
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 18 July 2023
Sec. Neuroenergetics and Brain Health
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1176349
This article is part of the Research TopicInsights in Neuroenergetics, Nutrition and Brain Health: 2023View all 9 articles
Background: Dietary interventions for migraine are receiving increasing attention. However, it remains unclear whether there is any relationship between migraine and selenium intake. The aim of this study was to investigate the association between selenium intake and migraine.
Methods: We used multivariate logistic regression equations to explore the association between selenium intake and migraine. Restricted cubic splines were used to examine the presence of non-linear relationships. Upon finding a non-linear relationship, a recursive algorithm was used to calculate the inflection point. Population differences were also explored through stratified analysis.
Results: In the model adjusted for all covariates, the ORs (95% CI) for the association between selenium intake and migraine were 0.96 (0.88, 1.04), which was no statistical significance. However, the result of the linear trend test with quadrilles of selenium intake indicated the association between selenium intake and migraine may be non-linear. The restricted cubic splines confirmed this non-linear relationship, finding an inflection point (93.1 mcg/day), where the odds of migraine decreased with increasing selenium intake before the inflection point, and no statistically significant relationship was found after the inflection point. The association between selenium intake and migraine was non-linear in all strata except the obese.
Conclusion: We found a non-linear association between selenium intake and migraine in the general American population.
Migraine is a prevalent neurological disorder characterized by recurrent, mostly unilateral, moderate-to-severe throbbing headaches, often accompanied by nausea, vomiting, photophobia, and phonophobia (Buzzi et al., 2005; Barnett, 2019), and according to the 2016 Global Burden of Diseases (GBD) study, migraine is the second most common neurological disorders (Stovner et al., 2018). The annual prevalence of migraine is 14.4%, with 18.9% in women and 9.8% in men (Ford et al., 2017; Stovner et al., 2018). The annual prevalence of migraine is low in adolescents and older adults, with some studies showing an annual prevalence of about 5% in adolescents and people over 50 years of age, while new-onset migraine-like headaches in people > 50 years require vigilance for secondary headaches (Burch et al., 2019; Ferrari et al., 2022). Migraine has some family aggregation with a heritability rate of 42 (Ducros, 2021).
In the current era, drug research and development have reached a bottleneck, and dietary intervention for chronic diseases is increasingly receiving attention. Modern researches have shown that the antioxidant properties of diet have therapeutic value for neurological diseases (Rizwana et al., 2021; Davis et al., 2022). Flavonoids can reduce cognitive impairment, and relieve depressive moods (Park et al., 2021). Vitamin B12 can inhibit neuronal damage caused by endoplasmic reticulum stress and enhance neural repair and functional recovery after traumatic brain injury (Poole et al., 2022). The intake of the antioxidant zinc is negatively correlated with the incidence of migraine, which indicates that zinc is an important influencing factor for migraine (Liu et al., 2023). The cohort study of Chinese adults showed that dietary selenium was negatively correlated with stroke, indicating that dietary selenium intake should be increased to a certain level to prevent stroke (Shi et al., 2022).
It is well known that selenium is an important antioxidant and an essential trace element for human health, and dietary intake is the main form of body supplementation (Wang et al., 2017; Rayman, 2020). Previous researches have shown that selenium intake can help improve psychoneurological disorders such as stroke, Parkinson’s, and depression through its antioxidant properties (Li et al., 2018; Sun et al., 2022; Zhang et al., 2022). Some migraineurs have lower serum catalase activity, non-oxidative thiol concentrations, and total antioxidant capacity. Meanwhile, the activities of superoxide dismutase in platelets and red blood cells in migraine patients are reduced (Malkki, 2015; Togha et al., 2019). Until now, the association between selenium intake and migraine has never been explored, so we used data from the National Health and Nutrition Examination Survey (NHANES) to assess the association between dietary selenium intake and migraine in order to fill this knowledge gap and provide new evidence for dietary modifications to intervene in migraine.
We obtained data from the database for three cycles. The NHANES is a continuously conducted cross-sectional survey that provides self-reported health information on a nationally representative civilian, noninstitutional population of the U.S. All data collection was carried out through home visits, screening by mobile examination centers (MECs), and laboratory testing. The National Center for Health Statistics (NCHS) Ethics Review Committee authorized this cross-sectional study with all participants completing written informed consents. No additional ethical application is required for this study. We combined these data for analysis and obtained a total of 31,126 participants, and our study sample was restricted to adults aged 20 years or older (N = 15,332). After excluding participants with missing information on migraine (N = 12) and dietary selenium (N = 2,356), a total of 12,964 study participants remained in our study, of whom 2,645 had migraine (Figure 1).
Data on selenium intake were collected by a 24-h dietary recall interview, conducted by trained dietary interviewers in person. Standard measurement guidelines were provided to participants for reporting food size and quantity. In our study, all participants underwent only the first 24-h dietary recall from 1999 to 2004. We therefore assessed selenium intake based on the first 24-h dietary recall.
The assessment of migraine was based on the question: “During the past 3 months, did you have severe headaches or migraines?.” We were unable to find any other information about migraine in the NHANES. The American migraine prevalence and prevention study indicated that of the 17.4% of participants who reported “severe headache,” 11.8 and 4.6% met the International Classification of Headache Disorders (2nd edition) criteria for migraine and possible migraine, respectively (Buse et al., 2013). Only 1% were classified as “other severe headache.” Thus, we deemed it reasonable to assume that most participants with severe headache experience migraine, the assumption was also made by other researchers in this area (Huang and He, 2022; Li et al., 2022; Peng et al., 2022).
