- 1Facultad de Enfermería y Fisioterapia, Universidad de Salamanca, Unidad de Investigación de Atención Primaria de Salamanca (APISAL), Instituto de Investigación Biomédica de Salamanca (IBSAL), Red de Investigación en Cronicidad, Atención Primaria y Promoción de la Salud (RICAPPS), Salamanca, Spain
- 2Universidad de Salamanca, Unidad de Investigación de Atención Primaria de Salamanca (APISAL), Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca, Spain
- 3School of Nursing, College of Nursing, Taipei Medical University, Taipei, Taiwan
- 4College of Humanities and Social Sciences, Graduate Institute of Humanities in Medicine, Taipei Medical University, Taipei, Taiwan
- 5Facultad de Enfermería y Fisioterapia, Universidad de Salamanca, Instituto de Investigación Biomédica de Salamanca (IBSAL), Hospital Universitario de Salamanca, Salamanca, Spain
Objective: This systematic review and meta-analysis of randomized-clinical trials aims to analyze the effect of interventions incorporating surface neurofeedback techniques on self-perceived sleep quality and insomnia in patients with or without sleep disturbances.
Methods: The review was completed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement and was deposited in the Prospero international prospective registry of systematic reviews (CRD42024528401). Seven clinical trials with different main outcomes but with pre-post intervention records of self-perceived sleep quality or insomnia symptoms assessed by questionnaires met our inclusion criteria, including a publication date within the last 10 years. Five trials investigated sleep quality through scores on the Pittsburgh sleep quality Index (PSQI) and three trials signs of insomnia severity assessed with validated scales. The methodological quality of the included studies was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias and showed a high quality of them.
Results: A total of 5 studies that evaluated sleep quality with the PSQI total score were included in the meta-analysis. The results revealed that control conditions succeeded in improving PSQI-assessed sleep quality more than the analyzed Neurofeedback interventions (PSQI total score 0.57; 95% CI 0.13 to 1.01; p = 0.01). On the other hand, a total of 3 studies that evaluated insomnia severity with various insomnia scales were included in the meta-analysis The results revealed that neither the NF interventions nor the control conditions show a favorable outcome relative to each other (−0.13; 95% CI −0.44 to 0.18; p = 0.41).
Conclusion: The interventions studied mostly apply a neurofeedback training protocol based on maintaining alpha waves in a range between 8 and 12 Hz, with electrode positioning in the frontal area or in the sensorimotor cortex and with a number of neurofeedback sessions ranging from 8 to 20 sessions. The meta-analysis showed that interventions incorporating surface neurofeedback do not produce additional benefits in self-perception of sleep quality or insomnia compared to a wide variety of control conditions including cognitive behavioral treatment or other biofeedback modalities.
Systematic review registration: PROSPERO – International prospective register of systematic reviews – CRD42024528401 https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=528401.
1 Introduction
Sleep quality is a term used to define an individual’s self-satisfaction with all aspects of sleep, which can be categorized into sleep efficiency, sleep latency, sleep duration and wakefulness after sleep onset (Nelson et al., 2022). Possible determinants of sleep quality include lifestyle factors (physical activity, diet, toxic habits or time spent in front of screens), psychological factors (anxiety, depression or stress), presence of certain morbidities or medication, environmental and sociodemographic factors (family income, employment status, living conditions of the usual dwelling, ambient temperature) and other biological factors (e.g., the level of melatonin, cortisol or vitamin D) (Jiménez-Vaquero et al., 2023).
Good sleep quality has positive effects such as feeling rested, maintaining a good cognitive state and normal personal relationships. The consequences of poor sleep quality include fatigue, irritability, daytime dysfunction, slowed responses and increased caffeine/alcohol consumption, as well as possible impacts on aspects related to personal motivation and quality of life (Nelson et al., 2022). In addition, the sleep duration has shown an increased risk of cardiovascular disease and higher all-cause mortality rates, with a U-shaped association in those individuals with a daily total sleep time less than 6 h and more than 8 h (Wang et al., 2019). Meanwhile, insomnia, characterized by difficulty with sleep onset, maintenance, and subsequent daytime symptoms, is increasingly prevalent and increases the risk of other medical comorbidities (Paul and Salas, 2024).
The study of sleep quality includes a wide variety of methods, among which polysomnography stands out for its accuracy. However, polysomnography is a complex and costly test in economic terms, which makes it difficult to include in research studies. The proliferation in recent years of different actigraphy devices, which have a sensitivity of over 90%, means that these devices are used in longitudinal and epidemiological studies (Ibáñez et al., 2019; Zinkhan and Kantelhardt, 2016). However, preliminary sleep assessment is usually completed with sleep questionnaires or sleep scales. Sleep questionnaires are a very cheap and quick test and, moreover, they summarize quantitatively the patient’s (subjective) perception of his or her own sleep quality. However, their subjectivity does not necessarily make the questionnaires inaccurate, as several validation studies have shown. The accuracy of sleep questionnaires has been extensively studied (Silva et al., 2011). All of these studies used polysomnography as the gold standard. The reported sensitivity was in the range 73–98%, while the reported specificity was in the range 50–96%. In addition, for some variables such as total sleep duration, the difference between the results of a questionnaire and the actigraphy measurement seems to be minimal (Kanda et al., 2023).
Neurofeedback (NF) is a form of biofeedback training that uses the recording of brain activity through imaging techniques to achieve, through a process of feedback, control and regulation of brain activity patterns. Based on the principles of operant conditioning, patients learn gradually through positive reinforcement provided by feedback (Marzbani et al., 2016). There are several types of NF, being the most commonly used frequency/power NF, also known as “surface neurofeedback.” This technique consists of placing surface electrodes (usually 2–4 in number) on the individual’s head that record the brain’s electrical activity. This activity is analyzed by a computer program that converts the EEG waves into visual, auditory or tactile signals that it sends to the patient so that he/she learns to work in a specific wave range, thus achieving the regulation of brain activity. These techniques have been used to change the amplitude or speed of specific brain waves in specific brain locations to treat ADHD, anxiety or insomnia (Banerjee and Argáez, 2017).
The increase in the number of devices capable of performing NF, together with the reduction in the cost of their acquisition, has led to a notable increase in recent years in the number of studies that have addressed the effect of these techniques on sleep quality. Lambert-Beaudet et al. (2021) published a review analyzing the effect of NF techniques in the treatment of insomnia. They concluded that, although the studies concerning NF as a treatment for insomnia are encouraging, many methodological barriers remained to be resolved in order to prove its efficacy unequivocally. However, this was not a systematic review, it was focused on NF in the treatment of insomnia and did not analyze the quality of the studies.
The objective of this systematic review was to analyze the effect of interventions incorporating surface NF techniques on self-perceived sleep quality and severity of insomnia by analyzing the results and quality of recent randomized clinical trials (RCTs).
2 Materials and methods
This systematic review and meta-analysis of RCTs was carried out following the protocol described in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis: (PRISMA) statement (Moher et al., 2009). The protocol was registered with PROSPERO, the International Prospective Register of Systematic Reviews (Registration no. CRD42024528401). The review and meta-analysis were conducted between March and May 2024.
2.1 Literature sources
A structured search of electronic databases (Pubmed, Scopus, Cochrane Library, PsycInfo) was performed. Studies were restricted to the last 10 years (from March 2014 to March 2024). Two reviewers (MFC and JIR) performed a search to identify RCTs that studied the effect of NF on sleep quality and insomnia as primary or secondary outcome. The search strategy followed the PICO framework, using key words, free text, and MeSH terms as appropriate and combining Boolean operators of (AND/OR/NOT/quotation marks/brackets). More details of the terms used in the search can be found in Supplementary Table S1. Filters were applied to limit the search to RCTs and the English language.
