
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci., 12 May 2023
Sec. Gut-Brain Axis
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1174287
 Yangke Mao1,2†
Yangke Mao1,2† Pan Zhang1,2†
Pan Zhang1,2† Ruirui Sun1,2†
Ruirui Sun1,2† Xinyue Zhang1,2
Xinyue Zhang1,2 Yuqi He1
Yuqi He1 Siyang Li1
Siyang Li1 Tao Yin1,2*
Tao Yin1,2* Fang Zeng1,2,3*
Fang Zeng1,2,3*Background: Neuroimaging studies have identified aberrant activity patterns in multiple brain regions in functional dyspepsia (FD) patients. However, due to the differences in study design, these previous findings are inconsistent, and the underlying neuropathological characteristics of FD remain unclear.
Methods: Eight databases were systematically searched for literature from inception to October 2022 with the keywords “Functional dyspepsia” and “Neuroimaging.” Thereafter, the anisotropic effect size signed the differential mapping (AES-SDM) approach that was applied to meta-analyze the aberrant brain activity pattern of FD patients.
Results: A total of 11 articles with 260 FD patients and 202 healthy controls (HCs) were included. The AES-SDM meta-analysis demonstrated that FD patients manifested increased activity in the bilateral insula, left anterior cingulate gyrus, bilateral thalamus, right precentral gyrus, left supplementary motor area, right putamen, and left rectus gyrus and decreased functional activity in the right cerebellum compared to the HCs. Sensitivity analysis showed that all these above regions were highly reproducible, and no significant publication bias was detected.
Conclusion: The current study demonstrated that FD patients had significantly abnormal activity patterns in several brain regions involved in visceral sensation perception, pain modulation, and emotion regulation, which provided an integrated insight into the neuropathological characteristics of FD.
Functional dyspepsia (FD), a prevalent functional gastrointestinal disorder (FGID), is characterized by a group of long-term and fluctuating symptoms including postprandial fullness, early satiety, epigastric pain, and epigastric burning without a clear structural explanation (Enck et al., 2017). As one of the most common FGIDs, FD has a high incidence, affecting up to 10–16% of the individuals in the general population worldwide (Ford et al., 2020). According to previous studies, the population prevalence of FD ranges from 8% to 23% in Asia and approximately 12% in North America (Ghoshal et al., 2011; Aziz et al., 2018). Although not life-threatening or disabling, FD imposes a heavy medical burden on individuals and significantly reduces their quality of life (El-Serag and Talley, 2003; Miwa et al., 2022). However, due to the incomplete understanding of its pathophysiology and lack of specific biomarkers, the diagnosis and treatment of FD are largely restricted.
There is increasing evidence indicating that FD is a complex disease with multi-factorial interaction (Tack et al., 2004). Central homeostasis imbalance (Tait and Sayuk, 2021), gastroduodenal motility alteration (Oustamanolakis and Tack, 2012), gastroduodenal hypersensitivity (Mertz et al., 1998), and intestinal microbiota disorder (Shimura et al., 2016) have been considered as the potential pathogenesis of FD. Specifically, in the latest Rome IV criteria (Drossman et al., 2018), FD is defined as a functional disorder of brain–gut interaction, which indicates that the dysfunction of the alimentary tract and brain, as well as aberrant interaction patterns between them, play an important role in explaining the mechanism of FD. Within this framework, researchers utilized real-time neuroimaging technology to explore how the brain activity patterns of FD patients differ from that of healthy controls (HCs) under the resting state and stimulus conditions. With the application of functional neuroimaging technology, multiple aberrant brain regions have been found in FD patients, including but not limited to the insula (Zeng et al., 2012), thalamus (Liu et al., 2018), amygdala (Zeng et al., 2019), and dorsolateral prefrontal cortex (Liu et al., 2017). These functional brain abnormalities were identified in a systematic review (Lee et al., 2016). However, the differences in sample size, scanning methods, imaging modalities, data analysis, and other methodological limitations may lead to the heterogeneity of results, making it difficult to draw reliable and comprehensive conclusions. Therefore, it is necessary to use a meta-analysis method to make a comprehensive analysis of the previous findings.
Anisotropic effect size signed differential mapping (AES-SDM) is a potent method for coordinate-based meta-analysis of neuroimaging data (Müller et al., 2018). It provides a statistical technique for pooling different neuroimaging findings and integrating heterogeneous brain regions based on the peak coordinates and statistical parametric maps (Radua and Mataix-Cols, 2009; Radua et al., 2012, 2014). Compared with another commonly used method, activation likelihood estimation (ALE), AES-SDM could test publication bias, reproducibility, and robustness of the results (Radua et al., 2012). With the advantages of high sensitivity, good control of false positives, and lower imprecision than other coordinate-based methods, AES-SDM has been widely used and proven in recent studies (Ma et al., 2022; Zhang et al., 2023).
Therefore, the current study aimed to utilize the AES-SDM method to identify the functional brain alterations of FD patients under resting-state conditions. The results of this study would provide a clearer understanding of the underlying pathophysiological mechanism of FD and contribute to finding potential neural biomarkers, thus facilitating the advancement of FD diagnosis and treatment.
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021) and was registered at the International Prospective Register of Systematic Reviews of the University of York (PROSPERO, registration no. CRD42019134983).
A comprehensive search was conducted from the following eight electronic databases from inception to 15 October 2022: PubMed, Web of Science, Embase, Cochrane Database, China National Knowledge Infrastructure, Chongqing VIP Database, China Biology Medicine Disc Database, and Wanfang Database. The language was restricted to English or Chinese. The search terms of PubMed were as follows: (“functional dyspepsia” OR “indigestion” OR “FD”) AND (“neuroimaging” OR “magnetic resonance imaging” OR “MRI” OR “positron emission tomography” OR “PET” OR “single photon emission computed tomography” OR “SPECT” OR “amplitude of low-frequency fluctuation” OR “ALFF” OR “fractional amplitude of low-frequency fluctuation” OR “fALFF” OR “regional homogeneity” OR “ReHo” OR “independent component analysis” OR “functional connectivity”). The search strategies of the other seven databases were modified according to the above formula. After the electronic searches, we further screened the references of relevant reviews and included articles to find potential studies.
