
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 27 February 2023
Sec. Translational Neuroscience
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1085082
This article is part of the Research Topic Infection-Triggered Encephalopathy Syndromes as Emerging Pediatric Neuroinflammatory Diseases View all 8 articles
 Hiroshi Sakuma1*†
Hiroshi Sakuma1*† Jun-ichi Takanashi2†
Jun-ichi Takanashi2† Kazuhiro Muramatsu3
Kazuhiro Muramatsu3 Hidehito Kondo4
Hidehito Kondo4 Takashi Shiihara5
Takashi Shiihara5 Motomasa Suzuki6
Motomasa Suzuki6 Kazuo Okanari7
Kazuo Okanari7 Mariko Kasai8
Mariko Kasai8 Osamu Mitani9
Osamu Mitani9 Tomoyuki Nakazawa10
Tomoyuki Nakazawa10 Taku Omata11
Taku Omata11 Konomi Shimoda12
Konomi Shimoda12 Yuichi Abe13
Yuichi Abe13 Yoshihiro Maegaki14
Yoshihiro Maegaki14 Kei Murayama15
Kei Murayama15 Yuka Murofushi2
Yuka Murofushi2 Hiroaki Nagase16
Hiroaki Nagase16 Akihisa Okumura17
Akihisa Okumura17 Yasunari Sakai18
Yasunari Sakai18 Hiroko Tada1,19
Hiroko Tada1,19 Masashi Mizuguchi20 Japanese Pediatric Neuro-COVID-19 Study Group
Masashi Mizuguchi20 Japanese Pediatric Neuro-COVID-19 Study GroupBackground and objectives: To clarify whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection cause acute encephalopathy in children and which are the most common syndromes that cause them and what are the outcomes.
Methods: A nationwide web-based survey among all members of the Japanese Society of Child Neurology to identify pediatric patients aged < 18 years who developed acute encephalopathy in Japan between 1 January 2020 and 31 May 2022 associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection confirmed by polymerase chain reaction or antigen tests using pharyngeal swabs. Acute encephalopathy was defined as acute onset of impaired consciousness lasting > 24 h or an altered mental state; neurological symptoms arising within 2 weeks of onset of COVID-19 or multisystem inflammatory syndrome in children (MIS-C)/pediatric inflammatory multisystem syndrome (PIMS); evidence of SARS-CoV-2 infection; and reasonable exclusion of other diseases. Patients were divided into the known clinico-radiological acute encephalopathy syndrome group and unexplained or unclassifiable acute encephalopathy group. Outcomes were assessed by pediatric cerebral performance category (PCPC) score at hospital discharge.
Results: Of the 3,802 society members, 217 representing institutions responded, and 39 patients with suspected acute encephalopathy were reported, of which 31 met inclusion criteria. Of these patients, 14 were diagnosed with known clinico-radiological acute encephalopathy syndromes, with acute encephalopathy with biphasic seizures and late reduced diffusion (five patients) being the most common. Five developed acute encephalopathy associated with MIS-C/PIMS. Among 31 patients, 9 (29.0%) had severe sequelae or died (PCPC ≥ 4). Two of three patients with encephalopathy with acute fulminant cerebral edema and two with hemorrhagic shock and encephalopathy syndrome died. The PCPC scores were higher in the known clinico-radiological acute encephalopathy syndrome group than in the unexplained or unclassifiable acute encephalopathy group (P < 0.01).
Discussion: Acute encephalopathy related to SARS-CoV-2 infection was demonstrated to be more severe than that caused by other viruses in Japan. Acute encephalopathy syndromes characterized by specific neuroradiological findings was associated with poor clinical outcomes.
At present, the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains prevalent. Although the primary site of infection of SARS-CoV-2 is the respiratory system, with no evidence of direct central nervous system invasion, various neurological complications of COVID-19 have been reported (Ellul et al., 2020; Iadecola et al., 2020). Acute encephalopathy is known as a neurological complication of COVID-19, in addition to cerebrovascular disease, encephalitis, meningitis, anosmia/ageusia, and Guillain–Barré syndrome (Ellul et al., 2020; Iadecola et al., 2020). A study has reported that one of the most prevalent neurological syndromes caused by SARS-CoV-2 is acute encephalopathy, which was identified in 1,845 of 3,740 adult patients (49%) hospitalized with COVID-19 (Chou et al., 2021).
Acute encephalopathy triggered by infectious diseases has been widely reported, mainly in children, and most of these cases occur with common viral infections such as influenza virus and human herpes virus-6 (Mizuguchi et al., 2007). Patients present with various degrees of consciousness disorders soon after infection, which may occasionally be accompanied by seizures. Many cases have been reported from Japan and other East Asian countries, and Japanese children may be more susceptible to acute encephalopathy associated with viral infections (Hoshino et al., 2012; Kasai et al., 2020).
Therefore, we conducted a nationwide survey in Japan to determine the current status of pediatric acute encephalopathy associated with COVID-19. Herein, we report on the high incidence of severe, occasionally fatal, acute encephalopathy syndromes associated with COVID-19 in Japanese children.
