- 1The Unit of Regulatory and Molecular Biology, Research Divisions of Biochemistry and Molecular Biology, Salus University, Elkins Park, PA, USA
- 2Department of Physiology and Biophysics, Boston University School of Medicine, Boston, MA, USA
This monograph presents a historical perspective of cornerstone developments on the biochemistry and physiology of mammalian membrane guanylate cyclases (MGCs), highlighting contributions made by the authors and their collaborators. Upon resolution of early contentious studies, cyclic GMP emerged alongside cyclic AMP, as an important intracellular second messenger for hormonal signaling. However, the two signaling pathways differ in significant ways. In the cyclic AMP pathway, hormone binding to a G protein coupled receptor leads to stimulation or inhibition of an adenylate cyclase, whereas the cyclic GMP pathway dispenses with intermediaries; hormone binds to an MGC to affect its activity. Although the cyclic GMP pathway is direct, it is by no means simple. The modular design of the molecule incorporates regulation by ATP binding and phosphorylation. MGCs can form complexes with Ca2+-sensing subunits that either increase or decrease cyclic GMP synthesis, depending on subunit identity. In some systems, co-expression of two Ca2+ sensors, GCAP1 and S100B with ROS-GC1 confers bimodal signaling marked by increases in cyclic GMP synthesis when intracellular Ca2+ concentration rises or falls. Some MGCs monitor or are modulated by carbon dioxide via its conversion to bicarbonate. One MGC even functions as a thermosensor as well as a chemosensor; activity reaches a maximum with a mild drop in temperature. The complexity afforded by these multiple limbs of operation enables MGC networks to perform transductions traditionally reserved for G protein coupled receptors and Transient Receptor Potential (TRP) ion channels and to serve a diverse array of functions, including control over cardiac vasculature, smooth muscle relaxation, blood pressure regulation, cellular growth, sensory transductions, neural plasticity and memory.
Introduction
Initially relegated to the shadows of cyclic AMP signaling, cyclic GMP has slowly ascended to eminence as an important independent signal in the body, governed by the activities of soluble and membrane guanylate cyclases (MGCs) and phosphodiesterases. Here, we briefly outline the chronological development of the field of mammalian MGCs, focusing on how the modular design of the receptor enzyme adds layers of complexity to its biochemistry and physiological function. Variations in the modules confer flexibility and enable MGCs to command a pervasive signaling role over diverse functions throughout the body. Some of the advancements made by the authors and their collaborators are featured. For more comprehensive coverage, the reader is referred to the authors’ earlier reviews: (Sharma (1978, 1985, 2002, 2010); Pugh et al. (1997); Sharma et al. (2004, 2014); Duda et al. (2005b, 2014); Sharma and Duda (2012); Sharma and Duda (2014a,b)).
Cyclic GMP Is a Hormonal Second Messenger
An observation made five decades ago, that cyclic GMP exists in rat urine (Ashman et al., 1963) and the finding that its level in urine depends upon hormonal state (Hardman and Sutherland, 1969; Hardman et al., 1969) were early clues that cyclic GMP serves as a hormonal second messenger. Guided by a prototypic cyclic AMP template, this concept implicated a membrane receptor guanylate cyclase transduction system in vertebrates. In the beginning, there was passionate enthusiasm for this concept and in support, cyclic GMP was present and guanylate cyclase catalytic activity was detected in all tested tissues (Goldberg et al., 1969, 1973; Ishikawa et al., 1969). But with reports that guanylate cyclase catalytic activity was stimulated by a variety of non-hormonal ligands: polyunsaturated fatty acids, peroxides, hydroperoxides, free radicals, ascorbic acid, sodium nitroprusside, and cigarette smoke (Goldberg and Haddox, 1977; Murad et al., 1979), a notion took hold in which MGC was a non-specific enzyme regulated by the cell’s oxidation-reduction potential. In extreme views, no component of cyclic GMP turnover was regarded as linked directly to an intracellular signaling pathway. Where it was found, cyclic GMP turnover was believed to be subordinate to the cyclic AMP signaling system. As a case in point, the known cyclic GMP-dependent protein kinase activity showed some cross-reactivity with cyclic AMP and was thus interpreted as a means for augmenting cyclic AMP signaling (Gill and McCune, 1979).
A few groups pushed on, not willing to relinquish a primary role for cyclic GMP as a second messenger role in hormonal signal transduction (reviewed in Sharma, 1978, 1985; Sharma et al., 1988a,b; Pugh et al., 1997; Sharma and Duda, 1997, 2012, 2014a,b; Sharma, 2002, 2010; Duda et al., 2005b, 2014; Sharma et al., 2015). Two key observations proved to be instrumental in resolving the matter. First, an ACTH-sensitive MGC transduction system exists in rat adrenocortical carcinoma 494 cells and in isolated adrenal fasciculata cells (Perchellet and Sharma, 1980; Shanker and Sharma, 1980; Jaiswal and Sharma, 1986; Jaiswal et al., 1986; Venkataraman et al., 1998; reviewed in Sharma, 2010). Second, unlike adrenal homogenates, isolated adrenal fasciculate and adrenocortical carcinoma cells lack cyclic AMP phosphodiesterase activity. These systems were therefore ideal for testing whether cyclic AMP is the sole hormonal second messenger or whether ACTH evokes MGC activity (Kitabchi et al., 1971; Sharma, 1972).
Physiological levels of ACTH (1–10 μU) stimulate steroidogenesis, yet do not raise the level of cyclic AMP (Figure 1; Sharma et al., 1974). Moreover, there exists an excellent temporal relation between cyclic GMP formation (Sharma et al., 1974; Harrington et al., 1978), phosphorylation and steroidogenesis (Sharma, 1973; Sharma et al., 1976; Perchellet et al., 1978) at these hormone concentrations. Exogenous cyclic GMP mimics ACTH in stimulating protein kinase activity, followed by conversion of cholesterol to corticosterone (Sharma et al., 1976; Sharma and Sawhney, 1978). Eventually, direct evidence was obtained for the presence of ACTH/Ca2+-dependent MGC activity in adrenocortical and adrenocortical carcinoma plasma membranes (Nambi and Sharma, 1981a,b; Nambi et al., 1982a,b). At levels an order of magnitude higher, ACTH will elevate cyclic AMP and drive steroidogenesis further but these levels extend into the supra-physiological range (Sharma et al., 1974, 1976). Thus, cyclic GMP is the principal physiological second messenger of ACTH in isolated adrenal fasciculate and adrenocortical carcinoma cells and cyclic AMP is not the sole second messenger for hormonal signaling in the body.
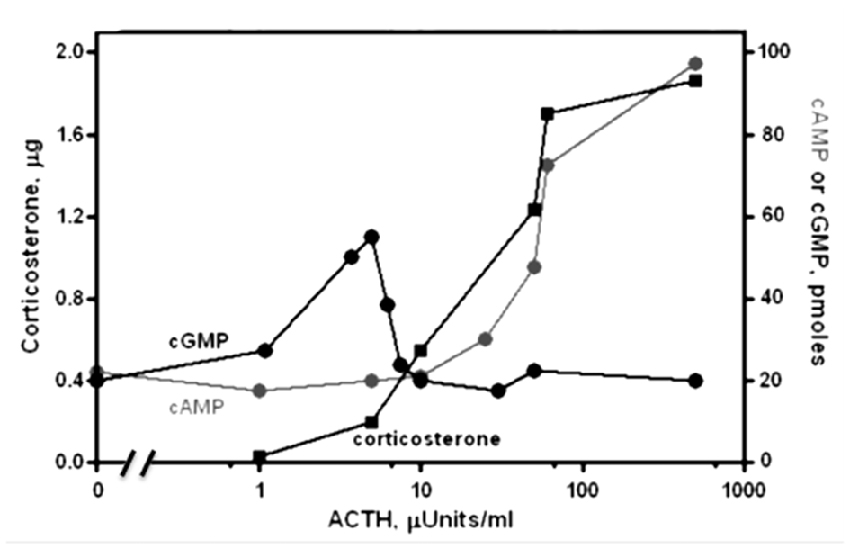
Figure 1. Dose-response relations for the levels of cyclic GMP, cyclic AMP and corticosterone as a function of ACTH in suspensions of isolated adrenal cells of rat. Cyclic GMP (black circles) and corticosterone (black squares) are produced at [ACTH] too low to affect cyclic AMP synthesis. At higher [ACTH], activation of a phosphodiesterase counteracts GMP synthesis and cyclic AMP levels (gray circles) rise to further increase steroidogenesis. Modified, with permission, from Sharma et al. (1974). One micro unit (μU) of ACTH is equal to 4.5 pg or 1.2 × 10−13 moles.
Two challenges were mounted against these ideas. First, a dibutyryl cyclic AMP analog was the most effective activator of cyclic AMP-dependent protein kinase, yet dibutyryl cyclic GMP evoked far less steroidogenic activity than its parent compound cyclic GMP (Hayashi et al., 1979). Second, incubation of adrenocortical cells with sodium nitroprusside and ascorbic acid incremented cyclic GMP levels without stimulating the production of corticosterone (Laychock and Hardman, 1978). Both challenges were overcome upon purification and characterization of the cyclic GMP-dependent protein kinase from the adrenal cortex (Ahrens et al., 1982). In a playful twist of NATURE, the cyclic GMP-dependent protein kinase differs from the cyclic AMP-dependent protein kinase in several curious ways. The most potent effector molecule for cyclic GMP-dependent protein kinase is 8-bromo cyclic GMP; dibutyryl cyclic GMP is almost totally ineffective (Ahrens et al., 1982). Perhaps more surprisingly, it turns out that nitroprusside and ascorbate inhibit cyclic GMP-dependent protein kinase activity (Sharma et al., 1988a,b).
After a heme-dependent soluble guanylate cyclase was purified from bovine lung (Ignarro et al., 1982a,b), it became clear that only the soluble guanylate cyclase is stimulated by free radical-, nitric oxide (NO)-generating agents, including heme; such agents produce little or no effect on MGC (Nambi et al., 1982b; Sharma et al., 1989b). It was thus established that guanylate cyclases exist in membrane and soluble forms each with distinct biochemical properties, and that their signaling roles do not necessarily overlap. In rat adrenal cortex, most (80%) of the guanylate cyclase is membrane-bound and only a minor amount (20%) is soluble (Nambi et al., 1982b; Sharma et al., 1989b). In the second challenge above, it was this minor fraction that was stimulated by sodium nitroprusside and ascorbic acid.
In its current status, the soluble form of the guanylate cyclase is encoded by two genes [the functionalities of β2 cloned from rat kidney and α2, from human fetal brain, remain to be determined (Denninger and Marletta, 1999)]. It is heterodimeric and requires heme for its activity (Figure 2; Allerston et al., 2013; Sharma and Duda, 2014a). As will be described below, MGCs are quite different. In mammals, MGCs are encoded by seven different genes and although dimerization is requisite for activity, it would appear that only homodimers are formed in situ.
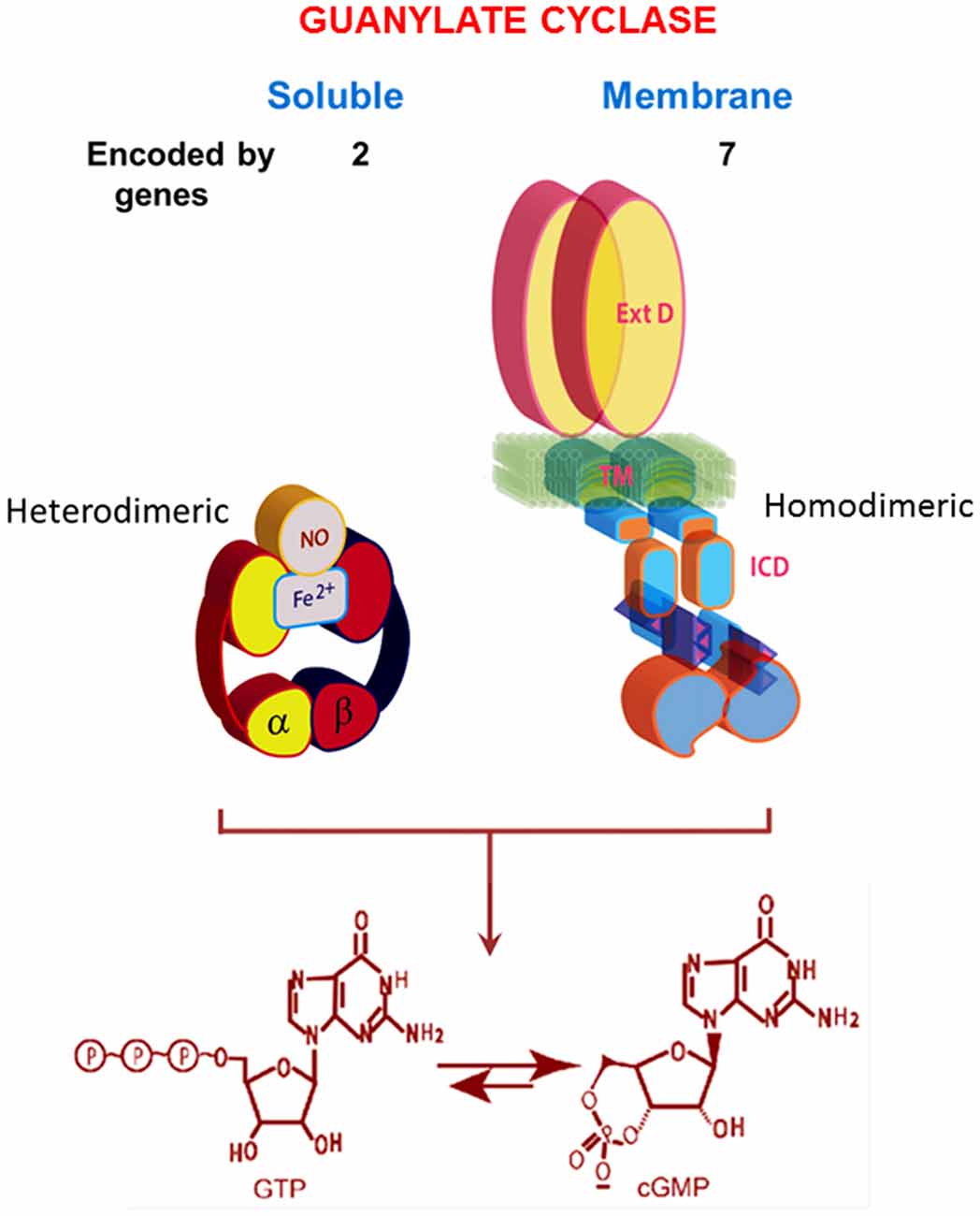
Figure 2. Comparison of soluble and membrane guanylate cyclases (MGCs). The soluble form is a heterodimer of α and β subunits with a single heme capable of binding nitric oxide (NO). The C-terminal segments of both subunits contribute to the catalytic center. The MGC is a single transmembrane spanning protein that is active as a homodimer. Heme binding does not occur. A transmembrane domain (TM) divides the protein into two roughly equal parts, an extracellular domain (ExtD) and an intracellular domain (ICD). Within the ICD, corresponding C-terminal segments from each monomer, arranged in an antiparallel orientation, form the catalytic center. Both types of cyclases are lyases (EC4.6.1.2) that catalyze synthesis of cyclic GMP from GTP. Note that the relative sizes of the modules are not drawn to scale. Redrawn, with permission, from Sharma and Duda (2014a).
Atrial Natriuretic Factor Receptor Guanylate Cyclase (ANF-RGC) Was the First Membrane Guanylate Cyclase to be Purified and Characterized
Purification and characterization of an MGC from rat adrenocortical carcinoma (Paul, 1986; Paul et al., 1987; Sharma, 1988; Sharma and Duda, 2014b) and subsequently from adrenal cortex (Takayanagi et al., 1987; Meloche et al., 1988) brought the discovery that it is a receptor for atrial natriuretic factor (ANF). This receptor MGC, named ANF-RGC, provides the basis for ANF-stimulated cyclic GMP and corticosterone synthesis (Jaiswal et al., 1986). Presumably a similar MGC responds to ACTH, but confirmation is not yet available. The original report noted, “coexistence of the ANF receptor and guanylate cyclase activities on a single polypeptide chain indicates that the mechanism of transmembrane signal transduction involving mediation by second messenger, cyclic GMP, is different from the well-established adenylate cyclase system. In hormone-dependent adenylate cyclase there is an assemblage of individual components—receptor, GTP binding protein, and catalytic moiety—for signal transduction. In contrast, the presence of dual activities—receptor binding and enzymatic—on a single polypeptide chain indicates that this transmembrane protein contains both the information for signal recognition and its translation into a second messenger” (Paul et al., 1987). Accordingly, a multimodal ANF-RGC signal transduction model was envisioned (Sharma et al., 1988a,b, 1989a) and refined (Sharma, 2002; Figure 2). Its central features were that a hormone receptor domain protruded into the extracellular space, a catalytic domain resided inside the cell and a transmembrane segment linked the two.
The same antibody used to purify ANF-RGC to homogeneity from adrenocortical carcinoma and from adrenal cortex indicated a much wider expression including testes (Marala and Sharma, 1988), neurons located in the ventral horn region of rat spinal cord, cerebellar Purkinje cells, and renal glomerular cells (Ballermann et al., 1988). The milestone discovery of ANF-RGC would revolutionize the basic cellular signal transduction field as well as the field of clinical cardiovascular physiology.
An intriguing finding of the early studies was the obligatory presence of Ca2+ for hormonal stimulation of steroidogenesis (Sayers et al., 1972; Haksar and Péron, 1973; Bowyer and Kitabchi, 1974), yet by itself, Ca2+ was ineffective. Only when present with the hormone, did it become an effective steroidogenic factor (Perchellet and Sharma, 1979). Although the underlying mechanism could have been indirect and has still not been clarified, Ca2+ would be found to play a profound role in regulating MGC activity and diversifying its functionality as the field of MGCs progressed.
MGCs Are Constructed with a Modular Design
About 3-years post-purification, the mRNA of ANF-RGC was cloned (Chinkers et al., 1989; Lowe et al., 1989; Pandey and Singh, 1990; Duda et al., 1991). Cloning and sequencing of two more family members soon followed (Chang et al., 1989; Schulz et al., 1989; Duda et al., 1993c). Like ANF-RGC (or GC-A), the new guanylate cyclases are receptors for hormones: type C-natriuretic peptide for one receptor and guanylin, uroguanylin and heat stable enterotoxin for the other, hence, they were named CNP-RGC (or GC-B) and STa-RGC (or GC-C), respectively.
Hydropathy analysis suggests that all three receptors are modular, with nearly identical topographies. The extracellular portion forms the hormone-binding domain and embodies the maximal structural diversity. The intracellular portion starts with a kinase-like domain that stretches up to a catalytic domain, (Duda et al., 1991). By applying recombinant tools to MGCs and guided by the theoretical ANF-RGC structural template it was possible to test the multi-modularity concept, map the domains and define their functional components and predict the transmembrane migration of the hormonal signal at a sub-molecular level.
The first task was to establish that the extracellular domain (ExtD) of ANF-RGC truly does constitute the hormone’s (ANF) signaling site. A genetic variant of ANF-RGC (GCα), cloned from the adrenal cortex, has a structure that varies in just two amino acid residues from the ExtD of the true ANF-RGC: His338 substitutes for Gln and Pro364 substitutes for Leu (Duda et al., 1991). GCα supports normal basal catalytic activity, yet it is not an ANF receptor. Transformation of its residues 338 and 364 to the true ANF-RGC residues confers ANF-binding and regulatory activities indicating the necessity for Gln338 and/or Leu364. Subsequent point mutation analyses single out Leu364 as the key residue for controlling both activities. Historically, GCα was the first naturally occurring ANF-RGC mutation functionally linked with hormonally-dependent core catalytic domain (CCD) activity (Duda et al., 1991).
The amino acid residues in CNP-RGC corresponding to Gln338 and Leu364 in ANF-RGC are Glu332 and Val358, respectively. For CNP-RGC, Glu332 in the ExtD is critical for C-type natriuretic peptide (CNP) binding and CNP-dependent catalytic regulatory activity (Duda et al., 1993c, 1994). As with Leu364 in ANF-RGC, the site has no influence on the basal catalytic activity of the guanylate cyclase. The folding patterns of the external domains of these two guanylate cyclases are nearly identical (Duda et al., 1994). Substitution of the Gln338 residue with Glu endows ANF-RGC with significant CNP signal transduction activity (Duda et al., 1995). Conversely, replacing Val358 with Leu generates significant ANF signal transduction activity in CNP-RGC. These two residues therefore perform equivalent functions in controlling the ligand specificities of ANF-RGC and CNP-RGC. Subsequent X-ray crystallographic analyses provided validation and revealed the ExtDs to exist as homo-dimers in a head to head conformation (He et al., 2001; Ogawa et al., 2004, 2009).
