
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 05 May 2023
Sec. Translational Neuroscience
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1144559
This article is part of the Research Topic Impacts of 2021 WHO Classification on the Precise Diagnosis and Management of Gliomas View all 8 articles
 Wenlin Chen1†
Wenlin Chen1† Shanmu Jin1,2†
Shanmu Jin1,2† Qianshu Liu1,3†
Qianshu Liu1,3† Hai Wang1†
Hai Wang1† Yu Xia1,3†
Yu Xia1,3† Xiaopeng Guo1,4†
Xiaopeng Guo1,4† Siying Guo1,3
Siying Guo1,3 Yaning Wang1
Yaning Wang1 Yixin Shi1,3
Yixin Shi1,3 Delin Liu1,3
Delin Liu1,3 Yilin Li1,2
Yilin Li1,2 Yuekun Wang1
Yuekun Wang1 Hao Xing1
Hao Xing1 Junlin Li1,3
Junlin Li1,3 Jiaming Wu1,3
Jiaming Wu1,3 Tingyu Liang1
Tingyu Liang1 Tian Qu1,3
Tian Qu1,3 Huanzhang Li1,3
Huanzhang Li1,3 Tianrui Yang1,3
Tianrui Yang1,3 Kun Zhang1,3
Kun Zhang1,3 Yu Wang1,4*
Yu Wang1,4* Wenbin Ma1,4*
Wenbin Ma1,4*Introduction: The fifth edition of the World Health Organization (WHO) classification of central nervous system (CNS) tumors released in 2021 formally defines pediatric-type diffuse gliomas. However, there is still little understanding of pediatric-type diffuse gliomas, and even less attention has been paid to adult patients. Therefore, this study describes the clinical radiological, survival, and molecular features of adult patients with pediatric-type glioma.
Methods: Adult patients who underwent surgery from January 2011 to January 2022, classified as pediatric-type glioma, were included in this study. Clinical, radiological, histopathological, molecular pathological, and survival data were collected for analysis.
Results: Among 596 adult patients, 20 patients with pediatric-type glioma were screened, including 6 with diffuse astrocytoma, MYB- or MYBL1-altered, 2 with diffuse midline glioma, H3 K27-altered, and 12 with diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype. Pediatric high-grade glioma (pHGG) frequently showed tumor enhancement, peritumoral edema, and intratumoral necrosis. Adult patients with pHGG showed a longer life expectancy than adult patients with glioblastoma. Common molecular alterations included chromosome alterations and CDKN2A/B, PIK3CA, and PTEN, while altered KMT5B and MET were found to affect the overall survival.
Conclusion: Our study demonstrated adult patients with pediatric-type glioma. Notably, our research aims to expand the current understanding of adult patients with pediatric-type diffuse gliomas. Furthermore, personalized therapies consisting of targeted molecular inhibitors for MET and VEGFA may exhibit beneficial effects in the corresponding population.
Diffuse gliomas, known as a group of astrocytic and oligodendroglial tumors with infiltrative growth patterns, predominate in malignant primary brain tumors and occur across all age groups, with an average annual incidence of 0.47, 1.84, and 8.72 per 100,000 in children, adolescents and young adults (AYA), and older adults (Ostrom et al., 2022). Even with the combined treatment of surgery, radiotherapy, chemotherapy, targeted therapy, and tumor treating fields, the overall prognosis remains dismal (Stupp et al., 2017). Given the poor prognosis status and intertumoral heterogeneity, researchers have been striving for a classification criterion to more precisely stratify treatment response and survival outcome. The 2016 World Health Organization (WHO) classification of central nervous system (CNS) tumors is based mainly on histology (Louis et al., 2016), while several prognostic molecular markers have been identified in recent years. Therefore, integrating molecular pathologic features into the 2021 WHO classification of CNS tumors updates our understanding of diffuse gliomas (Louis et al., 2021). For instance, IDH-wildtype diffuse astrocytoma or anaplastic astrocytoma with EGFR amplification, combined whole chromosome 7 gain and whole chromosome 10 loss (+7/−10) or TERT promoter mutation, resembles IDH-wildtype glioblastoma in the aggressive clinical course, underlining the importance of combining histopathology and molecular pathology in diffuse glioma diagnosis (Brat et al., 2018).
Pediatric-type diffuse gliomas, whose molecular alterations and survival outcomes are quite different from adult-type counterparts (Jones et al., 2012), are formally recognized in the latest classification and divided into pediatric low-grade glioma (pLGG) and pediatric high-grade glioma (pHGG) (Louis et al., 2021). pLGGs include diffuse astrocytoma, MYB- or MYBL1-altered; angiocentric glioma; polymorphous low-grade neuroepithelial tumor of the young; and diffuse low-grade glioma, MAPK pathway-altered. pHGGs include diffuse midline glioma (DMG), H3 K27-altered; diffuse hemispheric glioma, H3 G34-mutant; diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype; and infant-type hemispheric glioma. Besides, the denotations of adult-type and pediatric-type are not strictly limited to the population of the corresponding age stratum, giving us a hint on the classification of diffuse gliomas in adult patients. Adult cases of diffuse astrocytoma or anaplastic astrocytoma, IDH-wildtype failing to fall into glioblastoma, IDH-wildtype for lack of EGFR amplification, +7/−10 and TERT promoter mutation, should undergo additional molecular tests (e.g., MYB, MYBL1, BRAF, FGFR1, histone H3) and may be classified as certain subtype of pediatric-type diffuse glioma (Gritsch et al., 2022). Under the new classification, there is still a lack of clinical description of the pediatric type of diffuse glioma (Hong et al., 2022; Kalelioglu et al., 2022), and even less attention has been paid to adult patients in the pediatric type. Additionally, previous studies have tended to classify such patients as adult-type patients, with implications for both prognostic estimation and treatment. However, investigating adult patients with pediatric-type diffuse gliomas is essential for discriminating them from adult-type in clinical settings.
This research focused on and comprehensively analyzed the histological and molecular data of 20 adult patients with pediatric-type glioma according to the 2021 WHO classification of CNS tumors. Further studies were conducted to contribute preliminary knowledge regarding this population. Notably, we described their basic clinical information, radiological features, and molecular landscape. Likewise, the overall survival and possible prognostic molecular alterations were discussed.
Patients who underwent surgery at the Department of Neurosurgery at Peking Union Medical College Hospital (PUMCH) from January 2011 to January 2022 were screened (Guo et al., 2023). Adult patients (≥18 years old) classified as pediatric-type glioma according to the 2021 WHO classification of CNS tumors were enrolled in this study. Among them, patients with comprehensive clinical data were included for further analysis. The study was approved by the Institutional Ethics Review Board of PUMCH (S-424) and conformed to the requirements of the Declaration of Helsinki.