Children aged < 18 years who developed COVID-19-associated acute encephalopathy between 1 January 2020 and 31 May 2022 were included in the study. Acute encephalopathy was defined as a condition meeting all of the following four diagnostic criteria:
(1) acute onset of impaired consciousness (Glasgow Coma Scale < 11 or Japan Coma Scale > 20, lasting > 24 h) or altered mental state such as abnormal speech, behavior, or personality change (Mizuguchi et al., 2021)
(2) neurological symptoms arising in the acute phase (within 2 weeks from onset) of COVID-19 or COVID-19-associated multisystem inflammatory syndrome in children (MIS-C)/pediatric inflammatory multisystem syndrome (PIMS) (Jiang et al., 2020; Whittaker et al., 2020)
(3) SARS-CoV-2 infection confirmed by PCR or antigen tests using pharyngeal swabs; and
(4) reasonable exclusion of other diseases.
We conducted a web-based survey from June 7 to 30, 2022 among all members of the Japanese Society of Child Neurology. The collected data included age of onset, sex, provisional clinical diagnosis, vaccination history against SARS-CoV-2, and outcomes, which were provided by pediatric neurologists at the survey.
We defined five clinico-radiological acute encephalopathy syndromes associated with viral infection: acute encephalopathy with biphasic seizures and late reduced diffusion (AESD); encephalopathy with acute fulminant cerebral edema; acute necrotizing encephalopathy (ANE); hemorrhagic shock and encephalopathy syndrome (HSES); and mild encephalitis/ encephalopathy with a reversible splenial lesion (MERS) as shown in Supplementary Table 1 (Levin et al., 1983; Krishnan et al., 2021; Mizuguchi et al., 2021). We excluded encephalopathy secondary to apparent hypoxia or cerebrovascular disease, metabolic or toxic encephalopathy, acquired demyelinating syndromes, and autoimmune encephalitis. We also excluded patients with underlying genetic abnormalities that may affect the development of encephalopathy. Clinical variables were compared between the known clinico-radiological acute encephalopathy syndrome group and the unexplained or unclassifiable acute encephalopathy group. The severity of respiratory impairment was classified as mild, moderate-1, moderate-2, or severe according to the Japanese Ministry of Health, Labor and Welfare criteria (Supplementary Table 2; Kato, 2021). MRIs were mostly performed a few days after the onset because there is a delay between the onset of disease and the appearance of characteristic MRI findings in AESD and MERS Outcomes were assessed by pediatric cerebral performance category (PCPC) score (Zaritsky et al., 1995) at hospital discharge. If a patient had underlying disability and recovered to baseline status after the illness, the PCPC score was rated 1.
Some patient details have been submitted for publication as case reports by their treating physicians (Nishimura et al., 2022; Saito et al., 2022).
The Mann–Whitney test was used to determine statistical significance for discrete variables, and the chi square test or the Fisher exact test was used for binary variables. Significance was set at P < 0.05. Statistical analyses were performed using the R software, version 4.2.1 (R Project for Statistical Computing).
The study was approved by the Institutional Review Board of Tokyo Metropolitan Institute of Medical Science (#20–28). Informed consent for the presentation of neuroimages was obtained from all the patients or their guardians.
Altogether, 3,802 members of the Japanese Society of Child Neurology were surveyed, and 217 responded (response rate: 5.7%) (Figure 1). After excluding duplicates, response was obtained from 201 medical institutions. Of the respondents, 26 (12.0%) reported 39 cases of suspected acute encephalopathy. Six of these cases were excluded because they did not meet the criteria for acute encephalopathy: one because the duration of consciousness impairment was < 24 h, two because the time between infection and the appearance of neurological symptoms exceeded 14 days, and three because underlying chromosomal or genetic abnormalities [1p36 deletion syndrome, SCN8A encephalopathy, and MYRF variant with recurrent MERS (Saito et al., 2022)]. Hence, 33 patients fulfilled the definition of acute encephalopathy. One patient diagnosed with another immune neurological disease and another who did not provide consent for study participation were excluded. Finally, 31 patients were included in the analysis.
Of these, 14 were diagnosed with a known clinico-radiological acute encephalopathy syndrome (Figure 2). The causative diseases were AESD (Takanashi et al., 2006) (five patients), encephalopathy with fulminant acute cerebral edema (Krishnan et al., 2021) (three patients), MERS (Tada et al., 2004) (two patients), ANE (Mizuguchi et al., 1995) (two patients), and HSES (Levin et al., 1983) (two patients). The remaining 17 patients had acute encephalopathy of unexplained or unclassifiable cause.
