
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurosci. , 19 February 2025
Sec. Neurogenomics
Volume 19 - 2025 | https://doi.org/10.3389/fnins.2025.1531593
Adult-onset autosomal dominant leukodystrophy (ADLD) is a rare, slowly progressive, and fatal neurodegenerative disorder characterized by central nervous system white matter loss due to LMNB1 gene abnormalities encoding laminB1. However, not all LMNB1 mutations lead to ADLD. Currently, two genetic alterations have been identified in association with the pathogenesis of ADLD: LMNB1 gene tandem duplication and LMNB1 gene upstream deletions. We report a case of a 60-year-old man diagnosed with ADLD, with pyramidal tract dysfunction and autonomic abnormalities as the main clinical manifestations. MRI revealed bilateral symmetric high signal intensities in the white matter of the medulla oblongata, middle cerebellar peduncles, cerebral peduncle, periventricular white matter, centrum semi vale, and the pressure region of the corpus callosum. Whole exome sequencing results indicated 73.6Kb duplicate copy number variation signals in the 5q23.2 region of the proband’s chromosome. The Multiplex ligation-dependent probe amplification (MLPA) experiment results indicate recurrent mutations across all exons (exon1–11) of the LMNB1 gene. This is the eighth ADLD pedigree from China. We conducted a literature review of all ADLD pedigrees in China and summarized the characteristics of Chinese patients with ADLD to raise awareness of ADLD disease.
Adult-onset autosomal dominant leukodystrophy (ADLD) is a rare, slowly progressive, and fatal neurodegenerative disorder characterized by the loss of central nervous system (CNS) white matter (Neri et al., 2023), caused by either LMNB1 duplications or heterozygous deletions upstream of the LMNB1 promoter (Padiath et al., 2006; Giorgio et al., 2015). Both distinct mechanisms result in overexpression of LMNB1 and abnormal intracellular accumulation, contributing to selective progressive CNS demyelination in a way, but the specific pathological mechanisms remain unclear (Rolyan et al., 2015; Padiath, 2019). All reported Chinese families of ADLD are due to LMNB1 gene duplication. The LMNB1 gene, situated at chromosome 5q23.2, encodes the protein laminB1.Overexpression of LMNB1 protein in ADLD has been associated with increased nuclear rigidity in fibroblasts and dysregulation of alternative RNA splicing, affecting RNA splicing processes (Ferrera et al., 2014; Bartoletti-Stella et al., 2015). Studies also have found that overexpression of LMNB1 may lead to oligodendrocyte dysfunction and subsequent demyelination (Rolyan et al., 2015; Lin and Fu, 2009). Furthermore, LMNB1 overexpression can decrease the expression of lipid synthesis genes and myelin-enriched lipids via age-dependent epigenetic modifications (Rolyan et al., 2015; Padiath, 2016), partially accounting for the late onset. Recent studies indicate that LMNB1 overexpression may impair astrocytic function, diminishing their essential support to oligodendrocytes during myelination (Ratti et al., 2021a,b). Non-myelinating cells are vulnerable to LMNB1 overexpression, potentially playing a role in ADLD pathogenesis (Roy et al., 2023). The LMNB1 gene and its association with the disease is given in Supplementary Figure S1. ADLD is the sole central nervous system demyelinating disease linked to LMNB1 overexpression (Neri et al., 2023; Padiath and Fu, 2010), first identified by Eldridge et al. (1984).
Up to now, over 30 families have been diagnosed globally, but precise prevalence data remain unavailable (Raininko et al., 1993). Unlike most hereditary leukodystrophies that usually present in infancy or early childhood, ADLD typically manifests with classical clinical symptoms in the fourth or fifth decade of life (Coffeen et al., 2000). Affected individuals generally survive for one to two decades following clinical onset (Finnsson et al., 2015). Initial clinical symptoms frequently include autonomic abnormalities like bladder dysfunction, constipation, orthostatic hypotension, erectile dysfunction, and impaired sweating. Pyramidal tract and cerebellar involvement typically appear months to years later, resulting in spastic weakness of extremities, gait ataxia, nystagmus, dysmetria, intention tremor, etc. (Padiath and Fu, 2010). In the early stages of the disease, cognitive function is typically maintained or only slightly impaired, but cognitive decline and psychiatric issues may arise in the later stages (Raininko et al., 1993). MRI of the brain and spinal cord can detect diffuse, confluent, and symmetrical white matter lesions. An ADLD Chinese family with autonomic abnormalities and pyramidal tract dysfunction is presented, and to date, a total of 7 Chinese families with ADLD have been reported. We conducted a literature review of these case reports and observed the clinical characteristics of these Chinese families.
