- 1Neurology Medical Center II, Foresea Life Insurance Guangzhou General Hospital, Guangzhou, China
- 2Department of Medical Imaging, Foresea Life Insurance Guangzhou General Hospital, Guangzhou, China
- 3Center of Surgical Anesthesia, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 4Central Operating Room, Foresea Life Insurance Guangzhou General Hospital, Guangzhou, China
- 5Department of Cell Biology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China
Non-invasive vagus nerve stimulation (VNS) represents a transformative approach for managing a broad spectrum of inflammatory and autoimmune conditions, including rheumatoid arthritis and inflammatory bowel disease. This comprehensive review delineates the mechanisms underlying VNS, emphasizing the cholinergic anti-inflammatory pathway, and explores interactions within the neuro-immune and vagus-gut axes based on both clinical outcomes and pre-clinical models. Clinical applications have confirmed the efficacy of VNS in managing specific autoimmune diseases, such as rheumatoid arthritis, and chronic inflammatory conditions like inflammatory bowel disease, showcasing the variability in stimulation parameters and patient responses. Concurrently, pre-clinical studies have provided insights into the potential of VNS in modulating cardiovascular and broader inflammatory responses, paving the way for its translational application in clinical settings. Innovations in non-invasive VNS technology and precision neuromodulation are enhancing its therapeutic potential, making it a viable option for patients who are unresponsive to conventional treatments. Nonetheless, the widespread adoption of this promising therapy is impeded by regulatory challenges, patient compliance issues, and the need for extensive studies on long-term efficacy and safety. Future research directions will focus on refining VNS technology, optimizing treatment parameters, and exploring synergistic effects with other therapeutic modalities, which could revolutionize the management of chronic inflammatory and autoimmune disorders.
1 Introduction
Inflammation is a vital biological response that protects the body from harmful stimuli, such as pathogens, damaged cells, and irritants (Netea et al., 2017). This complex process, marked by the coordinated activation of immune and non-immune cells, is essential for pathogen clearance and tissue repair. However, inflammation can also be a double-edged sword. Acute inflammation is a rapid, self-limiting response critical for immediate defense and healing (Hannoodee and Nasuruddin, 2020). In contrast, chronic inflammation is a prolonged, dysregulated process that can persist for months or even years, often without an overt pathogen. Chronic inflammation is a key driver of numerous diseases, including autoimmune disorders, cardiovascular diseases, metabolic syndrome, neurodegenerative conditions, and various cancers (Furman et al., 2019; Christ et al., 2019; Dugan et al., 2023). Collectively, these inflammation-related conditions pose a significant global health burden, accounting for more than half of all deaths worldwide (Yu et al., 2024).
Despite the availability of various anti-inflammatory therapies, managing chronic inflammation effectively remains a formidable challenge (Blagov et al., 2024; Feehan and Gilroy, 2019). Current drug therapy, especially non-steroidal anti-inflammatory drugs (NSAIDs), can cause serious gastrointestinal complications such as peptic ulcers and gastritis, especially with long-term use. In addition to impairing organ function, they also increase the risk of cardiovascular disease, including heart attack and stroke. Biologic therapies, while targeted and effective against specific inflammatory pathways, also carry the risk of immunosuppression. This can make patients more susceptible to infections and, in some cases, lead to the development of malignancies. In addition, biologics may induce autoantibodies and, in rare cases, autoimmune diseases. The use of certain anti-inflammatory drugs also carries the risk of hepatotoxicity and renal dysfunction, and therefore requires regular monitoring and caution when used in patients with pre-existing liver or kidney disease. The urgent need for novel, targeted approaches that can modulate the immune response with greater precision and safety. In this context, VNS has emerged as a promising therapeutic strategy by leveraging the body's intrinsic neuroimmune pathways to control inflammation (Browning et al., 2017; Chen et al., 2023). Originally developed for neurological conditions such as epilepsy and depression, VNS has been repurposed to harness its anti-inflammatory potential (Bazoukis et al., 2023). By engaging multiple mechanisms—including the cholinergic anti-inflammatory pathway (Song et al., 2024; Bonaz et al., 2016b), the vagus-adrenal axis, and the vagus-gut axis—VNS offers a level of precision in modulating systemic inflammation that traditional therapies cannot achieve.
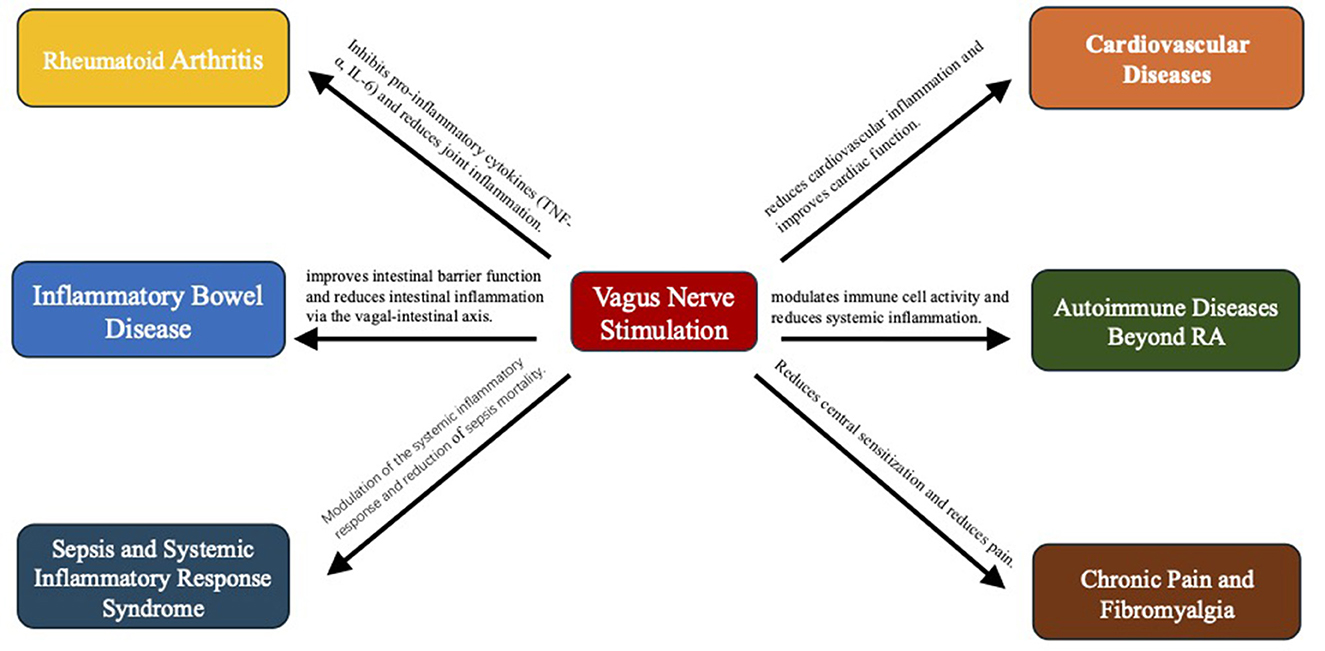
The clinical applications of VNS are rapidly expanding beyond its established role in treating rheumatoid arthritis and inflammatory bowel disease (Hays et al., 2023). Emerging evidence supports its efficacy across a wide range of inflammatory conditions, including sepsis, cardiovascular diseases, autoimmune disorders, and chronic pain syndromes (Zafeiropoulos et al., 2024; Dawson et al., 2021; Tao et al., 2020; Shao et al., 2023; Courties et al., 2021; Ridengnaxi and Wang, 2024). Innovations in VNS technology, such as non-invasive devices and personalized stimulation protocols, are further enhancing its therapeutic potential and accessibility. This review presents a comprehensive examination of the mechanistic pathways engaged by VNS, its evolving clinical applications from traditional to emerging inflammatory conditions, and the latest technological innovations that enhance its therapeutic potential. By integrating clinical outcomes with mechanistic insights from pre-clinical models, our review offers a unique synthesis aimed at advancing the integration of VNS into mainstream anti-inflammatory therapy.
2 Mechanisms of vagus nerve stimulation in anti-inflammatory therapy
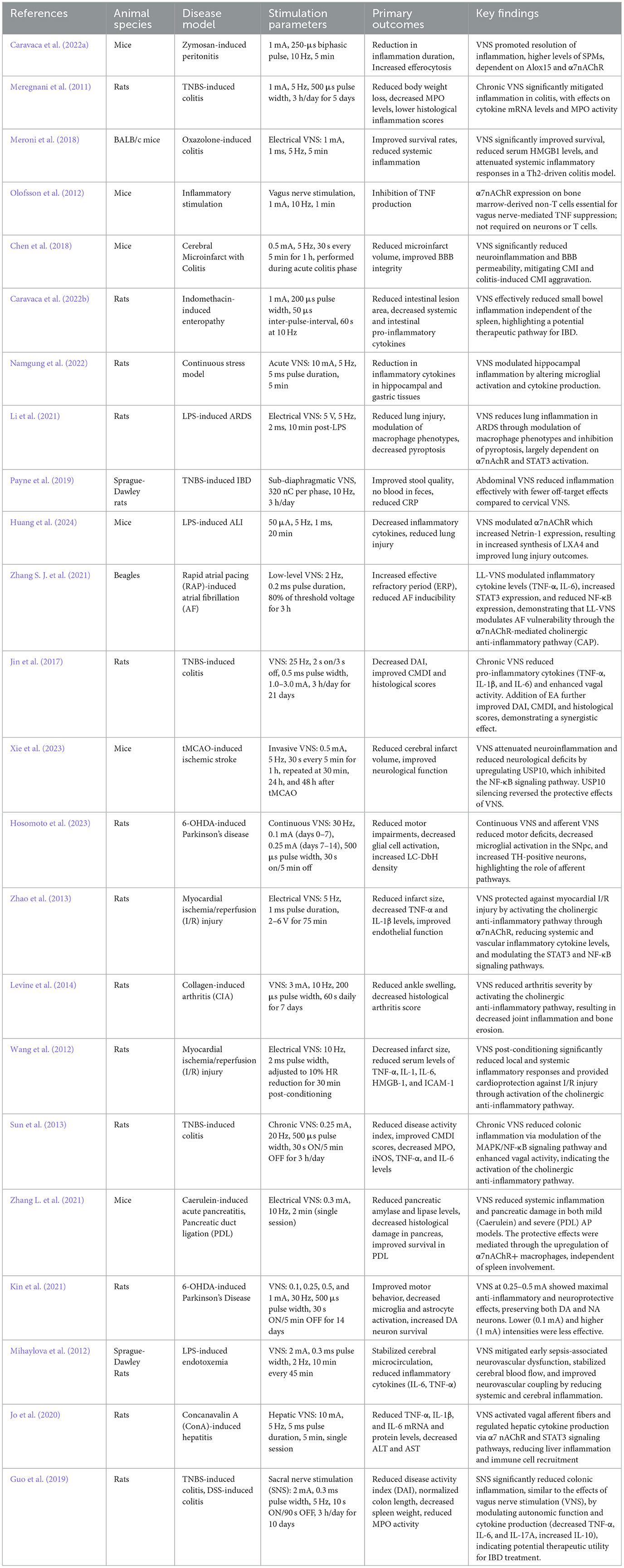
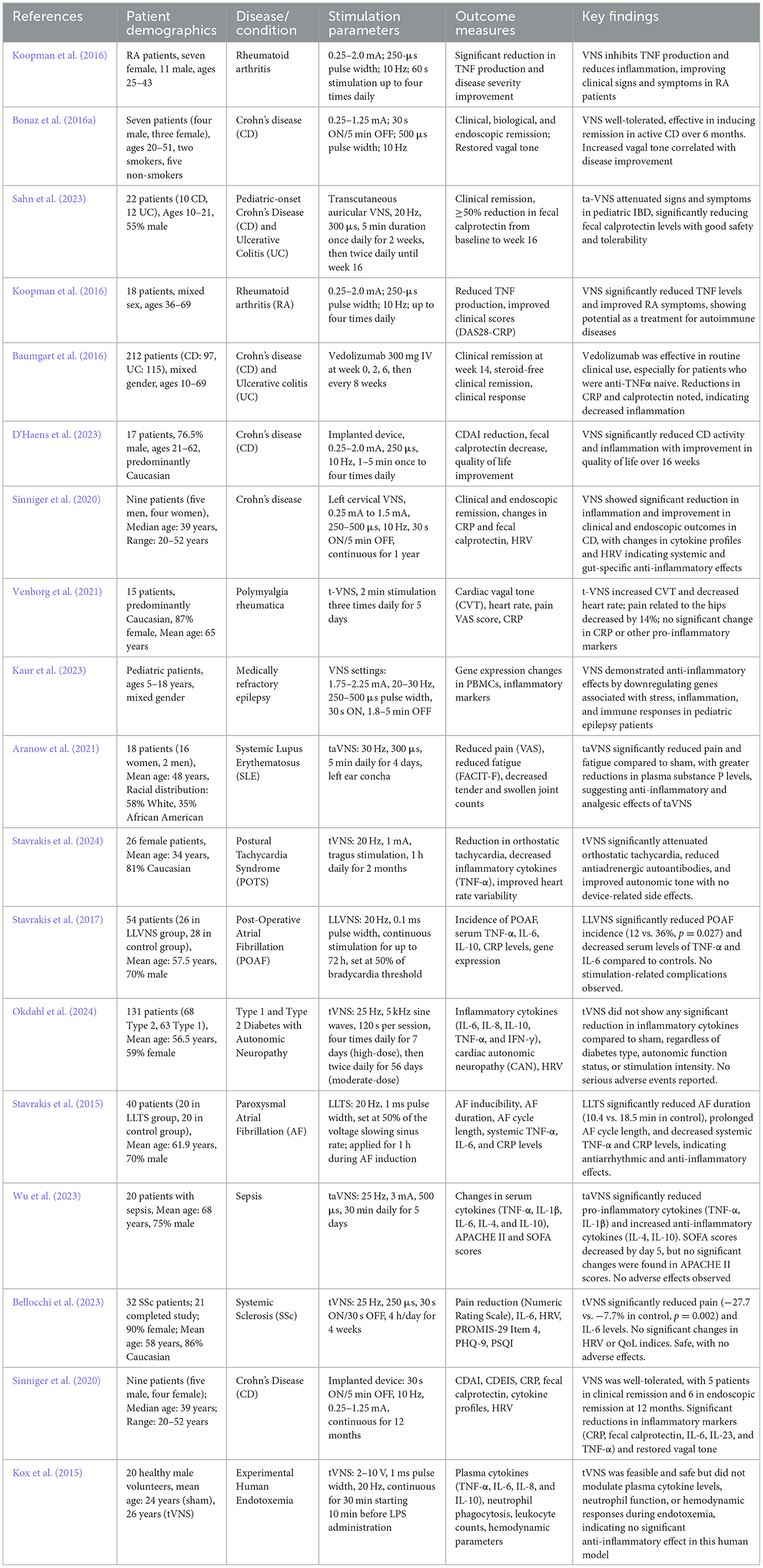
Recent advancements have expanded the clinical applications of vagus nerve stimulation (VNS) in treating inflammatory diseases. Table 1 summarizes key pre-clinical studies demonstrating the anti-inflammatory effects of VNS in various animal models. These foundational studies have paved the way for exploring VNS in clinical settings. Additionally, the clinical efficacy of VNS across different inflammatory diseases is outlined in Table 2, highlighting its therapeutic potential in conditions such as rheumatoid arthritis, inflammatory bowel disease, and others.
