- 1Department of Otorhinolaryngology, The Fifth Clinical Medical College of Shanxi Medical University, Taiyuan, China
- 2Graduate School, Shanxi Medical University, Taiyuan, China
- 3Department of Surgery, Children's Hospital of Shanxi Province, Taiyuan, China
Objective: The aim of this is to explore changes in cross-modal reorganization within the auditory–visual cortex after cochlear implantation, examining their influence on auditory and speech functions along with their underlying mechanisms.
Methods: Twenty prelingually deaf children who received cochlear implantation and rehabilitation training at our hospital between February 2022 and February 2023 comprised the prelingual deaf group. Simultaneously, 20 healthy children served as the control group. The prelingual deaf group underwent brain cortical activity assessment and evaluation of auditory-speech recovery pre-surgery, at postoperative weeks 1 and 2, and at months 1, 3, 6, 9, and 12. The control group underwent parallel assessments and evaluations. We analyzed the correlation between cortical activity in the auditory–visual cortex of patients and their auditory-speech functional recovery.
Results: The group with prelingual deafness displayed elevated levels of auditory and visual cortical electromagnetic intensity compared to the control group, both prior to and 9 months after surgery. However, by the 12-month mark post-surgery, there was no discernible distinction between the two groups. Following surgery, the prelingually deaf group exhibited a progressive improvement in both Categories of Auditory Performance (CAP) and Speech Intelligibility Rate (SIR), initially lagging behind the control group. Notably, a negative correlation emerged between auditory and visual cortical electromagnetic intensity values and CAP/SIR scores at the 12-month post-surgery assessment.
Conclusion: Cochlear implantation in prelingually deaf children results in elevated activity within the auditory and visual cortices, demonstrated by heightened electromagnetic intensity readings. Cross-modal reorganization is observed temporarily at 3 months post-surgery, which resolves to baseline levels by 12 months post-surgery. This phenomenon of reversal correlates with the restoration of auditory and speech functions in these children.
1 Introduction
Cochlear implant is a common way to treat deafness, which can replace the inner ear hair cells in the diseased area and cause direct electrical stimulation to the auditory nerve fibers to promote the production of hearing (Saki et al., 2022). Although cochlear implants can help hearing-impaired children recover hearing and speech function, the effect after implantation still varies greatly (Niparko et al., 2010; Geers et al., 2011), especially in noisy environments (Saksida et al., 2022). It is not clear why some children with cochlear implants have poor audiotory and speech perception after implantation. Many factors, such as strategy for rehabilitation of communication, age of onset of hearing loss, duration of deafness, age of cochlear implantation, experience of wearing hearing-Aids, and duration of use of cochlear implantation, have an impact on the outcome of auditory and speech perception, but the huge differences in the development of auditory skills in children with cochlear implantation remain unexplained (Zeng, 2004; Tomblin et al., 2005; Lin et al., 2008; Niparko et al., 2010; Tobey et al., 2013). In recent years, studies have found that after the loss of hearing function, the brain will use the auditory cortex to assist in processing when the visual cortex processes stimulus signals. Visual compensation causes changes in the cerebral cortex, which is clinically called brain cross-modal functional recombination (Stroh et al., 2022). This neuroplasticity may provide adaptive benefits after hearing deprivation by enhancing non-auditory skills, such as superior visual speech reading skills (Rouger et al., 2007). On the other hand, it has also been shown to be related to behavioral measures of speech performance (Strelnikov et al., 2013; Anderson et al., 2017; Fullerton et al., 2023). This adult cochlear implant research literature suggests that cross-modal plasticity may be another factor affecting speech perception outcomes in children with cochlear implants. However, the effects of cochlear implantation on the cross-modal plasticity of the auditory visual cortex in children with hearing loss have not yet been fully defined in clinic, and the mechanism of cochlear implantation affecting the recovery of hearing and speech function needs to be further explored. Therefore, this study aims to analyze this and provide a basis for clinical intervention, as reported below.
