
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 23 April 2024
Sec. Brain Imaging Methods
Volume 18 - 2024 | https://doi.org/10.3389/fnins.2024.1373264
A correction has been applied to this article in:
Corrigendum: Beyond nodes and edges: a bibliometric analysis on graph theory and neuroimaging modalities
Understanding the intricate architecture of the brain through the lens of graph theory and advanced neuroimaging techniques has become increasingly pivotal in unraveling the complexities of neural networks. This bibliometric analysis explores the evolving landscape of brain research by focusing on the intersection of graph theoretical approaches, neuroanatomy, and diverse neuroimaging modalities. A systematic search strategy was used that resulted in the retrieval of a comprehensive dataset of articles and reviews. Using CiteSpace and VOSviewer, a detailed scientometric analysis was conducted that revealed emerging trends, key research clusters, and influential contributions within this multidisciplinary domain. Our review highlights the growing synergy between graph theory methodologies and neuroimaging modalities, reflecting the evolving paradigms shaping our understanding of brain networks. This study offers comprehensive insight into brain network research, emphasizing growth patterns, pivotal contributions, and global collaborative networks, thus serving as a valuable resource for researchers and institutions navigating this interdisciplinary landscape.
The unraveling of the brain’s intricate architecture stands as one of the most profound scientific endeavors, perpetually evolving to grasp the enigmatic complexity of the human brain (Tononi et al., 1994; Ramón Y Cajal, 1995; Bota et al., 2003). Understanding the brain’s organizational principles, connectivity patterns, and dynamic interplay between its components holds the key to comprehending cognition, behavior, and various neurological disorders (Bassett and Bullmore, 2009; Rubinov and Sporns, 2010; van den Heuvel and Hulshoff Pol, 2010).
Graph theory, a mathematical discipline that is concerned with the study of graphs or networks, has emerged as a foundational framework for modeling and analyzing the complex systems inherent in fields that include neuroscience (Ramnani et al., 2004; Bullmore and Bassett, 2011; Sporns, 2013a). Graph theory offers a systematic and quantitative means of characterizing the intricate structural and functional connectivity patterns that underlie neural circuits (Sporns, 2011; Sporns, 2013a). Graph theory facilitates a rigorous assessment of network properties, such as efficiency, resilience, and modularity, through the abstraction of the brain’s architecture into a network comprised of nodes and edges (Bassett and Bullmore, 2006; Rubinov and Sporns, 2010; Stam, 2014; Ma et al., 2021; Zamani Esfahlani et al., 2021). Nodes within the brain network typically correspond to anatomically or functionally defined brain regions, and edges represent the connections between them. These connections that can be derived from structural data (e.g., diffusion magnetic resonance imaging (MRI) tractography) or functional data (e.g., correlations in the function magnetic resonance imaging (fMRI) signal) encode a complex web of interactions that govern brain function (Watts and Strogatz, 1998).
Neuroimaging techniques provide a complementary path for probing the structure and function of the brain in vivo. These techniques encompass a spectrum of modalities, which range from traditional anatomical imaging methods that include structural MRI to advanced functional imaging methods such as resting-state fMRI and task-based fMRI (Ogawa et al., 1990; Conturo et al., 1999; Bandettini and Cox, 2000; Le Bihan and Johansen-Berg, 2012). Neuroimaging modalities yield rich datasets amenable to analysis within the framework of graph theory by capturing neural activity and connectivity patterns with high spatiotemporal resolution (Sporns et al., 2004; Hagmann et al., 2008; Honey et al., 2009).
The convergence of graph theory and neuroimaging has ushered in a transformative paradigm in the study of brain organization and function (Sporns, 2013b; Preti et al., 2017). This interdisciplinary synergy enables researchers to conceptualize the brain as a complex network. With the application of graph theoretical techniques to neuroimaging data, researchers can uncover fundamental principles that govern brain network organization, such as the presence of highly connected “hub” regions and the modular organization of functional brain networks (Crossley et al., 2014; Preti et al., 2017). Pioneering studies by Bullmore and Sporns (2009) and Bullmore and Sporns (2012) have elucidated the economy of brain network organization and complex brain networks’ structural and functional systems, respectively. Further, this integrative approach provides insight into how alterations in brain network topology relate to cognitive processes, behavior, and neurological disorders, thus advancing our understanding of brain function in health and disease (Menon, 2011; Fornito et al., 2012; Rubinov and Bullmore, 2013; Uddin et al., 2013; Iturria-Medina et al., 2014; Fornito et al., 2015).
Bibliometrics constitutes the analysis of published information and their associated metadata, such as abstracts, keywords, and citations. Bibliometrics aims to depict and elucidate relationships among these published works by employing statistical methods (Broadus, 1987; Hicks et al., 2015). This approach hinges on the premise that the scholarly output within a research domain is encapsulated within its published literature (Ninkov et al., 2022; Funada et al., 2023; Miao et al., 2023). The methodology of a bibliometric analysis encompasses diverse comprehensive techniques, including mathematical methods, network analyses, and clustering algorithms. These methodologies serve to scrutinize the overarching profiles of published works, thus presenting an objective and quantitative overview of the current status and evolving trends within various fields (Ying et al., 2023). In recent years, bibliometric analyses have gained widespread traction due to accessible software tools such as CiteSpace (Chaomei, 2006) and VOSviewer (van Eck and Waltman, 2010). The availability of these tools, coupled with the exponential growth in published literature, has rendered bibliometric analysis instrumental in comprehensively assessing the development trajectories of numerous specialized research fields (Brandt et al., 2019; Akmal et al., 2020; Hassan et al., 2021; Ge et al., 2022; Li et al., 2022).
In this study, we perform exhaustive systematic searches and rigorous data curation to meticulously compile a comprehensive dataset comprised of a multitude of articles and reviews to effectively capture the evolutionary trends within this expansive multidimensional domain. The integration of advanced visualization and data mining methodologies signifies a pioneering approach within bibliometric analyses, particularly for the exploration of brain networks through the amalgamation of graph theory and diverse neuroimaging techniques (van den Heuvel and Hulshoff Pol, 2010). This intersection represents a relatively uncharted territory that holds immense promise for unveiling novel insight into the complex landscape of brain connectivity.
Our primary objective is the elucidation of the intellectual trajectory and unfolding trends within the realm of brain network investigations over several decades. Our secondary goals is to provide a comprehensive evaluation of the diverse research networks that span across countries, institutions, authors, and journals. This holistic assessment approach allows us to delve deeper into collaborative networks, investigate key research productivity metrics, and pinpoint pivotal gaps within this dynamic interdisciplinary domain.
