- 1Department of Neurosurgery, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, China
- 2Health Science Center, Shenzhen University, Shenzhen, China
- 3Department of Neurosurgery, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu Province, China
- 4Nursing Department, Shenzhen Shekou People's Hospital, Shenzhen, China
Objective: To evaluate the effects of exercise therapy on patients with poststroke cognitive impairment and compare the differences in the effect of this method when compared with conventional measures, providing evidence for a more standardized and effective clinical application of exercise therapy.
Methods: A search was conducted using 7 electronic databases, including PubMed, CINAHL, Web of Science, CENTRAL, CNKI, Wanfang, SinoMed, and clinical trials registry platforms for randomized controlled trials concerning exercise therapy on patients with poststroke cognitive impairment. Two researchers independently screened the literature, evaluated the quality, and extracted information. Meta-analysis was carried out using Review Manager 5.4 software.
Results: There were 11 studies with 1,382 patients. Meta-analysis showed that exercise therapy could improve cognitive function [SMD = 0.67, 95% CI (0.31, 1.04), P = 0.0003], motor function [SMD = 1.81, 95% CI (0.41, 3.20), P = 0.01], and the activities of daily living [MD = 8.11, 95% CI (3.07, 13.16), P = 0.002] in patients with poststroke cognitive impairment.
Conclusion: Exercise therapy can not only improve cognitive function in patients with poststroke cognitive impairment but also improve motor function and the activities of daily living. Medical staff should prioritize the management of patients with poststroke cognitive impairment and carry out exercise therapy actively to improve the cognitive function of patients with stroke.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42023397553.
Introduction
Stroke is an important cause of cognitive impairment and dementia (Zhao et al., 2018). Poststroke cognitive impairment (PSCI) refers to cognitive impairment or dementia after a stroke. It is common in patients with stroke and usually occurs within 6 months after the stroke. The prevalence of PSCI ranges from 20 to 80% and is one of the most common complications in patients with stroke (Sun et al., 2014). PSCI is an important factor that seriously affects patients' quality of life and survival time, and it has evolved into one of the hot topics in stroke research and intervention (Dong et al., 2017). Due to cognitive impairment, patients' cognitive abilities decline, and their adaptability to the external environment is disturbed. Therefore, patients are prone to emotional disorders such as anxiety and depression. It can also be characterized by impaired memory function, decreased computing power, and abstract thinking, which affects not only the daily lives of patients but also their rehabilitation of limbs and neurological functions. It can induce a secondary stroke and even threaten their lives, seriously affecting the overall rehabilitation process (Dong et al., 2021). Stroke survivors with moderate PSCI were six times more likely to progress to occasional dementia than stroke survivors without cognitive impairment, and up to 25% of patients with cognitive impairment were diagnosed with dementia within 3 years of stroke (Narasimhalu et al., 2009; Sachdev et al., 2009). Therefore, the rehabilitation of cognitive function in patients with stroke is an urgent issue.
Early intervention is particularly important for patients with PSCI. Studies have shown that there is a wide variation in the treatment of cognitive problems after stroke, including pharmacological and non-pharmacological interventions (Quinn et al., 2021). However, the long-term efficacy of pharmacological interventions is unclear and may be associated with adverse effects. For example, an analysis of the evidence suggests that actovegin and cerebrolysin are animal-derived nootropics that may have potential efficacy in the treatment of neurodegenerative diseases. It has a beneficial effect on improving cognitive function after stroke (Quinn et al., 2021). However, the most common adverse event was a recurrent ischemic stroke. Therefore, more researchers are inclined toward non-pharmacological interventions, such as exercise therapy, cognitive intervention, and acupuncture therapy. Early non-pharmacological exercise therapy for patients can delay the progression of the disease, sometimes even reverse the process of cognitive decline, and reduce the disability rate. Huang et al. (2022) conducted a network meta-analysis of the comparative effectiveness of different exercise interventions on cognitive function in patients with mild cognitive impairment or dementia and found that all types of exercise can effectively improve overall cognitive function in patients. However, there is a lack of effective evidence for exercise therapy in patients with PSCI.
Although routine rehabilitation training can delay the process of cognitive decline in patients and prevent the disease from progressing to dementia, there are shortcomings, such as a single form of training, low patient acceptance, and difficulty in conducting continuous and effective training, which are not conducive to the recovery of cognitive function (Yu et al., 2019). Some studies have found that exercise therapy can improve health by increasing oxygen and blood supply to the brain and indirectly improving cognitive impairment (Tang et al., 2020). Exercise therapy is defined as “a regimen or plan of physical activity designed and prescribed for specific therapeutic goals with the purpose of restoring normal physical function or reducing symptoms caused by disease or injury (Caspersen et al., 1985)”. The regimen includes aerobic exercise, resistance exercise, and multiple combination exercises, as well as some traditional Chinese medicine exercises such as Baduanjin. Traditional Chinese medicine exercise therapy has been found to improve cognitive function in elderly patients with mild cognitive impairment (MCI) by regulating cognition-related brain function and structure (Su et al., 2022). Although there are many studies on the use of exercise therapy to improve PSCI, there is a relative lack of consensus, and there is no meta-analysis on the effects of exercise therapy on patients with PSCI. This study evaluated the effects of exercise therapy on patients with PSCI through a meta-analysis, aiming to provide a new evidence-based basis for intervention in patients with PSCI.
This systematic evaluation program is registered in the PROSPERO database (CRD42023397553).
Materials and methods
Search strategy
Two researchers searched PubMed, CINAHL, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Biomedical Literature Service System (SinoMed), and the clinical trials registry platform. The search was conducted from the database creation date to January 2023. In addition, the search was conducted by combining free words and subject terms. The search formula was (stroke OR cerebrovascular OR hemiplegia OR cerebral hemorrhage OR cerebral infarction OR cerebral stroke OR acute stroke) AND (cognitive dysfunction OR cognitive impairment OR cognition disorders) AND (physical activity OR physiotherapy OR fitness OR aerobic OR exercise OR resistance training OR physical fitness OR exercise). We searched both the included references and the gray literature. The results were cross-checked after each of the two researchers had completed the search independently. In case of disagreement, the decision was discussed with a third researcher.
Study design and eligibility criteria
This systematic review was completed according to the Cochrane Collaboration methodology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Sachdev et al., 2009).
