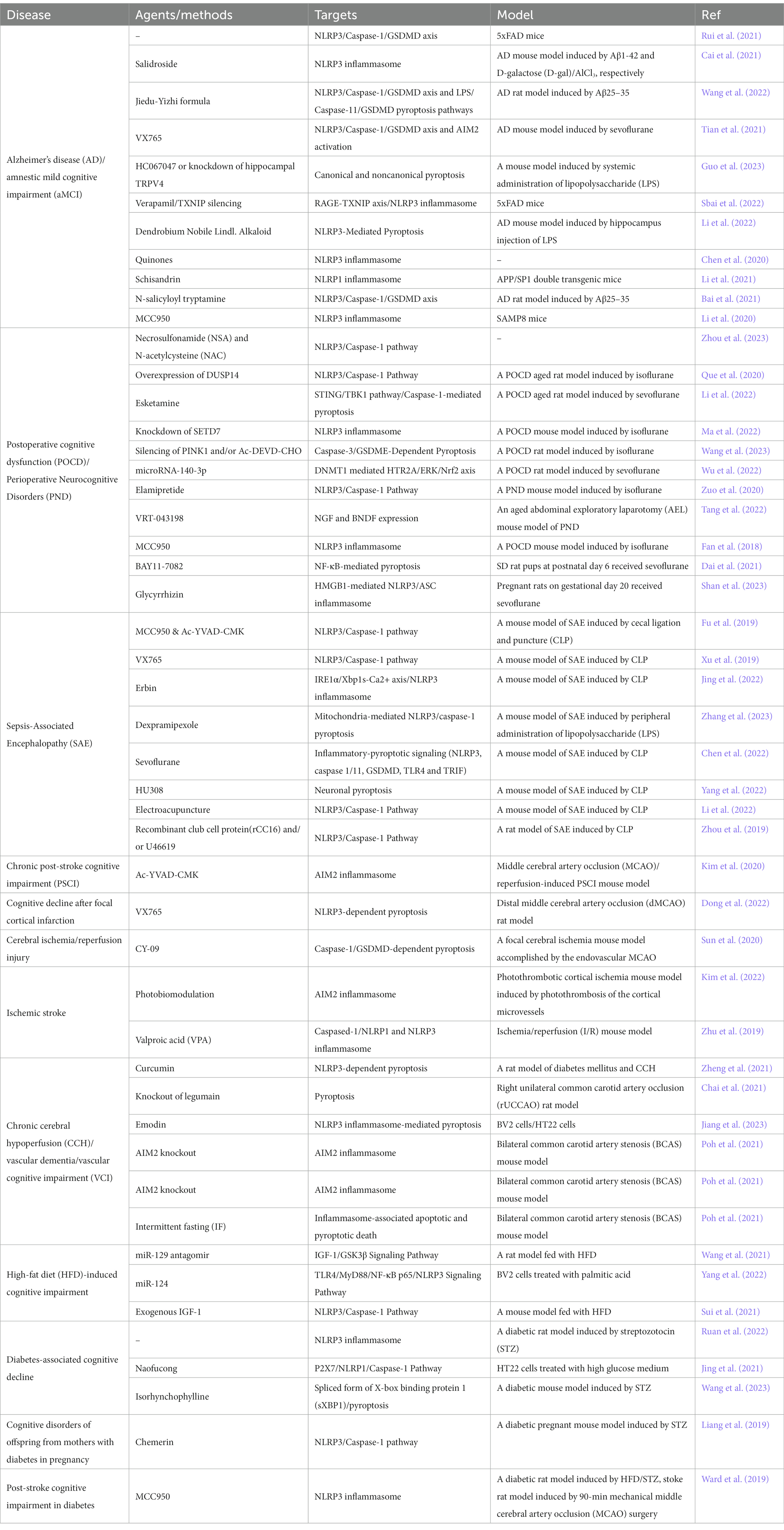
- 1Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Thoracic Surgery, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Cognitive impairment is a major global disease, manifests as a decline in cognitive functioning and endangers the health of the population worldwide. The incidence of cognitive impairment has increased rapidly with an increasingly aging population. Although the mechanisms of cognitive impairment have partly been elucidated with the development of molecular biological technology, treatment methods are very limited. As a unique form of programmed cell death, pyroptosis is highly pro-inflammatory and is closely associated with the incidence and progression of cognitive impairment. In this review, we discuss the molecular mechanisms of pyroptosis briefly and the research progress on the relationship between pyroptosis and cognitive impairment and its potential therapeutic values, to provide a reference for research in the field of cognitive impairment.
Introduction
Cognitive impairment is defined as a decline in cognitive functioning and endangers the health of the population especially elder people worldwide. The degree of cognitive impairment ranges from mild subjective cognitive impairment to severe dementia. Mild cognitive impairment (MCI) has a prevalence of approximately 6% in ages 60–64 and increases to approximately 25% in those ages 80–84 (Petersen et al., 2018). Approximately 5%–10% of people with MCI progress to dementia annually (Sanford, 2017). With an increasingly aging population, the incidence of cognitive impairment has increased rapidly. Although the mechanisms of cognitive impairment have partly been elucidated with the development of molecular biological technology, however, the tools and strategies for the treatment of cognitive impairment remained limited. To fully understand the neuropathological changes of cognitive impairment and develop effective therapeutic methods, there is still a long way to go.
The long-recognized modes of cell death are limited to apoptosis and necrosis. Apoptosis is mainly characterized by cell shriveling and the formation of apoptotic bodies, which are then rapidly engulfed by surrounding phagocytes without causing an inflammatory response. Necrosis was previously thought to be an unregulated and passive death process, but with further research, it has now been shown that some of the necrosis can be controlled and is called programmed necrosis (Tonnus et al., 2019), of which pyroptosis is one of the main forms. Recently increasing studies have revealed that pyroptosis played a significant role in cognitive impairment. This review summarized the molecular mechanisms of pyroptosis and focus on the function of pyroptosis involved in the pathogenesis and treatment of cognitive impairment-related diseases.
Molecular mechanisms of pyroptosis
Pyroptosis, a highly pro-inflammatory programmed cell death, was first observed in macrophages after a bacterial infection or treatment with bacterial toxins and was for a long time mistaken for a macrophage-specific cell death dependent on caspase-1, a pro-inflammatory protease cleaving interleukin 1β (IL-1β) (Thornberry et al., 1992). Subsequent studies revealed that intracytoplasmic pattern recognition receptors (PRRs) recognized exogenous pathogen-associated molecular patterns (PAMPs) or endogenous dangerous signaling to form inflammasomes that recruited and activated caspase-1, thus leading to pyroptosis (canonical pyroptosis pathway); murine caspase-11 and human caspase-4/5 could act directly as PRRs to recognize inflammasome assembled by polysaccharide-like lipid A and also result in pyroptosis (non-canonical pyroptosis pathway), which overturned the traditional concept of inflammasome (Fang et al., 2020). Recent studies have found that caspase-1 and caspase-11/4/5 both cleaved the common substrate gasdermin D (GSDMD) and led to pyroptosis (Kayagaki et al., 2015; Shi et al., 2015), gasdermin family proteins were identified as key effector molecules that mediate the onset of pyroptosis (Figure 1).
