
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 28 November 2023
Sec. Translational Neuroscience
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1288631
This article is part of the Research TopicDeveloping Personalized Treatment in Neurodegenerative Disorders: Role of Genomics and Novel Technologies in Identifying Actionable Targets and Developing Interventions in Rare-DiseasesView all 5 articles
 Ruwei Ou1
Ruwei Ou1 Chunyu Li1
Chunyu Li1 Qianqian Wei1
Qianqian Wei1 Kuncheng Liu1
Kuncheng Liu1 Junyu Lin1
Junyu Lin1 Tianmi Yang1
Tianmi Yang1 Yi Xiao1
Yi Xiao1 Qirui Jiang1
Qirui Jiang1 Yangfan Cheng1
Yangfan Cheng1 Yanbing Hou1
Yanbing Hou1 Lingyu Zhang1
Lingyu Zhang1 Wei Song1
Wei Song1 Xueping Chen1
Xueping Chen1 Xiaohui Lai2*
Xiaohui Lai2* Huifang Shang1*
Huifang Shang1*Objectives: Mutations in glucocerebrosidase (GBA1) can change the clinical phenotype of Parkinson’s disease (PD). This study aimed to explore the clinical characteristics of freezing of gait (FOG) in PD patients with GBA1 mutations.
Methods: A whole-exome sequencing analysis was used to identify the GBA1 mutations (pathogenic or likely pathogenic) and exclude other PD-related gene mutations. A forward binary logistic regression model was conducted to identify the associated factors of FOG. The stepwise multiple linear regression analysis models were used to explore the effect of FOG on quality of life.
Results: The prevalence of FOG in patients with GBA1 mutations (30/95, 31.6%) was significantly higher than those in patients without GBA1 mutations (152/760, 20%) (p = 0.009). A higher (i.e., worse) Unified PD Rating Scale part III score (OR = 1.126, 95%CI = 1.061–1.194, p < 0.001) and a lower (i.e., worse) Montreal Cognitive Assessment score (OR = 0.830, 95%CI = 0.713–0.967, p = 0.017) were significantly associated with FOG in PD patients with GBA1 mutations. The presence of FOG was significantly associated with the decreased (i.e., worse) score of PD Questionnaire 39 after adjustment for sex, age, disease duration, motor score, and non-motor score (B = 14.981, p = 0.001).
Conclusion: FOG is a relatively common disabling symptom in PD patients with GBA1 mutations, which is affected by motor disability and cognitive decline. Quality of life is reduced in patients with FOG and GBA1 mutations.
Freezing of gait (FOG) is a common symptom in Parkinson’s disease (PD) (Rogers, 1996), affecting approximately 60% of patients (Lamberti et al., 1997). FOG is regarded as a brief, episodic absence or marked reduction of forwarding progression of the feet despite the intention to walk (Giladi and Nieuwboer, 2008), which mainly occurs as start hesitation, upon turning or approaching a destination, or sometimes even when walking in an open walkway (Stern et al., 1980). FOG significantly impairs mobility and increases the risk of falls among PD patients (Giladi, 2001), which greatly reduces the quality of life (QoL) of patients (Ou et al., 2014).
Glucocerebrosidase (GBA1) mutations are common in PD and represent one of the most important risk factors yet discovered for PD (Schapira, 2015). Mutations in GBA1 can modify the clinical phenotype of PD (Davis A. A. et al., 2016). Compared to non-carriers, individuals with GBA1 mutations have been reported to exhibit earlier onset symptoms, are more likely to have affected relatives, and increased chances to have atypical clinical manifestations (Sidransky et al., 2009). Furthermore, GBA1 mutation carriers with PD are on a trajectory to cognitive decline (Liu et al., 2016), and they are more prone to present with the postural instability gait difficulty phenotype compared with non-carriers (Malek et al., 2018). However, the clinical symptoms of FOG in PD patients with GBA1 mutations remain largely unknown. Detailed descriptions regarding the clinical characteristics based on specific genotypes are important for precision medicine. The aim of this study is therefore to explore the clinical characteristics of FOG in a group of PD patients with GBA1 mutations.