2.1 Cholinergic anti-inflammatory pathway and neuro-immune reflexes
2.1.1 Cholinergic anti-inflammatory pathway
The cholinergic anti-inflammatory pathway (CAP) is central to the mechanism by which VNS modulates inflammation, primarily through acetylcholine (ACh) interactions with α7 nicotinic acetylcholine receptors (α7nAChR) on macrophages. This targeted interaction inhibits the release of pro-inflammatory cytokines such as Tumor necrosis factor alpha (TNF-α), Interleukin-1beta (IL-1β), and High Mobility Group Box 1 (HMGB1), effectively curtailing the inflammatory response, thus offering a more focused approach than systemic immunosuppressive therapies, which often result in broader immune suppression (Han et al., 2017; Kelly et al., 2022; Noviello et al., 2021). Introduced by Tracey in 2002, CAP has been extensively validated, showing its efficacy in reducing neuroinflammation and its beneficial impact on chronic conditions like autoimmune diseases and metabolic syndrome, with fewer associated side effects such as reduced risk of systemic infections, malignancy development, and organ toxicity typically seen with broader immunosuppressive treatments (Tracey, 2002; Qin et al., 2020; Lv et al., 2021). Additionally, CAP's interaction with biological pathways including those involving the gut microbiota and endocrine systems highlights its extensive regulatory influence on metabolic balance and energy homeostasis (Hilderman and Bruchfeld, 2020).
Moreover, while the direct modulation of inflammation via vagal nerve stimulation by CAP is well-established, recent research highlights a more complex mechanism involving the peripheral nervous system and immune cells. Specifically, the inflammatory reflex mediated by the vagus nerve critically regulates cytokine production, notably TNF-α, through acetylcholine signaling in spleen macrophages (Tracey, 2002). However, this pathway is not exclusively cholinergic; it also involves the activation of the sympathetic nervous system and β2-adrenergic receptors. VNS has been shown to activate splenic sympathetic nerve fibers, leading to the release of norepinephrine, which interacts with β2-adrenergic receptors on acetylcholine-producing T cells, thereby promoting their release of acetylcholine (Rosas-Ballina et al., 2011; Inoue et al., 2019). This circuitry is bolstered by the discovery of acetylcholine-producing, memory phenotype T cells in the spleen, which are pivotal due to the absence of enzymatic machinery for acetylcholine synthesis in spleen nerve fibers. Thus, the interplay between the parasympathetic and sympathetic nervous systems is crucial for modulating immune function via the cholinergic anti-inflammatory pathway. These T cells, activated by vagus nerve-induced action potentials, secrete acetylcholine to effectively control innate immune responses. This demonstrates a dynamic interplay between the nervous and immune systems, furthering our understanding of CAP's role in anti-inflammatory therapy. The advanced insights provided by these studies illustrate how neurotransmitters like acetylcholine are critical mediators between neural and immune regulation, expanding the potential therapeutic applications of CAP in reducing inflammatory diseases without the broader side effects associated with systemic immunosuppressive therapies.
2.1.2 Reflexive anti-inflammatory mechanisms
The Neuro-Immune Reflex represents a critical communication pathway between the nervous and immune systems, mediated through both afferent (sensory) and efferent (motor) branches. Central to the rapid modulation of inflammation through VNS are reflexive anti-inflammatory pathways (Pavlov and Tracey, 2012). The inflammatory reflex, initiated by afferent vagal signals from peripheral inflammation, is processed by the nucleus tractus solitarius (NTS) in the brainstem (Liu et al., 2024). The NTS then orchestrates efferent vagal outputs that inhibit pro-inflammatory cytokines, particularly TNF-α, via the cholinergic anti-inflammatory pathway (CAP). The combination of the afferent and efferent arms of this vagal-immune interaction is termed the “inflammatory reflex” (Kelly et al., 2022), this reflex enables quick and localized suppression of inflammation, essential in acute conditions like sepsis.VNS significantly reduces TNF levels in murine endotoxemia, an effect lost in animals with a severed vagus nerve (Borovikova et al., 2000). Additionally, VNS affects cardiovascular reflexes (Pavlov, 2021), such as the baroreflex, which regulates heart rate and vascular tone, thus indirectly moderating systemic inflammation. The interaction between these reflexive mechanisms enhances VNS's effectiveness in addressing both inflammatory and cardiovascular disturbances, providing a multifaceted approach to managing acute inflammatory responses.
2.1.3 Spleen-mediated immune modulation
Research indicates that the efferent arm of the inflammatory reflex is likely the sympathetic nervous system, not the CAP, as action potentials are transmitted through the sympathetic chain and splanchnic nerves to the splenic nerve, inhibiting cytokine release (Martelli et al., 2014). Although the spleen is not directly innervated by the vagus nerve, it is integral to its anti-inflammatory impact, with mediation occurring through splanchnic nerves and T cells (Rosas-Ballina et al., 2011, 2008). Recognized increasingly for its role in inflammation modulation, the spleen is stimulated by VNS via the splenic nerve. This stimulation leads to the release of acetylcholine, which binds to α7 nicotinic acetylcholine receptors (α7nAChRs) on splenic macrophages. This binding significantly curtails the release of pro-inflammatory cytokines like TNF-α, thereby diminishing systemic inflammation (Falvey et al., 2022; Wang et al., 2021). This pathway is particularly vital in chronic inflammatory diseases where persistent cytokine production drives disease activity. VNS offers a novel, non-invasive approach to managing conditions such as rheumatoid arthritis and lupus, providing a safer, more precise alternative to traditional immunosuppressive therapies and underscoring its potential for targeted immune modulation through the spleen.
2.1.4 Inflammatory reflex and baroreflex
The inflammatory reflex serves as a critical neuroimmune circuit, converting inflammation detection into reflexive inhibition of pro-inflammatory cytokine production, predominantly via vagal efferents through the cholinergic anti-inflammatory pathway (CAP; Pavlov and Tracey, 2012). This mechanism not only preserves immune homeostasis but also mitigates the risk of tissue damage from excessive inflammation (Caravaca et al., 2022a). Complementarily, the baroreflex, primarily recognized for cardiovascular regulation, adjusts heart rate and blood pressure in response to physiological stressors, thus influencing systemic inflammation. Recent studies underscore the therapeutic potential of these reflexes' synergy. Zhang et al. demonstrated that chronic VNS significantly improves autonomic control and attenuates heart failure development in a canine model, reflected by enhanced baroreflex sensitivity and reduced plasma levels of norepinephrine and angiotensin II, which are key mediators of cardiovascular and systemic inflammatory responses (Zhang et al., 2009). Simultaneous activation through VNS could enhance the management of inflammatory diseases with cardiovascular implications, such as atherosclerosis and myocardial infarction, broadening VNS's clinical applications. This integration of VNS in treating complex diseases showcases its dual therapeutic potential to modulate both inflammatory and cardiovascular dysfunctions.
2.2 Other neuro-immune mechanisms
2.2.1 Vagus-adrenal axis
The vagus-adrenal axis is a crucial pathway through which VNS modulates systemic inflammation. Stimulation of the vagus nerve activates the hypothalamic-pituitary-adrenal (HPA) axis via vagal afferent fibers (Bonaz et al., 2019), leading to the release of corticotropin-releasing factor (CRF) from the hypothalamus. CRF then triggers the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland, which in turn stimulates the adrenal glands to produce cortisol and adrenaline. Cortisol downregulates pro-inflammatory pathways, such as nuclear factor κB NF-κB, and enhances anti-inflammatory proteins like annexin A1, while adrenaline further reduces inflammation by promoting immune cell resolution through beta-adrenergic receptors (Bonaz et al., 2017a). These hormonal responses collectively mitigate inflammation (Thrivikraman et al., 2013), positioning the vagus-adrenal axis as a key mechanism by which VNS exerts its therapeutic effects, particularly in hyperinflammatory conditions such as sepsis and systemic inflammatory response syndrome (SIRS).
2.2.2 Neuropeptide-mediated pathways
Neuropeptides such as calcitonin gene-related peptide (CGRP) and vasoactive intestinal peptide (VIP) play pivotal roles in the anti-inflammatory effects of VNS. CGRP, secreted by sensory neurons, reduces pro-inflammatory cytokines and promotes vasodilation, aiding inflammation resolution (Fattori et al., 2021; Pavlov and Tracey, 2017). Conversely, VIP, by binding to immune cell receptors, diminishes the release of pro-inflammatory cytokines while boosting anti-inflammatory agents like IL-10 (Crosson and Talbot, 2023; Ganea et al., 2015). These interactions underscore the complexity and broad therapeutic potential of VNS in managing various inflammatory conditions.
2.3 VNS and metabolic regulation
2.3.1 Vagus-Gut Axis: gateway to metabolic modulation
The Vagus-Gut Axis plays a pivotal role in metabolic regulation through VNS, influencing both gut motility and the microbial landscape. VNS enhances gastrointestinal motility and secretion, thereby improving digestion and nutrient absorption, which are crucial for maintaining metabolic homeostasis (Bonaz, 2022). This enhanced motility also facilitates a more favorable gut environment, potentially reducing the proliferation of pathogenic bacteria and supporting beneficial microbiota that contribute to anti-inflammatory states (Matteoli and Boeckxstaens, 2013). Furthermore, VNS upregulates the release of neurotransmitters like acetylcholine, which binds to α7 nicotinic acetylcholine receptors on intestinal epithelial cells, fortifying the gut barrier and mitigating the translocation of endotoxins into the systemic circulation (Bonaz et al., 2021). This barrier enhancement is vital for preventing systemic inflammation, a known contributor to metabolic syndromes such as diabetes and obesity. By modulating these neural and microbial pathways, VNS through the Vagus-Gut Axis presents a promising therapeutic avenue for treating metabolic diseases by leveraging the interconnected roles of neural signals and gut microbiota, thus providing a multi-faceted approach to disease management that extends beyond traditional pharmacotherapy.