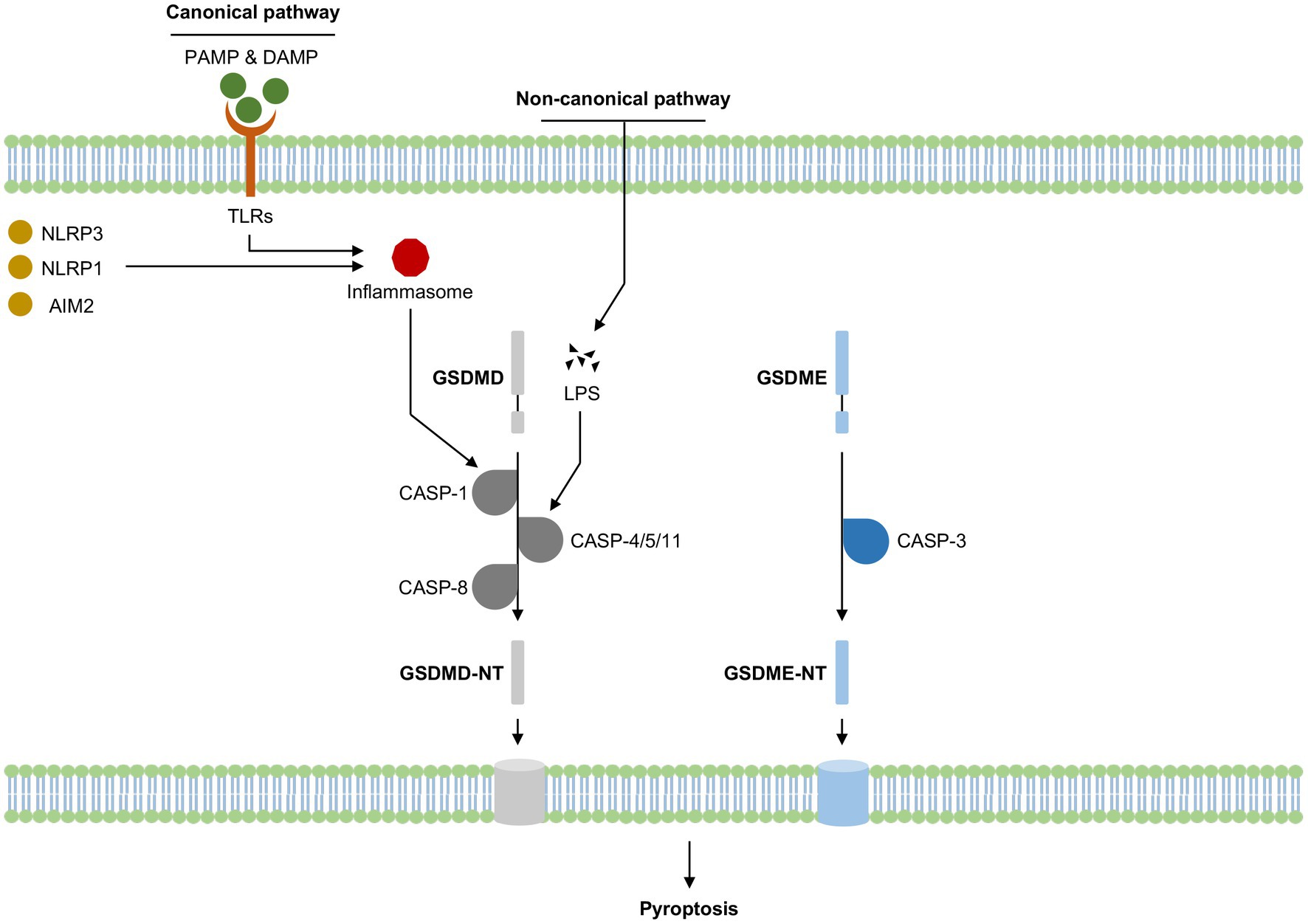
Figure 1. Summary of pyroptosis activation in cognitive impairment-related diseases. LPS, Lipopolysaccharide; CASP, Caspase; GZM, Granzyme; NT, N-terminal domain; PAMP, Pathogen-associated molecular patterns; DAMP, Damage-associated molecular patterns; TLRs, Toll-like receptors; NLRP3, nucleotide-binding oligomerization domain-like receptor protein 3; NLRP1, NLR family pyrin domain containing 1; AIM2, melanoma deficiency factor 2.
The canonical pyroptosis pathway was dependent on the activation of caspase-1, which exerted its effects via the inflammasome pathway. Inflammasome was first proposed in 2002 (Martinon et al., 2002) and was now defined as a class of multimeric protein complexes mainly composed of PRRs, apoptosis-associated speck-like protein containing CARD (ASC), and pro-caspase-1, to identify various irritating and damaging signals in natural immune responses and had a close relationship to the occurrence of cell death (Lamkanfi and Dixit, 2017). PRRs mainly included Toll-like receptors (TLRs), melanoma deficiency factor 2 (AIM2) and NOD-like receptors (NLRs), of which nucleotide-binding oligomerization domain-like receptor protein3 (NLRP3) got the most attention. PRRs could identify PAMPs or endogenous dangerous signaling to form inflammasome complex after two stages of priming and activation, which in turn activates its downstream caspase-1. Activated caspase-1 could cleave GSDMD to GSDMD-N terminal, forming pores in the cell membrane. Meanwhile, the pro-inflammatory factors pro-IL-1 and pro-IL-18 were also cleaved by activated caspase-1, transferred to mature IL-1β and IL-18, releasing from the cell membrane pores, thus inducing pyroptosis. Numerous studies have demonstrated that NLRP3/caspase-1 mediated pyroptosis signaling pathway played a crucial role and was the most common therapeutic target of cognitive impairment (Van Zeller et al., 2021; Li et al., 2022), any blockade of NLRP3/caspase-1 mediated pyroptosis may reverse the incidence or progression of cognitive impairment.
The non-canonical pyroptosis pathway was dependent on the activation of human caspase-4/-5 and homologous murine caspase-11 by intracellular lipopolysaccharides (LPS). LPS typically consisted of a hydrophobic domain known as lipid A (or endotoxin), a non-repeating “core” oligosaccharide, and a distal polysaccharide (or O-antigen) (Raetz and Whitfield, 2002). LPS could directly bind and activate caspase-4/-5/-11 protein to initiate pyroptosis (Zheng et al., 2021). Similar to the canonical pyroptosis pathway, activated caspase-4/-5/-11 cleaved GSDMD to GSDMD-N terminal, inducing pore formation in the cell membrane (Shi et al., 2017). During the process, LPS could also induce caspase-11-dependent cleavage of the pannexin-1 channel protein, activating the P2X purinoreceptor 7 (P2X7) receptor-dependent membrane pore opening and causing subsequent ATP release, K+ efflux, osmotic imbalance, leading to cell swelling and membrane rupture, and eventually resulting in pyroptosis (Shi et al., 2015; Liu et al., 2016; He et al., 2023). Furthermore, released ATP and K+ efflux through pannexin-1 transmembrane channel could activate NLRP3 inflammasome and IL-1β secretion, indicating that NLRP3 might be a crucial bridge between the canonical and non-canonical pyroptosis pathways (Karmakar et al., 2015; Yang et al., 2015; Karmakar et al., 2016).
GSDMD belonged to gasdermin family proteins and was primarily identified by two separate studies in 2015, Dixit et al. found that GSDMD played a key role in LPS-induced activation of the non-canonical inflammasome by screening chemically induced mouse mutants (Kayagaki et al., 2015), while Shao et al. performed a genome-wide screen of caspase-11 and caspase-1-induced pyroptosis pathways in cell lines, which revealed that GSDMD is the substrate of all inflammatory caspases and is the true executor of pyroptosis (Shi et al., 2015). Multiple caspases such as caspase-1/-4/-5/-8/-11 could cleave and activate GSDMD. Caspase-1 activated GSDMD through inflammasome complexes such as AIM2, NLRC4 or NLRP3 (Broz and Dixit, 2016). The activation of GSDMD by caspase-4 was reported to be regulated by interferon regulatory factor 2 (Benaoudia et al., 2019; Kayagaki et al., 2019). It was reported that caspase-8-dependent GSDMD cleavage relied on caspase-8 dimerization and autoprocessing (Demarco et al., 2020). LPS-triggered caspase-11-GSDMD signaling pathway was upregulated by IFN-γ and IFN-β (Brubaker et al., 2020; Zhang et al., 2020). Unlike GSDMD-mediated pyroptosis, gasdermin E (GSDME)-induced pyroptosis mainly relied on the activation of caspase-3. Traditionally caspase-3 is an apoptosis-related caspase, which could be activated under the treatment of TNF-α or chemotherapy drugs, inducing cell apoptosis. However, when GSDME existed, activated caspase-3 would cleave GSDME at the site of residue Asp270 and induce pyroptosis instead of apoptosis (Ouyang et al., 2023). The role of GSDMD and GSDME-mediated pyroptosis in cognitive impairment have been elucidated, but the mechanisms of other gasdermin family members such as GSDMA, GSDMB, GSDMC and DFNB59 in cognitive impairment remained unclear.
