
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Integr. Neurosci., 24 January 2020
Volume 13 - 2019 | https://doi.org/10.3389/fnint.2019.00075
This article is part of the Research TopicThe Importance of Interneurons in Neuronal CircuitryView all 11 articles
Spinal motoneurons exhibit sustained afterdischarges and plateau potentials following a brief high-frequency stimulation of Ia afferents. Also, there is evidence that spinal cord interneurons exhibit plateau potentials. However, to our knowledge, there are no reports about the possible afterdischarge behavior of lumbar spinal interneurons activated by Ia afferents. Given that there are spinal interneurons receiving monosynaptic inputs from Ia afferents, these cells could then be activated in parallel to motoneurons after repetitive muscle stretch. We explored this possibility in cats with a precollicular-postmammillary decerebration. We found that a brief high-frequency stimulation of Ia afferents produces afterdischarges that are highly correlated to a DC slow potential recorded at the cord dorsum. We conclude that in the cat spinal cord, not only the motoneurons but also the interneurons from the superficial and deep dorsal horn produce sustained afterdischarges, thus highlighting the importance of interneurons in the spinal neuronal circuitry. The significance of our finding is that it opens the possibility that the spinal cord interneurons activated by Ia afferents could also exhibit bistability, a relevant phenomenon well-characterized in the motoneurons.
The firing pattern of a spinal motoneuron can alternate between two stable states depending on the immediate previous history of such motoneuron; this is called bistability (Hounsgaard and Kiehn, 1985; Hounsgaard et al., 1988). Functionally, this property allows a motor unit to self-sustain its activity during the performance of long-lasting tonic motor tasks, such as postural tone maintenance (Eken and Kiehn, 1989; Gorassini et al., 1998). Also, under certain pathological circumstances, this ability gets anomalously increased in the motoneurons as it is believed to occur during spasticity after chronic spinal injury (Harvey et al., 2006a,b). A shift into a high-frequency firing state in the motoneurons can be achieved through the high-frequency stimulation of the homonymous muscle stretch receptors and specific brainstem nuclei (Granit et al., 1957; Mori et al., 1982; Crone et al., 1988; Bennett et al., 1998). The integrity of monoaminergic descendent pathways is fundamental for the activation of motoneuron bistable properties (Conway et al., 1988; Kiehn et al., 1996; Perrier and Delgado-Lezama, 2005; Perrier et al., 2013).
The capability to generate afterdischarges is shared by different neurons in the spinal cord. For instance, similarly to motoneurons, a class of spinal interneurons can exhibit sustained discharges after a brief exposure to electrical stimulation to the sensory pudendal nerve of female cats (Cueva-Rolón et al., 2002; Muñoz-Martínez and Delgado-Lezama, 2007). Also, interneuron afterdischarges can be related to the persistence of secondary pain (Price et al., 1978) and the triggering of the scratch pattern (Currie and Stein, 1990). In the same way, the capability to generate plateau potentials is shared by many neurons in the spinal cord (Abbinanti et al., 2012; Husch et al., 2014). For example, motoneurons and interneurons exhibit the property to generate plateau potentials (Russo and Hounsgaard, 1994, 1996; Abbinanti et al., 2012; Husch et al., 2014). Furthermore, the mechanisms that underlie plateau generation in both sets of spinal neurons are substantially similar (Hounsgaard and Kjaerulff, 1992; Svirskis and Hounsgaard, 1997; Abbinanti et al., 2012; Husch et al., 2014).
The present study aimed to examine whether a brief high-frequency stimulation of Ia afferents produces afterdischarges in spinal interneurons as was previously found for the spinal motoneurons (Hounsgaard et al., 1988; Lee and Heckman, 1998). Since there are spinal interneurons receiving monosynaptic and polysynaptic contacts from Ia afferents (Kanda, 1972; Brown, 1981; Harrison and Jankowska, 1985; Bannatyne et al., 2009), it is feasible that these cells are recruited in parallel to motoneurons after repetitive muscle stretch. Our results will highlight the importance of interneurons in the spinal neuronal circuitry.
Experiments were performed in seven adult cats whose weights ranged between 2.3 and 4.5 kg. We performed two types of experiments: (1) recording of the spinal cord dorsum in four cats; and (2) multiunit recording of spinal interneurons in four cats. In one cat, we performed both experimental paradigms. The protocol was approved by the ethics committee (CICUAL-Proyecto-00489) of the Benemérita Universidad Autónoma de Puebla. The guidelines stipulated in the Guide for the Care and Use of Laboratory Animals (Eighth Edition, revised in 2011) and the Mexican regulations (NOM-062-ZOO-1999) were carefully followed. Before surgery, anesthesia was induced by a volatile mix of isoflurane (3%–4%) and oxygen (96%–97%). After tracheostomy, anesthesia was maintained via a tracheal cannula until decerebration. Atropine (0.05 mg kg−1) and dexamethasone (2 mg kg−1) were administered at the beginning of the procedures. The radial vein and the common carotid artery were cannulated for liquid administration and blood pressure monitoring, respectively. We maintained the blood pressure between 80 and 120 mmHg by the administration of Dextran and glucose (5%) isotonic solution. Bicarbonate (100 mM) and glucose (5%) solution were delivered at a constant flux (5 ml h−1) throughout the whole experiment via the common carotid artery. The temperature was kept inside a physiological range through a heating pad over which the animal was laid and a radiant heat lamp. The decerebration method consisted of a precollicular-postmammillary transection. After the decerebration, anesthesia was gradually suspended. At the end of the experimental procedures, a lethal dose of pentobarbital sodium was administered intravenously.
In four cats, a laminectomy was performed to expose the lumbosacral spinal enlargement. We recorded the activity of the spinal cord dorsum using an array of 32 Ag-AgCl spherical electrodes (diameter of 200 μm), positioned over the surface of the lumbosacral spinal cord (Figure 1A). Such activity was acquired with the Synamps 2 amplifier (CompuMedics NeuroScan) at a sampling rate of 10 kHz and filters in DC mode. Simultaneously, the electroneurographic (ENG) activity was recorded from the gastrocnemius lateralis (GL) nerve, employing a bipolar hook silver wire electrode. GL activity was rectified and integrated. Pools were made at the hind limb exposed areas and filled with mineral oil.
