
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 21 February 2023
Sec. Neurodegeneration
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1117056
This article is part of the Research Topic Advances in understanding the pathogenetic mechanisms of neurodevelopmental disorders and neurodegenerative disease - The environment as a putative risk factor View all 12 articles
Objective: To determine the correlations between dietary and blood inflammation indices in elderly Americans and their effects on cognitive function.
Methods: This research extracted data from the 2011–2014 National Health and Nutrition Examination Survey for 2,479 patients who were ≥60 years old. Cognitive function was assessed as a composite cognitive function score (Z-score) calculated from the results of the Consortium to Establish a Registry for Alzheimer’s Disease Word Learning and Delayed Recall tests, the Animal Fluency test, and the Digit Symbol Substitution Test. We used a dietary inflammatory index (DII) calculated from 28 food components to represent the dietary inflammation profile. Blood inflammation indicators included the white blood cell count (WBC), neutrophil count (NE), lymphocyte count (Lym), neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), neutrophil–albumin ratio (NAR), systemic immune-inflammation index [SII, calculated as (peripheral platelet count) × NE/Lym], and systemic inflammatory response index [SIRI, calculated as (monocyte count) × NE/Lym]. WBC, NE, Lym, NLR, PLR, NAR, SII, SIRI, and DII were initially treated as continuous variables. For logistic regression, WBC, NE, Lym, NLR, PLR, NAR, SII, and SIRI were divided into quartile groups, and DII was divided into tertile groups.
Results: After adjusting for covariates, WBC, NE, NLR, NAR, SII, SIRI, and DII scores were markedly higher in the cognitively impaired group than in the normal group (p < 0.05). DII was negatively correlated with the Z-score when combined with WBC, NE, and NAR (p < 0.05). After adjusting for all covariates, DII was positively correlated with SII in people with cognitive impairment (p < 0.05). Higher DII with NLR, NAR, SII, and SIRI all increased the risk of cognitive impairment (p < 0.05).
Conclusion: DII was positively correlated with blood inflammation indicators, and higher DII and blood inflammation indicators increased the risk of developing cognitive impairment.
With the advent of an aging society, the cognitive decline of the elderly has become a social problem that is important to all humankind and needs to be solved (Hebert et al., 2013; Wang et al., 2021). The number of people living with dementia is increasing worldwide (Afzal et al., 2014). The increasing incidence rates of cognitive impairment and dementia will lead to an increased incidence of various geriatric diseases, which will greatly increase medical investment and impose a heavy socioeconomic burden (Mangialasche et al., 2009; Ruangritchankul et al., 2020). There is now evidence that cognitive impairment is related to human inflammation (Ferrucci and Fabbri, 2018; Irwin and Vitiello, 2019; Barter et al., 2021; Minhas et al., 2021). An adjustable, controlled intake of anti- or proinflammatory foods may be able to modulate the inflammatory state of the body, thereby positively impacting human cognitive function (Aquilani et al., 2020, 2022; van’t Klooster et al., 2020). The foods that people consume in daily life have complex constituents, which make it necessary to explore the impacts of certain combinations of dietary conditions on inflammation and cognitive impairment in humans (Watson et al., 2022). The dietary inflammatory index (DII) is calculated by combining various food components and is a recognized indicator of overall dietary inflammation (Shivappa et al., 2017, 2019; Ryu et al., 2019). There is evidence that DII is negatively correlated with cognitive function (Hayden et al., 2017; Frith et al., 2018; Shin et al., 2018), and studies have also found that DII has no significant effect on cognitive function (Zabetian-Targhi et al., 2021). There is a need to systematically explore the relationship between DII and cognitive function.
The blood inflammation indicators analyzed in the study were collected during physical examinations and recorded in the National Health and Nutrition Examination Survey (NHANES) database. Platelets, platelet–lymphocyte ratio (PLR), and neutrophil–lymphocyte ratio (NLR) have been found to be positively associated with the risks of cerebrovascular and cardiovascular disease (Trakarnwijitr et al., 2017; He et al., 2019). The systemic inflammatory response index [SIRI, calculated as neutrophil count (NE) × (monocyte count)/(lymphocyte count) (Lym)] and the systemic immune-inflammation index [SII, calculated as (peripheral platelet count) × NE/Lym] may be associated with age-related diseases such as those of the cerebrovascular and cardiovascular systems (Jin et al., 2021; Xu et al., 2021). However, there is little comprehensive evidence of the relationships between DII and the white blood cell count (WBC), NE, Lym, NLR, PLR, neutrophil–albumin ratio (NAR), SII, and SIRI, or of their synergistic effects on cognitive function.
We therefore used NHANES data to investigate the relationships of DII and a blood inflammation index with cognition in older Americans, and to explore possible ways to reduce the occurrence of cognitive impairment.
The data used in our study were derived from the NHANES public database in the United States. All participants provided written informed consent (Wu et al., 2021). There is a dedicated system management system that is responsible for data collection and updates in the NHANES, and the survey data and project information are updated regularly on the website and can be accessed by the public for free (Wu et al., 2021).
Data on the DII, blood inflammation index, and cognitive performance test scores were obtained from the NHANES for the period from 2011 to 2014 (Yang et al., 2020). All participants or their guardians signed an informed-consent form. We only included people aged 60 years or older, and after further exclusion screening, 2,479 cases were finally included (Figure 1).
