
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 02 February 2023
Sec. Neurodevelopment
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1113927
Introduction: Prenatal maternal stress (PNMS), including exposure to natural disasters, has been shown to serve as a risk factor for future child psychopathology and suboptimal brain development, particularly among brain regions shown to be sensitive to stress and trauma exposure. However, statistical approaches deployed in most studies are usually constrained by a limited number of variables for the sake of statistical power. Explainable machine learning, on the other hand, enables the study of high data dimension and offers novel insights into the prominent subset of behavioral phenotypes and brain regions most susceptible to PNMS. In the present study, we aimed to identify the most important child neurobehavioral and brain features associated with in utero exposure to Superstorm Sandy (SS).
Methods: By leveraging an explainable machine learning technique, the Shapley additive explanations method, we tested the marginal feature effect on SS exposures and examined the individual variable effects on disaster exposure.
Results: Results show that certain brain regions are especially sensitive to in utero exposure to SS. Specifically, in utero SS exposure was associated with larger gray matter volume (GMV) in the right caudate, right hippocampus, and left amygdala and smaller GMV in the right parahippocampal gyrus. Additionally, higher aggression scores at age 5 distinctly correlated with SS exposure.
Discussion: These findings suggest in utero SS exposure may be associated with greater aggression and suboptimal developmental alterations among various limbic and basal ganglia brain regions.
The prevalence of prenatal maternal stress (PNMS) has increased alarmingly; a recent large-scale study reported approximately 30% of pregnant women reported one or more types of stressors [Loomans et al., 2013; reviewed in Van den Bergh et al. (2020)]. Further, climate change events, in the form of hurricanes, tropical storms, wildfires, flooding, and droughts, are increasing in frequency and becoming more extreme in nature. These weather events cause serious disruptions in people’s lives and pose important threats to the mental health of individuals, especially among vulnerable populations such as pregnant women (Clemens et al., 2020; Zakrison et al., 2020). Considering the prevalence rate of PNMS, it is imperative to investigate its impact on early neurobehavioral development in their offspring (Monk et al., 2019).
An accumulating number of studies have established that various forms of PNMS serve as a risk factor for future child psychopathology (Monk et al., 2019; Lautarescu et al., 2020; Van den Bergh et al., 2020; Nomura et al., 2022). This included in utero maternal stress due to exposure to weather-related disasters, such as the Quebec Ice Storm, which led to greater externalizing and internalizing clinical behaviors (King et al., 2012) and Superstorm Sandy (SS), which led to greater clinical and adaptive behaviors (Nomura et al., 2022). Other studies have investigated the biophysiological consequences of suboptimal neurobehavioral development using magnetic resonance imaging (MRI) (Buss et al., 2010; El Marroun et al., 2016; Lebel et al., 2016; Davis et al., 2017; Wen et al., 2017; Mareckova et al., 2022, reviewed in Lautarescu et al., 2020). MRI serves as an unparalleled technology to pinpoint the structural and functional brain changes in various brain regions of the limbic system, such as the amygdala and hippocampus, and frontal lobe, such as the prefrontal cortex, among offspring exposed to PNMS and how those changes underlie the consequent neurobehavioral, emotional, and cognitive changes observed. Machine learning (ML), a rapidly developing technique in data science, is defined as a robust data-driven approach to automatically detect underlying patterns in high-dimensional data with high accuracy (Bi et al., 2019). Several studies have adopted ML algorithms and identified an enlarged amygdala as an important risk factor for early childhood anxiety (Qin et al., 2014), while other studies have discovered a wide range of biopsychosocial causal features using a predictive classification model (Saxe et al., 2017). However, research on the application of ML onto both neurobehavioral and brain volumetric data has been very limited in pediatric populations (Oskar and Stingone, 2020).
To date, there are a small number of ML studies pertaining to the association between prenatal stress or trauma exposure and altered neurobehavioral development and brain volumetric changes of offspring. One study demonstrated detection and classification of prenatal alcohol exposure (Rodriguez et al., 2021) and another identified the most affected regions of the brain (volumes) in children exposed to alcohol prenatally (Little and Beaulieu, 2020). However, a gold-standard of randomization is not possible among human populations, nor had any other work investigated disaster related PNMS in a quasi-experiment model in a human population. Quasi-experimental designs make it possible to pseudo-randomize prenatal stress independent of confounding personal attributes, such as genetic makeups and maternal psychological disorders status (Lafortune et al., 2021). SS, which hit a wide region in New York City (NYC), randomly “assigned” stressful conditions to pregnant women and their offspring, constructing an objective measure of stress independent of the mothers’ genetic background, psychopathology, and socioeconomic status. Thus, the Stress in Pregnancy (SIP) study, with its uniform exposure to a stressor, SS, enabled us to address the inherent bias that traditionally plagues studies assessing the impact of stress in a human population. As such, by leveraging the technique of explainable ML, this study attempts to identify important child neurobehavioral, and brain volumetric features associated with natural disaster-related PNMS; explainable ML makes it possible to uncover patterns of high-dimensional neurobehavioral and brain data of children exposed to such disasters.
The study capitalized on a longitudinal study that followed mother/child dyads from in utero to age 11, who were exposed, or not exposed, to Superstorm Sandy (SS) that hit metropolitan New York in 2012. An ML classifier was built to identify neurobehavioral and brain volumetric features that set a child with in utero exposure to SS apart from those without. The binary in utero exposure to a natural disaster was formulated as the target of the classification problem. ML can facilitate identification of features that dominate the classification process leading to high prediction accuracy. These features can be understood as the phenotypes most affected by natural disaster-related PNMS and studying them helps understand which behaviors and brain volumetric data in the selected brain regions are most affected as a result of SS exposure in utero. To this end, we trained a classification ML algorithm for features associated with SS exposure on a given participant and applied explainable artificial intelligence (AI) to identify features that can more accurately contribute to predicting structural changes in brain volume from the exposure.
