
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 28 April 2023
Sec. Neuroprosthetics
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1141567
 Fengyan Liang1,2
Fengyan Liang1,2 Shanshan Yu1
Shanshan Yu1 Siqi Pang1
Siqi Pang1 Xiao Wang1
Xiao Wang1 Jing Jie1
Jing Jie1 Fei Gao3
Fei Gao3 Zhenhua Song2
Zhenhua Song2 Binbin Li2
Binbin Li2 Wei-Hsin Liao4
Wei-Hsin Liao4 Ming Yin1*
Ming Yin1*Brain–computer interfaces (BCIs) have garnered extensive interest and become a groundbreaking technology to restore movement, tactile sense, and communication in patients. Prior to their use in human subjects, clinical BCIs require rigorous validation and verification (V&V). Non-human primates (NHPs) are often considered the ultimate and widely used animal model for neuroscience studies, including BCIs V&V, due to their proximity to humans. This literature review summarizes 94 NHP gait analysis studies until 1 June, 2022, including seven BCI-oriented studies. Due to technological limitations, most of these studies used wired neural recordings to access electrophysiological data. However, wireless neural recording systems for NHPs enabled neuroscience research in humans, and many on NHP locomotion, while posing numerous technical challenges, such as signal quality, data throughout, working distance, size, and power constraint, that have yet to be overcome. Besides neurological data, motion capture (MoCap) systems are usually required in BCI and gait studies to capture locomotion kinematics. However, current studies have exclusively relied on image processing-based MoCap systems, which have insufficient accuracy (error: ≥4° and 9 mm). While the role of the motor cortex during locomotion is still unclear and worth further exploration, future BCI and gait studies require simultaneous, high-speed, accurate neurophysiological, and movement measures. Therefore, the infrared MoCap system which has high accuracy and speed, together with a high spatiotemporal resolution neural recording system, may expand the scope and improve the quality of the motor and neurophysiological analysis in NHPs.
The past decades have seen the development of the lamprey (Grillner, 2003), cat (Clarac, 2008), rodent (McCrea and Rybak, 2008), sheep (Rizk et al., 2009), guinea pig (Sodagar et al., 2009), pig (Borton et al., 2011), and other animal models for locomotion. Specifically, non-human primates (NHPs) are critical in studying biomechanics, biodynamics, neurophysiology, pathophysiology, and evolution of humans, enabling the development of brain-computer interface (BCI) technologies.
NHPs have been widely selected as suitable subjects in neuroscience, especially BCI studies (Wessberg et al., 2000; Serruya et al., 2003; Griffith et al., 2008; Fitzsimmons et al., 2009; Foster et al., 2014; Yin et al., 2014) for several reasons. One is their unique phylogenetic proximity to humans (Lane, 2000). Thus, they offer a meaningful way to functionally evaluate neurotechnologies that have been designed for human subjects, enabling their effective translation to clinical settings (Badi et al., 2021). Second, NHPs and humans share similarities in functional brain structures. Third, NHPs share a similar diagonal interlimb synergy between the hindlimbs (legs) and forelimbs (arms) with humans, while nonprimate mammals have lateral sequence gait (Hildebrand, 1967; Courtine et al., 2005a,b). NHPs’ ability to walk bipedally like humans is of great interest in BCIs, as scientists seek to develop potential cures for gait deficits (Fitzsimmons et al., 2009). Last, NHPs can be trained to learn and perform more complicated tasksthan other animals, including reaching and grasping (Vargas-Irwin et al., 2010; Badi et al., 2021). In short, NHPs enable researchers to decode the relationship between intracortical activities and animal behaviors similar to humans (Zhang et al., 2012).
Neuroscience research, especially BCIs, the “brain-reading devices,” has been described as groundbreaking technology producing remarkable achievements. BCIs are promising to help restore movement, tactile sense, and communication in patients with paralysis (Hochberg et al., 2006, 2012; Collinger et al., 2013; Aflalo et al., 2015; Lebedev and Nicolelis, 2017; Willett et al., 2021; Drew, 2022). In 2004, BCI electrodes were embedded into the motor cortex of a human for the first time (Rapeaux and Constandinou, 2021). In 2021, a BCI that evoked tactile sensations and helped a patient with tetraplegia control prosthetic arms during reaching and grasping (Flesher et al., 2021), which is remarkable progress. In the same year, Willett et al. (2021) developed a BCI decoder to generate attempted handwriting, restoring communication in patients with paralysis. The study participant could type about 90 characters per minute, a speed comparable to the smartphone typing speeds of non-disabled individuals in the same age group. Behind all the achievements in humans, dozens of pilot neurophysiological experiments on NHPs were conducted for decades to decode how the brain senses and responds. In 1966, Evarts (1966) developed a technique for single-unit recording of pyramidal activities of tract neurons from awake, active NHPs for the first time. Georgopoulos et al. (1986) employed electrophysiological techniques to record single-neuron activities of NHPs during arm movements and found a strong correlation between the direction of reaching movement and a population of cortical neurons. In 2000, a population of cortical neurons in NHPs was processed to control a robotic arm in real-time (Wessberg et al., 2000).
Due to technological limitations, most NHP models for neural recordings are wired or tethered. The NHP is refined to a chair with its head and body fixed to protect the wire. Thus, only a few instructed arm movements can be studied. These models or paradigms are termed “head-fixed models” (Foster et al., 2014). However, arm movements are only a small subset of natural behaviors in NHPs. How the brain acts during other natural behaviors is still unclear and needs exploring.
The advent of wireless neural recording systems for NHPs has expanded motor and neurophysiological analysis, enabling challenging setups requiring large or total freedom, such as locomotion. In 2004, Wise et al. (2004) pioneered a wireless implantable electronic interface to record cortical neural information. In 2007, Santhanam et al. (2007) presented a dual-channel neural recording system named HermesB for NHPs and humans. Since 2008, researchers, including our group, have continuously designed wireless neurosensors for full-spectrum neural recordings to expand brain research (Chestek et al., 2008; Harrison et al., 2008; Yin and Ghovanloo, 2009; Miranda et al., 2010; Rouse et al., 2011; Borton et al., 2013; Schwarz et al., 2014; Yin et al., 2014). Wireless neural recordings enable freely-moving NHP models or paradigms (e.g., Figure 1). A freely-moving monkey’s motion is recorded synchronously by a fast-speed MoCap system along with a high spatiotemporal resolution neural recording system. However, the first three studies to design freely-moving NHP models to analyze cortical neurons and locomotion comprehensively were all until 2014 (Foster et al., 2014; Schwarz et al., 2014; Yin et al., 2014).
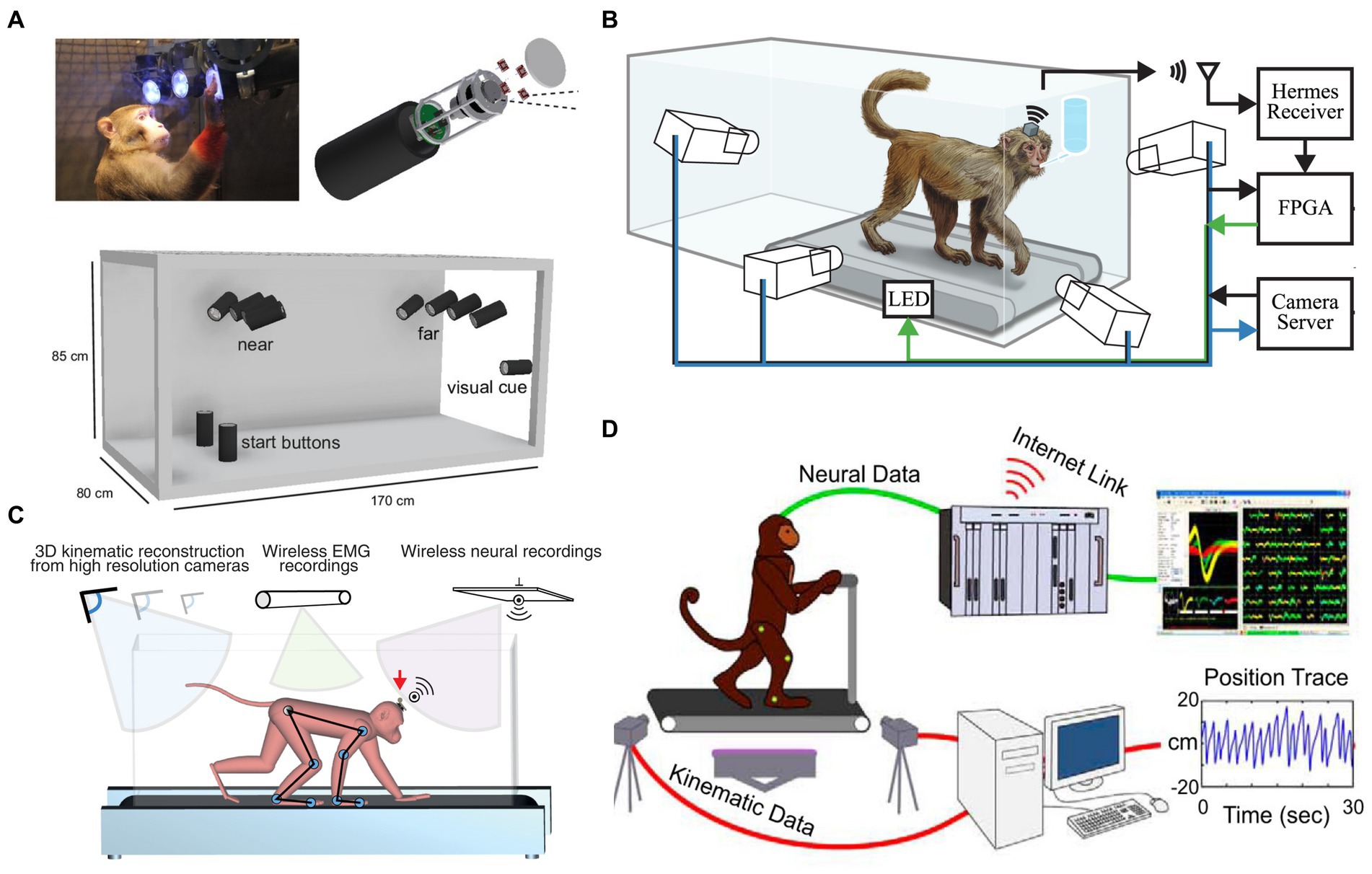
Figure 1. Freely-moving NHP models. (A) An unconstrained NHP in the Reach Cage [Reproduced with permission from Berger et al., 2020]. (B) Treadmill NHP model [Reproduced with permission from Foster et al., 2014]. (C) An unconstrained NHP on a treadmill [Reproduced with permission from Yin et al., 2014]. (D) Bipedal walking NHP model [Reproduced with permission from Lebedev and Nicolelis, 2017].