This study analyzed 28 of the 45 food components from the original DII: carbohydrates, protein, total fat, alcohol, fiber, cholesterol, saturated fat, MUFA, PUFA, n-3 fatty acids, n-6 fatty acids, niacin, vitamin A, thiamin (vitamin B1), riboflavin (vitamin B2), vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, Fe, Mg, zinc, selenium, folic acid, beta-carotene, caffeine, and energy. There is evidence that DII is still useful for predicting overall inflammation when only information on fewer food components is available (Shivappa et al., 2014a). DII calculations were based on a 24-h dietary recall interview or food record of the participant or their guardian (Shivappa et al., 2014b; Wirth et al., 2017). There are standard reference values for each food parameter in the world database. The 24-h dietary recall data were multiplied by standard food parameters from the world database to obtain individual dietary inflammation composite cognitive function scores (Z-scores) relative to the standard global average. We transformed this value into a percentile to reduce bias. Each percentile was doubled, and then 1 was subtracted from it. The percentage values for each food parameter were then multiplied by their respective “overall food parameter-specific inflammatory effect scores” to obtain individual food-specific DII scores. Finally, the DII scores for all individual food components were summed to obtain the “overall DII score” for each person (Shivappa et al., 2014a).
The cognitive function assessment consisted of the following four tests: Consortium to Establish a Registry for Alzheimer’s Disease Word Learning (CERAD-WL) test, Animal Fluency (AF) test, Digit Symbol Substitution Test (DSST), and CERAD Delayed Recall (CERAD-DR) test. The CERAD-WL test requires participants to recall as many words as possible after reading ten unrelated words aloud in different orders for a total of 30 points. The CERAD-DR test was administered after the AF test and DSST. Participants were asked to recall words on the CERAD-WL test, which was used to assess transient and delayed learning ability (Rosen, 1983).
The Animal Fluency (AF) tests. Participants were asked to name as many animals as possible within 1 min. The absolute verbal fluency and executive function of the participants were examined (Clark et al., 2009; Sutin et al., 2022).
In the DSST, we asked participants to copy the corresponding symbols into the boxes next to the numbers within 2 min for a total of 133 points (Brody et al., 2019). This test examines the executive function and working memory capacity of the participants.
To exclude uneven differences in individual cognitive scores, we used a Z-score consisting of the CERAD-WL test, CERAD-DR test, AF test, and DSST as the total globally standardized cognitive function score. The Z-score was calculated as Z = (x-m)/σ, where x is the raw score, m is the overall mean, and σ is the overall SD. A Z-score of<-1 is taken to indicate that the person has cognitive impairment (Wirth et al., 2017; Frith et al., 2018; Zhang et al., 2022).
Data on WBC, NE, Lym, NLR, PLR, NAR, SIRI, SII were extracted from the NHANES database or calculated using extracted peripheral blood counts (Wu et al., 2021).
The possible effects of the following confounders were assessed: age (continuous), sex (male and female), race (Mexican American, other Hispanic, non-Hispanic white persons, non-Hispanic black persons, and non-Hispanic American), marital status (married/living with a partner, widowed/divorced/separated, and unmarried), education level (less than 9th grade, 9–11th grade, high school graduate/GED or equivalent, some college or a degree, and college graduate or above), BMI (continuous), hypertension (yes and no), and diabetes (yes, borderline, and no).
We calculated new sample weights for the data analysis (Liu et al., 2013). If continuous variables did not conform to the normal distribution, they were represented by median (interquartile range) values; otherwise mean (SE) values were used. Regarding intergroup comparisons of baseline data, weighted-sample independent t-tests were used for continuous variables, while chi-square tests were used for categorical variables. WBC, NE, Lym, NLR, PLR, NAR, SII, SIRI, and DII were initially considered as continuous variables. In the logistic regression, WBC, NE, Lym, NLR, PLR, NAR, SII, and SIRI were divided into quartile groups (Q1, Q2, Q3, and Q4), and DII was divided into tertile groups (T1, T2, and T3) (Brody et al., 2019). The logistic regression model was adjusted for sex, age, race, marital status, education level, BMI, hypertension, and diabetes. Significant results were indicated by p < 0.05. All analyses were performed using R software.
The study finally included 2,479 individuals aged ≥60 years. The Z-scores indicated that 426 participants had low cognitive function and 2,053 had normal cognitive function. According to Z-scores, the low-cognitive-function group was older and had higher rates of non-Hispanic black persons, divorced/separated/widowed, lower education levels, hypertension, and diabetes than the normal group (Table 1).
No differences in LE and PLR were found between the cognitively impaired and normal groups. Patients in the cognitively impaired group had higher DII (p < 0.0001), WBC (p = 0.004), NE (p = 0.003), NLR (p = 0.008), NAR (p < 0.001), SII (p = 0.01), and SIRI (p = 0.009) than the normal group (Table 2).
