
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 09 June 2023
Sec. Perception Science
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1180434
This article is part of the Research TopicNeurobiological Mechanism of Acupuncture for Pain and Itch Volume IIView all 9 articles
Background: Functional magnetic resonance imaging (fMRI) has been widely used to investigate the brain effect of acupuncture point Stomach 36 (ST36, Zusanli). However, inconsistent results have hindered our understanding of the neural mechanisms of acupuncture at ST36.
Objective: To perform a meta-analysis of fMRI studies on acupuncture at ST36 to assess the brain atlas of acupuncture at ST36 from available studies.
Method: Based on a preregistered protocol in PROSPERO (CRD42019119553), a large set of databases was searched up to August 9, 2021, without language restrictions. Peak coordinates were extracted from clusters that showed significant signal differences before and after acupuncture treatment. A meta-analysis was performed using seed-based d mapping with permutation of subject images (SDM-PSI), a newly improved meta-analytic method.
Results: A total of 27 studies (27 ST36) were included. This meta-analysis found that ST36 could activate the left cerebellum, the bilateral Rolandic operculum, the right supramarginal gyrus, and the right cerebellum. Functional characterizations showed that acupuncture at ST36 was mainly associated with action and perception.
Conclusion: Our results provide a brain atlas for acupuncture at ST36, which, besides offering a better understanding of the underlying neural mechanisms, also provides the possibility of future precision therapies.
Acupuncture is an ancient Chinese treatment that involves inserting needles into specific acupuncture points to treat disease and has been practiced in East Asian countries for over 2000 years. A recent review of the high-quality evidence-based medical literature reveals that acupuncture could improve the functional communication of patients with post-stroke aphasia, relieve neck and shoulder pain and non-specific lower back pain, reduce the severity of vascular dementia symptoms, and improve the nasal symptoms of allergic rhinitis (Lu et al., 2022). Currently, acupuncture is widely used around the world, with particular attention being paid to its mechanisms. However, a combination of acupoints has often been used, which makes it difficult to understand the role of specific acupoints. Therefore, it is necessary to understand the mechanisms of individual acupoints for individualized treatment. Stomach 36 (ST36, Zusanli), located 3 cm below the knee joint on the anterior portion of the leg, is a commonly used acupoint and is widely used in clinical practice. Although many animal studies have been conducted to explore the mechanisms of ST36 (Zhang et al., 2018; Lu et al., 2019), the acupoints of animals and humans are different from one another. Thus, it is necessary to further understand its function based on human acupoints.
Functional magnetic resonance imaging (fMRI) provides an experimental window to observe the human brain (Buxton, 2013). It is not only completely noninvasive but also offers excellent temporal and spatial resolution and improved sensitivity to detect task activation in individual subjects through signal averaging (Detre and Wang, 2002). Indeed, the goal of functional neuroimaging is to map the activity of the living brain in space and time. In the last three decades, a great deal of research has been performed on the application of fMRI to acupuncture (Wu et al., 1999; Kang et al., 2013; Xiao et al., 2018; Cao et al., 2020). However, due to the high cost and time-consuming nature of fMRI scanning, the sample size of each study is small. In addition, the fMRI results for acupuncture at ST36 were heterogeneous.
Therefore, it is necessary to investigate the brain effect of acupuncture at ST36 based on previously published studies in a quantitative way. We first applied seed-based d mapping with permutation of subject images (SDM-PSI), a new generation algorithm for coordinate-based meta-analysis (CBMA), to determine the most prominent and replicable brain areas of the included acupoints. Then, we investigated the functional characterizations and co-activation patterns of significant clusters using behavioral domains, paradigm classes, and meta-analytic connectivity modeling (MACM) analysis, respectively.
The review process followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Moher et al., 2009), and the meta-analysis protocol was registered with PROSPERO1 (registration number: CRD42020204050).
Studies of acupuncture on some acupoints were searched from PubMed, Web of Science, Wanfang, and the Chinese National Knowledge Infrastructure (CNKI) from the inception of the databases up to August 2021 to identify relevant studies. The following search terms were used: (“acupuncture” or “acupuncture therapy” or “electroacupuncture” or “EA”) and (“zusanli” or “ST36”) and (“functional magnetic resonance imaging” or “fMRI” or “BOLD” or “ReHo” or “ALFF” or “fALFF”) NOT (“mice” or “rat” or “animal”). Additional articles were identified by cross-referencing the reference lists of the included articles.
In this review, we included all studies that used fMRI to investigate the effect of acupuncture on the human brain. To be included in this meta-analysis, research had to meet the following inclusion criteria: (1) it had to include healthy subjects only; (2) it had to include verum acupuncture only or verum versus sham acupuncture only using task-based fMRI with the whole-brain acquisition; (3) it only needed to report ST36; (4) it reported findings in 3D coordinates in the Montreal Neurological Institute (Evans et al., 1993) or Talairach space (Talairach and Tournoux, 1988); (5) it concerned manual acupuncture or electroacupuncture. Studies were excluded if they: (1) examined needling stimulation with tasks, such as finger movement; (2) investigated only region of interest (ROI) results or used functional connectivity, independent component analysis, and graph theory analytical methods; (3) Consisted of reviews, case reports, conferences, abstracts, and animal studies; (4) only included fMRI results between acupuncture and sham acupuncture; (5) had no effective value.
