- 1Department of Physiology and Pathophysiology, College of Medicine, Yanbian University, Yanji, Jilin, China
- 2Interdisciplinary Program of Biological Functional Molecules, College of Intergration Science, Yanbian University, Yanji, Jilin, China
- 3State Key Laboratory of Medical Neurobiology, Institutes of Brain Science, Fudan University, Shanghai, China
- 4Department of Environmental Science, Department of Chemistry, Yanbian University, Yanji, Jilin, China
Rapid progress in nanotechnology has advanced fundamental neuroscience and innovative treatment using combined diagnostic and therapeutic applications. The atomic scale tunability of nanomaterials, which can interact with biological systems, has attracted interest in emerging multidisciplinary fields. Graphene, a two-dimensional nanocarbon, has gained increasing attention in neuroscience due to its unique honeycomb structure and functional properties. Hydrophobic planar sheets of graphene can be effectively loaded with aromatic molecules to produce a defect-free and stable dispersion. The optical and thermal properties of graphene make it suitable for biosensing and bioimaging applications. In addition, graphene and its derivatives functionalized with tailored bioactive molecules can cross the blood–brain barrier for drug delivery, substantially improving their biological property. Therefore, graphene-based materials have promising potential for possible application in neuroscience. Herein, we aimed to summarize the important properties of graphene materials required for their application in neuroscience, the interaction between graphene-based materials and various cells in the central and peripheral nervous systems, and their potential clinical applications in recording electrodes, drug delivery, treatment, and as nerve scaffolds for neurological diseases. Finally, we offer insights into the prospects and limitations to aid graphene development in neuroscience research and nanotherapeutics that can be used clinically.
1. Introduction
Nanotechnology has nanometric resolution referring to the length of a billionth of a meter (Linda and Wade, 2017). This engineering technology allows device manipulation to a microscopic structure that suits the basic orders of biomolecule systems. With their controllable nanoscale properties, scientists have exploited various synthetic materials and devices for treating and monitoring pathological conditions in biomedicine.
A size change in a nanostructure induces variations in its functional properties (Berube, 2006), showing great potential for evaluating and solving several neurological problems, from neurons to neurological diseases. Nanomaterials can be used to construct biomimetic neural networks in vitro because of their physical and chemical properties (Malarkey and Parpura, 2010). Moreover, nanomaterials can be used for targeted drug delivery in vivo with minimal side effects through the fabrication method to improve the efficacy of cancer chemotherapy and enhance the efficiency of magnetic resonance imaging-guided gene delivery as radiation sensitizers and contrast enhancers (Nezakati et al., 2014). Driven by the lack of precise diagnosis and therapeutic strategies in the clinic (Shi et al., 2013), the development of neural nanotechnology and nanomaterials used for studying the mechanisms, diagnosis, and treatment of nervous system disorders has to breakthroughs in using carbon nanomaterials.
Two-dimensional (2D) materials have been extensively explored for biomedical applications (Convertino et al., 2020b). With super structural rigidity and a good surface-to-volume ratio, 2D materials provide maximal interaction between the environment and surfaces, allowing for high sensitivity and rapid performance with small sample volumes (Palumbo et al., 2018). Andre Geim and Konstantin Novoselov studied graphene and successfully separated it from graphite in 2004 (Novoselov et al., 2004). Graphene is the most widely studied nanomaterial for cell-based device platforms with peculiar properties, including its role as a polymeric conduit for nerve regeneration, carrier for targeted drug delivery, and for photo-thermal cancer therapy. Recently, highlighting the role of graphene in biomedical fields has inspired great interest in its analogs, such as transition metal dichalcogenide (Convertino et al., 2020b). However, compared with other nanomaterials, including carbon nanotubes and transition metal dichalcogenide, graphene has higher mechanical strength, chemical stability, and specific electrochemical properties for electrical signal detection and transmission. Furthermore, it can be used as a conductive stent or biosensor in the medical field. These characteristics make graphene and graphene-derived materials excellent candidates for detecting signals in the nervous system.
Previous studies have shown that graphene can aid in the adhesion and differentiation of neurons and can act as a neuroprotective agent. Graphene is a potential carrier to control drug release at specific disease sites, making it a valuable treatment strategy for neuronal diseases. Moreover, graphene and its derivatives can be used as scaffolds to enhance nerve repairing, establishing it as a next-generation candidate for advancing medical bioengineering. However, the safety of nanomaterials is essential to protect human health and ensure environmental safety. Thus, further studies are warranted to fill the knowledge gap on the limitations of carbon nanomaterials, including their toxicity and biocompatibility (Nezakati et al., 2014).
In this review, we focus on novel graphene applications in the nervous system. Graphene-based materials may be used clinically in the future; however, this article is limited to pre-clinical applications. We aimed to describe the properties of graphene-based materials applicable in neuroscience research, including using them as electrodes for neural recording and imaging, neural cell substrates, photothermal effects, and drug and gene deliveries. Furthermore, we aimed to outline the application of graphene-based materials in neurological diseases and neural tissue engineering. Finally, we aimed to discuss graphene’s cytotoxicity and provide an overview of its prospects.
2. Roles of graphene in neuroscience
Graphene is a sheet comprising a single layer of carbon atoms formed by stripping highly oriented pyrolytic graphite, like carbon nanofibers, another type of nanomaterial. In 2D, sp2 hybridized carbon atoms give graphene a hexagonal honeycomb lattice pattern with a thickness of approximately 0.335 nm [Figure 1 (left)]. Graphene normally can be synthesized by two main approaches, namely physical and chemical approaches. The chemical vapor deposition (CVD) technique has been commonly applied to produce large-scale, high-quality graphene film with no oxygen content, as well as defect-free hexagonal lattice (Reina et al., 2009). Hong et al. employed the CVD approach to generate large-scale and few-layer graphene films on a 300 nm-thick layer and then transferred them to a SiO2/Si substrate. Bea et al. developed monolayer graphene films of 30 inches by the CVD approach on a large copper substrate. The films showed low resistance of 125 Ω·m−1, superior to graphene grown on indium tin oxides. Unfortunately, this technique requires a very high temperature and vacuum environment, as well as exorbitant prices for only small-size devices. Many graphene-based nanomaterials are built up mostly by chemical reactions for covalent bonding, such as graphene oxide [GO; Figure 1 (middle)] and reduced graphene oxide [rGO; Figure 1 (right)]. The most popular approach to prepare graphene for bio-neurological studies is the chemical reduction of graphite GO to rGO, which involves severe oxidation of the material, followed by sonicated irritation to exfoliate the GO and reduction by chemical or thermal processes. Hummer’s method is widely used to produce GO by reacting graphite flakes with oxidizing agents/acids (Hummers and Offeman, 1958). In terms of health issues, especially in the biomedical field, green synthesis has gained considerable interest by using green reductants, such as L-ascorbic acid, D-glucose, and tea polyphenol (Xu et al., 2015).
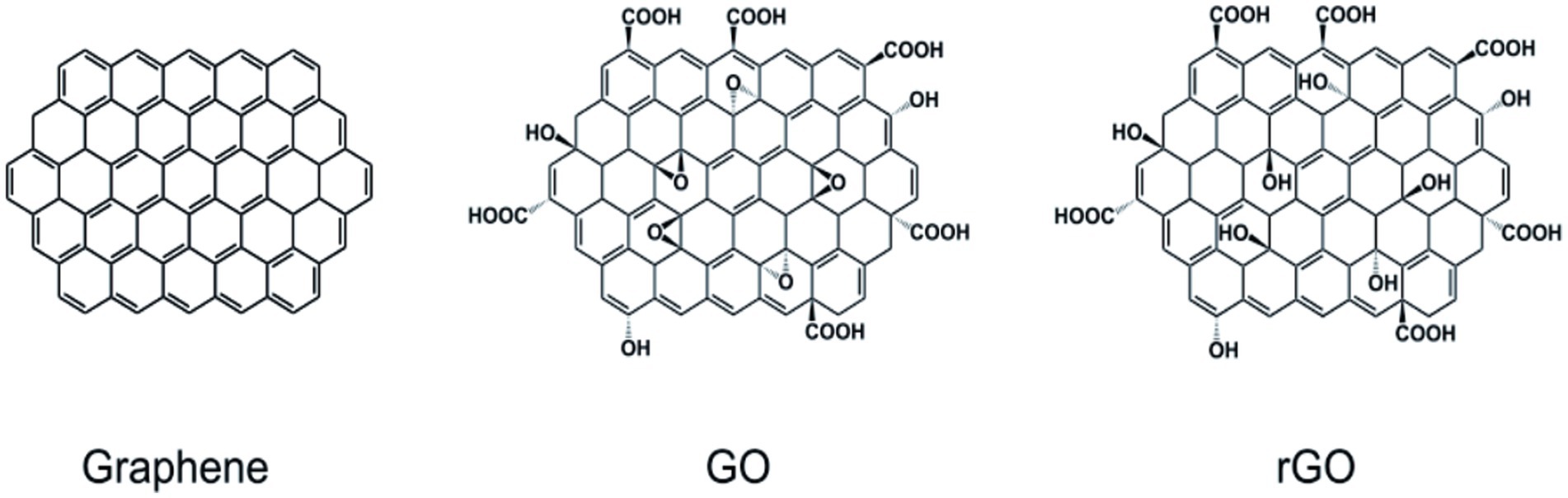
Figure 1. Structure diagram of graphene and other forms. (left) graphene; (middle) graphene oxide; (right) reduced graphene oxide. Kitko et al. (2018), Bramini et al. (2016), Ding et al. (2020), López-Dolado et al. (2016), and Serrano et al. (2018) with permission from Elsevier Ltd., copyright 2016.
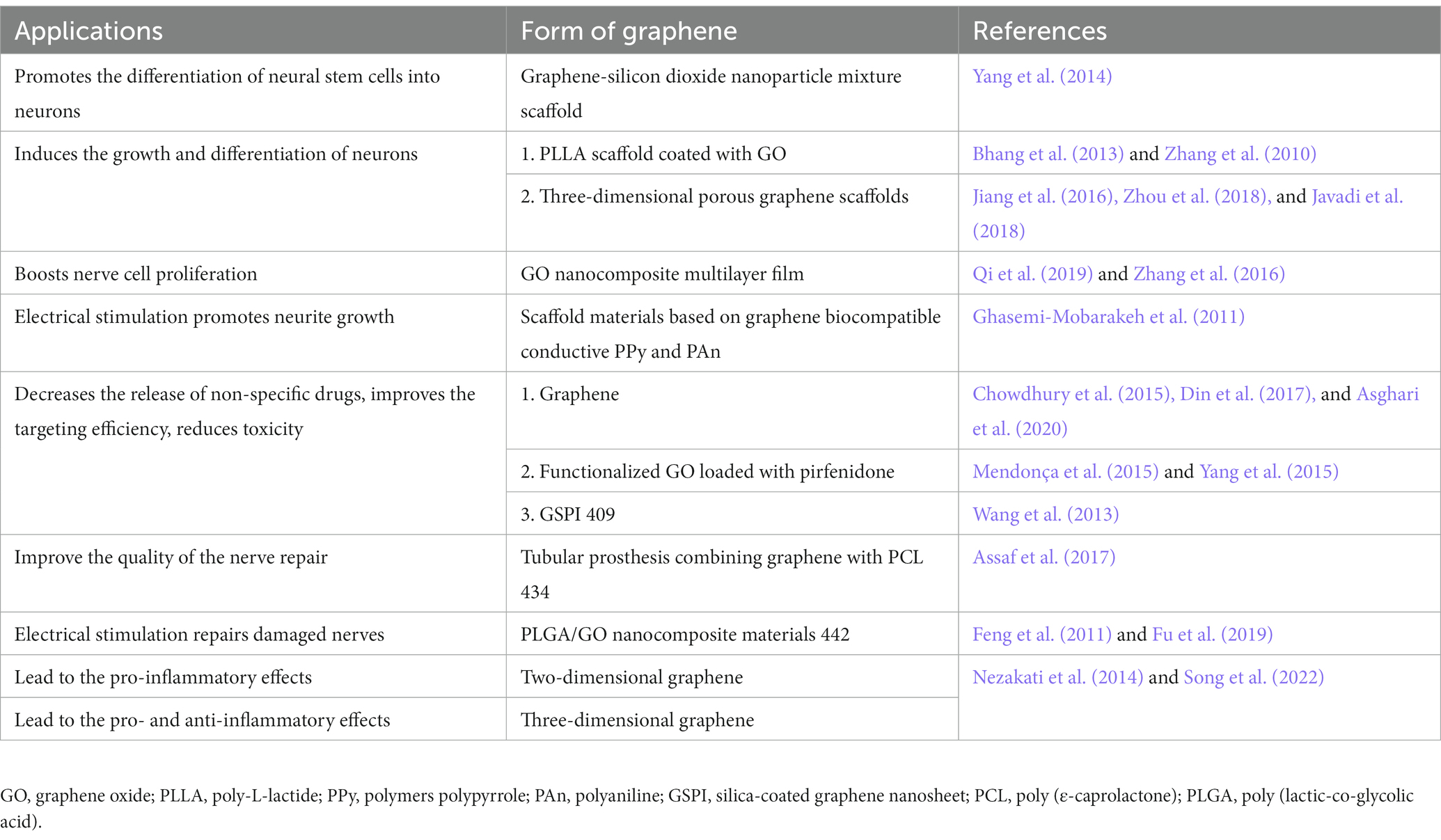
At room temperature, the interactions between carbon atoms in graphene are strong. Electrons in the carbon atoms are not easily affected or scattered by the movement of surrounding carbon atoms; its conductivity also contributes to electron transfer (Bramini et al., 2016). Additionally, its large surface area and potential for binding different biomolecules onto its surface make graphene a suitable nanomaterial for holding small-molecule drugs, genes, proteins, deoxyribonucleic acid (DNA), and small interfering ribonucleic acid (RNA) (Mousavi et al., 2019; Ma et al., 2014). In neuroscience, the electrical conductivity, biocompatibility, and mechanical, thermal, and optical properties of graphene (Table 1) are its most important characteristics. Graphene can enhance the properties of nanomaterials for various biomedical applications, including electrodes for neural recording, stimulation, and imaging (Sun et al., 2008; Wei and Wang, 2021), neural cell and tissue scaffolding (Lee et al., 2011), photothermal therapy (PTT) (Robinson et al., 2011; Yang et al., 2012a; Peng et al., 2023), and drug delivery (Mauro et al., 2020; Dash et al., 2021). Recently, researchers have discovered new applications of graphene in nerve tissue engineering (Magaz et al., 2021) and neurological diseases, such as brain tumors, Alzheimer’s disease (AD), and Parkinson’s disease (PD) (John et al., 2015; Cho et al., 2022). These properties and applications suggest that graphene has a promising role as a biomaterial in neuroscience.
3. Mechanical properties and electrical conductivity of graphene in neural electrodes
Neural interfaces are typically implanted with electrodes that can record or stimulate target tissues or cells in the brain, thereby establishing connections with the nervous system (Keefer et al., 2008). However, current electrode materials and technology have limitations. The formerly used metal electrode has poor stability, high hardness, and greater electrical noise, and its insertion may lead to mechanical damage of neurons and surrounding soft tissues, obstructing electrophysiological recording (Szarowski et al., 2003). The sp2 bond structure provides graphene with high resistance to elongation, with Young’s modulus of 1,100 GPa and fracture strength of 125 GPa (Lee et al., 2008). Bending the graphene sheet does not result in considerable distortion of these bonds. Thus, graphene-based electrodes are perfect for touching soft samples like nervous tissues (Palermo et al., 2016). Young’s modulus ranges from 100 Pa to 10 kPa, which results in great tensile stiffness and strength while remaining highly flexible. Moreover, the unique structure of conjugated sp2 bond in the graphene plane excites electrons from the valence to conduction bands with near-zero energy, like the zero band gap nature of metal, giving graphene an outstanding electrical conductivity (Jiang et al., 2007; Li et al., 2009). With great electrical conductivity and high mechanical flexibility, graphene can be a suitable biomaterial as a neuronal interface for stimulating and recording brain signals.
Graphene can be mechanically deformed beyond the linear regime (Lee et al., 2008); this is particularly important for increasing mechanical compliance in in vivo microscopy techniques performed on freely behaving animals. However, one of the major problems of using graphene in in vivo studies is that it is ultra-flexible and difficult to implant surgically. Kuzum et al. (2014) fabricated graphene electrodes on flexible polyimide substrates, increasing its stiffness for implantation the brain without causing discomfort. Applying oxygen plasma etching to pattern graphene could also provide stiffness to the electrode, allowing it to penetrate brain tissues. An alternative method is using a stiff carrier microneedle that attaches the flexible electrode, aiding it to reach the desired depth (Kim T. I. et al., 2013). The microneedle is disengaged from the flexible substrate and withdrawn from the tissue once it reaches the required depth. This procedure only causes minimal invasive operation; however, the back-and-forth motion may increase the risk of inflammation during insertion. Researchers have recently exploited temporary stiffening of graphene electrodes with silk fibroin, which provides a rigid structure that reaches the desired depth (Weltman et al., 2016). The silk fibroin coating dissolves quickly after insertion, enabling the graphene electrodes to return to their original flexibility.
Unfortunately, scaling down the microelectrode dimension while sustaining a high signal-to-signal ratio (SNR) for recording cellular activity is challenging. Studies have demonstrated effective in vivo neural recording by graphene electrodes compared to gold electrodes (Kuzum et al., 2014; Park et al., 2014). In these studies, the relatively low capacitance of a few graphene layers led to a high impedance with large noise, which is unfavorable for maintaining high SNR in microelectrode recording. Common methods for enhancing graphene conductivity use chemical doping approaches, such as micropatterning, to increase the number of active sites. Qu et al. (2010) demonstrated that using graphene doping with nitrogen-containing compounds could increase the electroactivity of graphene-based electrodes. Moreover, researchers have fabricated graphene with conducting polymers, such as polystyrene sulfonate, polypyrrole, and poly-3,4-ethylene dioxythiophene; the synergistic effect increases charge transfer on the graphene surface. With great mechano-electrical stability, this electrode accurately recorded electrophysiological signals of brain activity with increased SNR (Zhao et al., 2021; He et al., 2022). Recently, Bonaccini Calia et al. (2022) designed a linear array of graphene neural depth micro-transistors to increase electrical conductivity and demonstrated that this microelectrode could concurrently and stably record direct current-shifts and high-frequency neuron activity in awake rodents, which is consistent with the results of Masvidal-Codina et al. (2021). By decreasing the impedance between graphene and electrolyte, Chen et al. (2013) fabricated a graphene surface with a mild steam plasma, thus increasing the SNR during neural recording.