The participant originated from a northern Chinese family. All participants provided written informed consent for the publication of this case report. The study adhered to the Declaration of Helsinki and relevant Chinese policies.
The proband (II2) developed gait disturbances at age 54, which began with occasional foot-dragging while walking, and later developed spastic weakness in both lower limbs after prolonged walking. Worsening at age 58, he walked slowly and laboriously and needed support against the wall after walking short distances. Simultaneously, He began to develop a feeling of weakness in both upper limbs, accompanied by episodic dizziness and a top-heavy sensation. Autonomic symptoms, including sleep disorders, frequent urination, and constipation, appeared prior to pyramidal tract dysfunction. Neurological examination of the proband showed both lower limbs muscle strength of grade 4 and both upper limbs muscle strength of grade 5, mild muscular tension, brisk tendon reflexes, positive pathological reflexes, no cerebellar ataxia, and cognitive impairment. His resting blood pressure was 150/94 mmHg, but postural hypotension was not assessed at that time. The proband’s brain MRI revealed symmetric confluent long T2 signals in the medulla oblongata, middle cerebellar peduncles, cerebral peduncle, periventricular regions, centrum semi-oval, and corpus callosum (Figure 1A). Diffusion tensor magnetic resonance imaging (DTI) showed that the affected area of fiber bundles corresponded with MRI findings, with decreased fractional anisotropy (FA) values (Figure 1B). Magnetic resonance spectroscopy (MRS) revealed minor reductions in N-acetyl aspartate (NAA), choline (Cho), and creatine (Cr) within the lesion area relative to normal regions. The Cho/NAA ratio did not increase and no obvious proliferative changes were observed (Figure 1C).
The proband’s father (I1) also had similar gait disturbances for over 10 years, lacking a clear diagnosis or special treatment, and died of lung cancer at about 70 years old at last. His son (III1) had similar foot-dragging symptoms while walking, but did not undergo MRI evaluation. Relevant physical examinations have not revealed any positive signs, which may be related to his age, and regular follow-up observations are required. His nephew (III5) was diagnosed with white matter demyelinating lesions in other hospitals, highly suspected to be ADLD, but no significant clinical manifestations were observed and genetic testing was not undergone. His other relatives (II4, II5, III3, IV2) are asymptomatic and have not been examined.
Peripheral blood samples from the proband (II1) were collected for whole exome sequencing (WES) of genomic DNA. Peripheral blood DNA of the family members was extracted using the QIamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Whole exome capture for the proband’s DNA sample was performed using the xGen Exome Research Panel from Integrated DNA Technologies (Integrated DNA Technologies, Skokie, USA). The sequencing was conducted on the Illumina Novaseq 6,000 platform, with an average target sequencing depth of 200x. After filtering, the clean reads were aligned to the human reference genome (GRCh37, hg19) using BWA-MEM. Variant calling was guided by the GATK Best Practices. Variants with the “PASS” mark and coverage reads ≥20 were annotated using ANNOVAR integrated with available databases, such as RefSeq Gene, dbSNP150, ClinVar, and allele frequencies in populations from 1000G, ESP6500, and ExAC database. After the causative variants of the probands were found, Sanger sequencing was applied for the family members. Results indicated 73.6Kb duplicate copy number variation signals in the 5q23.2 region of the proband’s chromosome. The duplicate copy number variation region primarily contained LMNB1 and MARCHF3 genes on the genomic DNA of the patient. The Multiplex ligation-dependent probe amplification (MLPA) experiment results indicate recurrent mutations across all exons (exon1–11) of the LMNB1 gene. No duplication or deletion was found in NOTCH3 and PLP1 genes (Figure 2A). Therefore, he was diagnosed with LMNB1 duplication-mediated ADLD. The MLPA results of the proband’s son (III1) matched his father’s, also with recurrent mutations in the LMNB1 gene (Figure 2B). Unfortunately, further genetic testing was not performed on the proband’s other relatives. The family pedigree is given in Figure 3. Effective treatment strategies for ADLD are missing. These patients were treated with symptomatic therapy only.
