- 1Department of Biology, Faculty of Science, Hokkaido University, Sapporo, Japan
- 2Faculty of Pharmaceutical Science, Health Science University of Hokkaido, Tobetsu, Japan
- 3Centre for Mind/Brain Sciences, University of Trento, Rovereto, Italy
Equipped with an early social predisposition immediately post-birth, humans typically form associations with mothers and other family members through exposure learning, canalized by a prenatally formed predisposition of visual preference to biological motion, face configuration, and other cues of animacy. If impaired, reduced preferences can lead to social interaction impairments such as autism spectrum disorder (ASD) via misguided canalization. Despite being taxonomically distant, domestic chicks could also follow a homologous developmental trajectory toward adaptive socialization through imprinting, which is guided via predisposed preferences similar to those of humans, thereby suggesting that chicks are a valid animal model of ASD. In addition to the phenotypic similarities in predisposition with human newborns, accumulating evidence on the responsible molecular mechanisms suggests the construct validity of the chick model. Considering the recent progress in the evo-devo studies in vertebrates, we reviewed the advantages and limitations of the chick model of developmental mental diseases in humans.
1 Introduction
1.1 In search of valid animal models of developmental psychiatric disorders
Biological psychiatry and comparative psychology share a scientific question as for whether the apparent neurocognitive similarities among animals of different taxa (mostly vertebrates) could stem from common (homological) origins, namely that the underlying brain-behavior linkage evolved just once (Striedter, 2015). Alternatively, similar traits could arise as analogy via convergent evolution after multiple and independent events in each clade, therefore the challenges to find valid animal model of psychiatric disease are inevitably unsuccessful. Actually, the nervous system of vertebrates has held a highly conserved ground plan or “Bauplan” since the Cambrian period (Grillner and Robertson, 2016; Suryanarayana et al., 2021a,b); homologies are widely found from the level of composite molecules (transmitters, hormones, receptors, and second messengers) to neural pathways and cytoarchitectonic organizations at the macroscopic level (Güntürkün and Bugnyar, 2016; Shepherd and Grillner, 2018) [specifically for the mammal-bird homologies, see Güntürkün and Bugnyar (2016)]. Despite the conservative nature of the organization of central nervous system (CNS), huge phenotypic diversification occurs in the behavior and cognition of vertebrates through differentiated development (Bateson and Gluckman, 2011), complicating the untangling of the evolutionary processes. By using a common set of composite parts and wirings in the brain, animals of different taxa could have developed diverse machineries for adaptive behaviors, so that the seemingly identical phenotypes are due to convergent evolution, hence the analogy.
In the present review, with the aid of recent progresses in evo-devo studies, we argue a possibility that chicks and humans exhibit a homologous developmental trajectory leading to similar predisposition in their visual perception. Here, we propose a working hypothesis that taxonomically distinct animals could develop social perception via common ontogenetic processes during the prenatal embryonic period and the subsequent early post-natal period. It is stressed that the early-life experiences are under strong control of embryonic development of the social perception in both species. Neural events during the late embryonic stages set up the neonatal predisposition such as face and biological motion preferences, so that the babies appear to be “born knowing” without specific post-natal experiences. Some of the late embryonic events could thus have lasting neurobehavioral effects on social communication throughout life. Specifically, as encouraged by recent progresses of avian model studies (Csillag et al., 2022; Huang and Cheng, 2022), validity of the domestic chicks as an autism spectrum disorder (ASD) model animal will be examined on two aspects, the surface and construct validity as criteria of the depression animal model proposed by Willner (1984). The former (surface validity) includes an examination of the similarity of behaviors and developmental processes, particularly the perceptual predisposition to face, biological motion and animacy. The latter (construct validity) includes shared neural substrates and molecular cascades, particularly the potential roles of nicotinic acetylcholine (nAChR) transmission in the embryonic brain in controlling excitation-inhibition balance in neonates. If the ASD-like impairments found in chicks are homologous to the human ASD, we will find these construct molecular events are based on identical gene expression profiles and cellular events during the prenatal period. If otherwise, and the impaired visual perception in chicks is due to convergent evolution, we will find differences in the relevant brain regions and/or the molecular events.
1.2 Diverse environmental risk factors of ASD remain to be specified
ASD is the most prevalent developmental disorder primarily characterized by underdeveloped social interactions and communication and restricted and repetitive patterns of behavior and interests (Wing and Gould, 1979; American Psychiatric Association, 2013; World Health Organization, 2023). It is speculated that the heterogeneous diagnostic phenotypes, as well as the dimensional nature of this disorder, could be associated with a wide range of underlying genetic and environmental factors (Tchaconas and Adesman, 2013). In addition to apparent genetic risks (Sandin et al., 2014) [for an exhaustive hereditability study in Sweden; also see Iossifov et al. (2014) for de novo mutations and Gaugler et al. (2014) for common variations], exposure to environmental toxicants during pregnancy and the neonatal period remains a major social and scientific concern (Rossignol and Frye, 2014). For example, a large-scale twin study (Hallmayer et al., 2011) revealed high concordance rates among siblings, indicative of the role of genetic factors. However, the authors also reported that common environmental factors shared by these twins could substantially contribute to autism/ASD liability. Complex interactions are thought to occur between the genetic background of ASD susceptibility and the chemical agents acting during pregnancy and the early post-natal period.
However, it does not mean that these chemicals must be just eliminated. For example, valproic acid (VPA) has been identified as a risk factor for ASD (Christensen et al., 2013), but it remains an indispensable antiepileptic medication (Meador and Loring, 2013). The associated risks must be precisely evaluated in close consideration with known benefits. Studies using appropriate animal models are critical (Ergaz et al., 2016) because environmental risk management is difficult without reliable neurobehavioral measures of the symptoms. As epidemiological studies of diseases and epidemiological analyses are complimentary, it is critically important to find appropriate animal models. Rodents (mice and rats) are the most popular model animals because developed genetic tools are available (Mabunga et al., 2015; Chaliha et al., 2020). However, it must be noticed that rodents and primates have undergone distinct evolution since their separation during the late Cretaceous period over 66 million years ago.
As an alternative animal model, we have focused on newly hatched domesticated chicks (Gallus gallus domesticus) (Rosa-Salva et al., 2011; Vallortigara, 2012; Rosa-Salva et al., 2015; Versace et al., 2018). Birds are descendants of theropod dinosaurs, the major group of sauropsids, whereas primitive mammals diversified as a minor group of synapsids during the Carboniferous Period, ~ 300 million years ago. Considering such a taxonomically distinct animal as a valid model for human psychiatric disorders may sound unrealistic. If the adult phenotypes are solely compared, humans can never be chickens. However, as the hourglass bottleneck theory suggests (Irie and Kuratani, 2011), humans and chickens achieve developmental convergence during the prenatal/early neonatal periods in terms of specific neurocognitive aspects (Figure 1). It must be noticed that the hourglass model (Irie and Kuratani, 2011; Uesaka et al., 2019, 2020) has been proposed to explain the morphogenesis, rather than the CNS and the relevant behavioral traits. The phylotypical “bottleneck” period critical for the predisposition formation (namely, the responsive brain mechanisms) could therefore be different from that for the morphogenesis (i.e., mid-late embryonic stages). In order to determine whether certain animals are human-like in their perceptive natures, we must elucidate how phenotypic similarities arise through their respective development of the sensory system.
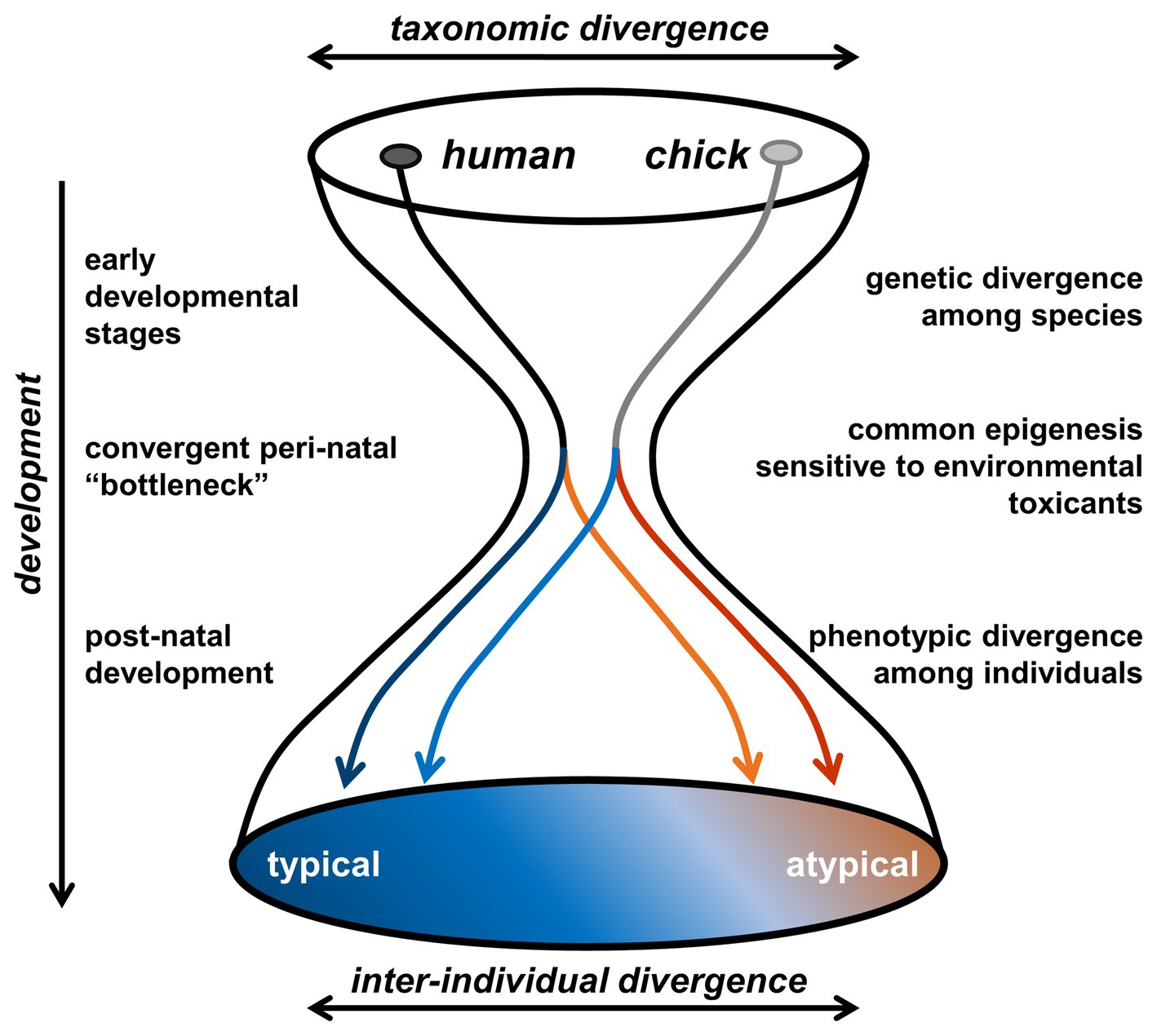
Figure 1. Hypothetical hourglass “bottleneck” model of the convergent evolution of peri-natal epigenetic control of social behaviors. During the early developmental stages, humans (primate) and chicks (birds) show a clear taxonomic divergence. Most organogenetic events are accomplished until the late embryonic period, namely before the third trimester in humans and the 14 days of incubation in domestic chicks. Convergent pre- and early post-natal “bottleneck” appears [i.e., phylotypic period (Irie and Kuratani, 2011)], and epigenetic processes (though mostly unspecified at present) would occur in the visual system responsible for the social perceptions. Subsequent developmental trajectory would be most sensitive to environmental toxicants during this “bottleneck” period, so that the inter-individual divergence overrides the taxonomic divergence.
