- Human Motion Analysis Laboratory, Department of Rehabilitation and Movement Science, University of Vermont, Burlington, VT, USA
Introduction and Purpose
The ability to maintain standing balance and orientation is crucial to mobility and independence. Standing balance is generally maintained by anticipatory postural adjustments associated with voluntary actions. However, inaccurate judgment or impaired anticipatory processes, as well as extrinsic postural perturbations–external forces not generated intrinsically due to voluntary movement (e.g., due to a slip, trip, push, etc.)–can render the need for reactive postural control to recover orientation and balance (Fasano et al., 2012).
For people with disorders of posture and balance associated with aging, neurodegeneration, or injury, an appropriately timed and coordinated postural response to extrinsically perturbed balance may represent the crucial difference between a harmless balance recovery and an aggravated pain condition or injurious fall. Indeed, postural responses to slips and sudden changes in load are common circumstances for incurring an episode of low back pain (LBP), a worldwide leading cause of disability (Manning et al., 1984; Andersson, 1999). In addition, falls associated with aging or neurological disorders are leading causes of injury, decreased activity participation, morbidity, and mortality (Grimbergen et al., 2004; Finlayson and Peterson, 2010; Batchelor et al., 2012). For these reasons research must detail the neurophysiology responsible for producing both healthy and impaired human postural responses to an extrinsically induced perturbation of standing balance.
A recently proposed neurophysiologic model of extrinsically induced postural responses reviewed initial evidence that the cerebral cortex influences these postural responses by (a) priming the most contextually accurate response during preparation, and (b) modifying late response phases (Jacobs and Horak, 2007). The involvement of the cerebral cortex during early response phases thus appears indirect and limited to priming sub-cortically generated synergies based on contextual features known prior to the perturbation, but the cortex can then directly participate in modifying the late response phases to improve response efficacy. Cortical functions associated with priming contextually appropriate responses are thus represented through pre-perturbation measures of cortical activity such as pre-movement potentials, and cortical functions associated with online modifications to late-phase responses are represented by measures of cortical activity following perturbation onset, such as perturbation evoked potentials (PEPs) (Adkin et al., 2008; Jacobs et al., 2008; Mochizuki et al., 2008, 2010). Further research remains needed, however, due to a paucity of data on neural mechanisms of human postural responses with disease or injury.
Understanding cortical function associated with impaired postural responses is essential given (a) the important role of the cerebral cortex for generating postural responses, (b) the potential that its influence may be enhanced to compensate for impaired automated processes of sub-cortical control, and (c) the accessibility of cortex for neuroplastic change with intervention. Unfortunately, because postural responses to extrinsic perturbations were historically considered reflexive and indicative of sub-cortical processing, the literature regarding cortical influence on extrinsically induced postural responses is not well developed. Whereas a more longstanding and developed literature exists on the role of the cerebral cortex for generating anticipatory postural adjustments with voluntary movement—both in the use of many methods of neurophysiologic recording and in the evaluation of people with health conditions (Gurfinkel and El'ner, 1988; Massion, 1992; Saitou et al., 1996; MacKinnon et al., 2007; Tsao et al., 2008; Jacobs et al., 2009a, 2010; Ng et al., 2012; Lomond et al., 2013; Papegaaij et al., 2014), the literature on postural responses to extrinsically induced perturbations is less extensive.
Therefore, the purpose of this opinion article is to focus on the cortical neurophysiology of impaired human postural responses to extrinsic perturbations of upright stance. This article will highlight the insights provided from rare studies of cortical function in people with impaired standing postural responses in order to demonstrate the need and potential value of future research focused on the cortical neurophysiology of impaired human postural responses to extrinsic perturbations.
