- 1Department of Neurology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 2School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou, China
- 3Research Units of Clinical Translation of Cell Growth Factors and Diseases Research, Chinese Academy of Medical Science, Wenzhou Medical University, Wenzhou, China
- 4Department of Neurology, The First Affiliated Hospital Hospital of Wenzhou Medical University, Wenzhou, China
- 5The People’s Hospital of Pingyang, Wenzhou, China
- 6The First People’s Hospital of Wenling, Taizhou, China
Introduction: Intracerebral hemorrhage (ICH) is the most prevalent cause of death. We sought to explore whether serum Fibroblast growth factor 21 (FGF21) is of substantial benefit in predicting poor prognosis in ICH patient.
Methods: A prospective, multicenter cohort analysis of serum FGF21 levels in 418 ICH patients was carried out. At three months following ICH start, the primary endpoint was death or major disability, whereas the secondary endpoint was death. We investigated the association between serum FGF21 and clinical outcomes. We added FGF21 to the existing rating scale to assess whether it enhanced the prediction ability of the original model. Effectiveness was determined by calculating the C-statistic, net reclassification index (NRI), absolute integrated discrimination improvement (IDI) index.
Results: Among 418 enrolled patients, 217 (51.9%) of the all subjects had death or significant disability. Compared with patients in the lowest quartile group, those in the first quartile group had higher risk of the primary outcome (Odds ratio, 2.73 [95%CI,1.42–5.26, p < 0.05]) and second outcome (Hazard ratio, 4.28 [95%CI,1.61–11.42, p < 0.001]). The integration of FGF21 into many current ICH scales improved the discrimination and calibration quality for the integrated discrimination index’s prediction of main and secondary findings (all p < 0.05).
Conclusion: Elevated serum FGF21 is associated with increased risks of adverse clinical outcomes at 3 months in ICH patients, suggesting FGF21 may be a valuable prognostic factor.
Introduction
More than 10% of all stroke hospitalizations are due to spontaneous intracerebral hemorrhage (ICH), the second most common kind of stroke (Sacco et al., 2009). It’s considered a major issue since it causes 40% death and significant disability rates, making it a significant public health concern (Qureshi et al., 2001, 2009). Hence, rehabilitation management for ICH patients must be guided by accurate prognoses and risk stratification. The original ICH score was the first commonly used and straightforward clinical grading system for ICH (Hemphill et al., 2001). New predictive ICH models and enhancements to the original ICH score (Gregório et al., 2018) have been implemented, such as the FUNC score (Rost et al., 2008), ICH-FOS score (Ji et al., 2013). However, none of these has achieved widespread acceptance and utilization in actual clinical practice (Schmidt et al., 2018). In recent decades, predictors of ICH prognostic models have been enhanced by adding novel laboratory indicators to clinical risk variables in order to increase the prediction capacity of the model (Cai et al., 2021).
Fibroblast growth factor 21 (FGF21) is a protein that is primarily produced in the liver and has many effects on how tissues balance their energy. It belongs to the subfamily of fibroblast growth factors (Chen et al., 2022). As it lacks heparin-binding domains, it is secreted into the bloodstream and exerts its endocrine effects there. It has been suggested that FGF21, a key regulator of glucose and lipid metabolism, might be used to treat metabolic illnesses (Geng et al., 2020). Furthermore, several epidemiological studies have found that FGF21 helps predict prognosis in cardiovascular disease and atherosclerosis (Chou et al., 2016; Li et al., 2016; Tabari et al., 2019). Nonetheless, significant population-based studies on the prognostic usefulness of serum FGF21 on functional outcome and death in patients with ICH are lacking. In light of this, the current study sought to evaluate the relationship between blood FGF21 levels and prognosis in ICH patients.
Materials and methods
Ethical statements
We performed this investigation utilizing data from a multicenter, prospective registry study done in five hospitals in the southeast coastline region of Zhejiang Province, China. For this predetermined biomarker analysis, blood samples were taken from study locations. The study was authorized by the institutional review boards of all research facilities, and we acquired written informed permission to participate in this study from legal representatives of patients. The study is registered at http://www.chictr.org.cn (ChiCTR2100051104).
Study population
The Stroke and Fibroblast Growth Factor Biomarkers Cohort Study (SFBCS) was established to investigate the determinants of stroke and prognosis in the coastal area of southeast Zhejiang Province, China. The SFBCS was a multicenter, prospective observational study of stroke biomarkers and outcomes that recruited 1,510 stroke patients (including ischemic stroke, transient ischemic attack, and primary ICH) within 24 h of symptom onset, from March 1, 2021, to August 31, 2022. With the exception of ischemic stroke and transient ischemic attack, this study included all patients over the age of 18 in our cohort who had ICH (International Classification of Diseases, Tenth Revision, I61) during their hospital stay.
Each facility reviews suspected ICH patients’ electronic medical records upon admission from the emergency room and the neurology/neurosurgery departments to rule out survival bias. Almost every patient had a clinically meaningful blood sample that could be tested in the laboratory. Hemorrhagic infarcts, traumatic hemorrhages, and hemorrhagic conversion of a recent ischemic stroke were excluded, as were patients with ICH caused by malignancies, irreversible coagulopathy, Dural venous sinus thrombosis, vascular malformations, aneurysms, tumors, or other causes.
Data collection
Age, sex, history of hypertension, diabetes, hyperlipidemia, atrial fibrillation, smoking, alcohol intake, were included in demographic and clinical information. One cigarette per day was classified as smoking. Drinking at least 80 grams of liquor per day was defined as alcohol usage. Hypertension and diabetes were identified based on self-reported medical histories. At admission, the Glasgow Coma Scale (GCS), the National Institutes of Health Stroke Scale (NIHSS), and the initial post-stroke systolic (SBP) and diastolic (DBP) blood pressure (mmHg) were measured. Laboratory data collection (blood glucose, blood lipids, blood creatinine, etc.) was conducted in each participating hospital. In ICH patients, baseline radiological data from brain CT or MRI scans were collected. The location of intracerebral hemorrhage was categorized into lobar (frontal, temporal, parietal, and occipital); deep (lenticular or caudate nucleus, thalamus, internal or external capsule); brainstem; and cerebellum. The volume of the hemorrhage was calculated according to the ABC/2 method (Kothari et al., 1996). Based on the CT scan, hematoma expansion was calculated as a threshold >33% or >6 mL (Dowlatshahi et al., 2011) and was analyzed as a binary variable. All images were assessed by one board-certified radiologist and were blinded to clinical data.