We collected demographic data, lifestyle habits, chronic disease status, body measurements, and biochemical examinations as covariates. Age, sex, race, education level, marital status, and income-to-poverty ratio were incorporated into the demographic variables. Smoking and drinking status were categorized as “current,” “former,” and “never.” The vigorous recreational activities were classified into two categories: yes and no. The medical comorbidities, such as stroke, CVD, hypertension, hyperlipemia, and diabetes, were ascertained by self-reported histories in the questionnaires. The BMI was measured and calculated by trained professionals. Overweight was defined as BMI ≥ 25 kg/m2 and < 30 kg/m2, and obese was defined with BMI > 30 kg/m2. Biochemical examinations include fasting glucose, uric acid, and C-reactive protein.
The study was a secondary analysis of a publicly accessible database. Categorical proportions (%) and means (standard errors, SE) are used for categorical variables and continuous variables, respectively. To compare differences between groups, weighted linear regression models (continuous variables) and weighted chi-square tests (categorical variables) were conducted. Weighted logistic regression analyses were used to explore the relationship between selenium intake and migraine. We used odds ratios (ORs) and 95% confidence interval (95% CI) to report results. Stratified analyses and linear trend-wise tests were also conducted to explore the stability and differences in this relationship across subgroups of the population. We used restricted cubic splines to assess whether there was a non-linear relationship between selenium intake and migraine. A recursive algorithm and a two-segment linear regression model were used to calculate the turning point upon detection of nonlinearity. Statistical analysis was undertaken using R (version 4.2.0) and Empower Stats. Statistical significance was defined as p < 0.05.
Table 1 shows the baseline characteristics of the study participants. The mean age of the study population was 46.33 years (95% CI, 45.78, 46.87). Women account for 47.37% of the total 12,964 participants, and 72.03% are white. Compared with people without migraine, migraine sufferers are younger, more likely to be female, have a higher BMI, C-reactive protein, and lower uric acid and income-to-poverty ratio, and are less likely to engage in recreational activities.
The association between selenium intake and migraine is shown in Table 2. In the model without all variables adjusted, the ORs (95% CI) were 0.90 (0.86, 0.94). However, after adjustment for all covariates, the ORs (95% CI) was 0.96 (0.88, 1.04), which was not statistically significant. Moreover, in the further trend test, the ORs (95% CI) of the association between selenium intake and migraine was Q2 (OR:0.87, 95CI%: 0.69, 1.10), Q3 (OR:0.78, 95CI%: 0.62, 0.99), Q4 (OR:0.81, 95CI%: 0.64, 1.03), and Q5 (OR:0.87, 95CI%: 0.67, 1.11), using Q1 as a reference, indicating that the relationship between selenium intake and migraine might be non-linear. When we performed a sex-stratified analysis, no statistically significant relationship was found. In the age stratification, the study population was not observed to be statistically significant. In the overweight population, the ORs (95% CI) were 0.98 (0.85, 1.12), with the ORs (95% CI) being 0.95 (0.86, 1.05) in the obese population.
To further explore the relationship between selenium intake and migraine, we used the restricted cubic spline (RCS). When all covariates were adjusted, we confirmed that the association between selenium intake and migraine was non-linear, which may account for the lack of statistical significance (Figure 2). Furthermore, we found the inflection point (93.1 mcg/day), and the relationship before and after the inflection point was reversed, with a negative correlation [0.64(0.49, 0.82)] before the inflection point and a non-statistically significant positive correlation [1.04 (0.90, 1.20)] after the inflection point (Table 3).
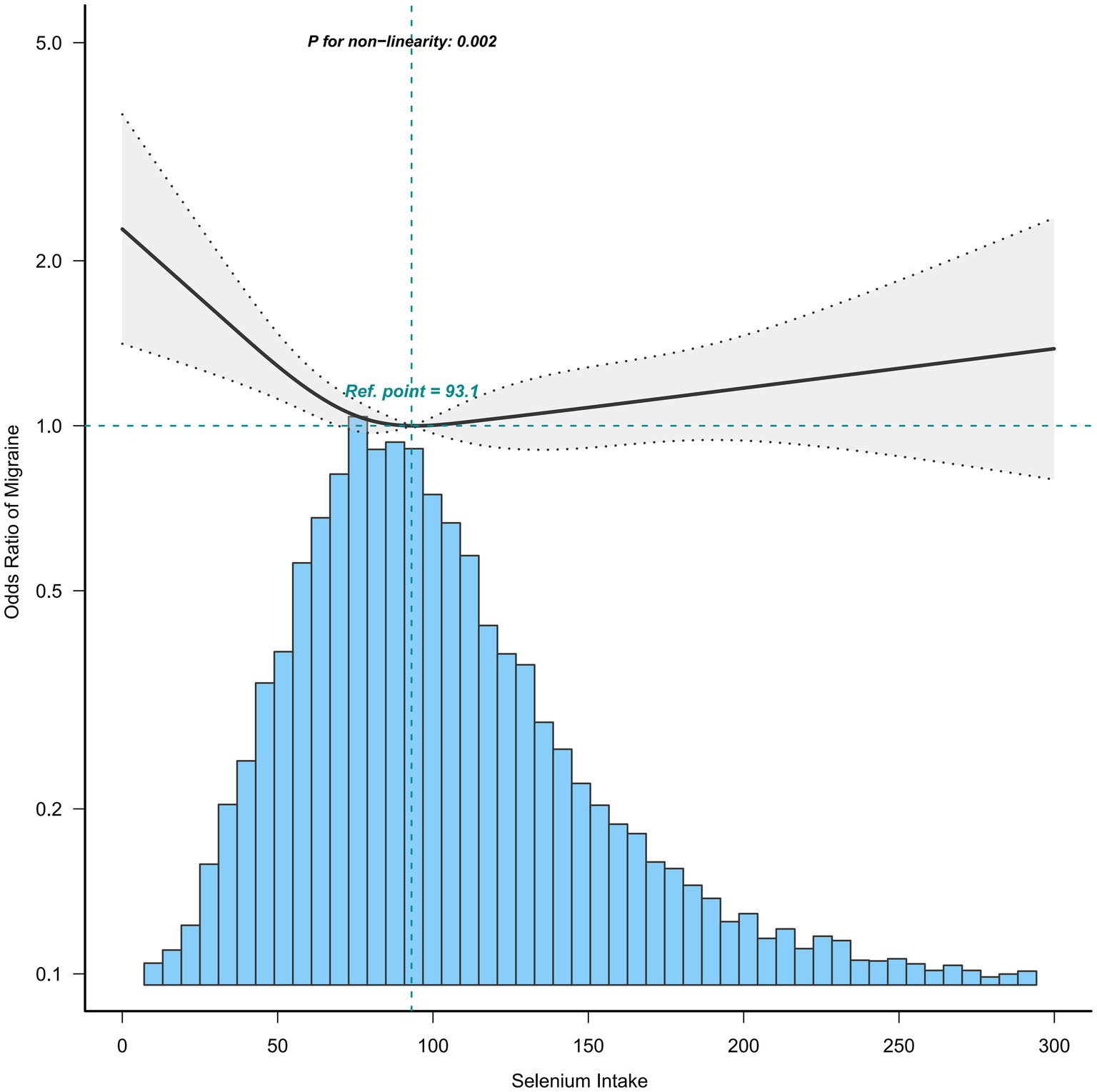
Figure 2. Association between selenium intake and migraine. Age, sex, race, income-to-poverty ratio, education level, marital status, smoking status, drinking status, hypertension, hyperlipemia, stroke, diabetes, CVD, vigorous recreational activity, BMI, fast glucose, uric acid, and CRP were adjusted.