Studies were included if they met the following criteria: (1) all subjects were above 18 years and patients met the Rome criteria for FD; (2) those that had taken resting-state functional neuroimaging as the imaging technique; (3) those that focused on the difference of voxel-wise brain activity between FD patients comparing to HCs; (4) results obtained with whole-brain analysis and reported in the Talairach or Montreal Neurological Institute (MNI) coordinates; and (5) original article published in a peer-viewed journal.
Studies were excluded if they had the following features: (1) reviews, protocols, conference articles, letters, animal studies, or case reports; (2) patients underwent any interventions or stimuli during or prior to neuroimaging scan; and (3) the enrolled FD patients were combined with other diseases.
Two authors independently screened the identified articles and then reviewed the full text to check whether the articles met the inclusion and exclusion criteria. Any disagreements or uncertainties were discussed in the consultation and resolved by the third author. The following study information was acquired: (1) basic information (name of the first author and publication year), (2) methodology (sample size of patients and HCs, diagnostic criteria of patients, and details of neuroimaging method including scanning device and imaging modality), and (3) difference in functional brain activity between FD patients and HCs (peak coordinates of different regions in Talairach or MNI space, cluster size, and statistical threshold). If the included study reported the altered brain regions without specific coordinates, we would contact the authors to request detailed information.
A 12-point checklist was adopted to assess the quality of all included studies according to previous neuroimaging meta-analyses (Du et al., 2014; Zhang et al., 2023) (Supplementary Table S1). The customized checklist focused on clinical and demographic information, neuroimaging methodology, and the quality of results. Quality assessment was conducted by two authors, respectively. The third author took the ultimate decision in the event of a disagreement.
The coordinate-based mate-analysis study was conducted with AES-SDM software version 5.15 (https://www.sdmproject.com/software). The detailed procedure was summarized as follows. First, the peak coordinates and effect sizes (T-values) for the clusters of difference between FD and HCs were extracted from the published results. Then, the effect size and variance maps of brain function differences with an anisotropic Gaussian kernel were recreated (Radua et al., 2014). In order to balance sensitivity and specificity, the full width at half maximum of the Gaussian kernel was set to 20 mm (Radua et al., 2012). Subsequently, the standard meta-analysis was carried out to generate a mean map through voxel-wise calculation of the random-effects mean of the study maps, taking sample size, intra-study variability, and inter-study heterogeneity into account (Tang et al., 2018). Thresholds were set at a P-value of < 0.005 (voxel level), with peak height threshold Z > 1 and cluster size threshold ≥20 voxels (Radua et al., 2010). Results were presented on the standardized template in MNI coordinates.
To test the reproducibility and robustness of the results, a jackknife sensitivity analysis was conducted. If a cluster remained significant in all or most of the sensitivity analysis, this brain region was thought to be highly replicable (Radua and Mataix-Cols, 2009). For publication bias, the Egger test was used to evaluate the asymmetry of funnel plots. Any result with a P-value of < 0.05 indicated a statistically significant publication bias.
A total of 927 articles were retrieved based on our search strategy, and 11 articles were finally qualified. The flowchart shows the procedure of retrieval and inclusion of studies (Figure 1). Since one of the studies included two subtypes of FD and performed between-group comparisons to HCs separately, it was split into two studies, as recommended in the previous study (Zhang et al., 2023). As a result, a total of 12 results were included in the meta-analysis to quantitatively investigate the brain abnormalities between FD patients and HCs. These studies included 260 FD patients (113 male subjects and 147 female subjects) and 202 HCs (84 male subjects and 118 female subjects). The detailed information on the included studies is displayed in Table 1.
The AES-SDM meta-analysis yielded a total of 12 clusters (Figure 2, Table 2). Compared to HCs, FD patients showed increased brain activity in the left insula/rolandic operculum, right insula, right precentral gyrus, left anterior cingulate/paracingulate gyrus (ACG), left supplementary motor area (SMA), right putamen, bilateral thalamus, and left rectus gyrus while decreased brain activity in the right crus I and the hemispheric lobule X of the right cerebellum.