Clinical and radiological data were collected retrospectively from the medical records. Clinical information collected for analysis included gender, age of diagnosis, course of the disease, baseline Karnofsky performance status (KPS) score, clinical symptoms, the extent of surgical resection (ESR), and postoperative treatments. The survival status was collected through outpatient and telephone follow-ups. Overall survival (OS) was defined as the time from surgery to the patient’s death or final follow-up (treated as censored values). Furthermore, median overall survival was defined as the overall survival achieved by 50% of included patients.
Radiological features were collected from the baseline magnetic resonance imaging (MRI), including tumor number, tumor location, involvement of functional areas, maximum diameter of tumor, maximum diameter of peritumoral edema, cyst cavity, necrotic centers, and tumor appearance on T1WI, T2WI, and T1-enhanced sequences. The evaluation of images was performed by a team of radiologists, including 2 junior residents responsible for specific feature extraction, and 1 chief radiologist responsible for re-examination of the results. All radiologists have board certification.
Tumor pathology classification was determined with the 2021 WHO classification of CNS tumor criteria, and histopathological and molecular pathological data were collected. Histopathological data were obtained from the pathological reports by PUMCH, mainly including the Ki-67 index and histological grade. For molecular pathology, we screened 60 molecular markers, including IDH1/2, MYB, MYBL1, EGFR, and CDKN2A/B, summarized from recent studies on tumorigenesis and prognosis of glioma. We analyzed the molecular alterations of each enrolled patient using next-generation sequencing (NGS), polymerase chain reaction (PCR)-based assays, and fluorescence in situ hybridization methods (FISH). The list of molecular markers included in this study is shown in Supplementary Table 1. Furthermore, we demonstrated the features of adult patients with each subgroup of pediatric-type glioma in terms of clinical information, radiological features, prognostic status, and molecular alterations.
Normally distributed variables were compared by Student’s t-test, and non-normally distributed variables were compared between groups by Kruskal-Wallis H test. Comparisons of categorical variables were performed by the chi-squared test. Additionally, survival analyses were performed using the Kaplan–Meier method and log-rank test and were presented by the Kaplan–Meier curves. p < 0.05 was considered to be statistically significant. All statistical analyses were performed with SPSS (version 26.0, IBM, USA) statistical software, and graphs were created using R Studio (PBC & Certified B Corp.®, USA) and GraphPad Prism (9, GraphPad Software, USA) software.
Among 596 adult patients, 20 patients with pediatric-type glioma were included in this study. Of these patients, 6 presented diffuse astrocytoma, MYB- or MYBL1-altered (all previously diagnosed as diffuse astrocytoma, IDH-wildtype), 2 with diffuse midline glioma, H3 K27-altered (both previously diagnosed as diffuse midline glioma, H3 K27M-mutant), and 12 with diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype (all previously diagnosed as anaplastic astrocytoma, IDH-wildtype).
Among the 20 patients enrolled, 13 were male, 7 were female, the mean age of diagnosis was 46 years. The median preoperative KPS of enrolled patients was 92.5. Regarding clinical symptoms, intracranial hypertension, neurological deficits, and epilepsy occurred in 5, 12, and 2 patients, respectively. For ESR, all patients experienced surgery, 55.0% (11/20) underwent gross total resection, 15.0% (3/20) went through subtotal resection, and 30.0% (6/20) received tumor biopsy only. For comprehensive postoperative therapy, 3 patients received the standard Stupp regimen (Stupp et al., 2009) (radiotherapy with concomitant temozolomide followed by up to six cycles of adjuvant temozolomide), while 7 did not. Furthermore, 10 patients were unable to be classified due to incomplete records. The specific information is shown in Table 1.
Neurological imaging is a crucial basis of diagnosis for intracranial tumors, and we summarized the radiological characteristics of patients with pediatric-type diffuse gliomas. After removing missing data, 6 patients with diffuse astrocytoma, MYB- or MYBL1-altered, 1 with diffuse midline glioma, H3 K27-altered, and 7 with diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype were included in the analysis, and representative radiological images are shown (Figure 1).
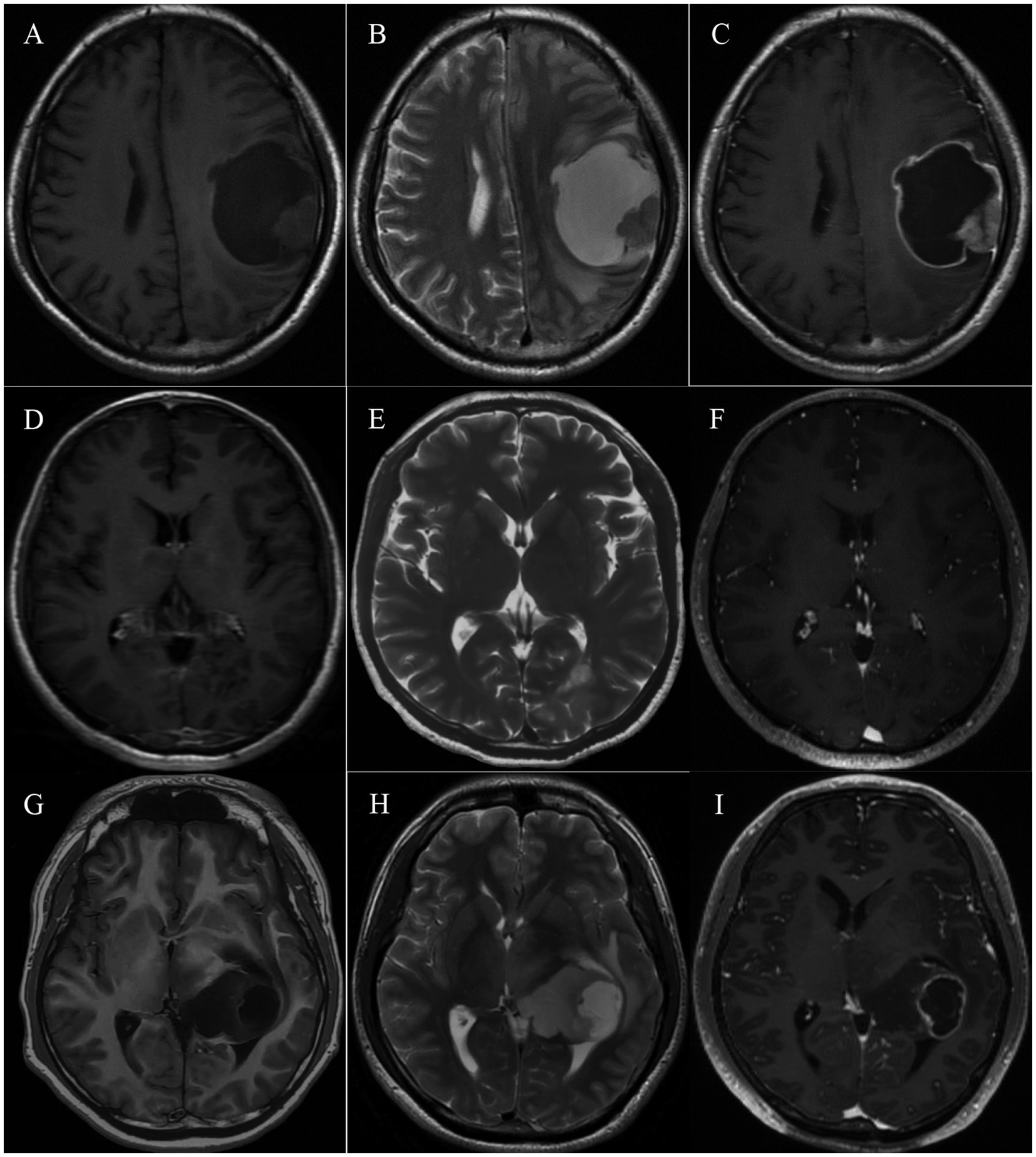
Figure 1. Typical MRI imaging of pediatric-type diffuse gliomas in adults. (A–C) MRI of a 32-year-old patient with primary diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype located in the left frontal and parietal lobe. The lesion shows a mixed signal on T1 and T2-weighted imaging and consists of an intermediately enhancing nodule and a ring-like enhancing cyst cavity. (D–F) MRI of a 47-year-old patient with diffuse astrocytoma, MYB- or MYBL1-altered, located in the left occipital lobe. The restricted lesion is hypointense on T1-weighted imaging, hyperintense on T2-weighted imaging, and not enhanced. (G–I) MRI of a 25-year-old patient with diffuse midline glioma, H3 K27-altered, located in the left thalamus and lateral ventricle. The cystic-solid lesion shows a mixed signal on T1 and T2-weighted imaging and ring-like enhancement.
