
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 24 January 2024
Sec. Neurogenomics
Volume 18 - 2024 | https://doi.org/10.3389/fnins.2024.1353759
This article is part of the Research Topic Neuroepigenomics Pathogenesis in Major Depressive Disorder View all 3 articles
Objective: Anxiety symptoms are prevalent neuropsychiatric manifestations in Parkinson’s disease (PD) and impact the development of motor complications. Our aim was to evaluate the association of GBA variants with the anxiety development in early PD cohort.
Methods: This cohort study used data from the Parkinson Progression Marker Initiative. The primary outcome anxiety was assessed by State–Trait Anxiety Inventory (STAI). The association between GBA and longitudinal change in the STAI total score was examined using linear mixed-effects model, and the association between GBA and anxiety progression was examined using Cox survival analysis.
Results: A total of 385 patients with PD were included in this study, 39 of them were GBA variant carriers and 346 were idiopathic PD without GBA variants. Patients with GBA variants had faster annual increase in anxiety score (β = 0.44; 95% CI, 0.18 to 0.71; p < 0.001) and were at higher risk of anxiety progression (HR 1.87; 95% CI, 1.03 to 3.41; p = 0.03,). Higher baseline scores for Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT), which indicated the autonomic dysfunction, also independently predicted faster increase in anxiety score (β = 0.48; 95%CI, 0.19 to 0.69; p < 0.001) and higher incidence of anxiety development (HR = 1.05; 95% CI, 1.01 to 1.08; p = 0.008).
Interpretation: These findings suggest that longitudinal anxiety symptoms worsening was faster in PD patients who were GBA variant carriers and have dysautonomia, and this association was enhanced if they have both.
Anxiety represents a highly prevalent and subjectively burdensome non-motor symptom in Parkinson’s disease (PD) (Blundell et al., 2023), with an average prevalence of 31% and a lifetime prevalence reaching up to 49% (Broen et al., 2016; Fan et al., 2022). Anxiety often emerges prior to the onset of motor symptoms in PD, suggesting its potential as an early manifestation of the disease (McGregor and Nelson, 2019; Carey et al., 2021). Anxiety in PD patients not only affects emotional wellbeing and daily functioning but also predict the severity of motor complications and cognitive decline, which further decreased health-related quality of life and increased more caregiver burden (Hinkle et al., 2021; Lo Buono et al., 2021).
Although the exact pathological mechanism of anxiety in Parkinson’s disease (PD) is not fully understood, researchers have proposed various predictors of anxiety in PD (Leentjens et al., 2011; Khedr et al., 2020). However, it is worth noting that most studies investigating this subject have been cross-sectional in nature (Dissanayaka et al., 2010; Cui et al., 2017). Only a few studies have incorporated analyses of longitudinal data to ascertain predictors of anxiety in PD, including baseline probable REM sleep behavior disorder (pRBD), cognitive impairment, daytime sleepiness, dysautonomia, and depressive symptoms (Rutten et al., 2017; Zhu et al., 2017; Wang et al., 2023), but the influence of genetic variants on anxiety development in PD patients over time has been poorly studied yet.
The GBA gene is responsible for encoding the lysosomal enzyme, β-glucocerebrosidase (GCase), which plays a crucial role in the metabolism of sphingolipids (Behl et al., 2021; Vázquez-Vélez and Zoghbi, 2021). GBA variants have emerged as the predominant genetic risk factors for PD on a global scale, and PD patients carrying GBA variants generally exhibit earlier onset, more severe motor and gait impairments, an increased risk of cognitive decline and depression, faster disease progression, and reduced survival rates (Neumann et al., 2009; Brockmann et al., 2015; Petrucci et al., 2020; Yang et al., 2023). However, the relationship between the GBA variants and anxiety progression remains unclear. Therefore, the aim of this study was to examine the associations between GBA variants, clinical covariates, and the anxiety progression in a large group of patients with early PD.
This study obtained data from the Parkinson’s Progression Markers Initiative (PPMI) database, and the PPMI study is an ongoing observational longitudinal study which aims to identify progression biomarkers for PD (Marek et al., 2018). Study protocols and manuals are available online at.1 Patients were recruited between 2010 and 2018 based on the strict inclusion/exclusion criteria that were previously described (Marek et al., 2018; Yang et al., 2022a). We downloaded data from the PPMI database in December 2022, and 5 years of follow-up data were included in further analysis.
A total of 385 idiopathic PD patients who had follow-up data were involved in the linear mixed model analysis, including 39 PD patients with GBA variants (GBA-PD) and 346 idiopathic PD without GBA variants (iPD). The GBA-PD group included those most common variants: N370S, L444P, T369M, and E326K (Straniero et al., 2020; Hoglinger et al., 2022; Pang et al., 2022).
DNA was extracted from the whole blood of the patients according to the study protocol as described in the PPMI biologics manual.2 Sanger sequencing was performed by PPMI to analyze and screen for variants within exons 1–11 of the GBA gene.
We obtained demographic and clinical data from the PPMI database for all subjects. Clinical evaluation was performed in the “off” treatment state. Motor symptoms were evaluated with the MDS-UPDRS III (Goetz et al., 2007). Global cognitive status was assessed using the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005). Autonomic dysfunction was assessed with the Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) (Rodriguez-Blazquez et al., 2010). Olfactory dysfunction was measured by the University of Pennsylvania Smell Identification Test (UPSIT) (Vengalil et al., 2016). Depressive symptoms were assessed with the short version of the Geriatric Depression Scale (GDS), and probable depression was defined as GDS >5 (Paudel et al., 2017). Excessive daytime sleepiness (EDS) was assessed with the Epworth Sleepiness Scale (ESS), and ESS > 10 was defined as EDS (Büchele et al., 2018). REM Sleep Behavior Disorder (RBD) was assessed by REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), and RBDSQ >5 was defined as probable REM Sleep Behavior Disorder (pRBD) (Berg et al., 2021).
The main outcome anxiety was assessed with State–Trait Anxiety Inventory (STAI). It consists of two subscales, the State subscale and the Trait subscale, with a total score for each subscale ranging from 20 to 80. The State subscale measures the current state of anxiety, whereas the Trait subscale evaluates relatively stable aspects of “anxiety proneness.” The STAI total score is a sum of the State subscale and the Trait subscale (Julian, 2011). It has been reported that the STAI had a good internal consistency among de novo PD patients (Yang et al., 2019), and clinically relevant anxiety is commonly defined as a STAI State score of ≥39 (Knight et al., 1983).