What We Can Learn from Cortical Neurophysiology During Impaired Postural Responses
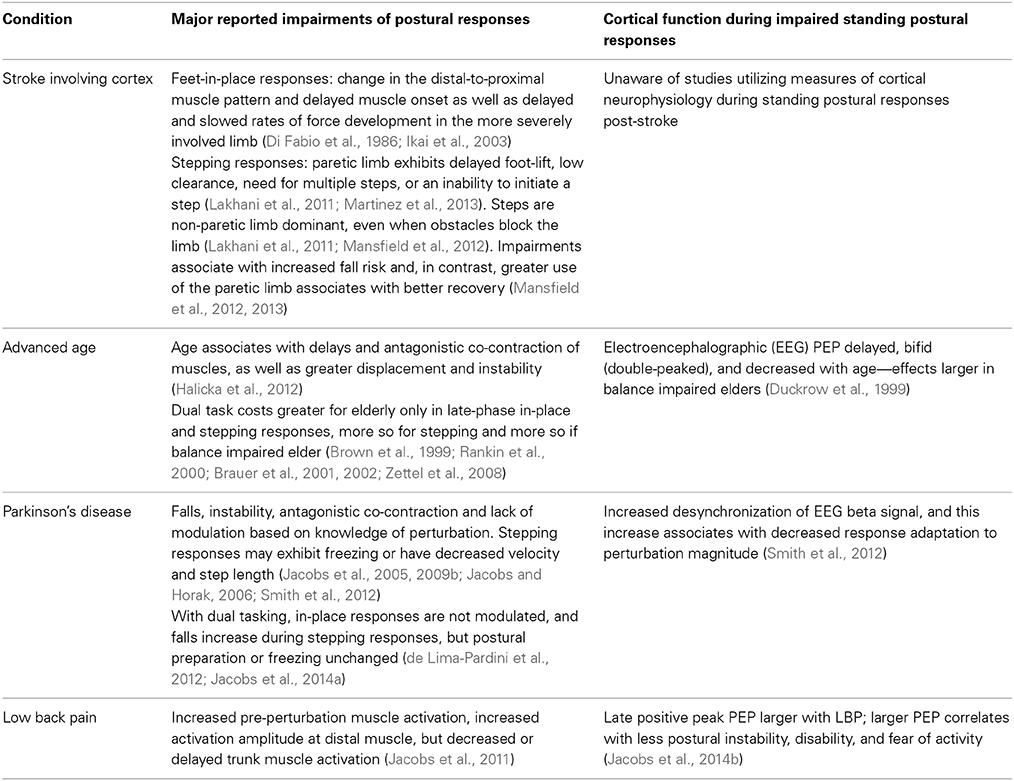
Table 1 highlights common movement impairments and our knowledge of cortical neurophysiology associated with human postural responses to perturbations of standing balance for selected example conditions of stroke, advanced age, Parkinson's disease (PD), and LBP. To demonstrate the case for this opinion article, these conditions represent samples of a much larger scope of postural disorders related to cortical injury, age- and disease-associated neurodegeneration, as well as chronic pain.
Stroke: Insights into Cortical Neurophysiology of Postural Responses Through Cortical Injury
Unfortunately, studies that measure cortical neurophysiology during standing postural responses of people with a history of stroke remain untested. Nevertheless, studies on stroke highlight the importance of the cortex for selecting and shaping postural response synergies and also highlight needs for understanding the neural mechanisms of altered postural responses following stroke.
Table 1 reinforces the importance of circuits involving the cortex for selecting an efficient and environmentally appropriate synergy (distal-to-proximal feet-in-place patterns and step selection in constrained conditions). The results also support a role for the cortex in generating the late-phase stepping response. Further, it is clear that mechanisms of both impairment and compensation exist, but such compensations (disuse of paretic limb) may represent increased fall risk and slowed recovery. A preliminary case study (Mansfield et al., 2011) suggests that compensatory step training can be beneficial, but such attempts at physical rehabilitation could be optimized if based in a more thorough understanding of the neural mechanisms responsible for these impairments and compensations. In addition, understanding neural mechanisms of impairment and compensation will better direct neurophysiologic treatments such as stimulation, pharmacology, or surgery. It is also unclear how the location and extent of post-stroke lesions affect different aspects of the postural response; the current literature does not clearly define lesion extent and includes injury of both cortical and subcortical regions. Thus, prognostics would benefit from being able to predict likely impairments from the lesion location and area. Clearly, too little is known about the effects of cortical injury on postural responses, and treatment options could be strongly influenced by a better understanding of cortical function during postural responses post-stroke.