2 Materials and methods
2.1 General demographic profile
The prelingually deaf group consisted of 20 children admitted to our hospital between February 2020 and February 2022, who underwent cochlear implantation and speech rehabilitation training. Additionally, a control group of 20 healthy children, who underwent physical examinations at our hospital during the same timeframe, was chosen. Inclusion criteria encompassed: (1) age between 2 and 7 years old; (2) prelingually deaf children with cochlear implants; (3) ability to cooperate with cortical activity detection, auditory, and visual function assessments; (4) normal hearing function in healthy children as indicated by standard auditory tests; (5) native Mandarin speakers; (6) informed consent obtained from parents or guardians. Exclusion criteria comprised: (1) acute inflammatory infections, acute/chronic renal failure, lung disease, immune disorders, coronary artery disease, chronic liver disease, or mental disorders; (2) abnormal cochlear electrode placement or cochlear abnormalities; (3) unwillingness to cooperate with examinations.
This research protocol has been approved by the ethics committee.
2.2 Detection and evaluation methods
The control group of children underwent assessment solely on the day of their physical examination. In contrast, the group diagnosed with prelingual deafness underwent evaluation prior to surgery, followed by assessments during the first and second weeks post-surgery, and subsequently at intervals of 1, 3, 6, 9, and 12 months post-surgery.
2.2.1 Brain cortical activity detection
The EGS400 electroencephalogram (EEG) system that caters to both pediatric and adult populations was manufactured by EGI, a US-based company, and was utilized for detection purposes. Prior to conducting examinations, the child and their guardians were provided with detailed instructions by the examiner. During testing, patients were comfortably seated while wearing an electrode cap.
1. Visual Stimulation Test: Each patient underwent an evaluation process when event-related potential (ERP) was triggered through visual stimulation tests. These tests employed silent or auditory pictures controlled by specialized psychological experiment software called Eprime 2.0. The pictures were displayed on a high-quality video graphic array computer screen whose dimensions were 350 × 350 pixels. Both types of pictures constituted independent stimulus sequences, with each stimulus lasting 1,000 ms. A total of 60 stimuli were presented at intervals ranging between 1,700 and 1,900 ms. Within each sequence, one deviant stimulus, differed from previous ones, was introduced after every 5 to 10 stimuli. Following the completion of each picture stimulation session, patients were given approximately 10–15 s of rest.
2. Data Processing: After collecting data using Brainstorm, each patient’s averaged ERP segments were generated using an ERP analysis software. These averaged data sets facilitated calculations pertaining to brain functional activity source localization. This process enabled the recording of changes in auditory and visual cortical activities through electromagnetic intensity values. In the calculation process, the average models of finite difference model (FDM), computed tomography (CT) and fMRI were used to reconstruct and determine the distribution of 2,447 dipoles in the cerebral cortex. Standardized lower solution brain electromagnetic tomography was used for the inverse method. In the inverse model, regularization levels are adjusted to stabilize the source location results. The source location results showed the electromagnetic intensity signal value, which was used to represent the cerebral cortex activity level and recorded the changes of auditory and visual cortex activity (electromagnetic intensity value) of each subject.
2.2.2 Assessment of auditory and language function recovery
The assessment tools utilized in this study to evaluate the recovery of auditory and speech functions include the Categories of Auditory Performance (CAP) questionnaire and the Speech Intelligibility Rate (SIR) questionnaire. The CAP assessment, as outlined by experts, delineates various levels of auditory performance through 0 to 9 (Albalawi et al., 2019). For this study, corresponding scores were allocated for each level, with higher CAP scores indicative of superior auditory behavior. Similarly, the SIR assessment establishes distinct levels of speech intelligibility (Wang et al., 2013). The SIR score corresponds directly to language proficiency, with a higher score denoting superior proficiency.
2.3 Statistical methods
The data analysis utilized SPSS 20.0 software. Count data were expressed as frequencies (percentages) [n (%)] and subjected to chi-square testing. For normally distributed continuous variables, descriptive statistics were reported as mean ± standard deviation ( ± s), with significance determined using t-tests. Pearson’s linear correlation analysis was employed to evaluate the relationship between auditory and visual cortical activities in pediatric patients with CAP and SIR scores. Statistical significance was set at p < 0.05.
