- 1Department of Rehabilitation Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 2Rehabilitation Medicine Center and Institute of Rehabilitation Medicine, West China Hospital, Sichuan University, Chengdu, China
- 3Innovation and Transformation Center, Fujian University of Traditional Chinese Medicine, Fuzhou, China
Introduction: This study focused on the research hotspots and development trends of the neuroimaging of attention deficit hyperactivity disorder (ADHD) in the past thirty years.
Methods: The Web of Science database was searched for articles about ADHD neuroimaging from January 1992 to September 2022. CiteSpace was used to analyze the co-occurrence of keywords in literature, partnerships between authors, institutions, and countries, the sudden occurrence of keywords, clustering of keywords over time, and analysis of references, cited authors, and cited journals.
Results: 2,621 articles were included. More and more articles have been published every year in the last years. These articles mainly come from 435 institutions and 65 countries/regions led by the United States. King's College London had the highest number of publications. The study identified 634 authors, among which Buitelaar, J. K. published the largest number of articles and Castellanos, F. X. was co-cited most often. The most productive and cited journal was Biological psychiatry. In recent years, burst keywords were resting-state fMRI, machine learning, functional connectivity, and networks. And a timeline chart of the cluster of keywords showed that “children” had the longest time span.
Conclusions: Increased attention has been paid to ADHD neuroimaging. This work might assist researchers to identify new insight on potential collaborators and cooperative institutions, hot topics, and research directions.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most prevalent neurodevelopmental disorder among children. The worldwide prevalence has been estimated between 2 and 7%, with an average of approximately 5% (Sayal et al., 2018). ADHD is manifested as poor sustained attention, impaired impulse control, and high hyperactivity (American Psychiatric Association, 2013). In addition, ADHD occurs throughout the patient's life cycle, sourcing from early childhood and extending to adulthood (Campbell et al., 2014; Franke et al., 2018).
A growing number of neuroimaging literature has started to explore the neural substrates and neuroimaging markers associated with ADHD. Some neuroimaging studies reported neurochemical, structural, and functional dysfunctions in the brain of patients with ADHD (Albajara Sáenz et al., 2019). Neuroimaging techniques are divided into two categories: structural and functional neuroimaging. Structural magnetic resonance imaging (sMRI) and diffusion tensor imaging (DTI) have been widely used in ADHD studies. Previous sMRI studies found that patients with ADHD had a decrease in overall brain volume (Narr et al., 2009; Nakao et al., 2011; Hoogman et al., 2017), as well as cortical thickness reductions, particularly in prefrontal, parietal, temporo-parietal, and occipital regions (Shaw et al., 2007; Wolosin et al., 2009; Almeida et al., 2010; Silk et al., 2016; Ambrosino et al., 2017). DTI study found an atypical junction of the corpus callosum in patients with ADHD (Aoki et al., 2018; Fuelscher et al., 2021).
Functional neuroimaging studies, including those employing functional MRI (fMRI), functional near-infrared spectroscopy (fNIRS), positron emission tomography (PET), single-positron emission computed tomography (SPECT), and magnetic resonance spectroscopy (MRS), have revealed abnormalities in brain function in patients with ADHD. fMRI is a non-invasive, high spatial resolution technology detecting functional changes in the brain through changes in cerebral blood oxygen levels. The complex pathophysiology of ADHD has been further investigated by resting-state MRI (rs-fMRI) and task-state MRI (task-MRI) functional magnetic resonance imaging studies (Plichta and Scheres, 2014; Massat et al., 2018; Cortese et al., 2021). rs-fMRI reflects the resting-state brain activity of children with ADHD. Task-fMRI can provide insight into the functional characteristics of the brain of children with ADHD during the performance of different tasks by employing various task paradigms such as motor responses (stop-signal task, go/no-go), interference suppression (Stroop, Simon, and Eriksen lateralization tasks) and transformation tasks (Hart et al., 2013; Lei et al., 2015; Hwang et al., 2019; Kolodny et al., 2020, 2022). fNIRS is a novel neuroimaging technique without any invasion and has become popular in recent years (Gossé et al., 2022). It detects neural activity by detecting oxygen and deoxyhemoglobin in the brain. fNIRS has a high tolerance for the head movements of subjects in experiments and is therefore particularly suitable for examining brain function in ADHD in a natural environment (Kohl et al., 2020). SPECT and PET are nuclear medicine techniques providing metabolic and functional information to examine the neurotransmitter dopamine in ADHD (Dubey et al., 1983; Gualtieri and Hicks, 1985). A meta-analysis study disclosed that the dopamine transporter density increased by 14% in patients with ADHD by analyzing nine studies on PET and SPECT (Fusar-Poli et al., 2012). MRS is a reliable method to detect neurotransmitters in vivo. Dysfunction in the metabolism of dopaminergic and glutamate has been considered to be a cause of ADHD (Perlov et al., 2009).
The application of the neuroimaging technique in ADHD has a history of more than 30 years (Zametkin et al., 1990), but the research hotspots and emerging trends have not been studied in a systematic manner. Thus, this study considered that comprehensively identifying the previous research hotspots and current research directions and defects was of high value for the subsequent research. Bibliometric analysis can analyze publications, such as books or journal articles quantitatively and qualitatively (Roldan-Valadez et al., 2019). Bibliometrics is chosen because it can analyze the contributions and cooperation of authors, institutions, countries, and journals, and judge the development and new trends of relevant literature based on current research.
To understand the current status of ADHD neuroimaging literature and identify new research directions, the software CiteSpace and Scimago Graphica were used to perform a bibliometric visualization analysis. The present study could provide a global perspective for clinical practitioners, neuroimaging experts, and researchers.
Methods
Retrieval method
This study was conducted by searching the Science Citation Index Expanded (SCI-Expanded) in the Web of Science (WoS). The relevant literature was published between 1 January 1992 and 20 September 2022, and the search subject terms were identified as (ADHD OR “Attention Deficit Disorder with Hyperactivity” OR “Hyperkinetic Syndrome” OR “hyperkinetic disorder” OR “Attention Deficit Hyperactivity Disorder”) AND (Neuroimaging OR “Functional Neuroimaging” OR “structural magnetic resonance imaging” OR “functional magnetic resonance imaging” OR fMRI OR “Magnetic resonance imaging” OR “Magnetic resonance*” OR MRS OR Tomography OR “Positron Emission Tomography” OR “Tomography, Emission-Computed” OR PET OR “Single-Photon” OR “Single Photon Emission Computed Tomography” OR PECT OR “functional near-infrared spectroscopy” OR fNIRS OR “Diffusion Tensor Imaging” OR DTI). The literature search was constrained to articles and review articles, eliminating other document types.
Data processing
Search results were processed using Microsoft Office Excel 2019 (Microsoft, USA), and a polynomial regression model was constructed, aiming to predict the number of articles in 2022. The data from the WoS were exported in “Full Record with Cited References” format and saved with the prefix “download.” “Node Types” select “Author,” “Institution,” “country,” and “Keyword” for visual analysis to generate a visual co-occurrence map. “Pruning” selects “Pathfinder” and “Pruning sliced networks” to prune each slice of the network to reduce the density of the network and improve its readability.
CiteSpace was employed for constructing a base map for hotspots and knowledge in nephrotoxicity research, and for centrality detection (Chen and Chen, 2005). The time-slicing was performed from January 1992 to September 2022 (1 year per slice), node type (1 per time), selection criteria (g-index: k = 15), and pruning (pathfinder and pruning sliced network). Scimago Graphica 1.0.25 visualized the collaborative relationships of authors from different countries. In the visual diagram, each node was deemed as an element, and its size referred to the frequency of occurrence. Cooperation or connection among various elements was marked with a connection line. The node with high betweenness centrality (>0.1) is the key point in this field, which is also a concern of CiteSpace. The purple ring represents centrality. Highly cited elements are often considered to be the research hotspots in this field. The flow diagram of this study is shown in Figure 1.
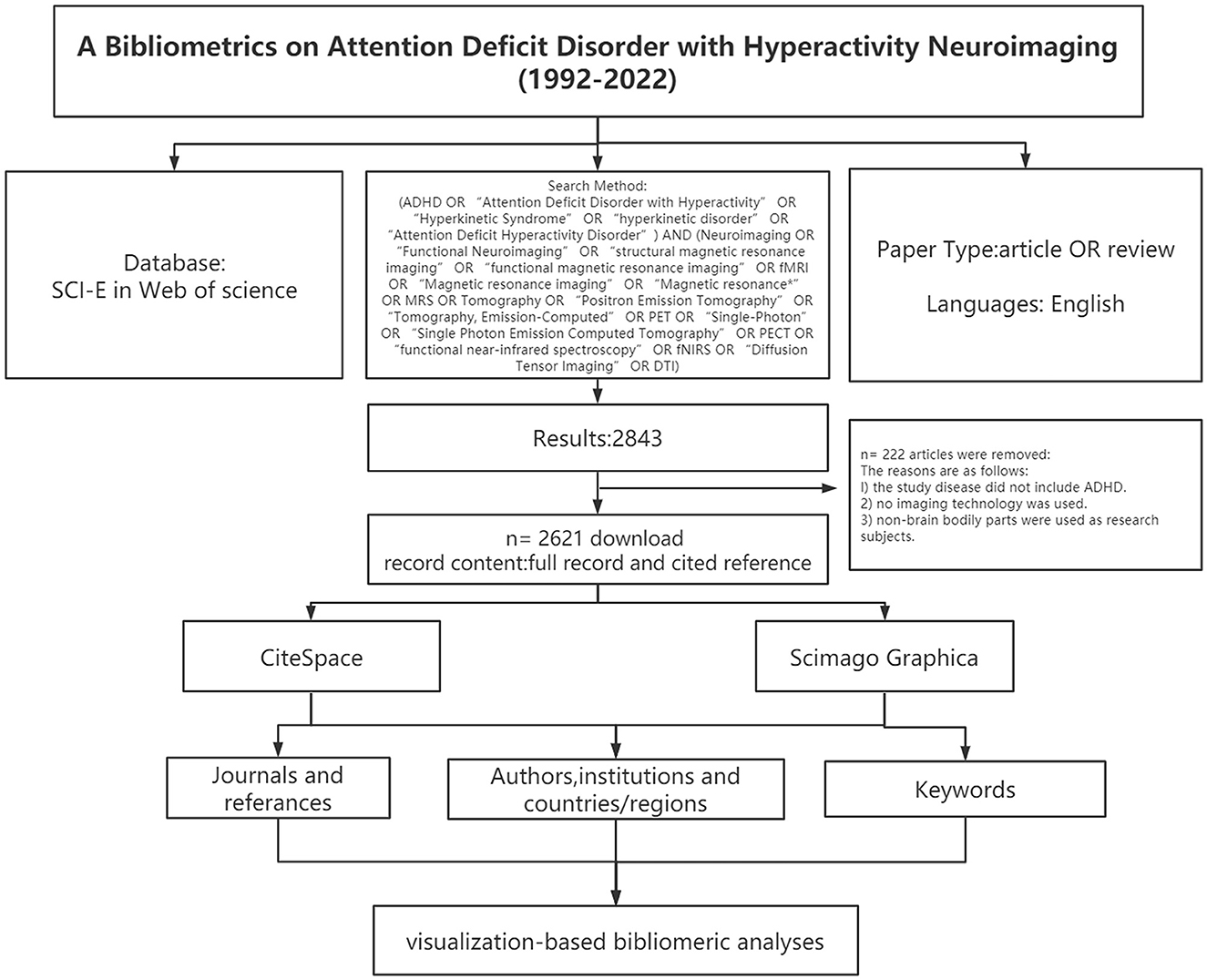
Figure 1. Frame flow diagram shows the detailed selection criteria and bibliometric analysis steps of ADHD neuroimaging.