Established in 2009, participants were drawn from the SIP study, a longitudinal study that follows mother/child dyads (from in utero to age 11) who were exposed, or not exposed, to SS (Finik and Nomura, 2017). Mothers of the current study cohort were originally recruited from antenatal OB/GYN clinics in New York City in efforts to understand how in utero exposure to adversity may alter fetal growth and development (Finik and Nomura, 2017). In 2013, a subsample of the SIP cohort, who were exposed or not exposed to SS in utero (N = 350), was analyzed to explore the trajectories of neurobehavioral development in offspring prospectively. Reflecting metropolitan New York, the children of the SIP study represent an urban population encompassing a diverse range of ethnic/racial backgrounds and socioeconomic strata; the majority of the cohort includes underrepresented races (Black, Hispanic, and/or Asian) and financial minorities (low socioeconomic status (SES), living below the poverty line) (Finik and Nomura, 2017). As a pilot study, a subsample of 30 were contacted for enrollment in MRI procedures. The MRI sample consisted of thirty school-aged children (n = 30) with a mean (SD) age of 8.50 (1.98). From the total sample, 21 (7 males and 14 females) were unexposed and 9 (1 male and 8 females) were exposed to SS in utero. All participants provided written consent and the protocol was approved by the Institutional Review Boards at the City University of New York (CUNY). Inclusion criteria for the original SIP study included being pregnant at the time of recruitment and planning to deliver the baby. Exclusion criteria for original participation included HIV infection, maternal psychosis, maternal age <15 years, life-threatening maternal medical complications, and congenital or chromosomal abnormalities in the fetus. Further details of the study can be found elsewhere (Finik and Nomura, 2017). Additionally, in this pilot, exclusion criteria for MRI participation included metal implants, devices, and/or objects in the body. Information on the handedness of the children was not collected. All participants provided written consent; the protocol was approved by the Institutional Review Boards at the City University of New York.
Child neurobehaviors were measured at age five (mean = 4.51, SD = 0.77) using the 2nd edition of the Behavioral Assessment for Children Parent-version (BASC-2P, Reynolds and Kamphaus, 2004). The BASC-2P produces eight clinical and four adaptive profiles. Based on the age and sex of the child, scores were standardized with a mean of 50 and a SD of 10 (Reynolds and Kamphaus, 2004). The eight clinical dimensions were Hyperactivity, Aggression, Anxiety, Depression, Somatization, Atypicality, Withdrawal, and Attention Problems. The four adaptive dimensions were Adaptability, Social Skills, Activities of Daily Living, and Functional Communication. Internal consistency in all 12 sub-dimensions were acceptable (α > 0.80) (Reynolds, 2010). The mean clinical and adaptive neurobehavioral scores by SS exposure status are shown in Table 1.
Magnetic resonance imaging images were acquired using a Siemens 3 Tesla Prisma MRI Scanner. 3D high-resolution T1-weighted images were collected using a magnetization with the following parameters: inversion time (TI)/repetition time (TR)/echo time (TE) = 1,070/2,500/2.9 msec, flip angle = 8.0 degrees, field of view = 256 mm × 256 mm, matrix = 256 × 256, and slice thickness = 1 mm without gap. The number of slices is 176. Real-time motion detection and correction was implemented using Volumetric Navigators (vNav) (Tisdall et al., 2012).
The FreeSurfer pipeline was used to generate cortical and subcortical volumetric measures (Dale et al., 1999). The skull was stripped from the T1 images and the interface between the white and gray matter was estimate and further refined to obtain the thickness of gray matter. Cortical surfaces were inflated and Talairach transformation was performed. The cortex was parcellated into different anatomical regions using Destrieux atlas. The brain regional volumes were normalized by the total intracranial volume. The mean gray matter volumes of the four brain regions most strongly associated with SS exposure are shown in Table 2.
The primary goal of the study is to evaluate whether certain features in behavioral and brain indices contribute to detecting in utero exposure to SS. The classification model of ML was implemented to identify which category (SS or non-SS exposure) the participant belonged to. Three common machine learning algorithms Random Forest (RF), XGBoost, and AdaBoost were evaluated for their ability to predict disaster exposure and identify the independent features that significantly contribute to the model’s decision. The evaluation can be found in Appendix. RF classifier was selected for this study due to its superior performance in prediction accuracy. RF classifier consists of multiple decision trees trained with different sub-portions of the trained dataset to ensure generalizability. The predictions from each subset are then voted for final classification result.
Our data suffers from the curse of dimensionality, where the sample size (n = 30) is relatively small compared to the number of variables (45 predictors). In ML implementations, dimensionality increases with each variable in the data. High dimensionality on a very small sample size leads to model overfitting. To avoid overfitting data, we applied a feature selection approach, Recursive Feature Elimination (RFE), to reduce dimensionality (Sartori et al., 2018). RFE starts with all features and eliminates the least important ones until the classifier reaches the best subset for the desired number of features. Reducing the number of features also helps the model to be more generalizable when used in independent data (Watts et al., 2021).
Our sample also suffers from class imbalance with a ratio of 30/70 with a disaster exposed minority group. With imbalance data, classifier models are prone to predict the majority class. We avoided such a problem by creating augmented data to balance both classes using the Synthetic Minority Oversampling Technique (SMOTE) (Chawla et al., 2002). SMOTE randomly perturbs a sample of the minority class based on its k-nearest neighbors to augment a new synthetic sample. Such operation is repeated until the data is balanced in target value classes.
The process of our classification model is summarized in Figure 1. Brain volumetric data and behavioral data were combined to form our dataset with a total of 45 features. The RF classifier was first trained with all features on the dataset for feature ranking. RFE was applied to extract the most important subset of features based on the ranking and RF was trained again with only these important predictors. We studied four settings with different combinations of data augmentation and feature selection for the best prediction outcome. The four settings are: Model 1.a without SMOTE using all features, Model 1.b with SMOTE using all features, Model 2.a without SMOTE using best feature subset, and Model 2.b with SMOTE using best feature subset. For the models with data oversampling, SMOTE was only applied to the training set.