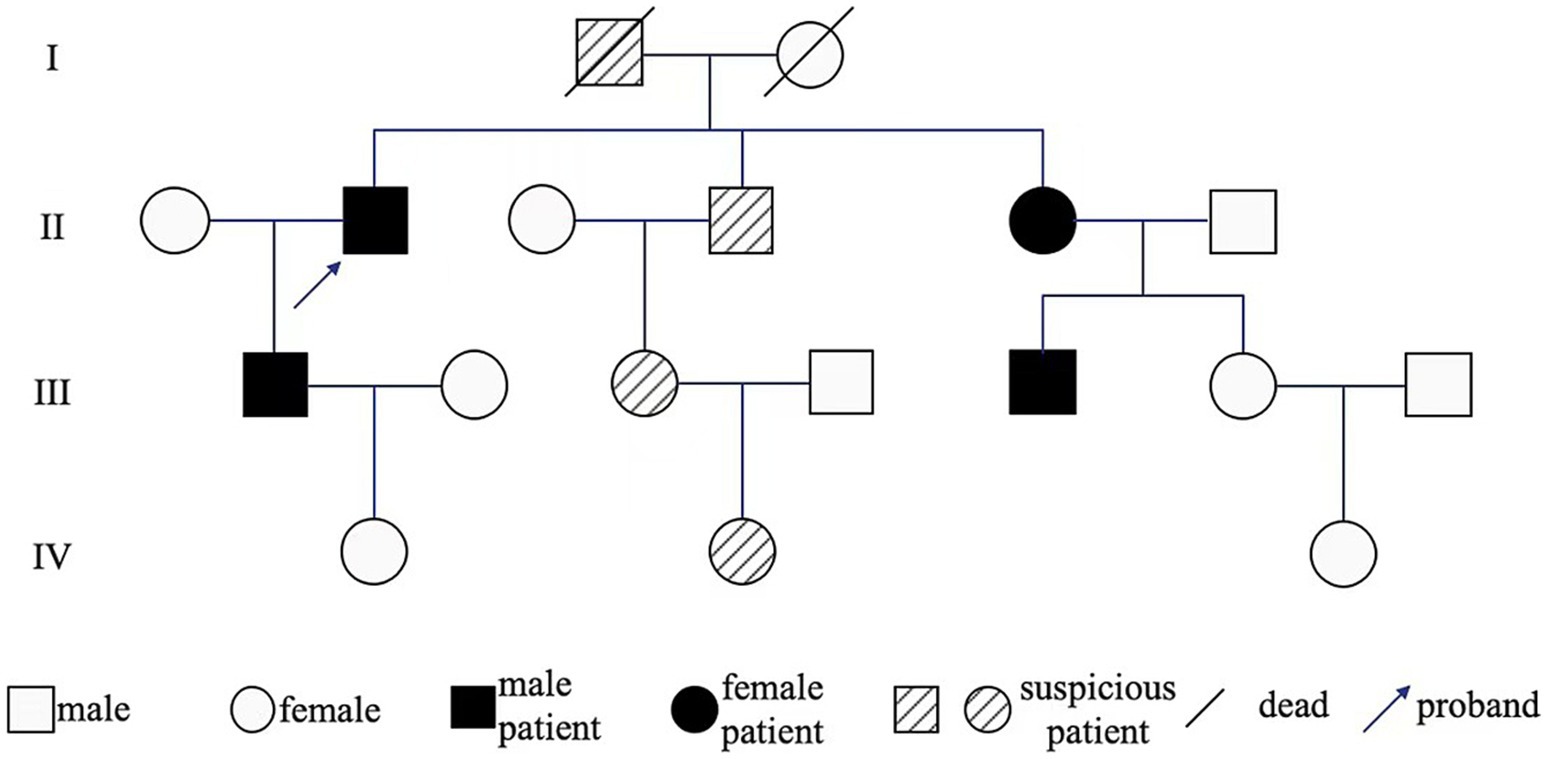
Figure 3. Genealogy map of ADLD. I1 developed gait disturbances in middle and old age and died of lung cancer at age 70; II2 constipation, gait disturbances appeared and gradually worsened at 54 years old; III1 occasional foot-dragging while walking; II4, II5, III3, IV2 are asymptomatic and have not been examined; III5 MRI examination revealed white matter demyelinating lesions, suggesting that II5 carried LMNB1 mutated gene.