Result
Analysis of publications a year
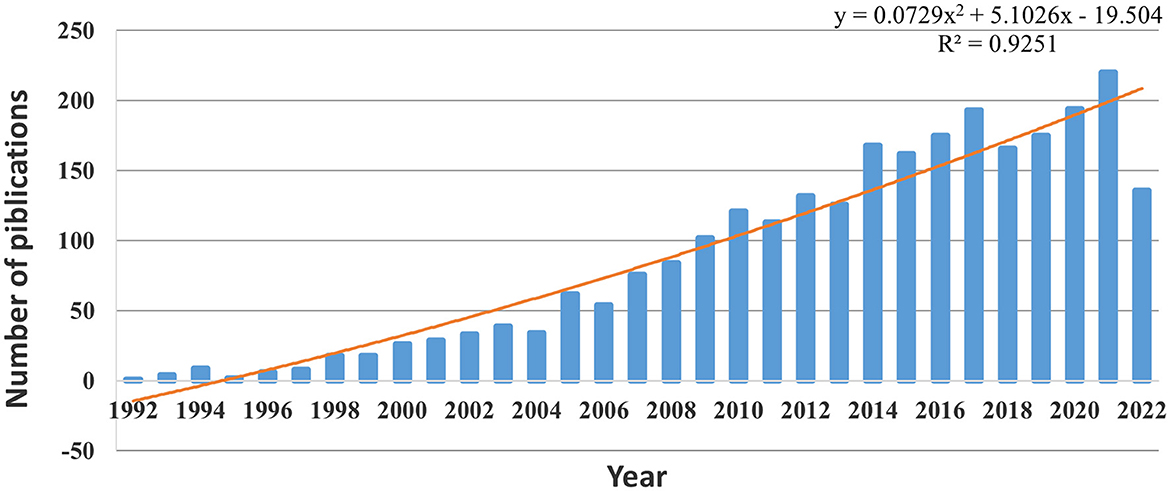
A total of 2621 articles were included. Figure 2 lists the number of publications broken down by year, illustrating an overall increase from 1992 to 2022. Since 2009, the number of publications has exceeded 100 each year. After fitting these data, the results showed that the statistical relationship between the year and the number of publications was R2 = 0.9251, indicating a significant correlation. Based on the fitting curve, this study predicts that the number of relevant articles on ADHD neuroimaging will reach 210 in 2022.
Analysis of countries/regions
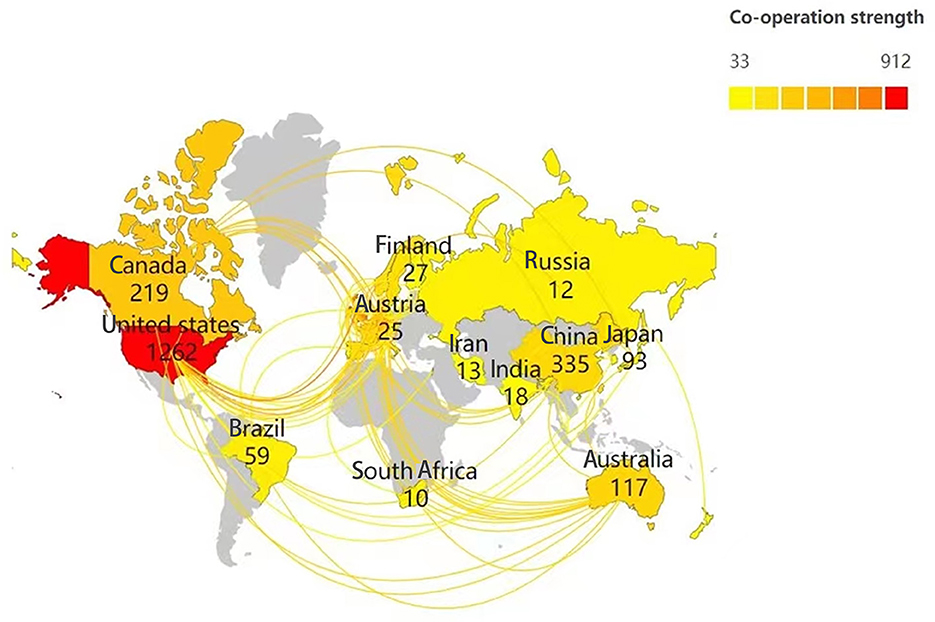
Scimago Graphica was used to analyze the cooperation of authors from various countries. As shown in Figure 3, retrieved articles on ADHD neuroimaging were from 65 countries including Asia, Africa, Europe, North America, and South America (with the greatest contribution).
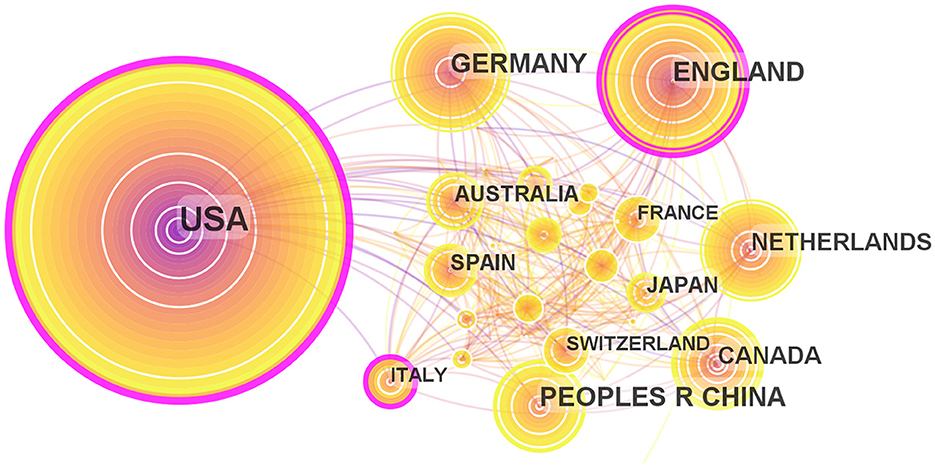
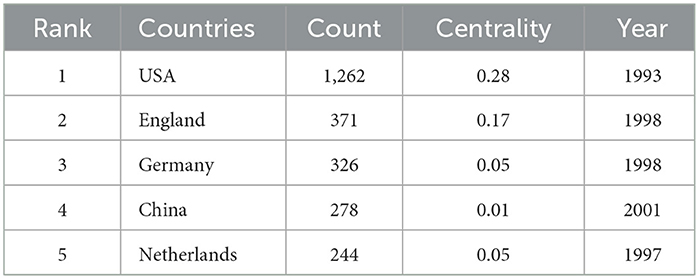
Maps drawn after the national visual analysis include 65 countries/regions and 326 links. As shown in Figure 4, these countries have greater centrality (>0.1) (purple rings). The top three countries with the greatest centrality are the United States (0.28), England (0.17), and Italy (0.16). These three countries are influential in the field of ADHD neuroimaging. The United States (n = 1,262, 48.15%), England (n = 371, 14.15%), and Germany (n = 326, 12.43%) published the most articles. The United States contributed almost half of all publications, with the first article published in 1993 which was earlier than any other country, showing great contribution in volume and importance. Table 1 quantifies the main findings. The top five countries (except the United States) published their first articles on ADHD neuroimaging around 1998.