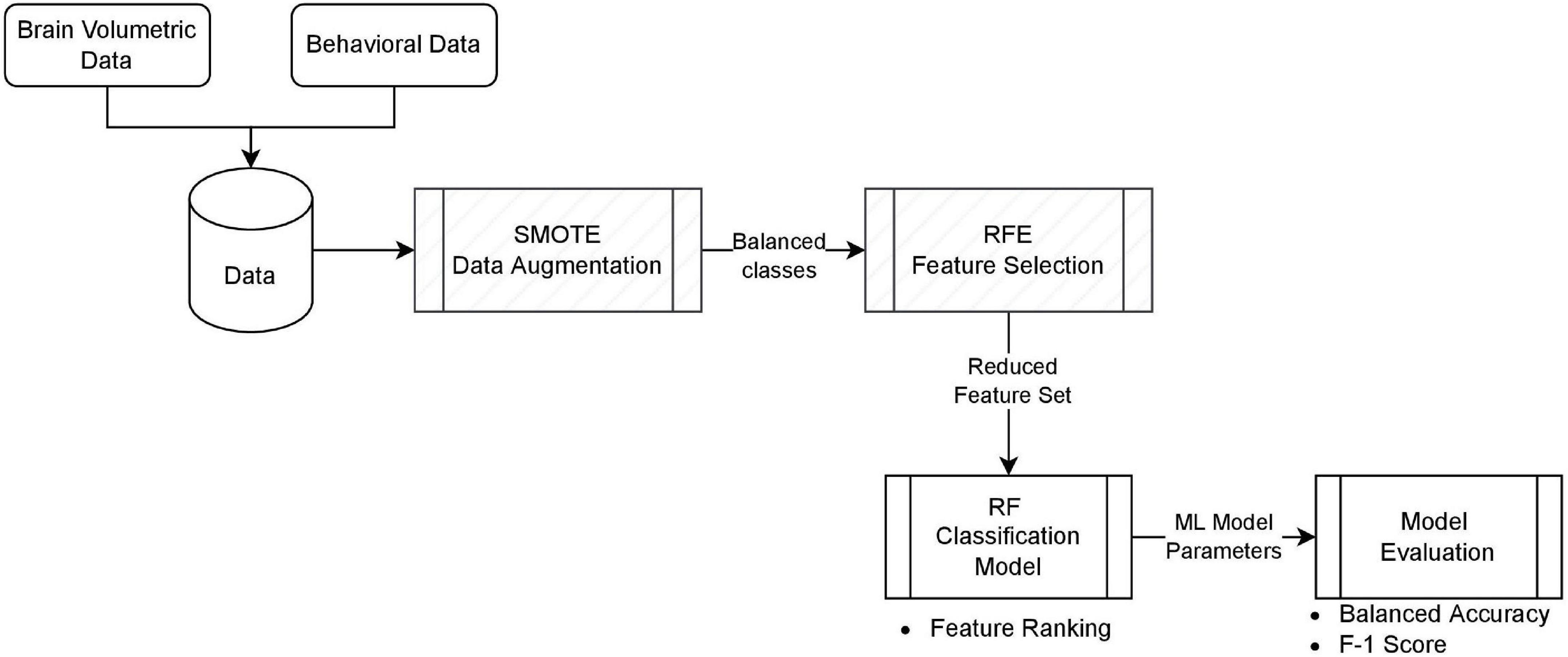
Figure 1. The classification model. Each block represents the implementation component, and the bullet of items is the outcome of that block. Hatch blocks are optional, depending on the model setting.
To facilitate interpretation of our classification model output, an explainable AI approach, Shapley additive explanations (SHAP), was used. Explainability in machine learning refers to the ability to understand and interpret the output of a complicated model. It is an important consideration when developing a machine learning model to ensure transparency to experts in the application domain. Specifically, Shapley Additive exPlanations (SHAP) is a method for explaining the output of machine learning models. It is a game-theoretic approach that assigns each feature of a model a “contribution” value, which represents the magnitude of the effect that the feature has on the model’s output. In other words, SHAP explains the correlation between a given feature and the prediction. In this way, we may call the result explainable ML. SHAP calculates the contribution of any given feature on the target value. For each individual feature x, a calculated SHAP value gives the direction of likelihood of the target value. We can also derive dependence plots from SHAP to show the effect of a single predictor on the prediction made by the model. The visualization of partial dependence plot helps to detect the point where the target value changes from 0 to 1 (or vice versa).
Given a small training dataset, the almost unbiased estimate of the true error can be obtained using Leave-One-Out Cross Validation (LOOCV). With a data size of n, LOOCV leaves 1 sample for testing and uses the other n-1 samples for training in each run, and the average accuracy of n runs is reported as the final evaluation score. It is important to note that, for Models 1.a and 2.a, SMOTE was applied only in the training set–there is no synthetic sample in our test data for the purpose of evaluation. We measured the model performance for each setting with balanced accuracy and F1 score results. Balanced accuracy was calculated as the average of sensitivity and specificity, where sensitivity = TP ÷ (TP + FN) and specificity = TN ÷ (TN + FP). The F1 score was calculated with the harmonic mean of precision and recall, where precision = TP ÷ (TP + FP) and recall = TP ÷ (TP + FN). TP = true positives, TN = true negatives, FP = false positives, and FN = false negatives. Here our TP is correctly predicted SS exposure and TN correctly predicted non-SS exposure. F1 score is preferred over balanced accuracy because the true negative correctly predicting non-Sandy exposure is not considered in the computation. Every case where SS exposure was not predicted correctly is penalized. For this reason, our RFE also applied F1-score to decide the best subset of features for feature selection.
Figure 2 shows the plots of ranked feature importance scores for different categories of features: neurobehavioral and brain volumetric data. The most relevant neurobehavioral feature that distinguished SS exposure was aggression. Moreover, the left amygdala, left hippocampus, and the right parahippocampal gyrus (PHG) were the three brain regions that distinguished SS exposure.
The best subset of six features chosen by RFE include aggression, the right caudate, right and left hippocampus, left amygdala, and right PHG. It is clear from the RFE selected features that brain volumetric data contributed more toward the prediction of the ML model.
Table 3 shows each model’s evaluation score. The best result was reached with an F1 score of 0.78 (see model 2.a), developed with the six features chosen by RFE. In Figure 3, the confusion matrix for model 2.a reveals that our model is fairly accurate at detecting both SS exposed and unexposed groups. Of note, when issues related to overfitting is controlled for, SMOTE is no longer effective.