3 Results
3.1 Comparison of auditory cortical activity between two groups
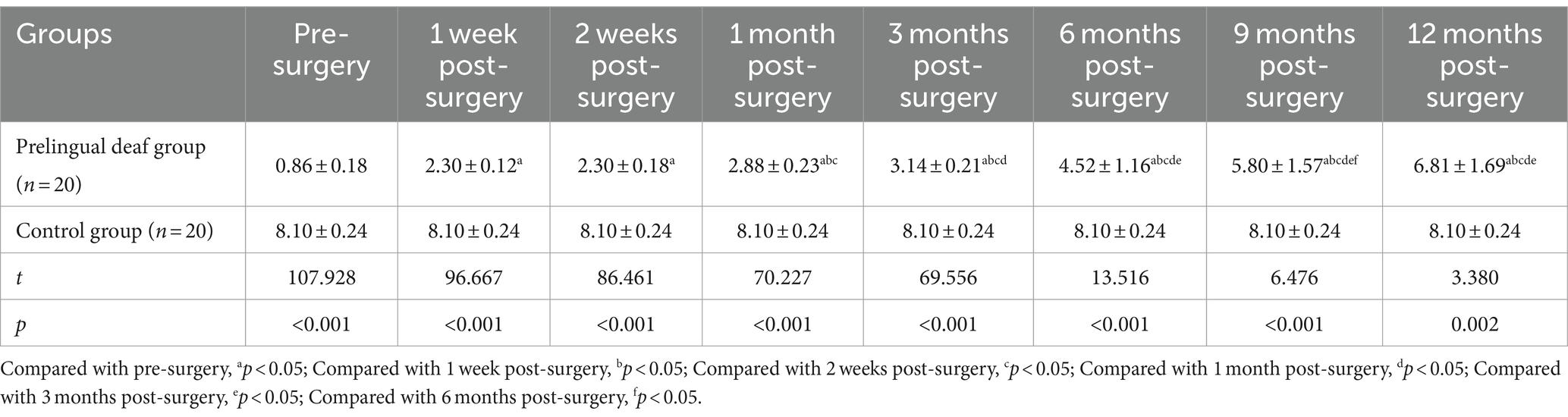
Since the control group consisted of typically developing children, only pertinent assessments were administered on the day of their physical examination to contrast their outcomes with those of the prelingually deaf group across various time periods. Notably, the auditory cortical electromagnetic intensity values in the prelingually deaf group showed a statistically significant rise compared to those in the control group from pre-surgery through 9 months post-surgery (p < 0.05).
However, there was no notable distinction in auditory cortical electromagnetic intensity values between the two groups at the 12-month mark post-surgery (p > 0.05). Within the prelingually deaf group, a gradual increase in auditory cortical electromagnetic intensity values was observed initially, from pre-surgery to 1 month post-surgery, followed by a subsequent gradual decline beginning at 3 months post-surgery, eventually stabilizing to a level akin to that observed in the control group at the 12-month post-surgery assessment (Table 1).
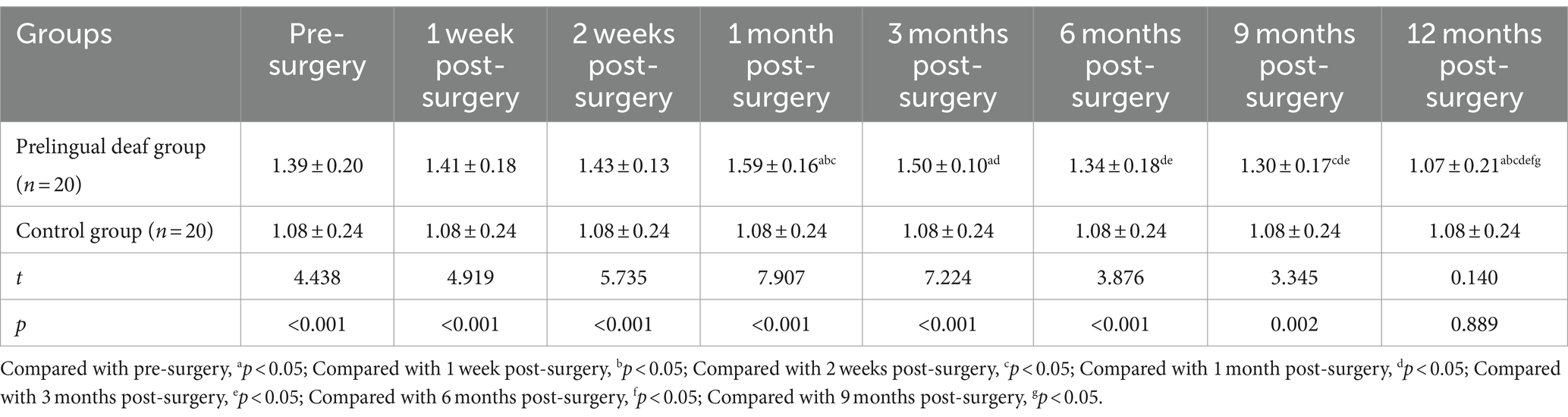
Table 1. Comparative analysis of auditory cortical electromagnetic intensity between the two groups (nA.m, x¯ ± s).