Two researchers manually extracted basic information and the peak voxel coordinates of the included studies, such as first author, year of publication, gender, mean age, number of subjects, field strength, analysis methods, stereoscopic template, and statistical threshold. Any discrepancies were discussed with the third researcher until a consensus was reached.
This meta-analysis was performed using SDM-PSI version 6.21 (Albajes-Eizagirre et al., 2019). Detailed SDM methods have been described on this website.2 First, text files of peak coordinates and effect sizes (e.g., t-values) of fMRI differences between post-treatment and pre-treatment were extracted. During the preprocessing, the lower and upper bounds of the possible effect-size values of the studies were recreated using the above files. Then, the mean map was generated by voxel-wise calculation of the random-effects mean of the study maps, weighted by the sample size, intra-study variability, and between-study heterogeneity. Family-wise error (FWE) correction (p < 0.05 and voxel extent ≥10) using the threshold-free cluster enhancement approach (TFCE) and 5,000 permutations was initially used.
Peak coordinate values were extracted for heterogeneity statistics and publication bias analyses. Heterogeneity between studies was assessed using the I2 statistic in a random-effects model, with I2 < 50% indicating low heterogeneity (Higgins et al., 2003).
Publication bias was assessed using funnel plots and Egger’s tests (Egger et al., 1997). An asymmetric plot and p < 0.05 were considered statistically significant.
Pain-related brain regions were searched,3 then common brain regions of activation of ST36 and pain-related brain regions were extracted.
Finally, co-activation pattern analysis and functional characterization were performed as described in detail in a previous study (Zhang et al., 2021).
A total of 27 studies met the inclusion criteria for the meta-analysis. The study selection process is shown in Figure 1. A total of 542 HC subjects reporting after vs. before contrast brain response, including ST36 (27), are shown in Table 1 and Figure 2.
The ST36 group showed hyperactivation in the bilateral cerebellum, hemispheric lobule VIII, bilateral Rolandic operculum (ROL), and right supramarginal gyrus (SMG.R). The results of the SDM analysis are summarized in Table 2 and Figure 3A.
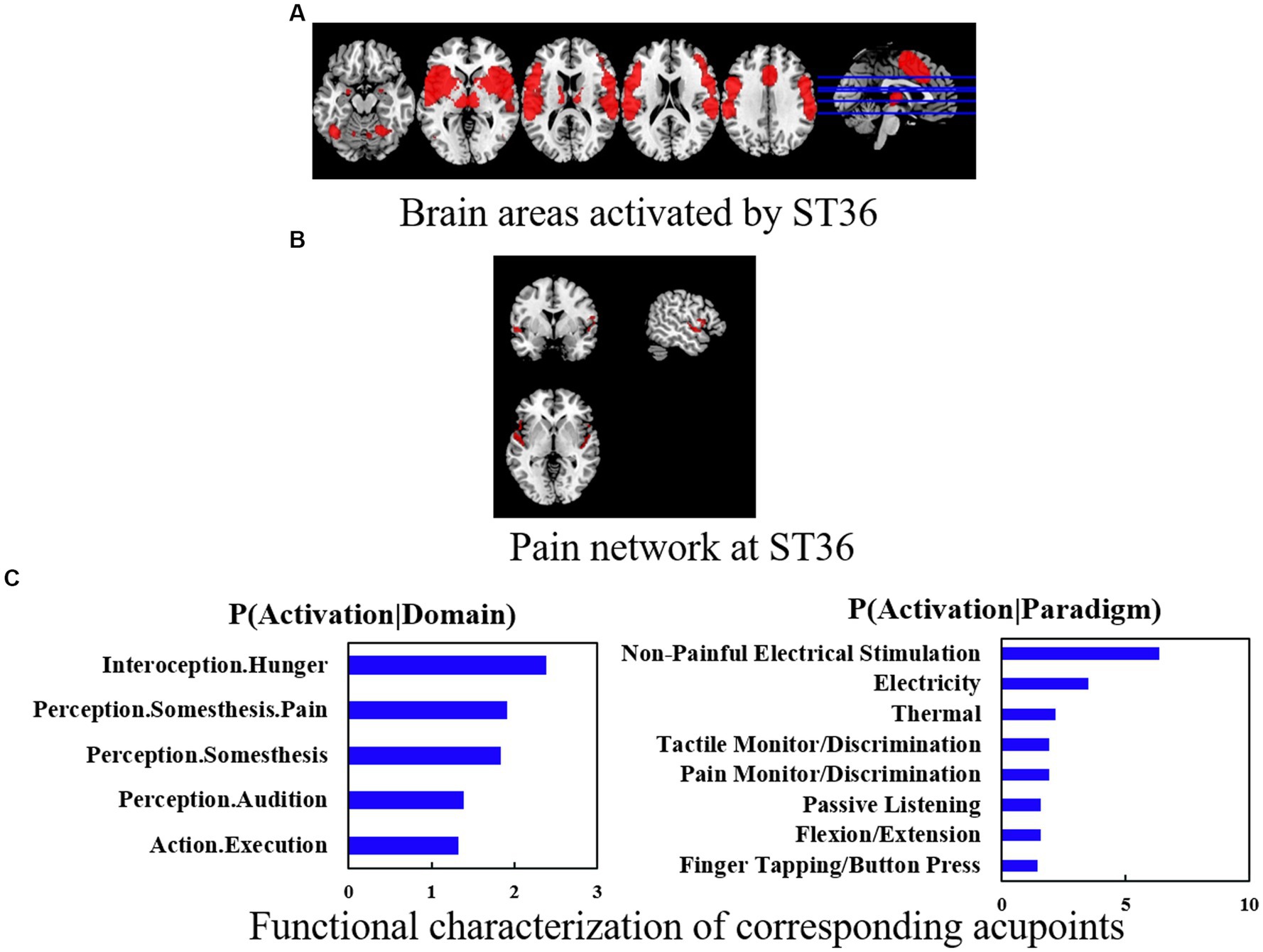
Figure 3. (A) Brain areas activated by ST36. (B) Pain network at ST36. (C) Functional characterization of corresponding acupoints. Coactivation connectivity of significance levels was thresholded at p < 0.05, corrected for multiple comparisons with FDR. Functional characterization of significance levels was thresholded at p < 0.05, corrected for multiple comparisons using FWE, clustering threshold at voxel-level p < 0.001.