Blood samples were collected from the vein immediately upon admission within 24 h, the 3rd and 7th day after the diagnosis. We calculated the cumulatively averaged FGF21 concentration using available FGF21 measurements to capture short-term longitudinal FGF21 patterns of participants. All samples were frozen at −80°C refrigerator in the central laboratory of Wenzhou Medical University until laboratory testing. The serum levels of FGF21 were determined using enzyme-linked immunosorbent assays (ELISAs) according to the manufacturer’s instructions. Routine blood analyses were performed in the hospital’s central laboratory.
Functional outcome assessment
Patient’s follow-up at 90-day was conducted by qualified personnel or physicians through telephone or in-person interview following a defined technique. Functional effects after ICH were evaluated using the modified Rankin Scale (mRS) with a range between 0 and 6, where a number of 0 means no illness and a number of 6 means death. At follow-up, the primary endpoints were composite events (mRS score 3–6), which comprised death from any cause (mRS score 6) or severe disability (mRS score 3–5). The secondary outcome was all-cause death.
Statistical analysis
All ICH patients were classified according to quartiles of FGF21 levels. Using the χ2 test for categorical data (such as sex and medical history) and ANOVA or the Kruskal-Wallis test for continuous variables (such as age), the baseline characteristics of patients were compared across quartiles of FGF21. Categorical variables are expressed as percentages, whereas continuous variables are represented as the mean (SD) or median (IQR). Missing data were imputed using the multiple imputation of chained equations approach. Utilizing univariable and multivariable analysis with logistic or Cox regression based on clinical confounding risk variables from clinical experience and literature, the association between FGF21 and adverse outcomes of ICH was evaluated. Comparing the high FGF21 quartiles to the low FGF21 quartiles, odds ratios (ORs) and 95% confidence intervals (CIs) for the primary or secondary outcome were calculated. Finally, Model 1 was adjusted for age (years) and sex (male or female); Model 2 