In terms of tumor distribution, it was prevalent for patients to have single tumors across different groups, and involvement of functional areas was relatively rare. The mean maximum tumor diameter was 4.19, 4.96, and 4.17 cm for diffuse astrocytoma, MYB- or MYBL1-altered, diffuse midline glioma, H3 K27-altered, and diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype, respectively. MRI in all groups generally showed low or mixed density on T1W1 and high or mixed density on T2W1. On T1-enhanced sequence, 6/7 (85.7%) patients with diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype tended to exhibit ring-like and heterogeneous enhancement. In contrast, fewer patients (3/6, 50%) with diffuse astrocytoma, MYB- or MYBL1-altered, showed enhancement. Patients with high-grade pediatric-type glioma showed a higher incidence of peritumoral edema (85.7% vs. 50%) and intratumoral necrosis (71.4% vs. 16.7%) but relatively smaller diameter in both radiological features (2.72 cm vs. 3.39 cm; 2.38 cm vs. 4.99 cm). Table 2 summarizes the comprehensive radiological features of adult patients with pediatric-type diffuse glioma.
We further explored the survival of adult patients with pediatric-type diffuse glioma and compared it with adult-type diffuse glioma. The median OS (mOS) of diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype was 35.5 months. In contrast, the mOS of diffuse astrocytoma, MYB- or MYBL1-altered, was 46.3 months, suggesting that adult patients with high-grade pediatric-type diffuse glioma are less likely to experience longer survival and better prognosis. Compared with glioblastoma (mOS = 14.3 months) and astrocytoma (mOS = 55.4 months), adult patients with pediatric-type high-grade glioma showed significantly better mOS than adult patients with glioblastoma (p = 0.047), while no statistical significance were observed when comparing pediatric-type high-grade glioma and WHO grade 2, 3, 4 astrocytoma. These results and specific survival information are presented in Figure 2.
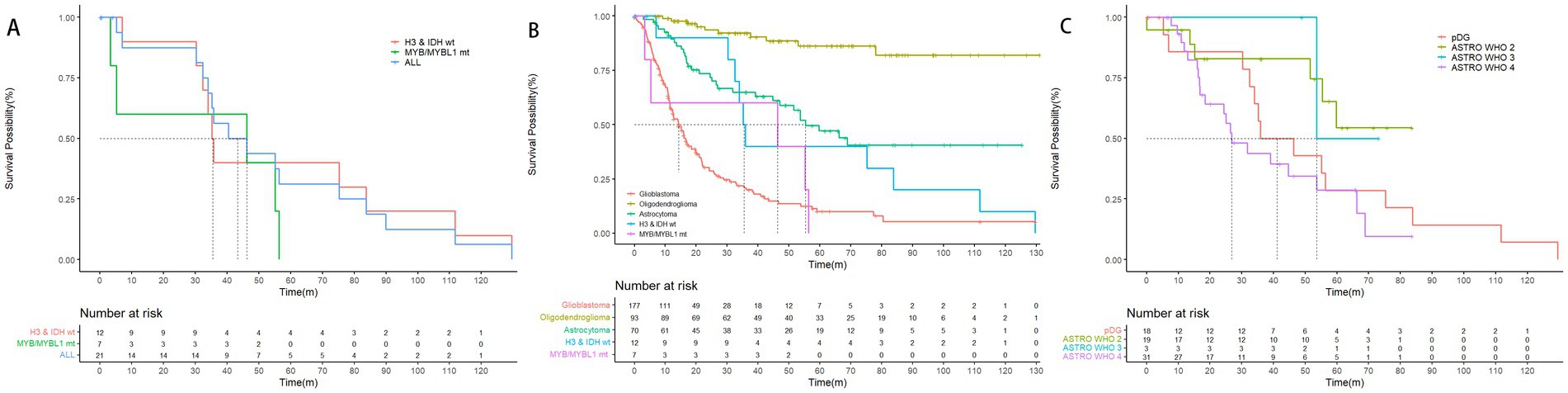
Figure 2. Overall survival of pediatric-type glioma based on the 2021 WHO classification of CNS tumors. (A) The mOS of pediatric-type glioma was 43.4 months, mOS of the 2 subtypes, diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype, and diffuse astrocytoma, MYB- or MYBL1-altered were 35.5 months, and 46.3 months (p = 0.43). (B) The mOS of glioblastoma, astrocytoma, diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype, and diffuse astrocytoma, MYB- or MYBL1-altered were 14.3 months, 55.4 months, 35.5 months, and 46.3 months. The mOS of oligodendroglioma cannot be calculated, as the survival possibility was above 50% at the time of follow-up. Notice that the mOS of glioblastoma was significantly shorter than diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype (p = 0.047). (C) The mOS of diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype and WHO grade 2, 3, and 4 diffuse astrocytoma were 35.5 months, NA, 53.6 months, and 26.9 months. Although the results did not indicate a statistically significant difference, there was a clear trend that mOS of patients decreased as the WHO grading increased. The mOS of patients with pediatric-type glioma was roughly between that of WHO grade 3 diffuse astrocytoma and WHO grade 4 diffuse astrocytoma.