The mechanical properties, electrical conductivity, and large surface area of graphene make it perfect material for developing ideal neural interfaces and more reliable electrodes, while highlighting the unique advantages of composites, demonstrating greater application potential in neural interfaces. However, more studies are required to investigate the long-term effects of graphene-based nanoelectrodes during in vivo recording validation.
4. The optical property of graphene in neural imaging and photothermal therapy
Traditional metal electrodes used for recording electrophysiological activities obstruct the vision field, produce optical shadows, and are more likely to introduce light-induced artifacts during the recording. Researchers have discovered that transparent graphene could solve this problem due to the unique band structure in the transmission spectrum of graphene that prevents the mentioned limitations. Theoretically, graphene allows the hot ballistic charge carriers produced by light (Gabor et al., 2011) to transfer their energy in a highly efficient carrier-to-carrier scattering process, with a mean path that can reach approximately 1 μm (Novoselov et al., 2004).
Graphene is highly transparent and absorbs 2.3% of incident visible light radiation (Kang et al., 2016), which allows for optimal optical access and avoids photoelectrical artifacts. These advantages are excellent in optical imaging applications, offering substantial improvements over conventional metal electrodes for the three-dimensional (3D) imaging application. Graphene enables spatial–temporal resolution for simultaneous imaging while recording physiological activity from the same target, especially for studying the wiring of neural circuits. Kuzum et al. (2014) recorded calcium fluorescence images via graphene electrodes in the dentate gyrus region without inducing artifacts from laser light. They observed overlapping neural cell bodies around the electrode while recording synaptic potential in vivo. Driscoll et al. (2021) used transparent graphene microelectrode arrays and successfully recorded simultaneous high-bandwidth electrophysiology and calcium imaging signals of brain network activity induced by epileptic seizures in vivo. Moreover, these cellular-scale methods enable the recording of electrophysiology and calcium fluorescence in CA1 pyramidal neurons in hippocampus seizures (Mulcahey et al., 2022). Moreover, inspired by the accomplishments of brain studies, integrating a transparent graphene sensor onto a designed abdominal window detected changes in the activity of the enteric nervous system through simultaneous optical and electrical recording in a live mouse (Rakhilin et al., 2016). From monitoring the activity of isolated neurons to forming intermuscular nerve plexus, this approach can help understand how the nervous system processes information. Thus, this technology can decipher and intervene in sequential spatiotemporal patterns of how the temporal progression of electrophysiology changes are associated with the spatial evolution of recruited core during brain disease onset and evolution.
Graphene exhibits a resonant response to any frequency photons in the ultra-broadband spectral spectrum from ultraviolet to infrared (IR) region (Wang et al., 2008). Recently, Savchenko et al. (2018) introduced a graphene-based optical actuator that uses light exposure to optically stimulate cells in a non-invasive manner, causing membrane depolarization. However, as a biomaterial, the optical absorption is low at 2.3%. Researchers have discovered that changing the physical architecture of graphene by increasing its flake or density can increase its optical absorption. For example, Rastogi et al. (2020) composed 3D fuzzy graphene with an out-of-plane shape, demonstrating great photothermal effects due to its high optical absorption in the near-IR (NIR) regime and non-invasively stimulating and mediating electrophysiological activity of neural spheroids. One practical concern about these optical stimulation methods is the possibility of off-target or adverse consequences due to the temperature increase in the surrounding neurons. Therefore, minimizing temperature fluctuations is preferable if the neurons are optically excitable. Anchoring graphene-based materials with appropriate biological ligands can specifically target different neural cells.
PTT has received widespread attention because it can thermally ablate cancer cells in a minimally invasive manner under an NIR laser. The broad optical absorption spectra are important in photothermal medical applications. Nanomaterials with strong absorption in the NIR regime can act as effective PTT absorbers that achieve therapeutic temperatures with less total light energy, reducing heat escaping from the target to healthy surrounding tissue and avoiding tissue damage. GO and rGO have been widely exploited as effective absorbers for NIR-based PTT in cancer therapy (Yang et al., 2012b; Akhavan and Ghaderi, 2013a). Clinical treatment requires specific and high-quality photothermal effects. Conjugating nanomaterials surface with modifying molecules, such as polymers and antibodies, can solve this problem. Yang et al. (2010) discovered that GO-polyethylene glycol (PEG) composite nanomaterials combined with the NIR fluorescent dye Cy7 could be successfully localized in target tumor in vivo and achieved ultra-efficient tumor ablation after laser treatment. Similarly, Akhavan et al. (2012) attached rGO-PEG with arginine-glycine-aspartic acid-based peptide and Cy3 to successfully target human glioblastoma cell line U87MG. This material exhibited high NIR absorption and resulted in 97% cell destruction in vitro. However, the rGO-PEG showed concentration-dependent cyto- and geno-toxicity. No toxic signal was found in the histological examination of GO-PEG during PTT by Liu operation. Another transition metal disulfide that is attracting more interest in the biomedical field is Tungsten disulfide because of its photothermal properties, high lubricity, and catalytic activity. Its high NIR absorption capacity has been applied to photothermal therapy of tumors (Appel et al., 2016). The development of graphene optical properties has promoted the in vitro and in vivo research of carbon nanomaterials (including graphene, carbon nanotubes, and diamond).
Photoacoustic and fluorescence imaging can also be combined with PTT for diagnosis. Photoacoustic imaging has few specific signals and cannot image deep tissues. Fluorescence imaging does not use ionizing radiation and has low tissue penetration. Thus, further research is needed to fully understand how to apply carbon nanomaterials better and fully in neurology, as their functions involve the interaction between their various properties. Therefore, improving the functionalization of nanomaterials, which can create better and less risky options for patients during clinical treatment of diseases, remains challenging.
5. Thermal property of graphene in photothermal therapy
Graphene has high thermal conductivity (κ) and great thermal management potential, indicating its development in nanoscale engineering for heat transport and management. Ma et al. (2017) found that the thermal conductivity of graphene significantly reduced with decreasing grain size by a thermal boundary of ~3.8 × 109 W m−2 K−1. Kumar et al. (2015) reported that large-area GO produced a very high thermal conductivity of 1,390 Wm−1 K−1.
Researchers have suggested various effective approaches for modulating the thermal conductivity of graphene. Depositing nanoparticles on the surface of graphene can mediate its thermal management (Wang J. et al., 2013; Sadrolhosseini et al., 2019; Sun et al., 2022). Chemical functionalization, such as through small coverage of fluorine (Chien et al., 2011; Pei et al., 2011), hydroxyapatite (Nie et al., 2022), polymer (Wang et al., 2020, Kang et al., 2022, Lei et al., 2022, Tan et al., 2022), hydrogen (Zhang et al., 2010, Jarrahian and Heidaryan, 2012, Zhang and Liu, 2019), and phenol (Bernal et al., 2018), has been theoretically used to significantly enhance graphene’s thermal conductivity.
As thermal stability improves, graphene use has risen in the fields of clinical biomedical application and tailored nanomedicine for neurologic diseases. For example, graphite lattices in the graphene family of nanomaterials fuse to form disparate forms of molecules, with a potential role in drug and gene delivery for treating neurological diseases. Using two complementary strategies for conferring static or spatial stability can improve the thermal stability of graphene and allow cells to occupy stable graphene sheets (Hong et al., 2012). In 2008, Liu et al. (2008) first synthesized GO-PEG composite to deliver the anticancer drug SN38 into the cancer cell interior. Zhang et al. (2010) further conjugated GO with folic acid and loaded it with multiple anticancer drugs to specifically target human MCF-7 cells. In addition to the delivery of therapeutic molecules, GO-conjugated polyethyleneimine (PEI) loaded with small interfering RNA or plasmid DNA could be used to reduce its target gene expression (Feng et al., 2011; Kim et al., 2011; Zhang et al., 2011). Additionally, Barrera et al. (2020) functionalized rGO with iron oxide nanoparticle deposition, which increased thermal temperature. GO functionalized with ferrimagnetic vortex-domain iron oxide also showed high thermal conversion efficiency in vivo (Liu et al., 2020). The composite rGO-Fe3O4 increased the temperature by approximately 50°C in 5 min, which is considered the cell-killing temperature, meaning it can accomplish advanced PPT. Cao et al. (2017) reported a system with GODs-PEG and porphyrin derivate (P). A GQD-PEG-P solution of 100 μg/mL increased the temperature to 53.6°C while maintaining the temperature of control water at 33.2°C. GOD-PEG-P combined with PTT demonstrated excellent efficiency in killing cancer cells in vivo and in vitro. AD is characterized by neuronal loss in the cerebral cortex and subcortical areas and amyloid β-protein (Aβ) accumulation. Recently, Wang et al. (2021) demonstrated that GO loaded with dauricine could inhibit the aggregation and misfolding of Aβ. Moreover, graphene-based PTT can effectively dissociate the Aβ fibrils upon NIR laser irradiation (Li et al., 2012).
These findings provided new ideas for combining PTT and graphene-based drug/gene delivery therapy and demonstrated a significant improvement over traditional surgical treatment method. Graphene-based materials showed high potential for the novel and efficient treatment of neurological disorders. However, the interaction between graphene and cells may interfere with the different cell viability reduction rates in thermal therapy (Barrera et al., 2020). Future research should focus on the molecular and cellular effects of graphene composites in thermal and delivery therapy, given its promising results.
6. Biocompatibility and toxicity of graphene in neural interaction
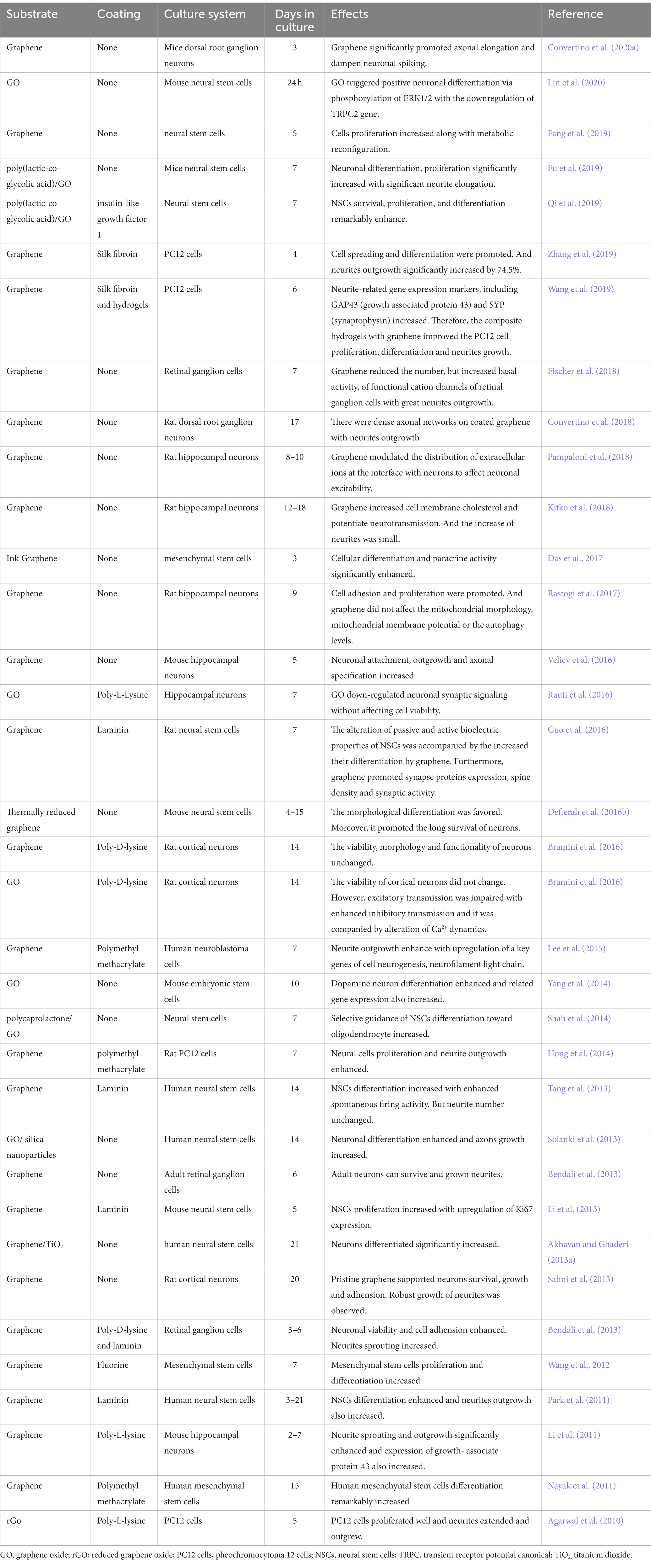
The first aspect to consider when developing graphene-based biomedical applications is their biocompatibility to avoid any adverse effects on living tissues (Li et al., 2011). Graphene is highly biocompatible, making it an ideal choice for neural interface materials in biological tissues (Sahni et al., 2013; Fanizza et al., 2022). Furthermore, graphene can improve the efficiency of implants or scaffold materials to enhance cell differentiation and proliferation owing to its unique biocompatibility (Lee et al., 2011; Ulloa Severino et al., 2016; D’Abaco et al., 2018). However, the biocompatibility of graphene depends on its functionalization, which can also improve the biocompatibility of graphene-based materials. For example, chitosan is another biocompatible material, and its composite, chitosan-GO, showed better biocompatibility than GO scaffolds alone, with enhanced cell infiltration and reduced inflammatory reactions (Vlasceanu et al., 2020). Additionally, the hydrogen bond interaction between chitosan-GO composites can enhance the regeneration and activity of nerve cells (López Tenorio et al., 2019). Hydrogel-containing graphene are synthetic materials similar to living tissues and can promote neuronal regeneration and differentiation, which is valuable in developing synthetic materials for engineering neuronal tissues (Javadi et al., 2018).
Graphene biocompatibility and its effects on the performance of living cells have shown that graphene on top of matrix substrates does not impede the interaction between neurons and substrates, thus maintaining neuronal viability (Fischer et al., 2018). Li et al. (2011) investigated how graphene substrates affect neuritis, an important structure for neural functions, during the maturation of mouse hippocampal cultures compared to tissue culture polystyrene (TCPS) substrates to address the detailed interaction between graphene and tissues/cells in the neural system. The results showed that graphene, as a biocompatible matrix, effectively stimulated neurite sprouting and outgrowth. Neurons started to extend their neurites toward the periphery after being cultured on the graphene substrates (Figure 2A). The phase-contrast micrograph indicates that the original trajectory extended along the neurite to calculate its length. As shown in Figure 2B, neurite numbers in each cell on the graphene substrate significantly increased between 2–7 days, compared with those with TCPS treatment. The neurite lengths also increased with graphene and TCPS, but the average length with graphene was significantly longer than that with TCPS (Figure 2C).
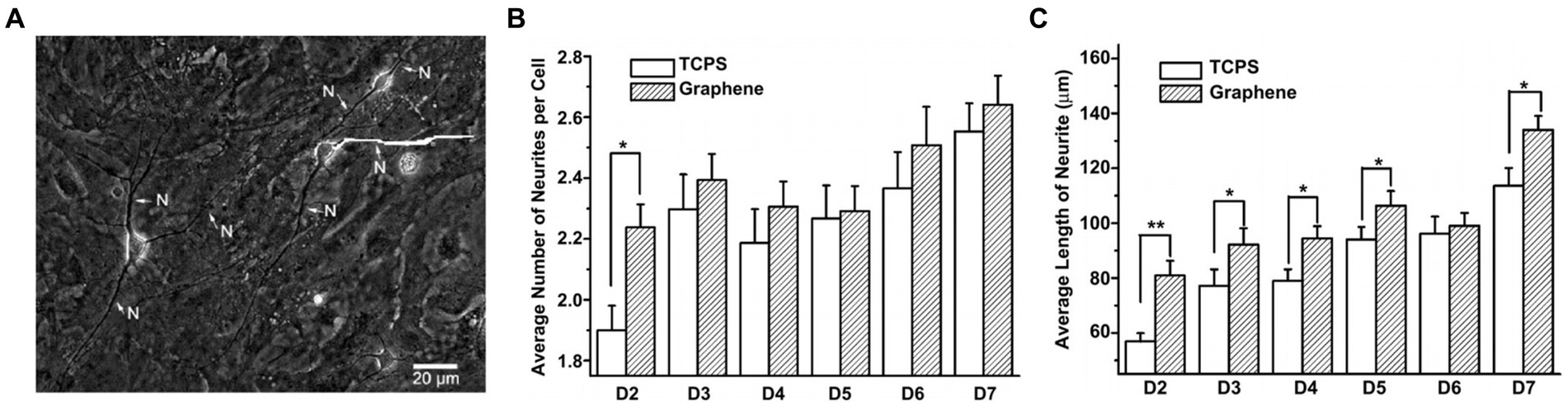
Figure 2. (A) Phase-contrast microscopic image of neurons. (B) The average number of neurites per cell on graphene and TCP. (C) The average length of neurite on graphene and TCP. Data are expressed as mean ± SEM (n = 288 for TCPS and n = 315 for graphene, **p < 0.01). Li et al. (2011) with permission from Elsevier Ltd., copyright 2011.