In terms of heterogeneity, I2 (0%) showed little heterogeneity in between-study variability on peak coordinates of ST36. In addition, Egger’s tests indicated no significant publication bias on the peak coordinates of ST36.
In terms of co-activation patterns and functional characterization, the bilateral cerebral, fusiform, postcentral, and temporal areas were co-activated with ST36 (Figures 3A,B). Functional characterization showed that the ST36 was mainly associated with action and perception. The PCs showed similar results (Figure 3C).
We investigated the pain network and non-pain network activation brains of ST36 and found that ST36 can activate pain-related networks including the left cerebellum, bilateral Rolandic operculum, left cerebellum crus 1, right supramarginal, and right postcentral. The specific-related network was also found in several brain areas like the right inferior frontal gyrus (IFG.R.), left superior temporal gyrus (STG.L), right cerebellum 8 (cerebellum 8R), and left cerebellum crus 2 (cerebellum crus 2L) (Table 3 and Figure 3B).
Functional characterization showed that acupuncture at ST36 was mainly associated with motor, cognition, learning and memory, self-awareness, and other aspects (Figure 3C).
In this study, we systematically reviewed task-based fMRI studies of acupuncture at ST36 in healthy subjects. First, we obtained the results of the brain regions activated by acupuncture at ST36 and then distinguished between pain-overlapping activation areas and non-pain activation areas based on the brain areas activated by acupuncture at ST36, where the pain-overlapping brain regions are also the pain sensations produced by the acupuncture itself, and the non-pain activation brain regions are also the specific function of acupuncture at ST36.
In this study, we found that bilateral cerebellum, hemispheric lobule VIII, SMG.R, bilateral Rolandic operculum (ROL), and right anterior thalamic projections were activated by acupuncture at ST36. However, this is inconsistent with a previous meta-analysis of ST36 (Zhang et al., 2019). The reasons for this could be explained by the following two points: first, the previous review investigated the effect of deqi when acupuncture at ST36 was applied on brain function; second, different statistical methods and thresholds may be available, this study is more rigorous in this regard.
Evidence suggests that the primary characteristic roles of the cerebellum not only include motor control function but also its non-motor function in cognitive control and learning processing (Moulton et al., 2010; Ruscheweyh et al., 2014; Moberget and Ivry, 2016). Although the cerebellum has often been shown to respond to painful stimuli, the current findings show that the cerebellum is more concerned with visceral than somatic pain (Claassen et al., 2020), which is consistent with the fact that ST36 is often used clinically to treat visceral pain (Fan et al., 2021). In addition, recent studies have also shown that electroacupuncture at ST36 can modulate cerebellar lobule VIII to treat comorbid brain regions of depression and pain (Lottering and Lin, 2021), further confirming ST36’s important role in the treatment of these conditions.
Studies also demonstrate that the right SMG.R has implications for cognitive functions such as temporal perception and attention, pitch memory performance (Wiener et al., 2010), emotion recognition (Wada et al., 2021), visual word recognition (Stoeckel et al., 2009), and episodic memory encoding (Rubinstein et al., 2021).
The Rolandic operculum processes integrated exteroceptive-interoceptive signals that are necessary for interoceptive awareness and bodily self-consciousness (Blefari et al., 2017). Several studies have reported that the Rolandic operculum not only plays a role in emotion processing but also in the sensory system for gustatory and visceral sensations together with the cingulate-operculum network (Eickhoff et al., 2006; Lelic et al., 2015). The gastrointestinal tract has been reported to be sensitive to emotion (depression, anxiety, and stress); the connection between the brain and gastrointestinal organs is explained by the theory of a gut-brain axis (top-down and bottom-up) (Kim and Shin, 2017).
Therefore, based on the functional characteristics of acupuncture at ST36, we found that this may be effective in the treatment of motor, cognition, learning and memory, self-awareness, and other aspects. Importantly, in clinical studies, ST36 is also often used for the treatment of gastrointestinal diseases and to improve constipation (Lu et al., 2019; Wu et al., 2020), pain (Zuo et al., 2019; Wan et al., 2021), cognition (Li Q. Q. et al., 2015; Yang et al., 2020), and other related diseases.
Acupuncture needles are applied to the body, which not only produces the sensation of pain but also has therapeutic effects. However, it is not clear whether this effect overlaps with the areas of the brain where the pain is generated or whether acupuncture is specific.