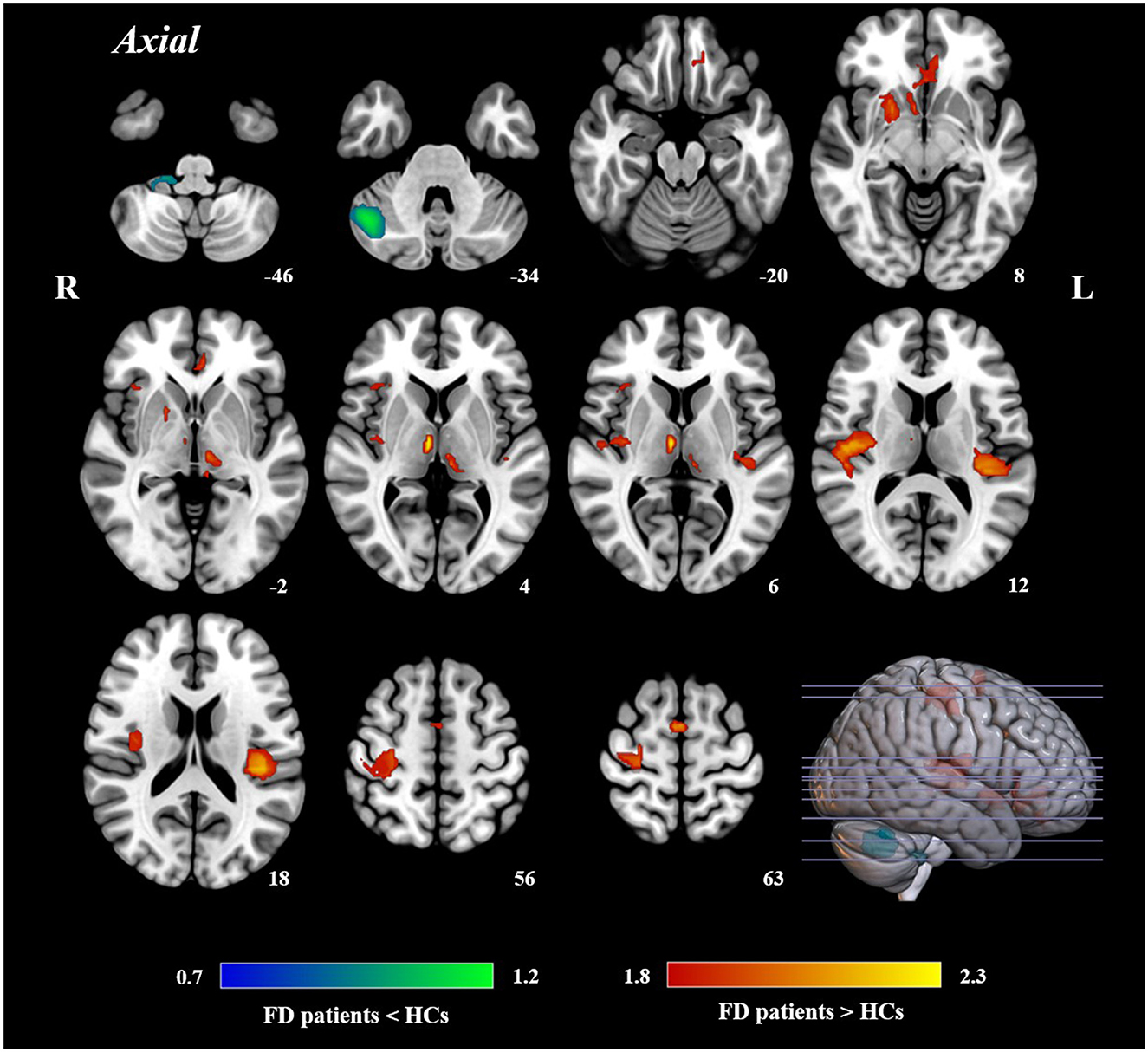
Figure 2. Results of AES-SDM of brain regions showing significant differences between FD patients and HCs.
The whole-brain jackknife sensitivity analysis revealed that all regions were highly reproducible (Table 3). Egger's test of funnel plot asymmetry showed no significant publication bias of abnormal regions in FD patients compared to HCs (Figure 3).
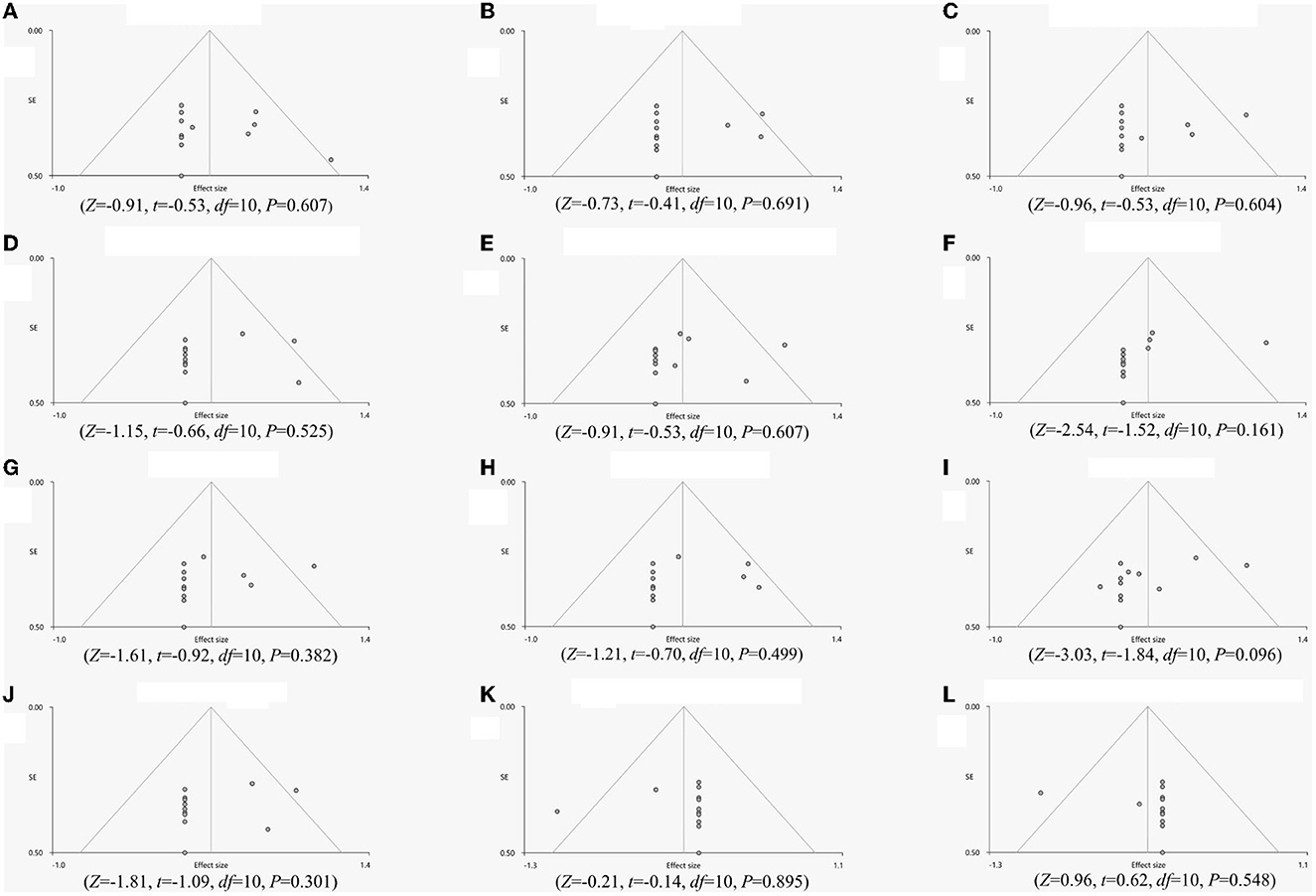
Figure 3. Egger's test of funnel plot asymmetry. (A) Left insula, (B) Right insula, (C) Right precentral gyrus, (D) Left anterior cingulate gyrus, (E) Left supplementary motor area, (F) Right putamen, (G) Left thalamus, (H), Right thalamus, (I), Right insula, (J), Left rectus gyrus, (K) Right cerebellum, curs I, (L) Right cerebellum, hemispheric lobule X.