3.2 Comparison of visual cortical activity between two groups
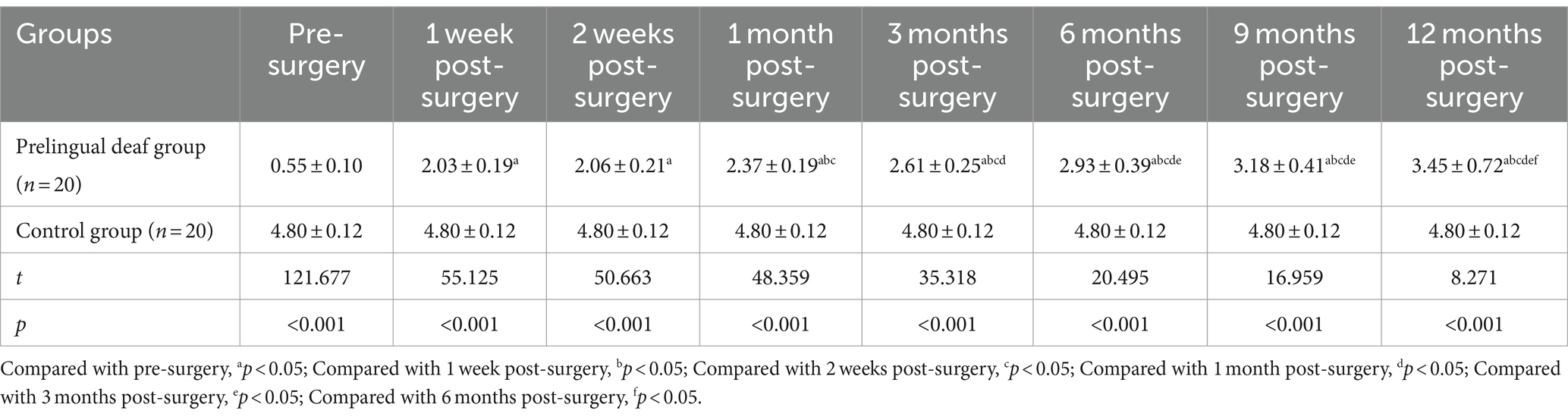
The prelingually deaf group demonstrated a notable rise in visual cortical electromagnetic intensity values compared to the control group from pre-surgery through 9 months post-surgery, as evidenced by statistically significant differences (p < 0.05). However, by the 12-month mark post-surgery, there was no significant variance in visual cortical electromagnetic intensity values between the two groups (p > 0.05). Specifically, within the prelingually deaf group, the peak in visual cortical electromagnetic intensity occurred at 1 month post-surgery, followed by a gradual decline from 3 months post-surgery onwards, eventually stabilizing near levels akin to those observed in the control group by the 12-month post-surgery mark (Table 2).