2 Surface validity
2.1 Visual predispositions canalize the development of social behaviors: common developmental features for the surface validity
It is speculated that the development of socialized behaviors during the early neonatal period depends on predisposed visual preference. In their inspiring report, Morton and Johnson (1991) argued that human babies are born to know visual characteristics of faces and denoted the information prior by term “CONSPEC.” With the aid of the “CONSPEC” information, neonates are supposed to gradually learn about the visual characteristics of conspecifics such as siblings and parents, and the device responsible for the subsequent learning to form social attachments was referred to as “CONLERN.” The predisposed “CONSPEC” information, or “core knowledge” (Spelke and Kinzler, 2007), could include a variety of visual features other than face (for reviews see Versace and Vallortigara, 2015; Di Giorgio et al., 2017; Lorenzi and Vallortigara, 2021; Rosa-Salva et al., 2021; Vallortigara, 2021a; Lemaire and Vallortigara, 2022), however, most studies have focused on face configuration, biological motion, and other cues of animacy.
2.1.1 Face-like configuration
Differential tuning to face configuration has been demonstrated in adolescents with ASD when compared with typically developing controls (for one of the earliest reviews, see Johnson, 1992; Johnson, 2005; also see Pavlova et al., 2017 for a recent study using pictures resembling the food-face paintings by Giuseppe Arcimboldo). Based on the finding that infants prefer faces at 2–4 months old (Mauer and Barrera, 1981; Mondloch et al., 1999), a predisposed face preference has been suggested as a reliable diagnostic indicator for ASD. Typically, a definitive ASD diagnosis can be achieved in toddlers aged ~3 years; reliable biomarkers at earlier ages, if available, could facilitate treatment at the early stages (Elsabbagh and Johnson, 2009). In addition to behavioral measures, physiological measures, such as electroencephalography (EEG), can be employed in newborn babies (Buiatti et al., 2019).
Actually, the visual preference to face-like configuration could arise as a predisposed prior early in life. Visually naïve monkeys deprived of social stimuli (including human faces) preferred faces (Sugita, 2008), indicating robust development of face perception. Even human embryos in the third trimester of pregnancy reportedly exhibit head-turn responses to upright face configurations (Reid et al., 2017). A follow-up study confirmed the preference to face-like configuration, but top-heavy bias (i.e., inversion effect) was not detected (Reissland et al., 2020); it is reported also that the maternal mental health had significant effects on the embryonic activity, thus suggesting further studies to confirm the findings.
Subcortical visual pathway (superior colliculus/pulvinar to the amygdala) is responsible for the proto-face configuration (three blobs configured at a low spatial frequency) in human neonates (Johnson, 1992). This subcortical (or tectofugal) pathway is functional throughout life and plays critical role in rapid perception of emotional (e.g., fearful) face (Pegna et al., 2005; Tamietto and de Gelder, 2010); see Inagaki et al. (2022) for the most reliable account of this pathway in adult rhesus monkeys. Consistently, damage to the amygdala has been shown to cause some ASD-like deficiencies according to the “theory of mind” [ToM (Stone et al., 2003)], although the causal link between the ToM and ASD remains controversial (Gernsbacher and Yergeau, 2019).
Considering that the subcortical face pathway is functional in the early prenatal period and critical for the typical development of social behavior (Baron-Cohen et al., 2000), we would expect to detect differentiated visual attention to the face in infants with familial risk of ASD, and more specifically, in those who are later diagnosed with ASD. However, the use of the “face pop-up” task in these infants revealed clear attention to face similar to that observed in control individuals (or even higher) at 7 and 14 months of age (Elsabbagh et al., 2013). Conversely, another longitudinal developmental study has reported a steady decline in selective eye-fixating behavior in infants who were subsequently diagnosed with ASD at the earlier developmental stage from 2 to 6 months of age (Jones and Klin, 2013). The predisposed face perception is not a fixed trait, and the developmental changes need to be carefully examined (Simion and Di Giorgio, 2015). More recently, it has been reported that newborns with a high familial risk of ASD (6–10 days of age) show a reduced face preference when compared with low-risk controls (Di Giorgio et al., 2016a); also see Di Giorgio et al. (2021a) for the follow-up longitudinal study on 4-month-old infants.
Visually naïve newly hatched chicks also exhibit an evident inborn preference for faces (Rosa-Salva et al., 2010, 2011, 2019). Exposure to VPA during the late embryonic stage substantially reduced the face preference score (Adiletta et al., 2021), possibly paralleling that observed in humans (Di Giorgio et al., 2016a). The subcortical visual pathway of birds (optic tectum and arcopallium/nucleus taeniae amygdala) functionally mature early, and newly hatched chicks start actively pecking small conspicuous objects at 1–2 days old. Furthermore, immediate early gene imaging studies revealed that telencephalic limbic nuclei are involved in the predisposition preference of chicks (Mayer et al., 2016, 2017, 2019). Note that traditional rodent models fail to spontaneously exhibit a comparable visual predisposition. On the other hand, tortoise hatchlings show visual preference to face-like configuration over comparable alternatives without any preceding visual experience (Versace et al., 2020). As these tortoises are solitary species without parental care after hatching, we may assume that the social preference to face could be dated back to the common ancestor of amniotic vertebrates, naturally including birds and mammals. In other words, the predisposed preference may not be a high faculty requiring cortical computations.
Issues on the eye contact deficits in ASD needs a careful consideration. Human eyes comprise a unique tool for social communications due to their morphological feature (Kobayashi and Kohshima, 1997); the exposed white sclera surrounds the dark iris, making it easier for others to detect what the subject is looking. The “eye contact” tactics could therefore be unique to humans, despite the subcortical pathway underlying orienting gaze control is evolutionarily conserved (Dean et al., 1989; Kardamakis et al., 2016). This morphology makes “eye contact” a powerful means to communicate socially in typically developing people, whereas adults with ASD often report adverse emotional responses to looking eyes of others and avoid “eye contact” (Trevisan et al., 2017). It is hypothesized that the excitatory/inhibitory imbalance occurs in the subcortical visual pathway of those infants with ASD, leading to hyper-activation of the limbic system (Hadjikhani et al., 2018). The “eye contact” aversion would most likely develop at later juvenile/adolescent stages following human-unique processes. However, as noted above (Elsabbagh et al., 2013), those infants at risk for ASD show clear attention to faces as high as typically developing controls at 7–14 months of age, suggesting that the deficient attention is not necessarily linked with “eye contact” aversion in ASD.
2.1.2 Biological motion
Biological motion (BM) preference comprises another aspect of the “CONSPEC” process; it is easily tested using highly reduced animations composed of relatively few light points (~ a dozen). Both human and chick neonates exhibit BM preference without visual experience (Simion et al. (2008) and Pavlova (2012) in human infants; Vallortigara et al. (2005) in chicks; also see Rugani et al. (2015) for the association of the BM preference with brain asymmetry). In addition to the commonality in their appearance at the early neonatal stage, both human infants and chicks show a clear inversion effect, that is, a preference for the upright walking motion over the inverted upside-down display [Troje and Westhoff (2006) in human infants; Vallortigara and Regolin (2006) and Chang and Troje (2009) in chicks]. Note also the recent report on the gravity prior, wherein naïve chicks preferred upward movements of single dot over the opposite downward (Bliss et al., 2023), in a manner similar to humans who judge upward motion more animate than the downward (Szego and Rutherford, 2008).
The motion characteristics of both local movements (such as the movements of the lower limbs) and global features (shape of the body) are critical in humans (Chang and Troje, 2009; Hirai et al., 2011). However, it remains unclear which of these visual components is associated with the development of social cognition. Bardi et al. (2011) reported that infants depend more on local cues, whereas Bidet-Ildei et al. (2014) highlighted the importance of translational displacement of the body. Hirai and Senju (2020) have proposed an integrative hypothesis of the two-process theory, wherein the “step detector” responsible for the local motions of feet below the body precedes the “bodily action evaluator” that processes the global processing of action types and styles. Several studies in both human adults and children have suggested the association between reduced sensitivity to BM and ASD (Blake et al., 2003; Rutherford et al., 2006; Wang et al., 2018; Kaliukhovich et al., 2021) [also see the recent meta-analysis (Federici et al., 2020)]. Importantly, it should be noted that, in our current context, visual preferences for social stimuli (face inversion, averted eye gaze, and BM) markedly differ between the two groups of infants with high and low familial risk of autism (Di Giorgio et al., 2016a). Further longitudinal studies are needed to determine the association between visual preference and the subsequent development of social interactions.
2.1.3 Animacy
In addition to the face and BM, both chicks and humans have a visual predisposition to other cues of animacy (Rosa-Salva et al., 2011; Vallortigara and Rosa-Salva, 2017; Vallortigara, 2021b for reviews). For example, living organisms are characterized by self-propelling animacy, a well-established preference in both human babies (Di Giorgio et al., 2016b) and visually naïve chicks (Rosa-Salva et al., 2016, 2018). Unpredictable speed changes in motion are a critical feature of animacy in newborn human babies (Di Giorgio et al., 2021b), as observed in newly hatched chicks with distinct inherited variability (Versace et al., 2017, 2019) (for involvement of septal and hypothalamic nuclei, see Lorenzi et al., 2017). Both variability in body orientation and the unpredictable temporal contingency of motion are critical in chicks (Lemaire et al., 2022; Rosa-Salva et al., 2023). It should also be noted that avoidance of looking at (threatening) objects is also considered to be innately predisposed (Hebert et al., 2019).
2.1.4 Imprinting and the early process of attachment formation
In chicks, the BM preference is functionally linked to filial imprinting. Imprinting is a complex process that involves predisposition and experience-based learning; thus, it may be homologous to the processes of attachment formation in human babies. Newly hatched domestic chicks and ducklings form lasting attachments, even when the first object seen is a non-biological artifact rather than a conspecific animal (Spalding, 1873; Lorenz, 1937). Artifacts such as rotating blue boxes were actually effective as imprinting objects (Horn, 2004). However, contrary to this popularly accepted idea, memorized preference for artifacts is short-lived and is gradually replaced by more naturalistic stimuli such as stuffed hens (Bolhuis et al., 1985; Johnson et al., 1985; Johnson and Horn, 1988). Accordingly, an innate predisposition gradually emerges after learned attachment fades. In contrast, BM preference emerges first and subsequently guides learning.
Perfectly naïve chicks show an apparent preference for BM, although with a relatively small effect size (Vallortigara et al., 2005). When imprinted by motion pictures, the BM preference is enhanced or “permissively induced” (Miura and Matsushima, 2012). The induction is nonspecific to the exposed stimulus, and any motion (even randomized point-light animation) is a similarly effective inducer. The memorized preference to the moving artifact, on the other hand, is determined by the visual features of the object such as color and shapes, thus is assumed to be “instructively induced.” However dissociable, the BM preference facilitates the memorized preference, or imprinting (Miura and Matsushima, 2016). BM animations were more effective than non-BM animations, and chicks with a higher BM preference exhibited higher imprinting scores.
Imprinting memory formation is functionally coupled with BM induction through enhanced thyroid hormone activity (Figure 2). Exposure to motion increases the expression of Dio2, which is responsible for the conversion of circulating thyroid hormone (T4) to its active form (T3) in the epithelial cells of telencephalic capillaries (Yamaguchi et al., 2012). The enhanced T3 influx into the dorsal pallium (intermediate medial mesopallium, IMM, an avian homolog of the mammalian neocortex, including the association areas) reopens the sensitive period and acutely strengthens learning and BM scores (Miura et al., 2018; Takemura et al., 2018). In aged chicks, T3 can reactivate the preference for animate objects (Lorenzi et al., 2021). Accordingly, imprinting allows chicks to remain imprintable for a prolonged period, guiding subsequent learning during the extended sensitive period to objects bearing BM features. Notably, these two aspects of imprinting (memory formation and induced predisposition) appeared tightly coupled and not dissociable (Miura et al., 2020).
