- 1Laboratory of Laser Sports Medicine, School of Physical Education and Sports Science, South China Normal University, Guangzhou, China
- 2Laboratory of Sports Rehabilitation, School of Physical Education and Sports Science, South China Normal University, Guangzhou, China
Fibromyalgia, a common and enduring pain disorder, ranks as the second most prevalent rheumatic disease after osteoarthritis. Recent years have witnessed successful treatment using non-invasive brain stimulation. Transcranial magnetic stimulation, transcranial direct current stimulation, and electroconvulsion therapy have shown promise in treating chronic pain. This article reviews the literature concerning non-invasive stimulation for fibromyalgia treatment, its mechanisms, and establishes a scientific basis for rehabilitation, and discusses the future directions for research and development prospects of these techniques are discussed.
1. Introduction
Fibromyalgia (FM) stands as a common chronic pain disorder (Giorgi et al., 2023), ranking second among rheumatic diseases after osteoarthritis (Clauw, 2014). Its prevalence remains consistent across races, approximately ranging from 2% to 4% (Sarzi-Puttini et al., 2020), with a female-to-male ratio of 7:1 (Neumann and Dan, 2003; Weir et al., 2006). FM is associated with various comorbidities (Robinson et al., 2004) and impacts global quality of life (Clauw, 2014; Chinn et al., 2016), though its pathogenesis remains partially understood (Sarzi-Puttini et al., 2020), implicating central sensitization, neurotransmitter imbalances, neurofunctional irregularities, and endocrine metabolic disturbances.
Pharmacological treatment remains predominant for FM; however, evidence suggests inadequacies in symptom alleviation and adverse reactions in some patients (Mease et al., 2013; Arnold et al., 2015; Gilron et al., 2016). Traditional analgesics, such as acetaminophen, yield inefficacy and severe side effects, leading some to consider antipsychotics for sleep improvement (Moldofsky et al., 1996). While quetiapine shows advantages in pain and sleep issues, its recommendation the level of evidence remains limited, suitable primarily for short-term FM treatment (Calandre et al., 2014; Walitt et al., 2016). A meta-analysis indicates partial symptom relief from drugs like amitriptyline, growth hormone, and sodium oxybate (Perrot and Russell, 2014). In summary, pharmacological treatments inadequately address FM symptoms, necessitating improved approaches (Rathore and Afridi, 2020). With the recognition of central pain system abnormalities in FM’s occurrence, non-invasive brain stimulation (NIBS) techniques emerge as potential non-pharmacological treatments (Fregni et al., 2006; Sampson et al., 2006; O'brien et al., 2018).
Non-invasive brain stimulation (NIBS), widely employed in treating depression, exhibits significant therapeutic effects. The co-occurrence of chronic pain and depression, likely stemming from functional impairment due to persistent pain (Thompson et al., 2016; Michaelides and Zis, 2019), underscores the potential of NIBS as a valuable intervention. Shared neuroanatomical structures and neurochemical phenomena further establish the link between depression and pain (Banks Sara and Kerns Robert, 1996), rendering NIBS not only applicable to depression but also to fibromyalgia (FM).
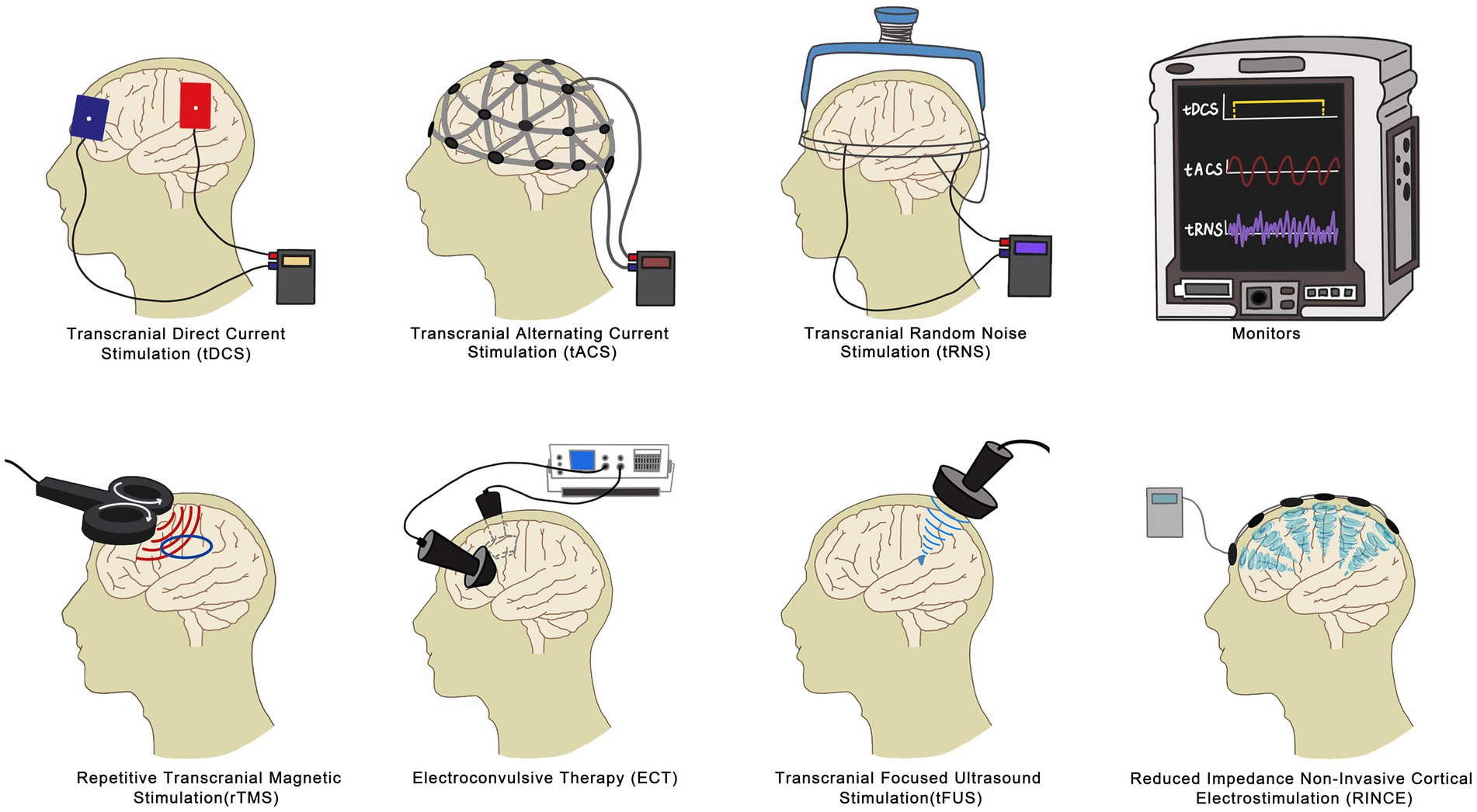
Among the commonly used and extensively studied NIBS techniques are repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), and electroconvulsive therapy (ECT). These techniques have shown efficacy in addressing FM symptoms, notably improving pain, fatigue, and sleep issues. However, there are other emerging branches of NIBS technology, such as Transcranial Alternating Current Stimulation (tACS), Reduced Impedance Non-Invasive Cortical Electrostimulation (RINCE), Transcranial Focused Ultrasound (tFUS), and Transcranial Random Noise Stimulation (tRNS). These methods possess their own unique characteristics and advantages. While they have fewer related studies compared to transcranial magnetic stimulation, tDCS, and electroconvulsive therapy, they hold significant promise for the future of FM relief and warrant further exploration. In this review, we focus on rTMS, tDCS, and ECT, delving into their mechanisms, clinical effects, and distinctions. This comprehensive examination establishes a solid scientific foundation for the rehabilitation of FM (Figure 1).