However, in vivo studies have demonstrated that the immune system is highly sensitive to graphene and quickly responds by increasing anti-inflammatory cytokine synthesis (Duch et al., 2011; Dudek et al., 2016). Low concentration of graphene, GO and rGO caused serious inflammation in rats (Wang Y. et al., 2011; Schinwald et al., 2012; Chortarea et al., 2022) and perturbed locomotor behavior in an in vivo zebrafish model (Cellot et al., 2020). Graphene functionalization is also used to minimize its adverse effects in in vivo settings. Fabricating graphene with poly(ε-caprolactone) caused an acceptable degree of immunological response in vivo (Wang W. et al., 2019). Hung et al. (2021) fabricated GO with gold and observed incredible immunological compatibility and anti-inflammatory effects in vivo. GO coated with biomimetic baicalin effectively ameliorate the inflammatory response in vivo (Guo et al., 2021). Furthermore, 500 mg/kg dose of dextran-coated GO had no significant effect on the immune system (Kanakia et al., 2014), and GO-PEG showed normal values of blood hematology analysis (Yang et al., 2013; Chong et al., 2014). Recently, Ding et al. (2020) reported a time- and dose-dependent, stealth-but-activating effect of GO-PEG on macrophages, and a low dosage of GO-PEG resulted in a mild, transient, and bearable immunological response in vivo. Feito et al. (2019) demonstrated the mechanism underlying macrophage response to GO-PEG labeled with fluorescein isothiocyanate. GO-PEG labeled with fluorescein isothiocyanate does not cause macrophage polarization and promote the M1/M2 balance, ensuring an appropriate immune response, which is consistent with in vivo studies (Lee et al., 2008, 2020; Hung et al., 2021; Zou et al., 2021). Kurapati et al. (2018) showed that graphene could be degraded by myeloperoxidase from active neutrophils in vivo. The biodistribution of graphene quantum dots (GODs) in in vivo mouse experiments did not accumulate in main organs with fast kidney clearance, probably due to their ultra-small size (Kanakia et al., 2014).
Of note, graphene-based materials in some medical and non-medical fields may exhibit toxicity in human cells, leading to cell death. For example, Jagiełło et al. (2019) investigated the in vitro effects of GO and rGO on human umbilical cord Wharton’s jelly-derived mesenchymal stem/stromal cells. GO had no cell toxicity despite its flake size. GO and rGO induced a prominent toxicity phenomenon in a dose- and time-dependent manner. Moreover, GO induced stronger toxicity to the cells than rGO (Wang K. et al., 2011; Kang et al., 2017). Interestingly, rGO with high reduction levels decreased cell proliferation and promoted cell necrosis. A study on graphene toxicity-induced cell necrosis reported that the mechanism underlying graphene toxicity is mainly related to reactive oxygen species production in cells, leading to DNA and protein damage and subsequently cell death through the necrosis pathway (Chatterjee et al., 2015; Sawosz et al., 2015). Functionalization of GO and rGO using different surfactants plays a significant role in reducing toxicity. Wojtoniszak et al. (2012) investigated GO and rGO toxicities using three surfactants, including PEG, sodium deoxycholate, and polyethylene glycol–polypropylene glycol–polyethylene glycol (Pluronic P123). GO functionalized with PEG had the lowest toxicity (36.3%) compared with other surfactants. Cytotoxicity analysis revealed that toxicity depends on the type of surfactants and concentration of nanomaterials to maintain stabilization and avoid agglomeration. Recently, Dybowska-Sarapuk et al. (2020) suggested that reducing the number of functionalized surfactants of graphene to a proper range could reduce the risk of induced cytotoxicity.
rGO injection at centration of 0.004 μg/μL into the core of olfactory bulb in vivo does not affect de novo neurogenesis and neuronal survival, nor produce immune response (Defteralı et al., 2016a). Intracerebral injection of GO in vivo does not induce neurotoxic effects and acute neuroinflammatory in brain structure (Portioli et al., 2020). Intratracheal injection of GO at low concentration in mice caused persistent and severe pulmonary injuries (Duch et al., 2011; Singh et al., 2011). Oral administration of GO at a dose of 0.5–100 μg/mL in Caenorhabditis elegans caused irreversible damage to the nervous system, which is closely related to reactive oxygen species production (Wu et al., 2013; Zhao et al., 2018). Overall, toxicity may be attributed to the interaction between some physical and chemical properties of graphene materials, such as the dose, size, shape, type of functional groups, reactive oxygen species production, and administration route (intravenous or oral).
Another popular member of the carbon nanomaterials family, carbon nanotubes demonstrated no major toxicity on cell lines, organotypic slice cultures, and dissociated primary cultures (Lovat et al., 2005; Fabbro et al., 2012; Lee et al., 2015). Similar to graphene, the toxicity of carbon nanotubes is associated with their soluble forms because of improper functionalization. In an in vitro study, Simon-Deckers et al. (2008) investigated the toxic effects of multi-walled carbon nanotubes, the most commonly used carbon nanotubes, on A549 human pneumocytes. This study demonstrated that nanotube toxicity, which is stronger than metal oxide nanoparticle toxicity, is mainly triggered by their entrance into cells and dispersion in the cytoplasm, regardless of their length. Similarly, in in vivo animal studies of multi-walled carbon nanotubes, exposure was induced through aspiration, inhalation, or intratracheal instillation, thereby inducing interstitial fibrosis and pulmonary inflammation (Porter et al., 2010; Poulsen et al., 2015). Moreover, Rahman et al. (2017) demonstrated that, due to their sizes, multi-walled carbon nanotubes can easily enter the pleural space, interstitium, and highly vascularized alveolar regions, demonstrating high-degree pulmonary biopersistence. These results indicate that the toxicity and safety of graphene must be considered.
Nonetheless, the results from in vitro or in vivo animal studies cannot be directly applied to humans. Graphene-based materials would be degraded to research tools if they only have good characteristics in experimental settings instead of being surgically applicable. Jakus et al. (2015) established 3D printable graphene fabricated with biodegradable polyester polylactide-co-glycolid containing 20–60% solid content and discovered that it is intraoperatively suitable for surgical procedures in a human cadaver, showing the possibility of graphene bio-ink as a standout candidate among emerging medical devices. However, in vivo pre-clinical studies for evaluating biocompatibility and toxicity to establish a detailed standard for elements of graphene-based materials for their potential clinical applications are lacking. In summary, suggestions for overcoming the disadvantages of graphene will promote the future development of graphene-based materials.
7. Applications of graphene in the nervous system
7.1. Functions of graphene in neurons
Electrical stimulation during the early stage of neuronal development indicates the role of graphene in cell differentiation and phenotypic maintenance (Kam et al., 2009; George et al., 2017). Biomaterial substrates have been investigated for supporting and controlling cellular growth in culture by mimicking natural neural cellular microenvironment. Moreover, various biomolecules can be added to promote neuronal growth and control neuronal survival. Several studies have demonstrated that graphene-based materials can act as biocompatible substrates to enhance neuronal growth, proliferation, and differentiation, as well as neurites sprouting and outgrowth, such as mesenchymal stem cells (MSCs), neural stem cells (NSCs), PC12 cells, dorsal root ganglion (DRG) neurons, retinal ganglion cells, and cortical neurons (Agarwal et al., 2010; Li et al., 2011, 2013; Nayak et al., 2011; Park et al., 2011; Wang et al., 2012, 2019b; Bendali et al., 2013; Sahni et al., 2013; Solanki et al., 2013; Tang et al., 2013; Akhavan and Ghaderi, 2013b; Hong et al., 2014; Shah et al., 2014; Yang et al., 2014; Lee et al., 2015; Bramini et al., 2016; Guo et al., 2016; Rauti et al., 2016; Veliev et al., 2016; Defteralı et al., 2016b; Das et al., 2017; Rastogi et al., 2017; Convertino et al., 2018, 2020b; Kitko et al., 2018; Fang et al., 2019; Fu et al., 2019; Qi et al., 2019; Zhang et al., 2019; Lin et al., 2020; Table 2). These outstanding results are probably the consequence of a complex interplay of electrical, mechanical, and chemical properties imposed by graphene, making it difficult to distinguish the microscopic origin of the effect of graphene on neurons. The topographic cues of graphene with many ripples and wrinkles may resemble the surrounding neural matrix, offering potential substrates for the growth of new neurons. The surface chemistry of graphene plays a part in cellular communication between neurons and substrates. Meanwhile, the electrical conductivity of graphene is widely believed to play a critical role in promoting the outgrowth of neurons and neurites (Cellot et al., 2009; Veliev et al., 2016; Pampaloni et al., 2018). However, a recent study found that both graphene films with high (below 1 kΩ−1) and low (up to 70 kΩ−1) conductivity favored neuron spreading without clear alteration, indicating that the high electrical conductivity of graphene is not the crucial property and the efficient range of its electric conductivity for graphene-based neuronal interfaces is broader than expected (Capasso et al., 2021). The guidance of neurites induced by the graphene edge may be due to its chemical composition. After in vivo implantation or long-term culture for several weeks, the chemical coating would eventually dissolve or degrade, which might expose toxicity concealed by the coating on the surface of the graphene (Bendali et al., 2013).
7.2. Chemical vapor deposition-grown graphene
Zhang et al. (2010) found that graphene sheets synthesized via the radio frequency catalytic CVD approach induced cytotoxic effects on neuronal PC12 cells in a concentration and shape-dependent manner. Time-dependent caspase3 activation after exposure to CVD-grown graphene at 10 μg/mL dosage shows evidence of apoptosis. However, Nayak et al. (2011) found CVD-grown graphene did not hamper hMSCs proliferation and accelerated their differentiation. Graphene film enhances the differentiation of hNSCs toward neurons (Park et al., 2011). CVD-grown graphene under low pressure promotes the adhesion and proliferation of hippocampal neurons but does not induce cell stress (Rastogi et al., 2017).
7.3. Graphene oxide and its derivative
GO can stimulate embryonic stem cells and NSCs to differentiate into neurons as well as promote the differentiation and growth of neuronal axons (Kim T. H. et al., 2013; Guo et al., 2017). GO exhibits a size-dependent effect on the differentiation of mouse NSCs (Lin et al., 2020) and hippocampal cells (Rauti et al., 2016). Some studies reported that GO impaired the excitatory transmission of primary and hippocampal neurons (Bramini et al., 2016; Rauti et al., 2016, 2019). However, Secomandi et al. (2020) found that GO decreased the cultured amygdalar neurons and transiently increased their excitatory transmission. Further experiments should be conducted to demonstrate the controversy over these different neurons after the GO application. Conductive materials for neural cells have been exploited, such as polyaniline, polythiophene, poly-3,4-ethylene dioxythiophene, and polypyrrole (Lei et al., 2014). Several studies functionalized GO with these conductive materials to enhance conductivity (Deng et al., 2011; Zhang and Zhao, 2012; Shah et al., 2014; Fu et al., 2019). Conductive graphene-based composites have been successfully applied to improve recording with substantial SNR and accelerate electrical stimulation of neural cells. Nearly all experiments were conducted on the graphene-based substrates for 1–3 weeks. This procedure is useful for deciphering the long-term effects of these substrates on neurons. However, neurites sprouting and hippocampal neuron outgrowth cultured on the substrates increased maximally during the second day of observation, which is consistent with the results of Convertino et al. (2020b), reaching a non-significant level compared with the control culture. Future studies should focus on exploring the early development of neurons on the substrates and determining the elements that influence neurites outgrowth based on culture time. In addition, the degradation effect of graphene-based substrates on neurons should be investigated.
Interestingly, few studies have investigated how neurons respond to graphene and its derivates. The nanoscale mechanisms underlying graphene-induced neurite outgrowth of neural cells remain unknown. Graphene can increase the local accumulation of nerve growth factors to boost the elongation of peripheral neurons (Convertino et al., 2020a). In previous studies, graphene increased the level of growth-associated protein-43, an efficient marker of axon outgrowth, in both hippocampal neurons (Li et al., 2011) and PC12 cells (Wang et al., 2019b), suggesting that central and peripheral neurons cultured on graphene-based substrates may be driven by the same mechanisms of neuritogenesis. Conductive materials enhance the cellular bioelectric property, through which electrical stimulation can facilitate and control neuritis outgrowth and axonal elongation (Uesaka et al., 2005; Meng, 2014). Moreover, the hippocampal neurons demonstrated increased cell firing, and this change might be due to the alteration of the membrane ion currents by the material interface (Pampaloni et al., 2018). Differentiated NSCs showed upregulated genes involved in the calcium signaling pathway, such as G protein-coupled receptors and Na/Ca exchangers (Park et al., 2011), and increased spontaneous Ca2+ spiking (Tang et al., 2013). Thus, primary neurons exposed to GO flakes demonstrated impaired Ca2+ signaling and several proteins associated with intracellular trafficking (Rauti et al., 2016). Moreover, cellular neurogenesis may be induced by graphene via focal adhesion kinase and p38 mitogen-activated protein kinase cascades (Lee et al., 2015), along with the activation of ERK1/2 phosphorylation (Lin et al., 2020). In addition, downregulating phosphatidylserines, which regulates the negative charge of the cytosolic side of the membranes, and upregulating phosphatidylethanolamines, which comprise membrane phospholipids of a synaptic vesicle, affect their fission and fusion (Gaffaney et al., 2008; Morita et al., 2012). The alteration in phosphatidylethanolamines/phosphatidylserines ratio may be involved in the change in synaptic transmission described previously. These studies are in the early stage; despite the different techniques of synthesizing graphene and various biological cells studied, the interaction between graphene and neural cells should be explored further for a better understanding and use of its biological efficacy. Overall, the understanding of graphene is advancing, providing a solid foundation for neuroscience research and the clinical application of graphene-based materials, given the advancement of biological tissue engineering and material science.
7.4. Drug delivery and graphene treatment for neurological diseases
Drug delivery to the brain may be ineffective for treating central tumors because the central nervous system is complex, increasing disease severity and major side effects without achieving satisfactory therapeutic effects (Meng et al., 2017; Fernandes et al., 2018; Saeedi et al., 2019). The current treatment modalities, including chemo- and photothermal-theory, have low efficacy and various side effects and risks and lack specific targeting. Nanocarriers are now superior approaches to cancer therapy. Graphene has been exploited as a drug loading material with effective potency and efficiency because of its π–π stacking, high specific surface, and hydrophobic and electrostatic interactions. Graphene and its derivatives have been developed into vectors of genes and drugs with functionalization as drug delivery systems because of its advantageous properties in achieving targeting and local delivery.
Graphene is a mode for intracellular administration. GO and rGO can deliver antigens that modify innate immune cells to trigger effective and adaptive immune responses for immunotherapy in vivo (Sinha et al., 2019; Wang X. et al., 2019; Parker et al., 2022). Yang et al. (2022) revealed that GO with antigen can be efficiently taken up by dendritic cells via receptor-mediated endocytosis, which enhances immune responses and increases delivery effectiveness both in vitro and in vivo. Meanwhile, Yan et al. (2019) loaded rGO with IDO inhibitor, which can trigger immune responses, and successfully induced antitumor immunity. Fang et al. (2022) assembled GO and metal–organic framework, Fe-porphyrin, of which the porous structure and large surface area enabled drug delivery. The use of a metal–organic framework as a photosensitizer under laser irradiation enables effective synergistic treatment through PTT. Moreover, a study functionalized graphene with PEI to deliver drugs. PEI is a non-viral gene carrier that can interact with negatively-charged DNA or RNA to form a complex. Synthesis of PEI-GO nanocomplex via π-π stowage and hydrophobic interaction produced higher transfection efficiency and less toxicity than PEI alone, delivering gene and achieving a high-drug load of insoluble drugs (Huang et al., 2016). These drug-delivery systems can improve targeting efficiency through functionalization with targeted genes (Chowdhury et al., 2015; Keles et al., 2016; Din et al., 2017; Asghari et al., 2020). For example, glioblastoma multiforme (GBM), a neurogenic tumor, is difficult to treat because of the intracranial location of the tumor. Jaworski et al. (2013, 2015) discovered that apoptosis occurred in GBM cells cultured on graphene and rGO platelets, indicating the possible effectiveness of graphene in anticancer therapy. Arginine-producing nitric oxide can affect reactive oxygen in mitochondria, cause cell apoptosis (Jeong et al., 2010), and have a high affinity for graphene (Hughes and Walsh, 2015). Sawosz et al. (2015) added arginine to rGO and examined the anticancer effect of the complex on GBM cells. They revealed that adding arginine to rGO increased rGO activity and led to GBM cell apoptosis. Another study used GO and rGO to transport microRNA to GBM cells. These complexes had less harmful effects on the viral carrier, but more efficiency of microRNA delivery than the viral carrier. GO and rGO functionalized with microRNA-induced microRNA deregulation stress, regulated relative gene expression to apoptosis, and increased GBM cell apoptosis (Kutwin et al., 2021). Wang Y. et al. (2013) designed an initial drug-delivery system that used chemo-photothermal treatment to target GBM cells. Coating graphene nanoparticles with mesoporous silica improved super absorption under NIR and drug loading efficiency compared with graphene nanosheets alone. A targeting peptide was designed and exploited as a GBM-cell targeting ligand; it modified mesoporous silica nanoparticles. Loading doxorubicin onto the targeting peptide conjugated-mesoporous silica covered-graphene system caused the specific and highest death rate of GBM cells with near-infrared irradiation than single photothermal- or chemotherapy (Figures 3A–E).