Further, our study seeks to identify prospective pathways, thus paving the way for potential directions and advancements in this multifaceted field. By critically evaluating collaborative networks and productivity metrics, our intent is not only to outline existing achievements, but also to identify crucial gaps that require further exploration and investigation. This multifaceted approach aims to significantly contribute to the understanding of brain network research dynamics, enabling the field to effectively navigate and chart future trajectories.
The data for the bibliometric analysis were obtained from the Clarivate Analytics’ Web of Science Core Collection (WOSCC), which included SCI-EXPANDED, SSCI, AHCI, ESCI, CCR-EXPANDED, IC, and search literature with the time span from January 1, 1995 through December 31, 2022. A bibliometric analysis of the retrieved documents was performed after searching in accordance with the abovementioned method. A total of 2,236 records, including 2,103 articles and 133 reviews were collected, and the records were exported in the “plain text” and “tab delimited file” format. Every document record included the title, author, keywords, abstract, year, organization, citation, and other relevant information.
VOSviewer 1.6.19 and CiteSpace 6.2.R5 were selected as the main bibliometric analysis tools to comprehensively analyze and summarize papers. To regulate the inclusion or exclusion of nodes in CiteSpace, the scale factor was adjusted to k = 25, the selection criteria was set to a top N = 50, and the time slice setting parameter was 2 years. Default configurations were maintained for all of the other settings (Chaomei, 2006). We conducted a cluster analysis and burst detection of keywords in order to understand the evolution of hotspots and predict the trends. Cluster labels were extracted from keyword lists using the log-likelihood ratio algorithm (p < 0.001). We identified the classical literature in the field using a co-citation analysis of the literature. Based on the contributions of countries, authors, and institutions, the cooperative co-occurrence graphs were drawn to analyze the connections between the elements. The flow chart of the study design is shown in Figure 1.
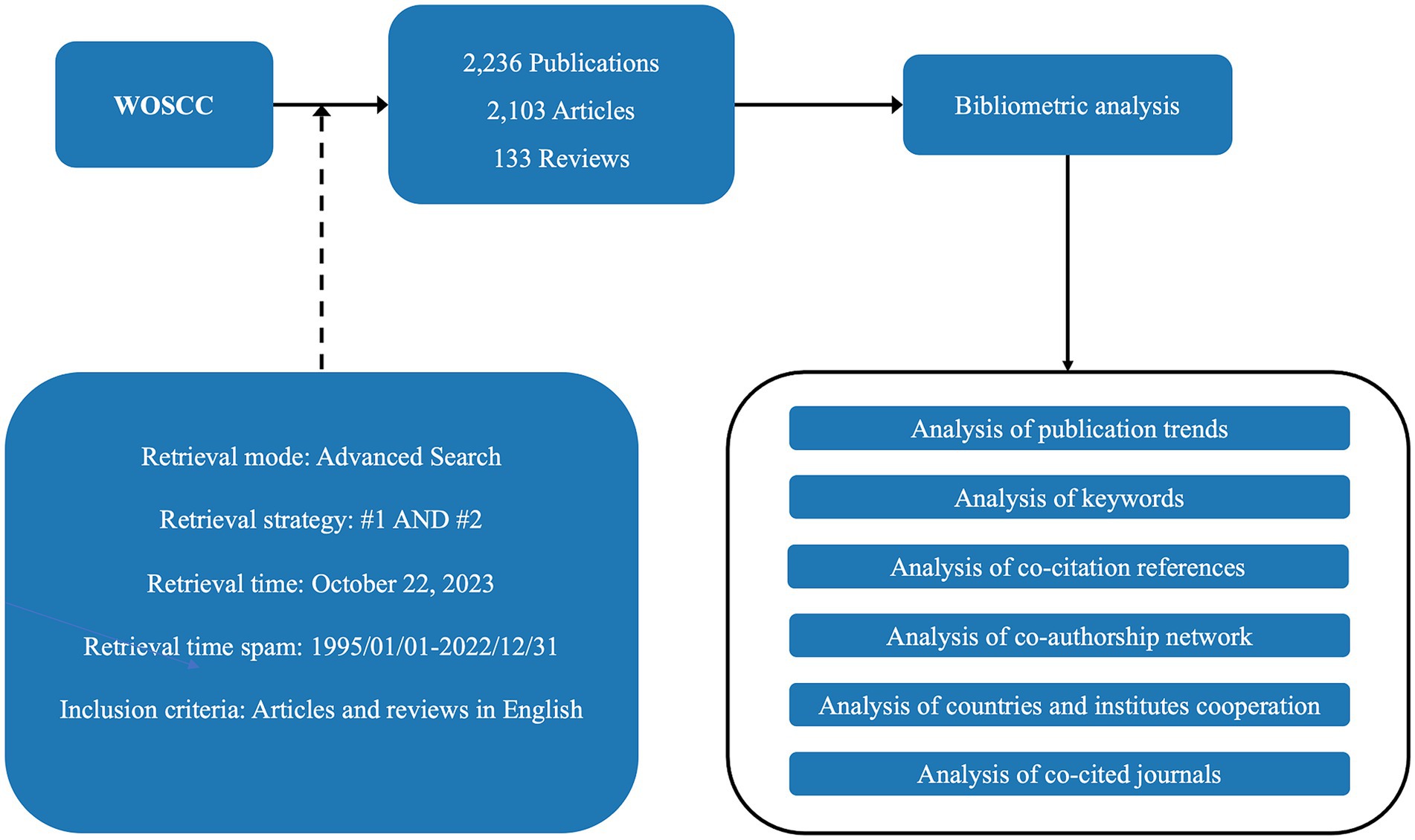
Figure 1. Flow chart of the study design. #1: [TS = (“Graph Theory” OR “Network Theory” OR “Network Analysis”) AND TS = (“brain” OR “Gray matter” OR “White matter” OR “Brain structure” OR “Brain anatomy” OR “brain connectivity” OR “Neural circuitry” OR “connectome”)]; #2: [TS = (“structural magnetic resonance imaging” OR “sMRI” OR “functional magnetic resonance imaging” OR “fMRI” OR “magnetic resonance spectroscopy” OR “MRS” OR “diffusion-tensor imaging” OR “DTI” OR “functional near-infrared spectroscopy” or “fNIRS” OR “single-photon emission computed tomography” OR “SPECT” OR “positron emission tomography” OR “PET”)]. WOSCC, web of science core collection.
From a modest beginning in 1995, the field has witnessed remarkable and sustained growth in scholarly output. The publication count surged significantly in recent decades, thus indicating a pivotal turning point in the domain’s development. This acceleration continued unabated throughout the next decade, with each year consistently surpassing the previous one in terms of the number of articles published. The climax in 2021, with a record-breaking 308 articles, underlines the growing importance of this multidisciplinary field. Although 2022 showed a slight reduction in publication numbers, it is essential to consider it within the context of the field’s overall trajectory (Figure 2).
