
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Integr. Neurosci. , 11 January 2021
Volume 14 - 2020 | https://doi.org/10.3389/fnint.2020.616778
This article is part of the Research Topic The Importance of Interneurons in Neuronal Circuitry View all 11 articles
 Zhiyun Zhang1,2
Zhiyun Zhang1,2 Dongsheng Xu2
Dongsheng Xu2 Jia Wang2
Jia Wang2 Jingjing Cui2
Jingjing Cui2 Shuang Wu2
Shuang Wu2 Ling Zou2
Ling Zou2 Yi Shen2
Yi Shen2 Xianghong Jing1,2*
Xianghong Jing1,2* Wanzhu Bai2*
Wanzhu Bai2*Objective: To investigate the sensory and sympathetic innervations associated with both acupoint “Shenshu” (BL23) and kidney in the rat for insight into the neuronal correlation between the Back-Shu Point and its corresponding visceral organ.
Methods: The BL23 and kidney were selected as the representative acupoint and visceral organ in this study, in which their local nerve fibers were examined by using double fluorescent immunohistochemistry with calcitonin gene-related peptide (CGRP) and tyrosine hydroxylase (TH). Meanwhile, their neuronal correlation in the dorsal root ganglia (DRGs), spinal cord, and sympathetic (paravertebral) chain were investigated using a double fluorescent neural tracing technique with Alexa Fluor 488 and 594 conjugates with cholera toxin subunit B (AF488/594-CTB).
Results: The local tissue of acupoint BL23 and the fibrous capsule of kidney distributed abundantly with CGRP- and TH-positive nerve fibers, corresponding to their sensory and sympathetic innervation. On the other hand, the sensory neurons associated with acupoint BL23 and kidney were labeled with AF488/594-CTB and distributed from thoracic (T) 11 to lumbar (L) 3 DRGs and from T10 to L2 DRGs, respectively, in which some of them in T12-T13 DRGs were simultaneously labeled with both AF488/594-CTB. Also, postganglionic neurons associated with both acupoint BL23 and kidney were found in the sympathetic chain at the same spinal segments but separately labeled with AF488-CTB and AF594-CTB.
Conclusion: Our study demonstrates the neural characteristics of the acupoint BL23 and kidney in the rat from the perspective of neurochemistry and neural pathways, providing an example for understanding the neuronal correlation between the Back-Shu Points and their corresponding visceral organs. These results suggest that the stimulation of the Back-Shu Points may regulate the activities of the target-organs via the periphery sensory and sympathetic pathways.
Back-Shu Points are the specific acupoints on the back, which are named as per their anatomical locations adjacent to visceral organs (in terms of Zang-Fu in Traditional Chinese Medicine; Cao et al., 2017). Although this kind of acupoints is commonly used for regulating the disorders of their corresponding visceral organs following the vicinal principle of acupoint selection, there is a lack of clear understanding of their inherent links (Cheng, 2011; Tu et al., 2019; Dai et al., 2020; Shou et al., 2020). Considering the diagnostic and therapeutic roles of Back-Shu Points playing in visceral diseases, viscerocutaneous reflexes have been paid more attention in this field. However, their neuronal correlation remains unclear (Cabioglu and Arslan, 2008; da Silva, 2010; Liu et al., 2010).
To reveal the inherent links between the Back-Shu Points and their corresponding visceral organs in detail, the acupoint “Shenshu” (BL23) and kidney were selected as the representative targets and examined with neuroanatomical approaches in the present study.
First, a double fluorescent immunohistochemistry with calcitonin gene-related peptide (CGRP) and tyrosine hydroxylase (TH) was employed to observe the sensory and sympathetic innervation in the local tissues of acupoint BL23 and the fibrous capsule of the kidney, respectively (Benarroch, 2011; Chakrabarty et al., 2013; Cui et al., 2015; Wang et al., 2019b). Second, the neural elements associated with acupoint BL23 and kidney were traced by using a double fluorescent neural tracing technique with Alexa Fluor 488 and 594 conjugates of cholera toxin subunit B (AF488/594-CTB) for figuring out the neuronal correlation between the acupoint BL23 and kidney. Through the injection of different neural tracers, sensory, sympathetic and motor neurons associated with BL23 and kidney can be observed. These two kinds of effective tools have been successfully applied in the field of acupuncture research (Xu et al., 2016; Wang et al., 2018b; Cui J. J. et al., 2019). By taking their advantages, we expect not only to determine the innervated characteristics of acupoint BL23 and kidney individually but also to outline the neuronal correlation between them via the sensory and sympathetic pathways. From the perspectives of neurochemistry and neural pathways, this research could provide valuable references for understanding the inherent links of Back-Shu Points and their corresponding visceral organs at the cellular level.
A total of eight young adult male Sprague–Dawley rats (8–10 weeks, weight 180–210 g) were used in the present study. Animals [license number SCXK(JING) 2017-0005] were provided by the National Institutes for Food and Drug Control. All animals were maintained with free access to water and food under controlled conditions with a 12 h light and dark cycle at a temperature of 24 ± 2°C. This study was approved by the ethics committee of the Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences (reference number 20160011). All surgical procedures were performed following guidelines for animal experiments by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, USA, 1996).
The BL23 and kidney were selected as the representative acupoint and visceral organ in this study. The corresponding site of BL23 on the rat was determined by relative anatomy (Xu et al., 2019). The BL23 locates at the same level as the inferior border of the spinous process of the second lumbar vertebra, 1.5 B-cun lateral to the posterior median line on the human (Huang and Huang, 2007). Under the respiratory anesthesia (1.5% isoflurane), a total of 4 μl 1% AF488-CTB (Invitrogen-Molecular Probes, Eugene, OR, USA) was injected subcutaneously and muscularly into the left side of BL23, while 2 μl 1% AF594-CTB was also injected into the left side of the fibrous capsule of the kidney that was exposed by local surgery. Hamilton micro-syringe was used for the injection. To prevent leakage of the solution, the syringe was left in place for an additional 5 min after injection and then withdrawn slowly.
Three days after injection, the rats were transcardially perfused with 100 ml of 0.9% saline followed by 300 ml of 4% paraformaldehyde in 0.1 M phosphate-buffered solution (PB, pH = 7.4). The local tissues of BL23 (at length of 5 mm and width of 3 mm) and the fibrous capsule of the kidney on the right side were taken for examining the nerve fibers in the regional distribution, while the spinal cord, dorsal root ganglia (DRGs), and sympathetic (paravertebral) chain on the left side were dissected out and stored in 25% sucrose in 0.1 M PB at 4°C for 2 days.
Serial transverse sections of the skin tissue of BL23 and longitudinal sections of the sympathetic chain were cut at a thickness of 20 μm on a cryostat (Thermo, Microm International FSE, Germany) and mounted on silane-coated glass slides, while the sections of the spinal cord and DRGs were cut in the transverse and sagittal pattern, respectively on a freezing microtome (Microm International HM 430, Thermo, Germany). Also, the fibrous capsule of the kidney was mounted on silane-coated glass slides in a whole-mount pattern.