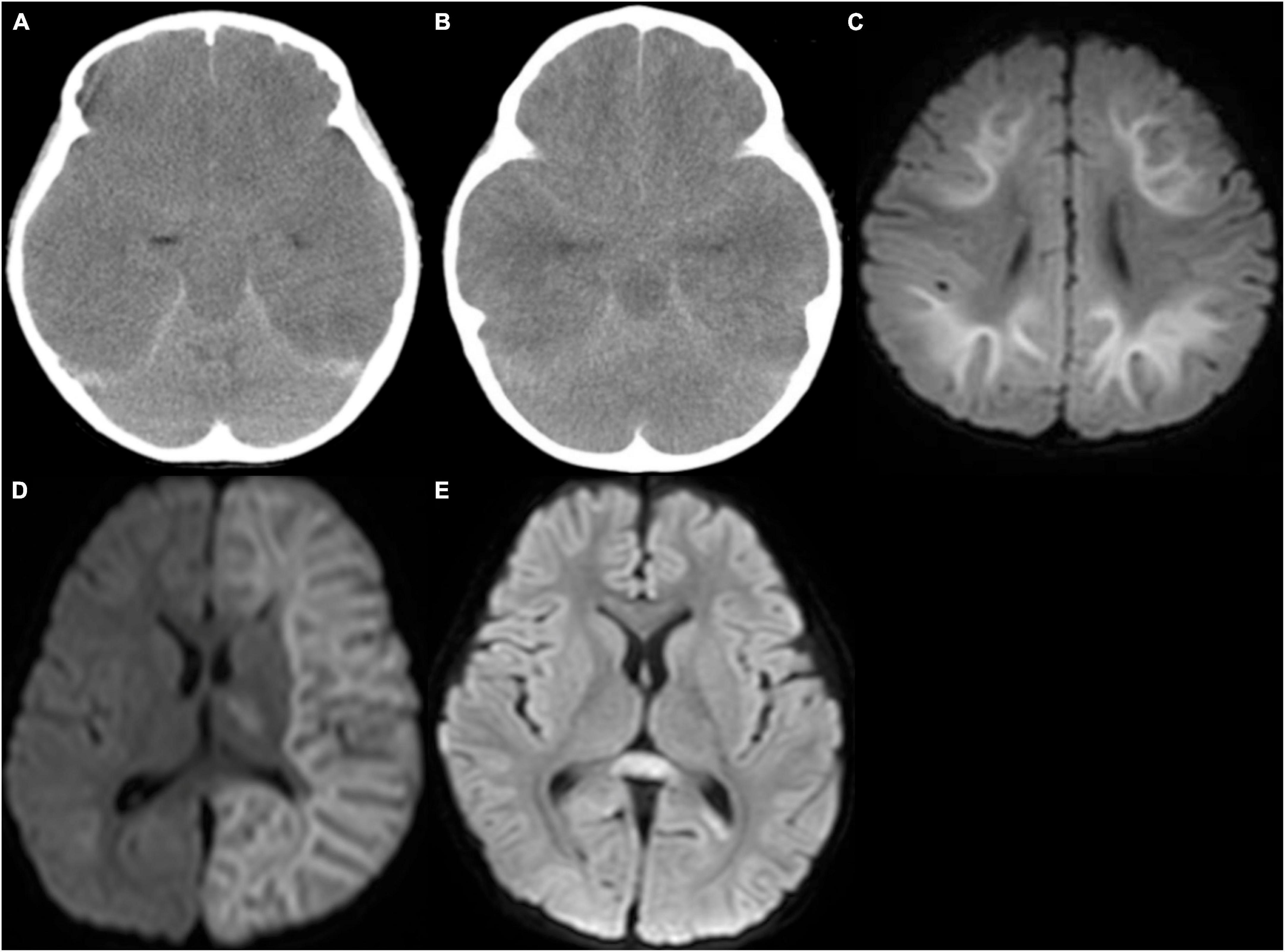
Figure 2. Computed tomography (CT) and magnetic resonance imaging. (A) CT in a patient with encephalopathy with fulminant acute cerebral edema revealed diffuse low density in the cerebrum with blurring of gray-white matter junction, and disappearance of ambient cistern, suggesting severe cerebral edema with probable downward herniation. (B) CT in a patient with hemorrhagic shock and encephalopathy syndrome revealed severe cerebral edema. (C) Diffusion-weighted image in a patient with acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) revealed high signal lesion in the subcortical white matter with sparing peri-Rolandic regions, so-called bright tree appearance. (D) Diffusion-weighted image of a patient with unilateral AESD revealed left-sided bright tree appearance (BTA) with a small lesion in the left ventral thalamus. (E) Diffusion-weighted image of a patient with mild encephalitis/encephalopathy with reversible splenial lesion (MERS) revealed a high signal lesion in the splenium of the corpus callosum.
A summary of the patient data is presented in Table 1. The median age of onset was 5 years (28 days–14 years), with a male-to-female ratio of 13:18. In Japan, Alpha and Delta strains were prevalent until December 2021, and the majority of patients had Omicron strains from January 2022 onward; 29 of the 31 patients developed the disease after January 2022. None of the patients were hospitalized prior to the onset of acute encephalopathy. Neurologic symptoms appeared 0–8 days (median 0 days) after the onset of COVID-19 that was defined as the appearance of either fever or respiratory symptoms, with main initial symptoms being seizures (n = 15), impaired consciousness (n = 8), and abnormal speech or behavior (n = 7). All patients presented these symptoms during the acute encephalopathic phase. Respiratory symptoms at the onset of neurological symptoms were mild in all patients, and no case was preceded by dyspnea due to pneumonia or other respiratory diseases. Only one patient had been vaccinated. Of the 31 patients, five developed acute encephalopathy associated with MIS-C/PIMS, and two of which developed MERS. Nineteen patients recovered to their pre-symptomatic state (PCPC = 1), one with mild disability (PCPC = 2), two with moderate disability (PCPC = 3), three with severe disability (PCPC = 4, including one with pre-existing developmental delay), two in a coma or vegetative state (PCPC = 5), and four died (PCPC = 6). In total, 10 (29.4%) had severe sequelae or died (PCPC ≥ 4) among the 31 patients.