2.2 Eligibility criteria
This study was guided by the participants, interventions, comparisons, outcomes and study design (PICOS) framework.
2.2.1 Study design
The eligible study design was randomized controlled trials or crossover trials with random assignment to the established sequence to the different arms of the study. Observational studies (case–control, prospective cohort, cross-sectional studies, case reports and case series) and non-randomized clinical trials or trials without a control group were excluded.
2.2.2 Population
The population of interest was adults or young people over 18 years of age, with no distinction of sex. Studies of patients with insomnia or sleep disturbances and studies that included individuals without the presence of these processes were included.
2.2.3 Intervention (exposure)
The exposures of interest included any interventions that included surface NF regardless of the protocol used, the number of sessions or the site of electrode placement. Interventions where other NF models such as Z-score neurofeedback or functional magnetic resonance imaging (fMRI) neurofeedback were performed were excluded.
2.2.4 Comparison
All studies with a control group were selected, regardless of the type of comparison, not only placebo control.
2.2.5 Outcomes
Eligible outcomes were evaluated for the presence of global sleep quality score data or insomnia severity score data assessed through questionnaires. We excluded studies that did not provide global data in both pre- and post-intervention assessments. In addition, other sleep quality-related indicators such as total sleep time, sleep latency, sleep efficacy, wakes after sleep onset and sleep satisfaction were analyzed, whenever present.
2.3 Study selection
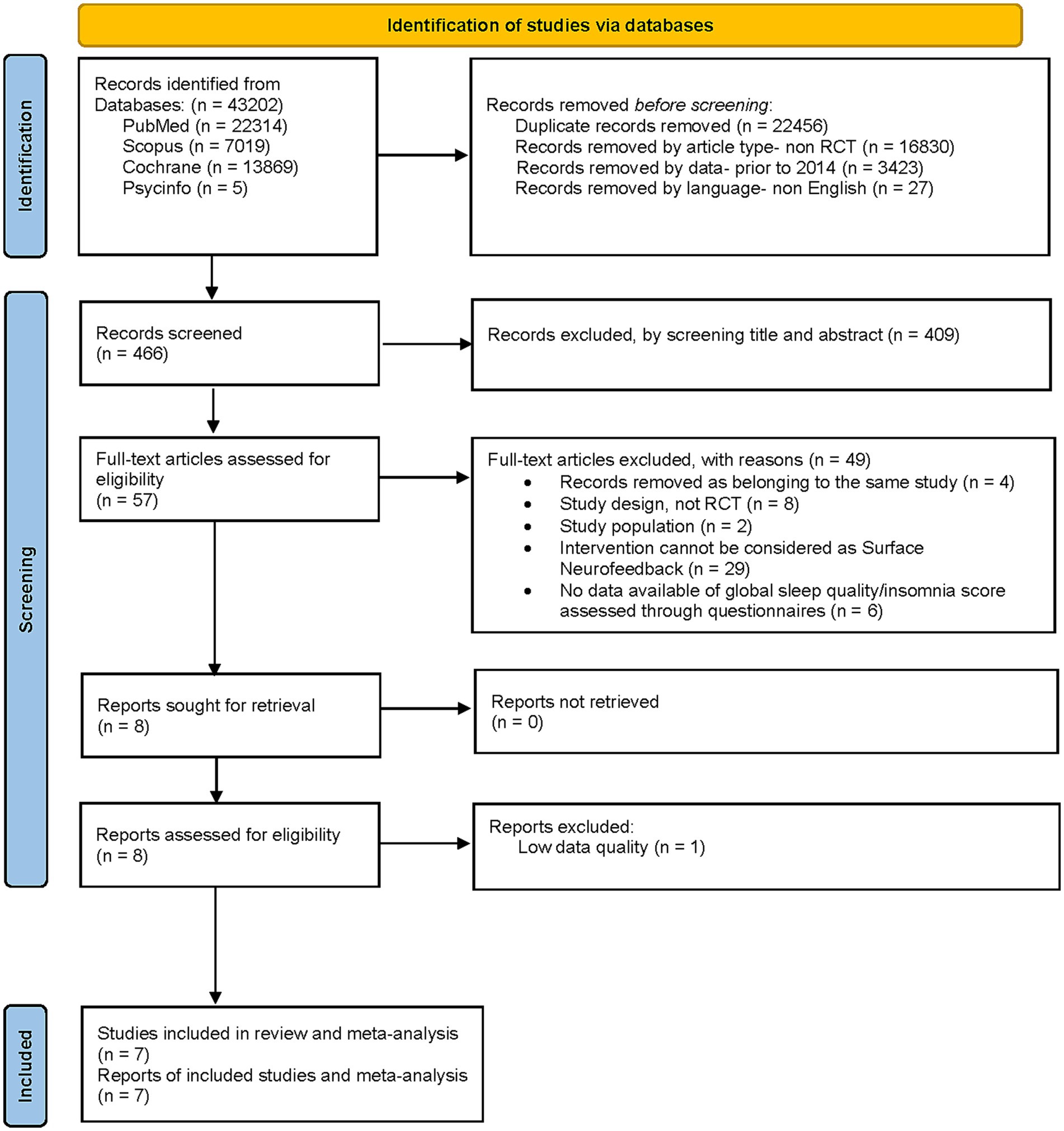
The titles and abstracts of the retrieved literature were first downloaded and imported into Endnote X9 (Clarivate; a reference management program) to eliminate duplicates. After removing duplicates and applying article type, data, and language filters, two reviewers (MFC and JIR) independently evaluated the article titles and abstracts. At least one reviewer selected studies for full-text review. The full texts of the included studies were independently reviewed by two reviewers (MFC and JIR). During this phase, records were excluded in application of the selection criteria described in the PICO framework. Disagreements were resolved by discussion and by decision of a third reviewer (IGY) when necessary (Figure 1).