2. The role of repetitive transcranial magnetic stimulation in fibromyalgia
2.1. Introduction to repetitive transcranial magnetic stimulation (rTMS)
Repetitive Transcranial Magnetic Stimulation (rTMS) employs an inductor and a capacitor to generate changing magnetic fields. These fields induce a reverse current in specific brain regions, affecting neuronal functions and electrical activities. The therapeutic impacts of rTMS rely on stimulation parameters, intervention duration, and targeted brain areas. Stimulation intensity typically ranges from 80 to 120% of the participant’s resting motor threshold. rTMS encompasses both excitatory (high-frequency rTMS, HF-rTMS, ≥5 Hz) and inhibitory (low-frequency rTMS, LF-rTMS, ≤1 Hz) modes, provoking neuronal excitation or reduction, respectively (Rosen et al., 2009; Ansari et al., 2021). The multipulse properties of this technique induce physiological neurological changes, which can have lasting effects for up to 15 min or more, even after the stimulation ends (Chen et al., 1997). In the case of fibromyalgia patients, they continued to experience significant pain relief following 10 or more consecutive sessions of transcranial magnetic stimulation, in contrast to the sham stimulation and placebo groups (Boyer et al., 2014; Altas et al., 2019). Notably, analgesic outcomes can extend for weeks (Moisset and Lefaucheur, 2019). In contrast to conventional pharmacological methods, rTMS offers a safer, lower side-effect approach for fibromyalgia treatment. Moreover, rTMS addresses concurrent symptoms in fibromyalgia patients, such as sleep disruptions, fatigue, and functional impairments (Su et al., 2021).
2.2. Application and mechanism of rTMS in fibromyalgia treatment
2.2.1. Analgesic effects of rTMS via primary motor cortex and dorsolateral prefrontal cortex
Studies by Tamura et al. (2004) and Sampson et al. (2006) reveal that applying LF-rTMS (1 Hz) to the primary motor cortex (M1) and dorsolateral prefrontal cortex (DLPFC) respectively, alleviates acute pain and offers potential pain regulatory effects in fibromyalgia treatment. Intriguingly, HF-rTMS also contributes to pain relief. Altas’ research demonstrates HF-rTMS (10 Hz) applied to M1 and DLPFC enhances physical function and emotional well-being in fibromyalgia patients (Altas et al., 2019). Furthermore, both high-frequency HF-rTMS and low-frequency LF-rTMS on the right DLPFC manifest analgesic and antidepressant outcomes (Ansari et al., 2021). Collectively, both HF-rTMS and LF-rTMS alleviate fibromyalgia pain through M1 and DLPFC modulation.
2.2.2. Analgesic effects of rTMS via neurotransmitter modulation
rTMS engages cortical neurons, affecting cortical excitability and brain activity to modulate pain processing. Disrupted neurotransmitter concentrations, notably glutamate N-methyl-D-aspartate (NMDA) receptor, contribute to central sensitization and fibromyalgia pain development. rTMS potentially mitigates pain by top-down neurotransmitter modulation (Dall’agnol et al., 2014).
Numerous investigations affirm rTMS’s engagement with the endogenous opioid system, generating analgesic effects. Dysregulation of the endogenous opioid peptide system and diminished opioid receptors characterize fibromyalgia patients (Ansari et al., 2021). Neuroimaging exposes pain-processing regions like the cingulate gyrus, orbitofrontal and prefrontal cortex, thalamus, and periaqueductal gray matter (Peyron et al., 2000; Apkarian et al., 2005). rTMS regulates dopamine, serotonin, and opioid peptide receptors, restoring homeostasis. Further, rTMS on rats’ cerebellar cortex reduces metabolic glutamate receptors and Protein kinase C synthesis, influencing neuronal activity and calcium levels, thus providing analgesic effects (Lee et al., 2014). HF-rTMS might alleviate pain via nitric oxide synthase (nNOS) down-regulation.