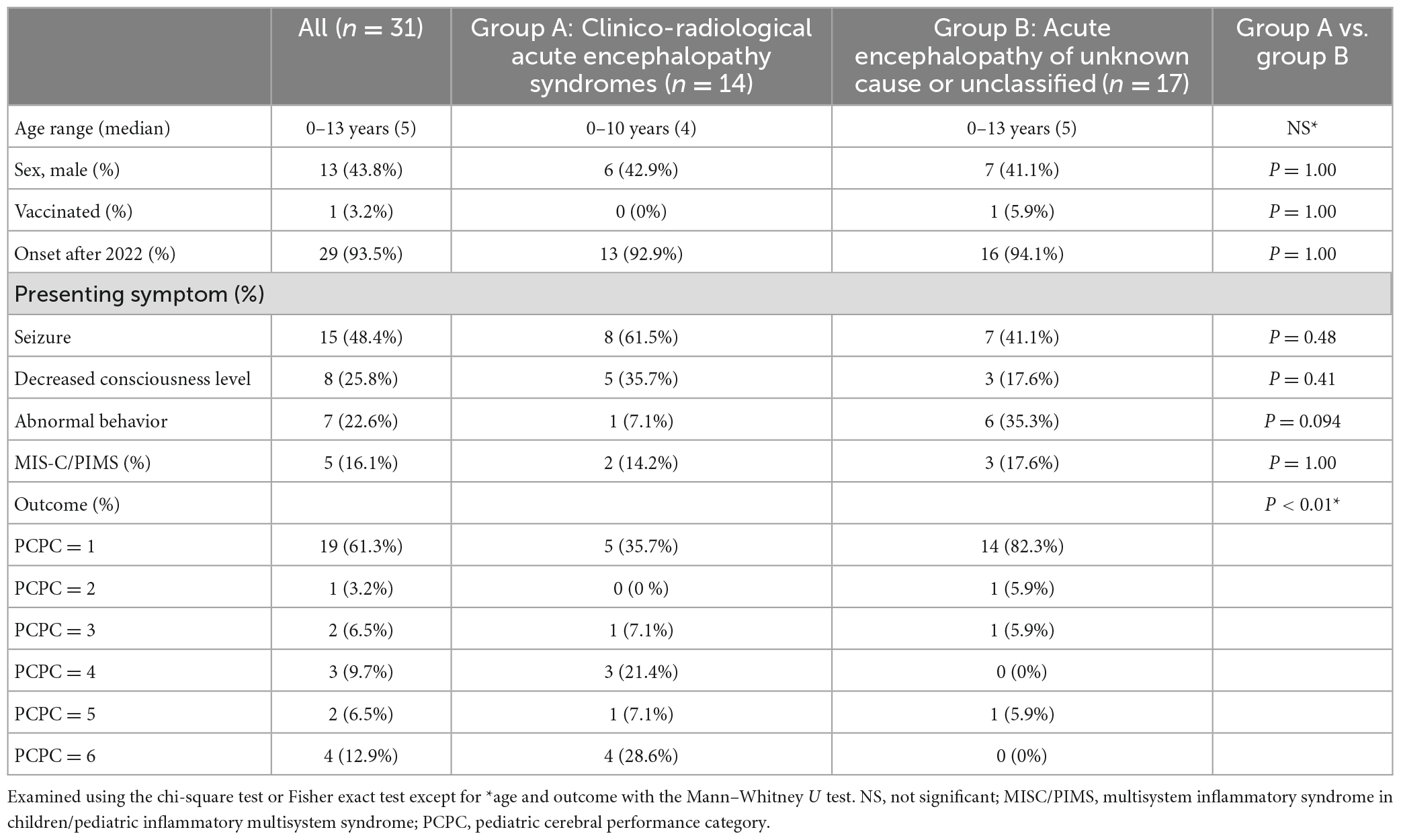
Table 1. Characteristics and outcomes of pediatric patients with COVID-19-related acute encephalopathy.
When patients were divided into two groups: the known clinico-radiological acute encephalopathy syndrome group (group A: n = 14) and the unexplained or unclassifiable acute encephalopathy group (group B: n = 17), the PCPC score was higher in group A than in group B (3.50 ± 2.06 vs. 1.41 ± 1.03, P < 0.01 by Mann–Whitney U test).
When group A was further classified into five clinico-radiological syndromes (Table 2), three patients developed encephalopathy with acute fulminant cerebral edema with two fatal outcomes and two patients developed HSES with fatal outcomes, suggesting that the prognosis for these two syndromes is particularly poor. Two patients with AESD and one with ANE had a PCPC score of 4, while all patients with MERS had a PCPC score of 1.
In this nationwide surveillance in Japan, 217 out of 3,802 members of the Japanese Society of Child Neurology reported 31 patients with acute encephalopathy. The response rate was low because we asked the representative of the facility to answer, and the membership included many pediatric practitioners and non-physician healthcare professionals. Twenty-nine of the 31 patients developed acute encephalopathy in 2022. By the end of 2021, approximately 240,000 patients aged < 20 years had COVID-19, while in 2022, approximately 1.98 million patients were recorded in a 5-month period. The number of patients with acute encephalopathy in each period was approximately proportional to the overall number of patients with COVID-19. The rapid increase in pediatric acute encephalopathy in 2022 may reflect this infection situation.
