- Center for Interdisciplinary Research in Biology (CIRB), College de France, CNRS, INSERM, Université PSL, Paris, France
Cell signaling based on homeoprotein transfer is a pathway with developmental and physiological functions. For a few transcription factors of this family, primarily ENGRAILED1, ENGRAILED2 and OTX2, their physiological functions have led to therapeutic strategies in animal models of human diseases, including Parkinson’s disease, amyotrophic lateral sclerosis, amblyopia and anxiety-related disorders. In mesencephalic dopaminergic neurons which degenerate in Parkinson’s disease, ENGRAILED1/2 have cell autonomous activities, but their transducing properties enables their use as therapeutic proteins. In contrast, in spinal alpha-motoneurons, which are lost in amyotrophic lateral sclerosis, ENGRAILED1 is supplied by V1 interneurons. Thus, its use as a therapeutic protein to protect alpha-motoneurons against degeneration mimics its normal non-cell autonomous neurotrophic activity. OTX2, synthesized and secreted by the choroid plexus, is transferred to parvalbumin interneurons and exerts regulatory functions controlling cerebral cortex plasticity. Understanding the latter OTX2 function has led to strategies for manipulating visual acuity and anxiety-like behavior in adult mice. In this review, we describe these cases and what is known about the involved molecular mechanisms. Because the transduction sequences are conserved in most of the few hundred homeoproteins, we argue how this family of molecules constitutes an important reservoir of physiological knowledge, with potential consequences in the search for new therapeutic strategies.
Introduction
The discovery of homeoprotein signaling
Transcription factor participation in signal transduction is normally cell autonomous. Homeoprotein (HP) transcription factors, discovered on the basis of their developmental functions but expressed throughout adulthood, provide an exception to the rule. Direct non-cell autonomous signaling by cell-to-cell HP transfer was first discovered in plants where KNOTTED1 travels through specific cell bridging structures called plasmodesmata (Lucas et al., 1995; Kim et al., 2002; Ruiz-Medrano et al., 2004; Bolduc et al., 2008). In animals, a first step in the discovery of HP transfer was the observation that their DNA-binding domain, or homeodomain (HD), is internalized by live cells and directly addressed to the cytoplasm and nucleus (Joliot et al., 1991). This finding was rapidly followed by the demonstration that full-length HPs are internalized and secreted through non-conventional mechanisms (Prochiantz and Di Nardo, 2015; Di Nardo et al., 2018, 2020). HP secretion and internalization domains are part of the highly conserved HD explaining why most of the 160 tested HPs can transfer (Lee et al., 2019). Despite this high number and the likeliness that this property is shared by the approximately 300 members of the HP family, the developmental and physiological functions associated with HP transfer have been studied for only a few of them, including ENGRAILED (EN), PAX6, VAX1 and OTX2. Before illustrating with EN1 and OTX2 how this novel signaling pathway is conducive to new therapeutic strategies, we will describe established HP signaling functions and the mechanisms involved.
Summary of established HP signaling functions in animals
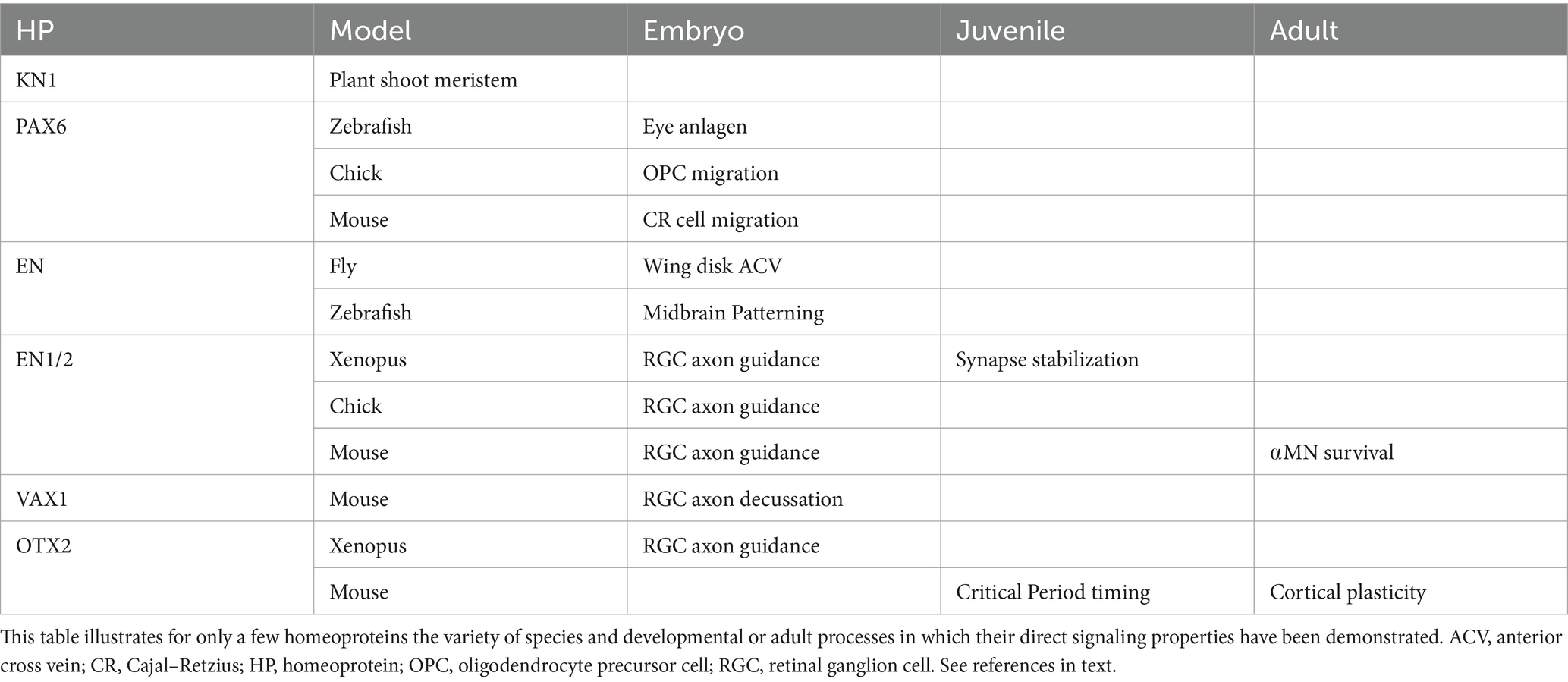
The main established functions are summarized in Table 1. During development, PAX6 signaling acts on cell migration, as shown for Cajal–Retzius cells in the embryonic mouse cerebral cortex (Kaddour et al., 2019), and for oligodendrocyte precursor cells (OPCs) in the chick spinal cord (Di Lullo et al., 2011). Still during development, EN signaling regulates anterior cross vein (ACV) formation in the Drosophila wing disk (Layalle et al., 2011), eye anlagen development and midbrain patterning in the zebrafish (Lesaffre et al., 2007; Rampon et al., 2015; Amblard et al., 2020b), and retinal ganglion cell (RGC) axon guidance and synaptic stability in the chick and frog (Brunet et al., 2005; Wizenmann et al., 2009; Yoon et al., 2012). In RGC growth cones, EN1 and EN2 (together EN1/2) activity involves the regulation of local mRNA translation (Brunet et al., 2005, 2007; Wizenmann et al., 2009). Some of these mRNAs encode mitochondrial complex I proteins and their EN1/2-induced translation results in transient ATP synthesis (Stettler et al., 2012). Also related to growth cone behavior, VAX1 was shown to regulate RGC decussation at the level of the optic chiasma (Kim et al., 2014; Min et al., 2023). In the adult, EN1 secreted by V1 interneurons in the mouse spinal cord is captured by motoneurons (MNs), and blocking this transfer induces αMN retrograde degeneration (Lebœuf et al., 2023). In the juvenile and adult mouse, OTX2 signaling regulates the opening and closure of cerebral cortex critical periods (CPs) of plasticity in the visual, auditory, and medial prefrontal cortices (Sugiyama et al., 2008). This regulation involves the secretion of OTX2 by the choroid plexus and its specific capture by parvalbumin interneurons (PV cells) localized in layer IV of the cerebral cortex (Beurdeley et al., 2012; Spatazza et al., 2013; Bernard et al., 2016). OTX2 internalization by PV cells induces their maturation and a shift in the excitatory/inhibitory (E/I) balance toward inhibition leading to heightened neural circuit plasticity (Sugiyama et al., 2008). In the visual system, blocking OTX2 signaling in the mouse retina within a week after eye opening delays CP onset (Sugiyama et al., 2008).
Mechanisms of intercellular transfer and co-signaling
Rapid insight into HP transfer mechanisms and specificity
Signaling requires HP secretion and internalization. Both processes involve specific domains within the HD and are unconventional in the sense that secretion is not through the ER-Golgi pathway while internalization does not require endocytosis and instead involves crossing the plasma membrane with direct access to the cytoplasm and nucleus. Despite years of research by several laboratories, including chemists and cell biologists, the internalization and secretion processes are not yet fully understood. Although transfer mechanisms were mainly studied with EN2 and OTX2, domain conservation suggests most HPs use similar strategies. EN2 secretion and internalization involve an interaction with phosphatidylinositol (4,5)-biphosphate (PIP2), and a recent report suggests that OTX2 secretion involves association with nuclear membrane buds and lysosomes (Amblard et al., 2020a; Park et al., 2023). From a therapeutic perspective, two main points are of particular interest. The first one is direct access to the cytoplasm, first demonstrated for the HP internalization domain defined by the third helix of the HD and known as Penetratin. Direct access may involve the formation of inverted micelles (Derossi et al., 1994, 1996, 1998; Berlose et al., 1996) and/or membrane hyperpolarization (Trofimenko et al., 2021). A second point is the specificity of cell targeting by HPs. In the case of EN1 and OTX2, the specific recognition of spinal αMNs and PV cells, respectively, is due to the interaction between glycosaminoglycans (GAGs) present at the cell surface and a GAG-binding motif overlapping with the HD first helix (Beurdeley et al., 2012; Lebœuf et al., 2023). Although two HPs are not sufficient to validate a “sugar code” hypothesis for in vivo specific targeting, it is of note that GAG binding domains are present at a similar sequence position in many HPs (Prochiantz and Di Nardo, 2015).
Co-signaling
An interesting aspect of HP signaling is that it can work synergistically with classical signaling. The best example is EN2 signaling that provokes the in vitro collapse of temporal retina RGC growth cones, similar to aggregated EphrinA5. In an experiment where temporal cones were given the choice to navigate on naive or EphrinA5-coated stripes, EphrinA5 avoidance was clear at 0.5 μg/mL but not at a 0.1 μg/mL, unless 75 nM EN2 was added to the culture medium (Wizenmann et al., 2009). The same cooperation was replicated by directly monitoring growth cone collapse, revealing that cooperative EN2/EphrinA5 signaling requires EN2 internalization and is mediated by local translation (Wizenmann et al., 2009). In fact, further experiments demonstrated that local translation is followed by a burst of ATP synthesis and that adenosine produced by extracellular ATP degradation activates Adenosine receptor A1, providing an intermediate step in EN2-potentiated EphrinA5 signaling (Stettler et al., 2012). Two other examples, not developed here, include the interaction between PAX6 and netrin signaling for OPC migration (Di Lullo et al., 2011), and that of EN and Decapentaplegic (DPP) signaling in the activation of Mothers Against DPP, resulting in ACV formation in the fly wing imaginal disc (Layalle et al., 2011). This concept is important to bear in mind for HP signaling mechanisms, as the exact active morphogen concentrations in vivo are unknown and HP transfer might thus be a co-signaling partner in several developmental and physiological situations.
Homeoprotein therapeutic activities in animal models of human diseases
The use of HPs and HP-derived tools in the regulation of physiological functions is recalled in Table 2. Potential HP-associated therapeutic pathways are summarized in Figure 1. They include local translation, transcription regulation, and chromatin organization with an impact on genome stability.