A total of 2,236 documents were examined, and a compilation of 287 author-generated keywords offered insight into the prevailing themes and research directions within this field. VOSviewer was used to produce an overlay visualization based on the average publication year (Figure 3A) to delineate keyword co-occurrences. The top 10 keywords, ranked by frequency, encapsulated the focal points of the current research endeavors: ‘graph theory,’ ‘functional connectivity,’ ‘fMRI,’ ‘connectivity,’ ‘organization,’ ‘brain networks,’ ‘resting-state fMRI,’ ‘cortex,’ ‘small-world,’ and ‘MRI’ (Supplementary Table S1). This analysis illuminated the thematic emphasis and prevalent areas of exploration within brain network research, outlining the dominant concepts and directions that have garnered significant attention among researchers.
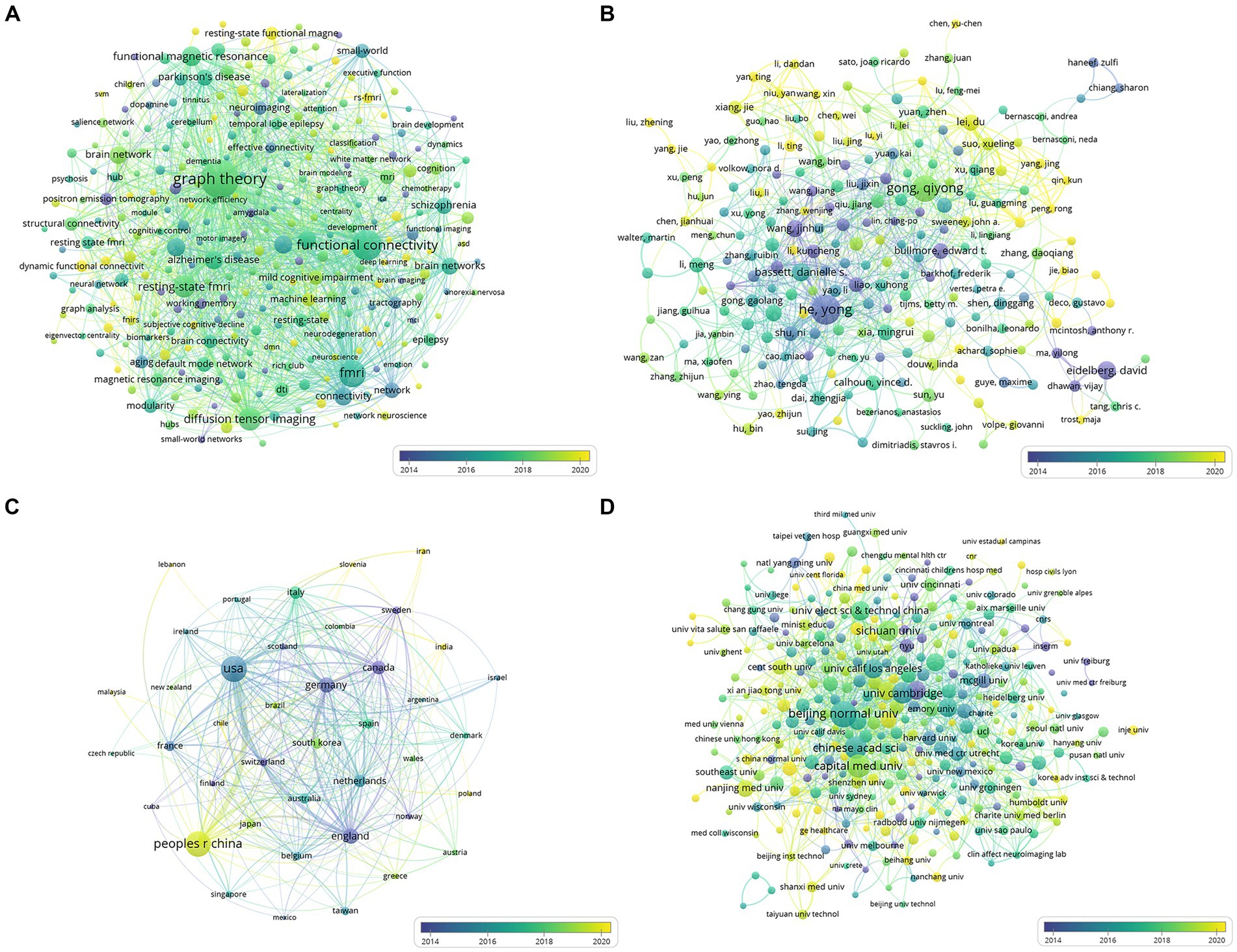
Figure 3. (A) Network of co-occurring author keywords; (B) Network of cooperation between authors; (C) Network of cooperation between countries/regions; (D) Network of cooperation between institutions. The size of a node is proportional to the frequency of its occurrence. The color of the node corresponds to the average year of publication.
A burst analysis was performed to identify the keywords that exhibited the most dynamic evolution over time. The top three keywords that demonstrated the strongest burst strength were ‘network analysis,’ ‘positron emission tomography,’ and ‘human brain.’ These keywords signified areas that have experienced significant surges in interest and focus within the literature.
Further, the keywords ‘significant difference’ and ‘machine learning’ displayed recent bursts, showing heightened activity specifically from 2020 to 2022 (Supplementary Table S2). This temporal analysis highlighted emerging areas of interest and reflected the evolving landscape within brain network research, indicating noteworthy shifts in attention and emphasis within the field during this period.
The time zone map for keywords visually depicts the temporal evolution of high-frequency keywords within the context of their appearance over time. This representation provides a clear visualization of the emergence periods of these keywords, enabling an understanding of their hotspots and potential future trends.
In this visualization, larger circles indicate keywords cited more frequently, reflecting heightened discussions and increased research activity around those specific terms. Supplementary Figure S1 shows a comprehensive overview of the prominence and temporal distribution of these keywords, serving as a valuable tool to identify the historical emergence and current significance of these terms within brain network research.
An analysis of the co-citation references revealed a rich landscape comprised of 23 distinct clusters that emphasized significant modularity (Q = 0.6946) and high silhouette scores (S = 0.879). These metrics underscored the reliability and distinctiveness of the identified clusters, with further scrutiny focused on 15 of these clusters for visualization within the co-citation references network (Figure 4). For detailed insights into each cluster’s thematic context, comprehensive descriptions are provided in Supplementary Table S3.