FM pain associates with gamma-aminobutyric acid (GABA) and glutamate (Glu) mechanism dysregulation (Mhalla et al., 2010). rTMS of the ipsilateral motor cortex significantly elevates inhibitory neurotransmitter GABA, with the contralateral side experiencing a decrease (Clauw, 2014). Consequently, impaired GABAergic neurotransmission correlates with FM pain. Motor cortex-targeted rTMS reinstates GABAergic and glutamatergic system balance, curbing excitatory and bolstering inhibitory neurotransmitter activity for fibromyalgia pain relief. To summarize, rTMS stands as a secure, non-invasive cortical stimulation technique.
3. The role and mechanism of transcranial direct current stimulation treatment in fibromyalgia
3.1. Introduction to transcranial direct current stimulation (tDCS)
tDCS employs small, constant currents (1–2 mA) externally to modulate brain neuronal activity. It has gained diverse applications in brain injury rehabilitation, cognitive and emotional regulation, and chronic pain management. Renowned for its affordability and tolerance, tDCS stands as a widely used non-invasive brain stimulation method (Saavedra et al., 2015). The stimulation modes categorize as anodal (a-tDCS), cathodal (c-tDCS), or sham (s-tDCS), each differing in action (Liu et al., 2021). Notably, while transcranial electrical stimulation adjusts neural network activity, transcranial magnetic stimulation triggers neuronal firing via suprathreshold stimulation. At the neuronal level, tDCS achieves effects by polarizing the resting membrane potential through different polarities, thus modulating cortical excitability. Notably, membrane polarization is the primary mechanism for transcranial electrical stimulation, with a-tDCS heightening cortical excitability and c-tDCS inhibiting it. The evidence-based recommendation for the application of tDCS in fibromyalgia is categorized as level B, according to the Standards of the European Federation of Neurological Societies (Lefaucheur et al., 2017).
3.2. Application and mechanism of tDCS in fibromyalgia treatment
3.2.1. tDCS and analgesic effects via targeting brain regions
Distinct tDCS types can be employed to mitigate fibromyalgia (FM) pain by focusing on diverse brain regions. Application of c-tDCS on S1, M1, or DLPFC reduces brain hyperexcitability, increasing pain thresholds and reducing sensitivity. However, targeting DLPFC for pain relief in FM patients is somewhat debated (Fregni et al., 2006). Nevertheless, research suggests c-tDCS applied to DLPFC could alleviate pain (Mariano et al., 2015). Valle’s study indicates a-tDCS on left M1 is more effective than left DLPFC in producing analgesic effects (Valle et al., 2009). Additionally, Fregni et al. (2006) find that a-tDCS targeting M1 substantially improves FM pain, sustaining analgesic effects for over 3 weeks. This suggests that M1 targeting is more effective. Still, the analgesic effects of c-tDCS on M1 remain debated (Steffen et al., 2018). Conversely, Roizenblatt et al. (2007) propose tDCS on M1 improves sleep in FM patients. Other studies suggest M1 stimulation could influence thalamus and basal ganglia function, thereby alleviating FM pain (Khedr et al., 2017). In summary, targeting M1 with tDCS seems more effective in alleviating FM pain symptoms.
3.2.2. tDCS and analgesic effects via neurotransmitter modulation
The analgesic effects of tDCS relate to neurotransmitter alterations. Studies underscore NMDA receptor activation’s role in pain generation and maintenance, a potential mechanism in fibromyalgia (FM). NMDA receptor non-competitive antagonist MK-801 reduces capsaicin-induced heat hyperalgesia and mechanical hypersensitivity. During tDCS, changes in membrane potential influence NMDA receptor expression and enhance γ-aminobutyric acid (GABA) release, thus easing pain (Foerster et al., 2015). N-acetylaspartate (NAA), an abundant brain metabolite, serves as a neuroprotective neurotransmitter, significant in pain management. Bradley R. Foerster et al. find tDCS decreases clinical pain scores, reduces glutamate and glutamine levels, and increases γ-GABA, hinting at enhanced neurotransmitter modulation to ease pain. Furthermore, tDCS elevates N-acetylaspartate levels, overall impacting neurotransmitters for pain relief. In addition, Kadetoff et al. (2012) observed elevated interleukin-8 (IL-8) in the cerebrospinal fluid of fibromyalgia (FM) patients, which is likely pivotal in the central sensitization process.
4. The role of electroconvulsive therapy in fibromyalgia treatment
4.1. Introduction to electroconvulsive therapy (ECT)
ECT involves applying electrical potential to the brain via stimulation electrodes affixed to the scalp, creating diverse electric fields based on electrode positioning. This induces synchronized neural cell oscillations and simultaneous autonomic nervous system excitation across extensive brain areas (Couvreur et al., 1989; Peterchev et al., 2010). Since Cerletti and Bini’s initial use of ECT for schizophrenia treatment in 1938(Kalinowsky, 1986), physicians have applied it to various conditions. Rasmussen et al. reviewed ECT’s pain treatment literature, concluding its efficacy in chronic pain management based on synthesized case reports spanning decades (Rasmussen and Rummans, 2002). Moreover, reports indicate ECT’s analgesic effects across different pain syndromes (Canavero and Bonicalzi, 2001; Wasan et al., 2004).