Pyroptosis and cognitive impairment
Alzheimer’s disease
The most common neurodegenerative condition affecting the aged population is Alzheimer’s disease (AD), which is characterized by a particular sequence of pathological alterations in the brain that cause neurodegeneration, loss of synaptic connections, progressive memory problems, and cognitive impairment (Masters et al., 2015). Unfortunately, despite a large number of studies on the mechanisms of AD, there were no approved therapies to halt or reverse its progression (Van Zeller et al., 2021). Recent studies elucidated the relationship between pyroptosis and AD, and some drugs targeting the pyroptosis pathway showed therapeutic potential.
Rui et al. (2021) detected the expression levels of NLRP3, caspase-1, GSDMD, and IL-1β and found all the above parameters were increased in the peripheral blood mononuclear cells (PBMCs) of amnestic mild cognitive impairment (aMCI) and AD patients, and IL-1β was positively associated with the disease, indicating the important role of pyroptosis in AD. Li et al. (2020) found that neuronal pyroptosis induced by the overexpression of NLRP3/caspase-1/GSDMD axis was the key cause of neuronal loss in AD, NLRP3 inhibitor MCC950 could inhibit neuronal pyroptosis by downregulating NLRP3/caspase-1/GSDMD axis, reduced the neurotoxicity of amyloid-β1-42 (Aβ1-42) in vitro, improved the spatial memory ability in vivo. NLRP3 inhibitor might be a potential therapeutic agent of AD. Tian et al. (2021) revealed that activated caspase-1 directly induced pyroptosis through NLRP3 and AIM2 activation in an AD mouse model induced by sevoflurane, caspase-1 small-molecule inhibitor VX-765 could significantly inhibit the pyroptosis pathway, suppress the release of IL-1β and IL-18 and downregulate tau phosphorylation, thus restoring neuron function of AD. Several studies focused on the ameliorative effects of traditional Chinese medicine in AD. Salidroside, the main pharmacological active ingredient isolated from Rhodiola rosea L. (Cai et al., 2021), Dendrobium nobile Lindl. Alkaloid (DNLA), the main active compound in Dendrobium nobile Lindl (Li et al., 2022), as well as Jiedu-Yizhi formula (Wang et al., 2022) were found to inhibit NLRP3-mediated or LPS-induced pyroptosis and improve AD. Li et al. (2021) revealed that Schisandrin, a representative lignan of Schisandra chinensis Bail., could also inhibit Aβ-induced NLRP-1-mediated neuronal pyroptosis and ameliorate cognitive impairment of AD. Quinones in Chinese Medicine could potentially prevent AD via modulating the NLRP3 inflammasomes, adopt molecular docking study indicated that purpurin and rhein might be the most promising NLRP3 inhibitors, however, further study was required to ascertain the preventive effect (Chen et al., 2020). Bai et al. (2021) elucidated that N-salicyloyl tryptamine derivatives could restore Aβ-induced pyroptosis through NLRP3/caspase-1/GSDMD axis and ameliorate cognitive function. It was reported that upregulated RAGE–TXNIP axis or activated transient receptor potential vanilloid 4 (TRPV4) participated in causing pyroptosis and result in cognitive impairment in AD, which could be the potential therapeutic target through suppression of overactivated pyroptosis (Sbai et al., 2022; Guo et al., 2023).
Perioperative neurocognitive disorder
Perioperative neurocognitive disorder (PND) is one of the common complications during the perioperative period and is mainly manifested as cognitive impairment. PNDs include acute postoperative delirium and relatively long-lasting postoperative cognitive dysfunction (Li et al., 2022). Age, surgical trauma, and anesthetics are the main risk factors of PND, however, the mechanisms of PND remained unclear. Many studies have elucidated the important role of pyroptosis in the pathogenesis of PND and targeting pyroptosis could be an effective method for PND treatment.
It was reported that NLRP3 inflammasome-mediated pyroptosis directly led to cognitive impairment in PND mice model induced by isoflurane, and NLRP3 inflammasome inhibitor MCC950 could inhibit overactivated pyroptosis and exert a neuroprotective effect, thus improving cognitive impairment (Fan et al., 2018). Zhou et al. demonstrated that pyroptosis, as well as the level of reactive oxygen species (ROS) was significantly upregulated in a postoperative cognitive dysfunction (POCD) mouse model induced by sevoflurane. Not only the pyroptosis inhibiter, necrosulfonamide (NSA), could improve cognitive impairment via suppressing pyroptosis, but also the ROS scavenger, N-acetylcysteine (NAC), could ameliorate POCD by reducing the level of ROS and pyroptosis through NLRP3 inflammasome pathway (Zhou et al., 2023). Caspase-1 inhibitor, VRT-043198, was reported to ameliorate PND in an aged mice model by inhibiting caspase-1-mediated pyroptosis (Tang et al., 2022). Zuo et al. (2020) revealed that elamipretide could also attenuate pyroptosis by inhibiting NLRP3/caspase-1 pathway and partly restore PND in aged mice. Que et al. (2020) demonstrated that isoflurane exposure resulted in cognitive dysfunction in aged rats, accompanied by decreased expression of DUSP14, and it was found that DUSP14 could regulate NLRP3-mediated pyroptosis, overexpression of DUSP14 inhibited pyroptosis and improved cognitive impairment, indicating that DUSP14 might be a new therapeutic target for POCD. Isoflurane induced hippocampal neuronal damage and cognitive impairment by upregulating SETD7 and activating pyroptosis in the hippocampus, knockdown of STED7 could inhibit the level of pyroptosis and the release of inflammatory cytokines, prevent hippocampus damage and improve POCD (Ma et al., 2022). Apart from NLRP3/caspase-1 axis, other pyroptosis pathways were revealed to be associated with PND. Wang et al. found that caspase-3 was activated in POCD, and activated caspase-3 could cleave gasdermin E (GSDME) to form GSDME-N terminal, forming pores in the cell membrane and inducing pyroptosis. Caspase-3 inhibitor Ac-DEVD-CHO (Ac-DC) could inhibit pyroptosis and improve cognitive impairment (Wang et al., 2023). NF-κB and HMGB1 induced pyroptosis were also elucidated in PND, NF-κB inhibitor or HMGB1 inhibitor treatment effectively improved PND by significantly inhibiting pyroptosis (Dai et al., 2021; Shan et al., 2023). Li et al. (2022) found that esketamine improved POCD in aged rats and alleviated the pyroptosis of astrocytes after LPS exposure, moreover, an underlying connection between STING/TBK1 signaling pathway and caspase-1-mediated pyroptosis was indicated, which required further research. MicroRNA-140-3p was found to improve POCD by repressing neuron pyroptosis via HTR2A/ERK/Nrf2 axis by targeting DNMT1 (Wu et al., 2022).