Figure 4 shows SHAP values of the top 15 features from the model trained with all features to demonstrate the model explainability. With the exception of aggression and adaptability, the features that were identified as important were brain regions. The top features included the right superior frontal gyrus, right caudate, right PHG, left PHG, left amygdala, right caudal middle frontal gyrus, right amygdala, right hippocampus, left superior frontal gyrus, left caudal anterior cingulate, aggression, adaptability, right caudal anterior cingulate, right lateral orbitofrontal gyrus, and the left caudal middle frontal gyrus. The features are ordered by their importance score. Five of the six features selected by RFE were also included and ranked in the same order. They are the caudate, right PHG, left amygdala, right hippocampus, and aggression. For the selected features from the RFE method, the SHAP plot revealed that higher aggression, a larger right caudate, smaller right PHG, larger right hippocampus, and a larger left amygdala distinctly showed higher likelihood of SS exposure. In contrast, we see that a larger left and right PHG have a higher chance of predicting non-SS exposure. Furthermore, lower aggression distinctly shows non-SS exposure.
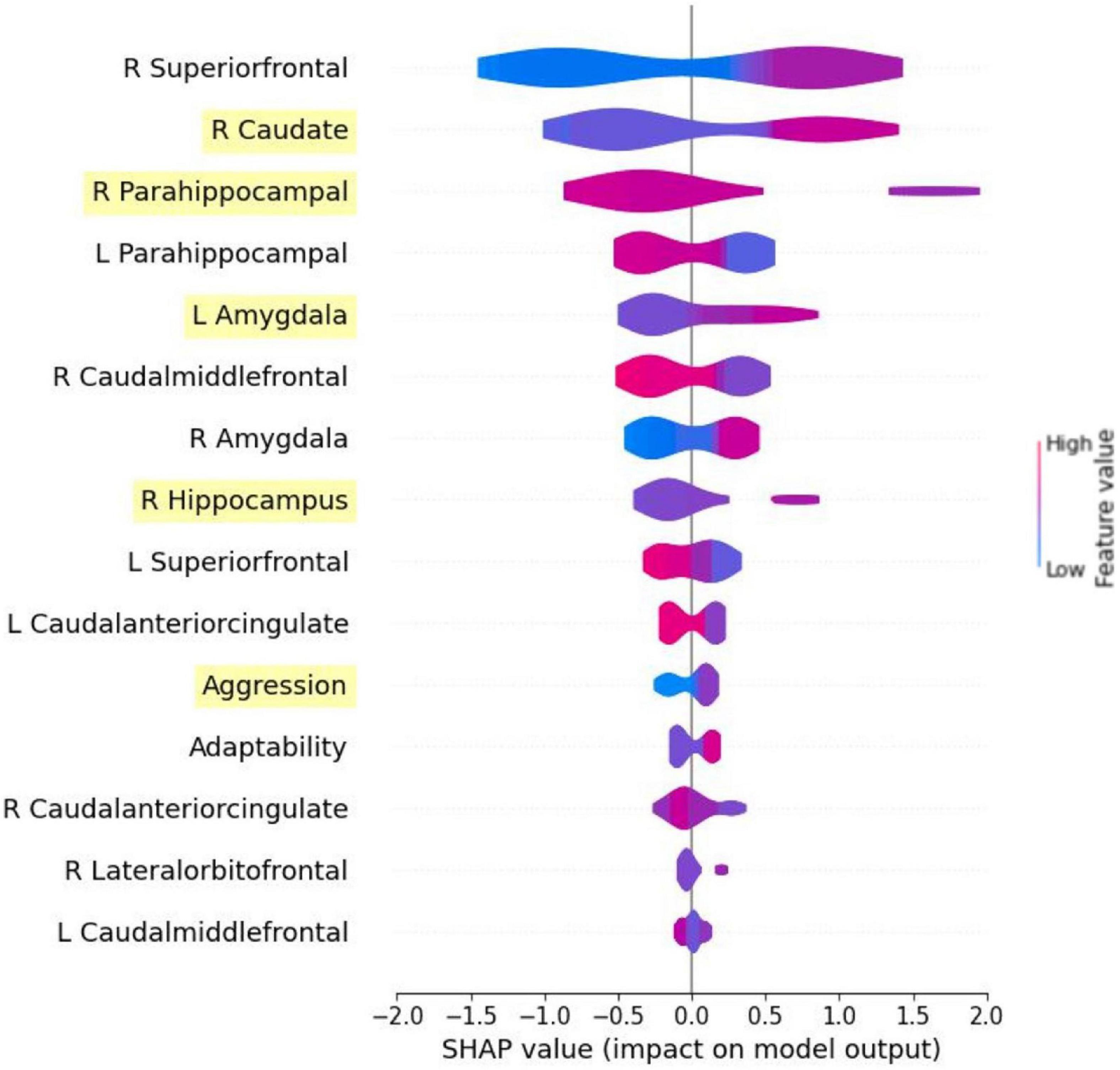
Figure 4. Summary plot in SHAP generated from the model using all features. Only the top 15 features are shown. Features highlighted are those selected from RFE method. To interpret the SHAP plot, the warmer color of the feature bars represents the higher feature values, and the cold color represents the lower feature values. When the SHAP value is higher and ¿0 the model is more likely to predict 1 (Sandy exposure), and when the value is lower and <0 the model is more likely to predict 0 (non-Sandy exposure).
In Figure 5, the dependence plots for the left and right hemispheres of both the amygdala and PHG (in volume) were given. For the right and left amygdala (top left and right), as the volume increases, the chance for SS exposure also increases. In contrast, for the right and left PHG (Figure 5, bottom left and right), the chance for SS exposure increases as the brain volume decreases. Aggression and adaptability dependence plots are shown in Figure 6. In both features, SS exposure is associated with a higher T score. Notably, for aggression scores, a more explicit difference is found between the SS exposed and unexposed groups.
