
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 28 February 2025
Sec. Autonomic Neuroscience
Volume 19 - 2025 | https://doi.org/10.3389/fnins.2025.1542224
Introduction: The gastrointestinal tract is the organ most extensively distributed by autonomic nerves, and researches have indicated a relationship between automatic nerves and the progression of gastrointestinal cancers. This study aimed to evaluate the autonomic nervous function in patients with gastrointestinal cancer and to explore its relationship with clinical characteristics.
Methods: We employed the Composite Autonomic Symptom Score 31 (COMPASS-31) questionnaire and cardiovascular autonomic reflex tests (CARTs) to evaluate autonomic nervous function, while also conducting a thorough analysis of clinical data.
Results: Our results showed that low white blood cell (WBC) count (OR = 0.461, 95% CI: 0.218–0.976, p = 0.043) and increased maximum tumor diameter (OR = 1.619, 95% CI: 1.025–2.555, p = 0.039) were risk factors for autonomic dysfunction according to the COMPASS-31 assessment. While hypertension (OR = 5.747, 95% CI: 1.186–27.862, p = 0.030) and elevated platelet-to-albumin ratio (PAR) (OR = 1.256, 95% CI: 1.025–1.540, p = 0.028) were identified as independent risk factors for autonomic dysfunction based on the CARTs results. Combining the findings from COMPASS-31 and CARTs revealed that older age (OR = 1.133, 95% CI: 1.015–1.264, p = 0.027) and vascular invasion (OR = 7.706, 95% CI: 1.391–42.684, p = 0.019) were also independent risk factors for autonomic dysfunction.
Conclusion: Our findings reveal that these specific factors related to gastrointestinal cancers significantly influence autonomic nervous function. It is essential to evaluate autonomic nervous function and its associated risk factors in patients with gastrointestinal malignancies, which provide new insights into the intervention strategies for cancer diseases.
Gastrointestinal cancers, especially gastric and colorectal cancers, pose a significant threat to public health due to their high incidence and mortality rates (Arnold et al., 2020). The global lifetime risks of developing and dying from gastrointestinal cancers from birth to death are 8.20 and 6.17% in 2020 from a population-based systematic analysis (Wang et al., 2024). This situation requires a better understanding of their pathophysiology and risk factors related to the tumors’ formation (Keum and Giovannucci, 2019). Recent studies have highlighted the role of the autonomic nervous system (ANS) in cancer progression, particularly in gastrointestinal tumors (Hanoun et al., 2015; Silverman et al., 2021; Zahalka and Frenette, 2020). Cancer can drive neurogenesis, as well as nerves that may fuel GI tumor progression. The ANS, including sympathetic and parasympathetic nerves, mainly innervates the gastrointestinal tract, playing a crucial role in regulating various physiological functions and tumor microenvironments. For instance, a study has shown that alterations in the sympathetic and parasympathetic branches can influence tumor growth, metastasis, and patient outcomes in malignancies (Wang et al., 2024; Li et al., 2023). It is known that some neurotransmitters and neuropeptides, such as epinephrine and acetylcholine, drive the activation of various oncogenic pathways downstream of neural receptors within cancer cells (Wan et al., 2022). Despite increasing awareness of this relationship, there is still a lack of clinical data describing the status of autonomic nervous function in gastrointestinal cancer patients. It remains unclear whether autonomic dysfunction can predict cancer prognosis and serve as a marker for risk stratification. Additionally, it is uncertain which factors are associated with autonomic dysfunction in cancer patients and whether these factors can be targeted for personalized interventions. As autonomic dysfunction can increase the risk of sudden death and impact patient prognosis (Balcıoğlu and Müderrisoğlu, 2015; Goldberger et al., 2019), it is essential to focus on both the autonomic function of patients and its associated risk factors. Therefore, this study aims to evaluate autonomic nervous function in patients with gastrointestinal cancers, using COMPASS-31 and CARTs, both of which are reliable tools for assessing autonomic function and have shown good consistency (Peng et al., 2021). Additionally, this study will correlate autonomic function with clinical characteristics to uncover potential associations that may inform clinical practice. Our research aims to improve the understanding of the relationship between gastrointestinal cancers and the autonomic nervous system, which could lead to innovative therapies targeting tumors and neurogenesis.