2.3.2 Vagus-Liver Axis: influencing glucose and lipid metabolism
The Vagus-Liver Axis plays a crucial role in the metabolic modulation exerted by VNS, specifically targeting key aspects of glucose and lipid metabolism that are central to metabolic syndrome pathogenesis. By activating the vagus nerve, VNS enhances glucose homeostasis through precise regulation of liver enzymes involved in gluconeogenesis and glycogenolysis, such as inhibiting phosphoenolpyruvate carboxykinase (PEPCK)—the rate-limiting enzyme in gluconeogenesis. This process, mediated through acetylcholine signaling via muscarinic receptors on hepatocytes, not only improves insulin sensitivity but also reduces hepatic glucose production, thereby offering a potent counter to hyperglycemia (Kawashima and Fujii, 2003; Pavlov and Tracey, 2012). Additionally, VNS influences lipid metabolism, potentially altering the expression of genes like SREBP-1c, a key regulator of fatty acid synthesis, thus affecting lipid transport and breakdown pathways. This neuromodulatory influence, while still under investigation for its direct mechanisms, suggests how VNS might mitigate dyslipidemia and lower cardiovascular disease risks associated with metabolic disturbances (Paton et al., 2013). Unlike traditional pharmacotherapies, which are often marred by side effects including gastrointestinal disturbances and cardiovascular risks, VNS provides a non-pharmacological, safer, and long-term therapeutic strategy for managing metabolic conditions like type 2 diabetes and hyperlipidemia, exemplifying a novel approach that leverages neural modulation to achieve metabolic balance with minimal adverse effects.
2.3.3 VNS and mitochondrial function
VNS significantly influences mitochondrial function, a key factor in controlling inflammation and optimizing energy production (Nesci et al., 2023). VNS reduces reactive oxygen species (ROS) and stabilizes mitochondrial membrane potential, thereby mitigating oxidative stress, a primary contributor to chronic inflammation and cellular damage (Zhang et al., 2024). It also regulates mitochondrial dynamics by balancing fusion and fission processes, modulating proteins such as Drp1 and Mfn1/2, especially in myocardial ischemia models (Prathumsap et al., 2022). This regulation is supported by the activation of the Nrf2/HO-1 signaling pathway, which strengthens cellular antioxidant defenses and protects mitochondria from oxidative harm (Zhang et al., 2024). Furthermore, VNS activates the AMPK signaling pathway, specifically through the M3R/CaMKKβ/AMPK axis (Xue et al., 2017), enhancing mitochondrial biogenesis and energy metabolism. This metabolic boost is complemented by increased glucose uptake and ATP production via upregulation of Glut4 and CPT1α (Luo et al., 2020), underscoring VNS's capacity to enhance mitochondrial energy efficiency. These mechanisms collectively position VNS as a potential therapeutic approach for conditions characterized by mitochondrial dysfunction and chronic inflammation.
2.3.4 Hormonal regulation through vagus nerve stimulation
VNS profoundly influences hormonal regulation, which plays a pivotal role in metabolic homeostasis and energy balance. By activating the vagus nerve, VNS can modulate the secretion of critical metabolic hormones, including insulin and glucagon, which are essential for glucose regulation. This modulation helps maintain glucose homeostasis, potentially benefiting conditions like diabetes by improving insulin sensitivity and reducing glycemic variability (Sorski and Gidron, 2023; Das, 2011). Moreover, VNS has been shown to affect the levels of ghrelin and leptin, hormones that regulate hunger and satiety. This regulation can help address obesity and its related metabolic complications by altering eating behaviors and energy expenditure (Obst et al., 2020). Additionally, VNS impacts the HPA axis, which plays a critical role in stress response and metabolic processes. By modulating this axis, VNS can mitigate the adverse effects of chronic stress, further contributing to metabolic regulation (Das, 2011). The hormonal adjustments induced by VNS illustrate its potential as a comprehensive treatment modality that extends beyond traditional pharmacological therapies, offering a holistic approach to managing metabolic and inflammatory diseases.
2.3.5 Integration of neuro-metabolic pathways in VNS
VNS uniquely integrates neuro-metabolic pathways, expanding its therapeutic applications beyond traditional neuromodulation by merging anti-inflammatory responses with metabolic regulation. It adjusts neurotransmitter and hormone secretion, influencing functions like insulin release, glucose homeostasis, lipid metabolism, and energy expenditure. Notably, VNS activates the cholinergic anti-inflammatory pathway, reducing pro-inflammatory cytokines and enhancing insulin sensitivity and glucose uptake. It also affects the hypothalamic-pituitary-adrenal axis and gut hormones such as ghrelin and leptin, linking neural activity with metabolic outcomes and impacting appetite and energy regulation. This comprehensive modulation offers a novel therapeutic approach for conditions like type 2 diabetes, obesity, and metabolic syndrome. While VNS can cause side effects such as voice alteration, throat discomfort, and coughing, it offers advantages over pharmacotherapies by potentially reducing systemic side effects like hypoglycemia, gastrointestinal issues, and weight gain, and by targeting multiple pathways simultaneously. Future research should delineate the specific neural circuits and mechanisms to optimize VNS protocols, potentially revolutionizing treatment strategies for metabolic and inflammatory disorders.
2.4 Emerging mechanisms and interactions
2.4.1 Microbiota-vagus interactions
The interaction between the gut microbiota and the vagus nerve is a dynamic area of research with substantial implications for the anti-inflammatory effects of VNS (Siopi et al., 2023; Bonaz et al., 2018). The gut microbiota maintains immune homeostasis, and its imbalance is associated with chronic inflammation in conditions like IBD and metabolic syndrome. VNS modifies the gut microbiota, promoting beneficial bacteria and reducing harmful ones (Han et al., 2022). This microbiota modulation by VNS introduces a novel therapeutic approach to reestablish immune equilibrium and decrease systemic inflammation, demonstrating the potential for microbiota-targeted VNS interventions in complex inflammatory disorders.
2.4.2 Crosstalk with central nervous system
The central nervous system (CNS) plays a dual role as both regulator and target of inflammation, with VNS exerting profound effects on neuroinflammatory pathways. VNS modulates neurotransmitter release and cytokine production, thereby reducing neuroinflammation and shielding neurons in conditions like multiple sclerosis, Parkinson's disease, and Alzheimer's disease. The interplay between VNS and the CNS illuminates its therapeutic potential for neuroinflammatory and neurodegenerative diseases. As insights into these interactions expand, VNS is poised to become integral in developing new management strategies for a broad spectrum of inflammatory conditions involving CNS dysfunction. A comprehensive overview of the mechanistic pathways by which vagus nerve stimulation exerts its anti-inflammatory effects is illustrated in Figure 1.
3 Expanded clinical applications of vagus nerve stimulation
3.1 Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by persistent synovial inflammation and progressive joint destruction, with a hallmark of immune dysregulation that leads to excessive production of pro-inflammatory cytokines, driving ongoing tissue damage (Smolen et al., 2016). Despite the relief provided by disease-modifying antirheumatic drugs (DMARDs) and biologics for many patients, these treatments often entail severe side effects and do not fully control the disease in all patients (Araújo et al., 2016). In this context, non-invasive VNS (nVNS) has emerged as an innovative adjunct therapy, showing promise in effectively modulating immune responses. Clinical studies report significant reductions in RA symptoms and inflammatory biomarkers, such as C-reactive protein (CRP), with nVNS, alongside decreases in joint pain and swelling and improvements in patient-reported quality of life, which underscores its capacity to enhance daily functioning (Marsal et al., 2020a, 2021; Mondal et al., 2021).
Some patients even maintain improved symptoms post-treatment, suggesting nVNS's potential for inducing lasting anti-inflammatory effects. Notably, nVNS reduces key pro-inflammatory cytokines like TNF-α and interleukin-6 (IL-6), which corresponds with overall health improvements (Drewes et al., 2021). Additionally, nVNS complements traditional RA therapies by enhancing their effectiveness (Marsal et al., 2020b), reducing necessary dosages, and minimizing adverse effects, establishing itself as a safe, effective, and viable alternative to conventional immunosuppressive therapies and offering a more targeted approach to managing this complex disease.
3.2 Inflammatory bowel disease
Inflammatory bowel disease, which includes Crohn's disease and ulcerative colitis (Hodson, 2016), is marked by a chronic, unregulated immune response within the gastrointestinal tract. The introduction of nVNS marks a significant advancement in treating IBD by targeting the vagal-intestinal axis (Mikami et al., 2022; Breit et al., 2018). This method enhances cholinergic signaling, diminishes mucosal inflammation, bolsters intestinal barrier integrity, and suppresses key pro-inflammatory cytokines like TNF-α and IL-6 (Bonaz, 2024). Clinical research highlights that reductions in fecal calprotectin—a critical inflammation marker—align with better clinical remission rates and enhanced life quality, establishing nVNS as a potent supplement to existing therapies, especially in treatment-resistant cases.
Moreover, nVNS mitigates inflammatory responses, boosts intestinal motility, and lessens reliance on conventional drugs, thus reducing their side effects and associated risks (Cirillo et al., 2022; Yeshi et al., 2020). Recent trials further validate nVNS's role in alleviating common IBD symptoms such as abdominal pain, diarrhea, and intestinal bleeding, supported by a robust safety profile with minimal adverse effects. Significant reductions in inflammatory biomarkers continue to substantiate nVNS's anti-inflammatory properties (D'Haens et al., 2023; Bonaz et al., 2017b). However, larger randomized controlled trials are necessary to definitively ascertain nVNS's long-term efficacy and safety. Research is also exploring how to optimize stimulation settings like frequency and intensity to tailor treatment protocols (Bonaz and Sinniger, 2023). In summary, nVNS is emerging as an essential tool in IBD management, offering a novel, effective option for patients unresponsive to standard treatments and potentially reshaping the landscape of translational medicine as investigations advance.
3.3 Sepsis and systemic inflammatory response syndrome
Sepsis and Systemic Inflammatory Response Syndrome (SIRS) are critical conditions characterized by an overwhelming immune response, leading to widespread inflammation, organ dysfunction, and high mortality (Chakraborty and Burns, 2024; Bosmann and Ward, 2013). At the onset of SIRS, immune cells like macrophages and lymphocytes release numerous inflammatory mediators, such as TNF-α and interleukins, initiating both local and systemic responses. This hyperinflammatory state often results in microcirculatory disorders, damaging microvasculature, decreasing blood flow, and causing tissue hypoxia that exacerbates organ dysfunction (Jentzer et al., 2020). Additionally, SIRS can disrupt immune regulation, lowering resistance to infections and elevating the risk of secondary complications.
Despite ongoing advancements in sepsis management, truly effective treatment options are still scarce. In this landscape, nVNS has shown promise in preclinical and early clinical studies as a method to control systemic inflammation and enhance survival outcomes (Komegae et al., 2018). nVNS works by mitigating the cytokine storm associated with septic shock, reducing levels of inflammatory mediators such as TNF-α, IL-1β, and HMGB1. Preliminary trials indicate that nVNS may also stabilize hemodynamic parameters and lessen the need for vasopressors (Van Beekum et al., 2021), underscoring its potential as a supportive therapy in critical care settings. This evolving evidence positions nVNS as a groundbreaking tool in managing these complex, life-threatening conditions.
3.4 Cardiovascular diseases
Chronic inflammation is central to the development of cardiovascular diseases such as atherosclerosis, myocardial infarction, and heart failure (Fearon and Fearon, 2008). Inflammatory processes initiate the accumulation of immune cells within arterial walls, causing endothelial damage and arterial thickening, which promote atherosclerosis and increase cardiovascular risk (Sohrab et al., 2023; Distler et al., 2021). Post-myocardial infarction, inflammation intensifies cardiac remodeling and contributes to heart failure progression. VNS, especially in its nVNS, offers a potential therapeutic approach by activating the cholinergic anti-inflammatory pathway, reducing key inflammatory markers such as C-reactive protein (CRP) and IL-6.