Analysis of institutions
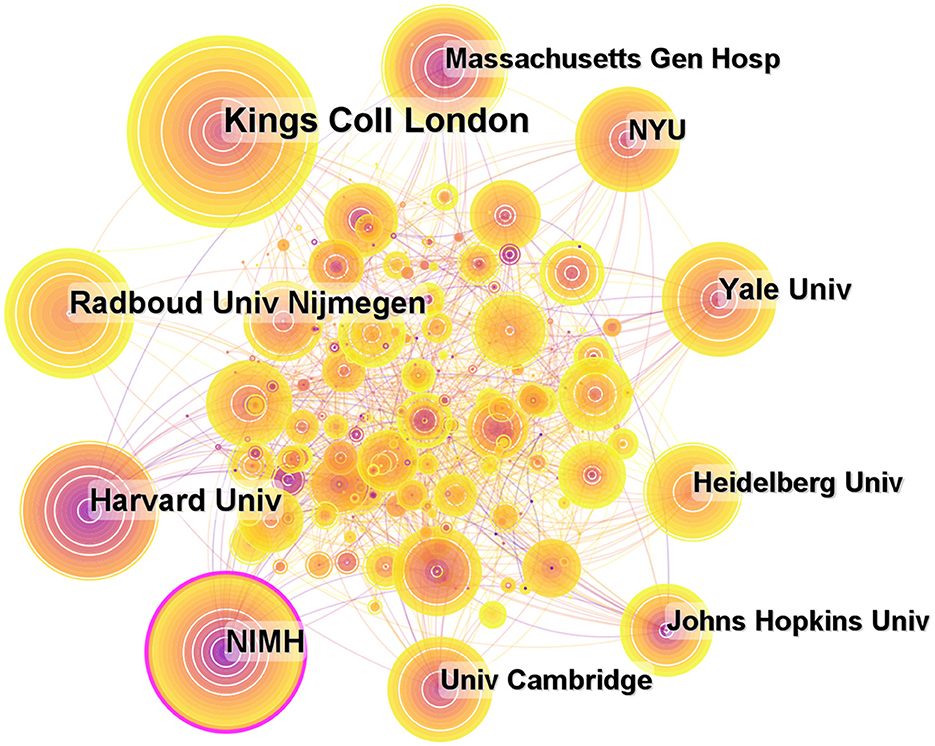
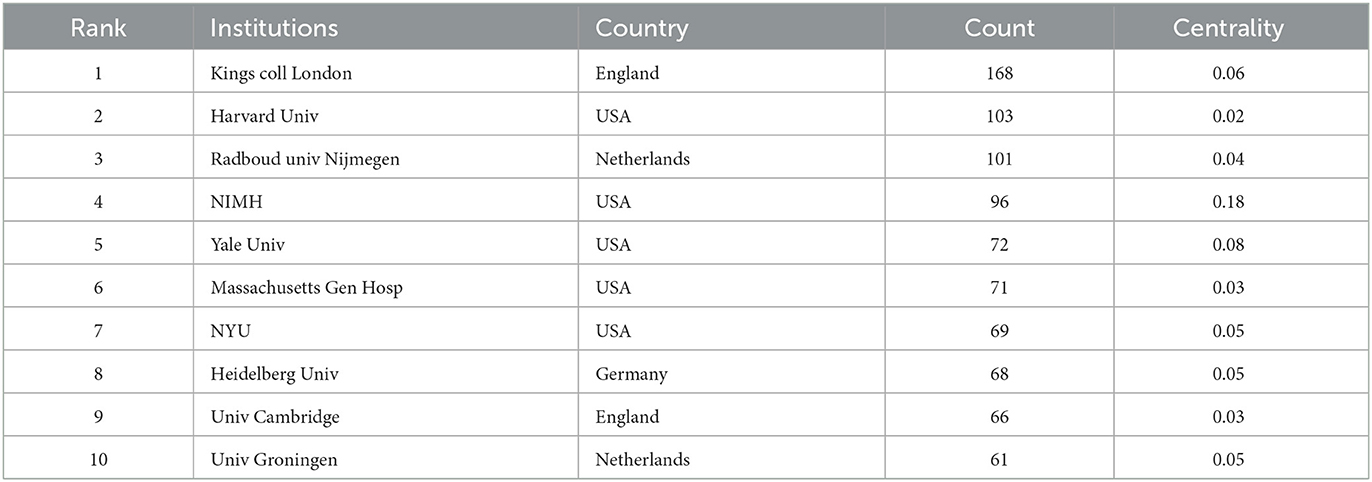
A total of 435 institutions contributed to publications on ADHD neuroimaging (Figure 5). The top 10 institutions contributed 875 articles (33.38%). Five American institutions are represented among the top 10 institutions: Kings College London (n = 168, 19.2%), Harvard University (n = 103, 11.8%), Radboud univ Nijmegen (n = 101, 11.5%), National Institute of Mental Health (n = 96, 10.8%), and Yale University (n = 72, 8.2%). It is worth noting that all these institutions are located in developed countries. The National Institute of Mental Health (NIMH) has a greater centrality (0.18). NIMH published the first article in 1993 about the child and adolescent psychopathology research (Table 2).
Authors and co-cited authors
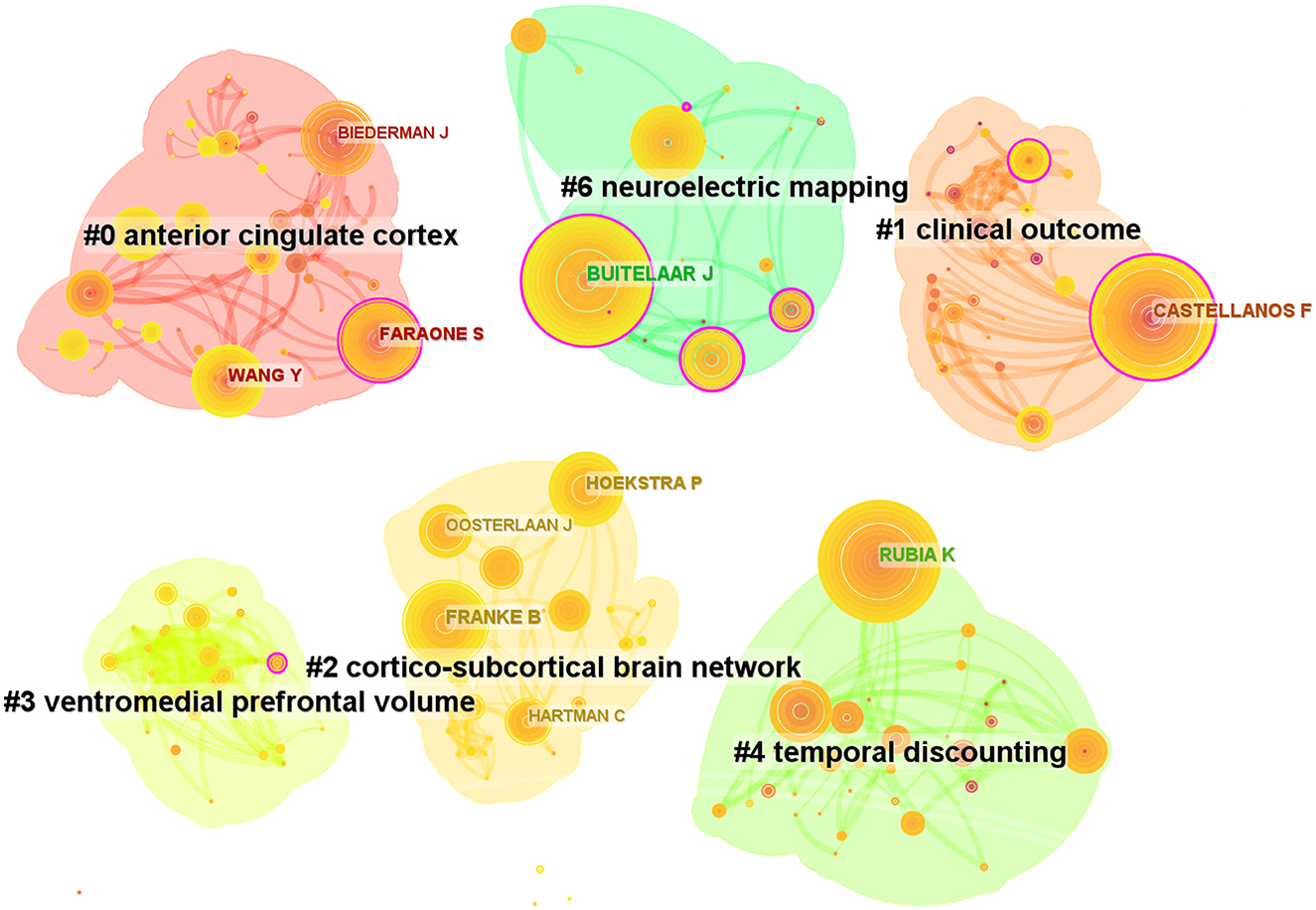
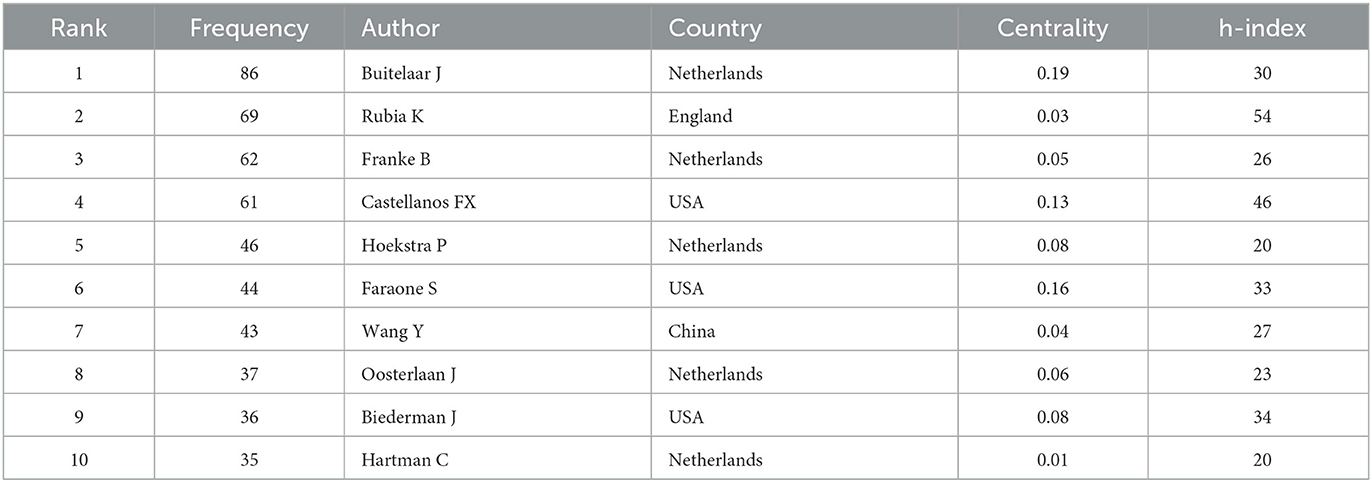
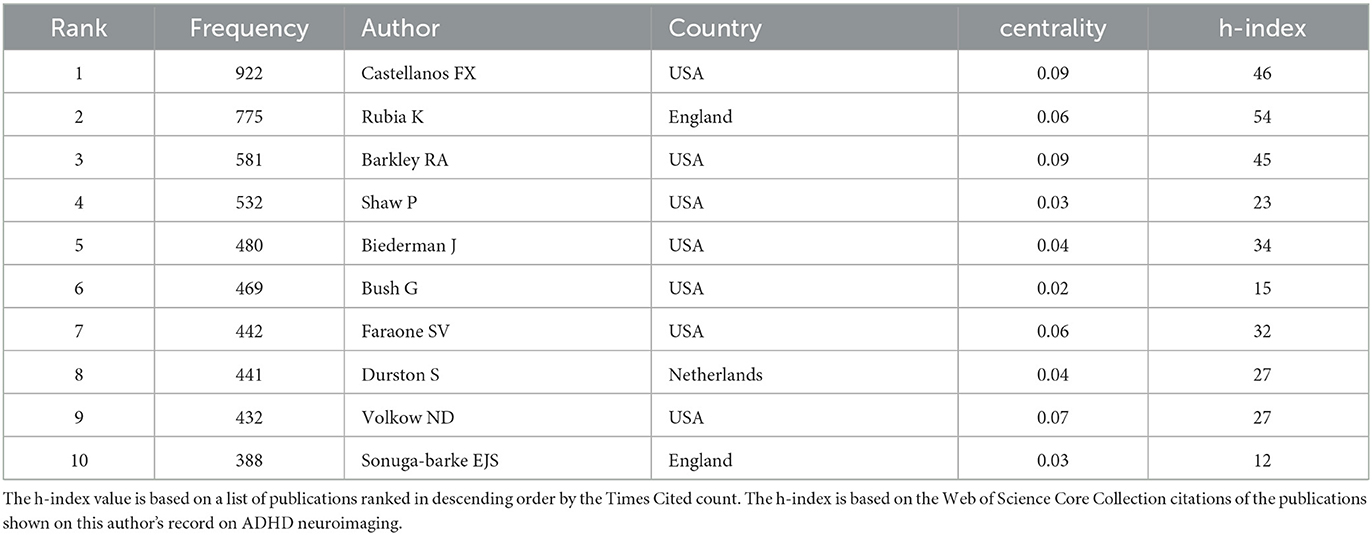
After analyzing the authors of these 2,621 articles, we obtained 634 nodes, 1,097 links, and six clusters (Figure 6). Among these authors, the top five authors were Buitelaar, J.K. (n = 86, 3%), Rubia, Katya (n = 69, 2.6%), Franke, Barbara (n = 62, 2.3%), Castellanos, Francisco Xavier (n = 61, 2.3%), and Hoekstra, Pieter J (n = 46, 1.7%; Table 3). Buitelaar, J.K. from Radboud University Nijmegen has the largest number of published papers, with an h-index of 112. Meanwhile, Buitelaar, J.K. has a greater centrality (0.19). His main research areas are Psychiatry, Neurosciences Neurology, and Psychology. He has published the