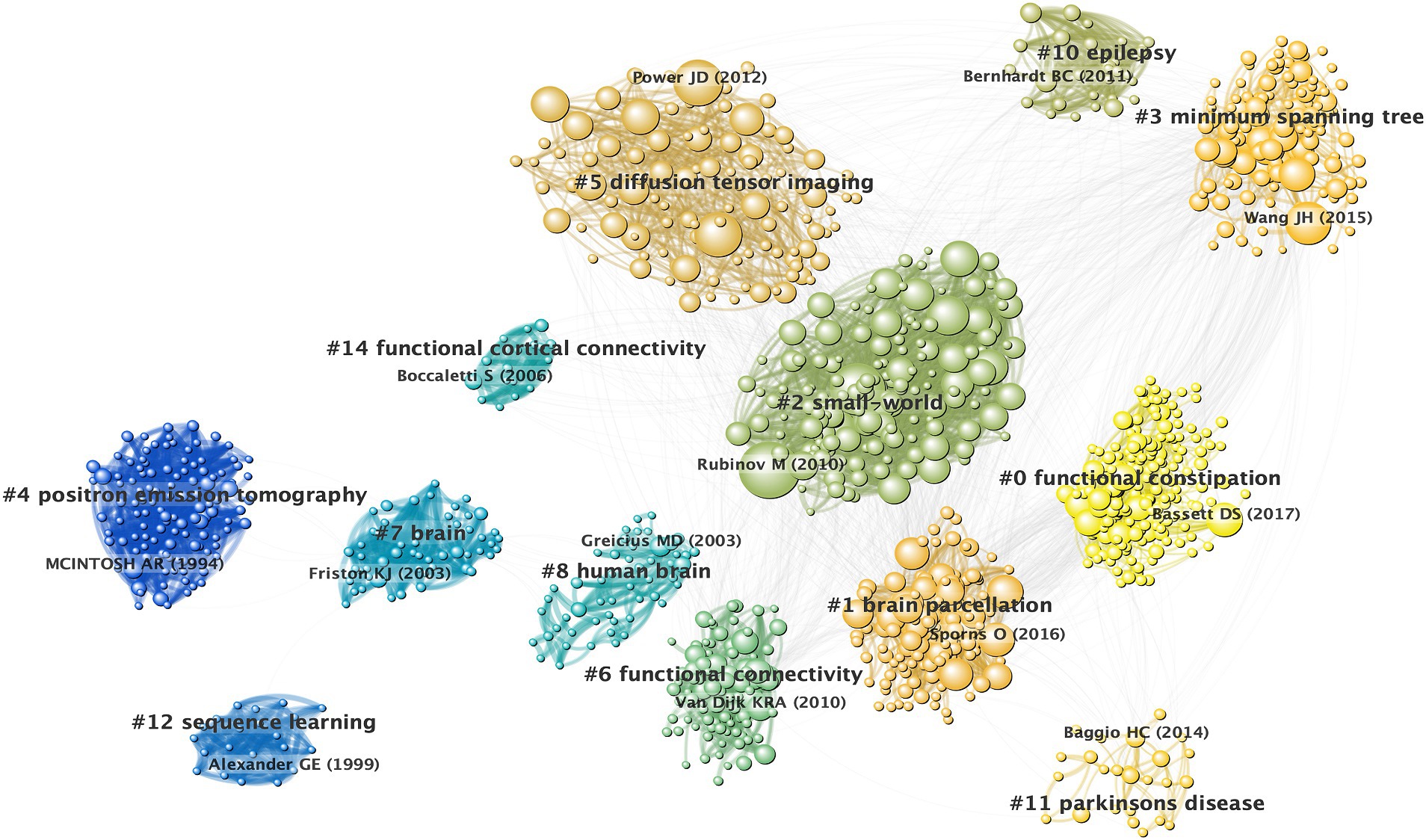
Figure 4. Co-citation references network and corresponding clustering visualization. A node represents a cited reference. The size of a node is proportional to its citation.
Each cluster exhibited a particularly high silhouette score indicative of a well-defined and distinct research focus within its domain. This comprehensive clustering analysis not only reinforced the reliability of the identified thematic clusters, but also offered a nuanced understanding of the diverse research avenues prevalent within brain network investigations.
The timeline map of the co-cited references was constructed by labeling the clusters using noun terms extracted from keywords. The arrangement of the node centers along the horizontal axis from left to right signifies the initial publication year of the cited documents, capturing the temporal evolution of the literature. This layout offered insights into the temporal characteristics and evolutionary trends of the referenced literature (Figure 5).
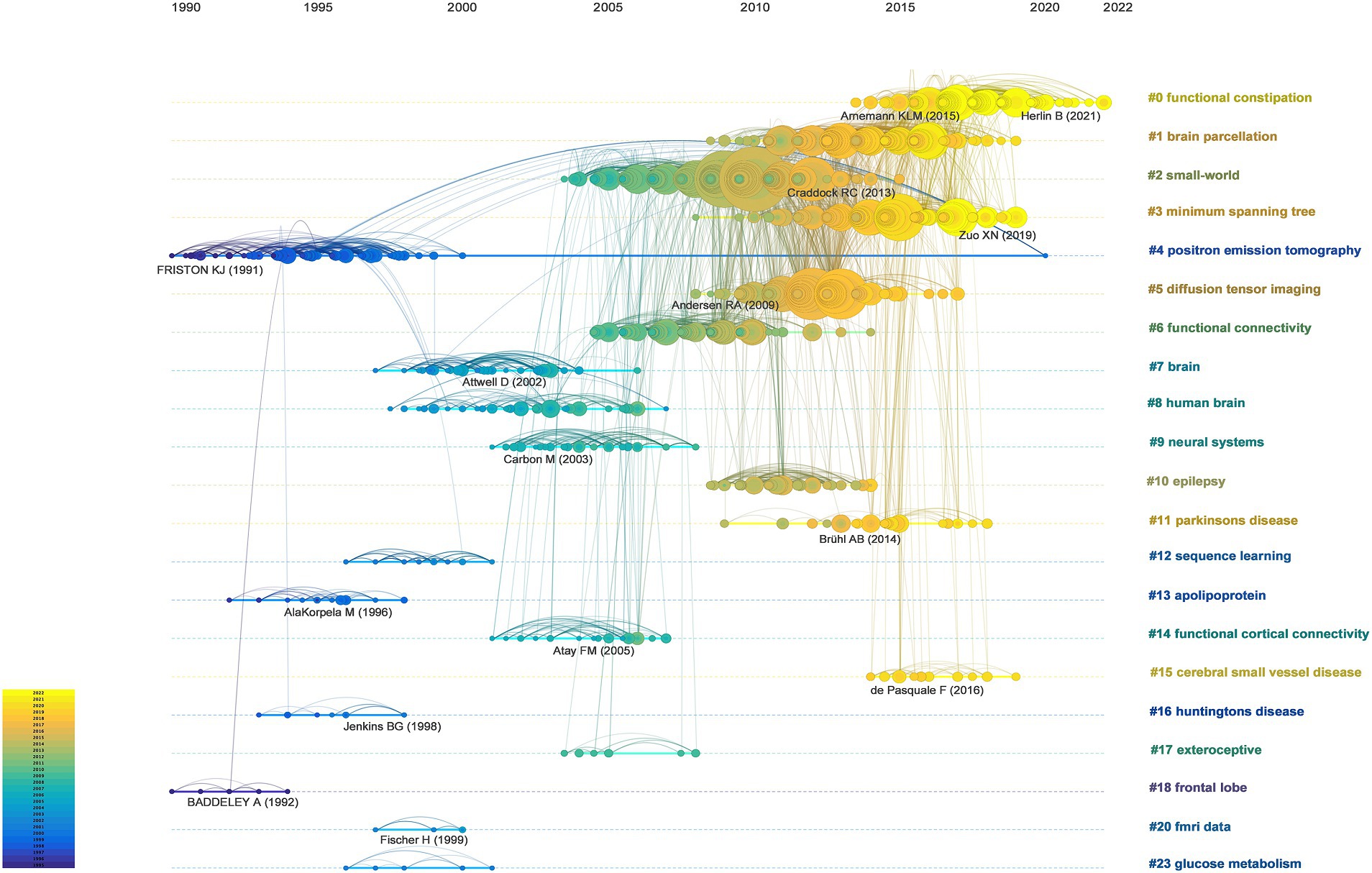
Figure 5. Timeline visualization of co-citation references network. A node represents a cited reference. For each cluster, nodes are organized by their year of publication on horizontal lines. The color of lines indicate the time of links between nodes or between clusters.
