- 1Neuropsychology Service, Department of Rehabilitation and Functional Recovery, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy
- 2Centre for Neurolinguistics and Psycholinguistics, Scientific Institute San Raffaele, Vita-Salute San Raffaele University, Milan, Italy
- 3Department of Neuroradiology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 4Department of Neurorehabilitation Sciences, Casa di Cura Igea, Milan, Italy
- 5Department of Neuroradiology, IRCCS San Raffaele Scientific Institute, Milan, Italy
Introduction: Anodal transcranial Direct Current Stimulation (tDCS) is a non-invasive, low-cost and environment-friendly brain neuromodulation technique that increases cortical excitability. In post-stroke aphasia, the role of the right hemisphere in language recovery remains debated. In this preliminary study, we aimed to investigate the efficacy of excitatory tDCS on the right hemisphere in chronic aphasic patients.
Methods: We applied anodal tDCS to the right homologous region of Broca’s area in four chronic aphasic patients while performing a one-month naming rehabilitation treatment. Longitudinal data on language assessment and naming performance were collected. Resting-state fMRI images were acquired before and after treatment to measure changes in functional connectivity.
Results: Results showed enhanced positive functional connectivity of the right Broca homologous with the left middle frontal and middle temporal gyri. Every patient showed improvements in language functions, but no major changes in naming performance.
Conclusion: These preliminary findings suggest that tDCS applied over the unaffected hemisphere may result in longitudinal inter-hemispheric functional neuroplastic changes that could specifically improve language recovery and could potentially be included in therapeutic neurorehabilitative plans.
Introduction
Post-stroke language recovery in aphasic patients is a complex and dynamic process influenced by many factors, including initial aphasia severity, brain lesion site and size, etiology, elapsed time from the stroke onset and the specific type of rehabilitation (Gerstenecker and Lazar, 2019). Transcranial Direct Current Stimulation (tDCS) has been used in clinical treatments and post-stroke aphasia rehabilitation protocols to prompt neuroplastic changes in the lesioned brain activity. A growing number of studies successfully applied excitatory (anodal) or inhibitory (cathodal) tDCS in combination with speech and language therapy and showed enhanced language recovery after treatment (Harvey and Hamilton, 2022). Most treatments applied anodal tDCS (A-tDCS, excitatory) on the left hemisphere (LH) in uni-cephalic montages. Other studies have focused on inhibiting the allegedly maladaptive activation of the RH using cathodal tDCS (C-tDCS) (Vines et al., 2009, 2011; Turkeltaub, 2015). Only few experimental designs have applied excitatory A-tDCS on the RH to promote functional reorganization. Vines and colleagues (Vines et al., 2011) reported improvement in verbal fluency after A-tDCS neuromodulation of the RH homologous of the lesioned left inferior frontal gyrus (IFG) in conjunction with Melodic Intonation Therapy (MIT), which facilitates language recovery. Flöel et al. (2011) administered A-tDCS (excitatory), C-tDCS (inhibitory) and sham tDCS (placebo stimulation) over the RH temporoparietal cortex in aphasic patients, while performing anomia training. The behavioral outcome resulted in enhanced performance in object naming in all the conditions.
In this preliminary study, we used resting state functional connectivity to investigate the efficacy of excitatory tDCS on the right homologous region of Broca’s area and the underlying mechanisms of neural plasticity in a small group of chronic aphasic patients.
Materials and methods
Participants
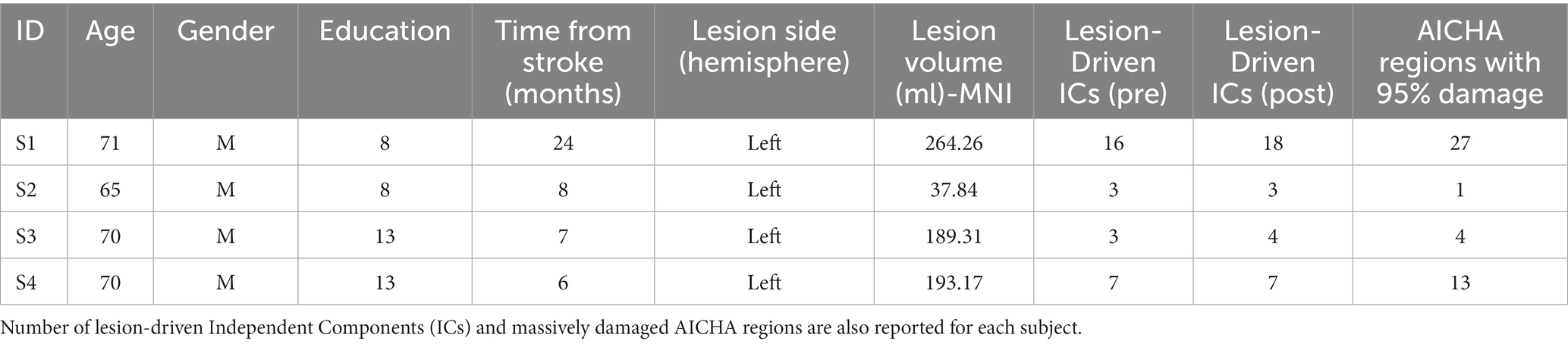
Four patients were included in the study (all males; mean age = 69 years; SD = 2.71; time elapsed from the stroke onset was at least 6 months—mean = 11.25; SD = 8.54) (see Table 1 for demographic and clinical characteristics of the study participants). All patients were diagnosed with chronic non-fluent aphasia with persistent and severe naming deficits through the Italian version of the Aachener Aphasie Test (AAT) (Luzzatti et al., 1994). The AAT examines the verbal performances in comprehension and expression, in the oral and written modality. The subtests are composed of 3–5 item sets, of 10 items each, for the examination of different linguistic units (phonemes, simple lexemes, complex lexemes produced according to morphological combination rules, and sentences). Individual item content is graded according to specified criteria of linguistic complexity, in order to differentiate aphasic disorders for a wide range of severity (Luzzatti et al., 2023).