2.4 Data extraction
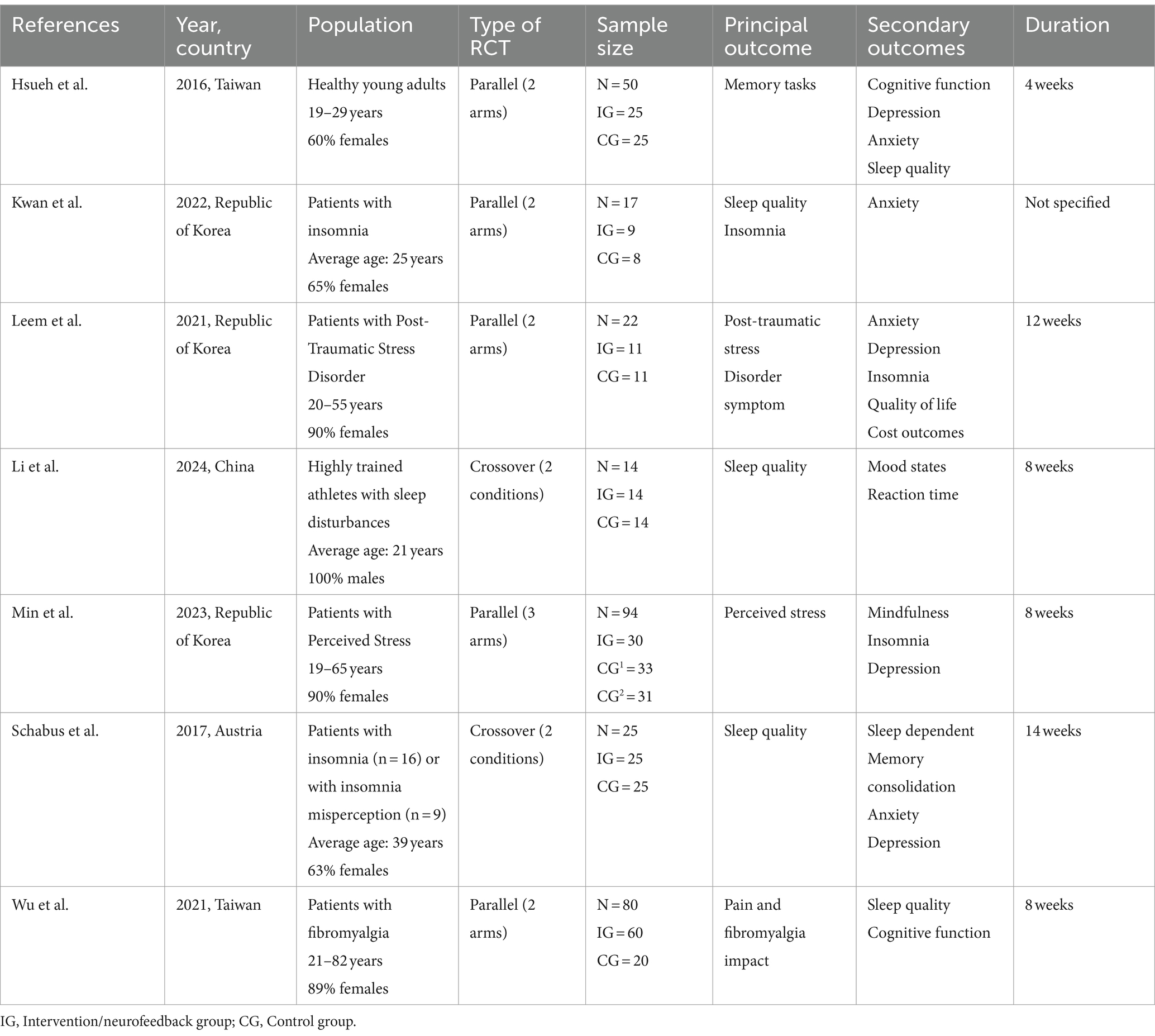
Three reviewers (MFC, JIR, and IGY) independently extracted data into a predesigned table in Microsoft Excel. If a study had multiple publications, the most recent one with completed data was selected. Study characteristics such as reference (first author), year, country, study population (health conditions, age, sex), type of RCT, sample size, main and secondary outcomes, and duration were extracted and recorded in Table 1.
2.5 Data analysis
Review Manager v.7.7.2 (The Cochrane Collaboration) was used to perform the statistical analyses. For studies with multiple measurements, only data from baseline and the immediate post-intervention time were extracted for analysis. If the necessary data were not reported, the first/corresponding authors of the relevant publication were contacted. For continuous outcomes, mean values and their SD were used in meta-analyses. Mean differences and 95% CI were used to assess the effect of NF interventions on the Pittsburgh Sleep Quality index total score while standardized mean differences (SMD) and 95% CI were calculated to analyze the effect of NF interventions on the insomnia scales total score, because this variable was collected through different tools in the studies analyzed. Finally, forest plots were generated.
Heterogeneity testing and the meta-analysis were conducted. A 2-sided p value <0.05 was considered statistically significant. Heterogeneity was evaluated using the χ2 test (with p < 0.10 indicating heterogeneity) and I2 test (with I2 > 50% indicating moderate heterogeneity and I2 > 75% indicating high heterogeneity). If I2 ≤ 50% and p > 0.10, a fixed-effect model was adopted for data merging and analysis; otherwise, a random-effects model was used.
2.6 Quality assessment (risk of bias)
Two evaluators independently assessed the methodological quality of the included studies using the Cochrane Collaboration’s tool for assessing the risk of bias (Higgins et al., 2023). For all RCTs, the following aspects were assessed: (Nelson et al., 2022) Randomization process (Jiménez-Vaquero et al., 2023) Deviations from the intended interventions (Wang et al., 2019) Missing outcome data (Paul and Salas, 2024) Measurement of the outcome (Ibáñez et al., 2019) Selection of the reported result. In addition, for crossover trials only, we assessed one more aspect: Bias arising from period and carryover effects. Each study was categorized as “low risk,” “uncertain risk,” or “high risk,” with disagreements resolved through consultation or discussion with a third researcher.
3 Results
3.1 Search results and selection
The preliminary search in all databases analyzed yielded 43,202 records. After removing 22,456 duplicated results and filtering by article-type, data and language, leaving 466 records for the screening. After the screening by title and abstract, 57 full-text articles were assessed for eligibility. A total of 49 of them were excluded by applying selection criteria: 4 records removed as belonging to the same study, 8 because of their study design, not RCT, 2 because of the characteristics of their study population, 29 because their intervention could not be considered as a “Surface neurofeedback” technique and 6 for not having available data of global sleep quality/insomnia score assessed through questionnaires. Finally, 8 records were assessed for eligibility although one was eliminated due to low quality data. A total of 7 records from 7 studies were included in the systematic review, 5 of which were included in the meta-analysis of the effect of NF techniques on the global sleep quality score assessed with the PSQI, and 3 of which were included in the meta-analysis of the effect of NF techniques on the total score on insomnia severity scales. The screening process is detailed in Figure 1.
3.2 Study characteristics
Table 1 summarizes the main characteristics of the included studies. These studies were all RCTs, 5 of them of parallel type (4 with 2 arms and one with 3 arms) and 2 of them of crossover type with 2 conditions and with randomization of the sequence. The studies are conducted in: The Republic of Korea (Wang et al., 2019), Taiwan (Jiménez-Vaquero et al., 2023), China (Nelson et al., 2022) and Austria (Nelson et al., 2022) between the years 2016, the oldest and 2024 the most recent. Three of these studies include population with insomnia or sleep disturbances. The rest of the studies included healthy population, with perceived stress or post-traumatic stress and people with fibromyalgia. The population range was variable but always older than 18 years, with a predominance of young or young-adult population, all of them having a percentage of women above 60%. Only 3 of the included studies had as principal outcome (Li et al., 2024; Kwan et al., 2022; Schabus et al., 2017) the change in sleep quality or insomnia, being present in the other 4 studies as secondary outcome (Hsueh et al., 2016; Leem et al., 2021; Min et al., 2023; Wu et al., 2021).