In this study, we found that the cerebellum, insula, superior temporal gyrus, and postcentral regions of the brain were implicated in the pain network, which is consistent with previous research by Chae et al. (2013) showing that acupuncture stimulation is associated with multidimensional pain.
In addition, many previous fMRI studies (Hui et al., 2000; Kong et al., 2007; Fang et al., 2009) have described the activation of these sensorimotor brain areas as a common feature of acupunctural stimulation. Thus, activation in sensorimotor brain areas such as the insula, thalamus, SI, and SII presumably reflects the involvement of the sensory pain-associated components of acupunctural needle stimulation. One previous meta-analysis suggested that brain response of acupuncture needle stimulation involved in sensory, cognitive, and affective dimensions of pain (Chae et al., 2013). Another study also demonstrated that regardless of the type of harmful stimulus, some brain regions such as the thalamus, insula, and ACC have a significant potential for activation, suggesting that acupuncture, as an external stimulation, could activate some important brain regions (Duerden and Albanese, 2013).
Importantly, we found that a specific-related network of acupuncture on ST36 was found in several brain areas, including IFG.R, STG.L, cerebellum 8R, and cerebellum crus 2L, which is consistent with a recent systematic review similar to this one that showed the opercular part of IFG.R, STG.L, and right median cingulate/paracingulate gyri (MCG.R) regions were positively activated following ST36 acupuncture (Huang et al., 2022).
The IFG.R has a role in modulating cognitive control in several domains, such as inhibitory control and the process of allocating attention (Or-Borichev et al., 2023). It also plays a causal role in the control of interference in memory retrieval (Stramaccia et al., 2017). STG.L plays an important role in auditory processing and social cognition (Bigler et al., 2007). The cerebellum is crucially involved in a wide spectrum of cognitive and affective functions (Jacobi et al., 2021), in addition to its well-established role in balance control and motor function (Chen et al., 2022). According to the above information, we could conclude that cognitive function is a common characteristic of specific activation patterns of ST36, which is consistent with previous research findings (Lu et al., 2014; Li F. et al., 2015). We speculate that acupuncture at ST36 could not only improve cognitive function but also regulate movement, auditory processing, and so on.
Although we found some interesting results from the current data, the interpretation of the results is complex because the central stimulation is not just a linear association. Therefore, it is difficult for us to determine the diseases that could be treated by acupuncture at ST36 through the activated brain region of acupuncture at ST36, so it needs to be combined with clinical practice to finally determine the brain map of acupuncture at ST36. Nonetheless, it still offers us some clinical guidance. In addition, although ST36 acupuncture produces pain-related brain regions linked to the physical stimulation of acupuncture, these cannot be ruled out as part of the treatment.
Our study has several limitations. First, the studies included in our meta-analysis differ with respect to experimental design, analytical methods, and software, which may lead to some heterogeneity. However, we did not find any heterogeneity in this study. Second, a previous study (Li Z. J. et al., 2014) found that the disease state is an important factor in the cerebral response to acupuncture stimulation. In this review, we only paid attention to healthy subjects, due to the small number of studies and sample size of acupuncture for patients. Third, the lack of control over the design of sham acupuncture reduces the reliability of the conclusions. Finally, due to the small number of included studies, the results need to be interpreted with caution.
Future studies should increase the sample size because a sufficient one is necessary to ensure sample power and obtain stable and reliable results.
In terms of brain response, the difference between verum acupoints and sham acupoints should be investigated in more studies to confirm the specificity of acupoints. In addition, the detailed acupoint operation of ST36 should be clearly described, and future acupuncture clinical trials should follow the STRICTA standards (MacPherson et al., 2002).
In terms of fMRI, there is some room for improvement, such as higher resolution and strict statistical analysis to obtain reliable results. Specifically, an appropriate experimental design for investigating acupuncture effects, such as a block- and event-related design, is crucial. Recently, a data-driven approach was considered suitable for describing response characteristics. Perhaps the most suitable method is to define the ROI to explore the efficacy of acupuncture based on the abnormal brain area of the corresponding disease. Finally, a strict threshold is also one of the important conditions to obtain reliable results. Furthermore, a checklist for the fMRI study should be followed in order to provide some reference for further research (Poldrack et al., 2008).
In conclusion, fMRI is a useful visualization tool for investigating the central mechanisms of acupoints. This study found that acupuncture at ST36 activated the bilateral cerebellum, hemispheric lobule VIII, bilateral Rolandic operculum, and right supramarginal gyrus, suggesting that acupuncture at ST36 could be used to treat motor, cognitive, learning and memory, and emotion-related disorders. This study provides new insights into the therapeutic mechanisms of acupuncture. In the future, it will be possible to map the brain altas of each acupoint, allowing it to achieve precise treatment of diseases.
