- 1Biomedical Research Center, QU Health, Qatar University, Doha, Qatar
- 2College of Sport Sciences, Sport Coaching Department, Qatar University, Doha, Qatar
- 3College of Dental Medicine, QU Health, Qatar University, Doha, Qatar
- 4Department of Pharmacology, BioHealth Institute Granada (IBs Granada), Neuroscience Institute (CIBM), School of Medicine, University of Granada, Granada, Spain
- 5College of Medicine, QU Health, Qatar University, Doha, Qatar
Background: Circadian rhythms and sleep patterns are important regulators of metabolic health. During Ramadan intermittent fasting (RIF), the sleep–wake cycles are often disrupted, which can affect physical activity (PA) and related metabolic responses. Limited knowledge is available on how sleep disruption influences PA in the general population during RIF. This pilot study aimed to examine the metabolic responses to moderate PA under normal and disrupted sleep patterns during RIF.
Methods: A pilot study was conducted on 12 participants comprising of individuals with normal (n = 5) and disrupted sleep patterns (n = 7). Blood samples were collected, and measurements of clinical traits, cytokines, homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic profiles were performed before and after intervention. Orthogonal partial least square – discriminant analysis (OPLS-DA) and linear regressions were performed to assess metabolic responses to PA during RIF under different patterns.
Results and conclusion: Fasting participants with normal sleep patterns exhibited lower HOMA-IR (β = −0.416, p = 0.047) in response to PA compared to those with disrupted sleep. Additionally, they demonstrated more efficient lipid utilization during PA, characterized by reduced diacylglycerol levels, which could enhance insulin sensitivity and lower the risk of type 2 diabetes. In contrast, fasting participants with disrupted sleep patterns experienced metabolic stress, marked by significant depletion of polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFAs), and plasmalogens in response to PA. These changes were associated with increased inflammation and oxidative stress, potentially leading to metabolic dysregulation.
1 Introduction
Human physiology varies throughout the 24-h cycle, encompassing changes at every stage, from the cellular (Cuesta et al., 2015) and metabolic (Chua et al., 2013) levels, to physiological and behavioral levels (Morris et al., 2015; Imeri and Opp, 2009). We have successfully evolved to synchronize metabolic activities to a 24-h day and sleep–wakefulness dependent systems and circadian rhythm (CR) are in charge of this 24-h variance. Sleep–wake systems and CR are essential for energy metabolism (Rynders et al., 2020; Zitting et al., 2018) and associated habits such as eating and exercise (Arble et al., 2015; Simon et al., 1998). For instance, growth hormone levels in the blood are mostly reliant on sleep, with the majority of secretion occurring during slow-wave sleep (Czeisler and Klerman, 1999). In contrast, cortisol levels peak in the early morning hours and are mostly regulated by the circadian cycle (Czeisler and Klerman, 1999). Similarly, energy expenditure peaks during waking hours and falls during sleeping hours (Jung et al., 2011).
Interestingly, the capacity of a human to predict and optimize metabolic processes is compromised if the circadian rhythms are disturbed or misaligned (Arble, 2023). The term “circadian misalignment” refers to a number of circumstances, including when sleep–wakefulness, and fasting-food intake cycles are not in sync with internal circadian time or external time, or when central and peripheral rhythms are not concurred (Chaput et al., 2023). Disturbances in the circadian rhythm have been associated with a number of cardiometabolic conditions, such as decreased insulin sensitivity, elevated markers of systemic inflammation, elevated blood pressure, decreased energy expenditure, and obesity (BaHammam and Almeneessier, 2020). Ramadan intermittent fasting (RIF) is an example of a pattern that can potentially disrupt the circadian rhythm and sleep–wake cycle as it involves a distinct pattern of dry and diurnal fasting for 29–30 days straight from sunrise to sunset followed by food-intake during the night hours (Patterson and Sears, 2017; Obaideen et al., 2022). Current literature has highlighted numerous advantageous metabolic, inflammatory, and anthropometric effects such as the improvement of body mass index (Jahrami et al., 2020), metabolic syndrome symptoms (Faris et al., 2020), inflammatory and stress markers (Faris et al., 2019), glucose metabolism (Faris et al., 2020), and promote longevity and healthier aging (Almuraikhy et al., 2024) during RIF.
Although, RIF has no direct effect on the circadian rhythm provided if the meals are strictly limited to the early evening and predawn hours and there is enough sleep during the night (BaHammam and Almeneessier, 2020). However, most individuals no longer follow the normal exposure to the natural sleep–wake cycle. People dine late into the night, remain active and exposed to light long after sunset, and wake up to alarm clocks hours later than they would ordinarily wake up. Moreover, there is evidence of reduced habitual physical activity of individuals during the fasting period (Farooq et al., 2021). Ramadan is typically regarded as a time of relative physical inactivity, increasing the risk of obesity and insulin resistance (AlMuraikhy et al., 2022). Both physical activity (PA) and IF have been postulated to have beneficial effects on health (Lessan et al., 2018). PA during fasting state is associated with increased fat metabolism compared to after food intake period, thereby emphasizing on the positive health outcomes of exercise performed when fasting (Lipert et al., 2021).