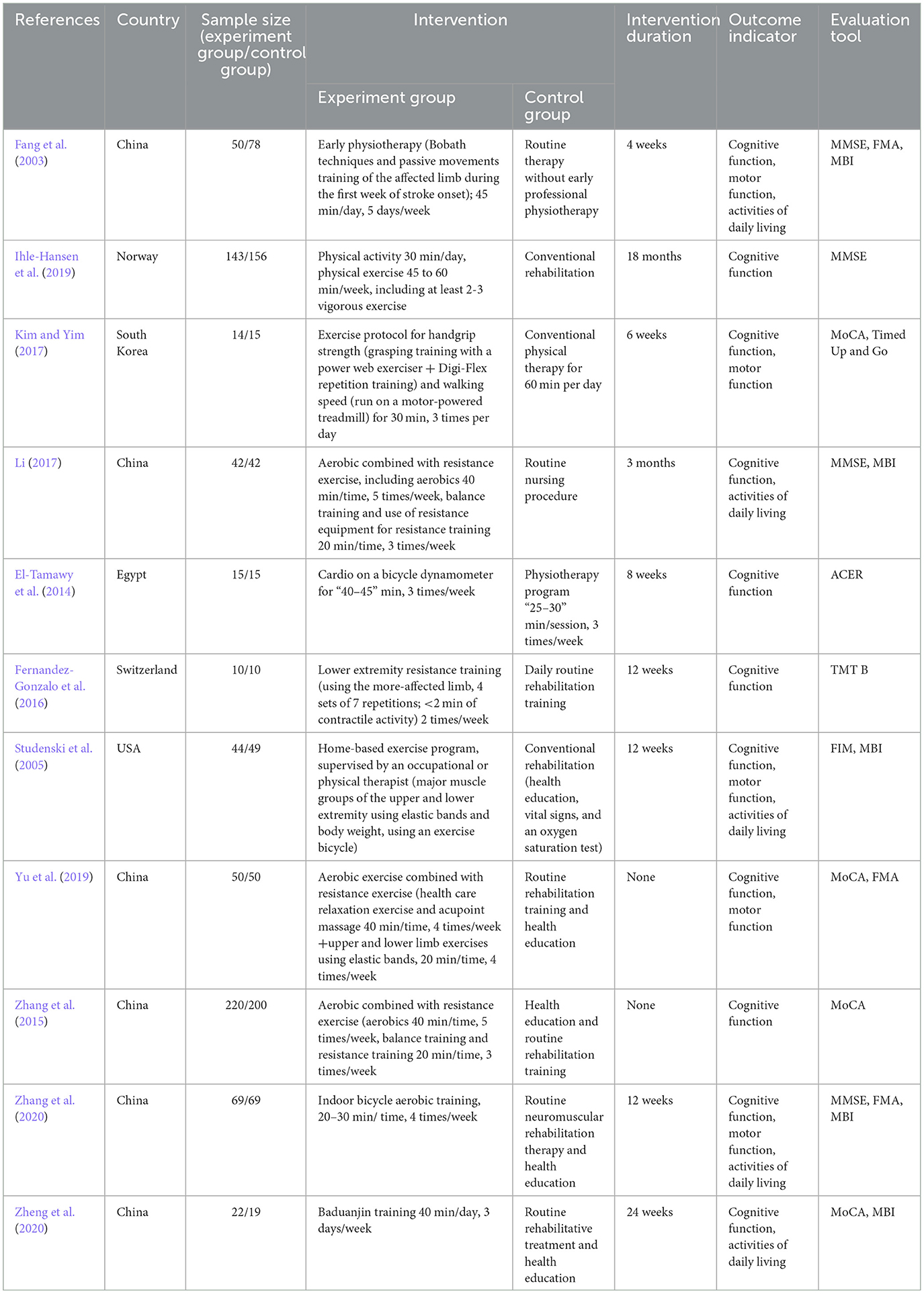
A participant-intervention-comparison-outcome (PICO) strategy was used to structure the research questions (Stone, 2002). The inclusion criteria were as follows: (1) Participants: patients who meet the diagnostic criteria adopted by the Fourth National Conference on Cerebrovascular Diseases and were diagnosed with ischemic or hemorrhagic stroke by CT or MRI examination, are over 18 years of age, and had a cognitive decline occurring within 6 months of stroke; (2) Intervention: exercise therapy, including aerobic exercise, resistance exercise, and multiple combination exercises; (3) Comparison: routine non-pharmacological intervention, including a balanced diet, health education, and routine rehabilitation training; (4) Outcome: the main outcome indicator was cognitive function, and the assessment tools were the Minimum Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA), and the secondary outcome indicators were motor function and activities of daily living, measured by the Fugl-Meyer Assessment (FMA) and the Modified Barthel Index (MBI). In addition, the study design must be a randomized controlled trial (RCT). The exclusion criteria were as follows: (1) data could not be extracted; (2) the full text could not be obtained; (3) the literature is a repeated publication; (4) the literature is a conference paper; and (5) the literature quality assessment was high risk.
Data extraction
Two researchers trained in evidence-based research independently searched the literature, imported the retrieved literature into the Endnote software, and deleted duplicate literature; they simultaneously and independently read the titles and abstracts for preliminary screening and carefully read the full text to determine the included literature according to the inclusion and exclusion criteria. The two researchers extracted information from the literature, including the year of publication, country, sample size, intervention measures of the experimental and control groups, intervention duration, outcome indicator, and evaluation tool. In case of disagreement, the decision was discussed with a third researcher. The researchers contacted the author by phone or email to request additional information.
Quality appraisal
The quality of included RCTs was assessed independently by two reviewers using the Cochrane Systematic Review Manual 5.1.0, with a third researcher consulted to reach a consensus in case of disagreement. The evaluation included (1) random sequence generation (selection bias), (2) allocation concealment (selection bias), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessment (detection bias), (5) incomplete outcome data (attrition bias), (6) selective reporting (reporting bias), and (7) other biases. The bias for the abovementioned 7 aspects is low bias, high bias, and unclear (lack of relevant information or bias situation is uncertain).
Statistical analysis
The RevMan software (version 5.4; Cochrane Collaboration, Copenhagen, Denmark) was used for meta-analysis. Based on the type of extracted data, we evaluated 95% confidence intervals (CIs) for continuous variables. For a continuous variable that was measured using different scales, we used standardized mean differences (SMD) as a measure for effect size; for a continuous variable that was measured using the same scale, we used mean differences (MD) for effect size. A P-value of < 0.05 (two-sided) was considered statistically significant in the estimation of effects. I2 was used to determine the heterogeneity of the results. If the P-value was > 0.1 and I2 was < 50%, indicating low heterogeneity, and if all studies were from a homogeneous population, the fixed-effects model was used for meta-analysis. If the P-value was ≤ 0.1 and I2 was ≥ 50%, indicating large heterogeneity, the source of heterogeneity was analyzed as far as possible. If the heterogeneity could not be reduced, the random-effects model was used for the meta-analysis. The inverse variance method was used to pool the effect-size measure. Clinical and methodological heterogeneity was addressed by sensitivity analysis, subgroup analysis, or descriptive analysis only.