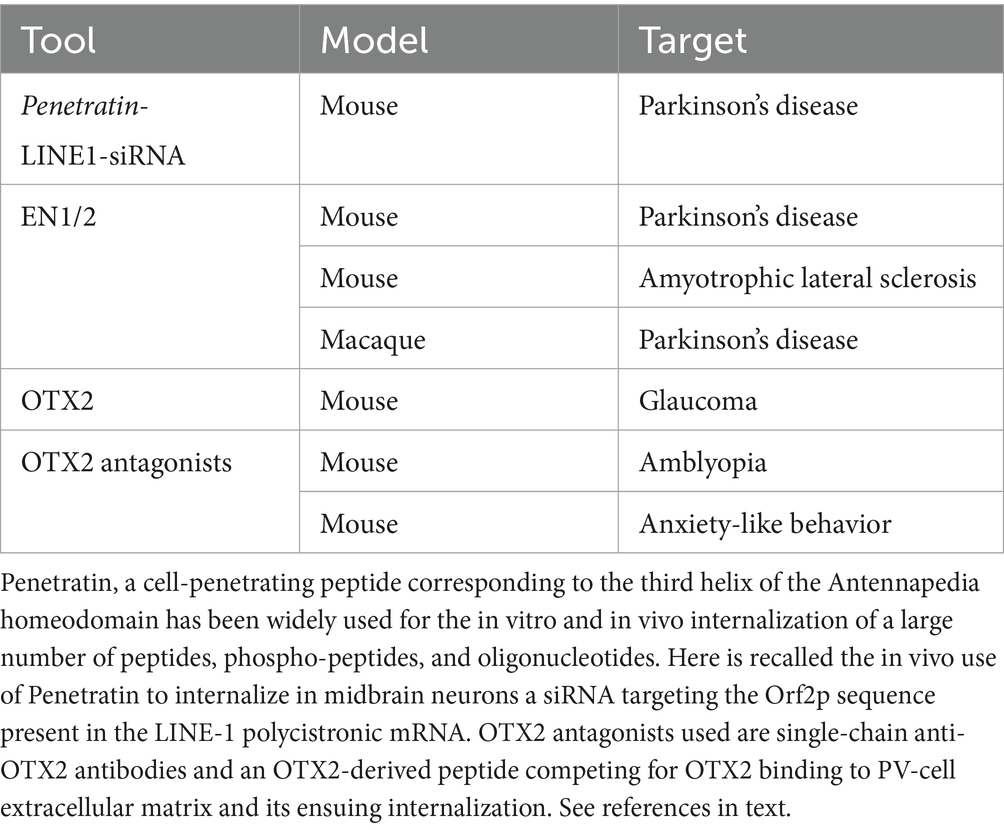
Table 2. Main utilizations of homeoproteins, homeoprotein-derived peptides and homeoprotein antagonists in the regulation of physiological functions.
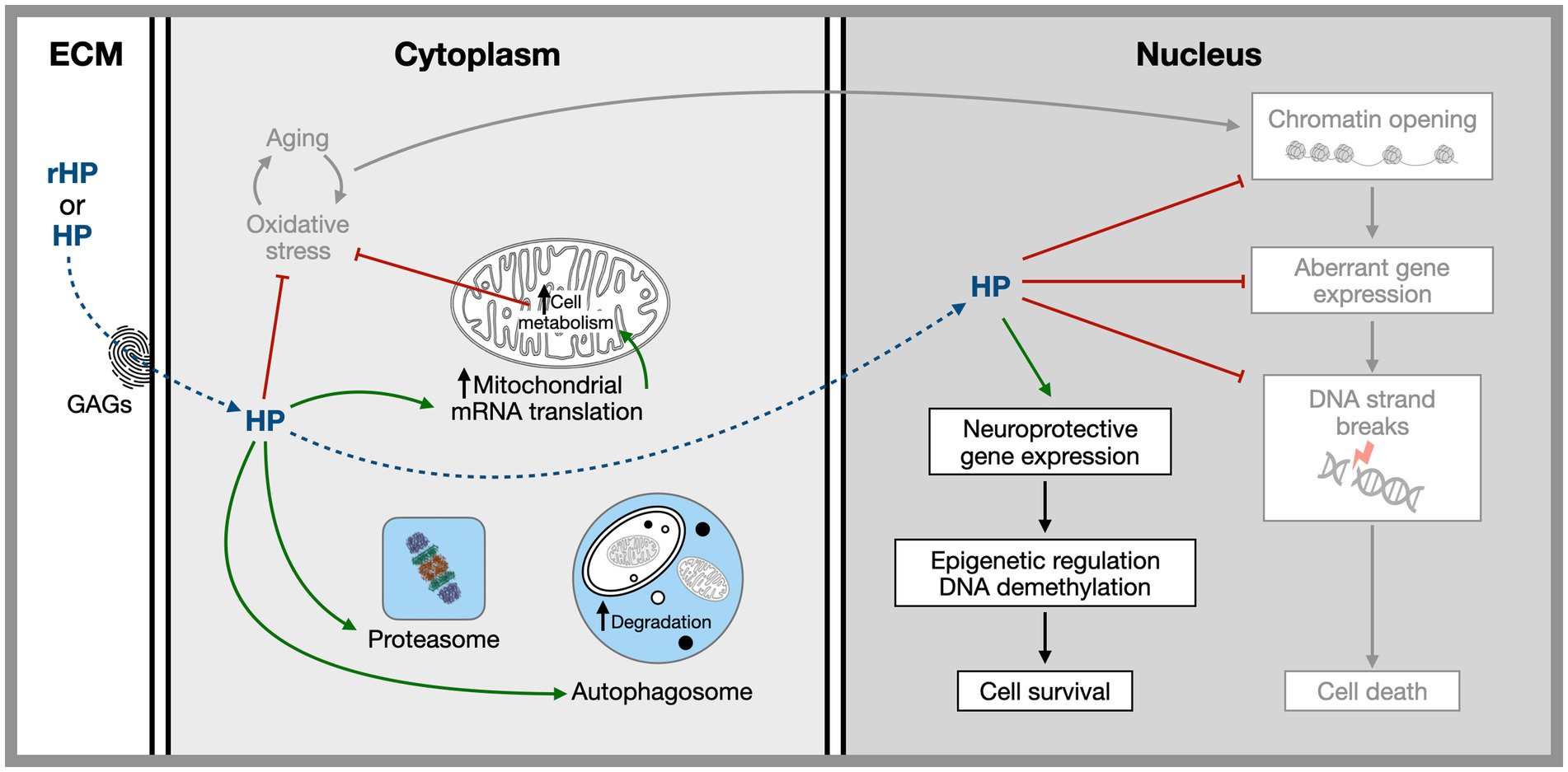
Figure 1. Hypothetical sites and mechanisms of homeoprotein non-cell autonomous activities based on the study of EN1, EN2, and OTX2. Homeoproteins secreted by physiological sources or injected in vivo gain access to specific target cells thanks to a glycosaminoglycan “fingerprint” recognition code. Once internalized they have both cytoplasmic and nuclear activities related to several aging hallmarks including, mitochondrial activity, protection against oxidative stress, regulation of proteostasis, the regulation of expression of neuroprotective genes and the protection of the chromatin landscape allowing for the repression within the heterochromatin of illegitimate genes presenting neuronal harming properties. References in the text.
