
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 17 February 2025
Sec. Neuroendocrine Science
Volume 19 - 2025 | https://doi.org/10.3389/fnins.2025.1502764
Introduction: In most mammals, paternal care is not mandatory for raising offspring. In house mice, experience with pups governs the extent and quality of paternal care. First-time fathers undergo a dramatic transition from ignoring or killing pups to caring for pups. The behavioral shift occurs together with changes in brain estrogen signaling as indicated by changes in estrogen receptor presence and distribution in multiple areas regulating olfaction, emotion, and motivation.
Methods: We measured changes in the expression of aromatase, the enzyme converting testosterone into estrogen, as an indirect measure of estrogen synthesis, in various areas of the limbic system in mice with increasing paternal experience.
Results: The amount of paternal experience (5 or 27 days) was associated with increased numbers of immunocytochemically-identified aromatase expressing cells in the medial and cortical amygdala, posterior piriform cortex, and ventromedial hypothalamus. Functionally, these changes can be related to the disappearance of aggression or neglect towards pups when first-time fathers or, even more, well-experienced fathers are handling their own pups. In the lateral septum, the anterior piriform cortex and to some extent in the medial preoptic area, parental experience increased the number of aromatase-positive cells only in fathers with 27 days of experience, and only in the right hemisphere. This represents a novel case of brain-functional lateralization triggered by experience. Nuclei/areas associated with maternal care (medial preoptic area, bed nucleus of stria terminalis, nucleus accumbens) exhibited a left-hemisphere advantage in aromatase expressing cells, both in pup-naïve and pup-experienced males. This newly found lateralization may contribute to the left-hemisphere dominant processing and perception of pup calls to release parental behavior.
Conclusion: In general, the experience-dependent changes in aromatase expression we observed in most brain areas did not mirror the previously reported changes in estrogen receptors (ERα) when pup-naïve males became pup-caring fathers. Hence, paternal behavior may depend, in a brain area-specific way, on the differential action of estrogen through its receptors and/or direct local modulation of neural processing.
Mammalian paternal care is facultative, and the behavioral and neural dynamics that result in becoming a caring father are less well understood compared to those of obligatory maternal care (e.g., Bales and Saltzman, 2016; Brunton and Russell, 2008; Dulac et al., 2014; Guoynes and Marler, 2020; Horrell et al., 2021; Lonstein and De Vries, 2000; Numan, 2020; Numan and Insel, 2003; Rilling and Mascaro, 2017; Woodroffe and Vincent, 1994). In rodents and primates, it is hypothesized that maternal care is induced by hormonal changes during pregnancy and parturition (onset phase), while its continuation (maintenance phase) is driven by sensory stimuli from the pups (Brunton and Russell, 2008; Fahrbach and Pfaff, 1982; Rosenblatt and Siegel, 1981). In-house mice, pup-naïve females (those with no sexual or pup-caring experience), males, and experienced fathers do not experience the onset phase. Instead, they require varying amounts of exposure to pups (from a few hours to several days of contact) before caring behavior is activated (Akther et al., 2015; Alsina-Llanes et al., 2015; Ehret and Buckenmaier, 1994; Ehret and Koch, 1989; Ehret et al., 1987, 1993; Elwood, 1985; Koch and Ehret, 1989a,b; Martín-Sánchez et al., 2015; Stolzenberg and Rissman, 2011; Tachikawa et al., 2013). Nonetheless, pup-naïve females engage in consummatory (pup retrieval) and appetitive (preference for pup ultrasounds) behaviors (Koch and Ehret, 1991; Numan, 2006) after fewer days of experience with pups (and sometimes spontaneously; Alsina-Llanes et al., 2015; Martín-Sánchez et al., 2015) compared to either pup-naïve males or previous fathers (Ehret and Buckenmaier, 1994; Ehret et al., 1987, 1993). Thus, pup naïve females appear to be more readily sensitized or motivated by pup cues to provide parental care than males and even experienced fathers. This observation indicates potential sex differences in the neural mechanisms underlying the activation of maternal versus paternal instincts.
Numerous studies have characterized the role of the limbic regions in the activation and regulation of parental behaviors. These regions include the medial preoptic area (MPOA), bed nucleus of the stria terminalis (BNST), lateral septum (LS), nucleus accumbens (NAC), nuclei of the amygdala, and ventromedial hypothalamus (VMH). Olfaction is believed to provide the main modulatory sensory input through pathways including the vomeronasal organ to the BNST and amygdala, the olfactory bulb to the amygdala, via the VMH to the LS, and via the piriform cortex (PIR) and hippocampus to the LS (e.g., Bridges, 2015; Brunton and Russell, 2008; Dulac et al., 2014; Ehret et al., 1991; Kohl and Dulac, 2018; Kohl et al., 2017; Numan, 2006, 2020). Estradiol treatment can activate both maternal and paternal instincts (Ehret and Koch, 1989; Koch and Ehret, 1989a,b; Poindron et al., 1988; Romero-Morales et al., 2018; Rosenblatt and Ceus, 1998; Rosenblatt et al., 1994; Siegel and Rosenblatt, 1975) and changes in brain estrogen signaling (through estrogen receptors) may be part of the underlying mechanism. In house mice, expression of parental behaviors coincides with changes in the distribution of estrogen receptor (ERα) positive cells in limbic brain areas of pup-naïve and pup-experienced females (Ehret and Buckenmaier, 1994; Koch, 1990) and males (Ehret et al., 1993). In pup-naïve male and female voles, high levels of spontaneous pup care are correlated with a higher number of Erα-positive cells in the MPOA (Li et al., 2015), potentially indicating convergent circuit dynamics. Research on the involvement of other estrogen receptors, such as ERβ and the membrane receptor GPER, in rodent paternal care remains very limited (Hyer et al., 2017; Torres et al., 2024). Consequently, their potential roles in this behavior have not yet been clearly established.
Low levels of circulating estrogens in males (Nilsson et al., 2015) suggest that brain-active estrogens are primarily synthesized locally in the brain from testosterone by the enzyme aromatase (e.g., Cornil et al., 2006, 2013; Garcia-Segura, 2008). Locally synthesized estrogens have been implicated in the onset of paternal behaviors in California mice (Trainor and Marler, 2002; Trainor et al., 2003). Aromatase is expressed in the limbic system of male mice (LS, BNST, MPOA, nuclei of the amygdala, VMH, and PIR) (Foidart et al., 1995; Schleicher et al., 1986; Stanić et al., 2014; Wozniak et al., 1992). In the present study, we sought to examine changes in aromatase expression during the onset and maintenance of paternal behavior of male mice (pup-naïve and fathers with increasing experience). We focused on LS, BNST, MPOA, nuclei of the amygdala, VMH, and PIR, which are areas in which ERα-positive cells have also been located in pup-experienced fathers (Ehret et al., 1993).
Adult male and female laboratory mice (outbred strain NMRI, 2–3 months old) with no previous sexual or parental experience were housed at the University of Ulm. As previously (Ehret et al., 1993), mice were randomly assigned to one of three treatment groups consisting of (1) naïve males without any experience with pups (N), (2) males co-caring with their female mate for their first litter for 5 days (5D), or (3) males co-caring with their female mate for their first litter until weaning and their second litter for 5 days. The total co-caring time of the latter males (first and second litter) was 26–27 days (27D) since after delivery of the first litter, females in post-partum estrus paired immediately, and, while nursing the first litter up to weaning at 21 days, delivered the second litter after 21–22 days of gestation. Mice were housed at 22°C and a 12 h light–dark cycle (starting at 7.00 h) in plastic cages (26.5 × 20 × 14 cm) either with other naïve males (group N; 4 brothers per cage) or with one female (groups 5D and 27D). Water and food were available ad libitum. The experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC) and were approved by the appropriate authority (Regierungspräsidium Tübingen, Germany).
Pup retrieval behavior was tested as previously described (Ehret and Koch, 1989; Ehret et al., 1993). Male mice (alone or with litter mate and pups) were placed overnight on a running board (length 110 cm, width 8 cm) with a central nest depression (Figure 1A) suspended in a soundproof and anechoic room. The running board was supplied with mouse chow and nest material from the cage and covered with a plexiglass hood with an opening for the tip of a suspended water bottle. The following day, retrieving tests were conducted under dim red lighting (<1 lx), ensuring that males relied on the sense of smell, audition, and touch to respond and retrieve pups. Before the start of testing, the hood and the female with the pups (groups 5D and 27D) were removed and placed back in their home cage outside the soundproof room. Seven pups were kept in the room to serve as targets for retrieving. The males were habituated for at least 30 min to the new situation after the hood (group N) or the hood and female with pups (groups 5D and 27D) were removed. Prior to testing males in the N group, 5-day-old foster pups were rubbed with the nest material of the respective males to provide a familiar scent. During the test, the seven pups (always 1–3 pups at a time) were randomly placed on the running board, at least 30 cm away from the nest. A male was scored as a ‘retriever’ if he retrieved all seven pups to the nest within 10 min. A male was scored as ‘non-retriever’ if he had not retrieved any pups after 10 min. The distinction between retriever and non-retriever was clear-cut because once a male had retrieved the first pup, he retrieved all. Retrieving tests were immediately stopped, and the pups were removed if a male attacked any of the pups (‘aggressor’).