3.3 Study parameters for surface neurofeedback and control conditions
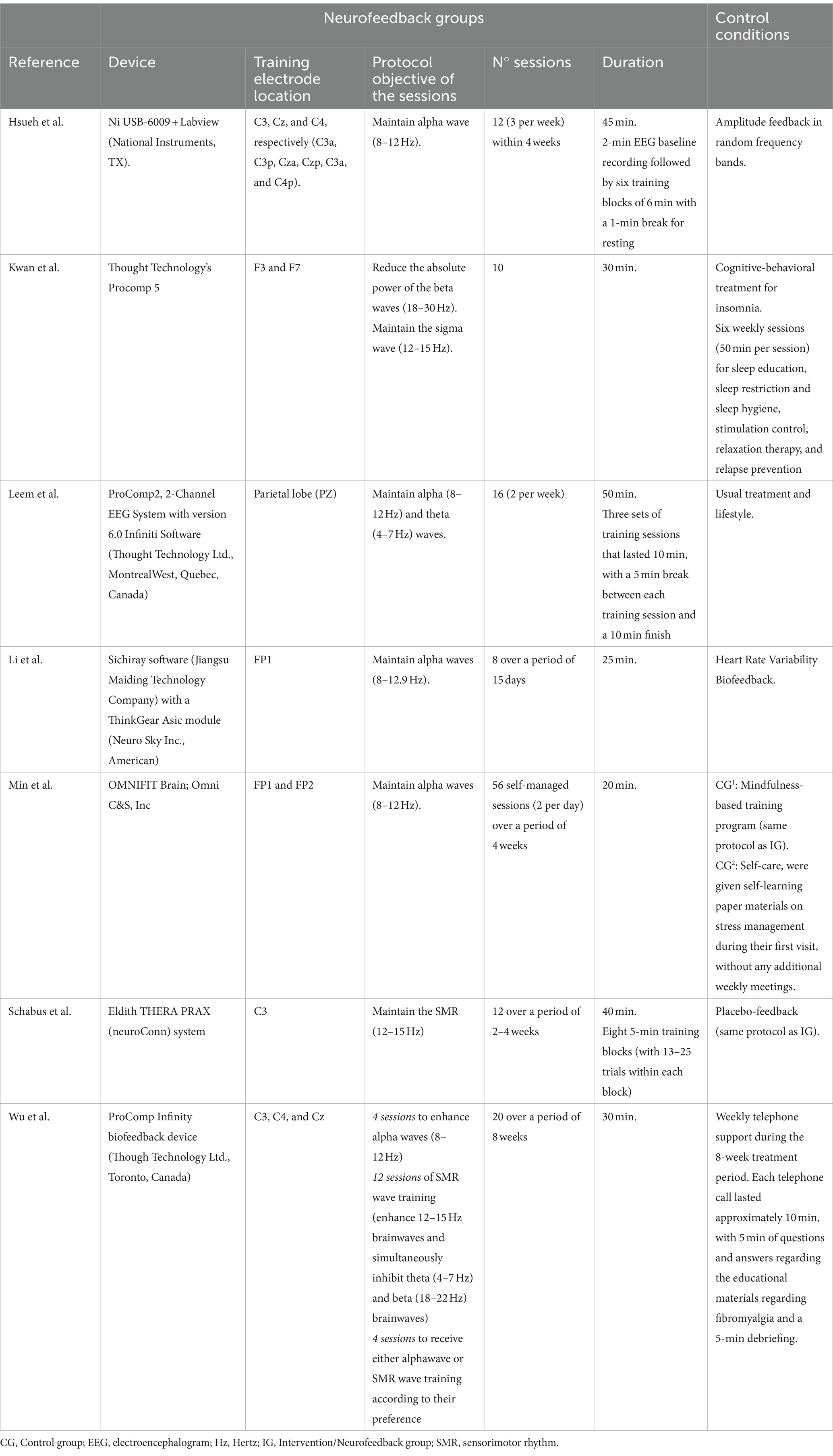
The included studies used a wide variety of devices to perform the NF sessions (Table 2). The placement of electrodes for these sessions, however, was grouped as follows: in three studies electrodes were placed in the sensorimotor cortex and in three other studies they were positioned in the frontal area. Only the work of Leem et al. (2021) used a placement in the parietal area. In relation to the objective of the sessions and the protocol used, the objective of maintaining alpha waves in a range between 8 and 12 Hz predominated, this objective being present in 5 of the 7 studies analyzed. Three of the studies reviewed included SMR wave training (enhance 12–15 Hz brainwaves) in isolation or in combination with the goal of inhibiting beta waves (18–30 Hz). The study by Wu et al. (2021) scheduled different sessions for both objectives (maintain alpha waves and SMR wave training). The number of NF sessions ranged from 8 to 20 sessions, with the exception of the study by Min et al. (2023) in which 56 self-managed sessions (2 per day) were performed. The mean duration of each session was between 20 and 50 min. In relation to the control conditions, the studies showed a great variability. Three studies compared the results of NF techniques against other types of biofeedback (random, HR feedback or placebo feedback) (Li et al., 2024; Schabus et al., 2017; Hsueh et al., 2016). The remaining studies compared against a wide variety of interventions including cognitive behavioral treatment, mindfulness training or usual treatment and lifestyle.
The participant interaction during NF sessions in each study to achieve the stated objectives is summarized in Supplementary Table S2.
3.4 Risk of bias
A quality assessment of the included studies was conducted using the Cochrane Collaboration’s tool (Figure 2). At least 4 of the 7 included studies were considered by the investigators as low risk of bias. The study by Kwan et al. (2022) and that by Li et al. (2024) present some questionable issues. In both studies, it is not known whether the randomization sequence was concealed until the allocation of the interventions and it is not known whether there was an analysis plan established by a research protocol or by the clinical trial registry. The study that raises the most doubts is that of Hsueh et al. (2016). There is little information on the concealment of the randomization sequence until the allocation of the interventions. There is also no information on whether or not the participants and caregivers were aware of the allocation. Nor is it known whether for the sleep variables studied, the statistical analyses were appropriate. It is also not known whether the statisticians in charge of the analysis were blinded or not, and it could be that the fact of knowing the assigned treatment could have influenced the evaluation of the results. Finally, a prior data analysis plan is also unknown in this study.
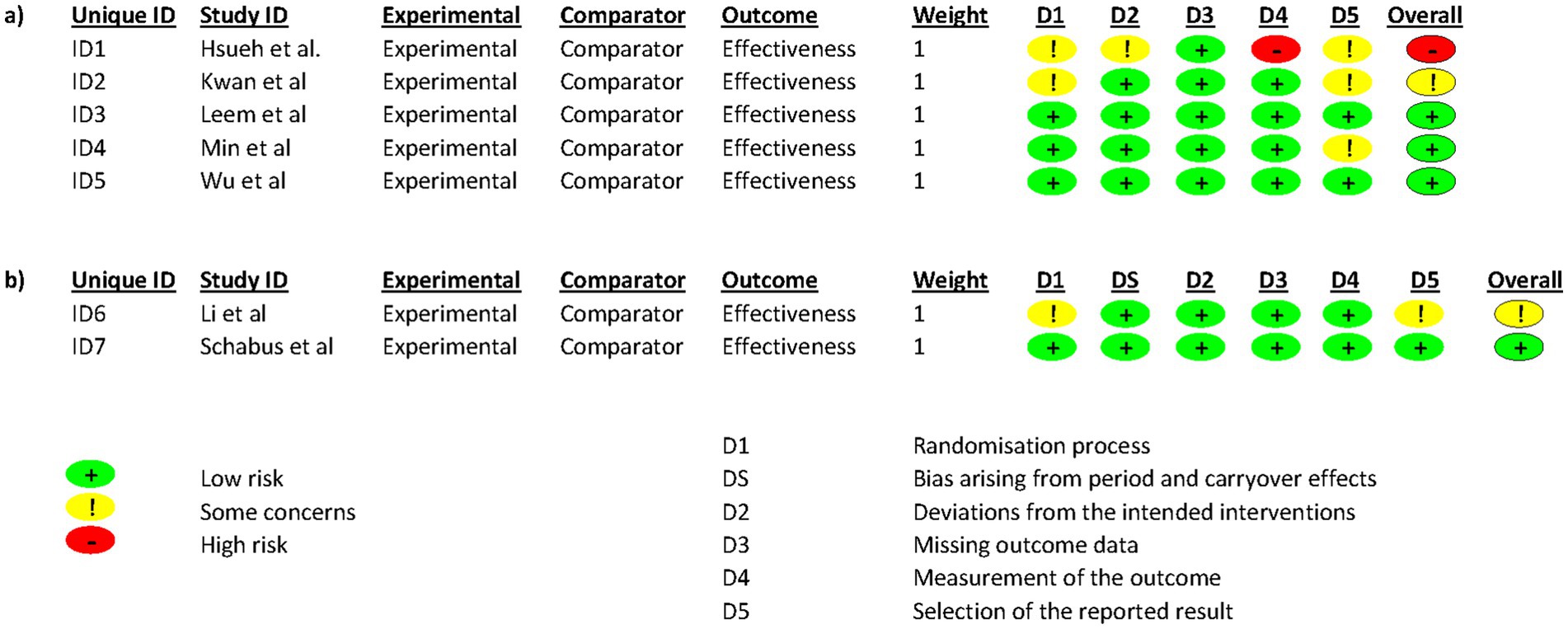
Figure 2. Risk of bias in each study. Red, green, and yellow colors indicate high, low, and unclear risk of bias, respectively.