The mounted sections of skin tissue of BL23 and the fibrous capsule of the kidney were simultaneously stained by using double fluorescent immunohistochemistry with CGRP and TH. First, the tissues were incubated in a blocking solution containing 3% normal donkey serum and 0.5% Triton X-100 in 0.1 M PB for 30 min and then transferred to mouse anti-CGRP monoclonal antibody (1:1,000, Abcam, Hong Kong) and rabbit anti-TH antibody (1:1,000, Abcam, Hong Kong) for overnight at 4°C. On the following day, after washing three times with 0.1 M PB, the tissues were exposed to donkey anti-mouse Alexa Fluor 594, donkey anti-rabbit Alexa Fluor 488 secondary antibodies (1:500, Molecular Probes, Eugene, OR, USA), and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1:40,000; Molecular Probes, Eugene, OR, USA) for 2 h of incubation. After washing, the tissues were coverslipped with 50% glycerin for observation. In contrast, the neuronal labeling with AF488/594-CTB in the sections of the spinal cord, DRGs, and sympathetic chain can be directly observed without further staining.
The anatomical structure of tissue sections from the spinal cord was based on Paxinos and Watson (2006). Samples were viewed and analyzed under a fluorescent microscope equipped with a digital camera (DP73, Olympus, Japan) or a laser scanning confocal microscope (FV1200, Olympus, Tokyo, Japan) equipped with a digital camera (DP70, Olympus, Tokyo, Japan). Three-dimensional reconstruction of CGRP- and TH-positive nerve fibers were performed using Imaris 7.7.1 software. Final images were processed with Adobe Photoshop/Illustrator CS5 (Adobe Systems, San Jose, CA, USA). The illustration was drawn with Adobe Illustrator CS5.
Twenty images captured by fluorescent microscope (10× magnification) were randomly selected to measure the length of nerve fibers in the local tissue of BL23 and the fibrous capsule of the kidney by cellSens dimension software. The density of CGRP- and TH-positive nerve fibers were compared according to the ratio of the length of nerve fibers to the local area. Data was expressed with mean ± standard error of the mean. The statistical analysis was performed by two-tailed Student’s t-test using the GraphPad Prism software 7.0.
By using double fluorescent immunohistochemistry with CGRP and TH, there were abundant CGRP- and TH-positive nerve fibers observed in acupoint BL23 and the fibrous capsule of the kidney (Figures 1A, 2A). These two kinds of nerve fibers ran together or separately. Although some of them are located closely in an intermingling pattern, by three-dimensional analysis, these two types of nerve fibers are distributed independently of each other (Figures 1B, 2B). Furthermore, the length of nerve fibers was quantitatively analyzed within the same twenty areas (2,790 μm × 2,091 μm). As a comparison, the density of TH-positive nerve fibers was significantly higher than that of CGRP-positive nerve fibers in both regions (Figures 1C, 2C).
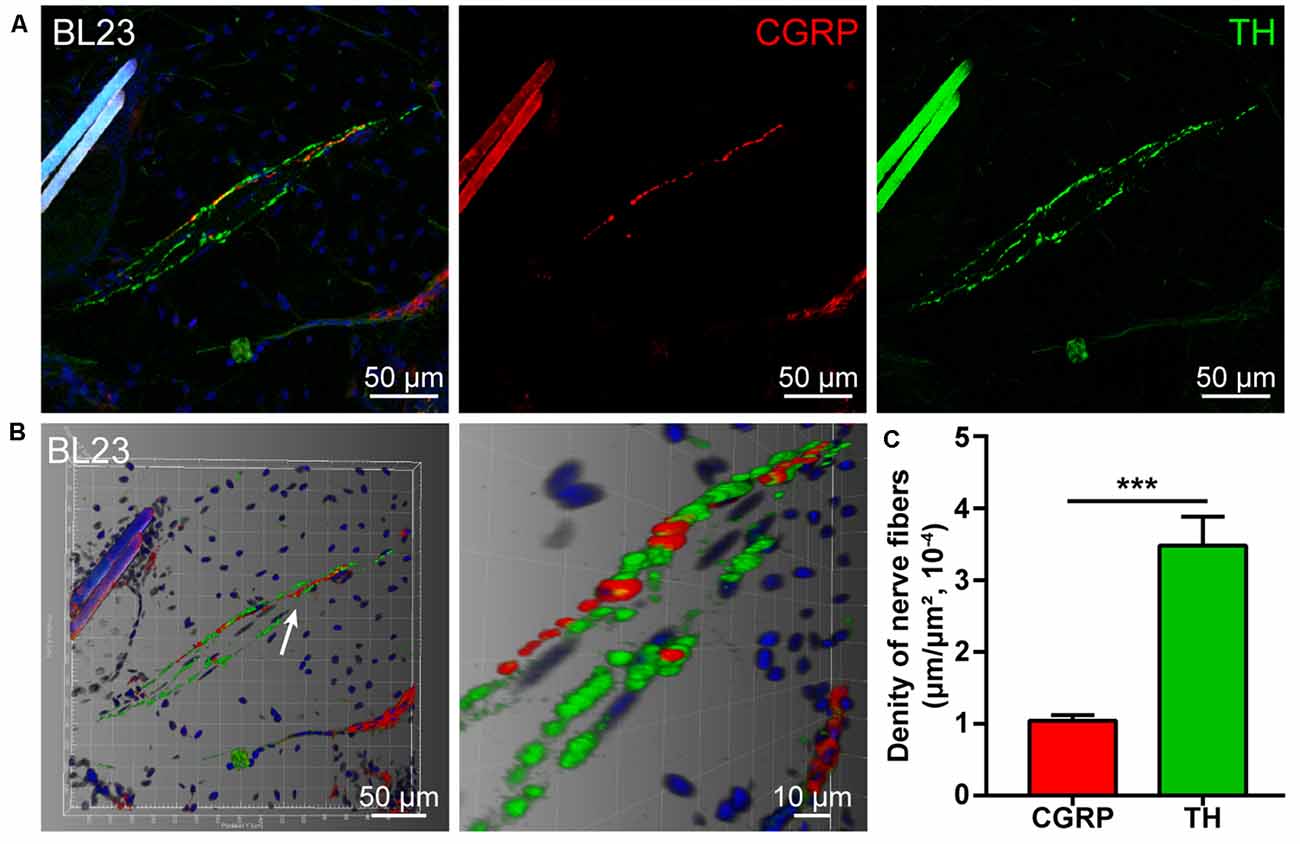
Figure 1. Distribution of the calcitonin gene-related peptide (CGRP)- and tyrosine hydroxylase (TH)-positive nerve fibers in the local tissue of BL23. (A) Representative images of CGRP- and TH-positive nerve fibers in the local tissue of BL23. (B) Adjusted images from (A) with three-dimensional reconstruction in a sloping pattern showing the CGRP- and TH-labeling, respectively. (C) The density of CGRP- and TH-positive nerve fibers distributed in the local tissue of BL23 ( ± SEM, n = 6). ***p < 0.001.