4.2. Application and mechanism of ECT in fibromyalgia treatment
4.2.1. ECT and analgesic effects via enhanced cerebral blood flow
Usui et al.’s study tracked regional cerebral blood flow (rCBF) changes pre and post ECT, revealing thalamic rCBF increase in fibromyalgia (FM) patients. This correlated with reduced tender points and pain scores (visual analog scale, VAS), indicating significant pain relief possibly tied to augmented rCBF (Usui et al., 2006). Neuroimaging verifies the prefrontal cortex, anterior cingulate cortex, insula, and amygdala’s roles in pain and emotion regulation (Rainville, 2002). Severe depression and chronic pain patients exhibit abnormal blood flow in these regions (Marsh et al., 1996), which ECT can normalize in severe depression (Elizagarate et al., 2001) and chronic pain (Fukui et al., 2002b). While some studies suggest ECT’s efficacy in low thalamic blood flow neuropathic pain (Fukui et al., 2002a, 2002b), other studies report mixed results (Salmon et al., 1988, McCance et al., 1996). Notably, ECT could raise brain-derived neurotrophic factor (BDNF) (Okamoto et al., 2008; Piccinni et al., 2009), pivotal in antidepressant actions through neural plasticity and linked to pain improvement.
4.2.2. ECT and analgesic effects via neurotransmitter modulation
ECT heightens responsiveness of serotonin, norepinephrine, and dopamine systems pivotal in central pain processing (King and Liston, 1990). ECT can stimulate inhibitory pathways by activating these neurotransmitter systems (Newman et al., 1998). Post-ECT treatment, neurotransmitter release increases, bolstering serotonin, norepinephrine, dopamine, and others. This enhances peripheral stimulus signal processing through central nervous system descending pathways, reinforcing brain inhibitory pain pathways (Canavero et al., 1994; Wasan et al., 2004).
ECT fosters central neurotransmitter systems’ new equilibrium through electrical stimulation. Past studies indicate repeated ECT boosts plasma beta-endorphin levels (Abrams, 2002). Insufficient beta-endorphin levels correlate with pain hypersensitivity. ECT might relieve fibromyalgia pain by promoting endorphin production, reducing pain sensitivity, and achieving analgesic effects. Okabe et al. note ECT’s potential to increase neuropeptide Y (NPY) expression (Okabe et al., 2010). NPY, a hormone with central and peripheral presence, plays diverse roles, including pain modulation. The mechanism by which ECT treats pain in patients with FM may involve promoting elevated NPY expression, thereby reducing pain sensitivity in patients and achieving analgesic effects.
5. Profiles of several non-invasive brain neurostimulation techniques
5.1. Transcranial alternating current stimulation (tACS)
tACS, a non-invasive brain stimulation technique, modulates neural activity by applying sinusoidal alternating current to the scalp, thus generating an electric field within the brain (Wischnewski et al., 2023). Much like direct current stimulation (tDCS), tACS effectively modulates cortical excitability (Sabel et al., 2020). However, when compared to tDCS, tACS demonstrates superior efficacy in precisely directing endogenous brain oscillations. It has the unique capability to mimic the natural alternation of brain oscillations and induce long-term synaptic plasticity, thereby effectively regulating brain function.
Despite being a more widely adopted non-invasive brain stimulation method, tACS is still relatively nascent in its development, particularly in the context of fibromyalgia. In recent years, researchers have successfully addressed the traditional limitations of tACS, which struggled with the precise localization of specific brain regions. They have accomplished this through innovative techniques, including high-definition tACS, phase-shifting tACS, amplitude-modulated tACS, temporal interference (TI) techniques, and intersecting short pulses (ISPs) (Wu et al., 2021). These advancements have propelled tACS into a more advanced stage of development and have expanded its potential in the treatment and alleviation of fibromyalgia symptoms.
5.2. Reduced impedance non-invasive cortical electrostimulation (RINCE)
RINCE is among the less explored methods of electrical stimulation within the realm of non-invasive brain stimulation. In RINCE, electrodes attached to the patient’s scalp generate a specific current frequency, allowing the current to penetrate deeper into the cortex by reducing the impedance of the skin and skull (O'Connell et al., 2018), potentially leading to improved stimulation effects. There are limited reports on RINCE therapy, and its adverse effects include transient mild head discomfort and localized cutaneous reactions (Szymoniuk et al., 2023). In an RCT investigating RINCE therapy for fibromyalgia control, it was found that mean pressure points and pressure pain thresholds improved in the active treatment group compared to the sham treatment group (Hargrove et al., 2012). Patients with fibromyalgia reported decreased pain VAS scores following RINCE treatment. Although there is limited current research on RINCE, these results are statistically and clinically significant, suggesting that RINCE offers advantages and potential for development in fibromyalgia treatment.
5.3. Transcranial focused ultrasound (tFUS)
tFUS emerges as a significant non-invasive brain stimulation technique (Aubry and Tanter, 2016). While it may not have received the same level of research attention as rTMS, tDCS, or other established methods, it boasts remarkable spatial precision, capable of targeting and stimulating deep brain regions with millimeter accuracy. This technique employs piezoelectric-element transducers that emit ultrasound pulses, effectively reaching and stimulating deep brain areas.
5.4. Transcranial random noise stimulation (tRNS)
tRNS, a non-invasive brain stimulation method, is renowned for its reduced discomfort levels in comparison to other tES techniques. In an analgesic study, tRNS demonstrated the capability to induce both immediate and sustained analgesia when applied to area m1 of the cerebral cortex. This analgesic effect was attributed to a reduction in pain anticipation (Yao et al., 2021). Furthermore, it exhibits potential for enhancing behaviors through long-term neuroplasticity effects (van der Groen et al., 2022). Nonetheless, the precise physiological mechanisms responsible for tRNS’s impact on nerves remain elusive.
6. Discussion
Limited studies have investigated the effects of tRNS, RINCE, and tFUS on fibromyalgia compared to tACS. In a double-blind randomized crossover study conducted by Bernardi et al., participants were randomly assigned to receive either transcranial alternating current stimulation (tACS) or random noise stimulation (RNS) treatment 5 days a week for 2 weeks. The intervention group received tACS, while the control group received RNS. The study defined three measurement time points: T0 (baseline), T1 (post-stimulation), and T2 (1 month or 4 weeks after stimulation) (Bernardi et al., 2021).