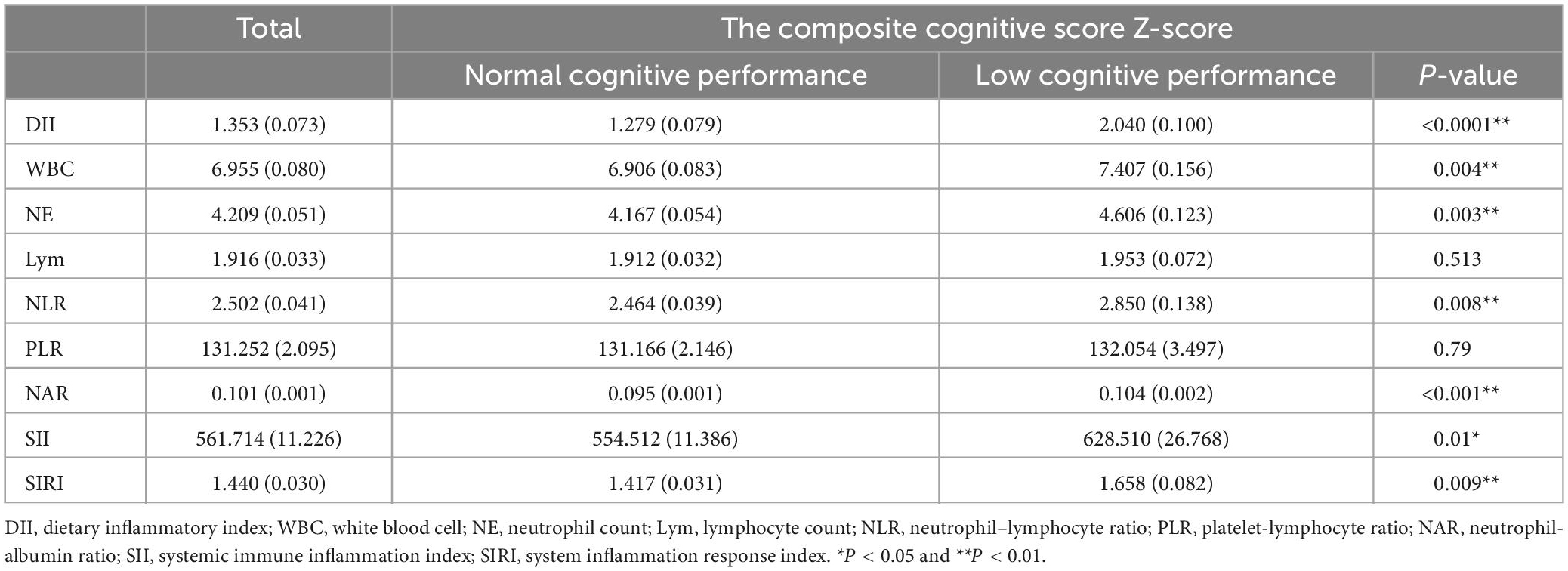
Table 2. Comparison of dietary inflammatory index and blood inflammatory indicators between the cognitive impairment group and the normal group.
Multiple linear regression analysis was performed to analyze the Correlations of DII, WBC, NE, Lym, NLR, PLR, NAR, SII, and SIRI with the Z-score. DII combined with Lym, NLR, PLR, SII, and SIRI, were no correlation with Z-scores after adjusting for all of the abovementioned covariates (p > 0.05). However, DII (β = −0.091, p < 0.0001) combined with WBC (β = −0.028, p = 0.012), NE (β = −0.036, p = 0.003), and NAR (β = −1.776, p < 0.001) were negatively correlated with Z-scores (Table 3).

Table 3. Relationship between DII and cognitive function after binding of each blood inflammatory index separately.
The relationship between DII and blood inflammation indices (WBC, NE, Lym, NLR, PLR, NAR, SII, and SIRI) in the cognitively impaired group was further investigated using multiple linear regression. After adjusting for covariates, DII was found to be positively correlated with SII (β = 27.476, p = 0.047). No significant associations were found between DII and the other inflammation indicators (p > 0.05) (Table 4).
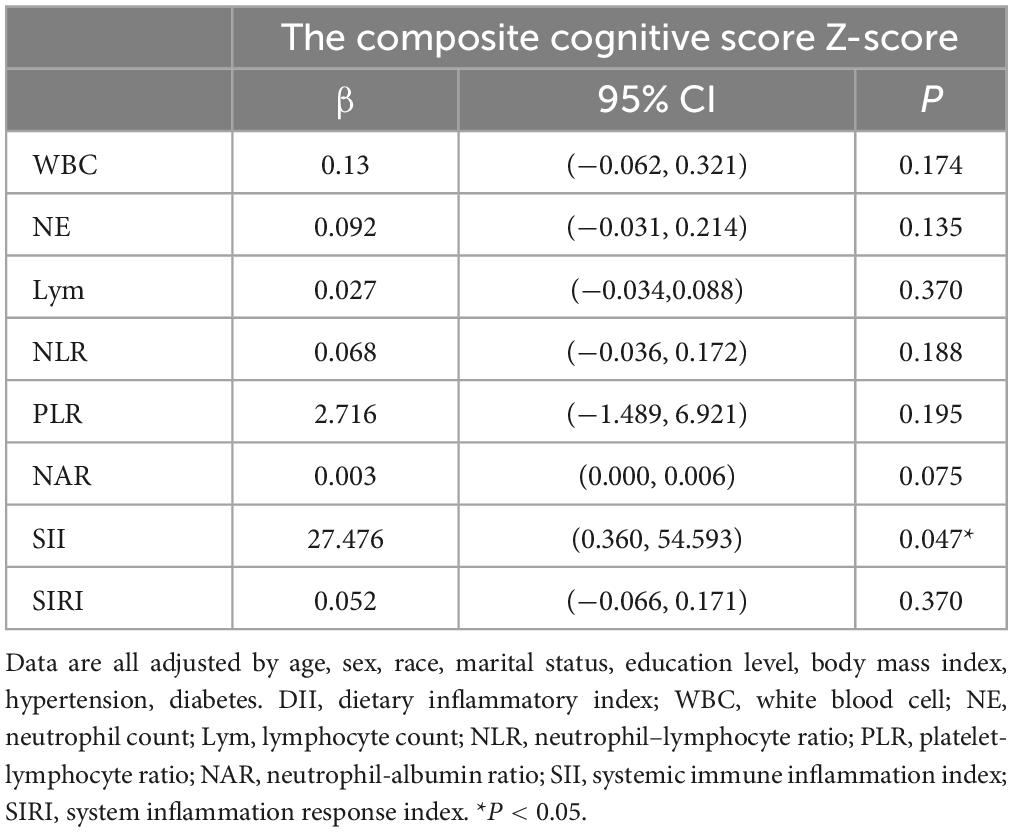
Table 4. Relationship between DII and each blood inflammatory index in a cognitively impaired population.