The association between GBA variants and longitudinal changes in STAI total scores was assessed using multivariate linear mixed-effects models in R 4.2.1 with the lme4 package. Development of anxiety was evaluated using Cox regression (package survival) and visualized in Kaplan–Meier plots (package survminer) in R 4.2.1. Patients were censored due to death, loss to follow-up, or last recorded visit. Models were adjusted for confounders, such as age at onset, sex, and education. All baseline variables with value of ps of less than 0.1 in the univariable Cox models were included in subsequent multivariable Cox models with a backward elimination procedure. The cutoff value of continuous variable such as baseline SCOPA-AUT scores that producing the maximum log rank was defined using the R package “maxstat.” Differences in demographic characteristics between the GBA-PD group and the iPD group were assessed using Student’s t-test or the Mann–Whitney U test for continuous variables and the χ2 test for categorical variables in IBM SPSS 25.0 (Armonk, NY, USA). All p-values were two-sided, and a p-value of less than 0.05 was regarded as statistically significant.
The demographic and clinical characteristics of the participants included in this study are presented in Table 1, including 39 PD patients with GBA variants (GBA-PD group) and 346 idiopathic PD without GBA variants (iPD group). At the baseline, the GBA-PD group showed higher RBDSQ scores (p = 0.006).
Linear mixed models demonstrated a more rapid Anxiety-STAI total score increase than non-carriers, with an annual increase in STAI total score of 1.21 point (95% CI, 0.41 to 2.1 point) versus 0.77 point (95% CI, 0.26 to 0.99 point), respectively. This difference was significant (β = 0.44; 95% CI, 0.18 to 0.71; p < 0.001) (Table 2, Figure 1A). A similar relationship was observed in the adjusted model 2 (including demographic factors, medication, cognition, motor symptoms, autonomic symptoms, sleep disorder, olfactory symptoms, and depression factor). Baseline SCOPA-AUT total score and depression and postural instability gait difficulty (PIGD) subtype were also significantly associated with faster increase in annual anxiety score in this linear mixed-effects model (Table 2). One point increase in SCOPA-AUT score was associated with an increase of 0.48 point in the STAI total score (p = 0.001). Baseline UPSIT score, MoCA score, and age at onset were also significantly independent predictors of annual change in STAI total score. One unit increase in age at onset, UPSIT score, and MoCA score was associated with a decrease of 0.22, 0.25, and 0.71 point in the anxiety score (p = 0.002, p = 0.02, and p = 0.03, respectively) (Table 2).
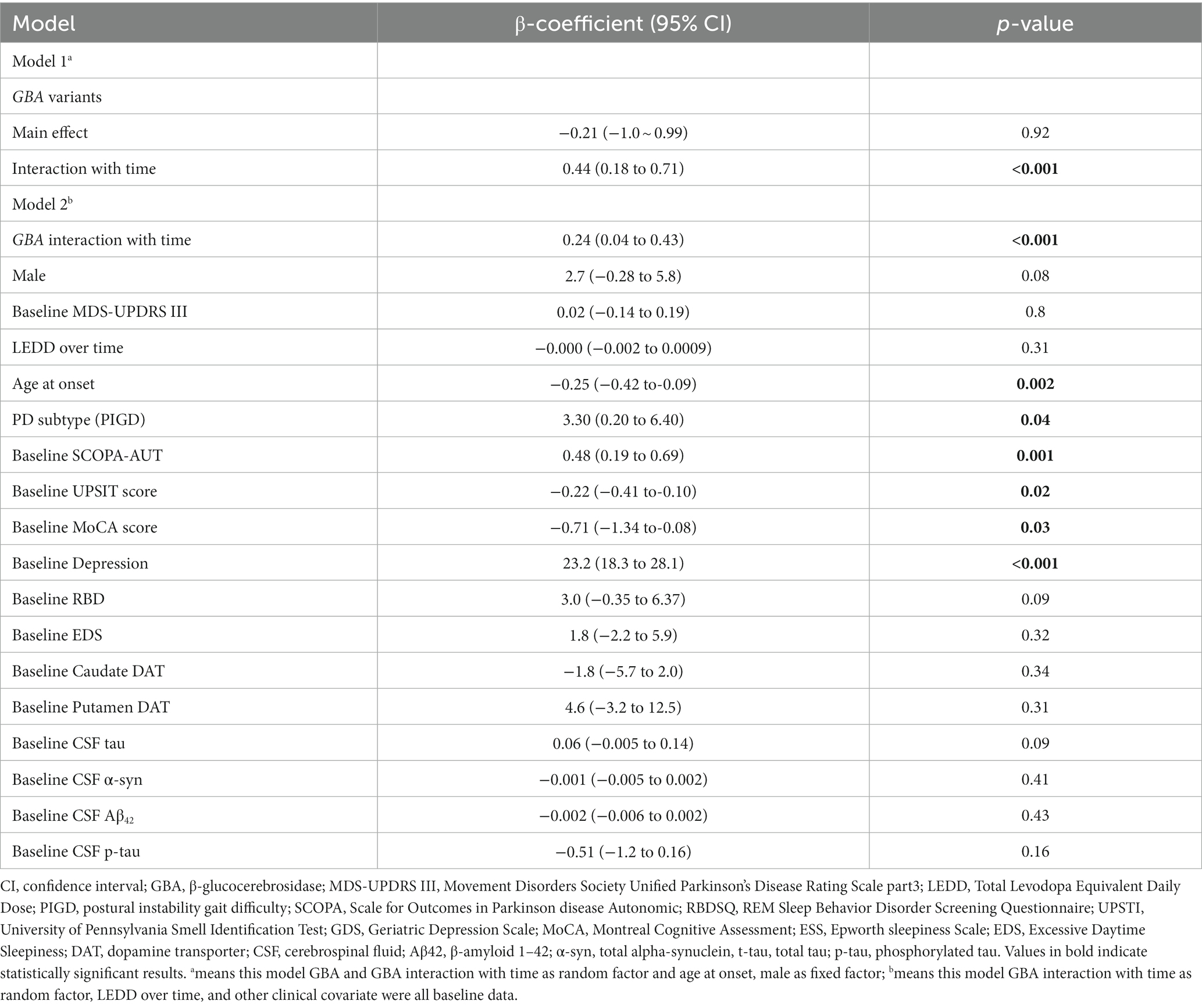
Table 2. Linear mixed model for predicting annual change in State–Trait Anxiety Inventory (STAI) score.
