysosomes to the adjacent disease cells, leading to long-term kidney preservation (Naphade et al., 2015). A similar mechanism accounts for the conservation of the cornea and thyroid in the Ctns−/− mice (Rocca et al., 2015; Gaide Chevronnay et al., 2016). An understanding of the role of this new communication system in quiescent cells, during the immune response and in pathological conditions may open new potential therapeutic opportunities to target these diseases with none-to-minimal side effects as the current scientific data suggests that TNTs are only minimally expressed under homeostatic conditions.
Another important question in the TNT field concerns the types of cargos being transported within the TNTs. Several reports support the idea that different types of TNTs, as categorized by size, content, and permeability, exist in different cells and under different conditions as well as presence or absence of gap junction channels. TNTs have been shown to mediate long-range transmission of Ca2+ signals between cells (Watkins and Salter, 2005; Hase et al., 2009; Wang et al., 2010, 2012; He et al., 2011; Smith et al., 2011; Wittig et al., 2012; Al Heialy et al., 2015; Osswald et al., 2015), a novel mechanism that adds to the known repertoire by which Ca2+ ions communicate information between cells. The mechanism through which this occurs is not well understood, but gap junctions are thought to play a role in mediating intercellular transmission of Ca2+ waves (Wang et al., 2010, 2012; Wittig et al., 2012; Lock et al., 2016). In other instances, TNT has been shown not to be permeable to Ca2+. TNTs in other systems allow transport of mitochondria and vesicles, suggesting that the internal pore size is large enough for the trafficking of these organelles (see review by Gerdes et al., 2007; Sherer et al., 2007). The observation that mitochondria can be exchanged between TNT-connected cells is extremely important because it could be one of the first demonstrations of cell to cell transfer of genetic material between non-dividing mammalian cells, suggesting that at least mitochondrial DNA (and potentially siRNA) is not cell type specific and can be shared between different types of cells connected by TNT-like structures (Li et al., 2014; Jackson et al., 2016; Jiang et al., 2016; Sinclair et al., 2016). It is still unclear whether multiple types of TNTs exist or whether the observed differences represent different maturation stages of the same processes. Also, the timing of gap junction formation in relation to the formation of TNT's is no known.
There are two hypotheses that describe how pathogens are sorted in TNTs in infected cells: First, that type of TNT determines the function of the tubular process and type of cargo transported and second, whether TNTs have the capability to sort the cargo at the initiating and terminating regions of the TNT. Both possibilities are feasible based on several scientific papers demonstrating differential TNT selectivity and transport properties (see Figure 1). For example, Drs. Osswald and Eugenin showed that gap junction channels are present in TNTs/TNT-like structures, suggesting that at least this type of TNT may have a cutoff of 1.2 kDa, such that only small molecules can be transferred between TNT connected cells expressing this kind of channel. Dr. Zurzolo showed that PrPSc (the pathogenic form of the prion protein) and other protein aggregates, as well as organelles and lysosomes, can be transmitted between the connected cells. Recent data from Drs. Lou, Pasquier, Osswald and Den Boer demonstrated that TNTs or TNT-like structures might also play a critical role in tumor growth, metastasis, and chemo-resistance, suggesting that TNT communication in tumors can exchange molecules which accelerate the spreading of disease and induce therapy resistance.
In conclusion, TNT's can transport a variety of products from second messengers (e.g., mRNA to large organelles), but the mechanism of selectivity, transport, and delivery are still unknown. Although myosin motors have been found inside TNTs and therefore likely to be involved in the movement of the different cargoes on the actin cables running inside TNTs, there are still many open questions relating to the identities of the specific motors; whether there is diffusion allowed, and regulation/determination of the different uni- or bi-directional transport mechanisms at play. To answer these questions, fundamental research is required (and should be actively encouraged) to better understand the biology of the structure and composition of TNTs and associated GJ channels, and their potential role in human disease.
Evidence of TNTs in vivo is the central requirement to further progression of research in this area. Literature evidence for the existence of these cellular protrusions has been limited to date, mainly because there are no known specific biomarkers of TNTs. However, a review of the literature revealed several examples of TNT-like structures that have been observed in vivo or ex vivo. These include the cytonemes found in Drosophila (Kornberg, 1999; Hsiung et al., 2005). TNT like structures between immune cells in lymph nodes (see review by Onfelt et al., 2004; Gerdes et al., 2007), and between MHC class II+ cells in the mouse cornea (Chinnery et al., 2008), as well as the bridges TNT-like structures observed in several models of malignant tumors (cancer) such as mesothelioma, lung cancer, ovarian cancer, and laryngeal cancer (Ady et al., 2014; Antanaviciute et al., 2014, 2015; Thayanithy et al., 2014b; Desir et al., 2016) or capable of crossing the dense tubular basement membrane in the kidney of the cystinosis mouse model (Naphade et al., 2015) or in their cornea and thyroid (Rocca et al., 2015; Gaide Chevronnay et al., 2016). One major issue in performing these in vivo and ex vivo studies is the difficulties in identifying the precise nature of the structures and clearly determining their role in the transfer. Nonetheless, several reports have provided in vivo evidence to support the role of TNTs in pathophysiology and several forms of the disease. Data from Drs. Osswald, Goodman, Lou, Eugenin and Den Boer reported evidence of TNT-like structures in brain tumors, and in ex vivo hematopoietic stem cells, lung, and ovarian cancers. Also, TNT-like structures were found in human macrophages present in lymph nodes obtained from HIV-infected individuals with HIV reactivation.