Hormonal Signal Transduction Is ATP-Regulated
An enigmatic observation was that despite binding ANF stoichiometrically, the catalytic activity of purified ANF-RGC was not stimulated (Paul et al., 1987). Two groups solved this puzzle (Chinkers et al., 1991; Marala et al., 1991); ATP is obligatory for ANF-dependent ANF-RGC activity. Neither ANF nor ATP alone is sufficient. Since two nonhydrolyzable analogs, ATPγS and AMP-PNP, mimic ATP with EC50s between 0.3–0.5 mM (Duda et al., 1993a), phosphorylation need not occur and ATP must be an allosteric regulator (Chinkers et al., 1991; Marala et al., 1991). With Hill coefficients near 1 (Duda et al., 2011b), it is not clear whether one ATP per dimer is adequate or whether two ATPs drive greater activity than one. Importantly, the identical situation exists for CNP transduction by recombinant CNP-RGC (Duda et al., 1993c).
Definition of the mechanism by which ATP controls transmission of the hormonal signal at the sub-molecular level was achieved through two decades of programmed study (reviewed in Sharma, 2010). A vaguely termed, “kinase homology domain (KHD)” of ANF-RGC extends from a juxtamembrane domain (JMD) to the CCD (Chinkers and Garbers, 1989; Marala et al., 1992). Within the KHD, a glycine rich cluster (GRC), Gly503-Arg-Gly-Ser-Asn-Tyr-Gly509 bestows ATP-dependence to ANF transduction (Goraczniak et al., 1992). Hence, this motif was more aptly renamed ATP-regulatory module (ARM). The ARM in CNP-RGC is Leu497-Arg-Gly499-Ser-Ser-Tyr-Gly503. If the ANF-RGC-ARM sequence is changed to its CNP-RGC counterpart, the ATP dependence of the ANF response is preserved (Duda et al., 1993a). Together with the earlier finding that the entire KHDs of ANF-RGC and CNP-RGC are interchangeable (Koller et al., 1992), the transduction mechanisms of these two MGCs appear to function identically. Evaluation of individual glycine residues in the ARM sequence homed in on Gly505 in ANF-RGC and the identically placed Gly499 in CNP-RGC as critical for ATP binding and ANF signal transduction (Duda et al., 1993b).
The ANF-RGC ARM domain was simulated through homology-based modeling, patterning it after the x-ray crystal structures of insulin receptor kinase and hematopoietic cell kinase. In turn, its functional elements were experimentally decoded through point mutation/expression, time-resolved tryptophan fluorescence and Forster Resonance Energy Transfer, reconstitution and mass spectroscopic studies (Figure 3A). The greater ARM boundaries stretch from residues 481–771. A smaller, 91 residue N-terminal lobe (496–586) connects to a larger, 185 residue C-terminal lobe (587–771; Duda et al., 2000; Sharma et al., 2001; Figure 3B). ATP binding partially amplifies the ANF signal by repositioning a W669-TAPELL675 motif in the larger lobe. This repositioning is critical for hormone-dependent activation of ANF-RGC; deletion of the WTAPELL motif results in an ANF-RGC that is unresponsive to ANF and ATP in the recombinant system (Duda et al., 2009) and in a genetically modified mouse model, causes hypertension and cardiac hypertrophy (Duda et al., 2013). Thus, the seven-residue encoded motif of ANF-RGC gene exerts total control over the hormonal regulation of blood pressure.
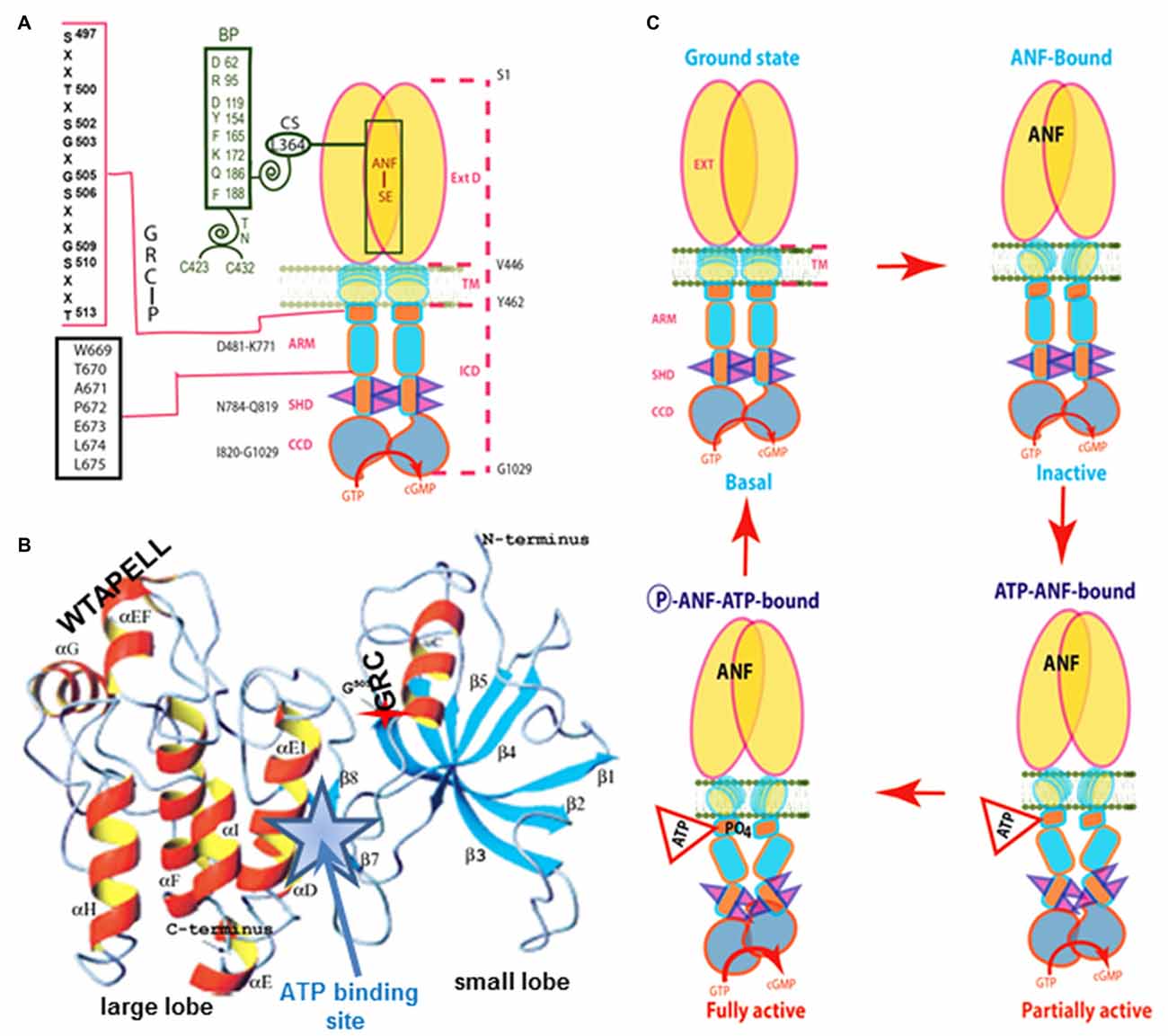
Figure 3. Model for signal transduction by atrial natriuretic factor-receptor guanylate cyclase (ANF-RGC). (A) Important module segments involved in activation. An ANF-signaling element (ANF-SE) resides in the ExtD. A Central Switch (CS), L364, controls the ANF binding site. A Binding Pocket (BP) is hinged with the CS (van den Akker et al., 2000; Ogawa et al., 2004, 2009). Two disulfide bridged cysteine residues act as a Transduction Node (TN) to guide the transmembrane migration of the ANF signal to an intracellular, ATP-Regulated Module (ARM; Duda et al., 2005b). ATP amplifies the ANF signal by bringing two critical domains to the surface: a glycine rich cluster (GRC), G-X-G505-X-X-X-G, making surrounding serine and threonine available for phosphorylation (GRC-P) and a 7-aa residue W669-TAPELL675 motif to activate the core catalytic domain (CCD). (B) Structure of the ARM in its apo form. Four antiparallel β strands and one helix constitute the small lobe. The large lobe is made up of eight α helices and two β strands. The positions of the key G505 residue of the GRC motif within the small lobe and of the W669-TAPELL675 motif within the large lobe are indicated. ATP binding is sandwiched between the two lobes (indicated by a star; modified, with permission, from Duda et al., 2000; Sharma et al., 2001). (C) Activation model for ANF-RGC. Binding of an ANF molecule to the ExtDs of the dimer primes the ANF-SE, by rotating TN. The twisting motion propagates through TM to prepare ARM for ATP binding (Ogawa et al., 2004; Parat et al., 2010). ATP binding triggers a cascade of temporal and spatial changes (Duda et al., 2001c). With G505 in GRC-P acting as a pivot, the ATP BP shifts its position and its floor rotates. There is movement of ARM’s β4 and β5 strands and the loop between them and movement of the αE and F helices that exposes the hydrophobic WTAPELL motif for interaction with CCD (Duda et al., 2009). These structural rearrangements initiate 50% maximal catalytic activity. Full activation is attained after multiple serines and threonines in GRC-P become phosphorylated (Duda et al., 2011b). The conformational changes wrought by ATP binding reduce the affinity of ANF-RGC for ANF and phosphorylation lowers the affinity for ATP binding. Dissociation of ANF and ATP return ANF-RGC to its ground state. Modified with permission, from Duda et al. (2011b).
Binding of ATP also causes buried serine and threonine residues in GRC-P within the smaller lobe of the ARM to shift to the surface, whereupon they are phosphorylated by a hypothetical kinase. Full amplification of the ANF signal is achieved by phosphorylation. Maximal activity is self-limiting, however, because ANF-RGC loses affinity for ANF upon ATP binding (Larose et al., 1991; Jewett et al., 1993; Duda and Sharma, 1995a,b) and loses affinity for ATP after phosphorylation.
Based on additional observations that dephosphorylation of ANF-RGC underlies homologous desensitization (Potter and Garbers, 1992), we propose a working model for activation (Figure 3C). ATP and ANF can bind ANF-RGC independently, but in the absence of ATP, ANF cannot stimulate cyclic GMP synthesis. ATP binding exposes six serines and threonines in the ARM, enabling them to be phosphorylated. Dephosphorylation can occur but the residues can then be re-phosphorylated. The conformational changes also lower the affinity for ANF binding. Phosphorylation promotes ATP release, which helps return of the receptor to the basal state by reducing activity and slowing the rate of phosphorylation. Dephosphorylation continues as long as ANF is bound. When ANF dissociates, the serines and threonines in ARM fold back into the receptor and lose access to phosphatase and kinase. If dephosphorylation is complete, then the receptor can bind ANF, but is completely inactive until it also binds ATP and is re-phosphorylated. But with residual phosphorylation, ANF binding alone elicits weak activity. Full activation requires ATP binding and phosphorylation of all residues. A central theme of the model is that it is dynamic. Notably, this operational mode selectively controls the regulatory activity of ANF without affecting the basal catalytic activity of ANF-RGC. It appears that the same model applies to the ligand-dependent activation of CNP-RGC and STa-RGC (Potter and Hunter, 1998; Bhandari et al., 2001; Jaleel et al., 2006).
MGCs Transduce Intracellular Ca2+ Signals
Phototransduction, the conversion of light into an electrical signal in the outer segments of rod and cone photoreceptors, marks the first step in the visual process. By the 1980s, it was known that both Ca2+ and cyclic GMP were critical cytosolic regulators of the photon response. Eventually, cyclic GMP was proven to be the second messenger of phototransduction when it was shown to increase directly the conductance of a new class of ion channels for which phosphorylation was not necessary (Fesenko et al., 1985). This discovery brought a major paradigm shift because until that time, cyclic nucleotides were thought to act exclusively through a kinase intermediary. However, critical issues remained: What was the source of the cyclic GMP? How did Ca2+ fit in? (reviewed in Pugh and Cobbs, 1986; Stryer, 1986).
To find answers, a search for the molecular identity of the photoreceptor ROS-GC was undertaken. After initial failures (Horio and Murad, 1991a,b; Shyjan et al., 1992), in a cutting edge finding, a protein was successfully purified from bovine rod outer segments (ROS) and its sequence was pieced together from protein fragments. Subsequent sequence-based molecular cloning established the true molecular structure of ROS-GC1 (Margulis et al., 1993; Goraczniak et al., 1994). Based on the bovine ROS-GC1 structure, the molecular structure of the original ret-GC (Shyjan et al., 1992) was corrected in 1995 to show that it is the human version of the bovine form (Lowe DG, accession number M92432). Notably, ROS-GC1 is the lone MGC to have its molecular identity established on the basis of protein sequence. Comparison of the results to those obtained by cloning led to the realization that ROS-GC is preceded by an N-terminal leader sequence (LS) that gets deleted post-translationally. The theoretical molecular mass of the protein with the 56-amino acid LS is 120, 361 Da; without it, it is 114, 360 Da (Goraczniak et al., 1994). The 114, 360 Da molecular mass was similar to the previously biochemically characterized bovine (Koch, 1991) and toad photoreceptor guanylate cyclases (Hayashi and Yamazaki, 1991). Unlike ANF-RGC and CNP-RGC (Paul et al., 1987; Chang et al., 1989; Chinkers et al., 1989; Duda et al., 1993c), ROS-GC1 is not modulated by natriuretic peptide hormone (Goraczniak et al., 1994). For that matter, no extracellular ligands have ever been identified, so ROS-GC1 remains an orphan surface receptor. A second ROS-GC (ROS-GC2) was discovered in bovine retina (Goraczniak et al., 1997), the human version was termed Ret-GC2 (Lowe et al., 1995). Recombinant ROS-GC1 and ROS-GC2 organize into homodimers and heterodimers, but few if any heterodimers form in bovine retina despite the co-expression of both guanylate cyclases in rods (Yang and Garbers, 1997).
In a groundbreaking discovery, a soluble bovine ROS fraction stimulated the catalytic activity of photoreceptor guanylate cyclase in the absence of Ca2+ (Koch and Stryer, 1988). Three separate groups purified two similar but molecularly distinct guanylate cyclase activating proteins (GCAPs): GCAP1 (Palczewski et al., 1994; Subbaraya et al., 1994; Gorczyca et al., 1995; Frins et al., 1996) and GCAP2 (Dizhoor et al., 1995). For ROS-GC1 expressed in a heterologous system of COS cells catalytic activity increases 4–5 fold with the addition of GCAP1 at 10 nM Ca2+. Activity then falls approximately 6-fold as Ca2+ is raised to 1 μM with an IC50 near 100 nM (Duda et al., 1996b; Figure 4A). The small discrepancy between these magnitudes of activity change comes about because GCAP becomes an inhibitor at high Ca2+ (Dizhoor and Hurley, 1996). ROS-GC1 in membranes of the photoreceptor outer segments membranes treated with exogenous GCAP1 or GCAP2 behaves similarly (Figure 4A). These results along with cross-linking experiments (Duda et al., 1996b) affirm the association of GCAP1 with ROS-GC1 at low and high Ca2+ levels, making GCAP1 a Ca2+-sensing subunit of the cyclase.
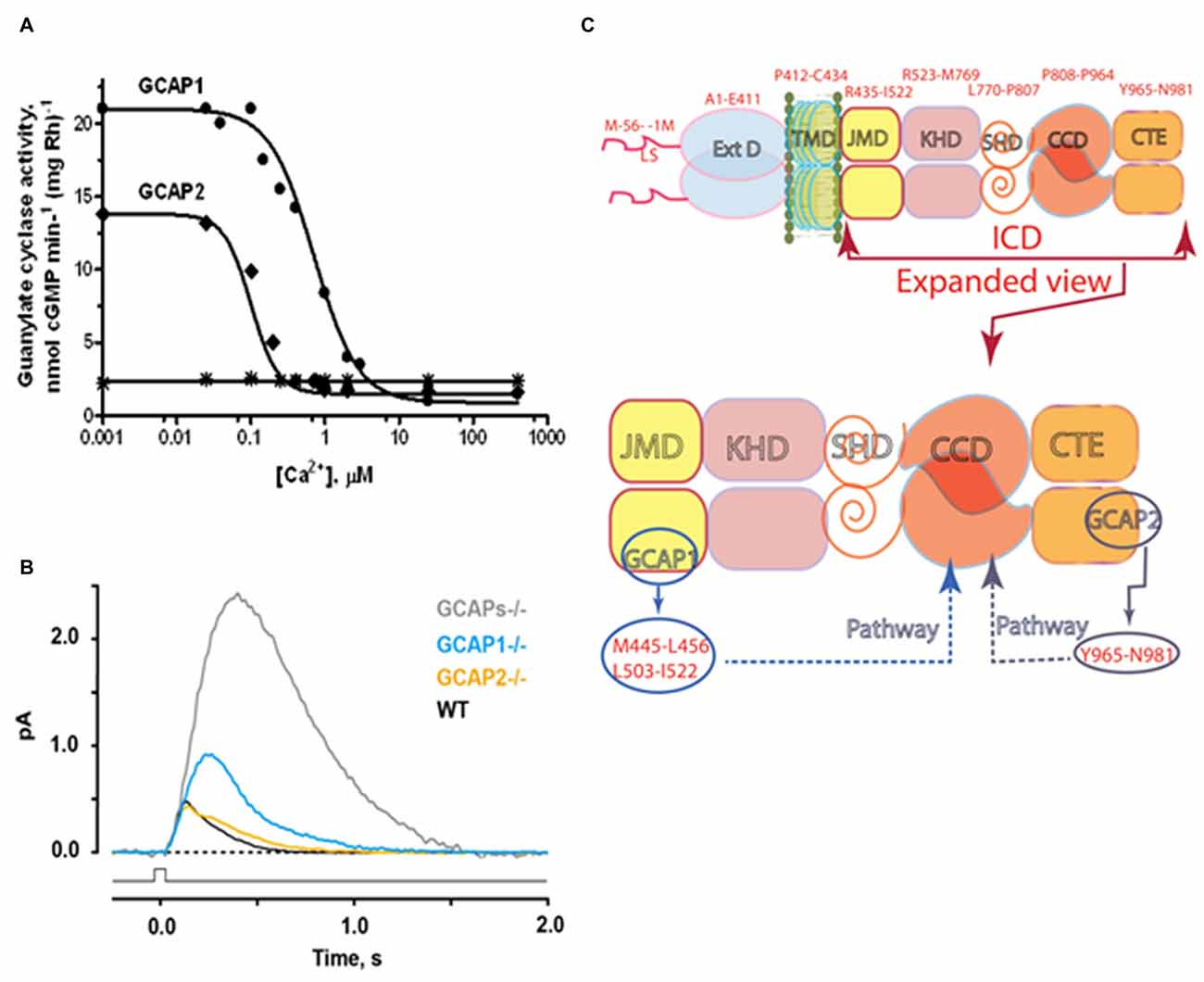
Figure 4. Ca2+- sensing subunits of rod outer segment-guanylate cyclase (ROS-GC). (A) Ca2+-dependence of ROS-GC1 activity in washed bovine ROS membranes. In the presence of recombinant guanylate cyclase activating protein (GCAP), activity is stimulated when Ca2+ is low and suppressed when Ca2+ is high. The “IC50” for Ca2+ was 707 ± 122 nM for GCAP1 and 100 ± 32 nM for GCAP2. The dose response curves were cooperative with Hill coefficients of 1.5 and 2.4 for GCAP1 and GCAP2, respectively. Results from Hwang et al. (2003). The difference in IC50 values for Ca2+ between GCAP1 and GCAP2, observed for the first time in this study, inspired the proposal of the “Ca2+ relay” model for the GCAP1 and GCAP2 modulation of ROS-GC activity (Koch and Dell’Orco, 2013). (B) Effects of GCAPs on the photon responses of rods from WT, GCAP1−/−, GCAP2−/− and GCAPs double knockout (GCAPs−/−) mice. In the absence of both GCAPs, the single photon response rises for a longer period to an amplitude ~5 fold larger than normal and recovers very slowly. Knockout of GCAP1 alone results in a compensatory overexpression of GCAP2. The rising phase of the photon response is not quite as prolonged as with GCAPs−/− but the amplitude is still ~2 fold larger than normal. GCAP1 levels appear to be normal after knockout of GCAP2. The photon response is normal in amplitude but takes somewhat longer to recover (Results from Wen et al., 2014). (C) Modular construction of the ROS-GC1 dimer. A 56 amino acid leader sequence (LS) precedes the ExtD in the nascent, immature protein. All signaling events occur in ICD, which is composed of: JMD, juxtamembrane domain; KHD, kinase homology domain; SHD, signaling helix domain; CCD, catalytic core domain; and CTE, C-terminal extension. Two specific switches for Ca2+ sensing subunits, one for GCAP1 in the JMD, and one for GCAP2 in the CTE, are located on opposing sides of the CCD. The MGC complex exists as a dimer of homodimers in which two ROS-GC1s combine either two GCAP1s or two GCAP2s.