The results showed that, in comparison to RNS, tACS led to an increase in EEG alpha1 activity [(8–10) Hz] at T1, a reduction in pain symptoms assessed through visual analog scales at T1, and enhancements in self-reported cognitive skills and neuropsychological scores at both T1 and T2. Notably, improvements were observed in both the tACS and RNS groups after receiving treatment. However, it’s important to note that this study had a small sample size and potential bias due to variations in participants’ medication regimens. To address this limitation, future studies should aim to validate this treatment approach using larger samples. Additionally, maintaining consistent medication regimens among study participants throughout the research is essential to mitigate potential bias from concurrent medication use.
7. Conclusion and future perspectives
The emergence of non-invasive brain stimulation techniques has revolutionized chronic pain treatment. Methods like rTMS, tDCS, and ECT have proven effective in addressing conditions such as fibromyalgia, showcasing considerable potential. Lesser-known techniques like rINCE, tFUS, tACS, and RNS, while having fewer studies behind them, exhibit promising traits and are poised for development. This paper delves into the intricacies of non-invasive brain stimulation mechanisms, identifies technical challenges, and outlines future research directions.
However, research on the application of non-invasive brain stimulation for fibromyalgia and analogous chronic pain conditions is still in its infancy. Several crucial considerations should guide our future endeavors:
1. The current clinical sample size for non-invasive brain stimulation in fibromyalgia remains relatively small. It is imperative to conduct comprehensive, large-scale, and standardized randomized controlled trials to determine optimal stimulation parameters and models for various non-invasive brain stimulation techniques (Hou et al., 2016).
2. Enhancing the credibility of experimental data necessitates supplementing subjective rating scales with objective measures. Furthermore, maintaining the integrity of participant blinding is of paramount importance.
3. An in-depth exploration is vital to pinpoint precise stimulation sites and understand how distinct parameters and target locations influence treatment outcomes. Utilizing neuroimaging and high-resolution electroencephalography can enhance localization, while integrating neurophysiological markers and diverse imaging modalities can refine the determination of stimulation intensity and duration.
4. Recognizing the synergy between non-invasive brain stimulation and pharmacology in fibromyalgia treatment is essential, given the frequent combination of these approaches. This synergy holds the promise of heightened therapeutic efficacy and adherence to rigorous technical standards, optimizing pain relief for fibromyalgia patients.
5. Currently, RINCE, tACS, tFUS, and tRNS, as branches of NIBS technology, have a relatively small number of studies compared with rTMS, tDCS, and ECT, and subsequent researchers have paid more attention to their development and deeper mechanistic studies. These developments are not only conducive to the overall development of non-invasive brain stimulation but will also play a greater potential role in the treatment of fibromyalgia disorders.
6. In light of the unique analgesic mechanisms of various non-invasive stimulation techniques, future research should explore their integration. This approach can overcome individual limitations, paving the way for personalized non-invasive stimulation methods and precision medicine. Such advancements will significantly contribute to the treatment of fibromyalgia and chronic pain, ultimately enhancing patient well-being and quality of life.
Author contributions
J-HZ: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Validation. JL: Conceptualization, Supervision, Validation, Writing – review & editing. Z-WY: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altas, E. U., Askin, A., Besiroglu, L., and Tosun, A. (2019). Is high-frequency repetitive transcranial magnetic stimulation of the left primary motor cortex superior to the stimulation of the left dorsolateral prefrontal cortex in fibromyalgia syndrome? Somatosens. Mot. Res. 36, 56–62. doi: 10.1080/08990220.2019.1587400
Ansari, A. H., Pal, A., Ramamurthy, A., Kabat, M., Jain, S., and Kumar, S. (2021). Fibromyalgia pain and depression: an update on the role of repetitive transcranial magnetic stimulation. ACS Chem. Neurosci. 12, 256–270. doi: 10.1021/acschemneuro.0c00785
Apkarian, A. V., Bushnell, M. C., Treede, R. D., and Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484. doi: 10.1016/j.ejpain.2004.11.001
Arnold, L. M., Sarzi-Puttini, P., Arsenault, P., Khan, T., Bhadra Brown, P., Clair, A., et al. (2015). Efficacy and safety of pregabalin in patients with fibromyalgia and comorbid depression taking concurrent antidepressant medication: a randomized, placebo-controlled study. J. Rheumatol. 42, 1237–1244. doi: 10.3899/jrheum.141196
Aubry, J. F., and Tanter, M. (2016). MR-guided transcranial focused ultrasound. Adv. Exp. Med. Biol. 880, 97–111. doi: 10.1007/978-3-319-22536-4_6
Banks Sara, M., and Kerns Robert, D. (1996). Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol. Bull. 119, 95–110. doi: 10.1037/0033-2909.119.1.95
Bernardi, L., Bertuccelli, M., Formaggio, E., Rubega, M., Bosco, G., Tenconi, E., et al. (2021). Beyond physiotherapy and pharmacological treatment for fibromyalgia syndrome: tailored tACS as a new therapeutic tool. Eur. Arch. Psychiatry Clin. Neurosci. 271, 199–210. doi: 10.1007/s00406-020-01214-y
Boyer, L., Dousset, A., Roussel, P., Dossetto, N., Cammilleri, S., Piano, V., et al. (2014). rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology 82, 1231–1238. doi: 10.1212/WNL.0000000000000280
Calandre, E. P., Rico-Villademoros, F., Galán, J., Molina-Barea, R., and Morillas-Arques, P. (2014). Quetiapine extended-release (Seroquel-Xr) versus amitriptyline monotherapy for treating patients with fibromyalgia: a 16-week, randomized, flexible-dose, open-label trial. Psychopharmacology 231, 2525–2531. doi: 10.1007/s00213-013-3422-0
Canavero, S., and Bonicalzi, V. (2001). Electroconvulsive therapy and pain. Pain 89, 301–302. doi: 10.1016/S0304-3959(00)00377-8
Canavero, S., Bonicalzi, V., and Pagni, C. A. (1994). Chronic pain, electroconvulsive therapy and reverberation. Pain 59:423. doi: 10.1016/0304-3959(94)90029-9
Chen, R., Classen, J., Gerloff, C., Celnik, P., Wassermann, E. M., Hallett, M., et al. (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 5, 1398–1403.