Individual raw scores and corresponding severity ranges (severe, moderate, mild, light) for repetition, written language, naming and comprehension were reported for each patient. Spontaneous speech was evaluated only in two patients (1 and 4), because of lack of spontaneous production in the other two. The study was approved by our local Ethic Committee and participants gave their informed consent, according to the Declaration of Helsinki.
Cases description
Patient 1
Seventy-one y.o. right-handed man who suffered from an ischemic stroke resulting in a massive left hemispheric lesion extended over a large network of cortical and subcortical regions. The Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) was used to define left hemispheric regions damaged at least at 50% of their volume: Inferior Frontal Gyrus (Pars Opercularis, Pars Triangularis); Rolandic Operculum; Insula; Inferior Parietal Lobule; Supramarginal Gyrus; Angular Gyrus; Putamen; Heschl’s Gyrus; Superior Temporal Gyrus; Middle Temporal Gyrus. Patient 1’s aphasia presented with mixed features. The patient was non-fluent and right hemiplegic. AAT scores showed a profile of non-fluent aphasia, with impairment in spontaneous speech, anomia, phonological errors and word fragments. Repetition, naming, written and oral comprehension were also moderately compromised. Reading aloud words and phrases was not evaluated.
Patient 2
Sixty-five y.o. right handed man who suffered from ischemic stroke after left internal carotid artery dissection (MR and CT Angiography) following a traumatic accident resulting in left posterior temporal and subcortical (mostly lenticular) lesions. Even if these lesions were much smaller compared with those of the other patients, the clinical assessment revealed a severe form of non-fluent aphasia, right hemiplegia and hemianopsia and was diagnosed by a neurologist with Broca’s aphasia. AAL regions damaged at least at 50% were the left Putamen and the left Globus Pallidus, with less damage over temporal and insular cortices. The patient had a very severe buccofacial apraxia. Repetition, written language and naming were severely impaired, while comprehension was compromised to a lesser extent.
Patient 3
Seventy y.o. right-handed man who suffered from a left middle cerebral artery (MCA) ischemic stroke, resulting in non-fluent aphasia, dysphagia, right spastic hemiplegia and buccofacial apraxia. The left frontal-temporal-insular lesion revealed by MR scan involved both cortical and subcortical areas. AAL regions damaged at least at 50% were: left Inferior Frontal Gyrus (Pars Opercularis); left Supramarginal gyrus; left Putamen; left Inferior Temporal gyrus. Patient 3’s aphasia presented with mixed features. The patient had severe deficits in naming (with prevalence of anomia, phonological errors and presence of perseveration) but had a spared prosody. Repetition, comprehension (both auditory and written) and written language were impaired. More specifically, the performance in word copying and in letter matching was severely impaired.
Patient 4
Seventy y.o. right-handed man who suffered from a left MCA occlusion, resulting in right hemiparesis and non-fluent aphasia. MRI and two CT scans showed a left frontal-temporal- insular lesion involving both cortical and subcortical (lenticular) areas. Left hemispheric AAL regions damaged at least at 50% were: Precentral Gyrus; Inferior Frontal Gyrus (Pars Opercularis; Pars Triangularis); Rolandic Operculum; Insula; Supramarginal Gyrus; Putamen; Heschl’s Gyrus; Superior Temporal Gyrus; Middle Temporal Gyrus. Patient 4’s aphasia presented with mixed features. The patient had severely compromised performance in naming (anomia), repetition and written comprehension, although auditory comprehension was only moderately impaired. Written language was completely damaged.
Study design and rehabilitation treatment
Treatment consisted in daily naming training sessions, 5 days a week, for 4 consecutive weeks. Each session lasted 45 min. During the first 20 min, anodal tDCS was administered simultaneously to picture naming training. A-tDCS was delivered by a constant-current stimulator (HDC-kit Newronika s.r.l. Milan, Italy) with a 1.5 mA intensity, through a pair of saline-soaked sponge electrodes (size = 5 cm × 7 cm; current density = 0.04 mA/cm2), anode over the right homologous region of Broca’s area (corresponding to the right inferior prefrontal cortex, the electrode was centered in the midline between F6-F8 of the international 10/20 system for EEG electrodes placement; Scrivener and Reader, 2022) and reference electrode (cathode) over the left deltoid.