Sepsis-associated encephalopathy
Sepsis-associated encephalopathy (SAE) is a frequent complication that leads to long-term cognitive impairments and psychiatric diseases in sepsis patients and has a close association with increased morbidity and mortality. The potential mechanisms of SAE are complex, including endothelial dysfunction, damage to the blood–brain barrier, oxidative stress, etc. However, the molecular changes in SAE required further research. Accumulating evidence has indicated that pyroptosis may be the bridge between SAE and overactivated neuroinflammation, especially the NLRP3/caspase-1 pathway. NLRP3 inhibitor MCC950 and the caspase-1 inhibitor Ac-YVAD-CMK or VX765 were used for the treatment of SAE and the results were exhilarating. Administration of the above inhibitors could repress overactivated pyroptosis and the release of pro-inflammatory cytokines, restore the synapse plasticity and preserve long-term potential, thus improving cognitive dysfunction (Fu et al., 2019; Xu et al., 2019). It was mentioned that electroacupuncture could improve 7-day survival rates and cognitive function by downregulating NLRP3/caspase-1/GSDMD pyroptosis pathway in an SAE mouse model (Li et al., 2022). As the most important pyroptosis pathway, the activity of NLRP3/caspase-1 signaling pathway was mediated by various cell components or molecules. Zhou et al. found that p38 MAPK and ERK signaling pathways might regulate NLRP3/caspase-1 pathway, and the phosphorylation of p38 MAPK and ERK was positively correlated with NLRP3, caspase-1, and inflammatory factor levels in SAE. Downregulation of p38 MAPK and ERK led to suppression of pyroptosis, however, the direct connection between these two pathways needed deeper exploration (Zhou et al., 2019). Jing et al. revealed the importance of IRE1α/Xbp1s-Ca2+ signaling in endoplasmic reticulum (ER) stress, which was involved in NLRP3 inflammasome activation. IRE1α/Xbp1s pathway was activated, promoting the ER Ca2+ influx to the cytoplasm and inducing NLRP3 inflammasome-mediated pyroptosis. The selective inhibitor STF083010 targeting IRE1α/Xbp1s could partly restore the process, and improved cognitive function by attenuating microglial pyroptosis (Jing et al., 2022). Mitochondria-mediated pyroptosis was reported in SAE, mitochondria impairment was associated with cognitive dysfunction. The mitochondrial protectant dexpramipexole (DPX) could sustain mitochondrial function and inhibit NLRP3/caspase-1 pyroptosis pathway, thus ameliorating neuroinflammation and cognitive impairment in SAE (Zhang et al., 2023). Sevoflurane was reported to activate HMGB1-induced NLRP3/ASC inflammasome, induce pyroptosis, and impair cognitive function in PND (Shan et al., 2023), however, in SAE, sevoflurane could act as protective role. Chen et al. (2022) demonstrated that sevoflurane could improve cognitive dysfunction by inhibiting the NLRP3-dependent caspase-1/11-GSDMD pathway, in which SIRT1 played a key role. Yang et al. showed that cannabinoid type 2 receptor (CB2R) helped to protect neurons and promote survival in SAE patients. Furthermore, it has been proven that the CB2R-specific agonist HU308 could repress neuronal pyroptosis, attenuate brain tissue damage and improve cognitive impairment in SAE (Yang et al., 2022).
Cerebrovascular diseases
Cerebrovascular diseases are also key contributors to the overall burden of cognitive impairment and mainly include acute ischemia stroke and chronic cerebral hypoperfusion (CCH). An acute ischemic stroke occurs due to the sudden interruption or reduction of blood supply in part of the brain, and the process is often combined with pre-existing microvascular and neurodegenerative changes, which results in a series of pathological changes leading to cognitive impairment (Rost et al., 2021). CCH is caused by chronic reduction of cerebral blood flow, which is a common pathophysiological process in cerebral vascular diseases such as atherosclerosis or arteriosclerosis, leading to a state of prolonged ischemia and hypoxia in the brain tissue, finally results in progressive and persistent cognitive impairment (He et al., 2023). Although the mechanisms of cerebrovascular disease-induced cognitive dysfunction have not been fully understood, it has been identified that pyroptosis was involved in the pathological process.
Kim et al. elucidated that AIM2 inflammasome, as well as caspase-1, IL-1β, IL-18, was significantly upregulated in the hippocampus and cortex in the mouse model of post-stroke cognitive impairment than in those of the sham group. AIM2 inflammasome-mediated pyroptosis could cause acute and chronic neuronal death after stroke, which might result in cognitive dysfunction. Moreover, knockout of AIM2 or inhibition of caspase-1 could improve cognitive function and partly reverse brain volume in the hippocampus compared to those in stroke mice (Kim et al., 2020). Furthermore, Kim et al. developed a miniaturized electronic device of photobiomodulation (PBM), consisting of packaged light-emitting diodes (LEDs) that incorporate a flexible substrate for in vivo brain PBM in a mouse model. The preventive and therapeutic effects of PBM affixed to the exposed skull of stroke mice model were evaluated, and the results showed that the PBM with 630 nm LED array could significantly attenuate the progression of cognitive impairment in the chronic poststroke phase via regulating AIM2 inflammasome activation and AIM2 inflammasome-mediated pyroptosis (Kim et al., 2022). It was reported that NLRP3/caspase-1-dependent pyroptosis participated in the cerebral ischemia/reperfusion injury and cognitive decline after focal cortical infarction, NLRP3 inhibitor and caspase-1 inhibitor could improve the symptoms, respectively (Sun et al., 2020; Dong et al., 2022). The antiepileptic drug valproic acid (VPA) was also reported to improve cerebral ischemia/reperfusion injury via modulating an apoptosis repressor with caspase recruitment domain (ARC)-mediated caspase-1-dependent pyroptosis pathway (Zhu et al., 2019).
The activation of AIM2 inflammasome contributed to the pathophysiology of chronic CCH-induced brain injury (Poh et al., 2021), and it was revealed and knockout of AIM2 attenuated pyroptosis in the cerebellum following CCH mainly by decreasing the production of proinflammatory cytokines (Poh et al., 2021). It was reported that curcumin and emodin could protect against CCH-induced cognitive dysfunction via inhibiting overactivated NLRP3-dependent pyroptosis (Zheng et al., 2021; Jiang et al., 2023). Chai et al. found that the level of legumain, a lysosomal cysteine protease, was significantly increased in the hippocampus of mice with CCH, considering the abnormal upregulation of legumain in mediating synaptic plasticity impairment and neuroinflammation, targeting legumain might be a potential therapy for CCH. Legumin knockout could partly restore synaptic plasticity and protect against cognitive impairment by decreasing the levels of inflammatory cytokines and the inflammasome complex and inhibiting pyroptosis (Chai et al., 2021). Interestingly, behavioral therapy also showed potential for the treatment of CCH-induced cognitive dysfunction. Poh et al. (2021) observed increased expression of inflammasome components and precursor IL-1β in the brain tissue following CCH, intermittent fasting (16 h food deprivation daily) could significantly reduce the expression levels of cleaved caspases-1/-8/-11 and maturation of both IL-1β and IL-18, inhibit proptosis and improve cognitive impairment, suggesting the therapeutic effect of non-pharmaceutical intervention.
Metabolic disorders
It has been well-recognized that metabolic disorders such as type 2 diabetes mellitus, obesity and cardiovascular diseases are associated with cognitive impairment (Zilliox et al., 2016; Sharma, 2021). Considerable molecular biological studies have elucidated the mechanisms of how diabetes and obesity/high-fat diet (HFD) caused cognitive impairment, among which pyroptosis played an important role.
