
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci. , 24 October 2023
Sec. Brain Imaging Methods
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1236069
 Mengqi Zhao1,2†
Mengqi Zhao1,2† Zeqi Hao1,2†
Zeqi Hao1,2† Mengting Li1,2
Mengting Li1,2 Hongyu Xi3
Hongyu Xi3 Su Hu1,2
Su Hu1,2 Jianjie Wen1,2
Jianjie Wen1,2 Yanyan Gao1,2
Yanyan Gao1,2 Collins Opoku Antwi1,2
Collins Opoku Antwi1,2 Xize Jia1,2
Xize Jia1,2 Yang Yu4*
Yang Yu4* Jun Ren1,2*
Jun Ren1,2*Background: Irritable bowel syndrome (IBS) is a brain-gut disorder with high global prevalence, resulting from abnormalities in brain connectivity of the default mode network and aberrant changes in gray matter (GM). However, the findings of previous studies about IBS were divergent. Therefore, we conducted a meta-analysis to identify common functional and structural alterations in IBS patients.
Methods: Altogether, we identified 12 studies involving 194 IBS patients and 230 healthy controls (HCs) from six databases using whole-brain resting state functional connectivity (rs-FC) and voxel-based morphometry. Anisotropic effect-size signed differential mapping (AES-SDM) was used to identify abnormal functional and structural changes as well as the overlap brain regions between dysconnectivity and GM alterations.
Results: Findings indicated that, compared with HCs, IBS patients showed abnormal rs-FC in left inferior parietal gyrus, left lingual gyrus, right angular gyrus, right precuneus, right amygdala, right median cingulate cortex, and left hippocampus. Altered GM was detected in the fusiform gyrus, left triangular inferior frontal gyrus (IFG), right superior marginal gyrus, left anterior cingulate gyrus, left rectus, left orbital IFG, right triangular IFG, right putamen, left superior parietal gyrus and right precuneus. Besides, multimodal meta-analysis identified left middle frontal gyrus, left orbital IFG, and right putamen as the overlapped regions.
Conclusion: Our results confirm that IBS patients have abnormal alterations in rs-FC and GM, and reveal brain regions with both functional and structural alterations. These results may contribute to understanding the underlying pathophysiology of IBS.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42022351342.
Irritable bowel syndrome (IBS) is a prevalent chronic functional gastrointestinal disorder manifested by recurrent abdominal pain and alterations in stool form or frequency (Ford et al., 2020). Previous studies have revealed that the brain-gut axis plays an important role in the occurrence of IBS (Koloski et al., 2016). This axis regulates gastrointestinal motility, visceral sensitivity, stress response, and cognitive function, and is closely related to central neural mechanism (Price et al., 2006). Moreover, a recent meta-analysis (Su et al., 2022) also confirmed that IBS patients had abnormal local spontaneous functional activity in several parts of the brain. Considering the high global prevalence (approximate 10–15%) (Drossman et al., 2002) and the significant impact of IBS on health-related quality of life (Gralnek et al., 2000), it is necessary to explore the pathological mechanism of IBS. However, the neural mechanisms of IBS remain to be further elucidated by examining brain functional and structural alterations.
Resting-state functional magnetic resonance imaging (rs-fMRI) is a promising task-independent tool which has been used to analyze the underlying neural mechanism of various diseases (including IBS) from the perspective of functional and structural abnormalities (Lowe et al., 2000; Brady et al., 2016; Bi et al., 2021a,b; Zhao et al., 2021). From the perspective of function, resting-state functional connectivity (rs-FC) is one of the most frequently used methods in rs-fMRI studies (Fox and Greicius, 2010). Quantizing functional connectivity between anatomically different brain regions, FC can identify the connectivity patterns of distributed brain networks, such as default mode network (DMN). DMN is a set of widely investigated brain regions in rs-fMRI studies and reflects the baseline mode of brain function (Raichle et al., 2001). As previous studies mentioned, DMN is preferentially affected by chronic pain, which is a core symptom of IBS (Farmer et al., 2012; Paine, 2021). Therefore, DMN may be an important influencing factor in the pathogenesis of IBS. Other studies also demonstrated the essential role played by DMN in IBS (Liu et al., 2016; Qi et al., 2016). Some important nodes in DMN play key roles in brain-gut interactions in functional gastrointestinal syndrome (Kano et al., 2018). And some researchers have already realized the significance of DMN in patients with IBS by conducting studies specifically focused on DMN. For instance, Qi et al. (2016) found that IBS patients exhibit non-optimized topological characteristics of the DMN compared with healthy controls (HCs), and the average FC of DMN correlates negatively with symptom severity. While Skrobisz et al. (2020) discovered that, compared with HCs, patients with IBS and functional dyspepsia showed abnormal connectivity in DMN.
In addition to functional alterations, abnormal gray matter (GM) structural changes in IBS patients have also been reported using voxel-based morphometry (VBM) (Zhao et al., 2018; Li et al., 2020; Öhlmann et al., 2021). VBM method can detect structural differences throughout the brain and is a time-efficient, automated and unbiased voxel-based method (Yasuda et al., 2010). Some studies found that GM structural changes in IBS patients might relate to pain perception and gastrointestinal symptoms. For example, a previous study found that greater gastrointestinal symptoms correlated with reduced GM volume in the occipital pole in patients with IBS (Öhlmann et al., 2021). Meanwhile, another study found that decreased GM volume in bilateral thalamus was associated with increased pain thresholds in girls with IBS (Bhatt et al., 2019).