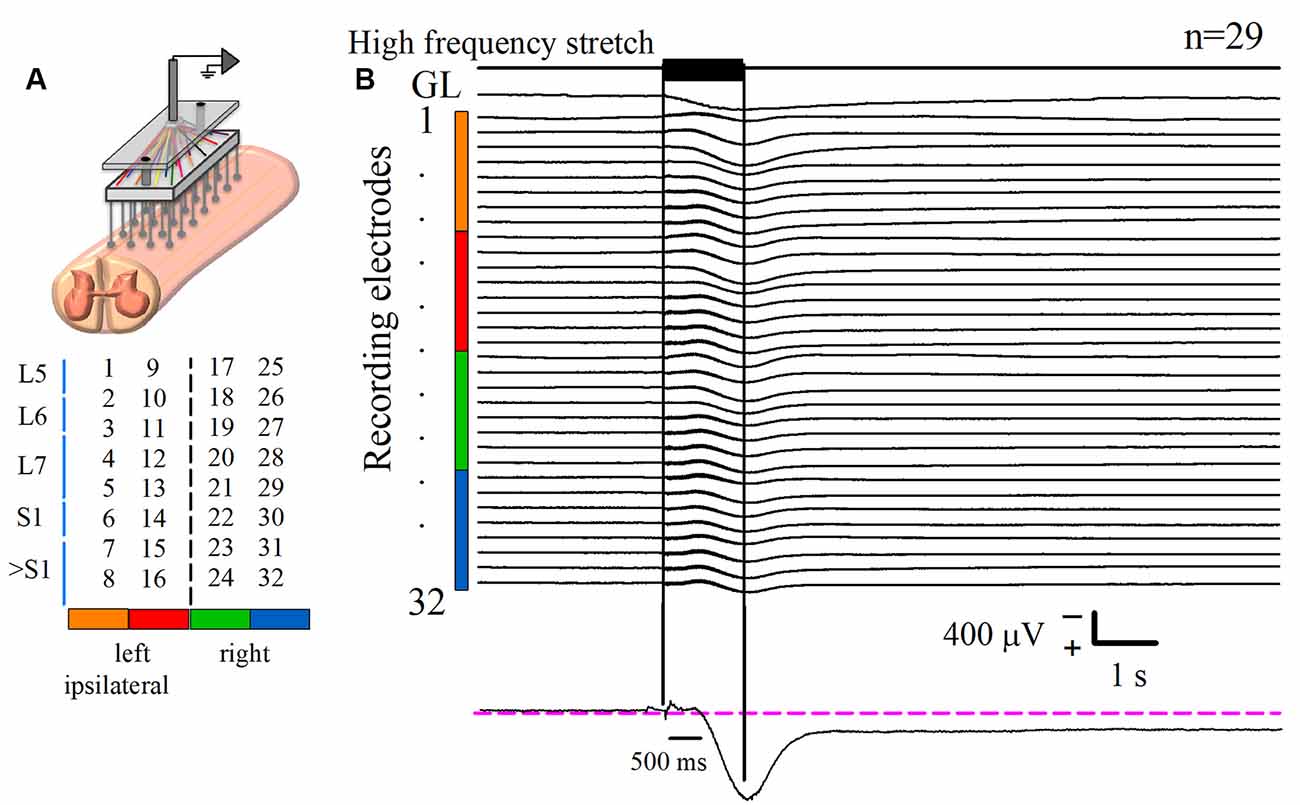
Figure 1. Recording of the DC potential on the spinal cord dorsum in response to a high-frequency stretch stimulus applied to the Achilles tendon. (A) Scheme of the recording paradigm, which consisted of an array of 32 (8 × 4) Ag-AgCl spherical electrodes positioned over the surface of the lumbosacral spinal cord. The array was coupled to a Synamps amplifier system in DC mode. The midline is represented by the dashed line that divides the two halves of the array. Spinal segments are specified at the left, according to Marcoux and Rossignol (2000). (B) The first upper trace is the high-frequency stretch stimulus applied on the left Achilles tendon. The two vertical black lines indicate the duration of such stimulus. The trace labeled with gastrocnemius lateralis (GL) is the rectified and integrated electroneurographic (ENG) signal recorded from the GL nerve. See how the GL activity experiences a sustained rise following the stimulus. The next 32 traces are the averaged DC potentials recorded from the spinal cord dorsum after several trials (n = 29) of stimulation. Such signals were obtained from the electrode array represented in (A); see color code. Note the marked positive DC shift that outlasts the mechanical stimulus duration for 6.4 ± 0.3 s (mean ± standard error). Observe that the sustained slow DC potential in the cord dorsum occurs concomitantly with the GL ENG activity. The recording in the lower panel is a zoom of a slow DC potential.
We also recorded the multiunit activity (MUA) of the superficial and deep dorsal horn interneurons of the L7 segment in four cats. These neurons did not respond to antidromic stimulation of the ventral roots. For this purpose, we employed quartz/platinum-tungsten fiber microelectrodes (1–2 MΩ) coupled with the Minimatrix system (Thomas Recording). Signals were acquired by a Digidata system (Molecular Devices, San Jose, CA, USA) at a sampling rate of 250 kHz. We used the “WaveClus” spike-sorting algorithm (Quiroga et al., 2004) to obtain unitary spikes from the raw signals.
The Achilles tendon of the left hind limb was dissected and coupled to a mechanical stimulator (Chubbuck, 1966). In this form, the mechanical stimulator administered vibrating stimuli to the triceps surae muscle. The stimulus consisted of high-frequency (200 Hz) and low-amplitude (200 μm) brief vibration (0.85–1.2 s). The stimulus characteristics ensured activation of the total amount of triceps surae Ia afferents (Lucas and Willis, 1974).
The instantaneous firing rate of the sorted units was measured and grouped into three intervals: “before,” “during,” and “after” the stimulus presentation. Time zero was defined as the instance of the beginning of stimulus presentation. The rates were compared by applying the nonparametric Friedman test and a post hoc Wilcoxon signed-rank test (R version 3.3.0, The R Project for Statistical Computing). The Pearson correlation coefficient between the DC signal and the relative counts of interneuronal spike times was computed. In all cases, the threshold for a type I error was set at 0.05.
Spinal cord dorsum activity was recorded in response to a brief high-frequency repetitive stretch of the Achilles tendon. After the stimulus presentation, the baseline cord dorsum potential recorded over the bilateral lumbosacral spinal cord exhibited a positive and slow DC shift. This slow DC potential was observed with a peak latency of 0.96 ± 0.004 s after the beginning of the stimulation. Figure 1B shows the averaged traces of 29 trials obtained from one cat. To characterize the slow DC potential, a double exponential model was fitted to these signals. A decay of 0.95 of the maximum amplitude was the criterion to determine the end of the DC potential. We found that the slow DC potential outlasted the duration of the stimulus by 6.4 ± 0.3 s (four cats, 108 trials); that is, we observed an “afterdischarge” in the cord dorsum activity due to the Ia afferent activation. More interestingly, this phenomenon occurred during the ENG sustained activity of the GL nerve (Figure 1B). This co-activation of spinal cord dorsum and motor output (GL) activity was seen in four animals.
We also found that the DC potential amplitude depended markedly on the recording site. The spatial distribution of the mean amplitude of the slow DC potential is presented in Figure 2. Each map of Figure 2A was obtained from one animal (four cats), whereas the map of Figure 2B is the grand average of the normalized maps shown in Figure 2A (four cats, 108 trials). In these maps, the dashed line represents the midline. We can observe that the amplitude of the DC potential was higher at the L6–L7 spinal segments ipsilateral to the stimulus presentation. This result was expected in accordance with the reported Ia afferent branching pattern, as discussed later.