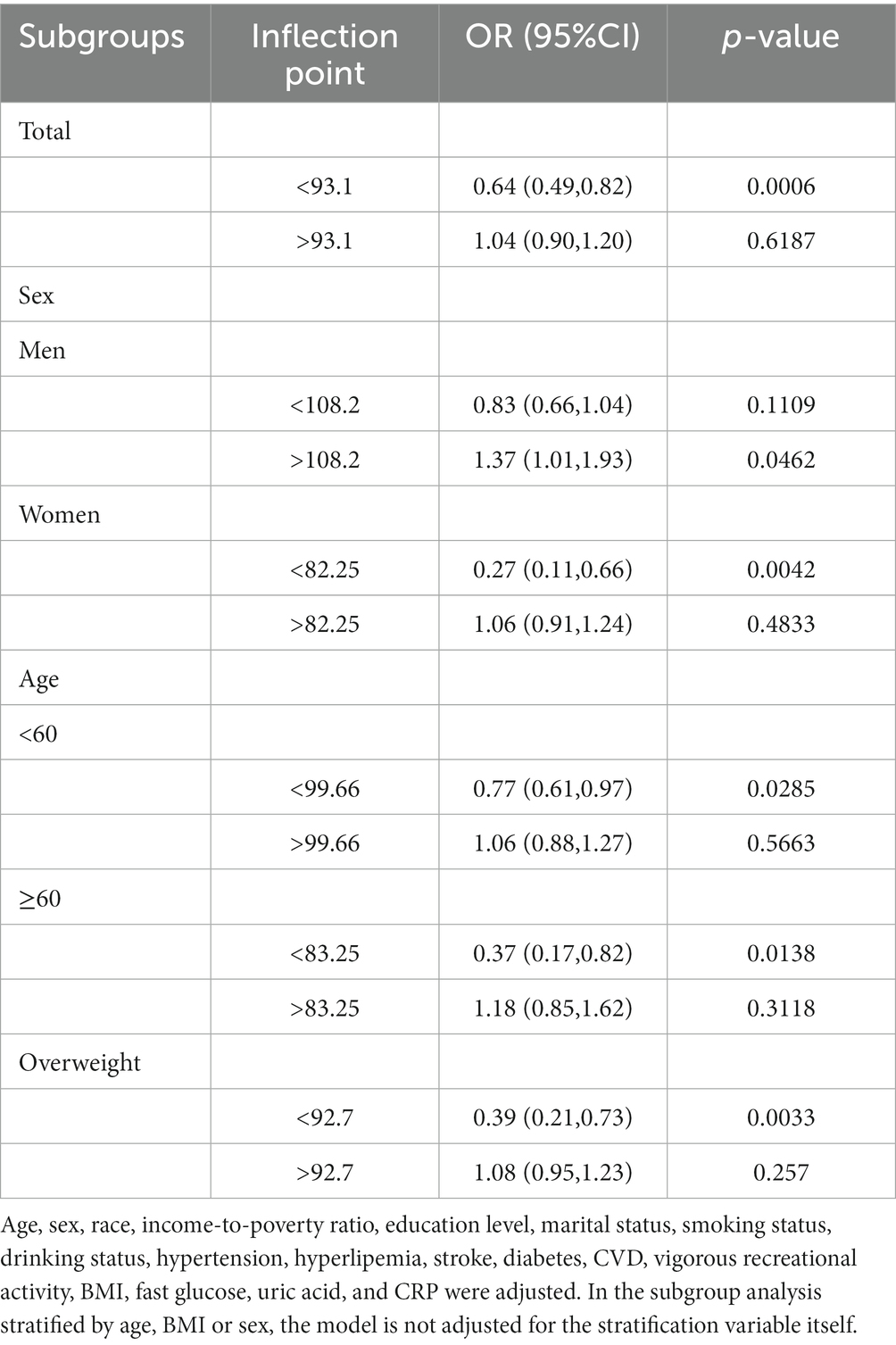
Table 3. Threshold effect analysis of selenium intake on migraine using a two-piecewise linear regression model.