HY, JX, and QH designed the whole study. JZ and YL analyzed the data. JZ wrote the manuscript. ZL searched and selected the studies. XH and HL participated in the discussion. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant nos. 62006220 and 62001462), and the Shenzhen Science and Technology Research Program (grant nos. JCYJ20180507182441903, JCYJ20200109114816594 and JCYJ20210324111206017).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Albajes-Eizagirre, A., Solanes, A., Vieta, E., and Radua, J. (2019). Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. NeuroImage 186, 174–184. doi: 10.1016/j.neuroimage.2018.10.077
Bai, L., Qin, W., Tian, J., Liu, P., Li, L. L., Chen, P., et al. (2009). Time-varied characteristics of acupuncture effects in fMRI studies. Hum. Brain Mapp. 30, 3445–3460. doi: 10.1002/hbm.20769
Bigler, E. D., Mortensen, S., Neeley, E. S., Ozonoff, S., Krasny, L., Johnson, M., et al. (2007). Superior temporal gyrus, language function, and autism. Dev. Neuropsychol. 31, 217–238. doi: 10.1080/87565640701190841
Blefari, M. L., Martuzzi, R., Salomon, R., Bello-Ruiz, J., Herbelin, B., Serino, A., et al. (2017). Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self-consciousness. Eur. J. Neurosci. 45, 1300–1312. doi: 10.1111/ejn.13567
Buxton, R. B. (2013). The physics of functional magnetic resonance imaging (fMRI). Rep. Prog. Phys. 76:096601. doi: 10.1088/0034-4885/76/9/096601
Cao, J., Tu, Y., Wilson, G., Orr, S. P., and Kong, J. (2020). Characterizing the analgesic effects of real and imagined acupuncture using functional and structure MRI. NeuroImage 221:117176. doi: 10.1016/j.neuroimage.2020.117176
Chae, Y., Chang, D. S., Lee, S. H., Jung, W. M., Lee, I. S., Jackson, S., et al. (2013). Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain 14, 215–222. doi: 10.1016/j.jpain.2012.11.011
Chen, F., Hu, Z., Liu, H., Zhen, F., Liu, C., and Li, Q. (2022). Altered homotopic connectivity in the cerebellum predicts stereopsis dysfunction in patients with Comitant Exotropia. Front. Hum. Neurosci. 16:917769. doi: 10.3389/fnhum.2022.917769
Claassen, J., Koenen, L. R., Ernst, T. M., Labrenz, F., Theysohn, N., Forsting, M., et al. (2020). Cerebellum is more concerned about visceral than somatic pain. J. Neurol. Neurosurg. Psychiatry 91, 218–219. doi: 10.1136/jnnp-2019-321025
Claunch, J. D., Chan, S. T., Nixon, E. E., Qiu, W. Q., Sporko, T., Dunn, J. P., et al. (2012). Commonality and specificity of acupuncture action at three acupoints as evidenced by FMRI. Am. J. Chin. Med. 40, 695–712. doi: 10.1142/S0192415X12500528
Detre, J. A., and Wang, J. J. (2002). Technical aspects and utility of fMRI using BOLD and ASL. Clin. Neurophysiol. 113, 621–634. doi: 10.1016/S1388-2457(02)00038-X
Dong, M., Zhao, L., Yuan, K., Zeng, F., Sun, J., Liu, J., et al. (2013). Length of acupuncture training and structural plastic brain changes in professional acupuncturists. PLoS One 8:e66591. doi: 10.1371/journal.pone.0066591
Duan, X., Liu, B., Hu, J., Liu, J., Long, Y., and Chen, Z. (2012). The brain effect of acupuncture at zusanli (ST36) by using regional homogeneity. Chin. Imag. J. Integr. Tradit. West. Med. 10:4. doi: 10.3969/j.issn.1672-0512.2012.01.003
Duerden, E. G., and Albanese, M. C. (2013). Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum. Brain Mapp. 34, 109–149. doi: 10.1002/hbm.21416
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Eickhoff, S. B., Lotze, M., Wietek, B., Amunts, K., Enck, P., and Zilles, K. (2006). Segregation of visceral and somatosensory afferents: an fMRI and cytoarchitectonic mapping study. NeuroImage 31, 1004–1014. doi: 10.1016/j.neuroimage.2006.01.023
Evans, A. C., Collins, D. L., Mills, S. R., Brown, E. D., Kelly, R. L., and Peters, T. M.. (1993). 3D statistical neuroanatomical models from 305 MRI volumes. In 1993 IEEE Conference Record Nuclear Science Symposium and Medical Imaging Conference.
Fan, J., Xu, P., Han, Y., Wen, C., Zhang, H., and Pan, X. (2021). Effects of electroacupuncture at Zusanli acupoint on visceral hypersensitivity in rats with functional dyspepsia. Chinese J. Tissue Eng. Res. 26, 663–668. doi: 10.13702/j.1000-0607.20210937
Fang, J., Jin, Z., Wang, Y., Li, K., Kong, J., Nixon, E. E., et al. (2009). The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Hum. Brain Mapp. 30, 1196–1206. doi: 10.1002/hbm.20583
Fang, J. L., et al. (2005). Magnetic resonance brain imaging of different central activation effects of true and false acupoints when twisting acupuncture. In Theses Collection of Celebration of 50th Anniversary of CATCM.