Notably, fundamental components of health are sleep and activity, and metabolic health is stressed by both circadian misalignment and insufficient sleep, which are linked to negative health consequences such as an elevated risk of obesity, cardiovascular disease, hypertension, dyslipidaemia and type 2 diabetes (Chaput et al., 2023; Chaput et al., 2020). While a recent study found that sleep disturbances during Ramadan negatively affect the physical performance of professional athletes (Lipert et al., 2021), there remains a significant knowledge gap regarding how disrupted sleep influences physical activity responses in the general population. To the best of our knowledge, no studies have addressed this issue outside of elite athletic contexts, and so the broader metabolic implications in non-athletic individuals are largely unexplored. We hypothesized that combining RIF and PA may increase the health advantages of fasting people, provided they adhere to their normal sleeping schedules. Sleep disturbances may attenuate these benefits by changing the metabolic pathways, such as disrupting lipid homeostasis, and promoting inflammation. This study aimed to assess the metabolic response to physical activity during RIF under both normal and disrupted sleep conditions which will provide novel insights into the role of circadian alignment in optimizing exercise-related metabolic responses. Given the observed reduction in daily physical activity during Ramadan (Farooq et al., 2021), particularly during fasting hours, our study aimed to assess the metabolic response to physical activity introduced during RIF under normal and disrupted sleep pattern in general population. By addressing this gap, our findings contribute to a growing field on the importance of sleep and circadian regulation in metabolic health and emphasize the need for lifestyle recommendations during Ramadan to maximize the benefits of fasting and exercise.
2 Methods
This study was conducted as a pilot study to investigate the metabolic responses to moderate physical activity in individuals with normal versus disrupted CR. The primary objective was to explore the preliminary changes that could be linked to variations in circadian rhythm and to analyze the study design’s viability for a more extensive investigation.
2.1 Participants
Twelve female students from Qatar University were enrolled during the month of Ramadan. These females (aged 20–30) volunteered to take part in the study. All participants provided consent form prior to participation. All protocols were approved by Qatar University (QU-IRB 1798-EA/23) as per regulations of Qatar Ministry of Public Health (MoPH). The inclusion criteria were as follows: BMI between 20 and 30 kg/m2, be free of cardiovascular disease, glaucoma, blood clots, muscle degeneration, cancer, and other autoimmune illnesses such as T2D or cancer. The disruption in circadian rhythm in our cohort was due to the habitual changes associated with Ramadan fasting.
In this study, participants self-reported their habitual sleep–wake cycles. Based on these reports, they were divided into two groups: those who maintained a normal circadian rhythm by sleeping during the night (n = 5) and those who exhibited a disrupted rhythm by sleeping during the day and remaining awake at night (n = 7). Groups were formed without intentional selection where age, health, and lifestyle were equally distributed by chance, allowing for fair comparison between the two groups. Clinical measurements and blood samples were taken before and after 4 weeks training period during Ramadan month.
2.2 Physical activity
A moderate intensity exercise (MIE) regimen was followed by all qualified individuals for three times per week during the afternoon (fasting hours) from 1 pm to 3 pm. Based on the American Society of American College of Sports Medicine (ACSM)’s recommendations (Haskell et al., 2007), training sessions included moderate (13–15 Borg scale, 50–70% VO2max) aerobic workouts (walking and jogging) which lasted for 30 min at a time. The intensity of the activities was determined by individual’s muscle and cardiorespiratory fitness. The six-minute walking test distance (6WT) is a submaximal assessment utilized to quantify the distance a participant can traverse on a flat surface within a six-minute duration. The Metabolic Equivalent of Task (MET) values were adjusted based on IPAQ responses to quantify daily activities. MET was utilized for intensity and energy expenditure, expressed similarly for individuals of different weights. The exercise intensity was evaluated using Borg scale test using the Borg rating of perceived exertion (RPE) (Borg, 1990). It was assessed for each participant following the 6WT and the training session. A person’s perception of their effort and exertion, dyspnoea, and exhaustion during an exercise can be measured with the Borg scale.
2.3 Metabolomics and statistical analysis
All participants’ serum samples were subjected to untargeted metabolomics utilizing Metabolon’s platform in accordance with established guidelines (Al-Khelaifi et al., 2018). The metabolomics data consisting of 259 unknown identities and 1,039 known identities were median scaled. The minimum value across batches from the median-scaled data was used to impute missing values before natural log transformation. The unknown metabolites were removed from further statistical analysis. To evaluate the data’s quality, principal component analysis (PCA) was used. Orthogonal partial least square-discriminant analysis (OPLS-DA) model was used to identify the greatest discriminant metabolites associated with physical activity under normal and disrupted CRs separately.
Univariate analyses were conducted in two stages: comparative analysis and interaction effects. The former included paired t-test between pre- and post- physical activity performed separately for normal and disrupted CRs to compare the change in the metabolite levels in different CR conditions. The latter analysis used a mixed-effects model to assess the interaction between CR and activity to determine if the effect of physical activity on metabolic profile differs by sleep pattern. The p-values were adjusted using false discovery rate (FDR) correction for both analyses. Additionally, functional enrichment analysis was performed on all nominally significant metabolites list from the comparative analysis using Wilcoxon sum of ranks and p-values were adjusted by the FDR correction. This was performed to investigate the over-represented sub-pathways using the metabolites in different CR conditions. The sub-pathways were previously predefined using Metabolon, and those with less than three top hits were dropped. Spearman’s correlation was performed between clinical parameters and relevant metabolites.
3 Results
3.1 Summary of participant clinical profile
Although none of the characteristics exhibited statistically significant variations between the two groups, HOMA-IR, LDL, TNF-α, total cholesterol, and triglycerides showed a decrease in normal group and an increase in the disrupted group (Table 1). Supplementary Table S3 shows the baseline comparison between the normal CR and disrupted CR groups.