Chinn, S., Caldwell, W., and Gritsenko, K. (2016). Fibromyalgia pathogenesis and treatment options update. Curr. Pain Headache Rep. 20:25. doi: 10.1007/s11916-016-0556-x
Clauw, D. J. (2014). Fibromyalgia: a clinical review. JAMA 311, 1547–1555. doi: 10.1001/jama.2014.3266
Couvreur, V., Ansseau, M., and Franck, G. (1989). Electroconvulsive therapy and its mechanism of action. Acta Psychiatr. Belg. 89, 96–109.
Dall’agnol, L., Medeiros, L. F., Torres, I. L., Deitos, A., Brietzke, A., Laste, G., et al. (2014). Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J. Pain 15, 845–855. doi: 10.1016/j.jpain.2014.05.001
Elizagarate, E., Cortes, J., Gonzalez Pinto, A., Gutierrez, M., Alonso, I., Alcorta, P., et al. (2001). Study of the influence of electroconvulsive therapy on the regional cerebral blood flow by HMPAO-SPECT. J. Affect. Disord. 65, 55–59. doi: 10.1016/S0165-0327(00)00200-7
Foerster, B. R., Nascimento, T. D., Deboer, M., Bender, M. A., Rice, I. C., Truong, D. Q., et al. (2015). Brief report: excitatory and inhibitory brain metabolites as targets of motor cortex transcranial direct current stimulation therapy and predictors of its efficacy in fibromyalgia. Arthritis Rheumatol. 67, 576–581. doi: 10.1002/art.38945
Fregni, F., Gimenes, R., Valle, A. C., Ferreira, M. J., Rocha, R. R., Natalle, L., et al. (2006). A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 54, 3988–3998. doi: 10.1002/art.22195
Fukui, S., Shigemori, S., and Nosaka, S. (2002a). Central pain associated with low thalamic blood flow treated by electroconvulsive therapy. J. Anesth. 16, 255–257. doi: 10.1007/s005400200036
Fukui, S., Shigemori, S., Yoshimura, A., and Nosaka, S. (2002b). Chronic pain with beneficial response to electroconvulsive therapy and regional cerebral blood flow changes assessed by single photon emission computed tomography. Reg. Anesth. Pain Med. 27, 211–213. doi: 10.1053/rapm.2002.31205
Gilron, I., Chaparro, L. E., Tu, D., Holden, R. R., Milev, R., Towheed, T., et al. (2016). Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain 157, 1532–1540. doi: 10.1097/j.pain.0000000000000558
Giorgi, V., Bazzichi, L., Batticciotto, A., Pellegrino, G., Di Franco, M., Sirotti, S., et al. (2023). Fibromyalgia: one year in review 2023. Clin. Exp. Rheumatol. 41, 1205–1213. doi: 10.55563/clinexprheumatol/257e99
Hargrove, J. B., Bennett, R. M., Simons, D. G., Smith, S. J., Nagpal, S., and Deering, D. E. (2012). A randomized placebo-controlled study of noninvasive cortical electrostimulation in the treatment of fibromyalgia patients. Pain Med. 13, 115–124. doi: 10.1111/j.1526-4637.2011.01292.x
Hou, W. H., Wang, T. Y., and Kang, J. H. (2016). The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology (Oxford) 55, 1507–1517. doi: 10.1093/rheumatology/kew205
Kadetoff, D., Lampa, J., Westman, M., Andersson, M., and Kosek, E. (2012). Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 242, 33–38. doi: 10.1016/j.jneuroim.2011.10.013
Kalinowsky, L. B. (1986). History of convulsive therapy. Ann. N. Y. Acad. Sci. 462, 1–4. doi: 10.1111/j.1749-6632.1986.tb51233.x
Khedr, E. M., Omran, E., Ismail, N. M., El-Hammady, D. H., Goma, S. H., Kotb, H., et al. (2017). Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul. 10, 893–901. doi: 10.1016/j.brs.2017.06.006
King, B. H., and Liston, E. H. (1990). Proposals for the mechanism of action of convulsive therapy: a synthesis. Biol. Psychiatry 27, 76–94. doi: 10.1016/0006-3223(90)90022-T
Lee, S. A., Oh, B. M., Kim, S. J., and Paik, N. J. (2014). The molecular evidence of neural plasticity induced by cerebellar repetitive transcranial magnetic stimulation in the rat brain: a preliminary report. Neurosci. Lett. 575, 47–52. doi: 10.1016/j.neulet.2014.05.029
Lefaucheur, J. P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92. doi: 10.1016/j.clinph.2016.10.087
Liu, X., Qiu, F., Hou, L., and Wang, X. (2021). Review of noninvasive or minimally invasive deep brain stimulation. Front. Behav. Neurosci. 15:820017. doi: 10.3389/fnbeh.2021.820017
Mariano, T. Y., Mascha, V. T. W., Garnaat, S. L., Rasmussen, S. A., and Greenberg, B. D. (2015). Transcranial direct current stimulation (tDCS) targeting left dorsolateral prefrontal cortex modulates task-induced acute pain in healthy volunteers. Pain Med. 17, 737–745. doi: 10.1093/pm/pnv042
Marsh, L., Lauriello, J., Sullivan, E. V., and Pfefferbaum, A. (1996). Neuroimaging in psychiatric disorders. In: Neuroimaging II, Ed. ED Bigler. New York: Plenum Press. 73–125. doi: 10.1007/978-1-4899-1769-0_4
Mccance, S., Hawton, K., Brighouse, D., and Glynn, C. (1996). Does electroconvulsive therapy (ECT) have any role in the management of intractable thalamic pain? Pain 68, 129–131. doi: 10.1016/S0304-3959(96)03169-7
Mease, P. J., Farmer, M. V., Palmer, R. H., Gendreau, R. M., Trugman, J. M., and Wang, Y. (2013). Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther. Adv. Musculoskelet Dis. 5, 113–126. doi: 10.1177/1759720X13483894
Mhalla, A., De Andrade, D. C., Baudic, S., Perrot, S., and Bouhassira, D. (2010). Alteration of cortical excitability in patients with fibromyalgia. Pain 149, 495–500. doi: 10.1016/j.pain.2010.03.009
Michaelides, A., and Zis, P. (2019). Depression, anxiety and acute pain: links and management challenges. Postgrad. Med. 131, 438–444. doi: 10.1080/00325481.2019.1663705
Moisset, X., and Lefaucheur, J. P. (2019). Non pharmacological treatment for neuropathic pain: invasive and non-invasive cortical stimulation. Rev. Neurol. (Paris) 175, 51–58. doi: 10.1016/j.neurol.2018.09.014
Moldofsky, H., Lue, F. A., Mously, C., Roth-Schechter, B., and Reynolds, W. J. (1996). The effect of zolpidem in patients with fibromyalgia: a dose ranging, double blind, placebo controlled, modified crossover study. J. Rheumatol. 23, 529–533.