was adjusted for age, sex, history of hypertension (yes or no), diabetes (yes or no), stroke (yes or no), and prior mRS (categorical); Model 3 was additionally adjusted for SBP (continuous), GCS score (continuous), location of hematoma (categorical), hematoma volume (continuous), and hematoma expansion (categorical). Kaplan–Meier survival curve was performed to show the cumulative incidence of death, where a log-rank test was stratified by FGF21 quartile. In addition, we assessed the predictive performance of the ICH score, ESSCN-ICH score, FUNC score, and ICH-FOS score, respectively. We appended FGF 21 to the four scales and calculated the difference in model performance using C statistics, the category-free net reclassification index (NRI), and integrated discrimination improvement (IDI). NRI and IDI are two alternatives to AUC for evaluating the performance improvement and usability of a new model (Pencina et al., 2008). To test the robustness of our findings, we conducted subgroup analyses stratified by sex, age, history of hypertension and diabetes, current smoking, and alcohol consumption. All data were analyzed with SAS version 9.4 software (SAS Institute Inc., Cary, NC) and R statistical software (Harrell Miscellaneous) version 4.2.1. p < 0.05 (two-sided) was considered statistically significant.
Results
Baseline characteristics
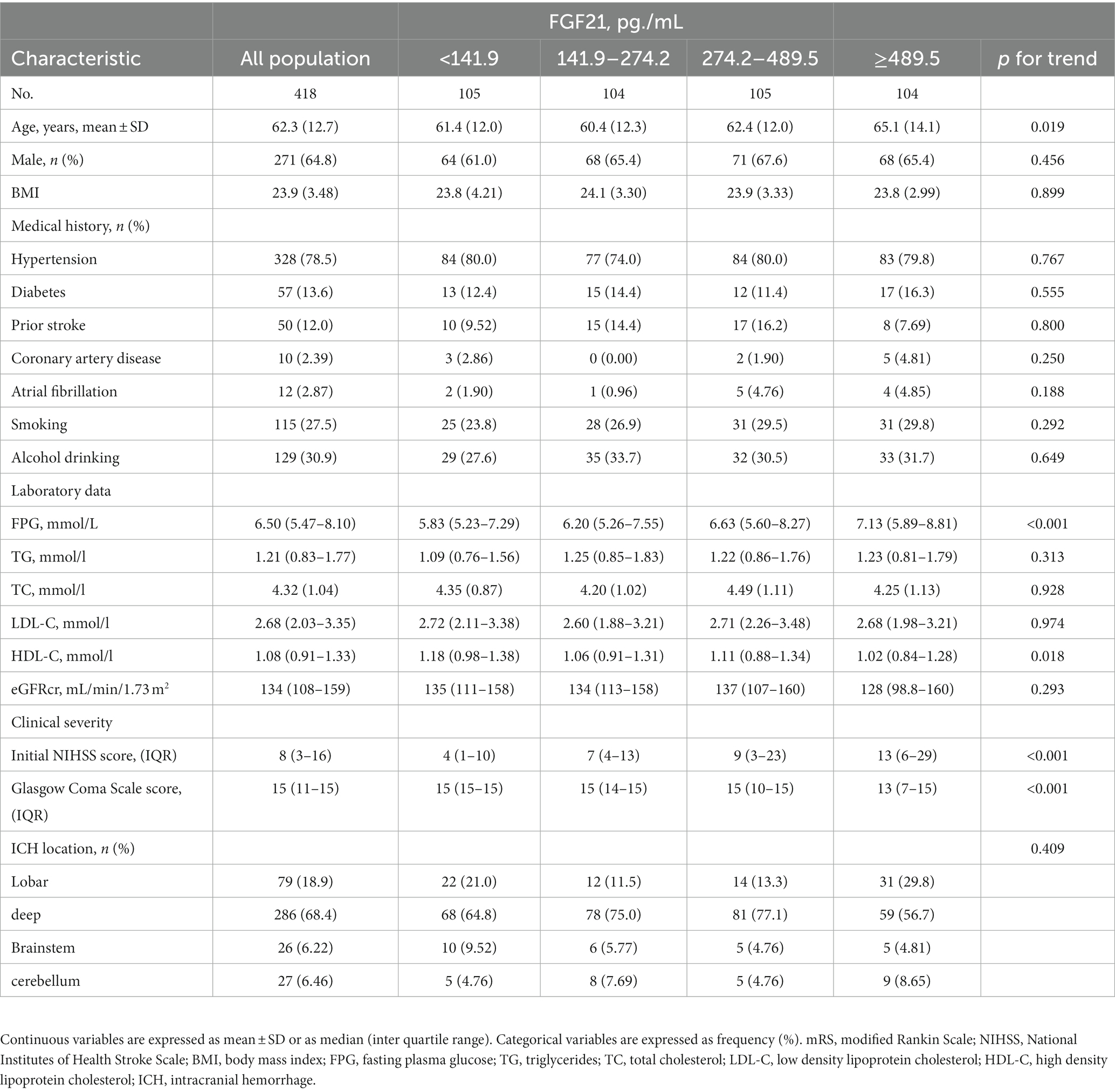
The final pooled cohort consisted of 418 patients (Figure 1). At 90-day, 86 (20.6%) of patients had died and 217 (51.9%) had an unsatisfactory outcome (mRS 3 to 6). The mean (SD) age was 62.3 (12.7) years, and 271 (64.8%) participants were male. Table 1 displays baseline characteristics by quartiles of FGF21. Patients with higher serum FGF21 were more likely to be older, have higher blood glucose, and have lower high-density lipoprotein than those with lower serum FGF21. Based on the neurological examination and radiological parameters upon admission, patients in the upper quartiles of FGF21 had a greater NIHSS score and a larger hematoma volume but lower GCS score.
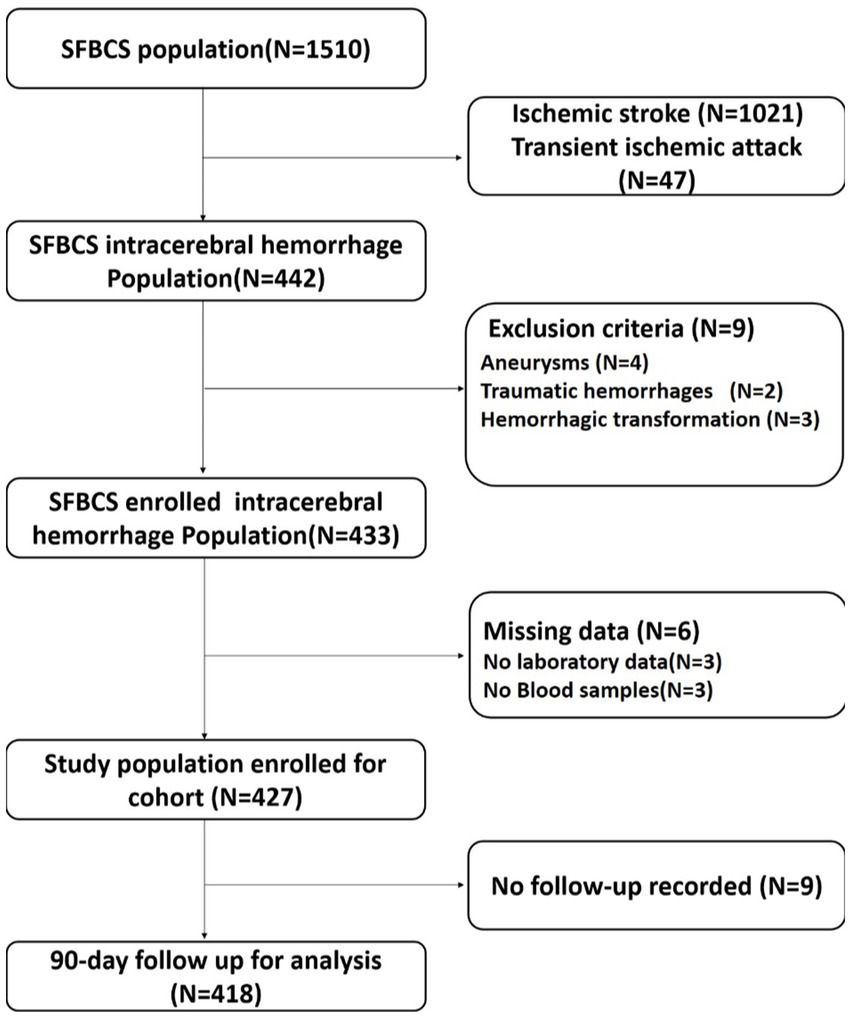
Figure 1. Flow chart of patient selection. SFBCS indicates stroke and fibroblast growth factor biomarkers cohort study.