The association between selenium intake and migraine was found to be non-linear in all subgroups of the population except the obese when the stratification was performed (Figures 3–5). In the sex stratification, the inflection point for men was 108.2 mcg/day, and the relationship was only statistically significant at 1.37 (1.01, 1.93) after the inflection point (Table 3). However, the negative association in women was observed only before the inflection point (82.25mcg/day; Table 3). The risk of depression decreased by 23% with increasing selenium intake in people aged < 60 only when selenium intake was less than 99.66mcg/day (Table 3). In the elderly population, the inflection point was 83.25 mcg/day (Table 3). In overweight adults, the relationship was statistically significant only before the inflection points [0.39 (0.210, 0.73)] (Table 3). There was no non-linear relationship in the obese population (P for non-linearity = 0.206; Figure 5B).
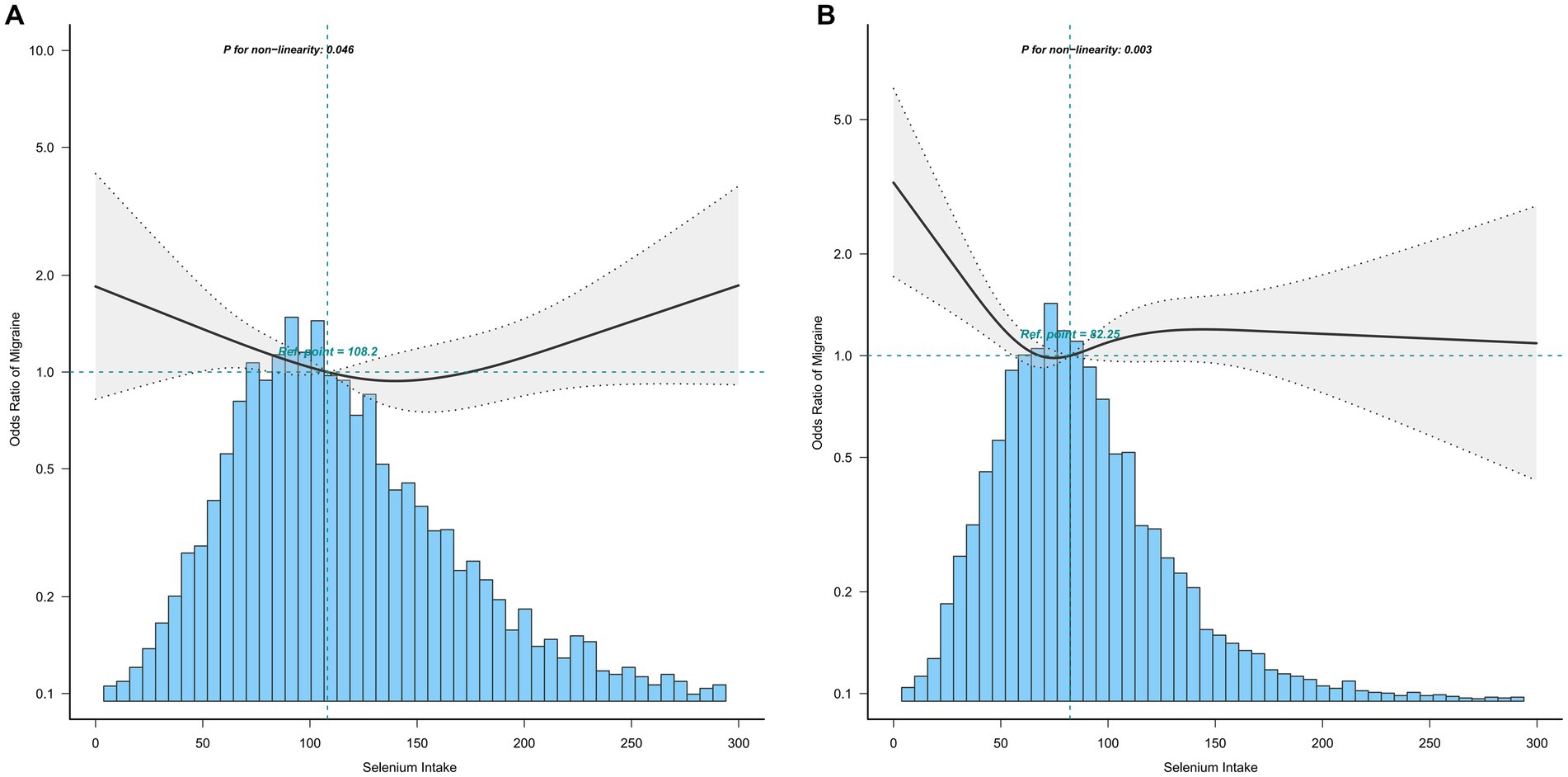
Figure 3. Association between selenium intake and migraine stratified by sex (A: men; B: women). In the subgroup analysis stratified by sex, the model is not adjusted for sex.
