
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 02 February 2023
Sec. Neurodevelopment
Volume 17 - 2023 | https://doi.org/10.3389/fnins.2023.1067833
Introduction: Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder for which early recognition is a major challenge. Autoantibodies against fetal brain antigens have been found in the blood of mothers of children with ASD (m-ASD) and can be transferred to the fetus where they can impact neurodevelopment by binding to fetal brain proteins. This study aims to identify novel maternal autoantibodies reactive against human fetal brain antigens, and explore their use as biomarkers for ASD screening and diagnosis.
Methods: A custom-made human fetal brain cDNA phage display library was constructed, and screened for antibody reactivity in m-ASD samples from the Simons Simplex Collection (SSC) of the Simons Foundation Autism Research Initiative (SFARI). Antibody reactivity against 6 identified antigens was determined in plasma samples of 238 m-ASD and 90 mothers with typically developing children (m-TD).
Results: We identified antibodies to 6 novel University Hasselt (UH)-ASD antigens, including three novel m-ASD autoantigens, i.e., ribosomal protein L23 (RPL23), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calmodulin-regulated spectrin-associated protein 3 (CAMSAP3). Antibody reactivity against a panel of four of these targets was found in 16% of m-ASD samples, compared to 4% in m-TD samples (p = 0.0049).
Discussion: Maternal antibodies against 4 UH-ASD antigens could therefore provide a novel tool to support the diagnosis of ASD in a subset of individuals.
Autism spectrum disorder (ASD) is a developmental disorder defined by pervasive and sustained impairments in social communication, and restricted, repetitive patterns of behavior, interest or activities (American Psychiatric Association, 2013). The prevalence of ASD is estimated at 1 in 54 children aged 8 years in the United States, 1 in 100 in Europe, and occurs four times more in males than in females (Delobel-Ayoub et al., 2020; Maenner et al., 2020).
Early recognition and diagnosis of children with ASD is essential to allow the start of an early intervention, which has been shown to lead to better outcomes (Zwaigenbaum et al., 2015). A combination of general developmental surveillance and ASD-specific early screening tests are used in primary care to identify children at risk for ASD (Hyman et al., 2020; Lipkin et al., 2020). The Modified Checklist for Autism in Toddlers (M-CHAT) and the Social Communication Questionnaire (SCQ) are some of the most used screening tests (Robins et al., 2001; Eaves et al., 2006), and are used in children between 18 and 42 months in low and high-risk populations (Baird et al., 2000). However, these tools are not designed for diagnostic purposes, and children who are identified by surveillance or screening require additional specialized evaluation for ASD diagnosis. The diagnosis of ASD is generally complex and requires a multidisciplinary approach using behavioral observations, based on the diagnostic criteria from the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Multiple questionnaires have been created to support the diagnostic process, the most used are the Autism Diagnostic Observational Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R), which score different behavioral patterns, such as eye contact, attention, or playing engagement (Lord et al., 1994, 2000; American Psychiatric Association, 2013).
Each of these diagnostic instruments still has limited sensitivity and specificity, and therefore only performs well, correctly identifying true positives and true negatives, in a clinical situation where the ASD prevalence is already high (Randall et al., 2018). This still leaves much room for improvement, especially when trying to identify children with ASD as early as possible. This can be achieved by improving screening, monitoring of high-risk populations, such as siblings of children with ASD, but also by the use of biomarkers which objectively measure biological characteristics of disease. Many types of ASD biomarkers have been described, ranging from structural parameters quantified using magnetic resonance imaging, physiological measurements such as electroencephalography, behavioral parameters such as eye tracking, but also a large diversity of molecular parameters, including metabolic, immune and genetic biomarkers (reviewed in Frye et al., 2019). Next to assisting diagnosis, screening, and presymptomatic detection, ASD biomarkers are potential tools to stratify ASD patients, and to provide understanding of the underlying disease mechanism at the molecular level.