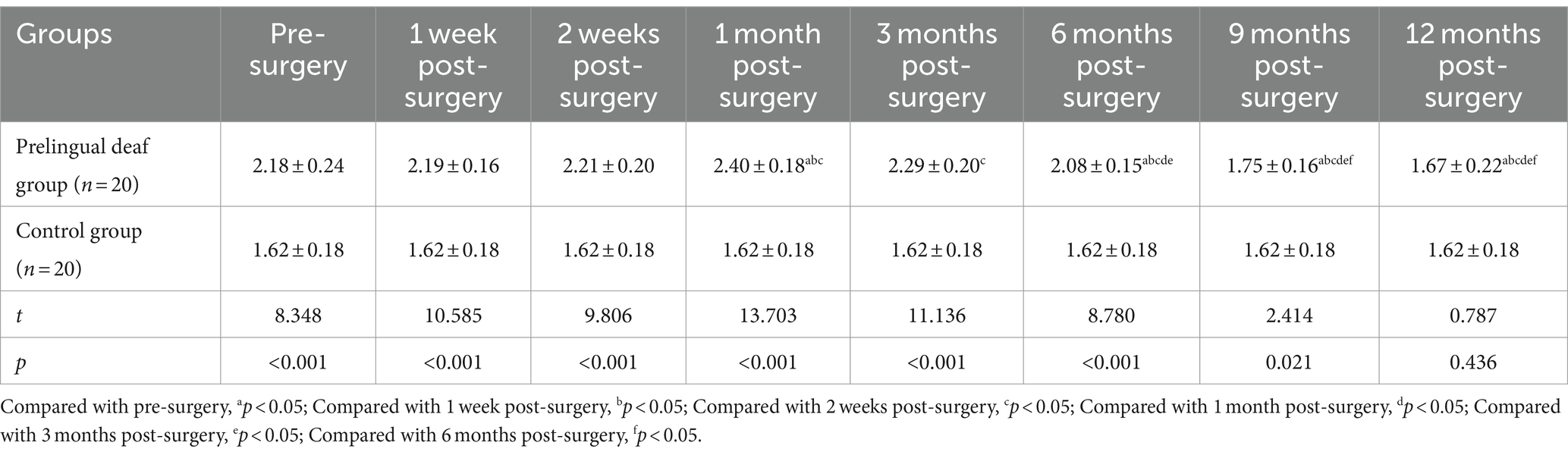
Table 2. Comparative analysis of visual cortical electromagnetic intensity between the two groups (nA.m, x¯ ± s).
3.3 Comparison of CAP scores between two groups
The prelingually deaf group demonstrated markedly lower CAP scores compared to the control group, both preoperatively and at the 12-month follow-up (p < 0.05). However, postoperative CAP scores in the prelingually deaf group displayed a progressive augmentation from 1 week to 12 months post-surgery, exceeding their preoperative levels (p < 0.05, Table 3).
3.4 Comparison of SIR scores between two groups
Before surgery and during the 12-month follow-up, the prelingually deaf group exhibited significantly lower SIR scores compared to the control group (p < 0.05). There was some noteworthy improvement in postoperative SIR scores in the prelingually deaf group from 1 week to 12 months after surgery (p < 0.05, Table 4).
3.5 Correlation analysis between auditory–visual cortical activity in prelingually deaf children and CAP/SIR scores
Significant fluctuations in auditory–visual cortical activity were noted among prelingually deaf children, with activity levels approaching those of the control group around 12 months post-surgery. Consequently, this study primarily investigates correlations among different indicators during this specific time frame. The results reveal a negative correlation between electromagnetic intensity values of auditory–visual cortical activity and CAP/SIR scores in these patients (p < 0.05, Table 5).
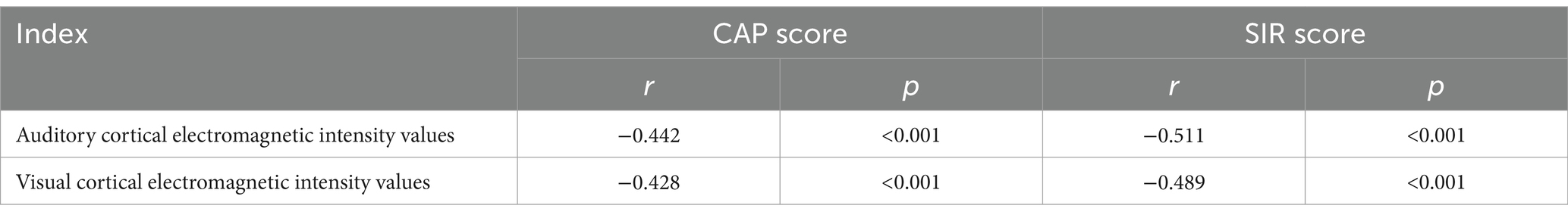
Table 5. Correlation analysis of auditory–visual cortical activity and CAP/SIR Scores in prelingually deaf children.