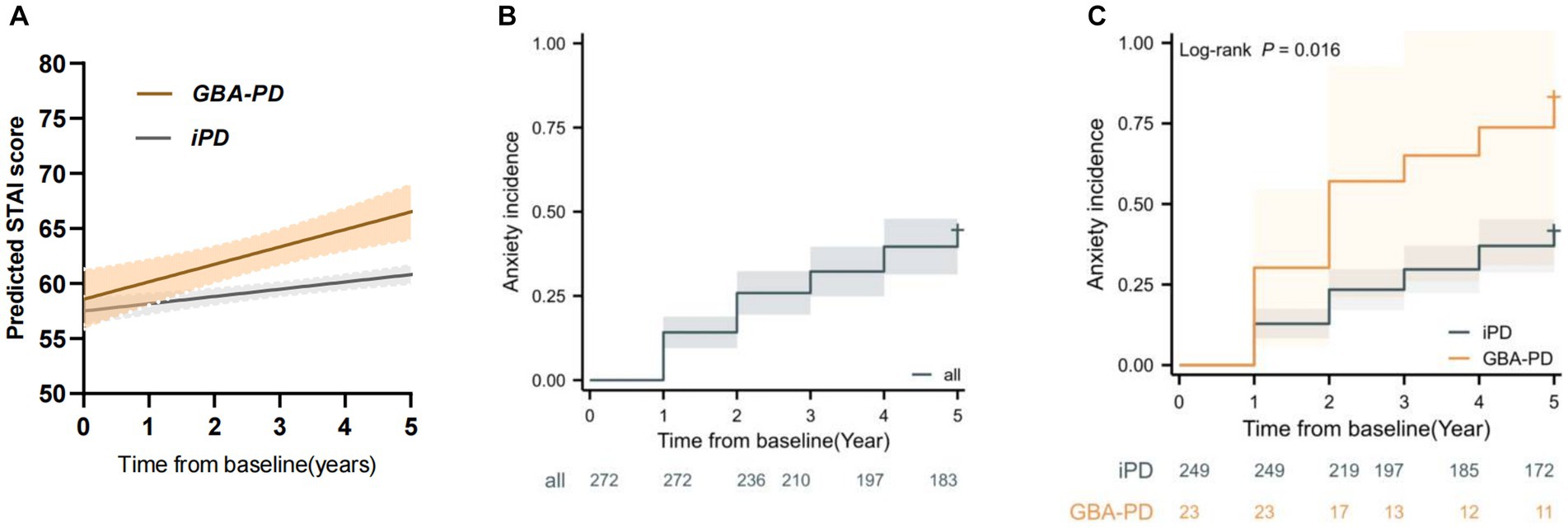
Figure 1. GBA variant carriers showed a faster increase in anxiety score and higher risk of anxiety progression. (A) Longitudinal trajectories of mean state–trait anxiety inventory (STAI) ratings in GBA variant carrier and non-carrier groups (B) Kaplan–Meier analyses of anxiety progression in Parkinson’s disease patients. (C) Kaplan–Meier analyses of anxiety progression in GBA variant carrier and non-carrier groups.
To further find the association between the clinical covariate changes over time and changes in anxiety scores during time, another linear mixed model was performed including those clinical covariate scores over time, and the cerebrospinal fluid total-tau (CSF t-tau), LEDD over time, and MDS-UPDRS motor score were associated with anxiety score (Table 3).
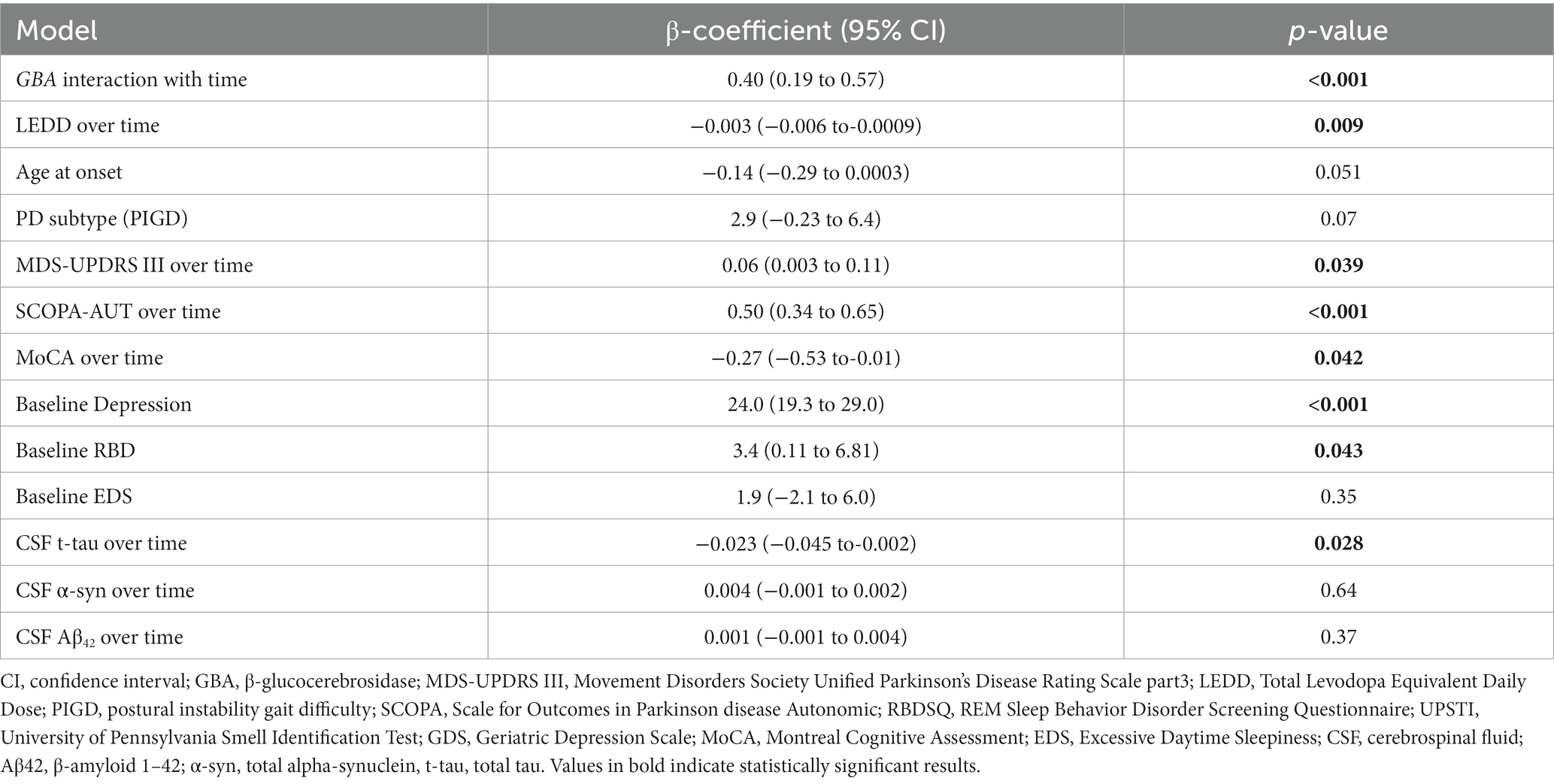
Table 3. Linear mixed model for predicting annual change in State–Trait Anxiety Inventory (STAI) score with clinical covariates change over time.