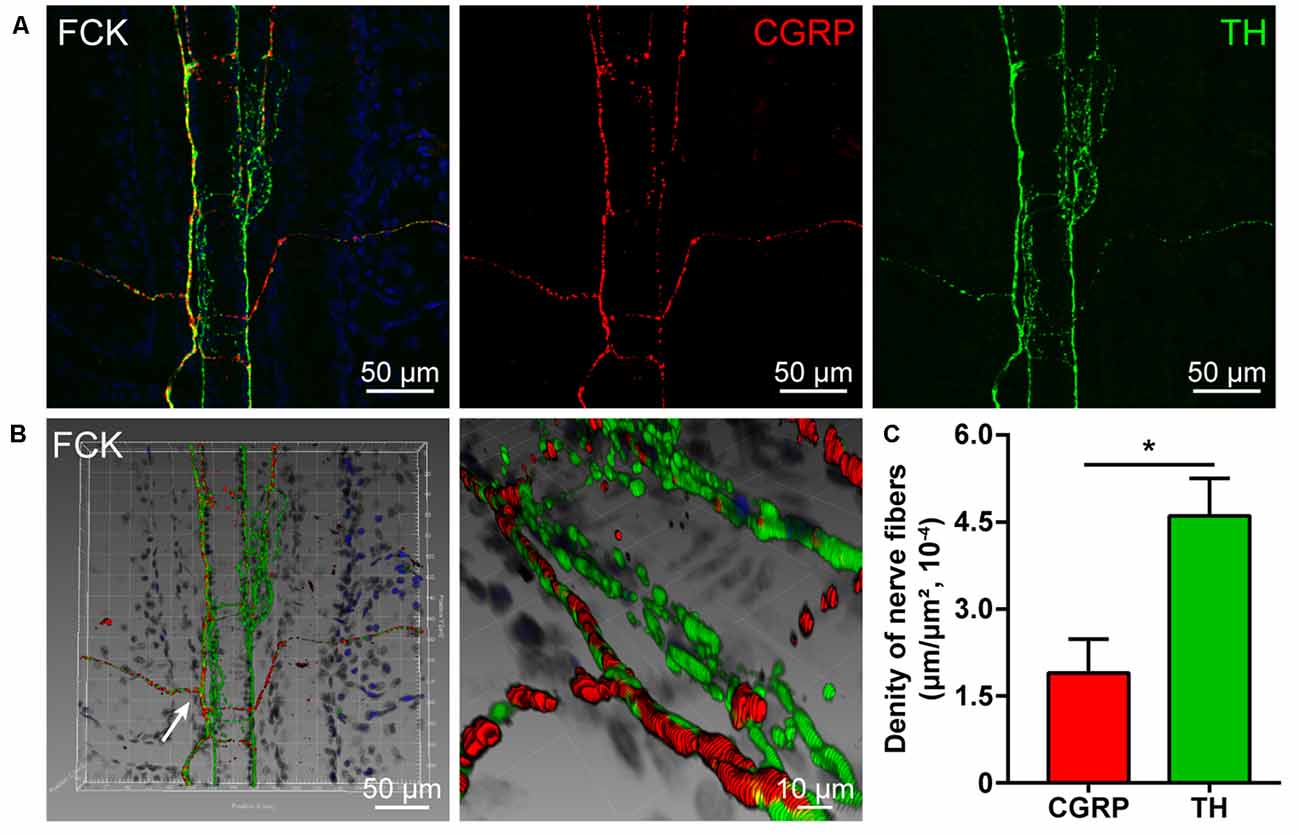
Figure 2. Distribution of the CGRP- and TH-positive nerve fibers in the fibrous capsule of the kidney. (A) Representative images of CGRP- and TH-positive nerve fibers in the fibrous capsule of the kidney. (B) Adjusted images from panel (A) with three-dimensional reconstruction in a sloping pattern showing the CGRP- and TH- labeling, respectively. (C) The density of CGRP- and TH-positive nerve fibers distributed in the fibrous capsule of the kidney ( ± SEM, n = 6). *p < 0.05. FCK refers to the fibrous capsule of the kidney.
The sensory neurons associated with acupoint BL23 were labeled with AF488-CTB and observed from thoracic (T) 10 to lumbar (L) 2 DRGs with a higher concentration in T12-T13 DRGs. While the sensory neurons related to the kidney were labeled with AF594-CTB and detected from T10 to L1 DRGs with a higher concentration in T13 DRG. Comparatively, some sensory neurons were simultaneously labeled with AF488/594-CTB in T12-T13 DRGs (Figures 3A,B).
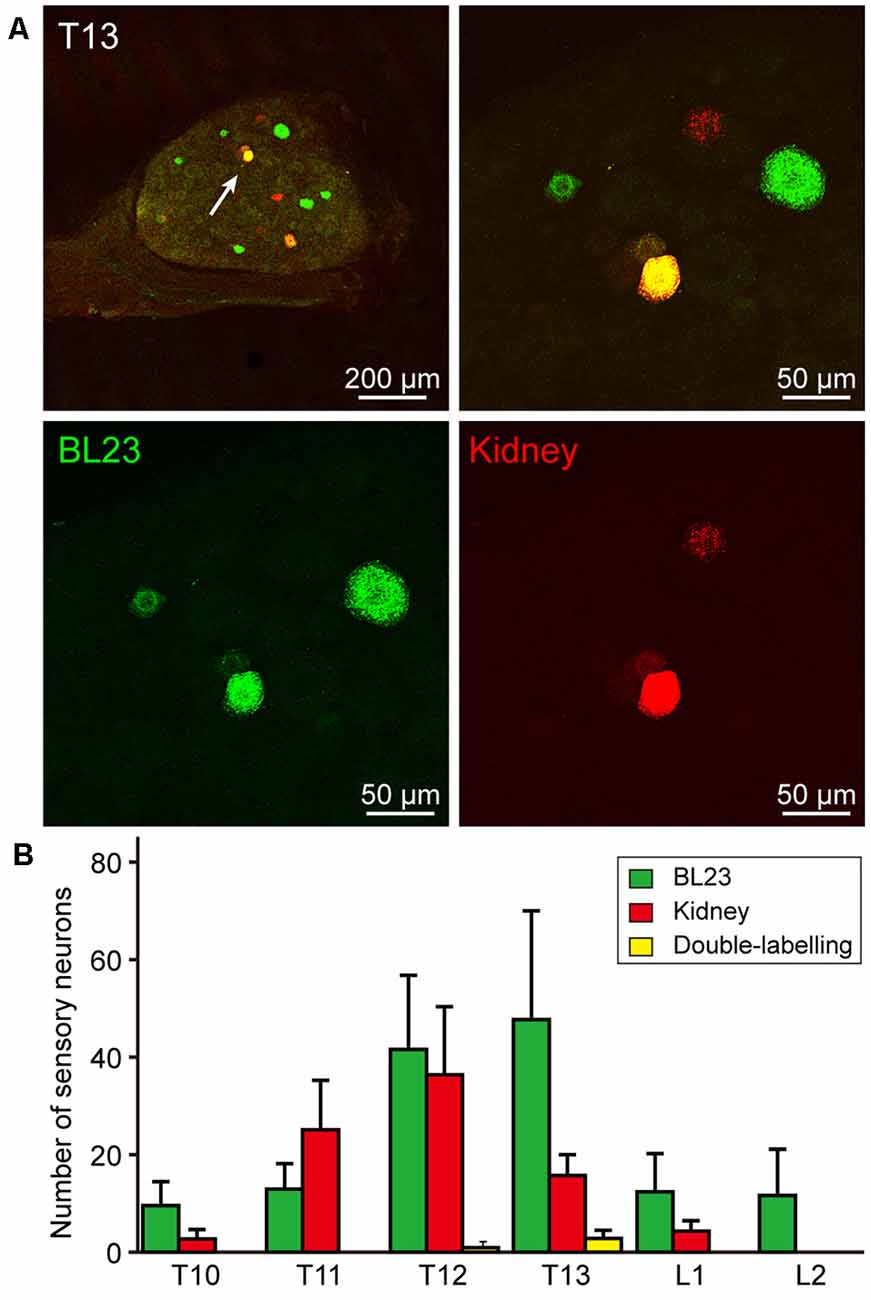
Figure 3. Sensory neurons associated with the acupoint BL23 and kidney in the dorsal root ganglia (DRGs). (A) A representative and magnified (arrowhead) photomicrograph showing the distribution of AF488/594-CTB labeled sensory neurons in T13 DRG. The double-labeled neurons with AF488/594-CTB presenting in yellow. (B) The number of labeled sensory neurons in the thoracic (T) and lumbar (L) DRGs ( ± SEM, n = 8).