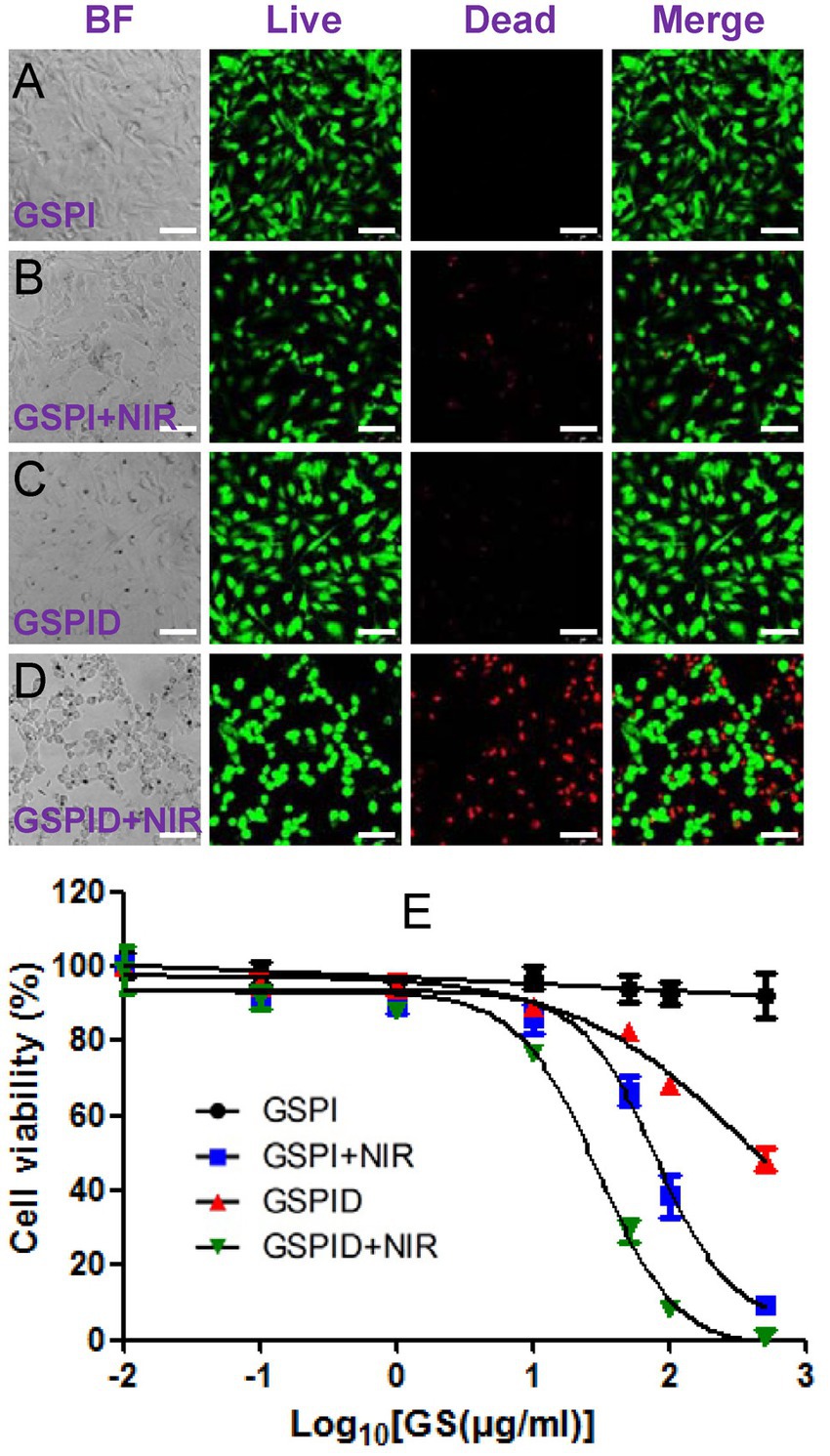
Figure 3. (A–E) Qualitative results of confocal microscopy showing the viability of glioma cells by LIVE-DEAD staining. BF, bright field images, Green: live cells. Red: dead cells; Wang et al. (2013) with permission from ACS Publications, copyright 2013.
Two points must be considered when nanocarriers deliver drugs to the central nervous system. First, drugs must have the ability to penetrate the blood–brain barrier (BBB) to ensure the delivery of sufficient dosage to kill cancer cells. Second, the delivery should specifically target cancer cells, avoiding harmful effects on normal tissues. For example, subarachnoid hemorrhage occurs in severe neurological diseases, and studies have demonstrated the feasibility of using functionalized GO (FGO) for effective drug delivery in such cases. The content of FGO nanotablets loaded with pirfenidone, which is used to treat subarachnoid hemorrhage, gradually increases with an increase in drug concentration (Figure 4A), making FGO a suitable medicine carrier that can be loaded with pirfenidone. The functionalization of transcription activator peptide and methoxy PEG introduced onto GO nanosheets aid BBB crossing and improve drug stability in blood circulation. Transcription activator peptide improved nanosheet ability to cross the BBB. Moreover, long-chain polyethers of methoxy PEG prolonged the lifetime of pirfenidone in circulation and increased the permeability of nanomaterials through blood vasculatures. Furthermore, pirfenidone was released from FGO at a pH of 5 within 72 h and not at a pH of 7 (Figure 4B). Subarachnoid hemorrhage may trigger an inflammatory response, and the inflammatory microenvironment is an acidic site with a pH of 5.5. Therefore, the effective release of pirfenidone amount under acidic conditions indicates that the FGO drug-delivery system has a satisfactory therapeutic effect on inflammatory lesions (Yang et al., 2015). The functionalization of graphene-based nanomaterials improves BBB penetration ability, increasing drug concentration gradients inside and outside blood vessels, and facilitating drug entry into the brain through capillary endothelium, providing a good carrier for targeted drug delivery in the brain. Similarly, Dong et al. (2016) examined the efficacy of targeting drug delivery with chemo-photothermal therapy on rats with GBM. Loaded onto transferring-conjugated GO-PEG, doxorubicin entered through the BBB and accumulated in the GBM region of the brain. Under NIR radiation, the tumor temperature significantly increased as the drug-delivery system is absorbed because of the GO’s aromatic structure. This photothermal effect could be mediated by applying the drug delivery later. The life of rats bearing GBM was also prolonged with targeted drug delivery utilizing nanomaterials. These characteristics indicate an admirable drug-delivery system for treating diseases. The saturable light absorption of graphene may also be useful for targeted delivery of improved graphene and its derivatives, which are accumulated in cancer cells after intravenous administration in treating neurogenic tumors. Thus, irradiating the tumor with NIR light will lead to the photothermal ablation of tumor cells, while retaining ordinary tissue with ambient temperature (He et al., 2016).
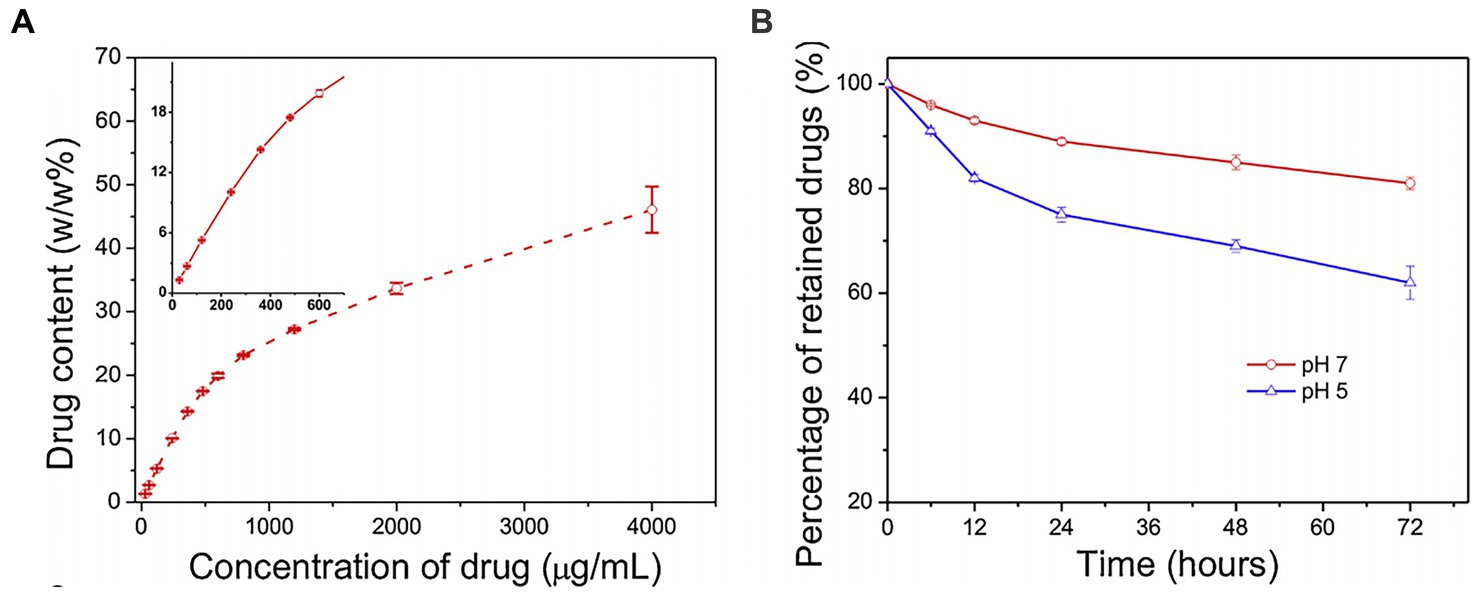
Figure 4. (A) The content of FGO loaded with pirfenidone was determined at the concentration of the drugs. (B) Graph of the release of pirfenidone from FGO by pH 5 and pH 7 in in a specific time point; Yang et al. (2015) with permission from Elsevier Ltd., copyright 2015.
Furthermore, neurodegeneration diseases of the nervous system, including AD and PD, lack accurate and economic clinical diagnostic tools. No drugs have successfully treated these diseases in clinical settings. Therefore, associated biomarkers must be detected and quantified for diagnosing these diseases early. One of the most important contributions to neurological diseases is the aggregation of amyloid fibrils. Graphene-based materials have been extensively explored to not only detect amyloid protein sensitively and quantitatively but also prevent the accumulation of amyloid, which might provide new insights into alternative diagnostics and therapy at the early stage of AD and PD. The conformation of Aβ peptides produced by β-secretase changes from alpha-helix to beta-sheet to create insoluble fibrils, within which heavy metal ions eventually result in amyloid plaques (Kayed et al., 2003). Beta-amyloid1-42 (Aβ1-42) is an important biomarker in AD and PD (He et al., 2018). Lopes et al. (2014) first demonstrated the progress of aggregating Aβ1-42 absorbed on the graphite electrode via its tyrosine oxidation record using differential pulse voltammetry. This experimental method offered a potential tool for detecting and preventing Aβ aggregation. Yousaf et al. (2019) reported a probe based on bovine-serum-albumin-capped fluorine functionalized GQDs that enabled spontaneous fluorescence-based monitoring of Aβ1-42 in vitro with a 10 times higher detection rate than that of conventional thioflavin dye and effective detection of amyloid plaques in vivo. Nangare and Patil (2022) decorated GO with chitosan, which offers sites for Aβ1-42 antibodies via layer-by-layer assembly on the surface of the plasmon resonance biosensor. This inimitable feature of biosensor achieved detection with a linear range of 2 fg/mL–400 ng/mL and a limit of 1.21 fg/mL. Yu et al. (2021) used a silver probe and magnetic GO substrate capturing the antigen, which was formed into a typical sandwich structure, thus achieving a detection linear range of Aβ1-42 from 100 pg. mL−1 to 10 fg mLd−1. Such a tool was successfully used and verified on AD human serum samples. However, the reported antibody against oligomers would possibly target monomers. Graphene-based electrochemical detection with a high specificity could also depend on DNA hybridization apart from antigen–antibody interactions. Bonanni et al. (2012) first examined the ability of GO to conjugate with DNA hybrids and discovered its promising role in DNA labeling. Song et al. (2022) conjugated GO with Aβ1-42-specific aptamer to generate a novel biosensor that could detect Aβ1-42 of 0.1 to 10 uM through surface-assisted laser desorption ionization mass spectrometry. MiRNA-137 was identified as a reliable biomarker for AD (Azimzadeh et al., 2017). Chang et al. (2021) modified GO with gold nanostar through an amine linker to capture miRNA-137 on the complementary sequence, achieving a detection limit at 10 fM with 1 fM sensitivity. The graphene surface can highly transfer the fluorescence quenching efficiency, making it a suitable broad-spectrum fluorescence quencher (Ma et al., 2017). Rahaie and Noroozi (2019) applied GO with SYBR green based on the hybridization chain reaction for detecting miR-137 and demonstrated that this designed biosensor achieved sensitive detection ranging from 0.05 nM to 5 nM with a limit at 82 pM. Moreover, Zhou et al. (2018) offered a strategy based on GO and entropy-driven strand displacement reaction. In this experiment, researchers released the bound Aβ1-42 oligomers using hairpin structure DNA probes, which hybridized with the fluorescein-labeled complementary probes. Continuous cycle reactions amplified the fluorescence signal until GO started to absorb the excess labeled probes. This method ensured ultrasensitive detection of Aβ1-42 oligomers at limit of 20 pM.
Inhibiting fibrillation is a prominent strategy for preventing or treating neurodegenerative diseases (Haataja et al., 2008). Graphene and its derivatives modulate the pattern of fibril formation. Gregory et al. (2020) showed that graphene effectively mitigates amyloid fibrillation using hen egg-white lysozyme, a classical model of amyloid-forming protein, using the interfacial charge transfer associated with hydrophobic and π-π interactions. This result was consistent with the discovery made by Ban et al. (2018) using graphene oxide quantum dots. Baweja et al. (2015) also discovered that GO and rGO decreased the beta-strand propensity of Aβ amino acid residues, inhibiting alpha helix to beta-sheet transition of Aβ peptide and the electrostatic interactions contributed to its adsorption on GO and rGO. However, researchers have suggested that the oxidative degree of GO surface influences the interplay between materials and filament growth. He et al. (2019) demonstrated that non-uniform GO in oxidation sites exhibited a remarkably stronger perturbation on Aβ fibril. Another study revealed that high oxidative GO-20 and GO-40 inhibited fibril elongation, while low oxidative GO-10 accelerated fibril elongation. The details of how the oxidation degree of GO causes different effects in in vitro experiments will provide new insights into the design strategy for amyloidosis therapy. A density functional theory study recently demonstrated that GO with 12.5% oxygen can effectively reduce Aβ plaque in AD (Liu and Luo, 2021). In in vivo experiments, Xiao et al. (2016) conjugated GODs with peptide glycine-proline-glutamate and administrated it to APP/PS1 transgenic mice. The deposition area of Aβ plaque reduced in the GODs with peptide glycine-proline-glutamate group compared with the control group. Besides, the learning and memory capacity was enhanced. Tak et al. (2020) synthesized GODs from the Clitoria ternatea flower and discovered that GODs effectively reduced AD-like symptoms in rodents. Many results support that graphene-based materials can be used as promising agents for treating neurodegenerative disease; however, there are limitations to consider. For example, several studies have demonstrated an apparent breakdown of Aβ fibril, and the resultant protein was not exclusively a monomer, raising concerns about the potential toxicity of the remaining smaller soluble oligomers. Further studies should explain the fate of these smaller oligomers in cells. The effect of graphene-based material concentration on neurodegenerative symptoms in vivo should also be determined.
In this review, we emphasized the potential, specific, and bright prospects of graphene for treating nervous system diseases. In the absence of ideal treatments for neurological diseases, such as brain cancer, AD, and PD, graphene-based nanotechnology can be used as a drug carrier to better control drug release, thereby improving drug delivery to the specific site and a tool to diagnose and treat these diseases. This technology should be further developed as matured gene or drug-targeted delivery systems and combined with physicochemical methods to obtain more satisfactory treatment results and provide a solid foundation for future neurotherapy development (Liao et al., 2018; Yao et al., 2019).
7.5. Graphene in nerve repair
The ability of graphene to repair nerves has been evaluated in several studies. The large surface area of graphene can increase its adsorption performance and act as an interface material for nerve repair (He et al., 2016; Domínguez-Bajo et al., 2017). For example, peripheral nerve injuries cause disconnections between spinal neurons and targeted organs. Autografting is a widely used approach if end-to-end neurorrhaphy is infeasible. With the recent developments in nanotechnology, Jakus et al. (2015) demonstrated that graphene-based nerve scaffold has great in vivo biocompatibility, as previously described. However, the nerve conduits were only implanted subcutaneously for 30 days, with no further neural expression performances. Moreover, Assaf et al. (2017) fabricated a tubular prosthesis made of graphene and polycaprolactone and examined the efficiency of the material on sciatic nerve repair in rats with disconnection. The prosthesis improved the number of myelinated axons in repaired nerves 12 weeks after surgery and contributed to a satisfactory regeneration outcome after a peripheral nerve injury. Furthermore, a relatively low mass ratio of the anterior tibialis muscle was observed, indicating the possibility of muscle atrophy. In addition, Qian et al. (2018b) conducted more detailed examinations to evaluate the long-term effects of graphene-related nerve conduit in sciatic nerve restoration, including counting myelinated axons, average myelinated axon diameter, the thickness of myelin sheath, and regenerated axon area. With the tubulization of the sciatic nerve defect in rats at 18 weeks after surgery, using polydopamine- and arginylglycylaspartic acid modified- and polycaprolactone functionalized-graphene via 3D printing fabrication, and layer-by-layer casting method, four parameters in the tubulization group demonstrated no distinct difference compared with those in the autograft group. In addition, the locomotor and sensory function recovery were examined. The conduit group loaded with cells also showed similar results as the autograft group, indicating that muti-layered 3D graphene can reverse muscle atrophy. Thus, these results demonstrated that graphene-based nanoscaffolds can cure long-term peripheral nerve defects by promoting axonal regeneration and nerve function recovery. Graphene foam/hydrogel scaffolds significantly promote the recovery of sciatic nerves in vivo with no obvious organ lesions or damage (Huang et al., 2022). Qian et al. (2018a) demonstrated that the GO/PCL nerve guidance conduit also successfully repaired sciatic nerve defects in rats, effectively promoting its functional and morphological recovery, and showed no obvious toxicity for the long-term. Loading graphene with brain-derived neurotrophic protein that contributes to axon pathfinding and neuronal migration can enhance the ability of graphene-based material in nerve repair. Huang et al. (2021) loaded a graphene/hydrogel scaffold with netrin-1, an axonal guidance cue, and successfully supported sciatic nerve regeneration. The performance of these kinds of graphene is superior to that of an autologous graft. Moreover, Pan et al. (2019) further immobilized PLGA/GO and brain-derived neurotrophic factor and insulin-like growth factor-1, which are involved in nerve regeneration, and demonstrated that PLGA/GO fabricated with brain-derived neurotrophic factor and insulin-like growth factor-1 significantly boosted the functional locomotor recovery and the counting of neurons located at the injury sites in animal models of spinal cord injury (SCI). GO-modified poly(D,L-lactide-co-caprolactone) significantly accelerates the functional recovery of sciatic nerve 8 weeks post-operation in vivo (Zhang et al., 2020). These results suggest that engineered graphene-based materials provide a novel therapeutic approach to cure SCI as a nerve implant.