Recombinant GCAP2 provides more powerful stimulation of recombinant ROS-GC1 at low Ca2+, but the EC50 of ROS-GC1 for GCAP2 of 6–8 μM is nearly an order of magnitude higher than the EC50 of ~0.8 μM for GCAP1 (Duda et al., 1996b; Goraczniak et al., 1998; Krishnan et al., 1998). ROS-GC2 is stimulated by GCAP2 at low Ca2+ by 12-fold with an EC50 of 1 μM. However, GCAP1 is not capable of stimulating ROS-GC2 or ANF-RGC at low Ca2+ (Duda et al., 1996b; Goraczniak et al., 1998). The binding sites on ROS-GC1 for the two Ca2+ sensing subunits were mapped by peptide competition and mutagenesis experiments (Figure 4C). Two short sequences, M445-L456 and L503-I522, in the JMD of ROS-GC1 are critical and specific for GCAP1 activation (Lange et al., 1999). The regulatory site for GCAP2, Y965-N981, is located in a C-terminal extension (CTE) of the CCD (Duda et al., 2005a). Despite their having binding sites positioned on opposite sides of the CCD, both GCAPs utilize the W657-TAPELL663 motif in ARM to amplify their stimulation of catalytic activity at low Ca2+ (Duda et al., 2011a). ATP binding is not obligatory, but will raise basal catalytic activity and GCAP1-stimulated activity at low Ca2+ by the same increment (Gorczyca et al., 1994; Aparicio and Applebury, 1996). Remarkably, ROS-GC exhibits intrinsic kinase activity and autophosphorylates four serine residues in its ARM (Aparicio and Applebury, 1996; Bereta et al., 2010). At present, the purpose of ROS-GC1 phosphorylation is a complete mystery; it produces no change in basal or GCAP1-stimulated activities and its occurrence is unrelated to Ca2+ regulation by GCAP1.
Rods and cones are not responsive to light in ROS-GC1/ROS-GC2 double knockout mice (Baehr et al., 2007) and guanylate cyclase activity loses its Ca2+ dependence in GCAP1/GCAP2 double knockout mice (Mendez et al., 2001). Expression of a third guanylate cyclase and a third GCAP linked with phototransduction is thereby excluded in mouse. In human retina there is a third GCAP capable of stimulating ROS-GC1 and ROS-GC2. It has Ca2+ regulatory properties similar to those of GCAP1 so its role in phototransduction is unclear (Haeseleer et al., 1999). Apparently mammalian photoreceptors utilize a Ca2+-modulated system composed of a pair of ROS-GCs and up to three GCAPs in their outer segments. Studies on mice deficient for either GCAP1 or GCAP2 (Makino et al., 2008, 2012) verify a relay model for how the two GCAPs operate in the rod (Koch and Dell’Orco, 2013). GCAP1 has the lower affinity for Ca2+ so it is the first responder to the light-induced fall in intracellular Ca2+. Over time after light onset and with higher intensities, the effect of GCAP1 saturates and stimulation of ROS-GC activity by GCAP2 becomes increasingly important. Together, the two GCAPs limit the growth of the photon response and accelerate the kinetics of the response recovery (Figure 4B). More complete accounts of the molecular properties of ROS-GCs and how ROS-GC systems control phototransduction may be found in Sharma and Duda (2014a); Wen et al. (2014); Koch and Dell’Orco (2015).
The discovery that intracellular Ca2+ regulates cyclic GMP synthesis in photoreceptor membranes shattered the concept that MGCs limit themselves to the transduction of hormones and other extracellular signals. Moreover it foreshadowed a greater complexity in signaling in other MGC transduction systems.
CD-GCAP Supports Bimodal Ca2+ Switching of ROS-GC Activity
Discovered contemporaneously with GCAPs, retinal CD-GCAP presented itself as another Ca2+-dependent regulator of ROS-GC (reviewed in Sharma et al., 2014). CD-GCAP turned out to be a conformational isomer of brain S100B (the only commercially available form; Pozdnyakov et al., 1995, 1997; Duda et al., 1996a, 2002; Margulis et al., 1996; Wen et al., 2012). But in contrast to the Zn2+-bound brain form, the retinal form has Ca2+-bound. The distinction is significant because the Zn2+-bound form inhibits while the Ca2+-bound form stimulates ROS-GC1 (Pozdnyakov et al., 1997). The rest of the review will concern itself only with the retinal form.
S100B can be chemically cross-linked to the ROS-GC1 dimer giving evidence for a direct interaction. In recombinant systems, S100B binds ROS-GC1 with a K1/2 of 198–395 nM (Duda et al., 2002) and stimulates cyclic GMP synthesis with a K1/2 for Ca2+ of ~400 nM (Duda et al., 1996a). Deletion constructs of ROS-GC1 and peptide competition studies map the interaction to C-terminal segments: aa962–981 and aa1030–1042. Within these segments, an R966-IHVNS972 motif is obligatory for binding while a flanking cluster, R1039–RQK1042, that does not contribute to S100B binding, promotes maximal ROS-GC1 activation (Duda et al., 2002). As a diminutive protein with an estimated molecular weight of 10 kDa and only 2 EF hands, it organizes into a tetrameric subunit of the MGC complex (Donaldson et al., 1995).
With the ability to couple to GCAPs and to S100B, ROS-GC1 could function as a bimodal Ca2+ switch in cells expressing all three components. The hypothesis was explored at the photoreceptor-bipolar synaptic region of the bovine retina where prior immunohistochemical studies had demonstrated the co-presence of ROS-GC1 with GCAP1 and S100B (Liu et al., 1994; Cooper et al., 1995; Duda et al., 2002). Indeed, ROS-GC1 activity is high in synaptosomal membranes from retina at 10 nM Ca2+, decreases as Ca2+ is raised to a few hundred nM and then rises again as Ca2+ exceeds 1 μM (Venkataraman et al., 2003; Figure 5).
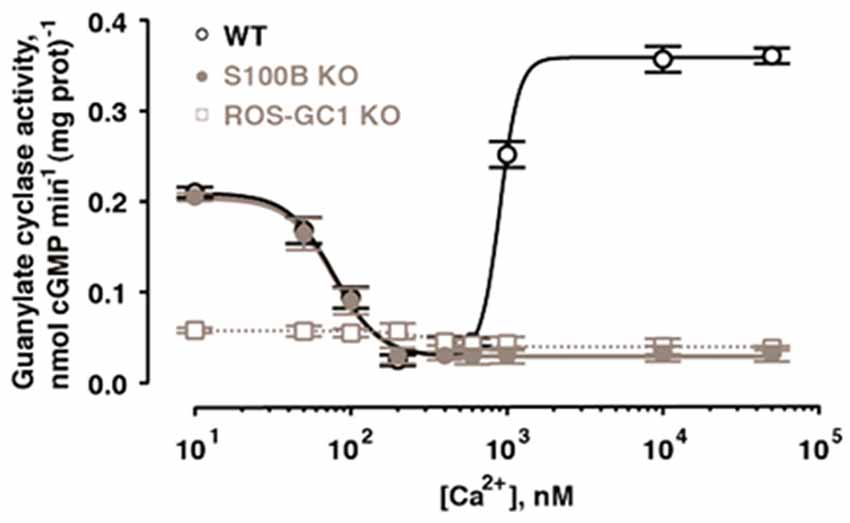
Figure 5. Bimodal Ca2+ switching in photoreceptor to bipolar cell synaptosomal membranes of WT mice. Stimulation of catalytic activity at high Ca2+ is missing from membranes of S100B−/− mice and from those of ROS-GC1−/− mice. Error bars show SEM. Modified with permission from Wen et al. (2012); copy right S. Karger AG., Basel.
The presence of ROS-GC1, GCAPs and S100B in the outer segments of the retinal photoreceptors (Cuenca et al., 1998; Kachi et al., 1999; Rambotti et al., 1999) implicated a role for bimodal switching in phototransduction. The possibility was probed with mouse knockout models: S100B−/−, GCAP1−/−, GCAP2−/−, GCAP1/GCAP2−/−, ROS-GC1−/−. Biochemical experiments on murine outer segments demonstrate functional linkage of S100B with ROS-GC1 but not ROS-GC2 at [Ca2+]i > 200 nM in the generation of cyclic GMP. Although these concentrations exceed the physiological range for Ca2+ in mouse ROS (Woodruff et al., 2002), they may be appropriate for cones, which sustain higher levels of Ca2+ in darkness. It therefore makes sense that the most recent immunocytochemistry co-localizes S100B and GCAP1 with ROS-GC1 in murine retinal cone outer segments but not ROS (Wen et al., 2012).
Bicarbonate Controls a Ca2+-Independent Mode in ROS-GC that is Interlaced with Ca2+-Sensing Modulators
In trailblazing studies, the MGC in olfactory neuroepithelium [olfactory neuroepithelial guanylate cyclase (ONE-GC), see below] was shown to be a sensor of atmospheric carbon dioxide (Hu et al., 2007; Guo et al., 2009). The mediator of the carbon dioxide signaling was bicarbonate. Bicarbonate did not stimulate recombinant ANF-RGC, CNP-RGC, STa-RGC, or ROS-GCs in these first studies, leading to the conclusion that among MGCs, bicarbonate was an activator unique to ONE-GC (Guo et al., 2009; Sun et al., 2009). Truncation mutants point to an interaction of bicarbonate directly with the broadly defined CCD to spur the generation of cyclic GMP (Sun et al., 2009; Duda and Sharma, 2010). The exclusive responsivity of ONE-GC to bicarbonate seemed odd because there is 85% structural conservation of its catalytic domain across MGCs. Revisiting the issue showed that the catalytic activities of recombinant bovine ROS-GCs are stimulated robustly by bicarbonate, ROS-GC1 with an ED50 of 27 mM and a Hill coefficient of 2.8, and ROS-GC2 with an ED50 of 39 mM and a Hill coefficient of 2.3 (Duda et al., 2015). The Hill coefficients >2 are consistent with one or more bicarbonate molecules binding to each monomer. Bicarbonate has a similar effect on ROS-GCs in photoreceptor outer segment membrane preparations (Figure 6A). ROS-GC’s catalytic activity remains constant over a range of pH from 7 to 9, ruling out a pH dependent mechanism for bicarbonate stimulation. The conflicting results from the first two studies may be related to the type of expression system because although bicarbonate lowers the activity of ANF-RGC expressed in CHO, the effect is not so marked with the same MGC expressed in E. coli or in HEK-293 (Guo et al., 2009; Sun et al., 2009).
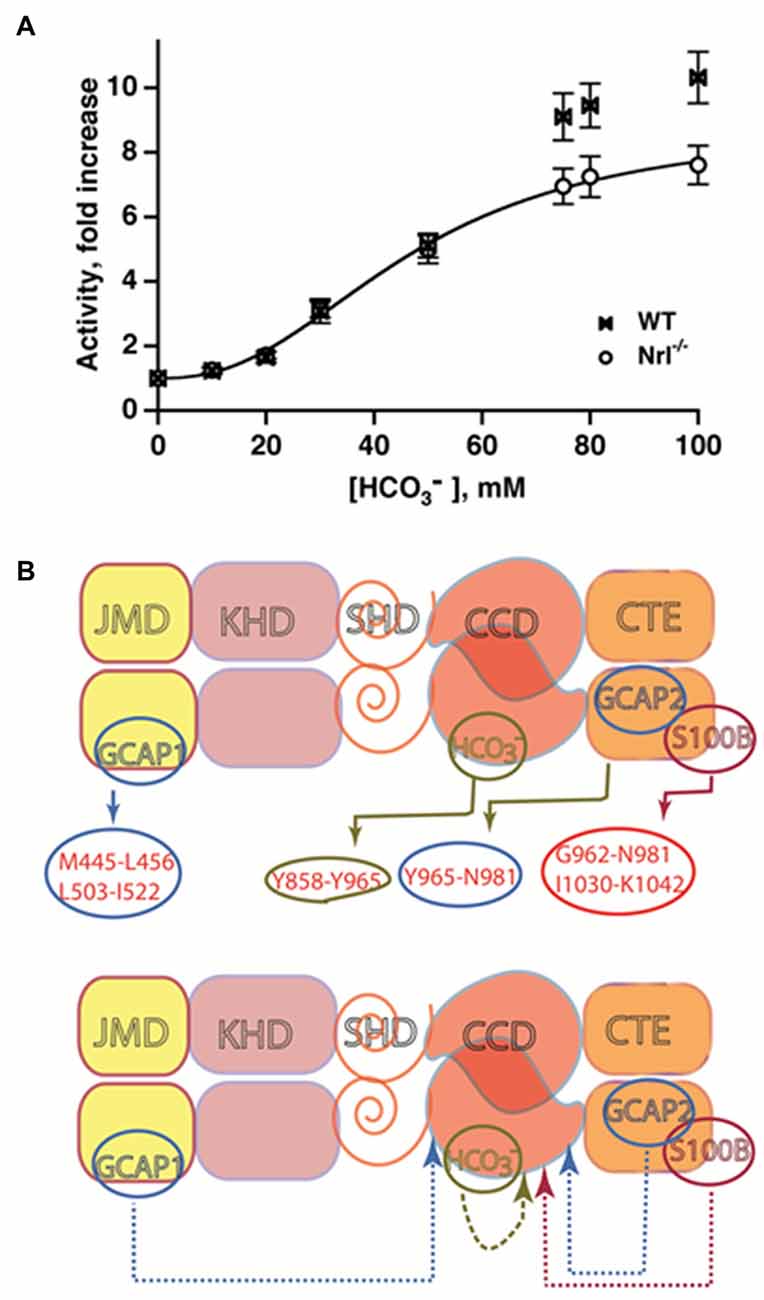
Figure 6. Bicarbonate modulation of ROS-GC activity. (A) Stimulation of ROS-GC in photoreceptor outer segment preparations from WT and neural retina leucine zipper transcription factor knockout (NRL−/−) mice. NRL−/− photoreceptors express ROS-GC1 and GCAP1 exclusively. The dependence of guanylate cyclase activity on bicarbonate is cooperative with an EC50 of 47 mM. The elevated activity at high bicarbonate concentration in WT outer segments is attributed to their additional expression of GCAP2. Error bars show SEM (Duda et al., 2015). (B) Ca2+-dependent and -independent modulators of ROS-GC 1 activity. Upper panel: three Ca2+ sensor proteins—GCAP1, GCAP2 and S100B— and one Ca2+-independent modulator, bicarbonate, target individually the indicated domains within the intracellular portion of ROS-GC1. Lower panel: the targeted domains are specific switches all of which signal activation of the catalytic domain. The signaling pathways are indicated as dashed arrows.
The bicarbonate signal transduction of ROS-GC1 occurs independently of [Ca2+]i. Yet, it synergizes with the Ca2+-sensors: GCAP1, GCAP2 and S100B to intensify Ca2+ modulation, particularly for GCAP2 (Duda et al., 2015, 2016). The effect on photoreceptors is to elevate the circulating current, decrease sensitivity to flashes and accelerate flash response recovery. As a charged molecule, bicarbonate does not freely cross membranes and gains access to ROS-GC in ROS by entering through the inner segment/synapse of intact rods. In contrast, bicarbonate can access ROS-GC from the inner and outer segments of red-sensitive cones. The basis is under active investigation. These findings clarify a large body of seemingly controversial studies surrounding bicarbonate and cyclic GMP synthesis in retinal photoreceptors and provided a clue that bicarbonate signaling would be characteristic of most, if not all MGCs. A model showing the interlaced Ca2+-dependent and -independent pathways in the photoreceptors is depicted in Figure 6B.
An F514S mutation in ROS-GC1 causes Leber’s congenital amaurosis type 1 blindness in human patients (Perrault et al., 1996, 1999; Rozet et al., 2001). There is a 10-fold loss in ROS-GC1 catalytic activity (Duda et al., 1999) that is almost totally insensitive to GCAP1 modulation, despite retention of GCAP1 binding to ROS-GC (Duda et al., 2016). It follows that the loss in GCAP1 modulation occurs at the signal transduction level and possibly resides in one or more of the catalytic core residues: D834, E874, D878, R925, C946, N953. In contrast, the mutation does not abolish Ca2+-modulation by GCAP2 or by S100B (Duda et al., 1999, 2016), even though the absolute activities are reduced in all conditions. The interaction of this disease-causing mutation with bicarbonate led to some insights into the intramolecular signaling pathways.
Bicarbonate partially raises basal catalytic as well as GCAP2- and S100B-stimulated activities of the F514S mutant but does little for the deficit in GCAP1 stimulation. The restorative capacity of bicarbonate indicates that it operates downstream or independently of the F514S mutation. At the basic level these findings support the earlier conclusion that the S100B- and GCAP2-modulated pathways within ROS-GC1 overlap (Duda et al., 2002) but that both are distinct from the GCAP1-modulated pathway (Duda et al., 1996a, 2012c; Krishnan et al., 1998; Koch et al., 2010; Koch and Dell’Orco, 2013).
At a clinical level, high enough bicarbonate levels could provide relief for patients expressing the F514S-mutant ROS-GC by restoring some basal and GCAP2-modulated guanylate cyclase activity in rods and cones. Mice stricken with the mutation would not be so fortunate, since their cones express GCAP1 to the exclusion of GCAP2 (Xu et al., 2013).
Another Ca2+ Sensor, Neurocalcin δ (NCδ) Is Expressed in Retinal Neurons
The failure of GCAP1 to recognize the KHD of ANF-GC and satisfy its Ca2+ requirement (Duda et al., 1996a) meant that other Ca2+-sensing subunits participate in MGC signaling. The neuronal calcium sensor (NCS) neurocalcin δ (NCδ) was tested as a potential ROS-GC regulator because it shares ~35% sequence identity with GCAPs and is expressed in the inner retina. Ca2+-free bovine brain NCδ has no effect on recombinant ROS-GC1, but the Ca2+-bound form stimulates it with a K1/2 for Ca2+ of 0.8 μM (Krishnan et al., 2004). Stimulation is ROS-GC1-specific; it does not occur with ROS-GC2. In these respects NCδ is functionally more similar to S100B than to GCAPs. NCδ bears four EF hands while S100B has only two. Despite the poor sequence identity of S100B to the C-terminal half of NCδ (GenBank accession numbers NP_001029727.1 and NP_776823.1, respectively), a comparison of their Ca2+-bound crystal structures reveals that the four helix-packing arrangements of their EF hands are very similar (Kumar et al., 1999). Thus, NCδ and S100B are structural and functional analogs.
NCδ purified from bovine retina exists as a homodimer of 20 kDa proteins (Krishnan et al., 2004; Venkataraman et al., 2008). Like other NCSs (Ladant, 1995; Frins et al., 1996), NCδ exhibits a Ca2+-dependent mobility shift. Of the four EF-hand motifs, only three—EF2, EF3, EF4—are predicted to bind Ca2+ (Okazaki et al., 1992; Terasawa et al., 1992; Vijay-Kumar and Kumar, 1999). The N-terminus is blocked, predominantly by myristoylation, a feature in common with all other members of the NCS-protein family except the Kv-channel interacting protein subfamily (Braunewell and Gundelfinger, 1999; Burgoyne and Weiss, 2001). Many members, NCδ included, operate as a Ca2+ myristoyl switch wherein the myristoyl group lies buried within a hydrophobic pocket in the absence of Ca2+, but is exposed when Ca2+ is bound to increase the membrane affinity of the protein (Lim et al., 2014). Without Ca2+, NCδ has no affinity for ROS-GC1. Yet from a functional standpoint, NCδ behaves as a true subunit of ROS-GC1, because the resting intracellular Ca2+ concentration of 100–200 nM is sufficient to keep NCδ membrane bound and associated with ROS-GC1. Stability enables a response of the MGC complex on a millisecond time scale. This concept has been experimentally validated. NCδ and ROS-GC1 are present together in the IPL under resting conditions and the addition of Ca2+ stimulates native ROS-GC1 activity (Krishnan et al., 2004); the myristoyl group of NCδ causes a 2-fold amplification of the saturation activity of ROS-GC1. The binding kinetic parameters of the Ca2+-bound NCδ with ROS-GC1 are (Krishnan et al., 2004): KA = 2.3 × 106 M−1 and KD = 4.6 × 10−7 M.
Mapping studies place binding of NCδ at aa732–962, a site that does not overlap with GCAP1-, GCAP2-, or S100B-modulated domains of ROS-GC1 (Krishnan et al., 2004). Finer analyses pinpoint the NCδ-modulated site between V836-L857 of ROS-GC1 (Note: the sequence numbering in the following reference is offset by one amino acid; Venkataraman et al., 2008), within the CCD. This segment is accessible to the solvent and fits comfortably in the V-shaped crevice of NCδ, bounded by the EF-1 hand residues. These residues form a special hydrophobic–hydrophilic patch, which may be a distinctive feature of the Ca2+-dependent signaling property of NCδ. It has a secondary structure of helix-loop-helix and is acidic in nature with a pI of 3.37.
Traditionally, the belief had been that the CCD, located at the C-terminus of the protein, was isolated from all of its ligands and depend on upstream modules to translate the ligand signals for its activation to generate cyclic GMP. The localization of NCδ binding, which predated work on the bicarbonate binding site, along with new insights on the antiparallel nature of the catalytic center (Duda et al., 2012c), called for a new signaling model in which the isolated core catalytic module by itself is dimeric in nature with intrinsic basal guanylate cyclase activity. The two chains of the catalytic module are antiparallel. Ca2+-bound NCδ interacts directly with the ROS-GC1 core catalytic site without need for the adjacent N-terminally located α-helical “dimerization domain” structural element (L770-P807). Because this dimerization domain plays a role in transmitting the signal from GCAP1 to the catalytic module (Zägel et al., 2013), it is now referred to as signaling helix domain (SHD).
ROS-GC1 couples with another NCS in the inner retina, most likely a GCAP, because cyclic GMP synthesis increases at very low Ca2+ (Krishnan et al., 2004). If the entire system is present in the same cells, then it could function as bimodal switch like that at the photoreceptor-bipolar cell synapse, except that NCδ replaces S100B.
