
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Integr. Neurosci., 21 November 2022
Volume 16 - 2022 | https://doi.org/10.3389/fnint.2022.989029
This article is part of the Research TopicMechanisms of Orofacial Pain and Sex Differences Volume IIView all 4 articles
Gliomas are the most common primary malignant brain tumors and are highly aggressive. Invasion and migration are the main causes of poor prognosis and treatment resistance in gliomas. As migration and invasion occur, patient survival and prognosis decline dramatically. MicroRNAs (miRNAs) are small, non-coding 21–23 nucleotides involved in regulating the malignant phenotype of gliomas, including migration and invasion. Numerous studies have demonstrated the mechanism and function of some miRNAs in glioma migration and invasion. However, the biological and clinical significance (including diagnosis, prognosis, and targeted therapy) of glioma migration and invasion-related miRNAs have not been systematically discussed. This paper reviews the progress of miRNAs-mediated migration and invasion studies in glioma and discusses the clinical value of migration and invasion-related miRNAs as potential biomarkers or targeted therapies for glioma. In addition, these findings are expected to translate into future directions and challenges for clinical applications. Although many biomarkers and their biological roles in glioma invasion and migration have been identified, none have been specific so far, and further exploration of clinical treatment is still in progress; therefore, we aimed to further identify specific markers that may guide clinical treatment and improve the quality of patient survival.
Glioma is the most common primary central malignancy, accounting for 80% of all malignant tumors of the brain (Siegel et al., 2015; Torre et al., 2015). The WHO classifies gliomas into low-grade groups (grades I and II) and high-grade groups (grades III and IV) (Huo et al., 2020). Fatal aggressiveness and a high recurrence rate are among its malignant features (Katakowski et al., 2010; Zhou et al., 2013). Despite the increasing sophistication of conventional treatments such as surgical resection and radiotherapy (de Groot et al., 2010), the prognosis is often extremely poor due to its malignant behavior, as well (Parker et al., 2013). Therefore, elucidation of the mechanisms of glioma progression is urgently needed to improve clinical outcomes.
High aggressiveness leads to poor prognosis and treatment resistance in patients with glioma. Clarifying the mechanisms of glioma aggressiveness will help improve patient prognosis. Important factors affecting tumor invasion and migration include Epithelial-to-mesenchymal transition (EMT), hypoxia, angiogenesis, and tumor microenvironment (TME). EMT is the process of epithelial to mesenchymal transition (Wurdinger et al., 2008). By regulating genetic programming, hypoxia allows cancer cells to acquire a more aggressive phenotype, which in turn allows body cells to adapt to the hypoxic environment (Kim et al., 2018). Angiogenesis is an important basis for the growth of solid tumors, including gliomas, and is closely related to the migration and invasion of gliomas (Folkman, 1971; Poleszczuk et al., 2015; Dai et al., 2018). Additionally, the tumors microenvironment is also crucial for tumors progression and invasion (Ren et al., 2014). Changes in cancer cell metabolism can increase tumors cell acid production, leading to normal cell death and protease hydrolase degradation of the extracellular matrix, thereby enhancing the migration and invasion ability of cancer cells (Williams et al., 1999; Lardner, 2001; Gatenby and Gawlinski, 2003). In addition, cell adhesion molecules, such as L1 cell adhesion molecule (L1CAM), can stimulate cell motility, proliferation, and invasion by interacting with two binding partners, integrins and fibroblast growth factor receptors (FGFRs) (Mohammadi et al., 1998; Bansal et al., 2003).
MicroRNAs (miRNA) are non-coding RNAs (ncRNAs) that are widely found in eukaryotes and are ~22 nucleotides in length (Takahashi et al., 2009; Su et al., 2014; Michailidi et al., 2015). Regulates cellular functions, including migration and invasion, by binding to target mRNAs (Yang et al., 2011; Bovell et al., 2013; Kumar et al., 2013; Yin et al., 2015). Several studies have shown that alterations in miRNA expression can regulate apoptosis, proliferation, tumorigenesis, invasion, and migration, and can also be involved in the occurrence, development, and recurrence of gliomas (Godlewski et al., 2008; Lin et al., 2013; Singh et al., 2013; Yeh et al., 2013; Zhang et al., 2019). In gliomas, miRNAs are involved in invasion and migration through cellular processes related to interactions with EMT, hypoxia, angiogenesis, and the TME. Additionally, miRNAs are able to exert pro- or anti-cancer effects in different signaling pathways, including classical wnt/β-catenin, epidermal growth factor receptor (EGFR), and transforming growth factor (TGF-β), but the exact details are not yet clear. It is imperative to elucidate the mechanisms of miRNAs associated with glioma invasion and migration, to clarify the biomarkers and therapeutic targets associated with glioma, and to provide a theoretical basis for clinical treatment. Therefore, in this study, we aimed to further identify specific markers that may guide clinical treatment and improve the quality of patient survival.
miRNAs influence tumors development by regulating various biological processes, such as cell proliferation, apoptosis, migration, and invasion (Hu et al., 2017; Li et al., 2017). Based on the extensive miRNA literature related to glioma invasion and migration, the cellular processes known to be associated with invasion and migration can be classified into four categories: EMT, angiogenesis, hypoxia, and TME interactions (Figures 1, 2). The dysregulated miRNAs with clear targets and corresponding cellular processes are listed in Table 1.
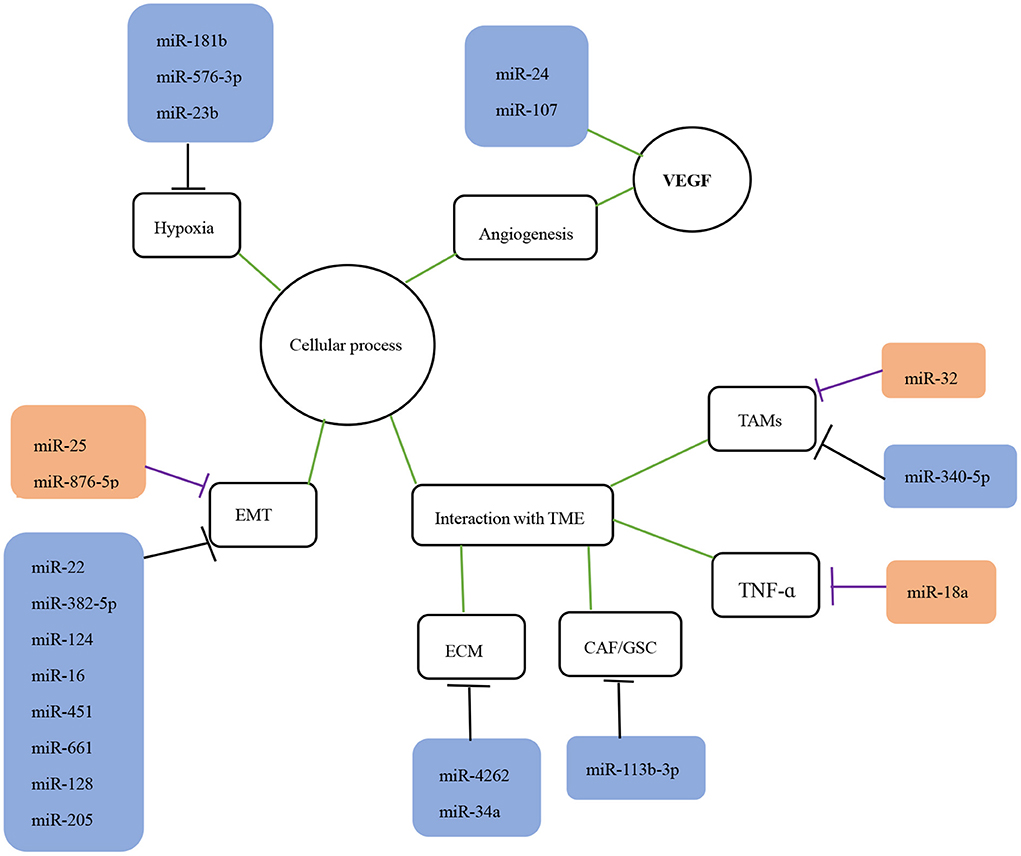
Figure 1. The role of miRNAs in the invasion and migration of glioma. The role of miRNAs in glioma migration and invasion is shown. The black lines indicate suppression while purple lines indicate promotion of downstream targets or processes. The green lines indicate interaction with corresponding processes or molecules. EMT, epithelial-mesenchymal transition; TME, tumor microenvironment; TAMS, tumor-associated macrophages; VEGF, vascular endothelial growth factor.
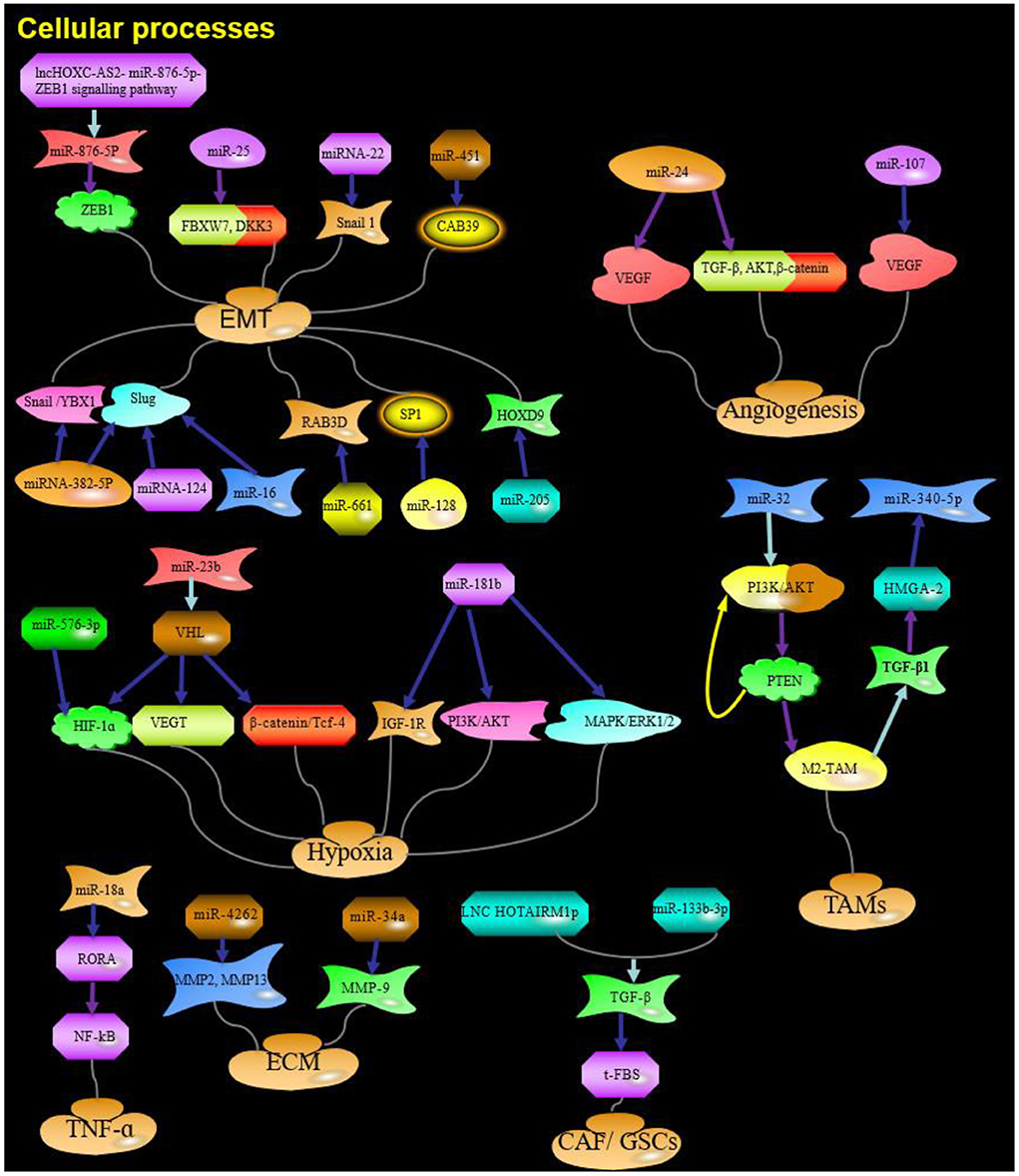
Figure 2. The role and targets of miRNA in cellular processes of glioma. Purple arrow indicates promotion; Blue arrows indicate suppression; Red arrows indicate positive feedback; Yellow arrows indicate negative feedback; Water green arrow indicates adjustment; ZEB1, zinc finger E-box binding homeobox 1;ZEB2, zinc finger E-box binding homeobox 2; F-box, F-box-containing; FBXW7, F-box-containing and WD repeat domain 7; DKK3, Dickkopf Wnt signaling pathway inhibitor 3; YBX1, Y box binding protein 1; HIF-1α, Hypoxia-inducible factor 1a; IGF-1R, insulin-like growth factor 1 receptor; HGS, hepatocyte growth factor-regulated tyrosine kinase substrate; TGF-β, transforming growth factor; PTEN, Phosphatase and tensin homolog; RORA, Retinoic acid receptor-related orphan receptor A; HOTAIRM1, HOXA transcript antisense RNA, myeloid-specific 1; ECM, extracellular matrix; Red and yellow arrows: Feedback.