Diabetes mellitus induced the risk and promoted the development of cognitive dysfunction mainly via the greater occurrence of small-/micro-vascular diseases or even stroke. Ward et al. found that diabetes could induce neuronal degeneration and blood–brain barrier disruption, thus impairing cognitive function. In the process amplified NLRP3 activation was observed, and NLRP3 inhibitor could ameliorate cognitive function and vascular integrity in a high-fat diet/streptozotocin-induced (HFD/STZ) diabetic male Wistar rat model with stroke. Although the role of pyroptosis was not deeply discussed, NLRP3 inhibitor showed therapeutic potential (Ward et al., 2019). Ruan et al. showed the effect of HECT domain E3 ubiquitin protein ligase 3 (HECTD3) in diabetes-related cognitive impairment. HECTD3 was upregulated, together with the increased levels of NLRP3/caspase-1/GSDMD pyroptosis pathway, in the hippocampus of STZ-induced diabetic rats and PC12 cells treated with high glucose medium. HECTD3 silencing could inhibit the activation of NLRP3 inflammasome, suppressed pyroptosis level and exerted a neuroprotective effect via MALT-mediated JNK signaling (Ruan et al., 2022). It was revealed that the P2X7-mediated NLRP1/Caspase-1 pyroptosis pathway, as well as apoptosis and oxidative stress, was overactivated in high glucose-induced hippocampal neuron injury. Naofucong, a compound preparation based on traditional Chinese medicine theory and modern pharmacology, was found to reduce both oxidative stress and pyroptosis by suppressing P2X7/NLRP1/caspase-1 pathway, finally improving cognitive impairment (Jing et al., 2021). Wang et al. elucidated the mechanism of how tetracyclic oxindole alkaloid isorhynchophylline (IRN) helped lessen diabetes-induced cognitive impairment. Spliced form of X-box binding protein 1 (sXBP1) played a crucial role in the process. IRN promoted sXBP1 translocation into the nucleus, and restored downstream high glucose-mediated impairment of insulin signaling, endoplasmic reticulum stress, and pyroptosis/apoptosis, thus improving cognitive dysfunction (Wang et al., 2023). Gestational diabetes mellitus (GDM) is defined as diabetes diagnosed for the first time during pregnancy and can lead to cognitive impairment in offspring. Liang et al. found that chemerin was significantly upregulated in the serum, placenta tissue, and umbilical cord blood of the diabetic mother, further study revealed that chemerin-induced diabetic pregnant disease via chemokine receptor-like 2 (CCRL2)-dependent enrichment of chemerin in the brain of offspring, which led to macrophage recruitment, activation of NLRP3/caspase-1 mediated pyroptosis, resulting in cognitive impairment. Chemerin exerted effects via chemerin receptor 23 (ChemR23), therefore targeting CCRL2 and ChemR23 could be effective for treating cognitive dysfunction in offspring of GDM (Liang et al., 2019).
Compared with cardiovascular diseases and diabetes mellitus, cognitive impairment is easy to be ignored in patients with obesity (Wang et al., 2017; Zhang et al., 2020). It is important to uncover the relationship between obesity/HFD and cognitive impairment. Sui et al. reported that under HFD conditions, neuronal pyroptosis was significantly increased, tau protein was hyperphosphorylated, Nrf-2/HO-1 signaling pathway was activated. Exogenous IGF-1 could improve cognitive impairment in a C57BL/6 J mice model fed with HFD by reversing the activity of the above signaling (Sui et al., 2021). MicroRNAs also showed potential in treating obesity/HFD-related cognitive impairment. Wang et al. found that HFD caused cognitive impairment following neuronal pyroptosis and a decrease of IGF-1/GSK3β signaling pathway in the midbrain and hippocampus tissues. Inhibition of miR-129 by miR-129 antagomir could attenuate NLRP3/caspase-1 mediated pyroptosis and improve cognitive impairment by activating IGF-1/GSK3β signaling pathway via directly targeting IGF-1 (Wang et al., 2021). Yang et al. used BV2 cells treated with palmitic acid to establish an in vitro model of HFD, the results showed TLR4/MyD88/NF-κB p65 signaling, together with NLRP3 expression, was upregulated in palmitic acid-treated BV2 cells. miR-124 could target TLR4/MyD88/NF-κB p65/NLRP3 signaling and reduce its activity, showing a protective effect against HFD-induced neuronal injury (Yang et al., 2022).
Discussion and perspectives
Although the effects and mechanisms of pyroptosis in cognitive impairment are not fully understood, numerous pieces of evidence showed that the various pyroptosis signaling pathway participated in the incidence and progression of cognitive dysfunction induced by different causes. Pyroptosis was regulated by canonical, non-canonical and gasdermin-dependent signaling pathways, forming a complex regulation network. The levels of pyroptosis signaling pathway were significantly upregulated in cognitive impairment, together with the release of large amounts of inflammatory substances such as IL-1β and IL-18, resulting in a cascade of inflammatory reactions, which could be harmful to brain tissues. Targeting and downregulation of the pyroptosis signaling pathway was an effective method to improve cognitive impairment, NLRP3 inhibitor and caspase-1 inhibitor were the most common agents to repress the activation of pyroptosis. Apart from the therapeutic methods/agents directly targeting the pyroptosis signaling pathway, different approaches have been also taken for the treatment of cognitive impairment (Table 1). Photobiomodulation (PBM) with 630 nm LED array affixed to the exposed skull of stroke mice model could significantly attenuate the progression of cognitive impairment in the chronic poststroke phase via regulating AIM2 inflammasome activation and AIM2 inflammasome-mediated pyroptosis (Kim et al., 2022). Electroacupuncture was also reported to improve 7-day survival rates and cognitive function by downregulating NLRP3/caspase-1/GSDMD pyroptosis pathway in an SAE mouse model (Li et al., 2022). Behavioral therapy such as intermittent fasting could also significantly inhibit overactivated proptosis and improve cognitive impairment (Poh et al., 2021). These attempts suggest the therapeutic effect of the non-pharmaceutical intervention and broaden the way to develop new therapy methods. The pathological changes of cognitive impairment were accompanied by the activation of the components of pyroptosis pathway, indicating the detection of pyroptosis components could be an early diagnostic biomarker. Therefore, pyroptosis played a crucial role in cognitive dysfunction, further research would be remarkably helpful for the prevention and protection of cognitive impairment.
Recent studies also focused on the relationship between mutation of gasdermin genes and specific diseases. Ruan et al. (2018) analyzed the structure of the GSDMA3 membrane pore with the help of cryo-electron microscopy and partly revealed mechanisms of how the cleavage of GSDMA3 formed membrane pores and the mechanisms of autoinhibition. Due to a loss of autoinhibition, disease-related mutations of GSDMA3 and its N terminal alone could initiate pyroptosis and had a close association with spontaneous alopecia and hyperkeratosis (Ding et al., 2016). Single nucleotide polymorphisms (SNPs) in GSDMA and GSDMB were reported to be related to childhood asthma and to a lesser extent to adult asthma (Zheng et al., 2020). Mutations of GSDME and DFNB59 could both induce deafness, but the mechanisms were different. GSDME mutations resulted in its overexpression and led to pyroptosis in HeLa cells (Wang et al., 2017), while DFNB59 mutations exerted function in a non-pyroptosis way (Delmaghani et al., 2006). Xia et al. (2021) reported the cryo-electron microscopy structures of the pore of GSDMD, elucidating the process of GSDMD-dependent membrane pore formation and GSDMD-mediated release of IL-1β. Liu et al. (2019) revealed the mechanisms of autoinhibition, lipid binding and oligomerization of GSDMD-N-terminal in virtue of the crystal structures. These studies of molecular structure gave an explanation for the actions of a number of mutant gasdermin family members (Ruan et al., 2018). However, the association between mutation of gasdermin genes and cognitive impairment required future exploration.
Given the unknown mechanisms of cognitive impairment, we are beginning to understand the molecular biological/pathological functions of cognitive impairment and pyroptosis. Further research towards elucidating new mechanisms of the pyroptosis signaling pathway will deepen our comprehension of cognitive impairment, and provide new ideas for developing more potent methods.