However, previous findings of rs-FC of DMN and VBM analyses in IBS patients were inconsistent. For example, both increased and decreased FC was found between right hippocampus and superior parietal gyrus (Li et al., 2013; Geng et al., 2021). For GM structural changes, some researchers only found decreased GM (Li et al., 2020), while others found both decreased and increased GM (Zhao et al., 2018; Öhlmann et al., 2021). These divergent results may be due to disease heterogeneity, small sample sizes, and sample selection differences (Fond et al., 2014; Li et al., 2020). Thus, it is necessary to conduct a meta-analysis to identify consistent abnormalities in IBS patients and to facilitate targeted treatments. In addition, since combining findings from different modalities can provide a more accurate differential analysis and can explore whether these functional changes indicate pure hemodynamic differences or reflect underlying morphological differences (Mayer et al., 2015), we integrate the multimodal findings to detect both functional and structural alterations in IBS patients.
Though a recent meta-analysis by Su et al. (2022) regarding regional spontaneous functional activity in IBS patients has been conducted, it focused exclusively on local brain activities instead of whole-brain FC. Moreover, this meta-analysis did not take GM changes into consideration. Therefore, the current study aims to seek consistent alterations in DMN connectivity as well as GM anatomic changes in IBS through whole-brain rs-FC and VBM. The findings of this meta-analysis might consolidate prior divergent results and facilitate the identification of potential IBS biomarkers in future studies.
This meta-analysis has been registered in the PROSPERO International Prospective Register of Systematic Reviews1 with the registration number “CRD42022351342” (available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=351342).
To conduct FC and VBM meta-analyses, a systematic literature search was performed according to the standard preferred reporting items for systematic reviews and meta-analyses (PRISMA) procedure (Moher et al., 2009; Wu et al., 2020; Yan et al., 2023). We searched in PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI), Wanfang Database (WF) and Scoups for published research up to August 12, 2023. For FC meta-analysis, the following keywords and their combinations were used: “resting state functional connectivity” or “resting state FC” or “rs-FC” or “ICA” or “independent component analysis” and “IBS” or “irritable bowel syndrome” and “DMN” or “default mode network.” For VBM analysis, the keywords included: “voxel-based morphometry,” “VBM,” “IBS,” and “irritable bowel syndrome.” In addition, the references lists of the retrieved studies were checked for potentially eligible studies.
The selection criteria were as follows: (1) Patients were diagnosed with irritable bowel syndrome based on any diagnostic criteria (Zhang et al., 2018); (2) Analysis was performed using seed-based FC or independent component analysis or VBM in the whole brain level; (3) A direct brain imaging comparison was conducted between IBS patients and healthy controls (HCs); (4) Peak coordinates (Talairach or Montreal Neurological Institute (MNI)) were reported, if the results showed significant statistical differences; and (5) The study was published in a peer-reviewed English or Chinese language journal.
Studies were excluded if they met one of the following conditions: (1) Studies were review articles, conference abstracts, or non-human research; (2) IBS patients diagnosed with comorbid neurological or psychiatric conditions; (3) The baseline comparison between IBS patients and HCs was not provided; (4) The analysis was not performed at the whole-brain level; and (5) If different studies had overlapping samples, only the study with the largest sample was included.
In addition, the quality of each study included in this meta-analysis was assessed using an 11-point checklist based on the previous meta-analyses, which include the assessment of demographic and clinical characteristics, scanner parameters, and analysis details (Cheng et al., 2020). According to these criteria, two researchers independently conducted the literature searches, study selection, data extraction, and quality evaluation. Extracted data include demographic and clinical characteristics of participants (sample size, age, gender, and illness duration), scanner parameters and basic methodological information (methods and software using for fMRI analysis, and statistical threshold information) (Table 1). We also extracted the coordinates of significant findings and statistical values related to effect size for SDM calculations. Any inconsistent opinions were discussed and settled through consultation. If no agreement could be reached, it was decided by a third author.
Functional connectivity and VBM meta-analyses were processed using the anisotropic effect-size signed differential mapping (AES-SDM) version 5.152 (Radua et al., 2014). First, the peak coordinates (both positive and negative) and corresponding effect sizes of statistically significant clusters were extracted from each included study. Then, an effect-size map of the individual study was created using an anisotropic unnormalized Gaussian kernel (Radua et al., 2014). Finally, the mean of the study maps was calculated with a random-effects model, and weighted by the sample size, the intra-study variance, and inter-study heterogeneity (Radua et al., 2012). We applied the recommended AES-SDM kernel size and thresholds, namely, 20 mm full width at half maximum (FWHM), voxel p < 0.005, peak height SDM-Z > 1, and cluster extent > 10 voxels (Radua et al., 2014). Images were displayed on a standardized anatomical template in the MNI space. Considering the influence of other important brain networks, we also conducted two additional meta-analyses for salience network and sensorimotor network (see Supplementary material).
A jackknife sensitivity analysis was performed to test the replicability of the results (Radua and Mataix-Cols, 2009). The identical analysis was repeated by discarding one of the included studies each time to assess if a result was robust and reliable. Besides, using a random-effect model with Q statistics, a heterogeneity analysis was performed to detect unexplained inter-study variability. And the threshold of this heterogeneity analysis was set at voxel p < 0.005, peak height Z > 1, and cluster extent > 10 voxels (Radua et al., 2014). Additionally, we implemented an Egger test to explore the probability of publication bias for each cluster through the values of the peaks in the main meta-analysis. Publication bias indicates the tendency to report statistically significant results more frequently than non-significant findings (Sterne et al., 2011). The results showing p < 0.05 are considered to be statistically significant (Egger et al., 1997).
To identify the overlapping brain regions that are significant in both functional and structural modalities, a multimodal approach was conducted by AES-SDM. The probability maps of VBM and functional response alterations would be combined to cross-validate the union alteration in both modalities (Nichols et al., 2005; Radua et al., 2013). The voxel-level threshold was set at p < 0.0025 as recommended (Radua et al., 2013).
Considering the influence of gender, meta-regression was conducted to examine the impact of gender on functional and structural alterations. The significant level was set at p < 0.0005, Z > 1, and cluster size > 10 voxels to reduce spurious findings (Radua and Mataix-Cols, 2009; Zhou et al., 2017).