Epithelial-to-mesenchymal transition is a well-studied physiological process, based on its typical features of imbalanced expression of epithelial markers (E-cadherin) and mesenchymal markers (N-cadherin and Vimentin) leading to a more aggressive or metastatic phenotype (Yang et al., 2008; Thiery et al., 2009), which is the reason they function as glioma-promoting genes. Studies have confirmed that EMT plays an important role in the migration and invasive activity of tumor cells (Wang Q. et al., 2014; Chen et al., 2019). Its transcription factors include zinc finger E-box binding homeobox 1 (ZEB1), Snail, Slug, and Twist 1 (Wellner et al., 2009; Myung et al., 2014). E-cadherin protein expression is downregulated upon binding of ZEB1 to its promoter, resulting in glioma cell separation and migration (Edwards et al., 2011). EMT can also be induced by miRNA-related signaling pathways. miR-25 represses the expression of F-box-containing and WD repeat domain 7 (FBXW7) and Dickkopf Wnt signaling pathway inhibitor 3 (DKK3), thereby promoting the proliferation and migration of glioma cells (Peng et al., 2019). miR-876-5p plays an important role in the lncHOXC-AS2 (LncRNA HOXC cluster antisense RNA 2)-miR-876-5p-ZEB1 signaling pathway to promote EMT in gliomas (Dong et al., 2019).
In contrast, some miRNAs inhibit invasion and migration by suppressing the EMT process.
Snail 1 is one of the zinc finger transcriptional repressors and its pathological expression is closely associated with the EMT program and tissue invasive activity of cancer cells. After transcription, miR-22 inhibits glioma cell growth by suppressing Snail 1 and induces glioma cell cycle arrest affecting their migration and invasion (Zhang et al., 2020). Conversely, overexpression of miR-382-5P also inhibits the expression of the glioma cell epithelial markers Snail and Slug, as well as possibly negatively regulates the Y box binding protein 1 (YBX1) gene, thereby suppressing glioma migration, invasion, and the EMT process (Wang J. et al., 2019). miR-124 and miR-16 can inhibit the EMT process by suppressing the transcriptional activity of Slug, further suppressing glioma migration and invasion (Xie et al., 2012; Wang Q. et al., 2014). Of course, some related miRNA signaling pathways can also inhibit EMT. miR-451 also reduces invasion, migration, and EMT in glioma cells by targeting CAB39, and thus, inhibiting the Phosphoinositide 3-kinase/protein kinase B/Snail (PI3K/Akt/Snail) signaling pathway (Nan et al., 2021a) miR-661 directly regulates the target gene RAB3D and inhibit AKT/GSK3β signaling, which in turn inhibits glioma cell migration, invasion and EMT (Jin et al., 2020). miR-128 plays a negative regulatory role in the SNAI1/miR-128/SP1 axis, counteracting this role of SNAI1 in promoting glioma progression and inhibiting glioma aggressiveness (Dong et al., 2014). miRNAs can cause downregulation of EMT expression by targeting and regulating their target genes. miR-205 inhibits glioma growth, invasion, and reverses EMT by downregulating its target HOXD9 (Dai et al., 2019).
As mentioned above, it has been shown that a large number of miRNAs play an important role in the EMT process, and therefore, prevention of EMT may be a promising approach to combat invasion and migration.
Hypoxia plays a driving role in tumor adaptation, promotion of tumor progression, and resistance to therapeutic effects (Valtorta et al., 2020). Hypoxia-inducible factor 1a (HIF-1a) is a major transcriptional regulator of hypoxia-induced gene expression (Pientka et al., 2012).
miR-576-3p overexpression, in turn, decreases the migratory and proangiogenic properties of hypoxia-treated glioma cells via suppressing HIF-1 expression (Hu et al., 2019). miR-23b affects the survival and invasion of glioma cells by targeting and modulating the von Hippel–Lindau (VHL), thereby inhibiting the Hypoxia-inducible factor 1a/vascular endothelial growth factor (HIF-1a/VEGF) pathway and b-catenin/Tcf-4 transcription (Chen et al., 2012a). Insulin-like growth factor 1 receptor (IGF-1R) is another major factor in hypoxia. Overexpression of miR-181b is able to target IGF-1R and its downstream signaling pathways PI3K/AKT and the RAS/RAF/MAP kinase-ERK kinase (MEK)/extracellular-signal-regulated kinase (ERK) (MAPK) (MAPK/ERK1/2), thereby inhibiting cell migration, invasion, and tumorigenesis (Shi et al., 2013).
The migration, invasion, and angiogenesis of glioma cells are closely related to hypoxia, and miRNAs targeting their association may become an important part of controlling tumor metastasis.
An important basis for solid tumor growth is angiogenesis and regulation by miRNAs (Dai et al., 2018). The angiogenic state is determined by the balance of anti-angiogenic and pro-angiogenic molecules and VEGF is regarded as the most essential angiogenic factor (Rudge et al., 2007; Yancopoulos, 2010). miRNAs are involved in regulating angiogenesis during glioma formation and affecting the aggressiveness of gliomas (Liu et al., 2014; Yao et al., 2014). miR-24 may be involved in glioma angiogenesis through upregulation of vascular endothelial growth factor and TGF-β expression levels as well as intracellular AKT and β-catenin signaling pathways (Dai et al., 2018). Glioma angiogenesis is dependent on the proliferation, migration, and tube formation of human brain microvascular endothelial cells (HBMVEC), and miR-107 overexpression may inhibit HBMVEC by downregulating VEGF expression, thereby suppressing cell invasion and migration (Chen et al., 2016).
Thus, maintaining a balance between pro- and anti-vascularity may help to inhibit cancer cell migration.
The TME plays an important role in tumor development and therapy and consists of different cell populations, such as immune cells, fibroblasts, tumor-associated macrophages (TAM), endothelial cells, signaling molecules, and extracellular matrix (ECM) components. It is also involved in regulating disease progression (Calabrese et al., 2007; Cheng et al., 2013; Matarredona and Pastor, 2019; Jeanmougin et al., 2020).
Tumor-associated macrophages are particularly important components of TME and are indispensable in the regulation of tumor development and antitumour immune responses (Roesch et al., 2018). In the TME, TAM promotes tumor angiogenesis and immunosuppression by secreting factors associated with stimulation of tumor invasion, survival, and proliferation (Qian and Pollard, 2010). Of these, M1-TAMs exhibit tumor suppression, whereas M2-TAMs exhibit tumor support (Chen et al., 2017). M2-TAMs promote glioma growth and invasion (Hambardzumyan et al., 2016). miR-32 promotes the transformation of M2 macrophages through the PI3K/AKT signaling pathway, thereby enhancing glioma proliferation and migration, mainly because inhibition of THP1 cells affects their internal phosphatase and tensin homolog (PTEN) expression, which in turn negatively regulates the PI3K/AKT signaling pathway (Bao and Li, 2019). In glioblastoma (GBM), M2-TAMs inhibit miR-340-5p by upregulating transforming growth factor β-1 and promoting basement membrane HMGA-2 expression (Liu Y. et al., 2019).
TNF-α is overexpressed and secreted in the TME and is a major regulator of inflammation (Ramaswamy et al., 2019). It can achieve glioma proliferation, migration, and treatment resistance through activation of the NF-kB signaling pathway (Guo et al., 2017; Geeviman et al., 2018). The TNF-α mediated NF-kB signaling pathway can be activated and lead to cell proliferation, invasion, and migration after retinoic acid receptor-related orphan receptor A (RORA) inhibition by miR-18a (Jiang et al., 2020).
Similarly, tumor-associated fibroblasts (CAF) and glioma stem cells (GSCs) show an active role in TME. LNC HOXA transcript antisense RNA myeloid-specific 1 (LNC HOTAIRM1), when directly bound to miR-133b-3p, is able to target and regulate its downstream target TGF-β, which is involved in the regulation of fibroblast transformation (transformation of fibroblasts, t-FBS) malignancy in TME of GSCs remodeling. It is also highly expressed in high-grade gliomas and t-FBS, representing a poor prognosis. The reduction of HOTAIRM1 inhibits the proliferation, invasion, migration, and even tumourigenicity of t-FB (Wang H. et al., 2021). Sarkar et al. (2006) showed that partial expression of matrix metalloproteinase (MMPs) can regulate glioma aggressiveness by affecting the degradation process of extracellular matrix (ECM) components. miR-4262 expression downregulation inhibits glioma proliferation and migration by suppressing the expression of MMP2 and MMP13 (Yang et al., 2020), and miR-34a inhibits glioma cell migration by regulating MMP-9 (Wang X. et al., 2019).
Therefore, clarifying the interactions between miRNAs and TME may be an important strategy to address the metastasis issue.
It is well documented that the migration and invasion of gliomas depend on different molecular mechanisms mediated by miRNAs. These miRNAs and their corresponding targets, as well as the corresponding molecular mechanisms, are shown in Table 2.
The wnt/β-catenin signaling pathway is one of the most important molecular pathways in the development of many human tumors, including gliomas, and it encompasses and is closely associated with the proliferation, migration, and invasion of tumor cells and their angiogenic processes (Wang et al., 2018; Gong et al., 2019; Huo et al., 2020). Upregulation of miR-1825 targets cell cycle protein-dependent kinase-14 (CDK14) via the Wnt/β-catenin protein pathway to inhibit invasion and migration (Lu F. et al., 2020). And miR-10a can promote glioma invasion and migration by targeting the 3′ untranslated region of myotubularin-related protein 3 (MTMR3), which regulates the wnt/β-catenin signaling pathway (Yan et al., 2019).