This study was approved by the Ethics Committee of West China Hospital of Sichuan University. All patients signed informed consent. A total of 95 PD patients with GBA1 mutations from the Department of Neurology, West China Hospital of Sichuan University were recruited between October 2010 and September 2021. The diagnosis of PD was based on the Unified Kingdom PD Society Brain Bank Clinical Diagnostic Criteria for PD (Hughes et al., 1992). Patients without GBA1 mutations, patients with severe dementia or hearing loss, and those who refused to be interviewed were excluded from this study. Additionally, we included 760 PD patients without GBA1 mutations as controls. These patients came from the same research center at the same time and underwent whole-exome sequencing (WES). All recruited patients conducted brain MRI scans to exclude other neurological disorders, such as cerebrovascular disease, brain tumors, and encephalitis.
Clinical data including age, age of onset, gender, disease duration, years of education, body mass index (BMI), treatment regimen, and motor complications were collected through in-person interviews by neurologists specializing in PD. The Unified PD Rating Scale (UPDRS) part III (Goetz et al., 2007) and Hoehn and Yahr (H&Y) stage (Hoehn and Yahr, 1967) were used to evaluate the motor severity. The quality of life (QoL) of PD patients was assessed using PD Questionnaire 39 (PDQ-39), which consists of eight domains (Mobility, Activities of daily life, Emotional well-being, Stigma, Social support, Cognitions, Communication, and Bodily discomfort) (Jenkinson et al., 1995). Non-motor symptoms (NMS) were measured using the Non-Motor Symptoms Scale (NMSS) (Wang et al., 2009). Cognition was evaluated utilizing the Frontal Assessment Battery (FAB) (Dubois et al., 2000) and Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005). Depression and anxiety were assessed using the Hamilton Depression Rating Scale (HAMD) (24 items) [20] and the Hamilton Anxiety Rating Scale (HAMA) [21], respectively.
Freezing episodes during the visit were observed by experienced neurologists or reported by the patients themselves, along with their family members or caregivers when FOG occurred at home or anywhere outside of the hospital. Patients were identified as experiencing FOG (freezers) based on a score (≥1) from item 1.3 of the FOG Questionnaire, which asked: “Do you feel that your feet get glued to the floor while walking, making a turn, or when trying to initiate walking?” [22]. The responses provided by patients were verified by their relatives or caregivers and cross-checked with clinical records for data accuracy.
Information regarding GBA1 mutations in PD patients is presented in Supplementary Table S1. Patients carrying GBA1 mutations (pathogenic or likely pathogenic) were identified and excluded if they carried mutations in other PD-related genes through WES analysis. Genomic DNA was extracted from peripheral blood leukocytes using standard phenol-chloroform procedures. A total of 5ug DNA was fragmented into an average size of 350 bp with a Covaris LE220-plus focused ultrasonicator, and the DNA library was constructed with the KAPA Library Amplification Kit. Then WES was performed routinely on the Illumina NovaSeq 6,000 system following manufacturer’s instructions. Pathogenicity categorization followed the American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al., 2015).
Data analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 for Windows. All statistical tests were two-tailed, with p values < 0.05 considered statistically significant. Frequencies and descriptive statistics were utilized to summarize the clinical features of samples. Data was reported as percentages for categorical variables and mean ± standard deviation (SD) for continuous variables. The Student’s T test, Chi-square test, Fisher exact test, and Wilcoxon rank-sum test were employed to compare clinical data between PD patients with and without FOG.
A binary logistic regression model was utilized to explore potential factors associated with FOG. The presence or absence of FOG was used as the dependent variable. The following parameters: sex (male/female), age, disease duration, motor fluctuation (yes/no), dyskinesia (yes/no), education, as well as the UPDRS III, HAMD, HAMA, MoCA, FAB, and NMSS scores, were set as covariables.
The stepwise multiple linear regression analysis models were used to explore the effect of FOG on QoL. The models were initially performed with adjustments for sex, age, disease duration, UPDRS III score, and NMSS score (Model 1), followed by additional adjustments for motor fluctuation and dyskinesia (Model 2), and further adjustments for HAMD, HAMA, MoCA, and FAB scores (Model 3). A collinearity test was conducted which revealed no collinearity among these variables.