Moreover, macrophage recruitment increases after nerve injury, highlighting the importance of immune responses in tissue regeneration. In the context of neural tissues, several studies have investigated the effect of graphene-based scaffolds for nerve repair on immune cells, which are important for driving wound healing and tissue regeneration. López-Dolado et al. (2016) demonstrated that implanting 3D rGO scaffolds in SCI rats induced immune modulation and angiogenic responses without causing systemic toxicity. In vivo studies using SCI rats showed that implanting 3D rGO scaffolds in the injured spinal cord improved the structured lesion zones more than those left untreated, indicating the contribution of scaffolds in improving sealing and injury stabilization. Pro-regenerative macrophages were more obviously visible at the interface and functional new blood vessels inside the scaffolds than lesion regions without scaffolds 30 days after surgery (López-Dolado et al., 2015, 2016). The immune signature may guide NSCs migration. Furthermore, whether graphene could elicit immune responses and how it affects NSC behaviors when interfaced with macrophages remain unclear. Thus, Jiang et al. (2016) demonstrated that 3D graphene evoked mild neuro-immunity and caused macrophages to secrete some inflammatory cytokines without changing their cell cycle profiles and viability. These results were consistent with the findings of Serrano et al. (2018), which showed that 3D graphene culture interfaced with macrophages promoted neurosphere formation and NSC migration from the neurospheres. In contrast, 2D graphene and TCPS interfacing with macrophages failed to achieve such results. Hence, the capacity of 3D graphene to govern NSCs migration might depend on its topographical structure by mildly activating macrophages.
The fabrication of graphene-based composite nanofibers can determine their function, providing versatile therapeutic approaches. Wang et al. (2019a) fabricated PLGA/GO nanofibers using methylene blue via physisorption, which is used to activate the autophagy signaling pathway and regulate the function of neural progenitor cells. They discovered that neural progenitor cells cultured on the PLGA/GO loaded with methylene blue entered a quiescence phase to avoid apoptosis and diminish tau phosphorylation. Unlike similar GO fabrication into various patterns through microcontact printing, Min et al. (2017) synthesized GO and magnetic nanoparticles, which were used as a force, to transfer the GO film into the desired substrate. A mixed mode of GO driven by magnetic force can precisely guide the formation of synaptic connections between neurons in different directions, providing a different method for curing SCI.
Graphene plays an indispensable role in various neuroscience applications by promoting nerve regeneration and neural restoration and treating some nerve diseases. Factors that promote the repair of neurite injury sites may vary with cell type. Therefore, more studies are required to overcome these problems and transform the successful repair of damaged nerves into functional recovery in clinical treatment (Liu and Jan, 2020).
8. Conclusion
Carbon nanomaterials have gained attention because of their large surface area, mechanical stability, electrical conductivity, and biocompatibility. Graphene family materials have been studied and used in several technical and scientific fields, including neurobiomedicine. In this review, we sketched the role of nanostructured carbon materials (particularly graphene) in neuroscience in in vitro and in vivo studies. A summary of the properties of graphene, including its mechanical and electrical properties, optical and thermal properties, biocompatibility, and toxicity, revealed that the characteristics of graphene and its derivatives vary from size, structural dimensions, and layer number-to-surface chemistry. Summarizing the applications of graphene and its derivatives, including neural culture, drug delivery, diagnosis and treatment of neuronal diseases, and nerve repair (Table 3), we found that their applications depend on the differences in functionalization, purification, and chemical and morphological formations. Therefore, many discrepancies exist between experimental results owing to various involved elements, such as potential toxicity and induced immune responses, which directly determine the possibility of further bioapplication. Fortunately, such experimental outcomes have increased enormously with great progress in scientific and clinical fields.
The observed discrepancies in in vitro and in vivo studies suggest that current studies have several limitations. First, compared with the number of in vitro studies, in vivo studies exploring the basic properties of graphene, specifically its biocompatibility and toxicity are limited. Lack of consistency between in vitro and in vivo results may downgrade graphene into only a research tool. Regarding in vivo study models, nearly all animal models are limited to mice, and no other species have been considered in exploring graphene applications. Second, most studies suggest that nanomaterials induce a mild immune response. However, researchers are yet to identify the standard method of administration. Furthermore, whether nanomaterials act the same way when eliciting an immune response via different routes of administration in vivo remains unknown, although the toxicity and pharmacokinetics of nanoparticles are closely dependent on the administration route. Third, most studies, whether in vitro or in vivo, did not perform in-depth investigations on the underlying mechanism involved in the relationship between cell differentiation and graphene-based substrates, such as induced morphologic interplay and cellular modulation pathways. Without these fundamental definitions, determining the role of graphene in biomedical applications may be challenging.
The following are proposals for future experiments for improving graphene and its derivatives development: First, the main consideration of nanomaterials in biomedical application is their biocompatibility. Further studies must focus more on biocompatibility and toxicity in vivo because the results from in vitro studies cannot be directly applied to humans. Moreover, the graphene-based implant as an electrode or scaffold should also be further investigated to observe its long-term effect in vivo. More animal models should be considered to clarify the relationship between different species and graphene. Second, graphene can exhibit long-term and short-term toxic effects on the same cell and can potentially cause long-term damage to cellular structures. This review highlighted that graphene toxicity is highly dependent on excretion, functionalization, size, and structural dimensions. Therefore, comparing graphene toxicity between different experiments is difficult, given its diversity in shape, surface, size, and fabrication. Thus, the fabrication and terminology of graphene and the examination of toxicological methodologies must be standardized. This will better aid the understanding of the physicochemical properties and potential toxicity of graphene in vivo and in intro, allowing its practical application in humans. Third, future studies should incorporate graphene with various optical components to probe deeper brain tissues and understand how brain circuits process information because a graphene electrode with a two-photon microscope can record the whole cortical surface in neuroscience investigation. Moreover, electrical stimulation has been clinically applied in rehabilitation after nerve injury. Graphene-based conduits have shown great potential in nerve repair as scaffolds. Future studies should measure the potential of combining electrical stimulation and graphene-based conductive scaffolds in clinical nerve repair.
This review sheds light on the safety and biological properties of graphene that must be considered before the utilization of graphene-based therapy for human benefit. This may pave the way for using graphene and its derivatives as powerful materials with versatile scientific and clinical applications.
Author contributions
TY and YY wrote the first draft of the manuscript. L-YM and YL wrote sections of the manuscript. JB, F-YW, and LZ reviewed the literature. All authors contributed to manuscript revisions as well as read and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundations of China (81560231; 81860251; 51703192), the Innovation Team Project of Yanbian University (China), and the International Cooperation Project of Science and Technology Department of Jilin Province (20210402004GH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
2D, two-dimensional; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; PTT, photothermal therapy; AD, Alzheimer’s disease; PD, Parkinson’s disease; SNR, signal-to-signal ratio; 3D, three-dimensional; IR, infrared; NIR, near-IR; GO, graphene oxide; *rGO, reduced graphene oxide; PEG, polyethylene glycol; PEI, polyethyleneimine; Aβ, amyloid β-protein; TCPS, tissue culture polystyrene; DRG, dorsal root ganglion; GAP-43, growth-associate protein-43; GBM, glioblastoma multiforme; BBB, blood–brain barrier; FGO, functionalized GO; SCI, spinal cord injury; NSCs, neural stem cells.
References
Agarwal, S., Zhou, X., Ye, F., He, Q., Chen, G. C., Soo, J., et al. (2010). Interfacing live cells with nanocarbon substrates. Langmuir 26, 2244–2247. doi: 10.1021/la9048743
Akhavan, O., and Ghaderi, E. (2013a). Flash photo stimulation of human neural stem cells on graphene/TiO2 heterojunction for differentiation into neurons. Nanoscale 5, 10316–10326. doi: 10.1039/c3nr02161k
Akhavan, O., and Ghaderi, E. (2013b). Graphene nanomesh promises extremely efficient in vivo photothermal therapy. Small 9, 3593–3601. doi: 10.1002/smll.201203106
Akhavan, O., Ghaderi, E., and Emamy, H. (2012). Nontoxic concentrations of PEGylated graphene nanoribbons for selective cancer cell imaging and photothermal therapy. J. Mater. Chem. 22:20626. doi: 10.1039/c2jm34330d
Appel, J. H., Li, D. O., Podlevsky, J. D., Debnath, A., Green, A. A., Wang, Q. H., et al. (2016). Low cytotoxicity and Genotoxicity of two-dimensional MoS(2) and WS(2). ACS Biomater Sci. Eng. 2, 361–367. doi: 10.1021/acsbiomaterials.5b00467
Asghari, S., Rezaei, Z., and Mahmoudifard, M. (2020). Electrospun nanofibers: a promising horizon toward the detection and treatment of cancer. Analyst 145, 2854–2872. doi: 10.1039/C9AN01987A
Assaf, K., Leal, C. V., Derami, M. S., de Rezende Duek, E. A., Ceragioli, H. J., and de Oliveira, A. L. R. (2017). Sciatic nerve repair using poly(ε-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene. Brain Behav. 7:e00755. doi: 10.1002/brb3.755
Azimzadeh, M., Nasirizadeh, N., Rahaie, M., and Naderi-Manesh, H. (2017). Early detection of Alzheimer’s disease using a biosensor based on electrochemically-reduced graphene oxide and gold nanowires for the quantification of serum microRNA-137. RSC Adv. 7, 55709–55719. doi: 10.1039/C7RA09767K
Ban, D. K., Somu, P., and Paul, S. (2018). Graphene oxide quantum dot alters Amyloidogenicity of hen egg White lysozyme via modulation of protein surface character. Langmuir 34, 15283–15292. doi: 10.1021/acs.langmuir.8b02674
Barrera, C. C., Groot, H., Vargas, W. L., and Narváez, D. M. (2020). Efficacy and molecular effects of a reduced Graphene oxide/Fe(3)O(4) Nanocomposite in Photothermal therapy against Cancer. Int. J. Nanomedicine 15, 6421–6432. doi: 10.2147/IJN.S256760
Baweja, L., Balamurugan, K., Subramanian, V., and Dhawan, A. (2015). Effect of graphene oxide on the conformational transitions of amyloid beta peptide: a molecular dynamics simulation study. J. Mol. Graph. Model. 61, 175–185. doi: 10.1016/j.jmgm.2015.07.007
Bayer, I. S. (2017). Thermomechanical Properties of Polylactic Acid-Graphene Composites: A State-of-the-Art Review for Biomedical Applications. Materials (Basel) 10. doi: 10.3390/ma10070748
Bendali, A., Hess, L. H., Seifert, M., Forster, V., Stephan, A. F., Garrido, J. A., et al. (2013). Purified neurons can survive on peptide-free graphene layers. Adv. Healthc. Mater. 2, 929–933. doi: 10.1002/adhm.201200347
Bernal, M. M., Pierro, A. D., Novara, C., Giorgis, F., Mortazavi, B., Saracco, G., et al. (2018). Edge-grafted molecular junctions between Graphene Nanoplatelets: Applied chemistry to enhance heat transfer in Nanomaterials. Adv. Funct. Materials, 1706951–1706954. doi: 10.1002/adfm.201706954
Berube, D. M. (2006). A review of Nano-hype: the truth behind the nanotechnology buzz. Nanotechnol. Law Bus. 3:375. doi: 10.1177/1075547006298660
Bhang, S. H., Kwon, S. H., Lee, S., Kim, G. C., Han, A. M., Kwon, Y. H., et al. (2013). Enhanced neuronal differentiation of pheochromocytoma 12 cells on polydopamine-modified surface. Biochem. Biophys. Res. Commun. 430, 1294–1300. doi: 10.1016/j.bbrc.2012.11.123
Bonaccini Calia, A., Masvidal-Codina, E., Smith, T. M., Schäfer, N., Rathore, D., Rodríguez-Lucas, E., et al. (2022). Full-bandwidth electrophysiology of seizures and epileptiform activity enabled by flexible graphene microtransistor depth neural probes. Nat. Nanotechnol. 17, 301–309. doi: 10.1038/s41565-021-01041-9
Bonanni, A., Chua, C. K., Zhao, G., Sofer, Z., and Pumera, M. (2012). Inherently electroactive graphene oxide nanoplatelets as labels for single nucleotide polymorphism detection. ACS Nano 6, 8546–8551. doi: 10.1021/nn301359y
Bramini, M., Sacchetti, S., Armirotti, A., Rocchi, A., Vázquez, E., León Castellanos, V., et al. (2016). Graphene oxide Nanosheets disrupt lipid composition, Ca(2+) homeostasis, and synaptic transmission in primary cortical neurons. ACS Nano 10, 7154–7171. doi: 10.1021/acsnano.6b03438
Cao, Y., Dong, H., Yang, Z., Zhong, X., Chen, Y., Dai, W., et al. (2017). Aptamer-conjugated Graphene quantum dots/Porphyrin derivative Theranostic agent for intracellular Cancer-related MicroRNA detection and fluorescence-guided Photothermal/photodynamic synergetic therapy. ACS Appl. Mater. Interfaces 9, 159–166. doi: 10.1021/acsami.6b13150
Capasso, A., Rodrigues, J., Moschetta, M., Buonocore, F., Faggio, G., Messina, G., et al. (2021). Neuronal networks: interactions between primary neurons and Graphene films with different structure and electrical conductivity. Adv. Funct. Mater. 11:31.