4 Discussion
Childhood deafness exerts a profound impact on both language and auditory development, posing significant challenges for affected families and society at large (Zheng, 2019). From recent data, it can be seen that there is a sizable population of approximately 427,000 disabled children aged 0–14 in China, the majority of whom grapple with severe disabilities necessitating early intervention strategies (Meng and Zhang, 2021). Among the interventions, cochlear implantation stands out as a widely adopted approach for individuals with hearing impairments, necessitating postoperative speech rehabilitation to bolster vocabulary acquisition and facilitate the recuperation of hearing and speech capabilities (Alzhrani et al., 2021; Aljazeeri et al., 2022). Yet, the precise mechanisms through which cochlear implantation fosters the restoration of auditory and speech functions in prelingually deaf children, who have experienced hearing loss before developing language skills, remain elusive.
It is becoming evident from emerging research that during the initial phases of cochlear implantation, the auditory cortex undergoes adaptations in visual processing, indicating potential alterations in brain activity across auditory and visual cortices among patients (Zhao et al., 2020). Given the pivotal importance of the first-year post-implantation for individuals with prelingual deafness in terms of hearing and speech rehabilitation, this study aims to examine fluctuations in cortical activity, alongside changes in auditory and speech functions during this critical period.
The primary objective of the study is to explore potential associations between cortical activity patterns and the restoration of auditory and speech functions in these patients, with the overarching goal of advancing our understanding of the underlying mechanisms. The results of this study indicate a notable increase in auditory and visual cortical electromagnetic intensity values in the prelingually deaf group following cochlear implant surgery, compared to the control group.
This increase peaked at 1 month post-surgery, with subsequent gradual normalization observed over the following months. These findings suggest the presence of abnormal activity in both auditory and visual cortices during the early stages of cochlear implantation in children with hearing loss. However, this aberrant activity diminishes over time, indicating a reversal of cross-modal functional reorganization within the brain. This phenomenon may arise from the initial inability of the auditory mechanisms of these children to compensate for the original visual system when utilizing cochlear implants, resulting in abnormal cortical activity during this period.
Nevertheless, usage of cochlear implant for more than a month prompts rapid development and heightened plasticity in the auditory cortex, leading to a reversal of cross-modal functional reorganization and adjustments in both auditory and visual cortical activities. Furthermore, prolonged speech training facilitates gradual normalization in both auditory and visual cortical activities.
The findings indicate that the visual cortex does not play a direct role in compensatory mechanisms. Thus, post-cochlear implantation, auditory input may shift central functional control away from vision, leading to a gradual decrease in visual cortical activity and promoting cross-modal functional reorganization in the brain (Xin et al., 2022). Consequently, prelingually deaf children initially display abnormal cortical activity after cochlear implantation, followed by a reversal phenomenon and a gradual return to normalcy.
This investigation uncovered a positive correlation between the duration of cochlear implantation in pediatric patients and the degree of improvement in auditory and speech functions, consistent with prior studies (Wang et al., 2022; Zhou et al., 2022). Subsequent examination of the interplay between cortical activity in children’s brains and the restoration of auditory and speech functions revealed a strong relationship between electromagnetic intensity values within auditory and visual cortices, alongside CAP and SIR scores. This underscores the critical role of cortical activity in fostering auditory and visual recovery.
Some scholars suggest that overreliance on visual aids post-cochlear implantation could impede optimal auditory function recovery. The restructuring of postoperative auditory cortex activity is pivotal in promoting auditory restoration, highlighting the impact of auditory cortical activity on auditory function (Sun et al., 2021). The reversal and restructuring of cross-modal functions in the cerebral cortex following cochlear implantation surgery are associated with the recovery of auditory and speech functions in deaf patients.
The potential normalization of abnormal activities in the auditory and visual cortices post-cochlear implantation may constitute one of the mechanisms influencing the efficacy of hearing and speech recovery. Despite previous reports on the effects of cochlear implantation on hearing, the underlying mechanisms remain elusive. This study brings forth potential mechanisms to offer evidence-based support for clinical interventions.