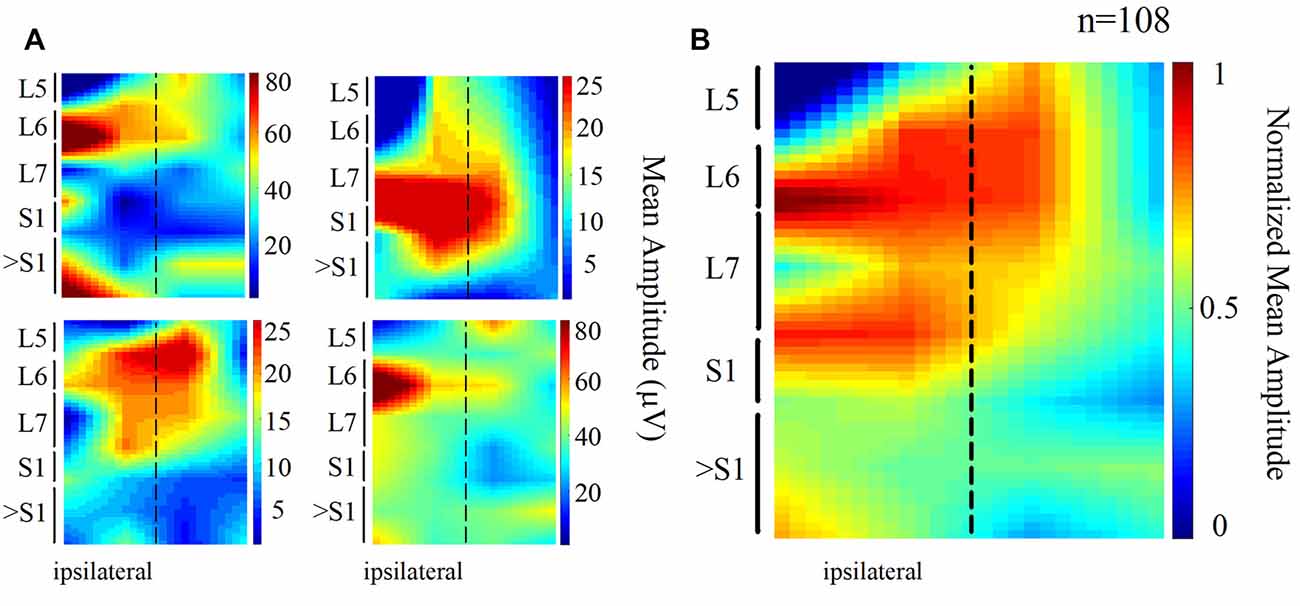
Figure 2. Topographic maps obtained from the spatial distribution of the DC potentials recorded from the cord dorsum. (A) Each map was obtained from one animal. Again, the dashed line represents the midline. Larger amplitudes tend to focus at the ipsilateral (left) L6 and L7 segments. (B) Grand average of the four normalized maps in (A) n = 108 trials, four cats.
The MUA was recorded at the L7 spinal segment from the sites drawn as white and black circles in Figure 3A. The distribution of the depth of recording sites is roughly bimodal, with the first mode around 2,000 μm and the second one about 2,800 μm (Figure 3B). In such locations, we found 37 interneurons that responded with unitary firing activity to muscle stretch. We recorded 20 superficial neurons (see white circles in Figure 3A) whose firing activity exhibited a low firing rate (Figure 3C) and 17 deep interneurons (see black with circles in Figure 3A) whose firing activity exhibited a high firing rate (Figure 3C). The pooled activity of both classes of interneurons is displayed in Figures 4A,B, in which each dot represents a single spike and the rows stand for different interneurons. The two black vertical lines indicate the stimulus duration. Sustained afterdischarges were observed after the cessation of the stimulus for up to 7 s; see the rasters of firing activity following the stimulation compared to the basal activity. Also, see the yellow bars in the histograms of Figure 4 in comparison to the blue bars; note that the height of the yellow bars remains well above the basal activity (dashed horizontal line). To quantify these changes, the instantaneous firing rate was measured and compared between the following intervals: “before” (−7 to 0 s), “during” (0–0.93 s), and “after” (0.93–7.93 s). For all of the neurons (37), the mean firing rate was calculated considering the values of all interneurons throughout the corresponding time window. The instantaneous firing rates were time-averaged to obtain mean values of 22.2 ± 0.6 Hz, 27.2 ± 1.2 Hz, and 26.15 ± 0.74 Hz, respectively. We found statistical differences among these intervals (Friedman test, p = 0.012). Additionally, a post hoc comparison yielded statistical differences when applied to “before” vs. “during” (p = 0.002) and to “before” vs. “after” (p = 0.014) intervals. Finally, we found a strong correlation between the grand average of the DC potential recorded on the cord dorsum and the relative counts of the interneuronal spikes (Pearson correlation coefficient r = 0.78, p = 2.9 × 10−12).
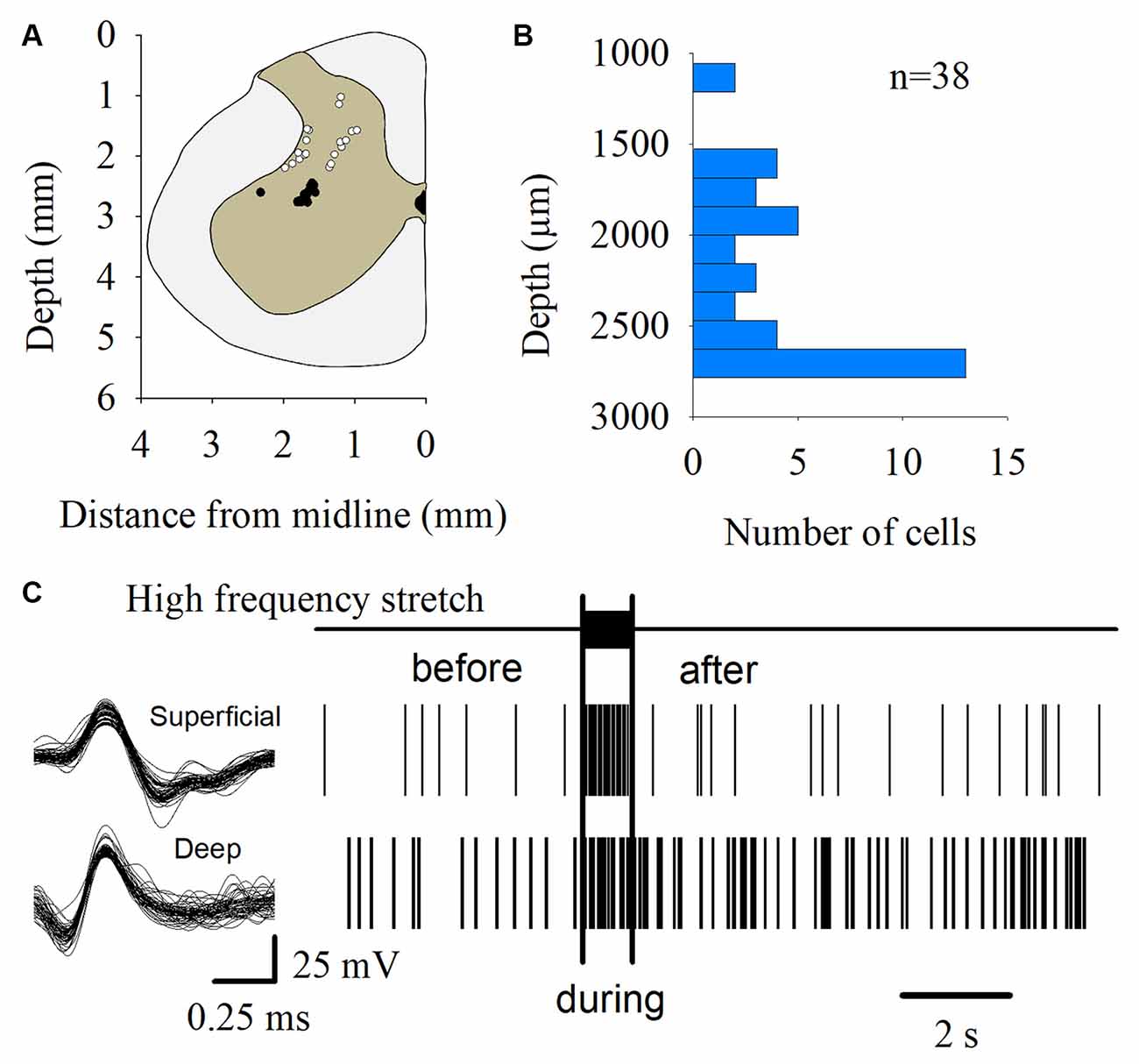
Figure 3. Multiunit recording of lumbar interneurons from the superficial and deep dorsal horn. (A) The white and black circles represent the sites from which the multiunit recordings of the spinal neurons were performed. The white circles represent the superficial dorsal horn neurons. The black circles represent the deep dorsal horn neurons. (B) Distribution of the depth of the recorded interneurons (n = 37) shown in (A). (C) Scheme representing the spike-sorting for both the superficial and deep dorsal horn neurons, as indicated. Note that the superficial dorsal horn neurons exhibited a slower firing rate than the deep dorsal horn neurons. The signals from single interneurons were obtained from the raw multiunit activity (MUA). The waveforms illustrated in the left panel are superimposed traces of two interneurons from two different animals. The right panel shows the raster plots of such neurons in response to the high-frequency stretch of the Achilles tendon (uppermost trace and vertical bars).