As we mentioned in the method part, clinically relevant anxiety is commonly defined as a STAI State score of ≥39; 97 patients from the iPD group and 16 from the GBA-PD group were found to have anxiety at baseline and were excluded from further survival analysis. Finally, 272 participants were enrolled in the survival analysis, including 23 patients from the GBA-PD group and 249 patients from the iPD group. The baseline characteristics of those 272 patients are presented in Supplementary Table S1.
During the 5-year follow-up, 98 patients (36.02%) reported developing anxiety (Figure 1B), 13 patients were from the GBA-PD group and 85 patients from the iPD group. Kaplan–Meier estimates showed that the GBA-PD group had a significantly higher incidence of anxiety than the iPD group (log rank 5.844, p = 0.016) (Figure 1C).
The results of the Cox regression analyses are shown in Table 4. In the univariable Cox regression analyses, the GBA variants, dysautonomia, pRBD, cognitive impairment, and olfactory dysfunction were significantly associated with anxiety progression. In multivariable Cox model, GBA variants remained to be a significant predictor of anxiety progression (HR 1.87; 95% CI 1.03 to 3.41; p = 0.03, Table 4) and dysautonomia (HR = 1.05; 95% CI 1.01 to 1.08; p = 0.008, Table 4), independently increasing the risk of anxiety development.
Based on the linear mixed models and multivariable Cox model results, higher baseline SCOPA-AUT scores predicted the future anxiety progression and higher anxiety score during time. To further support the association between baseline SCOPA-AUT score and anxiety progression, SCOPA-AUT score cutoff values were defined as 18 based on the producing maximum log-rank score. Kaplan–Meier analyses showed that the “SCOPA-AUT ≥ 18” group (“high-level” group) had a significantly higher incidence of anxiety compared with the “SCOPA-AUT < 18” group (“low-level” group) (log-rank 12.8, p < 0.001) (Figure 2A), and the “high-level” group also had a faster rate of annual increase in STAI total score compared with the “low-level” group (Figures 2B,C).
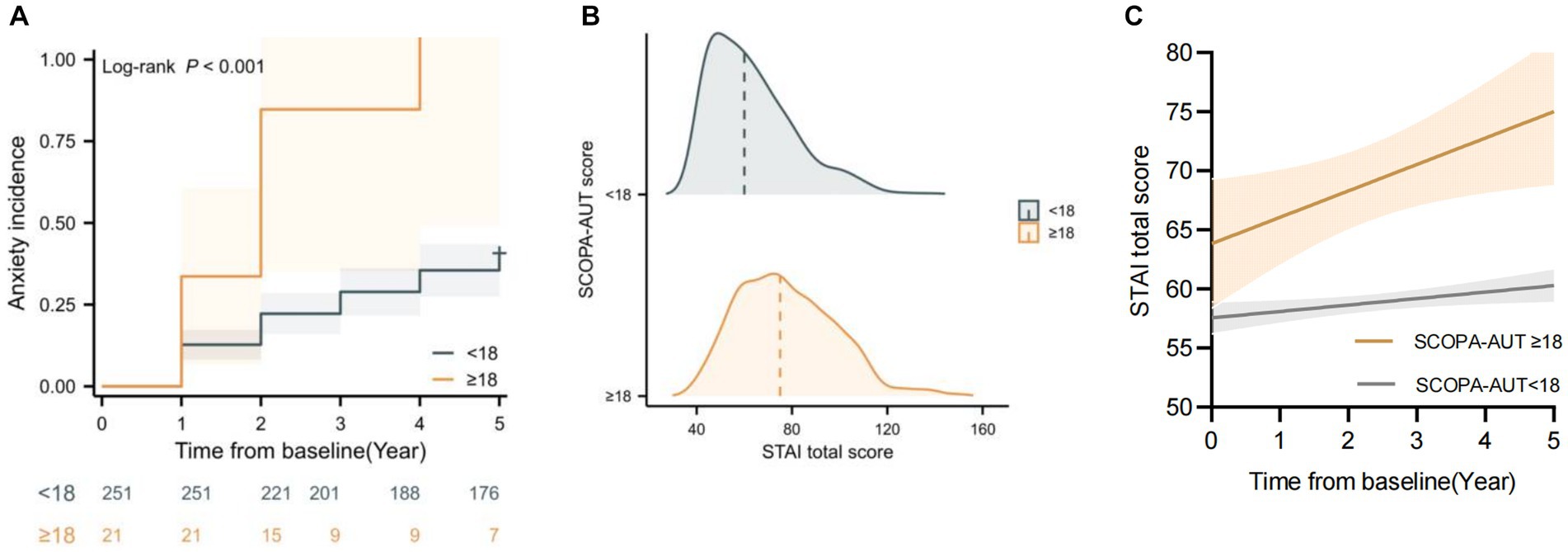
Figure 2. Baseline Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) was associated with State–Trait Anxiety Inventory (STAI) change and anxiety progression over time. (A) Kaplan–Meier analyses of anxiety progression in different SCOPA-AUT level groups. (B) Raincloud plots showing the expected increase in STAI score based on the SCOPA-AUT cutoff. (C) Longitudinal trajectories of mean STAI changes in different SCOPA-AUT level groups.