Neumann, L., and Dan, B. (2003). Epidemiology of fibromyalgia. Curr. Pain Headache Rep. 7, 362–368. doi: 10.1007/s11916-003-0035-z
Newman, M. E., Gur, E., Shapira, B., and Lerer, B. (1998). Neurochemical mechanisms of action of ECS: evidence from in vivo studies. J. ECT 14, 153–171. doi: 10.1097/00124509-199809000-00002
O'brien, T. A., Bertolucci, F., Torrealba-Acosta, G., Huerta, R., Fregni, F., and Thibaut, A. (2018). Non-invasive brain stimulation for fine motor improvement after stroke: a meta-analysis. Eur. J. Neurol. 25, 1017–1026. doi: 10.1111/ene.13643
O'connell, N. E., Marston, L., Spencer, S., Desouza, L. H., and Wand, B. M. (2018). Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 4:Cd008208. doi: 10.1002/14651858.CD008208.pub4
Okabe, T., Sato, C., Matsumoto, K., Ozawa, H., and Sakamoto, A. (2010). Electroconvulsive stimulation (ECS) increases the expression of neuropeptide Y (NPY) in rat brains in a model of neuropathic pain: a quantitative real-time polymerase chain reaction (RT-PCR) study. Pain Med. 10, 1460–1467. doi: 10.1111/j.1526-4637.2009.00678.x
Okamoto, T., Yoshimura, R., Ikenouchi-Sugita, A., Hori, H., Umene-Nakano, W., Inoue, Y., et al. (2008). Efficacy of electroconvulsive therapy is associated with changing blood levels of homovanillic acid and brain-derived neurotrophic factor (BDNF) in refractory depressed patients: a pilot study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 32, 1185–1190. doi: 10.1016/j.pnpbp.2008.02.009
Perrot, S., and Russell, I. J. (2014). More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur. J Pain 18, 1067–1080. doi: 10.1002/ejp.564
Peterchev, A. V., Rosa, M. A., Deng, Z. D., Prudic, J., and Lisanby, S. H. (2010). Electroconvulsive therapy stimulus parameters: rethinking dosage. J. ECT 26, 159–174. doi: 10.1097/YCT.0b013e3181e48165
Peyron, R., Laurent, B., and García-Larrea, L. (2000). Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol. Clin. 30, 263–288. doi: 10.1016/S0987-7053(00)00227-6
Piccinni, A., Debbio, A. D., Medda, P., Bianchi, C., Roncaglia, I., Veltri, A., et al. (2009). Plasma brain-derived neurotrophic factor in treatment-resistant depressed patients receiving electroconvulsive therapy. Eur. Neuropsychopharmacol. 19, 349–355. doi: 10.1016/j.euroneuro.2009.01.002
Rainville, P. (2002). Brain mechanisms of pain affect and pain modulation. Curr. Opin. Neurobiol. 12, 195–204. doi: 10.1016/S0959-4388(02)00313-6
Rasmussen, K. G., and Rummans, T. A. (2002). Electroconvulsive therapy in the management of chronic pain. Curr. Pain Headache Rep. 6, 17–22. doi: 10.1007/s11916-002-0019-4
Rathore, F. A., and Afridi, A. (2020). Is combination pharmacotherapy effective for management of fibromyalgia in adults? A Cochrane review summary with commentary. J. Musculoskelet. Neuronal Interact. 20, 297–300.
Robinson, R. L., Birnbaum, H. G., Morley, M. A., Sisitsky, T., Greenberg, P. E., and Wolfe, F. (2004). Depression and fibromyalgia: treatment and cost when diagnosed separately or concurrently. J. Rheumatol. 31, 1621–1629.