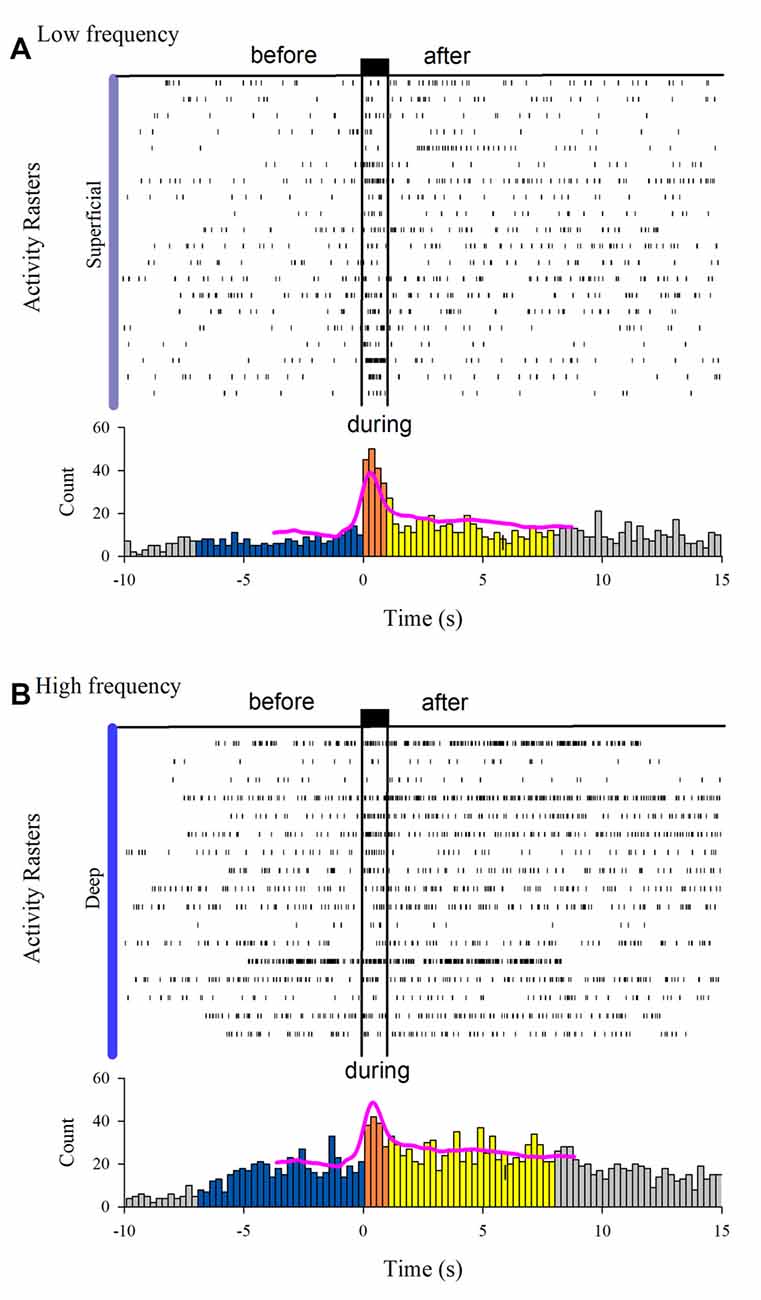
Figure 4. Interneuron afterdischarges of superficial and deep dorsal horn neurons. (A) Rasters of spiking activity from 20 superficial dorsal horn interneurons. Note the increase in the activity during and after the stimulation compared to the basal activity before stretch. The uppermost trace depicts the high-frequency stretch stimulus applied to the Achilles tendon. The duration of the stimulus is indicated by the two vertical bars. (B) The same as (A) but for 17 deep dorsal horn interneurons. The relative count histograms from the activity rasters are illustrated with the superimposed recording of the DC cord dorsum potential.
We compared the firing rate for both the superficial and the deep dorsal horn neurons. Figure 5A shows a graph of the depth vs. the firing rate (frequency) of such neurons before and after the vibratory stimuli. Note that the superficial neurons exhibit a slower firing rate and less frequency dispersion than the deep neurons. Figure 5B illustrates significant differences in the mean firing rate between the superficial and deep dorsal horn neurons for both conditions before and after the vibratory stimuli. Figure 5C shows that both groups of neurons exhibit a similar dispersion in the after/before ratio of their firing rate, irrespective of their depth.