Aging: A Lesson in How Recording Neurophysiology Enhances Knowledge Beyond Inference from Behavior
Research on aging and cortical function during postural responses has largely been inferred through dual-task costs on the postural response under the assumption that a second task requires cortical resources and, therefore, any effects on postural responses reflect use of cortical resources during the response (Jacobs and Horak, 2007; Maki and McIlroy, 2007). As identified in Table 1, this assumption is compatible with findings that dual-task costs are most evident during the late phase of feet-in-place responses and the swing phase of stepping responses, which are thought to be cortically influenced (Jacobs and Horak, 2007). It remains unclear whether the age-related increase in dual-task costs represents greater compensation by cortical resources when sub-cortical processes of two tasks compete, or whether the cortex has greater influence on postural responses in any condition, which is revealed by competition for cortical resources when dual tasking. Knowing the difference would strongly influence interventions to target sub-cortical vs. cortical physiology as well as to determine the scope of circumstances in which impaired postural responses are a concern.
Recordings of cortical function during postural responses could provide key insight for identifying the mechanisms and circumstances of impairment, which could subsequently direct more optimized intervention. Unfortunately, only one study has attempted to compare EEG potentials evoked by perturbations of standing balance in young and older adults (Duckrow et al., 1999). Findings suggest that age associates with delayed, diminished and prolonged central sensory-motor processing at the cortex. This diminished and prolonged neural processing might explain the lack of a rapid and contextually optimized response. It remains unclear whether the cortex is the source of impairment or if its altered evoked potentials are subsequent to sub-cortical impairment, but the prolonged time to process the potentials at the cortex could explain the increased use of cortical resources suggested by dual-task studies and provides better focus for targets of further study and intervention that couldn't have been derived through only behavioral inference. Further study would benefit from high-resolution neurophysiologic recordings under single- vs. dual-task conditions in order to enhance understanding of the source and timing of impairments to ultimately direct more efficacious interventions.
Parkinson's Disease: A Lesson in How Recordings of Cortical Function Reveal Unexpected Insights
In addition to the instability and falls associated with the impaired postural responses of people with PD, one of the more striking aspects is the lack of response modification based on contextual information about the upcoming perturbation's characteristics (Table 1). In addition, dual tasking during stepping responses does not alter early postural preparation, but does induce more falls, which suggests an ineffective step subsequent to the postural preparation (Jacobs et al., 2014a). The inability to optimize postural responses based on knowledge of perturbation characteristics and dual-task costs on only the late swing phase of stepping responses again suggest cortical involvement.
Because PD is often characterized as a disorder of diminished movement and cortical excitation during voluntary action, a contextually unmodified postural response might be predicted to associate with diminished preparatory potentials suggestive of a cortical incapacity to generate such potentials. Insights from EEG recordings of preparatory cortical function (contingent negative variation and event related desynchronization), however, demonstrate that people with PD fail to modulate their postural response by over-responding to small perturbations while concomitantly exhibiting increased desynchronization of upper beta (20–29 Hz) EEG signals prior to small perturbations. Further, larger desynchronization corresponds with less modulation of the postural response between small and large perturbation magnitudes (Smith et al., 2012). Beta desynchronization prior to movement is thought to represent motor preparation, inhibition of tonic activation, and/or anticipation of an impending need for movement within circuits that involve motor regions of the cortex (Jenkinson and Brown, 2011; Smith et al., 2012). Thus, recordings of cortical neurophysiology unexpectedly revealed that people with PD over-modulate their preparatory EEG activity prior to generating an unmodulated hypermetric response to small perturbations, rather than exhibiting diminished or unmodulated cortical preparation that coincides with the unmodulated response.
Given that preparatory cortical functions appear intact and over-responsive rather than incapacitated, these insights have significant ramifications for the potential to utilize behavioral and physical rehabilitation in order to train contextual response modulation. In addition, pharmacological or stimulation interventions would be directed differently for an over-responsive vs. under-responsive neurophysiologic condition. Therefore, this example in PD demonstrates the important value of recording cortical function during postural responses, because the neural mechanisms of a response may not be as expected based on behavioral inference alone.