Feng, Y., Bai, L., Zhang, W., Xue, T., Ren, Y., Zhong, C., et al. (2011). Investigation of acupoint specificity by multivariate granger causality analysis from functional MRI data. J. Magn. Reson. Imaging 34, 31–42. doi: 10.1002/jmri.22585
Fu, H., Yin, C., Zhang, Z., Sun, J., and Wang, L. (2013). fMRI imaging difference between acupuncture and wheat moxibustion at Zusanli point. Jiangsu J. Tradit. Chin. Med. 45, 54–56. doi: CNKI:SUN:JSZY.0.2013-01-038
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Huang, H. M., Yue, X., Huang, X., Long, W., Kang, S., Rao, Y., et al. (2022). Brain activities responding to acupuncture at ST36 (zusanli) in healthy subjects: a systematic review and Meta-analysis of task-based fMRI studies. Front. Neurol. 13:930753. doi: 10.3389/fneur.2022.930753
Hui, K. K., Liu, J., Makris, N., Gollub, R. L., Chen, A. J. W., Moore, C. I., et al. (2000). Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum. Brain Mapp. 9, 13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F
Hui, K. K., Liu, J., Marina, O., Napadow, V., Haselgrove, C., Kwong, K. K., et al. (2005). The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. NeuroImage 27, 479–496. doi: 10.1016/j.neuroimage.2005.04.037
Jacobi, H., Faber, J., Timmann, D., and Klockgether, T. (2021). Update cerebellum and cognition. J. Neurol. 268, 3921–3925. doi: 10.1007/s00415-021-10486-w
Jiang, Y., Liu, S., Xiao, X., Sun, G., Yang, X., and Li, B. (2006). Functional magnetic resonance imaging of acupoint acupuncture: a controlled study of true, false and tactile stimulation. Chin J Clinicians (Electronic Edition) 6, 6374–6378. doi: 10.3877/cma.j.issn.1674-0785.2012.20.104
Jiang, Y., Hao, Y., Zhang, Y., Liu, J., Wang, X., Han, J., et al. (2012). Thirty minute transcutaneous electric acupoint stimulation modulates resting state brain activities: a perfusion and BOLD fMRI study. Brain Res. 1457, 13–25. doi: 10.1016/j.brainres.2012.03.063
Jiang, Y., Wang, H., Liu, Z., Dong, Y., Dong, Y., Xiang, X., et al. (2013). Manipulation of and sustained effects on the human brain induced by different modalities of acupuncture: an fMRI study. PLoS One 8:e66815. doi: 10.1371/journal.pone.0066815
Jiang, H., Wang, J., and Zhao, X. (2010). Observation of the central mechanism of the brain effect of acupuncture at Zusanli with the supplementation method and the reduction method by functional magnetic resonance imaging. Chin. J. Med. Imaging Technol. 26, 635–638.
Jin, L. M., Qin, C. J., Lan, L., Sun, J. B., Zeng, F., Zhu, Y. Q., et al. (2014). Local anesthesia at ST36 to reveal responding brain areas to deqi. Evid. Based Complement. Alternat. Med. eCAM:987365 doi: 10.1155/2014/987365
Kang, O. S., Kim, S. Y., Jahng, G. H., Kim, H., Kim, J. W., Chung, S. Y., et al. (2013). Neural substrates of acupuncture in the modulation of cravings induced by smoking-related visual cues: an fMRI study. Psychopharmacology 228, 119–127. doi: 10.1007/s00213-013-3015-y
Kim, Y. K., and Shin, C. (2017). The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr. Neuropharmacol. 16, 559–573. doi: 10.2174/1570159X15666170915141036
Kong, J., Gollub, R. L., Webb, J. M., Kong, J. T., Vangel, M. G., and Kwong, K. (2007). Test-retest study of fMRI signal change evoked by electroacupuncture stimulation. NeuroImage 34, 1171–1181. doi: 10.1016/j.neuroimage.2006.10.019
Lelic, D., Nissen, T. D., Brock, C., Aziz, Q., and Drewes, A. M. (2015). Rapid balloon distension as a tool to study cortical processing of visceral sensations and pain. Neurogastroenterol. Motil. 27, 832–840. doi: 10.1111/nmo.12557
Li, Q. Q., Shi, G. X., Yang, J. W., Li, Z. X., Zhang, Z. H., He, T., et al. (2015). Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiol. Behav. 139, 482–490. doi: 10.1016/j.physbeh.2014.12.001
Li, F., Yan, C. Q., Lin, L. T., Li, H., Zeng, X. H., Liu, Y., et al. (2015). Acupuncture attenuates cognitive deficits and increases pyramidal neuron number in hippocampal CA1 area of vascular dementia rats. BMC Complement. Altern. Med. 15:133. doi: 10.1186/s12906-015-0656-x
Li, C., Yang, J., Park, K., Wu, H., Hu, S., Zhang, W., et al. (2014). Prolonged repeated acupuncture stimulation induces habituation effects in pain-related brain areas: an FMRI study. PLoS One 9:e97502. doi: 10.1371/journal.pone.0097502
Li, Z. J., Zeng, F., Yang, Y., Chen, Y., Zhang, D., Sun, J., et al. (2014). Different cerebral responses to puncturing at ST36 among patients with functional dyspepsia and healthy subjects. Forsch. Komplementmed. 21, 99–104. doi: 10.1159/000360804
Li, L., Lv, F., Guo, Z., Song, Y., Xie, H., Tang, X., et al. (2013). Effect of acupuncture stimulation of Zusanli (ST36) on cerebral regional homogeneity in volunteer subjects with different constitutions: a resting-state fMRI study. Acupunct. Res. 38, 306–313. doi: 10.13702/j.1000-0607.2013.04.016
Liu, J. (2012). Application of fMRI study on ST36 and correlative research of brain functional area. Shandong Traditional Chinese Medicine University.