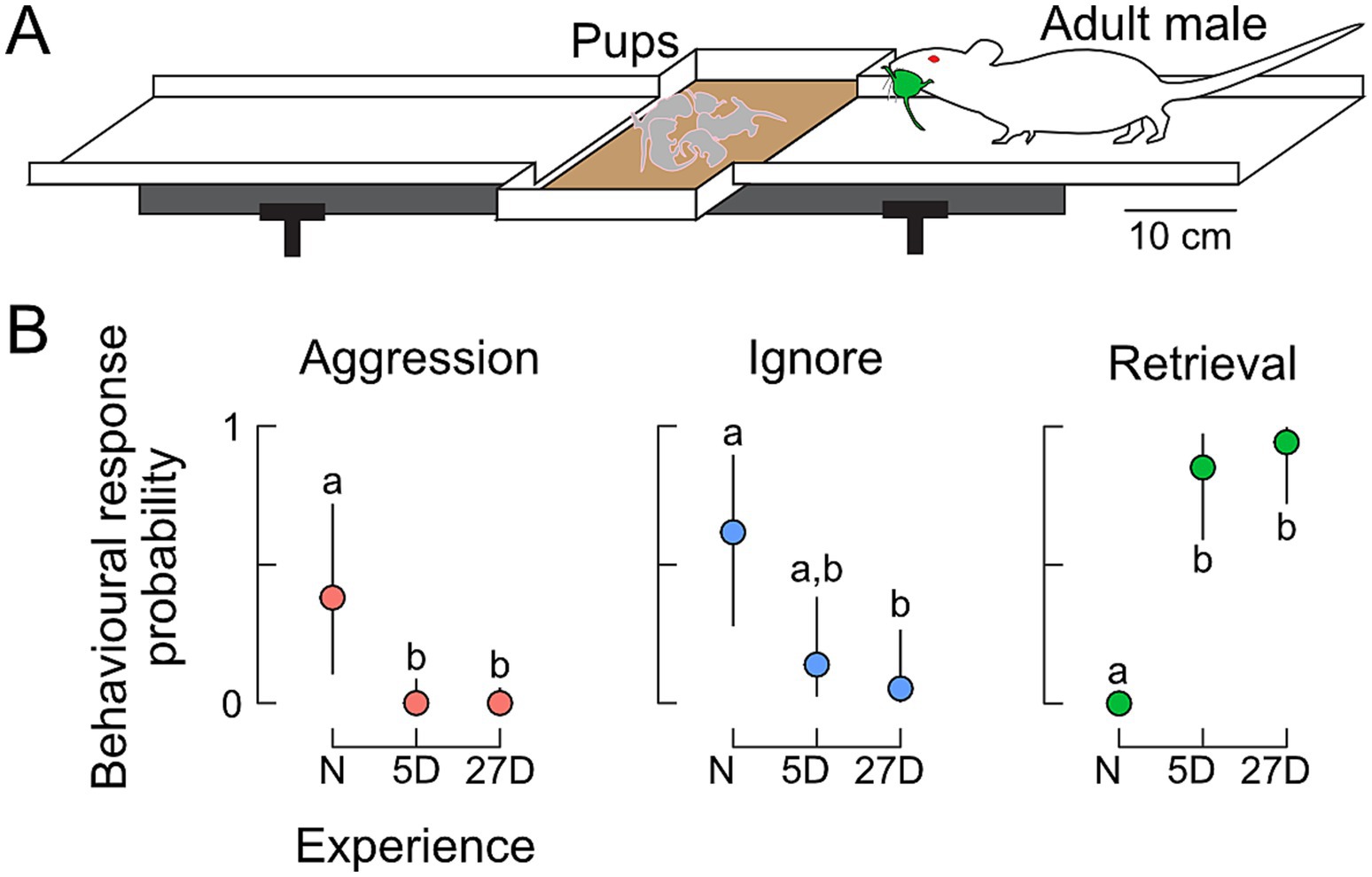
Figure 1. Behavioral responses toward pups. (A) Experimental setting for the pup-retrieving tests on a running board with a central nest depression. Responses were scored as aggression (attacking pups), ignoring pups, or retrieval of pups (returning pups to the nest). (B) Probabilities of behavioral expression in naïve (N) males and in fathers with five (5D) or 27 days of experience (27D). Aggression declined immediately from N to 5D fathers. Although fathers occasionally still ignored pups, the probability of retrieval was very high. Different letters indicate statistical differences between the groups of males. The sample size was n = 8 for N, n = 12 for 5D, and n = 12 for 27D.
Immediately following the pup retrieval test, males were euthanized by cervical dislocation, and their brains were quickly dissected and frozen over liquid nitrogen. Brains were mounted using O.C.T. compound, and frontal sections were serially cut (30 μm) using a cryostat (Thermo Scientific Microm HM560) and immediately mounted on slides to keep track of the left and right sides of the brain. Sections were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH = 7.4) for 60 min. Sections were rinsed thrice for 5 min with 0.1 M PB between subsequent processing steps. Slides were incubated with 0.2% Triton X-100 (Sigma) in 0.1 M PB for 45 min to permeabilize cells and with 1% H2O2 (Merck) in 0.1 M PB for 20 min to block endogenous peroxidase. In a humidified chamber, the sections were treated with 2% normal goat serum in 0.1 M PB for 60 min to block non-specific staining, then with the polyclonal rabbit anti-aromatase antibody (Abcam Cat# ab35604, RRID: AB_867729; 0.002 mg/mL in 2% normal goat serum) at 4°C for 72 h. Sections were then incubated with the secondary antibody from the goat against rabbit horseradish peroxidase (Dako, Cat# PO448, 0.00125 mg/mL in 0.1 M phosphate buffer) at room temperature for 60 min. Immunoreactivity was revealed by incubating the sections in 0.015% diaminobenzidine tetrachloride (DAB; Sigma), 0.023% NiCl, and 0.013% H2O2 in 0.1 M PB for 7 min. Sections were rinsed in PB, dehydrated in a series of alcohols and xylene, and coverslipped with Entellan (Merck). At the time of purchase, the primary antibody was recommended for aromatase immunohistochemistry on mouse tissue, and the supplier provided positive quality controls for mouse ovarian tissue. Dutta et al. (2014) also performed a Western blot with mouse tissue that confirmed the specificity of the antibody. Previous studies have used this antibody to detect aromatase immunoreactivity in mouse ovaries (Laws et al., 2014), brain stem (Ji et al., 2017), and auditory cortex (Charitidi and Canlon, 2010). We tested the specificity of the secondary antibody labeling by incubating sections with PB instead of the primary antibody. Immunoreactivity was completely absent in all these endogenous tissue background control sections (Figure 2A).
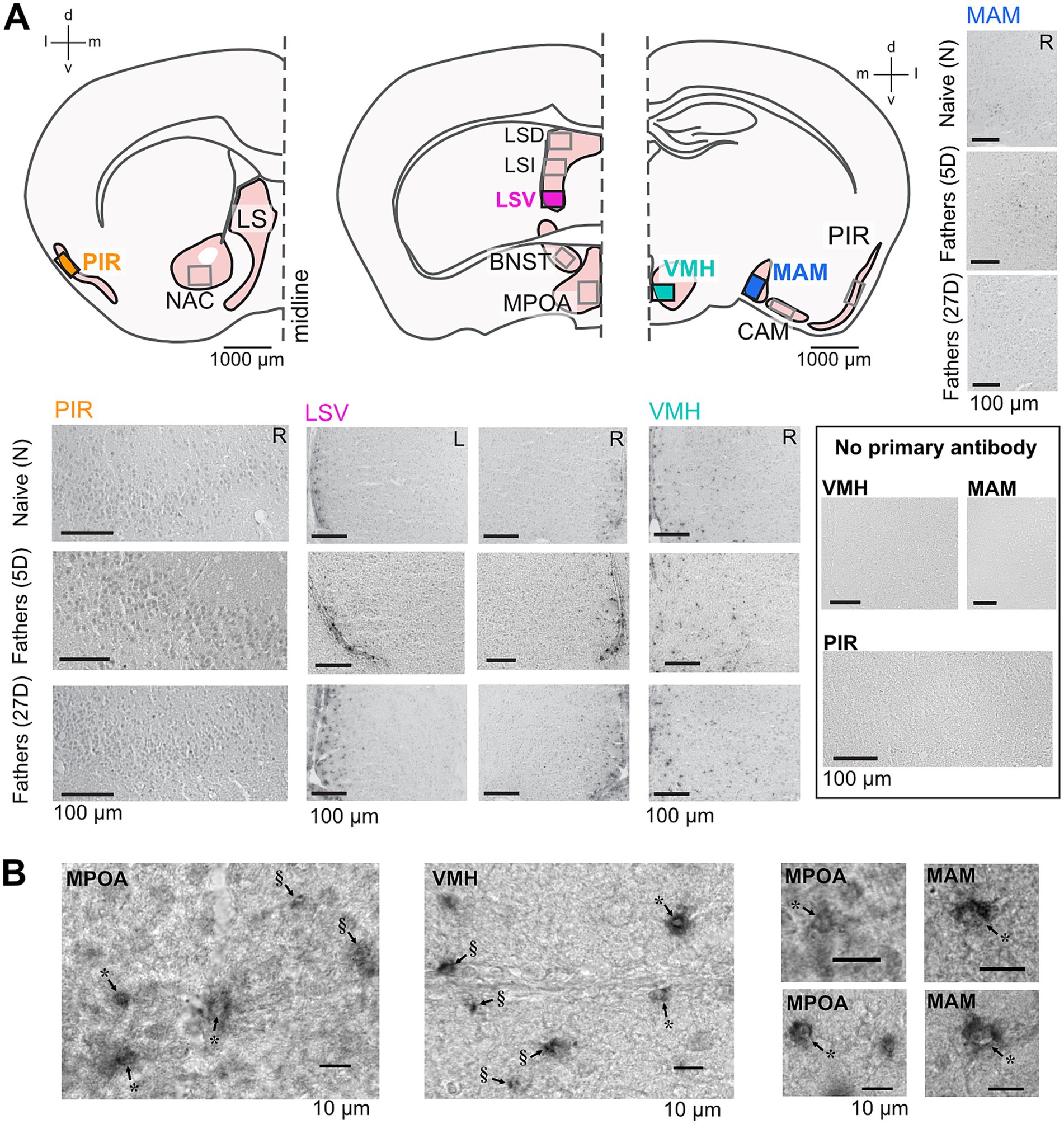
Figure 2. Aromatase immunolabeling in the paternal brain. Cross sections (A) of the brain areas analyzed here (Bregma coordinates: 0.98 mm, 0.14 mm, and − 1.34 mm). Boxes indicate approximate areas that were counted. Representative images from areas highlighted in (A) show aromatase immunostaining in naïve and experienced fathers (5D and 27D) in the left (L) and right (R) hemispheres and a lack of immunostaining in the no primary antibody controls. High-magnification (B) images of examples of aromatase-expressing cells in the MPOA, VMH, and MAM showing cytoplasmic expression and black granulae. In cases where the granulae were located perinuclear to an identifiable cell nucleus (marked with *), the cells were counted as aromatase-positive. In cases in which the granulae could not be associated with a cell nucleus (marked with §), the structure was not counted as an aromatase-positive cell. Additional images of aromatase immunolabeling are presented in Supplementary Figure S1.
Sections were independently analyzed by two experimenters who were blinded to the experimental conditions. To accommodate the variable intensity of the immunolabeling, they initially reviewed multiple sections from all animal groups to establish consensus on criteria for aromatase-specific labeling. Brain areas were identified using a 10x objective, while aromatase-positive cells were located with a 40x objective. Cells were classified as expressing aromatase if they (1) displayed more intense gray staining than the background and (2) contained black granulae typically clustered around the cell nucleus (Figure 2B). Structures presenting single or multiple black granulae without the associated gray cellular structure were not counted as aromatase-positive (Figure 2B). This procedure led to (a) a high agreement between the experimenters’ counts, (b) the identification of unequivocally aromatase-positive cells, and (c) relatively low counts of aromatase-positive cells per evaluated brain area in each section.
Sections were analyzed using a light microscope (Axiophot, Zeiss, Germany). The brain areas in which aromatase-positive cells were counted are listed in Table 1. Areas were matched to the mouse brain atlas plates and associated Bregma coordinates (Paxinos and Franklin, 2001) and anatomically matched across all experimental animals. These areas largely overlap with those showing ERα-positive cell dynamics during the acquisition of pup-caring experience in male (Ehret et al., 1993) and female (Ehret and Buckenmaier, 1994) mice. Aromatase-positive cells were counted separately in the right and left hemispheres of the brain. Counts per section for a given animal, brain area, and hemisphere were averaged between the maximum number of consecutive sections that could be matched across all animals, i.e., two sections for LS, BNST, VMH, and nucleus accumbens (NAC), or three sections for PIR, MPOA, cortical amygdala (CAM), and medial amygdala (MAM). These average counts from the individual animals were used in statistical analyses.

Table 1. Brain areas analyzed for aromatase expression, their Bregma coordinates and plate numbers from the mouse brain atlas (Paxinos and Franklin, 2001), and sample sizes per group per brain area.
All statistical analyses were carried out using R v4.2.0 (R Core Team, 2022).
Male behavior in response to pup presentation was classified as ignoring, attacking, or retrieving a pup. The outcomes of behavioral trials were analyzed as the multinomial probability of exhibiting a given response to pup presentation (aggression, ignore, retrieval) as a function of paternal experience (experience as an ordered factor, N < 5D < 27D). To perform pairwise comparisons between the groups for each behavior, we calculated the probability that the difference in the posterior predictive intervals between groups was significantly directional. We then corrected for multiple comparisons using the false discovery rate.