Early animal studies underscore the benefits of chronic VNS in enhancing long-term survival, preventing cardiac pump failure, and mitigating remodeling (Alfaddagh et al., 2020). Recent focus has shifted to low-level VNS (LL-VNS) as a non-invasive technique to improve cardiovascular autonomic function and suppress inflammation (Sorriento and Iaccarino, 2019). Although VNS has shown potential in animal models for managing hypertension and enhancing cardiovascular outcomes, clinical trials like NECTAR-HF and INOVATE-HF have produced mixed results, highlighting the necessity for better patient selection and protocol optimization (Cucu, 2022; Anzai, 2018). As such, continuing research is vital to fully realize the therapeutic promise of VNS in cardiovascular disease management, confirming its efficacy and safety in hypertensive patients through detailed, rigorous randomized controlled trials (Gierthmuehlen et al., 2020; Li et al., 2024). This ongoing investigation will be crucial in integrating VNS into broader cardiovascular treatment frameworks.
3.5 Autoimmune diseases beyond RA
The therapeutic reach of nVNS extends beyond rheumatoid arthritis (RA), benefitting other autoimmune diseases such as systemic lupus erythematosus (SLE), multiple sclerosis (MS), and psoriasis (Ramkissoon et al., 2021; Tynan et al., 2022). In SLE, nVNS has demonstrated effectiveness in reducing autoantibody titers and mitigating disease flares by influencing B-cell activity and cytokine profiles, presenting a viable strategy for managing systemic inflammation (Bazoukis et al., 2023). Similarly, early evidence suggests that nVNS may alleviate neuroinflammation and decelerate disease progression in MS, attributes likely linked to its neuroprotective effects and ability to modulate immune responses (Marrosu et al., 2007).
In the case of psoriasis, nVNS has shown potential in clinical trials to lessen the severity of skin lesions by suppressing Th17-related cytokines, pivotal in driving inflammation in this condition (Genovese et al., 2020). These insights are supported by broader VNS mechanisms, including the activation of the cholinergic anti-inflammatory pathway and direct modulation of immune cells like macrophages and lymphocytes (Fang et al., 2023). The diverse applications of nVNS highlight its role as a safer and more targeted alternative to conventional immunosuppressive therapies, offering significant promise for enhancing patient outcomes across a spectrum of inflammatory autoimmune disorders. This breadth of applicability underscores nVNS's potential as a transformative tool in autoimmune disease management.
3.6 Chronic pain and fibromyalgia
Chronic pain and fibromyalgia, characterized by heightened central sensitization and dysregulated inflammatory responses (Bentley et al., 2018), often pose significant challenges to traditional pharmacological treatments, which frequently offer limited success and notable side effects (Martinez and Guimarães, 2024; Cohen et al., 2021). Non-invasive VNS presents a promising alternative, effectively targeting both peripheral and central inflammatory pathways. In fibromyalgia, significant efficacy has been observed with nVNS in reducing pain intensity, enhancing sleep quality, and improving overall wellbeing due to the suppression of pro-inflammatory cytokines and neuropeptides such as substance P and CGRP, along with normalization of autonomic dysfunction (Shao et al., 2023). Clinical studies support these outcomes, indicating substantial reductions in pain levels and improvements in symptoms associated with fibromyalgia, such as fatigue and anxiety (Johnson and Wilson, 2018; Demircioglu et al., 2024).
The therapeutic mechanisms of nVNS extend beyond the alleviation of symptoms, encompassing the reduction of systemic inflammation and the modulation of neurotransmitter release (Silberstein et al., 2020). These processes are of particular significance in the context of fibromyalgia pathology. Furthermore, the treatment maintains a favorable safety profile, with most side effects being mild and temporary, including throat discomfort and minor heart rate changes (Woodbury and Staats, 2024). The efficacy and safety of VNS in managing chronic pain have been confirmed by systematic review and meta-analysis, leading to its FDA approval for certain pain conditions (Toffa et al., 2020; Cai et al., 2024). However, further research is needed to optimize treatment parameters for individual patients and to explore nVNS's long-term effectiveness and safety in fibromyalgia and other chronic pain conditions (Ananda et al., 2023). Additionally, future investigations should consider the benefits of integrating nVNS with other therapeutic modalities, such as pharmacotherapy and psychotherapy, to enhance patient outcomes.
3.7 Asthma and chronic obstructive pulmonary disease
Asthma and chronic obstructive pulmonary disease (COPD) are chronic respiratory conditions marked by persistent airway inflammation and hyperresponsiveness (Abbaszadeh et al., 2022). While traditional treatments such as corticosteroids are effective, they often entail significant long-term side effects (Dey et al., 2022). nVNS has emerged as a promising adjunctive therapy, showing potential to mitigate both local and systemic inflammation (Bowles et al., 2022). In asthma, nVNS has been found to reduce eosinophil counts and lower levels of key cytokines such as IL-4 and IL-5, leading to improved respiratory outcomes (Mulders et al., 2015). Similarly, in COPD, nVNS has proven effective in reducing systemic inflammation, enhancing lung function, and decreasing exacerbation frequency (Hilz, 2022). These benefits are likely mediated by nVNS's ability to modulate the inflammatory response, enhance lung function, and restore autonomic balance.
Furthermore, nVNS holds promise for treating other respiratory diseases, such as acute respiratory distress syndrome (ARDS), where it can help alleviate hypoxemia and pulmonary edema by tempering excessive inflammatory responses (Kaniusas et al., 2020). Advances in selective VNS techniques have enabled more precise modulation of the vagus nerve, improving therapeutic efficacy while minimizing side effects (Mastitskaya et al., 2021), notably in conditions like sleep-disordered breathing (Toffa et al., 2020; Kim et al., 2022). Despite these promising developments, extensive clinical trials are essential to confirm nVNS's long-term effectiveness and safety across various respiratory conditions. Future research should aim to clarify the molecular mechanisms by which nVNS influences respiratory inflammation, conduct rigorous randomized controlled trials, and develop tailored treatment protocols to optimize outcomes for patients with chronic respiratory diseases.
4 Innovations and future directions in non-invasive VNS
4.1 Advances in device technology
The field of non-invasive VNS has experienced significant advancements in device technology, which have enhanced patient adherence and improved therapeutic outcomes. Traditional nVNS devices typically deliver stimulation through skin electrodes (Thompson et al., 2021) and have demonstrated efficacy in managing conditions like epilepsy and depression by reducing seizure frequency and improving mood states (Ryvlin et al., 2021). Recent technological developments have introduced devices equipped with real-time feedback mechanisms that monitor vital physiological markers such as heart rate variability and cytokine levels, allowing for the dynamic adjustment of stimulation parameters to optimize treatment efficacy (Goggins et al., 2022).
Additionally, ear-based VNS (taVNS) devices, which target specific areas within the ear, have been recognized for increasing patient compliance and have shown potential to boost cognitive functions and mood, especially in elderly or cognitively impaired individuals (Owens et al., 2024; Morris et al., 2019). Moreover, neck-based nVNS devices, focusing on cervical region stimulation, have the advantage of directly influencing multiple brain functions, although they may cause discomfort at the stimulation site (Toffa et al., 2020; Yap et al., 2020). The miniaturization and enhanced portability of these devices have broadened their use beyond traditional clinical environments, facilitating continuous or on-demand therapy that can be customized to meet individual patient needs. These technological innovations mark a substantial progression in the application of nVNS, establishing it as a more feasible and appealing option for the long-term management of chronic inflammatory conditions.
4.2 Precision neuromodulation
Precision neuromodulation stands at the cutting edge of neurostimulation technology, steering the progression of non-invasive VNS toward more tailored and efficacious therapies (Owolabi et al., 2023). This advanced approach enhances therapeutic outcomes by specifically targeting distinct portions of the vagus nerve (Bowles et al., 2022), achieving greater efficacy and minimizing side effects compared to traditional whole-nerve stimulation methods. Precision VNS operates by selectively stimulating different fiber bundles within the vagus nerve (Mourdoukoutas et al., 2018), enabling exact control over physiological pathways responsible for functions like heart rate regulation (Wernisch et al., 2024), without impacting unrelated systems such as digestion or immune responses (Ahmed et al., 2022).
Technological innovations have further refined this approach by incorporating real-time feedback mechanisms that adjust stimulation parameters—intensity and frequency—based on real-time physiological data from the patient (Austelle et al., 2022). This adaptation enhances treatment flexibility and effectiveness, allowing for a responsive and dynamic therapeutic process. The integration of various biomarkers, including cytokine levels, heart rate variability, and EEG patterns, into treatment protocols enables clinicians to continuously customize VNS therapy to meet the specific, changing needs of each patient (Sauer et al., 2024). As precision neuromodulation evolves, it promises to significantly shape the future of nVNS, positioning it as a pivotal element of personalized medicine and expanding its application across a broad spectrum of inflammatory and neurological disorders.
4.3 Combination therapies
The integration of non-invasive VNS with other therapeutic modalities offers a promising avenue to enhance efficacy and tackle treatment resistance in complex inflammatory conditions (Abdullahi et al., 2023; Yu and Wang, 2023). Combining nVNS with pharmacological treatments, such as anti-inflammatory drugs or biologics, has been shown to produce synergistic effects that amplify therapeutic outcomes. For example, nVNS could enhance the effects of TNF inhibitors in rheumatoid arthritis or reduce the necessary dosages of corticosteroids in asthma, thus minimizing drug-related side effects and improving overall patient outcomes (Sauer et al., 2024).
Additionally, nVNS can be effectively paired with behavioral interventions, like cognitive-behavioral therapy (CBT), to improve outcomes in conditions such as depression and anxiety (Hays et al., 2023). Research indicates that VNS can boost the therapeutic effects of behavioral therapies by modulating neurochemicals that facilitate neuroplasticity, thereby enhancing cognitive functions such as learning and memory (Engineer et al., 2017). Moreover, when combined with other neuromodulatory techniques like transcranial magnetic stimulation (TMS) or transcutaneous electrical nerve stimulation (TENS), nVNS may offer complementary benefits, further improving autonomic balance and effectively controlling chronic inflammation (Sackeim, 2021).
Despite the promising potential of these combination therapies, several challenges remain in their clinical implementation, including the necessity for tailored treatment plans, enhanced patient education, and robust regulatory support (Güvenç Paltun et al., 2021). However, exploring these multimodal strategies opens new pathways for maximizing the therapeutic potential of nVNS, offering a flexible and comprehensive approach to managing complex inflammatory and neurological conditions.
5 Challenges and considerations for clinical implementation
Despite the promising advancements and broad therapeutic potential of non-invasive VNS, integrating this therapy into mainstream clinical practice faces several challenges.
5.1 Regulatory and accessibility challenges
The clinical integration of non-invasive VNS faces substantial regulatory and accessibility challenges that hinder its widespread adoption. Regulatory approval processes for nVNS devices exhibit significant variability across different regions (Kaç et al., 2023; Forum on et al., 2015), where stringent regulatory demands can delay market access and restrict patient availability. This inconsistency is compounded by the absence of standardized guidelines for the evaluation of nVNS's efficacy and safety, which often extends approval timelines and escalates costs for manufacturers (Amon et al., 2024). Furthermore, the high initial investment required for nVNS devices, along with their ongoing maintenance and replacement costs (Morris et al., 2016), constitutes a considerable financial barrier, particularly in resource-constrained settings. The situation is exacerbated by inconsistent insurance coverage, which limits accessibility for the populations that stand to benefit the most from this non-invasive, potentially cost-effective therapy.
To navigate these obstacles, it is imperative to foster coordinated actions aimed at streamlining regulatory pathways, reducing costs through technological innovations and economies of scale, and advocating for more comprehensive insurance coverage. Such strategic measures are crucial for enhancing the accessibility of nVNS, rendering it a more feasible option across diverse healthcare environments, and potentially revolutionizing its role in clinical practice.
5.2 Patient compliance and education
Patient compliance is crucial for the success of nVNS therapy (Jin et al., 2008). Unlike pharmacological treatments, nVNS requires active patient participation, which typically involves daily or on-demand use of the device. Ensuring adherence to prescribed treatment regimens is vital for achieving optimal outcomes, yet it presents a significant challenge without proper education and support (Thummak et al., 2023). Educational programs are essential in providing patients with comprehensive knowledge about how nVNS works, its expected benefits, and the critical importance of consistent use. Healthcare providers play a central role in this educational effort by offering thorough device training, delivering clear usage instructions, and addressing patient concerns to improve adherence (Gold and McClung, 2006). Moreover, incorporating nVNS into comprehensive care plans that include regular follow-ups, monitoring, and feedback can significantly boost patient engagement and compliance. Advances in user-friendly device design, minimal maintenance requirements, and integration with mobile health technology are additional strategies that can facilitate easier use and enhance patient compliance.