Certain study topics within the timeline map exhibited shorter durations, such as cluster (#12) that focuses on sequence learning. Conversely, other clusters, namely (#0) functional constipation and (#1) brain parcellation, continued to demonstrate ongoing activity, highlighting persistent research hotspots within this field. This visualization enables the identification of both transient and enduring research trends, providing a comprehensive temporal perspective on the evolution of cited literature and highlighting focal areas of ongoing interest and investigation within brain network research.
We meticulously identified the top 10 most cited references within the field, representing pivotal works that offer substantial insights into the subject matter. The comprehensive list of these references is presented in Table 1, signifying their significant contributions to the field’s advancement. One study has garnered exceptional attention and recognition: ‘Complex Network Measures of Brain Connectivity: Uses and Interpretations’ authored by Rubinov and Sporns (2010), and it stands out as the most frequently cited reference, having amassed an impressive citation count of 201.
Among these top references, three papers exhibited higher centrality, indicating their substantial influence on the field’s trajectory (Supplementary Table S4). These included ‘A Network Analysis of the Default Mode Hypothesis’ by Greicius et al. (2003), offering evidence for the cohesive existence of the default mode network; a comprehensive review by McIntosh (1999), elucidating neural systems and their correlation with cognition; and a clinical trial conducted by McIntosh et al. (1996), revealing changes in limbic and prefrontal functional interactions. These papers, distinguished by their higher centrality, underscore their profound impact and influential contributions within the domain of brain network research.
A network analysis of the co-cited authors revealed substantial modularity and silhouette scores (Q = 5,004; S = 0.7459), as depicted in Supplementary Figure S2. Among the clusters identified, Cluster #0, titled ‘Diffusion Tensor Imaging,’ emerged as the most significant and central cluster, emphasizing its pivotal role within the network. Supplementary Table S5 provides detailed descriptions for each cluster, offering comprehensive insights into their thematic focus and significance.
The top three most cited authors within this network were Rubinov M (n = 1,101 citations), Bullmore ET (n = 995), and Sporns O (n = 734), showcasing their significant contributions to the field. Further, the top three authors with the strongest betweenness centrality, indicating their pivotal role in connecting different segments of the network, were Friston KJ (0.08), Horwitz (0.07), and Raichle ME (0.05), as outlined in Supplementary Table S6. McIntosh AR emerged as the author with the strongest burst strength, indicating a notable surge in citations and an active contribution from 1995 to 2010, detailed in Supplementary Table S7.
Further, an analysis of the collaborative network of citing authors delineated influential cooperative groups, prominently led by Gong QY, He Y, Lei D, Suo XL, and Shu N (Figure 3B). These authors played instrumental roles within the network, significantly contributing to collaborative efforts and advancing research in the field, showcasing the importance of collaborative endeavors in shaping the landscape of brain network research.
An analysis of the co-cited journals highlighted the top three most cited publications: Neuroimage (n = 2,146 citations), Human Brain Mapping (n = 1787), and the Journal of Neuroscience (n = 1,657), demonstrating their significant influence within neuroimaging and neuroscience research. These journals have served as pivotal platforms for disseminating cutting-edge research in the field.
Further, the top three journals with the highest centrality, signifying their critical role in connecting various segments of the network, were Experimental Brain Research (0.05), Archives of Neurology (0.04), and Alzheimer Disease and Associated Disorders (0.04), as detailed in Supplementary Table S6. These journals have played instrumental roles in fostering the exchange and dissemination of neuroimaging and brain graph analysis research, contributing significantly to the field’s advancement.
It is worthy to note that NeuroReport emerged as the journal with the strongest burst, signaling a substantial increase in citations and continued activity from 1995 to 2012. Further, recent surges were observed in Scientific Reports, Nature Communications, Network Neuroscience, Frontiers in Aging Neuroscience, and Dialogues in Clinical Neuroscience, as indicated in Supplementary Table S8. These journals represent recent avenues of prominence, offering potential outlets for researchers engaged in neuroimaging and graph analysis to consider for their publications.
Figures 3C,D illustrate the cooperative networks among countries and institutions, respectively. Our analysis encompassed 41 countries or regions. The top three countries that contributed the most papers were the United States (873 papers, 39.04%), the People’s Republic of China (812 papers, 36.31%), and England (212 papers, 9.48%). In terms of citations, the United States was the most cited country (n = 62,156), followed by England (n = 25,325) and the People’s Republic of China (n = 22,970), as detailed in Supplementary Table S8.
Moreover, our dataset encompassed 322 institutions. Beijing Normal University emerged as the institution with the highest number of publications (n = 114), followed by Capital Medical University (n = 85) and the Chinese Academy of Sciences (n = 80). In terms of citations, Beijing Normal University secured the second position (n = 8,853), with the University of Cambridge leading the citations (n = 15,643), as depicted in Supplementary Table S9.