To the best of our knowledge, this was the first meta-analysis to investigate the alterations of brain activity in FD patients. The results showed with high reproducibility that FD patients had abnormal activity in several brain regions, including the bilateral insula, left ACG, bilateral thalamus, right precentral gyrus, left SMA, right putamen, left rectus gyrus, and right cerebellum.
Visceral sensation-related brain regions are thought to be key features of central pathological changes in FD. According to the results of the current study, FD patients showed significant brain abnormalities related to visceral sensation, especially in the insula, ACG, and thalamus. Among these areas, the alteration of the bilateral insula was the most notable finding, suggesting that the abnormal activity patterns of the insula may be one of the most important central pathological features of FD. The insula is a core region dealing with visceral sensation (Craig, 2002; Zubieta et al., 2005; Namkung et al., 2017). As shown in the previous studies, FD patients not only showed increased activity in the insula under resting-state conditions (Van Oudenhove et al., 2010b; Zeng et al., 2011) but also manifested abnormally higher activation during stimuli such as gastric distension (Van Oudenhove et al., 2010a). In addition, studies also found that aberrant functional connectivity strength in the insula correlated with disease duration (Liu et al., 2018), indicating that the chronic and recurrent symptoms of FD may exacerbate the abnormal visceral sensation. The cingulate cortex is considered to have a direct relationship with the internal neural of the gastrointestinal tract (Vogt, 2013). Thus, it is not unexpected that the increased activity of ACG was found in FD patients, which was in line with the previous functional magnetic resonance imaging and positron emission computed tomography studies (Zeng et al., 2011; Nan et al., 2015; Liu et al., 2018). For example, Nan et al. (2014) found that the regional homogeneity of the ACG was significantly strengthened and was positively associated with the severity of dyspeptic symptoms in FD patients. Furthermore, the altered brain activity in the thalamus, a subcortical region closely communicated with the ACG (Lee et al., 2016), was also detected in FD patients. As a crucial part of the subcortical network, the thalamus is a pivot for integrating and relaying sensations, especially visceral sensory signals (Sherman, 2007; Elvsåshagen et al., 2021), which plays a role in gastrointestinal sensory processing (Iannilli et al., 2014). It receives and primarily processes the afferent signals from the periphery and then relays them to higher-class brain areas such as the insula for sensory integration which correlates with visceral pain (Moisset et al., 2010; Mayer et al., 2015). In a recent resting-state study, FD patients had higher spontaneous activity in the homeostatic-afferent network which includes the thalamus and relates to visceral homeostatic-afferent information (Qi et al., 2020). Another task-state study observed robust bilateral thalamic activation during gastric distention (Ladabaum et al., 2001).
Interestingly, the insula, ACG, and thalamus are viewed as pivotal components of the “gastric sensation neuro-matrix” (Nagai et al., 2007; Van Oudenhove et al., 2008). Furthermore, the insula and ACG are also thought to be the essential part of the “pain matrix” (Jones, 1999; Naliboff et al., 2006), which play an important role in the pain process and regulation (Zubieta et al., 2005). They all receive gastrointestinal tract signals via the spinal/vagal pathway and are mainly involved in the processing of visceral sensations (Aziz et al., 2000). Given the close connection between the insula, ACG, thalamus, and visceral sensation, it is conceivable that the abnormal activity of the insula, ACG, and thalamus may be caused by the adverse sensation of chronic and recurrent gastroduodenal symptom attacks and thus further exacerbated the central dysregulation of dyspeptic symptoms.
There is also evidence proving that the putamen is combined with the thalamus in nociceptive information processing (Starr et al., 2011) and could contribute to the processing of sensory and motor aspects of pain (Bingel et al., 2004). The putamen was frequently activated during painful stimuli (Starr et al., 2011). In some chronic pain disorders, such as irritable bowel syndrome (IBS) and fibromyalgia, abnormal putamen was observed, suggesting that the putamen is strongly associated with the processing of pain-related motor responses (Song et al., 2006; Schmidt-Wilcke et al., 2007; Seminowicz et al., 2010). The cerebellum has been suggested to be involved in visceral activity as well as somatic balance and affective behavior (Allen et al., 1997). Many FD studies manifested abnormal activity patterns in the cerebellum. For example, Vandenberghe et al. (2007) found that FD patients showed activations in the bilateral cerebellum during painful gastric distension. Other FD studies also reported significant abnormalities of cerebellum activity in the resting state (Zhou et al., 2013; Chen et al., 2018). Similarly, some studies found similar altered cerebellar activations in patients with chronic visceral pain such as IBS (Guleria et al., 2017). These abnormal brain regions above implied that an altered pain network may be another crucial feature of FD. The abnormal brain activity above may represent an adverse response to nociceptive visceral projections, such as chronic pain and burning sensation from the upper abdomen. In addition, this inappropriate response possibly led to the dysregulation of pain sensation in the pain network.