This is a retrospectively descriptive study.
A total of 55 newly diagnosed patients with gastrointestinal cancers (1 duodenal cancer, 1 jejunal cancer, and 53 colorectal cancer) at the Department of Oncology, Southern Hospital, Southern Medical University, during June 2022 to October 2023, were included. The exclusion criteria included: (1) patients who had already received chemotherapy or radiotherapy; (2) patients with diabetes, rheumatism or chronic heart disease; (3) abnormal nerve conduction study indicated the presence of prior peripheral neuropathy.
The COMPASS-31 is a self-assessment instrument published by the Mayo Clinic in 2012 and includes 31 items assessing six domains of autonomic function: orthostatic hypohemia, vasomotor function, secretion function, gastrointestinal function, bladder function, and pupillary motor function. The total score is 100 (Sletten et al., 2012). In our previous study, the cut-off value for Chinese people to judge abnormal autonomic nervous function was >20 (Peng et al., 2021), which was also applied in this study.
Cardiovascular autonomic reflex tests (CARTs) are relatively objective tools for evaluating autonomic nervous function. CARTs include four tests: deep breathing, Valsalva maneuver, 30:15 heart rate ratio, and the decrease in systolic blood pressure after standing (Spallone et al., 2011). Each test is classified as normal, borderline, or abnormal, with scores of 0, 0.5, and 1, respectively. Therefore, the total score for CARTs ranges from 0.0 to 4.0, with nine possible grades. A score of ≥2.0 is classified as abnormal according to the American Diabetes Association (ADA) standard for diagnosing diabetic autonomic neuropathy (Spallone et al., 2011).
All participants were assessed for COMPASS and CARTs before receiving treatment. Then we collected clinical informations for all these participants, including: (1) demographic information such as gender, age, body mass index (BMI), history of hypertension, diabetes, smoking history, and Eastern Cooperative Oncology Group Performance Status (ECOG PS) score; (2) laboratory data including tumor markers, complete blood count (CBC), platelet/lymphocyte ratio (PLR), neutrophil/lymphocyte ratio (NLR), platelet/albumin ratio (PAR), C-reactive protein/albumin ratio (CAR), and systemic immune-inflammation index (SII); and (3) pathological data such as primary tumor site, degree of differentiation, tumor type, maximum diameter, regional lymph node metastasis, vascular invasion, and neural invasion. We divided these participants into normal or abnormal autonomic function groups according to the results of COMPASS-31 and CARTs, separately or combined. In the combined analysis, patients who scored abnormal on either COMPASS-31 or CARTs were classified into the abnormal group. After comparison between groups, univariate and multivariate regression analysis were performed to evaluate the risk factors of gastrointestinal cancer patients with abnormal autonomic nervous function.
Statistical analysis was conducted using SPSS 26.0 software. Quantitative data with a normal distribution were presented as (x ± s), while count data were expressed as percentages (%). Intergroup comparisons (data not shown) were made using analysis of variance. Correlation analysis was performed using both Pearson and Spearman methods. Univariate and multivariate logistic regression analyses were used to identify relevant risk factors for autonomic nerve dysfunction and their association with cancer. A p-value of less than 0.05 was considered statistically significant.
This study was authorized and supervised by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2022-362).
The study included 55 patients, consisting of 26 males and 29 females, with an average age of 53.4 ± 8.6 years. The average COMPASS-31 score was 14.3 ± 10.7, while the average CARTs score was 1.1 ± 0.8. The results indicated a significant positive correlation between COMPASS-31 and CARTs, with a correlation coefficient of r = 0.413 (p = 0.002).
According to whether the COMPASS-31 was >20, there were 40 cases in the normal group and 15 cases in the abnormal group. Univariate analysis showed that white blood cell (WBC) had statistical differences between groups, as shown in Table 1. Multivariate logistic regression analysis showed that decreased WBC and increased maximum tumor diameter were risk factors for autonomic dysfunction, as shown in Table 1.