Acute encephalopathy associated with COVID-19 reportedly occurs in the elderly in association with respiratory insufficiency. Among 707 patients infected by the SARS-CoV-2, 31 (4.4%) developed acute encephalopathy (64.6 ± 12.1 years). COVID-19 encephalopathy started 20.9 ± 8.1 days after COVID-19 symptom onset, and 28 of 31 (90%) patients had an acute respiratory distress syndrome (Uginet et al., 2021). In the present cohort of Japanese children, respiratory symptoms were mild in all patients, making respiratory compromise as the cause of the encephalopathy unlikely. Therefore, COVID-19-related acute encephalopathy observed in children is considered a different condition from that observed in older hospitalized patients. Moreover, neurological symptoms were the primary manifestation in these cases. To the best of our knowledge, no large case series on pediatric COVID-19 with predominant neurological manifestations has been conducted.
Meanwhile, several case series on acute encephalopathy complicated by COVID-19 in children have been reported. In a UK cohort, neurological manifestations were identified in 52 cases among 1,334 children and adolescents hospitalized with COVID-19, and 25 of them had encephalopathy (Ray et al., 2021). Another study has reported that 365 (22%) of 1,695 patients hospitalized with COVID-19 had documented neurologic involvement, among which 43 (12%) developed life-threatening conditions, including 15 with severe encephalopathy (LaRovere et al., 2021). In addition, at least 18 patients with acquired demyelinating syndromes were reported as neurological complications of COVID-19, of which NMOSD was the most common (n = 7) (Feizi et al., 2022).
Some infection-triggered acute encephalopathies are characterized by a stereotypical clinical course and specific neuroimaging findings, and thus we recognized these as specific clinico-radiological syndromes and analyzed the cases of acute encephalopathy from this perspective (Mizuguchi et al., 2007). Such infection-triggered encephalopathy syndromes include encephalopathy with fulminant acute cerebral edema (Krishnan et al., 2021), HSES (Levin et al., 1983), AESD (Takanashi et al., 2006), ANE (Mizuguchi et al., 1995), or MERS (Tada et al., 2004). In the present cohort, these clinico-radiological syndromes correlated with a poorer prognosis compared to those in the unclassified acute encephalopathy group.
Some of these acute encephalopathy syndromes have a hyperacute onset and are often difficult to rescue due to rapidly progressive brain edema and consequent loss of brain function. All four deaths in this study were characterized by fatal brain edema. Of note, encephalopathy with fulminant acute cerebral edema, often fatal due to rapidly progressive brain swelling, has been reported (Krishnan et al., 2021; LaRovere et al., 2021). This encephalopathy, observed in previously healthy children, may be relatively common in COVID-19. Two of four fatal cases met the diagnostic criteria for HSES. Most of the HSES cases reported in the past were infants, and similarities with heat stroke have been suspected (Bacon and Hall, 1992). However, since all of our two cases were older children and inappropriate temperature control was not reported, the case reported here as HSES may be a different disorder from conventional HSES. The two encephalopathy syndromes with acute cerebral edema are the most critical ones associated with COVID-19. Although the cause of this rapidly progressive brain edema is unknown, we postulate a disruption of the blood-brain barrier due to excessive uncontrolled inflammation. The involvement of cytokines and chemokines in the hemorrhagic shock and encephalopathy syndrome associated with viral infections other than COVID-19 has been demonstrated, (Yamaguchi et al., 2021) and interestingly, this is consistent with recent observations in COVID-19-related encephalopathy (Pilotto et al., 2020).
In addition to the four deaths, five patients, including two with AESD and one with ANE, were left with severe or profound disability (PCPC = 4 or 5). ANE is a severe encephalopathy characterized by symmetrical involvement of thalami, putamina, cerebral white matter, or brainstem (Mizuguchi et al., 1995). Familial ANE caused by RANBP2 gene mutations is known (Neilson et al., 2009), although the majority of cases in Japan are sporadic. AESD is a syndrome of clustered brief seizures and characteristic magnetic resonance imaging (MRI) diffusion-weighted imaging findings several days after initial prolonged status epilepticus (Takanashi et al., 2006). AESD is characterized by frontal lobe predominant lesions, which may suggest that the virus has an affinity for specific areas of the brain. In support of this, there is some evidence that SARS-CoV-2 could preferentially and directly target the frontal lobes (Toniolo et al., 2021). It is the most frequent acute encephalopathy syndrome in Japan and has been reported almost exclusively in Japan (Kasai et al., 2020). Some genetic susceptibility has been postulated, and a polymorphism of STK39 gene has recently been identified (Kasai et al., 2022).
COVID-19-related acute encephalopathy in children is often associated with MIS-C/PIMS. Splenial signal change is often observed in COVID-19-related MIS-C/PIMS (Abdel-Mannan et al., 2020; Lindan et al., 2021). MIS-C/PIMS is associated with 48% of pediatric COVID-19-related neurologic complications, of which 22 (46%) had acute encephalopathy and seven had signal changes in the splenium of the corpus callosum (SCC) (Ray et al., 2021). SCC lesions associated with viral infections, termed MERS, generally have a good prognosis, although not necessarily when associated with COVID-19. The few cases associated with MIS-C/PIMS and the majority of cases with encephalopathy in the acute phase of COVID-19 infection were characteristic of our cohort.