Understanding the role of the motor cortex during locomotion has been a main research focus in the past decades. But it is unclear and needs to be further explored. There are two views on the neural control of movement (Shenoy et al., 2013). On the one hand, some believe that the motor cortex codes higher-level movement parameters, such as the position of the end-effector. On the other hand, the motor cortex was believed to code muscle action. In NHP arm movement, a population of cortical neurons is found to strongly correlate with movement direction (Georgopoulos et al., 1986). Wessberg et al. (2000) supported this finding and used a population of cortical neurons to control a prosthetic limb. After that, dozens of methods were proposed to decode or model the relationship between motor functions and recorded neural activity, including linear Wiener filters (Wessberg et al., 2000; Carmena et al., 2003), principal component analysis (PCA, Churchland et al., 2012), the Kalman filters (Wu et al., 2002; Li et al., 2009) and long short-term memory neural network (LSTM, a commonly-used recurrent neural networks, Tseng et al., 2019; Glaser et al., 2020). However, locomotion differs from arm movements regarding autonomy. It is found that the contributions of the motor cortex to locomotion and reaching movements are different (Xing et al., 2019). Drew (1988) found that the motor cortical neurons adjust the flexor muscle in cats during quadrupedal walking. In 2017, recent findings in a mouse model suggested that the role of motor cortical output in treadmill walking is significantly different from that in reaching movements (Miri et al., 2017). However, Yakovenko and Drew (2015) proposed that the motor cortex of cats plays a similar role in reaching and walking movements. These contradictory conclusions show a gap remains in understanding the role of the motor cortex during locomotion because of the need for more evidence from NHP models.
NHP models or paradigms designed in neuroscience studies require simultaneous, high-speed, accurate neurophysiological and movement measurements (Foster et al., 2014). There are already several methods or sensors proposed for human motion capture (MoCap), including ultrasound (Akaka and Houck, 1980), infrared beam arrays (Clarke et al., 1985), Doppler radar (Martin and Unwin, 1980), gyroscopes (Giansanti et al., 2005), and accelerometers (Van der Kruk and Reijne, 2018). Based on their physics principle, nowadays, commercially available MoCap systems can be categorized into five classes: optoelectronic systems (OS), image processing systems (IPS), electromagnetic systems (EMS), ultrasonic localization systems (ULS), and inertial sensory systems (ISS). These MoCap systems have been widely used in sports, neuroscience, computer vision, and robotics. The OS, including active marker systems and passive marker systems, are based on fixed cameras to capture the light reflected or emitted by the marker. The OS benefits from the semiconductor industry. It currently has the highest precision compared to other systems and is often regarded as the gold standard (e.g., Vicon or Optotrak) in the literature (Corazza et al., 2010; Galna et al., 2014; Van der Kruk and Reijne, 2018; Albert et al., 2020) for accurate quantitative movement-based analysis. However, using marker-based MoCap systems on NHP models faces challenges in making the animals comply with the marker setup; very often they remove or even swallow the marker.
This paper reviews NHP models and systems for gait and correlated neurophysiological research, focusing on MoCap and BCI neural recording systems for NHPs, to help researchers choose suitable systems for their experimental setup. An extensive literature review of peer-reviewed papers on NHP models and systems for gait and neurophysiological analysis is conducted. This review thoroughly evaluates various MoCap systems and neural recording devices used in NHP BCI and gait studies in the past. Based on their performances, useability, and data quality, the advantages and limitations of recent BCI and gait studies have been discussed. Finally, the challenges and directions of future NHP BCI and gait experimental setups are concluded.
A systematic review was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Page et al., 2021).
A systematic literature search for neuroscientific studies of gait in NHP models was conducted in four main databases: Web of Science, Science Direct, PubMed, and IEEE, up until 1 June, 2022 (Figure 2). Some results searched in Google Scholar not covered by the four databases were also included as a supplement. The search strategy aimed to identify studies with NHP models for gait study, including the study that contains neuroscience. To exclude NHP gait studies that do not consider joint angles (e.g., only consider step numbers), we defined the search terms used as (gait OR walking OR locomotion) AND (primates OR monkey OR macaque OR rhesus OR ape) AND angle. Only publications in English were considered. The publication period investigated was from 2000 to June 2022.
First, the titles and abstracts of located papers were screened against the inclusion criteria. Then, the full texts of the papers were obtained and further screened for inclusion.
This review includes NHP models and systems for gait and neurophysiological analysis in peer-reviewed papers. It focuses on the MoCap device/system and neurophysiological device, especially the BCI device.
Book chapters, conference proceedings, review papers, and thesis works were excluded. The studies only considered NHP upper limb (not including locomotion) were also excluded.
The characteristics of the included studies, such as the MoCap device, authors, other sensors, the neuroscience device, and the measurements, were tabulated. Subgroups were formed based on whether neural signals were studied in the paper.
The database search initially returned 7,048 publications (Figure 2). We identified and excluded 4,931 duplicates. We assessed the remaining 2,117 publications for eligibility and ended up with 94 included studies. Nineteen studies were categorized into the subgroup as they also conducted neuroscience studies.
OS, IPS (including marker-based and markerless), footprint measurements, and observation and analysis from videos were employed in NHP studies (Table 1).
Infrared (IR)-based MoCap systems belonging to OS were used in a few studies (n = 11; 11.7%). Reflective or active markers were attached or painted on NHPs. Fixed infrared cameras emit the IR light and capture the light reflected by the markers to obtain the 3D locations of markers based on triangulation. Eleven studies employed three brands of IR-based MoCap systems: Qualisys (Blickhan et al., 2018, 2021; Ogihara et al., 2018; Oku et al., 2021), Optotrak (Xiang et al., 2007; Chen et al., 2009), and Vicon (Demes, 2011; Wei et al., 2016, 2018, 2019; Zhao et al., 2018). Qualisys and Vicon are passive marker systems, while Optotrak is an active marker system. IR light-emitting diodes (LEDs) emit IR light in the Optotrak system. How to put markers on NHPs is a challenging issue discussed in section 4.2.5.
In IPS, video-captured films or photos are digitally analyzed. IPS has difficulty recognizing images in real-time and might require high-quality, high-speed cameras at a high cost (Van der Kruk and Reijne, 2018). IPS has marker-based tracking and markerless tracking. Note that both the marker-based IPS and the OS require markers, but marker-based IPS uses high-contrast markers detected by visible light, while OS employs reflective markers and works with infrared light. Moreover, markerless IPS usually tracks 2D features, such as corners or edges, and is convenient to use, especially for NHP models. For example, “DeepLabCut” is an up-to-date open-source tool for markerless pose estimation based on transfer learning. Its feasibility on humans, mice, macaque, and Drosophila was validated (Mathis et al., 2018; Mathis and Mathis, 2020; Labuguen et al., 2021). Specifically, Labuguen proposed an open-source dataset of Macaques in the wild for the training of DeepLabCut (Labuguen et al., 2021).
A large proportion of the NHP studies chose IPS (n = 71; 75.5%) because of its convenience. Among them, Frame DIAS (21 studies: e.g., Mori et al., 2006; Higurashi et al., 2010; Goto et al., 2021), Simi Motion (five studies: Fitzsimmons et al., 2009; Capogrosso et al., 2016; Vouga et al., 2017; Tseng et al., 2019; Xing et al., 2019), Peak Motus software (four studies: Courtine et al., 2005a,b; Larson and Demes, 2011; Demes and O’Neill, 2013), and Xcitex (seven studies: e.g., O’Neill et al., 2015; Thompson et al., 2018; Young and Chadwell, 2020) were commonly used (n = 37; 52.1%). The remaining 34 studies used self-written programs based on development platforms, such as MATLAB (Reghem et al., 2013, 2014; Pontzer et al., 2014; Dunham et al., 2018) and C++ (Peikon et al., 2009), or manually observed and analyzed videos or photos to obtain spatial–temporal parameters (Kimura, 2000; Nakano, 2002; Ma et al., 2016; Hu et al., 2021).
Commercial IPS supports both marker-based and markerless tracking. Among the 71 studies using IPS, 27 studies used markerless tracking. For example, Demes and O’Neill (2013) and Young and Chadwell (2020) chose prominent anatomical landmarks, such as the eyes and the base of the tails, to obtain normal spatial–temporal parameters, such as step length and walking speeds. Hirasaki et al. (2019) chose anatomical landmarks, such as wrist, elbow, hip, and knee joints, to get joint angles. The remaining 44 studies adopted marker-based tracking. Reflective or high-contrast paint markers in white or black on the shaved skin of NHPs were reported by all studies, except for Ogihara et al. (2010), which adopted reflective tape markers. Some studies also stressed that the paint was non-toxic (Goto and Nakano, 2018; O’Neill et al., 2018; Shitara et al., 2022b) or water-soluble (O’Neill et al., 2018), and the NHPs in the study were highly trained (Nakatsukasa et al., 2004).
The systems with X-rays also belong to IPS. Schmidt (2005, 2008) used an X-ray system to obtain the kinematics based on uniplanar cineradiography. However, the kinematics were captured only when the animals ran perpendicularly to the X-ray beam. Gait analysis was performed in specialized software (Unimark, by R. Voss, Tübingen, Germany).
None of the 94 included studies were reported to employ EMS, ULS, or ISS systems, maybe because of the inconvenience.
Babu et al. (2007a,b, 2012) and Babu and Namasivayam (2008) did not employ cameras but footprint measurements to obtain spatial–temporal parameters. They painted non-toxic black ink on the feet of the NHPs. The animals left footprints on the white paper as they walked. But, this method is not reported in other NHP studies.
Different MoCap systems are with different accuracies (Table 2). The OS is believed to have the highest accuracy among other systems and is often regarded as the gold standard (e.g., Vicon or Optotrak) in the literature (Corazza et al., 2010; Galna et al., 2014; Van der Kruk and Reijne, 2018; Albert et al., 2020). For example, the Vicon system was reported to have an accuracy of 0.6 mm (Spörri et al., 2016) and 0.77 mm (listed in Table 2, Monnet et al., 2014). The OS outperformed IPS (Scaramuzza and Fraundorfer, 2011).
The feasibility and accuracy of markerless IPS still need to be improved due to the lack of proof in the literature (Ceseracciu et al., 2014). Ceseracciu et al. (2014) reported that markerless IPS has a root mean square error (RMSE) of 11.75°, 18.35% of the range of motion in the knee angle (in the sagittal plane), and an RMSE of 17.62°, 44.66% of the range of motion in the hip angle, compared with the gold standard in gait analysis. Similarly, significant differences in the knee angle (15.9°) during squats were reported by Perrott et al. (2017). Moreover, insufficient accuracy of the markerless system in the transverse plane is believed to hinder its application. For example, the Simi markerless system (Simi Reality Motion Systems GmbH, Unterschleißheim, Germany) was reported to have a standard deviation of 6.4° (±2.9°) in the sagittal plane hip angle, 9.2° (±3.8°) in the transverse plane hip angle and 20.6° (±28.3°) in elbow rotation (Ruß, 2016). The calculation error may be due to the camera’s optical distortion, the system’s processing errors, and skin artifacts (the system assumes that the model is rigid, whereas the skin is flexible during movement; Winiarski, 2003). Up to now, the Simi system’s accuracy or the validity of measurement is not yet validated by high-quality peer-reviewed studies.
Home-made IPS still has poor kinematics measurement. Foster et al. (2014) designed a markerless IPS with 8 video cameras for freely-moving NHPs. The estimated distance error for the wrist point is 26.1 mm. Nakamura et al. (2016) used four depth cameras (Kinect V1, Microsoft Corp., Redmond, WA, USA) to build a markerless system for NHPs. The estimated errors are reported as 4–14 cm for the position of different limbs and 35–43° for head rotation (listed in Table 2).