Interestingly, viruses, such as African Swine Fever, Ebola, Herpes Simplex, Marburg filoviruses, and Poxvirus Vaccinia, encode viral factors or alter cell activation to induce the formation of filopodia structures that allow viral trafficking between the extracellular matrix and environment into cells (Cudmore et al., 1995; Favoreel et al., 2005; Hartlieb and Weissenhorn, 2006; Jouvenet et al., 2006; Kolesnikova et al., 2007; Gill et al., 2008). These observations suggest that viruses have adapted to use TNT-like structures and GJ to promote viral spread. In conclusion, for TNTs to be considered a viable and functional mechanism for intercellular communications, generating compelling in vivo data that demonstrate a clear difference between healthy and disease states is critically important.
TNTs are considered to have two potential roles, as a mechanism for spreading disease-forming cargos (from prion to viruses) and/or as a means of spread chemotherapeutic agents, beneficial organelles or cellular molecules during stress and pathological conditions. In diseased cells, TNT levels are significantly elevated which may make it possible to specifically block TNT-like related pathways that are induced only in disease. Data from Drs. Lou, Pasquier and Den Boer proposed several models by which TNT formation and function between cancerous cells may be altered or modulated following response to chemotherapeutic drugs, the following exposure to clinically relevant tumor conditions such as hypoxia, micro-environmental-induced changes and/or following intercellular transfer of cellular organelles, such as mitochondria, microRNAs, and endosomal vesicles or even exosomes. Moreover, under normal conditions, disease states that promote inflammation (especially in cancer) could induce TNT formation in response to metabolic stress (Rustom et al., 2004; Abounit et al., 2016a,b).
TNT formation and induction has also been observed following injury, trauma or chronic tissue stresses. Here, they are thought to play a role in the exchange of energetic components and mitochondria (Wang et al., 2011; Zhang, 2011; Pasquier et al., 2013; Las and Shirihai, 2014; Li et al., 2014; Thayanithy et al., 2014b) to help compromised cells to survive stress. This possibility opens new potential therapeutic opportunities. For example, during the stroke, ischemia and reperfusion conditions regulating the formation of TNTs may provide a means of cell rescue. Furthermore, TNTs offer a novel delivery route for stem-cell based therapies against genetic conditions resulting in organelle dysfunction (Bruzauskaite et al., 2016; Antanavičiūtė et al., 2017) and for chemotherapeutic drugs that disrupt DNA replication, such as nucleoside analogs (Bruzauskaite et al., 2016; Antanavičiūtė et al., 2017). A study by Lou demonstrated that TNTs could facilitate the intercellular spread of therapeutic oncolytic viral vectors; furthermore, TNTs also mediated the bystander effect by facilitating distribution of therapeutic drugs (nucleoside analogs) activated by viral thymidine kinase. The study establishes TNTs as an alternate route, beyond gap junctions, for cells to amplify the effects of potential disease-targeting drugs, opening a new door to harnessing TNTs as potential cellular conduits for drug delivery (Bruzauskaite et al., 2016; Antanavičiūtė et al., 2017). Conversely, where infectious agents ‘hijack’ TNTs to spread their pathology, blocking TNTs by targeting specific TNT components could represent another therapeutic strategy in disease. Thus, further research in this area is required to help the scientific field to understand this dual nature of TNTs better.
There is a growing body of evidence that supports the critical role of TNT-like structures and gap junctions in development, immune response, and disease. The increased TNT formation in several pathogenic conditions provides a unique opportunity to pharmacologically modulate these processes to block or increase their formation to control the spread of pathogenic and healthy components communicated through TNTs.
An overview of recent scientific literature indicates that TNT-gap junctional research is in its early stage of research and there are still a number of outstanding questions relating to the mechanisms and signals driving the formation of TNTs, their morphology and detailed structural organization, their components (e.g., proteins and lipids), mechanisms determining their permeability and cargo, how TNTs collapse, biomarkers of TNT formation, and, most importantly, how all of these factors are associated with particular cellular functions (Figure 1). However, it is clear that the main function of TNTs during adulthood is to participate in the immune response and during several pathological conditions. To address these key TNT-related questions, a collaboration between leading TNT scientists is vital, and several aspects and questions of this emerging field are summarized in Table 1. Also, GJ not only communicate to neighboring cells but also potentially through TNTs over a long-range.