We also analyzed the SCOPA-AUT score over time and found that autonomic dysfunction increased over time (Figures 3A,B; Supplementary Figure S1). Dysautonomia was associated with higher anxiety score over time, and this trend was more significant in the GBA variant carriers (Figures 3C,D).
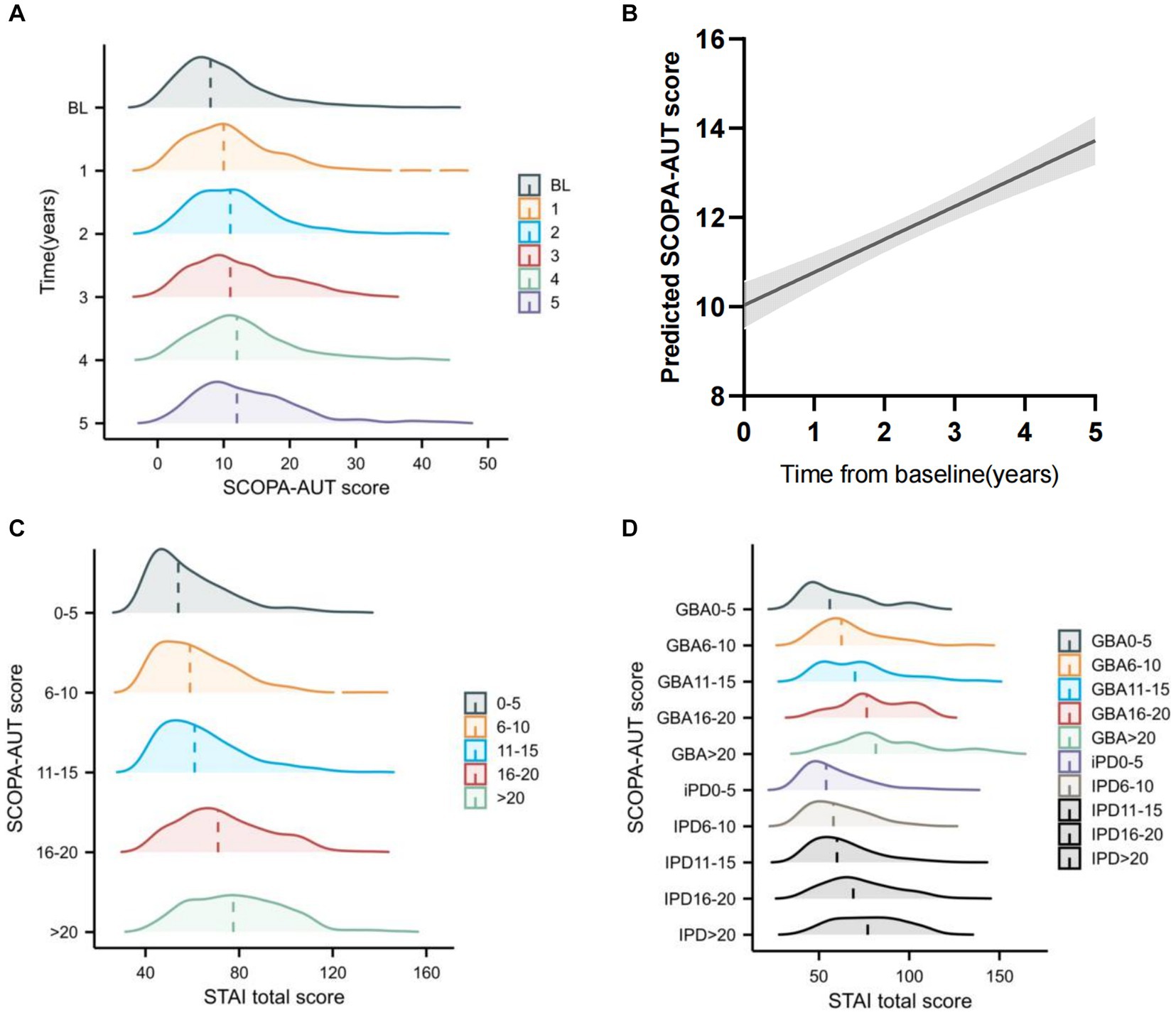
Figure 3. Longitudinal change of Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) and the association with State-Trait Anxiety Inventory (STAI) change over time (A) Raincloud plots showing the expected progressive increase in SCOPA-AUT score. (B) Longitudinal trajectories of SCOPA-AUT scores in patients with Parkinson’s disease. (C) Raincloud plots showing the expected STAI total score increase at different SCOPA-AUT score levels across all years. (D) Raincloud plots showing the expected STAI total score increase at GBA status and different SCOPA-AUT score levels across all years.
Next, we divided the patients into four groups based on the GBA variants and SCOPA-AUT level; the “GBA and SCOPA-AUT ≥ 18” group (“GBA/high” group) was at 7.5 times higher risk of progressing to anxiety than the “iPD and SCOPA-AUT < 18” group (“iPD/low” group), and the “iPD and SCOPA-AUT ≥ 18” group (“iPD/high” group) was at 2.3 times higher risk than the “iPD/low” group (log-rank 19.0, p = 0.004) (Figure 4A). Moreover, the anxiety score of the “GBA/high” and “iPD/high” group was predicted to increase much faster than the “GBA/low” and “iPD/low” groups (Figure 4B).

Figure 4. Longitudinal trajectories of the Mean State–Trait Anxiety Inventory (STAI) Rating (A) and Kaplan–Meier analyses of anxiety progression (B) in different groups according to the GBA variant carrier status and SCOPA-AUT level. “GBA/high” group: group with “GBA variants and SCOPA-AUT ≥ 18”; “GBA/low” group: group with “GBA variants and SCOPA-AUT < 18”; “iPD/high” group: group with “iPD and SCOPA-AUT ≥ 18”; “iPD/low” group: group with “iPD and SCOPA-AUT < 18”.