Marker-based IPS is usually more accurate than markerless IPS (Van der Kruk and Reijne, 2018). Simi system also supports marker-based tracking. The photogrammetric errors of the marker-based Simi system were reported as 11, 9, and 13 mm in the x, y, and z directions, respectively (Klous et al., 2010). An RMSE of 4° (±1°) in knee angle between Simi marker-based tracking and Qualisys (the gold standard) was reported during one gait cycle in race walking (Hanley et al., 2017). However, these accuracies are significantly lower than OS’ (listed in Table 2). Six studies adopted video observation and analysis but could only obtain the basic spatiotemporal parameters with poor accuracy. Four studies used footprint measurements in NHP’s locomotor analysis. This method is convenient and low-cost, but the accuracy and gait parameters obtained are not enough for the requirement of neuroscience studies.
Parameters involved in gait studies provide scales to quantify animal behaviors, including spatiotemporal parameters, joint angles, ground reaction force (GRF), dynamics, neurophysiological measurements, and others.
Commonly measured spatiotemporal parameters (SP) are stride time, step time, stance time, gait velocity, cadence, stride symmetry, and the number of steps. Most NHP studies measured spatiotemporal parameters (n = 92; 97.9%). Conversely, Ma et al. (2016) proposed a self-made score to quantify hindlimb locomotion in the NHP after spinal cord injury (SCI). The score was graded based on the manual video analysis. Revuelta et al. (2012) determined freezing of gait (FOG), a critical feature in Parkinson’s disease, in an NHP Parkinson’s model by reviewing the videos.
Of the 94 NHP studies, 68 (72.3%) measured joint angles, such as those of the hip, knee, and ankle angles. Joint angle is a critical feature in NHPs. For example, Demes (2011) researched three-dimensional kinematics of capuchin monkey bipedalism by putting reflective markers over the major joints of the animals and employing Peak Motus software (PEAK Performance Technologies Inc., Centennial, CO, USA) to calculate several angles (listed in Table 3 with definitions). Joint angles can quantify gait ability in SCI NHPs. Capogrosso et al. (2016) proposed a brain–spinal interface to alleviate gait disabilities in NHPs after SCI. The MoCap system, Simi, used 4–6 video cameras at 100 Hz along with reflected white paint markers on shaved skin to measure 7 landmarks on the animal’s right side. 3D spatial coordinates of the markers were obtained using the Simi motion tracking software and then the joint angles were computed accordingly. The increased knee angle in the paretic leg of NHPs showed the recovery of locomotion and demonstrated the feasibility of brain–spinal interface on NHPs and its potential application for humans.
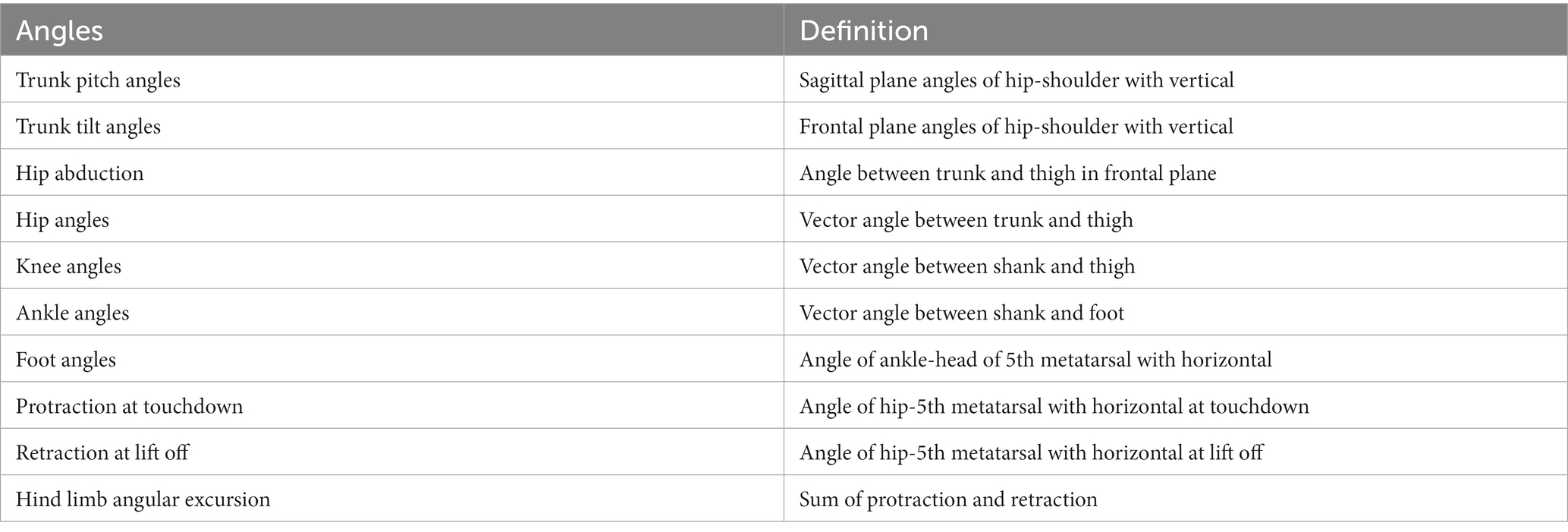
Table 3. Joint angles measured in Demes (2011).
One common way to obtain the dynamics in gait analysis is to measure the GRF during locomotion. Seventeen NHP studies adopted force plate(s) to measure GRF in the NHP experiments. Force plates can be combined with MoCap systems, such as Qualisys (Qualisys AB, Gothenburg, Sweden. Blickhan et al., 2018, 2021; Ogihara et al., 2018; Oku et al., 2021), Frame-DIAS (DKH, Tokyo, Japan. Higurashi et al., 2010; Ogihara et al., 2010, 2012; Shimada et al., 2017), and Xcitex (Xcitex Inc., Cambridge, MA, USA. Thompson et al., 2018; Young and Chadwell, 2020) for the calculation of joint dynamics by the system software. Some studies measured GRF not to obtain dynamics but rather the center of mass. Demes et al. (2015) used force plates to analyze the center of mass mechanics of an NHP during bipedal walking. Hanna et al. (2015) analyzed the center of mass during load-carrying locomotion in NHPs.
Neurophysiological measurements in NHP studies, such as electromyogram (EMG) and cortical neural recordings, are discussed in section 4.
NHP gait studies often involve gait parameter measurements and neurophysiological analysis; however, a few studies measure other locomotion-related signals. Nakatsukasa et al. (2004, 2006) employed an infrared gas analyzer in NHP studies to measure and analyze the energy consumption during bipedal and quadrupedal walking by measuring CO2 production.
In the discussion, we focus on NHP studies involving neuroscience research (19 studies, listed in Table 4), while those that do not measure any neurophysiological data were not considered. NHP neuroscience studies include intracortical neural recordings (i.e., BCI), EMG, motor-evoked potential (MEP), and somatosensory-evoked potential (SSEP).
NHP studies of intracortical neural recordings or BCI were based on home-made (e.g., HermesB) or commercial neural recording systems (e.g., Blackrock Microsystems, Salt Lake City, UT, USA; FHC Inc., Bowdoin, ME, USA). EMG studies employed implantable electrodes (Yin et al., 2014; Hu et al., 2021; Shitara et al., 2022b) or surface EMG sensors (Nakano, 2002; Goetz et al., 2012; Wei et al., 2019). Ma et al. (2016) adopted MEP and SSEP to evaluate spinal cord function electrophysiologically in NHP models.
There are 12 NHP BCI and gait studies from eight research teams (listed in Table 5). The BCIs in two of the 12 studies were implanted in the hippocampus, while BCIs in other studies were all in the motor cortex. Three studies by researchers at Duke University (Fitzsimmons et al., 2009; Peikon et al., 2009; Tseng et al., 2019) were based on the same tethered setup or paradigm. The NHPs walked bipedally on a treadmill with two hands fixed. Before the experiments, the NHPs were tattooed, and fluorescent, non-toxic markers were applied. A tethered multichannel neural acquisition system (Plexon, Inc., Dallas, TX, USA) was connected to headstages, enabling the simultaneous recording of neural activity and capture of movement during the experiments. But in 2014, Schwarz et al. (2014) from Duke University designed a wireless large-scale recording system to enable tethered and wireless freely-moving NHP models.
None of the 12 BCI and gait studies adopted OS but IPS. Because the OS faces challenges in making the animals comply with the marker setup; very often, they just remove or even swallow the marker. A better solution is needed to use OS, the gold standard with the highest accuracy in NHP’s BCI and gait studies.
Transmitting neural data wirelessly enables a wide range of natural behavior studies with full-body movements, including arm movements and locomotion (listed in Table 6). Compared with wired neural recording, wireless rexording does not need to tether the NHP, thus the NHP is freely-moving. Moreover, the training complexity in freely-moving NHPs is lower than in tethered ones.
A few studies (n = 6) performed wireless neural recordings (i.e., there is no wire between the BCI headstages and recording systems). For example, wireless modules designed by Yin et al. (2014; Figure 1C) enabled neural signal transmission to external receivers in the studies conducted at EPFL and Brown University (Yin et al., 2014; Capogrosso et al., 2016; Xing et al., 2019). Stanford University (Figure 1B; Foster et al., 2014) used home-designed HermesD and HermesE systems for the wireless transmission of neural data. Both Capogrosso et al. (2016) and Berger et al. (2020; Figure 1A) used a commercial neural recording system (CerePlex W, Blackrock Microsystems, Salt Lake City, UT, USA). The system offers 96 ch wideband neural recording with 16 b resolution, 30 kSps sampling rate, weighs only 33.5 g with battery, and measures 32.5 × 32.5 × 21 mm. It has a wireless transmission range of 3 m and 2 m for freely moving animals in rich multipath fading environments. But the system still requires improvement in coverage distance, battery life, and channel counts.
Studies undertaken at Duke University (Fitzsimmons et al., 2009; Peikon et al., 2009; Tseng et al., 2019), University of California (Courellis et al., 2019), Grenoble Institute Neurosciences (Goetz et al., 2012), and University of Toyama (Hazama and Tamura, 2019) were based on tethered NHP models, while other BCI studies enabled freely-moving NHPs (listed in Table 5). Fitzsimmons et al. (2009; Figure 1D) developed an NHP bipedal walking paradigm. Monkeys were trained to walk on the treadmill with arms holding a bar on the treadmill. A wired BCI system was used to obtain neural recordings from the M1 area. They reported that neurons in M1 modulate the firing rate to the timing of gait cycles. But monkeys’ arms holding a bar breaks the interlimb coordination between forelimbs and hindlimbs during nature gait. Conversely, freely-moving NHP models are promising to help understand how the brain changes with the environment and how to control prosthetic devices in response to neuron signals.
Due to the limitation of their wired recording systems, three studies at Duke University forced the NHPs to walk bipedally on a treadmill with two hands holding a bar. Therefore, only bipedal gait was analyzed. Moreover, fixing forelimbs may affect the nature of bipedal gait. By contrast, freely-moving NHP models in other studies enabled the study of bipedal and quadrupedal gait. Schwarz et al. (2014) and Xing et al. (2019) obtained bipedal, quadrupedal gait, and intracortical neural data.