5.3 Research gaps and future studies
While nVNS demonstrates significant promise in managing inflammatory conditions, there are several research gaps that need addressing to fully optimize its clinical application. Longitudinal studies are essential to determine the long-term efficacy and safety of chronic nVNS therapy (Ferstl et al., 2024; Lerman et al., 2019). Furthermore, a critical area of ongoing research is the optimization of stimulation parameters tailored to diverse patient populations (Thompson et al., 2021). Investigating biomarkers that can predict patient response to nVNS is pivotal for developing personalized treatment protocols (Ravan et al., 2017), which could significantly enhance both efficacy and safety. Additionally, exploring the potential of nVNS in novel applications, such as treating neurodegenerative diseases and cancer-related inflammation, could expand its therapeutic scope. Addressing these research gaps through comprehensive clinical trials and translational studies is fundamental to advancing the field of nVNS, paving the way for broader clinical adoption and enhanced patient outcomes.
6 Summary and outlook
This comprehensive review has synthesized the transformative potential of VNS in anti-inflammatory therapy, emphasizing its efficacy across a broad spectrum of inflammatory and autoimmune conditions as supported by robust clinical applications and pre-clinical models. VNS effectively mitigates symptoms in disorders such as rheumatoid arthritis, inflammatory bowel disease, and epilepsy, offering a viable alternative to pharmacological interventions by leveraging mechanisms such as the cholinergic anti-inflammatory pathway, vagus-adrenal axis, and vagus-gut axis. Technological advancements in precision neuromodulation and device design have not only enhanced therapeutic outcomes but also improved patient compliance, broadening VNS's appeal for long-term management of chronic inflammatory diseases.
However, the wider adoption of VNS faces challenges including regulatory complexities, high costs, and inconsistent insurance coverage, necessitating strategic approaches to overcome these barriers. Ensuring consistent and correct device use is critical for achieving optimal outcomes, with future research needing to focus on developing personalized protocols that enhance efficacy while minimizing side effects. By expanding VNS applications to include emerging conditions such as neurodegenerative diseases and integrating it into comprehensive treatment plans that include pharmacological and behavioral therapies, VNS can provide synergistic effects, enhancing anti-inflammatory benefits and patient quality of life. As the field progresses, VNS is poised to revolutionize the management of complex inflammatory disorders, promising a more effective and personalized therapeutic strategy that aligns with current scientific and clinical paradigms.
Author contributions
F-JL: Conceptualization, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. JW: Conceptualization, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. L-JG: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. H-SY: Conceptualization, Supervision, Validation, Writing – review & editing. HC: Conceptualization, Supervision, Validation, Writing – review & editing, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbaszadeh, H., Ghorbani, F., Abbaspour-Aghdam, S., Kamrani, A., Valizadeh, H., Nadiri, M., et al. (2022). Chronic obstructive pulmonary disease and asthma: mesenchymal stem cells and their extracellular vesicles as potential therapeutic tools. Stem Cell Res. Ther. 13:262. doi: 10.1186/s13287-022-02938-5
Abdullahi, A., Wong, T. W. L., and Ng, S. S. M. (2023). Putative role of non-invasive vagus nerve stimulation in cancer pathology and immunotherapy: can this be a hidden treasure, especially for the elderly? Cancer Med. 12, 19081–19090. doi: 10.1002/cam4.6466
Ahmed, U., Chang, Y. C., Zafeiropoulos, S., Nassrallah, Z., Miller, L., and Zanos, S. (2022). Strategies for precision vagus neuromodulation. Bioelectron. Med. 8:9. doi: 10.1186/s42234-022-00091-1
Alfaddagh, A., Martin, S. S., Leucker, T. M., Michos, E. D., Blaha, M. J., Lowenstein, C. J., et al. (2020). Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am. J. Prev. Cardiol. 4:100130. doi: 10.1016/j.ajpc.2020.100130
Amon, A., Marjenin, T., Duarte, R. V., Gilligan, C., Thomson, S. J., Eldabe, S., et al. (2024). Regulatory framework for implantable neurostimulation devices: comparison of systems in the US and European Union. Neuromodulation 27, 447–454. doi: 10.1016/j.neurom.2023.04.472
Ananda, R., Roslan, M. H. B., Wong, L. L., Botross, N. P., Ngim, C. F., and Mariapun, J. (2023). Efficacy and safety of vagus nerve stimulation in stroke rehabilitation: a systematic review and meta-analysis. Cerebrovasc. Dis. 52, 239–250. doi: 10.1159/000526470
Anzai, T. (2018). Inflammatory mechanisms of cardiovascular remodeling. Circ. J. 82, 629–635. doi: 10.1253/circj.CJ-18-0063
Aranow, C., Atish-Fregoso, Y., Lesser, M., Mackay, M., Anderson, E., Chavan, S., et al. (2021). Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann. Rheum. Dis. 80, 203–208. doi: 10.1136/annrheumdis-2020-217872
Araújo, F., Gonçalves, J., and Fonseca, J. E. (2016). Biosimilar DMARDs: what does the future hold? Drugs 76, 629–637. doi: 10.1007/s40265-016-0556-5
Austelle, C. W., O'Leary, G. H., Thompson, S., Gruber, E., Kahn, A., Manett, A. J., et al. (2022). A comprehensive review of vagus nerve stimulation for depression. Neuromodulation 25, 309–315. doi: 10.1111/ner.13528
Baumgart, D. C., Bokemeyer, B., Drabik, A., Stallmach, A., Schreiber, S., and Consortium, V. G. (2016). Vedolizumab induction therapy for inflammatory bowel disease in clinical practice–a nationwide consecutive German cohort study. Aliment. Pharmacol. Ther. 43, 1090–1102. doi: 10.1111/apt.13594
Bazoukis, G., Stavrakis, S., and Armoundas, A. A. (2023). Vagus nerve stimulation and inflammation in cardiovascular disease: a state-of-the-art review. J. Am. Heart Assoc. 12:e030539. doi: 10.1161/JAHA.123.030539
Bellocchi, C., Carandina, A., Torre, A. D., Turzi, M., Arosio, B., Marchini, M., et al. (2023). Transcutaneous auricular branch vagal nerve stimulation as a non-invasive add-on therapeutic approach for pain in systemic sclerosis. RMD Open 9:e003265. doi: 10.1136/rmdopen-2023-003265
Bentley, N., Awad, A. J., and Patil, P. G. (2018). “Chapter 43—physiology and pathophysiology of chronic pain,” in Neuromodulation, 2nd Edn, eds. E. S. Krames, P. H. Peckham, and A. R. Rezai (Cambridge, MA: Academic Press), 7.
Blagov, A. V., Summerhill, V. I., Sukhorukov, V. N., Zhigmitova, E. B., Postnov, A. Y., and Orekhov, A. N. (2024). Potential use of antioxidants for the treatment of chronic inflammatory diseases. Front. Pharmacol. 15:1378335. doi: 10.3389/fphar.2024.1378335
Bonaz, B. (2022). Anti-inflammatory effects of vagal nerve stimulation with a special attention to intestinal barrier dysfunction. Neurogastroenterol. Motil. 34:e14456. doi: 10.1111/nmo.14456
Bonaz, B. (2024). Unmet needs of drugs for irritable bowel syndrome and inflammatory bowel diseases: interest of vagus nerve stimulation and hypnosis. Inflammopharmacology 32, 1005–1015. doi: 10.1007/s10787-024-01446-7
Bonaz, B., Bazin, T., and Pellissier, S. (2018). The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12:49. doi: 10.3389/fnins.2018.00049
Bonaz, B., and Sinniger, V. (2023). Targeting the vagus nerve to treat inflammatory bowel disease? J. Crohn's Colitis 17, 1893–1894. doi: 10.1093/ecco-jcc/jjad149
Bonaz, B., Sinniger, V., Hoffmann, D., Clarençon, D., Mathieu, N., Dantzer, C., et al. (2016a). Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 28, 948–953. doi: 10.1111/nmo.12792
Bonaz, B., Sinniger, V., and Pellissier, S. (2016b). Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 594, 5781–5790. doi: 10.1113/JP271539
Bonaz, B., Sinniger, V., and Pellissier, S. (2017a). The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front. Immunol. 8:1452. doi: 10.3389/fimmu.2017.01452
Bonaz, B., Sinniger, V., and Pellissier, S. (2017b). Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 282, 46–63. doi: 10.1111/joim.12611
Bonaz, B., Sinniger, V., and Pellissier, S. (2019). Vagus nerve stimulation at the interface of brain-gut interactions. Cold Spring Harb. Perspect. Med. 9:a034199. doi: 10.1101/cshperspect.a034199
Bonaz, B., Sinniger, V., and Pellissier, S. (2021). Therapeutic potential of vagus nerve stimulation for inflammatory bowel diseases. Front. Neurosci. 15:650971. doi: 10.3389/fnins.2021.650971
Borovikova, L. V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G. I., Watkins, L. R., et al. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. doi: 10.1038/35013070
Bosmann, M., and Ward, P. A. (2013). The inflammatory response in sepsis. Trends Immunol. 34, 129–136. doi: 10.1016/j.it.2012.09.004
Bowles, S., Hickman, J., Peng, X., Williamson, W. R., Huang, R., Washington, K., et al. (2022). Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement. Neuron 110, 2867–2885.e7. doi: 10.1016/j.neuron.2022.06.017
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiat. 9:44. doi: 10.3389/fpsyt.2018.00044
Browning, K. N., Verheijden, S., and Boeckxstaens, G. E. (2017). The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology 152, 730–744. doi: 10.1053/j.gastro.2016.10.046
Cai, Y., Zhang, Y., Fang, Y., Hu, H., Li, X., and Fang, L. (2024). Evaluating the efficacy and acceptability of vagus nerve stimulation for fibromyalgia: a PRISMA-compliant protocol for a systematic review and meta-analysis. Front. Neurol. 15:1367295. doi: 10.3389/fneur.2024.1367295
Caravaca, A. S., Gallina, A. L., Tarnawski, L., Shavva, V. S., Colas, R. A., Dalli, J., et al. (2022a). Vagus nerve stimulation promotes resolution of inflammation by a mechanism that involves Alox15 and requires the α7nAChR subunit. Proc. Natl. Acad. Sci. U. S. A. 119:e2023285119. doi: 10.1073/pnas.2023285119
Caravaca, A. S., Levine, Y. A., Drake, A., Eberhardson, M., and Olofsson, P. S. (2022b). Vagus nerve stimulation reduces indomethacin-induced small bowel inflammation. Front. Neurosci. 15:730407. doi: 10.3389/fnins.2021.730407
Chakraborty, R. K., and Burns, B. (2024). Systemic Inflammatory Response Syndrome. StatPearls. Treasure Island (FL) Ineligible Companies. Disclosure: Bracken Burns Declares No Relevant Financial Relationships With Ineligible Companies. Treasure Island: StatPearls Publishing LLC.