EN1/2 as a therapeutic protein in animal models of Parkinson’s disease
Mesencephalic dopaminergic (mDA) neurons that innervate the striatum and degenerate in Parkinson’s disease (PD) express both EN1 and EN2. In the Swiss genetic background, En1 heterozygote (En1-Het) mice experience progressive mDA neuron retrograde degeneration and develop motor and non-motor PD-like symptoms (Sonnier et al., 2007; Alvarez-Fischer et al., 2011). Changes in epigenetic mark distribution and intensity, for marks such as H3K9me27, H3K9me3, Nucleolin, and LaminB2, are observed specifically in En1-Het mice mDA neurons, together with an increase in the number of γH2AX foci (DNA-breaks) and the expression of LINE-1A and LINE-1Tf/Gf retrotransposons (Rekaik et al., 2015; Blaudin de Thé et al., 2018). In keeping the observation that mDA neurons from En1-Het mice are more sensitive to oxidative stress, the internalization of recombinant EN1/2 by mDA neuron rescues them from oxidative stress induced either by a 6-OHDA stereotaxic injection in the mouse or by slow MPTP exposure in the non-human primate (Rekaik et al., 2015; Thomasson et al., 2019). EN1/2 injection, and internalization by mouse mDA neurons, 30 min after 6-OHDA administration, rescues the cells from degeneration and returns all nuclear marks back to normal (Rekaik et al., 2015). The hypothesis that a mechanism of EN1/2 “therapeutic activity” involves LINE-1A is supported by the finding that EN2 directly represses LINE-1A expression and binds to its promoter (Blaudin de Thé et al., 2018). Further evidence that substantiates the LINE-1A (and possibly Tf/Gf) hypothesis includes the protection against 6-OHDA by stavudine, a reverse-transcriptase inhibitor and siRNAs directed against LINE-1A ORF2 protein (Blaudin de Thé et al., 2018), and the protective activity of PIWIL1 protein (binds and inactivates LINE-1 transcripts) when overexpressed in the midbrain of En1-Het mice. These experiments suggest that in En1-Het or in WT mice exposed to oxidative stress, a loss of heterochromatin allows for toxic LINE-1 overexpression that can be repressed by EN1/2 gain of function, both directly at a transcriptional level and indirectly through heterochromatin restoration (Blaudin de Thé et al., 2018). It is of note that OTX2 exerts a similar protective activity on midbrain dopaminergic neurons and RGCs in a mouse glaucoma model (Torero-Ibad et al., 2011; Rekaik et al., 2015).
Neurotrophic protective activity of non-cell autonomous ENGRAILED1 for spinal cord alpha-motoneurons
In the ventral spinal cord, V1 interneurons, including Renshaw cells, express EN1 while the En1 locus is not active in αMNs (Wenner and O’Donovan, 1999; Sapir, 2004; Lebœuf et al., 2023). These large MNs receive synaptic input from V1 interneurons and capture secreted EN1 protein. When this transfer is blocked by the local expression of a secreted EN1-specific single-chain antibody (scFv-EN1), αMN retrograde degeneration is induced and muscular strength is partially lost (Lebœuf et al., 2023). A similar degenerative phenotype is observed in the Swiss En1-Het mouse (Lebœuf et al., 2023). Human recombinant EN1 (hEN1) injected intrathecally at lumbar 5 (L5) in mice gains access to the spinal cord parenchyma and is specifically captured by αMNs and γMNs, but not by any other cell type (Lebœuf et al., 2023). This specificity is dependent on a GAG-binding domain upstream of the HD, as was observed for the internalization of OTX2 by PV cells. A single 1 μg injection of hEN1 is sufficient to block αMN degeneration and restore endplate innervation with full neuromuscular strength for 3 months (Lebœuf et al., 2023). This long-lasting effect strongly suggests that EN1 activity engages epigenetic mechanisms, highly reminiscent of its activity in mDA neurons. Based on this similarity, a bioinformatic study was undertaken to identify genes expressed in human MNs that are differentially expressed in the Swiss En1-Het mouse substantia nigra and interact with one of the 4 main genes mutated in ALS familial forms (SOD1, FUS, TARDBP-43 and C9orf72; Rekaik et al., 2015; Lebœuf et al., 2023). This approach generated a list of 20 genes, including p62/SQSTM1 which was the only gene to interact with the 4 ALS genes (Lebœuf et al., 2023). p62/SQSTMI is mutated in some familial forms of ALS (Fecto et al., 2011; Shimizu et al., 2013; Hadano et al., 2016; Doherty and Baehrecke, 2018; Yilmaz et al., 2019; Foster and Rea, 2020) and encodes an autophagy protein that is considered a marker of aging (Hensley and Harris-White, 2015; Menzies et al., 2015; Tai et al., 2016; Leidal et al., 2018). p62/SQSTM1 expression increases with age in αMNs of WT mice and is increased in En1-Het mice or when EN1 transfer into αMNs is antagonized in WT mice (Lebœuf et al., 2023). In contrast, its expression is down-regulated following EN1 treatment of En1-Het mice (Lebœuf et al., 2023). Taken together, these results suggest that αMNs show accelerated aging in En1-Het mice and that EN1 is a “therapeutic” anti-aging protein working at an epigenetic level.