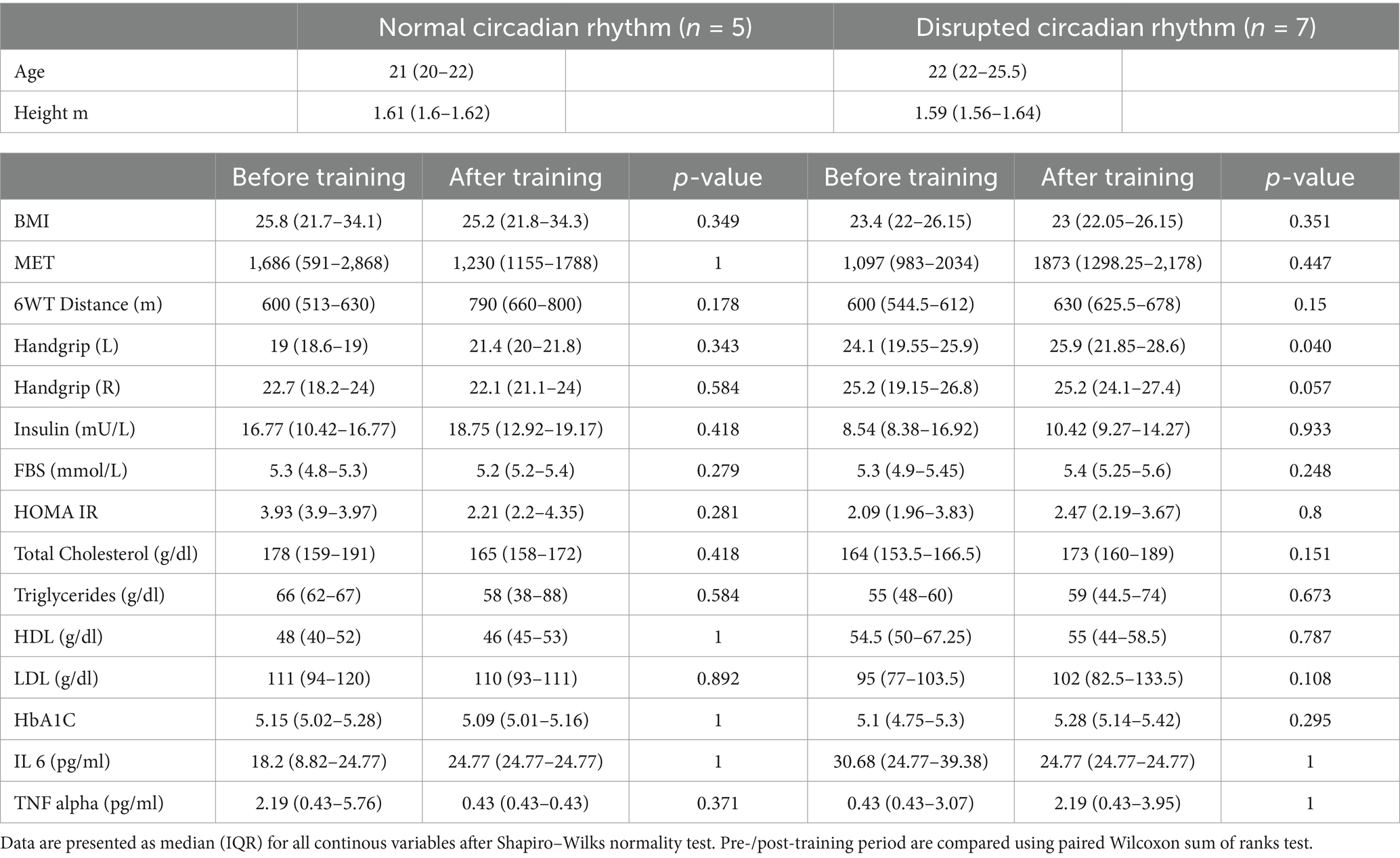
Table 1. General characteristics of participants categorized by circadian rhythm (normal vs. disrupted) and training (before vs. after).
3.2 Global metabolic signature of physical activity in individuals with normal and disrupted circadian rhythms
Orthogonal partial least squares-discriminant analysis (OPLS-DA) was performed separately for individuals with normal circadian rhythm and disrupted circadian rhythm to determine the change in the metabolic profile after moderate physical activity (Figure 1). The models performed in both CR groups showed Q2 > 0.4 conveying reliable reproducibility of the model.