A logistic regression approach was used to explore the association between inflammation scores and cognitive impairment risk. Q1 was the reference for all comparisons. First, after adjusting for all the confounding factors that we accounted for, the relationship between inflammatory indicators (DII, WBC, NE, Lym, NLR, PLR, NAR, SII, and SIRI) and the risk of cognitive impairment risk was examined independently (Table 5). The results indicated that T2 and T3 of DII (T2: OR = 1.879, 95% CI = 1.242–2.842; T3: OR = 2.661, 95% CI = 1.745–4.058), Q4 of WBC (OR = 1.778, 95% CI = 1.022–3.096), Q4 of NLR (OR = 1.671, 95% CI = 1.162–2.403), Q4 of NAR (OR = 1.656, 95% CI = 1.094–2.509), Q4 of SII (OR = 1.717, 95% CI = 1.092–2.700), and Q4 of SIRI (OR = 1.563, 95% CI = 1.082–2.258) were risk factors for cognitive impairment. DII combined with WBC, NE, Lym, NLR, PLR, NAR, SII, and SIRI were tested in models 1–8, respectively. Although the combinations of DII and WBC, NE, Lym, and PLR were not found to be significantly associated with the risk of cognitive impairment, higher DII with NLR, NAR, SII, and SIRI significantly increased the risk of cognitive impairment (p < 0.05) (Table 6).
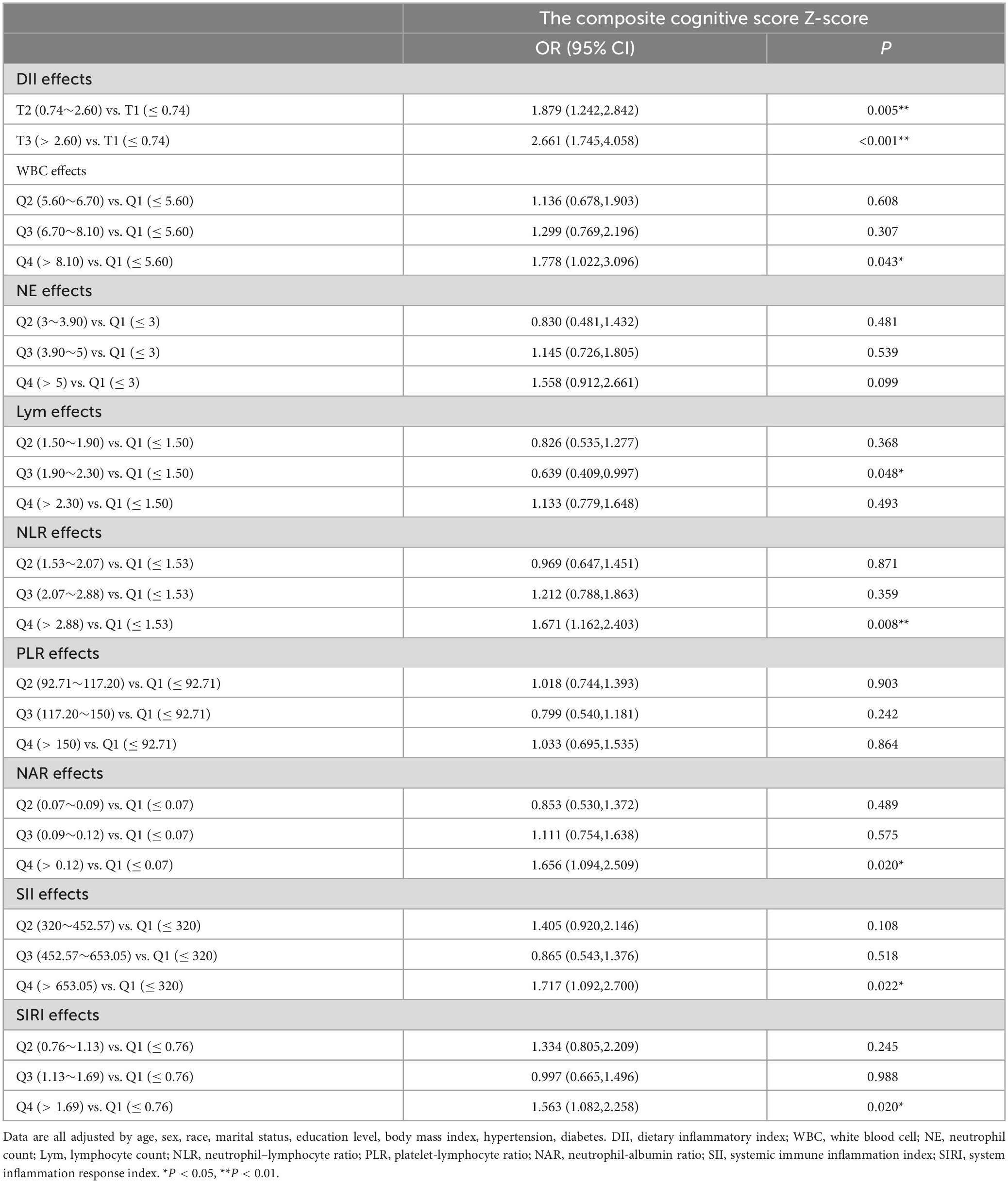
Table 5. The effect of dietary inflammatory index and blood inflammation indicators on cognitive function was analyzed by logistic regression.