Cellot, G., Cilia, E., Cipollone, S., Rancic, V., Sucapane, A., Giordani, S., et al. (2009). Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat. Nanotechnol. 4, 126–133. doi: 10.1038/nnano.2008.374
Cellot, G., Vranic, S., Shin, Y., Worsley, R., Rodrigues, A. F., Bussy, C., et al. (2020). Graphene oxide nanosheets modulate spinal glutamatergic transmission and modify locomotor behaviour in an in vivo zebrafish model. Nanoscale Horiz. 5, 1250–1263. doi: 10.1039/C9NH00777F
Chang, W., Zhao, J., Liu, L., Xing, X., Zhang, C., Meng, H., et al. (2021). Graphene oxide-Gold star construct on triangular electrodes for Alzheimer’s disease identification. J. Anal. Methods Chem. 2021:6661799. doi: 10.1155/2021/6661799
Chatterjee, N., Yang, J. S., Park, K., Oh, S. M., Park, J., and Choi, J. (2015). Screening of toxic potential of graphene family nanomaterials using in vitro and alternative in vivo toxicity testing systems. Environ. Health Toxicol. 30:e2015007. doi: 10.5620/eht.e2015007
Chen, C. H., Lin, C. T., Hsu, W. L., Chang, Y. C., Yeh, S. R., Li, L. J., et al. (2013). A flexible hydrophilic-modified graphene microprobe for neural and cardiac recording. Nanomedicine 9, 600–604. doi: 10.1016/j.nano.2012.12.004
Chien, S.-K., Yang, Y.-T., and Chen, C. o.-K. (2011). Influence of hydrogen functionalization on thermal conductivity of graphene: nonequilibrium molecular dynamics simulations. Appl. Phys. Lett. 98:033107. doi: 10.1063/1.3543622
Cho, Y. W., Lee, K. H., and Kim, T. H. (2022). Graphene-based materials for efficient neurogenesis. Adv. Exp. Med. Biol. 1351, 43–64. doi: 10.1007/978-981-16-4923-3_3
Chong, Y., Ma, Y., Shen, H., Tu, X., Zhou, X., Xu, J., et al. (2014). The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 35, 5041–5048. doi: 10.1016/j.biomaterials.2014.03.021
Chortarea, S., Kuru, O. C., Netkueakul, W., Pelin, M., Keshavan, S., Song, Z., et al. (2022). Hazard assessment of abraded thermoplastic composites reinforced with reduced graphene oxide. J. Hazard. Mater. 435:129053. doi: 10.1016/j.jhazmat.2022.129053
Chowdhury, S. M., Surhland, C., Sanchez, Z., Chaudhary, P., Suresh Kumar, M. A., Lee, S., et al. (2015). Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine 11, 109–118. doi: 10.1016/j.nano.2014.08.001
Convertino, D., Fabbri, F., Mishra, N., Mainardi, M., Cappello, V., Testa, G., et al. (2020a). Graphene promotes axon elongation through local stall of nerve growth factor signaling endosomes. Nano Lett. 20, 3633–3641. doi: 10.1021/acs.nanolett.0c00571
Convertino, D., Luin, S., Marchetti, L., and Coletti, C. (2018). Peripheral neuron survival and outgrowth on Graphene. Front. Neurosci. 12:1. doi: 10.3389/fnins.2018.00001
Convertino, D., Mishra, N., Marchetti, L., Calvello, M., Viegi, A., Cattaneo, A., et al. (2020b). Effect of chemical vapor deposition WS(2) on viability and differentiation of SH-SY5Y cells. Front. Neurosci. 14:592502. doi: 10.3389/fnins.2020.592502
D’Abaco, G. M., Mattei, C., Nasr, B., Hudson, E. J., Alshawaf, A. J., Chana, G., et al. (2018). Graphene foam as a biocompatible scaffold for culturing human neurons. R. Soc. Open Sci. 5:171364. doi: 10.1098/rsos.171364
Das, S. R., Uz, M., Ding, S., Lentner, M. T., Hondred, J. A., Cargill, A. A., et al. (2017). Electrical differentiation of Mesenchymal stem cells into Schwann-cell-like phenotypes using inkjet-printed Graphene circuits. Adv. Healthc. Mater. 6:1087. doi: 10.1002/adhm.201601087
Dash, B. S., Jose, G., Lu, Y. J., and Chen, J. P. (2021). Functionalized reduced Graphene oxide as a versatile tool for Cancer therapy. Int. J. Mol. Sci. 22:2989. doi: 10.3390/ijms22062989
Defteralı, Ç., Verdejo, R., Majeed, S., Boschetti-de-Fierro, A., Méndez-Gómez, H. R., Díaz-Guerra, E., et al. (2016a). In vitro evaluation of biocompatibility of uncoated thermally reduced Graphene and carbon nanotube-loaded PVDF membranes with adult neural stem cell-derived neurons and glia. Front. Bioeng. Biotechnol. 4:94. doi: 10.3389/fbioe.2016.00094
Defteralı, Ç., Verdejo, R., Peponi, L., Martín, E. D., Martínez-Murillo, R., López-Manchado, M., et al. (2016b). Thermally reduced graphene is a permissive material for neurons and astrocytes and de novo neurogenesis in the adult olfactory bulb in vivo. Biomaterials 82, 84–93. doi: 10.1016/j.biomaterials.2015.12.010
de Melo-Diogo, D., Lima-Sousa, R., Alves, C. G., Costa, E. C., Louro, R. O., and Correia, I. J. (2018). Functionalization of graphene family nanomaterials for application in cancer therapy. Colloids. Surf. B Biointerfaces 171, 260–275. doi: 10.1016/j.colsurfb.2018.07.030
Deng, M., Yang, X., Silke, M., Qiu, W., Xu, M., Borghs, G., et al. (2011). Electrochemical deposition of polypyrrole/graphene oxide composite on microelectrodes towards tuning the electrochemical properties of neural probes. Sensors Actuators B Chem. 158, 176–184. doi: 10.1016/j.snb.2011.05.062
Din, F. U., Aman, W., Ullah, I., Qureshi, O. S., Mustapha, O., Shafique, S., et al. (2017). Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomedicine 12, 7291–7309. doi: 10.2147/IJN.S146315
Ding, Z., Luo, N., Yue, H., Gao, Y., Ma, G., and Wei, W. (2020). In vivo immunological response of exposure to PEGylated graphene oxide via intraperitoneal injection. J. Mater. Chem. B 8, 6845–6856. doi: 10.1039/D0TB00499E
Domínguez-Bajo, A., González-Mayorga, A., López-Dolado, E., and Serrano, M. C. (2017). Graphene-derived materials interfacing the spinal cord: outstanding in vitro and in vivo findings. Front. Syst. Neurosci. 11:71. doi: 10.3389/fnsys.2017.00071
Dong, H., Jin, M., Liu, Z., Xiong, H., Qiu, X., Zhang, W., et al. (2016). In vitro and in vivo brain-targeting chemo-photothermal therapy using graphene oxide conjugated with transferrin for Gliomas. Lasers Med. Sci. 31, 1123–1131. doi: 10.1007/s10103-016-1955-2
Driscoll, N., Rosch, R. E., Murphy, B. B., Ashourvan, A., Vishnubhotla, R., Dickens, O. O., et al. (2021). Multimodal in vivo recording using transparent graphene microelectrodes illuminates spatiotemporal seizure dynamics at the microscale. Commun. Biol. 4:136. doi: 10.1038/s42003-021-01670-9
Duch, M. C., Budinger, G. R., Liang, Y. T., Soberanes, S., Urich, D., Chiarella, S. E., et al. (2011). Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 11, 5201–5207. doi: 10.1021/nl202515a
Dudek, I., Skoda, M., Jarosz, A., and Szukiewicz, D. (2016). The molecular influence of Graphene and Graphene oxide on the immune system under in vitro and in vivo conditions. Arch. Immunol. Ther. Exp. 64, 195–215. doi: 10.1007/s00005-015-0369-3
Dybowska-Sarapuk, L., Sosnowicz, W., Krzeminski, J., Grzeczkowicz, A., Granicka, L. H., Kotela, A., et al. (2020). Printed Graphene layer as a base for cell Electrostimulation-preliminary results. Int. J. Mol. Sci. 21:7865. doi: 10.3390/ijms21217865
Fabbro, A., Villari, A., Laishram, J., Scaini, D., Toma, F. M., Turco, A., et al. (2012). Spinal cord explants use carbon nanotube interfaces to enhance neurite outgrowth and to fortify synaptic inputs. ACS Nano 6, 2041–2055. doi: 10.1021/nn203519r
Fang, Z., Yang, E., Du, Y., Gao, D., Wu, G., Zhang, Y., et al. (2022). Biomimetic smart nanoplatform for dual imaging-guided synergistic cancer therapy. J. Mater. Chem. B 10, 966–976. doi: 10.1039/D1TB02306C
Fang, Q., Zhang, Y., Chen, X., Li, H., Cheng, L., Zhu, W., et al. (2019). Three-dimensional Graphene enhances neural stem cell proliferation through metabolic regulation. Front. Bioeng. Biotechnol. 7:436. doi: 10.3389/fbioe.2019.00436
Fanizza, C., Stefanelli, M., Risuglia, A., Bruni, E., Ietto, F., Incoronato, F., et al. (2022). In vitro and in vivo biocompatibility studies on engineered fabric with Graphene Nanoplatelets. Nanomaterials (Basel) 12:1405. doi: 10.3390/nano12091405
Feito, M. J., Diez-Orejas, R., Cicuéndez, M., Casarrubios, L., Rojo, J. M., and Portolés, M. T. (2019). Characterization of M1 and M2 polarization phenotypes in peritoneal macrophages after treatment with graphene oxide nanosheets. Colloids Surf. B Biointerfaces 176, 96–105. doi: 10.1016/j.colsurfb.2018.12.063
Feng, L., Zhang, S., and Liu, Z. (2011). Graphene based gene transfection. Nanoscale 3, 1252–1257. doi: 10.1039/c0nr00680g
Fernandes, L. F., Bruch, G. E., Massensini, A. R., and Frézard, F. (2018). Recent advances in the therapeutic and diagnostic use of liposomes and carbon Nanomaterials in ischemic stroke. Front. Neurosci. 12:453. doi: 10.3389/fnins.2018.00453
Fischer, R. A., Zhang, Y., Risner, M. L., Li, D., Xu, Y., and Sappington, R. M. (2018). Impact of Graphene on the efficacy of neuron culture substrates. Adv. Healthc. Mater. 7:e1701290. doi: 10.1002/adhm.201701290
Fu, C., Pan, S., Ma, Y., Kong, W., Qi, Z., and Yang, X. (2019). Effect of electrical stimulation combined with graphene-oxide-based membranes on neural stem cell proliferation and differentiation. Artif. Cells Nanomed. Biotechnol. 47, 1867–1876. doi: 10.1080/21691401.2019.1613422
Gabor, N. M., Song, J. C., Ma, Q., Nair, N. L., Taychatanapat, T., Watanabe, K., et al. (2011). Hot carrier-assisted intrinsic photoresponse in graphene. Science 334, 648–652. doi: 10.1126/science.1211384
Gaffaney, J. D., Dunning, F. M., Wang, Z., Hui, E., and Chapman, E. R. (2008). Synaptotagmin C2B domain regulates Ca2+−triggered fusion in vitro: critical residues revealed by scanning alanine mutagenesis. J. Biol. Chem. 283, 31763–31775. doi: 10.1074/jbc.M803355200
George, P. M., Bliss, T. M., Hua, T., Lee, A., Oh, B., Levinson, A., et al. (2017). Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials 142, 31–40. doi: 10.1016/j.biomaterials.2017.07.020
Ghasemi-Mobarakeh, L., Prabhakaran, M. P., Morshed, M., Nasr-Esfahani, M. H., Baharvand, H., Kiani, D., et al. (2011). Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 5, e17–35. doi: 10.1002/term.383
Gregory, W. E., Sharma, B., Hu, L., Raghavendra, A. J., and Podila, R. (2020). Interfacial charge transfer with exfoliated graphene inhibits fibril formation in lysozyme amyloid. Biointerphases 15:031010. doi: 10.1116/6.0000019
Guo, B., Feng, X., Wang, Y., Wang, X., and He, Y. (2021). Biomimetic and immunomodulatory baicalin-loaded graphene oxide-demineralized bone matrix scaffold for in vivo bone regeneration. J. Mater. Chem. B 9, 9720–9733. doi: 10.1039/D1TB00618E
Guo, X., and Mei, N. (2014). Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 22, 105–115. doi: 10.1016/j.jfda.2014.01.00
Guo, W., Qiu, J., Liu, J., and Liu, H. (2017). Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci. Rep. 7:5678. doi: 10.1038/s41598-017-06051-z
Guo, R., Zhang, S., Xiao, M., Qian, F., He, Z., Li, D., et al. (2016). Accelerating bioelectric functional development of neural stem cells by graphene coupling: implications for neural interfacing with conductive materials. Biomaterials 106, 193–204. doi: 10.1016/j.biomaterials.2016.08.019
Haataja, L., Gurlo, T., Huang, C. J., and Butler, P. C. (2008). Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 29, 303–316. doi: 10.1210/er.2007-0037
He, Z., Li, J., Chen, S. H., and Zhou, R. (2019). Surface inhomogeneity of Graphene oxide influences dissociation of Aβ(16-21) peptide assembly. J. Phys. Chem. B 123, 9098–9103. doi: 10.1021/acs.jpcb.9b07359
He, R., Yan, X., Guo, J., Xu, Q., Tang, B., and Sun, Q. (2018). Recent advances in biomarkers for Parkinson’s disease. Front. Aging Neurosci. 10:305. doi: 10.3389/fnagi.2018.00305
He, Z., Zhang, S., Song, Q., Li, W., Liu, D., Li, H., et al. (2016). The structural development of primary cultured hippocampal neurons on a graphene substrate. Colloids Surf. B Biointerfaces 146, 442–451. doi: 10.1016/j.colsurfb.2016.06.045
He, E., Zhou, Y., Luo, J., Xu, S., Zhang, K., Song, Y., et al. (2022). Sensitive detection of electrophysiology and dopamine vesicular exocytosis of hESC-derived dopaminergic neurons using multifunctional microelectrode array. Biosens. Bioelectron. 209:114263. doi: 10.1016/j.bios.2022.114263
Hong, B. J., Compton, O. C., An, Z., Eryazici, I., and Nguyen, S. T. (2012). Successful stabilization of graphene oxide in electrolyte solutions: enhancement of biofunctionalization and cellular uptake. ACS Nano 6, 63–73. doi: 10.1021/nn202355p
Hong, S. W., Lee, J. H., Kang, S. H., Hwang, E. Y., Hwang, Y. S., Lee, M. H., et al. (2014). Enhanced neural cell adhesion and neurite outgrowth on graphene-based biomimetic substrates. Biomed. Res. Int. 2014:212149. doi: 10.1155/2014/212149
Huang, Q., Cai, Y., Yang, X., Li, W., Pu, H., Liu, Z., et al. (2022). Graphene foam/hydrogel scaffolds for regeneration of peripheral nerve using ADSCs in a diabetic mouse model. Nano Res. 15, 3434–3445. doi: 10.1007/s12274-021-3961-3
Huang, Q., Cai, Y., Zhang, X., Liu, J., Liu, Z., Li, B., et al. (2021). Aligned Graphene mesh-supported double network natural hydrogel conduit loaded with Netrin-1 for peripheral nerve regeneration. ACS Appl. Mater. Interfaces 13, 112–122. doi: 10.1021/acsami.0c16391
Huang, Y. P., Hung, C. M., Hsu, Y. C., Zhong, C. Y., Wang, W. R., Chang, C. C., et al. (2016). Suppression of breast Cancer cell migration by Small interfering RNA delivered by Polyethylenimine-functionalized Graphene oxide. Nanoscale Res. Lett. 11:247. doi: 10.1186/s11671-016-1463-0
Hughes, Z. E., and Walsh, T. R. (2015). What makes a good graphene-binding peptide? Adsorption of amino acids and peptides at aqueous graphene interfaces. J. Mater. Chem. B 3, 3211–3221. doi: 10.1039/C5TB00004A
Hummers, W. S. Jr., and Offeman, R. E. (1958). Preparation of graphitic oxide. J. Am. Chem. Soc. 80:1339. doi: 10.1021/ja01539a017
Hung, H. S., Kung, M. L., Chen, F. C., Ke, Y. C., Shen, C. C., Yang, Y. C., et al. (2021). Nanogold-carried Graphene oxide: anti-inflammation and increased differentiation capacity of Mesenchymal stem cells. Nanomaterials (Basel) 11:2046. doi: 10.3390/nano11082046
Jagiełło, J., Sekuła-Stryjewska, M., Noga, S., Adamczyk, E., Dźwigońska, M., Kurcz, M., et al. (2019). Impact of Graphene-based surfaces on the basic biological properties of human umbilical cord Mesenchymal stem cells: implications for ex vivo cell expansion aimed at tissue repair. Int. J. Mol. Sci. 20:561. doi: 10.3390/ijms20184561
Jakus, A. E., Secor, E. B., Rutz, A. L., Jordan, S. W., Hersam, M. C., and Shah, R. N. (2015). Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 9, 4636–4648. doi: 10.1021/acsnano.5b01179
Jarrahian, A., and Heidaryan, E. (2012). A novel correlation approach to estimate thermal conductivity of pure carbon dioxide in the supercritical region. J. Supercrit. Fluids 64, 39–45. doi: 10.1016/j.supflu.2012.02.008
Javadi, M., Gu, Q., Naficy, S., Farajikhah, S., Crook, J. M., Wallace, G. G., et al. (2018). Conductive tough hydrogel for bioapplications. Macromol. Biosci. 18:270. doi: 10.1002/mabi.201700270
Jaworski, S., Sawosz, E., Grodzik, M., Winnicka, A., Prasek, M., Wierzbicki, M., et al. (2013). In vitro evaluation of the effects of graphene platelets on glioblastoma multiforme cells. Int. J. Nanomedicine 8, 413–420. doi: 10.2147/IJN.S39456
Jaworski, S., Sawosz, E., Kutwin, M., Wierzbicki, M., Hinzmann, M., Grodzik, M., et al. (2015). In vitro and in vivo effects of graphene oxide and reduced graphene oxide on glioblastoma. Int. J. Nanomed. 10, 1585–1596. doi: 10.2147/IJN.S77591
Jeong, B. S., Hu, W., Belyi, V., Rabadan, R., and Levine, A. J. (2010). Differential levels of transcription of p53-regulated genes by the arginine/proline polymorphism: p53 with arginine at codon 72 favors apoptosis. FASEB J. 24, 1347–1353. doi: 10.1096/fj.09-146001
Jiang, Z., Song, Q., Tang, M., Yang, L., Cheng, Y., Zhang, M., et al. (2016). Enhanced migration of neural stem cells by microglia grown on a three-dimensional Graphene scaffold. ACS Appl. Mater. Interfaces 8, 25069–25077. doi: 10.1021/acsami.6b06780
Jiang, Z., Zhang, Y., Tan, Y. W., Stormer, H. L., and Kim, P. (2007). Quantum hall effect in graphene. Solid State Commun. 143, 14–19. doi: 10.1016/j.ssc.2007.02.046
John, A. A., Subramanian, A. P., Vellayappan, M. V., Balaji, A., Mohandas, H., and Jaganathan, S. K. (2015). Carbon nanotubes and graphene as emerging candidates in neuroregeneration and neurodrug delivery. Int. J. Nanomed. 10, 4267–4277. doi: 10.2147/IJN.S83777
Kam, N. W., Jan, E., and Kotov, N. A. (2009). Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 9, 273–278. doi: 10.1021/nl802859a
Kanakia, S., Toussaint, J. D., Mullick Chowdhury, S., Tembulkar, T., Lee, S., Jiang, Y. P., et al. (2014). Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials 35, 7022–7031. doi: 10.1016/j.biomaterials.2014.04.066