Also, postganglionic neurons associated with acupoint BL23 and kidney were found in the sympathetic chain at the same lumbar segments, but they were separately labeled with AF488-CTB or AF594-CTB (Figure 4A). By counting the labeled neurons, the number of the postganglionic neurons associated with the kidney was higher than that of BL23 (Figure 4B).
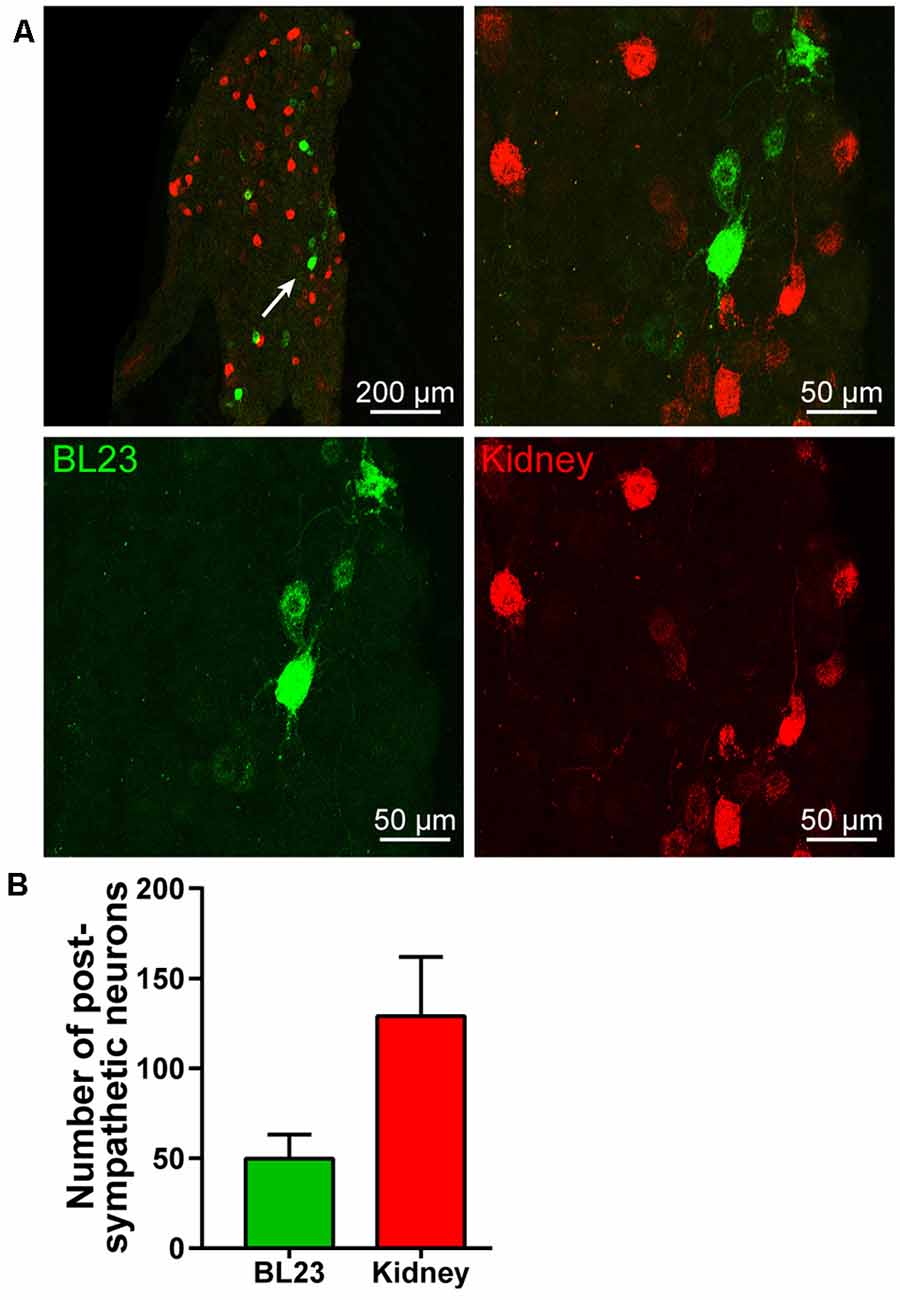
Figure 4. Postympathetic neurons associated with the acupoint BL23 and kidney in the sympathetic chain. (A) A representative and magnified (arrowhead) photomicrograph showing the distribution of AF488/594-CTB labeled post sympathetic neurons in the sympathetic chain at the lumbar segments. (B) The number of labeled post sympathetic neurons in the sympathetic chain ( ± SEM, n = 8).
Besides the sensory and sympathetic innervation, the motor innervation was also examined. The motor neurons associated with the acupoint BL23 were also observed in the spinal ventral horn at cervical, thoracic, and lumbar segments, respectively (Figure 5). The number of labeled motor neurons was not further counted in the present study. As a technical limitation, parasympathetic innervation was not demonstrated in the present study.
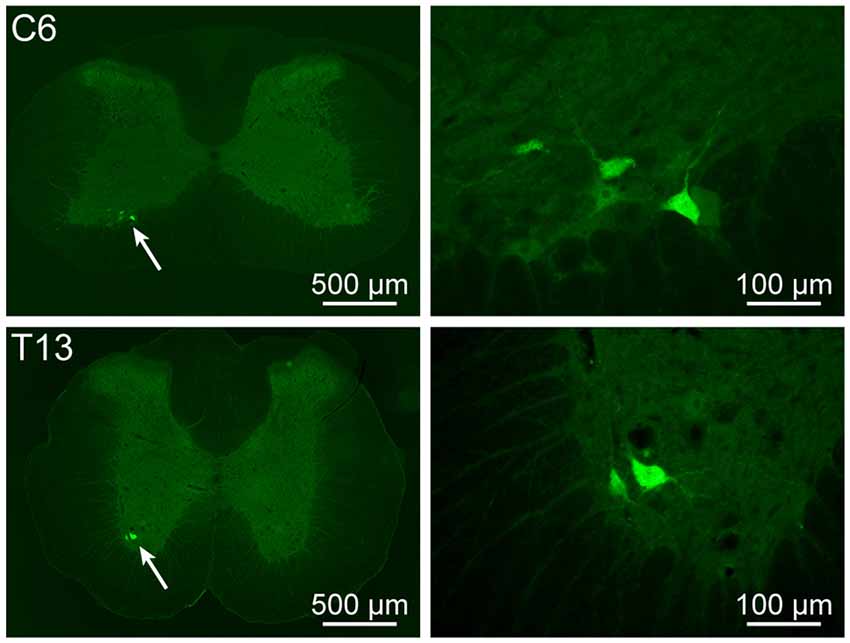
Figure 5. Motor neurons associated with the acupoint BL23 in the spinal ventral horn. The representative and magnified (arrowhead) photomicrographs showing the distribution of AF488-CTB labeled motor neurons at cervical (C) 6 and thoracic (T) 13 segments.