Epidermal growth factor receptor signaling pathway is one of the multiple oncogenic pathways regulated by miRNAs that control cell proliferation, invasion, migration, angiogenesis, and apoptosis (Xu et al., 2021). Activation of the EGFR signaling pathway also initiates its downstream signaling pathways, including the PI3K (phosphatidyl-inositol-3 Kinase)/ATK (protein kinase B)/mTOR (mammalian target of rapamycin) and Ras (rat sarcoma virus)/Raf (rapidly accelerated fibrosarcoma)/MEK (mitogen-activated protein-kinase)/ERK (extracellular signal-regulated kinase) pathways (Ivliev et al., 2010; Mazzoleni et al., 2010; Motomura et al., 2011). Mutant forms of EGFR associated with glioma exhibit constitutive kinase activity, long-term stimulation of RAS signaling to promote cell cycle progression, and activation of the PI3K/AKT pathway to promote cell proliferation and migration (Rong et al., 2009; Ivliev et al., 2010; Mazzoleni et al., 2010).
The RAS oncogene family includes three members: K-RAS, N-RAS, and H-RAS. Let-7a affects cell proliferation, apoptosis, migration, and invasion by regulating its functional target, K-RAS, and thus, its downstream PI3K/AKT signaling pathway (Wang et al., 2013). miR-143 overexpression inhibits the proliferation, migration, invasion, and angiogenesis process of glioma cells by suppressing its target gene N-RAS; thus, activating the downstream PI3K/AKT signaling pathway (Wang L. et al., 2014).
AKT plays an important role as a serine-threonine protein kinase in human malignancies, causing many downstream effects of PI3K, phosphorylating multiple substrates involved in regulating tumorigenesis, and promoting glioma cell survival through activation of mTOR, TSC2, and S6. It also promotes the invasion of glioma cells while increasing the expression of MMPs (Pu et al., 2004; Zhang et al., 2009). Moreover, AKT is not only negatively regulated by PTEN but also activated by several growth factors and their receptors, especially EGFR (Narita et al., 2002; Choe et al., 2003; Golding et al., 2009). AKT in glioblastoma is activated in the presence of EGFR overexpression or mutations. The deletion of PTEN in gliomas is closely associated with AKT activation (Zhang et al., 2010). A study confirmed that the PI3K/AKT signaling pathway might contribute to glioma cell proliferation and invasion by inactivating apoptosis-related signals (Chaudhuri et al., 2018). miR-92a overexpression promotes glioma cell proliferation through the KLF4 (Kruppel-like factor 4)/AKT/mTOR signaling pathway (Liu P. J. et al., 2019). miR-24 promotes glioma development by acting on the caudal-type homeobox 1 (CDX1) target to activate the PI3K/Akt signaling pathway, promote cell proliferation, and induce apoptosis (Lei et al., 2021). A disintegrin and Metalloprotease 17(ADAM17), the target of miR-145, is also a major upstream component of several EGFR precursor ligands (Zheng et al., 2007; Lu et al., 2013), which activate MEK/ERK and PI3K/Akt when EGFR binds to the ligands, leading to aggressive and other malignant phenotypes (Tsatas et al., 2002). MMP-2 and MMP-9 protein expression can be significantly upregulated upon miR-221/222 overexpression and promote proliferation and invasion of glioma cells in the presence of the Akt downstream pathway (Zhang et al., 2010).
TGF-β has a dual role, acting as a suppressor in the early stages of tumors growth and displaying a malignant phenotype induced by oncogenic factors during tumors development (Bruna et al., 2007; Yang et al., 2012). The TGF-β signaling pathway is mediated by SMAD proteins (Duan and Chen, 2016). TGF-β is able to promote glioma cell growth through the SMAD and ERK1/2 pathways, thereby activating Nodal expression (Sun et al., 2014). TGF-β concentration and miR-132 levels in glioma cells are positively correlated and promote each other. miR-132 enhances the activation of TGF-β signaling by inhibiting Smad7 expression in glioma cells (Wang et al., 2015). TGF-β also acts as a downstream target of lysine demethylase 2A (KDM2A), which regulates cell proliferation, while upregulation of miR-663a inhibits migration and invasion by suppressing KDM2A (Wang L. et al., 2021).
Aberrant activation of NF-κB is observed in many types of human tumors, including gliomas, and is regulated by IJBS kinase (IKK-β), which is involved in cell proliferation, cell migration, and angiogenesis (Goldbrunner et al., 1999; Tektonidis et al., 2011; Yang et al., 2014). miR-451 is able to significantly inhibit the proliferation, invasion, and migration of glioma cells by targeting IKK-β regulated NF-κB (Nan et al., 2021b). A key negative regulator of NF-κB signaling is cylindromatosis (CYLD) deubiquitinase (Kovalenko et al., 2003; Trompouki et al., 2003; Sun, 2010) and miR-182 is able to directly inhibit the ubiquitin binding of CYLD, a component of the NF-κB signaling pathway, thereby inducing an aggressive phenotype in glioma cells (Song et al., 2012).
The Notch signaling pathway is a conserved pathway that controls cell fate and growth evolution (Leong and Karsan, 2006; Sivasankaran et al., 2009; Chen et al., 2012b). In mammals, Notch2 is one of the four members of the Notch receptor family (Notch 1–4) (Wu and Bresnick, 2007) and is involved in the proliferation, invasion, and metastasis of a variety of tumors cells, such as uveal melanoma, gastric cancer, and medulloblastoma (Fan et al., 2004; Boulay et al., 2007; Sivasankaran et al., 2009; Asnaghi et al., 2012; Tseng et al., 2012). Although miR-107 expression is down-regulated in glioma tissues and cell lines, its overexpression can directly target and regulate Notch2, which inhibits the migratory and invasive capacity of glioma cells, a known target for Tenascin-C and COX-2 trans-activation (Chen et al., 2013). miR-524-5p exerts tumors suppression by directly negatively targeting Jagge-1 and Hes-1, two key components of the Notch pathway, which are direct functional targets of miR-524-5p (Chen et al., 2012b).
The activity of Janus kinase (JAK) and signal transducers and activators of transcription (STAT) pathway is a major signaling mechanism for many growth factors and cytokines (Rawlings et al., 2004). It has also been shown that the development of human gliomas is closely related to the JAK/STAT pathway (Tu et al., 2011). miR-133a inhibits connective tissue growth factor (CTGF) expression and JAK/STAT activation, thereby suppressing glioma cell migration and invasion (Zhang P. et al., 2019). The miR-221/222 cluster may affect the angiogenic process in glioblastoma cells by regulating the activation of the JAK/STAT pathway through the regulation of the suppressor of cytokine signaling-3 (SOCS3) (Xu et al., 2019). Oncogene transcription factor signaling and activator of transcription 3 (STAT3), a component of the JAK/STAT3 signaling pathway, is also particularly important in many physiological and pathophysiological processes, such as tumors growth, invasion, and metastasis (Bromberg and Darnell, 2000; Yu and Jove, 2004; Doucette et al., 2012).
The long noncoding RNAs (lncRNA)/miRNA/mRNA axis pair is indispensable in tumor invasion and metastasis. lncRNA acts as a transcriptional regulator of mRNA by competitively binding miRNA, a process known as the sponge effect. New studies of the lncRNA/miRNA/mRNA regulatory network are important for understanding the mechanisms of tumor invasion and migration. LncRNA PVT1 affects the downstream proteins BMP2 and BMP4, which regulate the bone morphogenetic protein (BMP) signaling pathway, by interacting with miR-128-3p and regulating Gremlin 1(GREM1), thereby affecting glioma cell proliferation, invasion, and migration (Fu et al., 2018). Long non-coding RNA NCK1-AS1 (LncRNA NCK1-AS1) binds to miR-138-2-3P and regulates the indirect target of NCK1-AS1 containing tripartite pattern 24 (TRIM24) to activate its downstream Wnt/β catenin, which in turn affects glioma cell invasion and migration (Huang et al., 2020). Therefore, further studies on non-coding RNAs can reveal new mechanisms that may help us further understand epigenetic modifications and also provide more relevant information on the pathogenesis of human gliomas (Bian et al., 2016).
DNA methylation is an important mechanism of miRNA deregulation in tumors. Epigenetic modifications are closely related to gene expression. Transcriptional silencing may lead to the methylation of DNA promoters located at or near CpG islands observed during tumorigenesis, with subsequent manipulation of chromatin structure and gene transcription (Ozsolak et al., 2008). Promoter hypermethylation is thought to be a mechanism for the downregulation of tumor suppressor genes in human cancers (Esteller et al., 2001). DNA methylation is established by DNA methyltransferases, in which DNA (cytosine-5-)-methyltransferase 1 (DNMT1) maintains the methylation pattern and can be downregulated post-transcriptionally by the miR-148 family (Li et al., 2019). DNMT1 mediates promoter methylation, leading to downregulation of miR-141 gene expression, targeting regulation of spindle and kinetochore-associated complex subunit 2 (SKA2), and promoting glioma invasion and metastasis (Bian et al., 2016).
Currently, the diagnosis of glioma is based on imaging, such as computed tomography (CT) and magnetic resonance (MRI) (Kopkova et al., 2019), which are the most widely used tools but are expensive, do not improve early diagnosis (Kreisl et al., 2009), and have limited resolution (Server et al., 2009; Zakaria et al., 2014). Additionally, histological examination is fundamental for the diagnosis and prognosis of gliomas, but this requires access to tumor tissue through invasive neurosurgery (Ma et al., 2018). Given this situation, there is an urgent need for an improved method for the minimally invasive and rapid diagnosis and monitoring of gliomas, and miRNA may be such an ideal biomarker (Wei et al., 2016; Ma et al., 2018). Circulating specific miRNAs, such as plasma or serum, are more stable and can be detected in clinical specimens, showing great potential as convenient and non-invasive biomarkers (Chen et al., 2008; Mitchell et al., 2008). Interestingly, many studies have found that circulating miRNAs are potential diagnostic or prognostic biomarkers for cancer and other disease classifications, including prostate cancer (Selth et al., 2013), breast cancer (McDermott et al., 2014), and gastric cancer (Zhu et al., 2014). Recently, a study explored the feasibility of using aberrant single miRNAs as diagnostic or prognostic biomarkers for glioma, such as miR-205 (Yue et al., 2016), miR-128 (Sun et al., 2015), and miR-210 (Lai et al., 2015). These results suggest that this technology has promising applications.
Studies have confirmed that miRNAs are involved in the pathogenesis of several human cancers (Wang and Chen, 2014) and that they may play a role in biological processes by regulating the expression of key genes at the post-transcriptional level (Deng et al., 2018). A large number of miRNAs can be detected in extracellular fluids, including plasma, serum, urine, cerebrospinal fluid (CSF), saliva, and other human body fluids (Turchinovich et al., 2011). Circulating miR-182 overexpression may be a useful non-invasive biomarker for early diagnosis and prediction of clinical prognosis in gliomas (Xiao et al., 2016). miR-205 in serum was determined to be a new and valuable diagnostic biomarker for glioma and an independent prognostic indicator for overall survival (OS) in advanced tumors (Yue et al., 2016).
Furthermore, in complex exosomes, miRNAs are relevant biomarkers for the diagnosis and prediction of cancer due to their role as regulators of tumors, both as oncogens and carcinogens (Jonas and Izaurralde, 2015; Touat et al., 2015). Abnormal expression of serum exosomal-(exo) miR-210 is positively correlated with glioma grade in patients with glioma (Lan et al., 2020). In contrast, exosomal miR-2276-5p is negatively correlated with tumor grade (Sun et al., 2021). And the expression of serum exosome miR-301a may serve as a new biomarker for the diagnosis of glioma and may predict the prognosis of patients (Lan et al., 2018).