Kang, M., Jeong, H., Park, S. W., Hong, J., Lee, H., Chae, Y., et al. (2022). Wireless graphene-based thermal patch for obtaining temperature distribution and performing thermography. Sci. Adv. 8:eabm6693. doi: 10.1126/sciadv.abm6693
Kang, Y., Liu, J., Wu, J., Yin, Q., Liang, H., Chen, A., et al. (2017). Graphene oxide and reduced graphene oxide induced neural pheochromocytoma-derived PC12 cell lines apoptosis and cell cycle alterations via the ERK signaling pathways. Int. J. Nanomed. 12, 5501–5510. doi: 10.2147/IJN.S141032
Kang, P., Wang, M. C., and Nam, S. W. (2016). Bioelectronics with two-dimensional materials. Microelectron. Eng. 161, 18–35. doi: 10.1016/j.mee.2016.04.003
Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469
Keefer, E. W., Botterman, B. R., Romero, M. I., Rossi, A. F., and Gross, G. W. (2008). Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 3, 434–439. doi: 10.1038/nnano.2008.174
Keles, E., Song, Y., Du, D., Dong, W. J., and Lin, Y. (2016). Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 4, 1291–1309. doi: 10.1039/C6BM00441E
Kim, T. H., Lee, K. B., and Choi, J. W. (2013). 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials 34, 8660–8670. doi: 10.1016/j.biomaterials.2013.07.101
Kim, T. I., McCall, J. G., Jung, Y. H., Huang, X., Siuda, E. R., Li, Y., et al. (2013). Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216. doi: 10.1126/science.1232437
Kim, H., Namgung, R., Singha, K., Oh, I. K., and Kim, W. J. (2011). Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug. Chem. 22, 2558–2567. doi: 10.1021/bc200397j
Kitko, K. E., Hong, T., Lazarenko, R. M., Ying, D., Xu, Y. Q., and Zhang, Q. (2018). Membrane cholesterol mediates the cellular effects of monolayer graphene substrates. Nat. Commun. 9:796. doi: 10.1038/s41467-018-03185-0
Kumar, P., Shahzad, F., Yu, S., Hong, S. M., Kim, Y.-H., and Koo, C. M. (2015). Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 94, 494–500. doi: 10.1016/j.carbon.2015.07.032
Kurapati, R., Mukherjee, S. P., Martín, C., Bepete, G., Vázquez, E., Pénicaud, A., et al. (2018). Degradation of single-layer and few-layer Graphene by neutrophil myeloperoxidase. Angew. Chem. Int. Ed. Engl. 57, 11722–11727. doi: 10.1002/anie.201806906
Kutwin, M., Sosnowska, M. E., Strojny-Cieślak, B., Jaworski, S., Trzaskowski, M., Wierzbicki, M., et al. (2021). MicroRNA delivery by Graphene-based complexes into Glioblastoma cells. Molecules 26:804. doi: 10.3390/molecules26195804
Kuzum, D., Takano, H., Shim, E., Reed, J. C., Juul, H., Richardson, A. G., et al. (2014). Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 5:5259. doi: 10.1038/ncomms6259
Lee, B. C., Lee, J. Y., Kim, J., Yoo, J. M., Kang, I., Kim, J. J., et al. (2020). Graphene quantum dots as anti-inflammatory therapy for colitis. Sci. Adv. 6:eaaz2630. doi: 10.1126/sciadv.aaz2630
Lee, W. C., Lim, C. H., Shi, H., Tang, L. A., Wang, Y., Lim, C. T., et al. (2011). Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 5, 7334–7341. doi: 10.1021/nn202190c
Lee, J. S., Lipatov, A., Ha, L., Shekhirev, M., Andalib, M. N., Sinitskii, A., et al. (2015). Graphene substrate for inducing neurite outgrowth. Biochem. Biophys. Res. Commun. 460, 267–273. doi: 10.1016/j.bbrc.2015.03.023
Lee, C., Wei, X., Kysar, J. W., and Hone, J. (2008). Measurement of the elastic properties and intrinsic strength of monolayer Graphene. Science 321, 385–388. doi: 10.1126/science.1157996
Lei, Z., Liu, W., Xing, W., Zhang, Y., Liu, Y., Tao, P., et al. (2022). Developing thermal regulating and electromagnetic shielding nacre-inspired Graphene-conjugated conducting polymer film via apparent Wiedemann-Franz law. ACS Appl. Mater. Interfaces 14, 49199–49211. doi: 10.1021/acsami.2c14805
Lei, W., Si, W., Xu, Y., Gu, Z., and Hao, Q. (2014). Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim. Acta 181, 707–722. doi: 10.1007/s00604-014-1160-6
Li, P., Xu, T., Wu, S., Lei, L., and He, D. (2017). Chronic exposure to graphene-based nanomaterials induces behavioral deficits and neural damage in Caenorhabditis elegans. J. Appl. Toxicol. 37, 1140–1150. doi: 10.1002/jat.3468
Li, M., Yang, X., Ren, J., Qu, K., and Qu, X. (2012). Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s disease. Adv. Mater. 24, 1722–1728. doi: 10.1002/adma.201104864
Li, N., Zhang, Q., Gao, S., Song, Q., Huang, R., Wang, L., et al. (2013). Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 3:1604. doi: 10.1038/srep01604
Li, N., Zhang, X., Song, Q., Su, R., Zhang, Q., Kong, T., et al. (2011). The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 32, 9374–9382. doi: 10.1016/j.biomaterials.2011.08.065
Li, X., Zhu, Y., Cai, W., Borysiak, M., Han, B., Chen, D., et al. (2009). Transfer of large-area Graphene films for high-performance transparent conductive electrodes. Nano Lett. 9:2623. doi: 10.1021/nl902623y
Liao, G., Hu, J., Chen, Z., Zhang, R., Wang, G., and Kuang, T. (2018). Preparation, properties, and applications of Graphene-based hydrogels. Front. Chem. 6:450. doi: 10.3389/fchem.2018.00450
Lin, L., Zhuang, X., Huang, R., Song, S., Wang, Z., Wang, S., et al. (2020). Size-dependent effects of suspended Graphene oxide nanoparticles on the cellular fate of mouse neural stem cells. Int. J. Nanomedicine 15, 1421–1435. doi: 10.2147/IJN.S225722
Liu, H. H., and Jan, Y. N. (2020). Mechanisms of neurite repair. Curr. Opin. Neurobiol. 63, 53–58. doi: 10.1016/j.conb.2020.02.010
Liu, C., and Luo, X. (2021). Potential molecular and graphene oxide chelators to dissolve amyloid-β plaques in Alzheimer’s disease: a density functional theory study. J. Mater. Chem. B 9, 2736–2746. doi: 10.1039/D0TB02985H
Liu, Z., Robinson, J. T., Sun, X., and Dai, H. (2008). PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 130, 10876–10877. doi: 10.1021/ja803688x
Liu, X., Yan, B., Li, Y., Ma, X., Jiao, W., Shi, K., et al. (2020). Graphene oxide-grafted magnetic Nanorings mediated Magnetothermodynamic therapy favoring reactive oxygen species-related immune response for enhanced antitumor efficacy. ACS Nano 14, 1936–1950. doi: 10.1021/acsnano.9b08320
Lopes, P., Xu, M., Zhang, M., Zhou, T., Yang, Y., Wang, C., et al. (2014). Direct electrochemical and AFM detection of amyloid-β peptide aggregation on basal plane HOPG. Nanoscale 6, 7853–7857. doi: 10.1039/C4NR02413C
López Tenorio, D., Valencia, C. H., Valencia, C., Zuluaga, F., Valencia, M. E., Mina, J. H., et al. (2019). Evaluation of the biocompatibility of CS-Graphene oxide compounds in vivo. Int. J. Mol. Sci. 20:1572. doi: 10.3390/ijms20071572
López-Dolado, E., González-Mayorga, A., Gutiérrez, M. C., and Serrano, M. C. (2016). Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials 99, 72–81. doi: 10.1016/j.biomaterials.2016.05.012
López-Dolado, E., González-Mayorga, A., Portolés, M. T., Feito, M. J., Ferrer, M. L., Del Monte, F., et al. (2015). Subacute tissue response to 3D Graphene oxide scaffolds implanted in the injured rat spinal cord. Adv. Healthc. Mater. 4, 1861–1868. doi: 10.1002/adhm.201500333
Lovat, V., Pantarotto, D., Lagostena, L., Cacciari, B., Grandolfo, M., Righi, M., et al. (2005). Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 5, 1107–1110. doi: 10.1021/nl050637m
Ma, T., Liu, Z., Wen, J., Gao, Y., Ren, X., Chen, H., et al. (2017). Tailoring the thermal and electrical transport properties of graphene films by grain size engineering. Nat. Commun. 8:14486. doi: 10.1038/ncomms14486
Ma, W. G., Lu, S. Q., Zhu, D. B., Xing, X. B., and Su, B. H. (2014). Isothermal detection of RNA transcription levels using graphene oxide-based SYBR Green I fluorescence platform. Commun. Stat. Theory Methods 45, 3036–3052. doi: 10.1016/j.cclet.2013.12.013
Magaz, A., Li, X., Gough, J. E., and Blaker, J. J. (2021). Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 119:111632. doi: 10.1016/j.msec.2020.111632
Malarkey, E. B., and Parpura, V. (2010). Carbon nanotubes in neuroscience. Acta Neurochir. Suppl. 106, 337–341. doi: 10.1007/978-3-211-98811-4_62
Masvidal-Codina, E., Smith, T. M., Rathore, D., Gao, Y., Illa, X., Prats-Alfonso, E., et al. (2021). Characterization of optogenetically-induced cortical spreading depression in awake mice using graphene micro-transistor arrays. J. Neural Eng. 18:abecf3. doi: 10.1088/1741-2552/abecf3
Mauro, N., Scialabba, C., Agnello, S., Cavallaro, G., and Giammona, G. (2020). Folic acid-functionalized graphene oxide nanosheets via plasma etching as a platform to combine NIR anticancer phototherapy and targeted drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 107:110201. doi: 10.1016/j.msec.2019.110201
Mendonça, M. C., Soares, E. S., de Jesus, M. B., Ceragioli, H. Y., Ferreira, M., Catharino, R. R., et al. (2015). Reduced graphene oxide induces transient blood-brain barrier opening: an in vivo study. J. Nanobiotechnology 13:78. doi: 10.1186/s12951-015-0143-z
Meng, S. (2014). Nerve cell differentiation using constant and programmed electrical stimulation through conductive non-functional graphene nanosheets film. Tissue Eng. Regen. Med. 11, 274–283. doi: 10.1007/s13770-014-0011-1
Meng, J., Agrahari, V., and Youm, I. (2017). Advances in targeted drug delivery approaches for the central nervous system tumors: the inspiration of Nanobiotechnology. J. Neuroimmune Pharmacol. 12, 84–98. doi: 10.1007/s11481-016-9698-1
Min, K. J., Kim, T. H., and Choi, J. W. (2017). Magnetic force-driven Graphene patterns to direct synaptogenesis of human neuronal cells. Materials (Basel) 10:1151. doi: 10.3390/ma10101151
Monaco, A. M., and Giugliano, M. (2014). Carbon-based smart nanomaterials in biomedicine and neuroengineering. Beilstein J. Nanotechnol. 5, 1849–1863. doi: 10.3762/bjnano.5.196
Morita, S. Y., Shirakawa, S., Kobayashi, Y., Nakamura, K., Teraoka, R., Kitagawa, S., et al. (2012). Enzymatic measurement of phosphatidylserine in cultured cells. J. Lipid Res. 53, 325–330. doi: 10.1194/jlr.D021808
Mousavi, S. M., Soroshnia, S., Hashemi, S. A., Babapoor, A., Ghasemi, Y., Savardashtaki, A., et al. (2019). Graphene nano-ribbon based high potential and efficiency for DNA, cancer therapy and drug delivery applications. Drug Metab. Rev. 51, 91–104. doi: 10.1080/03602532.2019.1582661
Mulcahey, P. J., Chen, Y., Driscoll, N., Murphy, B. B., Dickens, O. O., Johnson, A. T. C., et al. (2022). Multimodal, multiscale insights into hippocampal seizures enabled by transparent, Graphene-based microelectrode arrays. eNeuro 9:2022. doi: 10.1523/ENEURO.0386-21.2022
Nangare, S., and Patil, P. (2022). Chitosan mediated layer-by-layer assembly based graphene oxide decorated surface plasmon resonance biosensor for highly sensitive detection of β-amyloid. Int. J. Biol. Macromol. 214, 568–582. doi: 10.1016/j.ijbiomac.2022.06.129
Nayak, T. R., Andersen, H., Makam, V. S., Khaw, C., Bae, S., Xu, X., et al. (2011). Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 5, 4670–4678. doi: 10.1021/nn200500h
Nezakati, T., Cousins, B. G., and Seifalian, A. M. (2014). Toxicology of chemically modified graphene-based materials for medical application. Arch. Toxicol. 88, 1987–2012. doi: 10.1007/s00204-014-1361-0
Nie, W., Dai, X., Copus, J. S., Kengla, C., Xie, R., Seeds, M., et al. (2022). Rapid mineralization of graphene-based 3D porous scaffolds by semi-dry electrodeposition for photothermal treatment of tumor-induced bone defects. Acta Biomater. 153, 573–584. doi: 10.1016/j.actbio.2022.09.019
Novoselov, K. S., Geim, A. K., Morozov, S. V., Jiang, D., Zhang, Y., Dubonos, S. V., et al. (2004). Electric field effect in atomically thin carbon films. Science 306, 666–669. doi: 10.1126/science.1102896
Palermo, V., Kinloch, I. A., Ligi, S., and Pugno, N. M. (2016). Nanoscale mechanics of Graphene and Graphene oxide in composites: a scientific and technological perspective. Adv. Mater. 28, 6232–6238. doi: 10.1002/adma.201505469
Palumbo, A., Tourlomousis, F., Chang, R. C., and Yang, E.-H. (2018). Influence of transition metal Dichalcogenide surfaces on cellular morphology and adhesion. ACS Appl. Bio Mat. 1, 1448–1457. doi: 10.1021/acsabm.8b00405
Pampaloni, N. P., Lottner, M., Giugliano, M., Matruglio, A., D’Amico, F., Prato, M., et al. (2018). Single-layer graphene modulates neuronal communication and augments membrane ion currents. Nat. Nanotechnol. 13, 755–764. doi: 10.1038/s41565-018-0163-6
Pan, S., Qi, Z., Li, Q., Ma, Y., Fu, C., Zheng, S., et al. (2019). Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif Cells Nanomed. Biotechnol. 47, 651–664. doi: 10.1080/21691401.2019.1575843
Park, S. Y., Park, J., Sim, S. H., Sung, M. G., Kim, K. S., Hong, B. H., et al. (2011). Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 23, H263–H267. doi: 10.1002/adma.201101503
Park, D. W., Schendel, A. A., Mikael, S., Brodnick, S. K., Richner, T. J., Ness, J. P., et al. (2014). Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 5:5258. doi: 10.1038/ncomms6258
Parker, H., Gravagnuolo, A. M., Vranic, S., Crica, L. E., Newman, L., Carnell, O., et al. (2022). Graphene oxide modulates dendritic cell ability to promote T cell activation and cytokine production. Nanoscale 14, 17297–17314. doi: 10.1039/D2NR02169B
Pattammattel, A., Pande, P., Kuttappan, D., Puglia, M., Basu, A. K., Amalaradjou, M. A., et al. (2017). Controlling the Graphene-Bio Interface: Dispersions in Animal Sera for Enhanced Stability and Reduced Toxicity. Langmuir 33, 14184–14194. doi: 10.1021/acs.langmuir.7b02854
Pei, Q.-X., Sha, Z.-D., and Zhang, Y.-W. (2011). A theoretical analysis of the thermal conductivity of hydrogenated graphene. Carbon 49, 4752–4759. doi: 10.1016/j.carbon.2011.06.083
Peng, Z., Chang, Q., Xing, M., and Lu, F. (2023). Active hydrophilic Graphene oxide Nanocomposites delivery mediated by adipose-derived stem cell for elevated Photothermal therapy of breast Cancer. Int. J. Nanomed. 18, 971–986. doi: 10.2147/IJN.S380029
Porter, D. W., Hubbs, A. F., Mercer, R. R., Wu, N., Wolfarth, M. G., Sriram, K., et al. (2010). Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 269, 136–147. doi: 10.1016/j.tox.2009.10.017
Portioli, C., Bussy, C., Mazza, M., Lozano, N., Jasim, D. A., Prato, M., et al. (2020). Intracerebral injection of Graphene oxide Nanosheets mitigates microglial activation without inducing acute neurotoxicity: a pilot comparison to other Nanomaterials. Small :e2004029
Poulsen, S. S., Saber, A. T., Williams, A., Andersen, O., Købler, C., Atluri, R., et al. (2015). MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol. Appl. Pharmacol. 284, 16–32. doi: 10.1016/j.taap.2014.12.011
Qi, Z., Guo, W., Zheng, S., Fu, C., Ma, Y., Pan, S., et al. (2019). Enhancement of neural stem cell survival, proliferation and differentiation by IGF-1 delivery in graphene oxide-incorporated PLGA electrospun nanofibrous mats. RSC Adv. 9, 8315–8325. doi: 10.1039/C8RA10103E
Qian, Y., Song, J., Zhao, X., Chen, W., Ouyang, Y., Yuan, W., et al. (2018a). 3D fabrication with integration molding of a Graphene oxide/Polycaprolactone Nanoscaffold for Neurite regeneration and angiogenesis. Adv. Sci. 5:1700499. doi: 10.1002/advs.201700499
Qian, Y., Zhao, X., Han, Q., Chen, W., Li, H., and Yuan, W. (2018b). An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 9:323. doi: 10.1038/s41467-017-02598-7
Qu, L., Liu, Y., Baek, J. B., and Dai, L. (2010). Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 4, 1321–1326. doi: 10.1021/nn901850u
Rahaie, M., and Noroozi, S. K. (2019). A nanobiosensor based on graphene oxide and DNA binding dye for multi-microRNAs detection. Biosci. Rep. 39:1404. doi: 10.1042/BSR20181404
Rahman, L., Jacobsen, N. R., Aziz, S. A., Wu, D., Williams, A., Yauk, C. L., et al. (2017). Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: investigating the mechanisms of pulmonary carcinogenesis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 823, 28–44. doi: 10.1016/j.mrgentox.2017.08.005
Rakhilin, N., Barth, B., Choi, J., Muñoz, N. L., Kulkarni, S., Jones, J. S., et al. (2016). Simultaneous optical and electrical in vivo analysis of the enteric nervous system. Nat. Commun. 7:11800. doi: 10.1038/ncomms11800
Rastogi, S. K., Garg, R., Scopelliti, M. G., Pinto, B. I., Hartung, J. E., Kim, S., et al. (2020). Remote nongenetic optical modulation of neuronal activity using fuzzy graphene. Proc. Natl. Acad. Sci. U. S. A. 117, 13339–13349. doi: 10.1073/pnas.1919921117