Results
Study selection
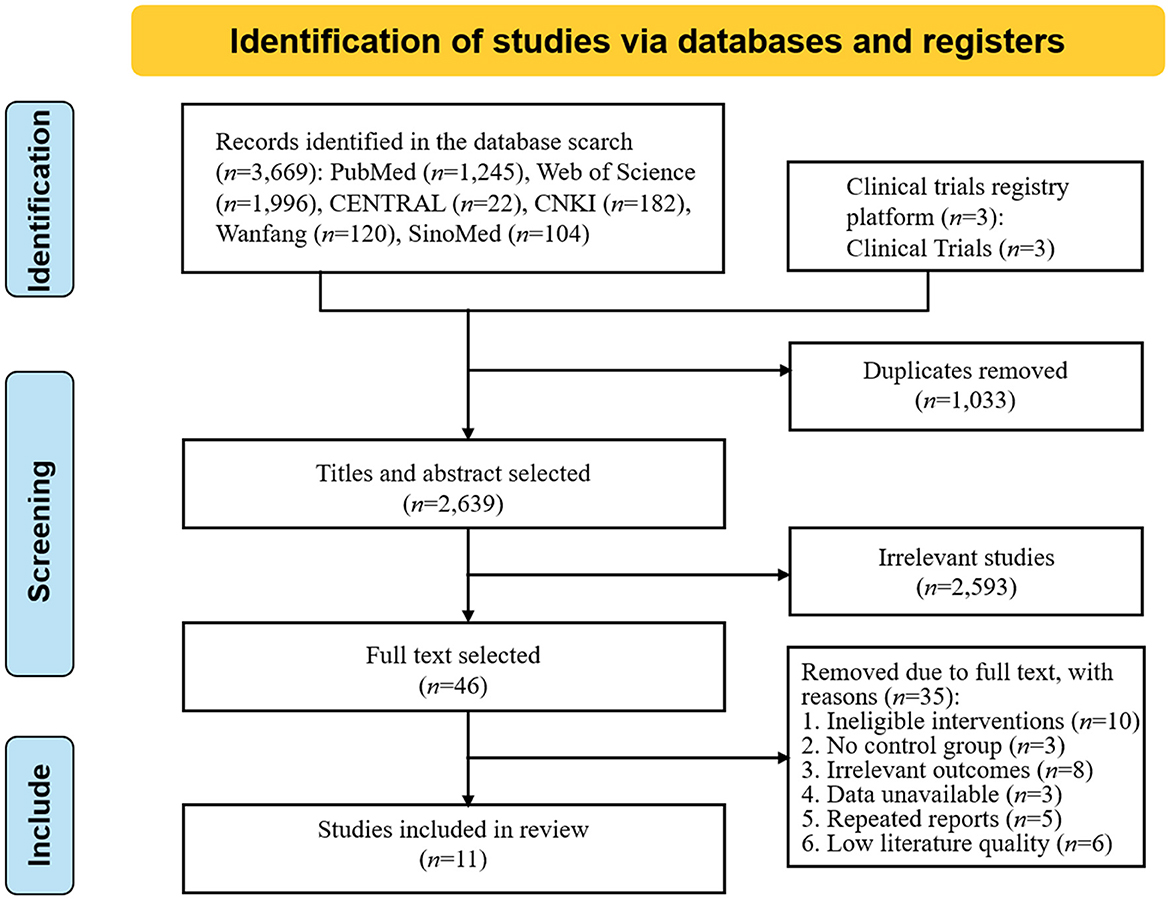
According to the search strategy, 3,672 studies were preliminarily searched, 1,033 duplicate studies were excluded, and 11 studies (Fang et al., 2003; Studenski et al., 2005; El-Tamawy et al., 2014; Zhang et al., 2015, 2020; Fernandez-Gonzalo et al., 2016; Kim and Yim, 2017; Li, 2017; Ihle-Hansen et al., 2019; Yu et al., 2019; Zheng et al., 2020) were finally included after the preliminary screening of the title abstract and reading the full text. The literature screening process and results are shown in Figure 1.
Characteristics of the included studies
A total of 11 studies were included in this review, and the characteristics of each study are shown in Table 1. Only Chinese and English literature were included in this review, and literature in other languages was not considered. The sample sizes of the RCTs ranged from 20 to 420 participants. The total sample size for the meta-analysis was 1,382 participants, including both experimental (n = 679) and control (n = 703) participants.
Quality assessment
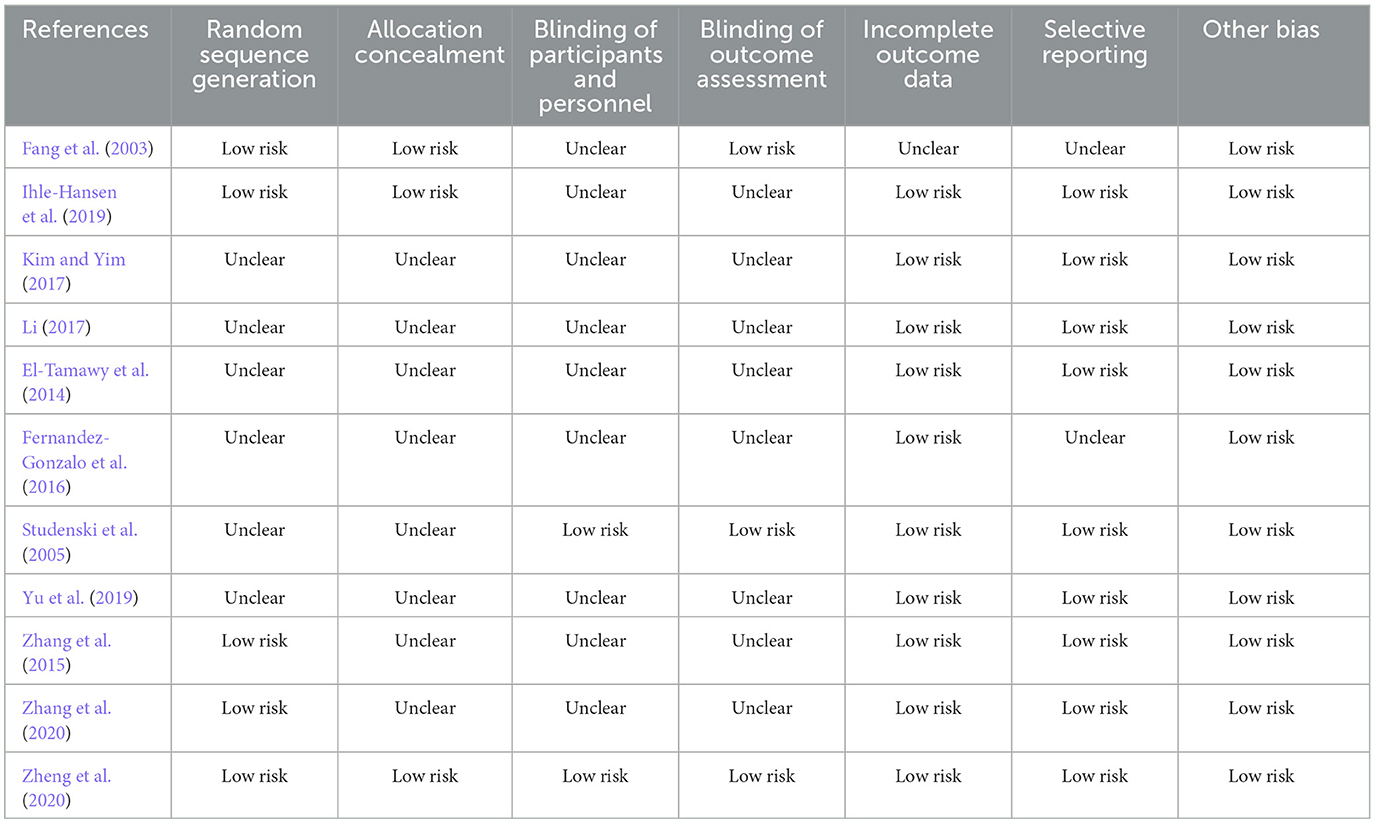
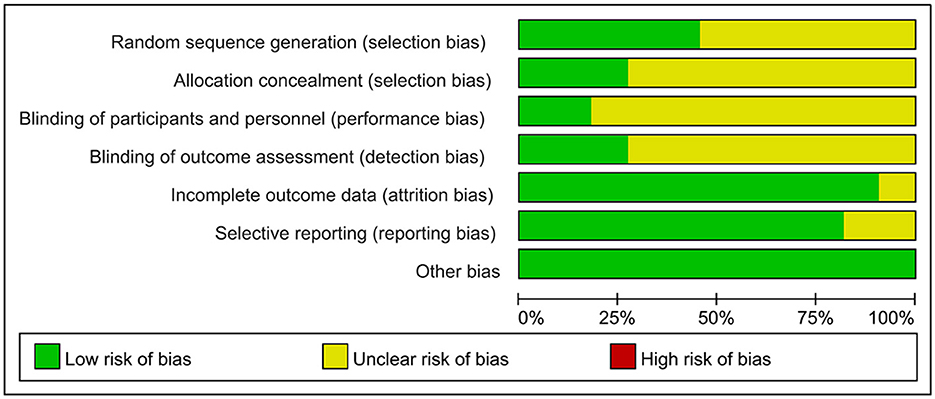
The 11 studies included in this analysis were RCTs, all of which had clearly defined inclusion and exclusion criteria for subjects and were comparable at baseline. The tools used to measure outcomes in the experimental and control groups were consistent, and the same statistical methods were used. The method of random sequence generation was explained in 5 studies (Fang et al., 2003; Zhang et al., 2015, 2020; Ihle-Hansen et al., 2019; Zheng et al., 2020), and allocation concealment was described in detail in only 3 studies (Fang et al., 2003; Ihle-Hansen et al., 2019; Zheng et al., 2020). The blinding of outcome evaluators was explicitly described in 3 studies (Fang et al., 2003; Studenski et al., 2005; Zheng et al., 2020), and the blinding of subjects and intervention implementers was explicitly described in 2 studies (Studenski et al., 2005; Zheng et al., 2020). Only 1 study (Fang et al., 2003) had missing data, and it did not explain the method of handling missing data; no other sources of bias were found in all studies. The literature quality assessment is shown in Table 2, Figure 2.
Results of meta-analysis