Currently, histopathology is the gold standard for the diagnosis of glioma staging and grading, but the histopathology of tumor specimens obtained by resection or stereotactic biopsy relies heavily on specific structural similarities between tumor cells and non-tumorigenic glial cells (Riemenschneider et al., 2010). Tumor staging and follow-up after glioma treatment rely on neuroimaging, of which MRI is particularly important. For cancer patients, blood biomarkers are highly effective in screening, early diagnosis, detection of efficacy, and predictive review (Sawyers, 2008). Hence, the search for non-invasive objective biomarkers, especially in serum or plasma, is crucial for the diagnosis of glioma.
The development of glioma is closely related to the expression levels of miRNAs and predicts the prognosis of patients. Therefore, detecting the expression levels of miRNAs in patients with glioma may assist with the selection of optimal tumor treatment. Some miRNAs associated with invasion and migration are considered potential markers to predict the prognosis of patients with glioma. Low expression of miR-135a-5p suggests a poor prognosis for patients with glioma (Luo et al., 2019). The expression of miR-497 in patients with glioma is positively correlated with survival time (Feng et al., 2016). Decreased serum levels of miR-376a, miR-376b, and miR-376c in human patients with glioma indicate a poor prognosis (Huang et al., 2017). Since serum or plasma contains more miRNAs of non-tumor origin than CSF, CSF may be considered a more appropriate source of diagnostic tissue. Some reports also suggest that exosomal miRNAs are more likely to be of tumor origin than CSF, and therefore can be used to predict prognosis (Zhu et al., 2012). In addition, tumor-derived exosomes can convert fibroblasts and mesenchymal stem cells into myofibroblasts, thereby promoting tumor angiogenesis and metastasis (Liu et al., 2018). High expression of miR-454-3p in exosomes or low expression in tissues is strongly associated with poor prognosis (Shao et al., 2019).
Although miRNAs theoretically have promising applications as diagnostic or prognostic biomarkers for glioma, no specific miRNAs have been validated for glioma. Second, although fluorescent quantitative real-time PCR (qPCR) is the most commonly used techniques for quantifying miRNA, it still has limitations, such as low sensitivity and poor accuracy in detecting copy templates (Ma et al., 2013). Three-dimensional DNA nanomachine can be combined with toehold-mediated strand displacement reaction for sensitive electrochemical detection of miRNA (Lu H. et al., 2020).
A defined miRNA may have multiple different miRNA targets and a single target may be regulated by multiple miRNAs (Friedman et al., 2008). There is a growing interest in building large and complex regulatory networks for targeted therapies. miRNAs play an important role in both normal and abnormal physiological activities. Recently, a large number of miRNAs have been closely related to cancer; therefore, miRNAs have a promising future as targets for the treatment of different types of cancer (Musilova and Mraz, 2015).
To date, miRNAs have been shown to function as carcinogens (Calin and Croce, 2006) and likewise as tumor suppressors (Calin et al., 2002; Bonci et al., 2008). Therefore, activation of oncogenic factors or inhibition of oncogenic factors is the most basic miRNA therapeutic approach (Chi and Zhou, 2016). Although the envisioned results were not achieved by applying antisense miRNA oligonucleotide constructs (Gentner et al., 2009), by applying double-stranded miRNA mimics (De Guire et al., 2010), or viral/liposome delivery systems (Xie et al., 2019), these were inevitably confounded by toxic effects, including off-target and immune-related effects (Xuan et al., 2015). Fortunately, liposomes coated with polyethylene glycol (PEG) have been used as carriers for delivering miRNA, so that it could penetrate the blood-brain barrier and act on the target (Jonas et al., 2015). This new nanocarrier can wrap miRNA molecules and improve their stability, which helps to obtain higher transduction efficiency and effective bioactivity, promising the clinical application of this efficient and less toxic method of miRNA transfection. The nanocarrier polyethylene glycol methyl ester (mPEG-g-PEI) enhanced the transfection rate of miR-135a by improving the uptake impact of normal glial cells and glioma cells. It can considerably improve glioma patients' survival and prevent tumor development (Liang et al., 2017).
However, miRNA-targeted therapies still face several serious problems, from basic to clinical applications. First, cell membranes are negatively charged, and miRNA molecules cannot interact directly with them, but when miRNAs are wrapped by positively charged PEI, they can facilitate their uptake by cell membranes (Torchilin et al., 2003). Second, non-targeted effects of non-specific miRNAs, such as the development of less toxic miRNA mimics and inhibitors, have also achieved results beyond our expectations, but this requires in-depth studies on pharmacological, pharmacokinetic, and pharmacodynamic factors (Moody et al., 2017; Zhang et al., 2018). Third, the development of an efficient and less toxic gene delivery system is a major challenge for gene therapy. Non-viral vectors are potentially an attractive option, but there remains a need to improve their transfection efficiency and reduce their toxicity (Godbey et al., 1999). Finally, nanoparticle-based delivery systems may be a good application for miRNAs in organisms. Nanoparticles made from synthetic natural cationic polymers or cationic substances as one of the non-viral gene delivery vehicles have the potential to overcome the intracellular and extracellular barriers of the organism, ensuring that miRNAs are not degraded by serum nucleases and also increase uptake by target cells. After uptake, miRNA multimers can then be released from endosomes into the cytoplasm and into their pathways (Liang et al., 2017).
Dysregulation of miRNAs is involved in regulating the aggressive process of gliomas and leads to poor prognosis and treatment resistance. To improve the prognosis of patients, it is imperative to gain insight into the expression of miRNAs to partially control their tumor invasion and metastasis. In this study, we reviewed the progress of research on miRNAs related to glioma invasion and metastasis and discussed their roles. We have summarized the clinical significance (diagnosis, prognosis, and treatment) of some miRNAs in glioma, and have attempted to provide new ideas to further explore the role of miRNAs in glioma.
Aberrant expression of miRNAs plays an important role in the migration and invasion of gliomas. However, uniform standards and protocols are essential for miRNA studies, as the expression patterns may be inconsistent due to variations in criteria and methods between experiments. In addition, comprehensive genomic searches and bioinformatics analyses are necessary to find more miRNAs associated with glioma migration and invasion, thus, improving the accuracy of clinical applications.
Although miRNAs in many extracellular fluids, such as plasma, serum, urine, CSF, and saliva have profound implications in diagnosis or prediction of prognosis, their validity still requires further investigation. Therefore, a highly sensitive, specific, and low-cost method to detect miRNAs must be established. For example, miRNAs can be electrochemically detected when 3D DNA nanomachines are combined with toe-mediated SDR (Lu H. et al., 2020). It is also a promising tool for early screening by detecting miRNA-containing exosomes.
In recent years, miRNAs have emerged as promising targets for the treatment of cancer. miRNAs can regulate several mRNAs simultaneously and are more effective than targeting individual mRNAs (Yang et al., 2017). Recent advances in miRNA direct repair (miRNA mimics) and miRNA inhibition therapies (antisense oligonucleotides, antiglycans, locked nucleic acid anti-miRNA, and small molecule miRNA inhibitors) make miRNAs ideal candidates for entry into clinical trials for glioblastoma. The expression levels of miRNAs that are dysregulated in different diseases may be restored by the action of the aforementioned complexes. Moreover, Recent studies have identified the microbubbles Lipid-polymer hybrid nanoparticles (MBs-LPHNs-CRGD) delivery system as a potentially efficient targeted gene delivery system in GBM (Yang et al., 2021).
Overall, we reviewed the biological significance and clinical potential of miRNA in glioma, which can guide clinical diagnosis and treatment and provide some theoretical basis for further basic research. Identification of more sensitive miRNA biomarkers for the diagnosis and treatment of glioma is imperative for improving the quality of life and prognosis of patients.
XG and FM conceived, drafted, supervised, and revised the manuscript. XG collected and prepared the related references. HJ and LC drew the figures. All authors read and approved the final manuscript.
We would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Asnaghi, L., Ebrahimi, K. B., Schreck, K. C., Bar, E. E., Coonfield, M. L., Bell, W. R., et al. (2012). Notch signaling promotes growth and invasion in uveal melanoma. Clin. Cancer Res. 18, 654–665. doi: 10.1158/1078-0432.CCR-11-1406
Bansal, R., Magge, S., and Winkler, S. (2003). Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte-lineage cells. J. Neurosci. Res. 74, 486–493. doi: 10.1002/jnr.10773
Bao, L., and Li, X. (2019). MicroRNA-32 targeting PTEN enhances M2 macrophage polarization in the glioma microenvironment and further promotes the progression of glioma. Mol. Cell. Biochem. 460, 67–79. doi: 10.1007/s11010-019-03571-2
Bian, E. B., Ma, C. C., He, X. J., Wang, C., Zong, G., Wang, H. L., et al. (2016). Epigenetic modification of miR-141 regulates SKA2 by an endogenous 'sponge' HOTAIR in glioma. Oncotarget 7, 30610–30625. doi: 10.18632/oncotarget.8895
Bonci, D., Coppola, V., Musumeci, M., Addario, A., Giuffrida, R., Memeo, L., et al. (2008). The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 14, 1271–1277. doi: 10.1038/nm.1880
Boulay, J. L., Miserez, A. R., Zweifel, C., Sivasankaran, B., Kana, V., Ghaffari, A., et al. (2007). Loss of NOTCH2 positively predicts survival in subgroups of human glial brain tumors. PLoS ONE 2, e576. doi: 10.1371/journal.pone.0000576
Bovell, L. C., Shanmugam, C., Putcha, B. D., Katkoori, V. R., Zhang, B., Bae, S., et al. (2013). The prognostic value of microRNAs varies with patient race/ethnicity and stage of colorectal cancer. Clin. Cancer Res. 19, 3955–3965. doi: 10.1158/1078-0432.CCR-12-3302
Bromberg, J., and Darnell, J. E. Jr. (2000). The role of STA Ts in transcriptional control and their impact on cellular function. Oncogene 19, 2468–2473. doi: 10.1038/sj.onc.1203476
Bruna, A., Darken, R. S., Rojo, F., Ocaña, A., Peñuelas, S., Arias, A., et al. (2007). High TGFb-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 11, 147–160. doi: 10.1016/j.ccr.2006.11.023
Calabrese, C., Poppleton, H., Kocak, M., Hogg, T. L., Fuller, C., Hamner, B., et al. (2007). A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82. doi: 10.1016/j.ccr.2006.11.020
Calin, G. A., and Croce, C. M. (2006). MicroRNA signatures in human cancers. Nat. Rev. Cancer 6, 857–866. doi: 10.1038/nrc1997
Calin, G. A., Dumitru, C., Shimizu, M., Bichi, R., Zupo, S., Noch, E., et al. (2002). Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 9, 15524–15529. doi: 10.1073/pnas.242606799
Chaudhuri, S., Singh, M. K., Bhattacharya, D., Datta, A., Hazra, I., Mondal, S., et al. (2018). T11TS immunotherapy repairs PI3K-AKT signaling in T-cells:clues toward enhanced T-cell survival in rat glioma model. J. Cell. Physiol. 233, 759–770. doi: 10.1002/jcp.26047
Chen, L., Chen, X. R., Zhang, R., Li, P., Liu, Y., Yan, K., et al. (2013). MicroRNA-107 inhibits glioma cell migration and invasion by modulating Notch2 expression. J. Neurooncol. 112, 59–66. doi: 10.1007/s11060-012-1037-7
Chen, L., Han, L., Zhang, K., Shi, Z., Zhang, J., Zhang, A., et al. (2012a). VHL regulates the effects of miR-23b on glioma survival and invasion via suppression of HIF-1α/VEGF and β-catenin/Tcf-4 signaling. Neuro Oncol. 14, 1026–1036. doi: 10.1093/neuonc/nos122
Chen, L., Li, Z. Y., Xu, S. Y., Zhang, X. J., Zhang, Y., Luo, K., et al. (2016). Upregulation of miR-107 inhibits glioma angiogenesis and VEGF expression. Cell. Mol. Neurobiol. 36, 113–120. doi: 10.1007/s10571-015-0225-3
Chen, L., Zhang, W., Yan, W., Han, L., Zhang, K., Shi, Z., et al. (2012b). The putative tumor suppressor miR-524-5p directly targets Jagged-1 and Hes-1 in glioma. Carcinogenesis 33, 2276–2282. doi: 10.1093/carcin/bgs261
Chen, W., Huang, B., Wang, E., and Wang, X. (2019). MiR-145 inhibits EGF-induced epithelial-to-mesenchymal transition via targeting Smad 2 in human glioblastoma. OncoTargets Ther. 12, 3099–3107. doi: 10.2147/OTT.S202129
Chen, X., Ba, Y., Ma, L., Cai, X., Yin, Y., Wang, K., et al. (2008). Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 18, 997–1006. doi: 10.1038/cr.2008.282
Chen, X. W., Yu, T. J., Zhang, J., Li, Y., Chen, H. L., Yang, G. F., et al. (2017). CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene 36, 5045–5057. doi: 10.1038/onc.2017.118
Cheng, L., Huang, Z., Zhou, W., Wu, Q., Donnola, S., Liu, J. K., et al. (2013). Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153, 139–152. doi: 10.1016/j.cell.2013.02.021
Chi, Y., and Zhou, D. (2016). MicroRNAs in colorectal carcinoma—from pathogenesis to therapy. J. Exp. Clin. Cancer Res. 35, 43. doi: 10.1186/s13046-016-0320-4
Choe, G., Horvath, S., Cloughesy, T. F., Crosby, K., Seligson, D., Palotie, A., et al. (2003). Analysis of the phos-phatidylinositol 3'-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 63, 2742–2746.