As described above, COVID-19 was associated with various types of acute encephalopathy syndromes. This is similar, for example, to the acute encephalopathy syndrome associated with influenza, indicating that different viruses cause the same clinical phenotype (Mizuguchi et al., 2007). These observations can be explained by the hypothesis that a non-specific immune response to viral infection may trigger acute encephalopathy syndromes. In general, these infection-triggered encephalopathy syndromes have no evidence of virus infiltration in the brain and are considered to be different from primary encephalitis caused by a neurotropic virus.
Previous reports of virus-related acute encephalopathy outcomes from Japan indicated that PCPC = 1 in 56%, PCPC = 2 or 3 in 14%, PCPC = 4 or 5 in 12%, and PCPC = 6 in 5% (Kasai et al., 2020). Compared with these results, COVID-19-related acute encephalopathy may have a relatively poor prognosis, that is, 9/31 patients (29.0%) had severe sequelae or died (PCPC ≥ 4). The type of acute encephalopathy syndrome varied partly according to the causative virus: in influenza-associated encephalopathy, MERS (25%) and AESD (16%) predominated, while encephalopathy with fulminant acute cerebral edema (0.5%) and HSES (0%) were rare. The relatively high proportion of encephalopathy with fulminant acute cerebral edema and HSES in COVID-19-related acute encephalopathy syndromes may be associated with poor outcomes.
This study had several limitations. First, due to its retrospective nature, no standardized protocols for diagnosis and treatment were considered. Although clinical practice guidelines for acute encephalopathy syndromes are followed in Japan, different treatment strategies may have affected outcomes. Second, infection control measures often precluded MRI, which may have resulted in underdiagnosis of acute encephalopathy syndromes. Thirdly, detailed clinical course and laboratory data were not collected in the present survey and require further study in the future. Finally, the different methods used in the present and previous studies made it difficult to precisely compare the data between them.
COVID-19-related acute encephalopathy syndromes are characterized by a rapid onset of seizures and impaired/altered consciousness without severe respiratory symptoms and by potentially poor outcomes. These represent an important public health threat in children, and SARS-CoV-2 should be recognized as a pathogen causing acute encephalopathy syndrome.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Tokyo Metropolitan Institute of Medical Science (#20–28). Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
Division of Child Neurology and Child Psychiatry, Okinawa Prefectural Nanbu Medical Center and Children’s Medical Center, Okinawa, Japan: Tsuyoshi Matsuoka. Division of Pediatric Critical Care Medicine, Matsudo City General Hospital, Chiba, Japan: Hiroshi Oakada. Department of Pediatrics, Nagasaki University Hospital, Nagasaki, Japan: Tatsuharu Sato. Division of Neurology, Saitama Children’s Medical Center, Saitama, Japan: Kenjiro Kikuchi. Department of Pediatric Neurology, Fukuoka Children’s Hospital, Fukuoka, Japan: Satoshi Akamine. Department of Neurology, Tokyo Metropolitan Children’s Medical Center, Tokyo, Japan: Nanako Kawata. Department of Pediatrics and Adolescent Medicine, Tokyo Medical University, Tokyo, Japan: Shinichiro Morichi. Department of Pediatrics, Aichi Medical University School of Medicine, Nagakute, Aichi, Japan: Hideyuki Iwayama. Ibaraki Pediatric Education and Training Station, University of Tsukuba, Ibaraki, Japan: Ryuta Tanaka. Department of Pediatrics, Kurashiki Central Hospital, Okayama, Japan: Yoshiyuki Hanaoka. Department of Pediatrics, Yokohama Rosai Hospital, Kanagawa, Japan: Yuki Minamisawa. Department of Pediatric Neurology, Shizuoka Children’s Hospital, Shizuoka, Japan: Tatsuya Ema. Division of Neuropediatrics, Nagano Children’s Hospital, Nagano, Japan: Mitsuo Motobayashi. Department of Pediatrics, Sapporo City General Hospital, Hokkaido, Japan: Tomoshiro Ito. Department of Pediatrics, Faculty of Medicine, University of Yamanashi, Yamanashi, Japan: Fumikazu Sano.
HS, JT, and MM: concept and design. HS, JT, OM, KaM, MS, KO, MK, HK, KS, and TS: acquisition, analysis, and or interpretation of data. HS and JT: drafting of the manuscript. HS: statistical analysis, obtained funding, had full access to all the data in the study, takes responsibility for the integrity of the data, and the accuracy of the data analysis. AO and MM: supervision. All authors contributed to the critical revision of the manuscript for important intellectual content.
This study was funded by Grant-in-aid for Research on Measures for Intractable Diseases No. 21FC1005 (HS, JT, YA, YM, KeM, HN, AO, YS, and MM), a grant for Research on Emerging and Re-emerging Infectious Diseases and Immunization No. 22HA1003 (HS) from the Japanese Ministry of Health, Labor and Welfare, the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and development (AMED) No. 21fk0108436 (HS), and JSPS Kakenhi Grant No. 22K07831 (HS).