Liu, L., Zeng, D., Meng, Z., Shi, C., Liang, M., and Li, H. (2014). Regional homogeneity study of resting state functional magnetic resonance imaging of acupuncture at Zusanli (ST36) by using regional homogeneity. Biomed. Eng. Clin. Med. 18, 146–149. doi: 10.13339/j.cnki.sglc.2014.02.010
Long, Y., Liu, B., Liu, X., Yan, C., Chen, Z., Chen, J., et al. (2009). The effect of acupuncture at Zusanli point was evaluated by resting state functional magnetic resonance. Chin. J. Med. Imag. Technol. 25, 373–376. doi: 10.3321/j.issn:1003-3289.2009.03.013
Lottering, B., and Lin, Y. W. (2021). TRPV1 responses in the cerebellum lobules VI, VII, VIII using Electroacupuncture treatment for chronic pain and depression comorbidity in a murine model. Int. J. Mol. Sci. 22:5028. doi: 10.3390/ijms22095028
Lu, Y., Huang, Y., Tang, C., Shan, B., Cui, S., Yang, J., et al. (2014). Brain areas involved in the acupuncture treatment of AD model rats: a PET study. BMC Complement. Altern. Med. 14:178. doi: 10.1186/1472-6882-14-178
Lu, M. J., Yu, Z., He, Y., Yin, Y., and Xu, B. (2019). Electroacupuncture at ST36 modulates gastric motility via vagovagal and sympathetic reflexes in rats. World J. Gastroenterol. 25, 2315–2326. doi: 10.3748/wjg.v25.i19.2315
Lu, L. M., Zhang, Y., Tang, X., Ge, S., Wen, H., Zeng, J., et al. (2022). Evidence on acupuncture therapies is underused in clinical practice and health policy. BMJ 376:e067475. doi: 10.1136/bmj-2021-067475
MacPherson, H., White, A., Cummings, M., Jobst, K., Rose, K., Niemtzow, R., et al. (2002). Standards for reporting interventions in controlled trials of acupuncture: the STRICTA recommendations. STandards for reporting interventions in controlled trails of acupuncture. Acupunct. Med. 20, 22–25. doi: 10.1136/aim.20.1.22
Moberget, T., and Ivry, R. B. (2016). Cerebellar contributions to motor control and language comprehension: searching for common computational principles. Ann. N. Y. Acad. Sci. 1369, 154–171. doi: 10.1111/nyas.13094
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G., PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 3, e123–e130. doi: 10.1371/journal.pmed.1000097
Moulton, E. A., Schmahmann, J. D., Becerra, L., and Borsook, D. (2010). The cerebellum and pain: passive integrator or active participator? Brain Res. Rev. 65, 14–27. doi: 10.1016/j.brainresrev.2010.05.005
Napadow, V., Makris, N., Liu, J., Kettner, N. W., Kwong, K. K., and Hui, K. K. S. (2005). Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum. Brain Mapp. 24, 193–205. doi: 10.1002/hbm.20081
Nierhaus, T., Pach, D., Huang, W., Long, X., Napadow, V., Roll, S., et al. (2015). Differential cerebral response to somatosensory stimulation of an acupuncture point vs. two non-acupuncture points measured with EEG and fMRI. Front. Hum. Neurosci. 9:74. doi: 10.3389/fnhum.2015.00074
Or-Borichev, A., Gurevitch, G., Klovatch, I., Greental, A., Lerner, Y., Levy, D. J., et al. (2023). Neural and functional validation of fMRI-informed EEG model of right inferior frontal gyrus activity. NeuroImage 266:119822. doi: 10.1016/j.neuroimage.2022.119822
Poldrack, R. A., Fletcher, P. C., Henson, R. N., Worsley, K. J., Brett, M., and Nichols, T. E. (2008). Guidelines for reporting an fMRI study. NeuroImage 40, 409–414. doi: 10.1016/j.neuroimage.2007.11.048
Rubinstein, D. Y., Camarillo-Rodriguez, L., Serruya, M. D., Herweg, N. A., Waldman, Z. J., Wanda, P. A., et al. (2021). Contribution of left supramarginal and angular gyri to episodic memory encoding: an intracranial EEG study. NeuroImage 225:117514. doi: 10.1016/j.neuroimage.2020.117514
Ruscheweyh, R., Kühnel, M., Filippopulos, F., Blum, B., Eggert, T., and Straube, A. (2014). Altered experimental pain perception after cerebellar infarction. Pain 155, 1303–1312. doi: 10.1016/j.pain.2014.04.006
Stoeckel, C., Gough, P. M., Watkins, K. E., and Devlin, J. T. (2009). Supramarginal gyrus involvement in visual word recognition. Cortex 45, 1091–1096. doi: 10.1016/j.cortex.2008.12.004
Stramaccia, D. F., Penolazzi, B., Altoè, G., and Galfano, G. (2017). TDCS over the right inferior frontal gyrus disrupts control of interference in memory: a retrieval-induced forgetting study. Neurobiol. Learn. Mem. 144, 114–130. doi: 10.1016/j.nlm.2017.07.005
Sun, J., Zhu, Y., Jin, L., Yang, Y., von Deneen, K. M., Qin, W., et al. (2012). Partly separated activations in the spatial distribution between de-qi and sharp pain during acupuncture stimulation: an fMRI-based study. Evid. Based Complement. Alternat. Med. 934085. doi: 10.1155/2012/934085
Talairach, J., and Tournoux, P. A. (1988). Co-planar stereotaxic atlas of the human brain. 3D proportional system: an approach to cerebral imaging.