Dai, B., Zhou, G., Hu, Z., Zhu, G., Mao, B., Su, H., et al. (2019). MiR-205 suppresses epithelial-mesenchymal transition and inhibits tumor growth of human glioma through down-regulation of HOXD9. Biosci Rep. 39, BSR20181989. doi: 10.1042/BSR20181989
Dai, D., Huang, W., Lu, Q., Chen, H., Liu, J., Hong, B., et al. (2018). miR-24 regulates angiogenesis in gliomas. Mol. Med. Rep. 18, 358–368. doi: 10.3892/mmr.2018.8978
de Groot, J. F., Fuller, G., Kumar, A. J., Piao, Y., Eterovic, K., Ji, Y., et al. (2010). Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 12, 233–242. doi: 10.1093/neuonc/nop027
De Guire, V., Caron, M., Scott, N., Ménard, C., Gaumont-Leclerc, M., Chartrand, F., et al. (2010). Designing small multiple-target artificial RNAs. Nucleic Acids Res. 38, e140. doi: 10.1093/nar/gkq354
Deng, Y. H., Deng, Z. H., Hao, H., Wu, X. L., Gao, H., Tang, S. H., et al. (2018). MicroRNA-23a promotes colorectal cancer cell survival by targeting PDK4. Exp. Cell Res. 373, 171–179. doi: 10.1016/j.yexcr.2018.10.010
Dong, N., Guo, J., Han, S., Bao, L., Diao, Y., Lin, Z., et al. (2019). Positive feedback loop of lncRNA HOXC-AS2/miR-876-5p/ZEB1 to regulate EMT in glioma. Onco. Targets. Ther. 12, 7601–7609. doi: 10.2147/OTT.S216134
Dong, Q., Cai, N., Tao, T., Zhang, R., Yan, W., Li, R., et al. (2014). An axis involving SNAI1, microRNA-128 and SP1 modulates glioma progression. PLoS ONE. 9, e98651. doi: 10.1371/journal.pone.0098651
Doucette, T. A., Kong, L. Y., Yang, Y., Ferguson, S. D., Yang, J., Wei, J., et al. (2012). Signal transducer and activator of transcription 3 promotes angiogenesis and drives malignant progression in glioma. Neuro Oncol. 14, 1136–1145. doi: 10.1093/neuonc/nos139
Duan, Y., and Chen, Q. (2016). TGF-β1 regulating miR-205/miR-195 expression affects the TGF-β signal pathway by respectively targeting SMAD2/SMAD7. Oncol. Rep. 36, 1837–1844. doi: 10.3892/or.2016.5023
Edwards, L. A., Woolard, K., Son, M. J., Li, A., Lee, J., Ene, C., et al. (2011). Effect of brain- and tumor-derived connective tissue growth factor on glioma invasion. J. Natl. Cancer Inst. 103, 1162–1178. doi: 10.1093/jnci/djr224
Esteller, M., Corn, P. G., Baylin, S. B., and Herman, J. G. (2001). A gene hypermethylation profile of human cancer. Cancer Res. 61, 3225–3229. doi: 10.1007/BF02979467
Fan, X., Mikolaenko, I., Elhassan, I., Ni, X., Wang, Y., Ball, D., et al. (2004). Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 64, 7787–7793. doi: 10.1158/0008-5472.CAN-04-1446
Feng, F., Kuai, D., Wang, H., Li, T., Miao, W., Liu, Y., et al. (2016). Reduced expression of microRNA-497 is associated with greater angiogenesis and poor prognosis in human gliomas. Hum. Pathol. 58, 47–53. doi: 10.1016/j.humpath.2016.04.022
Folkman, J. (1971). Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186. doi: 10.1056/NEJM197111182852108
Friedman, R. C., Farh, K. K., Burge, C. B., and Bartel, D. P. (2008). Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105. doi: 10.1101/gr.082701.108
Fu, C., Li, D., Zhang, X., Liu, N., Chi, G., Jin, X., et al. (2018). LncRNA PVT1 facilitates tumorigenesis and progression of glioma via regulation of MiR-128-3p/GREM1 Axis and BMP signaling pathway. Neurotherapeutics 15, 1139–1157. doi: 10.1007/s13311-018-0649-9
Gatenby, R. A., and Gawlinski, E. T. (2003). The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical models. Cancer Res. 63, 3847–3854.
Geeviman, K., Babu, D., and Prakash Babu, P. (2018). Pantoprazole induces mitochondrial apoptosis and attenuates NF-kappAB signaling in glioma cells. Cell. Mol. Neurobiol. 38, 1491–1504. doi: 10.1007/s10571-018-0623-4
Gentner, B., Schira, G., Giustacchini, A., Amendola, M., Brown, B. D., Ponzoni, M., et al. (2009). Stable knockdown of microRNA in vivo by lentiviral vectors. Nat. Methods. 6, 63–66. doi: 10.1038/nmeth.1277
Godbey, W. T., Wu, K. K., and Mikos, A. G. (1999). Poly(ethylenimine) and its role in gene delivery. J. Control. Release. 60, 149–160. doi: 10.1016/S0168-3659(99)00090-5
Godlewski, J., Nowicki, M. O., Bronisz, A., Williams, S., Otsuki, A., Nuovo, G., et al. (2008). Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 68, 9125–9130. doi: 10.1158/0008-5472.CAN-08-2629
Goldbrunner, R. H., Bernstein, J. J., and Tonn, J. C. (1999). Cell-extracellular matrix interaction in glioma invasion. Acta Neurochir. 141, 295–305. doi: 10.1007/s007010050301
Golding, S. E., Morgan, R. N., Adams, B. R., Hawkins, A. J., Povirk, L. F., and Valerie, K. (2009). Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol. Ther. 8, 730–738. doi: 10.4161/cbt.8.8.7927
Gong, X., Liao, X., and Huang, M. (2019). lncRNA CASC7 inhibits the progression of glioma via regulating Wnt/β-catenin signaling pathway. Pathol. Res. Pract. 215, 564–570. doi: 10.1016/j.prp.2019.01.018
Guo, G., Gong, K., Ali, S., Ali, N., Shallwani, S., Hatanpaa, K. J., et al. (2017). A TNF-JNK-AXL-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat. Neurosci. 20, 1074–1084. doi: 10.1038/nn.4584
Hambardzumyan, D., Gutmann, D. H., and Kettenmann, H. (2016). The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27. doi: 10.1038/nn.4185
Hu, Q., Liu, F., Yan, T., Wu, M., Ye, M., Shi, G., et al. (2019). MicroRNA-576-3p inhibits the migration and proangiogenic abilities of hypoxia-treated glioma cells through hypoxia-inducible factor-1α. Int. J. Mol. Med. 43, 2387–2397. doi: 10.3892/ijmm.2019.4157
Hu, Y., Li, Y., Wu, C., Zhou, L., Han, X., Wang, Q., et al. (2017). MicroRNA-140-5p inhibits cell proliferation and invasion by regulating VEGFA/MMP2 signaling in glioma. Tumour Biol. 39, 1010428317697558. doi: 10.1177/1010428317697558
Huang, L., Li, X., Ye, H., Liu, Y., Liang, X., Yang, C., et al. (2020). Long non-coding RNA NCK1-AS1 promotes the tumorigenesis of glioma through sponging microRNA-138-2-3p and activating the TRIM24/Wnt/β-catenin axis. J. Exp. Clin. Cancer Res. 39, 63. doi: 10.1186/s13046-020-01567-1
Huang, Q., Wang, C., Hou, Z., Wang, G., Lv, J., Wang, H., et al. (2017). Serum microRNA-376 family as diagnostic and prognostic markers in human gliomas. Cancer Biomark. 19, 137–144. doi: 10.3233/CBM-160146
Huo, L. W., Wang, Y. F., Bai, X. B., Zheng, H. L., and Wang, M. D. (2020). circKIF4A promotes tumorogenesis of glioma by targeting miR-139-3p to activate Wnt5a signaling. Mol. Med. 26, 29. doi: 10.1186/s10020-020-00159-1
Ivliev, A. E., 't Hoen, P. A., and Sergeeva, M. G. (2010). Coexpression network analysis identifies transcriptional modules related to proastro cytic differentiation and sprouty signaling in glioma. Cancer Res. 70, 10060–10070. doi: 10.1158/0008-5472.CAN-10-2465
Jeanmougin, M., Håvik, A. B., Cekaite, L., Brandal, P., Sveen, A., Meling, T. R., et al. (2020). Improved prognostication of glioblastoma beyond molecular subtyping by transcriptional profiling of the tumor microenvironment. Mol. Oncol.14, 1016–1027. doi: 10.1002/1878-0261.12668
Jiang, Y., Zhou, J., Zhao, J., Hou, D., Zhang, H., Li, L., et al. (2020). MiR-18a-downregulated RORA inhibits the proliferation and tumorigenesis of glioma using the TNF-α-mediated NF-κB signaling pathway. EBioMedicine 52, 102651. doi: 10.1016/j.ebiom.2020.102651
Jin, T., Liu, M., Liu, Y., Li, Y., Xu, Z., He, H., et al. (2020). Lcn2-derived circular RNA (hsa_circ_0088732) inhibits cell apoptosis and promotes EMT in glioma via the miR-661/RAB3D axis. Front. Oncol. 10, 170. doi: 10.3389/fonc.2020.00170
Jonas, B., Larsen, T. B., Jolck, R. I., Eliasen, R., Holm, R., Gjetting, T., et al. (2015). Investigation of enzyme-sensitive lipid nanoparticles for delivery of siRNA to blood-brain barrier and glioma cells. Int J. Nanomed. 10, 5995–6008. doi: 10.2147/IJN.S87334
Jonas, S., and Izaurralde, E. (2015). Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 7, 421–433. doi: 10.1038/nrg3965
Katakowski, M., Buller, B., Wang, X., Rogers, T., and Chopp, M. (2010). Functional microRNA is transferred between glioma cells. Cancer Res. 70, 8259–8263. doi: 10.1158/0008-5472.CAN-10-0604
Kim, C. W., Oh, E. T., Kim, J. M., Park, J. S., Lee, D. H., Lee, J. S., et al. (2018). Hypoxia-induced microRNA-590-5p promotes colorectal cancer progression by modulating matrix metalloproteinase activity [published correction appears in Cancer Lett. 2019 Jul 28; 455, 73]. Cancer Lett. 416, 31–41. doi: 10.1016/j.canlet.2017.12.018