Thus, by blocking TNTs and/or gap junctional communication at long distances in infected cells and disrupting the transmission of infectious material to neighboring cells, this approach represents a unique therapeutic strategy for some hard-to-treat diseases which includes some retroviral and microbial infections, neurodegenerative disorders and metastasis in certain cancers.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Work in Ahmed's lab is supported by the National Institutes of Child Health and Human (NICHD and National Institute of Health (NIH); Work in MD lab is supported by a national grant from the Netherlands Organization for Scientific Research (“NWO-vici grant”), the KiKa Foundation and the Dutch Cancer Society; Work in SC lab (and SG) is supported by the Cystinosis Research Foundation, the National Institute of Mental Health grants R21-NS090066 and RO1-DK090058, the Sanford Stem Cell Clinical Center and the California Institute of Regenerative Medicine CLIN1-09230; Work in DH lab is funded by The National Institutes of Health grants, CA179087, GM115972, AI132378 and GM119948; Work in EAE lab is supported by The National Institute of Mental Health grant, MH096625, The National Institute of Neurological Disorders and Stroke, NS105584, PHRI funding and a GSK collaboration agreement; Work in KG Lab is supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number SC2GM111144 and a 2014 CSUPERB New Investigator Grant; Work of SK is supported by grants from the Research Foundation for Opto-Science and Technology and the Mochida Memorial Foundation for Medical and Pharmaceutical Research; Work in the EL lab has been supported by grants from The National Center for Advancing Translational Sciences of the National Institutes of Health (Award Number UL1TR000114), Institutional Research Grant #118198-IRG-58-001-52-IRG94 from the American Cancer Society, the National Pancreas Foundation, The Randy Shaver Cancer Research and Community Fund, The Litman Family Fund for Cancer Research, and the University of Minnesota Deborah E. Powell Center for Women's Health Interdisciplinary Seed Grant support (Grant #PCWH-2013-002); Work in the RM lab is funded by NIH/NIAID, U01-AI35041 04/01/1993—04/30/2019, Multicenter AIDS Cohort Study (RM is co-investigator); Work in the GP lab is supported by the Emmy-Noether-Programme of the Deutsche Forschungsgemeinschaft (PR905/1-2); Work in the VAS lab was supported by a grant (No. LIG-13/2012) from the Research Council of Lithuania; Work in the IS lab is funded by National Institutes of Health Grant GM 100201; Work in CZ lab is funded by Agence Nationale de la Recherche (ANR-14-JPCD-0002-01 and ANR-2016-CE160019), Equipe FRM (Fondation Recherche Médicale) 2014 (DEQ20140329557) and a GSK collaboration agreement.
Abounit, S., Bousset, L., Loria, F., Zhu, S., de Chaumont, F., Pieri, L., et al. (2016a). Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 35, 2120–2138. doi: 10.15252/embj.201593411
Abounit, S., Delage, E., and Zurzolo, C. (2015). Identification and characterization of tunneling nanotubes for intercellular trafficking. Curr. Protoc. Cell Biol. 67, 12 10 1–21. doi: 10.1002/0471143030.cb1210s67
Abounit, S., Wu, J. W., Duff, K., Victoria, G. S., and Zurzolo, C. (2016b). Tunneling nanotubes: a possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 10, 344–351. doi: 10.1080/19336896.2016.1223003
Abounit, S., and Zurzolo, C. (2012). Wiring through tunneling nanotubes–from electrical signals to organelle transfer. J. Cell Sci. 125, 1089–1098. doi: 10.1242/jcs.083279
Ady, J. W., Desir, S., Thayanithy, V., Vogel, R. I., Moreira, A. L., Downey, R. J., et al. (2014). Intercellular communication in malignant pleural mesothelioma: properties of tunneling nanotubes. Front. Physiol. 5:400. doi: 10.3389/fphys.2014.00400
Al Heialy, S., Zeroual, M., Farahnak, S., McGovern, T., Risse, P. A., Novali, M., et al. (2015). Nanotubes connect CD4+ T cells to airway smooth muscle cells: novel mechanism of T cell survival. J. Immunol. 194, 5626–5634. doi: 10.4049/jimmunol.1401718
Antanaviciute, I., Ereminiene, E., Vysockas, V., Rackauskas, M., Skipskis, V., Rysevaite, K., et al. (2015). Exogenous connexin43-expressing autologous skeletal myoblasts ameliorate mechanical function and electrical activity of the rabbit heart after experimental infarction. Int. J. Exp. Pathol. 96, 42–53. doi: 10.1111/iep.12109
Antanaviciute, I., Rysevaite, K., Liutkevicius, V., Marandykina, A., Rimkute, L., Sveikatiene, R., et al. (2014). Long-distance communication between laryngeal carcinoma cells. PLoS ONE 9:e99196. doi: 10.1371/journal.pone.0099196
Antanavičiūtė, I., Šimatonis, L., Ulčinas, O., Gadeikytė, A., Abakevičienė, B., Tamulevičius, S., et al. (2017). Femtosecond laser micro-machined polyimide films for cell scaffold applications. J. Tissue Eng. Regen. Med. doi: 10.1002/term.2376. [Epub ahead of print].