Association between FGF21 and ICH prognosis
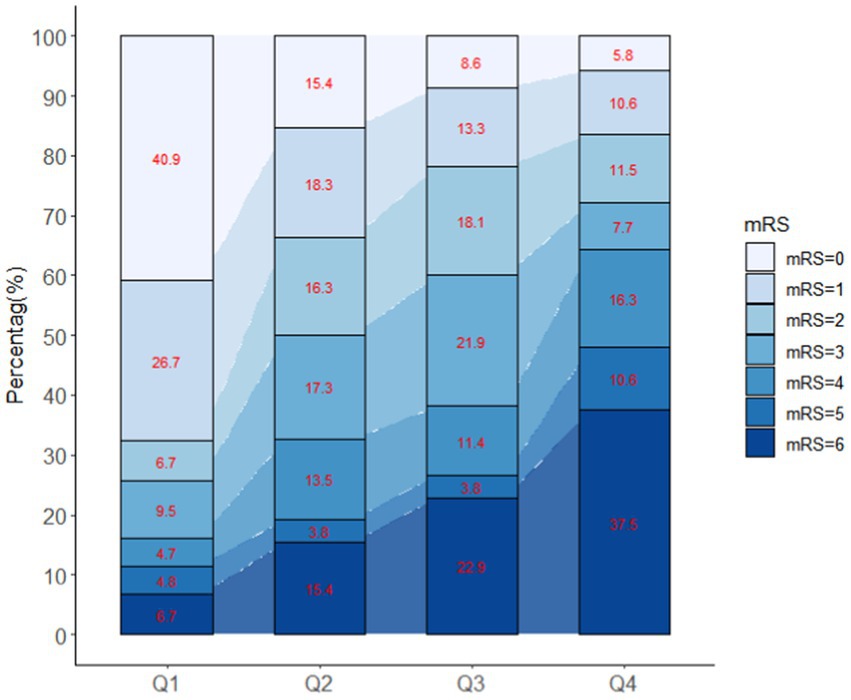
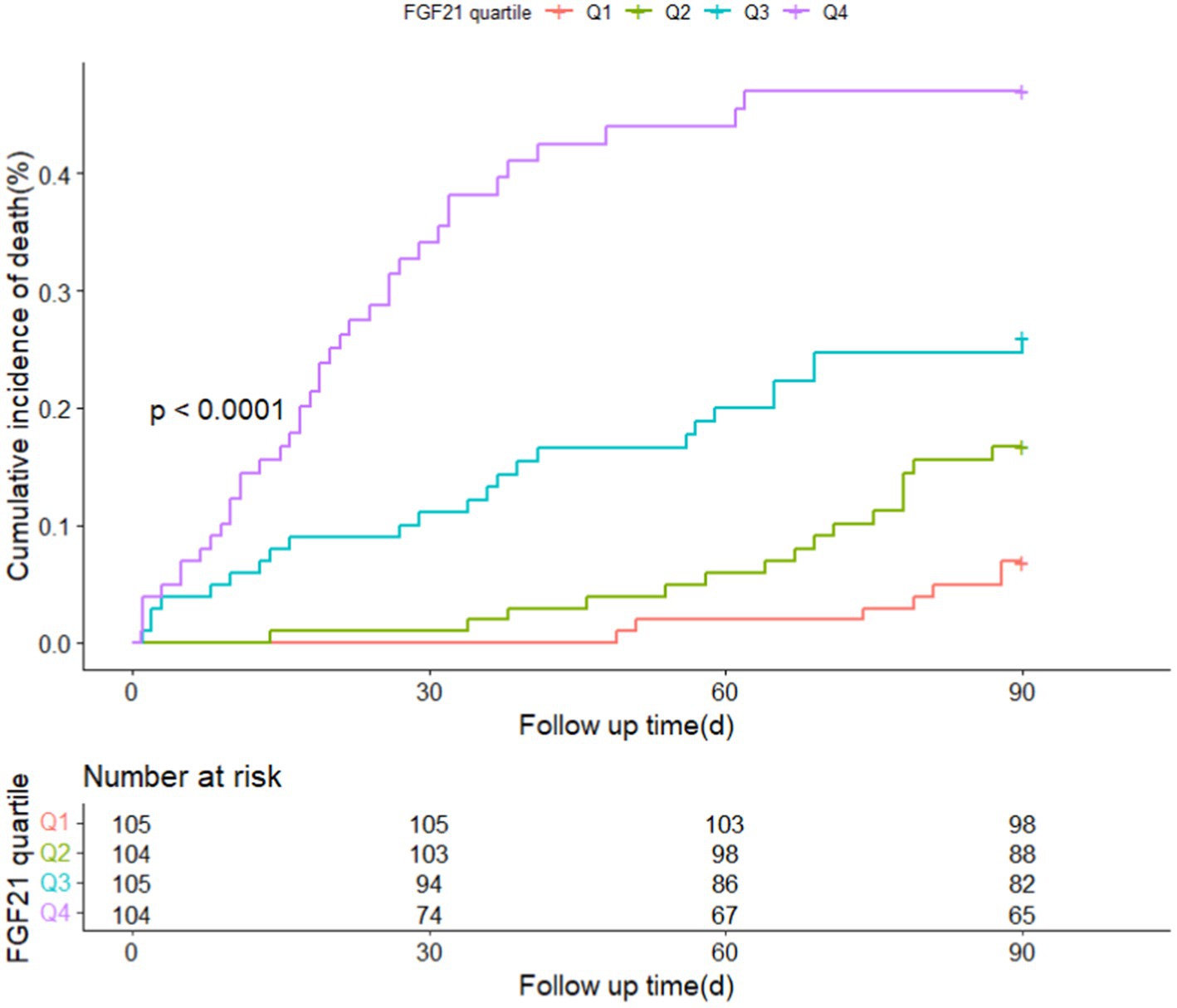
Patients with different quartiles of FGF21 varied in the distribution of mRS (Figure 2). The cumulative incidence rates of primary outcome at 3 months from the lowest quartile to the highest quartile of serum FGF21 were 25.7, 50.0, 60.0, and 72.1%, respectively. After adjustment for age, sex, history of hypertension, diabetes, stroke, prior mRS, SBP, GCS, hematoma location, hematoma volume, and hematoma expansion, the multivariable logistic regression analyses remained a significant association between FGF21 and unfavorable clinical outcomes [OR, 3.38 (95% CI, 1.41–8.06, p < 0.001); Table 2]. In subgroup analyses stratified by sex, age, current smoking, alcohol consumption, history of hypertension and diabetes, no significant interaction between serum FGF21 levels and these prespecified factors was observed (all p for interaction >0.05) and a substantial connection of FGF21 with risk of the primary outcome was seen in every subgroup (Supplementary Figure S1). Multivariable analyses between FGF21 and death seemed to have a relationship, however, after controlling all confounding variables, the connection was not significant (HR, 3.42 [95%CI, 0.65–17.93, p > 0.05]) (Supplementary Tables S1 in the Data Supplement). In addition, the cumulative incidence of 90-day death increased in higher FGF21 quartile groups (p < 0.001, log-rank test; Figure 3).