Aromatase cell counts were conducted in two data sets combined to increase statistical power for comparisons. The data sets were obtained using the same immunocytochemical protocols and chemicals; however, the experiments were performed in two cohorts. We observed some count variation between the data of the cohorts (e.g., for the PIR −1.34 data; see Supplementary Figure S2), and so each data set was standardized by the mean and variance of the paternal groups (5D and 27D; the groups for which the datasets were overlapping). Sections for some brain areas were missing in some mice. The pattern of missingness would have led to omitting eight of 56 individuals (14%). To not exclude these animals from the data analysis, we elected to impute the missing data (16 cases, 5.7% of 280 total region counts) using predictive mean matching (van Buuren and Groothuis-Oudshoorn, 2011; R package mice). We generated five such candidate data sets and integrated the resulting uncertainty.
Our statistical model of aromatase variation was designed to address non-independence arising from correlated variation within individuals among areas. Such non-independence could be an especially important factor when comparing identifiable subregions of an area, such as in the piriform cortex and each part of the lateral septum. Therefore, we examined variation in normalized cell counts among all brain areas simultaneously (multivariate ANOVA), treating individual mouse identification (ID) as a correlated group (random) effect to account for hemisphere differences within individual. Responses were modeled as t-distributed to increase robustness against outlier counts. The interaction of experience x hemisphere was examined together.
All models were fit using the R package brms (Bürkner, 2017) using four chains of 2500 iterations each, and a thinning rate of 5. We assumed uniform priors throughout. Model convergence was assessed by R̂ = 1 (Vehtari et al., 2021); the R̂ statistic was computed within each imputed data set. We calculated the posterior means of each data set based on the fitted statistical model (emmeans package; Lenth, 2022) and used these to examine the posterior distributions of contrasts between hemispheres (left minus right for each brain area and experimental group of animals) and between the experimental groups for each brain area and hemisphere, i.e., 5D minus N (5D-N), 27D minus N (27D-N), and 27D minus 5D (27D-5D). These data are plotted in Figure 3. The significance of each contrast was judged as a Bayesian probability of direction p > 0.975 (Makowski et al., 2019), which is an index of effect existence, i.e., our level of certainty in whether a reported change is different from zero, either positive or negative. This threshold closely corresponds to the two-tailed p-value of 0.05, so we report the p-value for simplicity. Plots were generated using the ggplot2 package (Wickham, 2016).
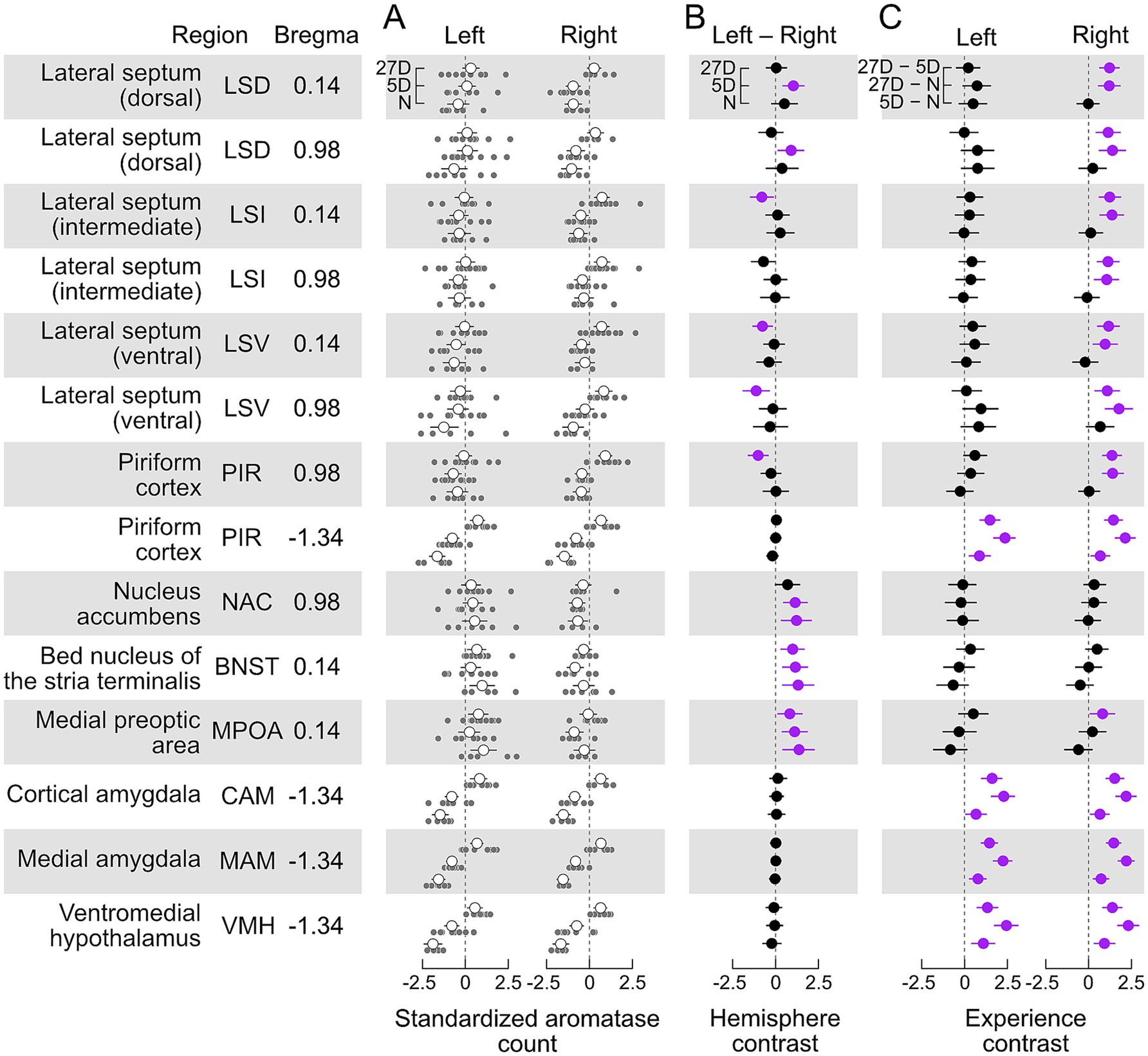
Figure 3. Aromatase expression differentially depends on paternal experience and brain hemisphere. (A) Standardized cell counts (light grey circles) and means with ±95% confidence interval (white circles) for each investigated brain area separately for the left and right sides of the brain and levels of pup experience (N, 5D, 27D). (B) Hemisphere contrast (left–right, with 95% confidence intervals) of the standardized counts of aromatase-positive cells for each brain area and experimental group. Contrasts with intervals significantly different (p < 0.05) from zero are highlighted in purple (see text for description). We found evidence for left-hemisphere-dominant lateralization (left minus right difference was positive) of aromatase cell counts, both independent of (BNST, MPOA, and most likely NAC) and dependent on pup experience (dorsal LS) and an experience-dependent right-hemisphere-dominant lateralization in the intermediate and ventral LS and anterior PIR (Bregma 0.98). (C). Experience contrast (5D–N; 27D–N, 27D–5D, with 95% confidence intervals) for the standardized counts of aromatase-positive cells, separately for each hemisphere and brain nucleus/area. We found that the occurrence of aromatase-positive cells in posterior (Bregma −1.34) PIR, CAM, MAM, and VMH in both brain hemispheres significantly increased with the experience level. The expression of aromatase in the LS and anterior PIR significantly increased only in the right hemisphere of the 27D group. No changes were observed in the NAC and BNST. Contrasts with intervals significantly different (p < 0.05) from zero are highlighted in purple (see text for description). Sample sizes are given in Table 1.
Residual correlations among areas represent relationships that are not explained by the other population- and group-level effects, including correlated expression and unexpected technical covariation. Inspection of residual correlations can, therefore, inform biological hypotheses. However, direct interpretation of the residual correlation matrix is challenging because the correlation between two brain areas could be due to mutual covariation with another area. We dichotomized relationships among brain areas by studying the matrix of partial correlations (de la Fuente et al., 2004; Shipley, 2000). We constructed an initial graph in which edges were created between all nodes with significant (p < 0.05) pairwise correlations. Edges were iteratively pruned by testing whether the association remained statistically significant after testing it against all other paths between two variables up to the third order. The independence test was determined from the posterior distribution of residual correlation matrices and implemented in the R package pcalg (Kalisch et al., 2012). The result is an undirected, acyclic graph representing a network of associations among brain areas. The graph is shown in Figure 4. The large number of tests may be conservative and may reject true associations, so we also report tentative links observed when setting a less stringent threshold of p < 0.10.
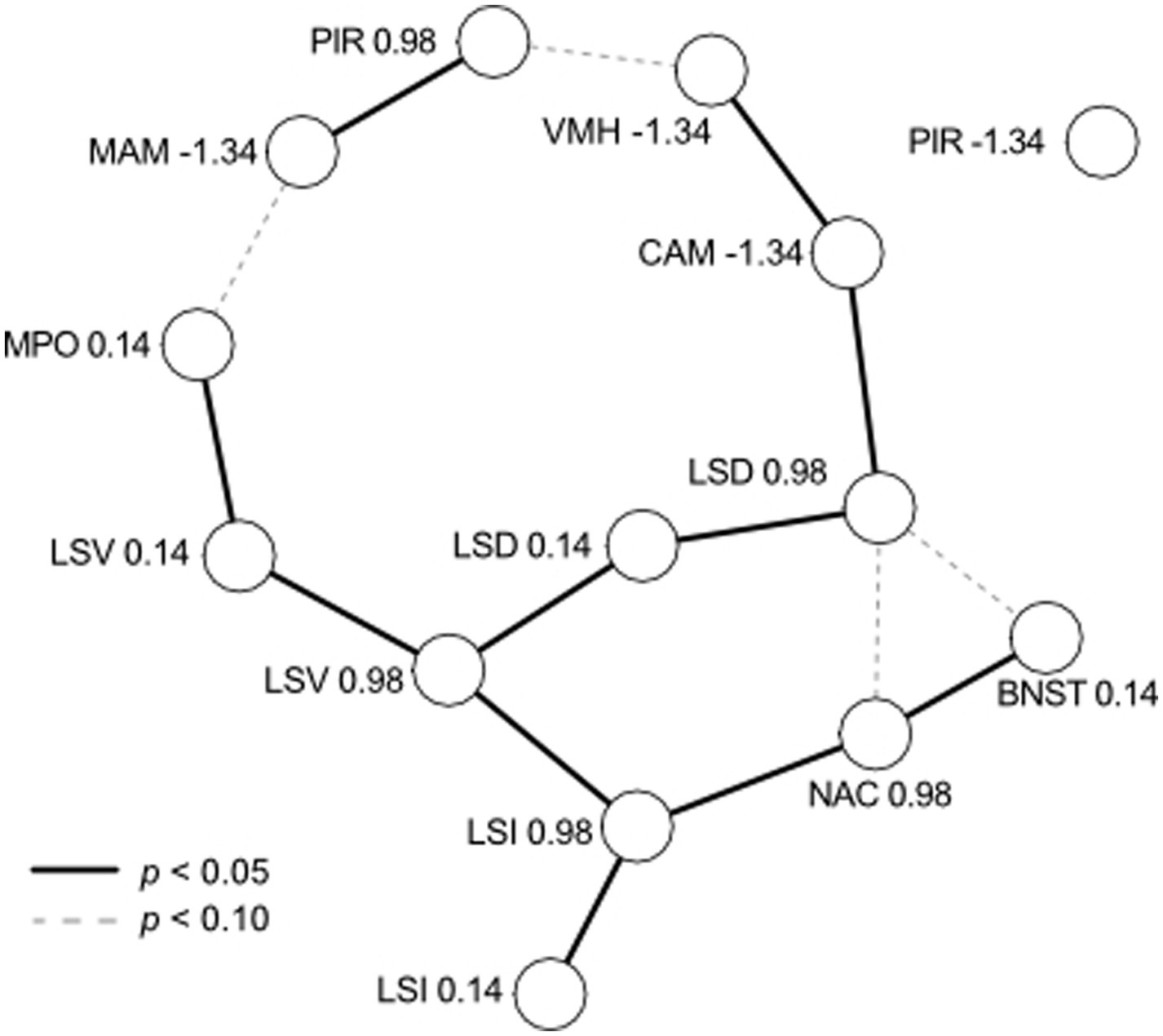
Figure 4. Residual correlations among brain nuclei/areas viewed as a network. Each nucleus/area at each Bregma coordinate (see Table 1) is represented by a node, with edges representing significant partial correlations (solid for p < 0.05, dashed for p < 0.10). Residual correlations were positive between all brain areas, with the sole exception of CAM (−1.34) and LSD (0.98), which were negatively correlated. PIR (−1.34) was not correlated with any other brain area.