In this study, we first comprehensively explored the relationship between GBA variants and long-term anxiety progression in newly diagnosed patients, with PD followed from the time of diagnosis. Taking advantage of this longitudinal data from a well-designed cohort, our linear mixed model and multivariable Cox regression model showed that GBA variant carriers experienced more than 57% greater annual increase in STAI total anxiety score and faster progression in anxiety compared with non-carriers.
Previous studies assessing anxiety in GBA-associated PD were mostly cross-sectional studies, and GBA variant carriers presented with a high prevalence of anxiety (DeBroff et al., 2023). A few studies using univariable Kaplan–Meier survival analysis demonstrated that the GBA-PD group developed anxiety earlier than the idiopathic PD group (Pachi et al., 2021). In contrast with those studies, we observed a clear association between GBA status and increase in anxiety score over time, and this association remained significant after adding other clinical risk factors in the linear mixed model. In this adjusted linear mixed model, dysautonomia, depressive symptoms, and cognitive decline, which have been proposed in other studies, were also demonstrated to be predictors of increase in anxiety score (Rutten et al., 2017; Gibson et al., 2023).
Apart from GBA variants, dysautonomia was another independent anxiety predictor that ascertained both by linear mixed model and multivariable Cox regression model in this study. Autonomic dysfunction increased over time; dysautonomia was associated with higher anxiety score over time, and this trend was more significant in the GBA variant carriers. Since a consensus cutoff score is not available for dysautonomia based on SCOPA-AUT, we calculated a SCOPA-AUT cutoff score of 18 based on the log-rank survival analysis and the post-hoc analysis. The “high-level” group showed faster anxiety progression and increase in anxiety score over time. Moreover, the “GBA/high” group showed the fastest increase in anxiety score.
A previous study found that CSF t-tau and a-syn changes were associated with the increase in anxiety score over time (Wang et al., 2023). Our result found the CSF t-tau level increased over time, but the baseline CSF t-tau level was associated with the annual change in anxiety score. Recently, studies showed that tau protein accumulation showed age-dependent anxiety behaviors and exacerbate stress responses (Li et al., 2017; Liu et al., 2023), which, to some extent, support the previous finding and our results. It is well known that most GBA variants reduce GCase activity (Gegg et al., 2012), and GBA-PD may have a burden of iPD and demonstrated decreased CSF levels of total α-Syn compared with non-carriers in other cross-sectional studies (Lerche et al., 2021). However, in our study, we did not find the association between CSF a-syn burden and development of anxiety. But studies showed that tau protein could also accumulated in GBA mutation model (Yang et al., 2022b), and one drug that targeted the GBA variants carriers cohort ambroxol could reverses tau accumulation in GBA mutation model, which might suggest the tau protein’s role in the GBA variants’ contribution to the anxiety development in our results.
Unlike other studies, we did not find pRBD to be associated with anxiety symptoms over time, which might be associated with the GBA variant that we add in linear mixed model and Cox model for the first time. Studies have shown that GBA variant carriers developed pRBD more frequently than patients without GBA variants (Gan-Or et al., 2018; Huang et al., 2022); the pRBD that found by a previous cohort might be not an independent predictor but caused by the effect of GBA variants. This also encouraged us to explore the contribution of more genetic factors to the anxiety progression in PD.
Several limitations should be taken into consideration in this study. First, the retrospective design of this study represents its primary limitation. To mitigate this limitation, we attempted to address it by utilizing the linear mixed models and Cox survival analysis, to analyze the disease-duration scale time, employing log-rank tests for comparing survival curves. In addition, more prospective study on the prodromal GBA cohort, that did not develop PD yet, would help us to elucidate the contribution of GBA variants more clearly. Second, those non-motor symptoms including anxiety and dysautonomia were difficult to measure due to their unpredictable nature and excessive subjectivity. In future studies, these finding could be further supported with diagnostic confirmation of anxiety through neuroimaging and dysautonomia through polysomnography. Third, this PPMI cohort mainly consists of Caucasian population, and the Chinese population carries different GBA variants, so more studies need to replicate those results in the Chinese cohort to confirm our findings.
Our finding provides evidence for the role of GBA variants in the rate of anxiety progression in the general PD population. We show that GBA variants and dysautonomia are independent predicators of faster increase in anxiety score and development. Considering this relationship, small molecules aimed at GBA variants and treatment for dysautonomia may help to reduce anxiety symptoms in people with PD. More studies need to be conducted to confirm our findings and reveal the precise mechanism, underlying the role of GBA variants in anxiety progression in the PD population and investigate the potential for anxiety reduction via the mechanism.
Publicly available datasets were analyzed in this study. This data can be found at: www.ppmi-info.org/access-ataspecimens/download-data.
The studies involving humans were approved by the ethics committee of First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SS: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft. YB: Formal analysis, Writing – review & editing. NY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (nos. 82301618 to NY, no. 82101940 to SS). Postdoctoral Research Foundation of China (no. 2021M702945) to NY. The Henan Medical Science and Technology Joint Building Program (nos. LHGJ20210300 to NY and no LHGJ20210303 to SS). Major project of Medical science and Technology in Henan Province (no. SBGJ202102173).