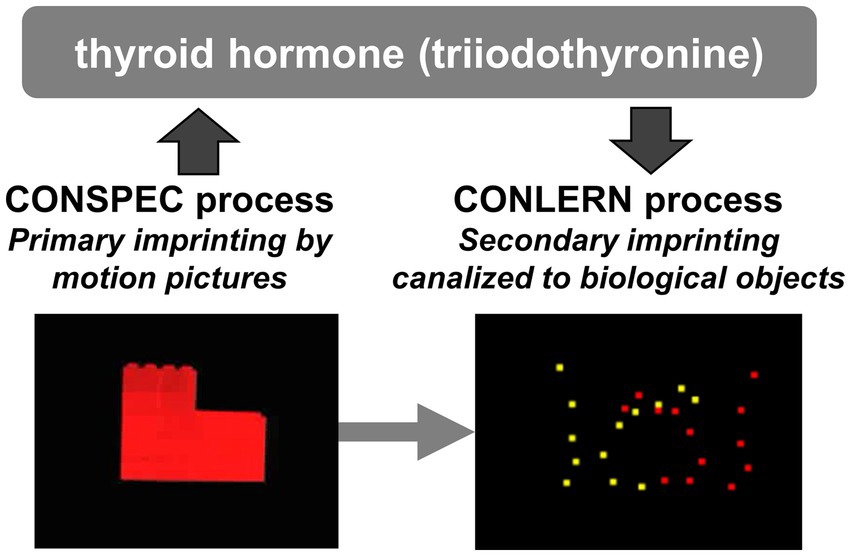
Figure 2. Thyroid hormone conversion in the central nervous system mediates the link between CONSPEC and CONLERN mechanisms toward adaptive socialization via visual canalization. Any motion pictures of artifact such as rotating red toy will effectively cause the primary imprinting selectively to the visual feature of the object. However, the primary imprinting is associated by CONSPEC mechanisms, wherein the biological motion (BM) preference is permissively induced if accompanied by enhanced Dio2 gene expression and a rapid inflow of thyroid hormone (T3) in the dorsal pallium. Thyroid hormone subsequently elongates the sensitive period for memory formation, allowing the secondary imprinting for a longer post-hatch period. With the aid of the induced BM preference, the secondary imprinting is canalized to images of conspecifics, and the chicks will form firm social attachments to conspecifics.
Newly hatched domestic chicks could serve as a valid animal model for studying environmental risk factors for ASD, at least at the surface phenomena level. Imprinting is also assumed to be a primary stage of emotional development in human infants (Mobbs et al., 2016). The following section examines how ASD-like deficiencies could arise in the chick model and whether the underlying mechanisms are shared.
What are the predisposed priors for? Why should animals, and humans as well, be born knowing before birth (Vallortigara, 2021a)? A recent progress in machine learning could give us a hint (Barabási et al., 2023). Most artificial neural network models require extensive updating of network-wide weights among neurons by intensive training. Even though any randomly connected initial network could give rise to target functions after intensive and long training (Amari, 2020), learning processes are often slow and require intensive set of training examples, yet risk overfitting. By implementing genetic connectome model (GCM) (Kovács et al., 2020), the author showed that predisposed wiring should serve priors through evolutionarily shaped processes, thus promoting the computational power (Barabási et al., 2023). Genetic identities of neurons, as determined by the single-cell RNA seq in developing brains, could therefore be a powerful means to disentangle the molecular events responsible for the construct validity.
3 Construct validity
3.1 Valproic acid, an anticonvulsant drug, mediates ASD-like impairment of social behavior development and acute suppression of spontaneous embryonic movements
Given that VPA was identified as an environmental risk factor for ASD during pregnancy (Moore et al., 2000; Rasalam et al., 2005), studies have attempted to clarify the underlying mechanisms using rodent models [see reviews (Meador and Loring, 2013; Ergaz et al., 2016; Nicolini and Fahnestock, 2018)]. Late embryonic exposure to VPA impairs social behavior also in chicks (Nishigori et al., 2013; Sgadò et al., 2018; Lorenzi et al., 2019; Adiletta et al., 2021). These studies have consistently reported impaired social behavior in newborns and hatchlings after embryonic VPA exposure, although drug-induced phenotype disorders were not necessarily identical, probably due to task-dependent variation among individuals. For example, Matsushima et al. (2022) reported low imprinting scores exclusively in individuals with a low BM preference. Specification of the ASD-like behavioral impairments by the embryonic VPA awaits further studies.
Actually, VPA has a wide spectrum of pharmacological effects, including actions on N-methyl-D-aspartate type glutamate receptors [NMDA-R (Gean et al., 1994)] and inhibitory GABA transmission (Winterer, 2003). Moreover, VPA is well-accepted as a potent inhibitor of histone deacetylases [HDACs (Phiel et al., 2001)]. Acute anticonvulsant action on the late embryos (the last week of the prehatch development) may induce ASD-like phenotypes, as VPA effectively suppresses embryonic motion (Matsushima et al., 2022). Spontaneous motion is ubiquitous among late embryos of vertebrates (Bekoff et al., 1975) (see also Bekoff, 2001; Blankenship and Feller, 2010 for more recent reviews), although its functional roles remain unclear. Nevertheless, suppression of embryonic movements per se fails to account for ASD-like deficiencies in chicks, since similarly effective suppressers (e.g., selective blockers of NMDA-R MK-801) failed to cause ASD-like symptoms (Matsushima et al., 2022).
The brain regions and molecular events that are responsible for the VPA-mediated ASD phenotypes remain elusive. In a rat model, VPA enhanced NMDA-R expression and synaptic potentiation in the hippocampus (Rinaldi et al., 2007), thereby causing an imbalance between excitatory and inhibitory transmission (E-I imbalance; see Uzunova et al., 2016; Lee et al., 2017 for comprehensive reviews). In support of the hyper-excitation hypothesis, post-natal blockade of NMDA-R by memantine (a drug prescribed for Alzheimer’s disease) rescued social interaction impairment (Kang and Kim, 2016). Consistent with studies performed in rodent models, administering bumetanide (a selective blocker of NKCC1 co-transporter) immediately before training could rescue chicks with VPA-induced impaired imprinting (Matsushima et al., 2022); the impact of bumetanide will be discussed below.
3.2 Selective impairment of BM predisposition via embryonic interference with nAChR receptors, including neonicotinoid insecticides
Pesticide chemicals, particularly considering the rapidly increasing consumption of neonicotinoid insecticides [NNs (Costas-Ferreira and Faro, 2021)], are another serious concern in the etiology of ASD. NNs are designed to selectively perturb cholinergic neurotransmission in the nervous system of insects through their agonistic nature, whereas NNs were assumed to have low toxicity in vertebrates. Early ecological reports have highlighted the population decline of insectivorous birds (Hallmann et al., 2014). Following concerns regarding the high persistence of NNs in plants and soil, NNs were found to impair the migratory ability of granivorous birds (Eng et al., 2017, 2019). Several recent epidemiological studies have reported the risk of maternal exposure to environmental NNs (Keil et al., 2014; Gunier et al., 2017). An early study (Keil et al., 2014) estimated the association between the indoor usage of imidacloprid (IMI; one of the most heavily used NNs for flea and tick treatment for pet animals) and ASD, detecting an alarming odds ratio of ~2.0. Prenatal exposure to agricultural pesticides was found to be associated with low intelligence quotient and verbal comprehension (Gunier et al., 2017). A large-scale study on the association between ambient pesticide usage (NNs included) and ASD in California’s agricultural region (Von Ehrenstein et al., 2019) detected considerable odd ratios for various pesticide chemicals; the effects of prenatal exposure were boosted by additional exposure in neonatal infants. A rodent model study assessing acetamiprid (ACE; another NNs) has reported the abnormal development of social and anxiety-related behaviors in males after prenatal and lactational exposure (Ongono et al., 2020).
Our study using a chick model (Sano et al., 2016) revealed high concordance with these reports in humans and rodents. Selective and non-selective blockade of nAChR (using tubocurarine and selective α7 subtype inhibitor), as well as perturbed nAChR transmission by IMI, could suppress embryonic movements and impair the BM preference of hatchlings. Notably, nAChR blockade did not impair imprinting memory formation, thus revealing distinct dimensions of social behavior malformation from those induced by VPA (Figure 3).
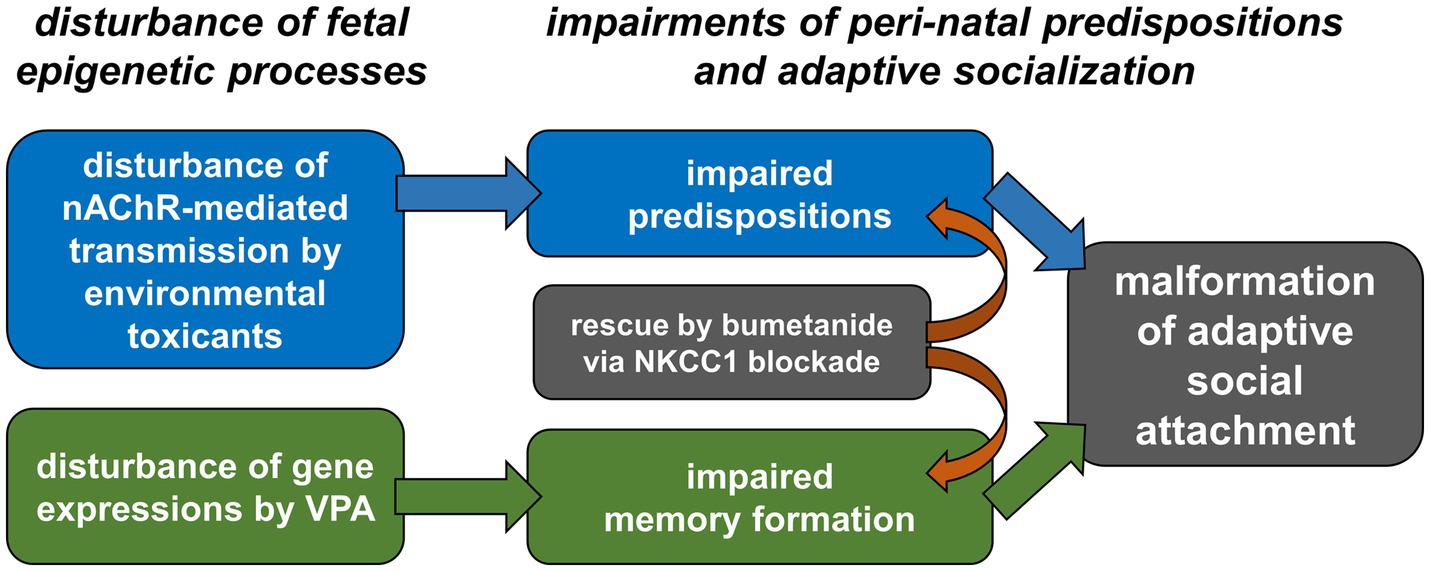
Figure 3. Disturbed epigenesis during the late embryonic stage leads to impaired predisposition critical for post-natal socialization through imprinting. Imidacloprid and other environmental toxicants disturbing nicotinic acetylcholine receptors (nAChR) would impair the induction of predisposed preference to biological motion. On the other hand, valproic acid (VPA) disturbs expression patterns of yet unspecified set of genes through inhibiting deacetylation of nuclear histone, leading to impaired memory formation. As both of the predisposition and the memory formation are critical for adaptive socialization, disturbance of either one of these would lead to social attachment hypoplasia. Despite distinct processes of disturbance, both impairments are rescued by bumetanide, a selective blocker of NKCC1 cotransporter.
3.3 Thyroid hormone, E-I imbalance in humans and chicks
Maternal hypothyroidism (gestational hypothyroxinemia) is another risk factor for ASD (Román et al., 2013; Berbel et al., 2014; Getahun et al., 2018). Circulating levels of thyroid hormones (THs, T4 in particular) could be a potential early biological marker for ASD. To date, no consensus has been reached regarding the role of THs in the development of ASD, given that both positive (Hoshiko et al., 2011) and negative (Ames et al., 2020) results have been reported. In addition to being a critical determinant of imprinting in chicks (Lemaire et al., 2022), TH plays critical roles in diverse neurodevelopmental processes (Batista and Hensch, 2019), particularly in the maturation of GABAergic transmission via the rapamycin (mTOR; mechanistic target of rapamycin) cascade (Westerholz et al., 2013). The mTOR-GABA cascade may mediate the acute facilitatory effects of THs in chicks (Yamaguchi et al., 2012; Batista et al., 2018; Lorenzi et al., 2021). T3 acutely enhances GABAergic transmission in slice preparations of the chick pallium (Saheki et al., 2022), although its functional link to the behavioral effects remains elusive.
Interestingly, in humans, symptomatic autism comorbid with fragile X syndrome and tuberous sclerosis complex (TSC) is accompanied by mutations in the mTOR signaling pathway. Rapamycin was shown to rescue social impairment in a mouse model of TSC (Sato et al., 2012). A more recent study addressing macrocephaly in infants with ASD has suggested that synaptic pathology related to the mTOR pathway is responsible for hyperconnectivity (Pagani et al., 2021).