Different subtypes of pediatric-type diffuse glioma showed distinct molecular characteristics, including mutations, amplifications, or deletions of chromosomes and genes. We analyzed the molecular alterations of tumors in each group of adult patients with pediatric-type diffuse gliomas. One patient was excluded because of an unaccomplished molecular pathological examination due to incomplete specimens. Figure 3 shows the alterations of molecular markers for each group of patients. Common genetic alterations in adult patients with pediatric-type diffuse gliomas mainly included chromosome alterations and CDKN2A/B (55 and 60%), PIK3CA (55%), and PTEN (55%).
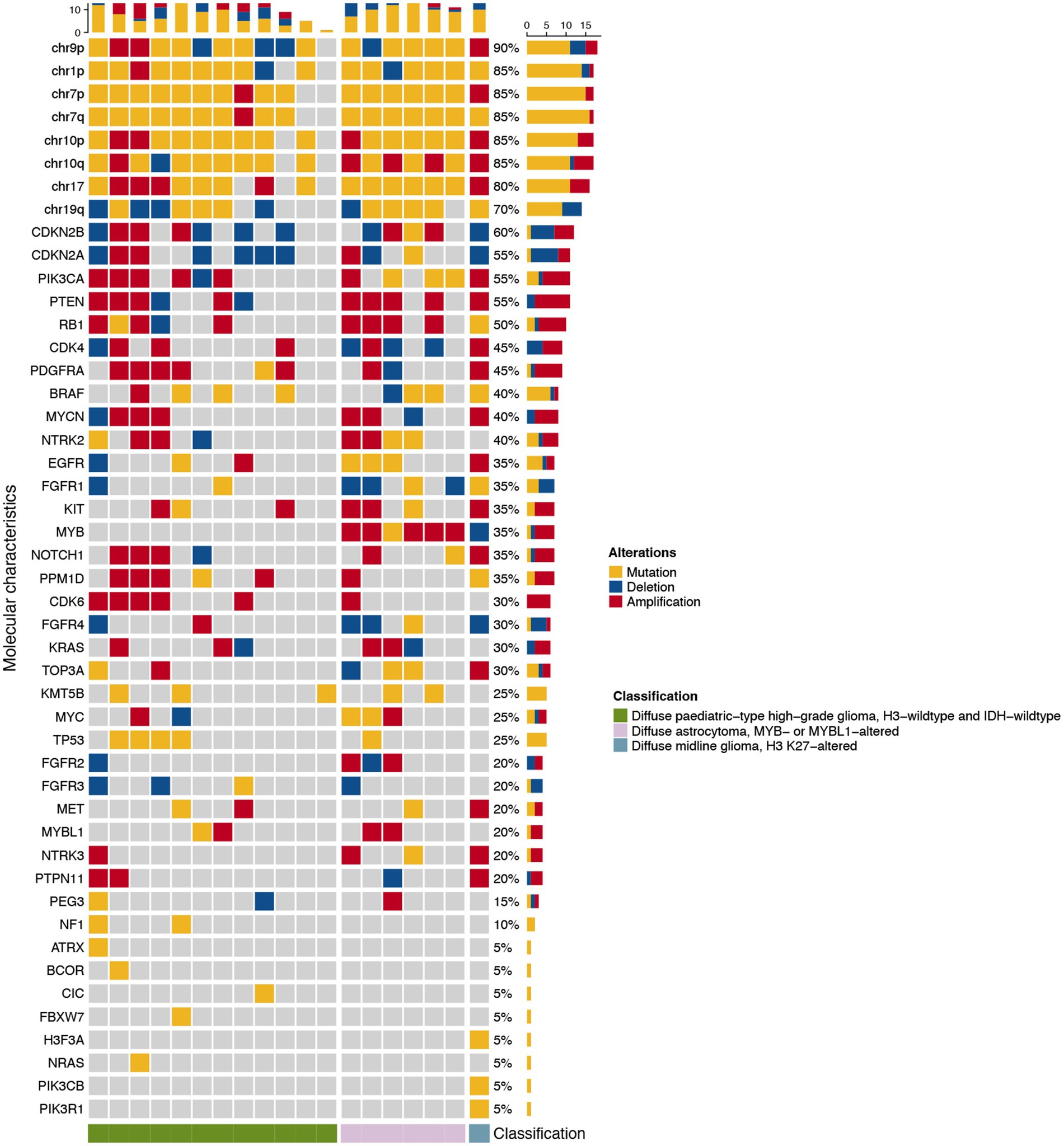
Figure 3. Waterfall heatmap of the molecular alterations of the 19 adult patients with pediatric-type glioma based on the 2021 WHO classification of CNS tumors. Each column represents an individual patient, and the cube’s color indicates the alteration status of each molecular characteristic.
Currently, molecular markers for adult glioma classification and prognosis include IDH1/2 mutations, homozygous deletion of CDKN2A and/or CDKN2B, EGFR amplification, and TERT promoter mutation. This study focused on the molecular pathology of adult patients with pediatric-type diffuse gliomas. Other clinical markers and molecular alterations associated with patient prognosis were also explored to provide clues for prognostic prediction and clinical decision-making. The results of multivariate regression analysis showed that alterations in KMT5B and MET were associated with shorter overall OS in adult patients with pediatric-type diffuse gliomas, with a value of p of 0.032 and 0.021, respectively (Figure 4).