Functional dyspepsia central alterations are characterized by disorders of multiple networks. In addition to visceral sensation and pain-related areas, pathological alterations involve emotion, cognition, etc. As reported in previous studies, FD patients always experience co-morbidity of gastrointestinal symptoms with emotional disturbances (Herrick et al., 2018). Liu et al. (2012) further found that FD patients with psychiatric disorders, especially anxiety and depression, had a higher glucose metabolism in the insula compared to FD patients without psychiatric disorders. Since the insula also mainly participated in emotion, homeostatic function, cognitive function, and affective function and not only visceral sensation (Cauda et al., 2011; Yu et al., 2020), it is plausible that the insula may largely be leading the process of visceral sensation and emotional modulation in FD patients.
Abnormal brain activity in the sensorimotor cortex, including the precentral gyrus and SMA, was found in this meta-analysis. The precentral gyrus, known as the primary motor area, as well as the SMA participate in movement control (Qi et al., 2020) and engage in somatic pain-related sensation (Morawetz et al., 2017; Li et al., 2021). Patients with somatic symptom disorder and pain symptoms demonstrated abnormalities in the precentral gyrus (Yoshino et al., 2014). Similarly, previous studies found activated SMA during visceral pain stimulation (Lotze et al., 2001; Kano et al., 2013). Therefore, these results suggested that the aberrant activity in the precentral gyrus and SMA may be attributed to the perception of abnormal gastrointestinal movements of FD patients.
As the current study utilized a relatively steady approach to identify the included studies, the results should be well representative of FD patients in the resting state. However, there were still several limitations. First, the limited number of resting-state neuroimaging studies of FD may introduce a risk of bias in the original results, which may require caution in interpreting the results. Second, the causality between FD and aberrant brain activity could not be verified. The alterations in brain regions are difficult to be elucidated because of one of the pathogenesis or the result of symptoms of FD due to the cross-sectional nature of the included studies. Third, language restrictions to English and Chinese might cause bias to some extent. Fourth, the voxel-wise meta-analysis was conducted according to peak coordinates and effect sizes rather than original brain images, which may affect the accuracy of the results. As a solution, the participant-level meta-analysis (Zunhammer et al., 2021) could be introduced in future studies.
The current meta-analysis demonstrated multiple brain regions with abnormal functional activity in FD patients, including the insula, ACG, thalamus, precentral gyrus, SMA, putamen, and cerebellum. The abnormal brain regions could be interpreted as a consequence of the interaction between recurrent gastrointestinal symptoms and abnormal brain regulation. Importantly, these results manifested that the central pathological mechanism of FD represented as multi-network imbalance mainly involved visceral sensation perception as well as pain and emotion regulation. This study contributed positively to the interpretation of the central pathological features of FD, which would advance the understanding of its pathogenesis and may combine with machine learning, thus facilitating the diagnosis and treatment of the disease in the future (He et al., 2022).
The data analyzed in this study is subject to the following licenses/restrictions: The datasets analyzed in the current study are available from the corresponding authors upon reasonable request. Requests to access these datasets should be directed to FZ, emVuZ2ZhbmdAY2R1dGNtLmVkdS5jbg==.
FZ and RS contributed to the conception and design of the study. PZ, XZ, YH, and SL retrieved studies and acquired data. YM conducted data analysis. YM and PZ wrote the article. TY and FZ revised the manuscript. All authors reviewed and approved the submitted version.
This study was financially supported by the National Science Fund for Distinguished Young Scholars (No. 82225050), the National Natural Science Foundation of China (Nos. 81973960, 81622052, and 81473602), and the Sichuan Science and Technology Program (Nos. 2019JDTD0011 and 15Q NJJ0008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1174287/full#supplementary-material
Allen, G., Buxton, R.B., Wong, E.C., and Courchesne, E. (1997). Attentional activation of the cerebellum independent of motor involvement. Science 275, 1940–1943. doi: 10.1126/science.275.5308.1940
Aziz, I., Palsson, O.S., Törnblom, H., Sperber, A.D., Whitehead, W.E., and Simrén, M. (2018). Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study. Lancet. 3, 252–262. doi: 10.1016/S2468-1253(18)30003-7
Aziz, Q., Schnitzler, A., and Enck, P. (2000). Functional neuroimaging of visceral sensation. J. Clin. Neurophysiol. 17, 604–612. doi: 10.1097/00004691-200011000-00006
Bingel, U., Gläscher, J., Weiller, C., and Büchel, C. (2004). Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cerebral Cortex 14, 1340–1345. doi: 10.1093/cercor/bhh094
Cauda, F., D'Agata, F., Sacco, K., Duca, S., Geminiani, G., and Vercelli, A. (2011). Functional connectivity of the insula in the resting brain. NeuroImage 55, 8–33. doi: 10.1016/j.neuroimage.2010.11.049
Chen, Y., Wang, R., Hou, B., Feng, F., Fang, X., Zhu, L., et al. (2018). Regional Brain Activity During Rest and Gastric Water Load in Subtypes of Functional Dyspepsia: A Preliminary Brain Functional Magnetic Resonance Imaging Study. J. Neurogastroenterol. Motil. 24, 268–279. doi: 10.5056/jnm17076
Craig, A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience 3, 655–666. doi: 10.1038/nrn894
Drossman, D.A., Tack, J., Ford, A.C., Szigethy, E., Törnblom, H., and Van Oudenhove, L. (2018). Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a rome foundation working team report. Gastroenterology 154, 1140–1171. doi: 10.1053/j.gastro.2017.11.279
Du, M., Liu, J., Chen, Z., Huang, X., Li, J., Kuang, W., et al. (2014). Brain grey matter volume alterations in late-life depression. J. Psychiatry Neurosci. 39, 397–406. doi: 10.1503/jpn.130275
El-Serag, H.B., and Talley, N.J. (2003). Health-related quality of life in functional dyspepsia. Alimentary Pharmacol. Therapeut. 18, 387–393. doi: 10.1046/j.1365-2036.2003.01706.x
Elvsåshagen, T., Shadrin, A., Frei, O., van der Meer, D., Bahrami, S., Kumar, V.J., et al. (2021). The genetic architecture of the human thalamus and its overlap with ten common brain disorders. Nat. Commun. 12, 2909. doi: 10.1038/s41467-021-23175-z
Enck, P., Azpiroz, F., Boeckxstaens, G., Elsenbruch, S., Feinle-Bisset, C., Holtmann, G., et al. (2017). Functional dyspepsia. Nat. Rev. Dis. Primers 3, 17081. doi: 10.1038/nrdp.2017.81
Ford, A.C., Mahadeva, S., Carbone, M.F., Lacy, B.E., and Talley, N.J. (2020). Functional dyspepsia. Lancet (London, England) 396, 1689–1702. doi: 10.1016/S0140-6736(20)30469-4
Ghoshal, U.C., Singh, R., Chang, F.-Y., Hou, X., Wong, B.C.Y., and Kachintorn, U. (2011). Epidemiology of uninvestigated and functional dyspepsia in Asia: facts and fiction. J. Neurogastroenterol. Motility 17, 235–244. doi: 10.5056/jnm.2011.17.3.235
Guan, Y., Cai, R., Wu, H., Li, C., and Shen, G. (2019a). Effects on the amplitude of low frequency fluctuation of resting-state brain function in the patients of functional dyspepsia with acupuncture at back-shu and front-mu points of stomach. China J. Tradit. Chin. Med. Phar. 34, 1993–1997.