In the FC meta-analysis, 6 studies were finally included, consisting of 194 patients with IBS (122 females and 72 males; mean age = 34.139) and 230 healthy controls (157 females and 73 males; mean age = 31.916). All studies had well-matched control groups. The mean quality score of FC studies was 10.25, and the mean score of VBM studies was 10.25. Quality assessment items that deducted the most scores of these studies were lack of desired information (including medication status, illness duration, comorbidity and severity of illness) and limitation discussion. Notably, the study of Gupta et al. (2014) and Icenhour et al. (2017) used ICA analysis, and the other four studies all applied a seed-based FC analysis. The DMN seeds used in FC analysis were shown in Table 1. As for the VBM meta-analysis, 6 studies were selected, with 189 IBS patients (150 females and 39 males) and 162 healthy controls (127 females and 35 males). The demographic and clinical characteristics, and analytic methods of included VBM studies are shown in Table 2. The process for literature identification and screening is presented in Figure 1.
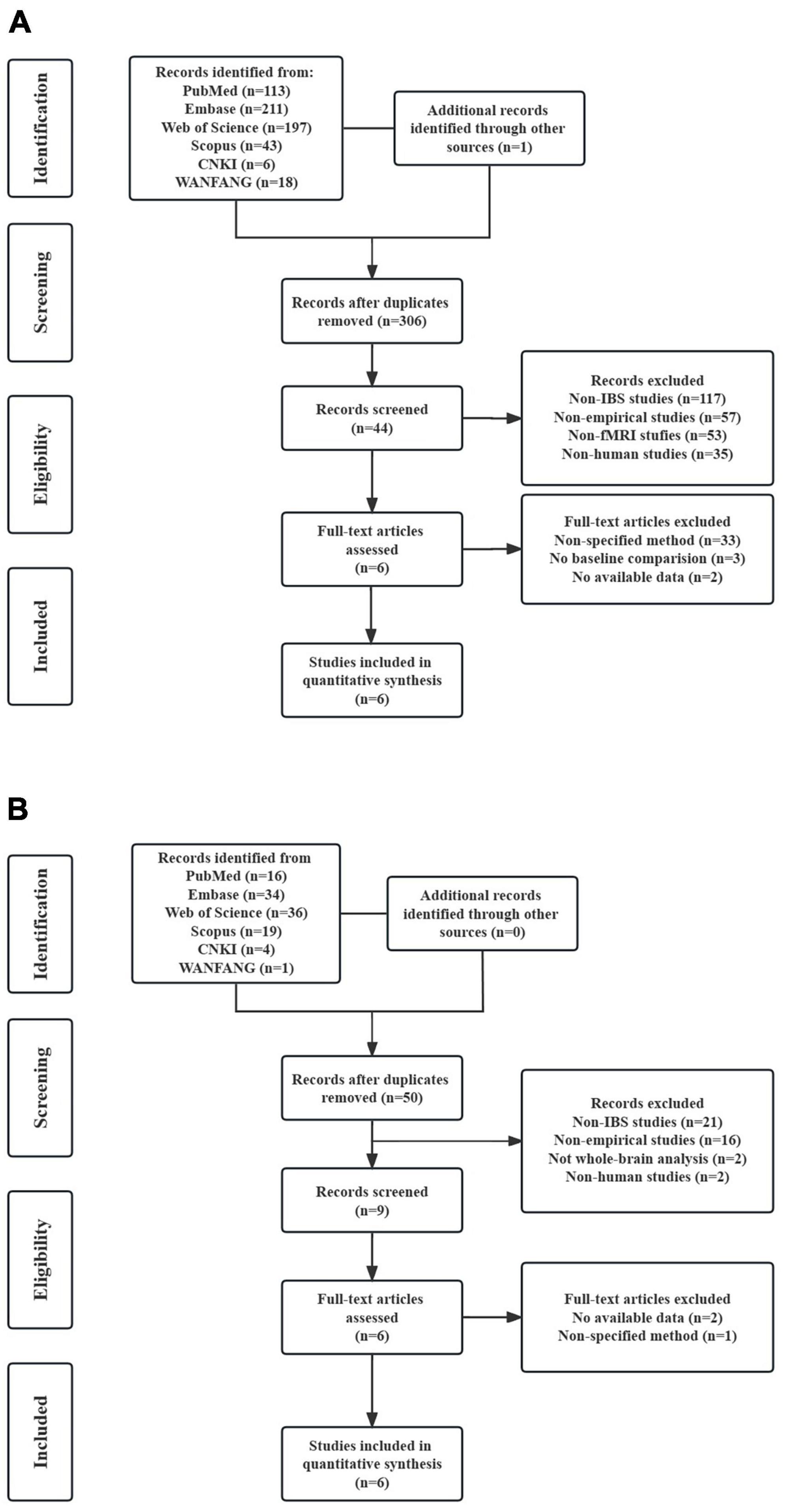
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram for literature identification and exclusion. (A) Flow diagram of study identification and selection for resting-state functional connectivity; (B) flow diagram of study identification and selection for voxel-based morphometry.
The meta-analysis revealed abnormal rs-FC between DMN and other brain regions. As presented in Table 3, patients with IBS showed increased rs-FC in left inferior parietal gyrus (IPG), left lingual gyrus, right angular gyrus, and right precuneus. Meanwhile, decreased rs-FC was detected in the right amygdala, right median cingulate gyrus (MCG), and left hippocampus.
Gray matter structural abnormalities were also observed in the meta-analysis. Bilateral fusiform gyrus, left triangular IFG and right superior marginal gyrus (SMG) showed increased GM in patients with IBS compared with HCs. However, the left anterior cingulate gyrus (ACG), left gyrus rectus, left orbital inferior frontal gyrus, right triangular IFG, right putamen, left superior parietal gyrus (SPG) and right precuneus showed decreased GM in patients with IBS.