Kopkova, A., Sana, J., Machackova, T., Vecera, M., Radova, L., Trachtova, K., et al. (2019). Cerebrospinal fluid microrna signatures as diagnostic biomarkers in brain tumors. Cancers 11, 1546. doi: 10.3390/cancers11101546
Kovalenko, A., Chable-Bessia, C., Cantarella, G., Israël, A., Wallach, D., and Courtois, G. (2003). The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424, 801–805. doi: 10.1038/nature01802
Kreisl, T. N., Kim, L., Moore, K., Duic, P., Royce, C., Stroud, I., et al. (2009). Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 27, 740–745. doi: 10.1200/JCO.2008.16.3055
Kumar, P., Luo, Y., Tudela, C., Alexander, J. M., and Mendelson, C. R. (2013). The c-Myc-regulated microRNA-17~92 (miR-17~92) and miR-106~a363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol. Cell Biol. 33, 1782–1796. doi: 10.1128/MCB.01228-12
Lai, N. S., Wu, D. G., Fang, X. G., Lin, Y. C., Chen, S. S., Li, Z. B., et al. (2015). Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br. J. Cancer. 112, 1241–1246. doi: 10.1038/bjc.2015.91
Lan, F., Qing, Q., Pan, Q., Hu, M., Yu, H., Yue, X., et al. (2018). Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell Oncol. 41, 25–33. doi: 10.1007/s13402-017-0355-3
Lan, F., Yue, X., and Xia, T. (2020). Exosomal microRNA-210 is a potentially non-invasive biomarker for the diagnosis and prognosis of glioma. Oncol. Lett. 19, 1967–1974. doi: 10.3892/ol.2020.11249
Lardner, A. (2001). The effects of extracellular pH on immune function. J. Leukoc. Biol. 69, 522–530. doi: 10.1189/jlb.69.4.522
Lei, D., Sun, H., and Zhang, B. (2021). MiR-24 promotes cell growth in human glioma by CDX1/PI3K/Akt signaling pathway. Cancer Biother. Radiopharm. 36, 588–599. doi: 10.1089/cbr.2020.3711
Leong, K. G., and Karsan, A. (2006). Recent insights into the role of Notch signaling in tumorigenesis. Blood 107, 2223–2233. doi: 10.1182/blood-2005-08-3329
Li, H., Yu, L., Liu, J., Bian, X., Shi, C., Sun, C., et al. (2017). miR-320a functions as a suppressor for gliomas by targeting SND1 and β-catenin, and predicts the prognosis of patients. Oncotarget 8, 19723–19737. doi: 10.18632/oncotarget.14975
Li, Y., Chen, F., Chu, J., Wu, C., Li, Y., Li, H., et al. (2019). miR-148-3p inhibits growth of glioblastoma targeting DNA methyltransferase-1 (DNMT1). Oncol. Res. 27, 911–921. doi: 10.3727/096504019X15516966905337
Liang, C., Sun, W., He, H., Zhang, B., Ling, C., Wang, B., et al. (2017). Antitumor effect of a new nano-vector with miRNA-135a on malignant glioma [published correction appears in Int J Nanomedicine. 2020 Jul 21; 15, 5179.]. Int. J. Nanomedicine. 13, 209–220. doi: 10.2147/IJN.S148142
Lin, Y. H., Liao, C. J., Huang, Y. H., Wu, M. H., Chi, H. C., Wu, S. M., et al. (2013). Thyroid hormone receptor represses miR-17 expression to enhance tumor metastasis in human hepatoma cells. Oncogene 32, 4509–4518. doi: 10.1038/onc.2013.309
Liu, C.-A., Chang, C.-Y., Hsueh, K.-W., Su, H.-L., Chiou, T.-W., Lin, S.-Z., et al. (2018). Migration/invasion of malignant gliomas and implications for therapeutic treatment. Int. J. Mol. Sci. 19, 1115. doi: 10.3390/ijms19041115
Liu, P. J., Ye, Y. X., Wang, Y. X., Du, J. X., Pan, Y. H., Fang, X. B., et al. (2019). MiRNA-92a promotes cell proliferation and invasion through binding to KLF4 in glioma. Eur. Rev. Med. Pharmacol. Sci. 23, 6612–6620. doi: 10.26355/eurrev_201908_18550
Liu, S., Yin, F., Zhang, J., Wicha, M. S., Chang, A. E., Fan, W., et al. (2014). Regulatory roles of miRNA in the human neural stem cell transformation to glioma stem cells. J. Cell. Biochem. 115, 1368–1380. doi: 10.1002/jcb.24786
Liu, Y., Li, X., Zhang, Y., Wang, H., Rong, X., Peng, J., et al. (2019). An miR-340-5p-macrophage feedback loop modulates the progression and tumor microenvironment of glioblastoma multiforme. Oncogene 38, 7399–7415. doi: 10.1038/s41388-019-0952-x
Lu, F., Li, C., Sun, Y., Jia, T., Li, N., and Li, H. (2020). Upregulation of miR-1825 inhibits the progression of glioblastoma by suppressing CDK14 though Wnt/β-catenin signaling pathway. World J. Surg. Oncol. 18, 147. doi: 10.1186/s12957-020-01927-3
Lu, H., Hailin, T., Yi, X., and Wang, J. (2020). Three-dimensional DNA nanomachine combined with toehold-mediated strand displacement reaction for sensitive electrochemical detection of MiRNA. Langmuir 36, 10708–10714. doi: 10.1021/acs.langmuir.0c01415
Lu, Y., Chopp, M., Zheng, X., Katakowski, M., Buller, B., Jiang, F., et al. (2013). MiR-145 reduces ADAM17 expression and inhibits in vitro migration and invasion of glioma cells. Oncol. Rep. 29, 67–72. doi: 10.3892/or.2012.2084
Luo, W., Sun, C., Zhou, J., Wang, Q., Yu, L., Bian, X. W., et al. (2019). miR-135a-5p functions as a glioma proliferation suppressor by targeting tumor necrosis factor receptor-associated factor 5 and predicts patients' prognosis. Am. J. Pathol. 189, 162–176. doi: 10.1016/j.ajpath.2018.08.019
Ma, C., Nguyen, H. P. T., Luwor, R. B., Stylli, S. S., Gogos, A., Paradiso, L., et al. (2018). A comprehensive meta-analysis of circulation miRNAs in glioma as potential diagnostic biomarker. PLoS ONE 13, e0189452. doi: 10.1371/journal.pone.0189452
Ma, J., Li, N., Guarnera, M., and Jiang, F. (2013). Quantification of plasma miRNAs by digital PCR for cancer diagnosis. Biomark. Insights. 8, 127–136. doi: 10.4137/BMI.S13154
Matarredona, E. R., and Pastor, A. M. (2019). Extracellular vesicle-mediated communication between the glioblastoma and its microenvironment. Cells 9, 96. doi: 10.3390/cells9010096
Mazzoleni, S., Politi, L. S., Pala, M., Cominelli, M., Franzin, A., Sergi Sergi, L., et al. (2010). Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res. 70, 7500–7513. doi: 10.1158/0008-5472.CAN-10-2353
McDermott, A. M., Miller, N., Wall, D., Martyn, L. M., Ball, G., Sweeney, K. J., et al. (2014). Identification and validation of oncologic miRNA biomarkers for luminal A-like breast cancer. PLoS ONE. 9, e87032. doi: 10.1371/journal.pone.0087032
Michailidi, C., Hayashi, M., Datta, S., Sen, T., Zenner, K., Oladeru, O., et al. (2015). Involvement of epigenetics and EMT-related miRNA in arsenic-induced neoplastic transformation and their potential clinical use. Cancer Prev. Res. 8, 208–221. doi: 10.1158/1940-6207.CAPR-14-0251
Mitchell, P. S., Parkin, R. K., Kroh, E. M., Fritz, B. R., Wyman, S. K., Pogosova-Agadjanyan, E. L., et al. (2008). Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 105, 10513–10518. doi: 10.1073/pnas.0804549105
Mohammadi, M., Froum, S., Hamby, J. M., Schroeder, M. C., Panek, R. L., Lu, G. H., et al. (1998). Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 17, 5896–5904. doi: 10.1093/emboj/17.20.5896
Moody, L., He, H., Pan, Y. X., and Chen, H. (2017). Methods and novel technology for microRNA quantification in colorectal cancer screening. Clin. Epigenetics 9, 119. doi: 10.1186/s13148-017-0420-9
Motomura, K., Natsume, A., Kishida, Y., Higashi, H., Kondo, Y., Nakasu, Y., et al. (2011). Benefits of interferon-β and temozolomide combination therapy for newly diagnosed primary glioblastoma with the unmethylated MGMT promoter: a multicenter study. Cancer 117, 1721–1730. doi: 10.1002/cncr.25637
Musilova, K., and Mraz, M. (2015). MicroRNAs in B-cell lymphomas: how a complex biology gets more complex. Leukemia 29, 1004. doi: 10.1038/leu.2014.351
Myung, J. K., Choi, S. A., Kim, S. K., Wang, K. C., and Park, S. H. (2014). Snail plays an oncogenic role in glioblastoma by promoting epithelial mesenchymal transition. Int. J.Clin. Exp. Pathol. 7, 1977–1987.
Nan, Y., Guo, L., Lu, Y., Guo, G., Hong, R., Zhao, L., et al. (2021a). miR-451 suppresses EMT and metastasis in glioma cells. Cell Cycle 20, 1270–1278. doi: 10.1080/15384101.2021.1933303
Nan, Y., Guo, L., Zhen, Y., Wang, L., Ren, B., Chen, X., et al. (2021b). miRNA-451 regulates the NF-κB signaling pathway by targeting IKKβ to inhibit glioma cell growth. Cell Cycle. 20, 1967–1977. doi: 10.1080/15384101.2021.1969496
Narita, Y., Nagane, M., Mishima, K., Huang, H. J., Furnari, F. B., and Cavenee, W. K. (2002). Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidyli-nositol 3-kinase/Akt pathway in glioblastomas. Cancer Res. 62, 6764–6769.