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). PPMI (a public-private partnership) is funded by the Michael J. Fox Foundation for Parkinson’s Research and Study partners. The authors should thank the Department of Translational Medicine Center of the First Affiliated Hospital of Zhengzhou University for their technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1353759/full#supplementary-material
Behl, T., Kaur, G., Fratila, O., Buhas, C., Judea-Pusta, C. T., Negrut, N., et al. (2021). Cross-talks among Gba mutations, glucocerebrosidase, and α-synuclein in Gba-associated Parkinson's disease and their targeted therapeutic approaches: a comprehensive review. Transl. Neurodegener. 10:4. doi: 10.1186/s40035-020-00226-x
Berg, D., Borghammer, P., Fereshtehnejad, S. M., Heinzel, S., Horsager, J., Schaeffer, E., et al. (2021). Prodromal Parkinson disease subtypes -key to understanding heterogeneity. Nat. Rev. Neurol. 17, 349–361. doi: 10.1038/s41582-021-00486-9
Blundell, E. K., Grover, L. E., Stott, J., and Schrag, A. (2023). The experience of anxiety for people with Parkinson's disease. NPJ Parkinsons Dis. 9:75. doi: 10.1038/s41531-023-00512-1
Brockmann, K., Srulijes, K., Pflederer, S., Hauser, A. K., Schulte, C., Maetzler, W., et al. (2015). Gba-associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov. Disord. 30, 407–411. doi: 10.1002/mds.26071
Broen, M. P., Narayen, N. E., Kuijf, M. L., Dissanayaka, N. N., and Leentjens, A. F. (2016). Prevalence of anxiety in Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 31, 1125–1133. doi: 10.1002/mds.26643
Büchele, F., Hackius, M., Schreglmann, S. R., Omlor, W., Werth, E., Maric, A., et al. (2018). Sodium oxybate for excessive daytime sleepiness and sleep disturbance in Parkinson disease: a randomized clinical trial. JAMA Neurol. 75, 114–118. doi: 10.1001/jamaneurol.2017.3171
Carey, G., Görmezoğlu, M., De Jong, J. J. A., Hofman, P. A. M., Backes, W. H., Dujardin, K., et al. (2021). Neuroimaging of anxiety in Parkinson's disease: a systematic review. Mov. Disord. 36, 327–339. doi: 10.1002/mds.28404
Cui, S. S., Du, J. J., Fu, R., Lin, Y. Q., Huang, P., He, Y. C., et al. (2017). Prevalence and risk factors for depression and anxiety in Chinese patients with Parkinson disease. BMC Geriatr. 17:270. doi: 10.1186/s12877-017-0666-2
Debroff, J., Omer, N., Cohen, B., Giladi, N., Kestenbaum, M., Shirvan, J. C., et al. (2023). The influence of Gba and Lrrk2 on mood disorders in Parkinson's disease. Mov. Disord. Clin. Pract. 10, 606–616. doi: 10.1002/mdc3.13722
Dissanayaka, N. N., Sellbach, A., Matheson, S., O'sullivan, J. D., Silburn, P. A., Byrne, G. J., et al. (2010). Anxiety disorders in Parkinson's disease: prevalence and risk factors. Mov. Disord. 25, 838–845. doi: 10.1002/mds.22833
Fan, J. Q., Lu, W. J., Tan, W. Q., Liu, X., Wang, Y. T., Wang, N. B., et al. (2022). Effectiveness of acupuncture for anxiety among patients with Parkinson disease: a randomized clinical trial. JAMA Netw. Open 5:e2232133. doi: 10.1001/jamanetworkopen.2022.32133
Gan-Or, Z., Liong, C., and Alcalay, R. N. (2018). Gba-associated Parkinson's disease and other Synucleinopathies. Curr. Neurol. Neurosci. Rep. 18:44. doi: 10.1007/s11910-018-0860-4
Gegg, M. E., Burke, D., Heales, S. J., Cooper, J. M., Hardy, J., Wood, N. W., et al. (2012). Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol. 72, 455–463. doi: 10.1002/ana.23614
Gibson, J. S., Flanigan, J. L., Patrie, J. T., Dalrymple, W. A., and Harrison, M. B. (2023). Predictors of anxiety in Parkinson's disease: results from a 3-year longitudinal cohort study. Neurol. Sci. 44, 547–556. doi: 10.1007/s10072-022-06427-8
Goetz, C. G., Fahn, S., Martinez-Martin, P., Poewe, W., Sampaio, C., Stebbins, G. T., et al. (2007). Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (Mds-Updrs): process, format, and clinimetric testing plan. Mov. Disord. 22, 41–47. doi: 10.1002/mds.21198
Hinkle, J. T., Perepezko, K., Gonzalez, L. L., Mills, K. A., and Pontone, G. M. (2021). Apathy and anxiety in De novo Parkinson's disease predict the severity of motor complications. Mov. Disord. Clin. Pract. 8, 76–84. doi: 10.1002/mdc3.13117
Hoglinger, G., Schulte, C., Jost, W. H., Storch, A., Woitalla, D., Kruger, R., et al. (2022). Gba-associated Pd: chances and obstacles for targeted treatment strategies. J. Neural Transm. (Vienna) 129, 1219–1233. doi: 10.1007/s00702-022-02511-7
Huang, J., Cheng, Y., Li, C., and Shang, H. (2022). Genetic heterogeneity on sleep disorders in Parkinson's disease: a systematic review and meta-analysis. Transl. Neurodegener. 11:21. doi: 10.1186/s40035-022-00294-1
Julian, L. J. (2011). Measures of anxiety: state-trait anxiety inventory (Stai), Beck anxiety inventory (Bai), and hospital anxiety and depression scale-anxiety (Hads-a). Arthritis. Care Res. 63, S467–S472. doi: 10.1002/acr.20561
Khedr, E. M., Abdelrahman, A. A., Elserogy, Y., Zaki, A. F., and Gamea, A. (2020). Depression and anxiety among patients with Parkinson’s disease: frequency, risk factors, and impact on quality of life. Egypt. J. Neurol. Psychiatr. Neurosurg. 56:5. doi: 10.1186/s41983-020-00253-5
Knight, R. G., Waal-Manning, H. J., and Spears, G. F. (1983). Some norms and reliability data for the state‐trait anxiety inventory and the Zung self‐rating depression scale. Br. J. Clin. Psychol. 22, 245–249. doi: 10.1111/j.2044-8260.1983.tb00610.x
Leentjens, A. F., Dujardin, K., Marsh, L., Martinez-Martin, P., Richard, I. H., and Starkstein, S. E. (2011). Symptomatology and markers of anxiety disorders in Parkinson's disease: a cross-sectional study. Mov. Disord. 26, 484–492. doi: 10.1002/mds.23528