The regulation of cerebral plasticity by OTX2 and its therapeutic outcomes
The cerebral cortex adapts to the surrounding environment during CPs of heightened plasticity that allow neural circuits to be remodeled by experience (Hensch, 2005). These CPs take place postnatally in different brain regions and involve many functions: visual, auditory, sensory-motor, linguistic, social, cognitive, etc. (Reh et al., 2020). Since the seminal studies of Hubel and Wiesel on binocular vision (Hubel and Wiesel, 1965, 1970; Wiesel and Hubel, 1965a,b; Wiesel, 1982), the CP for ocular dominance (OD) plasticity has garnered much attention. In the mouse, this CP opens at postnatal day 20 (P20), peaks around P28, and closes by P40, paralleling progressive PV cell maturation in response to OTX2 capture, which is mediated by specific binding to GAGs present within condensed extracellular matrix perineuronal nets (PNNs) that form around PV cells (Sugiyama et al., 2008; Beurdeley et al., 2012; Miyata et al., 2012). This capture of OTX2 is also progressive, with OTX2 levels being undetectable prior to CP onset, and then increasing in parallel with PNN levels (Sugiyama et al., 2008; Lee et al., 2017). OTX2 has a precise role in controlling CP timing. A gain of function of OTX2 at P17 results in peak plasticity at P20 and CP closure at P25, thus accelerating the entire maturation process (Sugiyama et al., 2008). Conversely, decreased OTX2 import into PV cells delays CP opening in the visual, auditory, and medial prefrontal cortices (Bernard et al., 2016; Lee et al., 2017). At the epigenetic level, OTX2 directly and rapidly upregulates Gadd45ß at CP onset, leading to changes in the pattern of CpG methylations that can impact transcription and chromatin structure (Apulei et al., 2018). OTX2 transfer from choroid plexus to PV cells is maintained throughout life, with maximal steady-state levels in the adult cortex (Spatazza et al., 2013). Long-term closing of one eye during CP, but not before or after, induces experimental amblyopia in the mouse, a condition also encountered in humans with juvenile monocular defects, such as strabismus or cataract, if not treated before 8 years of age (OD CP closure). Interestingly, transiently reopening plasticity by pharmacological OTX2 reduction in the adult cures experimental amblyopia in the mouse (Spatazza et al., 2013; Bernard et al., 2016; Apulei et al., 2018). Since OTX2 is captured by PV cells throughout the cortex (Spatazza et al., 2013), restoration of binocular vision in the amblyopic mouse is an example of a therapeutic protocol that may be of value for other neurodevelopmental diseases. Consistent with this idea, delaying OTX2 import interferes with the development of auditory tonotopic maps and mood-related behaviors (Lee et al., 2017). Strikingly, anxiety-like behaviors are attenuated in the Otx2-Het mouse and can be returned to normal in the adult by overexpressing OTX2 in the choroid plexus (Vincent et al., 2021). Conversely, reducing OTX2 levels in the cerebrospinal fluid installs a hypoanxious-like behavior implicating medial prefrontal cortex PV cells in the adult mouse (Vincent et al., 2021).
Concluding remarks on homeoproteins as time-controlling agents
Molecular studies in PD animal models, and the long-lasting activity of a single EN1 injection on αMN survival and activity, strongly suggest that EN1/2 exert important functions at an epigenetic level. This hypothesis has weight in the context of neurodegenerative diseases for which age is a major risk factor, even in familial forms provoked by mutations that remain silent for several years. The primary hallmarks of aging include genomic instability, epigenetic alterations, loss of proteostasis, disabled macro-autophagy, and telomere attrition (López-Otín et al., 2023). In neurons, telomere attrition is not operational but it is striking that in EN1/2 protective activities are associated with LINE-1 repression, the restoration of most epigenetic marks, and the restoration of autophagy by p62/SQSTM1 regulation. Given that chromatin modifications, loss of autophagy and the upregulation of mobile genetic elements of the LINE family are associated with aging (Laurent et al., 2010; Maxwell et al., 2011; Li et al., 2013; Meter et al., 2014; Krug et al., 2017; Simon et al., 2018, 2019; Valle et al., 2022), EN1/2 has potential as an anti-aging and even a reverse-aging therapeutic protein for mouse mDA neurons and αMNs. But timing does not only implicate aging, as illustrated by the importance for proper synchrony between circuit refinement and environmental information in postnatal learning. This need is well illustrated by how the exact timing of CP windows is essential for physiological alignment between environmental inputs and intrinsic programs of circuit maturation. This makes OTX2 transfer a key factor in determining when functional plasticity opens and closes during postnatal development, with the kinetics and epigenetic impact of OTX2 accumulation in PV cells providing temporal control of neural circuit maturation to define CP timing. In this context, the fact that decreasing OTX2 levels in PV cells reopens plasticity after CP closure suggests that manipulating the OTX2 pathway can be used to “reverse” cortical aging and phenocopy juvenile properties in the adult. It will thus be of high interest to evaluate if such time-controlling functions identified for EN1 and OTX2 are valid for the other HPs expressed in neuronal populations affected in several neurological and psychiatric pathologies.