Effects of exercise therapy on cognitive function in patients with PSCI
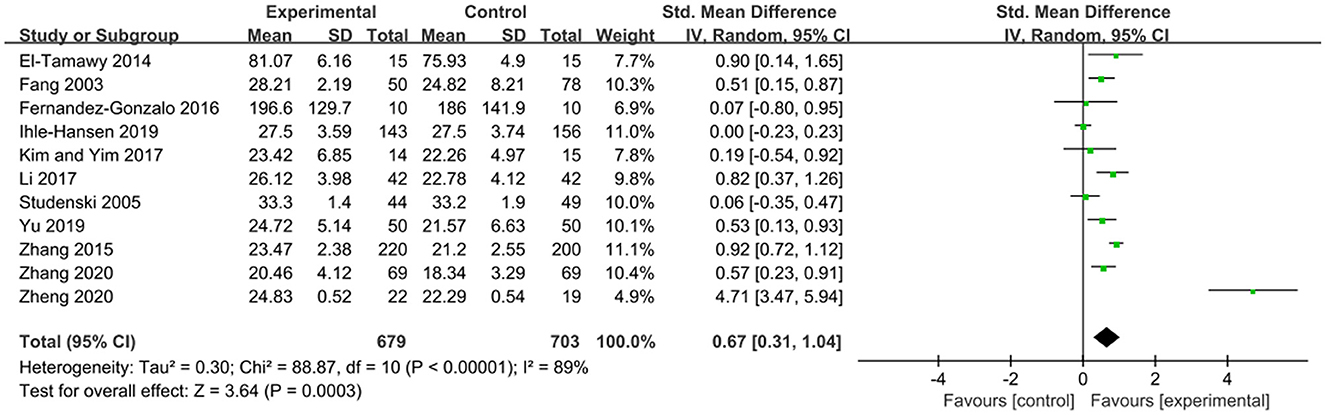
The MMSE scale and the MoCA scale are currently widely used cognitive function assessment scales in clinical practice, both of which can be used to assess patients' cognitive function (Jia et al., 2021). Both of them are effective in diagnosing dementia and cognitive impairment (Pinto et al., 2019). The effects of exercise therapy on cognitive function in patients with PSCI were reported in 11 studies (Fang et al., 2003; Studenski et al., 2005; El-Tamawy et al., 2014; Zhang et al., 2015, 2020; Fernandez-Gonzalo et al., 2016; Kim and Yim, 2017; Li, 2017; Ihle-Hansen et al., 2019; Yu et al., 2019; Zheng et al., 2020). SMD was selected for the combination of effect sizes, and the results showed significant heterogeneity (P < 0.01, I2 = 89%). When the study by Zheng et al. (2020) was removed using sensitivity analysis, the heterogeneity was reduced, and the 95% CI was (0.20, 0.74), but I2 was equal to 80%. The random-effects model was used for the meta-analysis. The results showed that the cognitive function of the experimental group was higher than that of the control group after the intervention, and the difference was statistically significant [SMD = 0.67, 95% CI (0.31, 1.04), P = 0.0003], as shown in Figure 3.
Subgroup analysis was performed based on the type of exercise: aerobic exercise was used in 4 studies (El-Tamawy et al., 2014; Ihle-Hansen et al., 2019; Zhang et al., 2020; Zheng et al., 2020); resistance exercise was used in 2 studies (Fang et al., 2003; Fernandez-Gonzalo et al., 2016); and aerobic exercise combined with resistance exercise was used in 5 studies (Studenski et al., 2005; Zhang et al., 2015; Kim and Yim, 2017; Li, 2017; Yu et al., 2019). In subgroup analysis (as shown in Figure 4), we found that each exercise type had a positive impact on cognitive function, with a p-value of < 0.05 for the overall effect for each exercise type. Moreover, aerobic exercise showed a large clinical effect in improving cognitive function [SMD = 1.32, 95%CI (0.30, 2.34)], while the other two exercise types exhibited a medium effect (SMD ≈ 0.5). However, the differences were not statistically significant (P = 0.29 from the test for subgroup differences) (Andrade, 2020).