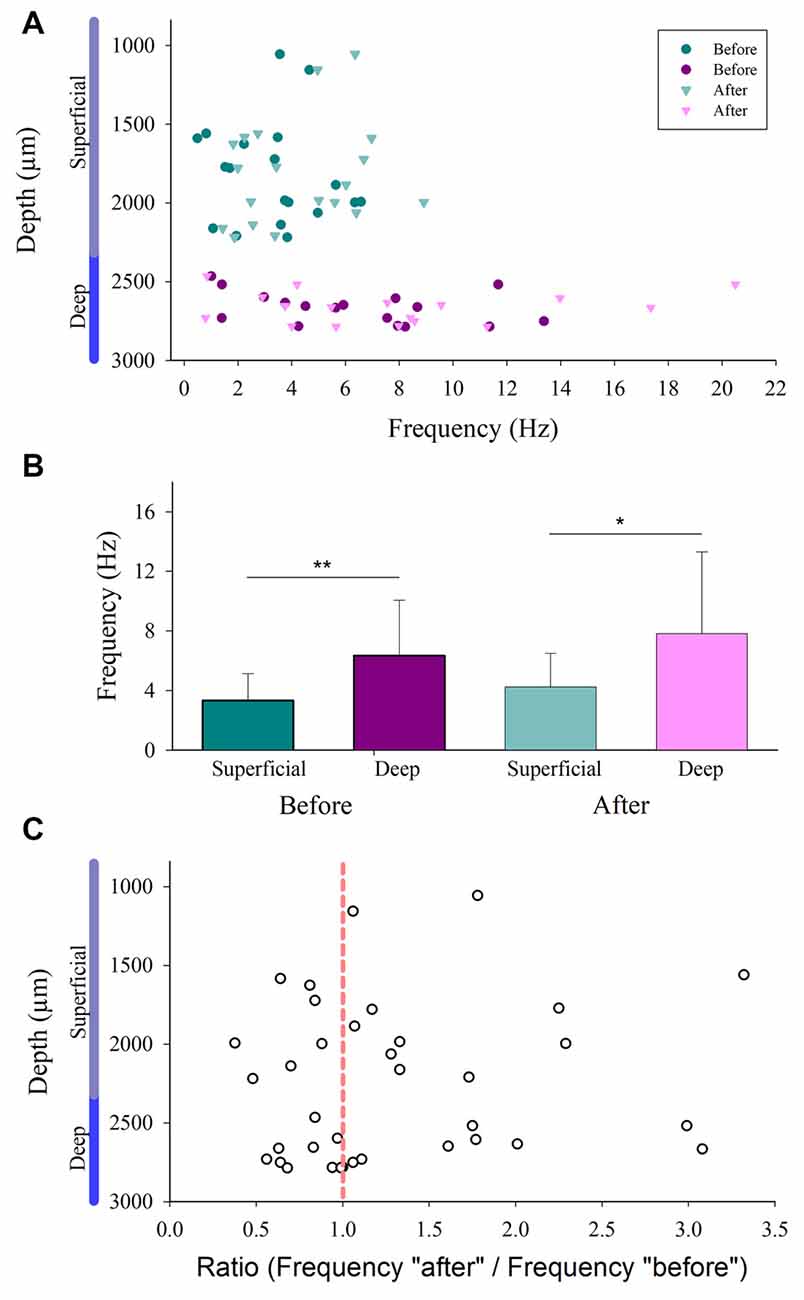
Figure 5. Firing rate distribution for the superficial and the deep dorsal horn neurons. (A) The depth vs. the firing rate (frequency in Hz) of superficial and the deep dorsal horn neurons before and after the vibratory stimuli. (B) Mean firing rate of the superficial and deep dorsal horn neurons for both conditions before and after the vibratory stimuli. (C) The after/before ratio of firing rate for the superficial and the deep dorsal horn neurons vs. the depth in which they were recorded.
Figure 6 shows the firing activity of other neurons, not exhibiting afterdischarges. We found 18 neurons that did not respond to the Ia stimulation. Moreover, we found three neurons responding to the Ia stimulation but not exhibiting sustained firing and two other neurons that were inhibited during the Ia stimulation.
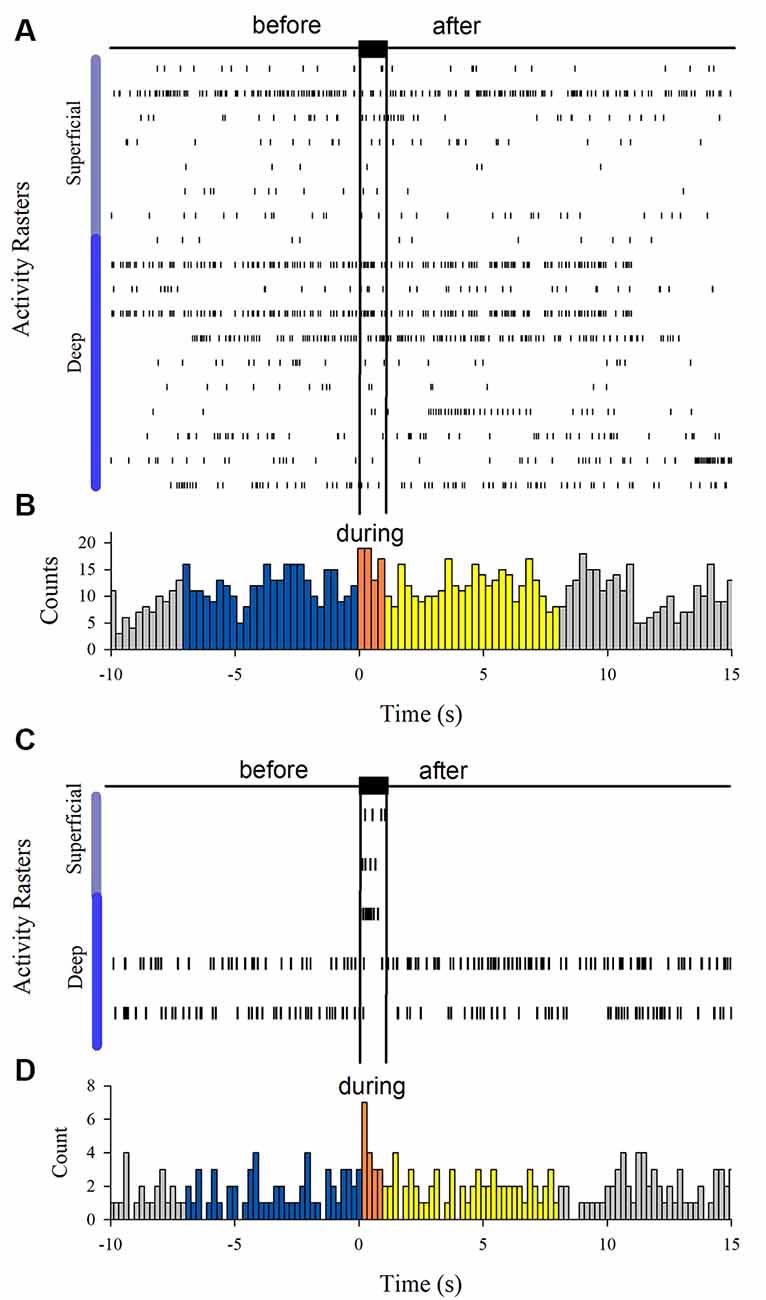
Figure 6. (A) Spiking activity of neurons not responding during the vibratory stimuli (n = 18). (C) Spiking activity of other neurons that only were excited (n = 3) or inhibited (n = 2) by the vibratory stimuli. (B,D) Histograms obtained from the raster displays in (A,C).