Author contributions
AD: Writing – original draft, Writing – review & editing. AP: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
AP is a co-founder and holds shares in BrainEver, a company developing HPs for therapeutic use.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alvarez-Fischer, D., Fuchs, J., Castagner, F., Stettler, O., Massiani-Beaudoin, O., Moya, K. L., et al. (2011). Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nat. Neurosci. 14, 1260–1266. doi: 10.1038/nn.2916
Amblard, I., Dupont, E., Alves, I., Miralvès, J., Queguiner, I., and Joliot, A. (2020a). Bidirectional transfer of homeoprotein EN2 across the plasma membrane requires PIP2. J. Cell Sci. 133:jcs244327. doi: 10.1242/jcs.244327
Amblard, I., Thauvin, M., Rampon, C., Queguiner, I., Pak, V. V., Belousov, V., et al. (2020b). H2O2 and Engrailed 2 paracrine activity synergize to shape the zebrafish optic tectum. Commun Biol 3:536. doi: 10.1038/s42003-020-01268-7
Apulei, J., Kim, N., Testa, D., Ribot, J., Morizet, D., Bernard, C., et al. (2018). Non-cell autonomous OTX2 Homeoprotein regulates visual cortex plasticity through Gadd45b/g. Cereb. Cortex 29, 2384–2395. doi: 10.1093/cercor/bhy108
Berlose, J., Convert, O., Derossi, D., Brunissen, A., and Chassaing, G. (1996). Conformational and associative Behaviours of the third Helix of Antennapedia homeodomain in membrane-mimetic environments. Eur. J. Biochem. 242, 372–386. doi: 10.1111/j.1432-1033.1996.0372r.x
Bernard, C., Vincent, C., Testa, D., Bertini, E., Ribot, J., Di Nardo, A. A., et al. (2016). A mouse model for conditional secretion of specific single-chain antibodies provides genetic evidence for regulation of cortical plasticity by a non-cell autonomous Homeoprotein transcription factor. PLoS Genet. 12:e1006035. doi: 10.1371/journal.pgen.1006035
Beurdeley, M., Spatazza, J., Lee, H. H. C., Sugiyama, S., Bernard, C., Di Nardo, A. A., et al. (2012). Otx2 binding to Perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci. 32, 9429–9437. doi: 10.1523/jneurosci.0394-12.2012
Blaudin de Thé, F.-X., Rekaik, H., Pezé-Heidsieck, E., Massiani-Beaudoin, O., Joshi, R. L., Fuchs, J., et al. (2018). Engrailed homeoprotein blocks degeneration in adult dopaminergic neurons through LINE-1 repression. EMBO J. 37:7374. doi: 10.15252/embj.201797374
Bolduc, N., Hake, S., and Jackson, D. (2008). Dual functions of the KNOTTED1 homeodomain: sequence-specific DNA binding and regulation of cell-to-cell transport. Sci. Signal. 1:pe28. doi: 10.1126/scisignal.123pe28
Brunet, I., Di Nardo, A. A., Sonnier, L., Beurdeley, M., and Prochiantz, A. (2007). The topological role of homeoproteins in the developing central nervous system. Trends Neurosci. 30, 260–267. doi: 10.1016/j.tins.2007.03.010
Brunet, I., Weinl, C., Piper, M., Trembleau, A., Volovitch, M., Harris, W., et al. (2005). The transcription factor Engrailed-2 guides retinal axons. Nature 438, 94–98. doi: 10.1038/nature04110
Derossi, D., Calvet, S., Trembleau, A., Brunissen, A., Chassaing, G., and Prochiantz, A. (1996). Cell internalization of the third Helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271, 18188–18193. doi: 10.1074/jbc.271.30.18188
Derossi, D., Chassaing, G., and Prochiantz, A. (1998). Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 8, 84–87. doi: 10.1016/s0962-8924(98)80017-2
Derossi, D., Joliot, A. H., Chassaing, G., and Prochiantz, A. (1994). The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 269, 10444–10450. doi: 10.1016/S0021-9258(17)34080-2
Di Lullo, E., Haton, C., Poupon, C. L., Volovitch, M., Joliot, A., Thomas, J. L., et al. (2011). Paracrine Pax6 activity regulates oligodendrocyte precursor cell migration in the chick embryonic neural tube. Development 138, 4991–5001. doi: 10.1242/dev.066282
Di Nardo, A. A., Fuchs, J., Joshi, R. L., Moya, K. L., and Prochiantz, A. (2018). The physiology of Homeoprotein transduction. Physiol. Rev. 98, 1943–1982. doi: 10.1152/physrev.00018.2017
Di Nardo, A. A., Joliot, A., and Prochiantz, A. (2020). Homeoprotein transduction in neurodevelopment and physiopathology. Sci. Adv. 6:eabc6374. doi: 10.1126/sciadv.abc6374
Doherty, J., and Baehrecke, E. H. (2018). Life, death and autophagy. Nat. Cell Biol. 20, 1110–1117. doi: 10.1038/s41556-018-0201-5
Fecto, F., Yan, J., Vemula, S. P., Liu, E., Yang, Y., Chen, W., et al. (2011). SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 68, 1440–1446. doi: 10.1001/archneurol.2011.250
Foster, A., and Rea, S. (2020). The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Neural Regen. Res. 15, 2186–2194. doi: 10.4103/1673-5374.284977
Hadano, S., Mitsui, S., Pan, L., Otomo, A., Kubo, M., Sato, K., et al. (2016). Functional links between SQSTM1 and ALS2 in the pathogenesis of ALS: cumulative impact on the protection against mutant SOD1-mediated motor dysfunction in mice. Hum. Mol. Genet. 25, 3321–3340. doi: 10.1093/hmg/ddw180
Hensch, T. K. (2005). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888. doi: 10.1038/nrn1787
Hensley, K., and Harris-White, M. E. (2015). Redox regulation of autophagy in healthy brain and neurodegeneration. Neurobiol. Dis. 84, 50–59. doi: 10.1016/j.nbd.2015.03.002
Hubel, D. H., and Wiesel, T. N. (1965). Binocular interaction in striate cortex of kittens reared with artificial squint. J. Neurophysiol. 28, 1041–1059. doi: 10.1152/jn.1965.28.6.1041
Hubel, D. H., and Wiesel, T. N. (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 206, 419–436. doi: 10.1113/jphysiol.1970.sp009022
Joliot, A., Pernelle, C., Deagostini-Bazin, H., and Prochiantz, A. (1991). Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA 88, 1864–1868. doi: 10.1073/pnas.88.5.1864