The etiology of ASD is complex and multifactorial in most individuals, compounding the influence of both genetic and environmental risk factors (reviewed by de la Torre-Ubieta et al., 2016; Modabbernia et al., 2017). In the last decade, the role of immune system dysfunction in the development of ASD has received increasing attention. This link between immune dysregulation and ASD even occurs during embryonic development, where different components of the maternal immune system have been described to impact prenatal brain development, leading to an increased risk of ASD in the child. This is the case in so-called maternal immune activation, where infections or increased inflammation during pregnancy have been shown to increase the risk for development of ASD in the child (Han et al., 2021). Here, components of the maternal innate immune system mediate a more general, non-antigen-specific immune response, mainly by pro-inflammatory cytokines such as IL-6 and IL-17A (Smith et al., 2007; Choi et al., 2016). On the other hand, maternal, antigen-specific immune responses, in the form of maternal autoantibodies targeting specific fetal brain proteins, have also been linked to the development of ASD in the child (m-ASD) (reviewed by Mazon-Cabrera et al., 2019). During pregnancy, these m-ASD autoantibodies are able to pass the placenta via transplacental transport (Kim et al., 2009; Palmeira et al., 2012), and to cross the fetal blood-brain barrier, which appears to be semi-permeable during this period (Kowal et al., 2015). Recent studies revealed lactate dehydrogenase A and B (LDH-A/B), Y-box-binding protein 1 (YBX1), cytosolic PSD-95 interactor (Cypin), collapsin response mediator protein 1 and 2 (CRMP1/2), stress-induced phosphoprotein 1 (STIP1), folate receptor alpha (FOLR1), and contactin-associated protein-like 2 (CASPR2) as the main antigens targeted by m-ASD autoantibodies (Braunschweig et al., 2013; Ramaekers et al., 2013; Brimberg et al., 2016). Using animal models, the active contribution of several of these m-ASD autoantibodies in the development of autistic features in the offspring has been demonstrated. Female mice which have been immunized with a mixture of immunogenic peptides from LDH-A/B, STIP1, and CRMP1 (Jones et al., 2020), or with the extracellular portion of CASPR2 (Bagnall-Moreau et al., 2020), and which subsequently form autoantibodies against these proteins during pregnancy, produce offspring with structural brain abnormalities and autism-related behavioral changes.
A better understanding of the different brain antigens that can be targeted by maternal autoantibodies, would allow to investigate the role of these target antigens in normal brain development, and how autoantibodies directed against them might impact the development of ASD. In addition, such antibodies might prove to be valuable early biomarkers for ASD. Therefore, in this study, an unbiased screening was performed to identify novel immunoglobulin G (IgG) m-ASD antibodies. To this end, a custom cDNA phage display library was created, which represents the diversity of antigens expressed in the human fetal brain during mid gestation, and used to screen for antibody reactivity in plasma samples of mothers who have a child with ASD (m-ASD). Next, antibody reactivity against selected antigens was determined in a large collection of m-ASD and control samples, and the possible added value in supporting ASD screening and diagnosis was studied.
Plasma samples from 268 mothers with a single child with ASD (m-ASD, Table 1) were obtained from the Simons Simplex Collection (SSC) of the Simons Foundation Autism Research Initiative (SFARI) in the United States (Fischbach and Lord, 2010). The children were diagnosed with ASD by their treating clinical psychologist using the DSM-IV diagnostic criteria. The maternal blood sample was obtained at ASD diagnosis of the child, at which time the child was between 3 and 7 years, and had no younger siblings. All children with ASD were white, of non-Hispanic descent, 223 (83%) were male. Relevant demographic and ASD-related clinical characteristics were obtained via SFARI Base (Fischbach and Lord, 2010), and are shown in Table 1.