Pup retrieval and aggression behaviors are completely colinear with the treatment groups (see below), so we could not examine behavioral differences simultaneously with experience. Pup ignoring, however, was observed in all experimental groups of mice. Therefore, we sought to find factors predicting why some males, regardless of experience, ignore pups (or why some males react to pups). Behavior toward pups was coded as reacting (aggressiveness or retrieval) or ignoring, and we then conducted a similar analysis to that described above for aromatase cell counts. All males were examined together without considering differences in experiences or hemispherical variation in aromatase cell counts.
Only males with paternal experience retrieved pups (Figure 1A). Figure 1B shows that naïve males with no previous sexual experience and pup contact mostly ignored the pups (n = 5/8) or were aggressive (n = 3/8). The majority of fathers with 5 days (5D) or 26–27 days (27D) of pup experience retrieved pups (n = 10/12 and 11/12, respectively), and only a few ignored pups (n = 2/12 and 1/12, respectively). No father was aggressive.
The probability of observing aggressive behavior toward pups was significantly higher in naïve males compared to fathers regardless of experience (5D and 27D, Figure 1B). The probability of other non-paternal behavior like ignoring pups was higher in naïve males compared to 27D fathers. Parental behavior measured as pup retrieval was significantly higher in fathers (5D and 27D) compared to naïve males. There were no significant differences in the probability of pup-retrieving between the groups of fathers, 5D and 27D (Figure 1B).
We found aromatase-positive cells in each examined area and with each experience level. In absolute terms, the largest numbers of aromatase-positive cells per section were seen in PIR, LSI, MAM, and CAM (see unstandardized data in Supplementary Figure S2). Aromatase labeling in the LSV, PIR, VMH, and MAM of the N and 27D groups showed qualitative differences between experimental groups and brain hemispheres (Figure 2). Standardized cell counts together with the group means are shown in Figure 3A along with our estimates of the posterior differences in aromatase counts by hemisphere (Left–Right, Figure 3B) and between levels of experience (Figure 3C).
We found evidence for significantly greater numbers of aromatase-positive cells in the left hemisphere of the MPOA and BNST in all three experimental groups (Left – Right >0, Figure 3B). The NAC exhibited a similar trend to MPOA and BNST, but with a just insignificant contrast in the 27D group. We did not detect significant lateralization of aromatase counts in any of the experimental groups in the amygdala (CAM and MAM), VMH, or posterior PIR (Bregma −1.34). In the other brain areas, the appearance of lateralization strongly depended on the level of pup experience. Long-term experience (27D) led to greater numbers of aromatase-positive cells in the right hemispheres of anterior PIR (Bregma 0.98), the LSV, and the anterior LSI (Bregma 0.14). Posterior LSI (Bregma 0.98) exhibited a similar trend that was just non-significant. In the LSD, pup experience had a short-term effect (5D) during which counts in the left hemisphere were greater. This effect was not present in the 27D group (Figure 3B).
In all cases in which we found significant changes in aromatase counts with pup experience, the number of aromatase-positive cells increased (Figure 3C). This experience-dependent increase was pervasive for the amygdala (CAM and MAM), the VMH, and the posterior PIR (Bregma −1.34). For these areas, the increase in aromatase positive cells was present in both hemispheres and with increasing experience, i.e., experience contrasts had significant positive values for both brain hemispheres and all experimental groups. In the LSD, LSI, and LSV (both Bregma coordinates) and the anterior PIR (Bregma 0.98), aromatase-positive cells increased significantly only in the right hemisphere of 27D males. Experience-dependent effects were absent in the NAC, the BNST, and the MPOA, except for a significant effect in the right MPOA of 27D males, i.e., 27D males had significantly more aromatase-positive cells than 5D males (Figure 3C).
We examined the unexplained (residual) variation in aromatase cell numbers among brain areas, as shown in Figure 4. We found positive residual correlations between all brain areas, with the sole exception of CAM (−1.34) and LSD (0.98), which were negatively correlated. Three subgroups of covariation are obvious. First, the LS is highly connected, and each subregion (D, I, V) is correlated across Bregma coordinates and with at least one other subregion. The LS appears to form the backbone of variation, to which other nodes are connected. This includes subgroups comprised of MPOA (connected to LSV), NAC and BNST (connected to LSI), and CAM and VMH (connected to LSD). Second, the subgroup of MAM and anterior PIR appears to be weakly connected to other parts of the graph (MPOA and VMH) because the edges are significant only with p < 0.10. Third, posterior PIR did not appear to be weakly correlated with any other analyzed brain area despite the increase in aromatase expression with experience similar to CAM, MAM, and VMH (see Figure 3).
The transition to fatherhood is distinctly marked by a reduction in aggressiveness toward pups and an increase in pup retrieval behavior (Figure 1). However, some males continue to ignore pups, and notably, one second-time father failed to retrieve pups during the pup retrieval test. To explore this further, we investigated whether variations in aromatase cell counts in any specific area could predict a male’s reaction to pups (contrast of reacting – ignoring mean cell counts, Figure 5). Our analysis revealed that males who reacted to pups had significantly higher numbers of aromatase cells in the MAM (p = 0.011) and exhibited a mild tendency toward higher cell counts in the CAM (p = 0.06).
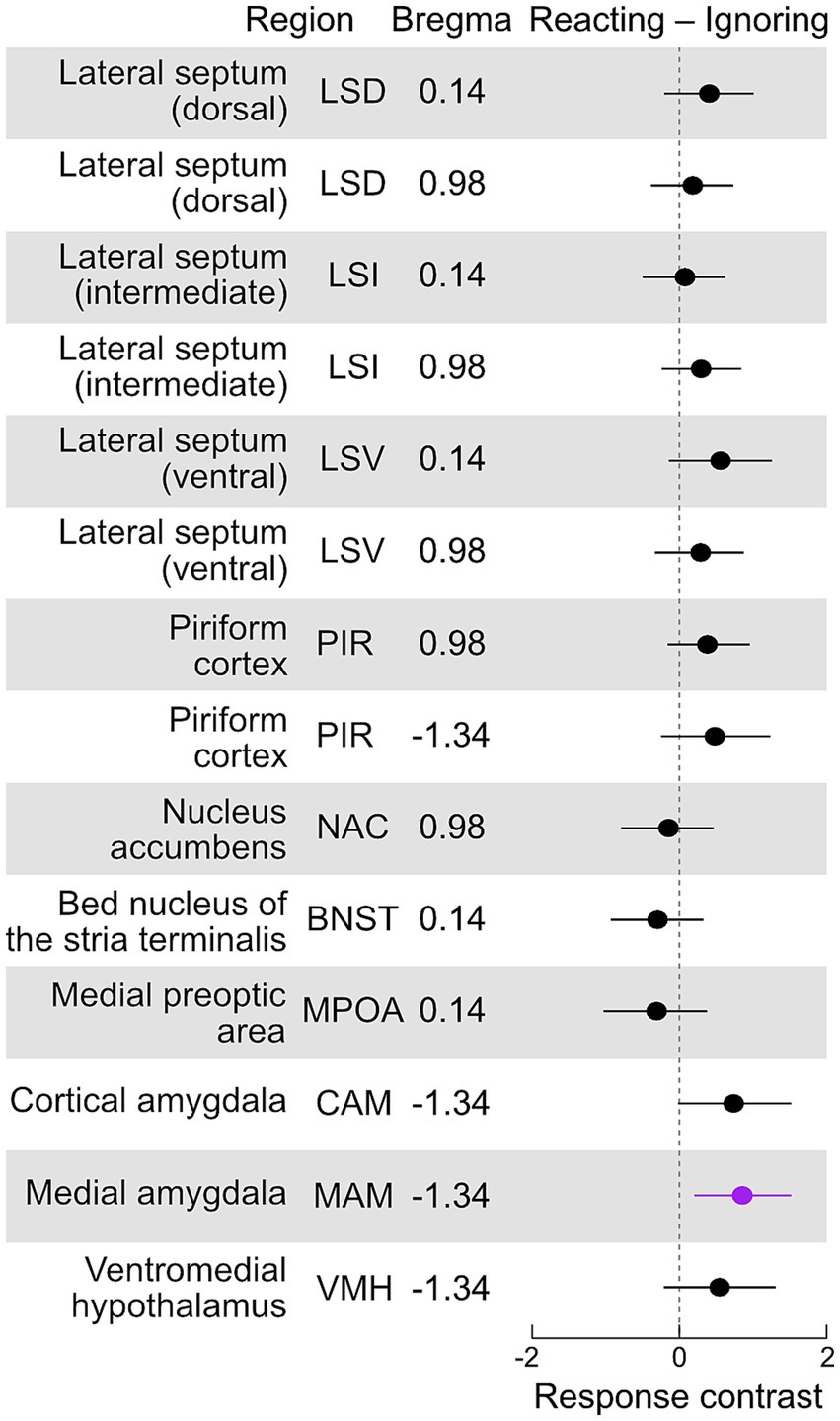
Figure 5. Aromatase expression in males that react to pups compared to those ignoring pups. Contrasts (reacting – ignoring, with 95% confidence intervals) of the standardized counts of aromatase-positive cells for each brain area. Contrasts with intervals significantly different (p < 0.05) from zero are highlighted in purple (see text for description). We found that reacting males had significantly higher numbers of aromatase cells in the MAM (p = 0.011) and a weak tendency to higher numbers in the CAM (p = 0.06).
In the present study, we found that aromatase, which catalyzes the conversion of testosterone to estrogen, is expressed throughout areas of the limbic system of male mice, regardless of their experience with pups. However, we identified important factors contributing to hierarchical variation in expression levels among individuals. First, the dramatic changes in behavior associated with paternal experience were associated with changes in aromatase expression in areas/nuclei of the limbic system. However, not only were these changes area-specific, but also hemisphere-specific (summarized in Figure 6). Finally, layered on these widespread patterns, we detected correlated aromatase levels among brain areas within individual mice. We briefly review the role of aromatase and the limbic areas to discuss how such expression patterns may influence, and be regulated by, paternal behaviors and experience.