Kaddour, H., Coppola, E., Di Nardo, A. A., Poupon, C. L., Mailly, P., Wizenmann, A., et al. (2019). Extracellular Pax6 regulates tangential Cajal–Retzius cell migration in the developing mouse neocortex. Cereb. Cortex 30, 465–475. doi: 10.1093/cercor/bhz098
Kim, N., Min, K. W., Kang, K. H., Lee, E. J., Kim, H.-T., Moon, K., et al. (2014). Regulation of retinal axon growth by secreted Vax1 homeodomain protein. eLife 3:e02671. doi: 10.7554/elife.02671
Kim, J. Y., Yuan, Z., Cilia, M., Khalfan-Jagani, Z., and Jackson, D. (2002). Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc. Natl. Acad. Sci. USA 99, 4103–4108. doi: 10.1073/pnas.052484099
Krug, L., Chatterjee, N., Borges-Monroy, R., Hearn, S., Liao, W.-W., Morrill, K., et al. (2017). Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLoS Genet. 13:e1006635. doi: 10.1371/journal.pgen.1006635
Laurent, G. S., Hammell, N., and McCaffrey, T. A. (2010). A LINE-1 component to human aging: do LINE elements exact a longevity cost for evolutionary advantage? Mech. Ageing Dev. 131, 299–305. doi: 10.1016/j.mad.2010.03.008
Layalle, S., Volovitch, M., Mugat, B., Bonneaud, N., Parmentier, M. L., Prochiantz, A., et al. (2011). Engrailed homeoprotein acts as a signaling molecule in the developing fly. Development 138, 2315–2323. doi: 10.1242/dev.057059
Lebœuf, M., Vargas-Abonce, S. E., Pezé-Hedsieck, E., Dupont, E., Jimenez-Alonso, L., Moya, K. L., et al. (2023). ENGRAILED-1 transcription factor has a paracrine neurotrophic activity on adult spinal α-motoneurons. EMBO Rep. 24:e56525. doi: 10.15252/embr.202256525
Lee, H. H. C., Bernard, C., Ye, Z., Acampora, D., Simeone, A., Prochiantz, A., et al. (2017). Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol. Psychiatry 22, 680–688. doi: 10.1038/mp.2017.1
Lee, E. J., Kim, N., Park, J. W., Kang, K. H., Kim, W., Sim, N. S., et al. (2019). Global analysis of intercellular homeodomain protein transfer. Cell Rep. 28, 712–722.e3. doi: 10.1016/j.celrep.2019.06.056
Leidal, A. M., Levine, B., and Debnath, J. (2018). Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 20, 1338–1348. doi: 10.1038/s41556-018-0235-8
Lesaffre, B., Joliot, A., Prochiantz, A., and Volovitch, M. (2007). Direct non-cell autonomous Pax6 activity regulates eye development in the zebrafish. Neural Dev. 17:2. doi: 10.1186/1749-8104-2-2
Li, W., Prazak, L., Chatterjee, N., Grüninger, S., Krug, L., Theodorou, D., et al. (2013). Activation of transposable elements during aging and neuronal decline in Drosophila. Nat. Publ. Group 16, 529–531. doi: 10.1038/nn.3368
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., and Kroemer, G. (2023). Hallmarks of aging: an expanding universe. Cell 186, 243–278. doi: 10.1016/j.cell.2022.11.001
Lucas, W. J., Bouché-Pillon, S., Jackson, D. P., Nguyen, L., Baker, L., Ding, B., et al. (1995). Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through Plasmodesmata. Science 270, 1980–1983. doi: 10.1126/science.270.5244.1980
Maxwell, P. H., Burhans, W. C., and Curcio, M. J. (2011). Retrotransposition is associated with genome instability during chronological aging. Proc. Natl. Acad. Sci. USA 108, 20376–20381. doi: 10.1073/pnas.1100271108
Menzies, F. M., Fleming, A., and Rubinsztein, D. C. (2015). Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 16, 345–357. doi: 10.1038/nrn3961
Meter, M. V., Kashyap, M., Rezazadeh, S., Geneva, A. J., Morello, T. D., Seluanov, A., et al. (2014). SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat. Commun. 5:5011. doi: 10.1038/ncomms6011
Min, K. W., Kim, N., Lee, J. H., Sung, Y., Kim, M., Lee, E. J., et al. (2023). Visuomotor anomalies in achiasmatic mice expressing a transfer-defective Vax1 mutant. Exp. Mol. Med. 55, 385–400. doi: 10.1038/s12276-023-00930-4
Miyata, S., Komatsu, Y., Yoshimura, Y., Taya, C., and Kitagawa, H. (2012). Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat. Neurosci. 15, 414–422. doi: 10.1038/nn.3023
Park, J. W., Lee, E. J., Moon, E., Kim, H.-L., Kim, I.-B., Hodzic, D., et al. (2023). Orthodenticle homeobox 2 is transported to lysosomes by nuclear budding vesicles. Nat. Commun. 14:1111. doi: 10.1038/s41467-023-36697-5
Prochiantz, A., and Di Nardo, A. A. (2015). Homeoprotein signaling in the developing and adult nervous system. Neuron 85, 911–925. doi: 10.1016/j.neuron.2015.01.019
Rampon, C., Gauron, C., Lin, T., Meda, F., Dupont, E., Cosson, A., et al. (2015). Control of brain patterning by Engrailed paracrine transfer: a new function of the Pbx interaction domain. Development 142, 1840–1849. doi: 10.1242/dev.114181
Reh, R. K., Dias, B. G., Nelson, C. A., Kaufer, D., Werker, J. F., Kolb, B., et al. (2020). Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. USA. 117, 23242–23251. doi: 10.1073/pnas.1820836117
Rekaik, H., Blaudin de Thé, F.-X., Fuchs, J., Massiani-Beaudoin, O., Prochiantz, A., and Joshi, R. L. (2015). Engrailed Homeoprotein protects mesencephalic dopaminergic neurons from oxidative stress. Cell Rep. 13, 242–250. doi: 10.1016/j.celrep.2015.08.076
Ruiz-Medrano, R., Xoconostle-Cazares, B., and Kragler, F. (2004). The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr. Opin. Plant Biol. 7, 641–650. doi: 10.1016/j.pbi.2004.09.012
Sapir, T. (2004). Pax6 and Engrailed 1 regulate two distinct aspects of Renshaw cell development. J. Neurosci. 24, 1255–1264. doi: 10.1523/jneurosci.3187-03.2004
Shimizu, H., Toyoshima, Y., Shiga, A., Yokoseki, A., Arakawa, K., Sekine, Y., et al. (2013). Sporadic ALS with compound heterozygous mutations in the SQSTM1 gene. Acta Neuropathol. 126, 453–459. doi: 10.1007/s00401-013-1150-5
Simon, M., Meter, M. V., Ablaeva, J., Ke, Z., Gonzalez, R. S., Taguchi, T., et al. (2018). Inhibition of retrotransposition improves health and extends lifespan of SIRT6 knockout mice. bioRxiv [Preprint]. 1–53.