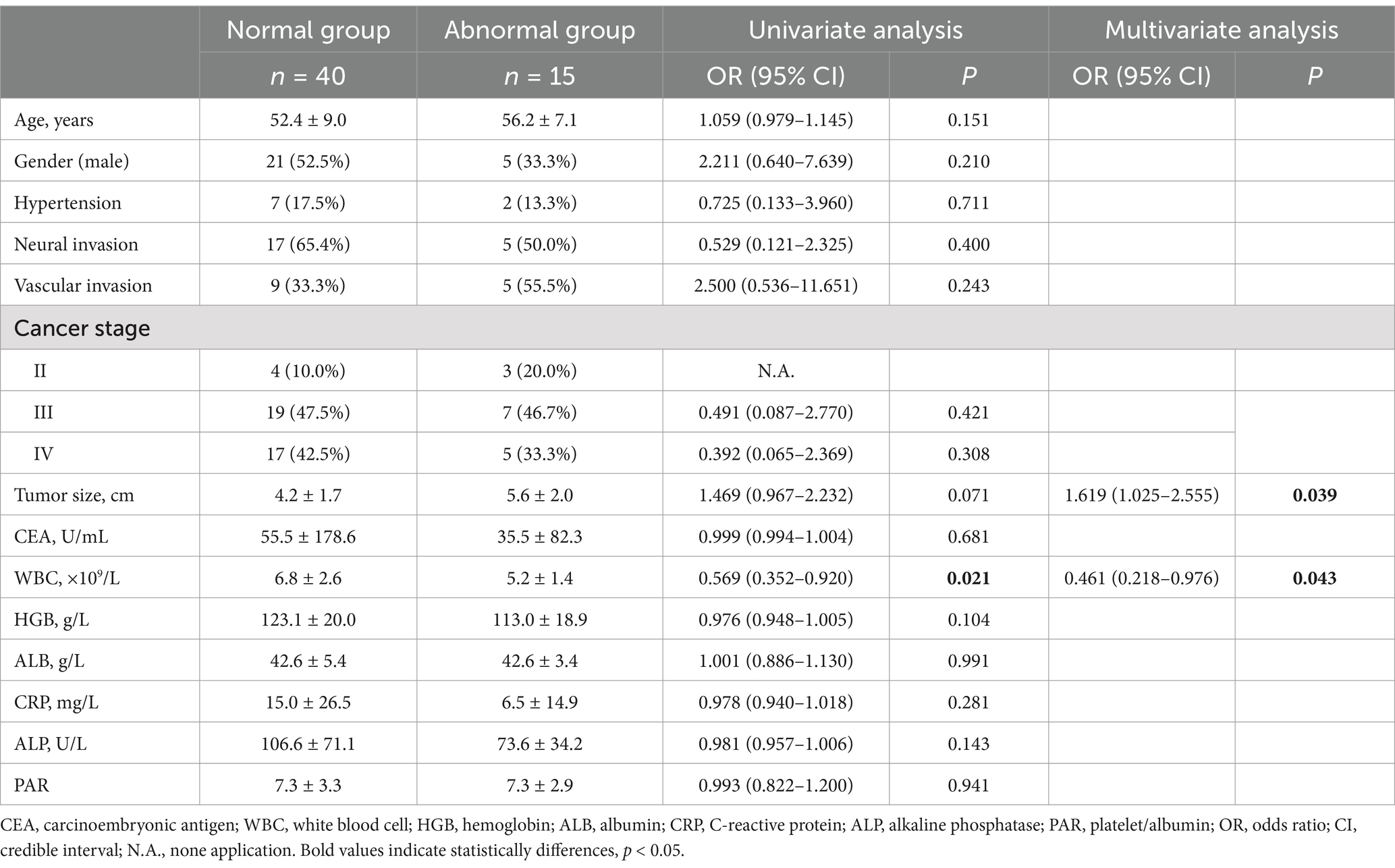
Table 1. Factors influencing autonomic nervous function in patients with gastrointestinal malignancies according to the COMPASS-31.
According to whether the CARTs were ≥2.0, there were 40 cases in the normal and 15 cases in the abnormal group (different from COMPASS-31). Univariate analysis showed that hypertension, Hemoglobin (HGB), albumin (ALB) and PAR had statistical differences between the groups, as shown in Table 2. Multivariate logistic regression analysis showed that hypertension and elevated PAR were risk factors for autonomic dysfunction, as shown in Table 2.