By applying the immunohistochemical and neural tracing techniques, we provide detailed information to insight into the sensory and sympathetic innervation associated with acupoint BL23 and kidney, including the nerve fibers in local tissues, sensory neurons in the DRGs, and sympathetic neurons in the paravertebral chain.
From a technical point of view, the double fluorescent immunohistochemistry and neural tracing techniques have been effectively used in the field of acupuncture research (Wu et al., 2015b; Wang et al., 2018a, 2019a). Taking the advantages of double fluorescent immunohistochemistry, it makes a sharp contrast between the CGRP- and TH-positive nerve fibers on the local tissue of acupoint BL23 and the fibrous capsule of the kidney. Since the innervations of both the kidney and its fibrous capsule come from the same origin by way of the renal plexus (Mulder et al., 2013; Mompeo et al., 2016; van Amsterdam et al., 2016), the nerve fibers in the kidney were not further examined in the present study. A previous study has shown that CGRP- and TH-positive nerve fibers were distributed widely in the renal parenchymal (Mulder et al., 2013).
For the neural tracing approach, AF488/594-CTB have been frequently used to compare the sensory and motor innervations of the different acupoints as well as the sensory and sympathetic innervations of visceral organs (Cui et al., 2013; Wu et al., 2015a; Zhang et al., 2015, 2018). Using the same approach, we further revealed the innervated characteristics of acupoint BL23 and kidney individually, and also outlined the neuronal correlation between them via the sensory and sympathetic pathways.
It should be noted here that the labeled neurons with AF594/488-CTB belong to the first-order neurons associated with the acupoint BL23 and kidney. Although numerous studies have shown that the neurons in the nucleus tractus solitarius can be activated by acupuncture stimulation (Fang et al., 2017; Xiao et al., 2018), these higher-order neurons cannot be traced in this study, which might be transsynaptically traced with neurotropic viruses (Wyss and Donovan, 1984; Weiss and Chowdhury, 1998; Holt et al., 2019). The other limitation of this study should be also emphasized here that, since non-acupoints are still a controversial issue, a control group of non-acupoints was not included in our study (Kim et al., 2017; Liu, 2019).
By this study, it is clearly shown that the chemical innervation of the local tissue of acupoint BL23 and the fibrous capsule of the kidney includes the CGRP- and TH-positive nerve fibers. As a comparison, CGRP and TH are expressed separately on different kinds of nerve fibers running in a parallel or individual pattern.
As a sensitive bio-marker, CGRP expresses on the sensory nerve fibers that are widely distributed in the skin and visceral tissues (Eftekhari et al., 2013; Carr and Frings, 2019; Li et al., 2019; Smolilo et al., 2020). Previous studies have shown that CGRP-positive nerve fibers originate from the sensory neurons in DRGs and distribute widely in the local skin tissues of acupoints and the visceral tissues (Ha et al., 2014; Fan et al., 2018; De Logu et al., 2019). Growing evidence indicates that upregulated CGRP can cause vasodilatation and neurogenic inflammation during the pain and plays a critical role in the development of peripheral and central sensitization through the nociceptive signaling pathway (Russell et al., 2014; Iyengar et al., 2017). For the TH, it is the rate-limiting enzyme in the biosynthesis of the catecholamines dopamine, noradrenaline (NA, also known as norepinephrine), and adrenaline (Briggs et al., 2013; Dickson and Briggs, 2013). In the sympathetic nervous system, NA stores in the postganglionic neurons and their nerve fibers with TH-positive expression, serving as the main neurotransmitter released from the sympathetic nerve endings for controlling vasoconstriction (Mukouyama, 2014; Connolly et al., 2015). Accordingly, TH-positive nerve fibers distribute extensively in cutaneous tissues and internal organs (Rodionova et al., 2016; Enríquez-Pérez et al., 2017; Zhang et al., 2020).
Taken together, it indicates that NA and CGRP are a pair of opposite messengers for regulating the vasoconstriction and vasodilatation in response to the local stimulation of the peripheral sensory and sympathetic nerve fibers in the skin (Hodges and Johnson, 2009; Thomas, 2011; Zhang et al., 2020). Although both NA and CGRP play significant roles in maintaining the balance of homeostasis, how the stimulation of the acupoint to affect the release of these chemical messengers in its corresponding visceral organ remains to be studied under the physiological and pathological conditions.
In parallel to observing the distribution of the sensory and sympathetic nerve fibers, the origins of sensory and postganglionic neurons associated with the acupoint BL23 and kidney were further determined with the neural tracing approach in this study. Even though most of the neurons were separately labeled with AF488-CTB or AF594-CTB, they locate adjacently in the DRGs and sympathetic chain at the same spinal segments, and some of the sensory neurons were simultaneously labeled with both AF488/594-CTB (Figure 6). These results indicate that there are a direct sensory correlation and an indirect sympathetic relationship between the acupoint BL23 and the kidney. Considering the sensory and sympathetic correlation, it is reasonable to speculate that stimulating signals from the acupoint BL23 area could be either directly or indirectly transported along the sensory and sympathetic pathways to the kidney, conversely, the disorders of the kidney might also be reflected on the body surface through the same routines. A similar neuronal correlation was also observed between the acupoint BL23 and the adrenal gland in our previous study (Zhang et al., 2018). In the present research, we provide another example to explain the neuronal correlation between the Back-Shu points and their corresponding visceral organs via the sensory and sympathetic pathways.
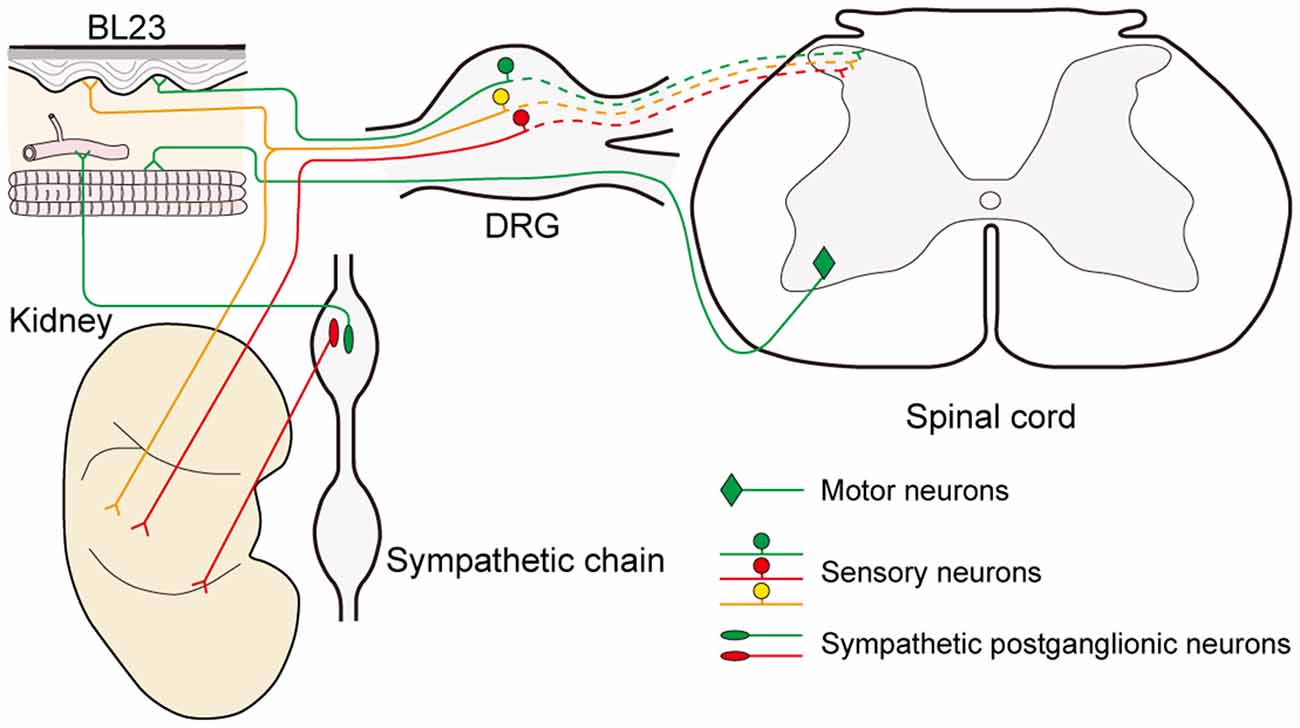
Figure 6. Illustration of the sensory, sympathetic, and motor correlation between the acupoint BL23 and kidney.