We found interneurons in the superficial and deep dorsal horn exhibiting afterdischarges following a brief high-frequency stimulation of Ia afferents. The superficial dorsal horn neurons exhibited a significantly lower firing rate compared with the deep dorsal horn neurons. The sustained interneuronal activity of both classes of neurons was correlated to the DC potential recorded on the lumbosacral spinal cord. However, although similar stimulation schemas have been used before, in mammals (Crone et al., 1988; Hounsgaard et al., 1988; Lee and Heckman, 1998) as well as in reptiles (Hounsgaard and Kjaerulff, 1992; Russo and Hounsgaard, 1996), attention was mainly focused on the motoneuron response.
The amplitude of the slow DC potential distributed spatially was consistent with anatomical data about the Ia afferent branching pattern. The Ia afferents of the soleus and gastrocnemius medialis muscles are mainly distributed through the ipsilateral spinal segment L7 but also give numerous branches to the L6 and S1 segments (Brown and Fyffe, 1978; Ishizuka et al., 1979).
Other slow waves, as the DC potential (also termed DC shift), can be recorded in other contexts. For instance, after the pudendal nerve stimulation in the female cat, it is possible to record a “delayed depolarization” in the hind limb motoneurons that outlasted the stimulus by up to 6 s. This is consistent with our observation of a DC shift in the cord dorsum potential that also outlasted the stimulus up to 6 s (Muñoz-Martínez and Delgado-Lezama, 2007). In other studies, it was found that previous to the alternating flexor-extensor activity during scratching, a positive DC shift occurs in the hind limb enlargement of decerebrate cats (Bayev and Kostyuk, 1981). Such DC shift is correlated to the tonic activity of interneurons from the deep dorsal horn and to the postural stage that precedes scratching (Cuellar et al., 2018). As stated in our “Results” section, we recorded a similar positive DC shift correlated to interneuron activity from the deep dorsal horn. It is possible that under certain circumstances (e.g., throughout the scratch postural stage or due to high-frequency muscle spindle stimulation), interneurons could exhibit shifts in their membrane potential in a bistable-like manner. In accordance with this idea, intermediate zone neurons located in the hind limb enlargement of an in vivo turtle preparation go into alternating shifts in their membrane potential in a phase-dependent manner during the scratch cycle (Berkowitz et al., 2006).
Recently, it was demonstrated that in mammals, intraspinal DC stimulation leads to the facilitation of several phenomena that outlasts the period of stimulation, including the monosynaptic reflex and the rise in excitability of cutaneous afferents (Bolzoni and Jankowska, 2015; see also Jankowska, 2017). Interestingly, in our in vivo preparation, both the GL-ENG activity and slow DC potential also exhibited facilitation after the brief high-frequency stimulation of Ia afferents. Therefore, we cannot exclude that the observed responses are due in part to the rise in excitability of muscle afferents or to the associated K+ release, among other factors, as potentially suggested by the DC potentials. In this context, it is essential to mention some limitations of our study.
The phenomenon described here is a first necessary step to highlight the importance of the afterdischarges in interneurons in spinal circuits. However, several elements will need to be addressed. First, we do not know the identity of these spinal interneurons other than that they receive Ia afferent inputs and that they are distributed in the superficial and deep dorsal horn. However, they may belong to a class of neurons exhibiting plateau potentials, which could be modulated by neuromodulators, as seen in motoneurons. This could be possible because there is a wide plethora of evidence suggesting that a plateau potential is a typical process inherent to afterdischarges in spinal motoneurons and interneurons (Russo and Hounsgaard, 1996; Svirskis and Hounsgaard, 1997; Abbinanti et al., 2012; Reali and Russo, 2013; Husch et al., 2014) and in other cell types of the nervous system (Llinás and Sugimori, 1980; Flatman et al., 1983). Also, there is evidence of the postnatal emergence of serotonin-induced plateau potentials in commissural interneurons of the mouse spinal cord (Abbinanti et al., 2012) and in adult spinal V2a interneurons (Husch et al., 2014). However, more experimental evidence is needed for unveiling the electrophysiological and pharmacological nature of the dorsal horn neurons exhibiting afterdischarges. Second, we do not know the underlying mechanisms in the relationship between the DC potential in the cord dorsum vs. the possible plateau potentials in interneurons. We can only speculate in this respect based on results from other animal preparations. For instance, turtle ventral horn interneurons seem not to depend on neuromodulator actions since neither serotonin receptor agonists nor other modulators need to be added to in vitro cord preparations to promote plateau potentials in these cells (Hounsgaard and Kjaerulff, 1992). Similarly, dorsal horn interneurons do not rely on neuromodulators for afterdischarge generation (Russo and Hounsgaard, 1996) as opposed to motoneurons in comparable settings (Perrier and Hounsgaard, 2003). Plateaus in both interneurons and motoneurons are highly sensitive to dihydropiridines, a phenomenon that suggests common mechanisms (Russo and Hounsgaard, 1996; Morisset and Nagy, 1999; Perrier and Hounsgaard, 2003).
We found that both the slow DC potential and the afterdischarge spiking activity of the spinal interneurons from the superficial and deep dorsal horn outlasted the duration of the brief high-frequency stimulation of Ia afferents around 6 s. We can compare this long-lasting activity with the activity of other cell types activated by sensory stimulation. For instance, spinal interneurons and motoneurons can exhibit similar sustained discharges after a brief exposure to electrical stimulation to the sensory pudendal nerve of female cats that outlasted the stimulus by up to 6 s (Muñoz-Martínez and Delgado-Lezama, 2007). Also, there is evidence that several types of interneurons can sustain long-lasting activity in the dorsal and ventral horns (Currie and Stein, 1990; Russo and Hounsgaard, 1994, 1996). Compared to other cell types, spinal neurons exhibit afterdischarges during especially prolonged periods: even in the tens of seconds scale. In the rodent cerebellar cortex, Purkinje cells undergo down-to-up transitions in their simple spike firing rate either spontaneously (Llinás and Sugimori, 1980) or in response to climbing fiber input. In this last case, after such an abrupt increase, the firing rate decays smoothly during a narrow 1 s time window (Loewenstein et al., 2005). In the cat, a neuronal population from the reticular nucleus of the thalamus exhibits plateau potentials during the occurrence of sleep spindles, about 1–2 s (Fuentealba et al., 2005). Cat hind limb motoneurons are said to be “fully bistable” whenever self-sustained firing lasts at least 3 s (Lee and Heckman, 1998), although there are reports of afterdischarges lasting as long as 1 min (Crone et al., 1988).
Functionally, particular interest has been paid to the so-called wide dynamic range (WDR) neurons. In the cat, WDR cells respond to low- as well as to high-threshold afferent stimulation (Salter and Henry, 1990). It is believed that WDR afterdischarges produce both secondary pain and non-nociceptive aftersensations (Price et al., 1978; Morisset and Nagy, 1998, 1999). The Ia information also reaches WDR neurons; however, its influence over these cells is mainly inhibitory (Pomeranz et al., 1968; Le Bars, 2002). Thus, we could exclude the involvement of this kind of cell in the phenomenon exposed here.