Language deficits were assessed with the AAT and the Snodgrass and Vanderwart picture naming test which included a set of 260 pictures of concrete objects (e.g., animals, tools, vegetables) (Snodgrass and Vanderwart, 1980). Treatment consisted in naming pictures showed by a computer. At the end of the 4-week treatment, patients were re-tested with the AAT and picture naming of all the figures, including the unnamed items.
Statistical analyses of the pre and post scores were performed using Wilcoxon non-parametric test. Data were considered significant when p ≥ 0.05. The commercially available software IBM SPSS Statistics v.23 (IBM Corp. ©) was used for statistical testing.
MRI acquisition
MRI scanning was performed before and after the four-week treatment with a 3 T Philips Ingenia CX MR scanner (Philips Medical Systems, Best, Netherlands) using a 32 channels SENSE head coil. In each run, a high-resolution MPRAGE (Magnetization Prepared Rapid Gradient Echo) T1-weighted anatomical image was acquired [Echo Time (TE) = 4 ms; Repetition Time (TR) = 8 ms; Flip Angle (FA) = 8°; Field of View (FOV) = 256 × 256 mm2; matrix size = 230 × 230; 150 axial slices; slice thickness = 2 mm; interslice gap = 1 mm; voxel size = 1 × 1 × 1 isotropic; PE direction = R/L; whole brain coverage]. Additionally, 7 min Resting-State functional scans were acquired with a fast speed echo Echo Planar Imaging (EPI) sequence [TE = 20 ms; TR = 2,500 ms; FA = 85°; number of volumes = 150; FOV = 240 × 240 mm2; matrix size = 84 × 84; 54 axial slices per volume; slice thickness = 3; interslice gap = 3; voxel size = 2.5 × 2.5 × 3; Phase Encoding direction (PE) = A/P; SENSE factor = 2; whole brain coverage]. Ten dummy scans preceded each run to optimize EPI image signal.
Preprocessing
Preprocessing of structural scans
Each subject’s structural T1 images were first oriented to match the AC-PC line and then aligned to each other with SPM12 v6685. Brain lesions were automatically identified with the Lesion Identification with Neighborhood Data Analysis (LINDA) toolkit, based on the Advanced Normalization Tools ANTs running on R (R Core Team, 2013). Binarized lesion masks in both native and in MNI space were computed and visually inspected to verify the correct lesion identification.
Enantiomorphic normalization was performed using the Clinical Toolbox. First, the T1 image was midline-aligned and mirrored over the lesioned hemisphere. This new reflected image was then co-registered to the native T1. The lesioned tissue was replaced by the corresponding tissue from the mirrored scan to create a chimeric image. This chimeric image was then normalized through the unified segmentation-normalization procedure. The resulting deformation fields were applied both to the native T1 scan and to the lesion mask.
The inverse spatial transform was used to create subject-specific native space regions of interest (ROIs) maps from the atlas of intrinsic connectivity of homotopic areas (AICHA) developed by Joliot et al. (2015), which segments the grey matter into 384 ROIs. Brain damage was then estimated as the number of regions interested by the lesion mask (95% volume overlap) (see Table 1). AAL atlas was also used to define anatomical regions damaged at least at 50% to more clearly express the structural damage.
Preprocessing of fMRI scans
First, functional images were slice time corrected, and then images were realigned and unwarped for motion and geometric distortions correction (Yourganov et al., 2017). Data from each session were then coregistered to the relative structural image. Finally, the images were spatially smoothed with a Gaussian Kernel with Full-Width at Half-Maximum (FWHM) = 6 mm. Preprocessing was done with SPM 12. Individual brain masks were estimated for each subject. Processed fMRI timecourses were entered into the CONN toolbox v17f (Whitfield-Gabrieli and Nieto-Castanon, 2012) and detrended via CompCor strategy using the mean signal from the white matter, obtained from the chimeric T1-weighted image; the six motion parameters from the realignment step; ramping effects at the beginning of each scanning session; linear, quadratic and cubic trends. We then applied a 0.01–0.1 Hz bandpass-filter to remove low-frequency drift and high-frequency noise. Global mean signal and chimeric cerebrospinal fluid (CSF) signal were not regressed out. Following the “Pipeline C” method (Yourganov et al., 2017), we used the nii_filter_lesion_ICs.m MATLAB script that identifies lesion-derived independent components and then regresses them out of the fMRI data. We performed probabilistic Independent Component Analysis (pICA) (Beckmann and Smith, 2004) on the previously denoised data, using Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) version 3.15, part of FSL. Previously estimated individual brain masks were used as a reference. The Z-scored IC spatial maps were thresholded at p < 0.05 and overlapped to the lesion masks of the patients. Significantly overlapping ICs (p < 0.05) were then regressed out using fsl_regfilt FSL script (number of ICs are reported in Table 1). ICA-filtered data were then re-entered into the CONN toolbox as raw data, skipping the initial preprocessing and denoising steps. BOLD time series were computed for each ROI of the AICHA atlas, masked by individual grey matter maps. First level ROI-to-ROI correlation analyses were used to determine the linear association of the BOLD time series between each pair of sources and a Fisher Z transformation was applied to the estimated R correlation coefficients. 384×384 ROI-to-ROI connectivity matrices were then computed for both the pre-rehabilitation and the post-rehabilitation conditions. ROI-to-ROI maps were entered into the second-level analysis to measure, group wise, the effect of rehabilitation on brain connectivity.