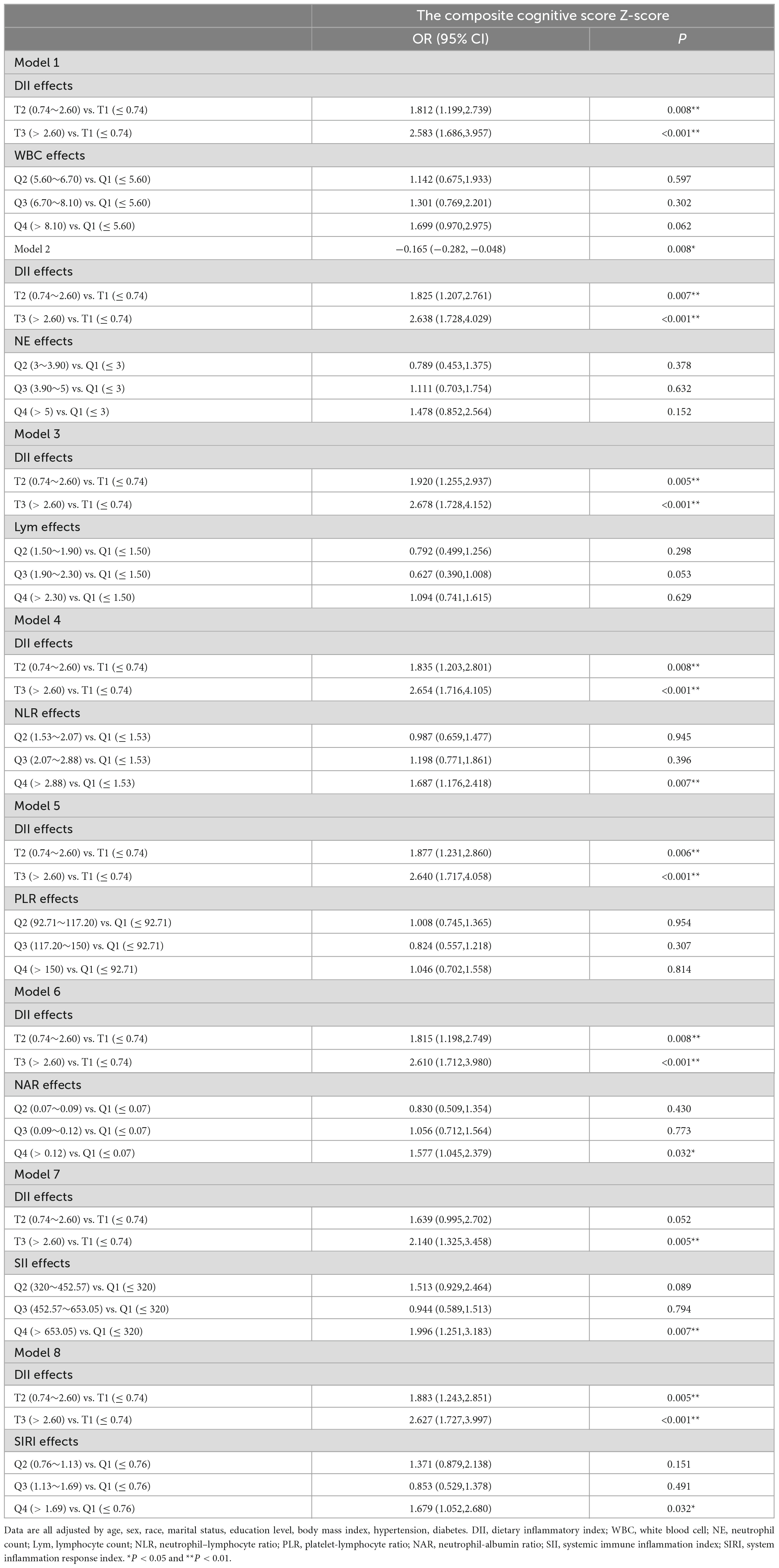
Table 6. Synergistic effects of dietary inflammatory index and blood inflammation indicators on cognitive function.
We systematically explored the relationships between dietary inflammation, blood inflammation indicators, and cognitive impairment. Our study found that DII combined with WBC, NE, NLR, NAR, SII, and SIRI were considerably higher in the low-cognitive-ability group than in the normal group. DII, WBC, NE, NLR, NAR, SII, and SIRI were negatively correlated with Z-scores. DII combined with WBC, NE, and NAR were all negatively correlated with Z-scores. DII was positively correlated with blood inflammation indicators. Older adults with higher levels of DII and blood inflammation indicators (NLR, NAR, DII, and SIRI) were at a higher risk of cognitive impairment.