NCδ Is a Ca2+ Sensor Modulating ANF-RGC
Based on the rationale that the binding site for NCδ lies in the catalytic center of ROS-GC, a domain that is conserved in all MGCs, NCδ was a prime candidate to be the Ca2+ sensor for ANF-RGC. The V836-L857 site in ROS-GC1 corresponds with the V851-L872 site of ANF-RGC with a sequence identity of 68.2%.
The first step was to test myristoylated NCδ against recombinant ANF-RGC. NCδ stimulates the catalytic activity of ANF-RGC in a dose-dependent fashion with an EC50 of 0.5 μM Ca2+ (Duda et al., 2012b). As is the case with ROS-GC1 and GCAPs, ATP/ANF is not needed for stimulation, further demonstrating that the Ca2+-modulated modes are distinct from that of hormonal, ANF signal transduction. These properties broke down the traditional concept that ANF-RGC was strictly a transducer of the natriuretic peptide hormone ANF. A second, Ca2+-modulated pathway regulates the same physiological processes of natriuresis, diuresis and blood pressure.
Only myristoylated NCδ stimulates the Ca2+-modulated ANF-RGC catalytic activity, the non-myristoylated form is ineffectual. It is now appreciated that in a previous study, Ca2+/NCδ did not stimulate ANF-GC (Kumar et al., 1999) because it was unmyristoylated, underscoring the importance of this posttranslational modification. Myristoylated NCδ controls the activity of ANF-RGC by lowering its Km for GTP substrate and by increasing its catalytic efficiency, kcat, from 6.5 ± 0.3 to 41.4 ± 0.5 pmol cyclic GMP/s. The functional unit of the Ca2+-bound complex is composed of NCδ dimer and ANF-RGC dimer.
To assess an overlap between the Ca2+- and the ANF-modulated pathways, ANF-RGC catalytic activity was analyzed first in the presence of 1 μM Ca2+ and 2 μM myristoylated NCδ and then with increasing concentrations of ANF, ranging from 10−11 to 10−6 M, and constant 0.8 mM ATP. The responses were enhanced 3.5- and 4.5-fold alone, and about 15-fold in combination. Thus the Ca2+-modulated and the hormone-modulated pathways can operate independently but when both are in play, the multiplicative power suggests a convergence in their control of ANF-RGC transduction activity.
To link cardiovascular biochemistry with physiology, a hemizygous NCδ knockout mouse, NCδ+/−, was generated (Duda et al., 2012b). We were unsuccessful in generating viable homozygous NCδ knockout mice. The adrenocortical zona glomerulusa was chosen as the subject of investigation because the ANF-RGC signal transduction system is present in glomerulosa cells (Takayanagi et al., 1987; Sharma et al., 1989b; Duda et al., 1991; Rondeau et al., 1995), and NCδ is expressed in the same layer (Nakano et al., 1993). The particulate fraction of the adrenal gland from NCδ+/− mice yields 50% of the normal ANF-RGC activity and addition of saturating amounts of exogenous NCδ and Ca2+ reconstitutes the full MGCs activity. Thus, the Ca2+-dependent NCδ-modulated ANF-RGC signaling pathway exists in the adrenal glands of the mice and, as anticipated, it is only functionally half as active in NCδ+/− mice compared to wild type mice.
ANF-RGC catalytic activity in the adrenal gland offsets the renin-angiotensin aldosterone system; it inhibits aldosterone synthesis and thereby lowers blood pressure (Burnett et al., 1984; Aoki et al., 2000; Shi et al., 2001). Aldosterone levels in the plasma of NCδ+/− mice are 27% higher over the control NCδ+/+ mice (Duda et al., 2014). Together with the evidence that corticosterone levels synthesized by fasciculata cells are unaffected by the absence of NCδ gene and immunohistochemical studies showing that NCδ and ANF-RGC are co-expressed in the glomerulosa cells of the mouse adrenal gland, the conclusion is that the Ca2+-modulated ANF-RGC transduction machinery resides specifically in aldosterone producing glomerulosa cells of the adrenal gland (Duda et al., 2012b). Importantly, it is physiologically linked with blood pressure regulation. Systolic blood pressure of the NCδ+/− mice is 38% higher than in wild type mice, 127 vs. 92 mm Hg.
In conclusion, ANF-RGC performs two transductions in some systems. One signal arrives externally in the form of potent hormones, ANF and brain natriuretic peptide (BNP), which bind to an external surface domain. The second signal is internal; a neurocalcin subunit monitors intracellular Ca2+ and directly modulates the catalytic domain (Figure 7). This Ca2+-modulation defines a new paradigm of ANF-RGC signaling and unveils an alternate pathway to the physiological control of the endocrine systems that avert hypertension. The NCS VILIP-1 can bind to ANF-RGC (Braunewell et al., 2001). If VILIP-1 modulates activity of ANF-RGC as it does for CNP-RGC, it may substitute for NCδ in other systems.
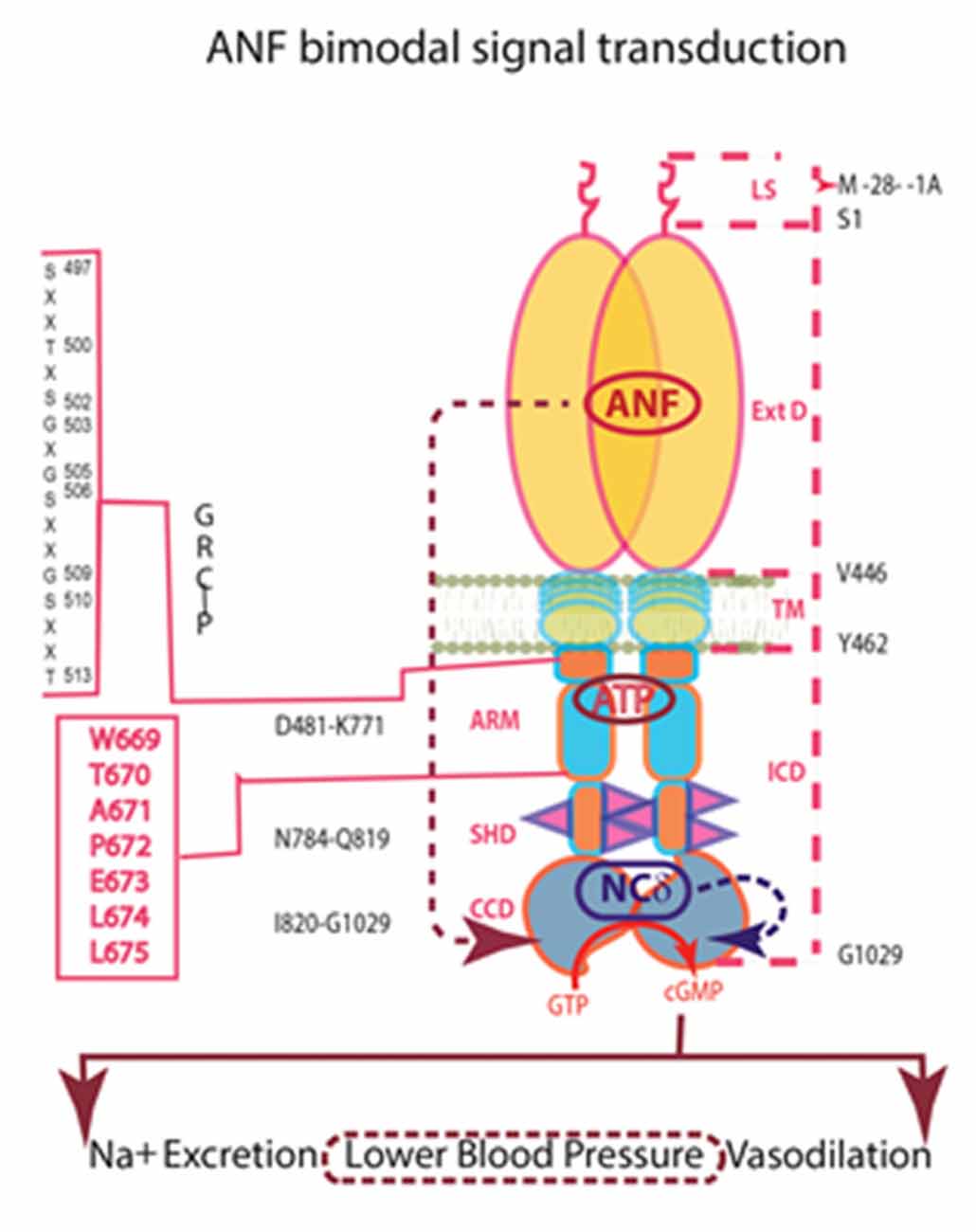
Figure 7. Independence of the signaling pathways of ANF/ATP and of NCδ. The trajectory of the ANF pathway (maroon dashed arrow) originates at the ExtD and passes through the TM, ARM and signaling helix domain (SHD) in its course to CCD. In contrast, the trajectory of the NCδ pathway (blue dashed arrow) lies within the CCD. The ANF-RGC dimer is thought to bind a dimer of NCδ, but only a single subunit is shown. Both pathways are the physiological regulators of the mouse blood pressure.
An MGC (ONE-GC, GC-D) Detects Odorants
Cloning of a MGC, termed GC-D, from a total olfactory cDNA library (Fulle et al., 1995) came at a time when olfaction was thought to occur solely through G protein coupled receptors and the cyclic AMP signaling pathway (Buck, 1995; Belluscio et al., 1998; Breer, 2003; Lai et al., 2005). Even though an odorant for GC-D was not yet known, there were clues for a cyclic GMP transduction system in olfaction. In situ hybridization and immunocytochemical studies indicate that GC-D co-expresses with a cyclic GMP stimulated phosphodiesterase, PDE2, that hydrolyzes cyclic GMP as well as cyclic AMP and an ion channel that is gated specifically by cyclic GMP (Fulle et al., 1995; Juilfs et al., 1997; Meyer et al., 2000). GC-D/PDE2 containing neurons constitute only a small subpopulation of neuroepithelial neurons for which components of the “standard” olfactory cyclic AMP signaling system: Golf, ACIII, PDE1C2, and the α3 and β1b subunits of the cyclic nucleotide gated ion channel, are missing. Moreover, they project to specific “necklace glomeruli” of the olfactory bulb. Interestingly, immunoprobes with different anti-GC-D antibodies uncover more extensive labeling of olfactory neuroepithelial cilia suggesting the possible expression of more than one type of MGC and/or a subsidiary role for cGMP in a majority of the olfactory receptors (Juilfs et al., 1997; Duda et al., 2001a).
A Ca2+-modulated ROS-GC1 system is absent from rat olfactory neuroepithelium and its ciliary subcompartment where odorants are transduced, so to assess the possibility that a novel MGC is involved, the authors cloned ONE-GC directly from a rat olfactory neuroepithelium cDNA library (Duda et al., 2001a). Sequence alignment analysis disclosed that ONE-GC has complete primary structural identity with the prior cloned GC-D (Juilfs et al., 1997), yet only partial 47.9% and 47.6% identity with ROS-GC1 and ROS-GC2, respectively. A polyclonal antibody raised against its unique 12 amino acid C-terminal epitope revealed that it shares no immunological identity with ROS-GC1 nor with ROS-GC2 (Duda et al., 2001a). Thus, ONE-GC and GC-D are the same guanylate cyclase. Because ONE-GC resides in the olfactory neuroepithelium compartment, the authors prefer the ONE-GC nomenclature over GC-D.
The Ca2+-modulated myr-NCδ-ONE-GC system meets five criteria set forth to secure its role as a genuine, Ca2+-modulated odorant transducer (Duda et al., 2001a, 2004; Duda and Sharma, 2008; Sharma and Duda, 2010): (1) ONE-GC responds to odorant in its native state. Uroguanylin is an extremely effective stimulus for ONE-GC expressing neurons (Leinders-Zufall et al., 2007), binding the ExtD of ONE-GC with an EC50 of 20 pM and saturating it at 500 pM (Duda and Sharma, 2008). Interestingly, STa-RGC binds uroguanylin and guanylin, but ONE-GC is more selective and does not recognize guanylin; (2) The response to odorant is relatively fast for an amplifying system; (3) ONE-GC resides within the membrane portion of the cilia; (4) It is modulated by physiological concentrations of free Ca2+ with a K1/2 of 700 nM, generating responses similar to that of odorant; and (5) The Ca2+-responsive system can be reconstituted with recombinant myr-NCδ and ONE-GC.
NCδ binds M880-L921 of ONE-GC, a segment encompassing the corresponding V836-L857 binding site in ROS-GC1 (see above), affording direct access to the catalytic domain (Duda and Sharma, 2008). The key 844MSEPIE849 motif in ROS-GC1 is present in ONE-GC as 908LSEPIE913. In vivo cell studies demonstrate that in resting cells, the NCδ-ONE-GC complex is exclusively at the plasma membrane region (Duda et al., 2004). Assessed by the SPR spectroscopy, kinetic parameters of the Ca2+-bound NCδ binding to ONE-GC are: KD = 2.8 × 10−7 M; kon = 5.7 × 103 M−1s−1; koff = 1.56 × 10−3 s−1.
Gene-knockout studies in mouse establish that in olfactory neuroepithelium another NCS, hippocalcin (Hpca; Krishnan et al., 2009) activates ONE-GC catalytic activity in the Ca2+ K1/2 range of 0.5–0.7 μM. Besides neurocalcin and Hpca, ONE-GC interacts with yet one more Ca2+ sensor, GCAP1 (Pertzev et al., 2010). The interaction was missed initially, because it is antithetical to that with ROS-GC in phototransduction. Instead of stimulating ONE-GC at the low nM range of free Ca2+ (i.e., in the Ca2+ free state), GCAP1 stimulates at the upper range of Ca2+ (i.e., in the Ca2+ bound state; Duda et al., 2006, 2012a; Figure 8). It was the first, and is to date, the only example of an NCS switching the directionality of its action with a change in binding partners. To add to the intrigue, stimulation of ONE-GC activity by GCAP1 occurs at an EC50 that is higher than the apparent IC50 for ROS-GC1 activity. Biochemical assays with antibodies indicate that 35% of the total ONE-GC transduction activity is controlled by GCAP1, 27% by NCδ, and 38% by Hpca (Krishnan et al., 2009; reviewed in Sharma and Duda, 2010).
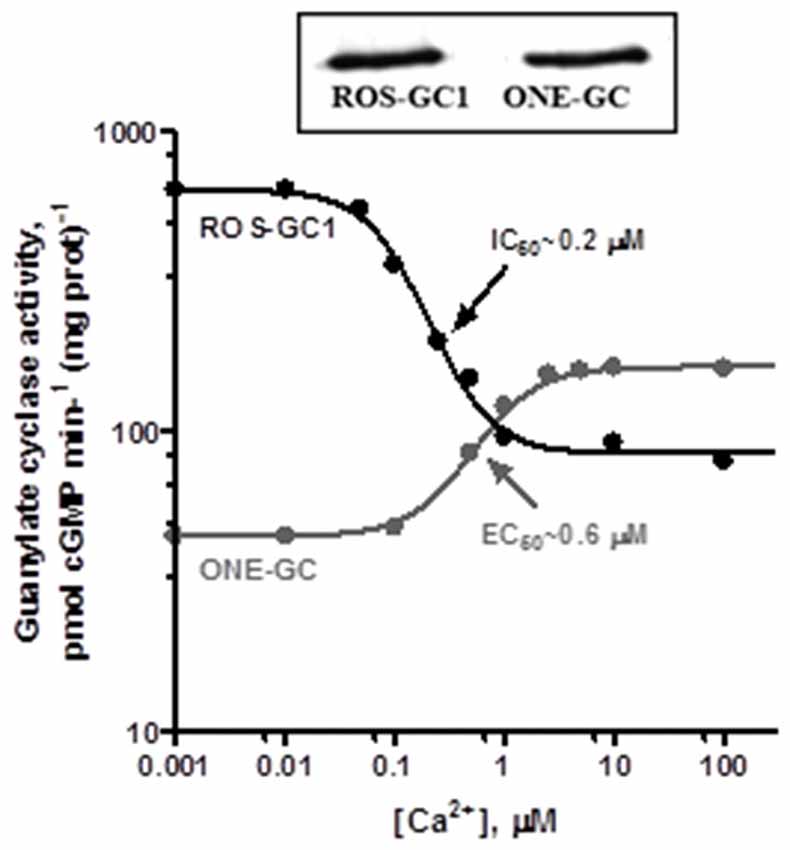
Figure 8. Antithetical Ca2+ modulation of ROS-GC1 and olfactory neuroepithelial guanylate cyclase (ONE-GC) activities by GCAP1. In the presence of GCAP1, the catalytic activity of recombinant ROS-GC1 decreases as Ca2+ is raised from 1 nM to 100 μM, but the catalytic activity of recombinant ONE-GC increases. Western blots confirming ROS-GC1 and ONE-GC expression are shown above. Redrawn from Duda et al. (2012a).
ONE-GC resembles the ANF-RGC in combining a surface hormone/odorant transduction with internal Ca2+ sensing. Yet, a primary sequence identity of only 28.1% with ANF-RGC but a sequence identity of 47.6% with ROS-GC makes it closer akin to the latter subfamily. Like photoreceptor ROS-GCs, ONE-GC is stimulated by bicarbonate (Guo et al., 2009; Sun et al., 2009). The target site of the bicarbonate signal resides within Y922-P1028 segment of the catalytic domain (Hu et al., 2007; Sun et al., 2009; Duda and Sharma, 2010).
Additional Ca2+ Sensors Couple to MGC in Hippocampus
The Ca2+ sensor frequenin (Frq, also termed NCS type-1, NCS-1) is the most primordial member of the NCS protein family with homologs already present in yeast (Fik-Rymarkiewicz et al., 2006; reviewed in Sharma and Duda, 2012). While frequenin supports Ca2+ modulation with numerous targets, its status with respect to MGCs was unknown. It was of interest to test MGCs because the results could provide clues about the timeline for the evolutionary appearance of NCS-MGC interactions. Bovine Frq has a subunit mass of 22 kDa and shows a Ca2+-dependent gel mobility shift, a characteristic of the NCS family (Ladant, 1995). Its structure is highly conserved on the evolutionary ladder: 100% identity with chicken (Nef et al., 1995; Olafsson et al., 1997; McFerran et al., 1999) rat, and human (Jeromin et al., 1999; Bourne et al., 2001); 98% with frog (Olafsson et al., 1995), 75% with C. elegans (De Castro et al., 1995), 72% with Drosophila (Pongs et al., 1993), and 60% with the yeast form (Hendricks et al., 1999). In common with the NCS family trait, there are four EF-hands, yet only three—2, 3 and 4—are functional in binding Ca2+. Frq is myristoylated but its operation as a Ca2+ myristoyl switch is somewhat controversial (Lim et al., 2011).
Co-immunoprecipitation experiments demonstrate that Frq and a ONE-GC-like MGC exist as a complex in bovine hippocampal neurons (Fik-Rymarkiewicz et al., 2006). When these studies were conducted, no Ca2+-modulated MGC transduction machinery had ever been reported in hippocampus. The existence of the complex is Ca2+-dependent, dissociating when the Ca2+ is depleted with EGTA. Interestingly, a small fraction of Frq immunoprecipitates with the hippocampal MGC even in the presence of 5 mM EGTA. The MGC and the native NCS from hippocampal neurons respond to [Ca2+] with a five-fold increase in activity and a K1/2 for Ca2+ of 0.7 μM. The EC50 of hippocampal MGC for recombinant myr-Frq is 0.7 μM, and at saturating levels of Ca2+, activity increases five-fold. Recombinant myr-Frq robustly stimulates the catalytic activity of recombinant ONE-GC 6-fold with an EC50 of 0.7 μM, only marginally (~1.5-fold) stimulates recombinant ROS-GC1, and utterly fails to stimulate ROS-GC2. The binding kinetics between Frq and the M836-C1110 segment of ONE-GC were determined through surface plasmon resonance spectroscopy: KD =0.43 μM; kon (the association rate constant) = 7.14 × 103M−1s−1; koff (dissociation rate constant) = 3.04 × 10−3s−1; and KA (equilibrium association constant) = 2.35 × 106 M−1. Frq therefore may be a soluble modulator rather than a true subunit of the hippocampal MGC. Functionally, a mixed distribution of Frq bound to a minor fraction of the hippocampal MGC and free Frq in the cytoplasm of resting neurons could serve to effectively raise the Ca2+ requirement, extend the Ca2+ range and lengthen the duration of the rise in Ca2+ necessary for stimulation of cyclic GMP synthesis. Modulation of an MGC by myr-Frq suggests that during evolution, Ca2+-sensors that stimulate MGCs at high Ca2+ probably arose before the GCAPs that stimulate at low Ca2+.
These results argue in favor of a ONE-GC-like MGC in hippocampus, but its identity remains elusive because GC-D is not expressed in bovine. Hippocalcin, which can couple with ONE-GC (vide supra, Krishnan et al., 2009), is potentially a second soluble Ca2+ modulator of MGCs in hippocampus. CNP-RGC is another hippocampal MGC, responding to CNP produced locally (Langub et al., 1995; Herman et al., 1996). Mutant rats with a defective response to CNP show disturbances in long term potentiation and long term depression (Barmashenko et al., 2014). These studies on hippocampus begin to unravel a role for MGCs in learning and memory.