Our study used an exhaustive bibliometric analysis to provide a comprehensive overview of brain network research dynamics. We highlighted the transformative synergy between graph theory and neuroimaging techniques, revealing diverse research clusters and showcasing the influential roles of countries, institutions, authors, and journals. Our findings underscored the multidimensional nature of contemporary investigations and the collaborative networks that drive advancements in this dynamic field.
The integration of graph theory methodologies with advanced neuroimaging techniques represents a pivotal advancement in neuroscience, fundamentally transforming our understanding of brain networks (Hagmann et al., 2008; Park and Friston, 2013; Bassett and Bullmore, 2017; Breakspear, 2017). Graph theory offers a robust mathematical framework for modeling complex relationships within brain networks, providing insight into their organizational principles (Watts and Strogatz, 1998; Bullmore and Sporns, 2009). When paired with cutting-edge neuroimaging modalities, such as fMRI and diffusion tensor imaging (DTI), this integration empowers researchers to visualize, analyze, and comprehend the brain’s intricate connectivity patterns in vivo (Biswal et al., 2010; Bullmore and Bassett, 2011; Deco et al., 2011; Le Bihan and Johansen-Berg, 2012; Sporns, 2013b). Studies by van den Heuvel and Sporns (2013) showcase how graph-based analyses unveil topological properties, small-world architectures, and decipher critical network hubs for information processing (Hagmann et al., 2008). He and Evans (2010) and Bullmore and Sporns (2012) emphasized that this integration transcends traditional neuroanatomical boundaries, fostering a holistic network-centric perspective. Such transformative integration, as outlined by researchers, has reshaped our understanding and shed light on how dynamic interactions between brain regions underpin cognitive processes, behavior, and neurological disorders (Bressler and Menon, 2010; Menon, 2011; Fornito et al., 2015; Bassett and Sporns, 2017).
Our comprehensive analysis unveiled dynamic trends and distinctive clusters that delineated the multifaceted landscape of brain network research. Among the identified clusters, sustained areas, such as functional connectivity and specific brain parcellation, emerged as enduring focal points. This was substantiated by their consistent activity and extensive exploration over time. For instance, functional constipation, that exhibited sustained prominence, signified its pivotal role in understanding the brain’s functional dynamics, garnering ongoing interest and exploration (Duan et al., 2021; Liu et al., 2021; Peihong et al., 2021; Yu et al., 2023). Conversely, emerging clusters, including sequence learning, showcased the field’s adaptability by embracing novel areas of investigation. Sequence learning’s recent surge in scholarly attention implies its potential for unraveling the brain’s cognitive mechanisms, and it is an evolving area of interest that will likely bring future breakthroughs (Yeo et al., 2011; Watanabe et al., 2019). These sustained and emerging clusters not only signify the depth and breadth of research in established domains, but also hint at the field’s receptiveness to novel methodologies, fostering continuous innovation and exploration within brain network investigations.
Further, recent advancements in neuroimaging technology have propelled the integration of machine learning methodologies into brain network analyses. Graph neural networks, for example, have demonstrated remarkable efficacy in capturing the intricate connectivity patterns within functional brain networks (Li et al., 2021). These networks offer a powerful framework for modeling brain dynamics by leveraging graph structures to represent complex relationships between brain regions. In our analysis, keywords such as “machine learning” and “deep learning” emerged (Figure 3A; Supplementary Figure S1; Supplementary Table S2), reaffirming the growing relevance of these methodologies in the realm of brain network research. This convergence of graph theory and machine learning holds significant promise for unraveling the intricacies of brain connectivity, paving the way for innovative approaches to understanding brain function and dysfunction.
The vibrant collaborative networks that span countries, institutions, authors, and journals underscore the dynamic interplay that molds brain network research. The strategic collaborations between countries such as the United States, the People’s Republic of China, and England signify a global alliance driving advancements in this field, highlighting the significance of international partnerships in accelerating research progress. Key institutions, such as the Beijing Normal University and the University of Cambridge, play pivotal roles, demonstrating their influential contributions in steering research trajectories and fostering cross-disciplinary investigations. Influential authors, exemplified by Rubinov and Bullmore (2013), not only spearhead collaborative networks, but they also contribute seminal works that shape the discourse within the field. Further, foundational references like Rubinov and Sporns (2010) work on complex network measures of brain connectivity stand as pillars in the field, amassing high citations and significantly influencing subsequent research directions. Esteemed journals, including Neuroimage, Human Brain Mapping, and the Journal of Neuroscience, serve as vital platforms for disseminating cutting-edge research, further solidifying their roles in propelling brain network investigations. These collaborative efforts, spanning diverse domains within brain network research, underscore the synergistic nature of collective contributions, affirming interdisciplinary growth and evolution within this dynamic field.
While our study provided valuable insights, certain limitations and gaps warrant consideration for future research endeavors. One notable limitation lies in the reliance on published literature, potentially overlooking unpublished or emerging research that could offer novel perspectives. The time span of our study may not have fully captured the most recent developments in the field. In addition, the scope of our analysis might have inadvertently omitted niche research or specific methodologies that could contribute substantially to the broader understanding of brain networks. Addressing these limitations could enrich future analyses and provide a more comprehensive view of the evolving landscape in this field.
Moving forward, the findings of this study suggested several promising paths for future investigations in brain network research. First, delving deeper into the functional implications of sustained clusters could unveil underlying mechanisms that drive brain functionality. Second, the exploration of emergent clusters is an exciting opportunity to uncover novel cognitive mechanisms. Further, the incorporation of interdisciplinary approaches that amalgamate graph theory with emerging neuroimaging techniques might offer fresh insight into brain connectivity. In addition, investigating the impact of neurological disorders on brain networks and exploring methodologies to characterize alterations in these conditions could pave the way for diagnostic and therapeutic advancements. Future studies could focus on fostering international collaborations to facilitate data sharing and standardization, fostering a more unified understanding of brain network dynamics across diverse populations. These proposed directions aim to bridge existing gaps, stimulate innovation, and further unravel the complexities of brain networks.
In summary, our comprehensive bibliometric analysis offers multifaceted insights into the intricate dynamics of brain network research. By elucidating evolving trends, collaborative networks, and key research clusters, our study underscored the transformative integration of graph theory with neuroimaging techniques, reshaping our understanding of brain connectivity. The sustained and emerging clusters identified revealed lasting areas of interest and indicated paths for future exploration, while collaborative networks among countries, institutions, authors, and journals highlighted the combined endeavors that can propel progress in this vibrant field. Importantly, the findings of this study serve as a foundation for future investigations, offering a panoramic view of brain network research dynamics. The implications of this study lie in its potential to guide future research directions, foster interdisciplinary collaborations, and inspire innovative methodologies, thus contributing significantly to unraveling the complexities of brain networks and advancing our understanding of cognitive processes, behaviors, and neurological disorders.