Author contributions
XY carried out the primary literature search, drafted, and revised the manuscript. ZT contributed to the drafting and revising of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer DW declared a past co-authorship with the author ZT to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, Y., Liu, D., Zhang, H., Wang, Y., Wang, D., Cai, H., et al. (2021). N-salicyloyl tryptamine derivatives as potential therapeutic agents for Alzheimer's disease with neuroprotective effects. Bioorg. Chem. 115:105255. doi: 10.1016/j.bioorg.2021.105255
Benaoudia, S., Martin, A., Puig Gamez, M., Gay, G., Lagrange, B., Cornut, M., et al. (2019). A genome-wide screen identifies IRF2 as a key regulator of caspase-4 in human cells. EMBO Rep. 20:e48235. doi: 10.15252/embr.201948235
Broz, P., and Dixit, V. M. (2016). Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420. doi: 10.1038/nri.2016.58
Brubaker, S. W., Brewer, S. M., Massis, L. M., Napier, B. A., and Monack, D. M. (2020). A rapid Caspase-11 response induced by IFNgamma priming is independent of guanylate binding proteins. iScience. 23:101612. doi: 10.1016/j.isci.2020.101612
Cai, Y., Chai, Y., Fu, Y., Wang, Y., Zhang, Y., Zhang, X., et al. (2021). Salidroside ameliorates Alzheimer's disease by targeting NLRP3 Inflammasome-mediated Pyroptosis. Front. Aging Neurosci. 13:809433. doi: 10.3389/fnagi.2021.809433
Chai, X., Li, X., Zhang, W., Tan, X., Wang, H., and Yang, Z. (2021). Legumain knockout improved cognitive impairment via reducing neuroinflammation in right unilateral common carotid artery occlusion mice. Life Sci. 285:119944. doi: 10.1016/j.lfs.2021.119944
Chen, D. B., Gao, H. W., Peng, C., Pei, S. Q., Dai, A. R., Yu, X. T., et al. (2020). Quinones as preventive agents in Alzheimer's diseases: focus on NLRP3 inflammasomes. J. Pharm. Pharmacol. 72, 1481–1490. doi: 10.1111/jphp.13332
Chen, H., Peng, Y., Wang, L., and Wang, X. (2022). Sevoflurane attenuates cognitive dysfunction and NLRP3-dependent caspase-1/11-GSDMD pathway-mediated pyroptosis in the hippocampus via upregulation of SIRT1 in a sepsis model. Arch. Physiol. Biochem. 128, 1413–1420. doi: 10.1080/13813455.2020.1773860
Dai, J., Li, X., Wang, C., Gu, S., Dai, L., Zhang, J., et al. (2021). Repeated neonatal sevoflurane induced neurocognitive impairment through NF-kappaB-mediated pyroptosis. J. Neuroinflammation 18:180. doi: 10.1186/s12974-021-02233-9
Delmaghani, S., del Castillo, F. J., Michel, V., Leibovici, M., Aghaie, A., Ron, U., et al. (2006). Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 38, 770–778. doi: 10.1038/ng1829
Demarco, B., Grayczyk, J. P., Bjanes, E., le Roy, D., Tonnus, W., Assenmacher, C. A., et al. (2020). Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv. 6:eabc3465. doi: 10.1126/sciadv.abc3465
Ding, J., Wang, K., Liu, W., She, Y., Sun, Q., Shi, J., et al. (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. doi: 10.1038/nature18590
Dong, D., Ren, A., Yang, Y., Su, J., Liu, L., Zhuo, W., et al. (2022). VX-765 alleviates beta-amyloid deposition and secondary degeneration in the ipsilateral hippocampus and ameliorates cognitive decline after focal cortical infarction in rats. J. Mol. Neurosci. 72, 2389–2397. doi: 10.1007/s12031-022-02088-6
Fan, Y., du, L., Fu, Q., Zhou, Z., Zhang, J., Li, G., et al. (2018). Inhibiting the NLRP3 inflammasome with MCC950 ameliorates isoflurane-induced pyroptosis and cognitive impairment in aged mice. Front. Cell. Neurosci. 12:426. doi: 10.3389/fncel.2018.00426
Fang, Y., Tian, S., Pan, Y., Li, W., Wang, Q., Tang, Y., et al. (2020). Pyroptosis: a new frontier in cancer. Biomed. Pharmacother. 121:109595. doi: 10.1016/j.biopha.2019.109595
Fu, Q., Wu, J., Zhou, X. Y., Ji, M. H., Mao, Q. H., Li, Q., et al. (2019). NLRP3/Caspase-1 pathway-induced pyroptosis mediated cognitive deficits in a mouse model of Sepsis-associated encephalopathy. Inflammation 42, 306–318. doi: 10.1007/s10753-018-0894-4
Guo, D., Xu, Y., Wang, Y., Zhong, X., Liu, Z., Li, S., et al. (2023). Hyperactivation of TRPV4 causes the hippocampal pyroptosis pathway and results in cognitive impairment in LPS-treated mice. Behav. Brain Res. 439:114223. doi: 10.1016/j.bbr.2022.114223
He, Y., Chen, X., Wu, M., Hou, X., and Zhou, Z. (2023). What type of cell death occurs in chronic cerebral hypoperfusion? A review focusing on pyroptosis and its potential therapeutic implications. Front. Cell. Neurosci. 17:1073511. doi: 10.3389/fncel.2023.1073511
Jiang, W., Liu, Z., Wu, S., Meng, T., Xu, L. L., Liu, J. F., et al. (2023). Neuroprotection of emodin by inhibition of microglial NLRP3 inflammasome-mediated pyroptosis. J. Integr. Neurosci. 22:48. doi: 10.31083/j.jin2202048
Jing, G., Wang, H., Nan, F., Liu, Y., and Zhang, M. (2021). Naofucong ameliorates high glucose induced hippocampal neuron injury through suppressing P2X7/NLRP1/Caspase-1 pathway. Front. Pharmacol. 12:647116. doi: 10.3389/fphar.2021.647116
Jing, G., Zuo, J., Fang, Q., Yuan, M., Xia, Y., Jin, Q., et al. (2022). Erbin protects against sepsis-associated encephalopathy by attenuating microglia pyroptosis via IRE1alpha/Xbp1s-ca(2+) axis. J. Neuroinflammation 19:237. doi: 10.1186/s12974-022-02598-5
Karmakar, M., Katsnelson, M. A., Dubyak, G. R., and Pearlman, E. (2016). Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat. Commun. 7:10555. doi: 10.1038/ncomms10555
Karmakar, M., Katsnelson, M., Malak, H. A., Greene, N. G., Howell, S. J., Hise, A. G., et al. (2015). Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J. Immunol. 194, 1763–1775. doi: 10.4049/jimmunol.1401624
Kayagaki, N., Lee, B. L., Stowe, I. B., Kornfeld, O. S., O'Rourke, K., Mirrashidi, K. M., et al. (2019). IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal. 12:eaax4917. doi: 10.1126/scisignal.aax4917
Kayagaki, N., Stowe, I. B., Lee, B. L., O’Rourke, K., Anderson, K., Warming, S., et al. (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. doi: 10.1038/nature15541
Kim, H., Kim, M. J., Kwon, Y. W., Jeon, S., Lee, S. Y., Kim, C. S., et al. (2022). Benefits of a skull-interfaced flexible and implantable multilight emitting diode array for photobiomodulation in ischemic stroke. Adv Sci (Weinh). 9:e2104629. doi: 10.1002/advs.202104629
Kim, H., Seo, J. S., Lee, S. Y., Ha, K. T., Choi, B. T., Shin, Y. I., et al. (2020). AIM2 inflammasome contributes to brain injury and chronic post-stroke cognitive impairment in mice. Brain Behav. Immun. 87, 765–776. doi: 10.1016/j.bbi.2020.03.011
Lamkanfi, M., and Dixit, V. M. (2017). In retrospect: the inflammasome turns 15. Nature 548, 534–535. doi: 10.1038/548534a
Li, D. D., Fan, H. X., Yang, R., Li, Y. Y., Zhang, F., Dendrobium, S. J. S., et al. (2022). Alkaloid suppresses NLRP3-mediated pyroptosis to alleviate LPS-induced neurotoxicity. Front. Pharmacol. 13:846541. doi: 10.3389/fphar.2022.846541