A Novel MGC (GC-G) Responds to Mild Cooling
A seventh MGC, GC-G, was cloned from rat with some suggestion for alternative splicing (Schulz et al., 1998). The realization that GC-G is expressed in selected, temperature- sensitive neurons of the Grueneberg Ganglion in the nasal cavity of mouse (Fleischer et al., 2009) led to the remarkable discovery that GC-G is a thermosensor (Chao et al., 2015), reminiscent of MGCs encoded by gcy-8, gcy-18 and gcy-23 in C. elegans (Takeishi et al., 2016). The molecular mechanism has not yet been defined but it would appear that dimerization of the CCD is optimal near 15°C with a sharp temperature dependence (Figure 9). GC-G is multifunctional—it also responds to the predator odorant 2,4,5-trimethylthiazoline (Chao et al., 2015) and to bicarbonate (Chao et al., 2010). Modulation by Ca2+ is not yet known. Activation of Grueneberg Ganglion neurons by mild cooling elicits ultrasound vocalization in pups, a behavior that is attenuated in GC-G knockouts (Chao et al., 2015).
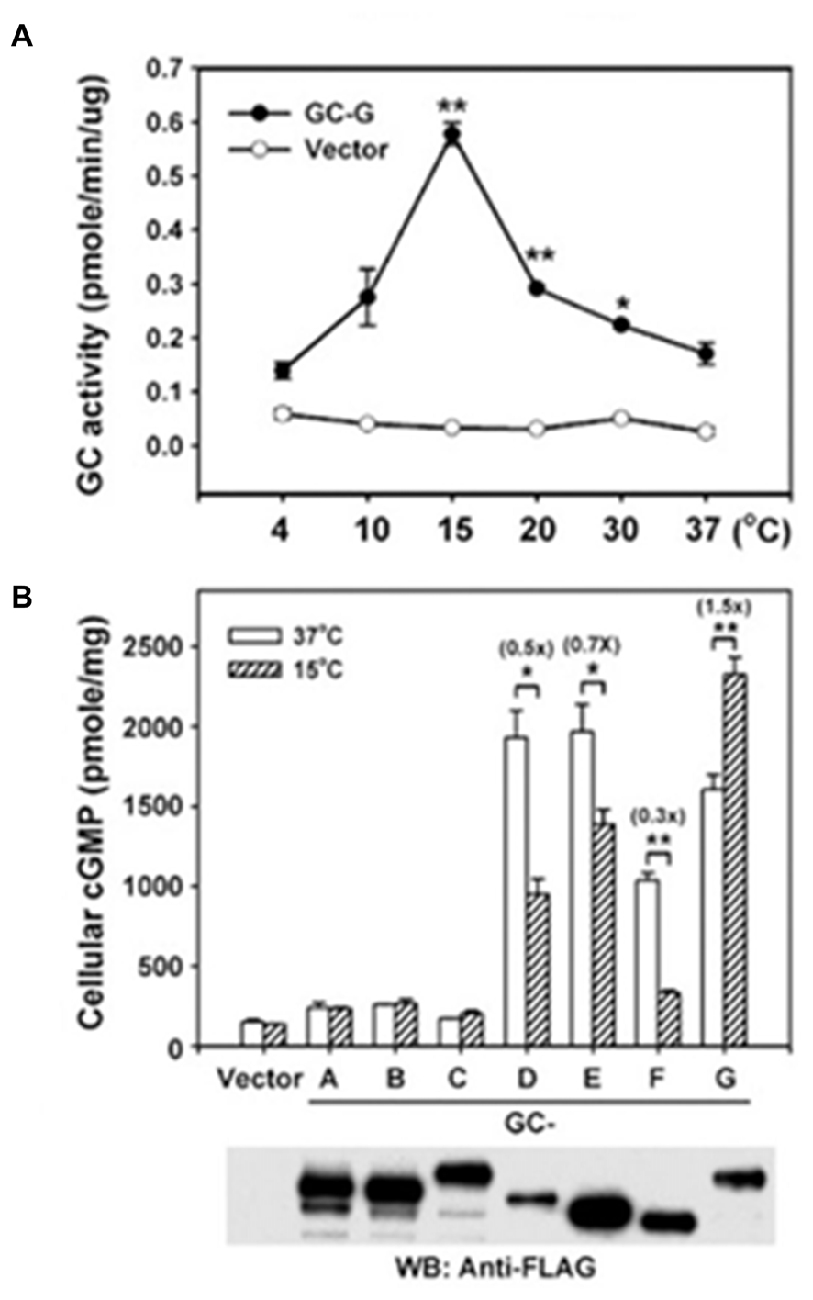
Figure 9. Thermosensing by GC-G. (A) Temperature dependence of GC-G activity. Membranes of HEK293T cells transfected with GC-G cDNA or empty vector were used to determine the guanylate cyclase activity at the indicated temperatures for 20 min. (B) Uniqueness of GC-G in its stimulation by cool temperature. Expression plasmids encoding FLAG-tagged versions of all known MGCs were transfected into HEK-293T cells and their membranes were used for guanylate cyclase activity assays at 37 and 15°C. ANF-RGC, CNP-RGC, and STa-RGC showed little change, whereas ROS-GC1, ROS-GC2 and ONE-GC showed greater activity at 37°C. Error bars show SD. *p < 0.05, **p < 0.01. Analysis by Western blot with anti-FLAG antibodies confirms the expression of each MGC. Reproduced with permission from Chao et al. (2015): Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J 34:294-306, © John Wiley and Sons, Inc.
New Functions of Ca2+-Interlocked MGCs Continue to Be Uncovered
GC-D has degraded to a pseudogene in most primates (Young et al., 2007), and GC-G appears to have lost its capacity to function as a cyclase in humans (Yang et al., 2010), nevertheless, there is growing appreciation for the extensive roles that the other MGCs have taken on throughout the body (Kuhn, 2016). A few examples that we have begun to explore are listed below.
In the pineal gland, adrenergic receptor activity is coupled to cyclic GMP synthesis by ROS-GC1 and changes in intracellular Ca2+ via S100B (Venkataraman et al., 1998). GCAP1 expression in the pineal gland raised the possibility for another bimodal switch like that present in photoreceptor-bipolar cell synapses (Venkataraman et al., 2000). However, immunohistochemical analyses distinguish two types of pinealocytes, one that is GCAP1-modulated and another that is S100B-modulated. Therefore, bimodal switching is precluded in this system. ANF, CNP and CNP-RGC are also expressed in pinealocytes (Olcese et al., 1994; Middendorff et al., 1996).
Expression of a Ca2+ signaling MGC transduction system in the anterior portion of the bovine gustatory epithelium has been demonstrated at the biochemical, molecular and functional levels, implicating a role in taste (Duda and Sharma, 2004). The system is composed of two components: the Ca2+-sensor protein, S100B and the transducer, ROS-GC1. Co-immunoprecipitation experiments reveal that ROS-GC1 and S100B physically interact with each other. The precise operational mechanism of this signal transduction mechanism has not yet been decoded at the physiological level.
Dissection and reconstitution of the biochemical and functional components of the rat olfactory bulb demonstrate that a GCAP1-modulated ROS-GC1 transduction system is present in its mitral cells. At the biochemical level, the machinery functions according to the principles established for phototransduction. Olfactory bulb ROS-GC1 senses free levels of Ca2+ via GCAP1 with an IC50 of 70 nM. The bulb neurons do not express GCAP2 Ca2+ sensor, nor do they express ROS-GC2. The olfactory bulb functions as a “processing unit” for post-odorant transduction events, directing the odorant impulses to the olfactory tract on their way to cortical centers of the brain for odorant perception (Figure 6; Duda et al., 2001b).
Numerous studies support second messenger roles for GMP and for the presence of an ANF-dependent ANF-RGC transduction system in the physiology of the testes: sperm motility, development of testicular germ cells, relaxation of peritubular lamina propia cells, testosterone synthesis in Leydig cells and dilation of testicular blood vessels (Marala and Sharma, 1988; Singh et al., 1988; Garbers, 1989; Pandey and Singh, 1990; Armstrong et al., 1994; Middendorff et al., 1997, 2000; Pandey et al., 1999; Mourdjeva et al., 2001).
Biochemical studies demonstrate a ROS-GC1 transduction system modulated by GCAP1 and S100B in plasma membrane of bovine testes (Jankowska et al., 2007, 2014). Immuno-stainings establish that these components co-localize in bovine and human sperm (Jankowska and Warchol, 2010). A convergence of molecular genetics, biochemistry and immunohistochemistry disclose NCδ in male gonads (Jankowska et al., 2014). Thus, three sensors: GCAP1, S100B and NCδ, capture and transmit Ca2+ signals to ROS-GC1 in this tissue. The transduction unit is expressed in all germinal cell types of the human testes: spermatogonias, spermatocytes, and spermatids. The spermatids residing close to the seminiferous tubule lumen embody the abundance of the transduction unit, suggesting involvement with the processes of spermatogenesis. Based on mouse-KO models, NCδ is the major (75%) and S100B the minor (25%) contributor of Ca2+ signaling by ROS-GC1. The contribution of GCAP1 has not been determined but there is evidence for bimodal Ca2+ operation in bovine and human spermatozoa (Jankowska and Warchol, 2010). Guanylate cyclase activity is minimal at 800 nM, but increases with a decline in Ca2+ with an IC50 of 200 nM and increases as Ca2+ is raised with an EC50 of 2 μM. GC-G expression in mouse sperm (Kuhn et al., 2004; Huang et al., 2006) could support thermotaxis and/or chemotaxis and contribute to the bicarbonate sensitivity. The homologous human gene is also expressed in sperm, yet it does not encode a true MGC because of termination codons in the KHD.
Conclusions
This monograph narrates an extraordinary tale of MGCs. It started about four decades ago, when the authors’ group made a key observation that a novel, epinephrine-sensitive MGC transduction system existed in rat adrenocortical carcinoma 494 cells that is absent from the normal adrenal cortex (Perchellet and Sharma, 1980; Shanker and Sharma, 1980). Over the ensuing years meticulous dissection of the MGC system resulted in its rise from anonymity to its present status as a preeminent cellular signal transducer generating the intracellular second messenger, cyclic GMP, to critically control countless physiological processes, including cardiac vascular tension, smooth muscle relaxation, blood volume, cellular growth, sensory transduction, neural plasticity, learning and memory (Figure 10).
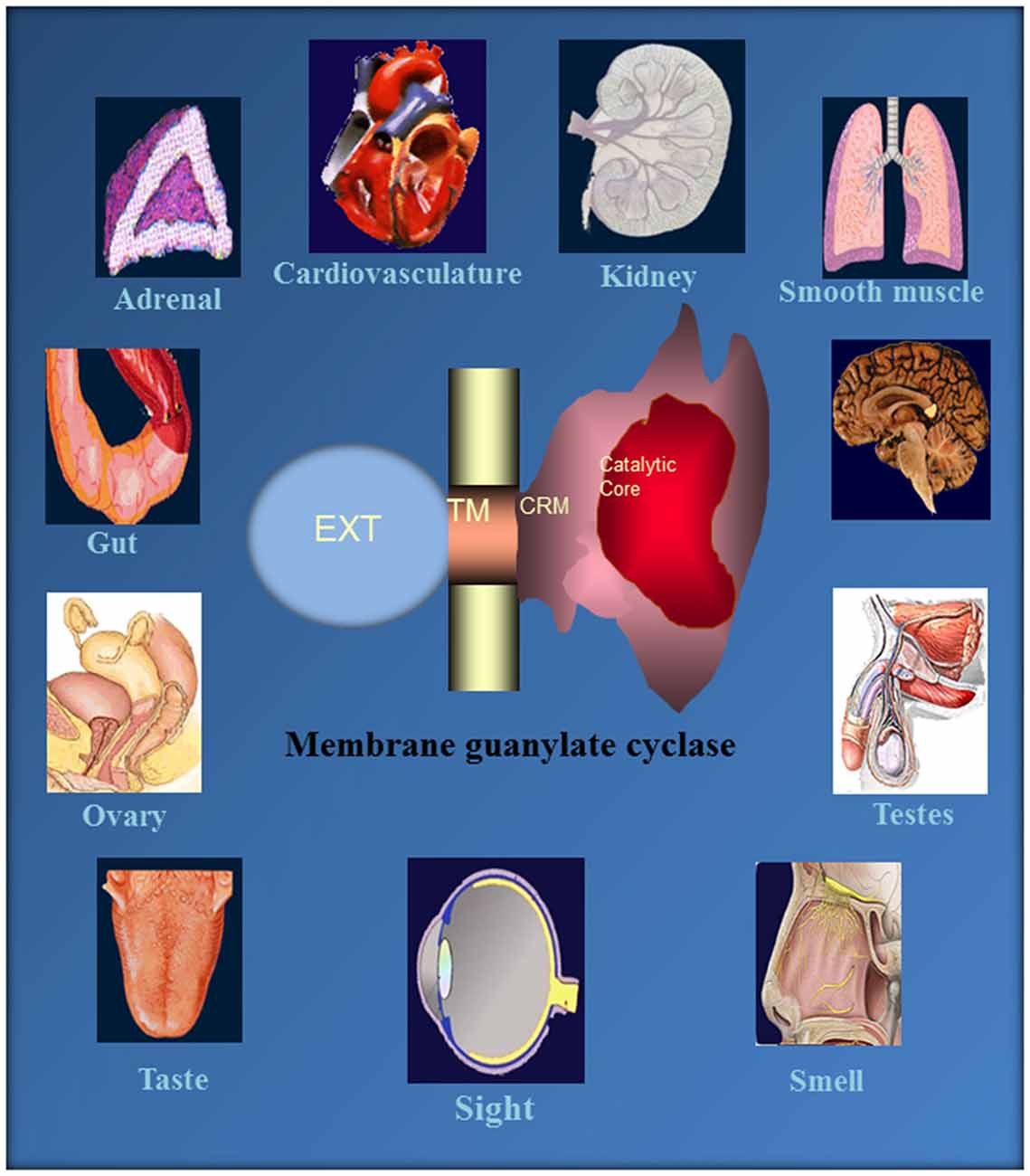
Figure 10. Ubiquitous expression of MGC signaling systems. MGCs is a multi-switching cyclic GMP generating machine linked with the physiology of cardio-vasculature, smooth muscle relaxation, sensory transduction, neuronal plasticity and memory in mammalian neurosensory, endocrine and peripheral tissues. Reproduced from Sharma et al. (2015).
How does it perform such diverse tasks? A modular structure makes the MGC extremely flexible and multimodal. Unlike the three-component design of its predecessor second messenger systems: adenylate cyclase and IP3, G-protein and G-protein coupled receptors; the MGC transduction system consists of a single entity, a trans-membrane-spanning protein that serves as both a receptor and a signal transducer. For all MGCs except for ROS-GC1 and 2, the ExtD binds hormones or small molecules. Some MGCs have subunits that attach to an intracellular domain (ICD) and confer Ca2+ sensitivity. The relation between Ca2+ and MGC activity is variable. With ANF-RGC, high Ca2+ stimulates activity without ligand binding whereas ROS-GC1 and ONE-GC can act as bimodal Ca2+ switches; activity is maximal as Ca2+ is depleted, it subsides at midrange [Ca2+]i, then rises once again as [Ca2+] reaches its highest levels. The basis for these different modes of action trace to the identity of the Ca2+ sensor involved: GCAPs, S100B, neurocalcin, frequenin. In a startling arrangement, the conduct of GCAP1 depends upon its MGC partner; it stimulates ROS-GCs at low Ca2+ and inhibits them at high Ca2+, but stimulates ONE-GC at high Ca2+. ROS-GC1 is capable of coupling with many different Ca2+ sensors (Figure 11), in part because of its CTE. In contrast, ROS-GC2 is quite selective even though it also has a CTE. Interestingly, the expansiveness of CTE in STa-RGC and in GC-G raises the question as to what other functions this structure performs. It is now recognized that the conserved CCD dimer is receptive to signals generated by both C-terminal and N-terminal modular blocks. In an extraordinary development, the CCD is also subject to direct tuning by Ca2+/neurocalcin and by bicarbonate. With conservation of the CCD, nearly every MGC responds to bicarbonate. The response of ROS-GC to bicarbonate and Ca2+-modulated GCAPs and S100B in combination exceeds the sum of the individual modulations affording a means to amplify the transductions. It remains to be seen whether bicarbonate can also amplify transduction of orthosteric ligand binding. Chemical modulation and regulation by phosphorylation of MGCs are summarized in Table 1. Besides sensing odorants and bicarbonate and Ca2+, GC-G has evolved a remarkable mechanism whereby dimerization and catalytic activity are maximal at a particular temperature in order to sense mild cooling. These transductions encroach on territories of GPCRs and TRP channels. In the former case, MGCs may provide for faster signaling and in the latter, case, amplification. The growing appreciation for MGC signaling throughout the body calls for reclassification of their Ca2+ sensors. NCδ and GCAPs can no longer be considered as strictly NCSs, as their expression is more widespread.
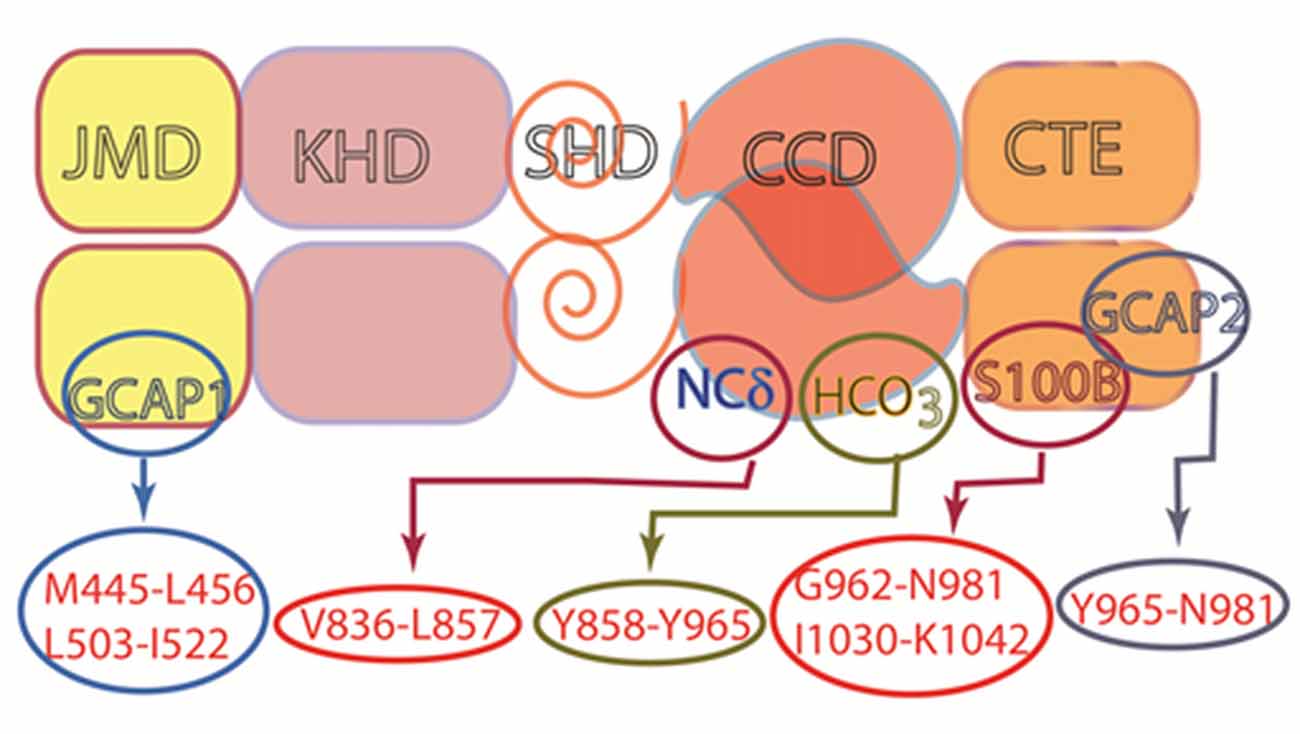
Figure 11. Multiple modulators of ROS-GC1 catalytic activity. ROS-GC is a multi-switch transduction unit with a site for HCO3− and Ca2+ sensor switching domains for GCAP1 and 2, S100B and NCδ. The sites for Frq and Hpca binding are not known, but based on studies with ONE-GC, the site for Frq is likely to be located in the CCD. This architecture provides it with cellular signaling specificity and elasticity in a variety of tissues.
Nomenclature
The reasoning for usage of the present nomenclature for the subtypes of MGCs was explained in our previous review (Sharma, 2010): “From its inception to its first discovery this enzyme has been termed ‘guanylate cyclase’… For unknown reasons some investigators, subsequently, have changed its name to ‘guanylyl cyclase’. The guanylate cyclases ANF-RGC, CNP-RGC and STa-RGC have been alternately named as GC-A, GC-B and GC-C, respectively; and ONE-GC, ROS-GC1 and ROS-GC2 as GC-D, GC-E and GC-F, respectively. This investigator has preferred the former functional nomenclature of the guanylate cyclases.”