Roizenblatt, S., Fregni, F., Gimenez, R., Wetzel, T., Rigonatti, S. P., Tufik, S., et al. (2007). Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled study. Pain Pract. 7, 297–306. doi: 10.1111/j.1533-2500.2007.00152.x
Rosen, A. C., Ramkumar, M., Nguyen, T., and Hoeft, F. (2009). Noninvasive transcranial brain stimulation and pain. Curr. Pain Headache Rep. 13, 12–17. doi: 10.1007/s11916-009-0004-2
Saavedra, L. C., Gebodh, N., Bikson, M., Diaz-Cruz, C., and Fregni, F. (2015). Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase ii open-label dose optimization. J Pain 17:14. doi: 10.1016/j.jpain.2015.09.009
Sabel, B. A., Thut, G., Haueisen, J., Henrich-Noack, P., Herrmann, C. S., Hunold, A., et al. (2020). Vision modulation, plasticity and restoration using non-invasive brain stimulation – an IFCN-sponsored review. Clin. Neurophysiol. 131, 887–911. doi: 10.1016/j.clinph.2020.01.008
Salmon, J. B., Hanna, M. H., Williams, M., Toone, B., and Wheeler, M. (1988). Thalamic pain--the effect of electroconvulsive therapy (ECT). Pain 33, 67–71. doi: 10.1016/0304-3959(88)90205-9
Sampson, S. M., Rome, J. D., and Rummans, T. A. (2006). Slow-frequency RTMS reduces fibromyalgia pain. Pain Med. 7, 115–118. doi: 10.1111/j.1526-4637.2006.00106.x
Sarzi-Puttini, P., Giorgi, V., Marotto, D., and Atzeni, F. (2020). Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 16, 645–660. doi: 10.1038/s41584-020-00506-w
Steffen, N., Josephine, B., Nina, T., Christoph, K., and Diener, H.-C. (2018). Polarity-specific modulation of pain processing by transcranial direct current stimulation – a blinded longitudinal FMRI study. J. Headache Pain 19:99. doi: 10.1186/s10194-018-0924-5
Su, Y.-C., Guo, Y.-H., Hsieh, P.-C., and Lin, Y.-C. (2021). Efficacy of repetitive transcranial magnetic stimulation in fibromyalgia: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 10:4669. doi: 10.3390/jcm10204669
Szymoniuk, M., Chin, J. H., Domagalski, Ł., Biszewski, M., JóŹwik, K., and Kamieniak, P. (2023). Brain stimulation for chronic pain management: a narrative review of analgesic mechanisms and clinical evidence. Neurosurg. Rev. 46:127. doi: 10.1007/s10143-023-02032-1
Tamura, Y., Okabe, S., Ohnishi, T., D, N. S., Arai, N., Mochio, S., et al. (2004). Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain 107, 107–115. doi: 10.1016/j.pain.2003.10.011
Thompson, T., Correll, C. U., Gallop, K., Vancampfort, D., and Stubbs, B. (2016). Is pain perception altered in people with depression? A systematic review and Meta-analysis of experimental pain research. J. Pain 17, 1257–1272. doi: 10.1016/j.jpain.2016.08.007
Usui, C., Doi, N., Nishioka, M., Komatsu, H., Yamamoto, R., Ohkubo, T., et al. (2006). Electroconvulsive therapy improves severe pain associated with fibromyalgia. Pain 121, 276–280. doi: 10.1016/j.pain.2005.12.025
Valle, A., Roizenblatt, S., Botte, S., Zaghi, S., Riberto, M., Tufik, S., et al. (2009). Efficacy of anodal transcranial direct current stimulation (TDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag 2, 353–361.
Van Der Groen, O., Potok, W., Wenderoth, N., Edwards, G., Mattingley, J. B., and Edwards, D. (2022). Using noise for the better: the effects of transcranial random noise stimulation on the brain and behavior. Neurosci. Biobehav. Rev. 138:104702. doi: 10.1016/j.neubiorev.2022.104702
Walitt, B., Klose, P., Üçeyler, N., Phillips, T., and Häuser, W. (2016). Antipsychotics for fibromyalgia in adults. Cochrane Database Syst. Rev. 6:Cd011804. doi: 10.1002/14651858.CD011804.pub2
Wasan, A. D., Artin, K., and Clark, M. R. (2004). A case-matching study of the analgesic properties of electroconvulsive therapy. Pain Med. 5, 50–58. doi: 10.1111/j.1526-4637.2004.04006.x
Weir, P. T., Harlan, G. A., Nkoy, F. L., Jones, S. S., Hegmann, K. T., Gren, L. H., et al. (2006). The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on international classification of diseases, 9th revision codes. J. Clin. Rheumatol. 12, 124–128. doi: 10.1097/01.rhu.0000221817.46231.18
Wischnewski, M., Alekseichuk, I., and Opitz, A. (2023). Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cogn. Sci. 27, 189–205. doi: 10.1016/j.tics.2022.11.013
Wu, L., Liu, T., and Wang, J. (2021). Improving the effect of transcranial alternating current stimulation (TACS): a systematic review. Front. Hum. Neurosci. 15:652393. doi: 10.3389/fnhum.2021.652393
Keywords: non-invasive brain stimulation (NIBS), fibromyalgia (FM), repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), electroconvulsive therapy (ECT), transcranial alternating current stimulation (tACS), reduced impedance non-invasive cortical electrostimulation (RINCE), transcranial focused ultrasound (tFUS)
Citation: Zhang J-H, Liang J and Yang Z-W (2023) Non-invasive brain stimulation for fibromyalgia: current trends and future perspectives. Front. Neurosci. 17:1288765. doi: 10.3389/fnins.2023.1288765
Edited by:
Mark H. Myers, University of Tennessee Health Science Center (UTHSC), United StatesReviewed by:
Benito de Celis Alonso, Meritorious Autonomous University of Puebla, MexicoQuanguang Zhang, Augusta University, United StatesLSU Health Sciences Center - Shreveport, United States
Copyright © 2023 Zhang, Liang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liang, MTk5NjEwODdAbS5zY251LmVkdS5jbg==; Zhong-Wei Yang, MTk5ODEwODBAbS5zY251LmVkdS5jbg==
 Jia-Hao Zhang
Jia-Hao Zhang Jian Liang
Jian Liang Zhong-Wei Yang
Zhong-Wei Yang