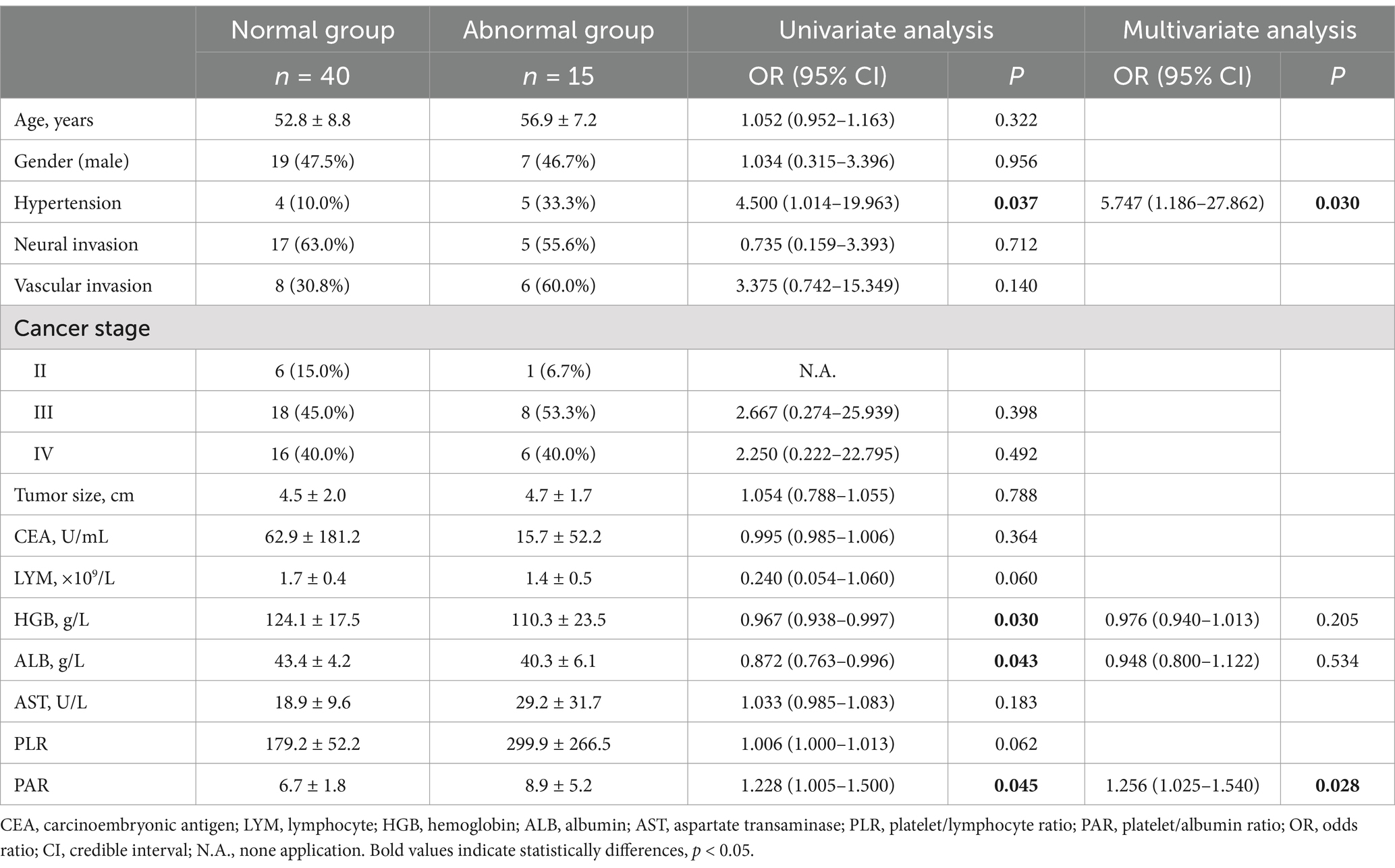
Table 2. Factors influencing autonomic nervous function in patients with gastrointestinal malignancies according to the CARTs.