Besides the sensory and sympathetic innervations, we also observed the motor neurons that are relative to acupoint BL23 only, and not to the kidney. Additionally, other parasympathetic elements, such as the vagal dorsal motor nucleus and nodose ganglion were not examined in the present study, but it is an important issue to be solved in the future study.
In general, the selection of acupoints along the course of meridians is the basic principle in the treatment of acupuncture. Unlike acupoints on the extremities to be orderly arranged in twelve meridians, it is an exception for the application of Back-Shu Points, because all of them belong to the bladder meridian of Foot-Taiyang, but each of them is responsible for reflecting and treating the disorder of visceral organs in corresponding to its name (Jia and Zhao, 2005). As it is known, certain areas of the skin sensitively responding to visceral diseases has been recorded in ancient Chinese medical texts (Cui X. et al., 2019), while the similar phenomenon has also been systematically investigated by the English neurologist Head (1861–1940) basing on the clinical findings of visceral disease associated with cutaneous manifestations as hyperalgesia or allodynia, and termed these areas as “Head zones” (Head, 1893). A recent study indicated that Back-Shu Points coincide spatially and functionally with the “maximum points” as described within the Head zones (Beissner et al., 2011; Beltrán Molano et al., 2014). According to the anatomo-functional correlation between the Back-Shu Points or the maximum points with their corresponding visceral organs, the most often considered explanation for their interactions is the viscerocutaneous reflexes (Gao et al., 2010; Rong et al., 2011). Although the exact mechanism behind viscerocutaneous reflexes is far from being fully understood, our neuroanatomical evidence supports the idea that viscerocutaneous reflexes could be an important approach for the neuronal interactions between the acupoint BL23 and kidney, which might be started as early as at the level of DRGs and sympathetic chain.
Coincidently, the “stimulating peripheral activity to relieve conditions (SPARC)” program to be funded by the National Institutes of Health is also aimed to investigate the complex pathways of nerve-organ interactions for ultimately promoting the precise treatment of diseases and conditions through peripheral electro-stimulation. Both acupuncture and SPARC are established by way of stimulating the peripheral nervous system. Therefore, figuring out the correlation between two types of peripheral nerves, somatic and visceral, could serve as a great source of inspiration for modern acupuncture research. As representative targets of acupoint and visceral organs, our study in the neuronal correlation of BL23 and kidney may provide an example to examine the interconnection between somatic and visceral nerves.
Increasing evidence shows that visceral diseases can induce the referred pain or sensitization on the body surface at their corresponding spinal segments in patients and experimental rats, which were closely correlated with the locations of traditional acupoints (Chen et al., 2014; Shi et al., 2018). In facing the sophisticated neuronal interconnection between the acupoints and visceral organs, from the viewpoint of the neural pathway, Back-Shu Points could be an important starting for observing the neuronal correlation with their corresponding visceral organs. The precise neural network how to serve the signal communication between the Back-Shu Points and their corresponding visceral organs might be a new issue in future studies.
In summary, by using the double fluorescent immunohistochemistry and neural tracing techniques, we have provided histological evidence to benefit the understanding of the sensory and sympathetic innervation of the acupoint BL23 and kidney. Considering the chemical characteristics and neural pathways, the acupoint BL23 and kidney establish a close sensory and sympathetic correlation between each other. Although present data cannot explain how the acupoint BL23 works on the kidney, this neuroanatomical connectivity may provide clues for exploring the mechanism of the role of neural pathways between the acupoint BL23 and kidney, which might also provide insights into the general rules underlying the other Back-Shu Points and their corresponding visceral organs.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by ethics committee of the Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences.
The manuscript presented here was carried out in collaboration between all authors. ZZ, DX, SW, YS, and LZ carried out the experiments. ZZ, DX, JW, and JC analyzed the data. XJ and WB designed the study. ZZ, JW, and WB wrote the article. All authors read and approved the final version of the manuscript accepted for publication.
This study was supported by the project of the National Key R&D Program of China (No. 2019YFC1709103 and 2019YFC1709002) and the National Natural Science Foundation of China (No. 81774211, 81774432, 81801561, and 82004492).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Beissner, F., Henke, C., and Unschuld, P. U. (2011). Forgotten features of head zones and their relation to diagnostically relevant acupuncture points. Evid. Based Complement. Alternat. Med. 2011:240653. doi: 10.1093/ecam/nen088
Beltrán Molano, M. L., Pinilla Bonilla, L. B., Beltrán Dussan, E. H., and Vasquez Londono, C. A. (2014). Anatomo-functional correlation between head zones and acupuncture channels and points: a comparative analysis from the perspective of neural therapy. Evid. Based Complement. Alternat. Med. 2014:836392. doi: 10.1155/2014/836392
Benarroch, E. E. (2011). CGRP: sensory neuropeptide with multiple neurologic implications. Neurology 77, 281–287. doi: 10.1212/WNL.0b013e31822550e2
Briggs, G. D., Nagy, G. M., and Dickson, P. W. (2013). Mechanism of action of salsolinol on tyrosine hydroxylase. Neurochem. Int. 63, 726–731. doi: 10.1016/j.neuint.2013.09.016
Cabioglu, M. T., and Arslan, G. (2008). Neurophysiologic basis of back-shu and huatuo-jiaji points. Am. J. Chin. Med. 36, 473–479. doi: 10.1142/S0192415X08005916
Cao, B. Y., Zhu, S. P., and Liu, T. (2017). Discussion on locating of back-shu points. Zhongguo Zhen Jiu 37, 851–855. doi: 10.13703/j.0255-2930.2017.08.013
Carr, R., and Frings, S. (2019). Neuropeptides in sensory signal processing. Cell Tissue Res. 375, 217–225. doi: 10.1007/s00441-018-2946-3
Chakrabarty, A., Liao, Z. H., and Smith, P. G. (2013). Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory pain. J. Pain. 14, 1053–1065. doi: 10.1016/j.jpain.2013.04.002