Ozsolak, F., Poling, L. L., Wang, Z., Liu, H., Liu, X. S., Roeder, R. G., et al. (2008). Chromatin structure analyses identify miRNA promoters. Genes Dev. 22, 3172–3183. doi: 10.1101/gad.1706508
Parker, N. R., Correia, N., Crossley, B., Buckland, M. E., Howell, V. M., Wheeler, H. R., et al. (2013). Correlation of microRNA 132 Up-regulation with an unfavorable clinical outcome in patients with primary glioblastoma multiforme treated with radiotherapy plus concomitant and adjuvant temozolomide chemotherapy. Transl. Oncol. 6, 742–748. doi: 10.1593/tlo.13553
Peng, G., Yang, C., Liu, Y., and Shen, C. (2019). miR-25-3p promotes glioma cell proliferation and migration by targeting FBXW7 and DKK3. Exp. Ther. Med. 18, 769–778. doi: 10.3892/etm.2019.7583
Pientka, F. K., Hu, J., Schindler, S. G., Brix, B., Thiel, A., Jöhren, O., et al. (2012). Oxygen sensing by the prolyl-4-hydroxylase PHD2 within the nuclear compartment and the influence of compartmentalisation on HIF-1 signalling. J. Cell Sci. 125(Pt 21), 5168–55176. doi: 10.1242/jcs.109041
Poleszczuk, J., Hahnfeldt, P., and Enderling, H. (2015). Therapeutic implications from sensitivity analysis of tumor angiogenesis models. PLoS ONE 10, e0120007. doi: 10.1371/journal.pone.0120007
Pu, P., Kang, C., Li, J., and Jiang, H. (2004). Antisense and dominant-negative AKT2 cDNA inhibits glioma cell invasion. Tumour Biol. 25, 172–178. doi: 10.1159/000081099
Qian, B. Z., and Pollard, J. W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51. doi: 10.1016/j.cell.2010.03.014
Ramaswamy, P., Goswami, K., Dalavaikodihalli Nanjaiah, N., Srinivas, D., and Prasad, C. (2019). TNF-α mediated MEK-ERK signaling in invasion with putative network involving NF-κB and STAT-6: a new perspective in glioma. Cell Biol. Int. 43, 1257–1266. doi: 10.1002/cbin.11125
Rawlings, J. S., Rosler, K. M., and Harrison, D. A. (2004). The JAK/STAT signaling pathway. J. Cell Sci. 117(Pt 8), 1281–1283. doi: 10.1242/jcs.00963
Ren, F., Fan, M., Mei, J., Wu, Y., Liu, C., Pu, Q., et al. (2014). Interferon-γ and celecoxib inhibit lung-tumor growth through modulating M2/M1 macrophage ratio in the tumor microenvironment. Drug Des. Devel. Ther. 8, 1527–1538. doi: 10.2147/DDDT.S66302
Riemenschneider, M. J., Jeuken, J. W., Wesseling, P., and Reifenberger, G. (2010). Molecular diagnostics of gliomas: state of the art. Acta Neuropathol. 120, 567–584. doi: 10.1007/s00401-010-0736-4
Roesch, S., Rapp, C., Dettling, S., and Herold-Mende, C. (2018). When Immune cells turn bad-tumor-associated microglia/macrophages in glioma. Int. J. Mol. Sci. 19, 436. doi: 10.3390/ijms19020436
Rong, Y., Belozerov, V. E., Tucker-Burden, C., Chen, G., Durden, D. L., Olson, J. J., et al. (2009). Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res. 69, 2540–2549. doi: 10.1158/0008-5472.CAN-08-1547
Rudge, J. S., Holash, J., Hylton, D., Russell, M., Jiang, S., Leidich, R., et al. (2007). VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc. Natl. Acad. Sci. USA. 104, 18363–18370. doi: 10.1073/pnas.0708865104
Sarkar, S., Nuttall, R. K., Liu, S., Edwards, D. R., and Yong, V. W. (2006). Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 66, 11771–11780. doi: 10.1158/0008-5472.CAN-05-0470
Selth, L. A., Townley, S. L., Bert, A. G., Stricker, P. D., Sutherland, P. D., Horvath, L. G., et al. (2013). Circulating microRNAs predict biochemical recurrence in prostate cancer patients. Br. J. Cancer 109, 641–650. doi: 10.1038/bjc.2013.369
Server, A., Kulle, B., Maehlen, J., Josefsen, R., Schellhorn, T., Kumar, T., et al. (2009). Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema. Acta Radiol. 50, 682–689. doi: 10.1080/02841850902933123
Shao, N., Xue, L., Wang, R., Luo, K., Zhi, F., Lan, Q., et al. (2019). miR-454-3p is an exosomal biomarker and functions as a tumor suppressor in glioma. Mol. Cancer Ther. 18, 459–469. doi: 10.1158/1535-7163.MCT-18-0725
Shi, Z. M., Wang, X. F., Qian, X., Tao, T., Wang, L., Chen, Q. D., et al. (2013). MiRNA-181b suppresses IGF-1R and functions as a tumor suppressor gene in gliomas. RNA 19, 552–560. doi: 10.1261/rna.035972.112
Siegel, R. L., Miller, K. D., and Jemal, A. (2015). Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29. doi: 10.3322/caac.21254
Singh, A., Happel, C., Manna, S. K., Acquaah-Mensah, G., Carrerero, J., Kumar, S., et al. (2013). Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Invest. 123, 2921–2934. doi: 10.1172/JCI66353
Sivasankaran, B., Degen, M., Ghaffari, A., Hegi, M. E., Hamou, M. F., Ionescu, M. C., et al. (2009). Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res. 69, 458–465. doi: 10.1158/0008-5472.CAN-08-2610
Song, L., Liu, L., Wu, Z., Li, Y., Ying, Z., Lin, C., et al. (2012). TGF-β induces miR-182 to sustain NF-κB activation in glioma subsets. J. Clin. Invest. 122, 3563–3578. doi: 10.1172/JCI62339
Su, Y., He, Q., Deng, L., Wang, J., Liu, Q., Wang, D., et al. (2014). MiR-200a impairs glioma cell growth, migration, and invasion by targeting SIM2-s. Neuroreport 25, 12–17. doi: 10.1097/WNR.0000000000000032
Sun, J., Liao, K., Wu, X., Huang, J., Zhang, S., and Lu, X. (2015). Serum microRNA-128 as a biomarker for diagnosis of glioma. Int. J. Clin. Exp. Med. 8, 456–463.
Sun, J., Liu, S.-z., Lin, Y., Cao, X.-p., and Liu, J.-m. (2014). TGF-bpromotes glioma cell growth via activating Nodal expression through Smad and ERK1/2 pathways, Bio-chem. biophysical Res. Commun. 443, 1066–1072. doi: 10.1016/j.bbrc.2013.12.097
Sun, J., Sun, Z., Gareev, I., Yan, T., Chen, X., Ahmad, A., et al. (2021). Exosomal miR-2276-5p in plasma is a potential diagnostic and prognostic biomarker in Glioma. Front. Cell. Dev. Biol. 9, 671202. doi: 10.3389/fcell.2021.671202
Sun, S. C. (2010). CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 17, 25–34. doi: 10.1038/cdd.2009.43
Takahashi, Y., Forrest, A. R., Maeno, E., Hashimoto, T., Daub, C. O., Yasuda, J., et al. (2009). MiR-107 and MiR-185 can induce cell cycle arrest in human nonsmall cell lung cancer cell lines. PloS ONE 4, e6677. doi: 10.1371/journal.pone.0006677
Tektonidis, M., Hatzikirou, H., Chauvière, A., Simon, M., Schaller, K., and Deutsch, A. (2011). Identification of intrinsic in vitro cellular mechanisms for glioma invasion. J. Theor. Biol. 287, 131–147. doi: 10.1016/j.jtbi.2011.07.012
Thiery, J. P., Acloque, H., Huang, R. Y., and Nieto, M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890. doi: 10.1016/j.cell.2009.11.007
Torchilin, V. P., Levchenko, T. S., Rammohan, R., Volodina, N., Papahadjopoulos-Sternberg, B., D'Souza, G. G., et al. (2003). Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl. Acad. Sci. USA. 100, 1972. doi: 10.1073/pnas.0435906100
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., Jemal, A., et al. (2015). Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108. doi: 10.3322/caac.21262
Touat, M., Duran-Peña, A., Alentorn, A., Lacroix, L., Massard, C., and Idbai, A. (2015). Emerging circulating biomarkers in glioblastoma: promises and challenges. Expert Rev. Mol. Diagn. 15, 1311–1323. doi: 10.1586/14737159.2015.1087315
Trompouki, E., Hatzivassiliou, E., Tsichritzis, T., Farmer, H., Ashworth, A., and Mosialos, G. (2003). CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by, TNFR family members. Nature 424, 793–796. doi: 10.1038/nature01803
Tsatas, D., Kanagasundaram, V., Kaye, A., and Novak, U. (2002). EGF receptor modifies cellular responses to hyaluronan in glioblas-toma cell lines. J. Clin. Neurosci. 9, 282–288. doi: 10.1054/jocn.2001.1063
Tseng, Y. C., Tsai, Y. H., Tseng, M. J., Hsu, K. W., Yang, M. C., Huang, K. H., et al. (2012). Notch2-induced COX-2 expression enhancing gastric cancer progression. Mol. Carcinog. 51, 939–951. doi: 10.1002/mc.20865
Tu, Y., Zhong, Y., Fu, J., Cao, Y., Fu, G., Tian, X., et al. (2011). Activation of JAK/STAT signal pathway predicts poor prognosis of patients with gliomas. Med. Oncol. 28, 15–23. doi: 10.1007/s12032-010-9435-1
Turchinovich, A., Weiz, L., Langheinz, A., and Burwinkel, B. (2011). Characterization of extracellular circulating microRNA. Nucleic Acids Res. 39, 7223–7233. doi: 10.1093/nar/gkr254
Valtorta, S., Salvatore, D., Rainone, P., Belloli, S., Bertoli, G., Moresco, R. M., et al. (2020). Molecular and cellular complexity of glioma. focus on tumour microenvironment and the use of molecular and imaging biomarkers to overcome treatment resistance. Int. J. Mol. Sci. 21, 5631. doi: 10.3390/ijms21165631
Wang, H., Li, H., Jiang, Q., Dong, X., Li, S., Cheng, S., et al. (2021). HOTAIRM1 promotes malignant progression of transformed fibroblasts in glioma stem-like cells remodeled microenvironment via regulating miR-133b-3p/TGFβ axis. Front. Oncol. 11, 603128. doi: 10.3389/fonc.2021.603128
Wang, J., Chen, C., Yan, X., and Wang, P. (2019). The role of miR-382-5p in glioma cell proliferation, migration and invasion. Onco. Targets. Ther. 12, 4993–5002. doi: 10.2147/OTT.S196322
Wang, L., Lang, B., Zhou, Y., Ma, J., and Hu, K. (2021). Up-regulation of miR-663a inhibits the cancer stem cell-like properties of glioma via repressing the KDM2A-mediated TGF-β/SMAD signaling pathway [published online ahead of print, 2021 Aug 23]. Cell Cycle. 1–18. doi: 10.1080/15384101.2021.1966962
Wang, L., Shi, Z. M., Jiang, C. F., Liu, X., Chen, Q. D., Qian, X., et al. (2014). MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget 5, 5416–5427. doi: 10.18632/oncotarget.2116
Wang, Q., Li, X., Zhu, Y., and Yang, P. (2014). MicroRNA-16 suppresses epithelial-mesenchymal transition-related gene expression in human glioma. Mol. Med. Rep. 10, 3310–3314. doi: 10.3892/mmr.2014.2583