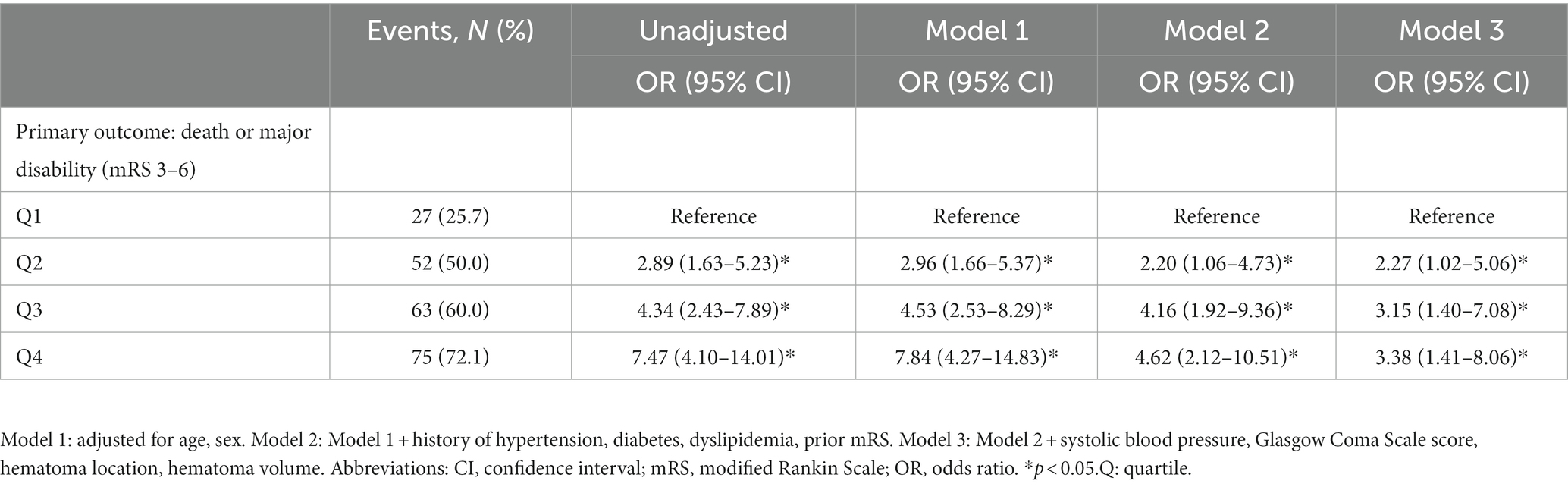
Table 2. Multivariable analyses of FGF21 to predict poor outcomes according to quartiles at baseline.
The inclusion of FGF21 enhances a model for predicting adverse results
We discovered whether adding serum FGF21 to the different recognized scales improved the risk prediction of adverse clinical outcomes with ICH (Table 3). Adding serum FGF21 to four prognostic scales (ICH, ESSCN-ICH, FUNC, ICH-FOS) considerably ameliorated their C-statistic performance (all p < 0.05). The range of NRI was from 5.91 to 13.35% in our study, which indicated that the addition of the serum FGF21 promotes the four recognized scores’ predictive performance for the primary outcome. For instance, the NRI of ESSCN-ICH plus FGF21 was 5.91% (0.39–11.44); the NRI of ICH-FOS score plus FGF21 was 9.40% (3.15–15.64); the NRI of FUNC score plus FGF21 was 13.35 (4.90–21.78). The NRI of ICH score plus FGF21 was 10.96% (4.14–17.79). The predictive ability of the new model assessed by IDI in our study can be clarified similarly to the explanation of NRI. For predictive ability of death, the performance of ICH score was significantly improved by adding serum FGF21 (C-statistics: 0.936 vs. 0.916, p = 0.003); (NRI = 6.89%, p = 0.033); (IDI =2.91%, p = 0.016). Adding serum FGF21 to FUNC score did significantly improve discriminatory power but did not improve risk reclassification for the death (NRI = −0.26%, p = 0.947; IDI =3.88%, p = 0.003) (Supplementary Table S2). However, no significant improvements were observed after the addition of serum FGF21 to the ESSCN-ICH, ICH-FOS scales (NRI and IDI, all p > 0.05) (Supplementary Table S2).