Either COMPASS-31 was >20, or CARTs were ≥2.0, there were 30 cases in the normal and 25 cases in the abnormal group. Univariate analysis showed that age, vascular invasion, lymphocyte (LYM), HGB had statistical differences between groups, as shown in Table 3. Multivariate logistic regression analysis showed that increased age and vascular invasion were independent risk factors for autonomic nervous dysfunction, as shown in Table 3.
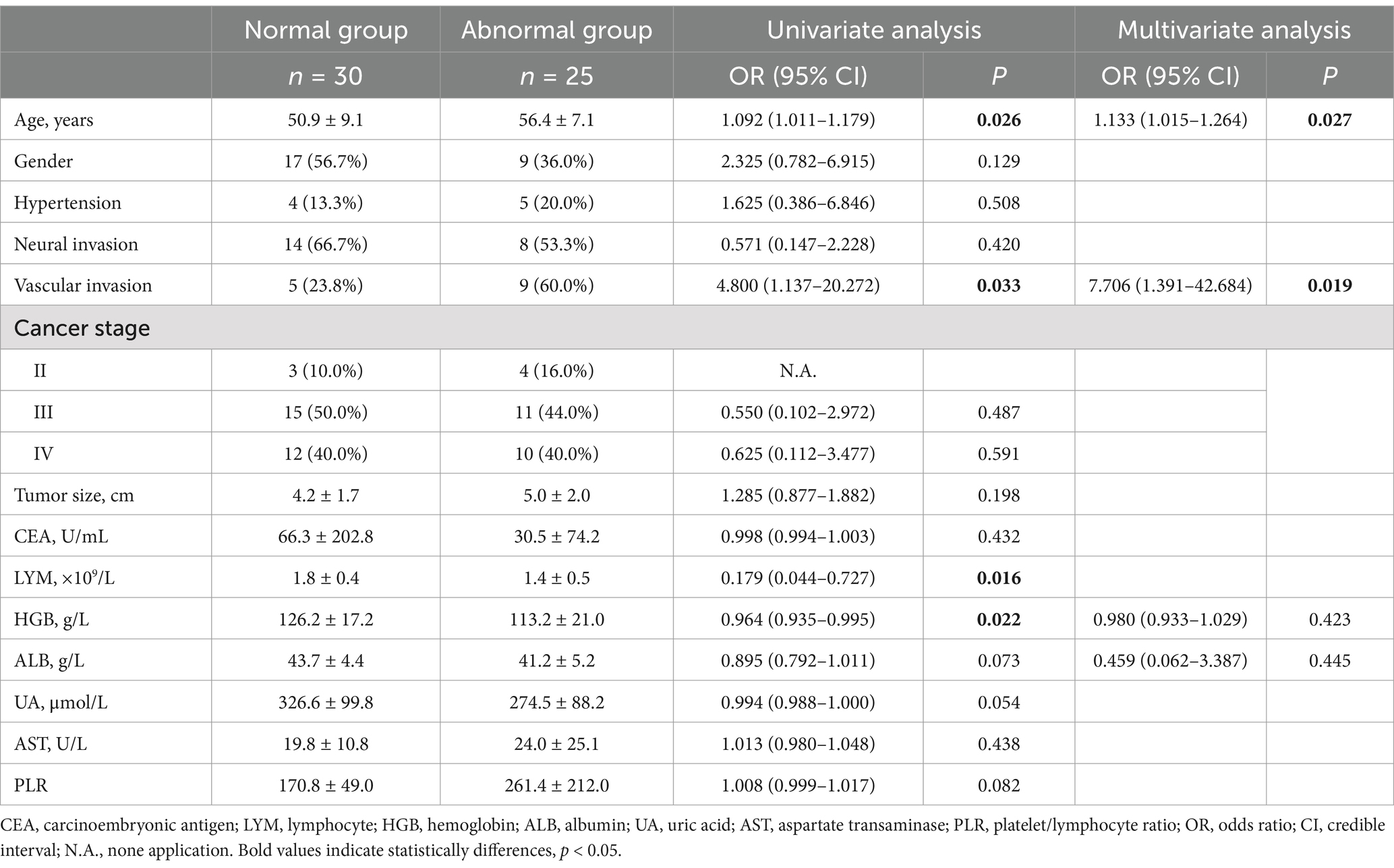
Table 3. Factors influencing autonomic nervous function in patients with gastrointestinal malignancies according to the combined COMPASS-31 and CARTs.