Guan, Y., Cai, R., Xiao, H., Chu, H., Li, C., and Shen, G. (2019b). Effect of acupuncture at Weishu combined with Zhongwan on the regional homogenity of resting-state brain function and electrogastrogram for patients with functional dyspepsia. J. Nanjing Univ. Tradit. Chin. Med. 35, 640–645. doi: 10.14148/j.issn.1672-0482.2019.0640
Guleria, A., Karyampudi, A., Singh, R., Khetrapal, C.L., Verma, A., Ghoshal, U.C., et al. (2017). Mapping of brain activations to rectal balloon distension stimuli in male patients with irritable bowel syndrome using functional magnetic resonance imaging. J. Neurogastroenterol. Motil. 23, 415–427. doi: 10.5056/jnm16148
He, Z., Lin, M., Xu, Z., Yao, Z., Chen, H., Alhudhaif, A., et al. (2022). Deconv-transformer (DecT): A histopathological image classification model for breast cancer based on color deconvolution and transformer architecture. Inform. Sci. 608, 1093–1112. doi: 10.1016/j.ins.2022.06.091
Herrick, L.M., Camilleri, M., Schleck, C.D., Zinsmeister, A.R., Saito, Y.A., and Talley, N.J. (2018). Effects of Amitriptyline and Escitalopram on Sleep and Mood in Patients With Functional Dyspepsia. Clin. Gastroenterol. Hepatol. 16, 401–406. doi: 10.1016/j.cgh.2017.10.021
Iannilli, E., Noennig, N., Hummel, T., and Schoenfeld, A.M. (2014). Spatio-temporal correlates of taste processing in the human primary gustatory cortex. Neuroscience 273, 92–99. doi: 10.1016/j.neuroscience.2014.05.017
Jones, A.K. (1999). The contribution of functional imaging techniques to our understanding of rheumatic pain. Rheumatic Dis. Clin. North America 25, 123–152. doi: 10.1016/S0889-857X(05)70058-2
Kano, M., Farmer, A.D., Aziz, Q., Giampietro, V.P., Brammer, M.J., Williams, S.C.R., et al. (2013). Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. Am. J. Physiol. Gastrointest Liver Physiol. 304, G687–G699. doi: 10.1152/ajpgi.00385.2012
Ladabaum, U., Minoshima, S., Hasler, W.L., Cross, D., Chey, W.D., and Owyang, C. (2001). Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology 120, 369–376. doi: 10.1053/gast.2001.21201
Lee, I.-S., Kullmann, S., Scheffler, K., Preissl, H., and Enck, P. (2018). Fat label compared with fat content: gastrointestinal symptoms and brain activity in functional dyspepsia patients and healthy controls. The Am. J. Clin. Nutr. 108, 127–135. doi: 10.1093/ajcn/nqy077
Lee, I.S., Wang, H., Chae, Y., Preissl, H., and Enck, P. (2016). Functional neuroimaging studies in functional dyspepsia patients: a systematic review. Neurogastroenterol. Motil. 28, 793–805. doi: 10.1111/nmo.12793
Li, L., Ma, J., Xu, J.-G., Zheng, Y.-L., Xie, Q., Rong, L., et al. (2021). Brain functional changes in patients with Crohn's disease: A resting-state fMRI study. Brain Behavior. 11, e2243. doi: 10.1002/brb3.2243
Liu, M.-L., Liang, F.-R., Zeng, F., Tang, Y., Lan, L., and Song, W.-Z. (2012). Cortical-limbic regions modulate depression and anxiety factors in functional dyspepsia: a PET-CT study. Ann. Nuclear Med. 26, 35–40. doi: 10.1007/s12149-011-0537-4
Liu, P., Fan, Y., Wei, Y., Zeng, F., Li, R., Fei, N., et al. (2018). Altered structural and functional connectivity of the insula in functional dyspepsia. Neurogastroenterol. Motil. 30, e13345. doi: 10.1111/nmo.13345
Liu, P., Qin, W., Wang, J., Zeng, F., Zhou, G., Wen, H., et al. (2013). Identifying neural patterns of functional dyspepsia using multivariate pattern analysis: a resting-state FMRI study. PLoS ONE 8, e68205. doi: 10.1371/journal.pone.0068205
Liu, P., Wang, G., Liu, Y., Zeng, F., Lin, D., Yang, X., et al. (2017). Disrupted intrinsic connectivity of the periaqueductal gray in patients with functional dyspepsia: A resting-state fMRI study. Neurogastroenterol. Motil. 29, e13060. doi: 10.1111/nmo.13060
Lotze, M., Wietek, B., Birbaumer, N., Ehrhardt, J., Grodd, W., and Enck, P. (2001). Cerebral activation during anal and rectal stimulation. NeuroImage 14, 1027–1034. doi: 10.1006/nimg.2001.0901
Ly, H.G., Ceccarini, J., Weltens, N., Bormans, G., Van Laere, K., Tack, J., et al. (2015). Increased cerebral cannabinoid-1 receptor availability is a stable feature of functional dyspepsia: a [F]MK-9470 PET study. Psychother. Psychosom. 84, 149–158. doi: 10.1159/000375454
Ma, T., Li, Z.-Y., Yu, Y., Yang, Y., Ni, M.-H., Xie, H., et al. (2022). Gray matter abnormalities in patients with complex regional pain syndrome: a systematic review and meta-analysis of voxel-based morphometry studies. Brain Sci. 12, 1115. doi: 10.3390/brainsci12081115