most papers in collaboration with Franke, Barbara, who ranks third in the number of papers, and he is also from Radboud University Nijmegen. Their first collaboration could be backdated to 2011. From the cluster summary, the authors focused on the anterior cingulate cortex, clinical outcome, cortico–subcortical brain network, ventromedial prefrontal volume, temporal discounting, and neuroelectric mapping. The map of the authors' country is shown in Tables 3, 4. Castellanos FX (n = 922) had the highest citation counts, followed by Rubia K (n = 775), Barkley RA (n = 581), Shaw P (n = 532), and Biederman J (n = 480; Table 4).
Analysis of journals and cited journals
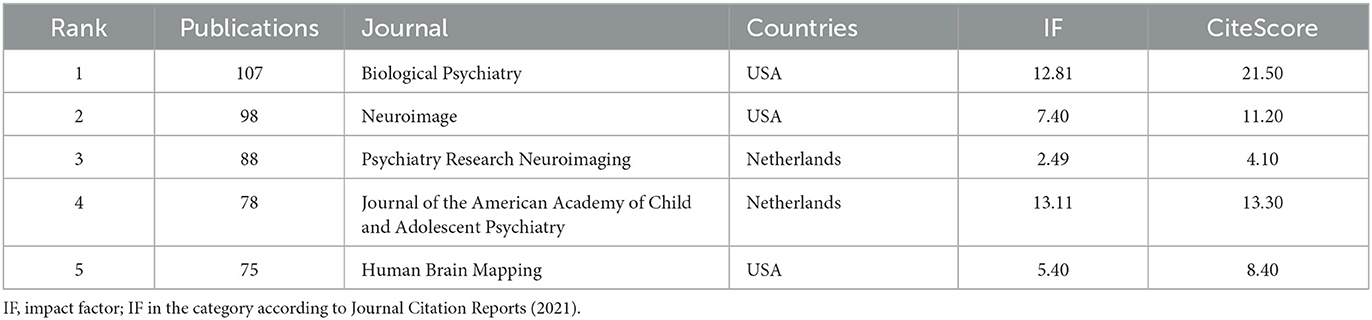
Journals publishing the most studies on ADHD neuroimaging are listed in Table 5 and are in the field of psychiatry and imaging. Biological Psychiatry published the most articles on ADHD neuroimaging, and the American Academy of Child and Adolescent Psychiatry occupied the highest impact factor.
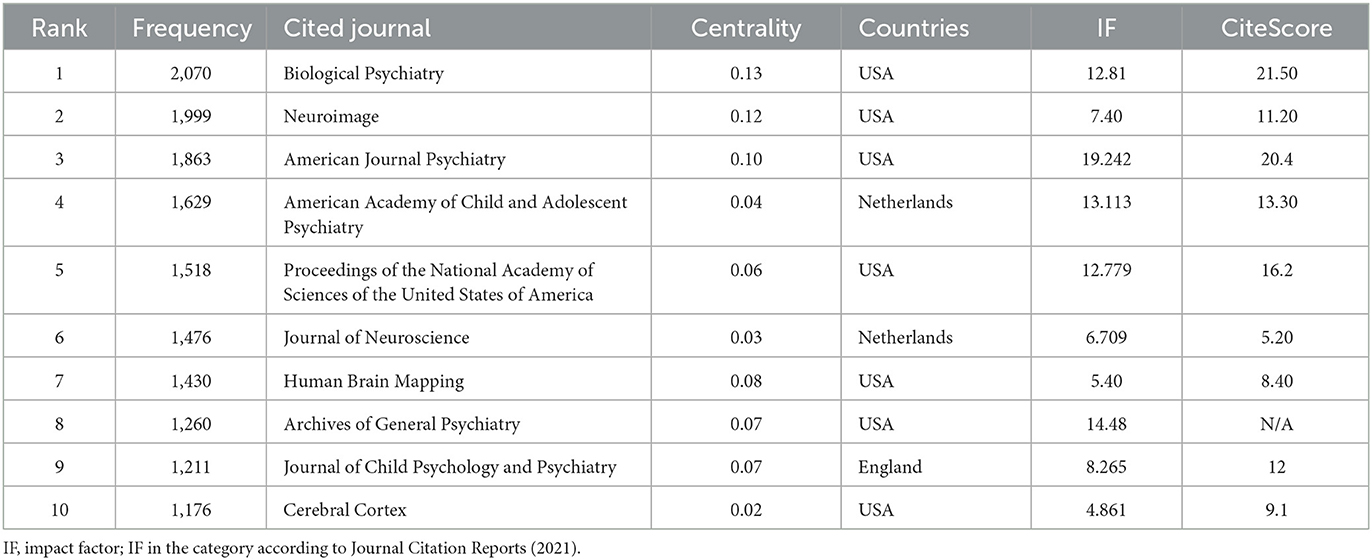
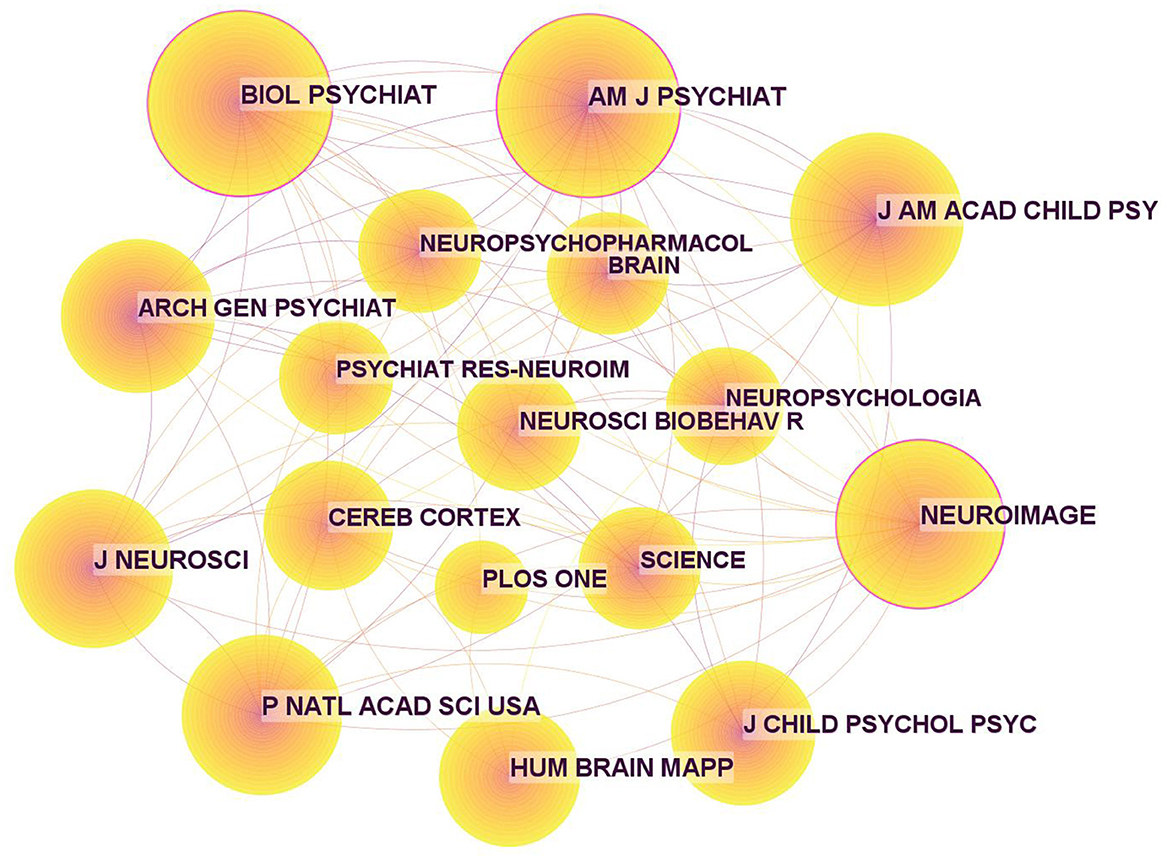
In addition, the journals on ADHD neuroimaging cited in these publications are shown in Table 6, and the corresponding map based on CiteSpace (Figure 7) generated 1,000 nodes and 5,948 links. Three nodes were marked with purple rings, indicating high centrality. The highest was 0.13 from Biological Psychiatry. The second highest centrality was Neuroimage with a centrality of 0.12. These two journals have a large number of publications and citations and play a pivotal role in ADHD neuroimaging.