In the elderly, the body becomes less functional and more susceptible to inflammation (Tangestani Fard and Stough, 2019). Human inflammation indicators (NLR, PLR, NAR, SII, and SIRI) have been indicated to be potentially related to various health hazards in the elderly, including cardiovascular and cerebrovascular diseases (Trakarnwijitr et al., 2017; He et al., 2019; Dong et al., 2020; Jin et al., 2021; Li et al., 2021; Xu et al., 2021). Platelets, PLR, NLR, and NAR are associated with the risks of stroke and cardiovascular disease (Trakarnwijitr et al., 2017; He et al., 2019). Our results found that DII combined with WBC, NE, NLR, NAR, SII, and SIRI were considerably higher in the cognitive-impairment group than in the normal group. DII, WBC, NE, Lym and NAR were negatively correlated with Z-scores, which was similar to the results of previous studies (Xu et al., 2021).
DII represents the combined inflammatory profile of the human diet, and the relationship between DII and many risk factors has been demonstrated for age-related diseases (Frith et al., 2018). Our study found that DII was significantly associated with SII. Although no significant correlation was found with other blood inflammation indicators, SII is a more-reliable and representative marker of inflammation, so we believe that the evidence here is sufficient and convincing. However, our conclusion differed from those of previous studies, including that of Wang et al. (2022), who found that DII was significantly correlated with SIRI but not with SII. The possible reasons for this are that the previous study involved Chinese subjects, and Chinese and American diets are very different, there are various racial differences, and the Montreal Cognitive Assessment scale differs from the Z-score calculated by our CERAD-WL, CERAD-DR, AF, and DSST. However, both studies suggested that DII contributes to chronic inflammation development in humans.
The correlation between DII and cognitive impairment has been previously explored in different regions. Hayden et al. (2017) found that DII scores were positively associated with the risk of developing cognitive impairment, Shin et al. (2018) found that higher DII indicated higher cognitive impairment risk, and Frith et al. (2018) also found that higher DII scores were negatively associated with cognitive impairment risk. Our study found that DII, WBC, NE, NLR, NAR, SII, and SIRI were all negatively associated with Z-scores. DII combined with WBC, NE, and NAR were negatively correlated with Z-scores. This suggests that DII and blood inflammation indices can synergistically serve to affect cognitive function.
Logistic regression was used to further investigate the synergistic effect of blood inflammation index and DII on cognitive impairment risk. The results indicated that older adults with higher DII and levels of blood inflammation indicators (NLR, NAR, DII, and SIRI) were at a higher risk of cognitive impairment, which was similar to the results of previous studies (Jin et al., 2021). The possible mechanism is that inflammation indicators can cross the blood–brain barrier to inflame nerves, leading to neurodegeneration (d’Avila et al., 2018; Godos et al., 2020; Leng and Edison, 2021). A comprehensive assessment of diet and blood inflammation can help us take early steps to develop a rational dietary intervention plan and protect cognitive function.
This study had some limitations. First, because the study had a cross-sectional design, it was not possible to infer causal relationships between dietary and blood inflammatory indicators, and cognition. Second, dietary inflammatory indicators calculated from dietary intake data obtained from 24-h dietary recall might not accurately reflect individual dietary intakes and are subjected to recall bias. Third, we did not identify the cause of any impairment, such as Alzheimer’s disease, Lewy-body dementia, or vascular dementia.
Our study also shows strengths and important originality. First of all, the study has a rich sample size and is analyzed only in older adults over 60 years of age, which has a strong social significance. In addition, the study used relatively accurate dietary data. Finally, the cognitive impairment composite -z score was created by summing the z scores [(individual test score - mean score)/SD] of these three individual tests (DSST, AFT, CERAD), with good sensitivity and avoiding ceiling and floor effects. For cognitive purposes our findings emphasize the importance of an anti-inflammatory diet with clinical implications.
In conclusion, we found that dietary and blood inflammation indicators were negatively associated with cognitive function in an elderly American population, and that dietary inflammation indicators were also negatively associated with cognitive function when combined with blood inflammation indicators. DII was positively correlated with blood inflammation. Older adults with higher DII and blood inflammation indicator levels were at a higher risk of cognitive impairment. An ideal dietary intake among older adults was associated with improved cognitive function, and future studies should therefore further investigate the interrelationships and the mechanisms underlying their effects on cognition.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
The data used in our study were derived from the NHANES public database in the United States. All participants provided written informed consent. The patients/participants provided their written informed consent to participate in this study.
WL, SL, and WZ: conceptualization. WL: methodology and data curation. YS: software. WL, SL, and GY: validation. WL, WZ, and ZC: writing – original draft preparation. WL and WZ: writing – review and editing. JL: visualization. All authors have read and agreed to the published version of the manuscript.
This study was supported by Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization (2021B1212040007), National Key R&D Program of China (2020YFC2005700), and Key Science & Technology Brainstorm Project of Guangzhou (202103000027).