Chen, X., He, X., Luo, S., Feng, Y., Liang, F., Shi, T., et al. (2018). Vagus nerve stimulation attenuates cerebral microinfarct and colitis-induced cerebral microinfarct aggravation in mice. Front. Neurol. 9:798. doi: 10.3389/fneur.2018.00798
Chen, Y., Zhang, Y., Wang, J., Li, S., Wang, Y., Zhang, Z., et al. (2023). Anti-neuroinflammation effects of transcutaneous auricular vagus nerve stimulation against depression-like behaviors via hypothalamic α7nAchR/JAK2/STAT3/NF-κB pathway in rats exposed to chronic unpredictable mild stress. CNS Neurosci. Ther. 29, 2634–2644. doi: 10.1111/cns.14207
Christ, A., Lauterbach, M., and Latz, E. (2019). Western diet and the immune system: an inflammatory connection. Immunity 51, 794–811. doi: 10.1016/j.immuni.2019.09.020
Cirillo, G., Negrete-Diaz, F., Yucuma, D., Virtuoso, A., Korai, S. A., De Luca, C., et al. (2022). Vagus nerve stimulation: a personalized therapeutic approach for Crohn's and other inflammatory bowel diseases. Cells 11:244103. doi: 10.3390/cells11244103
Cohen, S. P., Vase, L., and Hooten, W. M. (2021). Chronic pain: an update on burden, best practices, and new advances. Lancet 397, 2082–2097. doi: 10.1016/S0140-6736(21)00393-7
Courties, A., Berenbaum, F., and Sellam, J. (2021). Vagus nerve stimulation in musculoskeletal diseases. Joint Bone Spine 88:105149. doi: 10.1016/j.jbspin.2021.105149
Crosson, T., and Talbot, S. (2023). Decoding nociceptor-DC dialogues. Immunity 56, 906–908. doi: 10.1016/j.immuni.2023.04.016
Cucu, I. (2022). Signaling pathways in inflammation and cardiovascular diseases: an update of therapeutic strategies. Immuno 2, 630–650. doi: 10.3390/immuno2040039
Das, U. N. (2011). Vagus nerve stimulation as a strategy to prevent and manage metabolic syndrome. Med. Hypoth. 76, 429–433. doi: 10.1016/j.mehy.2010.11.013
Dawson, J., Liu, C. Y., Francisco, G. E., Cramer, S. C., Wolf, S. L., Dixit, A., et al. (2021). Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet 397, 1545–1553. doi: 10.1016/S0140-6736(21)00475-X
Demircioglu, G., Özden, A. V., and Genç, H. (2024). Comparison of the efficacy of auricular vagus nerve stimulation and conventional low back rehabilitation in patients with chronic low back pain. Complement. Ther. Clin. Pract. 56:101862. doi: 10.1016/j.ctcp.2024.101862
Dey, S., Eapen, M. S., Chia, C., Gaikwad, A. V., Wark, P. A. B., and Sohal, S. S. (2022). Pathogenesis, clinical features of asthma COPD overlap, and therapeutic modalities. Am. J. Physiol. Lung Cell Mol. Physiol. 322, 64–83. doi: 10.1152/ajplung.00121.2021
D'Haens, G., Eberhardson, M., Cabrijan, Z., Danese, S., Van Den Berg, R., Löwenberg, M., et al. (2023). Neuroimmune modulation through vagus nerve stimulation reduces inflammatory activity in Crohn's disease patients: a prospective open-label study. J. Crohns Colitis 17, 1897–1909. doi: 10.1093/ecco-jcc/jjad151
Distler, O., Ludwig, R. J., Niemann, S., Riemekasten, G., and Schreiber, S. (2021). Editorial: Precision medicine in chronic inflammation. Front. Immunol. 12:770462. doi: 10.3389/fimmu.2021.770462
Drewes, A. M., Brock, C., Rasmussen, S. E., Møller, H. J., Brock, B., Deleuran, B. W., et al. (2021). Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: results of a pilot study. Scand. J. Rheumatol. 50, 20–27. doi: 10.1080/03009742.2020.1764617
Dugan, B., Conway, J., and Duggal, N. A. (2023). Inflammaging as a target for healthy ageing. Age Ageing 52:afa328. doi: 10.1093/ageing/afac328
Engineer, C. T., Hays, S. A., and Kilgard, M. P. (2017). Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders. J. Neurodevelopment. Disord. 9:20. doi: 10.1186/s11689-017-9203-z
Falvey, A., Metz, C. N., Tracey, K. J., and Pavlov, V. A. (2022). Peripheral nerve stimulation and immunity: the expanding opportunities for providing mechanistic insight and therapeutic intervention. Int. Immunol. 34, 107–118. doi: 10.1093/intimm/dxab068
Fang, Y. T., Lin, Y. T., Tseng, W. L., Tseng, P., Hua, G. L., Chao, Y. J., et al. (2023). Neuroimmunomodulation of vagus nerve stimulation and the therapeutic implications. Front. Aging Neurosci. 15:1173987. doi: 10.3389/fnagi.2023.1173987
Fattori, V., Ferraz, C. R., Rasquel-Oliveira, F. S., and Verri, W. A. JR. (2021). Neuroimmune communication in infection and pain: friends or foes? Immunol. Lett. 229, 32–43. doi: 10.1016/j.imlet.2020.11.009
Fearon, W. F., and Fearon, D. T. (2008). Inflammation and cardiovascular disease. Circulation 117, 2577–2579. doi: 10.1161/CIRCULATIONAHA.108.772491
Feehan, K. T., and Gilroy, D. W. (2019). Is resolution the end of inflammation? Trends Mol. Med. 25, 198–214. doi: 10.1016/j.molmed.2019.01.006
Ferstl, M., Kühnel, A., Klaus, J., Lin, W. M., and Kroemer, N. B. (2024). Non-invasive vagus nerve stimulation conditions increased invigoration and wanting in depression. Comprehens. Psychiat. 132:152488. doi: 10.1016/j.comppsych.2024.152488
Forum on N. Nervous System D. Board on Health Sciences P. Institute of M. The National Academies of Sciences E. Medicine. (2015). Non-Invasive Neuromodulation of the Central Nervous System: Opportunities and Challenges: Workshop Summary. Washington, DC: National Academies Press.
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. doi: 10.1038/s41591-019-0675-0
Ganea, D., Hooper, K. M., and Kong, W. (2015). The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 213, 442–452. doi: 10.1111/apha.12427
Genovese, M. C., Gaylis, N. B., Sikes, D., Kivitz, A., Lewis Horowitz, D., Peterfy, C., et al. (2020). Safety and efficacy of neurostimulation with a miniaturised vagus nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: a two-stage multicentre, randomised pilot study. Lancet Rheumatol. 2, e527–e538. doi: 10.1016/S2665-9913(20)30172-7
Gierthmuehlen, M., Plachta, D. T. T., and Zentner, J. (2020). Implant-mediated therapy of arterial hypertension. Curr. Hypertens. Rep. 22:16. doi: 10.1007/s11906-020-1019-7
Goggins, E., Mitani, S., and Tanaka, S. (2022). Clinical perspectives on vagus nerve stimulation: present and future. Clin. Sci. 136, 695–709. doi: 10.1042/CS20210507
Gold, D. T., and McClung, B. (2006). Approaches to patient education: emphasizing the long-term value of compliance and persistence. Am. J. Med. 119, S32–S37. doi: 10.1016/j.amjmed.2005.12.021
Guo, J., Jin, H., Shi, Z., Yin, J., Pasricha, T., and Chen, J. D. Z. (2019). Sacral nerve stimulation improves colonic inflammation mediated by autonomic-inflammatory cytokine mechanism in rats. Neurogastroenterol. Motil. 31:e13676. doi: 10.1111/nmo.13676
Güvenç Paltun, B., Kaski, S., and Mamitsuka, H. (2021). Machine learning approaches for drug combination therapies. Brief. Bioinformat. 22:bbab293. doi: 10.1093/bib/bbab293
Han, B., Li, X., and Hao, J. (2017). The cholinergic anti-inflammatory pathway: an innovative treatment strategy for neurological diseases. Neurosci. Biobehav. Rev. 77, 358–368. doi: 10.1016/j.neubiorev.2017.04.002
Han, Y., Wang, B., Gao, H., He, C., Hua, R., Liang, C., et al. (2022). Vagus nerve and underlying impact on the gut microbiota-brain axis in behavior and neurodegenerative diseases. J. Inflamm. Res. 15, 6213–6230. doi: 10.2147/JIR.S384949
Hays, S. A., Rennaker, R. L. 2nd., and Kilgard, M. P. (2023). How to fail with paired VNS therapy. Brain Stimul. 16, 1252–1258. doi: 10.1016/j.brs.2023.08.009
Hilderman, M., and Bruchfeld, A. (2020). The cholinergic anti-inflammatory pathway in chronic kidney disease-review and vagus nerve stimulation clinical pilot study. Nephrol. Dial. Transplant. 35, 1840–1852. doi: 10.1093/ndt/gfaa200
Hilz, M. J. (2022). Transcutaneous vagus nerve stimulation—a brief introduction and overview. Auton. Neurosci. 243:103038. doi: 10.1016/j.autneu.2022.103038
Hosomoto, K., Sasaki, T., Yasuhara, T., Kameda, M., Sasada, S., Kin, I., et al. (2023). Continuous vagus nerve stimulation exerts beneficial effects on rats with experimentally induced Parkinson's disease: evidence suggesting involvement of a vagal afferent pathway. Brain Stimul. 16, 594–603. doi: 10.1016/j.brs.2023.03.003
Huang, Y., Dong, S., Li, X., Shi, J., Zhang, Y., Liu, S., et al. (2024). VNS-mediated α7nAChR signaling promotes SPM synthesis via regulation of netrin-1 expression during LPS-induced ALI [J]. FASEB J. 38:e9664. doi: 10.1096/fj.202301623R
Inoue, T., Abe, C., Kohro, T., Tanaka, S., Huang, L., Yao, J., et al. (2019). Non-canonical cholinergic anti-inflammatory pathway-mediated activation of peritoneal macrophages induces Hes1 and blocks ischemia/reperfusion injury in the kidney. Kidney Int. 95, 563–576. doi: 10.1016/j.kint.2018.09.020
Jentzer, J. C., Lawler, P. R., Van Diepen, S., Henry, T. D., Menon, V., Baran, D. A., et al. (2020). Systemic inflammatory response syndrome is associated with increased mortality across the spectrum of shock severity in cardiac intensive care patients. Circulation 13:e006956. doi: 10.1161/CIRCOUTCOMES.120.006956
Jin, H., Guo, J., Liu, J., Lyu, B., Foreman, R. D., Yin, J., et al. (2017). Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G192–G202. doi: 10.1152/ajpgi.00254.2016
Jin, J., Sklar, G. E., Min Sen Oh, V., and Chuen Li, S. (2008). Factors affecting therapeutic compliance: a review from the patient's perspective. Ther. Clin. Risk Manag. 4, 269–286. doi: 10.2147/TCRM.S1458
Jo, B. G., Kim, S. H., and Namgung, U. (2020). Vagal afferent fibers contribute to the anti-inflammatory reactions by vagus nerve stimulation in concanavalin A model of hepatitis in rats. Mol. Med. 26:119. doi: 10.1186/s10020-020-00247-2
Johnson, R. L., and Wilson, C. G. (2018). A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 11, 203–213. doi: 10.2147/JIR.S163248
Kaç, B., Hanoglu, L., and Tokaç, M. (2023). Possible consequences of reclassification of non-invasive brain stimulating as a class III medical devices in europe and its reflections to our country. J. Health Syst. Policies 5, 53–68. doi: 10.52675/jhesp.1279591
Kaniusas, E., Szeles, J. C., Kampusch, S., Alfageme-Lopez, N., Yucuma-Conde, D., Li, X., et al. (2020). Non-invasive auricular vagus nerve stimulation as a potential treatment for COVID-19-originated acute respiratory distress syndrome. Front. Physiol. 11:890. doi: 10.3389/fphys.2020.00890
Kaur, S., Selden, N. R., and Aballay, A. (2023). Anti-inflammatory effects of vagus nerve stimulation in pediatric patients with epilepsy. Front. Immunol. 14:1093574. doi: 10.3389/fimmu.2023.1093574
Kawashima, K., and Fujii, T. (2003). The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 74, 675–696. doi: 10.1016/j.lfs.2003.09.037
Kelly, M. J., Breathnach, C., Tracey, K. J., and Donnelly, S. C. (2022). Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 3:100696. doi: 10.1016/j.xcrm.2022.100696
Kim, J. S., Lee, D. E., Bae, H., Song, J. Y., Yang, K. I., and Hong, S. B. (2022). Effects of vagus nerve stimulation on sleep-disordered breathing, daytime sleepiness, and sleep quality in patients with drug-resistant epilepsy. J. Clin. Neurol. 18, 315–322. doi: 10.3988/jcn.2022.18.3.315
Kin, I., Sasaki, T., Yasuhara, T., Kameda, M., Agari, T., Okazaki, M., et al. (2021). Vagus nerve stimulation with mild stimulation intensity exerts anti-inflammatory and neuroprotective effects in Parkinson's disease model rats. Biomedicines 9:789. doi: 10.3390/biomedicines9070789
Komegae, E. N., Farmer, D. G. S., Brooks, V. L., Mckinley, M. J., Mcallen, R. M., and Martelli, D. (2018). Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav. Immun. 73, 441–449. doi: 10.1016/j.bbi.2018.06.005
Koopman, F. A., Chavan, S. S., Miljko, S., Grazio, S., Sokolovic, S., Schuurman, P. R., et al. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 113, 8284–8289. doi: 10.1073/pnas.1605635113
Kox, M., van Eijk, L. T., Verhaak, T., Frenzel, T., Kiers, H. D., Gerretsen, J., et al. (2015). Transvenous vagus nerve stimulation does not modulate the innate immune response during experimental human endotoxemia: a randomized controlled study. Arthritis Res. Ther. 17:150. doi: 10.1186/s13075-015-0667-5
Lerman, I., Davis, B., Huang, M., Huang, C., Sorkin, L., Proudfoot, J., et al. (2019). Noninvasive vagus nerve stimulation alters neural response and physiological autonomic tone to noxious thermal challenge. PLoS ONE 14:e0201212. doi: 10.1371/journal.pone.0201212
Levine, Y. A., Koopman, F. A., Faltys, M., Caravaca, A., Bendele, A., Zitnik, R., et al. (2014). Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS ONE 9:e104530. doi: 10.1371/journal.pone.0104530
Li, D., Li, X., Zhang, X., Chen, J., Wang, Z., Yu, Z., et al. (2024). Geniposide for treating atherosclerotic cardiovascular disease: a systematic review on its biological characteristics, pharmacology, pharmacokinetics, and toxicology. Chin. Med. 19:111. doi: 10.1186/s13020-024-00981-3
Li, S., Qi, D., Li, J.-N., Deng, X.-Y., and Wang, D.-X. (2021). Vagus nerve stimulation enhances the cholinergic anti-inflammatory pathway to reduce lung injury in acute respiratory distress syndrome via STAT3. Cell Death Discov. 7:63. doi: 10.1038/s41420-021-00431-1
Liu, T. T., Chen, S. P., Wang, S. J., and Yen, J. C. (2024). Vagus nerve stimulation inhibits cortical spreading depression via glutamate-dependent TrkB activation mechanism in the nucleus tractus solitarius. Cephalalgia 44:3331024241230466. doi: 10.1177/03331024241230466
Luo, B., Wu, Y., Liu, S.-L., Li, X.-Y., Zhu, H.-R., Zhang, L., et al. (2020). Vagus nerve stimulation optimized cardiomyocyte phenotype, sarcomere organization and energy metabolism in infarcted heart through FoxO3A-VEGF signaling. Cell Death Dis. 11:971. doi: 10.1038/s41419-020-03142-0
Lv, J., Ji, X., Li, Z., and Hao, H. (2021). The role of the cholinergic anti-inflammatory pathway in autoimmune rheumatic diseases. Scand. J. Immunol. 94:e13092. doi: 10.1111/sji.13092
Marrosu, F., Maleci, A., Cocco, E., Puligheddu, M., Barberini, L., and Marrosu, M. G. (2007). Vagal nerve stimulation improves cerebellar tremor and dysphagia in multiple sclerosis. Mult. Scler. 13, 1200–1202. doi: 10.1177/1352458507078399
Marsal, S., Corominas, H., De Agustin De Oro, J. J., Perez Garcia, C., Lopez Lasanta, M., Borrell, H., et al. (2020a). Non-invasive vagus nerve stimulation improves signs and symptoms of rheumatoid arthritis: results of a pilot study. Arthrit. Rheumatol. 3, e262–e269. doi: 10.1016/S2665-9913(20)30425-2
Marsal, S., Corominas, H., De Agustín, J. J., Pérez-García, C., López-Lasanta, M., Borrell, H., et al. (2021). Non-invasive vagus nerve stimulation for rheumatoid arthritis: a proof-of-concept study. Lancet Rheumatol. 3, e262–e269.