3.5 Meta-analysis
Sleep quality defined with the PSQI total score.
A total of 5 studies that evaluated sleep quality with the PSQI total score were included in the meta-analysis (Li et al., 2024; Kwan et al., 2022; Schabus et al., 2017; Hsueh et al., 2016; Wu et al., 2021). Since no significant heterogeneity was observed among the included studies (I2 = 28%; p = 0.22), a fixed-effect model was used for merging the data. The results revealed that control conditions succeeded in improving PSQI-assessed sleep quality more than the analyzed NF interventions (PSQI total score 0.57; 95% CI 0.13 to 1.01; p = 0.01), as shown in Figure 3a.
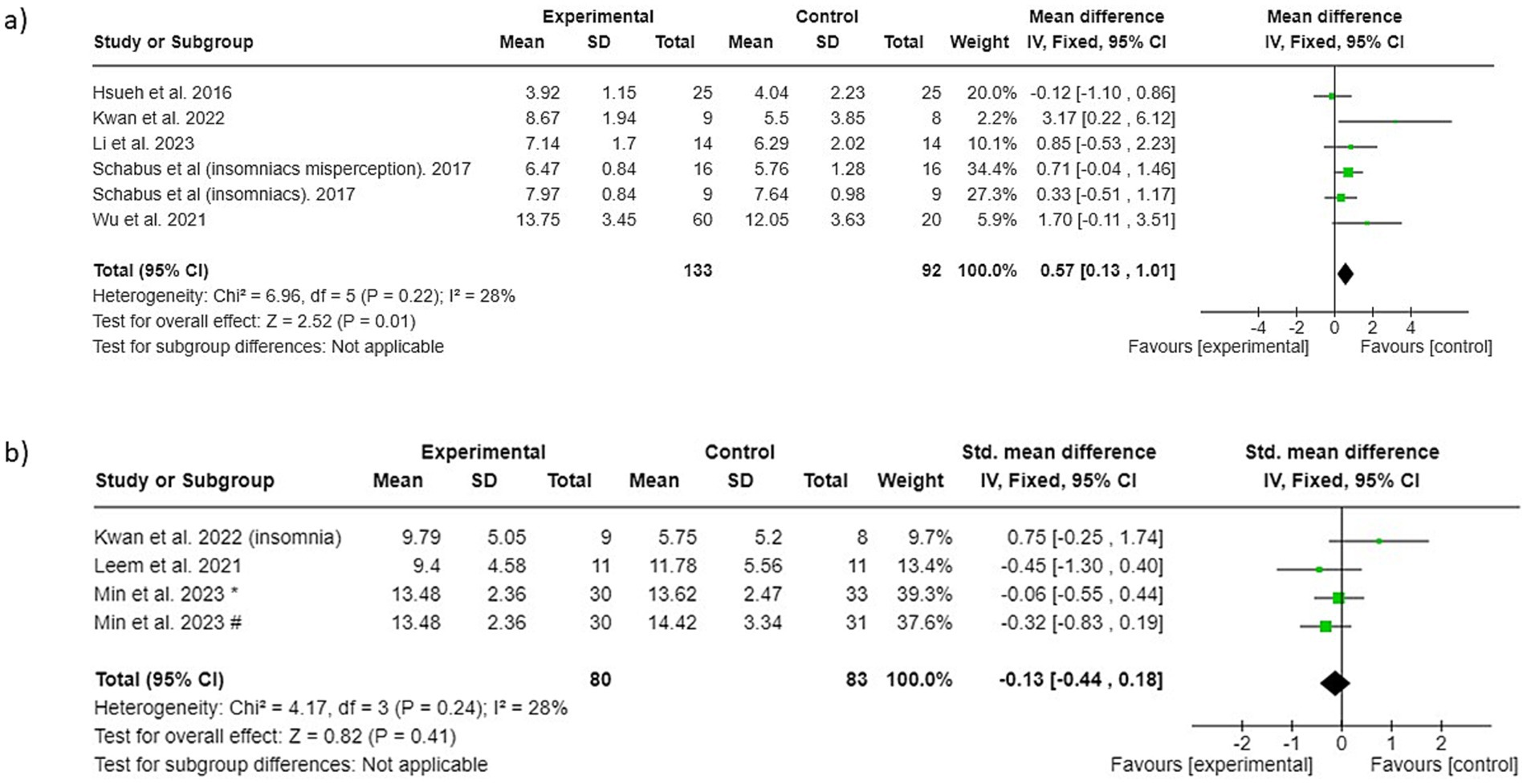
Figure 3. Forest plot of surface neurofeedback interventions versus control conditions: (a) sleep quality (b) insomnia. * NF groups vs. control condition 1, # NF groups vs. control condition 2.
The results of the mean scores on the PSQI pre and post-immediate time can be seen in Supplementary Table S3.
Insomnia severity defined with the insomnia scales total score.
A total of 3 studies that evaluated insomnia severity with various insomnia scales were included in the meta-analysis (Kwan et al., 2022; Leem et al., 2021; Min et al., 2023). Since no significant heterogeneity was observed among the included studies (I2 = 28%; p = 0.24), a fixed-effect model was used for merging the data. The results revealed that neither the NF interventions nor the control conditions show a favorable outcome relative to each other (−0.13; 95% CI −0.44 to 0.18; p = 0.41), as shown in Figure 3b.
The results of the mean scores on the insomnia scales pre and post-immediate time can be seen in Supplementary Table S4.