In the current study, we included 95 PD patients with GBA1 mutations and 30 of them (31.6%) reported FOG. Individuals with GBA1 mutations showed younger age than those without GBA1 mutations (48.9 ± 10.5 years vs. 51.6 ± 12.1 years, p = 0.020). No significant differences in male proportion (50.5% vs. 50.7%, p = 0.981), age of onset (44.8 ± 10.3 years vs. 46.7 ± 12.1 years, p = 0.096), and disease duration (4.0 ± 3.8 years vs. 4.8 ± 5.1 years, p = 0.066) were observed between the two groups. The prevalence of FOG in patients with GBA1 mutations was significantly higher than those in patients without GBA1 mutations (31.6% vs. 20%, p = 0.009).
The prevalence of FOG in male patients was 33.3% and the prevalence of FOG in female GBA1 patients was 29.8% (Figure 1A). In GBA1 patients with an age of onset <45 years, 35.6% patients reported FOG; while in patients with an age of onset >45 years, 25.0% patients were experiencing FOG (Figure 1B). Patients with higher H&Y stage or longer disease duration reported a higher prevalence of FOG (Figures 1C,D).
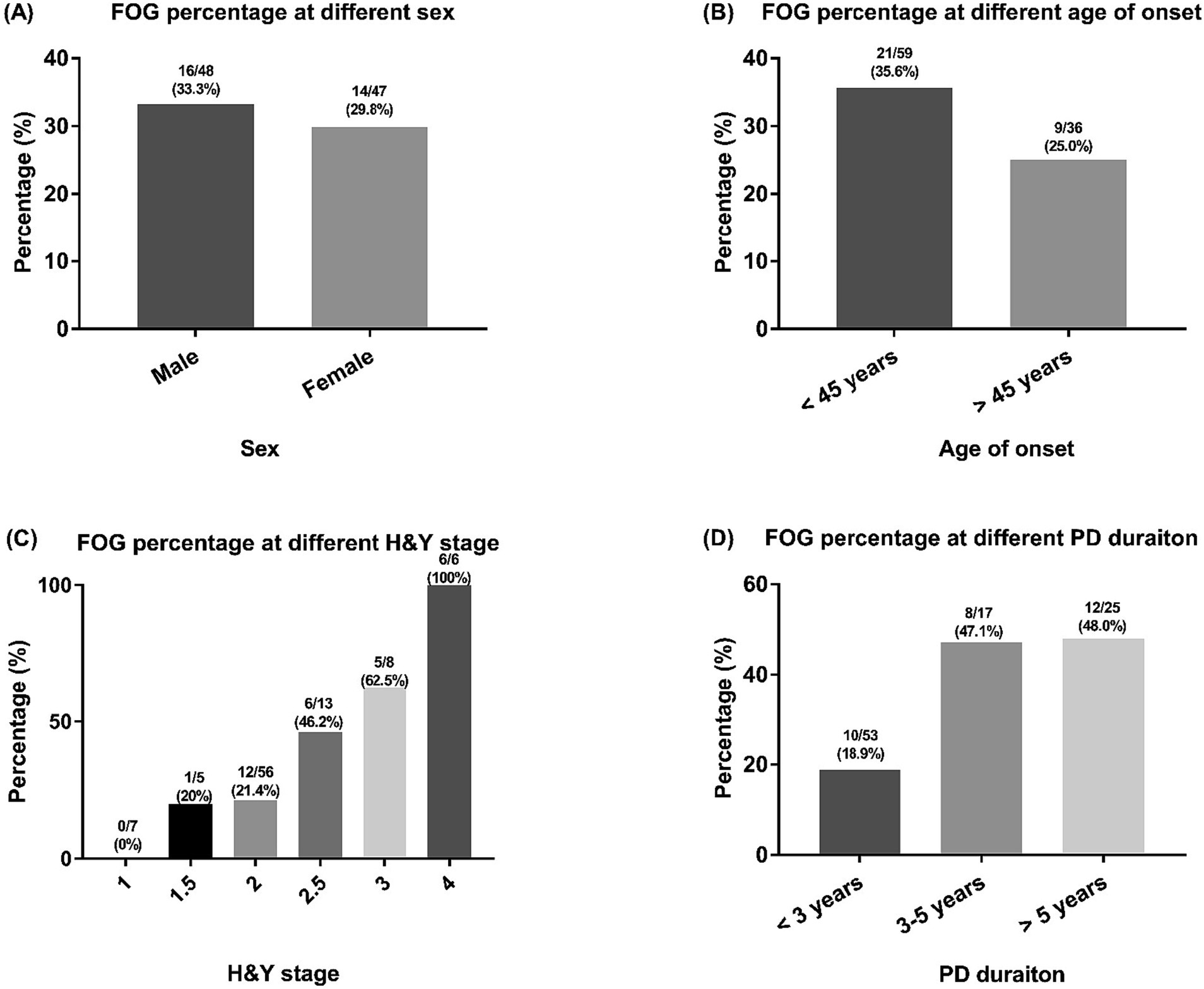
Figure 1. The FOG percentages of included PD patients with GBA1 mutations. FOG, freezing of gait; PD, Parkinson’s disease; GBA1, glucocerebrosidase. Comparisons of the percentage of FOG among PD patients across different categories, including sex (A) age at onset (B) H&Y stage (C) and PD duration (D) groups.