5 Conclusion
The timely implementation of cochlear implants in prelingually deaf children leads to distinctive auditory–visual cortical patterns. Over time, prolonged implantation triggers a reversal of cross-modal functional changes in the brain, gradually restoring typical activity. This phenomenon plays a crucial role in the restoration of auditory and speech capabilities in affected individuals. However, it is crucial to recognize the limitations of this study, including the possibility of subjective bias in scale assessments. Thus, there is a pressing need for more dependable methodologies to assess progress towards recovery.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by Ethics Committee of the Fifth Clinical Medical College of Shanxi Medical University (No. 2022-36). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
X-FQ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. L-DL: Investigation, Project administration, Supervision, Visualization, Writing – review & editing. L-YH: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. YC: Methodology, Project administration, Validation, Writing – review & editing. XL: Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Central Government guided local Science and Technology development Fund Project (Basic research of free exploration type; No. YDZJSX2022A070).
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CAP, Categories of auditory performance; SIR, Speech intelligibility rate; EEG, Electroencephalogram; ERP, Event-related potential.
References
Albalawi, Y., Nidami, M., Almohawas, F., Hagr, A., and Garadat, S. N. (2019). Categories of auditory performance and speech intelligibility ratings in prelingually deaf children with bilateral implantation. Am. J. Audiol. 28, 62–68. doi: 10.1044/2018_AJA-17-0112
Aljazeeri, I., Hamed, N., Abdelsamad, Y., Sharif, T., al-Momani, M., and Hagr, A. (2022). Anatomy-based frequency allocation in Cochlear implantation: the importance of Cochlear coverage. Laryngoscope 132, 2224–2231. doi: 10.1002/lary.30004
Alzhrani, F., Halawani, R., Basodan, S., and Hudeib, R. (2021). Investigating facial nerve stimulation after Cochlear implantation in adult and pediatric recipients. Laryngoscope 131, 374–379. doi: 10.1002/lary.28632
Anderson, C. A., Wiggins, I. M., Kitterick, P. T., and Hartley, D. E. H. (2017). Adaptive benefit of cross-modal plasticity following cochlear implantation in deaf adults. Proc. Natl. Acad. Sci. USA 114, 10256–10261. doi: 10.1073/pnas.1704785114
Fullerton, A. M., Vickers, D. A., Luke, R., Billing, A. N., McAlpine, D., Hernandez-Perez, H., et al. (2023). Cross-modal functional connectivity supports speech understanding in cochlear implant users. Cereb. Cortex 33, 3350–3371. doi: 10.1093/cercor/bhac277
Geers, A. E., Strube, M. J., Tobey, E. A., Pisoni, D. B., and Moog, J. S. (2011). Epilogue: factors contributing to long-term outcomes of cochlear implantation in early childhood. Ear Hear. 32, 84S–92S. doi: 10.1097/AUD.0b013e3181ffd5b5
Lin, F. R., Wang, N. Y., Fink, N. E., Quittner, A. L., Eisenberg, L. S., Tobey, E. A., et al. (2008). Assessing the use of speech and language measures in relation to parental perceptions of development after early cochlear implantation. Otol. Neurotol. 29, 208–213. doi: 10.1097/MAO.0b013e31812f6fa6
Meng, Q., and Zhang, T. Q. (2021). Research progress in the treatment of unilateral deafness in children. Shandong Med. J. 61, 109–112. doi: 10.3969/j.issn.1002-266X.2021.20.028
Niparko, J. K., Tobey, E. A., Thal, D. J., Eisenberg, L. S., Wang, N. Y., Quittner, A. L., et al. (2010). Spoken language development in children following cochlear implantation. JAMA 303, 1498–1506. doi: 10.1001/jama.2010.451