Chronic Pain: Demonstrating that Cortical Potentials to Postural Perturbations are Functionally Relevant
Chronic pain due to musculoskeletal injury such as LBP can significantly alter the central neural control of postural coordination. Although all phases of a postural response can be altered with LBP, the more consistent and significant findings are an enhanced muscle co-activation prior to perturbations and strongly diminished late-phase trunk muscle responses with concomitant increases in distal muscle responses (Table 1) (Jacobs et al., 2011). These pre-perturbation and late-phase alterations again implicate changes in cortical function during the postural response, and recent data demonstrate that late-phase evoked EEG potentials are enhanced with LBP. Interestingly, the enhancement appears compensatory because larger potential amplitudes correspond with less center-of-mass displacement, less disability, and less fear of physical activity (Jacobs et al., 2014b). Thus, these studies in LBP demonstrate two important lessons: (a) even peripheral musculoskeletal injuries alter the neural control of posture through the highest levels of the neural axis, and (b) cortical neurophysiology of postural responses can be relevant to the efficacy of the response and to clinical measures of disability.
Summary
The examples above demonstrate that cortical neurophysiology during postural responses to extrinsic perturbations of standing balance is critical for individuals with postural impairments such as advanced aging, neurodegeneration, and chronic pain. In addition, the little available literature emphasizes how recordings of cortical neurophysiology during extrinsically induced postural responses can offer crucial insights into mechanisms of impaired balance that are not available or unexpected based on behavioral inference alone. Lastly, cortical neurophysiology is functionally relevant to the stability of postural responses and, perhaps, to clinical disability associated with the health condition.
Despite the importance of understanding the neurophysiologic mechanisms of impaired postural responses to extrinsic perturbations of standing balance, very little neurophysiologic recording beyond the muscle has been attempted during these responses. With technologies such as EEG, near infrared spectroscopy, single photon emission computed tomography, transcranial magnetic or direct-current stimulation, etc., and with improved abilities to overcome technical challenges, the opportunity for expansive research on the neurophysiology of extrinsically induced postural responses exists in order to compliment parallel work on voluntary postural control. For any population with disorders of balance and posture, more research is needed to evaluate multiple measures that reflect unique neurophysiologic systems of both preparatory and evoked neural activation. In addition, these recordings should be undertaken across multiple contexts that vary predictability, perturbation characteristics, dual tasking, etc. to more accurately understand how environmental circumstances affect the neural control of postural responses. In so doing, crucial insights are likely to emerge that could support more efficacious interventions and clinical outcomes for those with balance disorders.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adkin, A. L., Campbell, A. D., Chua, R., and Carpenter, M. G. (2008). The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci. Lett. 435, 120–125. doi: 10.1016/j.neulet.2008.02.018
Andersson, G. B. (1999). Epidemiological features of chronic low-back pain. Lancet 354, 581–585. doi: 10.1016/S0140-6736(99)01312-4
Batchelor, F. A., Mackintosh, S. F., Said, C. M., and Hill, K. D. (2012). Falls after stroke. Int. J. Stroke 7, 482–490. doi: 10.1111/j.1747-4949.2012.00796.x
Brauer, S. G., Woollacott, M., and Shumway-Cook, A. (2001). The interacting effects of cognitive demand and recovery of postural stability in balance-impaired elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 56, M489–M496. doi: 10.1093/gerona/56.8.M489
Brauer, S., Woollacott, M., and Shumway-Cook, A. (2002). The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait Posture 15, 83–93. doi: 10.1016/S0966-6362(01)00163-1
Brown, L. A., Shumway-Cook, A., and Woollacott, M. H. (1999). Attentional demands and postural recovery: the effects of aging. J. Gerontol. A Biol. Sci. Med. Sci. 54, M165–M171.
de Lima-Pardini, A. C., Papegaaij, S., Cohen, R. G., Teixeira, L. A., Smith, B. A., and Horak, F. B. (2012). The interaction of postural and voluntary strategies for stability in Parkinson's disease. J. Neurophysiol. 108, 1244–1252. doi: 10.1152/jn.00118.2012
Di Fabio, R. P., Badke, M. B., and Duncan, P. W. (1986). Adapting human postural reflexes following localized cerebrovascular lesion: analysis of bilateral long latency responses. Brain Res. 363, 257–264.
Duckrow, R. B., Abu-Hasaballah, K., Whipple, R., and Wolfson, L. (1999). Stance perturbation-evoked potentials in old people with poor gait and balance. Clin. Neurophysiol. 110, 2026–2032.