In our study, we confirmed the eighth pedigree diagnosed with ADLD in China and summarized the clinical and imaging features of all ADLD pedigrees in the country (Table 1) (Dai et al., 2017; Zhang et al., 2019; He et al., 2021; Chen et al., 2022; Chen et al., 2023; Si-qi and Heng, 2023; Song et al., 2024). Five patients started with autonomic abnormalities and pyramidal signs, of which one patient reported pyramidal signs prior to autonomic abnormalities, which is consistent with typical ADLD onset mode but suggests potential individual differences. Varying degrees of cognitive impairment were observed in five patients, possibly related to disease progression. Two patients manifested double upper limb tremors, with one identifying tremor as the initial symptom. Combined with a head MRI, it could be inferred that the lesion involved the cerebellum. Two patients experienced sudden disturbance of consciousness and poor prognosis. It is reported that heat intolerance and false deterioration can appear in ADLD patients, manifesting as cognitive decline, disturbance of consciousness, and motor symptoms, but these changes are often reversible with disease improvement (Finnsson et al., 2015), and the disturbance of consciousness may also be associated with epilepsy. One patient presented with rare transient hypoglycemia and unilateral pupil dilation, potentially related to autonomic dysfunction caused by sympathetic nerve injury and adrenal medullary dysfunction (Terlizzi et al., 2016). All reported Chinese families are classic ADLD, but the cause of this feature remains unclear. This may be attributed to the rarity of ADLD, limiting its representativeness in the broader population, or it may reflect potential ethnic and genetic differences. Further global cases are needed to draw definitive conclusions. Non-classical clinical cases of LMNB1 upstream deletions reported in other countries have demonstrated late onset, lacking autonomic dysfunction and cerebellar ataxia (Mezaki et al., 2018).
MRI scanning is crucial for diagnosing ADLD, marked by diffuse, symmetrical leukodystrophy mainly affecting the frontal and parietal lobes, cerebellum, corpus callosum, and spinal cord, with minimal impact on periventricular white matter (Melberg et al., 2006; Sundblom et al., 2009). MRI abnormalities in the brain and spinal cord may emerge decades before clinical symptoms, with the extent of lesions correlating with the disease’s duration and severity (Finnsson et al., 2015; Zanigni et al., 2015). Notably, spinal cord MRI abnormalities can be obvious even when brain MRI changes are slight (Sundblom et al., 2009). In contrast to patients with classical ADLD, MRI findings in patients with nontypical ADLD showed selective white matter involvement, with less involvement in the cerebellum and medulla oblongata (Dimartino et al., 2024) (Supplementary Figure S2). Unfortunately, a spinal cord MRI was not performed on the proband. The imaging features of cases from China are consistent with those cases in other countries.
The onset age of ADLD is late, the disease progression is relatively slow. Early MRI examination and genetic detection can facilitate early intervention in the treatment of the disease. However, there is currently no effective treatment for ADLD, and any existing therapies primarily address clinical symptoms (Raininko et al., 1993). For example, the eight Chinese patients mentioned received dietary guidance, neurotrophic support, spasticity relief, functional exercise, cognitive improvement, and other treatments according to clinical symptoms. Recent studies suggest that reducing LMNB1 levels and restoring small molecules associated with nuclear abnormalities may prevent the occurrence and progression of the disease (Lin and Fu, 2009; Lin et al., 2014; Nmezi et al., 2020), and protein regulation may provide new therapeutic opportunities for ADLD (Giorgio et al., 2021).
