
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 10 July 2023
Sec. Neuroenergetics and Brain Health
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1163733
This article is part of the Research TopicInsights in Neuroenergetics, Nutrition and Brain Health: 2023View all 9 articles
 Xiaofan Yu1,2,3,4,5†
Xiaofan Yu1,2,3,4,5† Peicong Ge1,2,3,4,5*†
Peicong Ge1,2,3,4,5*† Yuanren Zhai1,2,3,4,5
Yuanren Zhai1,2,3,4,5 Wei Liu1,2,3,4,5
Wei Liu1,2,3,4,5 Qian Zhang1,2,3,4,5
Qian Zhang1,2,3,4,5 Xun Ye1,2,3,4,5
Xun Ye1,2,3,4,5 Xingju Liu1,2,3,4,5
Xingju Liu1,2,3,4,5 Rong Wang1,2,3,4,5
Rong Wang1,2,3,4,5 Yan Zhang1,2,3,4,5
Yan Zhang1,2,3,4,5 Jizong Zhao1,2,3,4,5,6
Jizong Zhao1,2,3,4,5,6 Dong Zhang1,2,3,4,5,7*†
Dong Zhang1,2,3,4,5,7*†Background and purpose: Urea cycle metabolites are expected to be the biomarkers for cerebrovascular diseases. However, the effects of circulating urea cycle metabolites on the risk of MMD and its subcategories remain unclear. The aim of this study was to prospectively investigate the association between plasma urea cycle metabolites and the risk of MMD and its subcategories.
Methods: We measured plasma urea cycle metabolite levels for 360 adult MMD patients and 89 matched healthy controls. Clinical and laboratory characteristics were obtained from the medical record. The study was conducted from July 2020 to December 2021.
Results: After multivariate adjustment, the risk of MMD increased with each increment in ornithine level (per natural log [ornithine] increment: OR, 3.893; 95% CI, 1.366–11.090). The risk of MMD decreased with each increment in arginine level (per natural log [arginine] increment: OR, 0.109; 95% CI, 0.028–0.427), urea level (per natural log [urea] increment: OR, 0.261; 95% CI, 0.072–0.940), and global arginine bioavailability ratio (GABR) level (per natural log [GABR] increment: OR, 0.189; 95% CI, 0.074–0.484). The addition of plasma arginine (integrated discrimination improvement: 1.76%, p = 0.021) or GABR (integrated discrimination improvement: 1.76%, p = 0.004) to conventional risk factors significantly improved the risk reclassification for MMD.
Conclusion: Plasma ornithine levels are positively associated with the risk of MMD. By contrast, the levels of arginine, urea, and GABR are inversely related to the risk of MMD. Plasma urea cycle metabolites might be potential biomarkers for the risk of MMD.
A rare cerebrovascular condition known as moyamoya disease (MMD) is characterized by the progression of intracranial carotid artery stenosis and an abnormal vascular network in the brain (Suzuki and Takaku, 1969; Scott and Smith, 2009).
The pathogenesis of MMD is presently not well understood. The onset and progression of MMD may be influenced by immune, inflammatory, and genetic causes (Fujimura et al., 2014; Bang et al., 2016). A significant correlation between RNF213 and MMD has been identified (Fujimura et al., 2014; Kim et al., 2016). A polymorphism in RNF213 was identified in 95% of familial patients with MMD and 79% of sporadic cases (Kim et al., 2016). The prevalence of this variant differed widely between MMD patients in Japan (90%) and China (23%) (Miyatake et al., 2012; Zhang et al., 2017). As a result, in addition to hereditary factors, other variables are also important in the onset of MMD in Chinese patients. Previous research has discovered that the risk of MMD is related to a number of conventionally modifiable risk variables, including albumin (ALB), homocysteine (Hcy), body mass index (BMI), and high-density lipoprotein cholesterol (HDL-C) (Ge et al., 2020). Nonetheless, the conventional risk variables are unable to fully account for the MMD risk. It is urgently necessary to identify new risk variables, especially modifiable risk variables.
The relationship between urea cycle metabolites and cardio-cerebrovascular diseases has been extensively studied (Martínez-González et al., 2015; Ahmad et al., 2016; Sun et al., 2019). Ornithine is an essential building block for the production of proline, polyamines, and citrulline and is crucial for the control of a number of metabolic activities (Sivashanmugam et al., 2017). Arginine is an amino acid associated with many biological processes, including protein synthesis, immune reaction, and the creation of nitric oxide. It has been reported that arginine may be a protective factor in ischemic cerebrovascular diseases (Ahmad et al., 2016; Grosse et al., 2020). Furthermore, the concept of “global arginine bioavailability ratio (GABR)” (defined as arginine/[ornithine + citrulline]), which was proposed in 2009, could be more predictive of the occurrence and development of cardio-cerebrovascular diseases (Tang et al., 2009). Although urea is the end product of the urea cycle, it has been associated with the development of stroke (You et al., 2018; Peng et al., 2021). Therefore, urea cycle metabolites are expected to be the biomarkers for cerebrovascular diseases. However, the effects of circulating urea cycle metabolites on the risk of MMD remain unclear. The purpose of this prospective study was to investigate the relationship between the concentrations of urea cycle metabolites and the risk of MMD and its subcategories.