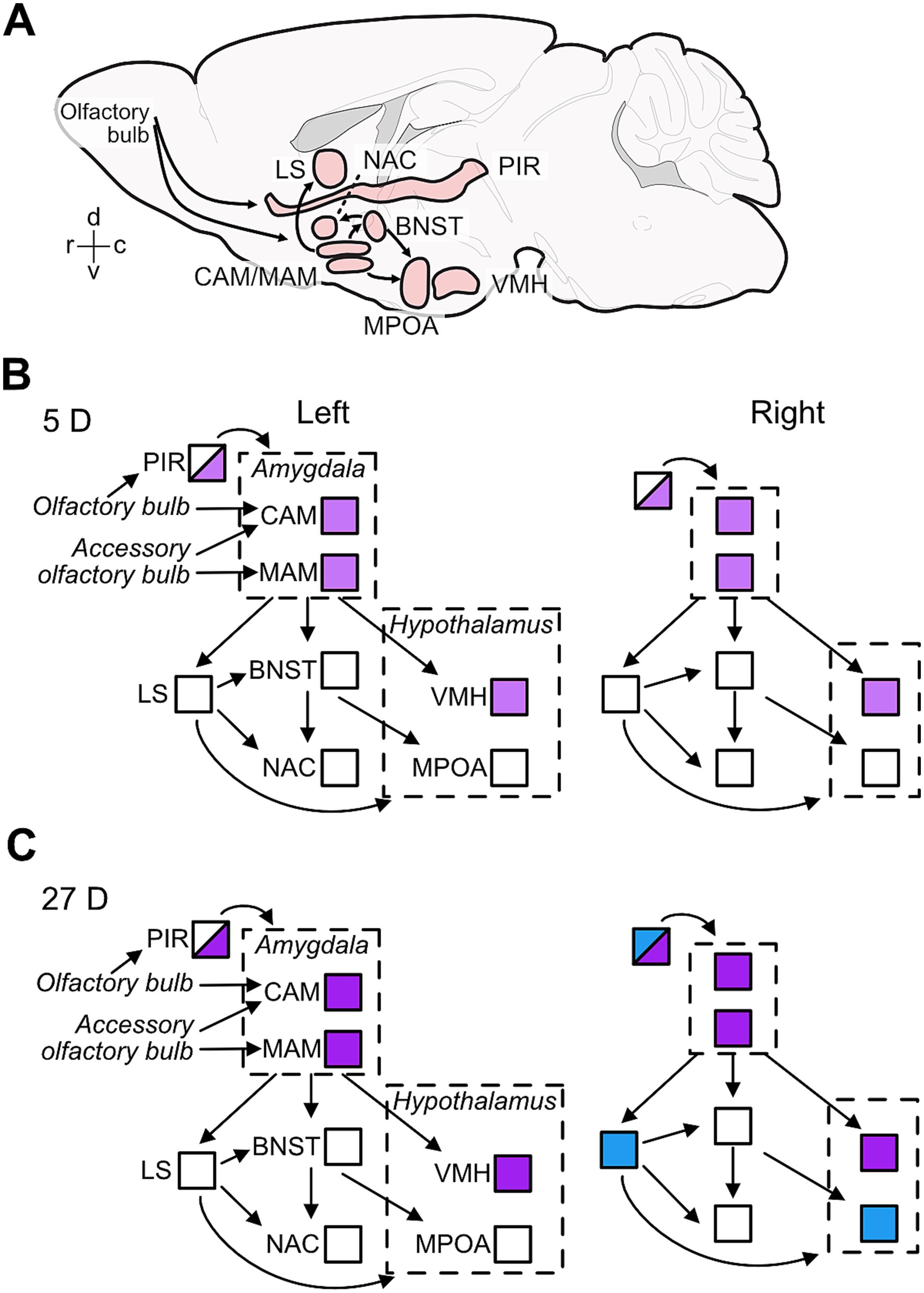
Figure 6. Effects of paternal experience on aromatase expression in areas of the limbic system. Sagittal view of the brain areas analyzed here (A) and summary of the changes in aromatase expression in 5D (B) and 27D (C) males in the left and right hemispheres of the brain nuclei/areas analyzed in the current study. (A) Positions and extensions of nuclei/areas are illustrative only and do not indicate anatomical relationships. The connectivity denoted in (A) shown by the arrows are exemplars for main information pathways (Numan, 2020; Kohl et al., 2017). (B) Pup experience in first-litter fathers (5D group) increased the numbers of aromatase-positive cells (indicated by light purple) relative to naïve males in the amygdala (CAM and MAM), hypothalamus (VMH) and in the posterior piriform cortex (PIR; half shading indicates these partial changes). (C) Second-litter fathers (27D group) had further increased aromatase expression in the same areas as 5D males (indicated as darker purple), i.e., the amygdala (CAM and MAM), hypothalamus (VMH), and the posterior piriform cortex (PIR) in both sides of the brain. In the right hemisphere, the number of aromatase-positive cells increased (indicated by blue) in the anterior PIR and lateral septum (LS) relative to naïve and 5D males and potentially in the MPOA relative to 5D males.
Aromatase expression in the male mouse brain has been shown via immunostaining with several protocols using different antibodies (Akther et al., 2015; Foidart et al., 1995), partly with enhancement through transgenic techniques (Stanić et al., 2014). In agreement with the published data, we found aromatase expression in all studied brain areas of our three experimental groups of male mice (Figure 3). Differences in the levels of aromatase expression between studies relate to differences in the staining methods, data analysis, and the social environment of the animals prior to and during the behavioral tests. For example, in our study, dynamics of paternal behavior and associated changes in aromatase levels have been examined while the fathers were continually housed with their female mate except during the pup-retrieval tests. This is different from the study of Akther et al. (2015), in which the relationship between pup-retrieving behavior in males and the dynamics of aromatase levels was tested throughout different ethological contexts (presence, absence, and reintroduction of their female mates and pups). In that previous study, brain aromatase expression was highest when the fathers were housed with their female partner but without their pups, followed by fathers housed with the whole family (Akther et al., 2015). Therefore, the observed changes in aromatase levels in that study can be related to male–female interaction. Nevertheless, the strong reduction of pup retrieval in fathers after injection with an aromatase inhibitor (letrozole; Akther et al., 2015) shows, along with the results of our present study, that proper levels of aromatase expression are integral to the display of paternal behavior in mice.
Interestingly, circulating testosterone is negatively correlated with paternal behavior in various rodent species (reviewed in Bales and Saltzman, 2016; Numan and Insel, 2003), and testosterone can inhibit paternal care in mice (Okabe et al., 2013). Our study did not examine the source of testosterone, the substrate of aromatase in the brain. However, previous studies have shown that changes in circulating testosterone may not be reflected locally in the brain. Neurosteroids can be locally produced, and levels in the brain may be independent of circulation or gonadal status (Giatti et al., 2019; Hojo et al., 2008, 2004).
In the following, we discuss the observed presence and changes of aromatase levels in the studied brain areas of the limbic system in order to see how these areas may contribute to changing male behavior towards pups from aggressive or ignoring in naïve males to caring in experienced fathers (Figure 1). Furthermore, we compare the dynamics of aromatase-positive cells with the dynamics of ERα-positive cells in the given brain areas to reveal the extent to which these align to support the hypothesis about aromatase influencing indirectly (via estrogen synthesis and ER binding) paternal behavior.
Three nuclei, the MAM, CAM, and VMH, were most affected by pup experience with increases in the number of aromatase-positive cells from N to 5D to 27D animals (Figures 3A,C; Supplementary Figure S2). These areas are associated with the regulation of aggression compared to sexual and parental behavior (e.g., Chen et al., 2019; Dwyer et al., 2022; Hashikawa et al., 2016; Lee et al., 2014; Sheehan et al., 2001; Unger et al., 2015; Yang et al., 2017). Important changes in aggression in the course of becoming a caring father are mediated by olfactory signals from the vomeronasal organ via the accessory olfactory bulb to the CAM and MAM (e.g., Hashikawa et al., 2016) and concern the switch from aggressive to caring behavior toward pups (Tachikawa et al., 2013). Concomitantly, aggressive motivation in fathers increases against male intruders to protect their own pups from potential pup killers, as shown in house mice (Shabalova et al., 2020), Californian mice (Trainor et al., 2008) and prairie voles (Getz et al., 1981). This increased aggression toward intruders was not mediated by changes in ERα or ERß expression in limbic centers but reflected in increased neuronal activation (FOS expression) in the MPOA, MAM, and, potentially, the VMH in California mice (Trainor et al., 2008). In house mice fathers, aggressive behavior toward intruders is regulated by oxytocin and activation of neurons in areas of the hypothalamus (periventricular and supraoptic nuclei; Shabalova et al., 2020). Further research could examine whether changes in aggression in naïve vs. fathers are also related to aromatase levels in the amygdala and VMH.
In contrast to aromatase expression (present study), the number of estrogen receptors (ERα) in MAM, CAM, and VMH did not change with parental experience in male mice (5D or 27D) (Ehret et al., 1993). Together, this indicates that the increased presence of local estrogen synthesis does not facilitate the expression of ERα, as has been shown in the VMH (Devidze et al., 2005; Malikov and Madeira, 2013), especially in combination with parental experience (Koch, 1990). Few studies have explored the role of other estrogen receptors in paternal care in rodents (Hyer et al., 2017; Torres et al., 2024). Other subtypes (ERβ and membrane receptor GPER) may be involved in the expression of parental behavior. Our study indicates that parental experience is associated with changes in aromatase expression, which can potentially affect the balance of androgen to estrogen levels in the brain. Further studies should also examine whether androgen receptors, expressed in the same brain areas as aromatase (Dart et al., 2024), are implicated in parental behavior e.g., (see research in Mongolian gerbils; Martínez et al., 2019). We suggest that the increase in aromatase expression in the MAM, CAM, and VMH in new (5D) and highly experienced (27D) fathers (Figure 6) supports the control of instinctive behavior (Honda et al., 2011), including inhibition of infanticide, via increased local synthesis of estrogens on neurons (e.g., Cornil et al., 2006, 2013).
In house mouse mothers, pup-retrieving performance does not depend on olfactory signals from the main olfactory bulb and its projections to the PIR (Koch and Ehret, 1991). Pup-naïve females, however, needed this olfactory information to learn to become as maternal as the mothers (Ehret and Buckenmaier, 1994; Koch and Ehret, 1991). While learning pup cues for becoming maternal, virgin females with maternal experience have an increase in ERα-positive cells in the posterior PIR compared to females without experience (also in the entorhinal cortex; Koch, 1990). Similarly, as males became caring fathers, ERα-positive cells became visible in the posterior PIR (also in the entorhinal cortex; Ehret et al., 1993). Given the significant increase of aromatase expression in the posterior PIR with increasing paternal experience (Figure 3C), it is possible that in PIR, unlike MAM, CAM, and VMH, estrogen may have induced its own receptors (tested for ERα) and enhanced olfactory learning of pup cues for increased paternal performance.