In the central nervous system, the metabolic control responsible for the balanced management of energy income and growth may underlie appropriate socialization during the early stages of life in humans and chicks. In addition, premature E-I balance (excitatory-inhibitory balance, or delayed GABA switch from depolarizing to hyperpolarizing response) could be a key event in neural maturation, affording a potential target for developing effective pharmacotherapies for ASD.
3.4 GABA switch, nicotinic transmission, and treatment using bumetanide and oxytocin
GABA exerts depolarizing transmission via GABA-A receptors in the embryonic and early post-natal stages, and the excitatory GABA is supposed to exert trophic functions for the functional maturation of the brain. During the perinatal period, excitation is converted to adult-type inhibitory neurotransmission [Ben-Ari (2002), Represa and Ben-Ari (2005), and Ben-Ari et al. (2007) for comprehensive reviews; Pfeffer et al. (2009) for the hippocampal development; also see Hyde et al. (2011) specifically for the schizophrenia etiology]; this conversion (referred to as the GABA switch) is mediated by a reduction in the intracellular chloride ion ([Cl−]i), which, in turn, can be attributed to the enhanced expression of cation-linked co-transporters responsible for the efflux of Cl− (KCC2 over NKCC1). To identify regulatory mechanisms underlying the GABA switch, nicotinic cholinergic transmission was found to be critical in the chick ciliary ganglion (Liu et al., 2006). Further analysis of the phosphorylation of KCC2 molecules revealed protein kinase C-mediated modulation by glutamatergic and serotonergic actions, as well as the activation of muscarinic acetylcholine receptors (Kahle et al., 2013) (for more recent comprehensive reviews see Kaila et al., 2014; Zhang et al., 2021). Cascade of causal molecular events is yet unspecified.
The association between GABA switch retardation and ASD was based on the finding that diazepam (an anxiolytic drug, a positive modulator of GABA-mediated inhibition used to relieve anxiety) could paradoxically increase aggressive behaviors in autistic children (Marrosu et al., 1987). Subsequent studies have shown that dysfunctional GABA inhibition could play a pivotal role in ASD etiology (Nelson and Valakh, 2015; Hadjikhani et al., 2018). Bumetanide has attracted attention as a potent candidate for ameliorating ASD symptoms, given that this agent selectively blocks the NKCC1 co-transporter responsible for Cl− influx (Delpire and Ben-Ari, 2022). Although initial open-label small-sized trials appeared positive and promising (Fernell et al., 2021; Wang et al., 2021), recent phase-2 trials failed to afford positive outcomes (Sprengers et al., 2021). A detailed follow-up analysis has identified heterogeneous phenotypes of neurocognitive impairment in patients with ASD, some of which were unaffected by bumetanide (Van Andel et al., 2023). In a chick model, bumetanide treatment immediately before training rescued impairments in both imprinting (by VPA) and BM preference (by nAChR blockade) (Matsushima et al., 2022). Further studies are required to identify underlying targets and pharmacology.
Oxytocin and related nonapeptides comprise another group of candidate drugs for ASD. In humans, intranasal application of oxytocin can acutely ameliorate social deficiencies such as BM perception and social communication in cases of relatively low severity (Kéri and Benedek, 2009; Parker et al., 2017). In typically developing individuals, oxytocin receptors peak during early childhood, whereas this peak is absent in those with ASD (Freeman et al., 2018). Similar facilitatory effects of oxytocin on prosocial behaviors have been observed in dogs (Nagasawa et al., 2015; Kovács et al., 2016), birds (Duque et al., 2018; Loveland et al., 2019; Seguchi et al., 2022) and fish (Nunes et al., 2020), suggesting its ubiquitous role in vertebrates. Considering the underlying mechanisms, a study using oxytocin-receptor knock-out mice has suggested that KCC2 is regulated by oxytocin (Leonzino et al., 2016). A more recent study in mice identified a link between the genetic risk of ASD and the oxytocinergic signaling pathway (Hörnberg et al., 2020). In chicks, intracranially administered mesotocin (an avian counterpart of oxytocin) enhanced the preference of naïve chicks (Loveland et al., 2019). The appropriately timed excitation/inhibition balance could play critical roles on functional maturation of the social brain network in both of the newly hatched chicks and the human neonates, thus forming another aspect of the convergent peri-natal “bottleneck” (Figure 1).
4 Perspectives
Do chick studies have future? Table 1 summarizes the validity and limitations of chicks as an ASD model animal, and some of these points will be discussed in detail. To conclude this review article, we will further discuss how neurocognitive homologies are to be defined. We will also discuss how we should address the homology issue on firm biological bases.
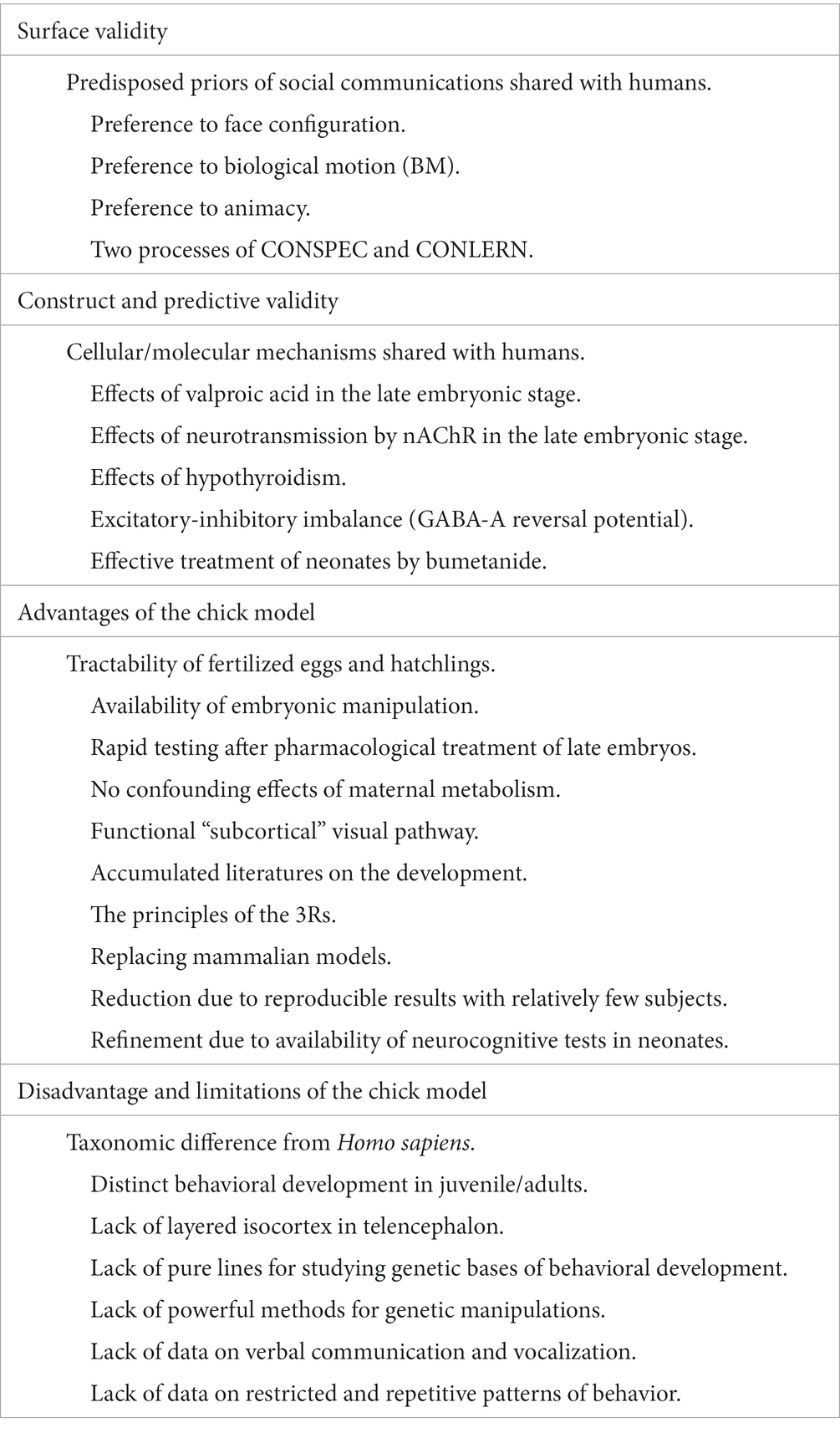
Table 1. Validity, advantages and limitations of the newly hatched domestic chicks as an ASD model animal.
4.1 Limitations and advantages of the domestic chick; comparisons with other novel ASD model animals
In terms of the taxonomic validity measured by the distance from Homo sapiens (Belzung and Lemoine, 2011), chicks are definitely inferior model of human disorders because they are not mammals. Non-human primates such as common marmosets (Watanabe et al., 2021) is one of the most promising options in this respect. Prenatal VPA treatment actually causes a deficient inequity aversion (Yasue et al., 2018), suggesting impaired social motivation or interest to conspecifics. Although marmosets have the ability to visually perceive BM (Brown et al., 2010), effects of prenatal VPA treatment have not been addressed in the neonatal visual perception. Genetic engineering technique using the CRISPR/Cas9 system has successfully generated marmosets that carry mutations in the fragile X mental retardation 1 (FMR1) (Abe et al., 2021). Though fragmental at present, these studies are expected to yield a promising model system, high in both face and construct validity in near future.
Zebrafish is another promising model to study ASD despite the inferior homological validity (Choi et al., 2021). Several technical advantages such as the large-scale pool of mutants, wide applicability of tractable genetic manipulation and high-resolution whole brain imaging, makes this fish a powerful candidate for studying the molecular basis of developmental disorders including ASD (Geng and Peterson, 2019; Torres-Pérez et al., 2023). High throughput screening of the environmental risk chemicals (Geng et al., 2022) is probably the most successful product so far.
Comparison with these two models clearly reveals limitations and advantages of domestic chicks as ASD model. As the genetic engineering is premature in birds, the chick model is inappropriate for experimental manipulation of relevant genes. The rodent models are superior to specify the genes involved in the ASD etiology. On the other hand, environmental risk chemicals are reliably searched for in chicks and fish because (1) chemicals can be quantitatively applied to eggs and (2) rapid development allows efficient screening. Furthermore, (3) complications due to maternal metabolisms are inevitable in mammals, while these are disregarded in studies using chick and fish models. The screening efficiency is much higher in fish than any other models, however, the marmoset and chick models are superior in their behavioral similarity to humans. To examine the BM predisposition, for example, similar video clips of point light animation are used for testing chicks (Vallortigara et al., 2005), marmosets (Brown et al., 2010) and human babies (Simion et al., 2008), whereas motion pictures of different kinetics are used in zebrafish (Larsch and Baier, 2018).
4.2 Is the similar social perception due to genuine homology or convergent evolution?
The construct validity of chicks as the ASD model could be assumed to be evidence in favor of the homology, however, the present list is inevitably not exhaustive. ASD is actually a spectrum composed of a variety of diverse phenotypes among individuals with different genetic and environmental backgrounds. Any animal model would therefore be partial, covering limited aspects of the disorder. It is therefore critically important to collect any pieces of disproof, namely the discrepancy between the model and the human cases in their etiology, and also between the model animals of different taxa, such as rodents, primates, birds and fish.
So far, two major points remain ambiguous, making the answer to this question difficult. One is to specify the responsible brain region for the priors, and another is the molecular events leading to the formation of the predisposed social perception. As for the first, we tentatively assume the subcortical visual pathway, however, its development during the late embryonic stages is largely unknown. For the second, as would be expected from the VPA effects, a variety of epigenetic processes are supposed to underlie, such as DNA methylation, histone modification and post-translational splicing (Eshraghi et al., 2018; Leung et al., 2023). It is also noticed that the phylotypic bottleneck of the vertebrate morphogenesis is characterized by a transition in chromatic accessibility (Uesaka et al., 2019). Comprehensive survey is expected on the developmental changes of chromatin environment in embryos of these assumed model animals, particularly in its late stage of development.