All 12 NHP BCI and gait studies used the IPS. However, the accuracy of IPS is not comparable with OS, as discussed in section 3.3. Goetz et al. (2012), Foster et al. (2014), and Berger et al. (2020) used markerless IPS systems, whose accuracy is outperformed by marker-based IPS. Simi motion is a marker-based IPS system commonly used in five BCI and gait studies. However, as mentioned in section 3.3, the accuracy of Simi is not yet validated by high-quality peer-reviewed studies. Thus, the reported error (4–20°) does not meet the requirement of high-speed, precise kinematics and neural decoding. However, Simi can be used in BCI and gait studies that do not need accurate kinematics due to its convenience. For example, Capogrosso et al. (2016) used Simi to compare the gait of the NHP SCI model before and after the intervention (brain–spinal interface). Joint angles, step height, and foot trajectory of the NHP were obtained and compared.
Up to now, no NHP BCI and gait studies have used OS, which has the highest accuracy as the gold standard (Corazza et al., 2010; Galna et al., 2014; Van der Kruk and Reijne, 2018; Albert et al., 2020). The OS requires the placement of rigid reflective markers on NHPs. Thus, attaching reflective markers on NHPs to enable OS in BCI and gait studies becomes a key issue.
In the six NHP BCI and gait studies that used marker-based IPS systems, reflective white paint or white fluorescent makeup was commonly used as markers on the NHPs. The reflective white paint provides high contrast to other colors on NHPs and thus can be easily captured by the IPS.
Although no NHP BCI and gait studies used OS, there is still some experience from 11 NHP gait studies that used OS (introduced in 3.2.1). To attach the rigid reflective markers to NHPs, one way (four studies) is to make NHPs walk on a treadmill with two forelimbs fixed (Wei et al., 2016, 2018, 2019; Zhao et al., 2018). This method disables the study of quadrupedal gait. Another way (seven studies) is to attach the markers with straps or tapes. For example, Velcro straps, double-sided tape, and children’s leggings were used by Blickhan et al. (2018, 2021), Ogihara et al. (2018), and Oku et al. (2021), whereas Xiang et al. (2007) and Cohen et al. (2009) adopted Optotrak active OS. In the experiments, IR LEDs (rigid bodies, or “markers”) were fixed on tapes and then wrapped around the head, chest, wrists, and ankles. Optotrak system requires training the NHPs not to eat or grasp the tape or the markers, and this issue may influence the experiment. Moreover, fixing forelimbs may affect the nature of bipedal gait. Both approaches have their weaknesses. A better solution is needed to use OS in BCI and gait studies. Finding replacements for markers, such as self-made surface markers, may be a solution. Recently, our group is setting up an OS-based freely-moving NHP model (Figure 3) with a high accuracy and fast speed MoCap system (Mars 4H, Nokov Science and Technology Co., Ltd., Beijing, China) along with a high spatiotemporal resolution neural recording system and wireless EMG system, to increase the accuracy of kinematics and thus expand motor and neurophysiological analysis in NHPs.
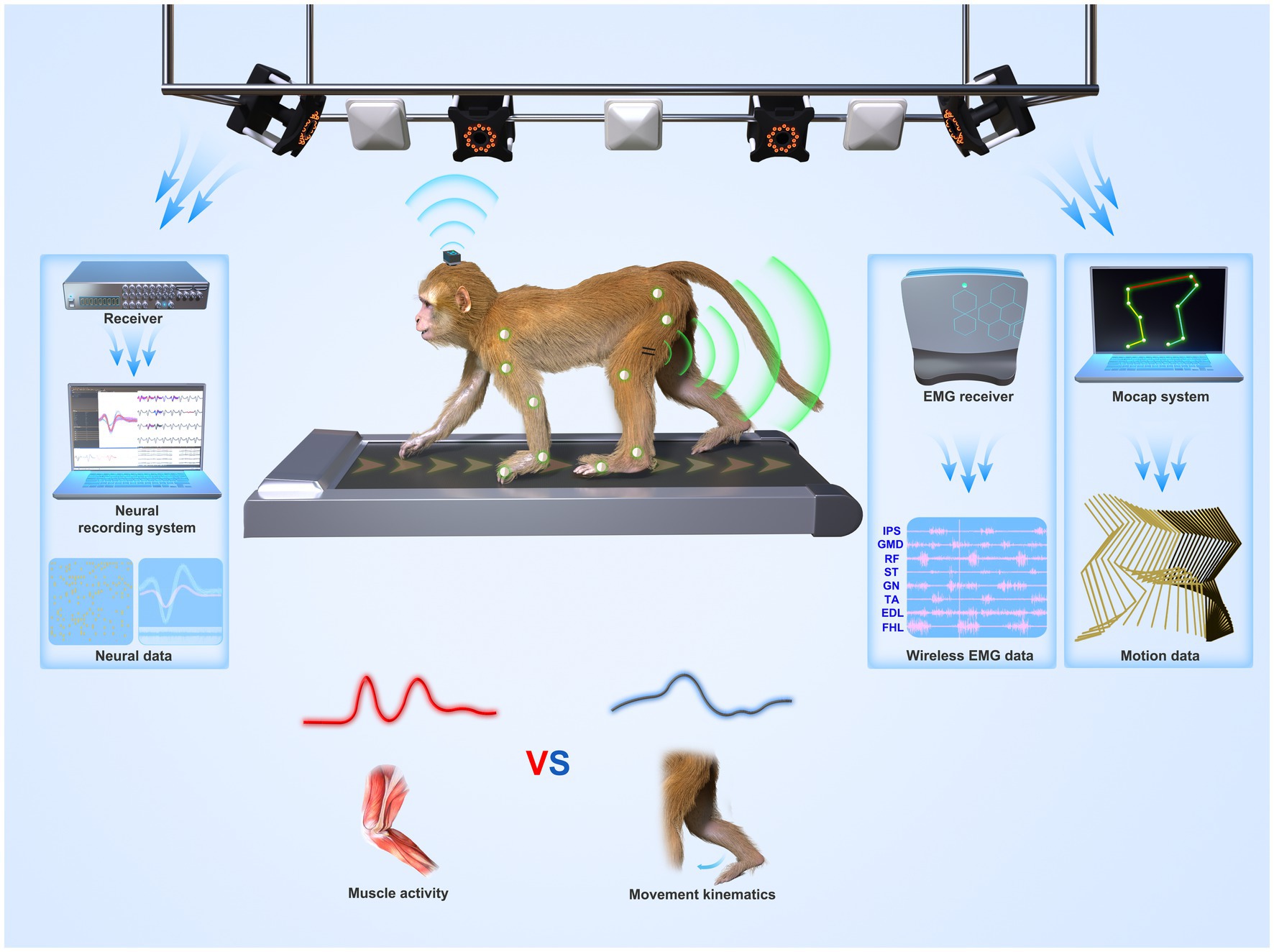
Figure 3. System overview of a suggested freely-moving (untethered) NHP model or paradigm. Wireless neural, EMG, and motion data from a monkey were recorded synchronously. The paradigm can be used to understand whether the motor cortex codes muscle activities (lower left panel) or movement parameters (lower right panel) during locomotion.
With the MoCap systems, 12 NHP BCI and gait studies have different findings or contributions. Capogrosso et al. (2016) proposed a brain-spinal interface to alleviate gait disabilities in NHPs after SCI. The increased knee angle obtained by the MoCap system in the paretic leg showed the recovery of locomotion and stressed the potential of the brain-spinal interface for humans. Three studies undertaken at Duke University established tethered NHP bipedal models that enabled online decoding of 3D kinematics from intracortical neural signals. Subsequently, a better computational decoder, the LSTM, was proposed to replace the Kalman filter (Tseng et al., 2019) because LSTM units have physiological features like neuron populations, such as neuronal dynamics, and directional tuning. In 2014, three studies of different universities all built freely-moving NHP models using self-developed wireless neural recording systems (Foster et al., 2014; Schwarz et al., 2014; Yin et al., 2014). Following those studies, Xing et al. (2019) simultaneously recorded bipedal and quadrupedal kinematics with intracortical data. They found that it was possible to decode kinematics from a limited number of neurons just as well as or better than from the neural population (18–80 neurons). However, the limited accuracy of the MoCap system (Simi, reported error: 4–20°) may influence the robustness of their findings. Berger et al. (2020) designed a free-moving NHP model based on a commercial-available wireless recording system (Blackrock Microsystems, Salt Lake City, UT, USA) and a markerless motion capture system to study goal-directed movements, including walk-and-reach.
Two studies analyzed the role of the hippocampus’s place cells in locomotion. Hazama and Tamura (2019) found that NHP’s place cells are more sensitive to locomotion velocity than direction. Courellis et al. (2019) reported that the correlation between the activities of place cells and θ oscillations in NHP is remarkably different from rodents.
The role of the motor cortex in NHP arm movement has been studied for a long time. Whether the motor cortex codes muscle activities or higher-level movement parameters such as limb trajectory is an open question (Figure 3). In other words, does the cortical activity correlate with the muscle EMG or with movement kinematics such as position and velocity? In 1986, a population of cortical neurons was found to have strong correlations with movement direction (Georgopoulos et al., 1986). But, Cisek (2006) argued that the motor system does not describe the motion but produces it. Moreover, evidence from cats suggested a direct contribution of the motor cortex to leg muscle adjustment during gait modifications (Drew and Marigold, 2015). However, studies from another view sought to describe the firing rate of motor neuron populations as the function of various kinematic parameters such as hand or joint kinematics, target locations, and the estimated endpoint error (Reimer and Hatsopoulos, 2009; Wang et al., 2010; Pearce and Moran, 2012). Locomotion movements are different from arm movements regarding autonomy. There is still a lack of consensus on the role of the motor cortex during locomotion due to the lack of related studies.
Due to technological limitations of neural recording systems, only three papers (Foster et al., 2014; Yin et al., 2014; Xing et al., 2019) studied the role of the motor cortex during NHP’s quadrupedal locomotion and only one of them (Xing et al., 2019) analyzed the role of the motor cortex regarding kinematics, not just spatial–temporal parameters. Our group (Yin et al., 2014) reported the reproducible cyclic ensemble modulation of NHP motor cortex across the entire gait cycle during natural locomotion. Foster et al. (2014) found that the modulation of NHP’s motor cortex correlates well with wrist speed during locomotion. However, neither Yin nor Foster gave insights into gait kinematics such as joint angles; their gait parameters are limited to spatial–temporal parameters. Xing et al. (2019) found that it was possible to decode hindlimb kinematics and gait phase from limited neurons just as well or as accurately as from the neural population (18–80 neurons). It is the first demonstration that the motor cortex can robustly decode kinematics and gait during NHP quadrupedal locomotion. But the limited accuracy of the MoCap system (Simi, reported error: 4–20°) used in their research may influence the robustness of their findings. Moreover, their findings lack evidence, considering they are the only study analyzing gait kinematics and neural recordings simultaneously during NHP quadrupedal locomotion.
Therefore, since the advent of wireless neural recording technology has paved the way for freely-moving BCI and gait studies in NHPs, one of the future study directions is to clarify the role of the motor cortex during locomotion, especially the accurate correlation or model between motor cortex activities and gait kinematics by using high spatiotemporal resolution neural recording along with high accuracy and fast speed MoCap systems.