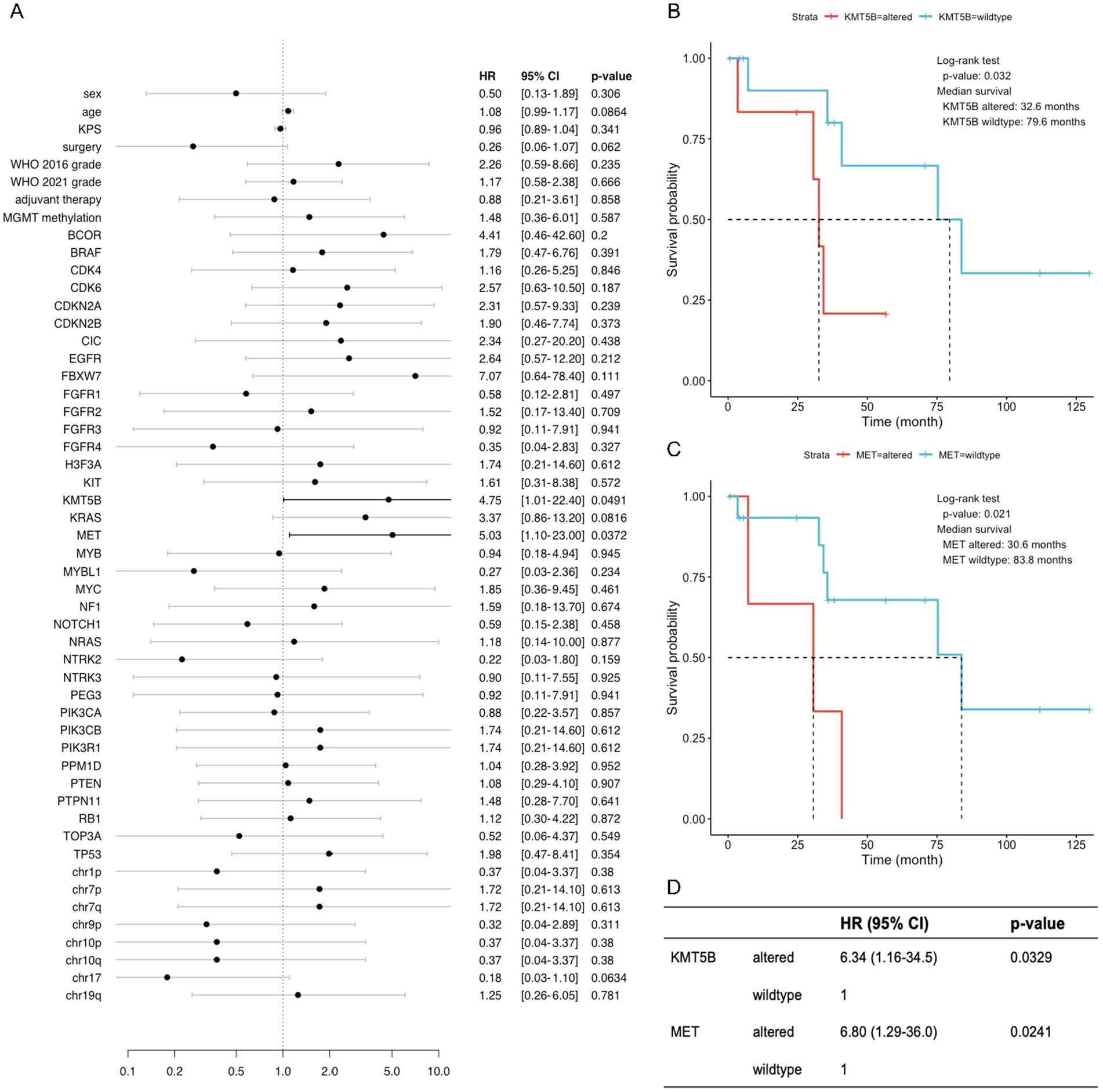
Figure 4. KMT5B and MET were identified as important prognostic factors for adult patients with pediatric-type glioma. (A) Forest plot of univariate Cox regressions of clinical and molecular variables. Only variables with calculable value of p and finite confidence intervals are shown. (B,C) Kaplan–Meier survival curves of two variables (KMT5B and MET) with significant results (p < 0.05) in univariate Cox analysis. (D) Multivariate Cox regression on KMT5B and MET, indicating that each factor exerts an independent effect on overall survival.
Since the new 2021 WHO classification of CNS tumors (Louis et al., 2021), studies on pediatric-type diffuse gliomas, especially in adult patients, have been lacking. Thus, this study included 20 adult patients with pediatric-type diffuse gliomas of 3 subtypes, and differential clinical features were discovered among these subgroups. For the radiological characteristics, tumor enhancement, peritumoral edema, and intratumoral necrosis were more frequently observed in the pHGG subgroup, corresponding to stronger invasiveness. Notably, adult patients with pHGG showed a relatively longer life expectancy than patients with glioblastoma. Additionally, CDKN2A/B, PIK3CA, and PTEN alterations were commonly observed in adult patients with pediatric-type diffuse gliomas. As for molecular alterations, altered KMT5B and MET were found to prominently affect overall survival.
In this study, we described and analyzed adult patients with pediatric-type diffuse gliomas, particularly diffuse astrocytoma, MYB- or MYBL1-altered, diffuse midline glioma, H3 K27-altered, and diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype. In this cohort, the median age is 46 years with a standard deviation (SD) of >10 years, which differs from the studies that suggested pediatric diffuse glioma occurs predominantly in children and young adults (Schramm et al., 2004). One of the possible reasons may be that our hospital receives mainly adult patients, and the absence of typical clinical symptoms may be related to their older age at diagnosis. At the same time, current researches focused mainly on pediatric cases, and the occurrence in the adult population may be expounded insufficiently. Patients with diffuse astrocytoma, MYB- or MYBL1-altered are mostly diagnosed with drug-resistant seizures, previous study suggested that 81% of these patients develop epilepsy in childhood and the median age of seizure onset is 10 years (Wefers et al., 2020). H3-wildtype and IDH-wildtype patients typically diagnosed in children, adolescents or young adults, and a meta-analysis of 190 patients found that the median age in this group was less than 12 years, although age of patients was broadly distributed with the oldest age being over 30 years (Mackay et al., 2017). For H3 K27-altered diffuse midline gliomas, the reported age of diagnosis is 30 years for adult patients, which is quite consistent with the patients we included that diagnosed as 45 and 25 years, respectively. More details shown in Supplementary Figure 1 and a review by statistician was accomplished (Meyronet et al., 2017; Dono et al., 2020; Enomoto et al., 2020; Lv et al., 2022). Twelve patients were initially diagnosed as anaplastic astrocytoma, IDH-wildtype, and reclassified as H3-wildtype and IDH-wildtype diffuse pediatric-type high-grade glioma according to the 2021 WHO classification of CNS tumors (Louis et al., 2016). Diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype can further be divided into 3 subtypes: diffuse pediatric-type high-grade glioma RTK2, diffuse pediatric-type high-grade glioma RTK1, and diffuse pediatric-type high-grade glioma MYCN (Korshunov et al., 2017; Ellison et al., 2019). Notably, H3-wildtype and IDH-wildtype pHGG show a relatively benign course compared to their adult counterparts. A multicenter study indicated a 2-year survival rate of 23.5% and mOS of 17.2 months (Mackay et al., 2017). In this study, survival analysis of different subtypes showed that H3-wildtype and IDH-wildtype pHGG have significantly longer mOS (35.5 months) than glioblastoma IDH-wildtype (14.3 months, p = 0.047). Prognostic risk factors for this subtype of pediatric-type glioma are currently unknown. However, a study conducted in 2022 analyzed 17 pediatric patients and concluded that TP53 mutation is a significant risk factor for poor prognosis (Hong et al., 2022).
Diffuse astrocytoma, MYB- or MYBL1-altered accounts for approximately 2% of all pediatric low-grade gliomas composed of monomorphic cells. MYB is a family of genes containing the MYB/SANT structural domain transcription factor, and the MYBL1 gene plays a similar role (Baker et al., 2009; Kim et al., 2018). Other possible molecular features include BRAF and FGFR1 alterations (Qaddoumi et al., 2016; Barinfeld et al., 2022). Previous reports of this pediatric type of tumor are scarce, especially studies focusing on adult patients. Wefers et al. analyzed the survival of 18 patients (mainly adults). They showed a good prognosis with a median follow-up of 2.5 years and only one recurrence during the follow-up period, which was subsequently eradicated by re-operation (Wefers et al., 2020). In our study, we described a total of 6 patients in terms of clinical, radiological, molecular, and prognostic characteristics, which were generally consistent with previous studies.