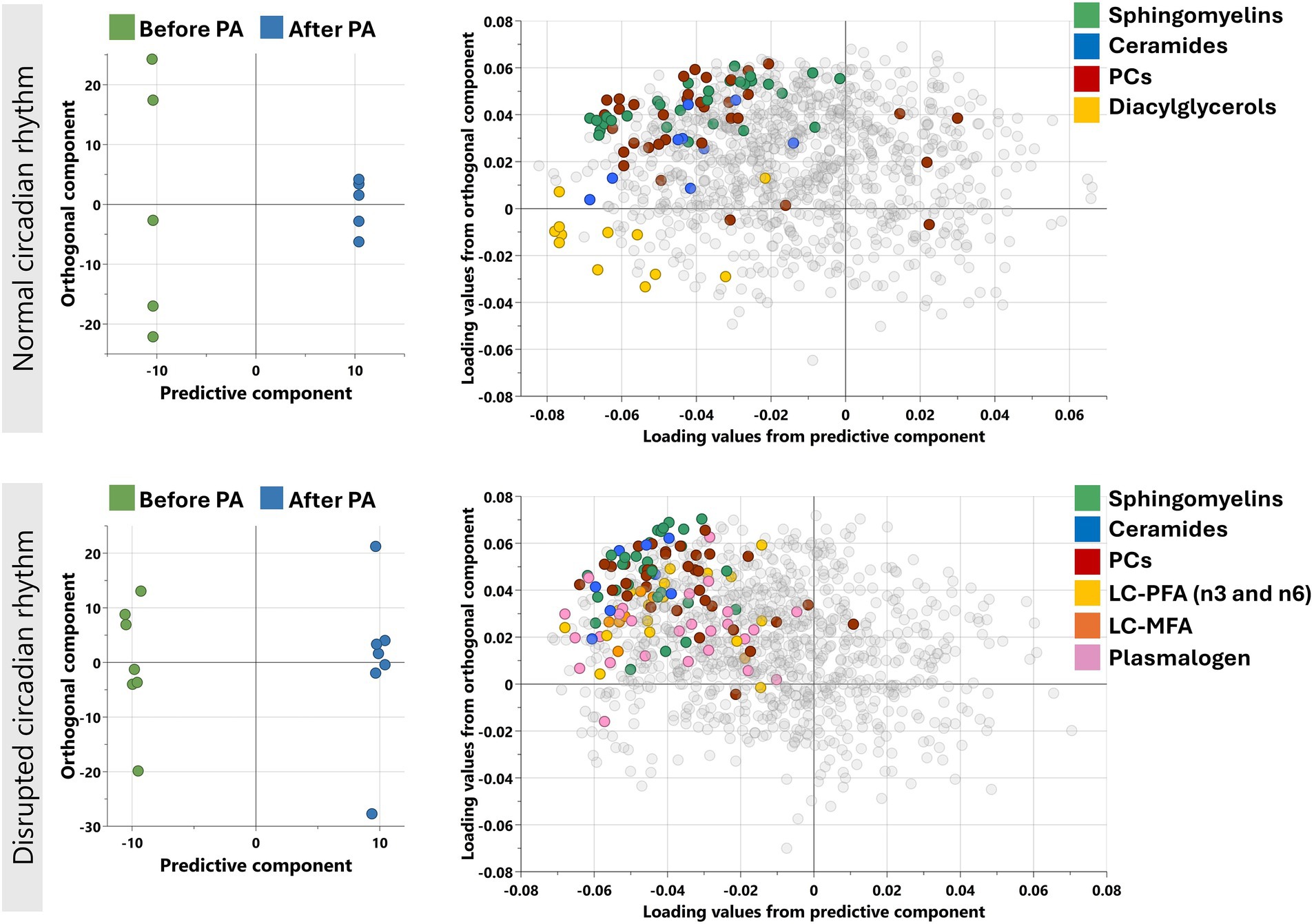
Figure 1. Orthogonal projections to latent structures discriminant analysis (OPLS-DA) performed for individuals with normal and disrupted circadian rhythm separately as shown. Model on normal CR individuals identified one predictive and five orthogonal components (R2X = 0.743: Q2 = 0.462) while on disrupted CR, the model identified 1 predictive and 3 orthogonal components (R2X = 0.42: Q2 = 0.564). The corresponding loadings plots (right) for normal and disrupted CR individuals as shown next to the scores plot (left). Enriched sub-pathways are colored in both OPLS-DA models, including LC-MFA, long chain monosaturated fatty acids; LC-PFA, long chain polyunsaturated fatty acid; PC, Phosphatidylcholines.
3.3 Metabolites associated with physical activity (PA) in normal and disrupted CR during Ramadan
The change in all metabolites before and after PA was performed one-by-one using paired t-test and FC analysis to investigate metabolites associated with PA in different CR conditions. Reductions in N-linoleoyl taurine in normal CR, and lysophospholipids [1-palmitoyl-GPE (16:0); 1-docosahexaenoyl-GPE (22:6)*] and diacylglycerol [palmitoyl-linoleoyl-glycerol (16:0/18:2) (Cuesta et al., 2015)*] in disturbed CR were observed. The results are displayed in Supplementary Table S1.
3.4 Functional enrichment analysis
Functional enrichment analysis was performed on all nominally significant metabolites from comparative analysis in normal and disrupted CR separately. The enriched pathways with FDR < 0.01 were considered statistically significant and are shown in Table 2. Lipids such as Phosphatidylcholine, Ceramides, and Sphingomyelins are enriched during Ramadan fasting irrespective of CR condition.
3.5 Evidence of CR related response to physical activity in Ramadan
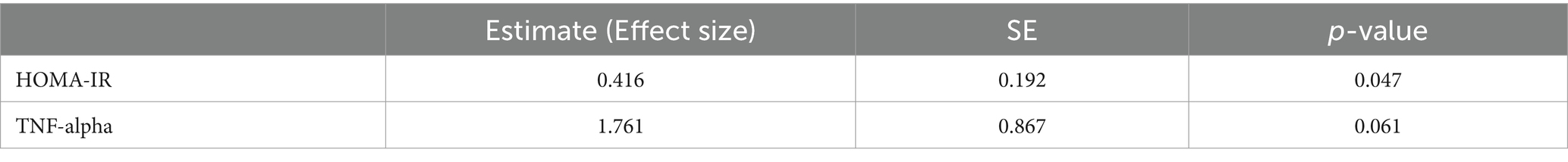
To investigate if the metabolic alteration observed due to physical activity is dependent on sleep pattern, mixed effects model was utilized to evaluate the interaction between PA and CR in all metabolites. Twenty-three metabolites were nominally significant, however, after multiple testing correction the significance was not retained. The nominally significant metabolites are displayed in Supplementary Table S2. Similar analysis was performed in clinical parameters and significant results are displayed in Table 3. Specifically, HOMA-IR demonstrated a significant increase of 0.416 (SE = 0.192, p = 0.047), indicating a negative impact of disrupted sleep on insulin resistance in fasting individuals engaging in physical activity. TNF-alpha levels, while not statistically significant, showed a trend toward increased inflammation, with an estimate of 1.761 (SE = 0.867, p = 0.061).