Mayer, E.A., Labus, J.S., Tillisch, K., Cole, S.W., and Baldi, P. (2015). Towards a systems view of IBS. Nat. Rev. 12, 592–605. doi: 10.1038/nrgastro.2015.121
Mertz, H., Fullerton, S., Naliboff, B., and Mayer, E.A. (1998). Symptoms and visceral perception in severe functional and organic dyspepsia. Gut 42, 814–822. doi: 10.1136/gut.42.6.814
Miwa, H., Nagahara, A., Asakawa, A., Arai, M., Oshima, T., Kasugai, K., et al. (2022). Evidence-based clinical practice guidelines for functional dyspepsia 2021. J. Gastroenterol. 57, 47–61. doi: 10.1007/s00535-021-01843-7
Moisset, X., Bouhassira, D., Denis, D., Dominique, G., Benoit, C., and Sabat,é, J.-M. (2010). Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur. J. Pain 14, 142–148. doi: 10.1016/j.ejpain.2009.04.011
Morawetz, C., Bode, S., Derntl, B., and Heekeren, H.R. (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 72, 111–128. doi: 10.1016/j.neubiorev.2016.11.014
Müller, V.I., Cieslik, E.C., Laird, A.R., Fox, P.T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Nagai, M., Kishi, K., and Kato, S. (2007). Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur. Psychiat. 22, 387–394. doi: 10.1016/j.eurpsy.2007.02.006
Naliboff, B.D., Berman, S., Suyenobu, B., Labus, J.S., Chang, L., Stains, J., et al. (2006). Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 131, 352–365. doi: 10.1053/j.gastro.2006.05.014
Namkung, H., Kim, S.-H., and Sawa, A. (2017). The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 40, 200–207. doi: 10.1016/j.tins.2017.02.002
Nan, J., Liu, J., Mu, J., Dun, W., Zhang, M., Gong, Q., et al. (2015). Brain-based Correlations Between Psychological Factors and Functional Dyspepsia. J. Neurogastroenterol. Motility 21, 103–110. doi: 10.5056/jnm14096
Nan, J., Liu, J., Zhang, D., Yang, Y., Yan, X., Yin, Q., et al. (2014). Altered intrinsic regional activity and corresponding brain pathways reflect the symptom severity of functional dyspepsia. Neurogastroenterol. Motility. 26, 660–669. doi: 10.1111/nmo.12311
Oustamanolakis, P., and Tack, J. (2012). Dyspepsia: organic versus functional. J. Clin. Gastroenterol. 46, 175–190. doi: 10.1097/MCG.0b013e318241b335
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi: 10.1136/bmj.n71
Qi, R., Shi, Z., Weng, Y., Yang, Y., Zhou, Y., Surento, W., et al. (2020). Similarity and diversity of spontaneous brain activity in functional dyspepsia subtypes. Acta Radiol. 61, 927–935. doi: 10.1177/0284185119883391
Radua, J., and Mataix-Cols, D. (2009). Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiat. 195, 393–402. doi: 10.1192/bjp.bp.108.055046
Radua, J., Mataix-Cols, D., Phillips, M.L., El-Hage, W., Kronhaus, D.M., Cardoner, N., et al. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiat. 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Radua, J., Rubia, K., Canales-Rodríguez, E.J., Pomarol-Clotet, E., Fusar-Poli, P., and Mataix-Cols, D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiat 5, 13. doi: 10.3389/fpsyt.2014.00013
Radua, J., van den Heuvel, O.A., Surguladze, S., and Mataix-Cols, D. (2010). Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch. General Psychiat. 67, 701–711. doi: 10.1001/archgenpsychiatry.2010.70
Schmidt-Wilcke, T., Luerding, R., Weigand, T., Jürgens, T., Schuierer, G., Leinisch, E., et al. (2007). Striatal grey matter increase in patients suffering from fibromyalgia–a voxel-based morphometry study. Pain 132, S109–S116. doi: 10.1016/j.pain.2007.05.010
Seminowicz, D.A., Labus, J.S., Bueller, J.A., Tillisch, K., Naliboff, B.D., Bushnell, M.C., et al. (2010). Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 139, 48–57. doi: 10.1053/j.gastro.2010.03.049
Sherman, S.M. (2007). The thalamus is more than just a relay. Curr. Opin. Neurobiol. 17, 417–422. doi: 10.1016/j.conb.2007.07.003
Shimura, S., Ishimura, N., Mikami, H., Okimoto, E., Uno, G., Tamagawa, Y., et al. (2016). Small intestinal bacterial overgrowth in patients with refractory functional gastrointestinal disorders. J. Neurogastroenterol. Motility 22, 60–68. doi: 10.5056/jnm15116
Song, G.H., Venkatraman, V., Ho, K.Y., Chee, M.W.L., Yeoh, K.G., and Wilder-Smith, C.H. (2006). Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 126, 79–90. doi: 10.1016/j.pain.2006.06.017
Starr, C.J., Sawaki, L., Wittenberg, G.F., Burdette, J.H., Oshiro, Y., Quevedo, A.S., et al. (2011). The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain J. Neurol. 134, 1987–2004. doi: 10.1093/brain/awr117
Tack, J., Bisschops, R., and Sarnelli, G. (2004). Pathophysiology and treatment of functional dyspepsia. Gastroenterology 127, 1239–1255. doi: 10.1053/j.gastro.2004.05.030