For abnormal regions in FC meta-analysis, jackknife sensitivity analysis revealed that all seven clusters remained significant in five combinations out of six datasets, as shown in Table 3. And heterogeneity analysis showed that left IPG and left lingual gyrus had significant heterogeneity. As for the Egger test, right angular gyrus and right precuneus showed the presence of publication bias.
Meanwhile, the major results of the VBM meta-analysis remain reliable after sensitivity analysis. Specifically, all clusters showing increased GM (including bilateral fusiform, left triangular IFG and right SMG) in patients with IBS were significant in all but one combination. Decreased GM in left ACG, left gyrus rectus and left orbital IFG were detected in all combinations, while right putamen was detected in five combinations, followed by right precuneus and right triangular IFG detected in four combinations. And left SPG was only significant in three combinations. All significant brain regions in VBM meta-analysis did not present significant publication bias and the presence of heterogeneity.
As presented in Figure 2, multimodal meta-analysis showed that hyper-connectivity converged with increased GM was significant in left middle frontal gyrus (MFG), and hyper-connectivity converged with decreased GM in left orbital IFG. Besides, right putamen showed hypoconnectivity combined with GM decrease.

Figure 2. The results of meta-analysis. (A) Clusters showing functional connectivity (FC) differences in patients with irritable bowel syndrome (IBS) compared with healthy controls (HCs) (red denotes increased FC, blue denotes decreased FC). (B) Clusters showing gray matter (GM) differences in IBS patients compared with HCs (red denotes increased GM, blue denotes decreased GM). (C) The overlap regions showed significant functional and gray matter structural changes between patients with IBS and HCs.
No significant associations were found between gender and functional as well as structural changes.
This meta-analysis synthesizes diverse results from rs-FC of DMN and VBM results in IBS patients. The voxel-based meta-analysis showed rs-FC changes mainly in DMN and limbic regions. In addition, abnormal structural brain changes were detected in regions mainly participating in pain and emotion processing (including the fusiform gyrus, IFG, SMG, ACG, rectus, SPG, putamen and precuneus). The multimodal analysis revealed that GM changes were closely integrated with functional alterations in regions responsible for attention, emotion and pain processing (i.e., MFG, orbital IFG and right putamen).
For FC meta-analysis, our results found that increased FC was mainly within DMN regions, including left IPG, right angular gyrus and right precuneus. Several studies have also found abnormal brain activities of DMN in patients with IBS (Qi et al., 2016; Bhatt et al., 2022), and Letzen et al. (2013) found that DMN plasticity was sensitive to analgesic effects which further confirms its important role in pain. Previous study demonstrated that DMN activity increased when attention is engaged in mind wandering and decreased when attention is captured by pain (Kucyi and Davis, 2015). Additionally, DMN is also involved in pain-related attention and information gathering process (Cavanna and Trimble, 2006; Ploner et al., 2011). Therefore, we speculated that increased FC within DMN could imply deficits in pain-related visceral attention and sensations. Besides, our study also found increased FC in visual area (left lingual gyrus), consistent with a previous study that also demonstrated abnormal brain activities in visual cortex in patients with IBS (Lee et al., 2012). Meanwhile, patients with chronic pain showed altered functional activity in visual cortical areas as well (Li et al., 2018; Messina et al., 2022). This could be associated with abnormality in pain perception in patients with IBS. To be noted that right angular gyrus and right precuneus showed significant publication bias, while left IPG and left lingual showed significant heterogeneity, which could be due to clinical differences or demographic information and should be interpreted with caution (Sterne et al., 2011).
In the present study, decreased connectivity was found between DMN and limbic system (including amygdala, hippocampus and MCG). The limbic system links visceral states and emotions to cognition and behavior, and its dysfunction is associated with emotion dysregulation, memory impairment and cognitive deficits (Catani et al., 2013). Previous studies have discovered dysfunction of the hippocampal-amygdala circuit and abnormal hippocampus activities during heterotopic stimulation and cognitive task in IBS patients compared with HCs (Wilder-Smith et al., 2004; Aizawa et al., 2012). Besides, previous studies found that MCG took part in the processing of emotional and cognitive responses to painful information, and the local brain activities in the MCG were related to the disease duration (Frot et al., 2008; Ma et al., 2015). Taken together, visceral pain was detected and adjusted inappropriately through emotional arousal and homeostatic afferent networks (including the amygdala and MCG regions), then the conditioned fear of pain will be formatted by the hippocampus, making this process a vicious circle (Tillisch et al., 2011; Murray et al., 2021).
As for VBM meta-analysis, changed GM was mainly detected in regions involved in pain processing, including pain perception, transmission and interpretation. The altered brain regions included a wide range of areas in the frontal, parietal and temporal lobes. Namely, SMG, SPG and precuneus belong to the parietal lobe, while fusiform belong to the temporal lobe, which is involved in sensory information perception, imagination and integration (Hyvärinen, 1982; Spagna et al., 2021). Besides, the gyrus rectus connects the sensory integration network and visceral-motor centers (Öngür and Price, 2000). Accordingly, the abnormal changes in these regions will not only influence the reception of pain sensory inputs but also disturb the visceromotor outputs. Previous studies have found reduced volumes and abnormal activities in these regions in IBS patients (Labus et al., 2014; Guleria et al., 2017), and pain sensitivity questionnaire scores were found to have a positive correlation with gray matter volume in fusiform gyrus (Ruscheweyh et al., 2018).