We appreciate the members of the Japanese Society of Child Neurology for their assistance in data collection for the study. This study was officially supported by the Committee of Collaborative Study Support in the Japanese Society of Child Neurology.
HS received a University–Industry Joint Research Fund from Meiji Co., Ltd. KK received research funds from Syneos Health Clinical Co., Ltd. for the clinical trial of Zogenix. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1085082/full#supplementary-material
Abdel-Mannan, O., Eyre, M., Lobel, U., Bamford, A., Eltze, C., Hameed, B., et al. (2020). Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 77, 1440–1445. doi: 10.1001/jamaneurol.2020.2687
Bacon, C. J., and Hall, S. M. (1992). Haemorrhagic shock encephalopathy syndrome in the British Isles. Arch. Dis. Child. 67, 985–993. doi: 10.1136/adc.67.8.985
Chou, S. H., Beghi, E., Helbok, R., Moro, E., Sampson, J., Altamirano, V., et al. (2021). Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID consortium and the energy consortium. JAMA Netw. Open 4, e2112131. doi: 10.1001/jamanetworkopen.2021.12131
Ellul, M. A., Benjamin, L., Singh, B., Lant, S., Michael, B. D., Easton, A., et al. (2020). Neurological associations of COVID-19. Lancet Neurol. 19, 767–783. doi: 10.1016/S1474-4422(20)30221-0
Feizi, P., Sharma, K., Pasham, S. R., Nirwan, L., Joseph, J., Jaiswal, S., et al. (2022). Central nervous system (CNS) inflammatory demyelinating diseases (IDDs) associated with COVID-19: A case series and review. J. Neuroimmunol. 371, 577939. doi: 10.1016/j.jneuroim.2022.577939
Hoshino, A., Saitoh, M., Oka, A., Okumura, A., Kubota, M., Saito, Y., et al. (2012). Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 34, 337–343. doi: 10.1016/j.braindev.2011.07.012
Iadecola, C., Anrather, J., and Kamel, H. (2020). Effects of COVID-19 on the nervous system. Cell 183, 16–27.e11. doi: 10.1016/j.cell.2020.08.028
Jiang, L., Tang, K., Levin, M., Irfan, O., Morris, S. K., Wilson, K., et al. (2020). COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 20, e276–e288. doi: 10.1016/S1473-3099(20)30651-4
Kasai, M., Omae, Y., Kawai, Y., Shibata, A., Hoshino, A., Mizuguchi, M., et al. (2022). GWAS identifies candidate susceptibility loci and microRNA biomarkers for acute encephalopathy with biphasic seizures and late reduced diffusion. Sci. Rep. 12:1332. doi: 10.1038/s41598-021-04576-y
Kasai, M., Shibata, A., Hoshino, A., Maegaki, Y., Yamanouchi, H., Takanashi, J. I., et al. (2020). Epidemiological changes of acute encephalopathy in Japan based on national surveillance for 2014-2017. Brain Dev. 42, 508–514. doi: 10.1016/j.braindev.2020.04.006
Kato, Y. (2021). Case management of COVID-19 (secondary version). JMA J. 4, 191–197. doi: 10.31662/jmaj.2021-0036
Krishnan, P., Glenn, O. A., Samuel, M. C., Sheriff, H., Foster-Barber, A., Sejvar, J. J., et al. (2021). Acute fulminant cerebral edema: A newly recognized phenotype in children with suspected encephalitis. J. Pediatr. Infect. Dis. Soc. 10, 289–294. doi: 10.1093/jpids/piaa063
LaRovere, K. L., Riggs, B. J., Poussaint, T. Y., Young, C. C., Newhams, M. M., Maamari, M., et al. (2021). Neurologic involvement in children and adolescents hospitalized in the united states for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 78, 536–547. doi: 10.1001/jamaneurol.2021.0504
Levin, M., Hjelm, M., Kay, J. D., Pincott, J. R., Gould, J. D., Dinwiddie, R., et al. (1983). Haemorrhagic shock and encephalopathy: A new syndrome with a high mortality in young children. Lancet 2, 64–67. doi: 10.1016/s0140-6736(83)90057-0
Lindan, C. E., Mankad, K., Ram, D., Kociolek, L. K., Silvera, V. M., Boddaert, N., et al. (2021). Neuroimaging manifestations in children with SARS-CoV-2 infection: A multinational, multicentre collaborative study. Lancet Child. Adolesc. Health 5, 167–177. doi: 10.1016/S2352-4642(20)30362-X
Mizuguchi, M., Abe, J., Mikkaichi, K., Noma, S., Yoshida, K., Yamanaka, T., et al. (1995). Acute necrotising encephalopathy of childhood: A new syndrome presenting with multifocal, symmetric brain lesions. J. Neurol. Neurosurg. Psychiatry 58, 555–561. doi: 10.1136/jnnp.58.5.555
Mizuguchi, M., Ichiyama, T., Imataka, G., Okumura, A., Goto, T., Sakuma, H., et al. (2021). Guidelines for the diagnosis and treatment of acute encephalopathy in childhood. Brain Dev. 43, 2–31. doi: 10.1016/j.braindev.2020.08.001