Author Contributions
All authors contributed in the organization, design and writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding was provided in part by the National Eye Institute (Grant no. EY023980), but the contents of this article are the sole responsibility of the authors and do not necessarily reflect the official views of the National Eye Institute. The authors gratefully acknowledge past support by numerous USPHS awards from the National Institutes of Health, the National Science Foundation and the Damon Runyon Walter Winchell Cancer Fund, the dedicated work of numerous postdoctoral fellows and graduate students, and wonderful collaborations with Drs. Ari Sitaramayya, Karl-W. Koch, Wolfgang Baehr, Anuradha Krishnan and Venkat Venkataraman.
References
Ahrens, H., Paul, A. K., Kuroda, Y., and Sharma, R. K. (1982). Adrenocortical cyclic GMP-dependent protein kinase: purification, characterization and modification of its activity by calmodulin and its relationship with steroidogenesis. Arch. Biochem. Biophys. 215, 597–609. doi: 10.1016/0003-9861(82)90121-7
Allerston, C. K., von Delft, F., and Gileadi, O. (2013). Crystal structures of the catalytic domain of human soluble guanylate cyclase. PLoS One 8:e57644. doi: 10.1371/journal.pone.0057644
Aoki, H., Richmond, M., Izumo, S., and Sadoshima, J. (2000). Specific role of the extracellular signal-regulated kinase pathway in angiotensin II-induced cardiac hypertrophy in vitro. Biochem. J. 347, 275–284. doi: 10.1042/bj3470275
Aparicio, J. G., and Applebury, M. L. (1996). The photoreceptor guanylate cyclase is an autophosphorylating protein kinase. J. Biol. Chem. 271, 27083–27089. doi: 10.1074/jbc.271.43.27083
Armstrong, V. L., Clulow, J., Murdoch, R. N., and Jones, R. C. (1994). Intracellular signal transduction mechanisms of rat epididymal spermatozoa and their relationship to motility and metabolism. Mol. Reprod. Dev. 38, 77–84. doi: 10.1002/mrd.1080380113
Ashman, D. F., Lipton, R., Melicow, M. M., and Price, T. D. (1963). Isolation of adenosine 3′, 5′-monophosphate and guanosine 3′, 5′-monophosphate from rat urine. Biochem. Biophys. Res. Commun. 11, 330–334. doi: 10.1016/0006-291x(63)90566-7
Baehr, W., Karan, S., Maeda, T., Luo, D. G., Li, S., Bronson, J. D., et al. (2007). The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J. Biol. Chem. 282, 8837–8847. doi: 10.1074/jbc.m610369200
Ballermann, B. J., Marala, R. B., and Sharma, R. K. (1988). Characterization and regulation by protein kinase C of renal glomerular atrial natriuretic peptide receptor-coupled guanylate cyclase. Biochem. Biophys. Res. Commun. 157, 755–761. doi: 10.1016/s0006-291x(88)80314-0
Barmashenko, G., Buttgereit, J., Herring, N., Bader, M., Ozcelik, C., Manahan-Vaughan, D., et al. (2014). Regulation of hippocampal synaptic plasticity thresholds and changes in exploratory and learning behavior in dominant negative NPR-B mutant rats. Front. Mol. Neurosci. 7:95. doi: 10.3389/fnmol.2014.00095
Belluscio, L., Gold, G. H., Nemes, A., and Axel, R. (1998). Mice deficient in G(olf) are anosmic. Neuron 20, 69–81. doi: 10.1016/s0896-6273(00)80435-3
Bereta, G., Wang, B., Kiser, P. D., Baehr, W., Jang, G. F., and Palczewski, K. (2010). A functional kinase homology domain is essential for the activity of photoreceptor guanylate cyclase 1. J. Biol. Chem. 285, 1899–1908. doi: 10.1074/jbc.m109.061713
Bhandari, R., Srinivasan, N., Mahaboobi, M., Ghanekar, Y., Suguna, K., and Visweswariah, S. S. (2001). Functional inactivation of the human guanylyl cyclase C receptor: modeling and mutation of the protein kinase-like domain. Biochemistry 40, 9196–9206. doi: 10.1021/bi002595g
Bourne, Y., Dannenberg, J., Pollmann, V., Marchot, P., and Pongs, O. (2001). Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1). J. Biol. Chem. 276, 11949–11955. doi: 10.1074/jbc.M009373200
Bowyer, F., and Kitabchi, A. E. (1974). Dual role of calcium in steroidogenesis in the isolated adrenal cell of rat. Biochem. Biophys. Res. Commun. 57, 100–105. doi: 10.1016/s0006-291x(74)80362-1
Braunewell, K. H., Brackmann, M., Schaupp, M., Spilker, C., Anand, R., and Gundelfinger, E. D. (2001). Intracellular neuronal calcium sensor (NCS) protein VILIP-1 modulates cGMP signalling pathways in transfected neural cells and cerebellar granule neurones. J. Neurochem. 78, 1277–1286. doi: 10.1046/j.1471-4159.2001.00506.x
Braunewell, K. H., and Gundelfinger, E. D. (1999). Intracellular neuronal calcium sensor proteins: a family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res. 295, 1–12. doi: 10.1007/s004410051207
Breer, H. (2003). Olfactory receptors: molecular basis for recognition and discrimination of odors. Anal. Bioanal. Chem. 377, 427–433. doi: 10.1007/s00216-003-2113-9
Buck, L. B. (1995). Unraveling chemosensory diversity. Cell 83, 349–352. doi: 10.1016/0092-8674(95)90110-8
Burgoyne, R. D., and Weiss, J. L. (2001). The neuronal calcium sensor family of Ca2+-binding proteins. Biochem. J. 353, 1–12. doi: 10.1042/bj3530001
Burnett, J. C. Jr., Granger, J. P., and Opgenorth, T. J. (1984). Effects of synthetic atrial natriuretic factor on renal function and renin release. Am. J. Physiol. 247, F863–F866.
Chang, M. S., Lowe, D. G., Lewis, M., Hellmiss, R., Chen, E., and Goeddel, D. V. (1989). Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature 341, 68–72. doi: 10.1038/341068a0
Chao, Y. C., Cheng, C. J., Hsieh, H. T., Lin, C. C., Chen, C. C., and Yang, R. B. (2010). Guanylate cyclase-G, expressed in the Grueneberg ganglion olfactory subsystem, is activated by bicarbonate. Biochem. J. 432, 267–273. doi: 10.1042/bj20100617
Chao, Y. C., Chen, C. C., Lin, Y. C., Breer, H., Fleischer, J., and Yang, R. B. (2015). Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J. 34, 294–306. doi: 10.15252/embj.201489652
Chinkers, M., and Garbers, D. L. (1989). The protein kinase domain of the ANP receptor is required for signaling. Science 245, 1392–1394. doi: 10.1126/science.2571188
Chinkers, M., Garbers, D. L., Chang, M. S., Lowe, D. G., Chin, H. M., Goeddel, D. V., et al. (1989). A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature 338, 78–83. doi: 10.1038/338078a0
Chinkers, M., Singh, S., and Garbers, D. L. (1991). Adenine nucleotides are required for activation of rat atrial natriuretic peptide receptor/guanylyl cyclase expressed in a baculovirus system. J. Biol. Chem. 266, 4088–4093.
Cooper, N., Liu, L., Yoshida, A., Pozdnyakov, N., Margulis, A., and Sitaramayya, A. (1995). The bovine rod outer segment guanylate cyclase, ROS-GC, is present in both outer segment and synaptic layers of the retina. J. Mol. Neurosci. 6, 211–222. doi: 10.1007/bf02736766
Cuenca, N., Lopez, S., Howes, K., and Kolb, H. (1998). The localization of guanylyl cyclase-activating proteins in the mammalian retina. Invest. Ophthalmol. Vis. Sci. 39, 1243–1250.
De Castro, E., Nef, S., Fiumelli, H., Lenz, S. E., Kawamura, S., and Nef, P. (1995). Regulation of rhodopsin phosphorylation by a family of neuronal calcium sensors. Biochem. Biophys. Res. Commun. 216, 133–140. doi: 10.1006/bbrc.1995.2601
Denninger, J. W., and Marletta, M. A. (1999). Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta 1411, 334–350. doi: 10.1016/s0005-2728(99)00024-9
Dizhoor, A. M., and Hurley, J. B. (1996). Inactivation of EF-hands makes GCAP-2 (p24) a constitutive activator of photoreceptor guanylyl cyclase by preventing a Ca2+-induced “activator-to-inhibitor” transition. J. Biol. Chem. 271, 19346–19350. doi: 10.1074/jbc.271.32.19346
Dizhoor, A. M., Olshevskaya, E. V., Henzel, W. J., Wong, S. C., Stults, J. T., Ankoudinova, I., et al. (1995). Cloning, sequencing and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J. Biol. Chem. 270, 25200–25206. doi: 10.1074/jbc.270.42.25200
Donaldson, C., Barber, K. R., Kay, C. M., and Shaw, G. S. (1995). Human S100b protein: formation of a tetramer from synthetic calcium-binding site peptides. Protein Sci. 4, 765–772. doi: 10.1002/pro.5560040416
Duda, T., Bharill, S., Wojtas, I., Yadav, P., Gryczynski, I., Gryczynski, Z., et al. (2009). Atrial natriuretic factor receptor guanylate cyclase signaling: new ATP-regulated transduction motif. Mol. Cell. Biochem. 324, 39–53. doi: 10.1007/s11010-008-9983-2
Duda, T., Fik-Rymarkiewicz, E., Venkataraman, V., Krishnan, A., and Sharma, R. K. (2004). Calcium-modulated ciliary membrane guanylate cyclase transduction machinery: constitution and operational principles. Mol. Cell. Biochem. 267, 107–122. doi: 10.1023/b:mcbi.0000049372.33965.4f
Duda, T., Fik-Rymarkiewicz, E., Venkataraman, V., Krishnan, R., Koch, K. W., and Sharma, R. K. (2005a). The calcium-sensor guanylate cyclase activating protein type 2 specific site in rod outer segment membrane guanylate cyclase type 1. Biochemistry 44, 7336–7345. doi: 10.1021/bi050068x
Duda, T., Venkataraman, V., Ravichandran, S., and Sharma, R. K. (2005b). ATP-regulated module (ARM) of the atrial natriuretic factor receptor guanylate cyclase. Peptides 26, 969–984. doi: 10.1016/j.peptides.2004.08.032
Duda, T., Goraczniak, R. M., and Sharma, R. K. (1991). Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc. Natl. Acad. Sci. U S A 88, 7882–7886. doi: 10.1073/pnas.88.17.7882
Duda, T., Goraczniak, R. M., and Sharma, R. K. (1993a). Core sequence of ATP regulatory module in receptor guanylate cyclases. FEBS Lett. 315, 143–148. doi: 10.1016/0014-5793(93)81151-o
Duda, T., Goraczniak, R. M., and Sharma, R. K. (1993b). The glycine residue of ATP regulatory module in receptor guanylate cyclases that is essential in natriuretic factor signaling. FEBS Lett. 335, 309–314. doi: 10.1016/0014-5793(93)80408-m
Duda, T., Goraczniak, R. M., Sitaramayya, A., and Sharma, R. K. (1993c). Cloning and expression of an ATP-regulated human retina C-type natriuretic factor receptor guanylate cyclase. Biochemistry 32, 1391–1395. doi: 10.1021/bi00057a001
Duda, T., Goraczniak, R. M., and Sharma, R. K. (1994). Glutamic acid-332 residue of the type C natriuretic peptide receptor guanylate cyclase is important for signaling. Biochemistry 33, 7430–7433. doi: 10.1021/bi00189a050
Duda, T., Goraczniak, R. M., and Sharma, R. K. (1995). Single amino acid residue-linked signaling shifts in the transduction activities of atrial and type C natriuretic factor receptor guanylate cyclases. Biochem. Biophys. Res. Commun. 212, 1046–1053. doi: 10.1006/bbrc.1995.2075
Duda, T., Goraczniak, R. M., and Sharma, R. K. (1996a). Molecular characterization of S100A1–S100B protein in retina and its activation mechanism of bovine photoreceptor guanylate cyclase. Biochemistry 35, 6263–6266. doi: 10.1021/bi960007m
Duda, T., Goraczniak, R., Surgucheva, I., Rudnicka-Nawrot, M., Gorczyca, W. A., Palczewski, K., et al. (1996b). Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry 35, 8478–8482. doi: 10.1021/bi960752z
Duda, T., Jankowska, A., Venkataraman, V., Nagele, R. G., and Sharma, R. K. (2001a). A novel calcium-regulated membrane guanylate cyclase transduction system in the olfactory neuroepithelium. Biochemistry 40, 12067–12077. doi: 10.1021/bi0108406
Duda, T., Venkataraman, V., Krishnan, A., Nagele, R. G., and Sharma, R. K. (2001b). Negatively calcium-modulated membrane guanylate cyclase signaling system in the rat olfactory bulb. Biochemistry 40, 4654–4662. doi: 10.1021/bi0027985
Duda, T., Yadav, P., Jankowska, A., Venkataraman, V., and Sharma, R. K. (2001c). Three dimensional atomic model and experimental validation for the ATP-regulated module (ARM) of the atrial natriuretic factor receptor guanylate cyclase. Mol. Cell. Biochem. 217, 165–172. doi: 10.1023/A:1007236917061
Duda, T., Koch, K. W., Venkataraman, V., Lange, C., Beyermann, M., and Sharma, R. K. (2002). Ca2+ sensor S100 beta-modulated sites of membrane guanylate cyclase in the photoreceptor-bipolar synapse. EMBO J. 21, 2547–2556. doi: 10.1093/emboj/21.11.2547
Duda, T., Krishnan, R., and Sharma, R. K. (2006). GCAP1 Antithetical calcium censor of ROS-GC transduction machinery. Calcium Bind. Proteins 1,102–107.
Duda, T., Pertzev, A., Koch, K. W., and Sharma, R. K. (2012a). Antithetical modes of and the Ca2+ sensors targeting in ANF-RGC and ROS-GC1 membrane guanylate cyclases. Front. Mol. Neurosci. 5:44. doi: 10.3389/fnmol.2012.00044
Duda, T., Pertzev, A., and Sharma, R. K. (2012b). Ca2+ modulation of ANF-RGC: new signaling paradigm interlocked with blood pressure regulation. Biochemistry 51, 9394–9405. doi: 10.1021/bi301176c
Duda, T., Pertzev, A., and Sharma, R. K. (2012c). Differential Ca2+ sensor guanylate cyclase activating protein modes of photoreceptor rod outer segment membrane guanylate cyclase signaling. Biochemistry 51, 4650–4657. doi: 10.1021/bi300572w
Duda, T., Pertzev, A., and Sharma, R. K. (2011a). 657WTAPELL663 motif of the photoreceptor ROS-GC1: a general phototransduction switch. Biochem. Biophys. Res. Commun. 408, 236–241. doi: 10.1016/j.bbrc.2011.03.134
Duda, T., Yadav, P., and Sharma, R. K. (2011b). Allosteric modification, the primary ATP activation mechanism of atrial natriuretic factor receptor guanylate cyclase. Biochemistry 50, 1213–1225. doi: 10.1021/bi1018978
Duda, T., Pertzev, A., and Sharma, R. K. (2013). The ANF-RGC gene motif (669)WTAPELL(675) is vital for blood pressure regulation: biochemical mechanism. Biochemistry 52, 2337–2347. doi: 10.1021/bi400175d
Duda, T., Pertzev, A., and Sharma, R. K. (2014). Atrial natriuretic factor receptor guanylate cyclase, ANF-RGC, transduces two independent signals, ANF and Ca2+. Front. Mol. Neurosci. 7:17. doi: 10.3389/fnmol.2014.00017
Duda, T., Pertzev, A., Makino, C. L., and Sharma, R. K. (2016). Bicarbonate and Ca2+ sensing modulators activate photoreceptor ROS-GC1 synergistically. Front. Mol. Neurosci. 9:5. doi: 10.3389/fnmol.2016.00005
Duda, T., and Sharma, R. K. (1995a). ATP bimodal switch that regulates the ligand binding and signal transduction activities of the atrial natriuretic factor receptor guanylate cyclase. Biochem. Biophys. Res. Commun. 209, 286–292. doi: 10.1006/bbrc.1995.1501
Duda, T., and Sharma, R. K. (1995b). ATP modulation of the ligand binding and signal transduction activities of the type C natriuretic peptide receptor guanylate cyclase. Mol. Cell. Biochem. 152, 179–183. doi: 10.1007/bf01076081
Duda, T., and Sharma, R. K. (2004). S100B-modulated Ca2+-dependent ROS-GC1 transduction machinery in the gustatory epithelium: a new mechanism in gustatory transduction. FEBS Lett. 577, 393–398. doi: 10.1016/j.febslet.2004.09.089
Duda, T., and Sharma, R. K. (2008). ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem. Biophys. Res. Commun. 367, 440–445. doi: 10.1016/j.bbrc.2007.12.153
Duda, T., and Sharma, R. K. (2010). Distinct ONE-GC transduction modes and motifs of the odorants: uroguanylin and CO2. Biochem. Biophys. Res. Commun. 391, 1379–1384. doi: 10.1016/j.bbrc.2009.12.068
Duda, T., Venkataraman, V., Goraczniak, R., Lange, C., Koch, K. W., and Sharma, R. K. (1999). Functional consequences of a rod outer segment membrane guanylate cyclase (ROS-GC1) gene mutation linked with Leber’s congenital amaurosis. Biochemistry 38, 509–515. doi: 10.1021/bi9824137
Duda, T., Wen, X. H., Isayama, T., Sharma, R. K., and Makino, C. L. (2015). Bicarbonate modulates photoreceptor guanylate cyclase (ROS-GC) catalytic activity. J. Biol. Chem. 290, 11052–11060. doi: 10.1074/jbc.m115.650408
Duda, T., Yadav, P., Jankowska, A., Venkataraman, V., and Sharma, R. K. (2000). Three dimensional atomic model and experimental validation for the ATP-Regulated Module (ARM) of the atrial natriuretic factor receptor guanylate cyclase. Mol. Cell. Biochem. 214, 7–14. doi: 10.1023/A:1007144328682
Fesenko, E. E., Kolesnikov, S. S., and Lyubarsky, A. L. (1985). Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313, 310–313. doi: 10.1038/313310a0
Fik-Rymarkiewicz, E., Duda, T., and Sharma, R. K. (2006). Novel frequenin-modulated Ca2+-signaling membrane guanylate cyclase (ROS-GC) transduction pathway in bovine hippocampus. Mol. Cell. Biochem. 291, 187–204. doi: 10.1007/s11010-006-9215-6
Fleischer, J., Mamasuew, K., and Breer, H. (2009). Expression of cGMP signaling elements in the Grueneberg ganglion. Histochem. Cell Biol. 131, 75–88. doi: 10.1007/s00418-008-0514-8
Frins, S., Bönigk, W., Müller, F., Kellner, R., and Koch, K. W. (1996). Functional characterization of a guanylyl cyclase-activating protein from vertebrate rods. Cloning, heterologous expression and localization. J. Biol. Chem. 271, 8022–8027. doi: 10.1074/jbc.271.14.8022
Fulle, H. J., Vassar, R., Foster, D. C., Yang, R. B., Axel, R., and Garbers, D. L. (1995). A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc. Natl. Acad. Sci. U S A 92, 3571–3575. doi: 10.1073/pnas.92.8.3571
Garbers, D. L. (1989). Molecular basis of signalling in the spermatozoon. J. Androl. 10, 99–107. doi: 10.1002/j.1939-4640.1989.tb00068.x
Gill, G. N., and McCune, R. W. (1979). Guanosine 3′,5′-monophosphate-dependent protein kinase. Curr. Top. Cell. Regul. 15, 1–45. doi: 10.1016/b978-0-12-152815-7.50005-3
Goldberg, N. D., Dietz, S. B., and O’Toole, A. G. (1969). Cyclic guanosine 3′,5′-monophosphate in mammalian tissues and urine. J. Biol. Chem. 244, 4458–4466.
Goldberg, N. D., and Haddox, M. K. (1977). Cyclic GMP metabolism and involvement in biological regulation. Annu. Rev. Biochem. 46, 823–896. doi: 10.1146/annurev.bi.46.070177.004135
Goldberg, N. D., O’Dea, R. F., and Haddox, M. K. (1973). Cyclic GMP. Adv. Cyclic Nucleotide Res. 3, 155–223.