Upon reasonable request, the corresponding authors will provide the data supporting the findings of this manuscript. From July 1, 2020, to December 31, 2021, we prospectively collected adult MMD patients (18 years ≤ age ≤ 60 years) at the Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University. Each participant signed informed consent. The Ethics Committee of Beijing Tiantan Hospital, Capital Medical University, accepted the study’s procedure (Ethical inspection No. 2021YFC2500502). The registration number of this study is ChiCTR2000031412.
According to the Japanese guidelines released in 2012, digital subtraction angiography (DSA) was used to diagnose all patients with MMD (Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis and Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases, 2012). A total of 500 patients with MMD-type cerebrovascular disease (including 418 adult patients) from across the nation received care at our facility from July 1, 2020, to December 31, 2021. Among the adult patients, 11 patients older than 60 years, 13 patients with moyamoya syndrome, and 34 patients without urea cycle metabolite data were excluded. Moyamoya syndrome refers to patients with MMD-type cerebrovascular disease accompanied by other basic diseases, such as arteriosclerosis, autoimmune disease, meningitis, and Down syndrome (Scott and Smith, 2009). Finally, 360 adult patients with comprehensive urea cycle metabolite data were included in this study (Supplementary Figure I). Among the 360 adult patients, 259 patients had ischemic-type MMD and 101 patients had hemorrhagic-type MMD. Age-matched healthy individuals who underwent regular checkups were recruited for the control group. The individuals in the control group did not suffer from heart disease or MMD, according to interviews with them and their families.
After a 15-min break spent seated, the participants’ right arms were examined for systolic blood pressure (SBP) and diastolic blood pressure (DBP) using a conventional mercury manometer. Additionally, electrocardiography heart rate (HR) data were captured. Weight (kg)/height (m2) was used to determine the BMI. Venous blood samples were collected from the participants after 12 h of fasting. White blood cells (WBC), lymphocytes (LY), platelets (PLT), glucose (Glu), ALB, creatinine (Cr), uric acid (UA), triglyceride (TG), total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), apolipoprotein A (apoA), apolipoprotein B (apoB), and Hcy were all measured in fasting blood. The RNF213 p.R4810K variant was detected, but the p.A4399T variant was not detected. The following primers were used: RNF213-4810F (rs112735431), 5′-GCCCTCCATTTCTAGCACAC-3′, and RNF21-4810R, 5′-AGCTGTGGCGAAAGCTTCTA-3′.
Data on MMD patients’ age, sex, and comorbidities, such as hypertension, coronary artery disease, smoking, drinking, diabetes mellitus, thyroid disorders, and initial clinical features (ischemia and hemorrhage), were gathered at the time of admission. Smokers were participants who smoked at least one cigarette per day for at least 1 year. Drinkers were individuals who drank at least 125 mL of wine per day for at least 1 year (D' Avanzo et al., 1996). Two neurosurgeons used DSA to evaluate the Suzuki stage blindly.
The plasma levels of urea cycle metabolites (ornithine, citrulline, arginine, aspartic acid, and urea) were quantified by ultra-performance liquid chromatography tandem mass spectrometry (Sciex QTRAP 6500 LC–MS/MS). An aliquot of 50 μL of the serum sample was transferred to a centrifuge tube, mixed with 250 μL of 20% acetonitrile/methanol, vortexed for 3 min, and centrifuged at 12,000 rpm for 10 min at 4°C. Then, 250 μL of the supernatant was transferred to a new centrifuge tube and placed in a freezer at −20°C for 30 min. Next, the supernatant was centrifuged at 12,000 rpm for 10 min at 4°C. After centrifugation, 180 μL of the supernatant was added to a protein precipitation plate for further LC–MS analysis.
Baseline characteristics are presented as medians (25th–75th percentile) for continuous variables and proportions for categorical variables. Chi-square and Mann–Whitney U tests were used for categorical and continuous variables in baseline features, respectively.
The participants were divided into four categories based on the quartile of change in ornithine, arginine, urea, or GABR index. P for trend was calculated using the quartile of change in ornithine, arginine, urea, or GABR index as the ordinal variable. The Cochran–Armitage test was used to assess trends in categorical variables, and one-way ANOVA was performed to analyze continuous variables.