Wessberg et al. (2000) employed a population of cortical neurons to control a prosthetic limb. After that, dozens of methods were proposed to decode or model the relationship between the motor of upper limbs and recorded neural activities in NHP’s arm movement tasks, including linear Wiener Filters (Wessberg et al., 2000; Carmena et al., 2003), PCA (Churchland et al., 2012), Kalman Filters (Wu et al., 2002; Li et al., 2009), and LSTM (Tseng et al., 2019; Glaser et al., 2020). Wessberg et al. (2000) employed a linear filter and an artificial neuron network (ANN) to decode neural population signals to estimate real-time three-dimensional hand movements and control a robotic device for the first time. No significant differences were reported between these two decoders. Churchland used PCA, the popular dimensionality reduction technique, to extract essential features at the single-neuron level from neuron populations during arm reaching in NHPs. Tseng et al. (2019) LSTM was reported to outperform Kalman Filter, the state-of-the-art method in decoding arm movements from neuron populations.
However, since the role of the motor cortex during locomotion is unclear, whether the decoding methods for arm movement can model neuron populations in the motor cortex and gait kinematics is unknown. Xing et al. (2019) successfully employed Poisson Linear Dynamical System (PLDS) to reconstruct limb kinematics from neuron populations in the motor cortex. He suggested that the state-of-art RNN may outperform PLDS. He also found a difference between the contributions or functions of the motor cortex to locomotion and reaching movements. Except for Xing’s work, no other studies tried to model the neuron populations in the motor cortex and gait kinematics. Moreover, the limited accuracy of the MoCap system (Simi, reported error: 4–20°) used in Xing’s study influenced the robustness of his conclusions.
Therefore, future studies need to: testify if motor neuron populations and gait kinematics can be modeled using previous decoding methods for arm movements such as PLDS and PCA; testify if the contributions or functions of the motor cortex in bipedal locomotion, quadrupedal locomotion, and arm movements are different; try to find an optimal decoding method to fully extract the gait kinematics from cortical neuron populations and give insights for the generation mechanism of locomotion.
While wireless neural recording devices can free animals and offer unprecedented opportunities for recording signals under untethered natural behavior, building a feasible wireless system for NHP BCI and gait research faces many technical challenges. The most critical ones include wireless coverage and quality, data throughput, size, and power consumption. It is even more challenging since all the above specs are cross related. Usually, NHP gait experimental space needs to be at least 1 m × 1 m × 1 m (Schwarz et al., 2014), with some extending up to a few cubic meters (CerePlex W, Blackrock Microsystems, Salt Lake City, UT, USA). Depending on the wireless recording system channel counts, a high data rate comes with high channel counts. For example, 96 channels with a 30kSps sample rate (to record high-frequency neural action potentials) and 16-bit resolution will produce roughly 50Mbps data throughput. With such a high data rate, wireless transmission with a few meters of reliable coverage and low power (in the 10s of mW range) is tough, especially when the applications require high-fidelity wireless communication with a minimum bit-error rate. Plus, a minimum bit-error rate often requires complicated power-hungry error detection and correction coding in wireless transceivers. With the increased system complexity and power dissipation, the systems need to use larger batteries and produce more heat. The former increase the size and weight of the device, hinder usability, and potentially biases the animal behavior; the latter could harm the animal. Therefore, low-power, reliable, high data rate short distance wireless communication is key to a successful wireless recording device for NHPs BCI and gait application.
Previously, Schwarz et al. (2014) built a custom wireless neural recording system to record high-frequency neural data from a maximum of 1792 channels. For every 128 channels, the data was transmitted through a commercial ISM band radio (Nordic nRF24L01+) with 1.33 Mbps data throughput after intensive data compression. To transmit all 1792 channels, they used 16 ISM radios. And the overall transceiver power reached 2 mW/ch, leading to 256 mW per 128 ch and over 4 W for the whole 1792 channel system. As a result, the battery and size of the entire system are fairly large (roughly 10 cm × 10 cm × 10 cm). Additionally, the original 16-bit sample data was shortened to 8-bit, and lookup table compression was used to free up extra bits further to leverage the limited 1.33 Mbps ISM radio data throughput. Foster et al. (2014) presented a wireless neural recording system (Hermes D and E) for a freely-moving monkey treadmill model. They used custom design ASIC and device to implement the system, which achieved 24 Mbps transmission of 128 ch data at 12.5 kSps/ch sampling rate. But the wireless power consumption is still high (125 mW) and the sampling rate is relatively low for Aps recordings (usually around 30 kSps/ch is the industrial standard). The entire device was enclosed in a 38 × 38 × 51 mm aluminum enclosure. Though its power dissipation, size, sampling rate, and data throughput still require further improvement, the system is small and light-weighted enough for NHPs applications. Yin et al. (2014) proposed a wireless system for locomotion analysis using custom wireless recording ASICs and devices, the power consumption of the system has been limited to around 82.5 mW for 100 channels, with the wireless communication part to be around 4 mW/15 mW (low and high output power modes). The size of the device was 52 × 44 × 30 mm and it can function over 48 h from a single 1.2 Ah one-half AA Li-SOCL2 primary battery. SIMO wireless communication technology was used to improve signal fidelity. Although the system has quite a few improvements in size, weight, power dissipation, and signal fidelity, it still experienced issues in wireless coverage distance and potential signal loss due to the multipath fading effect from the animals’ freely moving. Animals moving causes received signal strength to vary over a large scale. Capogrosso et al. (2016), Berger et al. (2020), and Silvernagel et al. (2021) all used CerePlex W system from Blackrock Microsystem. The system offers 96 ch wideband neural recording with 16 b resolution, 30 kSps sampling rate, weighs only 33.5 g with battery, and measures 32.5 × 32.5 × 21 mm. It has a wireless transmission range of 3 m and 2 m for freely-moving animals in rich multipath fading environments. It can operate for 3.5 h continuously without recharging the battery, which is mostly sufficient for applications like NHPs BCI and gait research. But the system still requires improvement in coverage distance, battery life, and channel counts.
Overall, wireless systems attract many researchers’ attention in the field due to the ability to free the animals and enable NHP research requiring natural behavior in an untethered setup, which provides more genuine un-bias neural data. However, because of technical challenges, such an ideal wireless recording system is yet to come and awaits further engineering.
Our understanding of the motor cortex remains incomplete (Shenoy et al., 2013), and further research is needed to understand its role in locomotion and neural control in NHP. This knowledge is essential to provide insights into the generation mechanism of walking in humans, thus giving implications for the control design of capable, accurate neural prostheses and biped robots (Shenoy et al., 2013) and for the rehabilitation of gait disorders such as stroke. NHP has been commonly selected as a suitable subject in neuroscience studies. This review summarizes 94 NHP studies with gait analysis, including 12 studies with BCI and gait studies. While wired neural recording systems have been used to acquire electrophysiological data in NHPs, the advent of wireless neural recording systems has expanded the scope of neuroscience research on freely-moving NHPs, enabling challenging experimental setups requiring large or total freedom, such as locomotion. Our group has continuously designed wireless neurosensors for full-spectrum neural recordings (Yin and Ghovanloo, 2008, 2009, 2011; Yin et al., 2013, 2014). However, with the harsh requirements for a complete NHP locomotion research setup, it was in 2014 that the first three studies came out with designs of freely-moving NHP models to comprehensively analyze cortical neurons and locomotion (Foster et al., 2014; Schwarz et al., 2014; Yin et al., 2014).
Limited research (Foster et al., 2014; Yin et al., 2014; Xing et al., 2019) has been conducted on the role of the motor cortex during NHP quadrupedal locomotion. The study of Xing et al.’s (2019) is the only study that attempts to decode hindlimb kinematics from a limited number of neurons in NHP quadrupedal locomotion; the low accuracy MoCap system used in his study compromised the robustness of his conclusions. Thus, the role of the motor cortex in gait generation, including bipedal and quadrupedal gait, remains unsolved, and the suitable methods to decode or model the neuron populations in the motor cortex and gait kinematics are also unclear. Future studies need to (1) clarify the role of the motor cortex during locomotion, especially the accurate correlation or model between motor cortex activities and gait kinematics; (2) quantify and compare the contributions or functions of the motor cortex in bipedal locomotion, quadrupedal locomotion, and arm movements; (3) investigate if motor neuron populations and gait kinematics can be modeled using previous decoding methods for arm movements such as PLDS and PCA; (4) testify if the state-of-art RNN may outperform previous decoding methods (as suggested by Xing et al.); (5) identify an optimal decoding method to fully extract the gait kinematics from cortical neuron populations for freely-moving bipedal and quadrupedal gait; (6) explore contributions from other cortical areas (such as posterior parietal cortex) to the control of locomotion.
The advent of wireless neural recording systems for NHPs enabled freely-moving NHP models and neuroscience research on NHP locomotion. However, current MoCap systems in BCI and gait studies are based on image processing and lack accuracy (error: ≥4° and 9 mm). Some home-made IPS systems used in NHP models have been reported even higher measurement errors (26.1 mm and > 40 mm). To address this issue, future BCI and gait studies require simultaneous, high-speed, and accurate measures of neural and movement data. Neuroscience studies may consider using a commercial infrared MoCap system (OS) that is considered the gold standard with the highest accuracy (Figure 3). However, the challenge is how to place markers on NHPs’ bodies as they may remove or even eat the marker. This review discussed the methods used in the literature and suggested that self-made surface markers may be a solution. Additionally, CerePlex W system (Blackrock Microsystem, Salt Lake City, UT, USA, Figure 3) is the most frequently-used and validated commercial wireless neural recording system in current BCI studies. But, there is still room for improvement in coverage distance, battery life, and channel counts. Home-made wireless neural recording systems should also consider signal quality, data throughput, working distance, size, and power constraints. Moreover, other measurement systems such as wireless EMG systems (Figure 3) and force plates can be combined with commercial MoCap systems to obtain EMG and gait dynamics, such as joint force and torque, to enhance neuroscience studies. Currently, our group is working to set up an OS-based NHP model and improve neural recording systems for NHP neuroscience studies.
FL and SY contributed to the conception, investigation, and original draft of the study. SP provided methodological input. XW and JJ provided scientific input and contributed to the manuscript writing. ZS and BL contributed to the manuscript editing. FL wrote the first draft of the manuscript. FG, W-HL, and MY supervised the whole project and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the Key R&D Project of Hainan Province (Grant Nos. ZDYF2022SHFZ302, ZDYF2022SHFZ275, and ZDYF2021SHFZ083), the High-level Talent Project of Natural Science Foundation of Hainan Province (Grant Nos. 322RC560 and 821RC532), the National Natural Science Foundation of China (No. 32160204), the Major Science and Technology Projects of Hainan Province (Grant No. ZDKJ2021032), and Hainan Province Clinical Medical Center (No: 0202067).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aflalo, T., Kellis, S., Klaes, C., Lee, B., Shi, Y., Pejsa, K., et al. (2015). Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 348, 906–910. doi: 10.1126/science.aaa5417
Akaka, W. H., and Houck, B. A. (1980). The use of an ultrasonic monitor for recording locomotor activity. Behav. Res. Methods Instrum. 12, 514–516. doi: 10.3758/BF03201825
Albert, J. A., Owolabi, V., Gebel, A., Brahms, C. M., Granacher, U., and Arnrich, B. (2020). Evaluation of the pose tracking performance of the azure kinect and kinect v2 for gait analysis in comparison with a gold standard: a pilot study. Sensors 20:5104. doi: 10.3390/s20185104
Babu, R. S., Anand, P., Jeraud, M., Periasamy, P., and Namasivayam, A. (2007a). Bipedal locomotion of bonnet macaques after spinal cord injury. Mot. Control. 11, 322–347. doi: 10.1123/mcj.11.4.322
Babu, R. S., and Namasivayam, A. (2008). Recovery of bipedal locomotion in bonnet macaques after spinal cord injury: footprint analysis. Synapse 62, 432–447. doi: 10.1002/syn.20513
Babu, R. S., Periasamy, P., Varadamurthy, S., Sethuraman, O. S., and Namasivayam, A. (2007b). Locomotor behavior of bonnet macaques after spinal cord injury. Mot. Control. 11, 322–347. doi: 10.1123/mcj.11.4.322
Babu, R. S., Sunandhini, R. L., Sridevi, D., Periasamy, P., and Namasivayam, A. (2012). Locomotor behavior of bonnet monkeys after spinal contusion injury: footprint study. Synapse 66, 509–521. doi: 10.1002/syn.21537
Badi, M., Borgognon, S., O’Doherty, J. E., and Shokur, S. (2021). “Cortical stimulation for somatosensory feedback: translation from nonhuman primates to clinical applications” in Somatosensory feedback for neuroprosthetics. ed. B. Güçlü (London, United Kingdom: Academic Press), 413–441.