4 Discussion
Circadian clock and quality sleep regulate important biological processes. A disruption of this clock raises the possibility of developing chronic illnesses. We have looked into Ramadan as a distinct entity in light of the increasing trend of recommending “intermittent fasting” for overall cardiometabolic health. Engaging in physical activity is also regarded as a vital habit for improved health (Warburton and Bredin, 2016). Routine physical activity is associated with a significant reduction in premature mortality risk and can help prevent over 25 chronic medical conditions (Warburton et al., 2016). Even though exercise induces beneficial changes, its positive outcomes may not be attained if the internal circadian rhythm is disrupted due to misplaced sleeping cycles (Kline, 2014).
The objective of this study was to examine the effect of disrupted circadian rhythms on physical activity during Ramadan intermittent fasting. The major findings reveal that: (i) homeostatic model assessment for insulin resistance (HOMA-IR) is improved, and decrease in inflammation marker TNF-α is observed in individuals adhering to normal sleeping cycles compared to their disturbed counterparts, (ii) disrupted CR individuals showed reduced plasmalogens, and long chain poly- and monosaturated fatty acids, while reduced diacylglycerols were observed in normal CR participants, and (iii) physical activity in Ramadan is associated with reduced plasma sphingomyelins, ceramides, and phosphatidylcholines regardless of circadian rhythm disruption. To our knowledge, this is the first study investigating the impact of disturbed sleep-wake cycle during Ramadan on physical activity response on a metabolomic level in general population. The interpretations of these findings are summarized below.
4.1 Evidence of impact of disturbed CR on insulin resistance
Our observations suggest that combined effect physical activity and normal CR has a greater reduction in HOMA-IR and an increase in disturbed CR, this is in line with studies that have demonstrated that poor sleep quality have significantly elevated HOMA-IR compared to well-rested counterparts (De Bernardi Rodrigues et al., 2016; Rawat et al., 2019). Physical activity can mitigate some negative effects of disturbed sleep; however, its effectiveness may be reduced. For example, young adults who are physically active but with poor sleep cycle may not achieve the same insulin sensitivity benefits as those who maintain both good sleep cycle and regular exercise (Rawat et al., 2019). Interestingly, TNF-α also shows a similar trend with higher reduction in normal CR and an increase in disrupted CR. Ramadan alone can cause reductions in TNF-α (Zeb et al., 2020), but accompanied with poor sleeping schedules shows the elevation of the inflammatory protein even in fasting state in this study. Systemic low-grade inflammation is a frequent underlying cause of obesity and the associated cardiometabolic diseases, such as, cardiovascular disease, type 2 diabetes, and non-alcoholic fatty liver disease is (Furman et al., 2019). Our data suggest that disturbed CR can contribute to obesity and metabolic disorders, even in the context of combined physical activity and fasting.
4.2 Lipid metabolism with respect to disturbed CR after physical activity
Findings from this study show distinct pathways that are specific to different CR conditions post physical activity. We observed a significant reduction in diacylglycerols (DAGs) in normal CR in addition to several lipid changes. Normal sleep patterns combined with exercise facilitates a reduction in DAG levels, potentially aiding metabolic health and recovery (Morton et al., 2020). Notably, circadian rhythms also govern the activity of several DAGs (Chua et al., 2013; Gooley, 2016), and as such could serve as a marker for both sleep debt and circadian time. The reduction of diacylglycerols in individuals with normal sleep patterns post-exercise may indicate a protective mechanism against metabolic disturbances (see also Supplementary Figure S1). This result is consistent with the literature that suggests that accumulation of certain DAGs in skeletal muscle exacerbates insulin resistance. This highlights the importance of both physical activity and optimal sleep cycle in maintaining healthy lipid profiles and overall metabolic health.
On the other hand, we observed reductions in plasmalogens, long chain poly-(PUFAs) and monosaturated fatty acids (MUFAs) in individuals with disturbed CR in response to PA. This relationship highlights the complex connection between lipid metabolism, sleep quality, and physical exertion. We have shown that plasmalogen levels decrease in individuals with disturbed sleep patterns. These specialized phospholipids are crucial for maintaining membrane integrity and cellular function (Bozelli et al., 2021). Lower levels of these lipids are associated with cognitive decline, as seen in aging and neurodegenerative disorders. A recent study showed that supplementation with plasmalogens can enhance cognitive performance and promote neurogenesis and synaptic plasticity in animal models (Gu et al., 2022). Plasmalogens also have anti-inflammatory responses (Bozelli et al., 2021). A reduction in these lipids, coupled with increase in TNF-α, seen in our study with disrupted CR individuals indicates a potential increase in inflammation due to misaligned CR (see also Supplementary Figure S2). Additionally, PUFAs and MUFAs, which are essential fatty acids, lower the production of inflammatory cytokines and eicosanoids (Calder, 2006) and are therapeutic to acute and chronic inflammatory conditions. This study has shown these lipids are also lowered in disrupted CR, which leads to inflammation and metabolic dysregulation and eventually leading to insulin resistance, obesity and overall reduction in cognitive health (Gooley, 2016).