Astanina, K., Koch, M., Jungst, C., Zumbusch, A., and Kiemer, A. K. (2015). Lipid droplets as a novel cargo of tunnelling nanotubes in endothelial cells. Sci. Rep. 5:11453. doi: 10.1038/srep11453
Austefjord, M. W., Gerdes, H. H., and Wang, X. (2014). Tunneling nanotubes: diversity in morphology and structure. Commun. Integr. Biol. 7:e27934. doi: 10.4161/cib.27934
Bilioni, A., Sanchez-Hernandez, D., Callejo, A., Gradilla, A. C., Ibanez, C., Mollica, E., et al. (2013). Balancing Hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and shifted/DmWif. Dev. Biol. 376, 198–212. doi: 10.1016/j.ydbio.2012.12.013
Bischoff, M., Gradilla, A. C., Seijo, I., Andres, G., Rodriguez-Navas, C., Gonzalez-Mendez, L., et al. (2013). Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat. Cell Biol. 15, 1269–1281. doi: 10.1038/ncb2856
Briscoe, J., and Vincent, J. P. (2013). Hedgehog threads to spread. Nat. Cell Biol. 15, 1265–1267. doi: 10.1038/ncb2878
Bruzauskaite, I., Bironaite, D., Bagdonas, E., Skeberdis, V. A., Denkovskij, J., Tamulevicius, T., et al. (2016). Relevance of HCN2-expressing human mesenchymal stem cells for the generation of biological pacemakers. Stem Cell Res. Ther. 7:67. doi: 10.1186/s13287-016-0326-z
Chinnery, H. R., Pearlman, E., and McMenamin, P. G. (2008). Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J. Immunol. 180, 5779–5783. doi: 10.4049/jimmunol.180.9.5779
Corvalan, L. A., Araya, R., Branes, M. C., Saez, P. J., Kalergis, A. M., Tobar, J. A., et al. (2007). Injury of skeletal muscle and specific cytokines induce the expression of gap junction channels in mouse dendritic cells. J. Cell. Physiol. 211, 649–660. doi: 10.1002/jcp.20971
Costanzo, M., Abounit, S., Marzo, L., Danckaert, A., Chamoun, Z., Roux, P., et al. (2013). Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J. Cell Sci. 126, 3678–3685. doi: 10.1242/jcs.126086
Cudmore, S., Cossart, P., Griffiths, G., and Way, M. (1995). Actin-based motility of vaccinia virus. Nature 378, 636–638. doi: 10.1038/378636a0
Davis, D. M., and Sowinski, S. (2008). Membrane nanotubes: dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 9, 431–436. doi: 10.1038/nrm2399
Delage, E., Cervantes, D. C., Penard, E., Schmitt, C., Syan, S., Disanza, A., et al. (2016). Differential identity of Filopodia and tunneling nanotubes revealed by the opposite functions of actin regulatory complexes. Sci. Rep. 6:39632. doi: 10.1038/srep39632
Delage, E., and Zurzolo, C. (2013). Exploring the role of lipids in intercellular conduits: breakthroughs in the pipeline. Front. Plant Sci. 4:504. doi: 10.3389/fpls.2013.00504
Desir, S., Dickson, E. L., Vogel, R. I., Thayanithy, V., Wong, P., Teoh, D., et al. (2016). Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget 7, 43150–43161. doi: 10.18632/oncotarget.9504
Dramsi, S., and Cossart, P. (1998). Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14, 137–166. doi: 10.1146/annurev.cellbio.14.1.137
Eugenin, E. A., Branes, M. C., Berman, J. W., and Saez, J. C. (2003). TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J. Immunol. 170, 1320–1328. doi: 10.4049/jimmunol.170.3.1320
Eugenin, E. A., Gaskill, P. J., and Berman, J. W. (2009a). Tunneling nanotubes (TNT): a potential mechanism for intercellular HIV trafficking. Commun. Integr. Biol. 2, 243–244. doi: 10.4161/cib.2.3.8165
Eugenin, E. A., Gaskill, P. J., and Berman, J. W. (2009b). Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell. Immunol. 254, 142–148. doi: 10.1016/j.cellimm.2008.08.005
Favoreel, H. W., Van Minnebruggen, G., Adriaensen, D., and Nauwynck, H. J. (2005). Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. U.S.A. 102, 8990–8995. doi: 10.1073/pnas.0409099102
Gaide Chevronnay, H. P., Janssens, V. P., Van Der Smissen, P., Rocca, C. J., Liao, X. H., Refetoff, S., et al. (2016). Hematopoietic stem cells transplantation can normalize thyroid function in a cystinosis mouse model. Endocrinology 157, 1363–1371. doi: 10.1016/S1525-0016(16)32972-0
Gerdes, H. H., Bukoreshtliev, N. V., and Barroso, J. F. (2007). Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 581, 2194–2201. doi: 10.1016/j.febslet.2007.03.071