3.6 Other sleep quality indicators
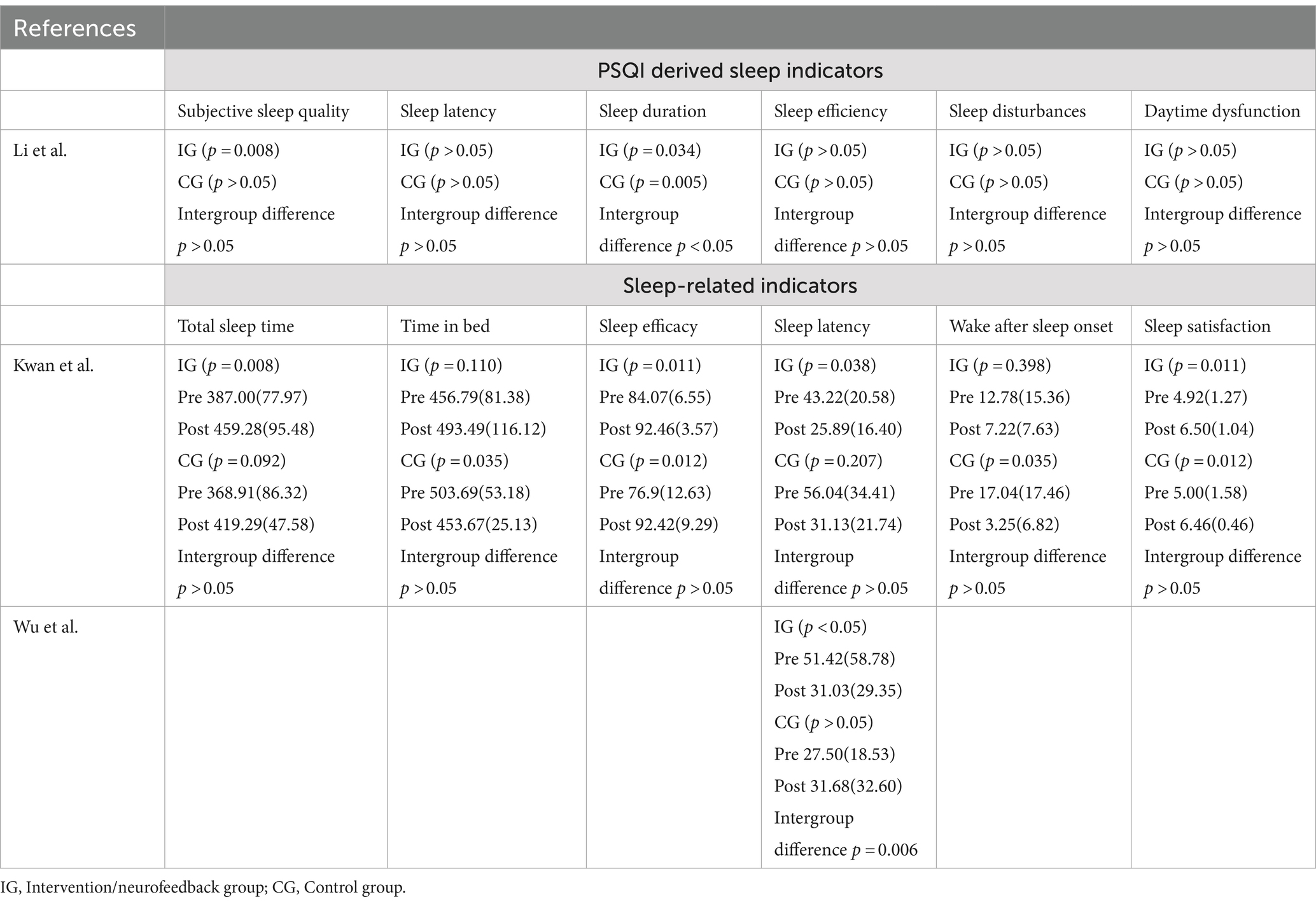
Table 3 shows the results and intra- and intergroup differences in other sleep quality indicators. Only the study by Li et al. (2024) analysed differences in the scoring of the dimensions of which the PSQI is composed, showing an intra-group difference within the NF intervention groups in the dimensions of sleep duration, with better scores in the post-intervention assessment.
Kwan et al. (2022) found no intergroup differences in the variables analysed, although intragroup differences within the NF groups were observed in the variables of total sleep time, sleep efficiency, sleep latency and sleep satisfaction. In contrast, the work of Wu et al. (2021) shows better sleep latency (time elapsed between turning off the light and the onset of the first sleep phase) at the post-intervention visit in the NF group compared to the control conditions.
4 Discussion
4.1 Principal findings
To our knowledge, this is the first study to conduct a systematic review and meta-analysis addressing the effect of surface NF techniques on self-perceived sleep quality and severity of insomnia by analyzing the results and quality of recent RCTs. The number of clinical trials selected and analysed is still limited, although it has grown in recent years. However, the selected studies are generally of good scientific quality, although small in relation to their sample size. The main results of the systematic review and meta-analysis do not show beneficial results of these surface NF techniques for the improvement of self-perceived sleep quality, with the groups used as control condition showing a slightly favorable relationship, as a consequence of the diversity and intensity of the interventions used as control. In relation to the self-perception of the severity of the signs of insomnia, neither the surface NF groups nor the groups used as control condition show a favorable relationship.
4.2 Characteristics of the analyzed studies
As shown by the heterogeneity results of the meta-analyses, the studies analyzed are moderately homogeneous and of contrasted quality. However, there are some issues that should be taken into consideration when making an adequate interpretation of the results. The first is that the number of studies analyzed (n = 7) and the total number of participants (n = 302) are still limited. In addition, most of the studies were conducted in 3 countries (Korea, Taiwan and China) with an Eastern population and only one study worked with a Western population. On the other hand, the health conditions of the populations included in the studies include both people with sleep disorders and people without these problems but with other processes that can affect the perception of sleep quality, such as perceived stress or fibromyalgia (Huang et al., 2024; Tafoya et al., 2023; Osorio et al., 2006; Wu et al., 2017). Finally, there is a clear deviation in the biological sex of the participants included in the different studies, with the majority being women.
4.3 Study parameters for surface neurofeedback and control conditions
Most of the studies analyzed (5 out of 7) use a NF training protocol based on maintaining alpha waves in a range between 8 and 12 Hz. The main characteristic of this brain rhythm is its association with the visual system, recorded mainly in the occipital area, which is clearly increased when we close our eyes (Morrone and Minini, 2023). Alpha brain waves are usually associated with relaxed and pleasant moods and are therefore used in the process of relaxation (muscle relaxation), which eventually leads to sleep. Alpha training is often used for the treatment of various conditions, such as pain relief, stress and anxiety reduction, memory improvement, mental performance enhancement and treatment of brain injuries (Lentz et al., 1999; Vernon, 2005).
There is no uniformity in the placement of electrodes for these sessions depending on the objectives of each particular study or the particularities of each device used. The placement of the electrodes for training is of vital importance to achieve appropriate results. It has been described, for example, that training along the right sensorimotor hemisphere of the right hemisphere (C4) can invoke feelings, emotions or calmness and increase concentration. Training on the opposite side (C3) could lead to undesired results such as a depletion of mental energy (Marzbani et al., 2016; Carrobles, 2016; Ribeiro et al., 2023).
Finally, none of the studies analyzed explored the minimum number of sessions necessary to achieve certain beneficial results in relation to sleep quality or the improvement of signs of insomnia. It does seem that all the interventions coincide in proposing an intensive protocol with at least 2 or 3 sessions per week for periods of at least 4 weeks. Interestingly, in this regard, the work of Min et al. (2023) planned a total of 56 self-managed sessions (2 per day) over a period of 4 weeks. The approach of self-managed sessions may increase adherence to the intervention, making it possible to implement this type of intervention in a wider population.
4.4 Sleep quality and insomnia
In the groups studied in this review that used NF, all of them obtained a discrete improvement in their final PSQI score. But when this change was compared against control conditions the results did not offer an additional benefit. On the contrary, the results of the meta-analysis conclude that, in the case of self-perceived sleep quality through the PSQI, the groups used as control conditions obtain more favorable results than the NF groups. This finding requires a deep reflection on the possible reasons underlying this relationship. In this meta-analysis, a total of 5 studies were included that compared each NF intervention against different control conditions in each study. These control conditions ranged from placebo feedback or heart rate feedback to intensive cognitive behavioral treatment interventions or intensive telephone support. Intensive interventions with cognitive behavioral treatment have shown very beneficial results on sleep quality when tested independently (De Niet et al., 2009; Barrios Araya et al., 2023; Schramm et al., 2016). The same has been reported for other interventions incorporating biofeedback, e.g., heart rate biofeedback (Li et al., 2022). Another aspect to consider is that the quality of sleep assessed with the PSQI total score reflects multiple aspects of sleep quality. And these aspects can have a very variable result if we analyze them independently. For example, the only study that analyzes the response of the interventions on the different dimensions of the PSQI is the work of Li et al. (2024). The intervention analyzed in this work obtains favorable results in subjective sleep quality and sleep duration. Therefore, future work should analyze which particular aspects of sleep quality may benefit from these NF techniques.