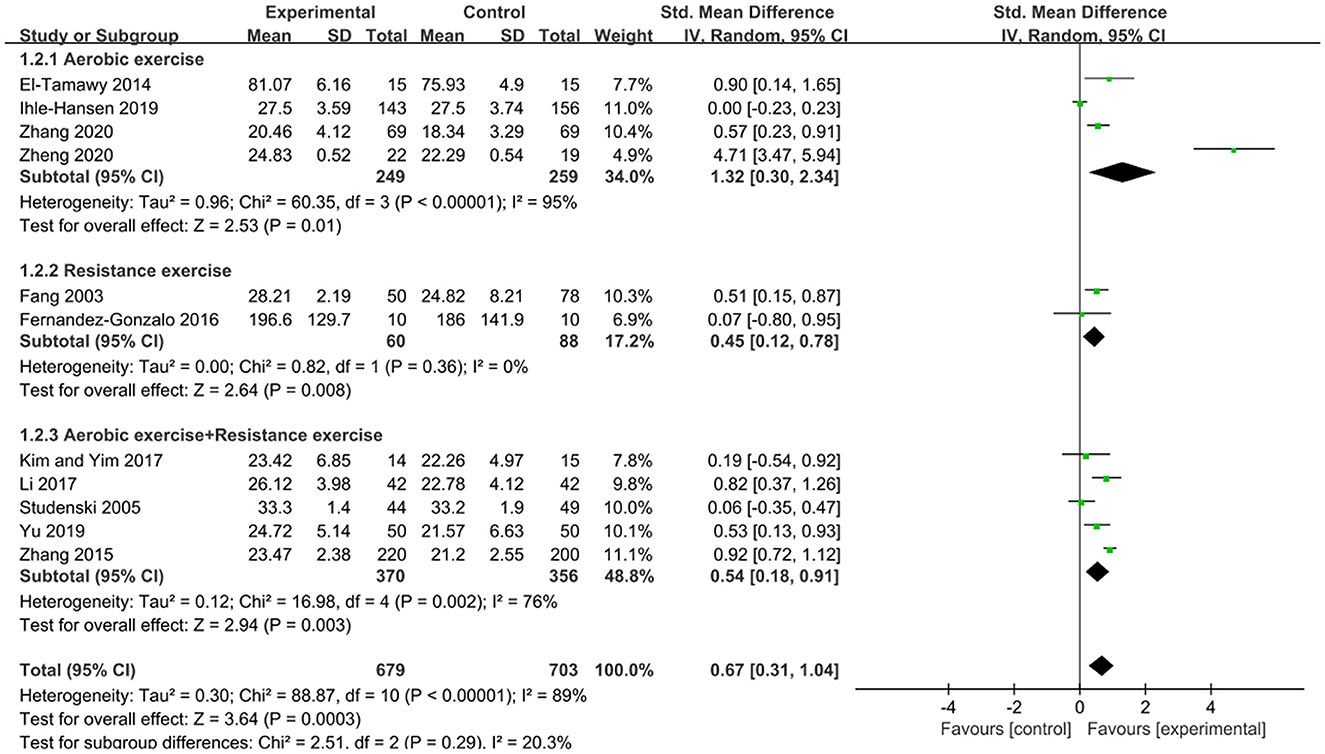
Figure 4. Subgroup analysis of the effects of different types of exercise on cognitive function in patients with PSCI.
Effects of exercise therapy on motor function in patients with PSCI
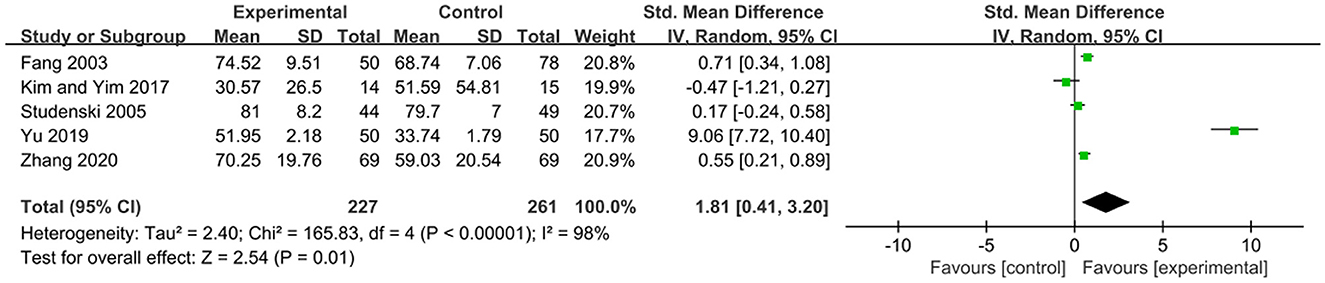
The FMA scale is considered by many in the field of stroke rehabilitation as one of the most comprehensive quantitative measures of motor impairment after stroke (Gladstone et al., 2002). The effects of exercise therapy on motor function in patients with PSCI were reported in 5 studies (Fang et al., 2003; Studenski et al., 2005; Kim and Yim, 2017; Yu et al., 2019; Zhang et al., 2020). SMD was selected for the combination of effect sizes, and the results showed significant heterogeneity (P < 0.01, I2 = 98%). When the study by Yu et al. (2019) was removed using sensitivity analysis, the heterogeneity was reduced, and the 95% CI was (−0.06, 0.72) but I2 was equal to 70%. The random-effects model was used for the meta-analysis. The results showed that the motor function of the experimental group was higher than that of the control group after the intervention, and the difference was statistically significant [SMD = 1.81, 95% CI (0.41, 3.20), P = 0.01], as shown in Figure 5.
Effects of exercise therapy on activities of daily living in patients with PSCI
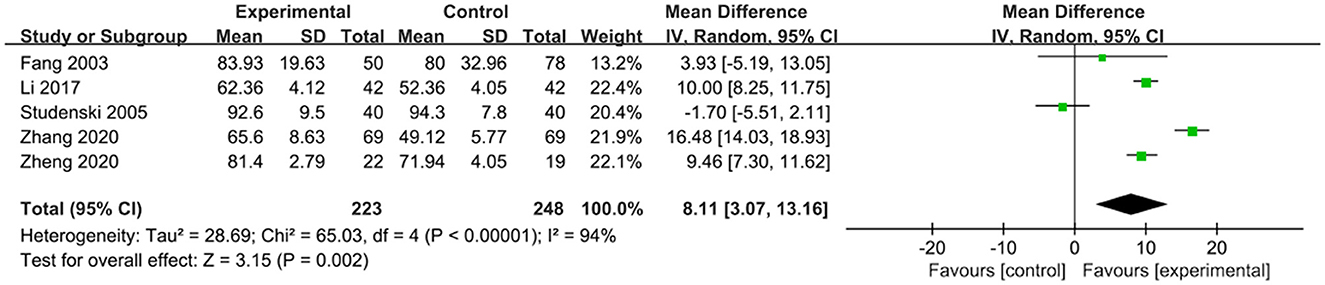
The effects of exercise therapy on the activities of daily living in patients with PSCI were reported in 5 studies (Fang et al., 2003; Studenski et al., 2005; Li, 2017; Zhang et al., 2020; Zheng et al., 2020). SMD was selected for the combination of effect sizes, and the results showed significant heterogeneity (P < 0.01, I2 = 94%). When the study by Studenski et al. (2005) was removed using sensitivity analysis, heterogeneity was reduced, and the 95% CI was (7.14, 14.86), but I2 was equal to 88%. The random-effects model was used for the meta-analysis. The results showed that the activities of daily living of the experimental group were higher than those of the control group after the intervention, and the difference was statistically significant [MD = 8.11, 95% CI (3.07, 13.16), P = 0.002], as shown in Figure 6.