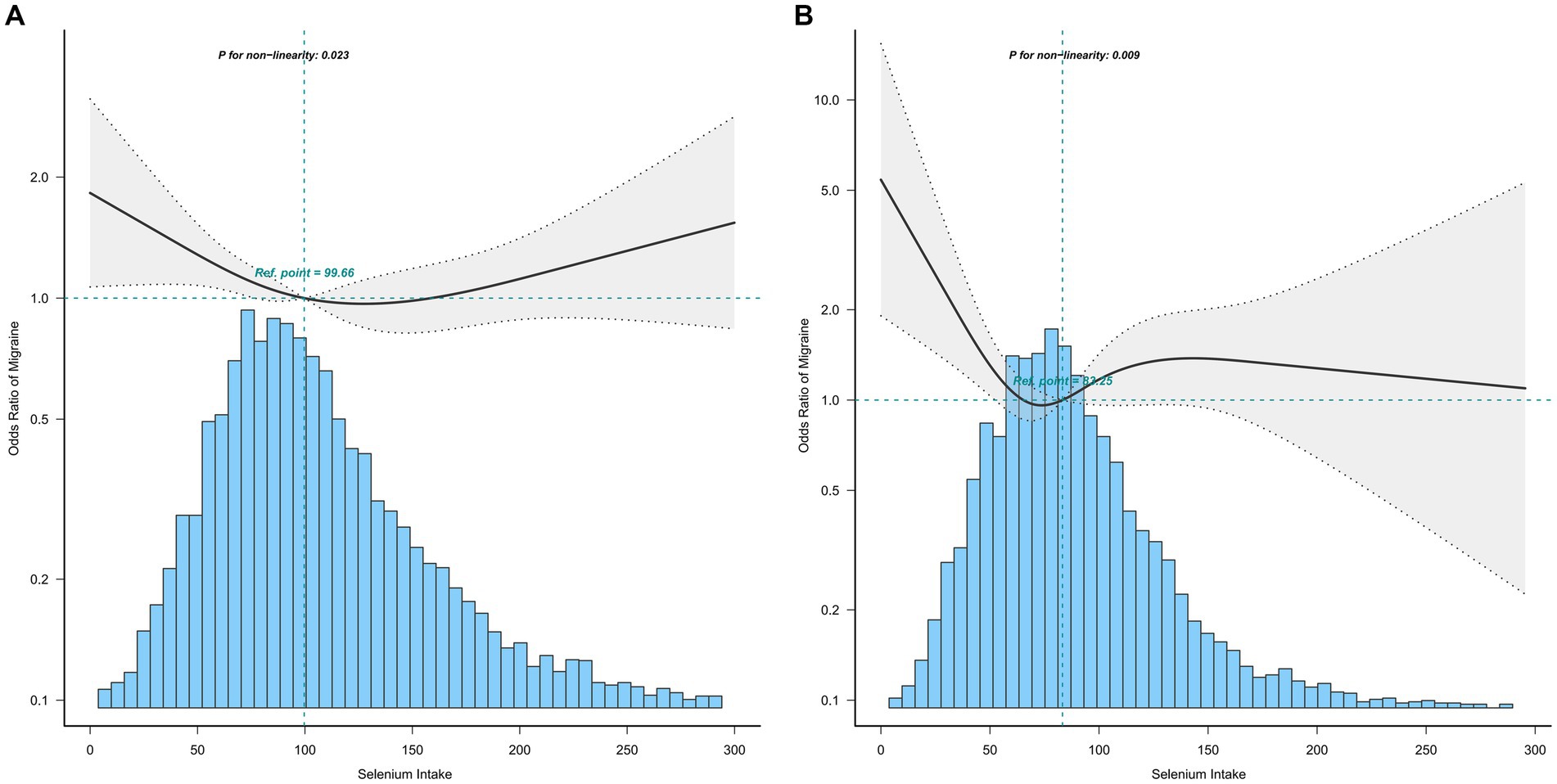
Figure 4. Association between selenium intake and migraine stratified by age (A: age < 60; B: age ≥ 60). In the subgroup analysis stratified by age, the model is not adjusted for age.
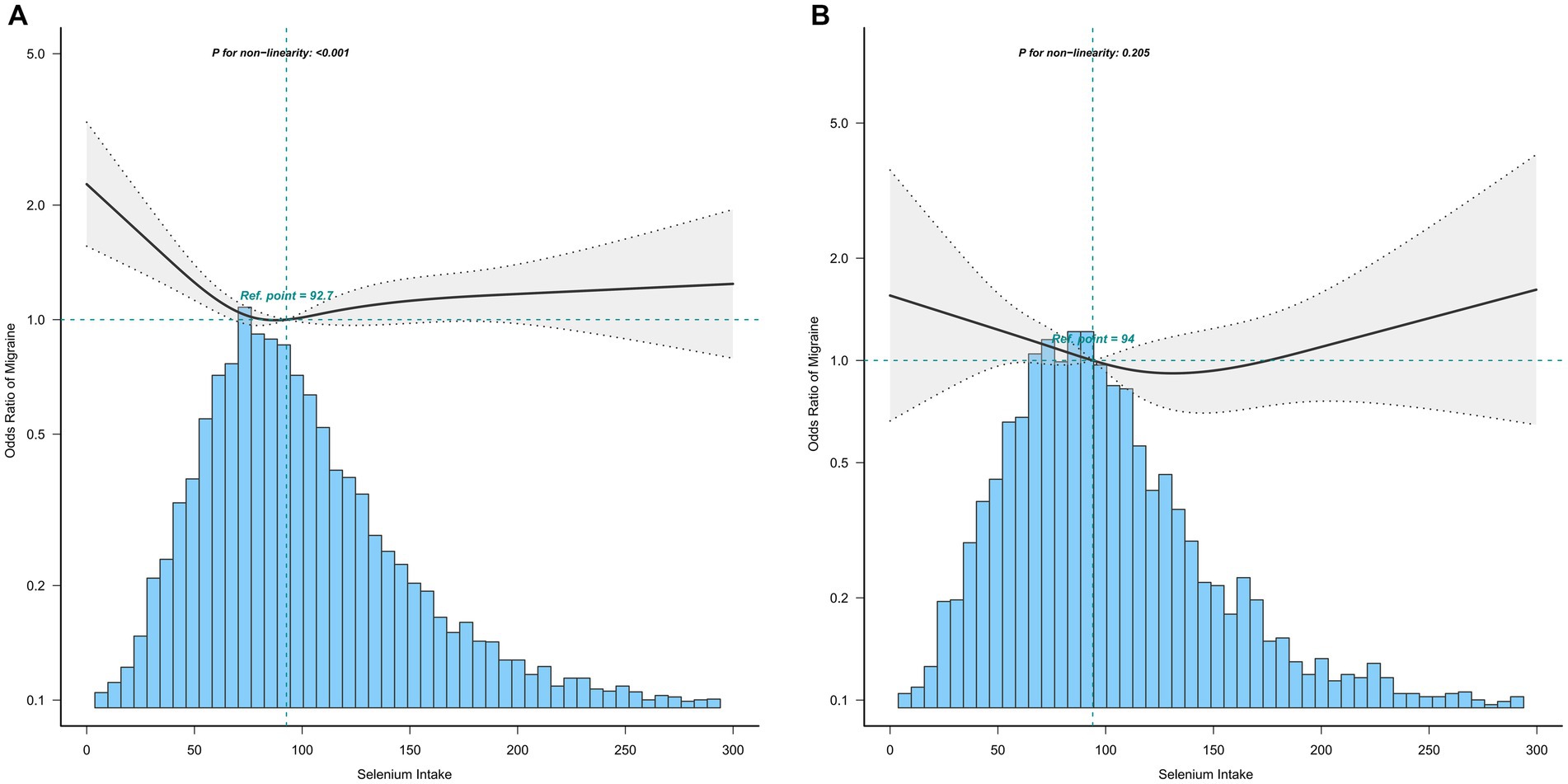
Figure 5. Association between selenium intake and migraine stratified by BMI (A: overweight; B: obese). In the subgroup analysis stratified by BMI, the model is not adjusted for BMI.