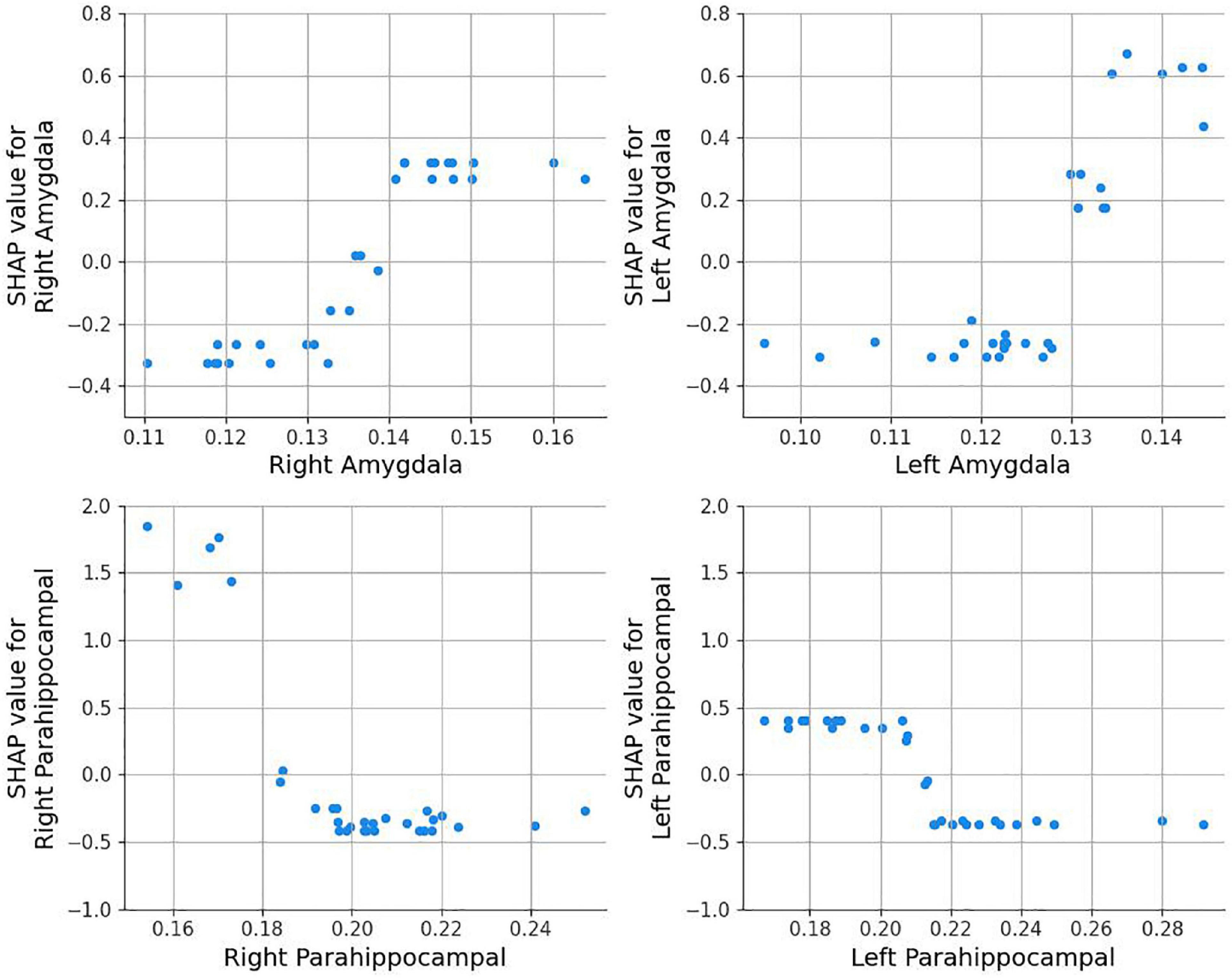
Figure 5. Amygdala and parahippocampal dependence plots. A higher SHAP value indicates that the model is more likely to predict 1 (Sandy exposure).
To the best of our knowledge, the current study is the first to show that the implementation of a machine learning model was predictive in identifying children exposed in utero to natural disasters based on various neurobehavioral phenotypes and brain regions, shown to be sensitive to stress and trauma exposure (Lautarescu et al., 2020; National Scientific Council on the Developing Child, 2020; Van den Bergh et al., 2020).
Our results showed distinctly higher aggression and adaptability scores among our SS exposed group, which, consistent with the work of King et al. (2012) and Nomura et al. (2022), suggests in utero maternal stress, from exposure to a weather-related disaster, is strongly associated with child neurobehavioral phenotypes. Our results demonstrate the stress a child experiences in utero increases the risk for future psychopathology but may also impact a child’s potential to acquire and express certain adaptive skills in their postnatal environment (Nomura et al., 2022).
In agreement with the findings from numerous earlier studies, our results suggest in utero stress exposure may be associated with a larger amygdala volume (Buss et al., 2012; Wen et al., 2017; Acosta et al., 2019; Jones et al., 2019) and a larger hippocampal volume (Cao-Lei et al., 2021), although less supported. It has been further suggested that prenatal stress exposure appears to accelerate the development of these regions, particularly the amygdala, as a means of allowing more rapid detection of incoming danger and threat (Lautarescu et al., 2020). Our results are consistent with the evolutional model of stress that suggests the exaggeration of automatic fear detection programmed prenatally to better prepare for the likely at stake environment postnatally (Glover, 2011).
Our results offer further, more novel, insights as in utero SS exposure was found to be linked with both a larger caudate and a smaller PHG. Enlargements in the caudate have been suggested to underlie the pathophysiology of various neurobehavioral and emotional disorders, characterized by repetitive and ritualistic tendencies, such as obsessive-compulsive disorder (OCD) and autism (Ring and Serra-Mestres, 2002). A reduced PHG was found to be associated with elevated prenatal maternal anxiety (Buss et al., 2010; Acosta et al., 2019). The caudate and PHG are two brain regions shown to influence emotion regulation and the formation of emotional memories (Bhatt et al., 2012; Zhu et al., 2019; Driscoll et al., 2022). Our findings on a larger caudate and a smaller PHG may extend the current literature and support the association between prenatal natural disaster-related maternal stress and altered child volumetric brain development, which may have long-term implications on child neurodevelopment and emotion regulation (Talge et al., 2007; Wu et al., 2022).