Analysis of keywords
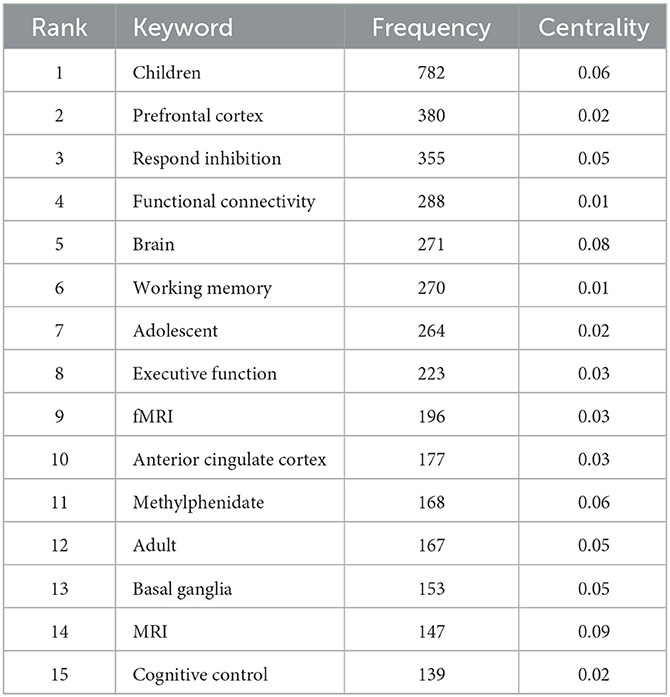
We used the frequency of certain keywords significantly increasing or even reaching the outbreak state within a given period of time as an indicator to analyze the new trend of cutting-edge topic activity (Chen and Chen, 2005). Based on the presence of these keywords, we generated an overall network graph (802 nodes and 4,229 links) to assess the current hottest topics. There were 8 keywords that occurred more than 200 times (Table 7).
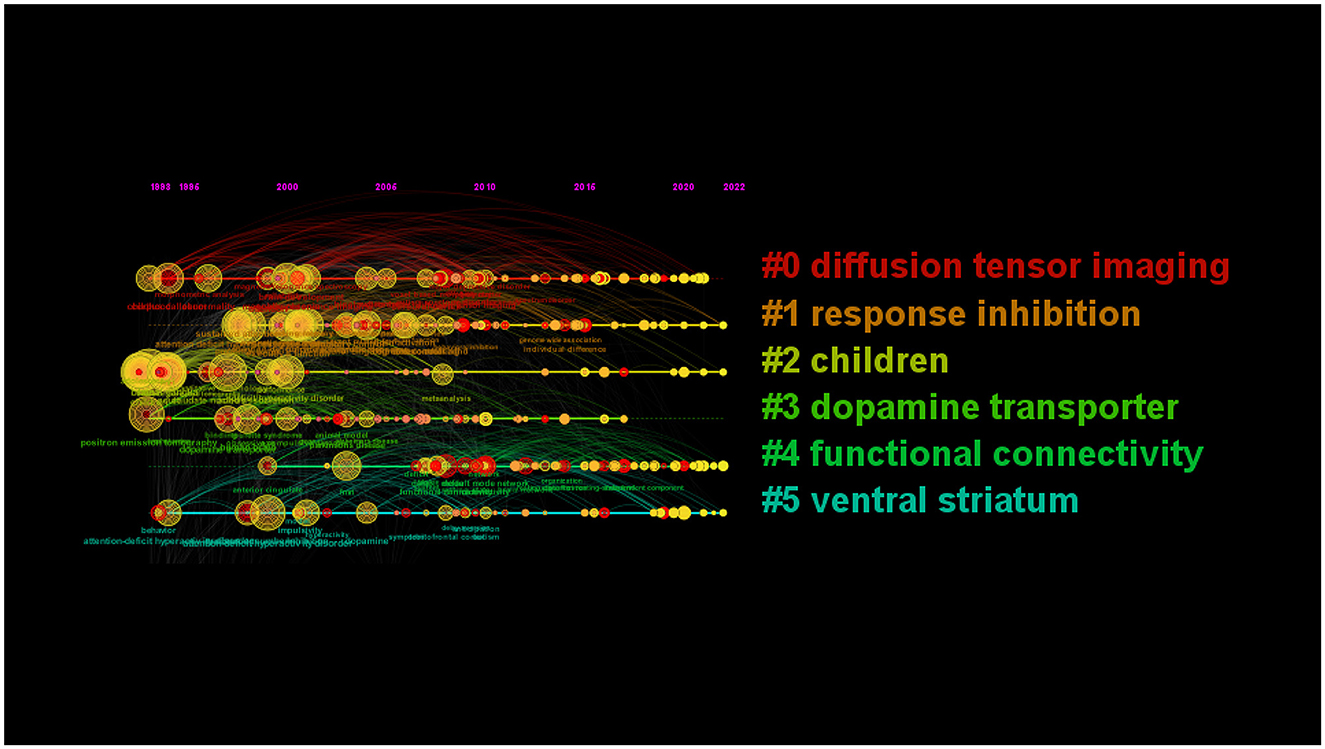
Nine clusters reflected topical trends, including “white matter,” “response inhibition,” “children,” “positron emission tomography,” “functional connectivity,” and “ventral striatum.” In the timeline view, keywords from one cluster are in the same horizontal line. The longer the time span is, the longer the cluster lasts, representing the rise, prosperity, and decline of research in different periods. The timeline view in Figure 8 shows the six clusters. Cluster#0 included “corpus callosum,” “white matter,” “voxel-based morphometry,” and so on; cluster#1 included “inhibitory control,” “prefrontal cortex,” “stroop task,” and so on; cluster#2 included “childhood onset,” “boy,” “child psychopathology,” and so on; cluster#4 included “resting state network,” “deep learning,” “default network,” and so on.
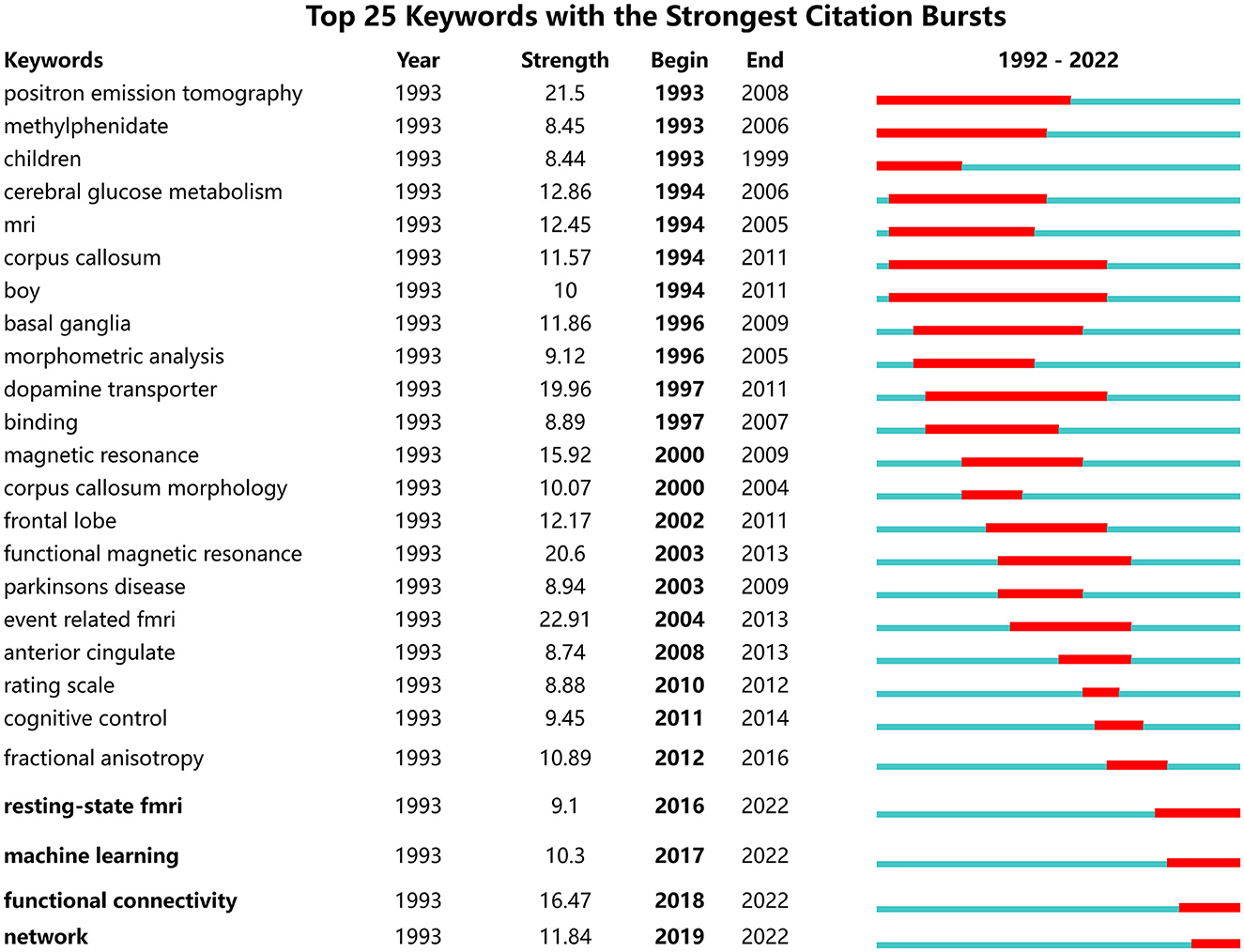
As shown in Figure 9, the green and red lines refer to 1992–2022 and the periods of each keyword, respectively. “Strength” indicates the emergence intensity of keywords. The ones with high outbreak intensity are: “event related fmri” (2004–2013, strength 22.84), “positron emission tomography” (1993–2008, strength 21.13), “functional magnetic resonance” (2003–2013, strength 20.55), “dopamine transporter” (1997–2011, strength 18.58), and “magnetic resonance” (2000–2009, strength 15.22).