Other possible candidates that are able to sustain long-lasting activity are the diverse types of interneurons that reside in the intermediate zone, which respond to muscle stretch (Harrison and Jankowska, 1985), and laminae V–VII interneurons that establish direct excitatory and inhibitory contacts with ipsilateral motoneurons (Bannatyne et al., 2009; Jankowska, 2015). We can speculate that the afterdischarges of excitatory interneurons would reinforce the effect of motoneuron intrinsic properties to keep active the already recruited units. It must be mentioned that a reverberating loop of interneuronal activity was proposed early besides the motoneuron intrinsic properties to explain sustained motor activity (Hultborn et al., 1975). Contrarily, afterdischarges of inhibitory interneurons would tend to finish plateau in motoneurons. Additionally, inhibitory input would contribute to reverse the “normal” order of the recruitment of motor units (Heckman and Binder, 1993).
Also, in in vitro turtle preparations, interneurons have been found within laminae VII and VIII with plateau potentials, which emit contralateral projections (Hounsgaard and Kjaerulff, 1992). In this last case, a role during alternating motor activities was suggested. Further characterization is needed to understand whether the cat interneurons exhibiting afterdischarges also emit contralateral projections.
In conclusion, interneurons of the superficial and deep dorsal horn in the cat remain active in a parallel manner to motoneurons after ipsilateral hind limb Ia stimulation. These neurons could be characterized by the stimulation of different segmental and descending afferents, as well as by the intrinsic properties measured from intracellular recordings; however, such types of experiments are challenging in our in vivo preparation in cats and are out of the scope of the present manuscript. We can only show the phenomenon of afterdischarges in dorsal horn neurons that are highly correlated to a DC slow potential at the cord dorsum. Morphological and physiological characterization of these cells would be of paramount importance for a deeper understanding of the functional role of the present phenomenon.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The animal study was reviewed and approved by Ethics committee (CICUAL) from the Benemérita Universidad Autónoma de Puebla.
EM and AF conceived and designed the experiments. EM and AM-F wrote the article. AM-F, MM-C and EM performed the experiments. AM-F, MM-C, NH, AF and EM performed the analysis. All of the authors revised and approved the manuscript.
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) grant under projects F1–153583, 229866, and PIFI-PROMEP-VIEP-Cátedra-Marcos Moshinsky (Fundación Marcos Moshinsky; EM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank John Reid for proofreading the English manuscript.
Abbinanti, M. D., Zhong, G., and Harris-Warrick, R. M. (2012). Postnatal emergence of serotonin-induced plateau potentials in commissural interneurons of the mouse spinal cord. J. Neurophysiol. 108, 2191–2202. doi: 10.1152/jn.00336.2012
Bannatyne, B. A., Liu, T. T., Hammar, I., Stecina, K., Jankowska, E., and Maxwell, D. J. (2009). Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J. Physiol. 587, 379–399. doi: 10.1113/jphysiol.2008.159129
Bayev, K. V., and Kostyuk, P. G. (1981). Primary afferent depolarization evoked by the activity of spinal scratching generator. Neuroscience 6, 205–215. doi: 10.1016/0306-4522(81)90056-7
Bennett, D. J., Hultborn, H., Fedirchuk, B., and Gorassini, M. (1998). Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J. Neurophysiol. 80, 2023–2037. doi: 10.1152/jn.1998.80.4.2023
Berkowitz, A., Yosten, G. L., and Ballard, R. M. (2006). Somato-dendritic morphology predicts physiology for neurons that contribute to several kinds of limb movements. J. Neurophysiol. 95, 2821–2831. doi: 10.1152/jn.01246.2005
Bolzoni, F., and Jankowska, E. (2015). Presynaptic and postsynaptic effects of local cathodal DC polarization within the spinal cord in anaesthetized animal preparations. J. Physiol. 593, 947–966. doi: 10.1113/jphysiol.2014.285940
Brown, A. G. (1981). “Afferent fibres from primary endings in muscle spindles,” in Organization in the Spinal Cord, (New York, NY: Springer), 154–171.
Brown, A. G., and Fyffe, R. E. (1978). The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J. Physiol. 274, 111–127. doi: 10.1113/jphysiol.1978.sp012137
Chubbuck, J. G. (1966). Small motion biological stimulator. Johns Hopkins APL Tech. Digest. 5, 18–23.
Conway, B. A., Hultborn, H., Kiehn, O., and Mintz, I. (1988). Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J. Physiol. 405, 369–384. doi: 10.1113/jphysiol.1988.sp017337
Crone, C., Hultborn, H., Kiehn, O., Mazieres, L., and Wigström, H. (1988). Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J. Physiol. 405, 321–343. doi: 10.1113/jphysiol.1988.sp017335
Cuellar, C. A., De La Torre Valdovinos, B., Huidobro, N., Delgado-Lezama, R., Ornelas-Kobayashi, R., and Manjarrez, E. (2018). The spinal neurons exhibit an ON-OFF and OFF-ON firing activity around the onset of fictive scratching episodes in the cat. Front. Cell. Neurosci. 12:68. doi: 10.3389/fncel.2018.00068
Cueva-Rolón, R., Delgado-Lezama, R., Raya, J. G., Raya, M., Tecuanhuey, R., and Muñoz-Martínez, E. J. (2002). Sustained firing of alpha and gamma hind limb motoneurons induced by stimulation of the pudendal nerve. J. Neurophysiol. 88, 3232–3242. doi: 10.1152/jn.00157.2002
Currie, S. N., and Stein, P. S. (1990). Cutaneous stimulation evokes long-lasting excitation of spinal interneurons in the turtle. J. Neurophysiol. 64, 1134–1148. doi: 10.1152/jn.1990.64.4.1134
Eken, T., and Kiehn, O. (1989). Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiol. Scand. 136, 383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x
Flatman, J. A., Schwindt, P. C., Crill, W. E., and Stafstrom, C. E. (1983). Multiple actions of N-methyl-D-aspartate on cat neocortical neurons in vitro. Brain Res. 266, 169–173. doi: 10.1016/0006-8993(83)91323-9
Fuentealba, P., Timofeev, I., Bazhenov, M., Sejnowski, T. J., and Steriade, M. (2005). Membrane bistability in thalamic reticular neurons during spindle oscillations. J. Neurophysiol. 93, 294–304. doi: 10.1152/jn.00552.2004
Gorassini, M. A., Bennett, D. J., and Yang, J. F. (1998). Self-sustained firing of human motor units. Neurosci. Lett. 247, 13–16. doi: 10.1016/s0304-3940(98)00277-8
Granit, R., Phillips, C. G., Skoglund, S., and Steg, G. (1957). Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J. Neurophysiol. 20, 470–481. doi: 10.1152/jn.1957.20.5.470
Harrison, P. J., and Jankowska, E. (1985). Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J. Physiol. 361, 379–401. doi: 10.1113/jphysiol.1985.sp015651