MM: Data curation, Formal analysis, Resources, Software, Visualization, Writing – original draft. ZW: Data curation, Resources, Software, Writing – review & editing. LJ: Data curation, Software, Visualization, Writing – review & editing. KH: Resources, Software, Visualization, Writing – review & editing. LL: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. YC: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1373264/full#supplementary-material
Akmal, M., Hasnain, N., Rehan, A., Iqbal, U., Hashmi, S., Fatima, K., et al. (2020). Glioblastome Multiforme: a bibliometric analysis. World Neurosurg. 136, 270–282. doi: 10.1016/j.wneu.2020.01.027
Bandettini, P. A., and Cox, R. W. (2000). Event-related fMRI contrast when using constant interstimulus interval: theory and experiment. Magn. Reson. Med. 43, 540–548. doi: 10.1002/(SICI)1522-2594(200004)43:4<540::AID-MRM8>3.0.CO;2-R
Bassett, D. S., and Bullmore, E. (2006). Small-world brain networks. Neuroscientist 12, 512–523. doi: 10.1177/1073858406293182
Bassett, D. S., and Bullmore, E. T. (2009). Human brain networks in health and disease. Curr. Opin. Neurol. 22, 340–347. doi: 10.1097/WCO.0b013e32832d93dd
Bassett, D. S., and Bullmore, E. T. (2017). Small-world brain networks revisited. Neuroscientist 23, 499–516. doi: 10.1177/1073858416667720
Bassett, D. S., and Sporns, O. (2017). Network neuroscience. Nat. Neurosci. 20, 353–364. doi: 10.1038/nn.4502
Biswal, B. B., Mennes, M., Zuo, X. N., Gohel, S., Kelly, C., Smith, S. M., et al. (2010). Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 107, 4734–4739. doi: 10.1073/pnas.0911855107
Bota, M., Dong, H. W., and Swanson, L. W. (2003). From gene networks to brain networks. Nat. Neurosci. 6, 795–799. doi: 10.1038/nn1096
Brandt, J. S., Hadaya, O., Schuster, M., Rosen, T., Sauer, M. V., and Ananth, C. V. (2019). A bibliometric analysis of top-cited journal articles in obstetrics and gynecology. JAMA Netw. Open 2:e1918007. doi: 10.1001/jamanetworkopen.2019.18007
Breakspear, M. (2017). Dynamic models of large-scale brain activity. Nat. Neurosci. 20, 340–352. doi: 10.1038/nn.4497
Bressler, S. L., and Menon, V. (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 14, 277–290. doi: 10.1016/j.tics.2010.04.004
Broadus, R. N. (1987). Toward a definition of “bibliometrics”. Scientometrics 12, 373–379. doi: 10.1007/BF02016680
Bullmore, E. T., and Bassett, D. S. (2011). Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 7, 113–140. doi: 10.1146/annurev-clinpsy-040510-143934
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Bullmore, E., and Sporns, O. (2012). The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349. doi: 10.1038/nrn3214
Chaomei, C. (2006). CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soci. Inform. Sci. Technol. 57, 359–377. doi: 10.1002/asi.20317
Conturo, T. E., Lori, N. F., Cull, T. S., Akbudak, E., Snyder, A. Z., Shimony, J. S., et al. (1999). Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. USA 96, 10422–10427. doi: 10.1073/pnas.96.18.10422
Crossley, N. A., Mechelli, A., Scott, J., Carletti, F., Fox, P. T., McGuire, P., et al. (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137, 2382–2395. doi: 10.1093/brain/awu132
Deco, G., Jirsa, V. K., and McIntosh, A. R. (2011). Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 12, 43–56. doi: 10.1038/nrn2961
Duan, S., Liu, L., Li, G., Wang, J., Hu, Y., Zhang, W., et al. (2021). Altered functional connectivity within and between salience and sensorimotor networks in patients with functional constipation. Front. Neurosci. 15:628880. doi: 10.3389/fnins.2021.628880
Fornito, A., Zalesky, A., and Breakspear, M. (2015). The connectomics of brain disorders. Nat. Rev. Neurosci. 16, 159–172. doi: 10.1038/nrn3901
Fornito, A., Zalesky, A., Pantelis, C., and Bullmore, E. T. (2012). Schizophrenia, neuroimaging and connectomics. NeuroImage 62, 2296–2314. doi: 10.1016/j.neuroimage.2011.12.090
Funada, S., Yoshioka, T., Luo, Y., Iwama, T., Mori, C., Yamada, N., et al. (2023). Global trends in highly cited studies in COVID-19 research. JAMA Netw. Open 6:e2332802. doi: 10.1001/jamanetworkopen.2023.32802
Ge, Y., Chao, T., Sun, J., Liu, W., Chen, Y., and Wang, C. (2022). Frontiers and hotspots evolution in psycho-cardiology: a bibliometric analysis from 2004 to 2022. Curr. Probl. Cardiol. 47:101361. doi: 10.1016/j.cpcardiol.2022.101361
Greicius, M. D., Krasnow, B., Reiss, A. L., and Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 100, 253–258. doi: 10.1073/pnas.0135058100
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., et al. (2008). Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. doi: 10.1371/journal.pbio.0060159
Hassan, W., Zafar, M., Duarte, A. E., Kamdem, J. P., and Teixeira da Rocha, J. B. (2021). Pharmacological research: a bibliometric analysis from 1989 to 2019. Pharmacol. Res. 169:105645. doi: 10.1016/j.phrs.2021.105645
He, Y., and Evans, A. (2010). Graph theoretical modeling of brain connectivity. Curr. Opin. Neurol. 23, 341–350. doi: 10.1097/WCO.0b013e32833aa567
Hicks, D., Wouters, P., Waltman, L., de Rijcke, S., and Rafols, I. (2015). Bibliometrics: the Leiden manifesto for research metrics. Nature 520, 429–431. doi: 10.1038/520429a
Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., et al. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. USA 106, 2035–2040. doi: 10.1073/pnas.0811168106
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., and Evans, A. C.and the Alzheimer's Disease Neuroimaging Initiative (2014). Epidemic spreading model to characterize misfolded proteins propagation in aging and associated neurodegenerative disorders. PLoS Comput. Biol. 10:e1003956. doi: 10.1371/journal.pcbi.1003956
Le Bihan, D., and Johansen-Berg, H. (2012). Diffusion MRI at 25: exploring brain tissue structure and function. NeuroImage 61, 324–341. doi: 10.1016/j.neuroimage.2011.11.006