Publication bias
A funnel plot analysis of the included literature with cognitive function, motor function, and activities of daily living as the outcome indicators showed that the distribution was generally symmetric, and the meta-analysis results were reliable (Figure 7).
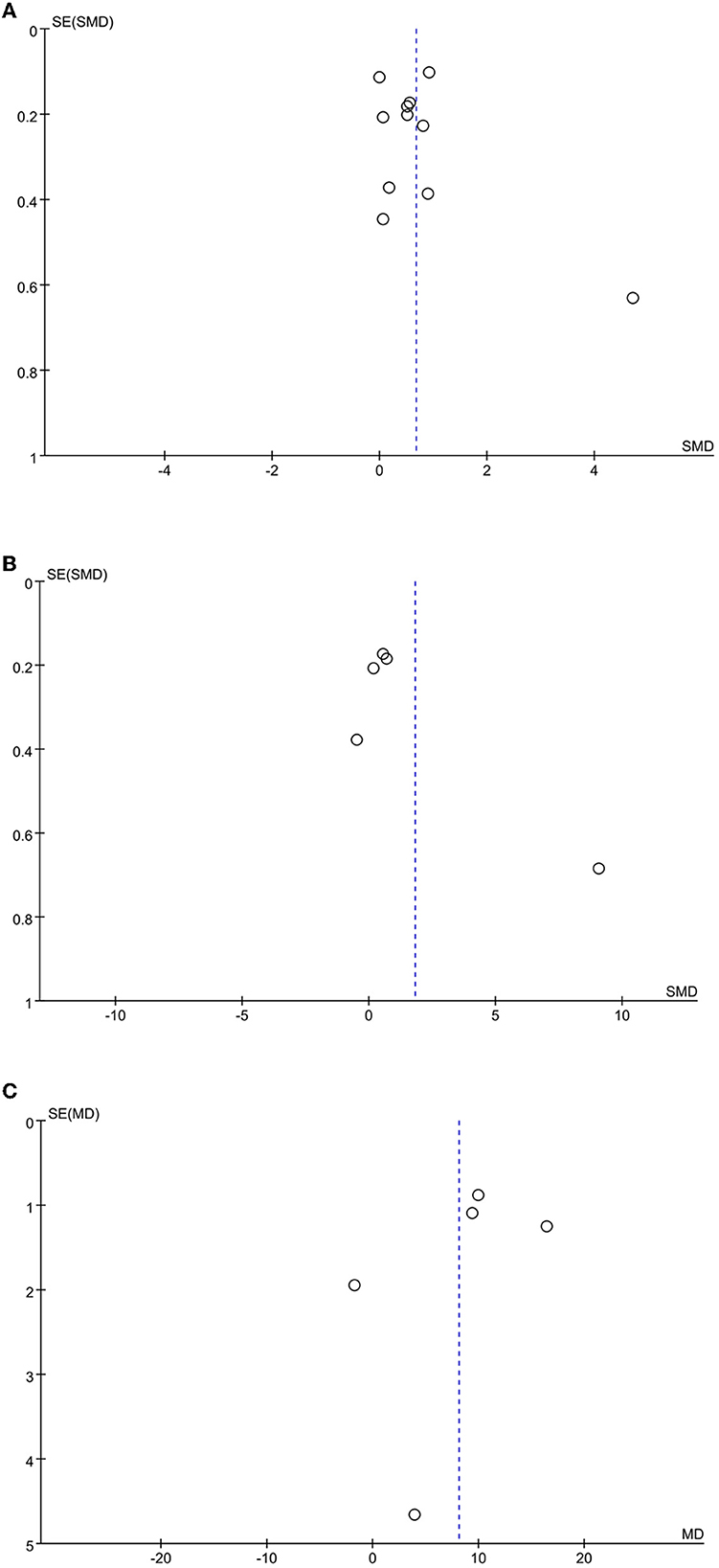
Figure 7. Funnel plot of the included studies. (A) cognitive function; (B) motor function; (C) activities of daily living.
Discussion
With the understanding of stroke, people pay attention not only to the problems of motion perception caused by stroke but also to the impact of stroke on cognitive function. After a stroke, the overall cognitive function of most patients is in a state of decline due to brain damage (Li et al., 2013). Physical activity has been shown to increase brain neuronutrients, improve cerebrovascular function and cerebral perfusion, reduce stress responses, and increase brain plasticity through synaptogenesis and nerve regeneration (Li et al., 2013). The 2019 Canadian Stroke Best Practice Guidelines recommend that exercise therapy be considered an adjunctive treatment for cognitive impairment, including attention, memory, and executive performance (Lanctot et al., 2020). In this study, we analyzed two current mainstream scales for comprehensive cognitive assessment and found that the cognitive function score in the exercise therapy group was higher than those in the control group, and the difference was statistically significant (P < 0.05). This study strongly demonstrated that exercise therapy can improve cognitive function in patients with PSCI, which is consistent with the findings of Ravichandran et al. (2020). Exercise therapy may improve PSCI for several reasons. Several studies have suggested that exercise can improve cognitive impairment in patients with mild PSCI, possibly because it can improve patients' cardiopulmonary function, reduce brain atrophy volume, increase cerebral blood flow, promote the establishment of brain neural networks, improve brain tissue metabolism, and stimulate central nervous system excitation (Szulc-Lerch et al., 2018). Moreover, exercise can also reduce or delay the occurrence of stroke risk factors such as coronary atherosclerotic heart disease, type 2 diabetes, hypertension, and other common diseases (Callisaya and Nosaka, 2017). A study found that exercise can improve cognitive performance, specifically memory and executive functions, and this was accompanied by an increase in plasma brain-derived neurotrophic factor (BDNF) levels (Sungkarat et al., 2018). In addition, exercise, especially aerobic exercise, significantly improved cortical connectivity and thus improved cognitive function in patients with PSCI (Ahlskog et al., 2011), which is consistent with the results of the subgroup analysis in this study. Studies have shown that exercise therapy can be used as a potentially effective technique to improve cognitive function in patients with PSCI.
In addition to cognitive impairment, motor impairment is a common consequence of stroke. Life becomes more difficult for patients with motor impairments who have PSCI. This study has shown that exercise therapy can improve motor function in patients with PSCI. The patients master the correct motor skills due to repeated, regular coordination training during the exercise. The movement of the cerebral cortex motor area is “set” through the input of repeated and intensified normal movement mode to the brain for stimulation so that the patient's body movements achieve maximum coordination and randomness and then promote the recovery of the affected limb movement ability and effectively reduce the occurrence of hemiplegic limb disuse and misuse atrophy deformation (Li et al., 2016). Many molecular signaling pathways are involved in this process, but among them, the brain-derived neurotrophic factor is a key promoter of neuroplasticity involved in motor learning and rehabilitation after stroke. Exercise, especially aerobic exercise, can upregulate neuronutrients (such as BDNF) to enhance the plasticity of the motor system (Mang et al., 2013). At the same time, exercise therapy can train the limbs of patients with PSCI, improve the condition of their movement impairment, and make their activities more coordinated.