In this exploration with a United States population, no significant association between selenium intake and migraine was found after adjusting for all covariates. In a further restricted cubic spline, we observed a non-linear relationship between selenium intake and migraine, with an inflection point of 93.1mcg/day, where the odds of migraine decreased with increasing niacin intake before the inflection point, and no statistically significant relationship was found after the inflection point. The association between selenium intake and migraine was non-linear in all strata except the obese. To our knowledge, this is the first study to explore the association between selenium intake and migraine and perform stratified analyses to explore differences among populations in subgroups.
The association between the antioxidant selenium and migraine is rarely studied. A case–control study indicated migraine patients had significantly lower selenium levels compared to healthy participants. Individuals in the lowest quartile of selenium levels were approximately 11 times more likely to have migraine than those in the highest quartile of selenium levels (Talaie et al., 2022). Animal experiments have shown that selenium supplementation improves migraine in model rats by modulating oxidative stress and antioxidant redox systems in the brain, with protective effects on MMCA, GSH, GSH-Px, and antioxidant vitamin concentrations (Naziroglu et al., 2015).
Stratified analysis was performed to further estimate the association between selenium intake and migraine in different groups. In the stratified analysis before performing restricted cubic splines, we did not find a significant relationship between selenium intake and migraine. Upon the restricted cubic splines, the relationships between selenium intake and migraine were non-linear in all subgroups of the population except the obese, and a clear relationship existed only before the inflection point in all subgroups of the population, except men and the obese. In men, the relationship was statistically significant both before and after the inflection point.
Selenium is an essential immune nutrient with anti-inflammatory, antioxidant, and immunomodulatory effects (Huang et al., 2012). However, from the toxicological viewpoint, selenium becomes extremely toxic when its concentration is slightly above its nutritional level (Huang et al., 2022). Selenium (especially organic selenium) provides important regulatory effects on neuromodulatory systems such as the cholinergic and glutamatergic systems, as well as enzymes such as delta-ALA-D and acetylcholinesterase (Dominiak et al., 2016; Naziroglu et al., 2020). On the other hand, the interaction of selenium derivatives with metal-induced toxicity has produced conflicting results. Although ameliorative and neutral effects of organic and inorganic selenium on non-essential metal toxicity are frequently reported, substantial evidence demonstrates the potentiating effect of selenium on the neurotoxicity of non-essential metals such as mercury, cadmium, and lead, which reduces the antioxidant capacity of neuronal cells, while increasing their susceptibility to further oxidative damage (Nogueira and Rocha, 2011; Lv et al., 2021; Naderi et al., 2021).
Selenium intake in certain amounts can help alleviate migraines, and the probable mechanism may be related to inflammation and oxidative stress. Migraine is recognized to be associated with increased mitochondrial energy metabolism (Cevoli et al., 2019). Mitochondrial oxidative stress is important in the pathophysiology of migraine, and selenium has a regulatory effect on mitochondrial oxidative stress in the brain (Fila et al., 2022). Oxidative stress is controlled by antioxidants, including selenium. Selenium plays an important role in the nervous system, including the brain, as a cofactor for glutathione peroxidase and is incorporated into selenoproteins involved in antioxidant defense. It is neuroprotective by modulating excessive ROS production, inflammation, and Ca2 + overload in several diseases, including inflammatory pain, allergic, atopic pain, diabetic neuropathic pain, and injurious pain (Steinbrenner and Sies, 2013; Hariharan and Dharmaraj, 2020).
The occurrence of migraine can be exacerbated when selenium is consumed in excess, although with no statistical significance. There is substantial evidence that excessive selenium intake may disrupt the normal function of various key proteins and signaling molecules engaged in oxidative stress and neuroinflammation, leading to an increased inflammatory response (Benton, 2002; Rayman et al., 2018; Zhao et al., 2022). Elevated selenium exposure is also suspected to be associated with neuropsychiatric disorders such as stroke, depression, and headaches (Colangelo et al., 2014; Hu et al., 2017).
Our research has some advantages. First, we adopted a large, nationally representative, and rigorously quality-controlled dataset, which increases the reliability of our results. Second, such a large sample size enables us to conduct further subgroup analysis, exploring the differences in the relationship between selenium intake and migraine among different populations, which is beneficial for formulating different intervention measures according to specific populations. Third, with the adjustment of potential confounders, our conclusions are more in line with reality. However, this study also has several limitations. First, as a cross-sectional study, due to the nature of observational studies, we cannot exclude reverse causality, nor can we reveal the causal relationship between the dependent and independent variables. Second, dietary data were collected from a 24-h dietary recall, so they cannot be used to represent long-term dietary intake, and it is not possible to determine whether the relationship between selenium intake and migraine will change over time. Third, the use of 24-h dietary recall depends on memory, so it is prone to over-reporting or under-reporting, and may not reflect habitual intake. Fourth, other potential confounders not adjusted in this study may still cause bias.