Resting state analysis
A between-session T-Test was performed to investigate directional treatment-induced differences in functional connectivity generalized across all the subjects. In order to examine post-treatment > pre-treatment effects and specifically investigate the supporting role of the right hemisphere following inferior PFC stimulation, we constrained our analyses to inter-hemispheric effects (target ROIs only in the LH) selecting three ROIs in the right IFG as seeds (S_Inf_Frontal-1-R; S_Inf_Frontal-2-R; and G_Frontal_Inf_Tri-1-R), with MNI centroid coordinates corresponding to right BA 44 and 45. This analysis allows to measure connectivity changes of the stimulated regions with the lesioned areas of the brain, thus helping to directly explore the strength and direction of the effect of excitatory stimulation of the contralateral hemisphere on the lesioned LH. Results were thresholded with a p < 0.05 threshold with a False Discovery Rate (FDR) analysis level correction for multiple comparisons. Finally, we explored between-ROI connectivity by means of Network Based Statistics (NBS) (Zalesky et al., 2010) with 10,000 iterations and applying a statistical threshold of p < 0.05 with Family Wise Error (FWE) correction for multiple comparisons.
Results
Neuropsychological tests
Aachener Aphasie Test and Snodgrass and Vanderwart picture naming scores are reported in Table 2. Every patient improved in the repetition and comprehension AAT subtests, although such result did not reach significance. Only a tendency for improvement was observed, for both repetition and comprehension results (p = 0.068 and p = 0.066, respectively).
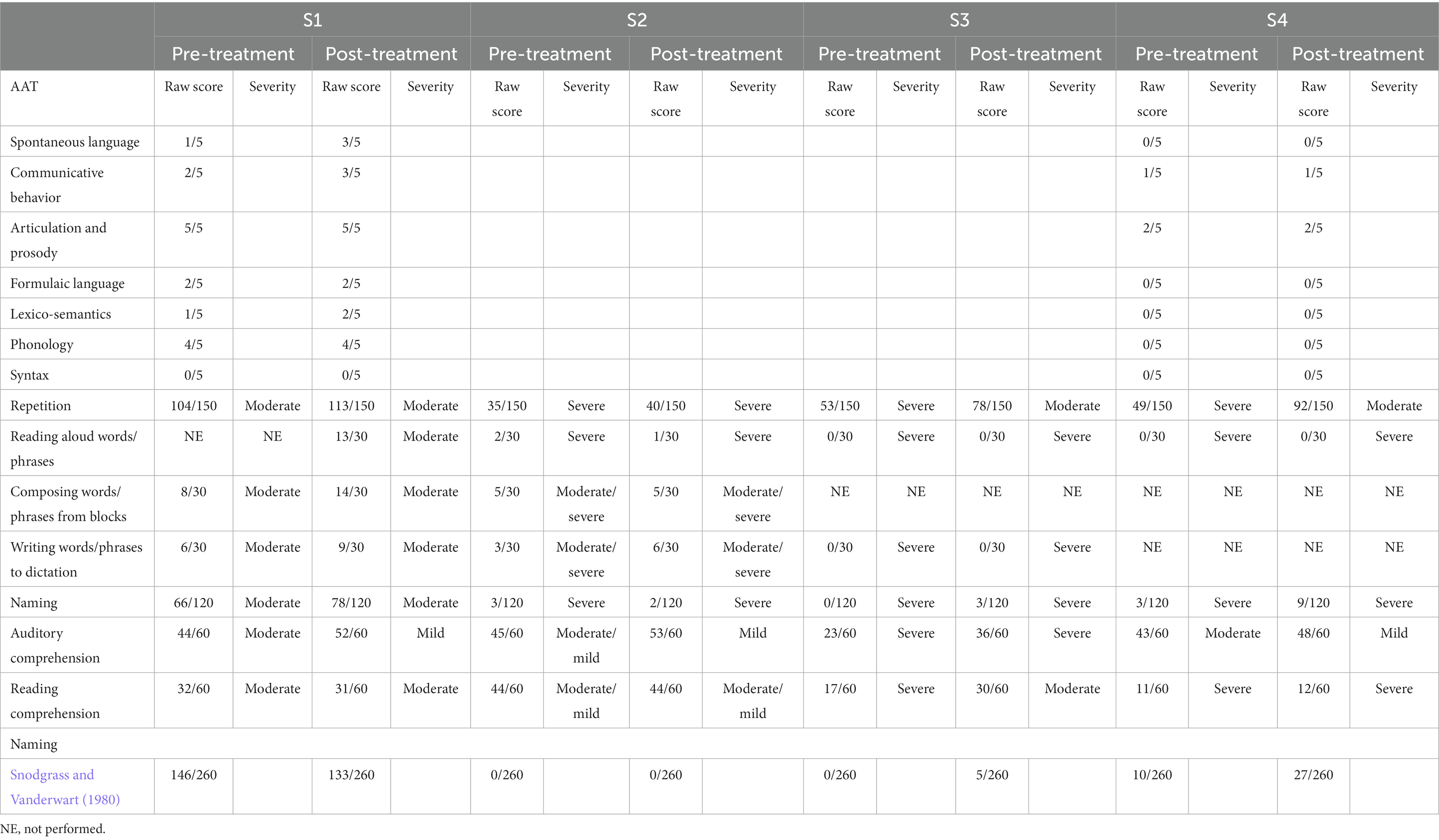
Table 2. Neuropsychological tests: pre and post treatment AAT and Snodgrass and Vanderwart picture naming scores.