After experiencing initial pain, this feeling could still be modified by top-down cognitive controls, which link to regions involved in high-level cognition and affects (Garcia-Larrea and Bastuji, 2018). And this also reflected in our results. ACG and IFG play important roles in cognitive control and negative affects, which are all influence factors for IBS development (Beer et al., 2006; Shackman et al., 2011; Obermann et al., 2013). A prior study pointed out that the reduction of gray matter volume in frontal gyrus was associated with impaired cognitive function (Luo et al., 2015). Previous studies investigating pain disorders found reduced GM in ACG as well (Obermann et al., 2013; Kregel et al., 2015). Putamen is another region that involved in modulation and emotional responses to pain (Scott et al., 2006; Borsook et al., 2010). Azqueta-Gavaldon et al. (2020) found that patients with complex regional pain syndrome had decreased gray matter density in the putamen and that increased connectivity between putamen and pre-/post-central gyrus was associated with more severe clinical pain. The interaction among these brain regions may provide a possible explanation for the abnormal visceral perception and visceral hypersensitivity in IBS.
Through multimodal conjunction analysis, the consistent regions which showed dysconnectivity converged with changed GM were detected, including hyper-connectivity combined with increased GM in left MFG, hyper-connectivity combined with decreased GM in left orbital IFG, and hypoconnectivity combined with decreased GM in the right putamen. Previous study pointed out that brain functional and microstructural changes in IBS patients were particularly located in regions associated with the processing, integration and modulation of sensory information (Mayer et al., 2015), which are consistent with our results. As illustrated above, IFG and putamen are related to pain integration and modulation, while MFG is a part of prefrontal cortex and implicated in attention modulation (Japee et al., 2015), and a previous meta-analysis of patients with IBS also found abnormal brain activities in this area (Su et al., 2022). Researchers also elucidated that its abnormal activity might be associated with selective attention to stomach sensations in patients with functional dyspepsia (Zeng et al., 2011). Our results showed functional as well as structural changes in MFG, and this could imply that the gastrointestinal symptoms in IBS patients may relate to inappropriate attention toward stomach sensation. On the whole, these results further indicated that IBS is a psychosomatic disorder, and psychological factors may be important moderators in patients with IBS (Fond et al., 2014).
Some limitations should be taken into account when interpreting the results in our study. First, the present meta-analysis only contains six studies of FC and six studies of VBM, which is a relatively small sample size. Considering the difficulty of recruiting eligible patients and the diversity of research methods in rs-fMRI studies, it is challenging to incorporate a large number of methods-specific articles and this seems to be a common dilemma in meta-analysis of fMRI studies. Nevertheless, our study could be seen as an exploratory study to provide the first meta-analytic evidence of DMN together with VBM alterations in IBS. Future studies should include more up-to-date studies and confirm these findings. Second, our VBM meta-analysis only includes studies focusing on gray matter changes, therefore, future studies should conduct meta-analysis of diffusion tensor imaging in IBS patients to explore white matter changes. Furthermore, our study only focused on DMN, future meta-analyses should take other important brain networks into consideration as well.
Our study presents evidence on dysconnectivity of DMN and aberrant changes of GM in IBS. IBS patients exhibited disrupted associations within DMN and between DMN and the limbic system. Besides, altered GMV was demonstrated in regions mostly associated with pain and emotion processing. And multimodal analysis found that hyper/hypo-connectivity converged with GM changes was located in brain regions involved in attention, emotion and pain processing. These results could help us to further understand the pathophysiology of IBS and motivate future studies to identify potential IBS biomarkers.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
JR, YY, and XJ contributed to the conception of the study. MZ, SH, and JW conducted screening, data extraction, and statistical analysis. ML and YG contributed to analysis and manuscript preparation. MZ and ZH wrote the first draft of the manuscript. ZH and CA helped review and editing the manuscript. All authors read and approve the final manuscript.
This research was supported by the National Natural Science Foundation of China (No. 82001898).
We would like to thank all authors in the research involved in the meta-analysis for use their data and coordinates.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1236069/full#supplementary-material
Aizawa, E., Sato, Y., Kochiyama, T., Saito, N., Izumiyama, M., Morishita, J., et al. (2012). Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology 143, 1188–1198. doi: 10.1053/j.gastro.2012.07.104
Azqueta-Gavaldon, M., Youssef, A. M., Storz, C., Lemme, J., Schulte-Göcking, H., Becerra, L., et al. (2020). Implications of the putamen in pain and motor deficits in complex regional pain syndrome. Pain 161, 595–608. doi: 10.1097/j.pain.0000000000001745
Beer, J. S., John, O. P., Scabini, D., and Knight, R. T. (2006). Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. J. Cogn. Neurosci. 18, 871–879. doi: 10.1162/jocn.2006.18.6.871
Bhatt, R. R., Gupta, A., Labus, J. S., Liu, C., Vora, P. P., Jean, S., et al. (2022). A neuropsychosocial signature predicts longitudinal symptom changes in women with irritable bowel syndrome. Mol. Psychiatry 27, 1774–1791.