Another aspect could be the blood–brain-barrier (BBB), because premature BBB of the embryonic brains could be responsible for the fragility to environmental toxicants, yet some of the BBB functions are conserved among vertebrates and even some invertebrate animals (O’Brown et al., 2018). At the same time, the endothelial epithelial cells of blood vessels in the brain are actively regulating the endocrine control, such as thyroid hormone in chicks (Yamaguchi et al., 2012) and juvenile hormone for cast differentiation in ants (Ju et al., 2023); BBB could play as an active regulator or “gatekeeper” of the humoral factors in the developing CNS, rather than immature underdeveloped barriers. In this context, epigenetic regulation of BBB in healthy and damaged brain needs more attention (Ihezie et al., 2021); for example, histone deacetylase inhibitor such as VPA (see above) could protect the BBB damages after stroke. Possible links connecting between BBB development and ASD etiology are to be searched for in future, with a close reference on the involvement of gut-brain axis in ASD (Morton et al., 2023).
5 Concluding remark
No single animal models suffice to understand the complex etiology, cellular/molecular mechanisms, responsible brain networks, behavioral traits and pharmacological therapeutics of ASD. Though inevitably fragmental, findings obtained from a variety of, but appropriately chosen animal models should be complementarily integrated for a proper understanding of a developmental disease with multiple causes and wide phenotypic spectrum. Developmental homology to human neonates would be most critical criteria of the valid animal models. In this respect, the domestic chick comprises a unique position for studying neurocognitive disorders.
A fundamental question remains. Why did chicks converge to human neonates despite distinct evolutionary separations over 300 million years? This remains an unresolved puzzle, which could be addressed by analyzing behavioral phenotypes and disorders (surface validity) and the underlying mechanisms (construct validity). Biological psychiatry and comparative psychology therefore ask a same question: What are we?
Author contributions
TM: Conceptualization, Funding acquisition, Investigation, Writing – original draft. TI: Conceptualization, Supervision, Writing – review & editing. GV: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was supported by grants funded by the Japan Society for Promotion of Science (JSPS Kakenhi) to TM (Fostering Joint International Research, #19KK0211) and to Koichi J. Homma [Grants-in-aid for Scientific Research (B), #23H02517]. This work was supported also by grants from the European Union’s Seventh Framework Programme (FP7/2007-2013) Grant ERC-2011-ADG_20110406, project no. 461 295517, PREMESOR and PRIN 2016 grant from MIUR (Ministero Istruzione Universita et Ricerca) to GV.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abe, Y., Nakao, H., Goto, M., Tamano, M., Koebis, M., Nakao, K., et al. (2021). Efficient marmoset genome engineering by autologous embryo transfer and CRISPR/Cas9 technology. Sci. Rep. 11:20234. doi: 10.1038/s41598-021-99656-4
Adiletta, A., Pedrana, S., Rosa-Salva, O., and Sgadò, P. (2021). Spontaneous visual preference for face-like stimuli is impaired in newly-hatched domestic chicks exposed to valproic acid during embryogenesis. Front. Behav. Neurosci. 15:733140. doi: 10.3389/fnbeh.2021.733140
Amari, S.-I. (2020). Any target function exists in a neighborhood of any sufficiently wide random network: a geometrical perspective. Neural Comput. 32, 1431–1447. doi: 10.1162/neco_a_01295
American Psychiatric Association. (2013). DSM-5 diagnostic and statistical manual of mental disorders. 5th Edn. Washington, DC: American Psychiatric Association.
Ames, J. L., Windham, G. C., Lyall, K., Pearl, M., Kharrazi, M., Yoshida, C. K., et al. (2020). Neonatal thyroid stimulating hormone and subsequent diagnosis of autism spectrum disorders and intellectual disability. Autism Res. 13, 444–455. doi: 10.1002/aur.2247
Barabási, D. L., Beynon, T., Katona, A., and Perez-Nieves, N. (2023). Complex computation from developmental priors. Nat. Commun. 14:2226. doi: 10.1038/s41467-023-37980-1
Bardi, L., Regolin, L., and Simion, F. (2011). Biological motion preference in humans at birth: role of dynamic and configural properties. Dev. Sci. 14, 353–359. doi: 10.1111/j.1467-7687.2010.00985.x
Baron-Cohen, S., Ring, H. A., Bullmore, E. T., Ashwin, C., and Williams, S. C. R. (2000). The amygdala theory of autism. Neurosci. Biobehav. Rev. 24, 355–364. doi: 10.1016/S0149-7634(00)00011-7
Bateson, P., and Gluckman, P. (2011). Plasticity, robustness, development and evolution. Oxford: Cambridge University Press
Batista, G., and Hensch, T. K. (2019). Critical period regulation by thyroid hormones: potential mechanisms and sex-specific aspects. Front. Mol. Neurosci. 12, 77. doi: 10.3389/fnmol.2019.00077
Batista, G., Johnson, J. L., Dominguez, E., Costa-Mattioli, M., and Pena, J. L. (2018). Regulation of filial imprinting and structural plasticity by mTORC1 in newborn chickens. Sci. Rep. 8:8044. doi: 10.1038/s41598-018-26479-1
Bekoff, A. (2001). Spontaneous embryonic motility: an enduring legacy. Int. J. Dev. Neurosci. 19, 155–160. doi: 10.1016/S0736-5748(00)00089-7
Bekoff, A., Stein, P. S. G., and Hamburger, V. (1975). Coordinated motor output in the hindlimb of the 7-day chick embryo. Proc. Nat. Acad. Sci. 72, 1245–1248. doi: 10.1073/pnas.72.4.1245
Belzung, C., and Lemoine, M. (2011). Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 1:9. doi: 10.1186/2045-5380-1-9
Ben-Ari, Y. (2002). Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739. doi: 10.1038/nrn920
Ben-Ari, Y., Gaiarsa, J. L., Tyzio, R., and Khazipov, R. (2007). GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87, 1215–1284. doi: 10.1152/physrev.00017.2006
Berbel, P., Navarro, D., and Román, G. C. (2014). An evo-devo approach to thyroid hormones in cerebral and cerebellar cortical development: etiological implications for autism. Front. Endocrinol. 5:146. doi: 10.3389/fendo.2014.00146
Bidet-Ildei, C., Kitromilides, E., Orliaguet, J.-P., Pavlova, M., and Gentaz, E. (2014). Preference for point-light biological motion in newborns: contribution of translational displacement. Dev. Psychol. 50, 113–120. doi: 10.1037/a0032956
Blake, R., Turner, L. M., Smoski, M. J., Pozdol, S. L., and Stone, W. L. (2003). Visual recognition of biological motion is impaired in children with autism. Psychiol. Sci. 14, 151–157. doi: 10.1111/1467-9280.01434
Blankenship, A. G., and Feller, M. B. (2010). Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci. 11, 18–29. doi: 10.1038/nrn2759
Bliss, L., Vasas, V., Freeland, L., Roach, R., Ferré, E. R., and Versace, E. (2023). A spontaneous gravity prior: newborn chicks prefer stimuli that move against gravity. Biol. Lett. 19:20220502. doi: 10.1098/rsbl.2022.0502
Bolhuis, J. J., Johnson, M. H., and Horn, G. (1985). Effects of early experience on the development of filial preferences in the domestic chick. Dev. Psychobiol. 18, 299–308. doi: 10.1002/dev.420180403"
Brown, J., Kaplan, G., Rogers, L. J., and Vallortigara, G. (2010). Perception of biological motion in common marmosets (Callithrix jacchus): by females only. Anim. Cogn. 13, 555–564. doi: 10.1007/s10071-009-0306-0
Buiatti, M., Di Giorgio, E., Piazza, M., Polloni, C., Menna, G., Taddei, F., et al. (2019). Cortical route for face like pattern processing in human newborns. Proc. Nat. Acad. Sci. 116, 4625–4630. doi: 10.1073/pnas.1812419116
Chaliha, D., Albrecht, M., Vaccarezza, M., Takechi, R., Lam, V., Al-Salami, H., et al. (2020). A systematic review of the valproic acid-induced rodent model of autism. Dev. Neurosci. 42, 12–48. doi: 10.1159/000509109
Chang, D. H. F., and Troje, N. F. (2009). Characterizing global and local mechanisms in biological motion perception. J. Vision 9, 1–10. doi: 10.1167/9.5.8
Choi, T. Y., Choi, T. I., Lee, Y. R., Choe, S. K., and Kim, C. H. (2021). Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 53, 310–317. doi: 10.1038/s12276-021-00571-5
Christensen, J., Grønborg, T. K., Sørensen, M. J., Schendel, D., Parner, E. T., Pedersen, L. H., et al. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703. doi: 10.1001/jama.2013.2270
Costas-Ferreira, C., and Faro, L. R. F. (2021). Neurotoxic effects of neonicotinoids on mammals: what is there beyond the activation of nicotinic acetylcholine receptors? - a systematic review. Int. J. Mol. Sci. 22:8413. doi: 10.3390/ijms22168413
Csillag, A., Ádám, A., and Zacher, G. (2022). Avian models for mechanisms underlying altered social behavior in autism. Front. Physiol. 13:1032046. doi: 10.3389/fphys.2022.1032046
Dean, P., Redgrave, P., and Westby, G. W. M. (1989). Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12, 137–147. doi: 10.1016/0166-2236(89)90052-0
Delpire, E., and Ben-Ari, Y. (2022). A wholistic view of how bumetanide attenuates autism spectrum disorders. Cell 11:2419. doi: 10.3390/cells11152419
Di Giorgio, E., Frasnelli, E., Rosa-Salva, O., Scattoni, M. L., Puopolo, M., Tosoni, D., et al. (2016a). Difference in visual social predispositions between newborns at low- and high-risk for autism. Sci. Rep. 6:26395. doi: 10.1038/srep26395
Di Giorgio, E., Loveland, J. L., Mayer, U., Rosa-Salva, O., Versace, E., and Vallortigara, G. (2017). Filial responses as predisposed and learned preferences: early attachment in chicks and babies. Behav. Brain Res. 325, 90–104. doi: 10.1016/j.bbr.2016.09.018
Di Giorgio, E., Lunghi, M., Simion, F., and Vallortigara, G. (2016b). Visual cues of motion that trigger animacy perception at birth: the case of self-propulsion. Dev. Sci. 20:e12394. doi: 10.1111/desc.12394
Di Giorgio, E., Lunghi, M., Vallortigara, G., and Simion, F. (2021b). Newborns’ sensitivity to speed changes as a building block for animacy perception. Sci. Rep. 11:542. doi: 10.1038/s41598-020-79451-3
Di Giorgio, E., Rosa-Salva, O., Frasnelli, E., Calcagni, A., Lunghi, M., Scattoni, M. L., et al. (2021a). Abnormal visual attention to simple social stimuli in 4-month-old infants at high risk for autism. Sci. Rep. 11:15785. doi: 10.1038/s41598-021-95418-4
Duque, J. F., Leichner, W., Ahmann, H., and Stevens, J. R. (2018). Mesotocin influences pinyon jay prosociality. Biol. Lett. 14:20180105. doi: 10.1098/rsbl.2018.0105
Elsabbagh, M., Gliga, T., Pickles, A., Hudry, K., Charman, T., Johnson, M. H., et al. (2013). The development of face orienting mechanisms in infants at-risk of autism. Behav. Brain Res. 251, 147–154. doi: 10.1016/j.bbr.2012.07.030
Elsabbagh, M., and Johnson, M. H. (2009). Getting answers from babies about autism. Trends Cogn. Sci. 14, 81–87. doi: 10.1016/j.tics.2009.12.005
Eng, M. L., Stutchbury, B. J. M., and Morrissey, C. A. (2017). Imidacloprid and chlorypyrifos insecticides impair migratory ability in a seed-eating songbird. Sci. Rep. 7:15176. doi: 10.1038/s41598-017-15446-x
Eng, M. L., Stutchbury, B. J. M., and Morrissey, C. A. (2019). A neonicotinoid insecticide reduces fueling and delays migration in songbirds. Science 365, 1177–1180. doi: 10.1126/science.aaw9419