We thank Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database. His outstanding work, nhanesR package and webpage, makes it easier for us to explore NHANES database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Afzal, S., Bojesen, S. E., and Nordestgaard, B. G. (2014). Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 10, 296–302. doi: 10.1016/j.jalz.2013.05.1765
Aquilani, R., Costa, A., Maestri, R., Cotta Ramusino, M., Perini, G., Boselli, M., et al. (2022). Is the brain undernourished in Alzheimer’s disease? Nutrients 14:1872. doi: 10.3390/nu14091872
Aquilani, R., Costa, A., Maestri, R., Cotta Ramusino, M., Pierobon, A., Dossena, M., et al. (2020). Mini nutritional assessment may identify a dual pattern of perturbed plasma amino acids in patients with Alzheimer’s disease: A window to metabolic and physical rehabilitation? Nutrients 12:1845. doi: 10.3390/nu12061845
Barter, J., Kumar, A., Bean, L., Ciesla, M., and Foster, T. C. (2021). Adulthood systemic inflammation accelerates the trajectory of age-related cognitive decline. Aging 13, 22092–22108. doi: 10.18632/aging.203588
Brody, D. J., Kramarow, E. A., Taylor, C. A., and Mcguire, L. C. (2019). Cognitive performance in adults aged 60 and over: National health and nutrition examination survey, 2011-2014. Natl Health Stat. Report. 126, 1–23.
Clark, L. J., Gatz, M., Zheng, L., Chen, Y. L., Mccleary, C., and Mack, W. J. (2009). Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 24, 461–468.
d’Avila, J. C., Siqueira, L. D., Mazeraud, A., Azevedo, E. P., Foguel, D., Castro-Faria-Neto, H. C., et al. (2018). Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J. Neuroinflammation 15:28. doi: 10.1186/s12974-018-1059-y
Dong, X., Nao, J., and Gao, Y. (2020). Peripheral monocyte count predicts outcomes in patients with acute ischemic stroke treated with rtPA thrombolysis. Neurotox. Res. 37, 469–477. doi: 10.1007/s12640-019-00103-0
Ferrucci, L., and Fabbri, E. (2018). Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522. doi: 10.1038/s41569-018-0064-2
Frith, E., Shivappa, N., Mann, J. R., Hébert, J. R., Wirth, M. D., and Loprinzi, P. D. (2018). Dietary inflammatory index and memory function: Population-based national sample of elderly Americans. Br. J. Nutr. 119, 552–558. doi: 10.1017/S0007114517003804
Godos, J., Currenti, W., Angelino, D., Mena, P., Castellano, S., Caraci, F., et al. (2020). Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 9:346. doi: 10.3390/antiox9040346
Hayden, K. M., Beavers, D. P., Steck, S. E., Hebert, J. R., Tabung, F. K., Shivappa, N., et al. (2017). The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women’s health initiative memory study. Alzheimers Dement. 13, 1187–1196. doi: 10.1016/j.jalz.2017.04.004
He, S., Lei, W., Li, J., Yu, K., Yu, Y., Zhou, L., et al. (2019). Relation of platelet parameters with incident cardiovascular disease (The Dongfeng-Tongji Cohort Study). Am. J. Cardiol. 123, 239–248. doi: 10.1016/j.amjcard.2018.10.016
Hebert, L. E., Weuve, J., Scherr, P. A., and Evans, D. A. (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80, 1778–1783. doi: 10.1212/WNL.0b013e31828726f5
Irwin, M. R., and Vitiello, M. V. (2019). Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 18, 296–306.
Jin, Z., Wu, Q., Chen, S., Gao, J., Li, X., Zhang, X., et al. (2021). The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: A ten-year follow-up study in 85,154 individuals. J. Inflamm. Res. 14, 131–140. doi: 10.2147/JIR.S283835
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 17, 157–172. doi: 10.1038/s41582-020-00435-y
Li, J., Yuan, Y., Liao, X., Yu, Z., Li, H., and Zheng, J. (2021). Prognostic significance of admission systemic inflammation response index in patients with spontaneous intracerebral hemorrhage: A propensity score matching analysis. Front. Neurol. 12:718032. doi: 10.3389/fneur.2021.718032
Liu, Y., Li, Z., Xiong, H., Gao, X., Wu, J., and Wu, S. (2013). Understanding and enhancement of internal clustering validation measures. IEEE Trans. Cybern. 43, 982–994. doi: 10.1109/TSMCB.2012.2220543
Mangialasche, F., Polidori, M. C., Monastero, R., Ercolani, S., Camarda, C., Cecchetti, R., et al. (2009). Biomarkers of oxidative and nitrosative damage in Alzheimer’s disease and mild cognitive impairment. Ageing Res. Rev. 8, 285–305. doi: 10.1016/j.arr.2009.04.002
Minhas, P. S., Latif-Hernandez, A., Mcreynolds, M. R., Durairaj, A. S., Wang, Q., Rubin, A., et al. (2021). Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 590, 122–128. doi: 10.1038/s41586-020-03160-0
Rosen, W. G. (1983). Clinical and neuropsychological assessment of Alzheimer disease. Adv. Neurol. 38, 51–64.