This study investigated the relationship between autonomic nervous function and gastrointestinal cancers in diagnosed patients, using the COMPASS-31 questionnaire and CARTs to evaluate their autonomic function status. We identified correlations with several risk factors, including advanced age, larger maximum tumor diameter, hypertension, elevated PAR, reduced WBC count, and vascular invasion. This study highlighted the importance of examining patients’ autonomic function and the risk factors that may contribute to dysfunction of the autonomic nervous system.
This study offered new insights into how the autonomic nervous system (ANS) relates to gastrointestinal cancers, specifically focusing on assessing autonomic function in cancer patients. Previous research primarily examined the molecular mechanisms of the ANS in regulating different aspects of tumor development (Hajiasgharzadeh et al., 2020; March et al., 2020; Shao et al., 2016). The clinical evaluations of autonomic nervous function in cancer patients were uncommon. COMPASS-31 and CARTs are useful tools for evaluating autonomic function. COMPASS-31 is a questionnaire that is easy to use but relatively subjective; CARTs rely on electrophysiological devices and are more objective. They have shown good agreement in the diagnosis of diabetic autonomic neuropathy (Peng et al., 2021). Our study established a foundational approach for clinically evaluating autonomic nervous function in cancer patients, which paved the way for further prognostic research.
Our findings showed that certain clinical features—such as older age, hypertension, high PAR, low WBC count, increased maximum tumor diameter and vascular invasion—were associated with autonomic dysfunction in patients with gastrointestinal cancer. Autonomic decline is associated with age, and it has been confirmed in our previous study that COMPASS-31 score is positively correlated with age (Peng et al., 2021). At the same time, aging is also a chronic inflammatory state of the whole body, which can induce neuroinflammation, resulting in impaired autonomic nervous function (Li et al., 2023). Hypertension can both cause and result from abnormal autonomic nervous function. Clinical studies have confirmed the dysregulation of autonomic nervous control in the cardiovascular system, which is usually associated with increased sympathetic tone and decreased parasympathetic tone, which leads to a variety of cardiovascular diseases, including hypertension (Mancia and Grassi, 2014). Our findings also confirm the association between hypertension and autonomic disorders in patients with gastrointestinal cancer. Elevated PAR indicates increased platelet number, which is also an indicator of systemic inflammation. Thrombocytosis may be related to sympathetic nerve activation. Studies have reported that noradrenaline (NE) can induce megakaryocyte adhesion, migration and proplatelet formation, and promote the production of platelets (Chen et al., 2016). Similarly, adrenergic receptor agonists can induce vasoconstriction and local decrease in blood flow, triggering rapid calcium signaling of white blood cells and stopping cell movement, so sympathetic activation can impair the mobility of white blood cells (Devi et al., 2021; Globig et al., 2023), which can explain the leukopenia in the group of patients with autonomic dysfunction in this study. Therefore, decreased WBC and elevated platelets may be related to overactivation of sympathetic nerve, and are associated with poor prognosis of various tumors (Proctor et al., 2011). Larger tumor diameter and vascular invasion certainly reflect advanced tumor progression, which also supported the positive correlation between tumor development and autonomic nervous dysfunction. This also supported the previous findings that systemic immune responses, closely linked to autonomic regulation, might be weakened in cancer patients, affecting their prognosis (Globig et al., 2023; Saloman et al., 2016).
However, the present research is primarily a preliminary clinical study, and it is crucial to recognize its limitations. First, the small sample size restricts how widely the findings can be applied to larger patient populations. Currently, we are evaluating a larger group of patients with gastrointestinal cancer. Additionally, we aim to explore the relationships between autonomic nerve function and other types of cancer. Second, the present study lacks long-term follow-up data. Although we recognize that autonomic nervous dysfunction can result in a poor prognosis, it is essential to directly observe the outcome differences between the two patient groups. We are currently following up these groups of patients and observing changes in their survival curves. Finally, the study did not include laboratory experiments to confirm the link between the observed risk factors and autonomic dysfunction. This omission raises questions about the mechanistic insights derived solely from clinical data. Collectively, these limitations highlight the necessity for future studies to incorporate larger, more representative cohorts and robust experimental designs to substantiate the clinical observations reported herein.