4.3 Lipid metabolism during Ramadan in relation to physical activity
Univariate and pathway enrichment analysis showed that metabolites from lipid pathways, mainly, sphingomyelins, ceramides, and phosphatidylcholines are significantly lower after exercise in both normal and disrupted CR individuals, indicating a central favorable lipid metabolism and energy regulation due to fasting and physical activity. Intermittent fasting is associated with metabolic transition from glucose to fatty acid-derived ketones, where there is a change from storing fat and synthesizing lipids and cholesterol to mobilizing fat through fatty acid oxidation and fatty acid-derived ketones, which maintain muscle mass and function (Anton et al., 2018). Recent studies have shown that RIF is accompanied by unique lipidomic fingerprints that set it apart from non-fasting days due to the notable dietary and lifestyle changes that coincide with the month of Ramadan, such as changes in macronutrients intake (Shatila et al., 2021), quality of sleep and duration (Faris et al., 2020), and physical activity (Almuraikhy et al., 2024; Farooq et al., 2021). Considering the mentioned factors, our data is expected to show a list of lipidomic changes, as such, our data demonstrates reduction in ceramides and sphingomyelins, and this is consistent with the recent literature which has demonstrated RIF is associated with reduced levels of sphingolipid/ceramide in circulation (Madkour et al., 2023). An excess of free fatty acids is frequently linked to fasting, and this may inhibit acid ceramidases (Chavez et al., 2005). Ceramides trigger β-cell death via producing free radicals, triggering endoplasmic reticulum stress, and suppressing Akt, contributing to insulin resistance (Galadari et al., 2013). Lower levels of these lipid species may improve metabolic function by increasing blood flow to skeletal muscle (Cantalupo et al., 2017). Additionally, studies have shown that reduced ceramide levels are beneficial as it ameliorates lipotoxicity, inflammation and oxidative stress, thereby contributing to improved insulin sensitivity by enhancing glucose utilization in muscle and liver tissues (Neeland et al., 2018; Haus et al., 2009).
We acknowledge the limited sample size used in this study, which may have contributed to the loss of statistical significance after applying multiple-testing correction methods. This underscores the exploratory nature of our study and highlights the need for larger-scale investigations to validate and expand upon our preliminary findings. We also acknowledge that the study’s focus on a specific cohort of female students from Qatar University limits the generalizability of the findings.
The absence of Borg rating of perceived exertion data is another limitation, which we plan to address in future studies. It’s worth emphasizing that the fasting duration was kept uniform for all participants throughout Ramadan. This deliberate decision was made to ensure consistency in fasting times and to promote adherence among all subjects. While participants were encouraged to maintain a well-balanced diet, they were not required to follow a specific, predetermined meal plan. This lack of dietary control could be viewed as an additional limitation of our research. Despite these limitations, this pilot study aimed to explore the metabolic responses to physical activity during RIF under normal and disrupted sleep patterns to provide initial insights into an under-researched area.
5 Conclusion
Human body is regulated by the central and peripheral circadian clocks, allowing alertness, energy consumption, nutritional processing, and activity to take place during the biological daytime due to low melatonin levels, and during the biological night, when melatonin levels are high, sleep, low energy metabolism, and restorative activities take place. Disrupted circadian rhythms have detrimental effects on health. Our data shows exercising during fasting hours have favorable lipid utilization and thereby suggesting a possible reversal of insulin resistance and lower risk of type 2 diabetes. However, under disrupted CR, the response to PA is accompanied by systemic stress due to depletion of PUFAs, MUFAs, and plasmalogens that are integral to lipid synthesis, membrane composition, and endoplasmic reticulum homeostasis, leading to increase in inflammation, oxidative stress, and metabolic dysregulation. Future research should validate current findings with larger, diverse cohorts and comprehensive assessments of sleep (polysomnography, actigraphy, questionnaires), diet (fasting protocols, nutritional composition), and physical performance (aerobic/anaerobic capacity, strength, endurance). Follow-up studies are also warranted to investigate the persistence and long-term impact of these metabolic changes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Qatar University (QU-IRB 1798-EA/23) as per regulations of Qatar Ministry of Public Health (MoPH). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NA: Software, Writing – original draft. KN: Writing – original draft. SA: Investigation, Methodology, Writing – review & editing. MS: Investigation, Methodology, Writing – review & editing. HA-A: Investigation, Methodology, Writing – review & editing. NP: Data curation, Formal analysis, Writing – review & editing. FT: Data curation, Formal analysis, Writing – review & editing. AA: Data curation, Formal analysis, Writing – review & editing. ME: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1542016/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Spearman’s correlation was performed between metabolic markers and diacylglycerols in the normal CR group post physical activity. * signifies a p-value <0.05.
SUPPLEMENTARY FIGURE S2 | Spearman’s correlation was performed between inflammatory markers vs. plasmalogens and PUFAs in the disrupted CR group post physical activity.
References
Al-Khelaifi, F., Diboun, I., Donati, F., Botrè, F., Alsayrafi, M., Georgakopoulos, C., et al. (2018). A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med Open 4:2. doi: 10.1186/s40798-017-0114-z
AlMuraikhy, S., Anwardeen, N., Naeem, A., Sellami, M., Domling, A., Agouni, A., et al. (2022). Comparing the metabolic profiles associated with fitness status between insulin-sensitive and insulin-resistant non-obese individuals. Int. J. Environ. Res. Public Health 19:12169. doi: 10.3390/ijerph191912169
Almuraikhy, S., Sellami, M., Naja, K., al-Amri, H., Anwardeen, N., Aden, A., et al. (2024). Joint effects of exercise and Ramadan fasting on telomere length: implications for cellular aging. Biomedicines 12:1182. doi: 10.3390/biomedicines12061182
Anton, S. D., Moehl, K., Donahoo, W., Marosi, K., Lee, S., Mainous, A. III, et al. (2018). Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity 26, 254–268. doi: 10.1002/oby.22065
Arble, D. M. (2023). “Disrupted circadian rhythms and metabolic function” in Biological implications of circadian disruption: a modern health challenge. eds. L. K. Fonken and R. J. Nelson (Cambridge: Cambridge University Press), 223–237.