Rouger, J., Lagleyre, S., Fraysse, B., Deneve, S., Deguine, O., and Barone, P. (2007). Evidence that cochlear-implanted deaf patients are better multisensory integrators. Proc. Natl. Acad. Sci. USA 104, 7295–7300. doi: 10.1073/pnas.0609419104
Saki, N., Bayat, A., Nikakhlagh, S., Zamani, P., Khaleghi, A., Karimi, M., et al. (2022). Acoustic voice analysis in postlingual deaf adult cochlear implant users: a within-group comparison study. J. Voice 36, 439.e1–439.e8. doi: 10.1016/j.jvoice.2020.06.005
Saksida, A., Ghiselli, S., Picinali, L., Pintonello, S., Battelino, S., and Orzan, E. (2022). Attention to speech and music in young children with bilateral cochlear implants: a pupillometry study. J. Clin. Med. 11:1745. doi: 10.3390/jcm11061745
Strelnikov, K., Rouger, J., Demonet, J. F., Lagleyre, S., Fraysse, B., Deguine, O., et al. (2013). Visual activity predicts auditory recovery from deafness after adult cochlear implantation. Brain 136, 3682–3695. doi: 10.1093/brain/awt274
Stroh, A. L., Grin, K., Rösler, F., Bottari, D., Ossandón, J., Rossion, B., et al. (2022). Developmental experiences alter the temporal processing characteristics of the visual cortex: evidence from deaf and hearing native signers. Eur. J. Neurosci. 55, 1629–1644. doi: 10.1111/ejn.15629
Sun, Z., Seo, J. W., Park, H. J., Lee, J. Y., Kwak, M. Y., Kim, Y., et al. (2021). Cortical reorganization following auditory deprivation predicts cochlear implant performance in postlingually deaf adults. Hum. Brain Mapp. 42, 233–244. doi: 10.1002/hbm.25219
Tobey, E. A., Thal, D., Niparko, J. K., Eisenberg, L. S., Quittner, A. L., Wang, N. Y., et al. (2013). Influence of implantation age on school-age language performance in pediatric cochlear implant users. Int. J. Audiol. 52, 219–229. doi: 10.3109/14992027.2012.759666
Tomblin, J. B., Barker, B. A., Spencer, L. J., Zhang, X., and Gantz, B. J. (2005). The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. J. Speech Lang. Hear. Res. 48, 853–867. doi: 10.1044/1092-4388(2005/059)
Wang, X. S., Li, T. Y., Wu, M., and Yang, C. Y. (2022). The effect of cochlear implant on hearing and speech rehabilitation of hearing-impaired children aged 14~16 years. Chinese Scientific Journal of Hearing and Speech Rehabilitation 20, 210–212.
Wang, Y., Pan, T., Mi, S., and Ma, F. (2013). The reliability analysis of the Chinese version of speech intelligibility rate (SIR). J. Audiol. Speech Pathol. 21, 465–468.
Xin, T. Y., Wang, Y. Y., Wei, C. G., and Liu, Y. H. (2022). Correlation between auditory cortex activity and hearing ability after Cochlear implantation. Chin. J. Otol. 20, 948–953.
Zeng, F. G. (2004). Trends in cochlear implants. Trends Amplif. 8, 1–34. doi: 10.1177/108471380400800102
Zhao, X. Y., Hu, J., and Xu, B. C. (2020). Advances in Cochlear implantation. Chin. J. Otol. 18, 1113–1118.
Zheng, H. M. (2019). Analysis of epidemiology and pathogenic factors of hearing impairment in children under 7 years old. Matern. Child Health Care China 34, 2105–2107.
Keywords: auditory function, auditory–visual cortical activity, cochlear implantation, cross-modal functional reorganization, speech function
Citation: Qiao X-F, Liu L-D, Han L-Y, Chen Y and Li X (2024) Exploring cross-modal plasticity in the auditory–visual cortex post cochlear implantation: implications for auditory and speech function recovery and mechanisms. Front. Neurosci. 18:1411058. doi: 10.3389/fnins.2024.1411058
Edited by:
Daniele De Seta, Sapienza University of Rome, ItalyReviewed by:
Carla Gentile Matas, University of São Paulo, BrazilLisheng Yu, Peking University People’s Hospital, China
Copyright © 2024 Qiao, Liu, Han, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Feng Qiao, cWlhb3hmZmZAMTYzLmNvbQ==
 Xiao-Feng Qiao
Xiao-Feng Qiao Lu-Dan Liu2
Lu-Dan Liu2