The significant increase of aromatase-positive cells only in the right hemisphere in the anterior PIR of 27D fathers does not directly correlate with any evidence. In studies on ERα expression in the limbic areas of female and male mice under various conditions of the reproductive cycle, experience with pups, and intact or lesioned olfaction, no hemisphere differences have been reported in any area (Ehret et al., 1993; Ehret and Buckenmaier, 1994; Koch, 1990; Koch and Ehret, 1991). The splitting of PIR in regions with experience-dependent lateralized (anterior PIR) and non-lateralized (posterior PIR) aromatase change shows compartmentalization of the PIR. Functional relationships need to be elucidated in further studies.
The main effects of pup-experience on aromatase expression in the LS were changes in the left-right balance (Figure 3B) and significant increase of aromatase-positive cells only in the right LS of highly experienced (27D) fathers (Figure 3C). As in the PIR, ERα-positive cells in the LS become visible in caring fathers compared to pup-naïve males (Ehret et al., 1993). Since hemisphere differences in ERα-positive cells have not been found, we cannot directly relate increased right-hemisphere aromatase expression with a non-lateralized increase of ERα expression in the LS of both hemispheres. Lateralized input to the rostral parts of the LSD and LSI comes from the hippocampal CA1 and subiculum region (work in mice and rats; Rizzi-Wise and Wang, 2021; Risold and Swanson, 1997). In rodents, the CA1 shows lateralization in synapse morphology and receptor distribution (Shinohara et al., 2008; Xiao and Jordan, 2002) and left–right dissociation of memory processes (Shipton et al., 2014) with right-hemisphere dominance of spatial memory in mice (Shinohara et al., 2012). Also, androgen receptor levels are higher in the left hippocampus in male rats (Xiao and Jordan, 2002). It is unclear, however, whether and how lateralized hippocampal input to the LS could have caused lateralized aromatase expression in the LS of 27D fathers (Figure 3C). Right hemisphere lateralization is associated with the regulation of mood, affect, and stress, and with sustained and global arousal and attention (e.g., Ehret, 2006; Palomero-Gallagher and Amunts, 2022). Speculatively, our observed right-hemisphere LS bias in 27D fathers could be related to the stress of continuous arousal and steady involvement in a routine of pup care over several weeks.
The MPOA and the BNST and their output via the NAC are essential for controlling rodent parental behavior (e.g., Numan, 2006; Bridges, 2015; Wu et al., 2014). Significant increases have been observed in the MPOA in both the number of and area occupied by ERα-positive cells in 5D and 27D pup-experienced father mice (Ehret et al., 1993) and, although absent in pup-naïve males, ERα-positive cells are present in the BNST of 27D fathers (Ehret et al., 1993; Koch and Ehret, 1989a,b). The number of ERα-positive cells in females is also higher in the MPOA and BNST of one-day pup-experienced virgin females (Ehret and Buckenmaier, 1994). In the current study, we did not observe changes in the number of aromatase-positive cells in the BNST and NAC and found only a small increase in the MPOA in only the 27D males relative to 5D. This suggests that changes in sensory processing and behavior with experience in MPOA, BNST, and NAC may be more dependent on the regulation of estrogen signaling through binding to ERα than on local estrogen action after synthesis via aromatase. However, further research on other estrogen receptors is needed. Brain areas that regulate social behavior, including parental care, are conserved in vertebrates (O’Connell and Hofmann, 2012). However, neuroendocrine ligand spatial profiles are more variable than receptor distributions within the social decision-making network among vertebrates, although there are regional differences (O’Connell and Hofmann, 2012). Our study supports the existence of experience-dependent regional differences, specifically indicating that the effects of the MPOA, BNST, and NAC on parental behavior may depend on changes in ERα distribution. In contrast, the influence of the VMH and the amygdala may be driven by changes in aromatase expression. Overall, our results highlight the area-specific regulation of receptor and ligand synthesis based on parental experience.
The brain areas MPOA, BNST, and NAC exhibited substantial lateralization of the presence of aromatase-positive cells, biased toward the left hemisphere (Figure 3B). Left-hemisphere dominance has been noted in numerous contexts, including maternal perception and processing of pup-ultrasounds, in the latter case, through cFos expression (Ehret, 1987; Geissler et al., 2016). Maternal pup retrieval behavior likewise is associated with left- but not right-hemisphere oxytocin receptor expression and action (Marlin et al., 2015). To what extent can these sources of left-hemisphere bias in females (primarily auditory cortex) inform our findings? The left-hemisphere bias in females has primarily been observed in the auditory cortex (Geissler et al., 2016; Marlin et al., 2015). Lateralization of cFos expression has not been observed in the neural responses to pup presence in the limbic system of female mice, MPOA and BNST included (Geissler et al., 2013). However, neural activation characterized by cFos labeling concerns the input side of neurons (e.g., Sheng et al., 1990, 1993). Provided such absence of hemisphere differences in activation at the input side of the MPOA and BNST also existed in males/fathers, we can hypothesize that the greater left hemisphere aromatase expression in the MPOA and BNST may lead to a left-hemisphere bias of activation at the output of the MPOA and BNST after local estrogen action (enabled via aromatase) has taken place. Whether such a functional bias exists and how it may contribute to the preferred processing of pup ultrasounds to activate appetitive pup-searching behavior and possible left-hemisphere-dominant ultrasound perception in pup-experienced male mice similar to mothers remains to be shown.
The brain’s connectivity suggests that counts of active cells (e.g., c-Fos indicative of brain activation) within individuals may be correlated across regions. For example, aromatase could be uniformly lower or higher in some individuals than others or vary highly non-uniformly across brain areas. We expect that such variation will contribute to individual differences in behavior, such as some fathers continuing to ignore pups (Figure 1B). Our study found significant residual correlated variation among brain areas (Figure 4), generally not depicted in the data shown in Figure 3. Our analysis points to a central role of the LS in the variation of aromatase expression in the mouse brain. The LS has a central functional role in the brain, integrating cognitive and emotional information in the regulation of social relationships and instincts (Besnard and Leroy, 2022; Menon et al., 2022; Rizzi-Wise and Wang, 2021), including parental behavior (Fleischer and Slotnick, 1978; Slotnick and Nigrosh, 1975). Interestingly, these partial correlations (shown in Figure 4) reflect the main neural connections of the subregions LSD and LSV. For example, there is input from the amygdala to the LS supporting the LSD-CAM partial correlation, and there is an output from the LS to the MPOA supporting the LSV-MPOA partial correlation (Besnard and Leroy, 2022; Menon et al., 2022; Rizzi-Wise and Wang, 2021; Risold and Swanson, 1997). Thus, residual variation in aromatase expression (Figure 4) appears to mirror functional connections and relationships of the LS. Notably, the posterior PIR and, to a lesser degree, the anterior PIR and the MAM remained outside this connectivity. Indeed, Supplementary Figure S2 shows that posterior PIR (Bregma −1.34) was the sole case in which we observed substantial divergence in overall cell counts between the two cohorts of animals. Together, these observations on correlated individual variations indicate both coherent relationships between brain areas in a functional network and the presence of possibly unknown additional factors intrinsic to given experiments. In our present case, such factors modulated the numbers of aromatase-positive cells in PIR and MAM. Therefore, our study shows the usefulness of correlative modeling in assessing and estimating the relationships between the contributions of the members of a neuronal network and its overall functional properties. This is another example of correlative modeling supporting the adequate interpretation of absolute data.
Our results demonstrate that pup retrieval and aggression behaviors are closely related to the paternal experience groups (5D and 27D). Consequently, we aimed to investigate why some fathers do not appear to respond to their own pups, continuing to ignore them much like naïve males. Our analysis revealed that males who engaged with pups exhibited a significantly greater number of aromatase-expressing cells in the amygdala. Although tentative, these results (Figure 5) suggest that the amygdala, particularly the medial amygdala (MAM), may mediate robust emotional responses to pups. The longitudinal responses of males—both naïve and experienced fathers—to pups could clarify the repeatability of responses and whether the tendency to ignore is a transitional phase between aggression and retrieval or a distinct behavioral state. Such research would require a considerably larger sample size than was feasible in this study to effectively capture the natural variation in individual behavior.
Aromatase expression in the male limbic brain is regulated by parental experience in an area-, hemisphere-, and experience-specific manner. Gaining experience in paternal pup care led to enhanced aromatase expression (number of aromatase-positive cells) in brain regions involved in processing olfactory information (PIR, CAM, and MAM) and in areas that regulate aggression, sexual behavior, and other social interactions (MAM, VMH, and LS). In centers essential for controlling parental behavior (MPOA, BNST, NAC), aromatase expression was instead present with left-hemisphere dominance and largely independent of experience with pups. In general, the experience-dependent changes in aromatase expression observed in the majority of the brain areas did not reflect the previously reported changes in estrogen receptors (ERα) when pup-naïve males became pup-caring fathers.
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding authors. Data are available on Figshare DOI: https://doi.org/10.6084/m9.figshare.23695983.v1.
The animal study was approved by Regierungspräsidium Tübingen, Referat 35. The study was conducted in accordance with the local legislation and institutional requirements.
PD-G: Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Writing – original draft, Validation, Visualization. DS: Formal analysis, Methodology, Software, Writing – review & editing, Funding acquisition, Visualization. AM: Formal analysis, Investigation, Writing – review & editing, Methodology. DG: Investigation, Methodology, Supervision, Writing – review & editing, Formal Analysis. GE: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Formal analysis, Validation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Deutsche Forschungsgemeinschaft, EH53/19 to GE. PDG was funded by a postdoctoral fellowship from the Fonds Quebecois Recherche Nature et Technologie. PDG is currently supported by the Canada Research Chair Program. DAS was partially funded by a postdoctoral fellowship from the Kavli Neuroscience Discovery Institute.
We thank H. Sabine Schmidt for assistance with animal care, immunocytochemistry, and microscopy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1502764/full#supplementary-material
BNST, bed nucleus of the stria terminalis; CAM, cortical amygdala; LS, lateral septum; LSD, lateral septum dorsal; LDI, lateral septum intermediate; LSV, lateral septum ventral; MAM, medial amygdala; MPOA, medial preoptic area; NAC, nucleus accumbens; PIR, piriform cortex; VMH, ventromedial nucleus of the hypothalamus.