Two adult patients with DMGs located in the brain’s midline, involving the temporal lobe, thalamus, and pons (Meyronet et al., 2017). Thalamic DMGs are rarely reported, representing 1–5% of pediatric brain tumors (Mackay et al., 2018; Roux et al., 2020). In terms of clinical manifestations, one patient had prominent characteristics of pyramidal tract injury (reported incidence was 51%), as well as signs of limb sensory abnormalities and intracranial hypertension (Hoffman et al., 2018). For the radiological characteristics, previous researches indicated that DSC-MRI parameters, ADC values, and the T2-FLAIR mismatch sign are valuable in diagnosis and classification of DMGs (Kurokawa et al., 2022). However, due to the small sample size and insufficient MRI sequence, we were unable to verify this conclusion. Notably, the patient was diagnosed as both H3 p.K28M (K27M) mutation (for H3 K27-mutant subtypes) and amplification of EGFR (for the EGFR-mutant subtype), though it is reported that EGFR-altered DMGs most often occur during childhood (Broniscer et al., 2018; Castel et al., 2018).
Generally, pediatric gliomas exhibit hypointensity or isointensity on T1 (McNamara et al., 2022), a finding that was also observed in all samples of our study. In diffuse astrocytoma, MYB- or MYBL1 altered, large cysts are commonly reported, however, in our study, cyst was only observed in one case, albeit with the largest cyst diameter among all samples. Diffuse midline gliomas, H3 K27-altered usually exhibit T2 hyperintensity, with a various enhancement pattern. In our study, the only one case of DMG exhibited ring-like enhancement. In diffuse pediatric-type high-grade gliomas, H3-wildtype and IDH-wildtype, peritumoral edema and intratumoral necrosis, which are common characteristics of high-grade gliomas, were mild but frequently observed. Notably, there was an heterogeneity observed in imaging characteristics of the 7 diffuse pediatric-type high-grade gliomas, H3-wildtype and IDH-wildtype in our study. As previously mentioned, diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype can be further classified into 3 subtypes based on DNA methylation characteristics, and this imaging heterogeneity may be explained by the fact that different subtypes have different imaging patterns. For example, it has been reported that MYCN may be better circumscribed, with only mild peritumoral edema and homogeneous contrast enhancement (Tauziède-Espariat et al., 2020). The imaging characteristics of the other 2 subtypes, RTK1 and RTK2, have not been reported. Although further case accumulation is needed to draw a definitive conclusion, this imaging heterogeneity could be a future direction of research.
As a member of histone lysine methyltransferases (KMTs), KMT5B converts H4K20me1 into H4K20me2 and functions in a tumor suppressor-like manner. Through epigenetic silencing of oncogenes such as IL13RA2, overexpression of KMT5B reduced glioblastoma cell proliferation, cell viability, clonogenic potential in vitro, and tumor growth in vivo (López et al., 2021; Hulen et al., 2022). Two inactivating mutations of KMT5B (R187* and R699*) were identified in glioblastoma and diffuse intrinsic pontine glioma (DIPG) samples, which abrogated DNA repair and increased invasion and migration in neighboring cells (Vinci et al., 2018). Notably, 5 of 6 patients with altered KMT5B in our study had the same mutation of p.Glu833_Asp835delinsSerProSer, including 3 diffuse astrocytomas, MYB- or MYBL1-altered and 2 diffuse pediatric-type high-grade gliomas, H3-wildtype and IDH-wildtype. Considering their significantly shorter overall survivals and the anti-tumoral effect of KMT5B, it could be suspected that this deletion–insertion in the C-terminal region may cause functional defect to KMT5B and de-repression of oncogenes in a subgroup of adult patients with pediatric-type glioma. At the same time, the underlying mechanisms guarantee further investigation on a molecular level.
MET is among the top three dysregulated receptor tyrosine kinases (RTKs) in glioma cells, along with EGFR and PDGFRA (Snuderl et al., 2011). Activating mutations in MET are key events during the progression of low-grade gliomas to a higher grade, while MET gain in diffuse astrocytoma is associated with shorter overall survival (Pierscianek et al., 2013; Hu et al., 2018). Therefore, our finding that adult pediatric-type glioma patients with MET alterations have worse prognoses could be supported by previous results. In our study, 2 patients with diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype and 1 patient with diffuse midline glioma, H3 K27-altered had MET alterations. MET amplification is a common (22.9%) alteration in diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype (Hong et al., 2022). In diffuse midline glioma, H3 K27-altered, MET alterations including amplification and activating point mutations have been reported to occur sporadically (8.6–15.0%) (Hoffman et al., 2016; Porkholm et al., 2018; Dufour et al., 2020). No correlation between MET status and survival has been discovered in either of these two pediatric-type high-grade glioma subtypes (Hoffman et al., 2016; Porkholm et al., 2018; Dufour et al., 2020; Hong et al., 2022). Consistent with previous literature (Fabbri et al., 2022; Kalelioglu et al., 2022), MET alterations were not detected in any of our diffuse astrocytoma, MYB- or MYBL1-altered patients. MET-altered patients may merit particular attention in clinical management, especially in tumor recurrence. Bevacizumab has now become a common practice for recurrent glioma patients. However, inhibiting VEGFA could negate its suppression of HGF-dependent MET phosphorylation and tumor cell migration and lead to a more invasive phenotype (Lu et al., 2012). In light of these findings, combining anti-VEGFA and anti-MET treatments might clinically benefit MET-altered adult patients with pediatric glioma.
Our study results must be interpreted while considering some limitations. First of all, the systematic bias raised by the small sample size should be taken into consideration. In addition, forms of molecular alteration are various, in which simply classifying the molecular features as normal and altered may cover up some significant changes. Also, methylation profiling analysis was not conducted in the molecular diagnoses. Therefore, diagnostic uncertainties might exist. Other sources of errors include systematic errors produced during the process of immunohistochemical staining, data measurement, and observation (including imaging and patient follow-up). Therefore, further studies are necessary to confirm these findings.
In this study, we made a summative description and preliminary analysis of adult patients with pediatric diffuse glioma in light of the novel 2021 WHO classification of CNS tumors and suggested the correlation between molecular features and patients’ prognosis. Our research aims to expand the current understanding of adult patients with pediatric-type diffuse gliomas and to complement the limited research. Furthermore, personalized therapies consisting of targeted molecular inhibitors for MET and VEGFA may exhibit beneficial effects in the corresponding population.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Institutional Ethics Review Board of PUMCH. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
WC and XG: study conception and design. WC, SJ, QL, HW, YX, XG, SG, YNW, YS, DL, YL, YKW, HX, JL, JW, TL, TQ, HL, TY, and KZ: data collection. WC, SJ, QL, HW, and YX: data analysis and figures and tables. WC, SJ, QL, HW, YX, and XG: manuscript drafting and revision. YW and WM: study supervision. All authors contributed to the article and approved the submitted version.