ADLD is a rare white matter disease of the central nervous system that progresses slowly and is inherited in an autosomal dominant manner. The possibility of LMNB1 gene-related ADLD should be considered when a patient presents with slow progressive limb weakness, especially spastic weakness and autonomic dysfunction, combined with MRI suggesting symmetric leukodystrophy and family members have similar clinical symptoms. A comprehensive family history and thorough physical examination are crucial. Timely diagnosis and intervention can slow disease progression. This study has enhanced our understanding of ADLD by summarizing the clinical and imaging features of the eight currently identified families with ADLD in China, which is helpful for the early identification of the disease through clinical symptoms and MRI findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Tianjin Huanhu Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YJ: Formal analysis, Visualization, Writing – original draft. HL: Visualization, Writing – original draft, Writing – review & editing. YL: Data curation, Visualization, Writing – review & editing. ZhZ: Supervision, Writing – review & editing. ZX: Writing – review & editing. ZiZ: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank Fujian Medical University for providing technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1531593/full#supplementary-material
Bartoletti-Stella, A., Gasparini, L., Giacomini, C., Corrado, P., Terlizzi, R., Giorgio, E., et al. (2015). Messenger RNA processing is altered in autosomal dominant leukodystrophy. Hum. Mol. Genet. 24, 2746–2756. doi: 10.1093/hmg/ddv034
Chen, S. Y., Jiang, T., Lian, H. W., Chen, N. H., Zhang, Z. H., Lu, M., et al. (2023). Adult-onset autosomal dominant leukodystrophy caused by LMNB1 gene tandem repetition (with 1 case report and literature review). Chin. J. Clin. Neurosci. 31:200–203.
Chen, S., Zou, J. L., He, S., Li, W., Zhang, J. W., and Li, S. J. (2022). Adult-onset autosomal dominant leukodystrophy caused by LMNB1 gene tandem repetition (with 1 case report and literature review). Chin. J. Clin. Neurosci. 31:200–203
Coffeen, C. M., McKenna, C. E., Koeppen, A. H., Plaster, N. M., Maragakis, M., Schwankhaus, J. D., et al. (2000). Genetic localization of an autosomal dominant leukodystrophy mimicking chronic progressive multiple sclerosis to chromosome 5q31. Hum. Mol. Genet. 9, 787–793. doi: 10.1093/hmg/9.5.787
Dai, Y., Ma, Y., Li, S., Banerjee, S., Liang, S., Liu, Q., et al. (2017). An LMNB1 duplication caused adult-onset autosomal dominant Leukodystrophy in Chinese family: clinical manifestations, neuroradiology and genetic diagnosis. Front. Mol. Neurosci. 10:215. doi: 10.3389/fnmol.2017.00215
Dimartino, P., Zadorozhna, M., Yumiceba, V., Basile, A., Cani, I., Melo, U. S., et al. (2024). Structural variants at the LMNB1 locus: deciphering Pathomechanisms in autosomal dominant adult-onset demyelinating Leukodystrophy. Ann. Neurol. 96, 855–870. doi: 10.1002/ana.27038
Eldridge, R., Anayiotos, C. P., Schlesinger, S., Cowen, D., Bever, C., Patronas, N., et al. (1984). Hereditary adult-onset leukodystrophy simulating chronic progressive multiple sclerosis. N. Engl. J. Med. 311, 948–953. doi: 10.1056/NEJM198410113111504
Ferrera, D., Canale, C., Marotta, R., Mazzaro, N., Gritti, M., Mazzanti, M., et al. (2014). Lamin B1 overexpression increases nuclear rigidity in autosomal dominant leukodystrophy fibroblasts. FASEB J. 28, 3906–3918. doi: 10.1096/fj.13-247635