The results extracted from this review show that, although the use of NF seems encouraging for the treatment of insomnia, there are few studies in this field of research. In the 3 studies analyzed, the groups that used NF techniques achieved more beneficial scores on self-perceived severity of insomnia after the intervention, but the changes in these scores were not significant with respect to the groups used as control conditions. In 2021, this relationship has already been analyzed in a review by Lambert-Beaudet et al. (2021) which concludes exactly the same as in this work. Lambert-Beaudet et al. (2021), however, did not perform a meta-analysis of RCTs nor did they perform a systematic review, but among the possible reasons described for not finding satisfactory results are a are many of the ones presented here: (1) Lack of consensus in the protocols used: In two of the three studies an alpha wave maintenance training protocol was used and in another study a maintain of the sigma waves protocol (12–15 Hz). In addition, electrode positioning was also variable between frontal and parietal electrode positioning. There was more agreement on the minimum time of each session, at least 10 min, but not on the number of sessions, with two papers with less than 20 sessions, despite some researchers suggesting that up to 40 sessions of NF are necessary to effectively change behavior and symptoms (Thibault et al., 2017) (2) Small sample sizes: With the exception of the study by Min et al. (2023) (n = 94), the rest of the studies had a very small sample (17 and 22 participants) (3) Insufficient placebo control: The control conditions, as with the studies included in the meta-analysis of sleep quality, were very variable. Only the work of Leem et al. (2021) compared their intervention against the usual treatment and it is in this work where the most favorable results for the NF groups are found. Only two of the included studies (Schabus et al., 2017; Hsueh et al., 2016) compared NF versus placebo feedback, although, as suggested in the review by Lambert-Beaudet et al. (2021) to develop a truly inactive placebo treatment will require improvements to those currently in use in research. (4) Possible bias: In contrast to the review by Lambert-Beaudet et al. (2021), in our meta-analysis the studies included, due to the selection criteria used, allow us to highlight the high quality of the RCTs.
4.5 Strengths and limitations
This systematic review and meta-analysis of RCTs was carried out following the protocol described in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis: (PRISMA) statement. The search for studies was carried out in several large databases with broad search terms related to possible papers dealing with the topic, although there may have been some papers that could not be found and analyzed. The review only included Surface neurofeedback-type interventions, so there may have been some mixed interventions or interventions not sufficiently described that were not included. However, this is the most common type of NF and with the widest range of devices available to carry it out. The inclusion of studies with data on sleep quality or self-perceived insomnia through validated questionnaires has allowed us to perform a meta-analysis and has made it possible to include studies with a moderate level of heterogeneity. However, these results are self-perceived by the participants, which entails possible biases in the response of the participants in each study. In six of the seven clinical trials included in this work, in addition to the self-reported sleep assessment questionnaires, physiological sleep assessments have been used, which might provide additional important information regarding the effectiveness of NF for sleep quality.
Finally, moderators could not be explored due to small sample sizes of the included RCTs, and inability to detect moderation effects. Also, the differences in electrode placement, NF protocols, and control groups across studies limit the ability to draw solid conclusions.
4.6 Implications for practice and future research
The results of this review and meta-analysis do not offer sufficient evidence to incorporate surface NF techniques as an alternative in the treatment of insomnia or the improvement of sleep quality due, among other reasons, to the control conditions with which they have been compared. It is therefore necessary to continue with this line of research by carrying out more quality RCTs, with a greater number of participants, with a more uniform protocol and objectives, with a minimum number of sessions or the possibility of using auto-guided sessions, prior training, and comparing against control conditions that include random placebo feedback and focusing on people with previous sleep disorders who may benefit from it, studying the implications on insomnia and all aspects present in the quality of sleep. Furthermore, as noted above, in addition to self-reported sleep assessment questionnaires, physiological sleep assessments have been used, which could provide important additional information on the efficacy of NF for sleep quality.
5 Conclusion
This systematic review and meta-analysis demonstrate that interventions incorporating surface neurofeedback do not produce additional benefits in self-perception of sleep quality or insomnia compared to a wide variety of control conditions including cognitive behavioral treatment or other biofeedback modalities. The interventions studied mostly apply a NF training protocol based on maintaining alpha waves in a range between 8 and 12 Hz, with electrode positioning in the frontal area or in the sensorimotor cortex and with a number of NF sessions ranging from 8 to 20 sessions. However, the number of studies found and analyzed is still scarce, and more RCTs with larger samples and with greater uniformity in their protocols and objectives are needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JR-R: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MF-C: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NS-A: Methodology, Visualization, Writing – original draft, Writing – review & editing. JG-S: Methodology, Visualization, Writing – original draft, Writing – review & editing. IG-Y: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. RA-D: Methodology, Visualization, Writing – original draft, Writing – review & editing. H-YC: Methodology, Visualization, Writing – original draft, Writing – review & editing. P-ST: Methodology, Visualization, Writing – original draft, Writing – review & editing. H-CL: Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. MR-G: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Gerencia Regional de Salud de Castilla y Leon, Spain (GRS2709/A1/2023) and Instituto de Investigación Biomédica de Salamanca-IBSAL (IBYE23_00001). Neither of the two institutions has no authority in the study design, collection, management, analysis, or interpretation of the data.
Acknowledgments
The researchers would like to thank the following institutions: to all the professors and researchers of the University of Salamanca who made it possible, to the José Castillejo Foreign Mobility Stays Program for young PhDs, call 2022 (CAS22/00084) of the Ministry of Science, Innovation and Universities of the Government of Spain. Finally, to the Taiwan Fellowship Program of the Ministry of Foreign Affairs of the Republic of China (Taiwan).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1450163/full#supplementary-material
References
Banerjee, S., and Argáez, C. (2017) Neurofeedback and biofeedback for mood and anxiety disorders: a review of clinical effectiveness and guidelines; pp. 1–26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/30299634
Barrios Araya, S. C., Masalan Apip, M. P., Ferrada Calvo, X. V., Campos-Romero, S. C., and Molina Muñoz, Y. P. (2023). Sleep quality and fatigue in construction workers: effect of a cognitive behavioral intervention. J. Occup. Environ. Med. 65, 235–241. doi: 10.1097/JOM.0000000000002716