Bhatt, R. R., Gupta, A., Labus, J. S., Zeltzer, L. K., Tsao, J. C., Shulman, R. J., et al. (2019). Altered brain structure and functional connectivity and its relation to pain perception in girls with irritable bowel syndrome. Psychosom. Med. 81, 146–154. doi: 10.1097/psy.0000000000000655
Bi, X. A., Li, L., Xu, R., and Xing, Z. (2021a). Pathogenic factors identification of brain imaging and gene in late mild cognitive impairment. Interdiscip. Sci. 13, 511–520. doi: 10.1007/s12539-021-00449-0
Bi, X. A., Wu, H., Xie, Y., Zhang, L., Luo, X., and Fu, Y. (2021b). The exploration of Parkinson’s disease: A multi-modal data analysis of resting functional magnetic resonance imaging and gene data. Brain Imaging Behav. 15, 1986–1996. doi: 10.1007/s11682-020-00392-6
Borsook, D., Upadhyay, J., Chudler, E. H., and Becerra, L. (2010). A key role of the basal ganglia in pain and analgesia–insights gained through human functional imaging. Mol. Pain 6:27. doi: 10.1186/1744-8069-6-27
Brady, R. O., Masters, G. A., Mathew, I. T., Margolis, A., Cohen, B. M., Öngür, D., et al. (2016). State dependent cortico-amygdala circuit dysfunction in bipolar disorder. J. Affect. Disord. 201, 79–87. doi: 10.1016/j.jad.2016.04.052
Catani, M., Dell’acqua, F., and Thiebaut de Schotten, M. (2013). A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 37, 1724–1737. doi: 10.1016/j.neubiorev.2013.07.001
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129(Pt. 3), 564–583. doi: 10.1093/brain/awl004
Chen, X. F., Guo, Y., Lu, X. Q., Qi, L., Xu, K. H., Chen, Y., et al. (2021). Aberrant intraregional brain activity and functional connectivity in patients with diarrhea-predominant irritable bowel syndrome. Front. Neurosci. 15:721822. doi: 10.3389/fnins.2021.721822
Cheng, S., Xu, G., Zhou, J., Qu, Y., Li, Z., He, Z., et al. (2020). A multimodal meta-analysis of structural and functional changes in the brain of tinnitus. Front. Hum. Neurosci. 14:28. doi: 10.3389/fnhum.2020.00028
Davis, K., Pope, G., Chen, J., Kwan, C., Crawley, A., and Diamant, N. J. N. (2008). Cortical thinning in IBS: Implications for homeostatic, attention, and pain processing. Neurology 70, 153–154.
Drossman, D. A., Camilleri, M., Mayer, E. A., and Whitehead, W. E. (2002). AGA technical review on irritable bowel syndrome. Gastroenterology 123, 2108–2131.
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Fang, J. (2013). Altered regional gray matter volume in patients with irritable bowel syndrome revealed by VBM. J. Clin. Radiol. 32, 1702–1705. doi: 10.13437/j.cnki.jcr.2013.12.004
Farmer, M. A., Baliki, M. N., and Apkarian, A. V. (2012). A dynamic network perspective of chronic pain. Neurosci. Lett. 520, 197–203. doi: 10.1016/j.neulet.2012.05.001
Fond, G., Loundou, A., Hamdani, N., Boukouaci, W., Dargel, A., Oliveira, J., et al. (2014). Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 264, 651–660. doi: 10.1007/s00406-014-0502-z
Ford, A. C., Sperber, A. D., Corsetti, M., and Camilleri, M. (2020). Irritable bowel syndrome. Lancet 396, 1675–1688. doi: 10.1016/S0140-6736(20)31548-8
Fox, M. D., and Greicius, M. (2010). Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 4:19. doi: 10.3389/fnsys.2010.00019
Frot, M., Mauguière, F., Magnin, M., and Garcia-Larrea, L. (2008). Parallel processing of nociceptive A-delta inputs in SII and midcingulate cortex in humans. J. Neurosci. 28, 944–952. doi: 10.1523/jneurosci.2934-07.2008
Garcia-Larrea, L., and Bastuji, H. (2018). Pain and consciousness. Prog. Neuropsychopharmacol. Biol. Psychiatry 87(Pt. B), 193–199. doi: 10.1016/j.pnpbp.2017.10.007
Geng, H., Weng, S.-J., Zhao, T.-T., Chen, L., Wu, X.-L., Zhou, J.-L., et al. (2021). Mind-regulating and spleen-strengthening needling technique improves abdominal hypersensitivity and emotion by enhancing functional connectivity between hippocampus and brain regions in diarrhea-predominant irritable bowel syndrome patients. Zhen Ci Yan Jiu 46, 318–325.
Gralnek, I. M., Hays, R. D., Kilbourne, A., Naliboff, B., and Mayer, E. A. (2000). The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 119, 654–660.
Guleria, A., Karyampudi, A., Singh, R., Khetrapal, C. L., Verma, A., Ghoshal, U. C., et al. (2017). Mapping of brain activations to rectal balloon distension stimuli in male patients with irritable bowel syndrome using functional magnetic resonance imaging. J. Neurogastroenterol. Motil. 23, 415–427. doi: 10.5056/jnm16148
Gupta, A., Kilpatrick, L., Labus, J., Tillisch, K., Braun, A., Hong, J. Y., et al. (2014). Early adverse life events and resting state neural networks in patients with chronic abdominal pain: Evidence for sex differences. Psychosom. Med. 76, 404–412. doi: 10.1097/psy.0000000000000089
Hubbard, C. S., Becerra, L., Heinz, N., Ludwick, A., Rasooly, T., Wu, R., et al. (2016). Abdominal pain, the adolescent and altered brain structure and function. PLoS One 11:e0156545. doi: 10.1371/journal.pone.0156545
Icenhour, A., Witt, S. T., Elsenbruch, S., Lowén, M., Engström, M., Tillisch, K., et al. (2017). Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. Neuroimage Clin. 15, 449–457. doi: 10.1016/j.nicl.2017.06.001
Japee, S., Holiday, K., Satyshur, M. D., Mukai, I., and Ungerleider, L. G. (2015). A role of right middle frontal gyrus in reorienting of attention: A case study. Front. Syst. Neurosci. 9:23. doi: 10.3389/fnsys.2015.00023