Wang, W. T., and Chen, Y. Q. (2014). Circulating miRNAs in cancer: from detection to therapy. J. Hematol. Oncol. 7, 86. doi: 10.1186/s13045-014-0086-0
Wang, X., Chen, X., Sun, L., Bi, X., He, H., Chen, L., et al. (2019). MicroRNA-34a inhibits cell growth and migration in human glioma cells via MMP-9. Mol. Med. Rep. 20, 57–64. doi: 10.3892/mmr.2019.10233
Wang, X. R., Luo, H., Li, H. L., Cao, L., Wang, X. F., Yan, W., et al. (2013). Overexpressed let-7a inhibits glioma cell malignancy by directly targeting K-ras, independently of PTEN. Neuro Oncol. 15, 1491–1501. doi: 10.1093/neuonc/not107
Wang, Y., Yang, Q., Cheng, Y., Gao, M., Kuang, L., and Wang, C. (2018). Myosin heavy chain 10 (MYH10) gene silencing reduces cell migration and invasion in the glioma cell lines U251, T98G, and SHG44 by inhibiting the Wnt/β-catenin pathway. Med. Sci. Monit. 24, 9110–9119. doi: 10.12659/MSM.911523
Wang, Z. H., Zhang, Q. S., Duan, Y. L., Zhang, J. L., Li, G. F., Zheng, D. L., et al. (2015). TGF-β induced miR-132 enhances the activation of TGF-β signaling through inhibiting SMAD7 expression in glioma cells. Biochem. Biophys. Res. Commun. 463, 187–192. doi: 10.1016/j.bbrc.2015.05.001
Wei, X., Chen, D., Lv, T., Li, G., and Qu, S. (2016). Serum MicroRNA-125b as a potential biomarker for glioma diagnosis. Mol. Neurobiol. 53, 163–170. doi: 10.1007/s12035-014-8993-1
Wellner, U., Schubert, J., Burk, U. C., Chmalhofer, O., Zhu, F., Sonntag, A., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495. doi: 10.1038/ncb1998
Williams, A. C., Collard, T. J., and Paraskeva, C. (1999). An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene 18, 3199–3204. doi: 10.1038/sj.onc.1202660
Wu, J., and Bresnick, E. H. (2007). Bare rudiments of notch signaling how receptor levels are regulated. Trends Biochem. Sci. 32, 477–485. doi: 10.1016/j.tibs.2007.09.002
Wurdinger, T., Tannous, B. A., Saydam, O., Skog, J., Grau, S., Soutschek, J., et al. (2008). miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell 14, 382–393. doi: 10.1016/j.ccr.2008.10.005
Xiao, Y., Zhang, L., Song, Z., Guo, C., Zhu, J., Li, Z., et al. (2016). Potential diagnostic and prognostic value of plasma circulating microRNA-182 in human glioma. Med. Sci. Monit. 22, 855–862. doi: 10.12659/MSM.897164
Xie, X., Pan, J., Han, X., and Chen, W. (2019). Downregulation of microRNA-532-5p promotes the proliferation and invasion of bladder cancer cells through promotion of HMGB3/Wnt/b-catenin signaling. Chem. Biol. Interact. 300, 73–81. doi: 10.1016/j.cbi.2019.01.015
Xie, Y. K., Huo, S. F., Zhang, G., Zhang, F., Lian, Z. P., Tang, X. L., et al. (2012). CDA-2 induces cell differentiation through suppressing Twist/SLUG signaling via miR-124 in glioma. J. Neurooncol. 110, 179–186. doi: 10.1007/s11060-012-0961-x
Xu, B., Mei, J., Ji, W., Huo, Z., Bian, Z., Jiao, J., et al. (2021). MicroRNAs involved in the EGFR pathway in glioblastoma. Biomed Pharmacother. 134, 111115. doi: 10.1016/j.biopha.2020.111115
Xu, C. H., Liu, Y., Xiao, L. M., Chen, L. K., Zheng, S. Y., Zeng, E. M., et al. (2019). Silencing microRNA-221/222 cluster suppresses glioblastoma angiogenesis by suppressor of cytokine signaling-3-dependent JAK/STAT pathway. J. Cell. Physiol. 234, 22272–22284. doi: 10.1002/jcp.28794
Xuan, Y., Yang, H., Zhao, L., Lau, W. B., Lau, B., Ren, N., et al. (2015). MicroRNAs in colorectal cancer: small molecules with big functions. Cancer Lett. 360, 89–105. doi: 10.1016/j.canlet.2014.11.051
Yan, Y., Yan, H., Wang, Q., Zhang, L., Liu, Y., Yu, H., et al. (2019). MicroRNA 10a induces glioma tumorigenesis by targeting myotubularin-related protein 3 and regulating the Wnt/β-catenin signaling pathway. FEBS J. 286, 2577–2592. doi: 10.1111/febs.14824
Yancopoulos, G. D. (2010). Clinical application of therapies targeting VEGF. Cell 143, 13–16. doi: 10.1016/j.cell.2010.09.028
Yang, G., Zhang, R., Chen, X., Mu, Y., Ai, J., Shi, C., et al. (2011). MiR-106a inhibits glioma cell growth by targeting E2F1 independent of p53 status. J. Mol. Med. 89, 1037–1050. doi: 10.1007/s00109-011-0775-x
Yang, J., Yu, D., Liu, X., Changyong, E., and Yu, S. (2020). LINC00641/miR-4262/NRGN axis confines cell proliferation in glioma. Cancer Biol. Ther. 21, 758–766. doi: 10.1080/15384047.2020.1776581
Yang, L., Liu, M., Deng, C., Gu, Z., and Gao, Y. (2012). Expression of transforming growth factor-b1 (TGF-b1) and E-cadherin in glioma. Tumor Biol. 33, 1477–1484. doi: 10.1007/s13277-012-0398-z
Yang, M. H., Wu, M. Z., Chiou, S. H., Chen, P. M., Chang, S. Y., Liu, C. J., et al. (2008). Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 10, 295–305. doi: 10.1038/ncb1691
Yang, Q., Zhou, Y., Chen, J., Huang, N., Wang, Z., Cheng, Y., et al. (2021). Gene therapy for drug-resistant glioblastoma via lipid-polymer hybrid nanoparticles combined with focused ultrasound. Int. J. Nanomedicine 16, 185–199. doi: 10.2147/IJN.S286221
Yang, T. Q., Lu, X. J., Wu, T. F., Ding, D. D., Zhao, Z. H., Chen, G. L., et al. (2014). MicroRNA-16 inhibits glioma cell growth and invasion through suppression of BCL2 and the nuclear factor-κB1/MMP9 signaling pathway. Cancer Sci. 105, 265–271. doi: 10.1111/cas.12351
Yang, W., Ma, J., Zhou, W., Zhou, X., Cao, B., Zhang, H., et al. (2017). Molecular mechanisms and clinical implications of miRNAs in drug resistance of esophageal cancer. Expert Rev. Gastroenterol. Hepatol. 11, 1151–1163. doi: 10.1080/17474124.2017.1372189
Yao, W., Guo, G., Zhang, Q., Fan, L., Wu, N., and Bo, Y. (2014). The application of multiple miRNA response elements enables oncolytic adeno-viruses to possess specificity to glioma cells. Virology 458-459, 69–82. doi: 10.1016/j.virol.2014.04.007
Yeh, C. T., Huang, W. C., Rao, Y. K., Ye, M., Lee, W. H., Wang, L. S., et al. (2013). A sesquiterpene lactone antrocin from Antrodia camphorata negatively modulates JAK2/STAT3 signaling via microRNA let-7c and induces apoptosis in lung cancer cells. Carcinogenesis 34, 2918–2928. doi: 10.1093/carcin/bgt255
Yin, F., Zhang, J. N., Wang, S. W., Zhou, C. H., Zhao, M. M., Fan, W. H., et al. (2015). MiR-125a-3p regulates glioma apoptosis and invasion by regulating Nrg1. PLoS ONE. 10, e0116759. doi: 10.1371/journal.pone.0116759
Yu, H., and Jove, R. (2004). The STA Ts of cancer-new molecular targets come of age. Nat. Rev. Cancer. 4, 97–105. doi: 10.1038/nrc1275
Yue, X., Lan, F., Hu, M., Pan, Q., Wang, Q., and Wang, J. (2016). Downregulation of serum microRNA-205 as a potential diagnostic and prognostic biomarker for human glioma. J. Neurosurg. 124, 122–128. doi: 10.3171/2015.1.JNS141577
Zakaria, R., Das, K., Radon, M., Bhojak, M., Rudland, P. R., Sluming, V., et al. (2014). Diffusion-weighted MRI characteristics of the cerebral metastasis to brain boundary predicts patient outcomes. BMC Med. Imaging. 14, 26. doi: 10.1186/1471-2342-14-26
Zhang, B., Gu, F., She, C., Guo, H., Li, W., Niu, R., et al. (2009). Reduction of Akt2 inhibits migration and invasion of glioma cells. Int. J. Cancer 125, 585–595. doi: 10.1002/ijc.24314
Zhang, H., Jiang, H., Zhang, H., Liu, J., Hu, X., Chen, L., et al. (2019). miR-4262, low level of which predicts poor prognosis, targets proto-oncogene CD163 to suppress cell proliferation and invasion in gastric cancer. Onco. Targets. Ther. 12, 599–607. doi: 10.2147/OTT.S187881
Zhang, J., Han, L., Ge, Y., Zhou, X., Zhang, A., Zhang, C., et al. (2010). miR-221/222 promote malignant progression of glioma through activation of the Akt pathway. Int. J. Oncol. 36, 913–920. doi: 10.3892/ijo_00000570
Zhang, N., Shi, X. M., Guo, H. Q., Zhao, X. Z., Zhao, W. W., Xu, J. J., et al. (2018). Gold nanoparticle couples with entropy-driven toehold-mediated DNA strand displacement reaction on magnetic beads: toward ultrasensitive energy-transfer-based photoelectrochemical detection of miRNA-141 in real blood sample. Anal. Chem. 90, 11892–11898. doi: 10.1021/acs.analchem.8b01966
Zhang, P., Chen, F. Z., Jia, Q. B., and Hu, D. F. (2019). Upregulation of microRNA-133a and downregulation of connective tissue growth factor suppress cell proliferation, migration, and invasion in human glioma through the JAK/STAT signaling pathway. IUBMB Life. 71, 1857–1875. doi: 10.1002/iub.2126
Zhang, Y., Tu, L., Zhou, X., and Li, B. (2020). MicroRNA-22 regulates the proliferation, drug sensitivity and metastasis of human glioma cells by targeting SNAIL1. J BUON. 25, 491–496.
Zheng, X., Jiang, F., Katakowski, M., Kalkanis, S. N., Hong, X., Zhang, X., et al. (2007). Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci. 98, 674–684. doi: 10.1111/j.1349-7006.2007.00440.x
Zhou, M., Wang, H., Zhou, K., Luo, X., Pan, X., Shi, B., et al. (2013). A novel EGFR isoform confers increased invasiveness to cancer cells. Cancer Res. 73, 7056–7067. doi: 10.1158/0008-5472.CAN-13-0194
Zhu, C., Ren, C., Han, J., Ding, Y., Du, J., Dai, N., et al. (2014). A five-microRNA panel in plasma was identified as potential biomarker for early detection of gastric cancer. Br. J. Cancer 110, 2291–2299. doi: 10.1038/bjc.2014.119
Keywords: glioma, miRNAs, migration and invasion, biological implications, clinical potential
Citation: Guo X, Jiao H, Cao L and Meng F (2022) Biological implications and clinical potential of invasion and migration related miRNAs in glioma. Front. Integr. Neurosci. 16:989029. doi: 10.3389/fnint.2022.989029
Received: 08 July 2022; Accepted: 07 November 2022;
Published: 21 November 2022.
Edited by:
Elizabeth B. Torres, Rutgers, The State University of New Jersey, United StatesReviewed by:
Saumya Patel, Gujarat University, IndiaCopyright © 2022 Guo, Jiao, Cao and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Facai Meng, bWVuZ2ZjQGZveG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.