Gerdes, H. H., and Carvalho, R. N. (2008). Intercellular transfer mediated by tunneling nanotubes. Curr. Opin. Cell Biol. 20, 470–475. doi: 10.1016/j.ceb.2008.03.005
Gerdes, H. H., Rustom, A., and Wang, X. (2013). Tunneling nanotubes, an emerging intercellular communication route in development. Mech. Dev. 130, 381–387. doi: 10.1016/j.mod.2012.11.006
Gill, M. B., Edgar, R., May, J. S., and Stevenson, P. G. (2008). A gamma-herpesvirus glycoprotein complex manipulates actin to promote viral spread. PLoS ONE 3:e1808. doi: 10.1371/journal.pone.0001808
Gousset, K., Marzo, L., Commere, P. H., and Zurzolo, C. (2013). Myo10 is a key regulator of TNT formation in neuronal cells. J. Cell Sci. 126, 4424–4435. doi: 10.1242/jcs.129239
Gousset, K., Schiff, E., Langevin, C., Marijanovic, Z., Caputo, A., Browman, D. T., et al. (2009). Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11, 328–336. doi: 10.1038/ncb1841
Han, H., Hu, J., Yan, Q., Zhu, J., Zhu, Z., Chen, Y., et al. (2016). Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 13, 1517–1524. doi: 10.3892/mmr.2015.4726
Handel, A., Yates, A., Pilyugin, S. S., and Antia, R. (2007). Gap junction-mediated antigen transport in immune responses. Trends Immunol. 28, 463–466. doi: 10.1016/j.it.2007.08.006
Hartlieb, B., and Weissenhorn, W. (2006). Filovirus assembly and budding. Virology 344, 64–70. doi: 10.1016/j.virol.2005.09.018
Hase, K., Kimura, S., Takatsu, H., Ohmae, M., Kawano, S., Kitamura, H., et al. (2009). M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427–1432. doi: 10.1038/ncb1990
He, K., Shi, X., Zhang, X., Dang, S., Ma, X., Liu, F., et al. (2011). Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc. Res. 92, 39–47. doi: 10.1093/cvr/cvr189
Hsiung, F., Ramirez-Weber, F. A., Iwaki, D. D., and Kornberg, T. B. (2005). Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature 437, 560–563. doi: 10.1038/nature03951
Huang, H., and Kornberg, T. B. (2016). Cells must express components of the planar cell polarity system and extracellular matrix to support cytonemes. Elife 5:e18979. doi: 10.7554/eLife.18979
Jackson, M. V., Morrison, T. J., Doherty, D. F., McAuley, D. F., Matthay, M. A., Kissenpfennig, A., et al. (2016). Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 34, 2210–2223. doi: 10.1002/stem.2372
Jiang, D., Gao, F., Zhang, Y., Wong, D. S., Li, Q., Tse, H. F., et al. (2016). Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 7:e2467. doi: 10.1038/cddis.2016.358
Jouvenet, N., Windsor, M., Rietdorf, J., Hawes, P., Monaghan, P., Way, M., et al. (2006). African swine fever virus induces filopodia-like projections at the plasma membrane. Cell. Microbiol. 8, 1803–1811. doi: 10.1111/j.1462-5822.2006.00750.x
Jung, E., Osswald, M., Blaes, J., Wiestler, B., Sahm, F., Schmenger, T., et al. (2017). Tweety-homologue 1 drives brain colonization of gliomas. J. Neurosci. 37, 6837–6850. doi: 10.1523/JNEUROSCI.3532-16.2017
Karlikow, M., Goic, B., Mongelli, V., Salles, A., Schmitt, C., Bonne, I., et al. (2016). Drosophila cells use nanotube-like structures to transfer dsRNA and RNAi machinery between cells. Sci. Rep. 6:27085. doi: 10.1038/srep27085
Kolesnikova, L., Bohil, A. B., Cheney, R. E., and Becker, S. (2007). Budding of Marburgvirus is associated with filopodia. Cell. Microbiol. 9, 939–951. doi: 10.1111/j.1462-5822.2006.00842.x
Kornberg, T. (1999). Pictures in cell biology. cytonemes. Trends Cell Biol. 9:434. doi: 10.1016/S0962-8924(99)01653-0
Kornberg, T. B. (2014). The contrasting roles of primary cilia and cytonemes in Hh signaling. Dev. Biol. 394, 1–5. doi: 10.1016/j.ydbio.2014.07.015
Kornberg, T. B., and Roy, S. (2014). Cytonemes as specialized signaling filopodia. Development 141, 729–736. doi: 10.1242/dev.086223
Kumar, A., Kim, J. H., Ranjan, P., Metcalfe, M. G., Cao, W., Mishina, M., et al. (2017). Influenza virus exploits tunneling nanotubes for cell-to-cell spread. Sci. Rep. 7:40360. doi: 10.1038/srep40360
La Boissiere, S., Izeta, A., Malcomber, S., and O'Hare, P. (2004). Compartmentalization of VP16 in cells infected with recombinant herpes simplex virus expressing VP16-green fluorescent protein fusion proteins. J. Virol. 78, 8002–8014. doi: 10.1128/JVI.78.15.8002-8014.2004