Tian, D. S., Xiong, J., Pan, Q., Liu, F., Wang, L., Xu, S. B., et al. (2014). De qi, a threshold of the stimulus intensity, elicits the specific response of acupoints and intrinsic change of human brain to acupuncture. Evid. Based Complement. Alternat. Med. 914878. doi: 10.1155/2014/914878
Wada, S., Honma, M., Masaoka, Y., Yoshida, M., Koiwa, N., Sugiyama, H., et al. (2021). Volume of the right supramarginal gyrus is associated with a maintenance of emotion recognition ability. PLoS One 16:e0254623. doi: 10.1371/journal.pone.0254623
Wan, C., Xu, Y., Cen, B., Xia, Y., Yao, L., Zheng, Y., et al. (2021). Neuregulin1-ErbB4 signaling in spinal cord participates in electroacupuncture analgesia in inflammatory pain. Front. Neurosci. 15:636348. doi: 10.3389/fnins.2021.636348
Wang, D. (2009). Study on fMRI brain map in healthy adult underinggoing hand-moving and needling at Zusanli (ST36). Shantou University.
Wiener, M., Hamilton, R., Turkeltaub, P., Matell, M. S., and Coslett, H. B. (2010). Fast forward: supramarginal gyrus stimulation alters time measurement. J. Cogn. Neurosci. 22, 23–31. doi: 10.1162/jocn.2009.21191
Wu, M. T., Hsieh, J. C., Xiong, J., Yang, C. F., Pan, H. B., Chen, Y. C. I., et al. (1999). Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain—preliminary experience. Radiology 212, 133–141. doi: 10.1148/radiology.212.1.r99jl04133
Wu, G. J., Xu, F., Sun, X. M., and Chen, J. D. Z. (2020). Transcutaneous neuromodulation at ST36 (zusanli) is more effective than transcutaneous tibial nerve stimulation in treating constipation. J. Clin. Gastroenterol. 54, 536–544. doi: 10.1097/MCG.0000000000001184
Wu, Z., et al. (2007). Effect of acupuncture zusanli on magnetic resonance functional brain imaging. Chin. J. Trad. Med. Sci. Technol. 14:3. doi: 10.3969/j.issn.1005-7072.2007.05.001
Wu, S. N., Chen, J., Liu, B., Shang, X., and Li, X. (2011). Brain functional imaging study on the follow-up effect of acupuncture at Zusanli point. J. Guangzhou Univ. Tradit. Chin. Med. 28:4. doi: 10.13359/j.cnki.gzxbtcm.2011.01.004
Xiao, L. Y., Wang, X. R., Ye, Y., Yang, J. W., Cao, Y., Ma, S. M., et al. (2018). Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation 21, 762–776. doi: 10.1111/ner.12724
Yang, N. N., Ma, S. M., Yang, J. W., Li, T. R., and Liu, C. Z. (2020). Standardizing therapeutic parameters of acupuncture in vascular dementia rats. Brain Behav. 10:e01781. doi: 10.1002/brb3.1781
Zhang, J., Liu, Y., Lan, K., Huang, X., He, Y., Yang, F., et al. (2021). Gray matter atrophy in amnestic mild cognitive impairment: a voxel-based meta-analysis. Front. Aging Neurosci. 13:627919. doi: 10.3389/fnagi.2021.627919
Zhang, Z., Shi, Y., Cai, D., Jin, S., Zhu, C., Shen, Y., et al. (2018). Effect of electroacupuncture at ST36 on the intestinal mucosal mechanical barrier and expression of occludin in a rat model of sepsis. Acupunct. Med. 36, 333–338. doi: 10.1136/acupmed-2016-011187
Zhang, R., Zou, Y., Huang, S., Chen, Z., Liang, B., Li, Y., et al. (2007). MRI cerebral function imaging following acupuncture at Hegu, Zusanli and Neiguan points and Sanyinjiao points. J. Clin. Rehabil. Tissue Eng. Res. 278, 4271–4274. doi: 10.3321/j.issn:1673-8225.2007.22.003
Zhang, z., Wang, Y., Sun, J., Cao, H., Hu, N., Ma, L., et al. (2019). The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. J. Clin Acupunct. Moxibustion 35, 45–49. doi: 10.3969/j.issn.1005-0779.2019.01.013
Zhong, Z., Liu, B., Wu, S., and Ye, Y. (2014). Study of central nervous network mechanism of acupuncturing Acupoint Zusanli by resting-state functional magnetic resonance imaging. J. New Chin. Med. 46:4. doi: 10.13457/j.cnki.jncm.2014.04.071
Keywords: ST36, functional magnetic resonance imaging, mechanism, acupuncture, systematic review
Citation: Zhang J, Liu Y, Li Z, Hu Q, Huang X, Lv H, Xu J and Yu H (2023) Functional magnetic resonance imaging studies of acupuncture at ST36: a coordinate-based meta-analysis. Front. Neurosci. 17:1180434. doi: 10.3389/fnins.2023.1180434
Received: 06 March 2023; Accepted: 15 May 2023;
Published: 09 June 2023.
Edited by:
Yong Tang, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Kiran Kumar Solingapuram Sai, Wake Forest University, United StatesCopyright © 2023 Zhang, Liu, Li, Hu, Huang, Lv, Xu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Yu, MTM2MDMwNjYwOThAMTYzLmNvbQ==; Jinping Xu, anAueHVAc2lhdC5hYy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.