Three patients (patients 1, 3 and 4) showed a better or similar performance in naming new untrained stimuli after the one-month training, although such result did not reach significance (p > 0.05).
Resting state functional connectivity
Our results showed a significant change in functional connectivity, with enhanced positive connectivity between the right Inferior Frontal Sulcus (S_Inf_Frontal-2-R) and the left Middle Frontal Gyrus (G_Frontal_Mid-4-L) [T(3) = 18.80; p = 0.0002] and between the right Inferior Frontal Sulcus (S_Inf_Frontal-2-R) and the posterior part of the left Middle Temporal Gyrus (G_Temporal_Mid-4-L) [T(3) = 17.53; p = 0.0002] (see Figure 1). The between-ROI network showed a significant increase of functional connectivity after treatment (size = 4; intensity = 72.66; p < 0.001).
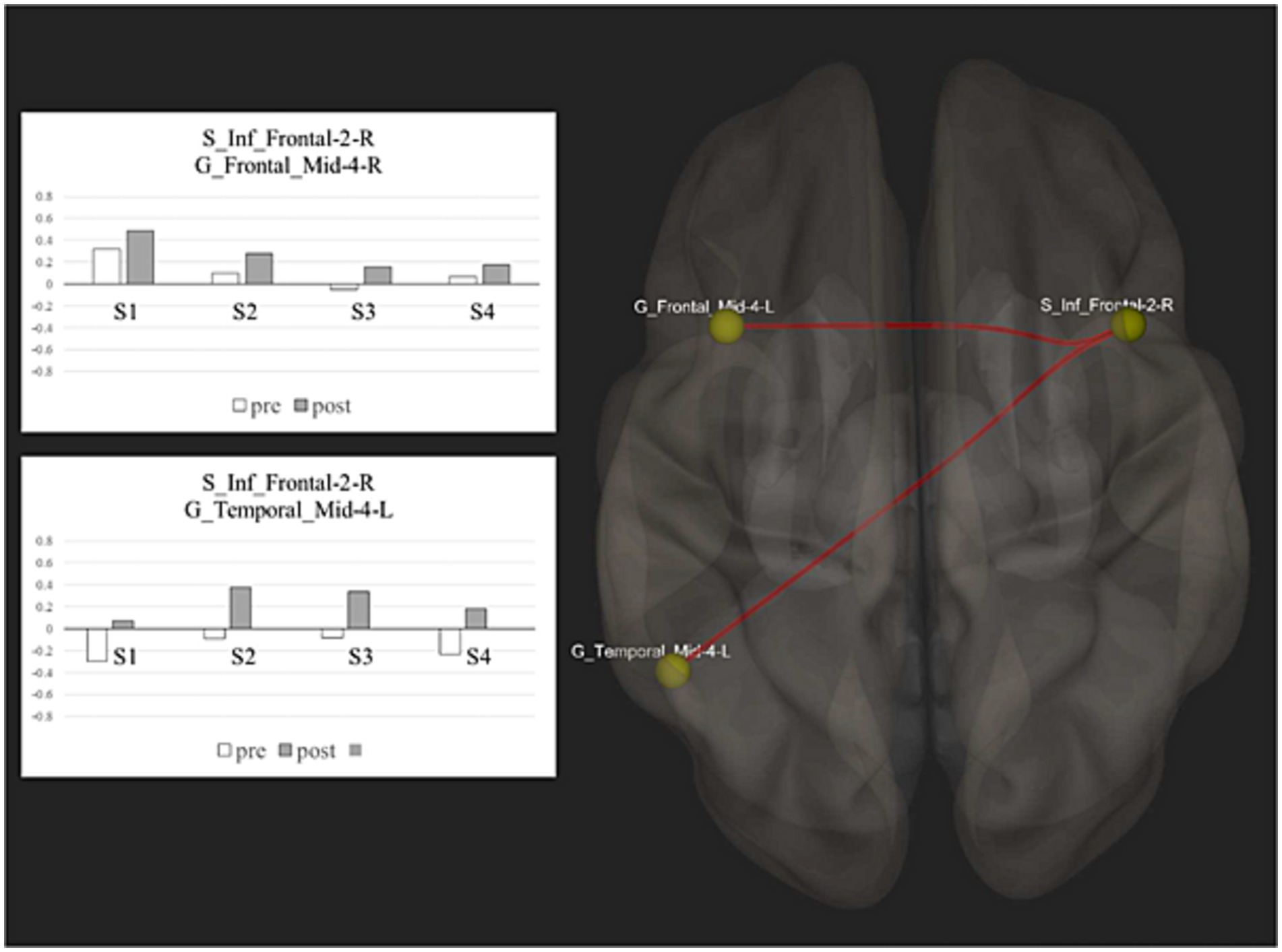
Figure 1. Resting state functional connectivity: this figure shows the spatial distribution of the network nodes and the correlation scores before and after treatment between the significant ROIs activity.