Li, Y., Li, Z., He, F., Qin, C., Fan, R., Zhang, F., et al. (2022). Electroacupuncture alleviates cognitive dysfunction and neuronal pyroptosis in septic mice. Acupunct. Med. 9645284221117847:096452842211178. doi: 10.1177/09645284221117847
Li, J., Li, L., He, J., Xu, J., and Bao, F. (2022). The NLRP3 inflammasome is a potential mechanism and therapeutic target for perioperative neurocognitive disorders. Front. Aging Neurosci. 14:1072003. doi: 10.3389/fnagi.2022.1072003
Li, Q., Wang, Q., Guan, H., Zhou, Y., and Liu, L. (2021). Schisandrin inhibits NLRP1 inflammasome-mediated neuronal pyroptosis in mouse models of Alzheimer's disease. Neuropsychiatr. Dis. Treat. 17, 261–268. doi: 10.2147/NDT.S279147
Li, Y., Wu, Z. Y., Zheng, W. C., Wang, J. X., Yue-Xin,, Song, R. X., et al. (2022). Esketamine alleviates postoperative cognitive decline via stimulator of interferon genes/TANK-binding kinase 1 signaling pathway in aged rats. Brain Res. Bull. 187, 169–180. doi: 10.1016/j.brainresbull.2022.07.004
Li, J., Zhuang, L., Luo, X., Liang, J., Sun, E., and He, Y. (2020). Protection of MCC950 against Alzheimer's disease via inhibiting neuronal pyroptosis in SAMP8 mice. Exp. Brain Res. 238, 2603–2614. doi: 10.1007/s00221-020-05916-6
Liang, Z., Han, L., Sun, D., Chen, Y., Wu, Q., Zhang, L., et al. (2019). Chemerin-induced macrophages pyroptosis in fetal brain tissue leads to cognitive disorder in offspring of diabetic dams. J. Neuroinflammation 16:226. doi: 10.1186/s12974-019-1573-6
Liu, Z., Wang, C., Yang, J., Zhou, B., Yang, R., Ramachandran, R., et al. (2019). Crystal structures of the full-length murine and human Gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51, 43–49.e4. doi: 10.1016/j.immuni.2019.04.017
Liu, X., Zhang, Z., Ruan, J., Pan, Y., Magupalli, V. G., Wu, H., et al. (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158. doi: 10.1038/nature18629
Ma, C., Yu, X., Li, D., Fan, Y., Tao, Q., Tang, Y., et al. (2022). Inhibition of SET domain-containing (lysine methyltransferase) 7 alleviates cognitive impairment through suppressing the activation of NOD-like receptor protein 3 inflammasome in isoflurane-induced aged mice. Hum. Exp. Toxicol. 41:096032712110614. doi: 10.1177/09603271211061497
Martinon, F., Burns, K., and Tschopp, J. (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426. doi: 10.1016/S1097-2765(02)00599-3
Masters, C. L., Bateman, R., Blennow, K., Rowe, C. C., Sperling, R. A., and Cummings, J. L. (2015). Alzheimer's disease. Nat. Rev. Dis. Primers. 1:15056. doi: 10.1038/nrdp.2015.56
Ouyang, X., Zhou, J., Lin, L., Zhang, Z., Luo, S., and Hu, D. (2023). Pyroptosis, inflammasome, and gasdermins in tumor immunity. Innate Immun. 29, 3–13. doi: 10.1177/17534259221143216
Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T. S. D., Ganguli, M., Gloss, D., et al. (2018). Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology 90, 126–135. doi: 10.1212/WNL.0000000000004826
Poh, L., Fann, D. Y., Wong, P., Lim, H. M., Foo, S. L., Kang, S. W., et al. (2021). AIM2 inflammasome mediates hallmark neuropathological alterations and cognitive impairment in a mouse model of vascular dementia. Mol. Psychiatry 26, 4544–4560. doi: 10.1038/s41380-020-00971-5
Poh, L., Rajeev, V., Selvaraji, S., Lai, M. K. P., Chen, C. L. H., Arumugam, T. V., et al. (2021). Intermittent fasting attenuates inflammasome-associated apoptotic and pyroptotic death in the brain following chronic hypoperfusion. Neurochem. Int. 148:105109. doi: 10.1016/j.neuint.2021.105109
Poh, L., Razak, S., Lim, H. M., Lai, M. K. P., Chen, C. L. H., Lim, L. H. K., et al. (2021). AIM2 inflammasome mediates apoptotic and pyroptotic death in the cerebellum following chronic hypoperfusion. Exp. Neurol. 346:113856. doi: 10.1016/j.expneurol.2021.113856
Que, Y. Y., Zhu, T., Zhang, F. X., and Peng, J. (2020). Neuroprotective effect of DUSP14 overexpression against isoflurane-induced inflammatory response, pyroptosis and cognitive impairment in aged rats through inhibiting the NLRP3 inflammasome. Eur. Rev. Med. Pharmacol. Sci. 24, 7101–7113. doi: 10.26355/eurrev_202006_21704
Raetz, C. R., and Whitfield, C. (2002). Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700. doi: 10.1146/annurev.biochem.71.110601.135414
Rost, N. S., Meschia, J. F., Gottesman, R., Wruck, L., Helmer, K., Greenberg, S. M., et al. (2021). Cognitive impairment and dementia after stroke: design and rationale for the DISCOVERY study. Stroke 52, e499–e516. doi: 10.1161/STROKEAHA.120.031611
Ruan, Z., Li, Y., and Chen, Y. (2022). HECTD3 promotes NLRP3 inflammasome and pyroptosis to exacerbate diabetes-related cognitive impairment by stabilising MALT1 to regulate JNK pathway. Arch. Physiol. Biochem. 1, 1–12. doi: 10.1080/13813455.2022.2093377
Ruan, J., Xia, S., Liu, X., Lieberman, J., and Wu, H. (2018). Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67. doi: 10.1038/s41586-018-0058-6
Rui, W., Xiao, H., Fan, Y., Ma, Z., Xiao, M., Li, S., et al. (2021). Systemic inflammasome activation and pyroptosis associate with the progression of amnestic mild cognitive impairment and Alzheimer's disease. J. Neuroinflammation 18:280. doi: 10.1186/s12974-021-02329-2
Sanford, A. M. (2017). Mild cognitive impairment. Clin. Geriatr. Med. 33, 325–337. doi: 10.1016/j.cger.2017.02.005
Sbai, O., Djelloul, M., Auletta, A., Ieraci, A., Vascotto, C., and Perrone, L. (2022). AGE-TXNIP axis drives inflammation in Alzheimer's by targeting Abeta to mitochondria in microglia. Cell Death Dis. 13:302. doi: 10.1038/s41419-022-04758-0
Shan, Y., Liu, P., Zhou, Y., Ding, X., Liu, H., and Yang, J. (2023). Prenatal sevoflurane exposure impairs the learning and memory of rat offspring via HMGB1-induced NLRP3/ASC inflammasome activation. ACS Chem. Neurosci. 14, 699–708. doi: 10.1021/acschemneuro.2c00620
Sharma, S. (2021). High fat diet and its effects on cognitive health: alterations of neuronal and vascular components of brain. Physiol. Behav. 240:113528. doi: 10.1016/j.physbeh.2021.113528
Shi, J., Gao, W., and Shao, F. (2017). Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254. doi: 10.1016/j.tibs.2016.10.004
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. doi: 10.1038/nature15514
Sui, G., Yang, C., Wang, L., Xiong, X., Guo, M., Chen, Z., et al. (2021). Exogenous IGF-1 improves tau pathology and neuronal pyroptosis in high-fat diet mice with cognitive dysfunction. Metab. Brain Dis. 36, 2079–2088. doi: 10.1007/s11011-021-00787-4
Sun, R., Peng, M., Xu, P., Huang, F., Xie, Y., Li, J., et al. (2020). Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J. Neuroinflammation 17:330. doi: 10.1186/s12974-020-01988-x
Tang, Q., Guo, Q., Li, K., and Fei, F. (2022). VRT-043198 ameliorates surgery-induced neurocognitive disorders by restoring the NGF and BNDF expression in aged mice. Neuropsychiatr. Dis. Treat. 18, 1027–1037. doi: 10.2147/NDT.S364250
Thornberry, N. A., Bull, H. G., Calaycay, J. R., Chapman, K. T., Howard, A. D., Kostura, M. J., et al. (1992). A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356, 768–774. doi: 10.1038/356768a0