Mizuguchi, M., Yamanouchi, H., Ichiyama, T., and Shiomi, M. (2007). Acute encephalopathy associated with influenza and other viral infections. Acta Neurol. Scand. 115(4 Suppl), 45–56. doi: 10.1111/j.1600-0404.2007.00809.x
Neilson, D. E., Adams, M. D., Orr, C. M., Schelling, D. K., Eiben, R. M., Kerr, D. S., et al. (2009). Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am. J. Hum. Genet. 84, 44–51. doi: 10.1016/j.ajhg.2008.12.009
Nishimura, W., Tomari, K., Matsuoka, T., Cho, Y., Kato, A., Kanno, K., et al. (2022). Multisystem inflammatory syndrome complicated by acute encephalopathy. Indian J. Pediatr. 89, 730. doi: 10.1007/s12098-022-04141-z
Pilotto, A., Odolini, S., Masciocchi, S., Comelli, A., Volonghi, I., Gazzina, S., et al. (2020). Steroid-responsive encephalitis in coronavirus disease 2019. Ann. Neurol. 88, 423–427. doi: 10.1002/ana.25783
Ray, S. T. J., Abdel-Mannan, O., Sa, M., Fuller, C., Wood, G. K., Pysden, K., et al. (2021). Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: A prospective national cohort study. Lancet Child. Adolesc. Health 5, 631–641. doi: 10.1016/S2352-4642(21)00193-0
Saito, M., Nakazawa, T., Toriumi, S., Takasu, M., Yagisawa, H., Murano, Y., et al. (2022). Case report: Mild encephalitis with a reversible splenial lesion associated with SARS-CoV-2 infection in a patient with MYRF variant. Front. Pediatr. 10:971432. doi: 10.3389/fped.2022.971432
Tada, H., Takanashi, J., Barkovich, A. J., Oba, H., Maeda, M., Tsukahara, H., et al. (2004). Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 63, 1854–1858. doi: 10.1212/01.wnl.0000144274.12174.cb
Takanashi, J., Oba, H., Barkovich, A. J., Tada, H., Tanabe, Y., Yamanouchi, H., et al. (2006). Diffusion MRI abnormalities after prolonged febrile seizures with encephalopathy. Neurology 66, 1304–1309; discussion 1291. doi: 10.1212/01.wnl.0000210487.36667.a5
Toniolo, S., Di Lorenzo, F., Scarioni, M., Frederiksen, K. S., and Nobili, F. (2021). Is the frontal lobe the primary target of SARS-CoV-2? J. Alzheimers Dis. 81, 75–81. doi: 10.3233/JAD-210008
Uginet, M., Breville, G., Assal, F., Lovblad, K. O., Vargas, M. I., Pugin, J., et al. (2021). COVID-19 encephalopathy: Clinical and neurobiological features. J. Med. Virol. 93, 4374–4381. doi: 10.1002/jmv.26973
Whittaker, E., Bamford, A., Kenny, J., Kaforou, M., Jones, C. E., Shah, P., et al. (2020). Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324, 259–269. doi: 10.1001/jama.2020.10369
Yamaguchi, H., Nishiyama, M., Tokumoto, S., Ishida, Y., Tomioka, K., Aoki, K., et al. (2021). Elevated cytokine, chemokine, and growth and differentiation factor-15 levels in hemorrhagic shock and encephalopathy syndrome: A retrospective observational study. Cytokine 137:155324. doi: 10.1016/j.cyto.2020.155324
Zaritsky, A., Nadkarni, V., Hazinski, M. F., Foltin, G., Quan, L., Wright, J., et al. (1995). Recommended guidelines for uniform reporting of pediatric advanced life support: The pediatric Utstein style. A statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Pediatrics 96(4 Pt 1), 765–779.
Keywords: infection-triggered encephalopathy syndrome (ITES), acute encephalopathy with biphasic seizures and late reduced diffusion (AESD), COVID-19, SARS-CoV-2, outcome, clinico-radiological syndrome, acute encephalopathy syndrome
Citation: Sakuma H, Takanashi J-i, Muramatsu K, Kondo H, Shiihara T, Suzuki M, Okanari K, Kasai M, Mitani O, Nakazawa T, Omata T, Shimoda K, Abe Y, Maegaki Y, Murayama K, Murofushi Y, Nagase H, Okumura A, Sakai Y, Tada H and Mizuguchi M (2023) Severe pediatric acute encephalopathy syndromes related to SARS-CoV-2. Front. Neurosci. 17:1085082. doi: 10.3389/fnins.2023.1085082
Received: 31 October 2022; Accepted: 07 February 2023;
Published: 27 February 2023.
Edited by:
Francesco Di Lorenzo, Santa Lucia Foundation (IRCCS), ItalyReviewed by:
Gautier Breville, Hôpitaux universitaires de Genève (HUG), SwitzerlandCopyright © 2023 Sakuma, Takanashi, Muramatsu, Kondo, Shiihara, Suzuki, Okanari, Kasai, Mitani, Nakazawa, Omata, Shimoda, Abe, Maegaki, Murayama, Murofushi, Nagase, Okumura, Sakai, Tada and Mizuguchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroshi Sakuma,  c2FrdW1hLWhzQGlnYWt1a2VuLm9yLmpw
c2FrdW1hLWhzQGlnYWt1a2VuLm9yLmpw
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.