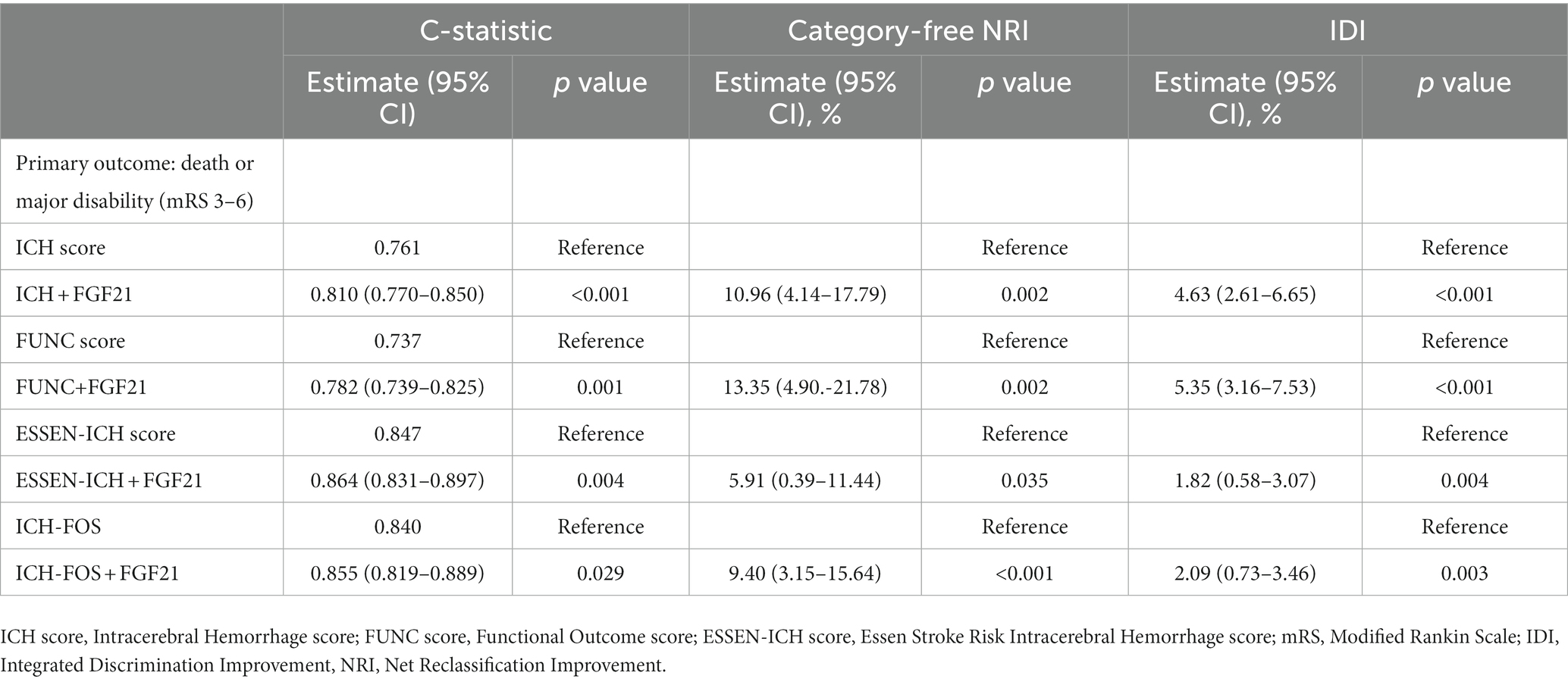
Table 3. Reclassification and discrimination statistics for 90-day outcomes by FGF21 among patients with ICH.
Discussion
This is the first research to shed light on the association between the level of FGF21 and ICH prognosis. In this prospective multicenter study, we established a significant association between elevated serum FGF21 levels and the composite outcome of death or major disability in ICH patients, and the association remained an independent risk factor even after adjusting for potential ICH risk factors.
FGF21 controls energy metabolism and functioned as a stress hormone in humans (Salminen et al., 2017). Several population-cohort studies focused on the role of FGF21 in diverse diseases’ prognosis. In a national multicenter cohort study, circulating FGF21 levels are inversely connected with muscular strength but are not independently correlated with muscle mass (Roh et al., 2021). Independently, the FGF21/adiponectin ratio predicted the emergence of pre-diabetes and diabetes in a prospective Shanghai Nicheng cohort study (Liu et al., 2021). Higher blood FGF21 levels were related to worse survival in hepatocellular carcinoma patients, according to a cohort study from Guangdong province (Liu et al., 2022). Additionally, Ong et al. (2019) and Wu et al. (2022) revealed that the amount of serum FGF21 was linked with the prognosis of several cardiovascular illnesses. Moreover, only one cohort study evaluated the prognostic value of serum FGF21 among patients with acute ischemic stroke (Zheng et al., 2021). Nevertheless, serum FGF21 and its association with unfavorable outcomes have yet to be assessed in a prospective multicenter cohort with ICH. In our population of Chinese patients with ICH, we discovered that higher levels of FGF21 were strongly related to worse long-term endpoints, even after controlling for established risk variables.
We added FGF21 to the prognostic models for predicting clinical outcomes in patients with ICH. Verified by C-statistic, IDI, and NRI across various prognostic ICH scales, the predictive performance of FGF21 for adverse outcomes at 90-day follow-up was enhanced. The development of scoring systems for ICH patients has gained momentum in the past and is still ongoing, such as the ICH score, Essen-ICH score, FUNC score, and ICH-FOS score from China. Until now, the ICH score (Wang et al., 2013), Essen-ICH score, and FUNC score have been validated in the Chinese population (Suo et al., 2018). However, they are limited by ignoring metabolic and physiologic blood parameters on patient’s initial arrival. The ICH-FOS demonstrated superior discrimination over existing ICH models for 3-month mortality and negative functional outcomes (Ji et al., 2022). Similar performance for predicting unfavorable outcomes and death was observed in our study. Meanwhile, with the addition of FGF21, the prognosis value of four scales was promoted for unfavorable functional outcome or death. Further studies are warranted to validate these findings and to explore the biological possible mechanism of FGF21 in ICH.
The direct targets and mechanisms linking FGF21 to unfavorable outcomes in ICH patients are still elusive. Traumatic mechanical compression from a hematoma, thrombin production, inflammation, excitotoxicity, and oxidative stress are only some of the secondary injuries that contribute to the overall pathological damage seen during ICH (Keep et al., 2012). Due to its extremely low heparin-binding affinity, FGF21 can pass the blood–brain barrier and control energy balance, glucose, and lipid homeostasis (Hsuchou et al., 2007). FGF21 protects tissues from increased oxidative stress damage, also increases its circulating concentration (Gómez-Sámano et al., 2017). Leng et al. (2015) focused on the neuroprotective role of FGF21, indicated that FGF21 can protect primary neurons from glutamate-induced excitotoxicity. Moreover, FGF21 reduces neuroinflammation and oxidative stress through regulating two pathways (Kang et al., 2020). In addition, FGF21 is related to hypertension, an important risk factor for ICH (Semba et al., 2013). FGF21 plays an important role in preventing angiotensin II-induced hypertension and vascular dysfunction (Pan et al., 2018). Above all, a higher circulating FGF21 level may occur after ICH because of a greater demand for its protective effects throughout the damage process. Further investigations are needed to gain a better insight into the physiological mechanism between FGF21 and ICH.