The demographic and clinical features of the PD patients with GBA1 mutaitons are listed in Table 1. The freezers showed a significantly higher (i.e., worse) UPDRS III score (p < 0.001), greater (i.e., worse) H&Y stage (p < 0.001), more frequent motor fluctuation (p = 0.015), higher (i.e., worse) NMSS score (p = 0.001), higher (i.e., worse) HAMD score (p < 0.001), higher (i.e., worse) HAMA score (p = 0.035), lower (i.e., worse) MoCA score (p < 0.001), and lower (i.e., worse) FAB scores (p = 0.044) than the non-freezers. The mean age, age of onset, disease duration, LEDD, and sex distribution were not significantly different between patients with and without FOG in GBA1 carriers (All p > 0.05).
The PDQ-39 results for the patients with and without FOG in PD patients with GBA1 mutations are listed in Table 2. The group experiencing freezing episodes demonstrated significantly higher (i.e., worse) total scores on the PDQ-39 (p < 0.001) as well as higher (i.e., worse) scores in the domains of mobility (p < 0.001), activities of daily life (p < 0.001), emotional well-being (p = 0.006), stigma (p = 0.029), communication (p < 0.001), and bodily discomfort (p = 0.020) compared to those without freezing episodes.
The potential factors associated with FOG in PD patients with GBA1 mutations are outlined in Table 3. The binary logistic regression model revealed that a higher UPDRS III score (OR = 1.126, 95%CI = 1.061–1.194, p < 0.001) and a lower MoCA score (OR = 0.830, 95%CI = 0.713–0.967, p = 0.017) were both significantly associated with FOG in PD patients with GBA1 mutations.
The impact of FOG on the QoL among patients with GBA1 mutations is presented in Table 4. Multiple regression analysis models indicated that the presence of FOG remained significantly associated with decreased QoL after adjusting for sex, age, disease duration, motor score, and non-motor score in Model 1, 2, and 3 (p < 0.05).
To the best of our knowledge, this is the first study to investigate the clinical characteristics of FOG in PD patients with GBA1 mutations. In the current study, FOG was observed in nearly 30% of PD patients with GBA1 mutations, which is a higher prevalence compared to those without GBA1 mutations. We found that FOG in individuals carrying GBA1 mutation was influenced by motor severity and cognitive decline. In addition, we also found that FOG significantly decreased the QoL for PD patients with GBA1 mutations.
In the current study, high frequency of the “severe” p.L444P/p.L483P GBA1 mutations were included, which accounted for almost half (45/95, 47.4%) of the pathogenic/likely pathogenic GBA1 cases. In a previous multi-ethnic Asian cohort with PD, p.L444P/p.L483P (11/496, 2.2%) was also detected as the most common variant in patients with GBA1 mutations (Lim et al., 2022). This suggests that our data are representative of the Asian PD population. Studies from Western populations usually find other GBA1 variants to be more common, such as p.E326K/p.E365K, p.T369M/p.T408M, and p.N370S/p.N409S (Malek et al., 2018), which on the other hand are usually absent in Asian populations (Lim et al., 2022). Therefore, our findings in the current study require further confirmation in the European PD population.