Kano, M., Dupont, P., Aziz, Q., and Fukudo, S. (2018). Understanding neurogastroenterology from neuroimaging perspective: A comprehensive review of functional and structural brain imaging in functional gastrointestinal disorders. J Neurogastroenterol. Motil. 24, 512–527. doi: 10.5056/jnm18072
Koloski, N. A., Jones, M., and Talley, N. J. (2016). Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: A 1-year population-based prospective study. Aliment. Pharmacol. Ther. 44, 592–600. doi: 10.1111/apt.13738
Kregel, J., Meeus, M., Malfliet, A., Dolphens, M., Danneels, L., Nijs, J., et al. (2015). Structural and functional brain abnormalities in chronic low back pain: A systematic review. Semin. Arthr. Rheum. 45, 229–237. doi: 10.1016/j.semarthrit.2015.05.002
Kucyi, A., and Davis, K. D. (2015). The dynamic pain connectome. Trends Neurosci. 38, 86–95. doi: 10.1016/j.tins.2014.11.006
Labus, J. S., Dinov, I. D., Jiang, Z., Ashe-McNalley, C., Zamanyan, A., Shi, Y., et al. (2014). Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain 155, 137–149. doi: 10.1016/j.pain.2013.09.020
Lee, H. F., Hsieh, J. C., Lu, C. L., Yeh, T. C., Tu, C. H., Cheng, C. M., et al. (2012). Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain 153, 1301–1310. doi: 10.1016/j.pain.2012.03.018
Letzen, J. E., Craggs, J. G., Perlstein, W. M., Price, D. D., and Robinson, M. E. (2013). Functional connectivity of the default mode network and its association with pain networks in irritable bowel patients assessed via lidocaine treatment. J. Pain 14, 1077–1087. doi: 10.1016/j.jpain.2013.04.003
Li, J., Huang, X., Sang, K., Bodner, M., Ma, K., and Dong, X. W. (2018). Modulation of prefrontal connectivity in postherpetic neuralgia patients with chronic pain: A resting-state functional magnetic resonance-imaging study. J. Pain Res. 11, 2131–2144. doi: 10.2147/jpr.S166571
Li, J., Yuan, B., Li, G., Lu, X., Guo, Y., Yang, Y., et al. (2020). Convergent syndromic atrophy of pain and emotional systems in patients with irritable bowel syndrome and depressive symptoms. Neurosci. Lett. 723:134865. doi: 10.1016/j.neulet.2020.134865
Li, S., Jiang, G., Ma, X., Su, H., Zhan, W., Tian, J., et al. (2013). [Functional connectivity of right hippocampus in irritable bowel syndrome during resting state]. J. Sun Yat-sen Univ (Med. Sci.) 34, 932–937. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2013.0164
Liu, X., Silverman, A., Kern, M., Ward, B. D., Li, S. J., Shaker, R., et al. (2016). Excessive coupling of the salience network with intrinsic neurocognitive brain networks during rectal distension in adolescents with irritable bowel syndrome: A preliminary report. Neurogastroenterol. Motil. 28, 43–53. doi: 10.1111/nmo.12695
Longarzo, M., Quarantelli, M., Aiello, M., Romano, M., Del Prete, A., Cimminiello, C., et al. (2017). The influence of interoceptive awareness on functional connectivity in patients with irritable bowel syndrome. Brain Imaging Behav. 11, 1117–1128. doi: 10.1007/s11682-016-9595-5
Lowe, M. J., Dzemidzic, M., Lurito, J. T., Mathews, V. P., and Phillips, M. D. (2000). Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage 12, 582–587. doi: 10.1006/nimg.2000.0654
Luo, W., Jiang, X., Wei, X., Li, S., and Li, M. (2015). A study on cognitive impairment and gray matter volume abnormalities in silent cerebral infarction patients. Neuroradiology 57, 783–789. doi: 10.1007/s00234-015-1535-3
Ma, X., Li, S., Tian, J., Jiang, G., Wen, H., Wang, T., et al. (2015). Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: A resting-state fMRI study. Clin. Neurophysiol. 126, 1190–1197. doi: 10.1016/j.clinph.2014.10.004
Mayer, E. A., Labus, J. S., Tillisch, K., Cole, S. W., and Baldi, P. (2015). Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 12, 592–605. doi: 10.1038/nrgastro.2015.121
Messina, R., Rocca, M. A., Valsasina, P., Misci, P., and Filippi, M. (2022). Clinical correlates of hypothalamic functional changes in migraine patients. Cephalalgia 42, 279–290. doi: 10.1177/03331024211046618
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Murray, I., Bhanot, G., and Bhargava, A. (2021). Neuron-glia-immune triad and cortico-limbic system in pathology of pain. Cells 10:1553. doi: 10.3390/cells10061553
Nichols, T., Brett, M., Andersson, J., Wager, T., and Poline, J. B. (2005). Valid conjunction inference with the minimum statistic. Neuroimage 25, 653–660. doi: 10.1016/j.neuroimage.2004.12.005
Obermann, M., Rodriguez-Raecke, R., Naegel, S., Holle, D., Mueller, D., Yoon, M. S., et al. (2013). Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 74, 352–358. doi: 10.1016/j.neuroimage.2013.02.029
Öhlmann, H., Koenen, L. R., Labrenz, F., Engler, H., Theysohn, N., Langhorst, J., et al. (2021). Altered brain structure in chronic visceral pain: Specific differences in gray matter volume and associations with visceral symptoms and chronic stress. Front. Neurol. 12:733035. doi: 10.3389/fneur.2021.733035
Öngür, D., and Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219. doi: 10.1093/cercor/10.3.206
Paine, P. (2021). Review article: Current and future treatment approaches for pain in IBS. Aliment. Pharmacol. Ther. 54(Suppl. 1), S75–S88. doi: 10.1111/apt.16550
Ploner, M., Lee, M. C., Wiech, K., Bingel, U., and Tracey, I. (2011). Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb. Cortex 21, 719–726. doi: 10.1093/cercor/bhq146
Price, D. D., Zhou, Q., Moshiree, B., Robinson, M. E., and Verne, G. N. (2006). Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J. Pain 7, 529–535.