Of note, no notable sex differences were observed between the assessed clinical and adaptive neurobehaviors nor between the GMV of the SS sensitive brain regions. In efforts to explore the association between the GMV of the SS sensitive brain regions and aggression scores, a post hoc analysis was conducted. Upon stratification of the sample by the SS exposure status, higher aggression scores were negatively associated with left amygdala GMV [r(19) = −0.45, p = 0.04] among the SS unexposed and marginally associated with left amygdala GMV among the SS exposed [r(7) = −0.60, p = 0.09]. No other notable associations between aggression and the GMV of SS sensitive brain regions were detected. We anticipated seeing a strong correlation between amygdala GMV and aggression scores as previous work among a sample of children, aged 6–9, found an association between smaller amygdala GMV and higher aggression scores (Thijssen et al., 2015). As validated by the aggression diathesis model, we speculate this strong correlation may be due to increased aggression leading to an imbalance between top down and bottom up control systems (Siever, 2008). Greater acts of aggression may lead top down systems, which are modulated by brain regions that are highly interconnected with the amygdala, such as the orbitofrontal cortex (OFC) and the anterior cingulate cortex (ACC), to fail to suppress aggressive acts in the presence of anger inducing stimuli (Siever, 2008). Moreover, increased aggression may induce hyperresponsivity of limbic brain regions, such as the amygdala, which provide the “drive” to respond to incoming danger and threat (Siever, 2008).
We developed an ensemble ML model to find brain and behavioral features that were associated with SS exposure on given participants. Our main goal was to see whether certain features contribute accurately to predicting structural changes in brain morphometry from exposure using ML. We achieved the best accuracy by using the feature selection method, RFE. We also examined the effect of oversampling by using SMOTE on all features. For high dimensional data, SMOTE generally performs better by avoiding overfitting (Dessie et al., 2021; Park et al., 2021). However, for data with a very small sample size (n = 30), involving augmented (simulation) data provides little benefit if we reduce feature dimensionality because overfitting is no longer a problem and oversampling can lead to underperformance due to a deteriorating sampling (Elreedy and Atiya, 2019). From our best results, we see that with a small data set, with very few positive cases, reducing the dimensionality, rather than introducing synthetic data, leads to higher model performance. Considering achieving greater generalizability is one of our aims, reducing the dimensionality would be one of the critical solutions. Our finding is consistent with prior studies using small sample sizes. Crippa et al. (2015) identified children with autism spectrum disorder (ASD) from a sample size of N = 30. Güven et al. (2020) predicted attention-deficit hyperactivity disorder (ADHD) children from a sample size of N = 44. Both studies implemented feature selection either manually or algorithmically.
Within the summary SHAP plot, most of the important features that affect our target value came from the brain volumetric data (i.e., the amygdala, hippocampus, and caudate) suggesting the brain’s particular sensitivity to stress exposure. Further, the dependence plots showed distinctly larger amygdala and smaller PHG brain volumes, respectively, increase the likelihood of SS exposure.
The study has various limitations. First, despite our prior research indicating the binary SS index (exposed vs. not exposed in utero) to be a significant and critical indicator of impairment, we acknowledge the use of binary, objective indices prevent the analysis of important, fine-grained measures, such as post-traumatic psychosocial reactions to SS. Second, the BASC-2P questionnaire used to assess child behavioral problems was based on parental report, which may lead to subjective bias. However, our study incorporated brain imaging to corroborate and enhance the objectivity and robustness of the findings. Third, information on the handedness of the children was not collected and fourth, the sample size was relatively small with mostly girls. Thus, the results should be interpreted with caution and warrant replication with bigger sample sizes in the future.
There are several strengths in this study. First, our study utilized a quasi-experimental design and rendered the pseudo-randomization of a natural disaster-related stress in utero. Currently, most of the studies that have investigated stress during pregnancy relied on the measurement of maternal psychopathology, trauma history or nominal pregnancy stress—which are likely to be confounded by factors, such as genetics, and heterogeneity of level of stress exerted. Second, the current study is part of a larger longitudinal project prospectively tracking and assessing the neurodevelopment of children, preventing the need to rely on retrospective parental reports. This eliminates the problem due to recall bias, which is common in cross-sectional research using information based on retrospective reports in part. Third, our study is the very first to deploy the technique of ML on neurobehavioral and brain imaging data in the research of in utero maternal stress. Future studies will aim to expand on our initial findings by tracking changes from childhood into adolescence.
Exploiting ML, we developed multiple models for classification and chose the RFE algorithm to extract the subset of features that contributed the most optimally to detection of natural disaster exposure in utero. The use of SHAP values assisted in model interpretation. SHAP plots provided critical insights into behavioral phenotypes and brain volumetric changes associated with natural disaster exposure in utero among offspring, especially within brain regions implicated in emotion regulation (the amygdala, caudate, and PHG). The goal of this study was to leverage advanced computational methodology to understand how a significant stressor connects brain and behavioral development among high-risk populations. Our preliminary results suggest that targeted intervention on behavioral phenotypes, such as aggression, and structural morphological alterations in the emotional centers of the brain can potentially play a key role in buffering the adverse impact of prenatal maternal stress on brain development and improve subsequent developmental outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the City University of New York University Integrated Institutional Review Board (protocol number: 2018-1305-QC). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
GD and C-LT led the study and oversaw the integrity of the ML implementation in the study. AS supervised the interpretation of the brain structure findings. YN supervised the interpretation of the behavioral findings. All authors contributed to the drafting, revising, and interpreting the data.
This study was supported in part by the Interdisciplinary Translational Grant (CUNY/CAB800, PIs. YN, AS, and C-LT).