Tian, D., Xing, Y., Gao, W., Zhang, H., Song, Y., Tian, Y., et al. (2021). Sevoflurane aggravates the progress of Alzheimer's disease through NLRP3/Caspase-1/Gasdermin D pathway. Front. Cell Dev. Biol. 9:801422. doi: 10.3389/fcell.2021.801422
Tonnus, W., Meyer, C., Paliege, A., Belavgeni, A., von Mässenhausen, A., Bornstein, S. R., et al. (2019). The pathological features of regulated necrosis. J. Pathol. 247, 697–707. doi: 10.1002/path.5248
Van Zeller, M., Dias, D., Sebastiao, A. M., and Valente, C. A. (2021). NLRP3 Inflammasome: a starring role in amyloid-beta- and tau-driven pathological events in Alzheimer's disease. J. Alzheimers Dis. 83, 939–961. doi: 10.3233/JAD-210268
Wang, Y., Gao, W., Shi, X., Ding, J., Liu, W., He, H., et al. (2017). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103. doi: 10.1038/nature22393
Wang, F., Wang, L., Sui, G., Yang, C., Guo, M., Xiong, X., et al. (2021). Inhibition of miR-129 improves neuronal pyroptosis and cognitive impairment through IGF-1/GSK3beta signaling pathway: an in vitro and in vivo study. J. Mol. Neurosci. 71, 2299–2309. doi: 10.1007/s12031-021-01794-x
Wang, J., Wang, X., Zhang, M., Lang, Y., Chen, B., Ye, Y., et al. (2023). The activation of spliced X-box binding protein 1 by isorhynchophylline therapy improves diabetic encephalopathy. Cell Biol. Toxicol. doi: 10.1007/s10565-022-09789-z
Wang, W., Zhao, B., Gao, W., Song, W., Hou, J., Zhang, L., et al. (2023). Inhibition of PINK1-mediated Mitophagy contributes to postoperative cognitive dysfunction through activation of Caspase-3/GSDME-dependent pyroptosis. ACS Chem. Neurosci. 14, 1249–1260. doi: 10.1021/acschemneuro.2c00691
Wang, F., Zhao, M., Han, Z., Li, D., Zhang, S., Zhang, Y., et al. (2017). Association of body mass index with amnestic and non-amnestic mild cognitive impairment risk in elderly. BMC Psychiatry 17:334. doi: 10.1186/s12888-017-1493-x
Wang, J., Zhu, X., Li, Y., Zhang, P., Wang, T., and Li, M. (2022). Jiedu-Yizhi formula improves cognitive impairment in an Abeta (25-35)-induced rat model of Alzheimer's disease by inhibiting pyroptosis. Evid. Based Complement. Alternat. Med. 2022:6091671. doi: 10.1155/2022/6091671
Ward, R., Li, W., Abdul, Y., Jackson, L. D., Dong, G., Jamil, S., et al. (2019). NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol. Res. 142, 237–250. doi: 10.1016/j.phrs.2019.01.035
Wu, Z., Tan, J., Lin, L., Zhang, W., and Yuan, W. (2022). microRNA-140-3p protects hippocampal neuron against pyroptosis to attenuate sevoflurane inhalation-induced post-operative cognitive dysfunction in rats via activation of HTR2A/ERK/Nrf2 axis by targeting DNMT1. Cell Death Dis. 8:290. doi: 10.1038/s41420-022-01068-4
Xia, S., Zhang, Z., Magupalli, V. G., Pablo, J. L., Dong, Y., Vora, S. M., et al. (2021). Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611. doi: 10.1038/s41586-021-03478-3
Xu, X. E., Liu, L., Wang, Y. C., Wang, C. T., Zheng, Q., Liu, Q. X., et al. (2019). Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain Behav. Immun. 80, 859–870. doi: 10.1016/j.bbi.2019.05.038
Yang, D., He, Y., Munoz-Planillo, R., Liu, Q., and Nunez, G. (2015). Caspase-11 requires the Pannexin-1 channel and the purinergic P2X7 pore to mediate Pyroptosis and Endotoxic shock. Immunity 43, 923–932. doi: 10.1016/j.immuni.2015.10.009
Yang, L., Li, Z., Xu, Z., Zhang, B., Liu, A., He, Q., et al. (2022). Protective effects of cannabinoid type 2 receptor activation against microglia overactivation and neuronal pyroptosis in sepsis-associated encephalopathy. Neuroscience 493, 99–108. doi: 10.1016/j.neuroscience.2022.04.011
Yang, C., Sui, G., Wang, L., Chen, Z., and Wang, F. (2022). MiR-124 prevents the microglial proinflammatory response by inhibiting the activities of TLR4 and downstream NLRP3 in palmitic acid-treated BV2 cells. J. Mol. Neurosci. 72, 496–506. doi: 10.1007/s12031-021-01921-8
Zhang, Y., Fu, Q., Ruan, J., Shi, C., Lu, W., Wu, J., et al. (2023). Dexpramipexole ameliorates cognitive deficits in sepsis-associated encephalopathy through suppressing mitochondria-mediated pyroptosis and apoptosis. Neuroreport 34, 220–231. doi: 10.1097/WNR.0000000000001882
Zhang, L., Wang, Z., Wang, X., Chen, Z., Shao, L., Tian, Y., et al. (2020). Prevalence of overweight and obesity in China: results from a cross-sectional study of 441 thousand adults, 2012-2015. Obes. Res. Clin. Pract. 14, 119–126. doi: 10.1016/j.orcp.2020.02.005
Zhang, C., Zhao, C., Chen, X., Tao, R., Wang, S., Meng, G., et al. (2020). Induction of ASC pyroptosis requires gasdermin D or caspase-1/11-dependent mediators and IFNbeta from pyroptotic macrophages. Cell Death Dis. 11:470. doi: 10.1038/s41419-020-2664-0
Zheng, X., Chen, W., Gong, F., Chen, Y., and Chen, E. (2021). The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: a review. Front. Immunol. 12:711939. doi: 10.3389/fimmu.2021.711939
Zheng, Z., Deng, W., Lou, X., Bai, Y., Wang, J., Zeng, H., et al. (2020). Gasdermins: pore-forming activities and beyond. Acta Biochim. Biophys. Sin. Shanghai 52, 467–474. doi: 10.1093/abbs/gmaa016
Zheng, Y., Zhang, J., Zhao, Y., Zhang, Y., Zhang, X., Guan, J., et al. (2021). Curcumin protects against cognitive impairments in a rat model of chronic cerebral hypoperfusion combined with diabetes mellitus by suppressing neuroinflammation, apoptosis, and pyroptosis. Int. Immunopharmacol. 93:107422. doi: 10.1016/j.intimp.2021.107422
Zhou, R., Yang, X., Li, X., Qu, Y., Huang, Q., Sun, X., et al. (2019). Recombinant CC16 inhibits NLRP3/caspase-1-induced pyroptosis through p38 MAPK and ERK signaling pathways in the brain of a neonatal rat model with sepsis. J. Neuroinflammation 16:239. doi: 10.1186/s12974-019-1651-9
Zhou, Y., Zhang, Y., Wang, H., Zhang, X., Chen, Y., and Chen, G. (2023). Microglial pyroptosis in hippocampus mediates sevolfurane-induced cognitive impairment in aged mice via ROS-NLRP3 inflammasome pathway. Int. Immunopharmacol. 116:109725. doi: 10.1016/j.intimp.2023.109725
Zhu, S., Zhang, Z., Jia, L. Q., Zhan, K. X., Wang, L. J., Song, N., et al. (2019). Valproic acid attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-pyroptosis pathways. Neurochem. Int. 124, 141–151. doi: 10.1016/j.neuint.2019.01.003
Zilliox, L. A., Chadrasekaran, K., Kwan, J. Y., and Russell, J. W. (2016). Diabetes and cognitive impairment. Curr. Diab. Rep. 16:87. doi: 10.1007/s11892-016-0775-x
Keywords: pyroptosis, cognitive impaiment, NLRP3 inflammasome, treatment, GSDMD
Citation: Yang X and Tang Z (2023) The role of pyroptosis in cognitive impairment. Front. Neurosci. 17:1206948. doi: 10.3389/fnins.2023.1206948
Edited by:
Fangyi Xu, University of Louisville, United StatesReviewed by:
Daoqi Wang, The Second Affiliated Hospital of Kunming Medical University, ChinaZhe Yu, Fujian Medical University, China
Copyright © 2023 Yang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Tang, enRhbmdAdGpoLnRqbXUuZWR1LmNu
 Xin Yang
Xin Yang Zhe Tang
Zhe Tang