Qi, R., Ke, J., Schoepf, U. J., Varga-Szemes, A., Milliken, C. M., Liu, C., et al. (2016). Topological reorganization of the default mode network in irritable bowel syndrome. Mol. Neurobiol. 53, 6585–6593. doi: 10.1007/s12035-015-9558-7
Radua, J., and Mataix-Cols, D. (2009). Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry 195, 393–402. doi: 10.1192/bjp.bp.108.055046
Radua, J., Mataix-Cols, D., Phillips, M. L., El-Hage, W., Kronhaus, D. M., Cardoner, N., et al. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Radua, J., Romeo, M., Mataix-Cols, D., and Fusar-Poli, P. (2013). A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Curr. Med. Chem. 20, 462–466.
Radua, J., Rubia, K., Canales-Rodríguez, E. J., Pomarol-Clotet, E., Fusar-Poli, P., and Mataix-Cols, D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry 5:13. doi: 10.3389/fpsyt.2014.00013
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. USA. 98, 676–682.
Ruscheweyh, R., Wersching, H., Kugel, H., Sundermann, B., and Teuber, A. (2018). Gray matter correlates of pressure pain thresholds and self-rated pain sensitivity: A voxel-based morphometry study. Pain 159, 1359–1365. doi: 10.1097/j.pain.0000000000001219
Scott, D. J., Heitzeg, M. M., Koeppe, R. A., Stohler, C. S., and Zubieta, J. K. (2006). Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J. Neurosci. 26, 10789–10795. doi: 10.1523/jneurosci.2577-06.2006
Seminowicz, D. A., Labus, J. S., Bueller, J. A., Tillisch, K., Naliboff, B. D., Bushnell, M. C., et al. (2010). Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 139, 48–U82. doi: 10.1053/j.gastro.2010.03.049
Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, A. S., Winter, J. J., and Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167.
Skrobisz, K., Piotrowicz, G., Naumczyk, P., Sabisz, A., Markiet, K., Rydzewska, G., et al. (2020). Imaging of morphological background in selected functional and inflammatory gastrointestinal diseases in fMRI. Front. Psychiatry 11:461. doi: 10.3389/fpsyt.2020.00461
Spagna, A., Hajhajate, D., Liu, J., and Bartolomeo, P. (2021). Visual mental imagery engages the left fusiform gyrus, but not the early visual cortex: A meta-analysis of neuroimaging evidence. Neurosci. Biobehav. Rev. 122, 201–217. doi: 10.1016/j.neubiorev.2020.12.029
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002. doi: 10.1136/bmj.d4002
Su, C., Liu, W., Wang, Q., Qiu, S., Li, M., Lv, Y., et al. (2022). Abnormal resting-state local spontaneous functional activity in irritable bowel syndrome patients: A meta-analysis. J. Affect. Disord. 302, 177–184. doi: 10.1016/j.jad.2022.01.075
Tillisch, K., Mayer, E. A., and Labus, J. S. (2011). Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140, 91–100. doi: 10.1053/j.gastro.2010.07.053
Wilder-Smith, C. H., Schindler, D., Lovblad, K., Redmond, S. M., and Nirkko, A. (2004). Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 53, 1595–1601. doi: 10.1136/gut.2003.028514
Wu, H., Yan, H., Yang, Y., Xu, M., Shi, Y., Zeng, W., et al. (2020). Occupational neuroplasticity in the human brain: A critical review and meta-analysis of neuroimaging studies. Front. Hum. Neurosci. 14:215. doi: 10.3389/fnhum.2020.00215
Yan, H., Wu, H., Cai, Z., Du, S., Li, L., Xu, B., et al. (2023). The neural correlates of apathy in the context of aging and brain disorders: A meta-analysis of neuroimaging studies. Front. Aging Neurosci. 15:1181558. doi: 10.3389/fnagi.2023.1181558
Yasuda, C. L., Betting, L. E., and Cendes, F. (2010). Voxel-based morphometry and epilepsy. Expert Rev. Neurother. 10, 975–984. doi: 10.1586/ern.10.63
Zeng, F., Qin, W., Liang, F., Liu, J., Tang, Y., Liu, X., et al. (2011). Abnormal resting brain activity in patients with functional dyspepsia is related to symptom severity. Gastroenterology 141, 499–506. doi: 10.1053/j.gastro.2011.05.003
Zhang, Q. E., Wang, F., Qin, G., Zheng, W., Ng, C. H., Ungvari, G. S., et al. (2018). Depressive symptoms in patients with irritable bowel syndrome: A meta-analysis of comparative studies. Int. J. Biol. Sci. 14, 1504–1512. doi: 10.7150/ijbs.25001
Zhao, L., Wang, Y., and Zhang, Y. (2018). Microstructural changes in the brain in elderly patients with irritable bowel syndrome. Aging Med. 1, 141–148. doi: 10.1002/agm2.12034
Zhao, T., Pei, L., Ning, H., Guo, J., Song, Y., Zhou, J., et al. (2021). Networks are associated with acupuncture treatment in patients with diarrhea-predominant irritable bowel syndrome: A resting-state imaging study. Front. Hum. Neurosci. 15:736512. doi: 10.3389/fnhum.2021.736512
Keywords: irritable bowel syndrome, meta-analysis, default mode network, functional connectivity, voxel-based morphometry
Citation: Zhao M, Hao Z, Li M, Xi H, Hu S, Wen J, Gao Y, Antwi CO, Jia X, Yu Y and Ren J (2023) Functional changes of default mode network and structural alterations of gray matter in patients with irritable bowel syndrome: a meta-analysis of whole-brain studies. Front. Neurosci. 17:1236069. doi: 10.3389/fnins.2023.1236069
Received: 07 June 2023; Accepted: 09 October 2023;
Published: 24 October 2023.
Edited by:
Nizhuan Wang, ShanghaiTech University, ChinaReviewed by:
Pan Zhang, Chengdu University of Traditional Chinese Medicine, ChinaCopyright © 2023 Zhao, Hao, Li, Xi, Hu, Wen, Gao, Antwi, Jia, Yu and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Ren, cmVuakB6am51LmNu; Yang Yu, eXV5YW5nMTAyM0B6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.