Analysis of references
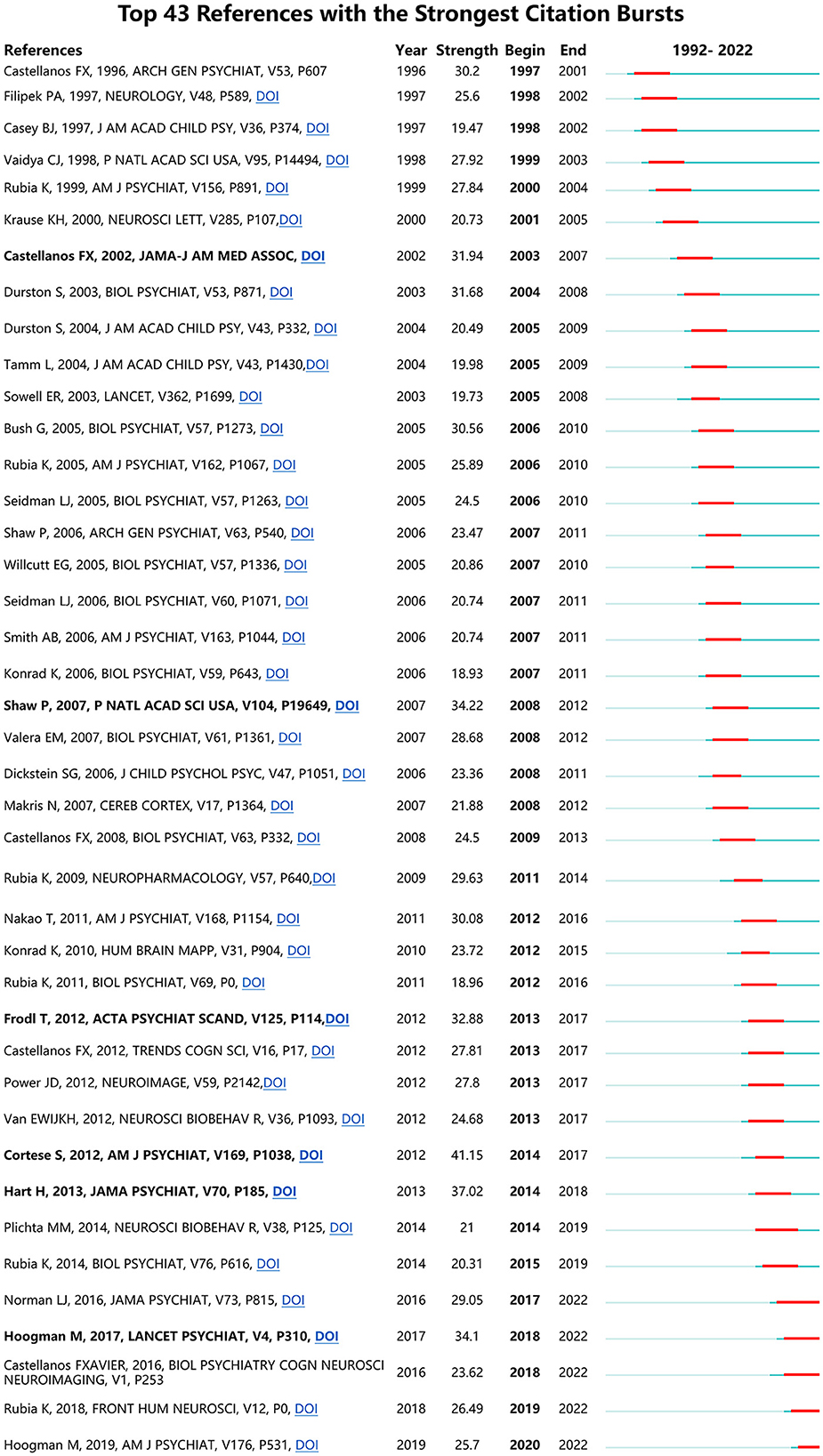
If a reference has the strongest citation bursts (CBs), it is deemed as a knowledge basis of the research frontiers (Fitzpatrick, 2005; Synnestvedt et al., 2005). Figure 10 shows ADHD neuroimaging references with the strongest CB from 1992 to 2022. CBs by the end of 2022 were led by Cortese et al. (2012) (41.15), followed by Shaw et al. (2007), Frodl and Skokauskas (2012), Hart et al. (2013), Hoogman et al. (2017), and Castellanos et al. (2002).
Discussion
The current study applied a visualized bibliometric method for analyzing the contributions and cooperations of authors, institutions, countries, journals, and emerging trends of neuroimaging in ADHD. From 1992 to 2022, the number of documents has shown a steady upward trend. A total of 2621 publications were collected from SCI-Expanded. All papers were published by 435 institutions from 65 countries/regions in 549 peer-reviewed journals.
In terms of the literature published by the country, the United States ranks first in both the number and centrality of literature, suggesting that the country plays a leading role, which is inextricably linked to the priority that the United States places on ADHD research. The National Institutes of Health (NIH)'s brain program, which was launched in the United States in 2013, has significantly increased funding and promoted ADHD neuroimaging research. The American periodical Biological Psychiatry (IF = 12.81) also has the most articles and citations. It is noteworthy that China, which ranks fourth in terms of the number of papers issued, is the only developing country in the top five. Research of the United Kingdom, Germany, and the Netherlands possess a central and significant position. Currently, most studies are conducted in developed countries such as Europe and the United States. Some scholars proposed that further research from less developed countries is needed to understand the interactions of neurobiological and socio-cultural elements in ADHD (Cortese and Coghill, 2018).
The number of papers published by King's College London ranked first accounting for 6.4% of the total number of studies. The Institute of Psychiatry, Psychology & Neuroscience (IoPPN) at King's College London is the premier for neurosciences research in Europe. Its cited publications about mental health were the most (the first 1%) (SciVal 2019). However, an analysis of these institutions showed that articles on ADHD were mostly published by research universities, including a small number of hospitals. ADHD will evolve toward precise clinical phenotypes in the next few years. Thus, relevant research units should establish cooperation with hospitals with accurate diagnostic capabilities as increasing clinicians publish literature on ADHD.
In this study, we learn more about the outstanding authors who contribute greatly to studies in this field. Author information can clearly identify the author's contribution to the field and establish potential partnerships. Buitelaar J, Rubia K, and Franke B contribute the greatest number of publications in the field of ADHD neuroimaging. Buitelaar J comes from the University of Bergen, and his research interests include ADHD, autism, and aggressiveness and impulsivity problems. Dr. Buitelaar takes an integrative strategy that includes clinical and phenotypic research, neuroimaging, cognitive research, genetics, pharmacology, and preclinical techniques. Rubia, K is a professor at King's College London, and his expertise is the pharmacological imaging of ADHD drugs and neurotransmitter manipulations, PET imaging in adults with ADHD, and so on. Franke B comes from Radboud University Nijmegen Medical Center. Dr. Franke B studies the influences of genetic factors on mental health problems and traits such as ADHD and focuses on understanding the biological mechanisms explaining the effects of such factors on the etiology of mental problems. In addition, we found that these three authors were members of the World Federation of ADHD and the ENIGMA-AHDH Working Group. Buitelaar J is the vice president of the World Federation of ADHD, while Franke B is a co-founder of ENIGMA. Through these organizations, researchers can strengthen communications and cooperation between different countries or institutions in this field. Scientific researchers including these three authors published the “The World Federation of ADHD International Consumption Statement” in 2021. Through an in-depth study of more than 2,000 participants, the authors came up with 208 specific descriptions of ADHD, which pointed out seven pieces of evidence-based evidence about the brain difference of ADHD (Faraone et al., 2021).
Keywords of “Children” and “prefrontal lobe” have the highest frequency and high centrality, which indicates that they are hot topics in research. ADHD is common in children whose brains are still developing, allowing scientists to conduct long-term longitudinal research on the brain structure of the whole life cycle. The prefrontal lobe is responsible for planning and organizing, as well as attention control, executive function, and other advanced processes. The prefrontal growth retardation in patients with ADHD leads to brain failure to effectively control behavioral activities, attention, and emotion. The development of fNIRS also promotes the research of the prefrontal lobe (Gossé et al., 2022).
In addition, the recent burst keywords are rs-fMRI, machine learning, functional connectivity, and network.
(1) The rs-fMRI can be used to investigate spontaneous brain neural activity during the rest state without requiring the preformation of any task (Anwar et al., 2016; Qian et al., 2018). Recently, different rs-fMRI computing approaches have been created based on functional segregation integration. ADHD has featured a lack of behavioral regulation, inattention, impulsivity, and hyperactivity. It might be difficult for participants with ADHD to complete task-fMRI scanning due to the symptom of hyperactivity. However, rs-fMRI does not require tasks, making it easier for participants with ADHD to comply and reducing the chance of head movement abnormalities. Thus, the rs-fMRI has been widely used in the mechanistic and clinical investigation of ADHD.