Arble, D. M., Bass, J., Behn, C., Butler, M., Challet, E., Czeisler, C., et al. (2015). Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep 38, 1849–1860. doi: 10.5665/sleep.5226
BaHammam, A. S., and Almeneessier, A. S. (2020). Recent evidence on the impact of Ramadan diurnal intermittent fasting, mealtime, and circadian rhythm on cardiometabolic risk: a review. Front. Nutr. 7:28. doi: 10.3389/fnut.2020.00028
Borg, G. (1990). Psychophysical scaling with applications in physical work and the perception of exertion. Scand. J. Work Environ. Health 16, 55–58. doi: 10.5271/sjweh.1815
Bozelli, J. C. Jr., Azher, S., and Epand, R. M. (2021). Plasmalogens and chronic inflammatory diseases. Front. Physiol. 12:730829. doi: 10.3389/fphys.2021.730829
Calder, P. C. (2006). n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases 2. Am. J. Clin. Nutr. 83, 1505S–1519S. doi: 10.1093/ajcn/83.6.1505S
Cantalupo, A., Gargiulo, A., Dautaj, E., Liu, C., Zhang, Y., Hla, T., et al. (2017). S1PR1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 70, 426–434. doi: 10.1161/HYPERTENSIONAHA.117.09088
Chaput, J. P., Dutil, C., Featherstone, R., Ross, R., Giangregorio, L., Saunders, T., et al. (2020). Sleep duration and health in adults: an overview of systematic reviews. Appl. Physiol. Nutr. Metab. 45, S218–s231. doi: 10.1139/apnm-2020-0034
Chaput, J. P., McHill, A., Cox, R., Broussard, J., Dutil, C., da Costa, B., et al. (2023). The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 19, 82–97. doi: 10.1038/s41574-022-00747-7
Chavez, J. A., Holland, W., Bär, J., Sandhoff, K., and Summers, S. (2005). Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 280, 20148–20153. doi: 10.1074/jbc.M412769200
Chua, E. C., Shui, G., Lee, I., Lau, P., Tan, L. C., Yeo, S. C., et al. (2013). Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 110, 14468–14473. doi: 10.1073/pnas.1222647110
Cuesta, M., Cermakian, N., and Boivin, D. B. (2015). Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J. 29, 1360–1370. doi: 10.1096/fj.14-265686
Czeisler, C. A., and Klerman, E. B. (1999). Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog. Horm. Res. 54, 97–130.
De Bernardi Rodrigues, A. M., da Silva, C. C., Vasques, A., Camilo, D., Barreiro, F., Cassani, R., et al. (2016). Association of Sleep Deprivation With Reduction in Insulin Sensitivity as Assessed by the Hyperglycemic Clamp Technique in Adolescents. JAMA Pediatr. 170, 487–494. doi: 10.1001/jamapediatrics.2015.4365
Faris, M. A. E., Jahrami, H., Alhayki, F., Alkhawaja, N., Ali, A., Aljeeb, S., et al. (2020). Effect of diurnal fasting on sleep during Ramadan: a systematic review and meta-analysis. Sleep Breath. 24, 771–782. doi: 10.1007/s11325-019-01986-1
Faris, A. E., Jahrami, H., Alsibai, J., and Obaideen, A. (2020). Impact of Ramadan diurnal intermittent fasting on the metabolic syndrome components in healthy, non-athletic Muslim people aged over 15 years: a systematic review and meta-analysis. Br. J. Nutr. 123, 1–22. doi: 10.1017/S000711451900254X
Faris, M. A., Jahrami, H., BaHammam, A., Kalaji, Z., Madkour, M., and Hassanein, M. (2020). A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on glucometabolic markers in healthy subjects. Diabetes Res. Clin. Pract. 165:108226. doi: 10.1016/j.diabres.2020.108226
Faris, M. E. A.-I. E., Jahrami, H., Obaideen, A., and Madkour, M. (2019). Impact of diurnal intermittent fasting during Ramadan on inflammatory and oxidative stress markers in healthy people: systematic review and meta-analysis. J. Nutr. Metabol. 15, 18–26. doi: 10.1016/j.jnim.2018.11.005
Farooq, A., Chamari, K., Sayegh, S., el, M., and al-Mohannadi, A. (2021). Ramadan daily intermittent fasting reduces objectively assessed habitual physical activity among adults. BMC Public Health 21:1912. doi: 10.1186/s12889-021-11961-9
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. doi: 10.1038/s41591-019-0675-0
Galadari, S., Rahman, A., Pallichankandy, S., Galadari, A., and Thayyullathil, F. (2013). Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 12:98. doi: 10.1186/1476-511X-12-98
Gooley, J. J. (2016). Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 75, 440–450. doi: 10.1017/S0029665116000288
Gu, J., Chen, L., Sun, R., Wang, J. L., Wang, J., Lin, Y., et al. (2022). Plasmalogens eliminate aging-associated synaptic defects and microglia-mediated neuroinflammation in mice. Front. Mol. Biosci. 9:815320. doi: 10.3389/fmolb.2022.815320
Haskell, W., Lee, I. M., Pate, R., Powell, K., Blair, S., Franklin, B., et al. (2007). Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 39, 1423–1434. doi: 10.1249/mss.0b013e3180616b27
Haus, J. M., Kashyap, S., Kasumov, T., Zhang, R., Kelly, K., DeFronzo, R., et al. (2009). Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58, 337–343. doi: 10.2337/db08-1228
Imeri, L., and Opp, M. R. (2009). How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 10, 199–210. doi: 10.1038/nrn2576