Carrobles, J. A. (2016). Bio/neurofeedback. Clínica y Salud. 27, 125–131. doi: 10.1016/j.clysa.2016.09.003
De Niet, G. J., Tiemens, B. G., Kloos, M. W., and Hutschemaekers, G. J. (2009). Review of systematic reviews about the efficacy of non-pharmacological interventions to improve sleep quality in insomnia. Int. J. Evid. Based Healthc. 7, 233–242. doi: 10.1111/j.1744-1609.2009.00142.x
Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., et al. (2023). Cochrane handbook for systematic reviews of interventions version 6.4 (updated august 2023). Cochrane [Internet]. Available at: www.training.cochrane.org/handbook
Hsueh, J.-J., Chen, T.-S., Chen, J.-J., and Shaw, F.-Z. (2016). Neurofeedback training of EEG alpha rhythm enhances episodic and working memory. Hum. Brain Mapp. 37, 2662–2675. doi: 10.1002/hbm.23201
Huang, W., Wen, X., Li, Y., and Luo, C. (2024). Association of perceived stress and sleep quality among medical students: the mediating role of anxiety and depression symptoms during COVID-19. Front. Psych. 15:1272486. doi: 10.3389/fpsyt.2024.1272486
Ibáñez, V., Silva, J., Navarro, E., and Cauli, O. (2019). Sleep assessment devices: types, market analysis, and a critical view on accuracy and validation. Expert Rev. Med. Devices 16, 1041–1052. doi: 10.1080/17434440.2019.1693890
Jiménez-Vaquero, C., Alonso-Dominguez, R., Garcia-Yu, I. A., Martín-Nogueras, A. M., Sánchez-Aguadero, N., Crespo-Sedano, A., et al. (2023). Analysis of the determinants of sleep quality in a Spanish population: a study protocol for a cross-sectional study. BMJ Open 13:e069444. doi: 10.1136/bmjopen-2022-069444
Kanda, K., Hirao, T., Ngatu, N. R., Murakami, A., Yamadori, Y., Yokoyama, K., et al. (2023). A comparison of sleep duration accuracy between questionnaire and accelerometer in middle childhood. Cureus. 15:e47236. doi: 10.7759/cureus.47236
Kwan, Y., Yoon, S., Suh, S., and Choi, S. (2022). A randomized controlled trial comparing Neurofeedback and cognitive-behavioral therapy for insomnia patients: pilot study. Appl. Psychophysiol. Biofeedback 47, 95–106. doi: 10.1007/s10484-022-09534-6
Lambert-Beaudet, F., Journault, W.-G., Rudziavic Provençal, A., and Bastien, C. H. (2021). Neurofeedback for insomnia: current state of research. World J. Psychiatry. 11, 897–914. doi: 10.5498/wjp.v11.i10.897
Leem, J., Cheong, M. J., Lee, H., Cho, E., Lee, S. Y., Kim, G.-W., et al. (2021). Effectiveness, cost-utility, and safety of Neurofeedback self-regulating training in patients with post-traumatic stress disorder: a randomized controlled trial. Healthcare (Basel, Switzerland) 9:1351. doi: 10.3390/healthcare9101351
Lentz, M. J., Landis, C. A., Rothermel, J., and Shaver, J. L. (1999). Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J. Rheumatol. 26, 1586–1592
Li, Q., Shi, M., Steward, C. J., Che, K., and Zhou, Y. (2024). A comparison between pre-sleep heart rate variability biofeedback and electroencephalographic biofeedback training on sleep in national level athletes with sleep disturbances. Appl. Psychophysiol. Biofeedback 49, 115–124. doi: 10.1007/s10484-023-09604-3
Li, Q., Steward, C. J., Cullen, T., Che, K., and Zhou, Y. (2022). Presleep heart-rate variability biofeedback improves mood and sleep quality in Chinese winter Olympic bobsleigh athletes. Int. J. Sports Physiol. Perform. 17, 1516–1526. doi: 10.1123/ijspp.2022-0037
Marzbani, H., Marateb, H. R., and Mansourian, M. (2016). Neurofeedback: a comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 7, 143–158. doi: 10.15412/J.BCN.03070208
Min, B., Park, H., Kim, J. I., Lee, S., Back, S., Lee, E., et al. (2023). The effectiveness of a Neurofeedback-assisted mindfulness training program using a Mobile app on stress reduction in employees: randomized controlled trial. JMIR Mhealth Uhealth 11:11. doi: 10.2196/42851
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. doi: 10.1136/bmj.b2535
Morrone, J., and Minini, L. (2023). The interlinking of alpha waves and visuospatial cognition in motor-based domains. Neurosci. Biobehav. Rev. 149:105152. doi: 10.1016/j.neubiorev.2023.105152
Nelson, K. L., Davis, J. E., and Corbett, C. F. (2022). Sleep quality: an evolutionary concept analysis. Nurs. Forum 57, 144–151. doi: 10.1111/nuf.12659
Osorio, C. D., Gallinaro, A. L., Lorenzi-Filho, G., and Lage, L. V. (2006). Sleep quality in patients with fibromyalgia using the Pittsburgh sleep quality index. J. Rheumatol. 33, 1863–1865
Paul, A. M., and Salas, R. E. (2024). Insomnia. Prim. Care 51, 299–310. doi: 10.1016/j.pop.2024.02.002
Ribeiro, T. F., Carriello, M. A., de Paula, E. P. J., Garcia, A. C., da Rocha, G. L., and Teive, H. A. G. (2023). Clinical applications of neurofeedback based on sensorimotor rhythm: A systematic review and meta-analysis. Front. Neurosci. 17:1195066. doi: 10.3389/fnins.2023.1195066
Schabus, M., Griessenberger, H., Gnjezda, M.-T., Heib, D. P. J., Wislowska, M., and Hoedlmoser, K. (2017). Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia. Brain 140, 1041–1052. doi: 10.1093/brain/awx011
Schramm, P. J., Zobel, I., Mönch, K., Schramm, E., and Michalak, J. (2016). Sleep quality changes in chronically depressed patients treated with mindfulness-based cognitive therapy or the cognitive behavioral analysis system of psychotherapy: a pilot study. Sleep Med. 17, 57–63. doi: 10.1016/j.sleep.2015.09.022
Silva, G. E., Vana, K. D., Goodwin, J. L., Sherrill, D. L., and Quan, S. F. (2011). Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-bang, and Epworth sleepiness scales. J. Clin. Sleep Med. 7, 467–472. doi: 10.5664/JCSM.1308
Tafoya, S. A., Aldrete-Cortez, V., Tafoya-Ramos, F., Fouilloux-Morales, C., and Díaz-Olavarrieta, C. (2023). Sleep and perceived stress: an exploratory mediation analysis of the role of self-control and resilience among university students. Int. J. Environ. Res. Public Health 20:6560. doi: 10.3390/ijerph20166560
Thibault, R. T., Lifshitz, M., and Raz, A. (2017). Neurofeedback or neuroplacebo? Brain 140, 862–864. doi: 10.1093/brain/awx033
Vernon, D. J. (2005). Can neurofeedback training enhance performance? An evaluation of the evidence with implications for future research. Appl. Psychophysiol. Biofeedback 30, 347–364. doi: 10.1007/s10484-005-8421-4
Wang, C., Bangdiwala, S. I., Rangarajan, S., Lear, S. A., AlHabib, K. F., Mohan, V., et al. (2019). Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur. Heart J. 40, 1620–1629. doi: 10.1093/eurheartj/ehy695
Wu, Y.-L., Chang, L.-Y., Lee, H.-C., Fang, S.-C., and Tsai, P.-S. (2017). Sleep disturbances in fibromyalgia: a meta-analysis of case-control studies. J. Psychosom. Res. 96, 89–97. doi: 10.1016/j.jpsychores.2017.03.011
Wu, Y.-L., Fang, S.-C., Chen, S.-C., Tai, C.-J., and Tsai, P.-S. (2021). Effects of Neurofeedback on fibromyalgia: a randomized controlled trial. Pain Manag. Nurs. 22, 755–763. doi: 10.1016/j.pmn.2021.01.004
Keywords: neurofeedback, sleep quality, insomnia, biofeedback, brain waves
Citation: Recio-Rodriguez JI, Fernandez-Crespo M, Sanchez-Aguadero N, Gonzalez-Sanchez J, Garcia-Yu IA, Alonso-Dominguez R, Chiu H-Y, Tsai P-S, Lee H-C and Rihuete-Galve MI (2024) Neurofeedback to enhance sleep quality and insomnia: a systematic review and meta-analysis of randomized clinical trials. Front. Neurosci. 18:1450163. doi: 10.3389/fnins.2024.1450163
Edited by:
Michael T. Smith, Johns Hopkins University, United StatesReviewed by:
Christopher Amalraj Vallaba Doss, Imam Abdulrahman Bin Faisal University, Saudi ArabiaHeather Altier, Johns Hopkins University, United States
Copyright © 2024 Recio-Rodriguez, Fernandez-Crespo, Sanchez-Aguadero, Gonzalez-Sanchez, Garcia-Yu, Alonso-Dominguez, Chiu, Tsai, Lee and Rihuete-Galve. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose I. Recio-Rodriguez, ZG9ucmVjaW9AdXNhbC5lcw==
 Jose I. Recio-Rodriguez
Jose I. Recio-Rodriguez Mei Fernandez-Crespo
Mei Fernandez-Crespo Natalia Sanchez-Aguadero1
Natalia Sanchez-Aguadero1 Hsin-Chien Lee
Hsin-Chien Lee