Li, D., Yu, D., Li, Y., and Yang, R. (2022). A bibliometric analysis of PROTAC from 2001 to 2021. Eur. J. Med. Chem. 244:114838. doi: 10.1016/j.ejmech.2022.114838
Li, X., Zhou, Y., Dvornek, N., Zhang, M., Gao, S., Zhuang, J., et al. (2021). BrainGNN: interpretable brain graph neural network for fMRI analysis. Med. Image Anal. 74:102233. doi: 10.1016/j.media.2021.102233
Liu, L., Hu, C., Hu, Y., Zhang, W., Zhang, Z., Ding, Y., et al. (2021). Abnormalities in the thalamo-cortical network in patients with functional constipation. Brain Imaging Behav. 15, 630–642. doi: 10.1007/s11682-020-00273-y
Ma, J., Zhang, J., Lin, Y., and Dai, Z. (2021). Cost-efficiency trade-offs of the human brain network revealed by a multiobjective evolutionary algorithm. NeuroImage 236:118040. doi: 10.1016/j.neuroimage.2021.118040
McIntosh, A. R. (1999). Mapping cognition to the brain through neural interactions. Memory 7, 523–548. doi: 10.1080/096582199387733
McIntosh, A. R., Grady, C. L., Haxby, J. V., Ungerrleider, L. G., and Horwitz, B. (1996). Changes in limbic and prefrontal functional interactions in a working memory task for faces. Cereb. Cortex 6, 571–584. doi: 10.1093/cercor/6.4.571
Menon, V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506. doi: 10.1016/j.tics.2011.08.003
Miao, Y. D., Quan, W., Dong, X., Gan, J., Ji, C. F., Wang, J. T., et al. (2023). A bibliometric analysis of ferroptosis, necroptosis, pyroptosis, and cuproptosis in cancer from 2012 to 2022. Cell Death Discov. 9:129. doi: 10.1038/s41420-023-01421-1
Ninkov, A., Frank, J. R., and Maggio, L. A. (2022). Bibliometrics: methods for studying academic publishing. Perspect. Med. Educ. 11, 173–176. doi: 10.1007/s40037-021-00695-4
Ogawa, S., Lee, T. M., Kay, A. R., and Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 87, 9868–9872. doi: 10.1073/pnas.87.24.9868
Park, H. J., and Friston, K. (2013). Structural and functional brain networks: from connections to cognition. Science 342:1238411. doi: 10.1126/science.1238411
Peihong, M., Tao, Y., Zhaoxuan, H., Sha, Y., Li, C., Kunnan, X., et al. (2021). Alterations of white matter network properties in patients with functional constipation. Front. Neurol. 12:627130. doi: 10.3389/fneur.2021.627130
Preti, M. G., Bolton, T. A., and Van De Ville, D. (2017). The dynamic functional connectome: state-of-the-art and perspectives. NeuroImage 160, 41–54. doi: 10.1016/j.neuroimage.2016.12.061
Ramnani, N., Behrens, T. E. J., Penny, W., and Matthews, P. M. (2004). New approaches for exploring anatomical and functional connectivity in the human brain. Biol. Psychiatry 56, 613–619. doi: 10.1016/j.biopsych.2004.02.004
Ramón Y Cajal, S., Histology of the nervous system of man and vertebrates. History of neuroscience. (1995), New York: Oxford University Press.
Rubinov, M., and Bullmore, E. (2013). Schizophrenia and abnormal brain network hubs. Dialogues Clin. Neurosci. 15, 339–349. doi: 10.31887/DCNS.2013.15.3/mrubinov
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Sporns, O. (2011). The human connectome: a complex network. Ann. N. Y. Acad. Sci. 1224, 109–125. doi: 10.1111/j.1749-6632.2010.05888.x
Sporns, O. (2013a). Structure and function of complex brain networks. Dialogues Clin. Neurosci. 15, 247–262. doi: 10.31887/DCNS.2013.15.3/osporns
Sporns, O. (2013b). Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 23, 162–171. doi: 10.1016/j.conb.2012.11.015
Sporns, O., Chialvo, D. R., Kaiser, M., and Hilgetag, C. C. (2004). Organization, development and function of complex brain networks. Trends Cogn. Sci. 8, 418–425. doi: 10.1016/j.tics.2004.07.008
Stam, C. J. (2014). Modern network science of neurological disorders. Nat. Rev. Neurosci. 15, 683–695. doi: 10.1038/nrn3801
Tononi, G., Sporns, O., and Edelman, G. M. (1994). A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 91, 5033–5037. doi: 10.1073/pnas.91.11.5033
Uddin, L. Q., Supekar, K., Lynch, C. J., Khouzam, A., Phillips, J., Feinstein, C., et al. (2013). Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 70, 869–879. doi: 10.1001/jamapsychiatry.2013.104
van den Heuvel, M. P., and Hulshoff Pol, H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534. doi: 10.1016/j.euroneuro.2010.03.008
van den Heuvel, M. P., and Sporns, O. (2013). Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696. doi: 10.1016/j.tics.2013.09.012
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
Watanabe, T., Rees, G., and Masuda, N. (2019). Atypical intrinsic neural timescale in autism. eLife 8:8. doi: 10.7554/eLife.42256
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of 'small-world' networks. Nature 393, 440–442. doi: 10.1038/30918
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. doi: 10.1152/jn.00338.2011
Ying, H., Zhang, X., He, T., Feng, Q., Wang, R., Yang, L., et al. (2023). A bibliometric analysis of research on heart failure comorbid with depression from 2002 to 2021. Heliyon 9:e13054. doi: 10.1016/j.heliyon.2023.e13054
Yu, X., Yu, J., Li, Y., Cong, J., Wang, C., Fan, R., et al. (2023). Aberrant intrinsic functional brain networks in patients with functional constipation. Neuroradiology 65, 337–348. doi: 10.1007/s00234-022-03064-y
Keywords: brain network, graph theory, neuroimaging, bibliometric analysis, research trends
Citation: Mamat M, Wang Z, Jin L, He K, Li L and Chen Y (2024) Beyond nodes and edges: a bibliometric analysis on graph theory and neuroimaging modalities. Front. Neurosci. 18:1373264. doi: 10.3389/fnins.2024.1373264
Received: 25 January 2024; Accepted: 08 April 2024;
Published: 23 April 2024.
Edited by:
Hao Zhang, Central South University, ChinaReviewed by:
Yangding Li, Hunan Normal University, ChinaCopyright © 2024 Mamat, Wang, Jin, He, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Li, bGlsaW41OTE3QG5qbXUuZWR1LmNu; Yiyong Chen, Y2hlbnlpeW9uZ0BuYnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.