Akther, S., Huang, Z., Liang, M., Zhong, J., Fakhrul, A. A. K. M., Yuhi, T., et al. (2015). Paternal retrieval behavior is regulated by brain estrogen synthetase (aromatase) in mouse sires that engage in communicative interactions with pairmates. Front. Neurosci. 9:450. doi: 10.3389/fnins.2015.00450
Alsina-Llanes, M., De Brun, V., and Olazábal, D. E. (2015). Development and expression of maternal behavior in naïve female C57BL/6 mice. Dev. Psychobiol. 57, 189–200. doi: 10.1002/dev.21276
Bales, K. L., and Saltzman, W. (2016). Fathering in rodents: neurobiological substrates and consequences for offspring. Horm. Behav. 77, 249–259. doi: 10.1016/j.yhbeh.2015.05.021
Besnard, A., and Leroy, F. (2022). Top-down regulation of motivated behaviors via lateral septum sub-circuits. Mol. Psychiatry 27, 3119–3128. doi: 10.1038/s41380-022-01599-3
Bridges, R. S. (2015). Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 36, 178–196. doi: 10.1016/j.yfrne.2014.11.007
Brunton, P. J., and Russell, J. A. (2008). The expectant brain: adapting for motherhood. Nat. Rev. Neurosci. 9, 11–25. doi: 10.1038/nrn2280
Bürkner, P. C. (2017). Brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28. doi: 10.18637/jss.v080.i01
Charitidi, K., and Canlon, B. (2010). Estrogen receptors in the central auditory system of male and female mice. Neuroscience 165, 923–933. doi: 10.1016/j.neuroscience.2009.11.020
Chen, P. B., Hu, R. K., Wu, Y. E., Pan, L., Huang, S., Micevych, P. E., et al. (2019). Sexually dimorphic control of parenting behavior by the medial amygdala. Cell 176, 1206–1221.e18. doi: 10.1016/j.cell.2019.01.024
Cornil, C. A., Ball, G. F., and Balthazart, J. (2006). Functional significance of the rapid regulation of brain estrogens: where do the estrogens come from? Brain Res. 1126, 2–26. doi: 10.1016/j.brainres.2006.07.098
Cornil, C. A., Seredynski, A. L., de Bournonville, C., Dickens, M. J., Charlier, T. D., Ball, G. F., et al. (2013). Rapid control of reproductive behaviour by locally synthesised oestrogens: focus on aromatase. J. Neuroendocrinol. 25, 1070–1078. doi: 10.1111/jne.12062
Dart, D. A., Bevan, C. L., Uysal-Onganer, P., and Jiang, W. G. (2024). Analysis of androgen receptor expression and activity in the mouse brain. Sci Rep. 14, 11115. doi: 10.1038/s41598-024-61733-9
de la Fuente, A., Bing, N., Hoeschele, I., and Mendes, P. (2004). Discovery of meaningful associations in genomic data using partial correlation coefficients. Bioinformatics 20, 3565–3574. doi: 10.1093/bioinformatics/bth445
Devidze, N., Mong, J. A., Jasnow, A. M., Kow, L. M., and Pfaff, D. W. (2005). Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proc. Natl. Acad. Sci. USA 102, 14446–14451. doi: 10.1073/pnas.0507144102
Dulac, C., O’Connell, L. A., and Wu, Z. (2014). Neural control of maternal and paternal behaviors. Science 345, 765–770. doi: 10.1126/science.1253291
Dutta, S., Mark-Kappeler, C. J., Hoyer, P. B., and Pepling, M. E. (2014). The steroid hormone environment during primordial follicle formation in perinatal mouse ovaries. Biol. Reprod. 91:68. doi: 10.1095/biolreprod.114.119214
Dwyer, J., Kelly, D. A., and Bergan, J. (2022). Brain-wide synaptic inputs to aromatase-expressing neurons in the medial amygdala suggest complex circuitry for modulating social behavior. eNeuro 9, ENEURO.0329–ENEU21.2021. doi: 10.1523/ENEURO.0329-21.2021
Ehret, G. (1987). Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature 325, 249–251. doi: 10.1038/325249a0
Ehret, G., and Buckenmaier, J. (1994). Estrogen-receptor occurrence in the female mouse brain: effects of maternal experience, ovariectomy, estrogen and anosmia. J. Physiol. Paris 88, 315–329. doi: 10.1016/0928-4257(94)90012-4
Ehret, G. (2006). “Hemisphere dominance of brain function - which functions are lateralized and why?” In 23 problems in systems neuroscience, eds. L. Hemmenvan and T. J. Sejnowski (New York, NY: Oxford University Press) 44–61.
Ehret, G., Jürgens, A., and Koch, M. (1993). Oestrogen receptor occurrence in the male mouse brain: modulation by paternal experience. Neuroreport 4, 1247–1250. doi: 10.1097/00001756-199309000-00009
Ehret, G., and Koch, M. (1989). Ultrasound-induced parental behaviour in house mice is controlled by female sex hormones and parental experience. Ethology 80, 81–93. doi: 10.1111/j.1439-0310.1989.tb00731.x
Ehret, G., Koch, M., Haack, B., and Markl, H. (1987). Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften 74:47. doi: 10.1007/BF00367047
Ehret, G., Koch, M., and Jirikowski, G. F. (1991). Hormone, ZNS und Fortpflanzungsverhalten. Allergologie 14, 383–394.
Elwood, R. W. (1985). Inhibition of infanticide and onset of paternal care in male mice (Mus musculus). J. Comp. Psychol. 99, 457–467. doi: 10.1037/0735-7036.99.4.457
Fahrbach, S. E., and Pfaff, D. W. (1982). “Hormonal and neural mechanisms underlying maternal behavior in the rat” in The physiological mechanisms of motivation. ed. D. W. Pfaff (New York, NY: Springer), 253–285.
Fleischer, S., and Slotnick, B. M. (1978). Disruption of maternal behavior in rats with lesions of the septal area. Physiol. Behav. 21, 189–200. doi: 10.1016/0031-9384(78)90041-0
Foidart, A., Harada, N., and Balthazart, J. (1995). Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res. 280, 561–574. doi: 10.1007/BF00318360
Garcia-Segura, L. M. (2008). Aromatase in the brain: not just for reproduction anymore. J. Neuroendocrinol. 20, 705–712. doi: 10.1111/j.1365-2826.2008.01713.x
Geissler, D. B., Sabine Schmidt, H., and Ehret, G. (2013). Limbic brain activation for maternal acoustic perception and responding is different in mothers and virgin female mice. J. Physiol. Paris 107, 62–71. doi: 10.1016/j.jphysparis.2012.05.006
Geissler, D. B., Schmidt, H. S., and Ehret, G. (2016). Knowledge about sounds—context-specific meaning differently activates cortical hemispheres, auditory cortical fields, and layers in house mice. Front. Neurosci. 10:98. doi: 10.3389/fnins.2016.00098
Getz, L. L., Carter, C. S., and Gavish, L. (1981). The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav. Ecol. Sociobiol. 8, 189–194. doi: 10.1007/BF00299829
Giatti, S., Diviccaro, S., Garcia-Segura, L. M., and Melcangi, R. C. (2019). Sex differences in the brain expression of steroidogenic molecules under basal conditions and after gonadectomy. J. Neuroendocrinol. 31:e12736. doi: 10.1111/jne.12736
Guoynes, C., and Marler, C. (2020). “Paternal behavior from a neuroendocrine perspective” in Oxford Research Encyclopedia of Neuroscience. Avaiable at: https://oxfordre.com/neuroscience/view/10.1093/acrefore/9780190264086.001.0001/acrefore-9780190264086-e-9 (Accessed 3 February, 2025).
Hashikawa, K., Hashikawa, Y., Falkner, A., and Lin, D. (2016). The neural circuits of mating and fighting in male mice. Curr. Opin. Neurobiol. 38, 27–37. doi: 10.1016/j.conb.2016.01.006
Hojo, Y., Hattori, T.-A., Enami, T., Furukawa, A., Suzuki, K., Ishii, H.-T., et al. (2004). Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 101, 865–870. doi: 10.1073/pnas.2630225100
Hojo, Y., Murakami, G., Mukai, H., Higo, S., Hatanaka, Y., Ogiue-Ikeda, M., et al. (2008). Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol. Cell. Endocrinol. 290, 31–43. doi: 10.1016/j.mce.2008.04.017
Honda, S. I., Wakatsuki, T., and Harada, N. (2011). Behavioral analysis of genetically modified mice indicates essential roles of neurosteroidal estrogen. Front. Endocrinol. 2:40. doi: 10.3389/fendo.2011.00040
Horrell, N. D., Acosta, M. C., and Saltzman, W. (2021). Plasticity of the paternal brain: effects of fatherhood on neural structure and function. Dev. Psychobiol. 63, 1499–1520. doi: 10.1002/dev.22097
Hyer, M. M., Khantsis, S., Venezia, A. C., Madison, F. N., Hallgarth, L., Adekola, E., et al. (2017). Estrogen-dependent modifications to hippocampal plasticity in paternal California mice (Peromyscus californicus). Horm. Behav. 96, 147–155. doi: 10.1016/j.yhbeh.2017.09.015
Ji, Y. X., Zhao, M., Liu, Y.-l., Chen, L.-s., Hao, P.-l., and Sun, C. (2017). Expression of aromatase and estrogen receptors in lumbar motoneurons of mice. Neurosci. Lett. 653, 7–11. doi: 10.1016/j.neulet.2017.05.017
Kalisch, M., Mächler, M., Colombo, D., Maathuis, M. H., and Bühlmann, P. (2012). Causal inference using graphical models with the R package pcalg. J. Stat. Softw. 47, 1–26. doi: 10.18637/jss.v047.i11
Koch, M. (1990). Effects of treatment with estradiol and parental experience on the number and distribution of estrogen-binding neurons in the ovariectomized mouse brain. Neuroendocrinology 51, 505–514. doi: 10.1159/000125384
Koch, M., and Ehret, G. (1989a). Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol. Behav. 45, 771–776. doi: 10.1016/0031-9384(89)90293-X
Koch, M., and Ehret, G. (1989b). Immunocytochemical localization and quantitation of estrogen-binding cells in the male and female (virgin, pregnant, lactating) mouse brain. Brain Res. 489, 101–112. doi: 10.1016/0006-8993(89)90012-7
Koch, M., and Ehret, G. (1991). Parental behavior in the mouse: effects of lesions in the entorhinal/piriform cortex. Behav. Brain Res. 42, 99–105. doi: 10.1016/S0166-4328(05)80044-0
Kohl, J., Autry, A. E., and Dulac, C. (2017). The neurobiology of parenting: a neural circuit perspective. BioEssays 39, 1–11. doi: 10.1002/bies.201600159
Kohl, J., and Dulac, C. (2018). Neural control of parental behaviors. Curr. Opin. Neurobiol. 49, 116–122. doi: 10.1016/j.conb.2018.02.002
Laws, M. J., Kannan, A., Pawar, S., Haschek, W. M., Bagchi, M. K., and Bagchi, I. C. (2014). Dysregulated estrogen receptor signaling in the hypothalamic-pituitary-ovarian axis leads to ovarian epithelial tumorigenesis in mice. PLoS Genet. 10:e1004230. doi: 10.1371/journal.pgen.1004230
Lee, H., Kim, D. W., Remedios, R., Anthony, T. E., Chang, A., Madisen, L., et al. (2014). Scalable control of mounting and attack by ESR1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. doi: 10.1038/nature13169
Lenth, R. (2022). Emmeans: estimated marginal means, aka least-squares means. R package version 1.8.0. Available at: https://CRAN.R-project.org/package=emmeans (Accessed February 3, 2025).