Studies have shown that exercise can improve patients' ability to undertake the activities of daily living and prevent the occurrence of falls in elderly people, especially exercise to maintain physical balance (Sherrington et al., 2017), which is consistent with the findings of this study. García-Rudolph et al. (2019) also found that physical activity can improve the quality of life after a stroke. Exercise promotes the recovery of motor function and improves the activities of daily living. Improving upper extremity function can improve the ability to eat, dress, and use utensils; rehabilitating the back, waist, and lower extremities can promote the rehabilitation of turning, sitting, and standing transfer abilities. Improvements in balance and gait can improve patients' ability to walk and climb stairs (Shiraishi et al., 2017). Therefore, medical staff can improve the activities of daily living in patients with stroke by strengthening exercise therapy.
Strengths and limitations
The main advantage of this meta-analysis review is that only RCTs were selected. RCTs have the highest level of research evidence, and most high-quality clinical trials use the RCT design method. At present, there are still limitations to this study. First, although we used a rigorous method to search and select literature, publication bias was inevitable because the eligible studies included were Chinese and English literature only. In addition, many Chinese studies on blind methods, allocation, and concealment provided insufficient information, which was a hidden danger. Second, since the study did not strictly screen patients for the time of onset and diagnosis of PSCI, our results may be influenced by inevitable heterogeneity. Third, due to the limited number of eligible studies, no further subgroup analysis of the effects of exercise duration was performed in this study, which could be considered more carefully in subsequent studies.
Conclusion
In conclusion, exercise therapy can not only significantly improve the cognitive function of patients with PSCI but also improve the motor function and activities of daily living of patients to some extent. However, as the intensity and frequency of exercise therapy are still heterogeneous, the intensity and frequency of exercise therapy should be further discussed. In addition, if exercise therapy has the advantages of feasibility, economy, and safety to improve the quality of life of patients with PSCI, it is worth exploring how it might be promoted further.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YZ, XQ, JC, CJ, FW, and DS contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by YZ, XQ, CJ, and CL under the supervision of LC and PY. The first draft of the manuscript was written by YZ, XQ, and JC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant number SJCX22_0815) and funding for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (grant number 2021-LCYJ-MS-05).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahlskog, J. E., Geda, Y. E., Graff-Radford, N. R., and Petersen, R. C. (2011). Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin. Proc. 86, 876–884. doi: 10.4065/mcp.2011.0252
Andrade, C. (2020). Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J. Clin. Psychiatry. 81, 20f.13681. doi: 10.4088/JCP.20f13681
Callisaya, M., and Nosaka, K. (2017). Effects of exercise on type 2 diabetes mellitus-related cognitive impairment and dementia. J. Alzheimers Dis. 59, 503–513. doi: 10.3233/JAD-161154
Caspersen, C. J., Powell, K. E., and Christenson, G. M. (1985). Physical-activity, exercise, and physical-fitness - definitions and distinctions for health-related research. Public Health Rep. 100, 126–131.
Dong, Q., Guo, Q. H., Luo, B. Y., and Xu, Y. (2017). Expert Consensus on the management of post-stroke cognitive impairment. Chin. J. Stroke. 12, 519–531. doi: 10.3969/j.issn.1673-5765.2017.06.011
Dong, X. F., Wang, M., Guo, Y. L., Lv, P. H., and Liu, Y. J. (2021). Meta-analysis of the effects of aerobic exercise on patients with cognitive impairment after stroke. Chin. Nurs. Manag. 21, 1676–1683. doi: 10.3969/j.issn.1672-1756.2021.11.017
El-Tamawy, M. S., Abd-Allah, F., Ahmed, S. M., Darwish, M. H., and Khalifa, H. A. (2014). Aerobic exercises enhance cognitive functions and brain derived neurotrophic factor in ischemic stroke patients. Neuro Rehabilitat. 34, 209–213. doi: 10.3233/NRE-131020
Fang, Y., Chen, X., Li, H., Lin, J., Huang, R., Zeng, I., et al. (2003). A study on additional early physiotherapy after stroke and factors affecting functional recovery. Clin. Rehabil. 17, 608–617. doi: 10.1191/0269215503cr655oa
Fernandez-Gonzalo, R., Fernandez-Gonzalo, S., Turon, M., Prieto, C., Tesch, P. A., García-Carreira, M. D. C., et al. (2016). Muscle, functional and cognitive adaptations after flywheel resistance training in stroke patients: a pilot randomized controlled trial. J. Neuroeng. Rehabil. 13:1–11. doi: 10.1186/s12984-016-0144-7
García-Rudolph, A., Sánchez-Pinsach, D., Salleras, E. O., and Tormos, J. M. (2019). Subacute stroke physical rehabilitation evidence in activities of daily living outcomes. Medicine. 98, e14501. doi: 10.1097/MD.0000000000014501
Gladstone, D. J., Danells, C. J., and Black, S. E. (2002). The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil. Neural Repair. 16, 232–240. doi: 10.1177/154596802401105171
Huang, X., Zhao, X., Li, B., Cai, Y., Zhang, S., Wan, Q., et al. (2022). Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: a systematic review and network meta-analysis. J. Sport Health Sci. 11, 212–223. doi: 10.1016/j.jshs.2021.05.003
Ihle-Hansen, H., Langhammer, B., Lydersen, S., Gunnes, M., Indredavik, B., Askim, T., et al. (2019). A physical activity intervention to prevent cognitive decline after stroke: secondary results from the Life After STroke study, an 18-month randomized controlled trial. J. Rehabil. Med. 51, 646–651. doi: 10.2340/16501977-2588
Jia, X., Wang, Z., Huang, F., Su, C., Du, W., Jiang, H., et al. (2021). A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry. 21, 1–13. doi: 10.1186/s12888-021-03495-6
Kim, J., and Yim, J. (2017). Effects of an exercise protocol for improving handgrip strength and walking speed on cognitive function in patients with chronic stroke. Med. Sci. Monitor . 23, 5402–5409. doi: 10.12659/MSM.904723
Lanctot, K. L., Lindsay, M. P., Smith, E. E., Sahlas, D. J., Foley, N., Gubitz, G., et al. (2020). Canadian stroke best practice recommendations: mood, cognition and fatigue following stroke, 6th edition update 2019. Int. J. Stroke. 15, 668–688. doi: 10.1177/1747493019847334