Tait, C., and Sayuk, G.S. (2021). The Brain-Gut-Microbiotal Axis: A framework for understanding functional GI illness and their therapeutic interventions. Eur. J. Internal Med. 84, 1–9. doi: 10.1016/j.ejim.2020.12.023
Tang, S., Lu, L., Zhang, L., Hu, X., Bu, X., Li, H., et al. (2018). Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine 36, 436–445. doi: 10.1016/j.ebiom.2018.09.010
Van Oudenhove, L., Dupont, P., Vandenberghe, J., Geeraerts, B., van Laere, K., Bormans, G., et al. (2008). The role of somatosensory cortical regions in the processing of painful gastric fundic distension: an update of brain imaging findings. Neurogastroenterol. Motil. 20, 479–487. doi: 10.1111/j.1365-2982.2007.01045.x
Van Oudenhove, L., Vandenberghe, J., Dupont, P., Geeraerts, B., Vos, R., Dirix, S., et al. (2010a). Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H(2)(15)O-PET study. Am. J. Gastroenterol. 105, 913–924. doi: 10.1038/ajg.2010.39
Van Oudenhove, L., Vandenberghe, J., Dupont, P., Geeraerts, B., Vos, R., Dirix, S., et al. (2010b). Regional brain activity in functional dyspepsia: a H(2)(15)O-PET study on the role of gastric sensitivity and abuse history. Gastroenterology 139, 36–47. doi: 10.1053/j.gastro.2010.04.015
Vandenberghe, J., Dupont, P., Van Oudenhove, L., Bormans, G., Demyttenaere, K., Fischler, B., et al. (2007). Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology 132, 1684–1693. doi: 10.1053/j.gastro.2007.03.037
Vogt, B.A. (2013). Inflammatory bowel disease: perspectives from cingulate cortex in the first brain. Neurogastroenterol. Motil. 25, 93–98. doi: 10.1111/nmo.12067
Yoshino, A., Okamoto, Y., Kunisato, Y., Yoshimura, S., Jinnin, R., Hayashi, Y., et al. (2014). Distinctive spontaneous regional neural activity in patients with somatoform pain disorder: a preliminary resting-state fMRI study. Psychiatry Res. 221, 246–248. doi: 10.1016/j.pscychresns.2013.12.006
Yu, H., Li, M.-L., Li, Y.-F., Li, X.-J., Meng, Y., Liang, S., et al. (2020). Anterior cingulate cortex, insula and amygdala seed-based whole brain resting-state functional connectivity differentiates bipolar from unipolar depression. J. Affect. Disor. 274, 38–47. doi: 10.1016/j.jad.2020.05.005
Zeng, F., Qin, W., Liang, F., Liu, J., Tang, Y., Liu, X., et al. (2011). Abnormal resting brain activity in patients with functional dyspepsia is related to symptom severity. Gastroenterology 141, 499–506. doi: 10.1053/j.gastro.2011.05.003
Zeng, F., Qin, W., Ma, T., Sun, J., Tang, Y., Yuan, K., et al. (2012). Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy. Am. J. Gastroenterol. 107, 1236–1247. doi: 10.1038/ajg.2012.53
Zeng, F., Song, W.-Z., Liu, X.-G., Xie, H.-J., Tang, Y., Shan, B.-C., et al. (2009). Brain areas involved in acupuncture treatment on functional dyspepsia patients: a PET-CT study. Neurosci. Lett. 456, 6–10. doi: 10.1016/j.neulet.2009.03.080
Zeng, F., Sun, R., He, Z., Chen, Y., Lei, D., Yin, T., et al. (2019). Altered Functional Connectivity of the Amygdala and Sex Differences in Functional Dyspepsia. Clin. Transl. Gastroenterol. 10, e00046. doi: 10.14309/ctg.0000000000000046
Zhang, X., Zhou, J., Guo, M., Cheng, S., Chen, Y., Jiang, N., et al. (2023). A systematic review and meta-analysis of voxel-based morphometric studies of migraine. J. Neurology 270, 152–170. doi: 10.1007/s00415-022-11363-w
Zhou, G., Liu, P., Wang, J., Wen, H., Zhu, M., Zhao, R., et al. (2013). Fractional amplitude of low-frequency fluctuation changes in functional dyspepsia: a resting-state fMRI study. Magn. Reson. Imaging 31, 19. doi: 10.1016/j.mri.2013.03.019
Zubieta, J.-K., Bueller, J.A., Jackson, L.R., Scott, D.J., Xu, Y., Koeppe, R.A., et al. (2005). Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 25, 7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005
Keywords: functional dyspepsia, neuroimaging, insula, fMRI, meta-analysis
Citation: Mao Y, Zhang P, Sun R, Zhang X, He Y, Li S, Yin T and Zeng F (2023) Altered resting-state brain activity in functional dyspepsia patients: a coordinate-based meta-analysis. Front. Neurosci. 17:1174287. doi: 10.3389/fnins.2023.1174287
Received: 26 February 2023; Accepted: 14 April 2023;
Published: 12 May 2023.
Edited by:
Shaoyong Yu, Johns Hopkins Medicine, United StatesCopyright © 2023 Mao, Zhang, Sun, Zhang, He, Li, Yin and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zeng, emVuZ2ZhbmdAY2R1dGNtLmVkdS5jbg==; Tao Yin, eWludGFvQGNkdXRjbS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.