Using the NHANES database study, a non-linear association between selenium intake and migraine was found with subgroup differences being explored. Our study may help clinicians and public health departments to better design relevant programs to help prevent and treat migraine. More basic experiments need to be conducted to explore the mechanisms by which selenium acts in migraine.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by National Center for Health Statistics (NCHS) Ethics Review Committee. The patients/participants provided their written informed consent to participate in this study.
LZ and JY: project development and research design and manuscript writing. XLu: responsibility for data analysis and interpretation, manuscript writing. XLi: manuscript editing and interpretation of the results. All authors contributed to the article and approved the submitted version.
This work was supported by Shandong Province Chinese Medicine Science and Technology Project (grant number 2021M150).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Benton, D. (2002). Selenium intake, mood and other aspects of psychological functioning. Nutr. Neurosci. 5, 363–374. doi: 10.1080/1028415021000055925
Burch, R. C., Buse, D. C., and Lipton, R. B. (2019). Migraine: epidemiology, burden, and comorbidity. Neurol. Clin. 37, 631–649. doi: 10.1016/j.ncl.2019.06.001
Buse, D. C., Loder, E. W., Gorman, J. A., Stewart, W. F., Reed, M. L., Fanning, K. M., et al. (2013). Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine prevalence and prevention (Ampp) study. Headache 53, 1278–1299. doi: 10.1111/head.12150
Buzzi, M. G., Cologno, D., and Formisano, R. (2005). Migraine disease: evolution and progression. J. Headache Pain 6, 304–306. doi: 10.1007/s10194-005-0215-9
Cevoli, S., Favoni, V., and Cortelli, P. (2019). Energy metabolism impairment in migraine. Curr. Med. Chem. 26, 6253–6260. doi: 10.2174/0929867325666180622154411
Colangelo, L. A., He, K., Whooley, M. A., Daviglus, M. L., Morris, S., and Liu, K. (2014). Selenium exposure and depressive symptoms: the coronary artery risk development in young adults trace element study. Neurotoxicology 41, 167–174. doi: 10.1016/j.neuro.2014.02.003
Davis, C. K., Bathula, S., Hsu, M., Morris-Blanco, K. C., Chokkalla, A. K., Jeong, S., et al. (2022). An antioxidant and anti-Er stress combo therapy decreases inflammation, secondary brain damage and promotes neurological recovery following traumatic brain injury in mice. J. Neurosci. 42, 6810–6821. doi: 10.1523/JNEUROSCI.0212-22.2022
Dominiak, A., Wilkaniec, A., Wroczynski, P., and Adamczyk, A. (2016). Selenium in the therapy of neurological diseases. Where is it going. Curr. Neuropharmacol. 14, 282–299. doi: 10.2174/1570159x14666151223100011
Ducros, A. (2021). Genetics of migraine. Rev. Neurol. (Paris) 177, 801–808. doi: 10.1016/j.neurol.2021.06.002
Ferrari, M. D., Goadsby, P. J., Burstein, R., Kurth, T., Ayata, C., Charles, A., et al. (2022). Migraine. Nat Rev Dis Primers 8:2. doi: 10.1038/s41572-021-00328-4
Fila, M., Jablkowska, A., Pawlowska, E., and Blasiak, J. (2022). DNA damage and repair in migraine: oxidative stress and beyond. Neuroscientist 29, 277–286. doi: 10.1177/10738584221090836
Ford, J. H., Jackson, J., Milligan, G., Cotton, S., Ahl, J., and Aurora, S. K. (2017). A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache 57, 1532–1544. doi: 10.1111/head.13202
Hariharan, S., and Dharmaraj, S. (2020). Selenium and Selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology 28, 667–695. doi: 10.1007/s10787-020-00690-x
Hu, X. F., Eccles, K. M., and Chan, H. M. (2017). High selenium exposure lowers the odds ratios for hypertension, stroke, and myocardial infarction associated with mercury exposure among Inuit in Canada. Environ. Int. 102, 200–206. doi: 10.1016/j.envint.2017.03.002
Huang, H., and He, K. (2022). The association between dietary Fiber intake and severe headaches or migraine in us adults. Front. Nutr. 9:1044066. doi: 10.3389/fnut.2022.1044066
Huang, Z., Rose, A. H., and Hoffmann, P. R. (2012). The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 16, 705–743. doi: 10.1089/ars.2011.4145
Huang, J., Xie, L., Song, A., and Zhang, C. (2022). Selenium status and its antioxidant role in metabolic diseases. Oxidative Med. Cell. Longev. 2022, 7009863–7009815. doi: 10.1155/2022/7009863
Li, D., Guo, Y., Xia, M., Zhang, J., and Zang, W. (2022). Dietary intake of thiamine and riboflavin in relation to severe headache or migraine: a cross-sectional survey. Headache 62, 1133–1142. doi: 10.1111/head.14384
Li, Z., Wang, W., Xin, X., Song, X., and Zhang, D. (2018). Association of Total Zinc, Iron, copper and selenium intakes with depression in the us adults. J. Affect. Disord. 228, 68–74. doi: 10.1016/j.jad.2017.12.004
Liu, H., Wang, Q., Dong, Z., and Yu, S. (2023). Dietary zinc intake and migraine in adults: a cross-sectional analysis of the National Health and nutrition examination survey 1999-2004. Headache 63, 127–135. doi: 10.1111/head.14431