Berger, M., Agha, N. S., and Gail, A. (2020). Wireless recording from unrestrained monkeys reveals motor goal encoding beyond immediate reach in frontoparietal cortex. elife 9:e51322. doi: 10.7554/eLife.51322
Blickhan, R., Andrada, E., Hirasaki, E., and Ogihara, N. (2018). Global dynamics of bipedal macaques during grounded and aerial running. J. Exp. Biol. 221:jeb178897. doi: 10.1242/jeb.178897
Blickhan, R., Andrada, E., Hirasaki, E., and Ogihara, N. (2021). Trunk and leg kinematics of grounded and aerial running in bipedal macaques. J. Exp. Biol. 224:jeb225532. doi: 10.1242/jeb.225532
Borton, D., Yin, M., Aceros, J., Agha, N., Minxha, J., Komar, J., et al. (2011). “Developing implantable neuroprosthetics: a new model in pig.” in 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. pp. 3024–3030. IEEE.
Borton, D. A., Yin, M., Aceros, J., and Nurmikko, A. (2013). An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 10:026010. doi: 10.1088/1741-2560/10/2/026010
Capogrosso, M., Milekovic, T., Borton, D., Wagner, F., Moraud, E. M., Mignardot, J. B., et al. (2016). A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288. doi: 10.1038/nature20118
Carmena, J. M., Lebedev, M. A., Crist, R. E., O'Doherty, J. E., Santucci, D. M., Dimitrov, D. F., et al. (2003). Learning to control a brain–machine interface for reaching and grasping by primates. PLoS Biol. 1:e42:E42. doi: 10.1371/journal.pbio.0000042
Ceseracciu, E., Sawacha, Z., and Cobelli, C. (2014). Comparison of markerless and marker-based motion capture technologies through simultaneous data collection during gait: proof of concept. PLoS One 9:e87640. doi: 10.1371/journal.pone.0087640
Chen, L., Wang, J., Li, S., Gao, L., Li, H., Wang, Z., et al. (2009). “Analysis of temporal and spatial gait parameters in children with spastic cerebral palsy.” in 2009 2nd International Conference on Biomedical Engineering and Informatics. pp. 1–4. IEEE.
Chestek, C. A., Gilja, V., Nuyujukian, P., Ryu, S. I., Shenoy, K. V., Kier, R. J., et al. (2008). “HermesC: RF wireless low-power neural recording system for freely behaving primates.” in 2008 IEEE International Symposium on Circuits and Systems. pp. 1752–1755. IEEE.
Churchland, M. M., Cunningham, J. P., Kaufman, M. T., Foster, J. D., Nuyujukian, P., Ryu, S. I., et al. (2012). Neural population dynamics during reaching. Nature 487, 51–56. doi: 10.1038/nature11129
Cisek, P. (2006). Integrated neural processes for defining potential actions and deciding between them: a computational model. J. Neurosci. 26, 9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006
Clarac, F. (2008). Some historical reflections on the neural control of locomotion. Brain Res. Rev. 57, 13–21. doi: 10.1016/j.brainresrev.2007.07.015
Clarke, R. L., Smith, R. F., and Justesen, D. R. (1985). An infrared device for detecting locomotor activity. Behav. Res. Methods Instrum. Comput. 17, 519–525. doi: 10.3758/BF03207645
Cohen, B., Xiang, Y., Yakushin, S. B., Kunin, M., Raphan, T., Minor, L., et al. (2009). Effect of canal plugging on quadrupedal locomotion in monkey. Ann. N. Y. Acad. Sci. 1164, 89–96. doi: 10.1111/j.1749-6632.2009.03845.x
Collinger, J. L., Wodlinger, B., Downey, J. E., Wang, W., Tyler-Kabara, E. C., Weber, D. J., et al. (2013). High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381, 557–564. doi: 10.1016/S0140-6736(12)61816-9
Corazza, S., Mündermann, L., Gambaretto, E., Ferrigno, G., and Andriacchi, T. P. (2010). Markerless motion capture through visual hull, articulated icp and subject specific model generation. Int. J. Comput. Vis. 87, 156–169. doi: 10.1007/s11263-009-0284-3
Courellis, H. S., Nummela, S. U., Metke, M., Diehl, G. W., Bussell, R., Cauwenberghs, G., et al. (2019). Spatial encoding in primate hippocampus during free navigation. PLoS Biol. 17:e3000546. doi: 10.1371/journal.pbio.3000546
Courtine, G., Roy, R. R., Hodgson, J., McKay, H., Raven, J., Zhong, H., et al. (2005a). Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate (Rhesus). J. Neurophysiol. 93, 3127–3145. doi: 10.1152/jn.01073.2004
Courtine, G., Roy, R. R., Raven, J., Hodgson, J., Mckay, H., Yang, H., et al. (2005b). Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 128, 2338–2358. doi: 10.1093/brain/awh604
Demes, B. (2011). Three-dimensional kinematics of capuchin monkey bipedalism. Am. J. Phys. Anthropol. 145, 147–155. doi: 10.1002/ajpa.21484
Demes, B., and O’Neill, M. C. (2013). Ground reaction forces and center of mass mechanics of bipedal capuchin monkeys: implications for the evolution of human bipedalism. Am. J. Phys. Anthropol. 150, 76–86. doi: 10.1002/ajpa.22176
Demes, B., Thompson, N. E., O’Neill, M. C., and Umberger, B. R. (2015). Center of mass mechanics of chimpanzee bipedal walking. Am. J. Phys. Anthropol. 156, 422–433. doi: 10.1002/ajpa.22667
Dorociak, R. D., and Cuddeford, T. J. (1995). Determining 3-D system accuracy for the VICON 370 system. Gait Posture 3:88. doi: 10.1016/0966-6362(95)93468-R
Drew, T. (1988). Motor cortical cell discharge during voluntary gait modification. Brain Res. 457, 181–187. doi: 10.1016/0006-8993(88)90073-X
Drew, L. (2022). The brain-reading devices helping paralysed people to move, talk and touch. Nature 604, 416–419. doi: 10.1038/d41586-022-01047-w
Drew, T., and Marigold, D. S. (2015). Taking the next step: cortical contributions to the control of locomotion. Curr. Opin. Neurobiol. 33, 25–33. doi: 10.1016/j.conb.2015.01.011
Dunham, N. T., McNamara, A., Shapiro, L., Hieronymus, T., and Young, J. W. (2018). A user's guide for the quantitative analysis of substrate characteristics and locomotor kinematics in free-ranging primates. Am. J. Phys. Anthropol. 167, 569–584. doi: 10.1002/ajpa.23686
Evarts, E. V. (1966). Pyramidal tract activity associated with a conditioned hand movement in the monkey. J. Neurophysiol. 29, 1011–1027. doi: 10.1152/jn.1966.29.6.1011
Fitzsimmons, N., Lebedev, M., Peikon, I., and Nicolelis, M. A. (2009). Extracting kinematic parameters for monkey bipedal walking from cortical neuronal ensemble activity. Front. Integr. Neurosci. 3:3. doi: 10.3389/neuro.07.003.2009
Flesher, S. N., Downey, J. E., Weiss, J. M., Hughes, C. L., Herrera, A. J., Tyler-Kabara, E. C., et al. (2021). A brain-computer interface that evokes tactile sensations improves robotic arm control. Science 372, 831–836. doi: 10.1126/science.abd0380
Foster, J. D., Nuyujukian, P., Freifeld, O., Gao, H., Walker, R., I Ryu, S., et al. (2014). A freely-moving monkey treadmill model. J. Neural Eng. 11:046020. doi: 10.1088/1741-2560/11/4/046020
Galna, B., Barry, G., Jackson, D., Mhiripiri, D., Olivier, P., and Rochester, L. (2014). Accuracy of the Microsoft Kinect sensor for measuring movement in people with Parkinson's disease. Gait Posture 39, 1062–1068. doi: 10.1016/j.gaitpost.2014.01.008
Georgopoulos, A. P., Schwartz, A. B., and Kettner, R. E. (1986). Neuronal population coding of movement direction. Science 233, 1416–1419. doi: 10.1126/science.3749885
Giansanti, D., Maccioni, G., and Macellari, V. (2005). The development and test of a device for the reconstruction of 3-D position and orientation by means of a kinematic sensor assembly with rate gyroscopes and accelerometers. IEEE Trans. Biomed. Eng. 52, 1271–1277. doi: 10.1109/TBME.2005.847404
Glaser, J. I., Benjamin, A. S., Chowdhury, R. H., Perich, M. G., Miller, L. E., and Kording, K. P. (2020). Machine learning for neural decoding. Eneuro 7:ENEU19.2020. doi: 10.1523/ENEURO.0506-19.2020
Goetz, L., Piallat, B., Thibaudier, Y., Montigon, O., David, O., and Chabardes, S. (2012). A non-human primate model of bipedal locomotion under restrained condition allowing gait studies and single unit brain recordings. J. Neurosci. Methods 204, 306–317. doi: 10.1016/j.jneumeth.2011.11.025
Goto, R., Grider-Potter, N., Kinoshita, Y., Oka, K., Shitara, T., and Nakano, Y. (2021). Paraspinal muscle activation during bipedal walking in orthograde primates and its implications for the evolution of trunk balance. Am. J. Phys. Anthropol. 174:41. doi: 10.1002/ajpa.24262
Goto, R., and Nakano, Y. (2018). A muscle synergy-based analysis of the trunk and hindlimb muscle activation patterns during quadrupedal and bipedal walking in Japanese macaque, white-handed gibbon, and human. Am. J. Phys. Anthropol. 165:103. doi: 10.1002/ajpa.23489
Griffith, D. A., Tamer Cavusgil, S., and Xu, S. (2008). Emerging themes in international business research. J. Int. Bus. Stud. 39, 1220–1235. doi: 10.1057/palgrave.jibs.8400412
Grillner, S. (2003). The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 4, 573–586. doi: 10.1038/nrn1137
Hanley, B., Tucker, C. B., and Bissas, A. (2017). Differences between motion analysis systems in calculating knee angle in elite race walking. Available at: http://ecss-congress.eu/2017/17/index.php/programme (Accessed September 20, 2022).