There are several shortcomings to this study that warrant discussion. First, our study only includes admission and in-hospitalization characteristics, which typically predict short-term outcomes. Second, this study only included Chinese people and assessed 90-day outcomes, longer follow-up periods, such as one year or more, require additional research. Third, no big enough collective of participants enrolled in this study, selection bias should be pay attention to. Yet, we adopted a pursuit approach to enroll almost all ICH patients with mild to severe symptoms. Considering the observational nature of this study, the potential occurrence of residual confounding represents a fourth constraint.
Conclusion
In conclusion, the present study provided evidence that elevated serum FGF21 was associated with unfavorable prognosis in ICH patients. Serum FGF21 may have potential prognostic value in risk stratification of ICH patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by institutional review boards of the Second Affiliated Hospital of Wenzhou Medical University.
Author contributions
KC was involved in the design of the study, data collection, and manuscript writing. XL took part in the design of the study, was a recipient of the obtained funding. HX, LR, YL, JW, and ZW were involved in data collection. XW and WH took part in the statistical analysis. LL and XL were a recipient of the obtained funding and was involved in the interpretation of the data and the manuscript revision.
Funding
This work was supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2023KY905; WKJ-ZJ-2130;2023KY307). The National Natural Science Foundation of China (No. 81971180).
Acknowledgments
The authors thank all staff members and participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1117057/full#supplementary-material
References
Cai, Y., Zhuang, Y. K., Wu, X. Y., Dong, X. Q., Du, Q., Yu, W. H., et al. (2021). Serum hypoxia-inducible factor 1alpha levels correlate with outcomes after intracerebral hemorrhage. Ther. Clin. Risk Manag. 17, 717–726. doi: 10.2147/TCRM.S313433
Chen, K., Rao, Z., Dong, S., Chen, Y., Wang, X., Luo, Y., et al. (2022). Roles of the fibroblast growth factor signal transduction system in tissue injury repair. Burns Trauma 10:tkac005. doi: 10.1093/burnst/tkac005
Chou, R. H., Huang, P. H., Hsu, C. Y., Chang, C. C., Leu, H. B., Huang, C. C., et al. (2016). Circulating fibroblast growth factor 21 is associated with diastolic dysfunction. Sci. Rep. 6:33953. doi: 10.1038/srep33953
Dowlatshahi, D., Demchuk, A. M., Flaherty, M. L., Ali, M., Lyden, P., and Smith, E. E. (2011). Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 76, 1238–1244. doi: 10.1212/WNL.0b013e3182143317
Geng, L., Lam, K. S. L., and Xu, A. (2020). The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat. Rev. Endocrinol. 16, 654–667. doi: 10.1038/s41574-020-0386-0
Gómez-Sámano, M. Á., Grajales-Gómez, M., Zuarth-Vázquez, J. M., Navarro-Flores, M. F., Martínez-Saavedra, M., Juárez-León, Ó. A., et al. (2017). Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 11, 335–341. doi: 10.1016/j.redox.2016.12.024
Gregório, T., Pipa, S., Cavaleiro, P., Atanásio, G., Albuquerque, I., Chaves, P. C., et al. (2018). Prognostic models for intracerebral hemorrhage: systematic review and meta-analysis. BMC Med. Res. Methodol. 18, 018–0613. doi: 10.1186/s12874-018-0613-8
Hemphill, J. C., Bonovich, D. C., Besmertis, L., Manley, G. T., and Johnston, S. C. (2001). The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32, 891–897. doi: 10.1161/01.str.32.4.891
Hsuchou, H., Pan, W., and Kastin, A. J. (2007). The fasting polypeptide FGF21 can enter brain from blood. Peptides 28, 2382–2386. doi: 10.1016/j.peptides.2007.10.007
Ji, R., Shen, H., Pan, Y., Wang, P., Liu, G., Wang, Y., et al. (2013). A novel risk score to predict 1-year functional outcome after intracerebral. Crit. Care 17:R275. doi: 10.1186/cc13130
Ji, R., Wang, W., Liu, X., Wang, L., Jiang, R., Zhang, R., et al. (2022). Head-to-head comparison of prognostic models of spontaneous intracerebral. Neurol. Res. 44, 146–155. doi: 10.1080/01616412.2021.1967678
Kang, K., Xu, P., Wang, M., Chunyu, J., Sun, X., Ren, G., et al. (2020). FGF21 attenuates neurodegeneration through modulating neuroinflammation and oxidant-stress. Biomed. Pharmacother. 129:110439. doi: 10.1016/j.biopha.2020.110439
Keep, R. F., Hua, Y., and Xi, G. (2012). Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 11, 720–731. doi: 10.1016/S1474-4422(12)70104-7
Kothari, R. U., Brott, T., Broderick, J. P., Barsan, W. G., Sauerbeck, L. R., Zuccarello, M., et al. (1996). The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27, 1304–1305. doi: 10.1161/01.str.27.8.1304
Leng, Y., Wang, Z., Tsai, L. K., Leeds, P., Fessler, E. B., Wang, J., et al. (2015). FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol. Psychiatry 20, 215–223. doi: 10.1038/mp.2013.192