Las, G., and Shirihai, O. S. (2014). Miro1: new wheels for transferring mitochondria. EMBO J. 33, 939–941. doi: 10.1002/embj.201488441
Li, X., Zhang, Y., Yeung, S. C., Liang, Y., Liang, X., Ding, Y., et al. (2014). Mitochondrial transfer of induced pluripotent stem cells-derived MSCs to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Respir. Cell Mol. Biol. 51, 455–465. doi: 10.1165/rcmb.2013-0529OC
Lock, J. T., Parker, I., and Smith, I. F. (2016). Communication of Ca2+ signals via tunneling membrane nanotubes is mediated by transmission of inositol trisphosphate through gap junctions. Cell Calcium 60, 266–272. doi: 10.1016/j.ceca.2016.06.004
Mailliard, R. B., Smith, K. N., Fecek, R. J., Rappocciolo, G., Nascimento, E. J., Marques, E. T., et al. (2013). Selective induction of CTL helper rather than killer activity by natural epitope variants promotes dendritic cell-mediated HIV-1 dissemination. J. Immunol. 191, 2570–2580. doi: 10.4049/jimmunol.1300373
Martinez, A. D., Eugenin, E. A., Branes, M. C., Bennett, M. V., and Saez, J. C. (2002). Identification of second messengers that induce expression of functional gap junctions in microglia cultured from newborn rats. Brain Res. 943, 191–201. doi: 10.1016/S0006-8993(02)02621-5
Matsue, H., Yao, J., Matsue, K., Nagasaka, A., Sugiyama, H., Aoki, R., et al. (2006). Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J. Immunol. 176, 181–190. doi: 10.4049/jimmunol.176.1.181
Mendoza-Naranjo, A., Saez, P. J., Johansson, C. C., Ramirez, M., Mandakovic, D., Pereda, C., et al. (2007). Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J. Immunol. 178, 6949–6957. doi: 10.4049/jimmunol.178.11.6949
Naphade, S., Sharma, J., Gaide Chevronnay, H. P., Shook, M. A., Yeagy, B. A., Rocca, C. J., et al. (2015). Brief reports: lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes. Stem Cells 33, 301–309. doi: 10.1002/stem.1835
Neijssen, J., Herberts, C., Drijfhout, J. W., Reits, E., Janssen, L., and Neefjes, J. (2005). Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434, 83–88. doi: 10.1038/nature03290
Ohno, H., Hase, K., and Kimura, S. (2010). M-Sec: emerging secrets of tunneling nanotube formation. Commun. Integr. Biol. 3, 231–233. doi: 10.4161/cib.3.3.11242
Onfelt, B., Nedvetzki, S., Benninger, R. K., Purbhoo, M. A., Sowinski, S., Hume, A. N., et al. (2006). Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 177, 8476–8483. doi: 10.4049/jimmunol.177.12.8476
Onfelt, B., Nedvetzki, S., Yanagi, K., and Davis, D. M. (2004). Cutting edge: membrane nanotubes connect immune cells. J. Immunol. 173, 1511–1513. doi: 10.4049/jimmunol.173.3.1511
Osswald, M., Jung, E., Sahm, F., Solecki, G., Venkataramani, V., Blaes, J., et al. (2015). Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98. doi: 10.1038/nature16071
Osswald, M., Solecki, G., Wick, W., and Winkler, F. (2016). A malignant cellular network in gliomas: potential clinical implications. Neuro-oncology 18, 479–485. doi: 10.1093/neuonc/now014
Pang, B., Neijssen, J., Qiao, X., Janssen, L., Janssen, H., Lippuner, C., et al. (2009). Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J. Immunol. 183, 1083–1090. doi: 10.4049/jimmunol.0900861
Pasquier, J., Guerrouahen, B. S., Al Thawadi, H., Ghiabi, P., Maleki, M., Abu-Kaoud, N., et al. (2013). Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 11:94. doi: 10.1186/1479-5876-11-94
Polak, R., de Rooij, B., Pieters, R., and den Boer, M. L. (2015). B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 126, 2404–2414. doi: 10.1182/blood-2015-03-634238
Quinn, T. A., Camelliti, P., Rog-Zielinska, E. A., Siedlecka, U., Poggioli, T., O'Toole, E. T., et al. (2016). Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc. Natl. Acad. Sci. U.S.A. 113, 14852–14857. doi: 10.1073/pnas.1611184114
Ramirez-Weber, F. A., and Kornberg, T. B. (1999). Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97, 599–607. doi: 10.1016/S0092-8674(00)80771-0