Hanna, J. B., Schmitt, D., Wright, K., Eshchar, Y., Visalberghi, E., and Fragaszy, D. (2015). Kinetics of bipedal locomotion during load carrying in capuchin monkeys. J. Hum. Evol. 85, 149–156. doi: 10.1016/j.jhevol.2015.05.006
Harrison, R. R., Kier, R. J., Kim, S., Rieth, L., Warren, D. J., Ledbetter, N. M., et al. (2008). “A wireless neural interface for chronic recording.” in 2008 IEEE Biomedical Circuits and Systems Conference. pp. 125–128. IEEE.
Hazama, Y., and Tamura, R. (2019). Effects of self-locomotion on the activity of place cells in the hippocampus of a freely behaving monkey. Neurosci. Lett. 701, 32–37. doi: 10.1016/j.neulet.2019.02.009
Higurashi, Y., Hirasaki, E., and Kumakura, H. (2010). Palmar and plantar pressure while walking on a horizontal ladder and single pole in Macaca fuscata. Int. J. Primatol. 31, 181–190. doi: 10.1007/s10764-010-9393-7
Hildebrand, M. (1967). Symmetrical gaits of primates. Am. J. Phys. Anthropol. 26, 119–130. doi: 10.1002/ajpa.1330260203
Hirasaki, E., Malaivijitnond, S., and Hamada, Y. (2019). Locomotor kinematics of two semi-wild macaque species (Macaca assamensis and Macaca arctoides) in Thailand. Folia Primatol. 90, 162–178. doi: 10.1159/000496024
Hochberg, L. R., Bacher, D., Jarosiewicz, B., Masse, N. Y., Simeral, J. D., Vogel, J., et al. (2012). Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375. doi: 10.1038/nature11076
Hochberg, L. R., Serruya, M. D., Friehs, G. M., Mukand, J. A., Saleh, M., Caplan, A. H., et al. (2006). Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171. doi: 10.1038/nature04970
Hu, D., Wang, S., Li, B., Liu, H., and He, J. (2021). Spinal cord injury-induced changes in encoding and decoding of bipedal walking by motor cortical ensembles. Brain Sci. 11:1193. doi: 10.3390/brainsci11091193
Kimura, T. (2000). Development of quadrupedal locomotion on level surfaces in Japanese macaques. Folia Primatol. 71, 323–333. doi: 10.1159/000021755
Klous, M., Müller, E., and Schwameder, H. (2010). Collecting kinematic data on a ski/snowboard track with panning, tilting, and zooming cameras: is there sufficient accuracy for a biomechanical analysis? J. Sports Sci. 28, 1345–1353. doi: 10.1080/02640414.2010.507253
Labuguen, R., Matsumoto, J., Negrete, S. B., Nishimaru, H., Nishijo, H., Takada, M., et al. (2021). MacaquePose: a novel “in the wild” macaque monkey pose dataset for markerless motion capture. Front. Behav. Neurosci. 14:581154. doi: 10.3389/fnbeh.2020.581154
Lane, M. A. (2000). Nonhuman primate models in biogerontology. Exp. Gerontol. 35, 533–541. doi: 10.1016/S0531-5565(00)00102-9
Larson, S. G., and Demes, B. (2011). Weight support distribution during quadrupedal walking in Ateles and Cebus. Am. J. Phys. Anthropol. 144, 633–642. doi: 10.1002/ajpa.21460
Lebedev, M. A., and Nicolelis, M. A. (2017). Brain-machine interfaces: from basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 97, 767–837. doi: 10.1152/physrev.00027.2016
Li, Z., O’Doherty, J. E., Hanson, T. L., Lebedev, M. A., Henriquez, C. S., and Nicolelis, M. A. (2009). Unscented Kalman filter for brain-machine interfaces. PLoS One 4:e6243. doi: 10.1371/journal.pone.0006243
Ma, Z., Zhang, Y. P., Liu, W., Yan, G., Li, Y., Shields, L. B., et al. (2016). A controlled spinal cord contusion for the rhesus macaque monkey. Exp. Neurol. 279, 261–273. doi: 10.1016/j.expneurol.2016.02.008
Martin, P. H., and Unwin, D. M. (1980). A microwave doppler radar activity monitor. Behav. Res. Methods Instrum. 12, 517–520. doi: 10.3758/BF03201826
Mathis, A., Mamidanna, P., Cury, K. M., Abe, T., Murthy, V. N., Mathis, M. W., et al. (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289. doi: 10.1038/s41593-018-0209-y
Mathis, M. W., and Mathis, A. (2020). Deep learning tools for the measurement of animal behavior in neuroscience. Curr. Opin. Neurobiol. 60, 1–11. doi: 10.1016/j.conb.2019.10.008
McCrea, D. A., and Rybak, I. A. (2008). Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 57, 134–146. doi: 10.1016/j.brainresrev.2007.08.006
Miranda, H., Gilja, V., Chestek, C. A., Shenoy, K. V., and Meng, T. H. (2010). HermesD: a high-rate long-range wireless transmission system for simultaneous multichannel neural recording applications. IEEE Trans. Biomed. Circuits Syst. 4, 181–191. doi: 10.1109/TBCAS.2010.2044573
Miri, A., Warriner, C. L., Seely, J. S., Elsayed, G. F., Cunningham, J. P., Churchland, M. M., et al. (2017). Behaviorally selective engagement of short-latency effector pathways by motor cortex. Neuron 95, 683–696.e11. doi: 10.1016/j.neuron.2017.06.042
Monnet, T., Samson, M., Bernard, A., David, L., and Lacouture, P. (2014). Measurement of three-dimensional hand kinematics during swimming with a motion capture system: a feasibility study. Sports Eng. 17, 171–181. doi: 10.1007/s12283-014-0152-4
Mori, F., Nakajima, K., Tachibana, A., and Mori, S. (2006). Obstacle clearance and prevention from falling in the bipedally walking Japanese monkey, Macaca fuscata. Age Ageing 35, ii19–ii23. doi: 10.1093/ageing/afl079
Nakamura, T., Matsumoto, J., Nishimaru, H., Bretas, R. V., Takamura, Y., Hori, E., et al. (2016). A markerless 3D computerized motion capture system incorporating a skeleton model for monkeys. PLoS One 11:e0166154. doi: 10.1371/journal.pone.0166154
Nakano, Y. (2002). The effects of substratum inclination on locomotor patterns in primates. Z. Morphol. Anthropol. 83, 189–199. doi: 10.1127/zma/83/2002/189
Nakatsukasa, M., Hirasaki, E., and Ogihara, N. (2006). Energy expenditure of bipedal walking is higher than that of quadrupedal walking in Japanese macaques. Am. J. Phys. Anthropol. 131, 33–37. doi: 10.1002/ajpa.20403
Nakatsukasa, M., Ogihara, N., Hamada, Y., Goto, Y., Yamada, M., Hirakawa, T., et al. (2004). Energetic costs of bipedal and quadrupedal walking in Japanese macaques. Am. J. Phys. Anthropol. 124, 248–256. doi: 10.1002/ajpa.10352
Ogihara, N., Hirasaki, E., Andrada, E., and Blickhan, R. (2018). Bipedal gait versatility in the Japanese macaque (Macaca fuscata). J. Hum. Evol. 125, 2–14. doi: 10.1016/j.jhevol.2018.09.001
Ogihara, N., Makishima, H., Hirasaki, E., and Nakatsukasa, M. (2012). Inefficient use of inverted pendulum mechanism during quadrupedal walking in the Japanese macaque. Primates 53, 41–48. doi: 10.1007/s10329-011-0265-3
Ogihara, N., Makishima, H., and Nakatsukasa, M. (2010). Three-dimensional musculoskeletal kinematics during bipedal locomotion in the Japanese macaque, reconstructed based on an anatomical model-matching method. J. Hum. Evol. 58, 252–261. doi: 10.1016/j.jhevol.2009.11.009
Oku, H., Ide, N., and Ogihara, N. (2021). Forward dynamic simulation of Japanese macaque bipedal locomotion demonstrates better energetic economy in a virtualised plantigrade posture. Commun. Biol. 4, 1–11. doi: 10.1038/s42003-021-01831-w
O’Neill, M. C., Demes, B., Thompson, N. E., and Umberger, B. R. (2018). Three-dimensional kinematics and the origin of the hominin walking stride. J. R. Soc. Interface 15:20180205. doi: 10.1098/rsif.2018.0205
O’Neill, M. C., Lee, L. F., Demes, B., Thompson, N. E., Larson, S. G., Stern, J. T. Jr., et al. (2015). Three-dimensional kinematics of the pelvis and hind limbs in chimpanzee (Pan troglodytes) and human bipedal walking. J. Hum. Evol. 86, 32–42. doi: 10.1016/j.jhevol.2015.05.012
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J. Clin. Epidemiol. 134, 103–112. doi: 10.1016/j.jclinepi.2021.02.003
Pearce, T. M., and Moran, D. W. (2012). Strategy-dependent encoding of planned arm movements in the dorsal premotor cortex. Science 337, 984–988. doi: 10.1126/science.1220642
Peikon, I. D., Fitzsimmons, N. A., Lebedev, M. A., and Nicolelis, M. A. (2009). Three-dimensional, automated, real-time video system for tracking limb motion in brain–machine interface studies. J. Neurosci. Methods 180, 224–233. doi: 10.1016/j.jneumeth.2009.03.010
Perrott, M. A., Pizzari, T., Cook, J., and McClelland, J. A. (2017). Comparison of lower limb and trunk kinematics between markerless and marker-based motion capture systems. Gait Posture 52, 57–61. doi: 10.1016/j.gaitpost.2016.10.020
Pontzer, H., Raichlen, D. A., and Rodman, P. S. (2014). Bipedal and quadrupedal locomotion in chimpanzees. J. Hum. Evol. 66, 64–82. doi: 10.1016/j.jhevol.2013.10.002
Rapeaux, A. B., and Constandinou, T. G. (2021). Implantable brain machine interfaces: first-in-human studies, technology challenges and trends. Curr. Opin. Biotechnol. 72, 102–111. doi: 10.1016/j.copbio.2021.10.001
Reghem, E., Chèze, L., Coppens, Y., and Pouydebat, E. (2013). Unconstrained 3D-kinematics of prehension in five primates: lemur, capuchin, gorilla, chimpanzee, human. J. Hum. Evol. 65, 303–312. doi: 10.1016/j.jhevol.2013.06.011
Reghem, E., Chèze, L., Coppens, Y., and Pouydebat, E. (2014). The influence of body posture on the kinematics of prehension in humans and gorillas (Gorilla gorilla). Exp. Brain Res. 232, 1047–1056. doi: 10.1007/s00221-013-3817-5
Reimer, J., and Hatsopoulos, N. G. (2009). The problem of parametric neural coding in the motor system. Adv. Exp. Med. Biol. 629, 243–259. doi: 10.1007/978-0-387-77064-2_12
Revuelta, G. J., Uthayathas, S., Wahlquist, A. E., Factor, S. A., and Papa, S. M. (2012). Non-human primate FOG develops with advanced parkinsonism induced by MPTP treatment. Exp. Neurol. 237, 464–469. doi: 10.1016/j.expneurol.2012.07.021
Rizk, M., Bossetti, C. A., Jochum, T. A., Callender, S. H., Nicolelis, M. A., Turner, D. A., et al. (2009). A fully implantable 96-channel neural data acquisition system. J. Neural Eng. 6:026002. doi: 10.1088/1741-2560/6/2/026002
Rouse, A. G., Stanslaski, S. R., Cong, P., Jensen, R. M., Afshar, P., Ullestad, D., et al. (2011). A chronic generalized bi-directional brain–machine interface. J. Neural Eng. 8:036018. doi: 10.1088/1741-2560/8/3/036018
Ruß, D. K. P. (2016). Evaluation of joint angle accuracy using markerless silhouette-based tracking and hybrid tracking against traditional marker tracking. Doctoral dissertation, Institute of Sport.