This work was funded by the Beijing Municipal Natural Science Foundation (7202150) and the National High-Level Hospital Clinical Research Funding (2022-PUMCH-A-019) for YW and by the National High-Level Hospital Clinical Research Funding (2022-PUMCH-B-113), the Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program (2019ZLH101), and the Beijing Municipal Natural Science Foundation (19JCZDJC64200[Z]) for WM.
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1144559/full#supplementary-material
Baker, A. M., Fu, Q., Hayward, W., Lindsay, S. M., and Fletcher, T. M. (2009). The Myb/SANT domain of the telomere-binding protein TRF2 alters chromatin structure. Nucleic Acids Res. 37, 5019–5031. doi: 10.1093/nar/gkp515
Barinfeld, O., Zahavi, A., Weiss, S., Toledano, H., Michowiz, S., and Goldenberg-Cohen, N. (2022). Genetic alteration analysis of IDH1, IDH2, CDKN2A, MYB and MYBL1 in pediatric Low-grade gliomas. Front. Surg. 9:880048. doi: 10.3389/fsurg.2022.880048
Brat, D. J., Aldape, K., Colman, H., Holland, E. C., Louis, D. N., Jenkins, R. B., et al. (2018). cIMPACT-NOW update 3: recommended diagnostic criteria for "diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV". Acta Neuropathol. 136, 805–810. doi: 10.1007/s00401-018-1913-0
Broniscer, A., Hwang, S. N., Chamdine, O., Lin, T., Pounds, S., Onar-Thomas, A., et al. (2018). Bithalamic gliomas may be molecularly distinct from their unilateral high-grade counterparts. Brain Pathol. 28, 112–120. doi: 10.1111/bpa.12484
Castel, D., Philippe, C., Kergrohen, T., Sill, M., Merlevede, J., Barret, E., et al. (2018). Transcriptomic and epigenetic profiling of 'diffuse midline gliomas, H3 K27M-mutant' discriminate two subgroups based on the type of histone H3 mutated and not supratentorial or infratentorial location. Acta Neuropathol. Commun. 6:117. doi: 10.1186/s40478-018-0614-1
Dono, A., Takayasu, T., Ballester, L. Y., and Esquenazi, Y. (2020). Adult diffuse midline gliomas: clinical, radiological, and genetic characteristics. J. Clin. Neurosci. 82, 1–8. doi: 10.1016/j.jocn.2020.10.005
Dufour, C., Perbet, R., Leblond, P., Vasseur, R., Stechly, L., Pierache, A., et al. (2020). Identification of prognostic markers in diffuse midline gliomas H3K27M-mutant. Brain Pathol. 30, 179–190. doi: 10.1111/bpa.12768
Ellison, D. W., Hawkins, C., Jones, D. T. W., Onar-Thomas, A., Pfister, S. M., Reifenberger, G., et al. (2019). cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF(V600E) mutation. Acta Neuropathol. 137, 683–687. doi: 10.1007/s00401-019-01987-0
Enomoto, T., Aoki, M., Hamasaki, M., Abe, H., Nonaka, M., Inoue, T., et al. (2020). Midline glioma in adults: clinicopathological, genetic, and epigenetic analysis. Neurol. Med. Chir. 60, 136–146. doi: 10.2176/nmc.oa.2019-0168
Fabbri, V. P., Caporalini, C., Asioli, S., and Buccoliero, A. (2022). Paediatric-type diffuse low-grade gliomas: a clinically and biologically distinct group of tumours with a favourable outcome. Pathologica 114, 410–421. doi: 10.32074/1591-951X-828
Gritsch, S., Batchelor, T. T., and Gonzalez Castro, L. N. (2022). Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 128, 47–58. doi: 10.1002/cncr.33918
Guo, X., Shi, Y., Liu, D., Li, Y., Chen, W., Wang, Y., et al. (2023). Clinical updates on gliomas and implications of the 5th edition of the WHO classification of central nervous system tumors. Front. Oncol. 13:1131642. doi: 10.3389/fonc.2023.1131642
Hoffman, L. M., DeWire, M., Ryall, S., Buczkowicz, P., Leach, J., Miles, L., et al. (2016). Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol. Commun. 4:1. doi: 10.1186/s40478-015-0269-0
Hoffman, L. M., Veldhuijzen van Zanten, S. E. M., Colditz, N., Baugh, J., Chaney, B., Hoffmann, M., et al. (2018). Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the international and European Society for Pediatric Oncology DIPG registries. J. Clin. Oncol. 36, 1963–1972. doi: 10.1200/jco.2017.75.9308
Hong, L., Shi, Z. F., Li, K. K., Wang, W. W., Yang, R. R., Kwan, J. S. H., et al. (2022). Molecular landscape of pediatric type IDH wildtype, H3 wildtype hemispheric glioblastomas. Lab. Investig. 102, 731–740. doi: 10.1038/s41374-022-00769-9
Hu, H., Mu, Q., Bao, Z., Chen, Y., Liu, Y., Chen, J., et al. (2018). Mutational landscape of secondary glioblastoma guides MET-targeted trial in brain tumor. Cells 175, 1665–1678.e18. doi: 10.1016/j.cell.2018.09.038
Hulen, J., Kenny, D., Black, R., Hallgren, J., Hammond, K. G., Bredahl, E. C., et al. (2022). KMT5B is required for early motor development. Front. Genet. 13:901228. doi: 10.3389/fgene.2022.901228
Jones, C., Perryman, L., and Hargrave, D. (2012). Paediatric and adult malignant glioma: close relatives or distant cousins? Nat. Rev. Clin. Oncol. 9, 400–413. doi: 10.1038/nrclinonc.2012.87
Kalelioglu, T., Rama, B., Cho, B. B., Lopes, B. M., and Patel, S. H. (2022). Pediatric-type diffuse low-grade glioma with MYB/MYBL1 alteration: report of 2 cases. Neuroradiol. J. 36, 232–235. doi: 10.1177/19714009221126015
Kim, J., Geyer, F. C., Martelotto, L. G., Ng, C. K. Y., Lim, R. S., Selenica, P., et al. (2018). MYBL1 rearrangements and MYB amplification in breast adenoid cystic carcinomas lacking the MYB-NFIB fusion gene. J. Pathol. 244, 143–150. doi: 10.1002/path.5006
Korshunov, A., Schrimpf, D., Ryzhova, M., Sturm, D., Chavez, L., Hovestadt, V., et al. (2017). H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 134, 507–516. doi: 10.1007/s00401-017-1710-1
Kurokawa, R., Kurokawa, M., Baba, A., Ota, Y., Kim, J., Capizzano, A., et al. (2022). Dynamic susceptibility contrast-MRI parameters, ADC values, and the T2-FLAIR mismatch sign are useful to differentiate between H3-mutant and H3-wild-type high-grade midline glioma. Eur. Radiol. 32, 3672–3682. doi: 10.1007/s00330-021-08476-7
López, V., Tejedor, J. R., Carella, A., García, M. G., Santamarina-Ojeda, P., Pérez, R. F., et al. (2021). Epigenetic deregulation of the histone methyltransferase KMT5B contributes to malignant transformation in glioblastoma. Front. Cell Dev. Biol. 9:671838. doi: 10.3389/fcell.2021.671838
Louis, D. N., Perry, A., Reifenberger, G., von Deimling, A., Figarella-Branger, D., Cavenee, W. K., et al. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820. doi: 10.1007/s00401-016-1545-1
Louis, D. N., Perry, A., Wesseling, P., Brat, D. J., Cree, I. A., Figarella-Branger, D., et al. (2021). The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251. doi: 10.1093/neuonc/noab106
Lu, K. V., Chang, J. P., Parachoniak, C. A., Pandika, M. M., Aghi, M. K., Meyronet, D., et al. (2012). VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22, 21–35. doi: 10.1016/j.ccr.2012.05.037
Lv, K., Chen, H., Cao, X., du, P., Chen, J., Liu, X., et al. (2022). Development and validation of a machine learning algorithm for predicting diffuse midline glioma, H3 K27-altered, H3 K27 wild-type high-grade glioma, and primary CNS lymphoma of the brain midline in adults. J. Neurosurg. 1–9. doi: 10.3171/2022.11.Jns221544 [Epub ahead of print].