Discussion
After excitatory A-tDCS over the right homologous region of Broca’s area, patients showed a tendency for improvement in comprehension and repetition abilities. Snodgrass and Vanderwart naming generalization performance was not consistent among all the patients, but three out of four patients (patients 1, 3, and 4) were able to name new untrained stimuli, possibly hinting an effect of generalization. Interestingly, the three patients who could perform effective naming generalization were those with more extended brain lesions, including severe damage of the left IFG (>50% of the AAL Pars Triangularis and Opercularis regions). Many studies suggested that the hyperexcitability observed in the RH after LH stroke would compensate for part of the damaged functions (Basso et al., 1989; Gainotti, 1993, 2015; Anglade et al., 2014). Excitatory A-tDCS stimulation over the right homologous region of Broca’s area conjointly with naming training might have had a beneficial effect on the behavioral performance of those patients with wide lesions including the left IFG. These claims are coherent with the connectivity results from resting state fMRI analysis. We found significantly increased interhemispheric connectivity between the seed region (right IFG) stimulated by A-tDCS, and two target areas located, respectively, in the left Middle Frontal Gyrus (MFG) and left Middle Temporal Gyrus (MTG). In post-stroke patients, behavioral impairment is frequently associated with disrupted interhemispheric connectivity that can affect distant regions and, more specifically, with reduced connectivity between homotopic areas (Grefkes and Fink, 2014; Baldassarre et al., 2016; Siegel et al., 2016; Klingbeil et al., 2019). Marangolo et al. (2016) investigated the effects of bi-cephalic tDCS (anode over left IFG, cathode over right IFG) and speech therapy on functional connectivity in aphasic patients, showing increased LH intrahemispheric connectivity. Our study inspected the impact of excitatory A-tDCS applied over the right homologous region of Broca’s area in aphasia rehabilitation. We showed significant changes in the functional connectivity proprieties of the selected target stimulated region, which is contrasting with the model of Transcallosal Inhibition. If the model held true, an excitatory stimulation would have facilitated cortical activation of the RH and therefore increased the detrimental inhibition of the LH. On the contrary, enhanced positive interhemispheric connectivity was found. Moreover, according to the Transcallosal Inhibition hypothesis (Netz et al., 1995), the maladaptive activation of the RH would have led to a decreased behavioral performance, which was not observed in this study. Indeed, repetition and comprehension equivalent scores increased after treatment in all patients, without reaching significance at the statistical analyses. Only a tendency for improvement was observed, for both repetition and comprehension results. The naming generalization performance also improved for 3 out of 4 patients, although it did not reach statistical significance. This latter result differs from previously reported in the literature (Flöel et al., 2011). However, it is important to note that in the study of Flöel et al., the speech training lasted 2 h per day. Significant improvements in naming were observed, also in the sham group, suggesting that the training itself might have been efficient enough to induce naming improvements, as it has been largely reported that the efficacy of language therapy depends on treatment intensity (Hartwigsen and Saur, 2019). It should also be pointed out that the two target areas (left MFG and left MTG) which resulted significantly more connected to the right stimulated region are spatially neighboring the damaged Broca and Wernicke areas in the left hemisphere. According to the model presented by Anglade et al. (2014), in efficient recovery, the functional language activity moves to the regions close to the lesion. The increased interhemispheric connectivity with these target regions could potentially clarify the improvement in repetition and comprehension scores. Patients were in a chronic stage (>6 months). In this phase, language functions’ recovery is mostly linked to the therapeutic intervention, while spontaneous recovery is minimal (Yamaji et al., 2021). Thus, we might hypothesize that neuroplasticity changes that we measured would be related to the stimulation/rehabilitation procedure and not to spontaneous recovery. Our results fit with the scheme of the mechanisms of disruption and reorganization in the language network proposed by Hartwigsen and Saur (Hartwigsen and Saur, 2019). They suggested that at chronic stages, neural plasticity reorganization would result in increased interhemispheric connectivity between the bilateral frontal areas and within the left frontal and left temporal regions. Furthermore, according to the authors, this network could also be effectively modulated by non-invasive brain stimulation. It is also probable that the picture naming training itself might have induced such neuroplastic changes as language therapy can significantly improve language functions in the chronic phase after stroke (Breitenstein et al., 2017). Previous studies reported increased activity in the right frontal cortex (Blasi et al., 2002) or in the right superior temporal gyrus and the left precuneus (Musso et al., 1999) after language training in chronic stroke patients, strongly supporting the compensatory role of the right hemisphere. Our results are in accordance with the hypothesis that both activation of homologous contralesional cortex and restoration of activity of left hemisphere regions (perilesionally and at distance) would constitute viable mechanisms for language recovery Blasi et al. (2002).