Odds ratios (ORs) of the risk of MMD (MMD overall, ischemic MMD, and hemorrhagic MMD) to plasma levels of ornithine, arginine, urea, or GABR (continuous and categorical variables) were assessed by a conditional logistic regression model, without and with modification for age, gender, HR, SBP, DBP, BMI, WBC, LY, PLT, Glu, Cr, UA, ALB, TG, TC, HDL-C, LDL-C, apoA, apoB, and Hcy.
Two statistical indices were used to measure the improvement in model performance resulting from the addition of new markers: net reclassification improvement and integrated discrimination improvement (Pencina et al., 2008). We created a conventional model (only including risk factors in model 2) and four novel models (including risk factors in model 2 and plasma ornithine, arginine, urea, or GABR) by using logistic regression. We evaluated net reclassification improvement and integrated discrimination improvement by comparing these models to determine whether adding plasma ornithine, arginine, urea, or GABR to risk factors might enhance the prediction ability for the risk of MMD.
All statistical analysis were carried out using IBM SPSS Statistics (version 22.0; IBM Corp.) and R software (version 4.2.0).1 p < 0.05 was considered statistically significant, and all tests were two-tailed.
The study was conducted from July 2020 to December 2021. In this study, we analyzed 360 MMD patients (259 patients with ischemic MMD and 101 patients with hemorrhagic MMD) and 89 age-matched controls with comprehensive urea cycle metabolite examinations (Supplementary Figure I). A total of 187 (41.65%) males and 262 (58.35%) females were enrolled, and the median age was 43.00 years (IQR, 33.00–50.00 years). The clinical and laboratory features of MMD and the controls are shown in Table 1. MMD patients had more risk variables for stroke compared with the healthy controls. MMD and its subcategories had higher levels of SBP, WBC count, TG, Hcy, and ornithine, and a higher prevalence of hypertension, hyperlipidemia, thyroid disease, smoking, and drinking (p < 0.05 for all). Moreover, patients with MMD had lower levels of HDL-C, apoA, urea, and GABR (p < 0.05 for all) (Figure 1). Patients with ischemic MMD had a lower level of arginine (p < 0.05). Patients with ischemic or hemorrhagic MMD had a higher prevalence of the RNF213 p.R4810K variant (p < 0.05 for all).
The correlations of plasma ornithine, arginine, urea, and GABR levels with the risk of MMD are presented in Tables 2–5. After adjustment for model 2, the risk of MMD increased with each increment in ornithine level (per natural log [ornithine] increment: OR, 3.893; 95% CI, 1.366–11.090). When plasma ornithine levels were divided into quartiles, those in the highest quartile had a noticeably higher risk of MMD than those in the lowest quartile (Q4 versus Q1: OR, 3.626; 95% CI, 1.334–9.855). Conversely, the risk of MMD decreased with each increment in arginine level (per natural log [arginine] increment: OR, 0.109; 95% CI, 0.028–0.427), urea level (per natural log [urea] increment: OR, 0.261; 95% CI, 0.072–0.940), and GABR level (per natural log [GABR] increment: OR, 0.189; 95% CI, 0.074–0.484) after adjustment for the risk variables in model 2. When plasma levels of arginine, urea, and GABR were divided into quartiles, those in the highest quartile had a noticeably lower risk of MMD than those in the lowest quartile (arginine, Q4 versus Q1: OR, 0.273; 95% CI, 0.114–0.652; urea, Q4 versus Q1: OR, 0.318; 95% CI, 0.136–0.745; GABR, Q4 versus Q1: OR, 0.264; 95% CI, 0.108–0.649). We can see the association of urea cycle metabolites and conventional risk factors in the Supplementary Materials (Supplementary Tables I–IV). Participants with higher ornithine concentrations tended to be male and had higher BMI, Glu, Cr, UA, apoB, and Hcy levels, lower PLT, HDL-C, and apoA levels, and a higher prevalence of hypertension, diabetes, coronary artery disease, smoking, and drinking. Participants with higher arginine concentrations tended to be male and had higher BMI, PLT, and LDL-C levels. Participants with higher urea concentrations tended to be male and had higher Cr and UA levels and a higher prevalence of coronary artery disease. Participants with higher GABR levels tended to be female and had higher PLT and HDL-C levels, lower Cr, UA, and Hcy levels, and a lower prevalence of smoking.
The Suzuki stage and RNF213 p.R4810K variant in patients with MMD are shown in Supplementary Table V. We did not detect the p.A4399T variant in the present study. The results suggested that MMD patients with lower plasma levels of citrulline or urea were more likely to have the RNF213 p.R4810K variant. Furthermore, MMD patients with higher levels of GABR tended to have a higher Suzuki stage level and the RNF213 p.R4810K variant (p < 0.05 for all). In addition, we further analyzed the association between GABR and the risk of hemorrhagic MMD in the overall MMD cases. After adjusting for all the variables in model 2, a higher trend of hemorrhagic MMD (OR, 2.432; 95% CI, 1.088–5.436) was found in MMD patients in Q4 compared with those in Q1 (Supplementary Tables VI).