Ruangritchankul, S., Peel, N. M., Shafiee Hanjani, L., and Gray, L. C. (2020). The changes in medication prescribing among the older people with cognitive impairment in the acute care setting. Clin. Interv. Aging 15, 865–876. doi: 10.2147/CIA.S252432
Ryu, S., Shivappa, N., Veronese, N., Kang, M., Mann, J. R., Hébert, J. R., et al. (2019). Secular trends in dietary inflammatory index among adults in the United States, 1999-2014. Eur. J. Clin. Nutr. 73, 1343–1351. doi: 10.1038/s41430-018-0378-5
Shin, D., Kwon, S. C., Kim, M. H., Lee, K. W., Choi, S. Y., Shivappa, N., et al. (2018). Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition 55–56, 56–62. doi: 10.1016/j.nut.2018.02.026
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R., and Hébert, J. R. (2014a). Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17, 1689–1696. doi: 10.1017/S1368980013002115
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R., Ma, Y., Ockene, I. S., et al. (2014b). A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. 17, 1825–1833. doi: 10.1017/S1368980013002565
Shivappa, N., Wirth, M. D., Hurley, T. G., and Hébert, J. R. (2017). Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the national health and nutrition examination survey-1999-2002. Mol. Nutr. Food Res. 61:1600630. doi: 10.1002/mnfr.201600630
Shivappa, N., Wirth, M. D., Murphy, E. A., Hurley, T. G., and Hébert, J. R. (2019). Association between the dietary inflammatory index (DII) and urinary enterolignans and C-reactive protein from the national health and nutrition examination survey-2003-2008. Eur. J. Nutr. 58, 797–805. doi: 10.1007/s00394-018-1690-5
Sutin, A. R., Luchetti, M., Stephan, Y., Strickhouser, J. E., and Terracciano, A. (2022). The association between purpose/meaning in life and verbal fluency and episodic memory: A meta-analysis of >140,000 participants from up to 32 countries. Int. Psychogeriatr. 34, 263–273. doi: 10.1017/S1041610220004214
Tangestani Fard, M., and Stough, C. (2019). A review and hypothesized model of the mechanisms that underpin the relationship between inflammation and cognition in the elderly. Front. Aging Neurosci. 11:56. doi: 10.3389/fnagi.2019.00056
Trakarnwijitr, I., Li, B., Adams, H., Layland, J., Garlick, J., and Wilson, A. (2017). Age modulates the relationship between platelet-to-lymphocyte ratio and coronary artery disease. Int. J. Cardiol. 248, 349–354. doi: 10.1016/j.ijcard.2017.06.127
van’t Klooster, C., van Der Graaf, Y., Ridker, P. M., Westerink, J., Hjortnaes, J., Sluijs, I., et al. (2020). The relation between healthy lifestyle changes and decrease in systemic inflammation in patients with stable cardiovascular disease. Atherosclerosis 301, 37–43. doi: 10.1016/j.atherosclerosis.2020.03.022
Wang, F., Fei, M., Hu, W. Z., Wang, X. D., Liu, S., Zeng, Y., et al. (2021). Prevalence of constipation in elderly and its association with dementia and mild cognitive impairment: A cross-sectional study. Front. Neurosci. 15:821654. doi: 10.3389/fnins.2021.821654
Wang, X., Li, T., Li, H., Li, D., Wang, X., Zhao, A., et al. (2022). Association of dietary inflammatory potential with blood inflammation: The prospective markers on mild cognitive impairment. Nutrients 14:2417. doi: 10.3390/nu14122417
Watson, S., Woodside, J. V., Winning, L., Wright, D. M., Srinivasan, M., and Mckenna, G. (2022). Associations between self-reported periodontal disease and nutrient intakes and nutrient-based dietary patterns in the UK Biobank. J. Clin. Periodontol. 49, 428–438. doi: 10.1111/jcpe.13604
Wirth, M. D., Shivappa, N., Davis, L., Hurley, T. G., Ortaglia, A., Drayton, R., et al. (2017). Construct validation of the dietary inflammatory index among African Americans. J. Nutr. Health Aging 21, 487–491. doi: 10.1007/s12603-016-0775-1
Wu, W. T., Li, Y. J., Feng, A. Z., Li, L., Huang, T., Xu, A. D., et al. (2021). Data mining in clinical big data: The frequently used databases, steps, and methodological models. Mil. Med. Res. 8:44. doi: 10.1186/s40779-021-00338-z
Xu, M., Chen, R., Liu, L., Liu, X., Hou, J., Liao, J., et al. (2021). Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: The Dongfeng-Tongji cohort study. Atherosclerosis 323, 20–29. doi: 10.1016/j.atherosclerosis.2021.02.012
Yang, J., Li, Y., Liu, Q., Li, L., Feng, A., Wang, T., et al. (2020). Brief introduction of medical database and data mining technology in big data era. J. Evid. Based Med. 13, 57–69. doi: 10.1111/jebm.12373
Zabetian-Targhi, F., Srikanth, V. K., Smith, K. J., Oddy, W. H., Beare, R., Moran, C., et al. (2021). Associations between the dietary inflammatory index, brain volume, small vessel disease, and global cognitive function. J. Acad. Nutr. Diet. 121, 915–924.e3. doi: 10.1016/j.jand.2020.11.004
Keywords: cognitive function, DII, blood inflammation indicators, NHANES, regression analysis
Citation: Li W, Li S, Shang Y, Zhuang W, Yan G, Chen Z and Lyu J (2023) Associations between dietary and blood inflammatory indices and their effects on cognitive function in elderly Americans. Front. Neurosci. 17:1117056. doi: 10.3389/fnins.2023.1117056
Received: 06 December 2022; Accepted: 07 February 2023;
Published: 21 February 2023.
Edited by:
Pamela J. Lein, University of California, Davis, United StatesReviewed by:
Sonal Agrawal, Rush University, United StatesCopyright © 2023 Li, Li, Shang, Zhuang, Yan, Chen and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Lyu,  bHl1anVuMjAyMEBqbnUuZWR1LmNu; Zhuoming Chen,
bHl1anVuMjAyMEBqbnUuZWR1LmNu; Zhuoming Chen,  em0xMjB0Y2h6bUBxcS5jb20=
em0xMjB0Y2h6bUBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.