(2) Machine learning: at present, guardian interview and clinical observation are mainly used to determine whether a child suffers from ADHD, which may vary greatly because of regional divergence. If a single scale or indicator is used to diagnose or predict ADHD, the reliability will be insufficient. Therefore, we suggest that some data (such as sociodemographic information and neuroimaging) can be used to assist except the scale and relevant indicators. Increasing evidence shows that machine learning (ML) can be applied to neuroimaging data for the diagnosis and efficacy prediction of ADHD (Li et al., 2019). The largest ADHD-related dataset adopted by ML-based classifiers is ADHD-200 Global Competition, which includes 776 ADHD-afflicted youngsters ages 7–21. The results also showed that few studies focused on the classifiers for adults with ADHD and that most studies were carried out with small sample sizes (<100 patients) based on the datasets (McLeod et al., 2014; Uddin et al., 2017). In addition, due to the presence of several psychopathologies, it is more challenging to diagnose adults with ADHD than individuals in childhood. Neuroimaging should be used in the future to identify brain biomarkers in ADHD adults, objectively quantify brain structure and function, and develop and implement effective machine learning methods that will aid in objectively diagnosing, monitoring treatment responses, and predicting results (Uddin et al., 2017).
(3) Functional connectivity: ADHD is considered a neurodevelopmental disease with “brain disconnection,” including structural and functional connectivity disorder. Functional connectivity (FC) analyses are a common method of analyzing fMRI data (McLeod et al., 2014). rsFC technology has been proven to be able to measure the correlation of brain activity in the resting brain region. Thus, scholars have commonly used it to analyze the abnormalities and interaction of brain functional networks in ADHD. Dynamic functional connectivity (DFC) is an extension of the traditional resting-state functional connectivity analysis, which refers to the phenomenon that the observed functional connectivity changes in a short time (Fateh et al., 2022). At present, DFC is associated with a variety of neurological diseases and is considered to be an accurate representation of the brain's functional network. The study found that children with subtypes of ADHD exhibited obvious and broad aberrations in static FC and DFC among the resting-state networks, forming the cortical and subcortical areas compared to typically developing children (Shaw et al., 2007). Another study concluded that the etiology of ADHD could be better interpreted by the changes in brain FC (Hoogman et al., 2019). However, there are few articles on DFC related to ADHD.
(4) Network: In recent years, there has been increasing acceptance that ADHD is a distributed functional network disorder. Patients with ADHD suffer from different degrees of alternation in default mode network (DMN), frontoparietal network (FPN), ventral attention network (VAN), dorsal attention network (DAN), affective network (AN), somatosensory network (SSN), and visual network (Rubia, 2018). However, results vary in different research. Some literature found that DMN showed weaker FC in patients with ADHD than that in healthy controls (Fair et al., 2010; Choi et al., 2013; Sripada et al., 2014), while other literature provided almost opposite results (McCarthy et al., 2013; Barber et al., 2015). Comparing these results can be difficult due to differences in analytic approaches and variances in nomenclature and functional network boundaries. A meta-analysis found that the abnormal activity of fronto–striato–cerebeller networks in patients with ADHD was related to neuropsychological impairment (Hart et al., 2013). The multilayer network is a new type of network model that is increasingly being employed in neuroscience to portray the dynamic interaction between different functional systems.
References with the strongest CB published recently may help us to investigate the research hotspots in this field. Listed in Figure 8, (1) Cortese et al. (2012) discussed the task-based functional MRI studies of ADHD using a meta-analysis, which included 55 studies (39 in children and 16 in adults). This study found that children with ADHD showed significant hyperactivation within the default and ventilatory networks compared with typically developing children. In adults, ADHD mainly is caused by deficits in visual, dorsal attention, and default networks. This article provided evidence of cognitive, sensorimotor, and other neurological disorders in patients with ADHD. At the same time, the study found that the early model of ADHD was concerned about the prefrontal–striatal circuits. (2) Hart et al. (2013) conducted a meta-analysis to analyze the fMRI images of participants with ADHD, aiming to explore the relationship between age and long-term stimulant medication use effect. The study found that participants with ADHD exhibited weaker ability in cognization in distinct fronto-basal ganglia-thalamic networks when they were required to complete some inhibition and attention-related actions. (3) Shaw et al. (2007) used computational neuroanatomic techniques to estimate the cortex thickness from 824 MRI images of 223 children with ADHD and 223 controls. This study found that ADHD was exhibited as a delayed cortical maturation. Furthermore, this delay was especially noticeable in prefrontal areas that controlled cognitive functions such as attention and motor planning.
The latest valuable cited literature provides valuable information for future research directions. Hoogman et al. (2019) used extensive research to pinpoint brain traits associated with ADHD and found that children with ADHD had widespread minor abnormalities in cortical surface area. Despite the presence of ADHD diagnosis, future longitudinal research should clarify individual lifespan trajectories that result in non-significant findings in adolescent and adult groups. Rubia (2018) focused on the fMRI-based cognitive neuroscience of ADHD and recent clinically relevant applications such as fMRI-based diagnostic classification or neuromodulation therapies, aiming to correct fMRI deficits with neurofeedback (NF) or brain stimulation.
Limitation
The current research is subjected to some limitations. First, only articles from the WoS and English articles were included, which might lead to bias in language and publication. Second, some studies unpublished or published newly were not included in this study. Third, the number of citations and centrality of the articles might vary depending on the particular time when the search was conducted. However, it is certainly believed that it has clarified the overall condition, emerging trends, and future directions of studies on ADHD, showing high reference values and providing some valuable suggestions for further study.
Conclusion
In conclusion, this study revealed the status of relevant studies, hot topics, and trends concerning neuroimaging in ADHD from 1992 to 2022. During the past three decades, the number of relevant articles has increased significantly, which suggests that neuroimaging in ADHD has a positive development prospect. Most ADHD neuroimaging articles are from developed countries, and the authors are encouraged to develop close cooperation with others from various institutions and countries. Notably, “resting-state Fmri,” “machine learning,” “functional connectivity,” and “network” may be the hot topics in this field. With the rapid development and maturation of brain imaging technology, scholars could conduct more relevant research about the diagnosis and prognosis of ADHD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JW and NS contributed to conceiving, designing, and revising the manuscript. HD and ZH contributed to the analysis and manuscript writing. ZL, LC, and YH contributed to data collection. JW and YZ contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
Acknowledgments
This study was supported by the Guiding Project of the Fujian Provincial Department of Science and Technology, China (Grant no. 2022Y0038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albajara Sáenz, A., Villemonteix, T., and Massat, I. (2019). Structural and functional neuroimaging in attention-deficit/hyperactivity disorder. Dev. Med. Child Neurol. 61, 399–405. doi: 10.1111/dmcn.14050
Almeida, L. G., Ricardo-Garcell, J., Prado, H., Barajas, L., Fernández-Bouzas, A., Ávila, D, and Martínez, R. B. (2010). Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. J. Psychiatr. Res. 44, 1214–1223. doi: 10.1016/j.jpsychires.2010.04.026
Ambrosino, S., de Zeeuw, P., Wierenga, L. M., van Dijk, S., and Durston, S. (2017). What can cortical development in attention-deficit/hyperactivity disorder teach us about the early developmental mechanisms involved? Cereb. Cortex 27, 4624–4634. doi: 10.1093/cercor/bhx182
American Psychiatric Association (ed.), . (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th Edn. Washington, DC: American Psychiatric Association.
Anwar, A. R., Hashmy, M. Y., Imran, B., Riaz, M. H., Mehdi, S. M. M., Muthalib, M., et al. (2016). “Complex network analysis of resting-state fMRI of the brain,” in 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (Orlando, FL), 3598–3601. doi: 10.1109/EMBC.2016.7591506
Aoki, Y., Cortese, S., and Castellanos, F. X. (2018). Research Review: Diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta-analyses and reflections on head motion. J. Child Psychol. Psychiatr. 59, 193–202. doi: 10.1111/jcpp.12778
Barber, A. D., Jacobson, L. A., Wexler, J. L., Nebel, M. B., Caffo, B. S., Pekar, J. J., et al. (2015). Connectivity supporting attention in children with attention deficit hyperactivity disorder. NeuroImage. Clin. 7, 68–81. doi: 10.1016/j.nicl.2014.11.011
Campbell, S. B., Halperin, J. M., and Sonuga-Barke, E. J. S. (2014). “A developmental perspective on attention-deficit/hyperactivity disorder (ADHD),” in Handbook of Developmental Psychopathology, eds M. Lewis and K. D. Rudolph (Boston, MA: Springer), 427–448. doi: 10.1007/978-1-4614-9608-3_22
Castellanos, F. X., Lee, P. P., Sharp, W., Jeffries, N. O., Greenstein, D. K., Clasen, L. S., et al. (2002). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 288, 1740–1748. doi: 10.1001/jama.288.14.1740
Chen, C., and Chen, Y. (2005). “Searching for clinical evidence in CiteSpace,” in Annual Symposium proceedings. AMIA Symposium, Vol. 2005, 121–125.