Goraczniak, R. M., Duda, T., and Sharma, R. K. (1992). A structural motif that defines the ATP-regulatory module of guanylate cyclase in atrial natriuretic factor signalling. Biochem. J. 282, 533–537. doi: 10.1042/bj2820533
Goraczniak, R., Duda, T., and Sharma, R. K. (1997). Structural and functional characterization of a second subfamily member of the calcium-modulated bovine rod outer segment membrane guanylate cyclase, ROS-GC2. Biochem. Biophys. Res. Commun. 234, 666–670. doi: 10.1006/bbrc.1997.6579
Goraczniak, R. M., Duda, T., and Sharma, R. K. (1998). Calcium modulated signaling site in type 2 rod outer segment membrane guanylate cyclase (ROS-GC2). Biochem. Biophys. Res. Commun. 245, 447–453. doi: 10.1006/bbrc.1998.8455
Goraczniak, R. M., Duda, T., Sitaramayya, A., and Sharma, R. K. (1994). Structural and functional characterization of the rod outer segment membrane guanylate cyclase. Biochem. J. 302, 455–461. doi: 10.1042/bj3020455
Gorczyca, W. A., Polans, A. S., Surgucheva, I. G., Subbaraya, I., Baehr, W., and Palczewski, K. (1995). Guanylyl cyclase activating protein. A calcium-sensitive regulator of phototransduction. J. Biol. Chem. 270, 22029–22036. doi: 10.1074/jbc.270.37.22029
Gorczyca, W. A., Van Hooser, J. P., and Palczewski, K. (1994). Nucleotide inhibitors and activators of retinal guanylyl cyclase. Biochemistry 33, 3217–3222. doi: 10.1021/bi00177a011
Guo, D., Zhang, J. J., and Huang, X. Y. (2009). Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry 48, 4417–4422. doi: 10.1021/bi900441v
Haeseleer, F., Sokal, I., Li, N., Pettenati, M., Rao, N., Bronson, D., et al. (1999). Molecular characterization of a third member of the guanylyl cyclase-activating protein subfamily. J. Biol. Chem. 274, 6526–6535. doi: 10.1074/jbc.274.10.6526
Haksar, A., and Péron, F. G. (1973). The role of calcium in the steroidogenic response of rat adrenal cells to adrenocorticotropic hormone. Biochim. Biophys. Acta 313, 363–371. doi: 10.1016/0304-4165(73)90036-6
Hardman, J. G., Davis, J. W., and Sutherland, E. W. (1969). Effects of some hormonal and other factors on the excretion of guanosine 3′,5′-monophosphate and adenosine 3′,5′-monophosphate in rat urine. J. Biol. Chem. 244, 6354–6362.
Hardman, J. G., and Sutherland, E. W. (1969). Guanyl cyclase, an enzyme catalyzing the formation of guanosine 3′,5′-monophosphate from guanosine triphosphate. J. Biol. Chem. 244, 6363–6370.
Harrington, C. A., Fenimore, D. C., and Farmer, R. W. (1978). Regulation of adrenocortical steroidogenesis by cyclic 3′-5′-guanosine monophosphate in isolated rat adrenal cells. Biochem. Biophys. Res. Commun. 85, 55–61. doi: 10.1016/s0006-291x(78)80010-2
Hayashi, K., Sala, G., Catt, K., and Dufau, M. L. (1979). Regulation of steroidogenesis by adrenocorticotropic hormone in isolated adrenal cells. The intermediate role of cyclic nucleotides. J. Biol. Chem. 254, 6678–6683.
Hayashi, F., and Yamazaki, A. (1991). Polymorphism in purified guanylate cyclase from vertebrate rod photoreceptors. Proc. Natl. Acad. Sci. U S A 88, 4746–4750. doi: 10.1073/pnas.88.11.4746
He, X.-L., Chow, D.-C., Martick, M. M., and Garcia, K. C. (2001). Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science 293, 1657–1662. doi: 10.1126/science.1062246
Hendricks, K. B., Wang, B. Q., Schnieders, E. A., and Thorner, J. (1999). Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat. Cell Biol. 1, 234–241. doi: 10.1038/12058
Herman, J. P., Dolgas, C. M., Rucker, D., and Langub, M. C. Jr. (1996). Localization of natriuretic peptide-activated guanylate cyclase mRNAs in the rat brain. J. Comp. Neurol. 369, 165–187. doi: 10.1002/(SICI)1096-9861(19960527)369:2<165::AID-CNE1 >3.0.CO;2-1
Horio, Y., and Murad, F. (1991a). Purification of guanylyl cyclase from rod outer segments. Biochim. Biophys. Acta 1133, 81–88. doi: 10.1016/0167-4889(91)90244-r
Horio, Y., and Murad, F. (1991b). Solubilization of guanylyl cyclase from bovine rod outer segments and effects of lowering Ca2+ and nitro compounds. J. Biol. Chem. 266, 3411–3415.
Hu, J., Zhong, C., Ding, C., Chi, Q., Walz, A., Mombaerts, P., et al. (2007). Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 317, 953–957. doi: 10.1126/science.1144233
Huang, Y. H., Wei, C. C., Su, Y. H., Wu, B. T., Ciou, Y. Y., Tu, C. F., et al. (2006). Localization and characterization of an orphan receptor, guanylyl cyclase-G, in mouse testis and sperm. Endocrinology 147, 4792–4800. doi: 10.1210/en.2005-1476
Hwang, J. Y., Lange, C., Helten, A., Hoppner-Heitmann, D., Duda, T., Sharma, R. K., et al. (2003). Regulatory modes of rod outer segment membrane guanylate cyclase differ in catalytic efficiency and Ca2+-sensitivity. Eur. J. Biochem. 270, 3814–3821. doi: 10.1046/j.1432-1033.2003.03770.x
Ignarro, L. J., Degnan, J. N., Baricos, W. H., Kadowitz, P. J., and Wolin, M. S. (1982a). Activation of purified guanylate cyclase by nitric oxide requires heme. Comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung. Biochim. Biophys. Acta 718, 49–59. doi: 10.1016/0304-4165(82)90008-3
Ignarro, L. J., Wood, K. S., and Wolin, M. S. (1982b). Activation of purified soluble guanylate cyclase by protoporphyrin IX. Proc. Natl. Acad. Sci. U S A 79, 2870–2873. doi: 10.1073/pnas.79.9.2870
Ishikawa, E., Ishikawa, S., Davis, J. W., and Sutherland, E. W. (1969). Determination of guanosine 3′,5′-monophosphate in tissues and of guanyl cyclase in rat intestine. J. Biol. Chem. 244, 6371–6376.
Jaiswal, N., Paul, A. K., Jaiswal, R. K., and Sharma, R. K. (1986). Atrial natriuretic factor regulation of cyclic GMP levels and steroidogenesis in isolated fasciculata cells of rat adrenal cortex. FEBS Lett. 199, 121–124. doi: 10.1016/0014-5793(86)81236-4
Jaiswal, N., and Sharma, R. K. (1986). Dual regulation of adenylate cyclase and guanylate cyclase: alpha 2-adrenergic signal transduction in adrenocortical carcinoma cells. Arch. Biochem. Biophys. 249, 616–619. doi: 10.1016/0003-9861(86)90041-x
Jaleel, M., Saha, S., Shenoy, A. R., and Visweswariah, S. S. (2006). The kinase homology domain of receptor guanylyl cyclase C: ATP binding and identification of an adenine nucleotide sensitive site. Biochemistry 45, 1888–1898. doi: 10.1021/bi052089x
Jankowska, A., Burczynska, B., Duda, T., Warchol, J. B., and Sharma, R. K. (2007). Calcium-modulated rod outer segment membrane guanylate cyclase type 1 transduction machinery in the testes. J. Androl. 28, 50–58. doi: 10.2164/jandrol.106.000182
Jankowska, A., Sharma, R. K., and Duda, T. (2014). Ca2+-modulated ROS-GC1 transduction system in testes and its presence in the spermatogenic cells. Front. Mol. Neurosci. 7:34. doi: 10.3389/fnmol.2014.00034
Jankowska, A., and Warchol, J. B. (2010). Ca2+-modulated membrane guanylate cyclase in the testes. Mol. Cell. Biochem. 334, 169–179. doi: 10.1007/s11010-009-0329-5
Jeromin, A., Shayan, A. J., Msghina, M., Roder, J., and Atwood, H. L. (1999). Crustacean frequenins: molecular cloning and differential localization at neuromuscular junctions. J. Neurobiol. 41, 165–175. doi: 10.1002/(SICI)1097-4695(19991105)41:2<165::AID-NEU1 >3.0.CO;2-9
Jewett, J. R., Koller, K. J., Goeddel, D. V., and Lowe, D. G. (1993). Hormonal induction of low affinity receptor guanylyl cyclase. EMBO J. 12, 769–777.
Juilfs, D. M., Fulle, H. J., Zhao, A. Z., Houslay, M. D., Garbers, D. L., and Beavo, J. A. (1997). A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc. Natl. Acad. Sci. U S A 94, 3388–3395. doi: 10.1073/pnas.94.7.3388
Kachi, S., Nishizawa, Y., Olshevskaya, E., Yamazaki, A., Miyake, Y., Wakabayashi, T., et al. (1999). Detailed localization of photoreceptor guanylate cyclase activating protein-1 and -2 in mammalian retinas using light and electron microscopy. Exp. Eye Res. 68, 465–473. doi: 10.1006/exer.1998.0629
Kitabchi, A. E., Wilson, D. B., and Sharma, R. K. (1971). Steroidogenesis in isolated adrenal cells of rat. II. Effect of caffeine on ACTH and cyclic nucleotide-induced steroidogenesis and its relation to cyclic nucleotide phosphodiesterase (PDE). Biochem. Biophys. Res. Commun. 44, 898–904. doi: 10.1016/0006-291X(71)90796-0
Koch, K. W. (1991). Purification and identification of photoreceptor guanylate cyclase. J. Biol. Chem. 266, 8634–8637.
Koch, K. W., and Dell’Orco, D. (2013). A calcium-relay mechanism in vertebrate phototransduction. ACS Chem. Neurosci. 4, 909–917. doi: 10.1021/cn400027z
Koch, K. W., and Dell’Orco, D. (2015). Protein and signaling networks in vertebrate photoreceptor cells. Front. Mol. Neurosci. 8:67. doi: 10.3389/fnmol.2015.00067
Koch, K. W., Duda, T., and Sharma, R. K. (2010). Ca2+-modulated vision-linked ROS-GC guanylate cyclase transduction machinery. Mol. Cell. Biochem. 334, 105–115. doi: 10.1007/s11010-009-0330-z
Koch, K. W., and Stryer, L. (1988). Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature 334, 64–66. doi: 10.1038/334064a0
Koller, K. J., de Sauvage, F. J., Lowe, D. G., and Goeddel, D. V. (1992). Conservation of the kinaselike regulatory domain is essential for activation of the natriuretic peptide receptor guanylyl cyclases. Mol. Cell. Biol. 12, 2581–2590. doi: 10.1128/mcb.12.6.2581
Krishnan, A., Duda, T., Pertzev, A., Kobayashi, M., Takamatsu, K., and Sharma, R. K. (2009). Hippocalcin, new Ca2+ sensor of a ROS-GC subfamily member, ONE-GC, membrane guanylate cyclase transduction system. Mol. Cell. Biochem. 325, 1–14. doi: 10.1007/s11010-008-0015-z
Krishnan, A., Goraczniak, R. M., Duda, T., and Sharma, R. K. (1998). Third calcium-modulated rod outer segment membrane guanylate cyclase transduction mechanism. Mol. Cell. Biochem. 178, 251–259. doi: 10.1023/A:1006860018300
Krishnan, A., Venkataraman, V., Fik-Rymarkiewicz, E., Duda, T., and Sharma, R. K. (2004). Structural, biochemical and functional characterization of the calcium sensor neurocalcin delta in the inner retinal neurons and its linkage with the rod outer segment membrane guanylate cyclase transduction system. Biochemistry 43, 2708–2723. doi: 10.1021/bi035631v
Kuhn, M. (2016). Molecular physiology of membrane guanylyl cyclase receptors. Physiol. Rev. 96, 751–804. doi: 10.1152/physrev.00022.2015
Kuhn, M., Ng, C. K., Su, Y. H., Kilić, A., Mitko, D., Bien-Ly, N., et al. (2004). Identification of an orphan guanylate cyclase receptor selectively expressed in mouse testis. Biochem. J. 379, 385–393. doi: 10.1042/bj20031624
Kumar, V. D., Vijay-Kumar, S., Krishnan, A., Duda, T., and Sharma, R. K. (1999). A second calcium regulator of rod outer segment membrane guanylate cyclase, ROS-GC1: neurocalcin. Biochemistry 38, 12614–12620. doi: 10.1021/bi990851n
Ladant, D. (1995). Calcium and membrane binding properties of bovine neurocalcin delta expressed in Escherichia coli. J. Biol. Chem. 270, 3179–3185.
Lai, P. C., Singer, M. S., and Crasto, C. J. (2005). Structural activation pathways from dynamic olfactory receptor-odorant interactions. Chem. Senses 30, 781–792. doi: 10.1093/chemse/bji070
Lange, C., Duda, T., Beyermann, M., Sharma, R. K., and Koch, K. W. (1999). Regions in vertebrate photoreceptor guanylyl cyclase ROS-GC1 involved in Ca2+-dependent regulation by guanylyl cyclase-activating protein GCAP-1. FEBS Lett. 460, 27–31. doi: 10.1016/s0014-5793(99)01312-5
Langub, M. C. Jr., Watson, R. E. Jr., and Herman, J. P. (1995). Distribution of natriuretic peptide precursor mRNAs in the rat brain. J. Comp. Neurol. 356, 183–199. doi: 10.1002/cne.903560205
Larose, L., McNicoll, N., Ong, H., and De Léan, A. (1991). Allosteric modulation by ATP of the bovine adrenal natriuretic factor R1 receptor functions. Biochemistry 30, 8990–8995. doi: 10.1021/bi00101a012
Laychock, S. G., and Hardman, J. G. (1978). Effects of sodium nitroprusside and ascorbic acid on rat adrenocortical cell cGMP levels and steroidogenesis. J. Cyclic Nucleotide Res. 4, 335–344.
Leinders-Zufall, T., Cockerham, R. E., Michalakis, S., Biel, M., Garbers, D. L., Reed, R. R., et al. (2007). Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc. Natl. Acad. Sci. U S A 104, 14507–14512. doi: 10.1073/pnas.0704965104
Lim, S., Dizhoor, A. M., and Ames, J. B. (2014). Structural diversity of neuronal calcium sensor proteins and insights for activation of retinal guanylyl cyclase by GCAP1. Front. Mol. Neurosci. 7:19. doi: 10.3389/fnmol.2014.00019
Lim, S., Strahl, T., Thorner, J., and Ames, J. B. (2011). Structure of a Ca2+-myristoyl switch protein that controls activation of a phosphatidylinositol 4-kinase in fission yeast. J. Biol. Chem. 286, 12565–12577. doi: 10.1074/jbc.M110.208868
Liu, X., Seno, K., Nishizawa, Y., Hayashi, F., Yamazaki, A., Matsumoto, H., et al. (1994). Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp. Eye Res. 59, 761–768. doi: 10.1006/exer.1994.1162
Lowe, D. G., Chang, M. S., Hellmiss, R., Chen, E., Singh, S., Garbers, D. L., et al. (1989). Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 8, 1377–1384.
Lowe, D. G., Dizhoor, A. M., Liu, K., Gu, Q., Spencer, M., Laura, R., et al. (1995). Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc. Natl. Acad. Sci. U S A 92, 5535–5539. doi: 10.1073/pnas.92.12.5535
Makino, C. L., Peshenko, I. V., Wen, X. H., Olshevskaya, E. V., Barrett, R., and Dizhoor, A. M. (2008). A role for GCAP2 in regulating the photoresponse. Guanylyl cyclase activation and rod electrophysiology in GUCA1B knock-out mice. J. Biol. Chem. 283, 29135–29143. doi: 10.1074/jbc.M804445200
Makino, C. L., Wen, X. H., Olshevskaya, E. V., Peshenko, I. V., Savchenko, A. B., and Dizhoor, A. M. (2012). Enzymatic relay mechanism stimulates cyclic GMP synthesis in rod photoresponse: biochemical and physiological study in guanylyl cyclase activating protein 1 knockout mice. PLoS One 7:e47637. doi: 10.1371/journal.pone.0047637
Marala, R., Duda, T., Goraczniak, R. M., and Sharma, R. K. (1992). Genetically tailored atrial natriuretic factor-dependent guanylate cyclase. Immunological and functional identity with 180 kDa membrane guanylate cyclase and ATP signaling site. FEBS Lett. 296, 254–258. doi: 10.1016/0014-5793(92)80298-u
Marala, R. B., and Sharma, R. K. (1988). Characterization of atrial-natriuretic-factor-receptor-coupled membrane guanylate cyclase from rat and mouse testes. Biochem. J. 251, 301–304. doi: 10.1042/bj2510301
Marala, R. B., Sitaramayya, A., and Sharma, R. K. (1991). Dual regulation of atrial natriuretic factor-dependent guanylate cyclase activity by ATP. FEBS Lett. 281, 73–76. doi: 10.1016/0014-5793(91)80361-6
Margulis, A., Goraczniak, R. M., Duda, T., Sharma, R. K., and Sitaramayya, A. (1993). Structural and biochemical identity of retinal rod outer segment membrane guanylate cyclase. Biochem. Biophys. Res. Commun. 194, 855–861. doi: 10.1006/bbrc.1993.1900
Margulis, A., Pozdnyakov, N., and Sitaramayya, A. (1996). Activation of bovine photoreceptor guanylate cyclase by S100 proteins. Biochem. Biophys. Res. Commun. 218, 243–247. doi: 10.1006/bbrc.1996.0343
McFerran, B. W., Weiss, J. L., and Burgoyne, R. D. (1999). Neuronal Ca2+ sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca2+-independent membrane association and interaction with binding proteins, suggesting a role in rapid Ca2+ signal transduction. J. Biol. Chem. 274, 30258–30265. doi: 10.1074/jbc.274.42.30258
Meloche, S., McNicoll, N., Liu, B., Ong, H., and De Léan, A. (1988). Atrial natriuretic factor R1 receptor from bovine adrenal zona glomerulosa: purification, characterization and modulation by amiloride. Biochemistry 27, 8151–8158. doi: 10.1021/bi00421a025
Mendez, A., Burns, M. E., Sokal, I., Dizhoor, A. M., Baehr, W., Palczewski, K., et al. (2001). Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. U S A 98, 9948–9953. doi: 10.1073/pnas.171308998
Meyer, M. R., Angele, A., Kremmer, E., Kaupp, U. B., and Muller, F. (2000). A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc. Natl. Acad. Sci. U S A 97, 10595–10600. doi: 10.1073/pnas.97.19.10595
Middendorff, R., Davidoff, M. S., Behrends, S., Mewe, M., Miethens, A., and Müller, D. (2000). Multiple roles of the messenger molecule cGMP in testicular function. Andrologia 32, 55–59.
Middendorff, R., Maronde, E., Paust, H. J., Müller, D., Davidoff, M., and Olcese, J. (1996). Expression of C-type natriuretic peptide in the bovine pineal gland. J. Neurochem. 67, 517–524. doi: 10.1046/j.1471-4159.1996.67020517.x
Middendorff, R., Müller, D., Wichers, S., Holstein, A. F., and Davidoff, M. S. (1997). Evidence for production and functional activity of nitric oxide in seminiferous tubules and blood vessels of the human testis. J. Clin. Endocrinol. Metab. 82, 4154–4161. doi: 10.1210/jc.82.12.4154
Mourdjeva, M., Russinova, A., Kyurkchiev, S., and Kehayov, I. (2001). Spatial and temporal distribution of atrial natriuretic factor in the rat testis. Biol. Cell 93, 301–307. doi: 10.1016/s0248-4900(01)01119-4
Murad, F., Arnold, W. P., Mittal, C. K., and Braughler, J. M. (1979). Properties and regulation of guanylate cyclase and some proposed functions for cyclic GMP. Adv. Cyclic Nucleotide Res. 11, 175–204.