Marsal, S., Corominas, H., Lopez Lasanta, M., Reina-Sanz, D., Perez-Garcia, C., Borrell Paños, H., et al. (2020b). Pilot clinical study of a non-invasive auricular vagus nerve stimulation device in patients with rheumatoid arthritis. Ann. Rheumat. Dis. 79, 1003–1004. doi: 10.1136/annrheumdis-2020-eular.3315
Martelli, D., Mckinley, M. J., and Mcallen, R. M. (2014). The cholinergic anti-inflammatory pathway: a critical review. Auton. Neurosci. 182, 65–69. doi: 10.1016/j.autneu.2013.12.007
Martinez, J. E., and Guimarães, I. (2024). Fibromyalgia—are there any new approaches? Best Pract. Res. Clin. Rheumatol. 38:101933. doi: 10.1016/j.berh.2024.101933
Mastitskaya, S., Thompson, N., and Holder, D. (2021). Selective vagus nerve stimulation as a therapeutic approach for the treatment of ards: a rationale for neuro-immunomodulation in COVID-19 disease. Front. Neurosci. 15:667036. doi: 10.3389/fnins.2021.667036
Matteoli, G., and Boeckxstaens, G. E. (2013). The vagal innervation of the gut and immune homeostasis. Gut 62, 1214–1222. doi: 10.1136/gutjnl-2012-302550
Meregnani, J., Clarençon, D., Vivier, M., Peinnequin, A., Mouret, C., Sinniger, V., et al. (2011). Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton. Neurosci. 160, 82–89. doi: 10.1016/j.autneu.2010.10.007
Meroni, E., Stakenborg, N., Gomez-Pinilla, P. J., De Hertogh, G., Goverse, G., Matteoli, G., et al. (2018). Functional characterization of oxazolone-induced colitis and survival improvement by vagus nerve stimulation. PLoS ONE 13:e0197487. doi: 10.1371/journal.pone.0197487
Mihaylova, S., Killian, A., Mayer, K., Pullamsetti, S. S., Schermuly, R., and Rosengarten, B. (2012). Effects of anti-inflammatory vagus nerve stimulation on the cerebral microcirculation in endotoxinemic rats. J. Neuroinflammation 9:183. doi: 10.1186/1742-2094-9-183
Mikami, Y., Tsunoda, J., Kiyohara, H., Taniki, N., Teratani, T., and Kanai, T. (2022). Vagus nerve-mediated intestinal immune regulation: therapeutic implications of inflammatory bowel diseases. Int. Immunol. 34, 97–106. doi: 10.1093/intimm/dxab039
Mondal, B., Choudhury, S., Banerjee, R., Roy, A., Chatterjee, K., Basu, P., et al. (2021). Non-invasive vagus nerve stimulation improves clinical and molecular biomarkers of Parkinson's disease in patients with freezing of gait. NPJ Parkinson's Dis. 7:46. doi: 10.1038/s41531-021-00190-x
Morris, J., Straube, A., Diener, H. C., Ahmed, F., Silver, N., Walker, S., et al. (2016). Cost-effectiveness analysis of non-invasive vagus nerve stimulation for the treatment of chronic cluster headache. J. Headache Pain 17:43. doi: 10.1186/s10194-016-0633-x
Morris, R., Yarnall, A. J., Hunter, H., Taylor, J. P., Baker, M. R., and Rochester, L. (2019). Noninvasive vagus nerve stimulation to target gait impairment in Parkinson's disease. Mov. Disord. 34, 918–919. doi: 10.1002/mds.27664
Mourdoukoutas, A. P., Truong, D. Q., Adair, D. K., Simon, B. J., and Bikson, M. (2018). High-resolution multi-scale computational model for non-invasive cervical vagus nerve stimulation. Neuromodulation 21, 261–268. doi: 10.1111/ner.12706
Mulders, D. M., De Vos, C. C., Vosman, I., and Van Putten, M. J. A. M. (2015). The effect of vagus nerve stimulation on cardiorespiratory parameters during rest and exercise. Seizure 33, 24–28. doi: 10.1016/j.seizure.2015.10.004
Namgung, U., Kim, K. J., Jo, B. G., and Park, J. M. (2022). Vagus nerve stimulation modulates hippocampal inflammation caused by continuous stress in rats. J. Neuroinflammation 19:33. doi: 10.1186/s12974-022-02396-z
Nesci, S., Spagnoletta, A., and Oppedisano, F. (2023). Inflammation, mitochondria and natural compounds together in the circle of trust. Int. J. Mol. Sci. 24:76106. doi: 10.3390/ijms24076106
Netea, M. G., Balkwill, F., Chonchol, M., Cominelli, F., Donath, M. Y., Giamarellos-Bourboulis, E. J., et al. (2017). A guiding map for inflammation. Nat. Immunol. 18, 826–831. doi: 10.1038/ni.3790
Noviello, C. M., Gharpure, A., Mukhtasimova, N., Cabuco, R., Baxter, L., Borek, D., et al. (2021). Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 184, 2121–2134.e13. doi: 10.1016/j.cell.2021.02.049
Obst, M. A., Heldmann, M., Alicart, H., Tittgemeyer, M., and Münte, T. F. (2020). Effect of short-term transcutaneous vagus nerve stimulation (tVNS) on brain processing of food cues: an electrophysiological study. Front. Hum. Neurosci. 14:206. doi: 10.3389/fnhum.2020.00206
Okdahl, T., Kufaishi, H., Kornum, D., Bertoli, D., Krogh, K., Knop, F. K., et al. (2024). Transcutaneous vagus nerve stimulation has no anti-inflammatory effect in diabetes. Sci. Rep. 14:21042. doi: 10.1038/s41598-024-72139-y
Olofsson, P. S., Katz, D. A., Rosas-Ballina, M., Levine, Y. A., Ochani, M., Valdés-Ferrer, S. I., et al. (2012). α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol. Med. 18, 539–543. doi: 10.2119/molmed.2011.00405
Owens, M. M., Jacquemet, V., Napadow, V., Lewis, N., and Beaumont, E. (2024). Brainstem neuronal responses to transcutaneous auricular and cervical vagus nerve stimulation in rats. J. Physiol. 602, 4027–4052. doi: 10.1113/JP286680
Owolabi, M. O., Leonardi, M., Bassetti, C., Jaarsma, J., Hawrot, T., Makanjuola, A. I., et al. (2023). Global synergistic actions to improve brain health for human development. Nat. Rev. Neurol. 19, 371–383. doi: 10.1038/s41582-023-00808-z
Paton, J. F., Sobotka, P. A., Fudim, M., Engelman, Z. J., Hart, E. C., Mcbryde, F. D., et al. (2013). The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064
Pavlov, V. A. (2021). The evolving obesity challenge: targeting the vagus nerve and the inflammatory reflex in the response. Pharmacol. Ther. 222:107794. doi: 10.1016/j.pharmthera.2020.107794
Pavlov, V. A., and Tracey, K. J. (2012). The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat. Rev. Endocrinol. 8, 743–754. doi: 10.1038/nrendo.2012.189
Pavlov, V. A., and Tracey, K. J. (2017). Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci. 20, 156–166. doi: 10.1038/nn.4477
Payne, S. C., Furness, J. B., Burns, O., Sedo, A., Hyakumura, T., Shepherd, R. K., et al. (2019). Anti-inflammatory effects of abdominal vagus nerve stimulation on experimental intestinal inflammation. Front. Neurosci. 13:418. doi: 10.3389/fnins.2019.00418
Prathumsap, N., Ongnok, B., Khuanjing, T., Arinno, A., Maneechote, C., Apaijai, N., et al. (2022). Vagus nerve stimulation exerts cardioprotection against doxorubicin-induced cardiotoxicity through inhibition of programmed cell death pathways. Cell. Mol. Life Sci. 80:21. doi: 10.1007/s00018-022-04678-4
Qin, Z., Xiang, K., Su, D. F., Sun, Y., and Liu, X. (2020). Activation of the cholinergic anti-inflammatory pathway as a novel therapeutic strategy for COVID-19. Front. Immunol. 11:595342. doi: 10.3389/fimmu.2020.595342
Ramkissoon, C. M., Güemes, A., and Vehi, J. (2021). Overview of therapeutic applications of non-invasive vagus nerve stimulation: a motivation for novel treatments for systemic lupus erythematosus. Bioelectr. Med. 7:8. doi: 10.1186/s42234-021-00069-5
Ravan, M., Sabesan, S., and D'Cruz, O. (2017). On quantitative biomarkers of VNS therapy using EEG and ECG signals. IEEE Trans. Biomed. Eng. 64, 419–428. doi: 10.1109/TBME.2016.2554559
Ridengnaxi, E., and Wang, Y. (2024). Vagus nerve stimulation for improvement of vascular cognitive impairment. Neuropsychiatr. Dis. Treat. 20, 1445–1451. doi: 10.2147/NDT.S465249
Rosas-Ballina, M., Ochani, M., Parrish, W. R., Ochani, K., Harris, Y. T., Huston, J. M., et al. (2008). Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U. S. A. 105, 11008–11013. doi: 10.1073/pnas.0803237105
Rosas-Ballina, M., Olofsson, P. S., Ochani, M., Valdés-Ferrer, S. I., Levine, Y. A., Reardon, C., et al. (2011). Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101. doi: 10.1126/science.1209985
Ryvlin, P., Rheims, S., Hirsch, L. J., Sokolov, A., and Jehi, L. (2021). Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. 20, 1038–1047. doi: 10.1016/S1474-4422(21)00300-8
Sackeim, H. A. (2021). Staging and combining brain stimulation interventions: vagus nerve stimulation and electroconvulsive therapy. J Ect. 37, 80–83. doi: 10.1097/YCT.0000000000000745
Sahn, B., Pascuma, K., Kohn, N., Tracey, K. J., and Markowitz, J. F. (2023). Transcutaneous auricular vagus nerve stimulation attenuates inflammatory bowel disease in children: a proof-of-concept clinical trial. Bioelectron. Med. 9:23. doi: 10.1186/s42234-023-00124-3
Sauer, V., Glaser, M., Ellwardt, E., Saryyeva, A., Krauss, J. K., Ringel, F., et al. (2024). Favorable combinations of antiseizure medication with vagus nerve stimulation to improve health-related quality of life in patients with epilepsy. Epilepsy Behav. 150:109562. doi: 10.1016/j.yebeh.2023.109562
Shao, P., Li, H., Jiang, J., Guan, Y., Chen, X., and Wang, Y. (2023). Role of vagus nerve stimulation in the treatment of chronic pain. Neuroimmunomodulation 30, 167–183. doi: 10.1159/000531626