This investigation revealed important connections between autonomic nerve dysfunction and different clinical features in patients with gastrointestinal cancers. Several independent risk factors were identified, including older age, hypertension, high PAR, low WBC, increased maximum tumor diameter and vascular invasion. These findings enhanced our understanding of the relationship between autonomic dysfunction and cancer characteristics. Furthermore, these findings pave the way for future research on therapeutic interventions targeting autonomic pathways.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Medical Ethics Committee of Nanfang Hospital of Southern Medical University (Approval number: NFEC-2022-362). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZX: Writing – original draft, Data curation, Formal analysis, Investigation, Methodology, Visualization. FQ: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. LC: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. JA: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. WZ: Data curation, Investigation, Resources, Writing – original draft. DQ: Data curation, Investigation, Resources, Writing – original draft. PY: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. WC: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Clinical Research Program of Nanfang Hospital, Southern Medical University (2021CR015) and Wu Jieping Medical Foundation (320.6750.2024-6-119).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arnold, M., Abnet, C. C., Neale, R. E., Vignat, J., Giovannucci, E. L., McGlynn, K. A., et al. (2020). Global burden of 5 major types of gastrointestinal Cancer. Gastroenterology 159, 335–349.e15. doi: 10.1053/j.gastro.2020.02.068
Balcıoğlu, A. S., and Müderrisoğlu, H. (2015). Diabetes and cardiac autonomic neuropathy: clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J. Diabetes 6, 80–91. doi: 10.4239/wjd.v6.i1.80
Chen, S., Du, C., Shen, M., Zhao, G., Xu, Y., Yang, K., et al. (2016). Sympathetic stimulation facilitates thrombopoiesis by promoting megakaryocyte adhesion, migration, and proplatelet formation. Blood 127, 1024–1035. doi: 10.1182/blood-2015-07-660746
Devi, S., Alexandre, Y. O., Loi, J. K., Gillis, R., Ghazanfari, N., Creed, S. J., et al. (2021). Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230.e7. doi: 10.1016/j.immuni.2021.03.025
Globig, A. M., Zhao, S., Roginsky, J., Maltez, V. I., Guiza, J., Avina-Ochoa, N., et al. (2023). The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature 622, 383–392. doi: 10.1038/s41586-023-06568-6
Goldberger, J. J., Arora, R., Buckley, U., and Shivkumar, K. (2019). Autonomic nervous system dysfunction: JACC focus seminar. J. Am. Coll. Cardiol. 73, 1189–1206. doi: 10.1016/j.jacc.2018.12.064
Hajiasgharzadeh, K., Somi, M. H., Sadigh-Eteghad, S., Mokhtarzadeh, A., Shanehbandi, D., Mansoori, B., et al. (2020). The dual role of alpha7 nicotinic acetylcholine receptor in inflammation-associated gastrointestinal cancers. Heliyon 6:e03611. doi: 10.1016/j.heliyon.2020.e03611
Hanoun, M., Maryanovich, M., Arnal-Estapé, A., and Frenette, P. S. (2015). Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 86, 360–373. doi: 10.1016/j.neuron.2015.01.026
Keum, N., and Giovannucci, E. (2019). Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 16, 713–732. doi: 10.1038/s41575-019-0189-8
Li, X., Li, C., Zhang, W., Wang, Y., Qian, P., and Huang, H. (2023). Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 8:239. doi: 10.1038/s41392-023-01502-8
Li, Y. T., Yuan, W. Z., and Jin, W. L. (2023). Vagus innervation in the gastrointestinal tumor: current understanding and challenges. Biochim. Biophys. Acta Rev. Cancer 1878:188884. doi: 10.1016/j.bbcan.2023.188884