Harvey, P. J., Li, X., Li, Y., and Bennett, D. J. (2006a). 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J. Neurophysiol. 96, 1158–1170. doi: 10.1152/jn.01088.2005
Harvey, P. J., Li, Y., Li, X., and Bennett, D. J. (2006b). Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J. Neurophysiol. 96, 1141–1157. doi: 10.1152/jn.00335.2005
Heckman, C. J., and Binder, M. D. (1993). Computer simulations of the effects of different synaptic input systems on motor unit recruitment. J. Neurophysiol. 70, 1827–1840. doi: 10.1152/jn.1993.70.5.1827
Hounsgaard, J., Hultborn, H., Jespersen, B., and Kiehn, O. (1988). Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J. Physiol. 405, 345–367. doi: 10.1113/jphysiol.1988.sp017336
Hounsgaard, J., and Kiehn, O. (1985). Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp. Brain Res. 57, 422–425. doi: 10.1007/bf00236551
Hounsgaard, J., and Kjaerulff, O. (1992). Ca2+-mediated plateau potentials in a subpopulation of interneurons in the ventral horn of the turtle spinal cord. Eur. J. Neurosci. 4, 183–188. doi: 10.1111/j.1460-9568.1992.tb00865.x
Hultborn, H., Wigström, H., and Wängberg, B. (1975). Prolonged activation of soleus motoneurones following a conditioning train in soleus Ia afferents—a case for a reverberating loop? Neurosci. Lett. 1, 147–152. doi: 10.1016/0304-3940(75)90030-0
Husch, A., Dietz, S. B., Hong, D. N., and Harris-Warrick, R. M. (2014). Adult spinal V2a interneurons show increased excitability and serotonin-dependent bistability. J. Neurophysiol. 113, 1124–1134. doi: 10.1152/jn.00741.2014
Ishizuka, N., Mannen, H., Hongo, T., and Sasaki, S. (1979). Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J. Comp. Neurol. 186, 189–211. doi: 10.1002/cne.901860206
Jankowska, E. (2015). On the distribution of information from muscle spindles in the spinal cord; how much does it depend on random factors? J. Anat. 227, 184–193. doi: 10.1111/joa.12331
Jankowska, E. (2017). Spinal control of motor outputs by intrinsic and externally induced electric field potentials. J. Neurophysiol. 118, 1221–1234. doi: 10.1152/jn.00169.2017
Kanda, K. (1972). Contribution of polysynaptic pathways to the tonic vibration reflex. Jpn. J. Physiol. 22, 367–377. doi: 10.2170/jjphysiol.22.367
Kiehn, O., Erdal, J., Eken, T., and Bruhn, T. (1996). Selective depletion of spinal monoamines changes the rat soleus EMG from a tonic to a more phasic pattern. J. Physiol. 492, 173–184. doi: 10.1113/jphysiol.1996.sp021299
Le Bars, D. (2002). The whole body receptive field of dorsal horn multireceptive neurones. Brain Res. Rev. 40, 29–44. doi: 10.1016/s0165-0173(02)00186-8
Lee, R. H., and Heckman, C. J. (1998). Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J. Neurophysiol. 80, 572–582. doi: 10.1152/jn.1998.80.2.572
Llinás, R., and Sugimori, M. (1980). Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J. Physiol. 305, 197–213. doi: 10.1113/jphysiol.1980.sp013358
Loewenstein, Y., Mahon, S., Chadderton, P., Kitamura, K., Sompolinsky, H., Yarom, Y., et al. (2005). Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat. Neurosci. 8, 202–211. doi: 10.1038/nn1393
Lucas, M. E., and Willis, W. D. (1974). Identification of muscle afferents which activate interneurons in the intermediate nucleus. J. Neurophysiol. 37, 282–293. doi: 10.1152/jn.1974.37.2.282
Marcoux, J., and Rossignol, S. (2000). Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. J. Neurosci. 20, 8577–8585. doi: 10.1523/jneurosci.20-22-08577.2000
Mori, S., Kawahara, K., Sakamoto, T., Aoki, M., and Tomiyama, T. (1982). Setting and resetting of level of postural muscle tone in decerebrate cat by stimulation of brain stem. J. Neurophysiol. 48, 737–748. doi: 10.1152/jn.1982.48.3.737
Morisset, V., and Nagy, F. (1998). Nociceptive integration in the rat spinal cord: role of non-linear membrane properties of deep dorsal horn neurons. Eur. J. Neurosci. 10, 3642–3652. doi: 10.1046/j.1460-9568.1998.00370.x
Morisset, V., and Nagy, F. (1999). Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J. Neurosci. 19, 7309–7316. doi: 10.1523/jneurosci.19-17-07309.1999
Muñoz-Martínez, E. J., and Delgado-Lezama, R. (2007). Pudendal nerve stimulation, interneurons post-discharge and delayed depolarization in hind limb motoneurons of the female cat. Brain Res. 1143, 126–131. doi: 10.1016/j.brainres.2007.01.064
Perrier, J. F., and Delgado-Lezama, R. (2005). Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J. Neurosci. 25, 7993–7999. doi: 10.1523/jneurosci.1957-05.2005
Perrier, J. F., and Hounsgaard, J. (2003). 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J. Neurophysiol. 89, 954–959. doi: 10.1152/jn.00753.2002
Perrier, J. F., Rasmussen, H. B., Christensen, R. K., and Petersen, A. V. (2013). Modulation of the intrinsic properties of motoneurons by serotonin. Curr. Pharm. Des. 19, 4371–4384. doi: 10.2174/13816128113199990341
Pomeranz, B., Wall, P. D., and Weber, W. V. (1968). Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J. Physiol. 199, 511–532. doi: 10.1113/jphysiol.1968.sp008666
Price, D. D., Hayes, R. L., Ruda, M., and Dubner, R. (1978). Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J. Neurophysiol. 41, 933–947. doi: 10.1152/jn.1978.41.4.933
Quiroga, R. Q., Nadasdy, Z., and Ben-Shaul, Y. (2004). Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 16, 1661–1687. doi: 10.1162/089976604774201631
Reali, C., and Russo, R. E. (2013). Neuronal intrinsic properties shape naturally evoked sensory inputs in the dorsal horn of the spinal cord. Front. Cell. Neurosci. 7:276. doi: 10.3389/fncel.2013.00276
Russo, R. E., and Hounsgaard, J. (1994). Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience 61, 191–197. doi: 10.1016/0306-4522(94)90222-4
Russo, R. E., and Hounsgaard, J. (1996). Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J. Physiol. 493, 39–54. doi: 10.1113/jphysiol.1996.sp021363
Salter, M. W., and Henry, J. L. (1990). Physiological characteristics of responses of wide dynamic range spinal neurones to cutaneously applied vibration in the cat. Brain Res. 507, 69–84. doi: 10.1016/0006-8993(90)90524-f
Keywords: interneuros, bistability, afterdischarges, DC shift, spinal cord
Citation: Méndez-Fernández A, Moreno-Castillo M, Huidobro N, Flores A and Manjarrez E (2020) Afterdischarges of Spinal Interneurons Following a Brief High-Frequency Stimulation of Ia Afferents in the Cat. Front. Integr. Neurosci. 13:75. doi: 10.3389/fnint.2019.00075
Received: 30 October 2019; Accepted: 16 December 2019;
Published: 24 January 2020.
Edited by:
Maria Gutierrez-Mecinas, University of Glasgow, United KingdomReviewed by:
Tuan Vu Bui, University of Ottawa, CanadaCopyright © 2020 Méndez-Fernández, Moreno-Castillo, Huidobro, Flores and Manjarrez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amira Flores, YW1pcmEuZmxvcmVzQGNvcnJlby5idWFwLm14
Elias Manjarrez, ZWxpYXNtYW5qYXJyZXpAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.