Silberstein, S. D., Yuan, H., Najib, U., Ailani, J., Morais, A. L., Mathew, P. G., et al. (2020). Non-invasive vagus nerve stimulation for primary headache: a clinical update. Cephalalgia 40, 1370–1384. doi: 10.1177/0333102420941864
Sinniger, V., Pellissier, S., Fauvelle, F., Trocmé, C., Hoffmann, D., Vercueil, L., et al. (2020). A 12-month pilot study outcomes of vagus nerve stimulation in Crohn's disease. Neurogastroenterol. Motil. 32:e13911. doi: 10.1111/nmo.13911
Siopi, E., Galerne, M., Rivagorda, M., Saha, S., Moigneu, C., Moriceau, S., et al. (2023). Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol. Psychiat. 28, 3002–3012. doi: 10.1038/s41380-023-02071-6
Smolen, J. S., Aletaha, D., and Mcinnes, I. B. (2016). Rheumatoid arthritis. Lancet 388, 2023–2038. doi: 10.1016/S0140-6736(16)30173-8
Sohrab, S. S., Raj, R., Nagar, A., Hawthorne, S., Paiva-Santos, A. C., Kamal, M. A., et al. (2023). Chronic inflammation's transformation to cancer: a nanotherapeutic paradigm. Molecules 28:114413. doi: 10.3390/molecules28114413
Song, G., Sclocco, R., Sharma, A., Guerrero-López, I., and Kuo, B. (2024). Electroceuticals and magnetoceuticals in gastroenterology. Biomolecules 14:70760. doi: 10.3390/biom14070760
Sorriento, D., and Iaccarino, G. (2019). Inflammation and cardiovascular diseases: the most recent findings. Int. J. Mol. Sci. 20:163879. doi: 10.3390/ijms20163879
Sorski, L., and Gidron, Y. (2023). The vagal nerve, inflammation, and diabetes-a holy triangle. Cells 12:121632. doi: 10.3390/cells12121632
Stavrakis, S., Chakraborty, P., Farhat, K., Whyte, S., Morris, L., Asad, Z. U. A., et al. (2024). Noninvasive vagus nerve stimulation in postural tachycardia syndrome: a randomized clinical trial. JACC Clin. Electrophysiol. 10, 346–355. doi: 10.1016/j.jacep.2023.10.015
Stavrakis, S., Humphrey, M. B., Scherlag, B., Iftikhar, O., Parwani, P., Abbas, M., et al. (2017). Low-level vagus nerve stimulation suppresses post-operative atrial fibrillation and inflammation: a randomized Study. JACC Clin. Electrophysiol. 3, 929–938. doi: 10.1016/j.jacep.2017.02.019
Stavrakis, S., Humphrey, M. B., Scherlag, B. J., Hu, Y., Jackman, W. M., Nakagawa, H, et al. (2015). Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 65, 867–875. doi: 10.1016/j.jacc.2014.12.026
Sun, P., Zhou, K., Wang, S., Li, P., Chen, S., Lin, G., et al. (2013). Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS ONE 8:e69424. doi: 10.1371/journal.pone.0069424
Tao, X., Lee, M. S., Donnelly, C. R., and Ji, R. R. (2020). Neuromodulation, specialized proresolving mediators, and resolution of pain. Neurotherapeutics 17, 886–899. doi: 10.1007/s13311-020-00892-9
Thompson, S. L., O'Leary, G. H., Austelle, C. W., Gruber, E., Kahn, A. T., Manett, A. J., et al. (2021). A review of parameter settings for invasive and non-invasive vagus nerve stimulation (VNS) applied in neurological and psychiatric disorders. Front. Neurosci. 15:709436. doi: 10.3389/fnins.2021.709436
Thrivikraman, K. V., Zejnelovic, F., Bonsall, R. W., and Owens, M. J. (2013). Neuroendocrine homeostasis after vagus nerve stimulation in rats. Psychoneuroendocrinology 38, 1067–1077. doi: 10.1016/j.psyneuen.2012.10.015
Thummak, S., Uppor, W., and Wannarit, L. O. (2023). Patient compliance: a concept analysis. Belitung. Nurs J. 9, 421–427. doi: 10.33546/bnj.2807
Toffa, D. H., Touma, L., El Meskine, T., Bouthillier, A., and Nguyen, D. K. (2020). Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review. Seizure Eur. J. Epilepsy 83, 104–123. doi: 10.1016/j.seizure.2020.09.027
Tynan, A., Brines, M., and Chavan, S. S. (2022). Control of inflammation using non-invasive neuromodulation: past, present and promise. Int. Immunol. 34, 119–128. doi: 10.1093/intimm/dxab073
Van Beekum, C. J., Willis, M. A., Von Websky, M. W., Sommer, N. P., Kalff, J. C., Wehner, S., et al. (2021). Electrical vagus nerve stimulation as a prophylaxis for SIRS and postoperative ileus. Auton. Neurosci. 235:102857. doi: 10.1016/j.autneu.2021.102857
Venborg, J., Wegeberg, A.-M., Kristensen, S., Brock, B., Brock, C., and Pfeiffer-Jensen, M. (2021). The effect of transcutaneous vagus nerve stimulation in patients with polymyalgia rheumatica. Pharmaceuticals 14:1166. doi: 10.3390/ph14111166
Wang, Q., Cheng, Y., Xue, F.-S., Yuan, Y.-J., Xiong, J., Li, R.-P., et al. (2012). Postconditioning with vagal stimulation attenuates local and systemic inflammatory responses to myocardial ischemia reperfusion injury in rats. Inflamm. Res. 61, 1273–1282. doi: 10.1007/s00011-012-0527-6
Wang, Y., Zhan, G., Cai, Z., Jiao, B., Zhao, Y., Li, S., et al. (2021). Vagus nerve stimulation in brain diseases: therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 127, 37–53. doi: 10.1016/j.neubiorev.2021.04.018
Wernisch, L., Edwards, T., Berthon, A., Tessier-Lariviere, O., Sarkans, E., Stoukidi, M., et al. (2024). Online Bayesian optimization of vagus nerve stimulation. J. Neural Eng. 21:ad33ae. doi: 10.1088/1741-2552/ad33ae
Woodbury, A., and Staats, P. (2024). Editorial: Non-invasive and minimally invasive vagus nerve stimulation for chronic pain. Front. Pain Res. 5:1402918. doi: 10.3389/fpain.2024.1402918
Wu, Z., Zhang, X., Cai, T., Li, Y., Guo, X., Zhao, X., et al. (2023). Transcutaneous auricular vagus nerve stimulation reduces cytokine production in sepsis: an open double-blind, sham-controlled, pilot study. Brain Stimul. 16, 507–514. doi: 10.1016/j.brs.2023.02.008
Xie, C., Gao, X., Liu, G., Tang, H., and Li, C. (2023). USP10 is a potential mediator for vagus nerve stimulation to alleviate neuroinflammation in ischaemic stroke by inhibiting NF-κB signalling pathway. Front. Immunol. 14:1130697. doi: 10.3389/fimmu.2023.1130697
Xue, R. Q., Sun, L., Yu, X. J., Li, D. L., and Zang, W. J. (2017). Vagal nerve stimulation improves mitochondrial dynamics via an M(3) receptor/CaMKKβ/AMPK pathway in isoproterenol-induced myocardial ischaemia. J. Cell Mol. Med. 21, 58–71. doi: 10.1111/jcmm.12938
Yap, J. Y. Y., Keatch, C., Lambert, E., Woods, W., Stoddart, P. R., and Kameneva, T. (2020). Critical review of transcutaneous vagus nerve stimulation: challenges for translation to clinical practice. Front. Neurosci. 14:284. doi: 10.3389/fnins.2020.00284
Yeshi, K., Ruscher, R., Hunter, L., Daly, N. L., Loukas, A., and Wangchuk, P. (2020). Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J. Clin. Med. 9:51273. doi: 10.3390/jcm9051273
Yu, H., Gao, R., Liu, Y., Fu, L., Zhou, J., and Li, L. (2024). Stimulus-responsive hydrogels as drug delivery systems for inflammation targeted therapy. Adv. Sci. 11:e2306152. doi: 10.1002/advs.202306152
Yu, Y., and Wang, Y. (2023). Editorial: invasive and non-invasive vagal nerve stimulation for the brain. Front. Neurol. 14:1269851. doi: 10.3389/fneur.2023.1269851
Zafeiropoulos, S., Ahmed, U., Bikou, A., Mughrabi, I. T., Stavrakis, S., and Zanos, S. (2024). Vagus nerve stimulation for cardiovascular diseases: is there light at the end of the tunnel? Trends Cardiovasc. Med. 34, 327–337. doi: 10.1016/j.tcm.2023.07.003
Zhang, L., Wu, Z., Tong, Z., Yao, Q., Wang, Z, and Li, W. (2021). Vagus nerve stimulation decreases pancreatitis severity in mice. Front. Immunol. 11:595957. doi: 10.3389/fimmu.2020.595957
Zhang, Q., Zhang, L., Lin, G., and Luo, F. (2024). The protective role of vagus nerve stimulation in ischemia-reperfusion injury. Heliyon 10:e30952. doi: 10.1016/j.heliyon.2024.e30952
Zhang, S. J., Huang, C. X., Zhao, Q. Y., Zhang, S. D., Dai, Z. X., Zhao, H. Y., et al. (2021). The role of α7nAChR-mediated cholinergic anti-inflammatory pathway in vagal nerve regulated atrial fibrillation. Int. Heart J. 62, 607–615. doi: 10.1536/ihj.18-510
Zhang, Y., Popovic, Z. B., Bibevski, S., Fakhry, I., Sica, D. A., Van Wagoner, D. R., et al. (2009). Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ. Heart Fail 2, 692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968
Keywords: vagus nerve stimulation (VNS), anti-inflammatory therapy, cholinergic anti-inflammatory pathway (CAP), neuro-immune reflex, clinical applications, precision neuromodulation, non-invasive therapy, chronic inflammation
Citation: Liu F-J, Wu J, Gong L-J, Yang H-S and Chen H (2024) Non-invasive vagus nerve stimulation in anti-inflammatory therapy: mechanistic insights and future perspectives. Front. Neurosci. 18:1490300. doi: 10.3389/fnins.2024.1490300
Received: 02 September 2024; Accepted: 24 October 2024;
Published: 13 November 2024.
Edited by:
Peter S. Staats, National Spine and Pain Centers, United StatesReviewed by:
Alexandre Kanashiro, University of Wisconsin, United StatesAli Veysel Ozden, Bahçeşehir University, Türkiye
Copyright © 2024 Liu, Wu, Gong, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Shuai Yang, aHNodWFpeWFuZ0AxNjMuY29t; Huan Chen, aHVhbmNoZW4zMzgzQDE2My5jb20=
 Fu-Jun Liu
Fu-Jun Liu Jing Wu
Jing Wu Li-Jun Gong
Li-Jun Gong Hong-Shuai Yang
Hong-Shuai Yang Huan Chen
Huan Chen