Lv, Q., Liang, X., Nong, K., Gong, Z., Qin, T., Qin, X., et al. (2021). Advances in research on the toxicological effects of selenium. Bull. Environ. Contam. Toxicol. 106, 715–726. doi: 10.1007/s00128-020-03094-3
Malkki, H. (2015). Headache: chronic migraine linked to reduced antioxidant capacity. Nat. Rev. Neurol. 11:426. doi: 10.1038/nrneurol.2015.125
Naderi, M., Puar, P., Zonouzi-Marand, M., Chivers, D. P., Niyogi, S., and Kwong, R. W. M. (2021). A comprehensive review on the neuropathophysiology of selenium. Sci. Total Environ. 767:144329. doi: 10.1016/j.scitotenv.2020.144329
Naziroglu, M., Celik, O., Uguz, A. C., and Butun, A. (2015). Protective effects of riboflavin and selenium on brain microsomal Ca2+-Atpase and oxidative damage caused by glyceryl Trinitrate in a rat headache model. Biol. Trace Elem. Res. 164, 72–79. doi: 10.1007/s12011-014-0199-x
Naziroglu, M., Oz, A., and Yildizhan, K. (2020). Selenium and neurological diseases: focus on peripheral pain and Trp channels. Curr. Neuropharmacol. 18, 501–517. doi: 10.2174/1570159X18666200106152631
Nogueira, C. W., and Rocha, J. B. (2011). Toxicology and pharmacology of selenium: emphasis on synthetic Organoselenium compounds. Arch. Toxicol. 85, 1313–1359. doi: 10.1007/s00204-011-0720-3
Park, S. J., Jaiswal, V., and Lee, H. J. (2021). Dietary intake of flavonoids and carotenoids is associated with anti-depressive symptoms: epidemiological study and in silico-mechanism analysis. Antioxidants (Basel) 11:53. doi: 10.3390/antiox11010053
Peng, C., Gao, L., Wu, K., Jiang, X., Chen, X., Li, C., et al. (2022). Association between the prognostic nutritional index and severe headache or migraine: a population-based study. Nutr. Neurosci., 1–10. doi: 10.1080/1028415X.2022.2143958
Poole, J., Jasbi, P., Pascual, A. S., North, S., Kwatra, N., Weissig, V., et al. (2022). Ischemic stroke and dietary vitamin B12 deficiency in old-aged females: impaired motor function, increased ischemic damage size, and changed metabolite profiles in brain and cecum tissue. Nutrients 14:2960. doi: 10.3390/nu14142960
Rayman, M. P. (2020). Selenium intake, status, and health: a complex relationship. Hormones (Athens) 19, 9–14. doi: 10.1007/s42000-019-00125-5
Rayman, M. P., Winther, K. H., Pastor-Barriuso, R., Cold, F., Thvilum, M., Stranges, S., et al. (2018). Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 127, 46–54. doi: 10.1016/j.freeradbiomed.2018.02.015
Rizwana, N., Agarwal, V., and Nune, M. (2021). Antioxidant for neurological diseases and neurotrauma and bioengineering approaches. Antioxidants (Basel) 11:72. doi: 10.3390/antiox11010072
Shi, W., Su, L., Wang, J., Wang, F., Liu, X., and Dou, J. (2022). Correlation between dietary selenium intake and stroke in the National Health and nutrition examination survey 2003-2018. Ann. Med. 54, 1395–1402. doi: 10.1080/07853890.2022.2058079
Steinbrenner, H., and Sies, H. (2013). Selenium homeostasis and antioxidant Selenoproteins in brain: implications for disorders in the central nervous system. Arch. Biochem. Biophys. 536, 152–157. doi: 10.1016/j.abb.2013.02.021
Stovner, L. J., Nichols, E., Steiner, T. J., Abd-Allah, F., Abdelalim, A., al-Raddadi, R. M., et al. (2018). Global, regional, and National Burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 17, 954–976. doi: 10.1016/S1474-4422(18)30322-3
Sun, C., Du, Z., Liu, X., Yang, Y., Zhou, S., Li, C., et al. (2022). Selenium forms and dosages determined their biological actions in mouse models of Parkinson’s disease. Nutrients 15:11. doi: 10.3390/nu15010011
Talaie, A., Jafary, H., Faraji, F., and Malekirad, A. A. (2022). The serum oxidative stress biomarkers and selenium levels in a Group of Migraine Patients Compared with healthy controls: a case-control study. Biol. Trace Elem. Res. 200, 4250–4255. doi: 10.1007/s12011-021-03024-2
Togha, M., Razeghi Jahromi, S., Ghorbani, Z., Ghaemi, A., and Rafiee, P. (2019). An investigation of oxidant/antioxidant balance in patients with migraine: a case-control study. BMC Neurol. 19:323. doi: 10.1186/s12883-019-1555-4
Wang, N., Tan, H. Y., Li, S., Xu, Y., Guo, W., and Feng, Y. (2017). Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxidative Med. Cell. Longev. 2017, 7478523–7478513. doi: 10.1155/2017/7478523
Zhang, H., Qiu, H., Wang, S., and Zhang, Y. (2022). Association of Habitually low Intake of dietary selenium with new-onset stroke: a retrospective cohort study (2004-2015 China health and nutrition survey). Front. Public Health 10:1115908. doi: 10.3389/fpubh.2022.1115908
Keywords: association, selenium intake, migraine, non-linear, subgroup analysis
Citation: Zhao L, Yin J, Li X and Lu X (2023) Association between selenium intake and migraine: a nationwide cross-sectional study. Front. Neurosci. 17:1176349. doi: 10.3389/fnins.2023.1176349
Received: 28 February 2023; Accepted: 22 June 2023;
Published: 18 July 2023.
Edited by:
David Mokler, University of New England, United StatesReviewed by:
Tiantong Niu, Capital Medical University, ChinaCopyright © 2023 Zhao, Yin, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xixue Lu, YmFuc2hhbl8xOTcxQDEyNi5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.