Simon, M., Meter, M. V., Ablaeva, J., Ke, Z., Gonzalez, R. S., Taguchi, T., et al. (2019). LINE1 Derepression in aged wild-type and SIRT6-deficient mice drives inflammation. Cell Metab. 29, 871–885.e5. doi: 10.1016/j.cmet.2019.02.014
Sonnier, L., Le Pen, G., Hartmann, A., Bizot, J.-C., Trovero, F., Krebs, M.-O., et al. (2007). Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1. J. Neurosci. 27, 1063–1071. doi: 10.1523/jneurosci.4583-06.2007
Spatazza, J., Lee, H. H. C., Di Nardo, A. A., Tibaldi, L., Joliot, A., Hensch, T. K., et al. (2013). Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 3, 1815–1823. doi: 10.1016/j.celrep.2013.05.014
Stettler, O., Joshi, R. L., Wizenmann, A., Reingruber, J., Holcman, D., Bouillot, C., et al. (2012). Engrailed homeoprotein recruits the adenosine A1 receptor to potentiate ephrin A5 function in retinal growth cones. Development 139, 215–224. doi: 10.1242/dev.063875
Sugiyama, S., Di Nardo, A. A., Aizawa, S., Matsuo, I., Volovitch, M., Prochiantz, A., et al. (2008). Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 508–520. doi: 10.1016/j.cell.2008.05.054
Tai, H., Wang, Z., Gong, H., Han, X., Zhou, J., Wang, X., et al. (2016). Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy 13, 99–113. doi: 10.1080/15548627.2016.1247143
Thomasson, N., Pioli, E., Friedel, C., Monseur, A., Lavaur, J., Moya, K. L., et al. (2019). Engrailed-1 induces long-lasting behavior benefit in an experimental Parkinson primate model. Mov. Disord. 34, 1082–1084. doi: 10.1002/mds.27714
Torero-Ibad, R., Rheey, J., Mrejen, S., Forster, V., Picaud, S., Prochiantz, A., et al. (2011). Otx2 promotes the survival of damaged adult retinal ganglion cells and protects against excitotoxic loss of visual acuity in vivo. J. Neurosci. 31, 5495–5503. doi: 10.1523/jneurosci.0187-11.2011
Trofimenko, E., Homma, Y., Fukuda, M., and Widmann, C. (2021). The endocytic pathway taken by cationic substances requires Rab14 but not Rab5 and Rab7. Cell Rep. 37:109945. doi: 10.1016/j.celrep.2021.109945
Valle, F. D., Reddy, P., Yamamoto, M., Liu, P., Saera-Vila, A., Bensaddek, D., et al. (2022). LINE-1 RNA causes heterochromatin erosion and is a target for amelioration of senescent phenotypes in progeroid syndromes. Sci. Transl. Med. 14:eabl6057. doi: 10.1126/scitranslmed.abl6057
Vincent, C., Gilabert-Juan, J., Gibel-Russo, R., Alvarez-Fischer, D., Krebs, M.-O., Le Pen, G., et al. (2021). Non-cell-autonomous OTX2 transcription factor regulates anxiety-related behavior in the mouse. Mol. Psychiatry 26, 6469–6480. doi: 10.1038/s41380-021-01132-y
Wenner, P., and O’Donovan, M. J. (1999). Identification of an Interneuronal population that mediates recurrent inhibition of Motoneurons in the developing Chick spinal cord. J. Neurosci. 19, 7557–7567. doi: 10.1523/jneurosci.19-17-07557.1999
Wiesel, T. N. (1982). Postnatal development of the visual cortex and the influence of environment. Nature 299, 583–591. doi: 10.1038/299583a0
Wiesel, T. N., and Hubel, D. H. (1965a). Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol. 28, 1029–1040. doi: 10.1152/jn.1965.28.6.1029
Wiesel, T. N., and Hubel, D. H. (1965b). Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 28, 1060–1072. doi: 10.1152/jn.1965.28.6.1060
Wizenmann, A., Brunet, I., Lam, J. S. Y., Sonnier, L., Beurdeley, M., Zarbalis, K., et al. (2009). Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo. Neuron 64, 355–366. doi: 10.1016/j.neuron.2009.09.018
Yilmaz, R., Müller, K., Brenner, D., Volk, A. E., Borck, G., Hermann, A., et al. (2019). SQSTM1/p62 variants in 486 familial ALS patients from Germany and Sweden. Neurobiol. Aging 87, 139.e9–139.e15. doi: 10.1016/j.neurobiolaging.2019.10.018
Keywords: homeoproteins, development, neurological diseases, psychiatric diseases, protein therapy
Citation: Di Nardo AA and Prochiantz A (2024) Therapeutic value of homeoprotein signaling pathways. Front. Neurosci. 18:1359523. doi: 10.3389/fnins.2024.1359523
Edited by:
Jia-Ren Liu, Boston Children’s Hospital and Harvard Medical School, United StatesReviewed by:
Jaspreet Kaur, University of Copenhagen, DenmarkCarlos Vicario, Spanish National Research Council (CSIC), Spain
Copyright © 2024 Di Nardo and Prochiantz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ariel A. Di Nardo, YXJpZWwuZGluYXJkb0Bjb2xsZWdlLWRlLWZyYW5jZS5mcg==; Alain Prochiantz, YWxhaW4ucHJvY2hpYW50ekBjb2xsZWdlLWRlLWZyYW5jZS5mcg==
 Ariel A. Di Nardo
Ariel A. Di Nardo Alain Prochiantz
Alain Prochiantz