Chen, S. P., Wang, S. B., Rong, P. J., Wang, J. Y., Qiao, L. N., Feng, X. M., et al. (2014). Acupuncture for visceral pain: neural substrates and potential mechanisms. Evid. Based Complement. Alternat. Med. 2014:609594. doi: 10.1155/2014/609594
Cheng, K. J. (2011). Neuroanatomical characteristics of acupuncture points: relationship between their anatomical locations and traditional clinical indications. Acupunct. Med. 29, 289–294. doi: 10.1136/acupmed.2011.010056
Connolly, K., Jackson, D., Pullen, C., and Fenning, A. (2015). Alpha-adrenoceptor antagonism by Crassostrea gigas oyster extract inhibits noradrenaline-induced vascular contraction in Wistar rats. J. Integr. Med. 13, 194–200. doi: 10.1016/S2095-4964(15)60167-4
Cui, J. J., Ha, L. J., Zhu, X. L., Wang, F. C., Jing, X. H., and Bai, W. Z. (2013). Specificity of sensory and motor neurons associated with BL40 and GB30 in the rat: a dual fluorescent labeling study. Evid. Based Complement. Alternat. Med. 2013:643403. doi: 10.1155/2013/643403
Cui, J. J., Wang, J., and Bai, W. Z. (2019). Innervated properties of acupuncture points LI 4 and LR 3 in the rat: neural pathway tracing with cholera toxin subunit B. Med. Acupunct. 31, 169–175. doi: 10.1089/acu.2019.1334
Cui, J. J., Zhu, X. L., Shi, H., Su, Y. S., Jing, X. H., and Bai, W. Z. (2015). The expression of calcitonin gene-related peptide on the neurons associated Zusanli (ST 36) in rats. Chin. J. Integr. Med. 21, 630–634. doi: 10.1007/s11655-015-2111-8
Cui, X., Zhang, W., Sun, J. H., He, X., Fu, Y., Wang, J., et al. (2019). Correlation between referred pain distribution and acupoint sensitization in patients with intestinal diseases. Zhongguo Zhen Jiu 39, 1193–1198. doi: 10.13703/j.0255-2930.2019.11.016
da Silva, M. A. H. (2010). A neurosegmental perspective of the classical Back Shu points. Med. Acupunct. 22, 257–264. doi: 10.1089/acu.2010.0762
Dai, L., Ooi, V. V., Zhou, W. J., and Guang, J. (2020). Acupoint embedding therapy improves nonalcoholic fatty liver disease with abnormal transaminase: a PRISMA-compliant systematic review and meta-analysis. Medicine 99:e18775. doi: 10.1097/MD.0000000000018775
De Logu, F., Nassini, R., Landini, L., and Geppetti, P. (2019). Pathways of CGRP release from primary sensory neurons. Handb. Exp. Pharmacol. 255, 65–84. doi: 10.1007/164_2018_145
Dickson, P. W., and Briggs, G. D. (2013). Tyrosine hydroxylase: regulation by feedback inhibition and phosphorylation. Adv. Pharmacol. 68, 13–21. doi: 10.1016/B978-0-12-411512-5.00002-6
Eftekhari, S., Warfvinge, K., Blixt, F. W., and Edvinsson, L. (2013). Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J. Pain 14, 1289–1303. doi: 10.1016/j.jpain.2013.03.010
Enríquez-Pérez, I. A., Galindo-Ordoñez, K. E., Pantoja-Ortíz, C. E., Martínez-Martínez, A., Acosta-González, R. I., Muñoz-Islas, E., et al. (2017). Streptozocin-induced type-1 diabetes mellitus results in decreased density of CGRP sensory and TH sympathetic nerve fibers that are positively correlated with bone loss at the mouse femoral neck. Neurosci. Lett. 655, 28–34. doi: 10.1016/j.neulet.2017.06.042
Fan, Y., Kim, D. H., Ryu, Y., Chang, S., Lee, B. H., Yang, C. H., et al. (2018). Neuropeptides SP and CGRP underlie the electrical properties of acupoints. Front. Neurosci. 12:907. doi: 10.3389/fnins.2018.00907
Fang, J. F., Du, J. Y., Shao, X. M., Fang, J. Q., and Liu, Z. (2017). Effect of electroacupuncture on the NTS is modulated primarily by acupuncture point selection and stimulation frequency in normal rats. BMC Complement Altern. Med. 17:182. doi: 10.1186/s12906-017-1690-7
Gao, J. H., Wang, Y. M., Cui, J. J., Ma, S. H., Cui, H. F., Fu, W. X., et al. (2010). On the scientific foundation that the tissue structure of acupoints can decide and affect specificity of acupoints-organs effects. Zhongguo Zhen Jiu 30, 293–295. doi: 10.13703/j.0255-2930.2010.04.010
Ha, L. J., Cui, J. J., Wang, F. C., Jing, X. H., and Bai, W. Z. (2014). The expression of calcitonin gene-related peptide in the sensory and motor neurons associated with “hegu” (LI 4) in the rat. Zhen Ci Yan Jiu 39, 112–116. doi: 10.13702/j.1000-0607.2014.02.005
Head, H. (1893). On disturbances of sensation with especial reference to the pain of visceral disease. Brain 16, 1–133. doi: 10.1093/brain/16.1-2.1
Hodges, G. J., and Johnson, J. M. (2009). Adrenergic control of the human cutaneous circulation. Appl. Physiol. Nutr. Metab. 34, 829–839. doi: 10.1139/H09-076
Holt, M. K., Pomeranz, L. E., Beier, K. T., Reimann, F., Gribble, F. M., and Rinaman, L. (2019). Synaptic inputs to the mouse dorsal vagal complex and its resident preproglucagon neurons. J. Neurosci. 39, 9767–9781. doi: 10.1523/JNEUROSCI.2145-19.2019
Huang, L. X., and Huang, Y. M. (2007). Evidence-Based Surface Anatomy for Acupuncture-Acupuncture Integrated With Surface Anatomy and Imaging. Beijing: People’s Medical Publishing House.
Iyengar, S., Ossipov, M. H., and Johnson, K. W. (2017). The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 158, 543–559. doi: 10.1097/j.pain.0000000000000831
Jia, J., and Zhao, J. S. (2005). Relation of indications of back-shu points of zang- and fu-organs with the bladder channel of foot-taiyang. Zhongguo Zhen Jiu 25, 414–416.
Kim, D. H., Ryu, Y., Hahm, D. H., Sohn, B. Y., Shim, I., Kwon, O. S., et al. (2017). Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci. Rep. 7:15214. doi: 10.1038/s41598-017-14359-z
Li, Y. Z., Miao, R. P., Yu, T. Y., Bai, W. Z., Cui, J. J., Lu, M. Q., et al. (2019). Mild mechanic stimulate on acupoints regulation of CGRP-positive cells and microglia morphology in spinal cord of sciatic nerve injured rats. Front. Integr. Neurosci. 13:58. doi: 10.3389/fnint.2019.00058
Liu, B. (2019). Non-acupoint effects: based on the analysis of traditional acupuncture-moxibustion theory. Zhong Guo Zhen Jiu 39, 161–165. doi: 10.13703/j.0255-2930.2019.02.014
Liu, J. L., Wang, J. Y., Chen, S. P., Gao, Y. H., Qiao, L. N., and Han, Y. J. (2010). Progress in the study on the mechanism underlying the correlation between acupoints/meridians and Zangfu organs. Zhen Ci Yan Jiu 35, 71–77. doi: 10.13702/j.1000-0607.2010.01.003
Mompeo, B., Maranillo, E., Garcia-Touchard, A., Larkin, T., and Sanudo, J. (2016). The gross anatomy of the renal sympathetic nerves revisited. Clin. Anat. 29, 660–664. doi: 10.1002/ca.22720
Mukouyama, Y. S. (2014). Vessel-dependent recruitment of sympathetic axons: looking for innervation in all the right places. J. Clin. Invest. 124, 2855–2857. doi: 10.1172/JCI76622
Mulder, J., Hökfelt, T., Knuepfer, M. M., and Kopp, U. C. (2013). Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R675–R682. doi: 10.1152/ajpregu.00599.2012
Paxinos, G., and Watson, C. (2006). The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Amsterdam, Netherlands: Elsevier.