We examined whether adding plasma urea cycle metabolites (ornithine, arginine, urea, and GABR) to the conventional model (all risk factors in model 2) could improve its ability to predict the risk of MMD. As shown in Table 6, the addition of plasma arginine (net reclassification improvement: 54.18%, p = 0.000; integrated discrimination improvement: 1.76%, p = 0.021) or GABR (net reclassification improvement: 27.72%, p = 0.018; integrated discrimination improvement: 1.76%, p = 0.004) to conventional risk factors significantly improved the risk reclassification for overall MMD. The addition of plasma arginine (net reclassification improvement: 59.82%, p = 0.000; integrated discrimination improvement: 2.92%, p = 0.004) or GABR (net reclassification improvement: 38.58%, p = 0.001; integrated discrimination improvement: 3.80%, p = 0.003) to conventional risk factors also significantly improved the risk reclassification for ischemic MMD. Furthermore, the addition of GABR (net reclassification improvement: 33.80%, p = 0.017; integrated discrimination improvement: 3.95%, p = 0.005) to conventional risk factors significantly improved the risk reclassification for hemorrhagic MMD.
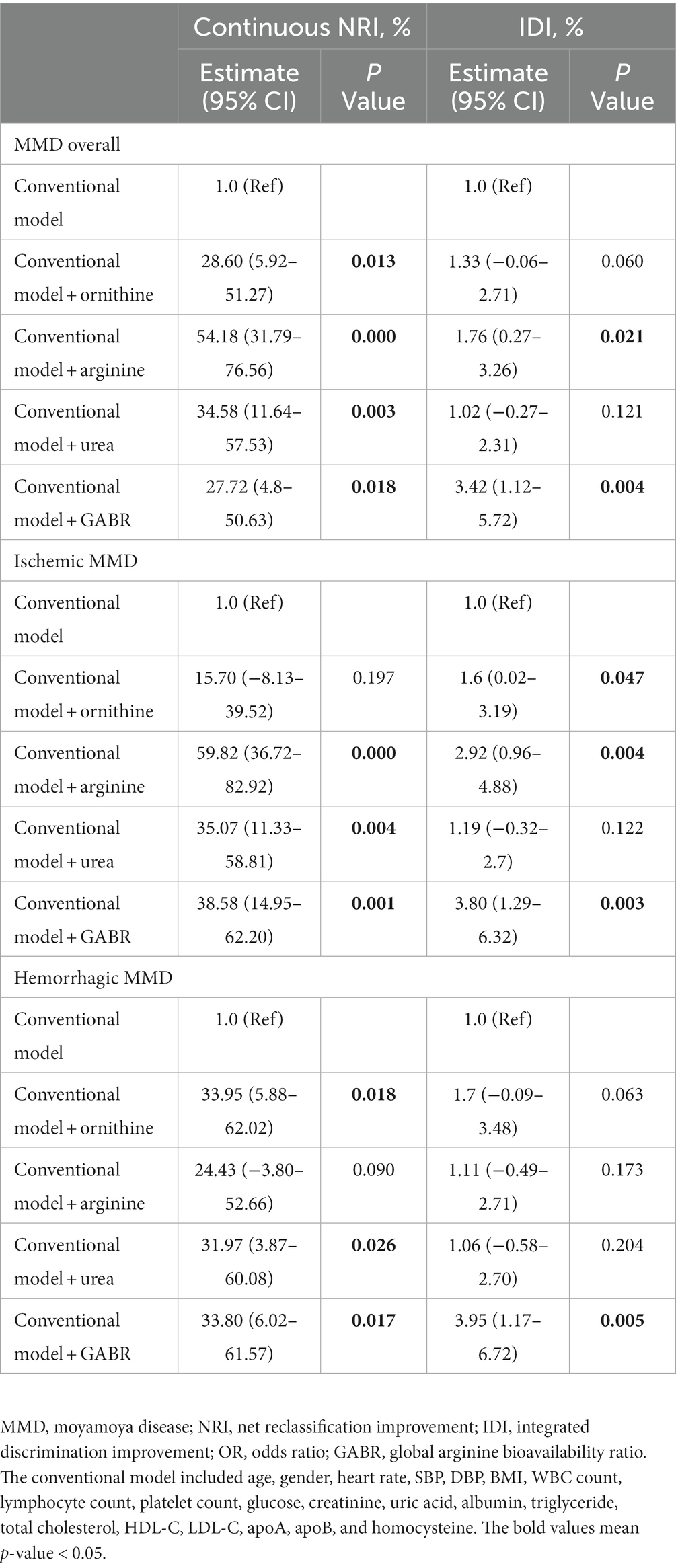
Table 6. Performance of models with plasma urea cycle metabolites at predicting the risk of MMD and its subtypes.