Ergaz, Z., Weinstein-Fudim, L., and Ornoy, A. (2016). Genetic and non-genetic animal models for autism spectrum disorders (ASD). Reprod. Toxicol. 64, 116–140. doi: 10.1016/j.reprotox.2016.04.024
Eshraghi, A. A., Liu, G., Kay, S.-I. S., Eshraghi, R. S., Mittal, J., Moshiree, B., et al. (2018). Epigenetics and autism spectrum disorder: is there a correlation? Front. Cell. Neurosci. 12:78. doi: 10.3389/fncel.2018.00078
Federici, A., Parma, V., Vicovaro, M., Radassao, L., Casartelli, L., and Ronconi, L. (2020). Anomalous perception of biological motion in autism: a conceptual review and meta-analysis. Sci. Rep. 10:4576. doi: 10.1038/s41598-020-61252-3
Fernell, E., Gustafsson, P., and Gillberg, C. (2021). Bumetanide for autism: open-label trial in six children. Acta Paediatr. 110, 1548–1553. doi: 10.1111/apa.15723
Freeman, S. M., Palumbo, M. C., Lawrence, R. H., Smith, A. L., Goodman, M. M., and Bales, K. L. (2018). Effect of age and autism spectrum disorder on oxytocin receptor density in the human basal forebrain and midbrain. Transl. Psychiatry 8:257. doi: 10.1038/s41398-018-0315-3
Gaugler, T., Klei, L., Sanders, S. J., Bodea, C. A., Goldberg, A. P., Lee, A. B., et al. (2014). Most genetic risk for autism resides with common variation. Nat. Genet. 46, 881–885. doi: 10.1038/ng.3039
Gean, P.-W., Huang, C.-C., Hung, C.-R., and Tsai, J.-J. (1994). Valproic acid suppresses the synaptic response mediated by the NMDA receptors in rat amygdalar slices. Brain Res. Bull. 33, 333–336. doi: 10.1016/0361-9230(94)90202-X
Geng, Y., and Peterson, R. T. (2019). The zebrafish subcortical social brain as a model for studying social behavior disorders. Dis. Models Mech. 12:dmm039446. doi: 10.1242/dmm.039446
Geng, Y., Zhang, T., Alonzo, I. G., Godar, S. C., Yates, C., Pluimer, B. R., et al. (2022). Top2a promotes the development of social behavior via PRC2 and H3K27me3. Sci. Adv. 8:eabm7069. doi: 10.1126/sciadv.abm7069
Gernsbacher, M. A., and Yergeau, M. (2019). Empirical failures of the claim that autistic people lack a theory of mind. Arch. Sci. Psychol. 7, 102–118. doi: 10.1037/arc0000067
Getahun, D., Jacobsen, S. J., Fassett, M. J., Wing, D. A., Xiang, A. H., Chiu, V. Y., et al. (2018). Association between maternal hypothyroidism and autism spectrum disorders in children. Pediatr. Res. 83, 580–588. doi: 10.1038/pr.2017.308
Grillner, S., and Robertson, B. (2016). The basal ganglia over 500 million years. Curr. Biol. 26, R1088–R1100. doi: 10.1016/j.cub.2016.06.041
Gunier, R. B., Bradman, A., Harley, K. G., Kogut, K., and Eskenazi, B. (2017). Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environ. Health Perspect. 125:057002. doi: 10.1289/EHP504
Güntürkün, O., and Bugnyar, T. (2016). Cognition without cortex. Trends Cogn. Sci. 20, 291–303. doi: 10.1016/j.tics.2016.02.001
Hadjikhani, N., Johnels, J. Å., Lassalle, A., Zürcher, N. R., Hippolyte, L., Gillberg, C., et al. (2018). Bumetanide for autism: more eye contact, less amygdala activation. Sci. Rep. 8:3602. doi: 10.1038/s41598-018-21958-x
Hallmann, C. A., Foppen, R. P. B., van Turnhaut, C. A. M., de Kroon, H., and Jongejans, E. (2014). Declines in insectivorous birds are associated with high neonicotinoid concentration. Nature 511, 341–343. doi: 10.1038/nature13531
Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 68, 1095–1102. doi: 10.1001/archgenpsychiatry.2011.76
Hebert, M., Versace, E., and Vallortigara, G. (2019). Inexperienced preys know when to flee or to freeze in front of a threat. Proc. Nat. Acad. Sci. 116, 22918–22920. doi: 10.1073/pnas.1915504116
Hirai, M., Saunders, D. R., and Troje, N. F. (2011). Allocation of attention to biological motion: local motion dominates global shape. J. Vision 11, 1–11. doi: 10.1167/11.3.4
Hirai, M., and Senju, A. (2020). The two-process theory of biological motion processing. Neurosci. Biobehav. Rev. 111, 114–124. doi: 10.1016/j.neubiorev.2020.01.010
Horn, G. (2004). Pathway of the past: the imprint of memory. Nat. Rev. Neurosci. 5, 108–120. doi: 10.1038/nrn1324
Hörnberg, H., Pérez-Garci, E., Schreiner, D., Hatstatt-Burklé, L., Magara, F., Baudouin, S., et al. (2020). Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature 584, 252–256. doi: 10.1038/s41586-020-2563-7
Hoshiko, S., Grether, J. K., Windham, G. C., Smith, D., and Fessel, K. (2011). Are thyroid hormone concentrations at birth associated with subsequent autism diagnosis? Autism Res. 4, 456–463. doi: 10.1002/aur.219
Huang, X., and Cheng, H.-W. (2022). Perspective: chicken models for studying the ontogenetic origin of neuropsychiatric disorders. Biomedicine 10:1155. doi: 10.3390/biomedicines10051155”
Hyde, T. M., Lipska, B. K., Ali, T., Mathew, S. V., Law, A. J., Metitiri, O. E., et al. (2011). Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 31, 11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011
Ihezie, S. A., Mathew, I. E., McBride, D. W., Dienel, A., Blackburn, S. L., and Pandit, P. K. T. (2021). Epigenetics in blood-brain barrier disruption. Fluids Barriers CNS 18:17. doi: 10.1186/s12987-021-00250-7
Inagaki, M., Inoue, K. I., Tanabe, S., Kimura, K., Takada, M., and Fujita, I. (2022). Rapid processing of threatening faces in the amygdala of non-human primates: subcortical inputs and dual roles. Cereb. Cortex 33, 895–915. doi: 10.1093/cercor/bhac109
Iossifov, I., O’Roak, B. J., Sanders, S. J., Ronemus, M., Krumm, N., Levy, D., et al. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221. doi: 10.1038/nature13908
Irie, N., and Kuratani, S. (2011). Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat. Commun. 2:248. doi: 10.1038/ncomms1248
Johnson, M. H. (1992). Imprinting and development of face recognition: from chick to man. Curr. Direct. Psychol. Sci. 1, 52–55.
Johnson, M. H. (2005). Subcortical face processing. Nat. Rev. Neurosci. 6, 766–774. doi: 10.1038/nrn1766
Johnson, M. H., Bolhuis, J. J., and Horn, G. (1985). Interaction between acquired preferences and developing predispositions during imprinting. Anim. Behav. 33, 1000–1006. doi: 10.1016/S0003-3472(85)80034-8
Johnson, M. H., and Horn, G. (1988). Development of filial preferences in dark-reared chicks. Anim. Behav. 36, 675–683. doi: 10.1016/S0003-3472(88)80150-7
Jones, W., and Klin, A. (2013). Attention to eye is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature 504, 427–431. doi: 10.1038/nature12715
Ju, L., Glastad, K. M., Sheng, L., Gospocic, J., Kingwell, C. J., Davidson, S. M., et al. (2023). Hormonal gatekeeper via the blood-brain barrier governs caste-specific behavior in ants. Cell 186, 1–21. doi: 10.1016/j.cell.2023.08.002
Kahle, K. T., Deeb, T. Z., Puskarjov, M., Silayeva, L., Liang, B., Kaila, K., et al. (2013). Modulation of neuronal activity by phosphorylation of the K-cl cotransporter KCC2. Trends Neurosci. 36, 726–737. doi: 10.1016/j.tins.2013.08.006
Kaila, K., Price, T. J., Payne, J. A., Puskarjov, M., and Voipio, J. (2014). Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 15, 637–654. doi: 10.1038/nrn3819
Kaliukhovich, D. A., Manyakov, N. V., Bangerter, A., Ness, S., Skalkin, A., Boice, M., et al. (2021). Visual preference for biological motion in children and adults with autism spectrum disorder: an eye-tracking study. J. Autism. Dev. Disord. 51, 2369–2380. doi: 10.1007/s10803-020-04707-w
Kang, J., and Kim, E. (2016). Suppression of NMDA receptor function in mice prenatally exposed to valproic acid improves social deficits and repetitive behaviors. Front. Mol. Neurosci. 8:17. doi: 10.3389/fnmol.2015.00017
Kardamakis, A. A., Pérez-Fernández, J., and Grillner, S. (2016). Spatiotemporal interplay between multisensory excitation and recruited inhibition in the lamprey optic tectum. elife 5:e16472. doi: 10.7554/eLife.16472
Keil, A. P., Daniels, J. L., and Hertz-Picciotto, I. (2014). Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (childhood autism risks from genetics and Envinronment) case-control study. Environ. Health 13:3. doi: 10.1186/1476-069X-13-3
Kéri, S., and Benedek, G. (2009). Oxytocin enhances the perception of biological motion in humans. Cogn. Affect,. Behav. Neurosci. 9, 237–241. doi: 10.3758/CABN.9.3.237
Kobayashi, H., and Kohshima, S. (1997). Unique morphology of the human eye. Nature 387, 767–768. doi: 10.1038/42842
Kovács, I. A., Barabási, D. L., and Barabási, A.-L. (2020). Uncovering the genetic blueprint of the C. elegans nervous system. Proc. Nat. Acad. Sci. 117, 33570–33577. doi: 10.1073/pnas.2009093117
Kovács, K., Kis, A., Kanizsár, O., Hernádi, A., Gácsi, M., and Topál, J. (2016). The effect of oxytocin on biological motion perception in dogs (Canis familiaris). Anim. Cogn. 19, 513–522. doi: 10.1007/s10071-015-0951-4
Larsch, J., and Baier, H. (2018). Biological motion as an innate perceptual mechanism driving social affiliation. Curr. Biol. 28, 3523–3532. doi: 10.1016/j.cub.2018.09.014
Lee, E., Lee, J., and Kim, E. (2017). Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 81, 838–847. doi: 10.1016/j.biopsych.2016.05.011
Lemaire, B., Rosa-Salva, O., Fraja, M., Lorenzi, E., and Vallortigara, G. (2022). Spontaneous preference for unpredictability in the temporal contingencies between agents’ motion in naïve domestic chicks. Proc. R. Soc. B 289:20221622. doi: 10.1098/rspb.2022.1622
Lemaire, B., and Vallortigara, G. (2022). Life is in motion (through a chick’s eye). Anim. Cogn. 26, 129–140. doi: 10.1007/s10071-022-01703-8
Leonzino, M., Busnelli, M., Antonucci, F., Verderio, C., Mazzanti, M., and Chini, B. (2016). The timing of the excitatory-to-inhibitory GABA switch is regulated by the oxytocin receptor via KCC2. Cell Rep. 15, 96–103. doi: 10.1016/j.celrep.2016.03.013
Leung, C. S., Rosenzweig, S. J., Yoon, B., Marinelli, N. A., Hollingsworth, E. W., Maguire, A. M., et al. (2023). Dysregulation of the chromatic environment leads to differential alternative splicing as a mechanism of disease in a human model of autism spectrum disorder. Human Mol. Genet. 32, 1634–1646. doi: 10.1093/hmg/ddad002
Liu, Z., Neff, R. A., and Berg, D. K. (2006). Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 314, 1610–1613. doi: 10.1126/science.1134246
Lorenzi, E., Lemaire, B. S., Versace, E., Matsushima, T., and Vallortigara, G. (2021). Resurgence of an inborn attraction for animate objects via thyroid hormone T3. Frontiers Behav Neurosci. 15:675994. doi: 10.3389/fnbeh.2021.675994
Lorenzi, E., Mayer, U., Rosa-Salva, O., and Vallortigara, G. (2017). Dynamic features of animate motion activate septal and preoptic areas in visually naïve chicks (Gallus gallus). Neuroscience 354, 54–68. doi: 10.1016/j.neuroscience.2017.04.022
Lorenzi, E., Pross, A., Rosa-Salva, O., Versace, E., Sgadò, P., and Vallortigara, G. (2019). Embryonic exposure to valproic acid affects social predispositions for dynamic cues of animate motion in newly-hatched chicks. Front. Physiol. 10:501. doi: 10.3389/fphys.2019.00501
Lorenzi, E., and Vallortigara, G. (2021). “Evolutionary and neural bases of the sense of Animacy” in The Cambridge handbook of animal cognition. eds. A. Kaufman, J. Call, and J. Kaufman (New York: Cambridge University Press), 295–321.