Fasano, A., Plotnik, M., Bove, F., and Berardelli, A. (2012). The neurobiology of falls. Neurol. Sci. 33, 1215–1223. doi: 10.1007/s10072-012-1126-6
Finlayson, M. L., and Peterson, E. W. (2010). Falls, aging, and disability. Phys. Med. Rehabil. Clin. N. Am. 21, 357–373. doi: 10.1016/j.pmr.2009.12.003
Grimbergen, Y. A., Munneke, M., and Bloem, B. R. (2004). Falls in Parkinson's disease. Curr. Opin. Neurol. 17, 405–415. doi: 10.1097/01.wco.0000137530.68867.93
Gurfinkel, V. S., and El'ner, A. M. (1988). [Participation of the secondary motor area of the frontal lobe of the brain in organizing postural components of human voluntary movement]. Neirofiziologiia 20, 7–15.
Halicka, Z., Lobotkova, J., Bzduskova, D., and Hlavacka, F. (2012). Age-related changes in postural responses to backward platform translation. Physiol. Res. 61, 331–335.
Ikai, T., Kamikubo, T., Takehara, I., Nishi, M., and Miyano, S. (2003). Dynamic postural control in patients with hemiparesis. Am. J. Phys. Med. Rehabil. 82, 463–469. doi: 10.1097/01.PHM.0000069192.32183.A7
Jacobs, J. V., and Horak, F. B. (2006). Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson's disease. Neuroscience 141, 999–1009. doi: 10.1016/j.neuroscience.2006.04.014
Jacobs, J. V., and Horak, F. B. (2007). Cortical control of postural responses. J. Neural Transm. 114, 1339–1348. doi: 10.1007/s00702-007-0657-0
Jacobs, J. V., Dimitrova, D. M., Nutt, J. G., and Horak, F. B. (2005). Can stooped posture explain multidirectional postural instability in patients with Parkinson's disease? Exp. Brain Res. 166, 78–88. doi: 10.1007/s00221-005-2346-2
Jacobs, J. V., Fujiwara, K., Tomita, H., Furune, N., Kunita, K., and Horak, F. B. (2008). Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin. Neurophysiol. 119, 1431–1442. doi: 10.1016/j.clinph.2008.02.015
Jacobs, J. V., Henry, S. M., Jones, S. L., Hitt, J. R., and Bunn, J. Y. (2011). A history of low back pain associates with altered electromyographic activation patterns in response to perturbations of standing balance. J. Neurophysiol. 106, 2506–2514. doi: 10.1152/jn.00296.2011
Jacobs, J. V., Henry, S. M., and Nagle, K. J. (2010). Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clin. Neurophysiol. 121, 431–440. doi: 10.1016/j.clinph.2009.11.076
Jacobs, J. V., Lou, J. S., Kraakevik, J. A., and Horak, F. B. (2009a). The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience 164:877–885. doi: 10.1016/j.neuroscience.2009.08.002
Jacobs, J. V., Nutt, J. G., Carlson-Kuhta, P., Allen, R., and Horak, F. B. (2014a). Dual tasking during postural stepping responses increases falls but not freezing in people with Parkinson's disease. Parkinsonism Relat. Disord. 20, 779–781. doi: 10.1016/j.parkreldis.2014.04.001
Jacobs, J. V., Nutt, J. G., Carlson-Kuhta, P., Stephens, M., and Horak, F. B. (2009b). Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp. Neurol. 215, 334–341. doi: 10.1016/j.expneurol.2008.10.019
Jacobs, J. V., Roy, C. L., Hitt, J. R., Popov, R. E., and Henry, S. M. (2014b). “Neural mechanisms and functional correlates of altered postural responses to perturbed standing balance with chronic low back pain,” in Society for Neuroscience Annual Meeting (Washington, DC).
Jenkinson, N., and Brown, P. (2011). New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 34, 611–618. doi: 10.1016/j.tins.2011.09.003
Lakhani, B., Mansfield, A., Inness, E. L., and McIlroy, W. E. (2011). Compensatory stepping responses in individuals with stroke: a pilot study. Physiother. Theory Pract. 27, 299–309. doi: 10.3109/09593985.2010.501848
Lomond, K. V., Henry, S. M., Jacobs, J. V., Hitt, J. R., Horak, F. B., Cohen, R. G., et al. (2013). Protocol to assess the neurophysiology associated with multi-segmental postural coordination. Physiol. Meas. 34:N97–N105. doi: 10.1088/0967-3334/34/10/N97
MacKinnon, C. D., Bissig, D., Chiusano, J., Miller, E., Rudnick, L., Jager, C., et al. (2007). Preparation of anticipatory postural adjustments prior to stepping. J. Neurophysiol. 97, 4368–4379. doi: 10.1152/jn.01136.2006
Maki, B. E., and McIlroy, W. E. (2007). Cognitive demands and cortical control of human balance-recovery reactions. J. Neurol Transm. 114, 1279–1296. doi: 10.1007/s00702-007-0764-y
Manning, D. P., Mitchell, R. G., and Blanchfield, L. P. (1984). Body movements and events contributing to accidental and nonaccidental back injuries. Spine 9, 734–739.