To our knowledge, this is the first clinical study to examine the prospective association between plasma urea cycle metabolites and the risk of adult MMD. In this study, we found that increased plasma ornithine levels were independently related to an increased risk of adult MMD. By contrast, increased arginine, urea, and GABR levels were associated with a decreased risk of adult MMD. These findings suggest that plasma urea cycle metabolites might be potential biomarkers for the risk of MMD.
The urea cycle is a metabolic pathway for the elimination of excess nitrogen and ammonia, which contain a range of amino acids and key enzymes. As a byproduct of the urea cycle, ornithine serves as a crucial building block for the production of proline, polyamines, and citrulline (Sivashanmugam et al., 2017). In this study, we discovered a positive correlation between plasma ornithine levels and the risk of MMD and its subcategories. Although the interaction between ornithine and MMD is unclear, numerous hypotheses can be put forth. First, the mitochondria in endothelial cells from MMD patients have aberrant morphology and function, according to the available information. This finding raises the possibility that MMD is a mitochondrial disease (Choi et al., 2018). Therefore, the activity of key enzymes in mitochondria related to the urea cycle (carbamoyl phosphate synthetase 1 and ornithine transcarbamylase) may be reduced in patients with MMD. Second, the urea cycle also occurs in enterocytes (Su et al., 2021). Numerous pro-inflammatory and anti-inflammatory cytokines affect the key enzymes of the urea cycle in non-hepatic cells (Morris 2002). Moreover, it has been demonstrated that systemic inflammation is critical in the pathophysiology of MMD (Han et al., 2020). Thus, it is reasonable to believe that elevated inflammatory factors in the circulation of patients with MMD may affect the activity of key enzymes of the urea cycle.
Arginine is an amino acid associated with many biological processes, including protein synthesis, immune reaction, the urea cycle, and the creation of nitric oxide (Morris, 2016). The correlation between plasma arginine levels and cardio-cerebrovascular diseases has been extensively studied. Many studies have indicated that higher levels of arginine in plasma were related to a lower incidence of cardio-cerebrovascular diseases (Ahmad et al., 2016; Yu et al., 2017; Grosse et al., 2020). In this study, we also discovered that plasma arginine levels are negatively related to the risk of MMD and its subcategories. Multiple potential pathophysiologic routes have been hypothesized for these inverse relationships. First, several studies have shown that arginine has anti-inflammatory effects. Qiu et al. found that arginine could inhibit the inflammatory response and oxidative stress induced by lipopolysaccharide (Qiu et al., 2019). In addition, a recent study suggested that arginine can reduce inflammation by preventing nuclear factor-κB activation (Liang et al., 2022). It has been demonstrated that the enhancement of nuclear factor-κB activation is involved in the pathogenesis of MMD (Takeda et al., 2020). Second, patients with mitochondrial disorders have nitric oxide deficiency (Almannai and El-Hattab, 2021). A case–control study indicated that nitric oxide deficiency may play a critical role in the onset of MMD (Park et al., 2014). Endothelial NO prevents platelet and leukocyte adhesion, attenuates inflammatory mediators, and causes endothelium-dependent vasorelaxation (Greco et al., 2018). As a result, it seems sensible to assume that arginine may prevent MMD through the production of nitric oxide. Third, another potential mechanism that appears to be present is the microbiome. In the case of Crohn’s disease, a higher prevalence of Ruminococcus gnavus and a decrease in Roseburia inulinivorans were observed, similar to what was observed in patients with MMD. Furthermore, there were elevated levels of microbial arginine and isoprene pathways in the gut microbiome of individuals with Crohn’s disease, indicating a possible association between the gut microbiome and uric cycle metabolism (Hu et al., 2021; Mineharu et al., 2022).
Although urea is the end product of the urea cycle, it has been associated with the development of stroke (You et al., 2018; Peng et al., 2021). Geng et al. showed in their metabolomic study that urea was lower in patients with MMD than in controls (Geng et al., 2020). In this study, we discovered that plasma urea levels were negatively related to the risk of MMD and its subcategories. As mentioned above, decreased activity of key enzymes in the urea cycle can lead to abnormal production of urea cycle metabolites, which also includes a decrease in the end product. A previous study suggested that the concept of GABR could be more predictive of the occurrence and development of cardio-cerebrovascular diseases. Our study also provides further evidence that GABR can also be a reliable predictor of MMD. Moreover, our results indicate that MMD patients with lower plasma levels of citrulline or urea and higher levels of GABR tend to have the RNF213 p.R4810K variant. Considering that GABR was inversely associated with MMD and was positively associated with the p.R4810K mutation, the relationship between the p.R4810K mutation and the urea cycle is still unclear. However, previous reports in this area are scarce and warrant further research.