The authors would like to acknowledge the Magnetic Resonance Imaging Facility of City University of New York Advanced Science Research Center for instrument use and technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1113927/full#supplementary-material
Acosta, H., Tuulari, J. J., Scheinin, N. M., Hashempour, N., Rajasilta, O., Lavonius, T. I., et al. (2019). Maternal pregnancy-related anxiety is associated with sexually dimorphic alterations in amygdala volume in 4-year-old children. Front. Behav. Neurosci. 13:175. doi: 10.3389/fnbeh.2019.00175
Bhatt, M. A., Lohrenz, T., Camerer, C. F., and Montague, P. R. (2012). Distinct contributions of the amygdala and parahippocampal gyrus to suspicion in a repeated bargaining game. Proc. Natl. Acad. Sci. U.S.A. 109, 8728–8733. doi: 10.1073/pnas.1200738109
Bi, Q., Goodman, K. E., Kaminsky, J., and Lessler, J. (2019). What is machine learning? A primer for the epidemiologist. Am. J. Epidemiol. 188, 2222–2239. doi: 10.1093/aje/kwz189
Buss, C., Davis, E. P., Muftuler, L. T., Head, K., and Sandman, C. A. (2010). High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 35, 141–153. doi: 10.1016/j.psyneuen.2009.07.010
Buss, C., Davis, E. P., Shahbaba, B., Pruessner, J. C., Head, K., and Sandman, C. A. (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. U.S.A. 109, E1312–E1319. doi: 10.1073/pnas.1201295109
Cao-Lei, L., Yogendran, S., Dufoix, R., Elgbeili, G., Laplante, D. P., and King, S. (2021). Prenatal maternal stress from a natural disaster and hippocampal volumes: Gene-by-environment interactions in young adolescents from project ice storm. Front. Behav. Neurosci. 15:706660. doi: 10.3389/fnbeh.2021.706660
Clemens, V., von Hirschhausen, E., and Fegert, J. M. (2020). Report of the intergovernmental panel on climate change: Implications for the mental health policy of children and adolescents in Europe—a scoping review. Eur. Child Adolesc. Psychiatry 31, 701–713. doi: 10.1007/s00787-020-01615-3
Chawla, N. V., Bowyer, K. W., Hall, L. O., and Kegelmeyer, W. P. (2002). SMOTE: Synthetic minority over-sampling technique. J. Artif. Intel. Res. 16, 321–357.
Crippa, A., Salvatore, C., Perego, P., Forti, S., Nobile, M., Molteni, M., et al. (2015). Use of machine learning to identify children with autism and their motor abnormalities. J. Autism Dev. Disord. 45, 2146–2156. doi: 10.1007/s10803-015-2379-8
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194.
Davis, E. P., Head, K., Buss, C., and Sandman, C. A. (2017). Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology 75, 56–63. doi: 10.1016/j.psyneuen.2016.10.005
Dessie, E. Y., Tsai, J. J., Chang, J. G., and Ng, K. L. (2021). A novel miRNA-based classification model of risks and stages for clear cell renal cell carcinoma patients. BMC Bioinformatics 22:270. doi: 10.1186/s12859-021-04189-2
Driscoll, M. E., Bollu, P. C., and Tadi, P. (2022). “Neuroanatomy, nucleus caudate,” in StatPearls. Treasure Island, FL: StatPearls Publishing. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/32491339
El Marroun, H., Tiemeier, H., Muetzel, R. L., Thijssen, S., van der Knaap, N. J., Jaddoe, V. W., et al. (2016). Prenatal exposure to maternal and paternal depressive symptoms and brain morphology: A population-based prospective neuroimaging study in young children. Depress. Anxiety 33, 658–666. doi: 10.1002/da.22524
Elreedy, D., and Atiya, A. F. (2019). A comprehensive analysis of synthetic minority oversampling technique (SMOTE) for handling class imbalance. Inform. Sci. 505, 32–64.
Finik, J., and Nomura, Y. (2017). Cohort profile: Stress in pregnancy (SIP) study. Int. J. Epidemiol. 46, 1388–1388k. doi: 10.1093/ije/dyw264
Glover, V. (2011). Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. J. Child Psychol. Psychiatry 52, 356–367. doi: 10.1111/j.1469-7610.2011.02371.x
Güven, A., Altınkaynak, M., Dolu, N., Izzetoglu, M., Pektas, F., Ozmen, S., et al. (2020). Combining functional near-infrared spectroscopy and EEG measurements for the diagnosis of attention-deficit hyperactivity disorder. Neural Comput. Appl. 32, 8367–8380. doi: 10.1007/s00521-019-04294-7
Jones, S. L., Dufoix, R., Laplante, D. P., Elgbeili, G., Patel, R., Chakravarty, M. M., et al. (2019). Larger amygdala volume mediates the association between prenatal maternal stress and higher levels of externalizing behaviors: Sex specific effects in project ice storm. Front. Hum. Neurosci. 13:144. doi: 10.3389/fnhum.2019.00144
King, S., Dancause, K., Turcotte-Tremblay, A. M., Veru, F., and Laplante, D. P. (2012). Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res. Part C Embryo Today Rev. 96, 273–288. doi: 10.1002/bdrc.21026
Lafortune, S., Laplante, D. P., Elgbeili, G., Li, X., Lebel, S., Dagenais, C., et al. (2021). Effect of natural disaster-related prenatal maternal stress on child development and health: A meta-analytic review. Int. J. Environ. Res. Public Health 18:8332. doi: 10.3390/ijerph18168332
Lautarescu, A., Craig, M. C., and Glover, V. (2020). Prenatal stress: Effects on fetal and child brain development. Int. Rev. Neurobiol. 150, 17–40.
Lebel, C., Walton, M., Letourneau, N., Giesbrecht, G. F., Kaplan, B. J., and Dewey, D. (2016). Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol. Psychiatry 80, 859–868. doi: 10.1016/j.biopsych.2015.12.004
Little, G., and Beaulieu, C. (2020). Multivariate models of brain volume for identification of children and adolescents with fetal alcohol spectrum disorder. Hum. Brain Mapp. 41, 1181–1194. doi: 10.1002/hbm.24867
Loomans, E. M., Van Dijk, A. E., Vrijkotte, T. G., Van Eijsden, M., Stronks, K., Gemke, R. J., et al. (2013). Psychosocial stress during pregnancy is related to adverse birth outcomes: Results from a large multi-ethnic community-based birth cohort. Eur. J. Public Health 23, 485–491. doi: 10.1093/eurpub/cks097
Mareckova, K., Miles, A., Liao, Z., Andryskova, L., Brazdil, M., Paus, T., et al. (2022). Prenatal stress and its association with amygdala-related structural covariance patterns in youth. NeuroImage Clin. 34:102976. doi: 10.1016/j.nicl.2022.102976
Monk, C., Lugo-Candelas, C., and Trumpff, C. (2019). Prenatal developmental origins of future psychopathology: Mechanisms and pathways. Annu. Rev. Clin. Psychology 15:317.