In our previous study conducted on 474 general PD patients with an average duration of 4.8 ± 4.0 years, we reported a high prevalence of FOG among the Chinese PD population at 46.6% (Ou et al., 2014). Although our current study included patients with shorter disease duration (mean 4.0 ± 3.8 years), approximately one-third of those with GBA1 mutations experienced FOG. The prevalence of FOG observed in our study aligns closely with the result from a recent meta-analysis reporting a weighted prevalence for overall early-stage PD (≤ 5 years) at 37.9% (Zhang et al., 2021). A previous small sample size study involving only 22 GBA1 mutation carriers revealed that FOG was present in as many as 72.2% of PD patients who had an average disease duration of 12.1 years (Da Silva et al., 2017). Discrepancies between these studies may be attributed to differences in sample sizes, inclusion criteria, and genetic backgrounds. Furthermore, our findings indicate that the incidence of FOG was significantly higher in the GBA1 population than in the non-GBA1 population. This novel finding requires further confirmation through large-sample and multi-center studies.
In the present study, after adjusting for age, disease duration, H&Y stage, and NMSS, our PD patients with FOG exhibited a reduced QoL, particularly in terms of bodily discomfort. These findings are consistent with those reported in an Israeli study (Moore et al., 2007), highlighting the significant impact of FOG on QoL of PD patients beyond its effect on gait. Currently, only a limited number of studies have investigated how the mechanism of FOG affects QoL, and a previous study suggested that the episodic nature of FOG may play a role (Moore et al., 2007).
Our GBA1 mutation carriers with FOG demonstrated more severe motor disability and more frequent motor complications in PD, aligning with our previous observational study (Ou et al., 2014) as well as several studies conducted on Caucasian populations (Giladi et al., 1992; Lamberti et al., 1997; Giladi et al., 2001). The association between motor severity and FOG in our GBA1 mutation population is consistent with the findings from general PD populations in America and Germany [8, 24]. In addition, a prospective study revealed that GBA1 mutations were associated with accelerated motor decline in PD (Davis M. Y. et al., 2016). However, contrary to previous studies conducted on Caucasian populations [5, 6, 8, 23, 24], we did not find an association between disease duration and FOG. Additionally, no correlation was identified between dopaminergic drugs and FOG among patients with GBA1 mutations—a finding inconsistent with some prior studies involving the general PD population [6, 8, 24].
In addition, the association between FOG and cognitive decline in our sample is consistent with several previous studies (Amboni et al., 2008, 2010; Nantel et al., 2012), which have demonstrated that FOG was linked to executive dysfunction and visuospatial deficits. The pathogenesis of FOG remains unclear. A resting-state fMRI study (Tessitore et al., 2012b) revealed that disruption in connectivity within the “executive attention” and visual neural networks may be associated with FOG in the general PD population. One voxel-based morphometry study found an association between FOG and posterior gray matter atrophy (Tessitore et al., 2012a). Another voxel-based morphometry found that PD patients with FOG exhibited frontal and parietal atrophy, suggesting the involvement of executive dysfunction and perception deficits (Kostic et al., 2012). Our studies highlight the significance of cognitive circuits and motor pathways in relation to FOG among patients with GBA1 mutations. Further investigations into its pathophysiology can provide valuable insights.
Some limitations should be acknowledged. (1) There may exist recall bias. (2) The cross-sectional analysis conducted in this study can only indicate possible associations but cannot reveal causality. (3) We did not determine whether patients experienced FOG during ON or OFF states. (4) The sample size was relatively small. (5) Sanger sequencing was not performed to verify GBA1 mutations. (6) The function and severity of certain GBA1 mutations still require further confirmation.
FOG is a relatively common disabling symptom observed among PD patients with GBA1 mutations. Motor disability and cognitive decline are likely contributors to the occurrence of FOG among carriers of GBA1 mutations. QoL is diminished for individuals with GBA1 mutations. Our findings provide similar results regarding FOG in patients with GBA1 mutations compared to previous reports on the overall PD population.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
RO: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CL: Data curation, Investigation, Methodology, Validation, Writing – review & editing. QW: Data curation, Investigation, Validation, Writing – review & editing. KL: Data curation, Investigation, Methodology, Validation, Writing – review & editing. JL: Data curation, Investigation, Writing – review & editing. TY: Investigation, Methodology, Writing – review & editing. YX: Investigation, Methodology, Writing – review & editing. QJ: Investigation, Methodology, Writing – review & editing. YC: Investigation, Methodology, Writing – review & editing. YH: Investigation, Methodology, Writing – review & editing. LZ: Investigation, Methodology, Writing – review & editing. WS: Investigation, Methodology, Writing – review & editing. XC: Investigation, Methodology, Writing – review & editing. XL: Validation, Visualization, Writing – review & editing, Conceptualization, Data curation, Methodology, Project administration, Software, Supervision. HS: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the funding of the National Science Fund of China (No. 82271272) and the Sichuan Science and Technology Program (No. 2022ZDZX0023).