Collateral vessels and the potential for angiogenesis differ among MMD subcategories (Zhao et al., 2019). However, the pathophysiological mechanisms of MMD subcategories remain unclear. A recent study showed that arginine can improve ventricular function by inducing neovascularization (Ranjbar, 2022). Our study demonstrated that increased GABR levels were associated with the risk of hemorrhagic MMD in overall MMD cases. In addition, MMD patients with higher GABR levels tended to have a higher Suzuki stage level. Therefore, GABR levels may be associated with the formation of collateral vessels in MMD. However, high GABR decreased the risk of MMD in the present study. We speculated that the possible reason for this contradictory conclusion is that the MMD cases we collected were at different Suzuki stages. Therefore, our next step was to perform a stratified analysis of MMD patients at different Suzuki stages. Another study has shown that homozygous or heterozygous RNF213 p.R4810K may be a potential biomarker for categorizing various clinical subcategories of MMD (Wang et al., 2021). The relationship between the p.R4810K mutation and the urea cycle remains to be explored further. One study showed that p.A4399T was associated with hemorrhagic MMD in a Chinese population (Wu et al., 2012). However, we did not detect the p.A4399T variant in the present study. We will continue to clarify the relationship between p.A4399T and GABR in MMD in future studies.
This study has several restrictions. First, this study was an observational study; the number of participants was not equal between the groups. Second, these findings could not be extended to children or other races, as only Chinese adult patients with MMD were included. Third, we only measured preoperative plasma urea cycle metabolite concentrations during hospitalization; this prevented us from studying the relationship between dynamic variations in plasma urea cycle metabolites and the prognosis of MMD. Finally, this is a prospective study using clinical information from a single institute. The applicability of the results is constrained, necessitating additional validation in a different cohort.
In conclusion, increased plasma ornithine levels were independently related to an increased risk of adult MMD. By contrast, increased arginine, urea, and GABR levels were associated with a decreased risk of adult MMD. Adding arginine and GABR to conventional risk factors could improve risk prediction for MMD. These findings suggest that plasma urea cycle metabolites might be potential biomarkers for the risk of MMD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Committee of Beijing Tiantan Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
XiY: data curation (lead) and writing of the original draft (lead). PG and DZ: conceptualization (equal) and methodology (equal). YuZ and WL: visualization (equal) and investigation (equal). QZ, XuY, and JZ: supervision (equal). XL, RW, and YaZ: software (equal) and validation (equal). All authors contributed to the article and approved the submitted version.
This study was supported by the National Key Technology Research and Development Programme of the Ministry of Science and Technology of China (2021YFC2500502).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1163733/full#supplementary-material
Ahmad, A. S., Shah, Z. A., and Doré, S. (2016). Protective role of arginase II in cerebral ischemia and excitotoxicity. J. Neurol. Neurosci. 7:88. doi: 10.21767/2171-6625.100088
Almannai, M., and El-Hattab, A. W. (2021). Nitric oxide deficiency in mitochondrial disorders: the utility of arginine and citrulline. Front. Mol. Neurosci. 14:682780. doi: 10.3389/fnmol.2021.682780
Bang, O. Y., Fujimura, M., and Kim, S. K. (2016). The pathophysiology of moyamoya disease: an update. J. Stroke 18, 12–20. doi: 10.5853/jos.2015.01760
Choi, J. W., Son, S. M., Mook-Jung, I., Moon, Y. J., Lee, J. Y., Wang, K. C., et al. (2018). Mitochondrial abnormalities related to the dysfunction of circulating endothelial colony-forming cells in moyamoya disease. J. Neurosurg. 129, 1151–1159. doi: 10.3171/2017.5.JNS17147
D' Avanzo, B., La Vecchia, C., Katsouyanni, K., Negri, E., and Trichopoulos, D. (1996). Reliability of information on cigarette smoking and beverage consumption provided by hospital controls. Epidemiology 7, 312–315. doi: 10.1097/00001648-199605000-00018