Li, Y., Lian, Z., Wang, B., Tai, F., Wu, R., Hao, P., et al. (2015). Natural variation in paternal behavior is associated with central estrogen receptor alpha and oxytocin levels. J. Comp. Physiol. A 201, 285–293. doi: 10.1007/s00359-015-0979-6
Lonstein, J. S., and De Vries, G. J. (2000). Sex differences in the parental behavior of rodents. Neurosci. Biobehav. Revs. 24, 669–686. doi: 10.1016/S0149-7634(00)00036-1
Makowski, D., Ben-Shachar, M. S., and Lüdecke, D. (2019). bayestestR: describing effects and their uncertainty, existence and significance within the Bayesian framework. J. Open Source Softw. 4:1541. doi: 10.21105/joss.01541
Malikov, V., and Madeira, M. D. (2013). Regulation of ERα protein expression by 17β-estradiol in cultured neurons of hypothalamic ventromedial nucleus. Neurochem. Res. 38, 82–89. doi: 10.1007/s11064-012-0891-1
Marlin, B. J., Mitre, M., D’amour, J. A., Chao, M. V., and Froemke, R. C. (2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504. doi: 10.1038/nature14402
Martínez, A., Arteaga-Silva, M., Bonilla-Jaime, H., Cárdenas, M., Rojas-Castañeda, J., Vigueras-Villaseñor, R., et al. (2019). Paternal behavior in the Mongolian gerbil, and its regulation by social factors, T, ERα, and AR. Physiol. Behav. 199, 351–358. doi: 10.1016/j.physbeh.2018.12.001
Martín-Sánchez, A., Valera-Marín, G., Hernández-Martínez, A., Lanuza, E., Martínez-García, F., and Agustín-Pavón, C. (2015). Wired for motherhood: induction of maternal care but not maternal aggression in virgin female CD1 mice. Front. Behav. Neurosci. 9:197. doi: 10.3389/fnbeh.2015.00197
Menon, R., Süß, T., Oliveira, V., Neumann, I. D., and Bludau, A. (2022). Neurobiology of the lateral septum: regulation of social behavior. Trends Neurosci. 45, 27–40. doi: 10.1016/j.tins.2021.10.010
Nilsson, M. E., Vandenput, L., Tivesten, Å., Norlén, A. K., Lagerquist, M. K., Windahl, S. H., et al. (2015). Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology 156, 2492–2502. doi: 10.1210/en.2014-1890
Numan, M. (2006). Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav. Cogn. Neurosci. Rev. 5, 163–190. doi: 10.1177/1534582306288790
Numan, M. (2020). The parental brain: Mechanisms, development, and evolution. New York: Oxford University Press.
O’Connell, L. A., and Hofmann, H. A. (2012). Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. doi: 10.1126/science.1218889
Okabe, S., Kitano, K., Nagasawa, M., Mogi, K., and Kikusui, T. (2013). Testosterone inhibits facilitating effects of parenting experience on parental behavior and the oxytocin neural system in mice. Physiol. Behav. 118, 159–164. doi: 10.1016/j.physbeh.2013.05.017
Palomero-Gallagher, N., and Amunts, K. (2022). A short review on emotion processing: a lateralized network of neuronal networks. Brain Struct. Funct. 227, 673–684. doi: 10.1007/s00429-021-02331-7
Paxinos, G., and Franklin, K. (2001). The mouse brain in stereotaxic coordinates. 2nd Edn. San Diego: Academic Press.
Poindron, P., Lévy, F., and Krehbiel, D. (1988). Genital, olfactory, and endocrine interactions in the development of maternal behaviour in the parturient ewe. Psychoneuroendocrinology 13, 99–125. doi: 10.1016/0306-4530(88)90009-1
R Core Team (2022). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rilling, J. K., and Mascaro, J. S. (2017). The neurobiology of fatherhood. Curr. Opin. Psychol. 15, 26–32. doi: 10.1016/j.copsyc.2017.02.013
Risold, P. Y., and Swanson, L. W. (1997). Connections of the rat lateral septal complex. Brain Res. Brain Res. Revs. 24, 115–195. doi: 10.1016/S0165-0173(97)00009-X
Rizzi-Wise, C. A., and Wang, D. V. (2021). Putting together pieces of the lateral septum: multifaceted functions and its neural pathways. eNeuro 8, ENEURO.0315–ENEU21.2021. doi: 10.1523/ENEURO.0315-21.2021
Romero-Morales, L., Martínez-Torres, M., Cárdenas, M., Álvarez, C., Carmona, A., Cedillo, B., et al. (2018). An increase in estradiol facilitates the onset of paternal behavior in the dwarf hamster (Phodopus campbelli). Horm. Behav. 99, 35–40. doi: 10.1016/j.yhbeh.2018.02.002
Rosenblatt, J. S., and Ceus, K. (1998). Estrogen implants in the medial preoptic area stimulate maternal behavior in male rats. Horm. Behav. 33, 23–30. doi: 10.1006/hbeh.1997.1430
Rosenblatt, J. S., and Siegel, H. I. (1981). “Factors governing the onset and maintenance of maternal behavior among nonprimate mammals” in Parental Care in Mammals. eds. D. J. Gubernick and P. H. Klopfer (Boston, MA: Springer), 13–76.
Rosenblatt, J. S., Wagner, C. K., and Morrell, J. I. (1994). Hormonal priming and triggering of maternal behavior in the rat with special reference to the relations between estrogen receptor binding and ER mRNA in specific brain regions. Psychoneuroendocrinology 19, 543–552. doi: 10.1016/0306-4530(94)90039-6
Schleicher, G., Stumpf, W. E., Morin, J. K., and Drews, U. (1986). Sites of aromatization of [3H]testosterone in forebrain of male, female and androgen receptor-deficient Tfm mice: an autoradiographic study. Brain Res. 397, 290–296. doi: 10.1016/0006-8993(86)90630-X
Shabalova, A. A., Liang, M., Zhong, J., Huang, Z., Tsuji, C., Shnayder, N. A., et al. (2020). Oxytocin and CD38 in the paraventricular nucleus play a critical role in paternal aggression in mice. Horm. Behav. 120:104695. doi: 10.1016/j.yhbeh.2020.104695
Sheehan, T., Paul, M., Amaral, E., Numan, M. J., and Numan, M. (2001). Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience 106, 341–356. doi: 10.1016/S0306-4522(01)00286-X
Sheng, H. Z., Fields, R. D., and Nelson, P. G. (1993). Specific regulation of immediate early genes by patterned neuronal activity. J. Neurosci. Res. 35, 459–467. doi: 10.1002/jnr.490350502
Sheng, M., McFadden, G., and Greenberg, M. E. (1990). Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 4, 571–582. doi: 10.1016/0896-6273(90)90115-V
Shinohara, Y., Hirase, H., Watanabe, M., Itakura, M., Takahashi, M., and Shigemoto, R. (2008). Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc. Natl. Acad. Sci. USA 105, 19498–19503. doi: 10.1073/pnas.0807461105
Shinohara, Y., Hosoya, A., Yamasaki, N., Ahmed, H., Hattori, S., Eguchi, M., et al. (2012). Right-hemispheric dominance of spatial memory in split-brain mice. Hippocampus 22, 117–121. doi: 10.1002/hipo.20886
Shipley, B. (2000). Cause and correlation in biology: A User’s guide to path analysis, structural equations, and causal inference in R. Cambridge, UK: Cambridge University Press.
Shipton, O. A., el-Gaby, M., Apergis-Schoute, J., Deisseroth, K., Bannerman, D. M., Paulsen, O., et al. (2014). Left–right dissociation of hippocampal memory processes in mice. Proc. Natl. Acad. Sci. USA 111, 15238–15243. doi: 10.1073/pnas.1405648111
Siegel, H. I., and Rosenblatt, J. S. (1975). Estrogen-induced maternal behavior in hysterectomized-ovariectomized virgin rats. Physiol. Behav. 14, 465–471. doi: 10.1016/0031-9384(75)90012-8
Slotnick, B. M., and Nigrosh, B. J. (1975). Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J. Comp. Physiol. Psychol. 88, 118–127. doi: 10.1037/h0076200
Stanić, D., Dubois, S., Chua, H. K., Tonge, B., Rinehart, N., Horne, M. K., et al. (2014). Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One 9:e90451. doi: 10.1371/journal.pone.0090451
Stolzenberg, D. S., and Rissman, E. F. (2011). Oestrogen-independent, experience-induced maternal behaviour in female mice. J. Neuroendocrinol. 23, 345–354. doi: 10.1111/j.1365-2826.2011.02112.x
Tachikawa, K. S., Yoshihara, Y., and Kuroda, K. O. (2013). Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J. Neurosci. 33, 5120–5126. doi: 10.1523/JNEUROSCI.2364-12.2013
Torres, T., Adam, N., Mhaouty-Kodja, S., and Naulé, L. (2024). Reproductive function and behaviors: an update on the role of neural estrogen receptors alpha and beta. Front. Endocrinol. 15:1408677. doi: 10.3389/fendo.2024.1408677
Trainor, B. C., Bird, I. M., Alday, N. A., Schlinger, B. A., and Marler, C. A. (2003). Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology 78, 36–44. doi: 10.1159/000071704
Trainor, B. C., Finy, M. S., and Nelson, R. J. (2008). Paternal aggression in a biparental mouse: parallels with maternal aggression. Horm. Behav. 53, 200–207. doi: 10.1016/j.yhbeh.2007.09.017
Trainor, B. C., and Marler, C. A. (2002). Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. R. Soc. Lond. B 269, 823–829. doi: 10.1098/rspb.2001.1954
Unger, E. K., Burke, K. J., Yang, C. F., Bender, K. J., Fuller, P. M., and Shah, N. M. (2015). Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10, 453–462. doi: 10.1016/j.celrep.2014.12.040
van Buuren, S., and Groothuis-Oudshoorn, K. (2011). Mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67. doi: 10.18637/jss.v045.i03
Vehtari, A., Gelman, A., Simpson, D., Carpenter, B., and Bürkner, P. C. (2021). Rank-normalization, folding, and localization: an improved Rˆ for assessing convergence of MCMC (with discussion). Bayesian Anal. 16, 667–718. doi: 10.1214/20-BA1221
Woodroffe, R., and Vincent, A. (1994). Mother’s little helpers: patterns of male care in mammals. Trends Ecol. Evol. 9, 294–297. doi: 10.1016/0169-5347(94)90033-7
Wozniak, A., Hutchison, R. E., and Hutchison, J. B. (1992). Localisation of aromatase activity in androgen target areas of the mouse brain. Neurosci. Lett. 146, 191–194. doi: 10.1016/0304-3940(92)90075-I
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M., and Dulac, C. G. (2014). Galanin neurons in the medial preoptic area govern parental behavior. Nature 509, 325–330. doi: 10.1038/nature13307
Xiao, L., and Jordan, C. L. (2002). Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat Hippocampus. Horm. Behav. 42, 327–336. doi: 10.1006/hbeh.2002.1822
Keywords: brain plasticity, estrogen, hemisphere lateralization, infanticide, limbic system, paternal care
Citation: Duarte-Guterman P, Skandalis DA, Merkl A, Geissler DB and Ehret G (2025) Brain aromatase and its relationship with parental experience and behavior in male mice. Front. Neurosci. 19:1502764. doi: 10.3389/fnins.2025.1502764
Received: 27 September 2024; Accepted: 27 January 2025;
Published: 17 February 2025.
Edited by:
Gustavo M. Somoza, Instituto Tecnológico de Chascomús (CONICET-UNSAM), ArgentinaReviewed by:
María José Sánchez-Catalán, University of Jaume I, SpainCopyright © 2025 Duarte-Guterman, Skandalis, Merkl, Geissler and Ehret. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula Duarte-Guterman, cGR1YXJ0ZWdAYnJvY2t1LmNh; Günter Ehret, Z3VlbnRlci5laHJldEB1bmktdWxtLmRl
†These authors have contributed equally to this work
‡Present addresses: Paula Duarte-Guterman, Department of Psychology and Centre for Neuroscience, Brock University, St. Catharines, ON, Canada
Dimitri A. Skandalis, Department of Psychology and Centre for Neuroscience, Brock University, St. Catharines, ON, Canada; Psychological and Brain Sciences and Johns Hopkins Kavli Neuroscience Discovery Institute, Johns Hopkins University, Baltimore, MD, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.