The authors thank the patients and their families for their participation in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1288631/full#supplementary-material
Amboni, M., Barone, P., Picillo, M., Cozzolino, A., Longo, K., Erro, R., et al. (2010). A two-year follow-up study of executive dysfunctions in parkinsonian patients with freezing of gait at on-state. Mov. Disord. 25, 800–802. doi: 10.1002/mds.23033
Amboni, M., Cozzolino, A., Longo, K., Picillo, M., and Barone, P. (2008). Freezing of gait and executive functions in patients with Parkinson’s disease. Mov. Disord. 23, 395–400. doi: 10.1002/mds.21850
Da Silva, C. P., De, M. A. G., Cabello Acero, P. H., Campos, M. J., Pereira, J. S., De, A. R. S. R., et al. (2017). Clinical profiles associated with LRRK2 and GBA mutations in Brazilians with Parkinson’s disease. J. Neurol. Sci. 381, 160–164. doi: 10.1016/j.jns.2017.08.3249
Davis, A. A., Andruska, K. M., Benitez, B. A., Racette, B. A., Perlmutter, J. S., and Cruchaga, C. (2016). Variants in GBA, SNCA, and MAPT influence Parkinson disease risk, age at onset, and progression. Neurobiol. Aging 37, 209.e1–209.e7. doi: 10.1016/j.neurobiolaging.2015.09.014
Davis, M. Y., Johnson, C. O., Leverenz, J. B., Weintraub, D., Trojanowski, J. Q., Chen-Plotkin, A., et al. (2016). Association of GBA mutations and the E326K polymorphism with motor and cognitive progression in Parkinson disease. JAMA Neurol. 73, 1217–1224. doi: 10.1001/jamaneurol.2016.2245
Dubois, B., Slachevsky, A., Litvan, I., and Pillon, B. (2000). The FAB: a frontal assessment battery at bedside. Neurology 55, 1621–1626. doi: 10.1212/WNL.55.11.1621
Giladi, N., Mcmahon, D., Przedborski, S., Flaster, E., Guillory, S., Kostic, V., et al. (1992). Motor blocks in Parkinson’s disease. Neurology 42, 333–339. doi: 10.1212/WNL.42.2.333
Giladi, N., and Nieuwboer, A. (2008). Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov. Disord. 23, S423–S425. doi: 10.1002/mds.21927
Giladi, N., Treves, T. A., Simon, E. S., Shabtai, H., Orlov, Y., Kandinov, B., et al. (2001). Freezing of gait in patients with advanced Parkinson’s disease. J. Neural Transm. 108, 53–61. doi: 10.1007/s007020170096
Goetz, C. G., Fahn, S., Martinez-Martin, P., Poewe, W., Sampaio, C., Stebbins, G. T., et al. (2007). Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov. Disord. 22, 41–47. doi: 10.1002/mds.21198
Hoehn, M. M., and Yahr, M. D. (1967). Parkinsonism: onset, progression and mortality. Neurology 17, 427–442. doi: 10.1212/WNL.17.5.427
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Jenkinson, C., Peto, V., Fitzpatrick, R., Greenhall, R., and Hyman, N. (1995). Self-reported functioning and well-being in patients with Parkinson’s disease: comparison of the short-form health survey (SF-36) and the Parkinson’s disease questionnaire (PDQ-39). Age Ageing 24, 505–509. doi: 10.1093/ageing/24.6.505
Kostic, V. S., Agosta, F., Pievani, M., Stefanova, E., Jecmenica-Lukic, M., Scarale, A., et al. (2012). Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology 78, 409–416. doi: 10.1212/WNL.0b013e318245d23c
Lamberti, P., Armenise, S., Castaldo, V., De Mari, M., Iliceto, G., Tronci, P., et al. (1997). Freezing gait in Parkinson’s disease. Eur. Neurol. 38, 297–301. doi: 10.1159/000113398
Lim, J. L., Lohmann, K., Tan, A. H., Tay, Y. W., Ibrahim, K. A., Abdul Aziz, Z., et al. (2022). Glucocerebrosidase (GBA) gene variants in a multi-ethnic Asian cohort with Parkinson’s disease: mutational spectrum and clinical features. J. Neural Transm. 129, 37–48. doi: 10.1007/s00702-021-02421-0