Fujimura, M., Sonobe, S., Nishijima, Y., Niizuma, K., Sakata, H., Kure, S., et al. (2014). Genetics and biomarkers of moyamoya disease: significance of RNF213 as a susceptibility gene. J. Stroke 16, 65–72. doi: 10.5853/jos.2014.16.2.65
Ge, P., Zhang, Q., Ye, X., Liu, X., Deng, X., Wang, J., et al. (2020). Modifiable risk factors associated with moyamoya disease: a case-control study. Stroke 51, 2472–2479. doi: 10.1161/STROKEAHA.120.030027
Geng, C., Cui, C., Guo, Y., Wang, C., Zhang, J., Han, W., et al. (2020). Metabolomic profiling revealed potential biomarkers in patients with moyamoya disease. Front. Neurosci. 14:308. doi: 10.3389/fnins.2020.00308
Greco, R., Demartini, C., Zanaboni, A. M., Blandini, F., Amantea, D., and Tassorelli, C. (2018). Endothelial nitric oxide synthase inhibition triggers inflammatory responses in the brain of male rats exposed to ischemia-reperfusion injury. J. Neurosci. Res. 96, 151–159. doi: 10.1002/jnr.24101
Grosse, G. M., Schwedhelm, E., Worthmann, H., and Choe, C. U. (2020). Arginine derivatives in cerebrovascular diseases: mechanisms and clinical implications. Int. J. Mol. Sci. 21:1798. doi: 10.3390/ijms21051798
Han, W., Jin, F., Zhang, H., Yang, M., Cui, C., Wang, C., et al. (2020). Association of brain-gut peptides with inflammatory cytokines in moyamoya disease. Mediat. Inflamm. 2020, 1–9. doi: 10.1155/2020/5847478
Hu, S., Png, E., Gowans, M., Ong, D. E. H., de Sessions, P. F., Song, J., et al. (2021). Ectopic gut colonization: a metagenomic study of the oral and gut microbiome in Crohn's disease. Gut Pathog. 13:13. doi: 10.1186/s13099-021-00409-5
Kim, E. H., Yum, M. S., Ra, Y. S., Park, J. B., Ahn, J. S., Kim, G. H., et al. (2016). Importance of RNF213 polymorphism on clinical features and long-term outcome in moyamoya disease. J. Neurosurg. 124, 1221–1227. doi: 10.3171/2015.4.JNS142900
Liang, M., Wang, Z., Li, H., Liu, B., and Yang, L. (2022). L-arginine prevents 4-hydroxy-2-nonenal accumulation and depresses inflammation via inhibiting NF-κB activation. J. Biochem. Mol. Toxicol. 36:e23087. doi: 10.1002/jbt.23087
Martínez-González, M., Ruiz-Canela, M., Hruby, A., Liang, L., Trichopoulou, A., and Hu, F. B. (2015). Intervention trials with the mediterranean diet in cardiovascular prevention: understanding potential mechanisms through metabolomic profiling. J. Nutr. 146, 913s–919s. doi: 10.3945/jn.115.219147
Mineharu, Y., Nakamura, Y., Sato, N., Kamata, T., Oichi, Y., Fujitani, T., et al. (2022). Increased abundance of Ruminococcus gnavus in gut microbiota is associated with moyamoya disease and non-moyamoya intracranial large artery disease. Sci. Rep. 12:20244. doi: 10.1038/s41598-022-24496-9
Miyatake, S., Miyake, N., Touho, H., Nishimura-Tadaki, A., Kondo, Y., Okada, I., et al. (2012). Homozygous c.14576G>a variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology 78, 803–810. doi: 10.1212/WNL.0b013e318249f71f
Morris, S. M. Jr. (2002). Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 22, 87–105. doi: 10.1146/annurev.nutr.22.110801.140547
Morris, S. M. Jr. (2016). Arginine metabolism revisited. J. Nutr. 146, 2579s–2586s. doi: 10.3945/jn.115.226621
Park, Y. S., Jeon, Y. J., Kim, H. S., Han, I. B., Choi, J. U., Kim, D. S., et al. (2014). The roles of methylenetetrahydrofolate reductase 677C>T and 1298A>C polymorphisms in moyamoya disease patients. Childs Nerv. Syst. 30, 1687–1695. doi: 10.1007/s00381-014-2495-3
Pencina, M. J., D'Agostino, R. B. Sr., D'Agostino, R. B. Jr., and Vasan, R. S. (2008). Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 27, 157–172. doi: 10.1002/sim.2929
Peng, R., Liu, K., Li, W., Yuan, Y., Niu, R., Zhou, L., et al. (2021). Blood urea nitrogen, blood urea nitrogen to creatinine ratio and incident stroke: the Dongfeng-Tongji cohort. Atherosclerosis 333, 1–8. doi: 10.1016/j.atherosclerosis.2021.08.011
Qiu, Y., Yang, X., Wang, L., Gao, K., and Jiang, Z. (2019). L-arginine inhibited inflammatory response and oxidative stress induced by lipopolysaccharide via arginase-1 signaling in IPEC-J2 cells. Int. J. Mol. Sci. 20:1800. doi: 10.3390/ijms20071800