Reichert, D., Scheinpflug, J., Karbanova, J., Freund, D., Bornhauser, M., and Corbeil, D. (2016). Tunneling nanotubes mediate the transfer of stem cell marker CD133 between hematopoietic progenitor cells. Exp. Hematol. 44, 1092.e2–1112.e2. doi: 10.1016/j.exphem.2016.07.006
Rocca, C. J., Kreymerman, A., Ur, S. N., Frizzi, K. E., Naphade, S., Lau, A., et al. (2015). Treatment of inherited eye defects by systemic hematopoietic stem cell transplantation. Invest. Ophthalmol. Vis. Sci. 56, 7214–7223. doi: 10.1167/iovs.15-17107
Rojas-Rios, P., Guerrero, I., and Gonzalez-Reyes, A. (2012). Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 10:e1001298. doi: 10.1371/journal.pbio.1001298
Roy, S., Hsiung, F., and Kornberg, T. B. (2011). Specificity of Drosophila cytonemes for distinct signaling pathways. Science 332, 354–358. doi: 10.1126/science.1198949
Roy, S., Huang, H., Liu, S., and Kornberg, T. B. (2014). Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science 343:1244624. doi: 10.1126/science.1244624
Rupp, I., Sologub, L., Williamson, K. C., Scheuermayer, M., Reininger, L., Doerig, C., et al. (2011). Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut. Cell Res. 21, 683–696. doi: 10.1038/cr.2010.176
Rustom, A., Saffrich, R., Markovic, I., Walther, P., and Gerdes, H. H. (2004). Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. doi: 10.1126/science.1093133
Schiller, C., Diakopoulos, K. N., Rohwedder, I., Kremmer, E., von Toerne, C., Ueffing, M., et al. (2013). LST1 promotes the assembly of a molecular machinery responsible for tunneling nanotube formation. J. Cell Sci. 126, 767–777. doi: 10.1242/jcs.114033
Sherer, N. M., Lehmann, M. J., Jimenez-Soto, L. F., Horensavitz, C., Pypaert, M., and Mothes, W. (2007). Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 9, 310–315. doi: 10.1038/ncb1544
Sinclair, K. A., Yerkovich, S. T., Hopkins, P. M., and Chambers, D. C. (2016). Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res. Ther. 7:91. doi: 10.1186/s13287-016-0354-8
Smith, I. F., Shuai, J., and Parker, I. (2011). Active generation and propagation of calcium signals within tunneling membrane nanotubes. Biophys. J. 100, L37–L39. doi: 10.1016/j.bpj.2011.03.007
Sowinski, S., Jolly, C., Berninghausen, O., Purbhoo, M. A., Chauveau, A., Kohler, K., et al. (2008). Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10, 211–219. doi: 10.1038/ncb1682
Tardivel, M., Begard, S., Bousset, L., Dujardin, S., Coens, A., Melki, R., et al. (2016). Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 4:117. doi: 10.1186/s40478-016-0386-4
Thayanithy, V., Babatunde, V., Dickson, E. L., Wong, P., Oh, S., Ke, X., et al. (2014a). Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp. Cell Res. 323, 178–188. doi: 10.1016/j.yexcr.2014.01.014
Thayanithy, V., Dickson, E. L., Steer, C., Subramanian, S., and Lou, E. (2014b). Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl. Res. 164, 359–365. doi: 10.1016/j.trsl.2014.05.011
Wang, X., Bukoreshtliev, N. V., and Gerdes, H. H. (2012). Developing neurons form transient nanotubes facilitating electrical coupling and calcium signaling with distant astrocytes. PLoS ONE 7:e47429. doi: 10.1371/journal.pone.0047429
Wang, X., and Gerdes, H. H. (2012). Long-distance electrical coupling via tunneling nanotubes. Biochim. Biophys. Acta 1818, 2082–2086. doi: 10.1016/j.bbamem.2011.09.002
Wang, X., Veruki, M. L., Bukoreshtliev, N. V., Hartveit, E., and Gerdes, H. H. (2010). Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. U.S.A. 107, 17194–17199. doi: 10.1073/pnas.1006785107
Wang, X., Yu, X., Xie, C., Tan, Z., Tian, Q., Zhu, D., et al. (2016). Rescue of brain function using tunneling nanotubes between neural stem cells and brain microvascular endothelial cells. Mol. Neurobiol. 53, 2480–2488. doi: 10.1007/s12035-015-9225-z
Wang, Y., Cui, J., Sun, X., and Zhang, Y. (2011). Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 18, 732–742. doi: 10.1038/cdd.2010.147
Watkins, S. C., and Salter, R. D. (2005). Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 23, 309–318. doi: 10.1016/j.immuni.2005.08.009
Wehland, J., and Carl, U. D. (1998). The sophisticated survival strategies of the pathogen Listeria monocytogenes. Int. Microbiol. 1, 11–18.