Loveland, J., Stewart, M., and Vallortigara, G. (2019). Effects of oxytocin-family peptides and substance P on locomotor activity and filial preferences in visually naïve chicks. Eur. J. Neurosci. 50, 3674–3687. doi: 10.1111/ejn.14520
Mabunga, D. F. N., Gonzales, E. L. T., Kim, J.-W., Kim, K. C., and Shin, C. Y. (2015). Exploring the validity of valproic acid animal model of autism. Exp. Neurobiol. 24, 285–300. doi: 10.5607/en.2015.24.4.285
Marrosu, F., Marrosu, G., Rachel, M. G., and Biggio, G. (1987). Paradoxical reactions elicited by diazepam in children with classical autism. Funct. Neurol. 2, 355–361.
Matsushima, T., Miura, M., Patzke, N., Toji, N., Wada, K., Ogura, Y., et al. (2022). Fetal blockade of nicotinic acetylcholine transmission causes autism-like impairment of biological motion preference in the neonatal chick. Cereb. Cortex Commun. 3:tgac041. doi: 10.1093/texcom/tgac041
Mauer, D., and Barrera, M. (1981). Infant’s perception of natural and distorted arrangements of a schematic face. Child Dev. 52, 196–202. doi: 10.2307/1129230
Mayer, U., Rosa-Salva, O., Lorenzi, E., and Vallortigara, G. (2016). Social predisposition dependent neuronal activity in the intermediate medial mesopallium of domestic chicks (Gallus gallus domesticus). Behav. Brain Res. 310, 93–102. doi: 10.1016/j.bbr.2016.05.019
Mayer, U., Rosa-Salva, O., Loveland, J. L., and Vallortigara, G. (2019). Selective response of the nucleus taeniae of the amygdala to a naturalistic social stimulus in visually naive domestic chicks. Sci. Rep. 9:9849. doi: 10.1038/s41598-019-46322-5
Mayer, U., Rosa-Salva, O., Morbioli, F., and Vallortigara, G. (2017). The motion of a living conspecific activates septal and preoptic areas in naïve domestic chicks (Gallus gallus). Eur. J. Neurosci. 45, 423–432. doi: 10.1111/ejn.13484
Meador, K. J., and Loring, D. W. (2013). Risks of in utero exposure to valproate. JAMA 309, 1730–1731. doi: 10.1001/jama.2013.4001
Miura, M., Aoki, N., Yamaguchi, S., Homma, K.-J., and Matsushima, T. (2018). Thyroid hormone sensitizes the imprinting-associated induction of biological motion preference in domestic chicks. Front. Physiol. 9:1740. doi: 10.3389/fphys.2018.01740
Miura, M., and Matsushima, T. (2012). Preference for biological motion in domestic chicks: sex-dependent effect of early visual experience. Anim. Cogn. 15, 871–879. doi: 10.1007/s10071-012-0514-x
Miura, M., and Matsushima, T. (2016). Biological motion facilitates imprinting. Anim. Behav. 116, 171–180. doi: 10.1016/j.anbehav.2016.03.025
Miura, M., Nishi, D., and Matsushima, T. (2020). Combined predisposed preferences for colour and biological motion make robust development of social attachment through imprinting. Anim. Cogn. 23, 169–188. doi: 10.1007/s10071-019-01327-5
Mobbs, E. J., Mobbs, G. A., and Mobbs, A. E. D. (2016). Imprinting, latchment and displacement: a mini review of early instinctual behaviour in newborn infants influencing breastfeeding success. Acta Paediatr. 105, 24–30. doi: 10.1111/apa.13034
Mondloch, C. J., Lewis, T. L., Budreau, D. R., Maurer, D., Dannemiller, J. L., Stephens, B. R., et al. (1999). Face perception during early infancy. Psychol. Sci. 10, 419–422. doi: 10.1111/1467-9280.00179
Moore, S. J., Turnpenny, P., Quinn, A., Glover, S., Lloyd, D. J., Montogomery, T., et al. (2000). A clinical study of 57 children with fetal anticonvulsant syndrome. J. Med. Genet. 37, 489–497. doi: 10.1136/jmg.37.7.489
Morton, J. T., Jin, D. M., Mills, R. H., Shao, Y., Rahman, G., McDonald, D., et al. (2023). Multi-level analysis of the gut-brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 26, 1208–1217. doi: 10.1038/s41593-023-01361-0
Morton, J., and Johnson, M. H. (1991). CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychol. Rev. 98, 164–181. doi: 10.1037/0033-295X.98.2.164
Nagasawa, M., Mitsui, S., En, S., Ohtani, N., Ohta, M., Sakuma, Y., et al. (2015). Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 348, 333–336. doi: 10.1126/science.1261022
Nelson, S. B., and Valakh, V. (2015). Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87, 684–698. doi: 10.1016/j.neuron.2015.07.033
Nicolini, C., and Fahnestock, M. (2018). The valproic acid-induced rodent model of autism. Exp. Neurol. 299, 217–227. doi: 10.1016/j.expneurol.2017.04.017
Nishigori, H., Kagami, K., Takahashi, A., Tezuka, Y., Sanbe, A., and Nishigori, H. (2013). Impaired social behavior in chicks exposed to sodium valproate during the last week of embryogenesis. Psychopharmacol 227, 393–402. doi: 10.1007/s00213-013-2979-y
Nunes, A. R., Carreira, L., Anbalagan, S., Blechman, J., Levkowitz, G., and Oliveira, R. F. (2020). Perceptual mechanisms of social affiliation in zebrafish. Sci. Rep. 10:3642. doi: 10.1038/s41598-020-60154-8
O’Brown, N. M., Pfau, S. J., and Gu, C. (2018). Bridging barriers: a comparative look at the blood-brain barrier across organisms. Genes Dev. 32, 466–478. doi: 10.1101/gad.309823.117
Ongono, J. S., Béranger, R., Baghdadli, A., and Mortamais, M. (2020). Pesticides used in Europe and autism spectrum disorder risk: can novel exposure hypotheses be formulated beyond organophosphates, organochlorines, organochlorines, pyrethroids and carbamates? – a systematic review. Environ. Res. 187:209646. doi: 10.1016/j.envres.2020.109646
Pagani, M., Barsotti, N., Bertero, A., Trakoshis, S., Ulysse, L., Locarno, A., et al. (2021). mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat. Commun. 12:6084. doi: 10.1038/s41467-021-26131-z
Parker, K. J., Oztan, O., Libove, R. A., and Harden, A. Y. (2017). Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Nat. Acad. Sci. 114, 8119–8124. doi: 10.1073/pnas.1705521114
Pavlova, M. A. (2012). Biological motion processing as a hallmark of social cognition. Cereb. Cortex 22, 981–995. doi: 10.1093/cercor/bhr156
Pavlova, M. A., Guerreschi, M., Tagliavento, L., Gitti, F., Sokolov, A. N., Fallgatter, A. J., et al. (2017). Social cognition in autism: face tuning. Sci. Rep. 7:2734. doi: 10.1038/s41598-017-02790-1
Pegna, A. J., Khateb, A., Lazeyras, F., and Seghier, M. L. (2005). Discriminating emotional faces without primary visual cortices involves the right amygdala. Nat. Neurosci. 8, 24–25. doi: 10.1038/nn1364
Pfeffer, C. K., Stein, V., Keating, D. J., Maier, H., Rinke, I., Rudhard, Y., et al. (2009). NKCC1-dependent GABAergic excitation drives synaptic network maturation during early hippocampal development. J. Neurosci. 29, 3419–3430. doi: 10.1523/JNEUROSCI.1377-08.2009
Phiel, C. J., Zhang, F., Huang, E. Y., Guenther, M. G., Lazar, M. A., and Klein, P. S. (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 28, 36734–36741. doi: 10.1074/jbc.M101287200
Rasalam, A. D., Hailey, H., Moore, S. J., Turnpenny, P. D., Lloyd, D. J., and Dean, J. C. S. (2005). Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 47, 551–555. doi: 10.1017/S0012162205001076
Reid, V. M., Dunn, K., Young, R. J., Amu, J., Donovan, J., and Reissland, N. (2017). The human foetus preferentially engages with face-like visual stimuli. Curr. Biol. 27, 1825–1828. doi: 10.1016/j.cub.2017.05.044
Reissland, N., Wood, R., Einbeck, J., and Lane, A. (2020). Effects of maternal health on fetal visual preference for face-like compared non-face like light stimulation. Early Hum. Dev. 151:105227. doi: 10.1016/j.earlhumdev.2020.105227
Represa, A., and Ben-Ari, Y. (2005). Tropic actions of GABA on neuronal development. Trends Neurosci. 28, 278–283. doi: 10.1016/j.tins.2005.03.010
Rinaldi, T., Kulangara, K., Antoniello, K., and Markram, H. (2007). Elevated NMDA receptor levels and enhanced post-synaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc. Nat. Acad. Sci. 104, 13501–13506. doi: 10.1073/pnas.0704391104
Román, G. C., Ghassabian, A., Bongers-Schokking, J. J., Jaddoe, V. W. V., Hofman, A., de Rijke, Y. B., et al. (2013). Association of gestational maternal hypothyroxinemia and increased autism risk. Ann. Neurol. 74, 733–742. doi: 10.1002/ana.23976
Rosa-Salva, O., Farroni, T., Regolin, L., Vallortigara, G., and Johnson, M. H. (2011). The evolution of social orienting: evidence from chicks (Gallus gallus) and human newborns. PLoS One 6:e18802. doi: 10.1371/journal.pone.0018802
Rosa-Salva, O., Grassi, M., Lorenzi, E., Regolin, L., and Vallortigara, G. (2016). Spontaneous preference for visual cues of animacy in naïve domestic chicks: the case of speed changes. Cognition 157, 49–60. doi: 10.1016/j.cognition.2016.08.014
Rosa-Salva, O., Hernik, M., Broseghini, A., and Vallortigara, G. (2018). Visually-naïve chicks prefer agents that move as if constrained by a bilateral body-plan. Cognition 173, 106–114. doi: 10.1016/j.cognition.2018.01.004
Rosa-Salva, O., Hernik, M., Fabbroni, M., Lorenzi, E., and Vallortigara, G. (2023). Naïve chicks do not prefer objects with stable body orientation, though they may prefer behavioural variability. Anim. Cogn. 26, 1177–1189. doi: 10.1007/s10071-023-01764-3
Rosa-Salva, O., Mayer, U., and Vallortigara, G. (2015). Roots of a social brain: developmental models of emerging animacy-detecting mechanisms. Neurosci. Biobehav. Rev. 50, 150–168. doi: 10.1016/j.neubiorev.2014.12.015
Rosa-Salva, O., Mayer, U., and Vallortigara, G. (2019). Unlearned visual preferences for the head region in domestic chicks. PLoS One 14:e0222079. doi: 10.1371/journal.Pone.0222079
Rosa-Salva, O., Mayer, U., Versace, E., Hebert, M., Lemaire, B. S., and Vallortigara, G. (2021). Sensitive periods for social development: interactions between predisposed and learned mechanisms. Cognition 213:104552. doi: 10.1016/j.cognition.2020.104552
Rosa-Salva, O., Regolin, L., and Vallortigara, G. (2010). Faces are special for newly hatched chicks: evidence for inborn domain-specific mechanisms underlying spontaneous preferences for face-like stimuli. Dev. Sci. 13, 565–577. doi: 10.1111/j.1467-7687.2009.00914.x
Rossignol, D. A., and Frye, R. E. (2014). Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry 4:e360. doi: 10.1038/tp.2014.4
Rugani, R., Rosa-Salva, O., Regolin, L., and Vallortigara, G. (2015). Brain asymmetry modulates perception of biological motion in newborn chicks (Gallus gallus). Behav. Brain Res. 290, 1–7. doi: 10.1016/j.bbr.2015.04.032
Rutherford, M. D., Pennington, B. F., and Rogers, S. J. (2006). The perception of animacy in young children with autism. J. Autism Dev. Disord. 36, 983–992. doi: 10.1007/s10803-006-0136-8
Saheki, Y., Aoki, N., Homma, K. J., and Matsushima, T. (2022). Suppressive modulation of the chick forebrain network for imprinting by thyroid hormone: an in vitro study. Front. Physiol. 13:881947. doi: 10.3389/fphys.2022.881947
Sandin, S., Lichtenstein, P., Kuja-Halkola, R., Larsson, H., Hultman, C. M., and Reichenberg, A. (2014). The familial risk of autism. JAMA 311, 1770–1777. doi: 10.1001/jama.2014.4144
Sano, K., Isobe, T., Yang, J., Win-Shwe, T., Yoshikane, M., Nakayama, S. F., et al. (2016). In utero and lactational exposure to acetamiprid induces abnormalities in socio-sexual and anxiety-related behaviors of male mice. Front. Neurosci. 10:228. doi: 10.3389/fnins.2016.00228
Sato, A., Kasai, S., Kobayashi, T., Takamatsu, Y., Hino, O., Ikeda, K., et al. (2012). Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat. Commun. 3:1292. doi: 10.1038/ncomms2295
Seguchi, A., Mogi, K., and Izawa, E.-I. (2022). Measurement of urinary mesotocin in large-billed crows by enzyme-linked immunosorbent assay. J. Vet. Med. Sci. 84, 520–524. doi: 10.1292/jvms.21-0635
Sgadò, P., Rosa-Salva, O., Versace, E., and Vallortigara, G. (2018). Embryonic exposure to valproic acid impairs social predispositions of newly-hatched chicks. Sci. Rep. 8:5919. doi: 10.1038/s41598-018-24202-8
Shepherd, G. M., and Grillner, S. (2018). Handbook of brain microcircuit. Oxford: Oxford University Press.