Ranjbar, K. (2022). Improved cardiac function following ischemia reperfusion injury using exercise preconditioning and L-arginine supplementation via oxidative stress mitigation and angiogenesis amelioration. Cardiovasc. Toxicol. 22, 736–745. doi: 10.1007/s12012-022-09752-8
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis and Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases (2012). Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med. Chir. (Tokyo) 52, 245–266. doi: 10.2176/nmc.52.245
Scott, R. M., and Smith, E. R. (2009). Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 360, 1226–1237. doi: 10.1056/NEJMra0804622
Sivashanmugam, M., Jaidev, J., Umashankar, V., and Sulochana, K. N. (2017). Ornithine and its role in metabolic diseases: an appraisal. Biomed. Pharmacother. 86, 185–194. doi: 10.1016/j.biopha.2016.12.024
Su, X., Li, X., Wang, H., and Cai, Z. (2021). Simultaneous determination of methionine cycle metabolites, urea cycle intermediates and polyamines in serum, urine and intestinal tissue by using UHPLC-MS/MS. Talanta 224:121868. doi: 10.1016/j.talanta.2020.121868
Sun, R., Li, Y., Cai, M., Cao, Y., and Piao, X. (2019). Discovery of a new biomarker pattern for differential diagnosis of acute ischemic stroke using targeted metabolomics. Front. Neurol. 10:1011. doi: 10.3389/fneur.2019.01011
Suzuki, J., and Takaku, A. (1969). Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 20, 288–299. doi: 10.1001/archneur.1969.00480090076012
Takeda, M., Tezuka, T., Kim, M., Choi, J., Oichi, Y., Kobayashi, H., et al. (2020). Moyamoya disease patient mutations in the RING domain of RNF213 reduce its ubiquitin ligase activity and enhance NFκB activation and apoptosis in an AAA+ domain-dependent manner. Biochem. Biophys. Res. Commun. 525, 668–674. doi: 10.1016/j.bbrc.2020.02.024
Tang, W. H., Wang, Z., Cho, L., Brennan, D. M., and Hazen, S. L. (2009). Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J. Am. Coll. Cardiol. 53, 2061–2067. doi: 10.1016/j.jacc.2009.02.036
Wang, Y., Yang, L., Wang, X., Zeng, F., Zhang, K., Zhang, Q., et al. (2021). Meta-analysis of genotype and phenotype studies to confirm the predictive role of the RNF213 p.R4810K variant for moyamoya disease. Eur. J. Neurol. 28, 823–836. doi: 10.1111/ene.14635
Wu, Z., Jiang, H., Zhang, L., Xu, X., Zhang, X., Kang, Z., et al. (2012). Molecular analysis of RNF213 gene for moyamoya disease in the Chinese Han population. PLoS One 7:e48179. doi: 10.1371/journal.pone.0048179
You, S., Zheng, D., Zhong, C., Wang, X., Tang, W., Sheng, L., et al. (2018). Prognostic significance of blood urea nitrogen in acute ischemic stroke. Circ. J. 82, 572–578. doi: 10.1253/circj.CJ-17-0485
Yu, E., Ruiz-Canela, M., Hu, F. B., Clish, C. B., Corella, D., Salas-Salvadó, J., et al. (2017). Plasma arginine/asymmetric dimethylarginine ratio and incidence of cardiovascular events: a case-cohort study. J. Clin. Endocrinol. Metab. 102, 1879–1888. doi: 10.1210/jc.2016-3569
Zhang, Q., Liu, Y., Zhang, D., Wang, R., Zhang, Y., Wang, S., et al. (2017). RNF213 as the major susceptibility gene for Chinese patients with moyamoya disease and its clinical relevance. J. Neurosurg. 126, 1106–1113. doi: 10.3171/2016.2.JNS152173
Zhao, Y., Li, J., Lu, J., Zhang, Q., Zhang, D., Wang, R., et al. (2019). Predictors of neoangiogenesis after indirect revascularization in moyamoya disease: a multicenter retrospective study. J. Neurosurg. 132, 98–108. doi: 10.3171/2018.9.JNS181562
Keywords: moyamoya disease, urea cycle, ornithine, arginine, risk factors
Citation: Yu X, Ge P, Zhai Y, Liu W, Zhang Q, Ye X, Liu X, Wang R, Zhang Y, Zhao J and Zhang D (2023) Plasma urea cycle metabolite levels and the risk of moyamoya disease. Front. Neurosci. 17:1163733. doi: 10.3389/fnins.2023.1163733
Received: 11 February 2023; Accepted: 21 June 2023;
Published: 10 July 2023.
Edited by:
Carla Ines Tasca, Federal University of Santa Catarina, BrazilReviewed by:
Yohei Mineharu, Kyoto University, JapanCopyright © 2023 Yu, Ge, Zhai, Liu, Zhang, Ye, Liu, Wang, Zhang, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peicong Ge, Z2VwZWljb25nQDE2My5jb20=; Dong Zhang, emhhbmdkb25nMDY2MEBhbGl5dW4uY29t
†ORCID: Xiaofan Yu, https://orcid.org/0000-0001-9665-7805
Peicong Ge, https://orcid.org/0000-0002-5963-9680
Dong Zhang, https://orcid.org/0000-0002-0801-4971
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.