Choi, J., Jeong, B., Lee, S. W., and Go, H. -J. (2013). Aberrant development of functional connectivity among resting state-related functional networks in medication-na?ve ADHD children. PLoS ONE. 8, e83516. doi: 10.1371/journal.pone.0083516
Cortese, S., Aoki, Y. Y., Itahashi, T., Castellanos, F. X., and Eickhoff, S. B. (2021). Systematic review and meta-analysis: resting-state functional magnetic resonance imaging studies of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 60, 61–75. doi: 10.1016/j.jaac.2020.08.014
Cortese, S., and Coghill, D. (2018). Twenty years of research on attention-deficit/hyperactivity disorder (ADHD): looking back, looking forward. Evid. Based Mental Health 21, 173–176. doi: 10.1136/ebmental-2018-300050
Cortese, S., Kelly, C., Chabernaud, C., Proal, E., Di Martino, A., Milham, M. P., et al. (2012). Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI Studies. Am. J. Psychiatry 169, 1038–1055. doi: 10.1176/appi.ajp.2012.11101521
Dubey, D. R., O'Leary, S. G., and Kaufman, K. F. (1983). Training parents of hyperactive children in child management: a comparative outcome study. J. Abnorm. Child Psychol. 11, 229–245. doi: 10.1007/BF00912088
Fair, D. A., Posner, J., Nagel, B. J., Bathula, D., Dias, T. G. C., Mills, K. L., et al. (2010). A typical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol. Psychiatr. 68, 1084-1091. doi: 10.1016/j.biopsych.2010.07.003
Faraone, S. V., Banaschewski, T., Coghill, D., Zheng, Y., Biederman, J., Bellgrove, M. A., et al. (2021). The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 128, 789–818. doi: 10.1016/j.neubiorev.2021.01.022
Fateh, A. A., Huang, W., Mo, T., Wang, X., Luo, Y., Yang, B., et al. (2022). Abnormal insular dynamic functional connectivity and its relation to social dysfunctioning in children with attention deficit/hyperactivity disorder. Front. Neurosci. 16, 890596. doi: 10.3389/fnins.2022.890596
Fitzpatrick, R. B. (2005). Essential science indicatorsSM. Med. Ref. Serv. Q. 24, 67–78. doi: 10.1300/J115v24n04_05
Franke, B., Michelini, G., Asherson, P., Banaschewski, T., Bilbow, A., Buitelaar, J. K., et al. (2018). Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 28, 1059–1088. doi: 10.1016/j.euroneuro.2018.08.001
Frodl, T., and Skokauskas, N. (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta psychiatrica Scandinavica. 125, 114–126. doi: 10.1111/j.1600-0447.2011.01786.x
Fuelscher, I., Hyde, C., Anderson, V., and Silk, T. J. (2021). White matter tract signatures of fiber density and morphology in ADHD. Cortex 138:329–340. doi: 10.1016/j.cortex.2021.02.015
Fusar-Poli, P., Rubia, K., Rossi, G., Sartori, G., and Balottin, U. (2012). Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am. J. Psychiatry 169, 264–272. doi: 10.1176/appi.ajp.2011.11060940
Gossé, L. K., Bell, S. W., and Hosseini, S. M. H. (2022). Functional near-infrared spectroscopy in developmental psychiatry: a review of attention deficit hyperactivity disorder. Eur. Arch. Psychiatry Clin. Neurosci. 272, 273–290. doi: 10.1007/s00406-021-01288-2
Gualtieri, C. T., and Hicks, R. E. (1985). Neuropharmacology of methylphenidate and a neural substrate for childhood hyperactivity. Psychiatr. Clin N. Am. 8, 875–892.
Hart, H., Radua, J., Nakao, T., Mataix-Cols, D., and Rubia, K. (2013). Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 70, 185. doi: 10.1001/jamapsychiatry.2013.277
Hoogman, M., Bralten, J., Hibar, D. P., Mennes, M., Zwiers, M. P., Schweren, L. S. J., et al. (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 4, 310–319. doi: 10.1016/S2215-0366(17)30049-4
Hoogman, M., Muetzel, R., Guimaraes, J. P., Shumskaya, E., Mennes, M., Zwiers, M. P., et al. (2019). Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am. J. Psychiatry 176, 531–542. doi: 10.1176/appi.ajp.2019.18091033
Hwang, S., Meffert, H., Parsley, I., Tyler, P. M., Erway, A. K., Botkin, M. L., et al. (2019). Segregating sustained attention from response inhibition in ADHD: an fMRI study. Neuroimage Clin. 21, 101677. doi: 10.1016/j.nicl.2019.101677
Kohl, S. H., Mehler, D. M. A., Lührs, M., Thibault, R. T., Konrad, K., and Sorger, B. (2020). The potential of functional near-infrared spectroscopy-based neurofeedback—a systematic review and recommendations for best practice. Front. Neurosci. 14, 594. doi: 10.3389/fnins.2020.00594
Kolodny, T., Mevorach, C., Stern, P., Ankaoua, M., Dankner, Y., Tsafrir, S., et al. (2022). Are attention and cognitive control altered by fMRI scanner environment? Evidence from Go/No-go tasks in ADHD. Brain Imaging Behav. 16, 1003–1013. doi: 10.1007/s11682-021-00557-x
Kolodny, T., Mevorach, C., Stern, P., Biderman, N., Ankaoua, M., Tsafrir, S., et al. (2020). Fronto-parietal engagement in response inhibition is inversely scaled with attention-deficit/hyperactivity disorder symptom severity. Neuroimage Clin. 25, 102119. doi: 10.1016/j.nicl.2019.102119
Lei, D., Du, M., Wu, M., Chen, T., Huang, X., Du, X., et al. (2015). Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology 29, 874–881. doi: 10.1037/neu0000200
Li, X., Guo, N., and Li, Q. (2019). Functional neuroimaging in the new era of big data. Genomics Proteomics Bioinform. 17, 393–401. doi: 10.1016/j.gpb.2018.11.005
Massat, I., Slama, H., Villemonteix, T., Mary, A., Baijot, S., Albajara Sáenz, A., et al. (2018). Hyperactivity in motor response inhibition networks in unmedicated children with attention deficit-hyperactivity disorder. World J. Biol. Psychiatry 19, 101–111. doi: 10.1080/15622975.2016.1237040
McCarthy, H., Skokauskas, N., Mulligan, A., Donohoe, G., Mullins, D., Kelly, J., et al. (2013). Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatr. (2013) 70, 1329–1337. doi: 10.1001/jamapsychiatry.2013.2174
McLeod, K. R., Langevin, L. M., Goodyear, B. G., and Dewey, D. (2014). Functional connectivity of neural motor networks is disrupted in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. Neuroimage Clin. 4, 566–575. doi: 10.1016/j.nicl.2014.03.010
Nakao, T., Radua, J., Rubia, K., and Mataix-Cols, D. (2011). Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry 168, 1154–1163. doi: 10.1176/appi.ajp.2011.11020281
Narr, K. L., Woods, R. P., Lin, J., Kim, J., Phillips, O. R., Del'Homme, M., et al. (2009). Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 48, 1014–1022. doi: 10.1097/CHI.0b013e3181b395c0
Perlov, E., Philipsen, A., Matthies, S., Drieling, T., Maier, S., Bubl, E., et al. (2009). Spectroscopic findings in attention-deficit/hyperactivity disorder: review and meta-analysis. World J. Biol. Psychiatry 10, 355–365. doi: 10.1080/15622970802176032
Plichta, M. M., and Scheres, A. (2014). Ventral–striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 38, 125–134. doi: 10.1016/j.neubiorev.2013.07.012
Qian, L., Zheng, L., Shang, Y., Zhang, Y., and Zhang, Y. (2018). Intrinsic frequency specific brain networks for identification of MCI individuals using resting-state fMRI. Neurosci. Lett. 664, 7–14. doi: 10.1016/j.neulet.2017.10.052
Roldan-Valadez, E., Salazar-Ruiz, S. Y., Ibarra-Contreras, R., and Rios, C. (2019). Current concepts on bibliometrics: a brief review about impact factor, Eigenfactor score, CiteScore, SCImago Journal Rank, Source-Normalised Impact per Paper, H-index, and alternative metrics. Ir. J. Med. Sci. 188, 939–951. doi: 10.1007/s11845-018-1936-5
Rubia, K. (2018). Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front. Hum. Neurosci. 12, 100. doi: 10.3389/fnhum.2018.00100
Sayal, K., Prasad, V., Daley, D., Ford, T., and Coghill, D. (2018). ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry 5, 175–186. doi: 10.1016/S2215-0366(17)30167-0
Shaw, P., Eckstrand, K., Sharp, W., Blumenthal, J., Lerch, J. P., Greenstein, D., et al. (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U.S.A. 104, 19649–19654. doi: 10.1073/pnas.0707741104
Silk, T. J., Beare, R., Malpas, C., Adamson, C., Vilgis, V., Vance, A., et al. (2016). Cortical morphometry in attention deficit/hyperactivity disorder: contribution of thickness and surface area to volume. Cortex 82, 1–10. doi: 10.1016/j.cortex.2016.05.012
Sripada, C., Kessler, D., Fang, Y., Welsh, R. C., Prem Kumar, K., and Angstadt, M. (2014). Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder: Disrupted Network Architecture in ADHD. Hum. Brain. Mapp. 35, 4693–4705. doi: 10.1002/hbm.22504
Synnestvedt, M. B., Chen, C., and Holmes, J. H. (2005). “CiteSpace II: visualization and knowledge discovery in bibliographic databases,” in AMIA Annual Symposium proceedings. AMIA Symposium, Vol. 2005, 724–728.
Uddin, L. Q., Dajani, D. R., Voorhies, W., Bednarz, H., and Kana, R. K. (2017). Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl. Psychiatry 7, e1218–e1218. doi: 10.1038/tp.2017.164
Wolosin, S. M., Richardson, M. E., Hennessey, J. G., Denckla, M. B., and Mostofsky, S. H. (2009). Abnormal cerebral cortex structure in children with ADHD. Hum. Brain Mapp. 30, 175–184. doi: 10.1002/hbm.20496
Keywords: attention-deficit hyperactivity disorder, neuroimaging, bibliometric, web of science, visualization
Citation: Deng H, Huang Z, Li Z, Cao L, He Y, Sun N, Zeng Y and Wu J (2023) Systematic bibliometric and visualized analysis of research hotspots and trends in attention-deficit hyperactivity disorder neuroimaging. Front. Neurosci. 17:1098526. doi: 10.3389/fnins.2023.1098526
Received: 15 December 2022; Accepted: 17 February 2023;
Published: 28 March 2023.
Edited by:
Nader Perroud, University of Geneva, SwitzerlandReviewed by:
Jie Wang, Innovation Academy for Precision Measurement Science and Technology (CAS), ChinaLi Nuo, Nanhai Maternity and Children's Hospital Affiliated to Guangzhou University of Traditional Chinese Medicine, China
Copyright © 2023 Deng, Huang, Li, Cao, He, Sun, Zeng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingsong Wu, jingsongwu01@163.com
 Haiyin Deng
Haiyin Deng Zhenming Huang
Zhenming Huang Zhaoying Li1
Zhaoying Li1 Youze He
Youze He Ning Sun
Ning Sun Jingsong Wu
Jingsong Wu