Liu, G., Boot, B., Locascio, J. J., Jansen, I. E., Winder-Rhodes, S., Eberly, S., et al. (2016). Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann. Neurol. 80, 674–685. doi: 10.1002/ana.24781
Malek, N., Weil, R. S., Bresner, C., Lawton, M. A., Grosset, K. A., Tan, M., et al. (2018). Features of GBA-associated Parkinson’s disease at presentation in the UK tracking Parkinson’s study. J. Neurol. Neurosurg. Psychiatry 89, 702–709. doi: 10.1136/jnnp-2017-317348
Moore, O., Peretz, C., and Giladi, N. (2007). Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov. Disord. 22, 2192–2195. doi: 10.1002/mds.21659
Nantel, J., Mcdonald, J. C., Tan, S., and Bronte-Stewart, H. (2012). Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson’s disease. Neuroscience 221, 151–156. doi: 10.1016/j.neuroscience.2012.07.007
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Ou, R., Guo, X., Song, W., Cao, B., Yang, J., Wei, Q., et al. (2014). Freezing of gait in Chinese patients with Parkinson disease. J. Neurol. Sci. 345, 56–60. doi: 10.1016/j.jns.2014.07.002
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. doi: 10.1038/gim.2015.30
Rogers, M. W. (1996). Disorders of posture, balance, and gait in Parkinson’s disease. Clin. Geriatr. Med. 12, 825–845. doi: 10.1016/S0749-0690(18)30203-9
Schapira, A. H. (2015). Glucocerebrosidase and Parkinson disease: recent advances. Mol. Cell. Neurosci. 66, 37–42. doi: 10.1016/j.mcn.2015.03.013
Sidransky, E., Nalls, M. A., Aasly, J. O., Aharon-Peretz, J., Annesi, G., Barbosa, E. R., et al. (2009). Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661. doi: 10.1056/NEJMoa0901281
Stern, G. M., Lander, C. M., and Lees, A. J. (1980). Akinetic freezing and trick movements in Parkinson’s disease. J. Neural Transm. Suppl. 16, 137–141.
Tessitore, A., Amboni, M., Cirillo, G., Corbo, D., Picillo, M., Russo, A., et al. (2012a). Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am. J. Neuroradiol. 33, 1804–1809. doi: 10.3174/ajnr.A3066
Tessitore, A., Amboni, M., Esposito, F., Russo, A., Picillo, M., Marcuccio, L., et al. (2012b). Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat. Disord. 18, 781–787. doi: 10.1016/j.parkreldis.2012.03.018
Wang, G., Hong, Z., Cheng, Q., Xiao, Q., Wang, Y., Zhang, J., et al. (2009). Validation of the Chinese non-motor symptoms scale for Parkinson’s disease: results from a Chinese pilot study. Clin. Neurol. Neurosurg. 111, 523–526. doi: 10.1016/j.clineuro.2009.02.005
Keywords: Parkinson’s disease, freezing of gait, GBA1 mutation, quality of life, gene mutation
Citation: Ou R, Li C, Wei Q, Liu K, Lin J, Yang T, Xiao Y, Jiang Q, Cheng Y, Hou Y, Zhang L, Song W, Chen X, Lai X and Shang H (2023) Freezing of gait in Parkinson’s disease with glucocerebrosidase mutations: prevalence, clinical correlates and effect on quality of life. Front. Neurosci. 17:1288631. doi: 10.3389/fnins.2023.1288631
Received: 04 September 2023; Accepted: 13 November 2023;
Published: 28 November 2023.
Edited by:
Sabina Vatovec, Novartis (Slovenia), Mengeš, SloveniaReviewed by:
Vladimira Vuletic, University of Rijeka, CroatiaCopyright © 2023 Ou, Li, Wei, Liu, Lin, Yang, Xiao, Jiang, Cheng, Hou, Zhang, Song, Chen, Lai and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Lai, bHhobmV1QGFsaXl1bi5jb20=; Huifang Shang, aGZzaGFuZzIwMDJAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.