This preliminary study shows increased inter-hemispheric connectivity following excitatory A-tDCS stimulation over the right homologous region of Broca’s area in chronic aphasic patients, suggesting a possibly beneficial effect of excitatory stimulation of the preserved hemisphere in chronic patients with extensive LH lesions. Therefore, future studies, including a wider sample of patients, a more intense daily training, a control group and groups with different tDCS montages should be carried out to provide stronger evidence to these promising results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the IRCCS San Raffaele Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FA: Investigation, Methodology, Writing – original draft. DF: Formal analysis, Investigation, Writing – original draft. AM: Formal analysis, Investigation, Writing – review & editing. EH: Methodology, Writing – review & editing. PD: writing – review & editing. FZ: writing – review & editing. DE: writing – review & editing. EP: writing – review & editing. MC: Resources, Supervision, Writing – review & editing. SI: Resources, Supervision, Writing – review & editing. JA: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anglade, C., Thiel, A., and Ansaldo, A. I. (2014). The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Inj. 28, 138–145. doi: 10.3109/02699052.2013.859734
Baldassarre, A., Ramsey, L. E., Siegel, J. S., Shulman, G. L., and Corbetta, M. (2016). Brain connectivity and neurological disorders after stroke. Curr. Opin. Neurol. 29, 706–713. doi: 10.1097/WCO.0000000000000396
Basso, A., Gardelli, M., Grassi, M. P., and Mariotti, M. (1989). The role of the right hemisphere in recovery from aphasia. Two case studies. Cortex J. Devoted Study Nerv. Syst. Behav. 25, 555–566. doi: 10.1016/s0010-9452(89)80017-6
Beckmann, C. F., and Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152. doi: 10.1109/TMI.2003.822821
Blasi, V., Young, A. C., Tansy, A. P., Petersen, S. E., Snyder, A. Z., and Corbetta, M. (2002). Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron 36, 159–170. doi: 10.1016/s0896-6273(02)00936-4
Breitenstein, C., Grewe, T., Flöel, A., Ziegler, W., Springer, L., Martus, P., et al. (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet 389, 1528–1538. doi: 10.1016/S0140-6736(17)30067-3
Flöel, A., Meinzer, M., Kirstein, R., Nijhof, S., Deppe, M., Knecht, S., et al. (2011). Short-term anomia training and electrical brain stimulation. Stroke 42, 2065–2067. doi: 10.1161/STROKEAHA.110.609032
Gainotti, G. (1993). The riddle of the right hemisphere’s contribution to the recovery of language. Eur. J. Disord. Commun. J. Coll. Speech Lang. Ther. Lond. 28, 227–246. doi: 10.3109/13682829309060038
Gainotti, G. (2015). Contrasting opinions on the role of the right hemisphere in the recovery of language. A critical survey. Aphasiology 29, 1–18. doi: 10.1080/02687038.2015.1027170
Gerstenecker, A., and Lazar, R. M. (2019). Language recovery following stroke. Clin. Neuropsychol. 33, 928–947. doi: 10.1080/13854046.2018.1562093
Grefkes, C., and Fink, G. R. (2014). Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 13, 206–216. doi: 10.1016/S1474-4422(13)70264-3
Hartwigsen, G., and Saur, D. (2019). Neuroimaging of stroke recovery from aphasia - insights into plasticity of the human language network. Neuroimage 190, 14–31. doi: 10.1016/j.neuroimage.2017.11.056
Harvey, D. Y., and Hamilton, R. (2022). Noninvasive brain stimulation to augment language therapy for poststroke aphasia. Handb. Clin. Neurol. 185, 241–250. doi: 10.1016/B978-0-12-823384-9.00012-8
Joliot, M., Jobard, G., Naveau, M., Delcroix, N., Petit, L., Zago, L., et al. (2015). AICHA: an atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods 254, 46–59. doi: 10.1016/j.jneumeth.2015.07.013
Klingbeil, J., Wawrzyniak, M., Stockert, A., and Saur, D. (2019). Resting-state functional connectivity: an emerging method for the study of language networks in post-stroke aphasia. Brain Cogn. 131, 22–33. doi: 10.1016/j.bandc.2017.08.005
Luzzatti, C., De Bleser, R., Scola, I., Frustaci, M., and Willmes, K. (2023). Update on the psychometric properties for the Italian version of the Aachen aphasia test (IT-AAT). Aphasiology 37, 658–695. doi: 10.1080/02687038.2022.2037501
Luzzatti, C., Willmes, K., Bleser, R., Bianchi, A., Chiesa, G., and De Tanti, A. (1994). Nuovi dati normativi per la versione italiana dell’Aachener Aphasia test. Arch. Psicol. Neurol. Psichiatr. 55, 1086–1131.