Li, W., Huang, R., Shetty, R. A., Thangthaeng, N., Liu, R., Chen, Z., et al. (2013). Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol. Dis. 59, 18–25. doi: 10.1016/j.nbd.2013.06.014
Li, X., Xie, J. L., Hou, Y. J., Chen, Z. T., Liang, Q. Y., Guo, C. N., et al. (2016). Application of EEG characteristic analysis in clinical study of Alzheimer's disease. Chin J Biomed Eng. 35, 234–240. doi: 10.3969/j.issn.0258-8021.2016.02.015
Li, Y. (2017). Effects of aerobic combined balance exercise on cognitive impairment after cerebral infarction. J Clin Nurs Pract. 3, 120–123. doi: 10.11997/nitcwm.2017012035
Mang, C. S., Campbell, K. L., Ross, C. J., and Boyd, L. A. (2013). Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys. Ther. 93, 1707–1716. doi: 10.2522/ptj.20130053
Narasimhalu, K., Ang, S., De Silva, D. A., Wong, M. C., Chang, H. M., Chia, K. S., et al. (2009). Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology. 73, 1866–1872. doi: 10.1212/WNL.0b013e3181c3fcb7
Pinto, T. C., Machado, L., Bulgacov, T. M., Rodrigues-Júnior, A. L., Costa, M. L., Ximenes, R. C., et al. (2019). Is the montreal cognitive assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's Disease (AD) in the elderly? Int. Psychogeriatr. 31, 491–504. doi: 10.1017/S1041610218001370
Quinn, T. J., Richard, E., Teuschl, Y., Gattringer, T., Hafdi, M., O'Brien, J. T., et al. (2021). European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur Stroke J. 6, I–XXXVIII. doi: 10.1177/23969873211042192
Ravichandran, H., Sharma, H. R., Haile, T. G., Gelaw, A. Y., Gebremeskel, B. F., Janakiraman, B., et al. (2020). Effects of trunk exercise with physioball to improve trunk balance among subjects with stroke: a systematic review and meta-analysis. J Exerc Rehabil. 16, 313–324. doi: 10.12965/jer.2040292.146
Sachdev, P. S., Chen, X., Brodaty, H., Thompson, C., Altendorf, A., and Wen, W. (2009). The determinants and longitudinal course of post-stroke mild cognitive impairment. J. Int. Neuropsychol. Soc. 15, 915–923. doi: 10.1017/S1355617709990579
Sherrington, C., Michaleff, Z. A., Fairhall, N., Paul, S. S., Tiedemann, A., Whitney, J., et al. (2017). Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Brit. J. Sport. Med. 51, 1750–1758. doi: 10.1136/bjsports-2016-096547
Shiraishi, N., Suzuki, Y., Matsumoto, D., Jeong, S., Sugiyama, M., Kondo, K., et al. (2017). Effects of a Self-Exercise program on activities of daily living in patients after acute stroke: a propensity score analysis based on the japan association of rehabilitation database. Arch. Phys. Med. Rehabil. 98, 434–441. doi: 10.1016/j.apmr.2016.08.467
Stone, P. W. (2002). Popping the (PICO) question in research and evidence-based practice. Appl. Nursing Res. 15, 197–198. doi: 10.1053/apnr.2002.34181
Studenski, S., Duncan, P. W., Perera, S., Reker, D., Lai, S. M., Richards, L., et al. (2005). Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke. 36, 1764–1770. doi: 10.1161/01.STR.0000174192.87887.70
Su, K., Yuan, J., Liu, H., Luo, M., Li, Q., Liu, S., et al. (2022). The comparative effectiveness of traditional chinese medicine exercise therapies in elderly people with mild cognitive impairment: a systematic review and network Meta-Analysis. Front. Neurology. 13. doi: 10.3389/fneur.2022.775190
Sun, J. H., Tan, L., and Yu, J. T. (2014). Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2, 80. doi: 10.3978/j.issn.2305-5839.2014.08.05
Sungkarat, S., Boripuntakul, S., Kumfu, S., Lord, S. R., and Chattipakorn, N. (2018). Tai chi improves cognition and plasma BDNF in older adults with mild cognitive impairment: a randomized controlled trial. Neurorehab. Neural Res. 32, 142–149. doi: 10.1177/1545968317753682
Szulc-Lerch, K. U., Timmons, B. W., Bouffet, E., Laughlin, S., Medeiros, d. e., Skocic, C. B., et al. (2018). Repairing the brain with physical exercise: cortical thickness and brain volume increases in long-term pediatric brain tumor survivors in response to a structured exercise intervention. Neuroimage Clin. 18, 972–985. doi: 10.1016/j.nicl.2018.02.021
Tang, T., Huang, H. L., Zhu, L. W., Li, H. Y., Zheng, T. T., Zhang, J. Y., et al. (2020). Research progress of TCM rehabilitation for post-stroke cognitive impairment. World J Integr Tradit Western Med. 15, 977–980. doi: 10.13935/j.cnki.sjzx.200546
Yu, X. Y., Wen, M. Y., and Chen, X. Y. (2019). Effects of aerobic exercise combined with resistance exercise on neural function, limb function and cognitive function in patients with non-dementia cognitive impairment after stroke. J. Clin. Nurs. 18, 33–36. doi: 10.3969/j.issn.1671-8933.2019.05.012
Zhang, J., Lv, D., and Li, J. (2015). Effects of aerobic combined impedance and balance exercise on cognitive function in patients with non-dementia cognitive impairment after stroke. Chin J Prac Nurs. 31, 2435–2438. doi: 10.3760/cma.j.issn.1672-7088.2015.32.005
Zhang, X. X., Zheng, Y., Ma, L., Xu, Y. Q., Zhang, H. M., Li, H. Y., et al. (2020). Effects of aerobic exercise training on insulin resistance and cognitive level in stroke patients. Chin J Med. 55, 434–437. doi: 10.3969/j.issn.1008-1070.2020.04.026
Zhao, L., Biesbroek, J. M., Shi, L., Liu, W., Kuijf, H. J., Chu, W. W., et al. (2018). Strategic infarct location for post-stroke cognitive impairment: a multivariate lesion-symptom mapping study. J. Cereb. Blood Flow Metab. 38, 1299–1311. doi: 10.1177/0271678X17728162
Keywords: stroke, cognitive impairment, exercise therapy, systematic review, meta-analysis
Citation: Zhang Y, Qiu X, Chen J, Ji C, Wang F, Song D, Liu C, Chen L and Yuan P (2023) Effects of exercise therapy on patients with poststroke cognitive impairment: A systematic review and meta-analysis. Front. Neurosci. 17:1164192. doi: 10.3389/fnins.2023.1164192
Received: 12 February 2023; Accepted: 09 March 2023;
Published: 06 April 2023.
Edited by:
Song Ge, University of Houston–Downtown, United StatesReviewed by:
Peijin Han, University of Michigan, United StatesChao Yang, University of Texas MD Anderson Cancer Center, United States
Copyright © 2023 Zhang, Qiu, Chen, Ji, Wang, Song, Liu, Chen and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Chen, Z3ljYzIwMTFAMTI2LmNvbQ==; Ping Yuan, NDEwOTc1MTQxQHFxLmNvbQ==
†These authors share first authorship
 Yuanxing Zhang
Yuanxing Zhang Xichenhui Qiu
Xichenhui Qiu Jinghao Chen
Jinghao Chen Cuiling Ji3
Cuiling Ji3 Dan Song
Dan Song Lu Chen
Lu Chen