Santhanam, G., Linderman, M. D., Gilja, V., Afshar, A., Ryu, S. I., Meng, T. H., et al. (2007). HermesB: a continuous neural recording system for freely behaving primates. IEEE Trans. Biomed. Eng. 54, 2037–2050. doi: 10.1109/TBME.2007.895753
Scaramuzza, D., and Fraundorfer, F. (2011). Visual odometry [tutorial]. IEEE Robotics and Automation Magazine 18, 80–92.
Schmidt, M. (2005). Quadrupedal locomotion in squirrel monkeys (Cebidae: Saimiri sciureus): a cineradiographic study of limb kinematics and related substrate reaction forces. Am. J. Phys. Anthropol. 128, 359–370. doi: 10.1002/ajpa.20089
Schmidt, M. (2008). Forelimb proportions and kinematics: how are small primates different from other small mammals? J. Exp. Biol. 211, 3775–3789. doi: 10.1242/jeb.019802
Schwarz, D. A., Lebedev, M. A., Hanson, T. L., Dimitrov, D. F., Lehew, G., Meloy, J., et al. (2014). Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat. Methods 11, 670–676. doi: 10.1038/nmeth.2936
Serruya, M., Hatsopoulos, N., Fellows, M., Paninski, L., and Donoghue, J. (2003). Robustness of neuroprosthetic decoding algorithms. Biol. Cybern. 88, 219–228. doi: 10.1007/s00422-002-0374-6
Shenoy, K. V., Sahani, M., and Churchland, M. M. (2013). Cortical control of arm movements: a dynamical systems perspective. Annu. Rev. Neurosci. 36, 337–359. doi: 10.1146/annurev-neuro-062111-150509
Shimada, H., Kanai, R., Kondo, T., Yoshino-Saito, K., Uchida, A., Nakamura, M., et al. (2017). Three-dimensional kinematic and kinetic analysis of quadrupedal walking in the common marmoset (Callithrix jacchus). Neurosci. Res. 125, 11–20. doi: 10.1016/j.neures.2017.06.005
Shitara, T., Goto, R., Ito, K., Hirasaki, E., and Nakano, Y. (2022b). Hip medial rotator action of gluteus medius in Japanese macaque (Macaca fuscata) and implications to adaptive significance for quadrupedal walking in primates. J. Anat. 241, 407–419. doi: 10.1111/joa.13658
Shitara, T., Ito, K., Fujiwara, T., Goto, R., Hirasaki, E., and Nakano, Y. (2022a). How was the abductor function of gluteus medius muscle acquired in the evolution of bipedalism? A comparison of the moment arms of gluteus medius during quadrupedal and bipedal walking in Japanese macaque (Macaca fuscata). Am. J. Biol. Anthropol. 177:167. doi: 10.1002/ajpa.24514
Silvernagel, M. P., Ling, A. S., and Nuyujukian, P., Brain Interfacing Laboratory (2021). A markerless platform for ambulatory systems neuroscience. Science. Robotics 6:eabj7045. doi: 10.1126/scirobotics.abj7045
Sodagar, A. M., Perlin, G. E., Yao, Y., Najafi, K., and Wise, K. D. (2009). An implantable 64-channel wireless microsystem for single-unit neural recording. IEEE J. Solid State Circuits 44, 2591–2604. doi: 10.1109/JSSC.2009.2023159
Spörri, J., Schiefermüller, C., and Müller, E. (2016). Collecting kinematic data on a ski track with optoelectronic stereophotogrammetry: a methodological study assessing the feasibility of bringing the biomechanics lab to the field. PLoS One 11:e0161757. doi: 10.1371/journal.pone.0161757
Thompson, N. E., O’Neill, M. C., Holowka, N. B., and Demes, B. (2018). Step width and frontal plane trunk motion in bipedal chimpanzee and human walking. J. Hum. Evol. 125, 27–37. doi: 10.1016/j.jhevol.2018.09.006
Tseng, P. H., Urpi, N. A., Lebedev, M., and Nicolelis, M. (2019). Decoding movements from cortical ensemble activity using a long short-term memory recurrent network. Neural Comput. 31, 1085–1113. doi: 10.1162/neco_a_01189
Van der Kruk, E., and Reijne, M. M. (2018). Accuracy of human motion capture systems for sport applications; state-of-the-art review. Eur. J. Sport Sci. 18, 806–819. doi: 10.1080/17461391.2018.1463397
Vargas-Irwin, C. E., Shakhnarovich, G., Yadollahpour, P., Mislow, J. M., Black, M. J., and Donoghue, J. P. (2010). Decoding complete reach and grasp actions from local primary motor cortex populations. J. Neurosci. 30, 9659–9669. doi: 10.1523/JNEUROSCI.5443-09.2010
Vouga, T., Zhuang, K. Z., Olivier, J., Lebedev, M. A., Nicolelis, M. A., Bouri, M., et al. (2017). EXiO—A brain-controlled lower limb exoskeleton for rhesus macaques. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 131–141. doi: 10.1109/TNSRE.2017.2659654
Wang, W., Chan, S. S., Heldman, D. A., and Moran, D. W. (2010). Motor cortical representation of hand translation and rotation during reaching. J. Neurosci. 30, 958–962. doi: 10.1523/JNEUROSCI.3742-09.2010
Wei, R. H., Song, W., Zhao, C., Zhao, W., Li, L. F., Ji, R., et al. (2016). Influence of walking speed on gait parameters of bipedal locomotion in rhesus monkeys. J. Med. Primatol. 45, 304–311. doi: 10.1111/jmp.12235
Wei, R. H., Zhao, C., Rao, J. S., Zhao, W., Wei, Y. Q., Zhou, X., et al. (2019). Neuromuscular control pattern in rhesus monkeys during bipedal walking. Exp. Anim. 68, 341–349. doi: 10.1538/expanim.18-0180
Wei, R. H., Zhao, C., Rao, J. S., Zhao, W., Zhou, X., Tian, P. Y., et al. (2018). The kinematic recovery process of rhesus monkeys after spinal cord injury. Exp. Anim. 67, 431–440. doi: 10.1538/expanim.18-0023
Wessberg, J., Stambaugh, C. R., Kralik, J. D., Beck, P. D., Laubach, M., Chapin, J. K., et al. (2000). Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature 408, 361–365. doi: 10.1038/35042582
Willett, F. R., Avansino, D. T., Hochberg, L. R., Henderson, J. M., and Shenoy, K. V. (2021). High-performance brain-to-text communication via handwriting. Nature 593, 249–254. doi: 10.1038/s41586-021-03506-2
Winiarski, S. (2003). Human locomotion analysis technique with SIMI motion. Acta Bioeng. Biomech. 5, 544–550.
Wise, K. D., Anderson, D. J., Hetke, J. F., Kipke, D. R., and Najafi, K. (2004). Wireless implantable microsystems: high-density electronic interfaces to the nervous system. Proc. IEEE 92, 76–97. doi: 10.1109/JPROC.2003.820544
Wu, W., Black, M., Gao, Y., Serruya, M., Shaikhouni, A., Donoghue, J., et al. (2002). Neural decoding of cursor motion using a Kalman filter. Adv. Neural Inf. Proces. Syst. 15, 133–140.
Xiang, Y., John, P., Yakushin, S. B., Kunin, M., Raphan, T., and Cohen, B. (2007). Dynamics of quadrupedal locomotion of monkeys: implications for central control. Exp. Brain Res. 177, 551–572. doi: 10.1007/s00221-006-0707-0
Xing, D., Aghagolzadeh, M., Truccolo, W., and Borton, D. (2019). Low-dimensional motor cortex dynamics preserve kinematics information during unconstrained locomotion in nonhuman primates. Front. Neurosci. 13:1046. doi: 10.3389/fnins.2019.01046
Yakovenko, S., and Drew, T. (2015). Similar motor cortical control mechanisms for precise limb control during reaching and locomotion. J. Neurosci. 35, 14476–14490. doi: 10.1523/JNEUROSCI.1908-15.2015
Yin, M., Borton, D. A., Aceros, J., Patterson, W. R., and Nurmikko, A. V. (2013). A 100-channel hermetically sealed implantable device for chronic wireless neurosensing applications. IEEE Trans. Biomed. Circuits Syst. 7, 115–128. doi: 10.1109/TBCAS.2013.2255874
Yin, M., Borton, D. A., Komar, J., Agha, N., Lu, Y., Li, H., et al. (2014). Wireless neurosensor for full-spectrum electrophysiology recordings during free behavior. Neuron 84, 1170–1182. doi: 10.1016/j.neuron.2014.11.010
Yin, M., and Ghovanloo, M. (2008). “A clockless ultra low-noise low-power wireless implantable neural recording system.” in 2008 IEEE International Symposium on Circuits and Systems (ISCAS). pp. 1756–1759. IEEE.
Yin, M., and Ghovanloo, M. (2009). “A flexible clockless 32-ch simultaneous wireless neural recording system with adjustable resolution.” in 2009 IEEE international solid-state circuits conference-digest of technical papers. pp. 432–433. IEEE.
Yin, M., and Ghovanloo, M. (2011). A low-noise clockless simultaneous 32-channel wireless neural recording system with adjustable resolution. Analog Integ. Circuit Sig Process 66, 417–431.
Young, J. W., and Chadwell, B. A. (2020). Not all fine-branch locomotion is equal: grasping morphology determines locomotor performance on narrow supports. J. Hum. Evol. 142:102767. doi: 10.1016/j.jhevol.2020.102767
Zhang, Q., Zhang, S., Hao, Y., Zhang, H., Zhu, J., Zhao, T., et al. (2012). Development of an invasive brain-machine interface with a monkey model. Chin. Sci. Bull. 57, 2036–2045. doi: 10.1007/s11434-012-5096-0
Keywords: non-human primate, gait, BCI, neurophysiological analysis, motor cortex
Citation: Liang F, Yu S, Pang S, Wang X, Jie J, Gao F, Song Z, Li B, Liao W-H and Yin M (2023) Non-human primate models and systems for gait and neurophysiological analysis. Front. Neurosci. 17:1141567. doi: 10.3389/fnins.2023.1141567
Received: 10 January 2023; Accepted: 11 April 2023;
Published: 28 April 2023.
Edited by:
Reza Lashgari, Shahid Beheshti University, IranReviewed by:
Solaiman Shokur, Swiss Federal Institute of Technology Lausanne, SwitzerlandCopyright © 2023 Liang, Yu, Pang, Wang, Jie, Gao, Song, Li, Liao and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Yin, bWluZ195aW5AaGFpbmFudS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.