Marangolo, P., Fiori, V., Sabatini, U., De Pasquale, G., Razzano, C., Caltagirone, C., et al. (2016). Bilateral transcranial direct current stimulation language treatment enhances functional connectivity in the left hemisphere: preliminary data from aphasia. J. Cogn. Neurosci. 28, 724–738. doi: 10.1162/jocn_a_00927
Musso, M., Weiller, C., Kiebel, S., Müller, S. P., Bülau, P., and Rijntjes, M. (1999). Training-induced brain plasticity in aphasia. Brain J. Neurol. 122, 1781–1790. doi: 10.1093/brain/122.9.1781
Netz, J., Ziemann, U., and Hömberg, V. (1995). Hemispheric asymmetry of transcallosal inhibition in man. Exp. Brain Res. 104, 527–533. doi: 10.1007/BF00231987
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/
Scrivener, C. L., and Reader, A. T. (2022). Variability of EEG electrode positions and their underlying brain regions: visualizing gel artifacts from a simultaneous EEG-fMRI dataset. Brain Behav. 12:e2476. doi: 10.1002/brb3.2476
Siegel, J. S., Ramsey, L. E., Snyder, A. Z., Metcalf, N. V., Chacko, R. V., Weinberger, K., et al. (2016). Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc. Natl. Acad. Sci. USA 113, E4367–E4376. doi: 10.1073/pnas.1521083113
Snodgrass, J. G., and Vanderwart, M. (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. 6, 174–215. doi: 10.1037/0278-7393.6.2.174
Turkeltaub, P. E. (2015). Brain stimulation and the role of the right hemisphere in aphasia recovery. Curr. Neurol. Neurosci. Rep. 15:72. doi: 10.1007/s11910-015-0593-6
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
Vines, B. W., Norton, A. C., and Schlaug, G. (2009). “Stimulating music: combining melodic intonation therapy with transcranial DC stimulation to facilitate speech recovery after stroke” in Transmitters and modulators in health and disease. eds. S. Shioda, I. Homma, and N. Kato (Tokyo: Springer Japan), 103–114.
Vines, B. W., Norton, A. C., and Schlaug, G. (2011). Non-invasive brain stimulation enhances the effects of melodic intonation therapy. Front. Psychol. 2:230. doi: 10.3389/fpsyg.2011.00230
Whitfield-Gabrieli, S., and Nieto-Castanon, A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. doi: 10.1089/brain.2012.00
Yamaji, C., and Maeshima, S. (2021). Spontaneous Recovery and Intervention in Aphasia. doi: 10.5772/intechopen.100851
Yourganov, G., Fridriksson, J., Stark, B., and Rorden, C. (2017). Removal of artifacts from resting-state fMRI data in stroke. Neuroimage Clin. 17, 297–305. doi: 10.1016/j.nicl.2017.10.027
Keywords: aphasia, transcranial direct current stimulation, resting state fMRI, language, stroke
Citation: Alemanno F, Fedeli D, Monti A, Houdayer E, Della Rosa PA, Zangrillo F, Emedoli D, Pelagallo E, Corbo M, Iannaccone S and Abutalebi J (2024) Increased interhemispheric functional connectivity after right anodal tDCS in chronic non-fluent aphasia: preliminary findings. Front. Neurosci. 18:1346095. doi: 10.3389/fnins.2024.1346095
Edited by:
Aneta Szymaszek, Polish Academy of Sciences, PolandReviewed by:
Mikio Kubota, Washington University in St. Louis, United StatesKatarzyna Polanowska, Institute of Psychiatry and Neurology (IPiN), Poland
Copyright © 2024 Alemanno, Fedeli, Monti, Houdayer, Della Rosa, Zangrillo, Emedoli, Pelagallo, Corbo, Iannaccone and Abutalebi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Alemanno, YWxlbWFubm8uZmVkZXJpY2FAaHNyLml0
 Federica Alemanno
Federica Alemanno Davide Fedeli
Davide Fedeli Alessia Monti
Alessia Monti Elise Houdayer
Elise Houdayer Pasquale Anthony Della Rosa5
Pasquale Anthony Della Rosa5 Daniele Emedoli
Daniele Emedoli Jubin Abutalebi
Jubin Abutalebi