Jahrami, H. A., Alsibai, J., Clark, C., and Faris, M. (2020). A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur. J. Nutr. 59, 2291–2316. doi: 10.1007/s00394-020-02216-1
Jung, C. M., Melanson, E., Frydendall, E., Perreault, L., Eckel, R., and Wright, K. (2011). Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J. Physiol. 589, 235–244. doi: 10.1113/jphysiol.2010.197517
Kline, C. E. (2014). The bidirectional relationship between exercise and sleep: implications for exercise adherence and sleep improvement. Am. J. Lifestyle Med. 8, 375–379. doi: 10.1177/1559827614544437
Lessan, N., Saadane, I., Alkaf, B., Hambly, C., Buckley, A., Finer, N., et al. (2018). The effects of Ramadan fasting on activity and energy expenditure. Am. J. Clin. Nutr. 107, 54–61. doi: 10.1093/ajcn/nqx016
Lipert, A., Kozłowski, R., Rasmus, P., Marczak, M., Timler, M., Timler, D., et al. (2021). Sleep quality and performance in professional athletes fasting during the month of Ramadan. Int. J. Environ. Res. Public Health 18:6890. doi: 10.3390/ijerph18136890
Madkour, M. I., Islam, M., Tippetts, T., Chowdhury, K., Lesniewski, L., Summers, S., et al. (2023). Ramadan intermittent fasting is associated with ameliorated inflammatory markers and improved plasma sphingolipids/ceramides in subjects with obesity: lipidomics analysis. Sci. Rep. 13:17322. doi: 10.1038/s41598-023-43862-9
Morris, C. J., Yang, J. N., Garcia, J. I., Myers, S., Bozzi, I., Wang, W., et al. (2015). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 112, E2225–E2234. doi: 10.1073/pnas.1418955112
Morton, S. J., Bergman, B. C., Zemski-Berry, K. A., Harrison, K. A., Schauer, I. E., Wright, K. P., et al. (2020). 0295 Skeletal muscle diacylglycerol accumulation and impaired insulin sensitivity during insufficient sleep. Sleep 43, A111–A112. doi: 10.1093/sleep/zsaa056.292
Neeland, I. J., Singh, S., McGuire, D., Vega, G., Roddy, T., Reilly, D., et al. (2018). Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas Heart Study. Diabetologia 61, 2570–2579. doi: 10.1007/s00125-018-4720-1
Obaideen, K., Abu, K., Madkour, M., and Faris, M. A. I. (2022). Seven decades of Ramadan intermittent fasting research: bibliometrics analysis, global trends, and future directions. Diabetes Metab. Syndr. 16:102566. doi: 10.1016/j.dsx.2022.102566
Patterson, R. E., and Sears, D. D. (2017). Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 37, 371–393. doi: 10.1146/annurev-nutr-071816-064634
Rawat, A., Gangwar, A. K., Tiwari, S., Kant, S., Garg, R. K., and Singh, P. K. (2019). Sleep quality and insulin resistance in adolescent subjects with different circadian preference: a cross-sectional study. J. Family Med. Prim. Care 8, 2502–2505. doi: 10.4103/jfmpc.jfmpc_400_19
Rynders, C. A., Morton, S. J., Bessesen, D. H., Wright, K. P., and Broussard, J. L. (2020). Circadian rhythm of substrate oxidation and hormonal regulators of energy balance. Obesity (Silver Spring) 28 Suppl 1, S104–s113. doi: 10.1002/oby.22816
Shatila, H., Baroudi, M., el, R., Chehab, R., Forman, M., Abbas, N., et al. (2021). Impact of Ramadan fasting on dietary intakes among healthy adults: a year-round comparative study. Front. Nutr. 8:689788. doi: 10.3389/fnut.2021.689788
Simon, C., Gronfier, C., Schlienger, J., and Brandenberger, G. (1998). Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J. Clin. Endocrinol. Metab. 83, 1893–1899. doi: 10.1210/jcem.83.6.4864
Warburton, D. E., and Bredin, S. S. (2016). Reflections on physical activity and health: what should we recommend? Can. J. Cardiol. 32, 495–504. doi: 10.1016/j.cjca.2016.01.024
Warburton, D. E., Bredin, S. S., Jamnik, V., Shephard, R. J., and Gledhill, N. (2016). Consensus on evidence-based preparticipation screening and risk stratification. Ann. Rev. Gerontol. Geriatr. 36:53.
Zeb, F., Wu, X., Chen, L., Fatima, S., Haq, I. U., Chen, A., et al. (2020). Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 123, 1216–1226. doi: 10.1017/S0007114519003428
Keywords: circadian rhythm, exercise, intermittent fasting, metabolomics, metabolism
Citation: Anwardeen NR, Naja K, Almuraikhy S, Sellami M, Al-Amri HS, Philip N, Tamimi F, Agil A and Elrayess MA (2025) The influence of circadian rhythm disruption during Ramadan on metabolic responses to physical activity: a pilot study. Front. Neurosci. 19:1542016. doi: 10.3389/fnins.2025.1542016
Edited by:
Krista Austin, Performance & Nutrition Coaching, United StatesReviewed by:
Mayur Doke, University of Miami Health System, United StatesAhmet Murat Günal, Haliç University, Türkiye
Copyright © 2025 Anwardeen, Naja, Almuraikhy, Sellami, Al-Amri, Philip, Tamimi, Agil and Elrayess. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed A. Elrayess, bS5lbHJheWVzc0BxdS5lZHUucWE=
 Najeha Rizwana Anwardeen
Najeha Rizwana Anwardeen Khaled Naja
Khaled Naja Shamma Almuraikhy
Shamma Almuraikhy Maha Sellami
Maha Sellami Hadaia Saleh Al-Amri2
Hadaia Saleh Al-Amri2 Faleh Tamimi
Faleh Tamimi Mohamed A. Elrayess
Mohamed A. Elrayess