Finnsson, J., Sundblom, J., Dahl, N., Melberg, A., and Raininko, R. (2015). LMNB1-related autosomal-dominant leukodystrophy: clinical and radiological course. Ann. Neurol. 78, 412–425. doi: 10.1002/ana.24452
Giorgio, E., Pesce, E., Pozzi, E., Sondo, E., Ferrero, M., Morerio, C., et al. (2021). A high-content drug screening strategy to identify protein level modulators for genetic diseases: a proof-of-principle in autosomal dominant leukodystrophy. Hum. Mutat. 42, 102–116. doi: 10.1002/humu.24147
Giorgio, E., Robyr, D., Spielmann, M., Ferrero, E., di Gregorio, E., Imperiale, D., et al. (2015). A large genomic deletion leads to enhancer adoption by the Lamin B1 gene: a second path to autosomal dominant adult-onset demyelinating leukodystrophy (ADLD). Hum. Mol. Genet. 24, 3143–3154. doi: 10.1093/hmg/ddv065
He, S., Chen, S., Li, W., Li, S. J., and Zhang, J. W. (2021). A family of adult-onset autosomal dominant leukodystrophy. Chin. J. Neuromed. 20, 695–699. doi: 10.3760/cma.j.cn115354-20200917-00742
Lin, S. T., and Fu, Y. H. (2009). miR-23 regulation of Lamin B1 is crucial for oligodendrocyte development and myelination. Dis. Model. Mech. 2, 178–188. doi: 10.1242/dmm.001065
Lin, S. T., Heng, M. Y., Ptáček, L. J., and Fu, Y. H. (2014). Regulation of myelination in the central nervous system by nuclear Lamin B1 and non-coding RNAs. Transl. Neurodegener. 3:4. doi: 10.1186/2047-9158-3-4
Melberg, A., Hallberg, L., Kalimo, H., and Raininko, R. (2006). MR characteristics and neuropathology in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. Am. J. Neuroradiol. 27, 904–911
Mezaki, N., Miura, T., Ogaki, K., Eriguchi, M., Mizuno, Y., Komatsu, K., et al. (2018). Duplication and deletion upstream of LMNB1 in autosomal dominant adult-onset leukodystrophy. Neurol. Genet. 4:e292. doi: 10.1212/NXG.0000000000000292
Neri, I., Ramazzotti, G., Mongiorgi, S., Rusciano, I., Bugiani, M., Conti, L., et al. (2023). Understanding the ultra-rare disease autosomal dominant Leukodystrophy: an updated review on Morpho-functional alterations found in experimental models. Mol. Neurobiol. 60, 6362–6372. doi: 10.1007/s12035-023-03461-1
Nmezi, B., Vollmer, L. L., Shun, T. Y., Gough, A., Rolyan, H., Liu, F., et al. (2020). Development and optimization of a high-content analysis platform to identify suppressors of Lamin B1 overexpression as a therapeutic strategy for autosomal dominant Leukodystrophy. SLAS Discov. 25, 939–949. doi: 10.1177/2472555220915821
Padiath, Q. S. (2016). Lamin B1 mediated demyelination: linking lamins, lipids and leukodystrophies. Nucleus 7, 547–553. doi: 10.1080/19491034.2016.1260799
Padiath, Q. S. (2019). Autosomal dominant Leukodystrophy: a disease of the nuclear Lamina. Front. Cell Dev. Biol. 7:41. doi: 10.3389/fcell.2019.00041
Padiath, Q. S., and Fu, Y. H. (2010). Autosomal dominant leukodystrophy caused by Lamin B1 duplications a clinical and molecular case study of altered nuclear function and disease. Methods Cell Biol. 98, 337–357. doi: 10.1016/S0091-679X(10)98014-X
Padiath, Q. S., Saigoh, K., Schiffmann, R., Asahara, H., Yamada, T., Koeppen, A., et al. (2006). Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat. Genet. 38, 1114–1123. doi: 10.1038/ng1872
Raininko, R., Gosky, M., and Padiath, Q. S., LMNB1-related autosomal dominant Leukodystrophy, in GeneReviews((R)), Adam, M. P., J. Feldman, G. M. Mirzaa, R. A. Pagon, S. E. Wallace, and A. Amemiya, Editors. (1993), Seattle (WA): University of Washington, Seattle.