Rodionova, K., Fiedler, C., Guenther, F., Grouzmann, E., Neuhuber, W., Fischer, M. J., et al. (2016). Complex reinnervation pattern after unilateral renal denervation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R806–R818. doi: 10.1152/ajpregu.00227.2014
Rong, P. J., Zhu, B., Li, Y. Q., Gao, X. Y., Ben, H., Li, Y. H., et al. (2011). Mechanism of acupuncture regulating visceral sensation and mobility. Front. Med. 5, 151–156. doi: 10.1007/s11684-011-0129-7
Russell, F. A., King, R., Smillie, S. J., Kodji, X., and Brain, S. D. (2014). Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142. doi: 10.1152/physrev.00034.2013
Shi, J., Wang, J., Wang, Y., Liu, K., Fu, Y., Sun, J. H., et al. (2018). Correlation between referred pain region and sensitized acupoints in patients with stable angina pectoris and distribution of sensitized spots in rats with myocardial ischemia. Zhen Ci Yan Jiu 43, 277–284. doi: 10.13702/j.1000-0607.180123
Shou, Y., Hu, L., Zhang, C. H., Xu, S. F., Jin, Q., Huang, L., et al. (2020). Efficacy of acupuncture at three nasal acupoints plus acupoint application for perennial allergic rhinitis: a multicenter, randomized controlled trial protocol. Trials 21:110. doi: 10.1186/s13063-019-4039-3
Smolilo, D. J., Hibberd, T. J., Costa, M., Wattchow, D. A., De Fontgalland, D., and Spencer, N. J. (2020). Intrinsic sensory neurons provide direct input to motor neurons and interneurons in mouse distal colon via varicose baskets. J. Comp. Neurol. 528, 2033–2043. doi: 10.1002/cne.24872
Thomas, G. D. (2011). Neural control of the circulation. Adv. Physiol. Educ. 35, 28–32. doi: 10.1152/advan.00114.2010
Tu, Q., Gan, J. H., Shi, J. L., Yu, H., He, S. L., and Zhang, J. (2019). Effect of transcutaneous electrical acupoint stimulation on postoperative analgesia after ureteroscopic lithotripsy: a randomized controlled trial. Urolithiasis 47, 279–287. doi: 10.1007/s00240-018-1056-8
van Amsterdam, W. A. C., Blankestijn, P. J., Goldschmeding, R., and Bleys, R. L. A. W. (2016). The morphological substrate for renal denervation: nerve distribution patterns and parasympathetic nerves. A post-mortem histological study. Ann. Anat. 204, 71–79. doi: 10.1016/j.aanat.2015.11.004
Wang, J., Cui, J. J., Ha, L. J., She, C., Xu, D. S., Jing, X. H., et al. (2019a). Review on application of neural tracing technique to experimental research of acupuncture. Zhen Ci Yan Jiu 44, 926–931. doi: 10.13702/j.1000-0607.180522
Wang, Y. L., Su, Y. S., He, W., and Jing, X. H. (2019b). Electroacupuncture relieved visceral and referred hindpaw hypersensitivity in colitis rats by inhibiting tyrosine hydroxylase expression in the sixth lumbar dorsal root ganglia. Neuropeptides 77:101957. doi: 10.1016/j.npep.2019.101957
Wang, J., Cui, J. J., She, C., Xu, D. S., Zhang, Z. Y., Wang, H., et al. (2018a). Differential innervation of tissues located at traditional acupuncture points in the rat forehead and face. Acupunct. Med. 36, 408–414. doi: 10.1136/acupmed-2017-011595
Wang, J., Gao, Q., Xu, D. S., Zhang, Z. Y., Cui, J. J., and Bai, W. Z. (2018b). Application of laser scanning confocal microscope to morphologic research of acupuncture and moxibustion. Zhen Ci Yan Jiu 43, 581–584. doi: 10.13702/j.1000-0607.171003
Weiss, M. L., and Chowdhury, S. I. (1998). The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res. 812, 227–241. doi: 10.1016/s0006-8993(98)00950-0
Wu, M. L., Cui, J. J., Xu, D. S., Zhang, K., Jing, X. H., and Bai, W. Z. (2015a). Neuroanatomical characteristics of deep and superficial needling using LI11 as an example. Acupunct. Med. 33, 472–477. doi: 10.1136/acupmed-2015-010882
Wu, M. L., Xu, D. S., Bai, W. Z., Cui, J. J., Shu, H. M., He, W., et al. (2015b). Local cutaneous nerve terminal and mast cell responses to manual acupuncture in acupoint LI4 area of the rats. J. Chem. Neuroanat. 68, 14–21. doi: 10.1016/j.jchemneu.2015.06.002
Wyss, J. M., and Donovan, M. K. (1984). A direct projection from the kidney to the brainstem. Brain Res. 298, 130–134. doi: 10.1016/0006-8993(84)91154-5
Xiao, L. Y., Wang, X. R., Yang, Y., Yang, J. W., Cao, Y., Ma, S. M., et al. (2018). Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation 21, 762–776. doi: 10.1111/ner.12724
Xu, D. S., She, C., Wang, J., Cui, J. J., Cai, H., and Bai, W. Z. (2016). Characteristics of distribution of blood vessels and nerve fibers in the skin tissues of acupoint “taichong” (LR 3) in the rat. Zhen Ci Yan Jiu 41, 486–491. doi: 10.13702/j.1000-0607.2016.06.002
Xu, D. S., Zhao, S., Cui, J. J., Ma, T. M., Xu, B., Yu, X. C., et al. (2019). A new attempt of re-mapping acupoint atlas in the rat. Zhen Ci Yan Jiu 44, 62–65. doi: 10.13702/j.1000-0607.180396
Zhang, B., Ma, S., Rachmin, I., He, M., Baral, P., Choi, S., et al. (2020). Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 577, 676–681. doi: 10.1038/s41586-020-1935-3
Zhang, K., Xu, D. S., Cui, J. J., Zhang, Z. Y., Jing, X. H., and Bai, W. Z. (2015). The expression of substance P in sensory neurons and nerve fibers associated with “sanyinjiao” (SP 6) region in the rat. Zhen Ci Yan Jiu 40, 449–454. doi: 10.13702/j.1000-0607.2015.06.004
Keywords: kidney, Shenshu (BL23), double fluorescent neural tracing technique, sensory neuron, postganglionic neuron
Citation: Zhang Z, Xu D, Wang J, Cui J, Wu S, Zou L, Shen Y, Jing X and Bai W (2021) Correlated Sensory and Sympathetic Innervation Between the Acupoint BL23 and Kidney in the Rat. Front. Integr. Neurosci. 14:616778. doi: 10.3389/fnint.2020.616778
Received: 13 October 2020; Accepted: 15 December 2020;
Published: 11 January 2021.
Edited by:
Eduardo Weruaga, University of Salamanca, SpainReviewed by:
Elias Manjarrez, Meritorious Autonomous University of Puebla, MexicoCopyright © 2021 Zhang, Xu, Wang, Cui, Wu, Zou, Shen, Jing and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianghong Jing, anhodGpiQDI2My5uZXQ=; Wanzhu Bai, d2Fuemh1YmFpc3lAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.