Mancia, G., and Grassi, G. (2014). The autonomic nervous system and hypertension. Circ. Res. 114, 1804–1814. doi: 10.1161/CIRCRESAHA.114.302524
March, B., Faulkner, S., Jobling, P., Steigler, A., Blatt, A., Denham, J., et al. (2020). Tumour innervation and neurosignalling in prostate cancer. Nat. Rev. Urol. 17, 119–130. doi: 10.1038/s41585-019-0274-3
Peng, Y., Liu, Y. S., Wu, M. Y., Chen, C. N., Li, C. Q., Jiang, A. Q., et al. (2021). Evaluation of the degree of agreement of four methods for diagnosing diabetic autonomic neuropathy. Front. Neurol. 12:637099. doi: 10.3389/fneur.2021.637099
Proctor, M. J., Morrison, D. S., Talwar, D., Balmer, S. M., Fletcher, C. D., O'Reilly, D. S., et al. (2011). A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur. J. Cancer 47, 2633–2641. doi: 10.1016/j.ejca.2011.03.028
Saloman, J. L., Albers, K. M., Rhim, A. D., and Davis, B. M. (2016). Can stopping nerves, Stop Cancer? Trends Neurosci. 39, 880–889. doi: 10.1016/j.tins.2016.10.002
Shao, J. X., Wang, B., Yao, Y. N., Pan, Z. J., Shen, Q., and Zhou, J. Y. (2016). Autonomic nervous infiltration positively correlates with pathological risk grading and poor prognosis in patients with lung adenocarcinoma. Thorac. Cancer 7, 588–598. doi: 10.1111/1759-7714.12374
Silverman, D. A., Martinez, V. K., Dougherty, P. M., Myers, J. N., Calin, G. A., and Amit, M. (2021). Cancer-associated neurogenesis and nerve-Cancer cross-talk. Cancer Res. 81, 1431–1440. doi: 10.1158/0008-5472.CAN-20-2793
Sletten, D. M., Suarez, G. A., Low, P. A., Mandrekar, J., and Singer, W. (2012). COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin. Proc. 87, 1196–1201. doi: 10.1016/j.mayocp.2012.10.013
Spallone, V., Bellavere, F., Scionti, L., Maule, S., Quadri, R., Bax, G., et al. (2011). Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr. Metab. Cardiovasc. Dis. 21, 69–78. doi: 10.1016/j.numecd.2010.07.005
Spallone, V., Ziegler, D., Freeman, R., Bernardi, L., Frontoni, S., Pop-Busui, R., et al. (2011). Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab. Res. Rev. 27, 639–653. doi: 10.1002/dmrr.1239
Wan, C., Yan, X., Hu, B., and Zhang, X. (2022). Emerging roles of the nervous system in gastrointestinal Cancer development. Cancers 14:3722. doi: 10.3390/cancers14153722
Wang, H., Huo, R., He, K., Cheng, L., Zhang, S., Yu, M., et al. (2024). Perineural invasion in colorectal cancer: mechanisms of action and clinical relevance. Cell. Oncol. 47, 1–17. doi: 10.1007/s13402-023-00857-y
Wang, S., Zheng, R., Li, J., Zeng, H., Li, L., Chen, R., et al. (2024). Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 9, 229–237. doi: 10.1016/S2468-1253(23)00366-7
Keywords: gastrointestinal cancer, autonomic nervous function, COMPASS-31, CARTs, clinical characteristics
Citation: Xiwen Z, Qiyun F, Chuqiao L, Anqi J, Zhenzhen W, Qiong D, Yu P and Chunlin W (2025) The assessment of autonomic nervous function in patients with gastrointestinal malignancies and its relationship with clinical characteristics. Front. Neurosci. 19:1542224. doi: 10.3389/fnins.2025.1542224
Received: 10 December 2024; Accepted: 18 February 2025;
Published: 28 February 2025.
Edited by:
Herbert F. Jelinek, Khalifa University, United Arab EmiratesReviewed by:
Hadis Ashrafizadeh, Dezful University of Medical Sciences, IranCopyright © 2025 Xiwen, Qiyun, Chuqiao, Anqi, Zhenzhen, Qiong, Yu and Chunlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Chunlin, d2FuZ2NodW5sMDNAMTYzLmNvbQ==; Peng Yu, cGVuZ3l1X3B5QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.