Nakano, A., Terasawa, M., Watanabe, M., Okazaki, K., Inoue, S., Kato, M., et al. (1993). Distinct regional localization of neurocalcin, a Ca2+-binding protein, in the bovine adrenal gland. J. Endocrinol. 138, 283–290. doi: 10.1677/joe.0.1380283
Nambi, P., Aiyar, N. V., Roberts, A. N., and Sharma, R. K. (1982a). Relationship of calcium and membrane guanylate cyclase in adrenocorticotropin-induced steroidogenesis. Endocrinology 111, 196–200. doi: 10.1210/endo-111-1-196
Nambi, P., Aiyar, N. V., and Sharma, R. K. (1982b). Adrenocorticotropin-dependent particulate guanylate cyclase in rat adrenal and adrenocortical carcinoma: comparison of its properties with soluble guanylate cyclase and its relationship with ACTH-induced steroidogenesis. Arch. Biochem. Biophys. 217, 638–646. doi: 10.1016/0003-9861(82)90545-8
Nambi, P., and Sharma, R. K. (1981a). Adrenocorticotropic hormone-responsive guanylate cyclase in the particulate fraction of rat adrenal glands. Endocrinology 108, 2025–2027. doi: 10.1210/endo-108-5-2025
Nambi, P., and Sharma, R. K. (1981b). Demonstration of ACTH-sensitive particulate guanylate cyclase in adrenocortical carcinoma. Biochem. Biophys. Res. Commun. 100, 508–514. doi: 10.1016/s0006-291x(81)80206-9
Nef, S., Fiumelli, H., de Castro, E., Raes, M. B., and Nef, P. (1995). Identification of neuronal calcium sensor (NCS-1) possibly involved in the regulation of receptor phosphorylation. J. Recept. Signal Transduct. Res. 15, 365–378. doi: 10.3109/10799899509045227
Ogawa, H., Qiu, Y., Huang, L., Tam-Chang, S. W., Young, H. S., and Misono, K. S. (2009). Structure of the atrial natriuretic peptide receptor extracellular domain in the unbound and hormone-bound states by single-particle electron microscopy. FEBS J. 276, 1347–1355. doi: 10.1111/j.1742-4658.2009.06870.x
Ogawa, H., Qiu, Y., Ogata, C. M., and Misono, K. S. (2004). Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: rotation mechanism for transmembrane signal transduction. J. Biol. Chem. 279, 28625–28631. doi: 10.1074/jbc.m313222200
Okazaki, K., Watanabe, M., Ando, Y., Hagiwara, M., Terasawa, M., and Hidaka, H. (1992). Full sequence of neurocalcin, a novel calcium-binding protein abundant in central nervous system. Biochem. Biophys. Res. Commun. 185, 147–153. doi: 10.1016/s0006-291x(05)80968-4
Olafsson, P., Soares, H. D., Herzog, K. H., Wang, T., Morgan, J. I., and Lu, B. (1997). The Ca2+ binding protein, frequenin is a nervous system-specific protein in mouse preferentially localized in neurites. Brain Res. Mol. Brain Res. 44, 73–82. doi: 10.1016/s0169-328x(96)00188-x
Olafsson, P., Wang, T., and Lu, B. (1995). Molecular cloning and functional characterization of the Xenopus Ca2+-binding protein frequenin. Proc. Natl. Acad. Sci. U S A 92, 8001–8005. doi: 10.1073/pnas.92.17.8001
Olcese, J., Müller, D., Münker, M., and Schmidt, C. (1994). Natriuretic peptides elevate cyclic 3′,5′-guanosine monophosphate levels in cultured rat pinealocytes: evidence for guanylate cyclase-linked membrane receptors. Mol. Cell. Endocrinol. 103, 95–100. doi: 10.1016/0303-7207(94)90074-4
Palczewski, K., Subbaraya, I., Gorczyca, W. A., Helekar, B. S., Ruiz, C. C., Ohguro, H., et al. (1994). Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron 13, 395–404. doi: 10.1016/0896-6273(94)90355-7
Pandey, K. N., Oliver, P. M., Maeda, N., and Smithies, O. (1999). Hypertension associated with decreased testosterone levels in natriuretic peptide receptor-A gene-knockout and gene-duplicated mutant mouse models. Endocrinology 140, 5112–5119. doi: 10.1210/en.140.11.5112
Pandey, K. N., and Singh, S. (1990). Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J. Biol. Chem. 265, 12342–12348.
Parat, M., Blanchet, J., and De Léan, A. (2010). Role of juxtamembrane and transmembrane domains in the mechanism of natriuretic peptide receptor A activation. Biochemistry 49, 4601–4610. doi: 10.1021/bi901711w
Paul, A. K. (1986). Particulate Guanylate Cyclase from Adrenocortical Carcinoma 494. Purification, Biochemical and Immunological Characterization. [Dissertation]. Memphis, TN: University of Tennessee
Paul, A. K., Marala, R. B., Jaiswal, R. K., and Sharma, R. K. (1987). Coexistence of guanylate cyclase and atrial natriuretic factor receptor in a 180-kD protein. Science 235, 1224–1226. doi: 10.1126/science.2881352
Perchellet, J. P., Shanker, G., and Sharma, R. (1978). Regulatory role of guanosine 3′,5′-monophosphate in adrenocorticotropin hormone-induced steroidogenesis. Science 199, 311–312. doi: 10.1126/science.202028
Perchellet, J. P., and Sharma, R. K. (1979). Mediatory role of calcium and guanosine 3′,5′-monophosphate in adrenocorticotropin-induced steroidogenesis by adrenal cells. Science 203, 1259–1261. doi: 10.1126/science.34216
Perchellet, J. P., and Sharma, R. K. (1980). Ectopic α-adrenergic mediated accumulation of guanosine 3′,5′-monophosphate in isolated adrenocortical carcinoma cells. Endocrinology 106, 1589–1593. doi: 10.1210/endo-106-5-1589
Perrault, I., Rozet, J. M., Calvas, P., Gerber, S., Camuzat, A., Dollfus, H., et al. (1996). Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat. Genet. 14, 461–464. doi: 10.1038/ng1296-461
Perrault, I., Rozet, J. M., Gerber, S., Ghazi, I., Leowski, C., Ducroq, D., et al. (1999). Leber congenital amaurosis. Mol. Genet. Metab. 68, 200–208. doi: 10.1006/mgme.1999.2906
Pertzev, A., Duda, T., and Sharma, R. K. (2010). Ca2+ sensor GCAP1: a constitutive element of the ONE-GC-modulated odorant signal transduction pathway. Biochemistry 49, 7303–7313. doi: 10.1021/bi101001v
Pongs, O., Lindemeier, J., Zhu, X. R., Theil, T., Engelkamp, D., Krah-Jentgens, I., et al. (1993). Frequenin–a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron 1, 15–28. doi: 10.1016/0896-6273(93)90267-u
Potter, L. R., and Garbers, D. L. (1992). Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J. Biol. Chem. 267, 14531–14534.
Potter, L. R., and Hunter, T. (1998). Identification and characterization of the major phosphorylation sites of the B-type natriuretic peptide receptor. J. Biol. Chem. 273, 15533–15539. doi: 10.1074/jbc.273.25.15533
Pozdnyakov, N., Goraczniak, R., Margulis, A., Duda, T., Sharma, R. K., Yoshida, A., et al. (1997). Structural and functional characterization of retinal calcium-dependent guanylate cyclase activator protein (CD-GCAP): identity with S100β protein. Biochemistry 36, 14159–14166. doi: 10.1021/bi971792l
Pozdnyakov, N., Yoshida, A., Cooper, N. G., Margulis, A., Duda, T., Sharma, R. K., et al. (1995). A novel calcium-dependent activator of retinal rod outer segment membrane guanylate cyclase. Biochemistry 34, 14279–14283. doi: 10.1021/bi00044a002
Pugh, E. N. Jr., and Cobbs, W. H. (1986). Visual transduction in vertebrate rods and cones: a tale of two transmitters, calcium and cyclic GMP. Vision Res. 26, 1613–1643. doi: 10.1016/0042-6989(86)90051-9
Pugh, E. N. Jr., Duda, T., Sitaramayya, A., and Sharma, R. K. (1997). Photoreceptor guanylate cyclases: a review. Biosci. Rep. 17, 429–473. doi: 10.1023/A:1027365520442
Rambotti, M. G., Giambanco, I., Spreca, A., and Donato, R. (1999). S100B and S100A1 proteins in bovine retina:their calcium-dependent stimulation of a membrane-bound guanylate cyclase activity as investigated by ultracytochemistry. Neuroscience 92, 1089–1101. doi: 10.1016/s0306-4522(99)00074-3
Rondeau, J. J., McNicoll, N., Gagnon, J., Bouchard, N., Ong, H., and De Lean, A. (1995). Stoichiometry of the atrial natriuretic factor-R1 receptor complex in the bovine zona glomerulosa. Biochemistry 34, 2130–2136. doi: 10.1021/bi00007a005
Rozet, J. M., Perrault, I., Gerber, S., Hanein, S., Barbet, F., Ducroq, D., et al. (2001). Complete abolition of the retinal-specific guanylyl cyclase (retGC-1) catalytic ability consistently leads to leber congenital amaurosis (LCA). Invest. Ophthalmol. Vis. Sci. 42, 1190–1192.
Sayers, G., Beall, R. J., and Seelig, S. (1972). Isolated adrenal cells: adrenocorticotropic hormone, calcium, steroidogenesis and cyclic adenosine monophosphate. Science 175, 1131–1133. doi: 10.1126/science.175.4026.1131
Schulz, S., Singh, S., Bellet, R. A., Singh, G., Tubb, D. J., Chin, H., et al. (1989). The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell 58, 1155–1162. doi: 10.1016/0092-8674(89)90513-8
Schulz, S., Wedel, B. J., Matthews, A., and Garbers, D. L. (1998). The cloning and expression of a new guanylyl cyclase orphan receptor. J. Biol. Chem. 273, 1032–1037. doi: 10.1074/jbc.273.2.1032
Shanker, G., and Sharma, R. K. (1980). Characterization of ectopic α-adrenergic binding receptors of adrenocortical carcinoma cells. Endocrinology 106, 1594–1598. doi: 10.1210/endo-106-5-1594
Sharma, R. K. (1972). Studies on adrenocortical carcinoma of rat cyclic nucleotide phosphodiesterase activities. Cancer Res. 32, 1734–1736.
Sharma, R. K. (1973). Metabolic regulation of steroidogenesis in adrenocortical carcinoma cells of rat. Effect of adrenocorticotropin and adenosine cyclic 3′:5′-monophosphate on corticosteroidogenesis. Eur. J. Biochem. 32, 506–512. doi: 10.1111/j.1432-1033.1973.tb02635.x
Sharma, R. K. (1978). “Abnormal adrenocorticotropic hormone control in adrenocortical carcinoma,” in Endocrine Control in Neoplasia, eds. R. K. Sharma and W. E. Criss (New York, NY: Raven Press), 13–52.
Sharma, R. K. (1985). “Biochemical control in adrenocortical carcinoma,” in Hormonally Responsive Tumors, ed. V. P. Hollander (New York, NY: Academic Press), 185–217.
Sharma, R. K. (1988). Guanylate cyclase and the atrial natriuretic factor receptor. Reply. Science 240, 805–806. doi: 10.1126/science.240.4853.805-a
Sharma, R. K. (2002). Evolution of the membrane guanylate cyclase transduction system. Mol. Cell. Biochem. 230, 3–30. doi: 10.1007/978-1-4615-0927-1_1
Sharma, R. K. (2010). Membrane guanylate cyclase is a beautiful signal transduction machine: overview. Mol. Cell. Biochem. 334, 3–36. doi: 10.1007/s11010-009-0336-6
Sharma, R. K., Ahmed, N. K., and Shanker, G. (1976). Metabolic regulation of steroidogenesis in isolated adrenal cells of rat. Relationship of adrenocorticotropin-, adenosine 3′:5′-monophosphate-and guanosine 3′:5′-monophosphate-stimulated steroidogenesis with the activation of protein kinase. Eur. J. Biochem. 70, 427–433. doi: 10.1111/j.1432-1033.1976.tb11033.x
Sharma, R. K., Ahmed, N. K., Sutliff, L. S., and Brush, J. S. (1974). Metabolic regulation of steroidogenesis in isolated adrenal cells of the rat. ACTH regulation of cGMP and cAMP levels and steroidogenesis. FEBS Lett. 45, 107–110. doi: 10.1016/0014-5793(74)80822-7
Sharma, R. K., Baehr, W., Makino, C. L., and Duda, T. (2015). Ca2+ and Ca2+-interlocked membrane guanylate cyclase signal modulation of neuronal and cardiovascular signal transduction. Front. Mol. Neurosci. 8:7. doi: 10.3389/fnmol.2015.00007
Sharma, R. K., and Duda, T. (1997). Plasma membrane guanylate cyclase. A multimodule transduction system. Adv. Exp. Med. Biol. 407, 271–279. doi: 10.1007/978-1-4899-1813-0_41
Sharma, R. K., and Duda, T. (2010). Odorant-linked ROS-GC subfamily membrane guanylate cyclase transduction system. Mol. Cell. Biochem. 334, 181–189. doi: 10.1007/s11010-009-0333-9
Sharma, R. K., and Duda, T. (2012). Ca2+-sensors and ROS-GC: interlocked sensory transduction elements: a review. Front. Mol. Neurosci. 5:42. doi: 10.3389/fnmol.2012.00042
Sharma, R. K., and Duda, T. (2014a). Membrane guanylate cyclase, a multimodal transduction machine: history, present and future directions. Front. Mol. Neurosci. 7:56. doi: 10.3389/fnmol.2014.00056
Sharma, R. K., and Duda, T. (2014b). “Atrial natriuretic factor receptor guanylate cyclase. The journey,” in Natriuretic Peptides. Physiology, Molecular Biology and Clinical Applications, ed. K. N. Pandey (New York, NY: Nova Science Publishers), 109–118.
Sharma, R. K., Duda, T., and Marala, R. B. (1989a). “A novel model depicting the protein kinase C regulation of Atrial Natriuretic Factor-dependent 180-kDa membrane guanylate cyclase,” in Advances in Atrial Peptide Research, American Society of Hypertension, Symposium Series, (Vol. 3), eds. B. M. Brenner and J. H. Laragh (New York, NY: Raven Press), 43–52.
Sharma, R. K., Marala, R. B., and Duda, T. M. (1989b). Purification and characterization of the 180-kDa membrane guanylate cyclase containing atrial natriuretic factor receptor from rat adrenal gland its regulation by protein kinase C. Steroids 53, 437–460. doi: 10.1016/0039-128x(89)90024-x
Sharma, R. K., Duda, T., Venkataraman, V., and Koch, K.-W. (2004). Calcium-modulated mammalian membrane guanylate cyclase ROS-GC transduction machinery in sensory neurons: a universal concept. Curr. Topics Biochem. Res. 6, 111–144.
Sharma, R. K., Jaiswal, R. K., and Duda, T. (1988a). “Second messenger role of cyclic GMP in atrial natriuretic factor receptor mediated signal transduction: 180-kDa membrane guanylate cyclase, its coupling with atrial natriuretic factor receptor and its regulation by protein kinase C,” in Biological and Molecular Aspects of Atrial Factors. UCLA Symposia on Molecular and Cellular Biology, New Series, (Vol. 81), ed. P. Needleman (New York, NY: Alan R. Liss, Inc.), 77–96.
Sharma, R. K., Marala, R. B., and Paul, A. K. (1988b). “Mediatory role of cyclic GMP in receptor mediated signal transduction: membrane guanylate cyclase and its coupling with atrial natriuretic factor receptor,” in Advances in Atrial Peptide Research, American Society of Hypertension, Symposium series (Vol. 2), eds. B. M. Brenner and J. H. Laragh (New York, NY: Raven Press), 61–77.
Sharma, R. K., Makino, C. L., Hicks, D., and Duda, T. (2014). ROS-GC interlocked Ca2+-sensor S100B protein signaling in cone photoreceptors: review. Front. Mol. Neurosci. 7:21. doi: 10.3389/fnmol.2014.00021
Sharma, R. K., and Sawhney, R. S. (1978). Metabolic regulation of steroidogenesis in isolated adrenal cell. Investigation of the adrenocorticotropic hormone, guanosine 3′,5′-monophosphate and adenosine 3′,5′-monophosphate control step. Biochemistry 17, 316–321. doi: 10.1021/bi00595a019
Sharma, R. K., Yadav, P., and Duda, T. (2001). Allosteric regulatory step and configuration of the ATP-binding pocket in atrial natriuretic factor receptor guanylate cyclase transduction mechanism. Can. J. Physiol. Pharmacol. 79, 682–691. doi: 10.1139/y01-033
Shi, S. J., Nguyen, H. T., Sharma, G. D., Navar, L. G., and Pandey, K. N. (2001). Genetic disruption of atrial natriuretic peptide receptor-A alters renin and angiotensin II levels. Am. J. Physiol. Renal Physiol. 281, F665–F673.
Shyjan, A. W., de Sauvage, F. J., Gillett, N. A., Goeddel, D. V., and Lowe, D. G. (1992). Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron 9, 727–737. doi: 10.1016/0896-6273(92)90035-c
Singh, S., Lowe, D. G., Thorpe, D. S., Rodriguez, H., Kuang, W. J., Dangott, L. J., et al. (1988). Membrane guanylate cyclase is a cell-surface receptor with homology to protein kinases. Nature 334, 708–712. doi: 10.1038/334708a0
Stryer, L. (1986). Cyclic GMP cascade of vision. Annu. Rev. Neurosci. 9, 87–119. doi: 10.1146/annurev.neuro.9.1.87
Subbaraya, I., Ruiz, C. C., Helekar, B. S., Zhao, X., Gorczyca, W. A., Pettenati, M. J., et al. (1994). Molecular characterization of human and mouse photoreceptor guanylate cyclase-activating protein (GCAP) and chromosomal localization of the human gene. J. Biol. Chem. 269, 31080–31089.
Sun, L., Wang, H., Hu, J., Han, J., Matsunami, H., and Luo, M. (2009). Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc. Natl. Acad. Sci. U S A 106, 2041–2046. doi: 10.1073/pnas.0812220106
Takayanagi, R., Snajdar, R. M., Imada, T., Tamura, M., Pandey, K. N., Misono, K. S., et al. (1987). Purification and characterization of two types of atrial natriuretic factor receptors from bovine adrenal cortex: guanylate cyclase-linked and cyclase-free receptors. Biochem. Biophys. Res. Commun. 144, 244–250. doi: 10.1016/s0006-291x(87)80502-8
Takeishi, A., Yu, Y. V., Hapiak, V. M., Bell, H. W., O’Leary, T., and Sengupta, P. (2016). Receptor-type guanylyl cyclases confer thermosensory responses in. C. elegans. Neuron 90, 235–244. doi: 10.1016/j.neuron.2016.03.002
Terasawa, M., Nakano, A., Kobayashi, R., and Hidaka, H. (1992). Neurocalcin: a novel calcium-binding protein from bovine brain. J. Biol. Chem. 267, 19596–19599.
van den Akker, F., Zhang, X., Miyagi, M., Huo, X., Misono, K. S., and Yee, V. C. (2000). Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature 406, 101–104. doi: 10.1038/35017602
Venkataraman, V., Duda, T., Ravichandran, S., and Sharma, R. K. (2008). Neurocalcin delta modulation of ROS-GC1, a new model of Ca2+ signaling. Biochemistry 47, 6590–6601. doi: 10.1021/bi800394s
Venkataraman, V., Duda, T., and Sharma, R. K. (1998). The alpha(2D/A)-adrenergic receptor-linked membrane guanylate cyclase: a new signal transduction system in the pineal gland. FEBS Lett. 427, 69–73. doi: 10.1016/s0014-5793(98)00396-2
Venkataraman, V., Duda, T., Vardi, N., Koch, K. W., and Sharma, R. K. (2003). Calcium-modulated guanylate cyclase transduction machinery in the photoreceptor–bipolar synaptic region. Biochemistry 42, 5640–5648. doi: 10.1021/bi034025x
Venkataraman, V., Nagele, R., Duda, T., and Sharma, R. K. (2000). Rod outer segment membrane guanylate cyclase type 1-linked stimulatory and inhibitory calcium signaling systems in the pineal gland: biochemical, molecular and immunohistochemical evidence. Biochemistry 39, 6042–6052. doi: 10.1021/bi9929960
Vijay-Kumar, S., and Kumar, V. D. (1999). Crystal structure of recombinant bovine neurocalcin. Nat. Struct. Biol. 6, 80–88. doi: 10.10.1038/4956
Wen, X. H., Dizhoor, A. M., and Makino, C. L. (2014). Membrane guanylyl cyclase complexes shape the photoresponses of retinal rods and cones. Front. Mol. Neurosci. 7:45. doi: 10.3389/fnmol.2014.00045
Wen, X. H., Duda, T., Pertzev, A., Venkataraman, V., Makino, C. L., and Sharma, R. K. (2012). S100B serves as a Ca2+ sensor for ROS-GC1 guanylate cyclase in cones but not in rods of the murine retina. Cell. Physiol. Biochem. 29, 417–430. doi: 10.1159/000338496
Woodruff, M. L., Sampath, A. P., Matthews, H. R., Krasnoperova, N. V., Lem, J., and Fain, G. L. (2002). Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 542, 843–854. doi: 10.1113/jphysiol.2001.013987
Xu, J., Morris, L., Thapa, A., Ma, H., Michalakis, S., Biel, M., et al. (2013). cGMP accumulation causes photoreceptor degeneration in CNG channel deficiency: evidence of cGMP cytotoxicity independently of enhanced CNG channel function. J. Neurosci. 33, 14939–14948. doi: 10.1523/JNEUROSCI.0909-13.2013
Yang, R. B., Au, H. K., Tzeng, C. R., Tsai, M. T., Wu, P., Wu, Y. C., et al. (2010). Characterization of a novel cell-surface protein expressed on human sperm. Hum. Reprod. 25, 42–51. doi: 10.1093/humrep/dep359
Yang, R. B., and Garbers, D. L. (1997). Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J. Biol. Chem. 272, 13738–13742. doi: 10.1074/jbc.272.21.13738
Young, J. M., Waters, H., Dong, C., Fülle, H. J., and Liman, E. R. (2007). Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS One 2:e884. doi: 10.1371/journal.pone.0000884
Keywords: membrane guanylate cyclase, cyclic GMP, signal transduction, ANF-RGC, ROS-GC, ONE-GC, neuronal calcium sensor, bicarbonate
Citation: Sharma RK, Duda T and Makino CL (2016) Integrative Signaling Networks of Membrane Guanylate Cyclases: Biochemistry and Physiology. Front. Mol. Neurosci. 9:83. doi: 10.3389/fnmol.2016.00083
Received: 13 July 2016; Accepted: 29 August 2016;
Published: 15 September 2016.
Edited by:
Jean-Marc Taymans, INSERM, FranceCopyright © 2016 Sharma, Duda and Makino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rameshwar K. Sharma, cnNoYXJtYUBzYWx1cy5lZHU=
 Rameshwar K. Sharma
Rameshwar K. Sharma Teresa Duda
Teresa Duda Clint L. Makino
Clint L. Makino