Weil, S., Osswald, M., Solecki, G., Grosch, J., Jung, E., Lemke, D., et al. (2017). Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro-oncology 19, 1316–1326. doi: 10.1093/neuonc/nox070
Winkler, F. (2016). Tumour network in glioma. ESMO Open 1:e000133. doi: 10.1136/esmoopen-2016-000133
Wittig, D., Wang, X., Walter, C., Gerdes, H. H., Funk, R. H., and Roehlecke, C. (2012). Multi-level communication of human retinal pigment epithelial cells via tunneling nanotubes. PLoS ONE 7:e33195. doi: 10.1371/journal.pone.0033195
Yasuda, K., Khandare, A., Burianovskyy, L., Maruyama, S., Zhang, F., Nasjletti, A., et al. (2011). Tunneling nanotubes mediate rescue of prematurely senescent endothelial cells by endothelial progenitors: exchange of lysosomal pool. Aging 3, 597–608. doi: 10.18632/aging.100341
Zaccard, C. R., Rinaldo, C. R., and Mailliard, R. B. (2016). Linked in: immunologic membrane nanotube networks. J. Leukoc. Biol. 100, 81–94. doi: 10.1189/jlb.4VMR0915-395R
Zaccard, C. R., Watkins, S. C., Kalinski, P., Fecek, R. J., Yates, A. L., Salter, R. D., et al. (2015). CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity. J. Immunol. 194, 1047–1056. doi: 10.4049/jimmunol.1401832
Zhang, Y. (2011). Tunneling-nanotube: a new way of cell-cell communication. Commun. Integr. Biol. 4, 324–325. doi: 10.4161/cib.4.3.14855
Zhang, Y., Yu, Z., Jiang, D., Liang, X., Liao, S., Zhang, Z., et al. (2016). iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF- α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 7, 749–763. doi: 10.1016/j.stemcr.2016.08.009
Keywords: Alzheimer, inflammation, cancer, gap junctions, reactivation
Citation: Ariazi J, Benowitz A, De Biasi V, Den Boer ML, Cherqui S, Cui H, Douillet N, Eugenin EA, Favre D, Goodman S, Gousset K, Hanein D, Israel DI, Kimura S, Kirkpatrick RB, Kuhn N, Jeong C, Lou E, Mailliard R, Maio S, Okafo G, Osswald M, Pasquier J, Polak R, Pradel G, de Rooij B, Schaeffer P, Skeberdis VA, Smith IF, Tanveer A, Volkmann N, Wu Z and Zurzolo C (2017) Tunneling Nanotubes and Gap Junctions–Their Role in Long-Range Intercellular Communication during Development, Health, and Disease Conditions. Front. Mol. Neurosci. 10:333. doi: 10.3389/fnmol.2017.00333
Received: 06 August 2017; Accepted: 02 October 2017;
Published: 17 October 2017.
Edited by:
Guilherme Lucas, University of São Paulo, BrazilReviewed by:
Felix Scholkmann, University Hospital Zurich, SwitzerlandCopyright © 2017 Ariazi, Benowitz, De Biasi, Den Boer, Cherqui, Cui, Douillet, Eugenin, Favre, Goodman, Gousset, Hanein, Israel, Kimura, Kirkpatrick, Kuhn, Jeong, Lou, Mailliard, Maio, Okafo, Osswald, Pasquier, Polak, Pradel, de Rooij, Schaeffer, Skeberdis, Smith, Tanveer, Volkmann, Wu and Zurzolo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eliseo A. Eugenin, ZWxpc2VvLmV1Z2VuaW5AcnV0Z2Vycy5lZHU=
George Okafo, Z2VvcmdlLm4ub2thZm9AZ3NrLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.