Li, Q., Zhang, Y., Ding, D., Yang, Y., Chen, Q., Su, D., et al. (2016). Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J. Clin. Endocrinol. Metab. 101, 4886–4894. doi: 10.1210/jc.2016-2308
Liu, Z. Y., Luo, Y., Fang, A. P., Wusiman, M., He, T. T., Liu, X. Z., et al. (2022). High serum fibroblast growth factor 21 is associated with inferior hepatocellular. Liver Int. 42, 663–673. doi: 10.1111/liv.15100
Liu, D., Wu, L., Gao, Q., Long, X., Hou, X., Qian, L., et al. (2021). FGF21/adiponectin ratio predicts deterioration in glycemia: a 4.6-year. Cardiovasc. Diabetol. 20, 021–01351. doi: 10.1186/s12933-021-01351-1
Ong, K. L., Hui, N., Januszewski, A. S., Kaakoush, N. O., Xu, A., Fayyad, R., et al. (2019). High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the Treating to New Targets [TNT] Study). Metabolism 93, 93–99. doi: 10.1016/j.metabol.2018.11.006
Pan, X., Shao, Y., Wu, F., Wang, Y., Xiong, R., Zheng, J., et al. (2018). FGF21 prevents angiotensin II-induced hypertension and vascular dysfunction by activation of ACE2/Angiotensin-(1-7) axis in mice. Cell Metab. 27, 1323–1337.e5. doi: 10.1016/j.cmet.2018.04.002
Pencina, M. J., D'agostino, R. B. Sr., D'agostino, R. B., and Vasan, R. S. (2008). Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 27, 157–172. doi: 10.1002/sim.2929
Qureshi, A. I., Mendelow, A. D., and Hanley, D. F. (2009). Intracerebral haemorrhage. Lancet 373, 1632–1644. doi: 10.1016/S0140-6736(09)60371-8
Qureshi, A. I., Tuhrim, S., Broderick, J. P., Batjer, H. H., Hondo, H., and Hanley, D. F. (2001). Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 344, 1450–1460. doi: 10.1056/NEJM200105103441907
Roh, E., Hwang, S. Y., Yoo, H. J., Baik, S. H., Cho, B., Park, Y. S., et al. (2021). Association of plasma FGF21 levels with muscle mass and muscle strength in a national multicentre cohort study: Korean Frailty and aging Cohort Study. Age Ageing 50, 1971–1978. doi: 10.1093/ageing/afab178
Rost, N. S., Smith, E. E., Chang, Y., Snider, R. W., Chanderraj, R., Schwab, K., et al. (2008). Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 39, 2304–2309. doi: 10.1161/STROKEAHA.107.512202
Sacco, S., Marini, C., Toni, D., Olivieri, L., and Carolei, A. (2009). Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 40, 394–399. doi: 10.1161/STROKEAHA.108.523209
Salminen, A., Kaarniranta, K., and Kauppinen, A. (2017). Regulation of longevity by FGF21: interaction between energy metabolism and stress responses. Ageing Res. Rev. 37, 79–93. doi: 10.1016/j.arr.2017.05.004
Schmidt, F. A., Liotta, E. M., Prabhakaran, S., Naidech, A. M., and Maas, M. B. (2018). Assessment and comparison of the max-ICH score and ICH score by external validation. Neurology 91, e939–e946. doi: 10.1212/WNL.0000000000006117
Semba, R. D., Crasto, C., Strait, J., Sun, K., Schaumberg, D. A., and Ferrucci, L. (2013). Elevated serum fibroblast growth factor 21 is associated with hypertension in community-dwelling adults. J. Hum. Hypertens. 27, 397–399. doi: 10.1038/jhh.2012.52
Suo, Y., Chen, W. Q., Pan, Y. S., Peng, Y. J., Yan, H. Y., Zhao, X. Q., et al. (2018). The max-intracerebral hemorrhage score predicts long-term outcome of intracerebral hemorrhage. CNS Neurosci. Ther. 24, 1149–1155. doi: 10.1111/cns.12846
Tabari, F. S., Karimian, A., Parsian, H., Rameshknia, V., Mahmoodpour, A., Majidinia, M., et al. (2019). The roles of FGF21 in atherosclerosis pathogenesis. Rev. Endocr. Metab. Disord. 20, 103–114. doi: 10.1007/s11154-019-09488-x
Wang, W., Lu, J., Wang, C., Wang, Y., Li, H., and Zhao, X. (2013). Prognostic value of ICH score and ICH-GS score in Chinese intracerebral hemorrhage patients: analysis from the China National Stroke Registry (CNSR). PLoS One 8:0077421. doi: 10.1371/journal.pone.0077421
Wu, G., Wu, S., Yan, J., Gao, S., Zhu, J., Yue, M., et al. (2022). Fibroblast growth factor 21 predicts short-term prognosis in patients with acute heart failure: a prospective cohort study. Front. Cardiovasc. Med. 9:834967. doi: 10.3389/fcvm.2022.834967
Keywords: fibroblast growth factor-21, prognosis, predictive value, intracerebral hemorrhage, enhancement
Citation: Chen K, Huang W, Wang J, Xu H, Ruan L, Li Y-a, Wang Z, Wang X, Lin L and Li X (2023) Increased serum fibroblast growth factor 21 levels are associated with adverse clinical outcomes after intracerebral hemorrhage. Front. Neurosci. 17:1117057. doi: 10.3389/fnins.2023.1117057
Edited by:
Minghan Liu, Third Military Medical University, ChinaReviewed by:
Huihui Liu, Second Affiliated Hospital of Soochow University, ChinaPing Zhang, Jiangxi Provincial People's Hospital, China
Lin Lu, First Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2023 Chen, Huang, Wang, Xu, Ruan, Li, Wang, Wang, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Lin, bGlubGl3ekAxNjMuY29t; Xiaokun Li, WGlhb2t1bmxpQHdtdS5lZHUuY24=
†These authors have contributed equally to this work
 Keyang Chen
Keyang Chen Wenting Huang
Wenting Huang Jing Wang
Jing Wang Huiqin Xu
Huiqin Xu Lixin Ruan5
Lixin Ruan5 Li Lin
Li Lin