Mackay, A., Burford, A., Carvalho, D., Izquierdo, E., Fazal-Salom, J., Taylor, K. R., et al. (2017). Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32, 520–537.e5. doi: 10.1016/j.ccell.2017.08.017
Mackay, A., Burford, A., Molinari, V., Jones, D. T. W., Izquierdo, E., Brouwer-Visser, J., et al. (2018). Molecular, pathological, radiological, and immune profiling of non-brainstem pediatric high-grade glioma from the HERBY phase II randomized trial. Cancer Cell 33, 829–842.e5. doi: 10.1016/j.ccell.2018.04.004
McNamara, C., Mankad, K., Thust, S., Dixon, L., Limback-Stanic, C., D’Arco, F., et al. (2022). 2021 WHO classification of tumours of the central nervous system: a review for the neuroradiologist. Neuroradiology 64, 1919–1950. doi: 10.1007/s00234-022-03008-6
Meyronet, D., Esteban-Mader, M., Bonnet, C., Joly, M. O., Uro-Coste, E., Amiel-Benouaich, A., et al. (2017). Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 19, 1127–1134. doi: 10.1093/neuonc/now274
Ostrom, Q. T., Price, M., Neff, C., Cioffi, G., Waite, K. A., Kruchko, C., et al. (2022). CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 24, v1–v95. doi: 10.1093/neuonc/noac202
Pierscianek, D., Kim, Y. H., Motomura, K., Mittelbronn, M., Paulus, W., Brokinkel, B., et al. (2013). MET gain in diffuse astrocytomas is associated with poorer outcome. Brain Pathol. 23, 13–18. doi: 10.1111/j.1750-3639.2012.00609.x
Porkholm, M., Raunio, A., Vainionpää, R., Salonen, T., Hernesniemi, J., Valanne, L., et al. (2018). Molecular alterations in pediatric brainstem gliomas. Pediatr. Blood Cancer 65:e26751. doi: 10.1002/pbc.26751
Qaddoumi, I., Orisme, W., Wen, J., Santiago, T., Gupta, K., Dalton, J. D., et al. (2016). Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 131, 833–845. doi: 10.1007/s00401-016-1539-z
Roux, A., Pallud, J., Saffroy, R., Edjlali-Goujon, M., Debily, M. A., Boddaert, N., et al. (2020). High-grade gliomas in adolescents and young adults highlight histomolecular differences from their adult and pediatric counterparts. Neuro Oncol. 22, 1190–1202. doi: 10.1093/neuonc/noaa024
Schramm, J., Luyken, C., Urbach, H., Fimmers, R., and Blümcke, I. (2004). Evidence for a clinically distinct new subtype of grade II astrocytomas in patients with long-term epilepsy. Neurosurgery 55, 340–347; discussion 347-8. doi: 10.1227/01.neu.0000129546.38675.1b
Snuderl, M., Fazlollahi, L., Le, L. P., Nitta, M., Zhelyazkova, B. H., Davidson, C. J., et al. (2011). Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20, 810–817. doi: 10.1016/j.ccr.2011.11.005
Stupp, R., Hegi, M. E., Mason, W. P., van den Bent, M., Taphoorn, M. J., Janzer, R. C., et al. (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466. doi: 10.1016/s1470-2045(09)70025-7
Stupp, R., Taillibert, S., Kanner, A., Read, W., Steinberg, D. M., Lhermitte, B., et al. (2017). Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318, 2306–2316. doi: 10.1001/jama.2017.18718
Tauziède-Espariat, A., Debily, M. A., Castel, D., Grill, J., Puget, S., Roux, A., et al. (2020). The pediatric supratentorial MYCN-amplified high-grade gliomas methylation class presents the same radiological, histopathological and molecular features as their pontine counterparts. Acta Neuropathol. Commun. 8:104. doi: 10.1186/s40478-020-00974-x
Vinci, M., Burford, A., Molinari, V., Kessler, K., Popov, S., Clarke, M., et al. (2018). Functional diversity and cooperativity between subclonal populations of pediatric glioblastoma and diffuse intrinsic pontine glioma cells. Nat. Med. 24, 1204–1215. doi: 10.1038/s41591-018-0086-7
Wefers, A. K., Stichel, D., Schrimpf, D., Coras, R., Pages, M., Tauziède-Espariat, A., et al. (2020). Isomorphic diffuse glioma is a morphologically and molecularly distinct tumour entity with recurrent gene fusions of MYBL1 or MYB and a benign disease course. Acta Neuropathol. 139, 193–209. doi: 10.1007/s00401-019-02078-w
Keywords: glioma, 2021 WHO classification of central nervous system tumors, pediatric-type diffuse gliomas, adult patients, molecular pathology
Citation: Chen W, Jin S, Liu Q, Wang H, Xia Y, Guo X, Guo S, Wang Y, Shi Y, Liu D, Li Y, Wang Y, Xing H, Li J, Wu J, Liang T, Qu T, Li H, Yang T, Zhang K, Wang Y and Ma W (2023) Spotlights on adult patients with pediatric-type diffuse gliomas in accordance with the 2021 WHO classification of CNS tumors. Front. Neurosci. 17:1144559. doi: 10.3389/fnins.2023.1144559
Received: 14 January 2023; Accepted: 17 April 2023;
Published: 05 May 2023.
Edited by:
Arthur Maynart Pereira Oliveira, Universidade Federal de Sergipe, BrazilReviewed by:
Sandra Camelo-Piragua, University of Michigan, United StatesCopyright © 2023 Chen, Jin, Liu, Wang, Xia, Guo, Guo, Wang, Shi, Liu, Li, Wang, Xing, Li, Wu, Liang, Qu, Li, Yang, Zhang, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Wang, eXdhbmdAcHVtY2guY24=; Wenbin Ma, bWF3YjIwMDFAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.