National Scientific Council on the Developing Child (2020). Connecting the brain to the rest of the body: Early childhood development and lifelong health are deeply intertwined. Working paper No. 15. Available online at: www.developingchild.harvard.edu (accessed September 6, 2022).
Nomura, Y., Zhang, W., and Hurd, Y. L. (2022). Stress in pregnancy: Clinical and adaptive behavior of offspring following Superstorm Sandy. Dev. Psychopathol. 34, 1249–1259. doi: 10.1017/S0954579421000304
Oskar, S., and Stingone, J. A. (2020). Machine learning within studies of early-life environmental exposures and child health: Review of the current literature and discussion of next steps. Curr. Environ. Health Rep. 7, 170–184. doi: 10.1007/s40572-020-00282-5
Park, K. H., Batbaatar, E., Piao, Y., Theera-Umpon, N., and Ryu, K. H. (2021). Deep learning feature extraction approach for hematopoietic cancer subtype classification. Int. J. Environ. Res. Public Health 18:2197. doi: 10.3390/ijerph18042197
Qin, S., Young, C. B., Duan, X., Chen, T., Supekar, K., and Menon, V. (2014). Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol. Psychiatry 75, 892–900. doi: 10.1016/j.biopsych.2013.10.006
Reynolds, C. R., and Kamphaus, R. W. (2004). BASC-2: Behavior assessment system for children, Vol. 32, 2nd Edn. Circle Pines, MN: American Guidance Service, 121–124.
Ring, H. A., and Serra-Mestres, J. (2002). Neuropsychiatry of the basal ganglia. J. Neurol. Neurosurg. Psychiatry 72, 12–21.
Rodriguez, C. I., Vergara, V. M., Davies, S., Calhoun, V. D., Savage, D. D., and Hamilton, D. A. (2021). Detection of prenatal alcohol exposure using machine learning classification of resting-state functional network connectivity data. Alcohol 93, 25–34. doi: 10.1016/j.alcohol.2021.03.001
Sartori, J. M., Reckziegel, R., Passos, I. C., Czepielewski, L. S., Fijtman, A., Sodré, L. A., et al. (2018). Volumetric brain magnetic resonance imaging predicts functioning in bipolar disorder: A machine learning approach. J. Psychiatr. Res. 103, 237–243. doi: 10.1016/j.jpsychires.2018.05.023
Saxe, G. N., Ma, S., Ren, J., and Aliferis, C. (2017). Machine learning methods to predict child posttraumatic stress: A proof-of-concept study. BMC Psychiatry 17:223. doi: 10.1186/s12888-017-1384-1
Talge, N. M., Neal, C., and Glover, V. Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health (2007). Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J. Child Psychol. Psychiatry 48, 245–261. doi: 10.1111/j.1469-7610.2006.01714.x
Thijssen, S., Ringoot, A. P., Wildeboer, A., Bakermans-Kranenburg, M. J., El Marroun, H., Hofman, A., et al. (2015). Brain morphology of childhood aggressive behavior: A multi-informant study in school-age children. Cogn. Affect. Behav. Neurosci. 15, 564–577. doi: 10.3758/s13415-015-0344-9
Tisdall, M. D., Hess, A. T., Reuter, M., Meintjes, E. M., Fischl, B., and van der Kouwe, A. J. (2012). Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn. Reson. Med. 68, 389–399. doi: 10.1002/mrm.23228
Van den Bergh, B. R., van den Heuvel, M. I., Lahti, M., Braeken, M., de Rooij, S. R., Entringer, S., et al. (2020). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 117, 26–64.
Watts, D., Moulden, H., Mamak, M., Upfold, C., Chaimowitz, G., and Kapczinski, F. (2021). Predicting offenses among individuals with psychiatric disorders-A machine learning approach. J. Psychiatr. Res. 138, 146–154. doi: 10.1016/j.jpsychires.2021.03.026
Wen, D. J., Poh, J. S., Ni, S. N., Chong, Y. S., Chen, H., Kwek, K., et al. (2017). Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl. Psychiatry 7:e1103. doi: 10.1038/tp.2017.74
Wu, Y., Espinosa, K. M., Barnett, S. D., Kapse, A., Quistorff, J. L., Lopez, C., et al. (2022). Association of elevated maternal psychological distress altered fetal brain, and offspring cognitive and social-emotional outcomes at 18 months. JAMA Netw. Open 5:e229244. doi: 10.1001/jamanetworkopen.2022.9244
Zakrison, T. L., Valdés, D. M., and Shultz, J. M. (2020). The medical, public health, and emergency response to the impact of 2017 hurricane Irma in Cuba. Disaster Med. Public Health Prep. 14, 10–17. doi: 10.1017/dmp.2019.71
Keywords: machine learning, explainable AI, prenatal maternal stress, Superstorm Sandy, brain volume, child behavior
Citation: Demirci GM, DeIngeniis D, Wong WM, Shereen AD, Nomura Y and Tsai C-L (2023) Superstorm Sandy exposure in utero is associated with neurobehavioral phenotypes and brain structure alterations in childhood: A machine learning approach. Front. Neurosci. 17:1113927. doi: 10.3389/fnins.2023.1113927
Received: 01 December 2022; Accepted: 12 January 2023;
Published: 02 February 2023.
Edited by:
Ya Wang, Institute of Psychology (CAS), ChinaReviewed by:
Xin-lu Cai, Hangzhou Normal University, ChinaCopyright © 2023 Demirci, DeIngeniis, Wong, Shereen, Nomura and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chia-Ling Tsai,  Y3RzYWlAcWMuY3VueS5lZHU=
Y3RzYWlAcWMuY3VueS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.