Rastogi, S. K., Raghavan, G., Yang, G., and Cohen-Karni, T. (2017). Effect of Graphene on nonneuronal and neuronal cell viability and stress. Nano Lett. 17, 3297–3301. doi: 10.1021/acs.nanolett.7b01215
Rauti, R., Lozano, N., León, V., Scaini, D., Musto, M., Rago, I., et al. (2016). Graphene oxide Nanosheets reshape synaptic function in cultured brain networks. ACS Nano 10, 4459–4471. doi: 10.1021/acsnano.6b00130
Rauti, R., Medelin, M., Newman, L., Vranic, S., Reina, G., Bianco, A., et al. (2019). Graphene oxide flakes tune excitatory neurotransmission in vivo by targeting hippocampal synapses. Nano Lett. 19, 2858–2870. doi: 10.1021/acs.nanolett.8b04903
Reina, A., Jia, X., Ho, J., Nezich, D., Son, H., Bulovic, V., et al. (2009). Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 9, 30–35. doi: 10.1021/nl801827v
Robinson, J. T., Tabakman, S. M., Liang, Y., Wang, H., Casalongue, H. S., Vinh, D., et al. (2011). Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 133, 6825–6831. doi: 10.1021/ja2010175
Sadrolhosseini, A. R., Habibiasr, M., Shafie, S., Solaimani, H., and Lim, H. N. (2019). Optical and thermal properties of laser-ablated platinum nanoparticles Graphene oxide composite. Int. J. Mol. Sci. 20:6153. doi: 10.3390/ijms20246153
Saeedi, M., Eslamifar, M., Khezri, K., and Dizaj, S. M. (2019). Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 111, 666–675. doi: 10.1016/j.biopha.2018.12.133
Sahni, D., Jea, A., Mata, J. A., Marcano, D. C., Sivaganesan, A., Berlin, J. M., et al. (2013). Biocompatibility of pristine graphene for neuronal interface. J. Neurosurg. Pediatr. 11, 575–583. doi: 10.3171/2013.1.PEDS12374
Savchenko, A., Cherkas, V., Liu, C., Braun, G. B., Kleschevnikov, A., Miller, Y. I., et al. (2018). Graphene biointerfaces for optical stimulation of cells. Sci. Adv. 4:eaat0351. doi: 10.1126/sciadv.aat0351
Sawosz, E., Jaworski, S., Kutwin, M., Vadalasetty, K. P., Grodzik, M., Wierzbicki, M., et al. (2015). Graphene functionalized with arginine decreases the development of Glioblastoma Multiforme tumor in a gene-dependent manner. Int. J. Mol. Sci. 16, 25214–25233. doi: 10.3390/ijms161025214
Schinwald, A., Murphy, F. A., Jones, A., MacNee, W., and Donaldson, K. (2012). Graphene-based nanoplatelets: a new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS Nano 6, 736–746. doi: 10.1021/nn204229f
Secomandi, N., Franceschi Biagioni, A., Kostarelos, K., Cellot, G., and Ballerini, L. (2020). Thin graphene oxide nanoflakes modulate glutamatergic synapses in the amygdala cultured circuits: exploiting synaptic approaches to anxiety disorders. Nanomedicine 26:102174. doi: 10.1016/j.nano.2020.102174
Serrano, M. C., Feito, M. J., González-Mayorga, A., Diez-Orejas, R., Matesanz, M. C., and Portolés, M. T. (2018). Response of macrophages and neural cells in contact with reduced graphene oxide microfibers. Biomater. Sci. 6, 2987–2997. doi: 10.1039/C8BM00902C
Shah, S., Yin, P. T., Uehara, T. M., Chueng, S. T., Yang, L., and Lee, K. B. (2014). Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 26, 3673–3680. doi: 10.1002/adma.201400523
Shi, S., Yang, K., Hong, H., Valdovinos, H. F., Nayak, T. R., Zhang, Y., et al. (2013). Tumor vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials 34, 3002–3009. doi: 10.1016/j.biomaterials.2013.01.047
Simon-Deckers, A., Gouget, B., Mayne-L’hermite, M., Herlin-Boime, N., Reynaud, C., and Carrière, M. (2008). In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 253, 137–146. doi: 10.1016/j.tox.2008.09.007
Singh, S. K., Singh, M. K., Nayak, M. K., Kumari, S., Shrivastava, S., Grácio, J. J., et al. (2011). Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano 5, 4987–4996. doi: 10.1021/nn201092p
Sinha, A., Choi, Y., Nguyen, M. H., Nguyen, T. L., Choi, S. W., and Kim, J. (2019). A 3D macroporous alginate Graphene scaffold with an extremely slow release of a loaded cargo for in situ long-term activation of dendritic cells. Adv. Healthc. Mater. 8:e1800571. doi: 10.1002/adhm.201800571
Solanki, A., Chueng, S. T., Yin, P. T., Kappera, R., Chhowalla, M., and Lee, K. B. (2013). Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv. Mater. 25, 5477–5482. doi: 10.1002/adma.201302219
Song, G., Shui, R., Wang, D., Fang, R., Yuan, T., Li, L., et al. (2022). Aptamer-conjugated graphene oxide-based surface assisted laser desorption ionization mass spectrometry for selective extraction and detection of Aβ(1-42) in an Alzheimer’s disease SH-SY5 cell model. Front. Aging Neurosci. 14:993281. doi: 10.3389/fnagi.2022.993281
Sun, H., Jiang, Y., Hua, R., Huang, R., Shi, L., Dong, Y., et al. (2022). Graphene and 2D hexagonal boron nitride Heterostructure for thermal management in actively tunable manner. Nanomaterials (Basel) 12:4057. doi: 10.3390/nano12224057
Sun, X., Liu, Z., Welsher, K., Robinson, J. T., Goodwin, A., Zaric, S., et al. (2008). Nano-Graphene oxide for cellular imaging and drug delivery. Nano Res. 1, 203–212. doi: 10.1007/s12274-008-8021-8
Szarowski, D. H., Andersen, M. D., Retterer, S., Spence, A. J., Isaacson, M., Craighead, H. G., et al. (2003). Brain responses to micro-machined silicon devices. Brain Res. 983, 23–35. doi: 10.1016/S0006-8993(03)03023-3
Tak, K., Sharma, R., Dave, V., Jain, S., and Sharma, S. (2020). Clitoria ternatea mediated synthesis of Graphene quantum dots for the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 11, 3741–3748. doi: 10.1021/acschemneuro.0c00273
Tan, X., Liu, T. H., Zhou, W., Yuan, Q., Ying, J., Yan, Q., et al. (2022). Enhanced electromagnetic shielding and thermal conductive properties of polyolefin composites with a Ti(3)C(2)T(x) MXene/Graphene framework connected by a hydrogen-bonded Interface. ACS Nano 16, 9254–9266. doi: 10.1021/acsnano.2c01716
Tang, M., Song, Q., Li, N., Jiang, Z., Huang, R., and Cheng, G. (2013). Enhancement of electrical signaling in neural networks on graphene films. Biomaterials 34, 6402–6411. doi: 10.1016/j.biomaterials.2013.05.024
Uesaka, N., Hirai, S., Maruyama, T., Ruthazer, E. S., and Yamamoto, N. (2005). Activity dependence of cortical axon branch formation: a morphological and electrophysiological study using organotypic slice cultures. J. Neurosci. 25, 1–9. doi: 10.1523/JNEUROSCI.3855-04.2005
Ulloa Severino, F. P., Ban, J., Song, Q., Tang, M., Bianconi, G., Cheng, G., et al. (2016). The role of dimensionality in neuronal network dynamics. Sci. Rep. 6:29640. doi: 10.1038/srep29640
Veliev, F., Briançon-Marjollet, A., Bouchiat, V., and Delacour, C. (2016). Impact of crystalline quality on neuronal affinity of pristine graphene. Biomaterials 86, 33–41. doi: 10.1016/j.biomaterials.2016.01.042
Vlasceanu, G. M., Șelaru, A., Dinescu, S., Balta, C., Herman, H., Gharbia, S., et al. (2020). Comprehensive appraisal of Graphene-oxide ratio in porous biopolymer hybrids targeting bone-tissue regeneration. Nanomaterials (Basel) 10:1444. doi: 10.3390/nano10081444
Wang, X., Cao, F., Yan, M., Liu, Y., Zhu, X., Sun, H., et al. (2019). Alum-functionalized graphene oxide nanocomplexes for effective anticancer vaccination. Acta Biomater. 83, 390–399. doi: 10.1016/j.actbio.2018.11.023
Wang, W., Junior, J. R. P., Nalesso, P. R. L., Musson, D., Cornish, J., Mendonça, F., et al. (2019). Engineered 3D printed poly(ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 100, 759–770. doi: 10.1016/j.msec.2019.03.047
Wang, Y., Lee, W. C., Manga, K. K., Ang, P. K., Lu, J., Liu, Y. P., et al. (2012). Fluorinated graphene for promoting neuro-induction of stem cells. Adv. Mater. 24, 4285–4290. doi: 10.1002/adma.201200846
Wang, Y., Li, Z., Wang, J., Li, J., and Lin, Y. (2011). Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol. 29, 205–212. doi: 10.1016/j.tibtech.2011.01.008
Wang, L., Liu, X., Fu, J., Ning, X., Zhang, M., Jiang, Z., et al. (2019a). Release of methylene blue from graphene oxide-coated electrospun nanofibrous scaffolds to modulate functions of neural progenitor cells. Acta Biomater. 88, 346–356. doi: 10.1016/j.actbio.2019.02.036
Wang, K., Ruan, J., Song, H., Zhang, J., Wo, Y., Guo, S., et al. (2011). Biocompatibility of Graphene oxide. Nanoscale Res. Lett. 6:8. doi: 10.1007/s11671-010-9751-6
Wang, L., Song, D., Zhang, X., Ding, Z., Kong, X., Lu, Q., et al. (2019b). Silk-Graphene hybrid hydrogels with multiple cues to induce nerve cell behavior. ACS Biomater Sci. Eng. 5, 613–622. doi: 10.1021/acsbiomaterials.8b01481
Wang, K., Wang, L., Chen, L., Peng, C., Luo, B., Mo, J., et al. (2021). Intranasal administration of dauricine loaded on graphene oxide: multi-target therapy for Alzheimer’s disease. Drug Deliv. 28, 580–593. doi: 10.1080/10717544.2021.1895909
Wang, Y., Wang, K., Zhao, J., Liu, X., Bu, J., Yan, X., et al. (2013). Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 135, 4799–4804. doi: 10.1021/ja312221g
Wang, F., Zhang, Y., Tian, C., Girit, C., Zettl, A., Crommie, M., et al. (2008). Gate-variable optical transitions in graphene. Science 320, 206–209. doi: 10.1126/science.1152793
Wang, K., Zhou, Z., Zhang, J., Tang, J., Wu, P., Wang, Y., et al. (2020). Electrical and thermal and self-healing properties of Graphene-Thermopolyurethane flexible conductive films. Nanomaterials (Basel) 10:753. doi: 10.3390/nano10040753
Wang, J., Zhu, L., Chen, J., Li, B., and Thong, J. T. (2013). Suppressing thermal conductivity of suspended tri-layer graphene by gold deposition. Adv. Mater. 25, 6884–6888. doi: 10.1002/adma.201303362
Wei, W., and Wang, X. (2021). Graphene-based electrode materials for neural activity detection. Materials (Basel) 14:6170. doi: 10.3390/ma14206170
Weltman, A., Yoo, J., and Meng, E. (2016). Flexible, penetrating brain probes enabled by advances in polymer microfabrication. Micromachines (Basel) 7:180. doi: 10.3390/mi7100180
Wojtoniszak, M., Chen, X., Kalenczuk, R. J., Wajda, A., Łapczuk, J., Kurzewski, M., et al. (2012). Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B Biointerfaces 89, 79–85. doi: 10.1016/j.colsurfb.2011.08.026
Wu, Q., Yin, L., Li, X., Tang, M., Zhang, T., and Wang, D. (2013). Contributions of altered permeability of intestinal barrier and defecation behavior to toxicity formation from graphene oxide in nematode Caenorhabditis elegans. Nanoscale 5, 9934–9943. doi: 10.1039/c3nr02084c
Xiao, S., Zhou, D., Luan, P., Gu, B., Feng, L., Fan, S., et al. (2016). Graphene quantum dots conjugated neuroprotective peptide improve learning and memory capability. Biomaterials 106, 98–110. doi: 10.1016/j.biomaterials.2016.08.021
Xu, C., Shi, X., Ji, A., Shi, L., Zhou, C., and Cui, Y. (2015). Fabrication and characteristics of reduced Graphene oxide produced with different Green Reductants. PLoS One 10:e0144842. doi: 10.1371/journal.pone.0144842
Yan, M., Liu, Y., Zhu, X., Wang, X., Liu, L., Sun, H., et al. (2019). Nanoscale reduced Graphene oxide-mediated Photothermal therapy together with IDO inhibition and PD-L1 blockade synergistically promote antitumor immunity. ACS Appl. Mater. Interfaces 11, 1876–1885. doi: 10.1021/acsami.8b18751
Yang, J., Dong, X., Li, B., Chen, T., Yu, B., Wang, X., et al. (2022). Poria cocos polysaccharide-functionalized graphene oxide nanosheet induces efficient cancer immunotherapy in mice. Front. Bioeng. Biotechnol. 10:1050077. doi: 10.3389/fbioe.2022.1050077
Yang, K., Gong, H., Shi, X., Wan, J., Zhang, Y., and Liu, Z. (2013). In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 34, 2787–2795. doi: 10.1016/j.biomaterials.2013.01.001
Yang, K., Hu, L., Ma, X., Ye, S., Cheng, L., Shi, X., et al. (2012a). Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 24, 1868–1872. doi: 10.1002/adma.201104964
Yang, D., Li, T., Xu, M., Gao, F., Yang, J., Yang, Z., et al. (2014). Graphene oxide promotes the differentiation of mouse embryonic stem cells to dopamine neurons. Nanomedicine (Lond.) 9, 2445–2455. doi: 10.2217/nnm.13.197
Yang, K., Wan, J., Zhang, S., Tian, B., Zhang, Y., and Liu, Z. (2012b). The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 33, 2206–2214. doi: 10.1016/j.biomaterials.2011.11.064
Yang, L., Wang, F., Han, H., Yang, L., Zhang, G., and Fan, Z. (2015). Functionalized graphene oxide as a drug carrier for loading pirfenidone in treatment of subarachnoid hemorrhage. Colloids Surf. B Biointerfaces 129, 21–29. doi: 10.1016/j.colsurfb.2015.03.022
Yang, K., Zhang, S., Zhang, G., Sun, X., Lee, S. T., and Liu, Z. (2010). Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 10, 3318–3323. doi: 10.1021/nl100996u
Yao, Q., Liu, H., Lin, X., Ma, L., Zheng, X., Liu, Y., et al. (2019). 3D interpenetrated Graphene foam/58S bioactive glass scaffolds for electrical-stimulation-assisted differentiation of rabbit Mesenchymal stem cells to enhance bone regeneration. J. Biomed. Nanotechnol. 15, 602–611. doi: 10.1166/jbn.2019.2703
Yousaf, M., Ahmad, M., Bhatti, I. A., Nasir, A., Hasan, M., Jian, X., et al. (2019). In vivo and in vitro monitoring of amyloid aggregation via BSA@FGQDs multimodal probe. ACS Sens 4, 200–210. doi: 10.1021/acssensors.8b01216
Yu, D., Yin, Q., Wang, J., Yang, J., Chen, Z., Gao, Z., et al. (2021). SERS-based immunoassay enhanced with silver probe for selective separation and detection of Alzheimer’s disease biomarkers. Int. J. Nanomedicine 16, 1901–1911. doi: 10.2147/IJN.S293042
Zhang, Y., Ali, S. F., Dervishi, E., Xu, Y., Li, Z., Casciano, D., et al. (2010). Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 4, 3181–3186. doi: 10.1021/nn1007176
Zhang, L., Bai, Z., and Liu, L. (2016). Exceptional thermal conductance across hydrogen-bonded Graphene/polymer interfaces. Adv. Mater. Interfaces 3:1600211.
Zhang, C., Fan, S., Shao, H., Hu, X., Zhu, B., and Zhang, Y. (2019). Graphene trapped silk scaffolds integrate high conductivity and stability. Carbon 148, 16–27. doi: 10.1016/j.carbon.2019.03.042
Zhang, L., and Liu, L. (2019). Hierarchically hydrogen-bonded graphene/polymer interfaces with drastically enhanced interfacial thermal conductance. Nanoscale 11, 3656–3664. doi: 10.1039/C8NR08760A
Zhang, L., Lu, Z., Zhao, Q., Huang, J., Shen, H., and Zhang, Z. (2011). Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small 7, 460–464. doi: 10.1002/smll.201001522
Zhang, L., Xia, J., Zhao, Q., Liu, L., and Zhang, Z. (2010). Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 6, 537–544. doi: 10.1002/smll.200901680
Zhang, D., Yao, Y., Duan, Y., Yu, X., Shi, H., Nakkala, J. R., et al. (2020). Surface-anchored Graphene oxide Nanosheets on cell-scale micropatterned poly(d,l-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl. Mater. Interfaces 12, 7915–7930. doi: 10.1021/acsami.9b20321
Zhang, J., and Zhao, X. S. (2012). Conducting polymers directly coated on reduced Graphene oxide sheets as high-performance Supercapacitor electrodes. J. Phys. Chem. C 116, 5420–5426. doi: 10.1021/jp211474e
Zhao, L., Kong, J., Krasteva, N., and Wang, D. (2018). Deficit in the epidermal barrier induces toxicity and translocation of PEG modified graphene oxide in nematodes. Toxicol Res (Camb) 7, 1061–1070. doi: 10.1039/C8TX00136G
Zhao, Y., Zhang, S., Yu, T., Zhang, Y., Ye, G., Cui, H., et al. (2021). Ultra-conformal skin electrodes with synergistically enhanced conductivity for long-time and low-motion artifact epidermal electrophysiology. Nat. Commun. 12:4880. doi: 10.1038/s41467-021-25152-y
Zheng, Z., Huang, L., Yan, L., Yuan, F., Wang, L., Wang, K., et al. (2019). Polyaniline Functionalized Graphene Nanoelectrodes for the Regeneration of PC12 Cells via Electrical Stimulation. Int. J. Mol. Sci. 20. doi: 10.3390/ijms2008201
Zhou, J., Meng, L., Ye, W., Wang, Q., Geng, S., and Sun, C. (2018). A sensitive detection assay based on signal amplification technology for Alzheimer’s disease’s early biomarker in exosome. Anal. Chim. Acta 1022, 124–130. doi: 10.1016/j.aca.2018.03.016
Keywords: neuroscience, nanomaterials, graphene, neurons, treatment
Citation: Ye T, Yang Y, Bai J, Wu F-Y, Zhang L, Meng L-Y and Lan Y (2023) The mechanical, optical, and thermal properties of graphene influencing its pre-clinical use in treating neurological diseases. Front. Neurosci. 17:1162493. doi: 10.3389/fnins.2023.1162493
Edited by:
Gabriel A. Silva, University of California, San Diego, United StatesReviewed by:
Anna Mariano, Italian Institute of Technology, ItalyMatteo Moschetta, Italian Institute of Technology, Italy
Copyright © 2023 Ye, Yang, Bai, Wu, Zhang, Meng and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long-Yue Meng, lymeng@ybu.edu.cn; Yan Lan, lanyan@ybu.edu.cn
†These authors have contributed equally to this work
 Ting Ye
Ting Ye