Simion, F., and Di Giorgio, E. (2015). Face perception and processing in early infancy: inborn predispositions and developmental changes. Front. Psychol. 6:969. doi: 10.3389/fpsyg.2015.00969
Simion, F., Regolin, L., and Bulf, H. (2008). A predisposition for biological motion in the newborn baby. Proc. Nat. Acad. Sci. 105, 809–813. doi: 10.1073/pnas.0707021105
Spalding, D. (1873). Instinct with original observations on young animals. Br. J. Anim. Behav. 27, 282–293. doi: 10.1016/S0950-5601(54)80075-X
Spelke, E. S., and Kinzler, K. D. (2007). Core knowledge. Dev. Sci. 10, 89–96. doi: 10.1111/j.1467-7687.2007.00569.x
Sprengers, J. J., van Andel, D. M., Zuithoff, N. P. A., Keijzer-Veen, M. G., Schulp, A. J. A., Scheepers, F. E., et al. (2021). Bumetanide for core symptoms of autism spectrum disorder (BAMBI): a single center, double-blinded, participant-randomized, placebo-controlled, phase-2 superiority trial. J. Am. Acad. Child Adolesc. Psychiatry 60, 865–876. doi: 10.1016/j.jaac.2020.07.888
Stone, V. E., Baron-Cohen, S., Calder, A., Keane, J., and Young, A. (2003). Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia 41, 209–220. doi: 10.1016/S0028-3932(02)00151-3
Striedter, G. F. (2015). “Principles of brain evolution” in Evolutionary changes in brain region structure (Massachusetts, USA: Sinauer Associates Inc)
Sugita, Y. (2008). Face perception in monkeys reared with no exposure to faces. Proc. Nat. Acad. Sci. 105, 394–398. doi: 10.1073/pnas.0706079105
Suryanarayana, S. M., Brita, R. B., and Grillner, S. (2021b). The neural bases of vertebrate motor behaviour through the lens of evolution. Philos. Trans. R. Soc. B 377:20200521. doi: 10.1098/rstb.2020.0521
Suryanarayana, S. M., Pérez-Fernández, J., Brita, R. B., and Grillner, S. (2021a). The lamprey forebrain–evolutionary implications. Brain Behav. Evol. 2021:492. doi: 10.1159/000517492”
Szego, P. A., and Rutherford, M. D. (2008). Dissociating the perception of speed and the perception of animacy: a functional approach. Evol. Human Behav. 29, 335–342. doi: 10.1016/j.evolhumbehav.2008.04.002
Takemura, Y., Yamaguchi, S., Aoki, N., Miura, M., Homma, K.-J., and Matsushima, T. (2018). Gene expression of Dio2 (thyroid hormone converting enzyme) in telencephalon is linked with predisposed biological motion preference in domestic chicks. Behav. Brain Res. 349, 25–30. doi: 10.1016/j.bbr.2018.04.039
Tamietto, M., and de Gelder, B. (2010). Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 11, 697–709. doi: 10.1038/nrn2889
Tchaconas, A., and Adesman, A. (2013). Autism spectrum disorders: a pediatric overview. Curr. Opin. Pediatr. 25, 130–143. doi: 10.1097/MOP.0b013e32835c2b70
Torres-Pérez, J. V., Anagianni, S., Mech, A. M., Havelange, W., García-González, J., Fraser, S. E., et al. (2023). baz1b loss-of-function in zebrafish produces phenotypic alterations consistent with the domestication syndrome. iScience 26:105704. doi: 10.1016/j.isci.2022.105704
Trevisan, D. A., Roberts, N., Lin, C., and Birmingham, E. (2017). How do adults and teens with self-declared autism spectrum disorder experience eye contact? A quantitative analysis of first-hand account. PLoS One 12:e0188446. doi: 10.1371/journal.pone.0188446
Troje, N. F., and Westhoff, C. (2006). The inversion effect in biological motion perception: evidence for a “life detector”? Curr. Biol. 16, 821–824. doi: 10.1016/j.cub.2006.03.022
Uesaka, M., Kuratani, S., and Irie, N. (2020). The developmental hourglass model and recapitulation: an attempt to integrate the two models. J. Exp. Zool. B Mol. Dev. Evol. 338, 76–86. doi: 10.1002/jez.b.23027
Uesaka, M., Kuratani, S., Takeda, H., and Irie, N. (2019). Recapitulation-like developmental transition of chromatin accessibility in vertebrates. Zool. Lett. 5:33. doi: 10.1186/s40851-019-0148-9
Uzunova, G., Pallanti, S., and Hollander, E. (2016). Excitatory/inhibitory imbalance in autism spectrum disorders: implications for interventions and therapeutics. World J. Biol. Psychiatry 17, 174–186. doi: 10.3109/15622975.2015.1085597
Vallortigara, G. (2012). Core knowledge of object, number, and geometry: a comparative and neural approach. Cogn. Neuropsychol. 29, 213–236. doi: 10.1080/02643294.2012.654772
Vallortigara, G. (2021a). Born knowing. Imprinting and the Origins of Knowledge. Cambridge, MA: MIT Press.
Vallortigara, G. (2021b). “Animacy detection and its role on our attitudes towards other species” in Human/animal relationships in transformation–scientific, moral and legal perspectives. eds. S. Pollo and A. Vitale (London, UK: Palgrave)
Vallortigara, G., and Regolin, L. (2006). Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr. Biol. 16, R279–R280. doi: 10.1016/j.cub.2006.03.052
Vallortigara, G., Regolin, L., and Marconato, F. (2005). Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 3:e208. doi: 10.1371/journal.pbio.0030208
Vallortigara, G., and Rosa-Salva, O. (2017). “Toolkits for cognition: from core-knowledge to genes” in Neurophenome: Cutting-edge approaches and technologies in neurobehavioral genetics. ed. V. Tucci (New York: Wiley-Blackwell), 229–245.
Van Andel, D. M., Sprengers, J. J., Königs, M., de Jonge, M. V., and Bruining, H. (2023). Effects of bumetanide on neurocognitive functioning in children with autism spectrum disorder: secondary analysis of a randomized placebo-controlled trial. J. Autism. Dev. Disord. 2023:5841. doi: 10.1007/s10803-022-05841-3
Versace, E., Damini, S., and Stancher, G. (2020). Early preference for face-like stimuli in solitary species as revealed by tortoise hatchlings. Proc. Nat. Acad. Sci. 117, 24047–24049. doi: 10.1073/pnas.201145311
Versace, E., Fracasso, I., Baldan, G., Dalle, Z. A., and Vallortigara, G. (2017). Newborn chicks show inherited variability in early social predispositions for hen-like stimuli. Sci. Rep. 7:40296. doi: 10.1038/srep40296
Versace, E., Martinho-Truswell, A., Kacelnik, A., and Vallortigara, G. (2018). Priors in animal and artificial intelligence: where does learning begin? Trends Cogn. Sci. 22, 963–965. doi: 10.1016/j.tics.2018.07.005
Versace, E., Ragusa, M., and Vallortigara, G. (2019). A transient time window for early predispositions in newborn chicks. Sci. Rep. 9:18767. doi: 10.1038/s41598-019-55255-y
Versace, E., and Vallortigara, G. (2015). Origins of knowledge: insights from precocial species. Front. Behav. Neurosci. 9:338. doi: 10.3389/fnbeh.2015.00338
Von Ehrenstein, O. S., Ling, C., Cui, X., Cockburn, M., Park, A. S., Yu, F., et al. (2019). Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ 364:l962. doi: 10.1136/bmj.l962
Wang, T., Shan, L., Miao, C., Zhida, X., and Jia, F. (2021). Treatment effect of bumetanide in children with autism spectrum disorder: a systematic review and meta-analysis. Front. Psych. 12:751575. doi: 10.3389/fpsyt.2021.751575
Wang, Y., Wang, L., Xu, Q., Liu, D., Chen, L., Troje, N. F., et al. (2018). Heritable aspects of biological motion perception and its covariation with autistic traits. Proc. Nat. Acad. Sci. 115, 1937–1942. doi: 10.1073/pnas.1714655115
Watanabe, S., Kurotani, T., Oga, T., Noguchi, J., Isoda, R., Nakagami, A., et al. (2021). Functional and molecular characterization of a non-human primate model of autism spectrum disorder shows similarity with the human disease. Nat. Commun. 12:5388. doi: 10.1038/s41467-021-25487-6
Westerholz, S., de Lima, A. D., and Voigt, T. (2013). Thyroid hormone-dependent development of early cortical networks: temporal specificity and the contribution of trkB and mTOR pathways. Front. Cell Neurosci. 7:121. doi: 10.3389/fncel.2013.00121
Willner, P. (1984). The validity of animal models of depression. Psychopharmacology 83, 1–16. doi: 10.1007/BF00427414
Wing, L., and Gould, J. (1979). Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism. Dev. Disord. 9, 11–29. doi: 10.1007/BF01531288
Winterer, G. (2003). Valproate and GABAergic system effects. Neuropsychopharmacology 28, 2050–2051. doi: 10.1038/sj.npp.1300245
World Health Organization. (2023). International statistical classification of diseases and related health problems (ICD-11). Available at: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/437815624.
Yamaguchi, S., Aoki, N., Kitajima, T., Iikubo, E., Katagiri, S., Matsushima, T., et al. (2012). Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat. Commun. 3:1081. doi: 10.1038/ncomms2088
Yasue, M., Nakagami, A., Nakagami, K., Ichinohe, N., and Kawai, N. (2018). Inequity aversion is observed in common marmosets but not in marmoset models of autism induced by prenatal exposure to valproic acid. Behav. Brain Res. 343, 36–40. doi: 10.1016/j.bbr.2018.01.013
Keywords: autism spectrum disorder, domestic chicks, imprinting, biological motion, face perception, valproic acid, neonicotinoids, bumetanide
Citation: Matsushima T, Izumi T and Vallortigara G (2024) The domestic chick as an animal model of autism spectrum disorder: building adaptive social perceptions through prenatally formed predispositions. Front. Neurosci. 18:1279947. doi: 10.3389/fnins.2024.1279947
Edited by:
Mónica Santos, University of Coimbra, PortugalReviewed by:
Fernando Martinez-Garcia, University of Jaume I, SpainCarmen Agustín-Pavón, University of Valencia, Spain
Copyright © 2024 Matsushima, Izumi and Vallortigara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshiya Matsushima, bWF0c3VzaGltYXRvc2hpeWFAZ21haWwuY29t
 Toshiya Matsushima
Toshiya Matsushima Takeshi Izumi
Takeshi Izumi Giorgio Vallortigara
Giorgio Vallortigara