Ratti, S., Rusciano, I., Mongiorgi, S., Neri, I., Cappellini, A., Cortelli, P., et al. (2021a). Lamin B1 Accumulation's effects on autosomal dominant Leukodystrophy (ADLD): induction of reactivity in the astrocytes. Cells 10:2566. doi: 10.3390/cells10102566
Ratti, S., Rusciano, I., Mongiorgi, S., Owusu Obeng, E., Cappellini, A., Teti, G., et al. (2021b). Cell signaling pathways in autosomal-dominant leukodystrophy (ADLD): the intriguing role of the astrocytes. Cell. Mol. Life Sci. 78, 2781–2795. doi: 10.1007/s00018-020-03661-1
Rolyan, H., Tyurina, Y. Y., Hernandez, M., Amoscato, A. A., Sparvero, L. J., Nmezi, B. C., et al. (2015). Defects of lipid synthesis are linked to the age-dependent demyelination caused by Lamin B1 overexpression. J. Neurosci. 35, 12002–12017. doi: 10.1523/JNEUROSCI.1668-15.2015
Roy, V., Bienjonetti, I., Brodeur, A., Dion-Albert, L., Touzel-Deschênes, L., Gould, P. V., et al. (2023). LMNB1-duplication mediated nuclear architecture alteration and demyelination of cerebral white matter in a patient with ADLD. Neuropathol. Appl. Neurobiol. 50:e12947. doi: 10.1111/nan.12947
Si-qi, Z., and Heng, M. (2023) A case of acute onset hereditary leukodystrophy caused by duplication mutation of LMNB1 gene. Chinese Medical Care Repository, 05(01).
Song, X. H., Wang, Z. J., Song, Y., Wang, X. L., Li, X. Y., Wang, Z. D., et al. (2024). LMNB1-related adult-onset autosomal dominant leukodystrophy family analysis. Beijing Med. J. 46, 374–379. doi: 10.15932/j.0253-9713.2024.05.007
Sundblom, J., Melberg, A., Kalimo, H., Smits, A., and Raininko, R. (2009). MR imaging characteristics and neuropathology of the spinal cord in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. Am. J. Neuroradiol. 30, 328–335. doi: 10.3174/ajnr.A1354
Terlizzi, R., Calandra-Buonaura, G., Zanigni, S., Barletta, G., Capellari, S., Guaraldi, P., et al. (2016). A longitudinal study of a family with adult-onset autosomal dominant leukodystrophy: clinical, autonomic and neuropsychological findings. Auton. Neurosci. 195, 20–26. doi: 10.1016/j.autneu.2016.02.005
Zanigni, S., Terlizzi, R., Tonon, C., Testa, C., Manners, D. N., Capellari, S., et al. (2015). Brain magnetic resonance metabolic and microstructural changes in adult-onset autosomal dominant leukodystrophy. Brain Res. Bull. 117, 24–31. doi: 10.1016/j.brainresbull.2015.07.002
Keywords: autosomal dominant adult leukodystrophy, LMNB1 gene, Chinese family, whole exome sequencing, the multiplex ligation-dependent probe amplification, case report
Citation: Jiang Y, Han L, Li Y, Zhao Z, Xin Z and Zhu Z (2025) Case report: LMNB1 duplication-mediated autosomal dominant adult leukodystrophy in a Chinese family and literature review of Chinese patients. Front. Neurosci. 19:1531593. doi: 10.3389/fnins.2025.1531593
Received: 20 November 2024; Accepted: 30 January 2025;
Published: 19 February 2025.
Edited by:
Francesca Luisa Conforti, University of Calabria, ItalyReviewed by:
Paola Ruffo, National Institute on Aging (NIH), United StatesCopyright © 2025 Jiang, Han, Li, Zhao, Xin and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zilong Zhu, emh1emlsb25nMTk3NkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.