Lerche, S., Schulte, C., Wurster, I., Machetanz, G., Roeben, B., Zimmermann, M., et al. (2021). The mutation matters: Csf profiles of Gcase, sphingolipids, α-Synuclein in Pd (Gba). Mov. Disord. 36, 1216–1228. doi: 10.1002/mds.28472
Li, X., Wang, Z., Tan, L., Wang, Y., Lu, C., Chen, R., et al. (2017). Correcting miR92a-vgat-mediated Gabaergic dysfunctions rescues human tau-induced anxiety in mice. Mol. Ther. 25, 140–152. doi: 10.1016/j.ymthe.2016.10.010
Liu, H., Yang, Z., Yu, C., Dong, H., Wang, S., Wang, G., et al. (2023). Tau aggravates stress-induced anxiety by inhibiting adult ventral hippocampal neurogenesis in mice. Cereb. Cortex 33, 3853–3865. doi: 10.1093/cercor/bhac312
Lo Buono, V., Palmeri, R., De Salvo, S., Berenati, M., Greco, A., Ciurleo, R., et al. (2021). Anxiety, depression, and quality of life in Parkinson's disease: the implications of multidisciplinary treatment. Neural Regen. Res. 16, 587–590. doi: 10.4103/1673-5374.293151
Marek, K., Chowdhury, S., Siderowf, A., Lasch, S., Coffey, C. S., Caspell-Garcia, C., et al. (2018). The Parkinson's progression markers initiative (Ppmi) - establishing a Pd biomarker cohort. Ann. Clin. Transl. Neurol. 5, 1460–1477. doi: 10.1002/acn3.644
Mcgregor, M. M., and Nelson, A. B. (2019). Circuit mechanisms of Parkinson's disease. Neuron 101, 1042–1056. doi: 10.1016/j.neuron.2019.03.004
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, Moca: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Neumann, J., Bras, J., Deas, E., O'sullivan, S. S., Parkkinen, L., Lachmann, R. H., et al. (2009). Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 132, 1783–1794. doi: 10.1093/brain/awp044
Pachi, I., Koros, C., Simitsi, A. M., Papadimitriou, D., Bougea, A., Prentakis, A., et al. (2021). Apathy: an underestimated feature in Gba and Lrrk2 non-manifesting mutation carriers. Parkinsonism Relat. Disord. 91, 1–8. doi: 10.1016/j.parkreldis.2021.08.008
Pang, S. Y., Lo, R. C. N., Ho, P. W., Liu, H. F., Chang, E. E. S., Leung, C. T., et al. (2022). Lrrk2, Gba and their interaction in the regulation of autophagy: implications on therapeutics in Parkinson's disease. Transl. Neurodegener. 11:5. doi: 10.1186/s40035-022-00281-6
Paudel, M. L., Taylor, B. C., Vo, T. N., Kats, A. M., Schousboe, J. T., Lui, L. Y., et al. (2017). Sleep disturbances and risk of hospitalization and inpatient days among older women. Sleep 40:zsx037. doi: 10.1093/sleep/zsx037
Petrucci, S., Ginevrino, M., Trezzi, I., Monfrini, E., Ricciardi, L., Albanese, A., et al. (2020). Gba-related Parkinson's disease: dissection of genotype-phenotype correlates in a large Italian cohort. Mov. Disord. 35, 2106–2111. doi: 10.1002/mds.28195
Rodriguez-Blazquez, C., Forjaz, M. J., Frades-Payo, B., De Pedro-Cuesta, J., and Martinez-Martin, P. (2010). Independent validation of the scales for outcomes in Parkinson's disease-autonomic (Scopa-Aut). Eur. J. Neurol. 17, 194–201. doi: 10.1111/j.1468-1331.2009.02788.x
Rutten, S., Van Der Ven, P. M., Weintraub, D., Pontone, G. M., Leentjens, A. F. G., Berendse, H. W., et al. (2017). Predictors of anxiety in early-stage Parkinson's disease - results from the first two years of a prospective cohort study. Parkinsonism Relat. Disord. 43, 49–55. doi: 10.1016/j.parkreldis.2017.06.024
Straniero, L., Asselta, R., Bonvegna, S., Rimoldi, V., Melistaccio, G., Solda, G., et al. (2020). The Spid-Gba study: sex distribution, penetrance, incidence, and dementia in Gba-Pd. Neurol. Genet. 6:e523. doi: 10.1212/NXG.0000000000000523
Vázquez-Vélez, G. E., and Zoghbi, H. Y. (2021). Parkinson's disease genetics and pathophysiology. Annu. Rev. Neurosci. 44, 87–108. doi: 10.1146/annurev-neuro-100720-034518
Vengalil, S., Agadi, J. B., and Raghavendra, K. (2016). University of Pennsylvania smell identification test abnormalities in Parkinson's disease. J. Assoc. Physicians India 64, 32–36.
Wang, H., Zhao, Y., and Schrag, A. (2023). Development of anxiety in early Parkinson's disease: a clinical and biomarker study. Eur. J. Neurol. 30, 2661–2668. doi: 10.1111/ene.15890
Yang, H. J., Ahn, J. H., Lee, J., Lee, W. K., Lee, J., and Kim, Y. (2019). Measuring anxiety in patients with early-stage Parkinson's disease: Rasch analysis of the state-trait anxiety inventory. Front. Neurol. 10:49. doi: 10.3389/fneur.2019.00049
Yang, N., Sang, S., Peng, T., Hu, W., Wang, J., Bai, R., et al. (2023). Impact of Gba variants on longitudinal freezing of gait progression in early Parkinson's disease. J. Neurol. 270, 2756–2764. doi: 10.1007/s00415-023-11612-6
Yang, N. N., Sang, S. S., Peng, T., and Lu, H. (2022a). Snca rs3910105 is associated with development of rapid eye movement sleep behavior disorder in Parkinson's disease. Front. Neurosci. 16:832550. doi: 10.3389/fnins.2022.832550
Yang, S. Y., Taanman, J. W., Gegg, M., and Schapira, A. H. V. (2022b). Ambroxol reverses tau and alpha-synuclein accumulation in a cholinergic N370S Gba1 mutation model. Hum. Mol. Genet. 31, 2396–2405. doi: 10.1093/hmg/ddac038
Keywords: Parkinson’s disease, anxiety, GBA variants, dysautonomia, pathogenic mechanism
Citation: Sang S, Ba Y and Yang N (2024) Longitudinal faster anxiety progression of GBA variant carriers in the early Parkinson’s disease cohort. Front. Neurosci. 18:1353759. doi: 10.3389/fnins.2024.1353759
Received: 11 December 2023; Accepted: 02 January 2024;
Published: 24 January 2024.
Edited by:
Ke Ma, Shandong University of Traditional Chinese Medicine, ChinaReviewed by:
Kai Li, Second Affiliated Hospital of Soochow University, ChinaCopyright © 2024 Sang, Ba and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nannan Yang, eHl5bm4xOTkwQDE2My5jb20=; Yunpeng Ba, YmF5dW5wZW5nMTIwOUBzaW5hLmNvbQ==; Yunpeng Ba, YmF5dW5wZW5nMTIwOUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.