Mansfield, A., Inness, E. L., Komar, J., Biasin, L., Brunton, K., Lakhani, B., et al. (2011). Training rapid stepping responses in an individual with stroke. Phys. Ther. 91, 958–969. doi: 10.2522/ptj.20100212
Mansfield, A., Inness, E. L., Lakhani, B., and McIlroy, W. E. (2012). Determinants of limb preference for initiating compensatory stepping poststroke. Arch. Phys. Med. Rehabil. 93, 1179–1184. doi: 10.1016/j.apmr.2012.02.006
Mansfield, A., Inness, E. L., Wong, J. S., Fraser, J. E., and McIlroy, W. E. (2013). Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil. Neural Repair 27, 526–533. doi: 10.1177/1545968313478486
Martinez, K. M., Mille, M. L., Zhang, Y., and Rogers, M. W. (2013). Stepping in persons poststroke: comparison of voluntary and perturbation-induced responses. Arch. Phys. Med. Rehabil. 94, 2425–2432. doi: 10.1016/j.apmr.2013.06.030
Massion, J. (1992). Movement, posture and equilibrium: interaction and coordination. Prog. Neurobiol. 38, 35–56.
Mochizuki, G., Boe, S., Marlin, A., and McIlRoy, W. E. (2010). Perturbation-evoked cortical activity reflects both the context and consequence of postural instability. Neuroscience 170, 599–609. doi: 10.1016/j.neuroscience.2010.07.008
Mochizuki, G., Sibley, K. M., Esposito, J. G., Camilleri, J. M., and McIlroy, W. E. (2008). Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clin. Neurophysiol. 119, 1626–1637. doi: 10.1016/j.clinph.2008.03.020
Ng, T. H., Sowman, P. F., Brock, J., and Johnson, B. W. (2012). Neuromagnetic brain activity associated with anticipatory postural adjustments for bimanual load lifting. Neuroimage 66C, 343–352. doi: 10.1016/j.neuroimage.2012.10.042
Papegaaij, S., Taube, W., Baudry, S., Otten, E., and Hortobagyi, T. (2014). Aging causes a reorganization of cortical and spinal control of posture. Front. Aging Neurosci. 6:28. doi: 10.3389/fnagi.2014.00028
Rankin, J. K., Woollacott, M. H., Shumway-Cook, A., and Brown, L. A. (2000). Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 55, M112–M119. doi: 10.1093/gerona/55.3.M112
Saitou, K., Washimi, Y., Koike, Y., Takahashi, A., and Kaneoke, Y. (1996). Slow negative cortical potential preceding the onset of postural adjustment. Electroencephalogr. Clin. Neurophysiol. 98, 449–455.
Smith, B. A., Jacobs, J. V., and Horak, F. B. (2012). Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson's disease. Exp. Brain Res. 222, 455–470. doi: 10.1007/s00221-012-3232-3
Tsao, H., Galea, M. P., and Hodges, P. W. (2008). Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 131, 2161–2171. doi: 10.1093/brain/awn154
Keywords: automatic postural responses, balance, cortex, Parkinson's disease, low back pain, aging, stroke
Citation: Jacobs JV (2014) Why we need to better understand the cortical neurophysiology of impaired postural responses with age, disease, or injury. Front. Integr. Neurosci. 8:69. doi: 10.3389/fnint.2014.00069
Received: 27 June 2014; Accepted: 13 August 2014;
Published online: 29 August 2014.
Edited by:
Isaac Louis Kurtzer, New York Institute of Technology–College of Osteopathic Medicine, USAReviewed by:
Colum Doan MacKinnon, University of Minnesota, USACopyright © 2014 Jacobs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:amphY29ic0B1dm0uZWR1
 Jesse V. Jacobs
Jesse V. Jacobs