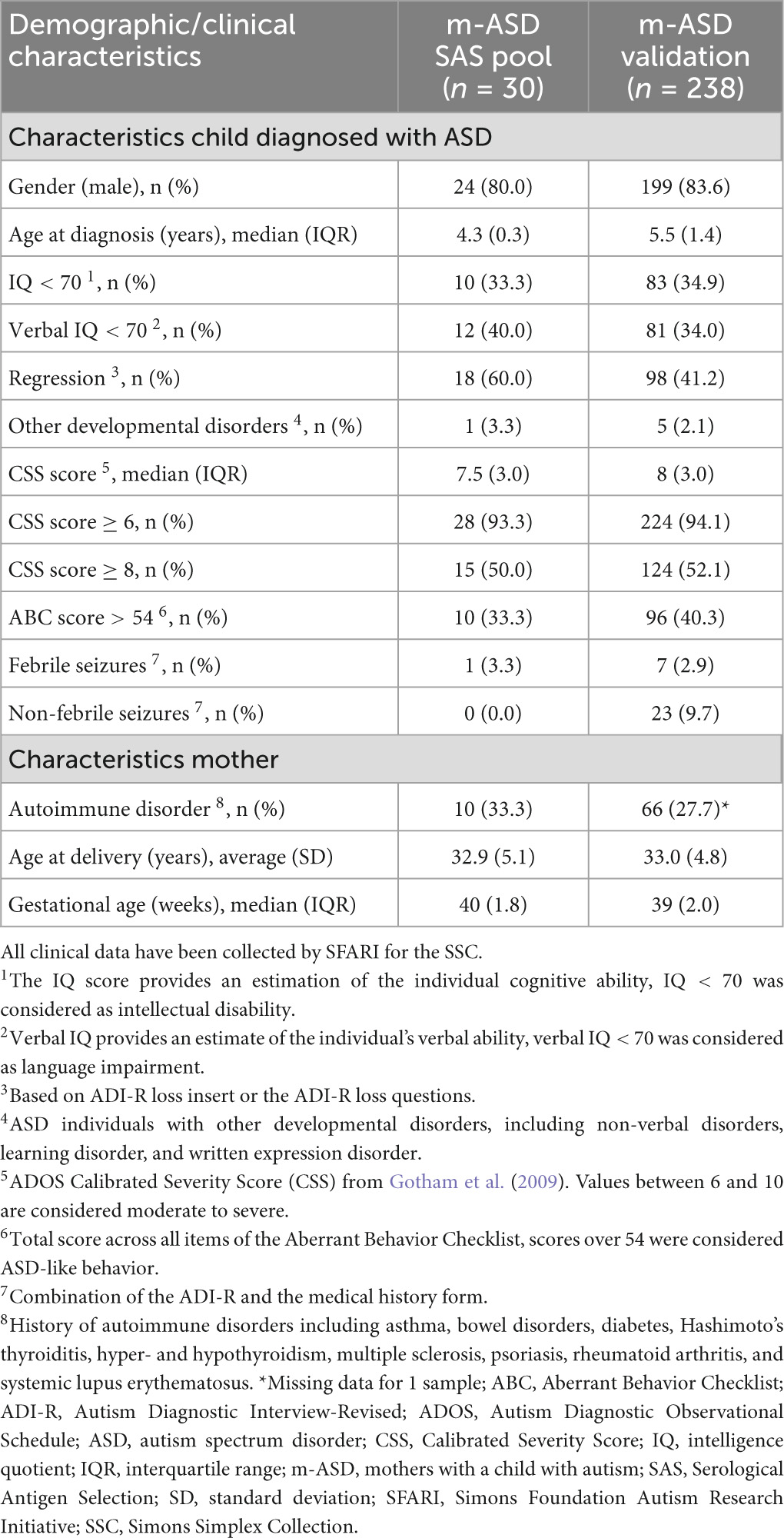
Table 1. Demographic and clinical characteristics of m-ASD samples from SSC used for SAS screening and validation.
In addition, control plasma samples from 90 mothers with typically developing children (m-TD) were collected in collaboration with University Biobank Limburg (UBiLim, Belgium) (Linsen et al., 2019). Demographic and clinical characteristics were obtained via a questionnaire. At the time of sampling, the women included in the study had a child between 3 and 7 years of age, resulting from their last pregnancy. The children of the m-TD mothers were white, of East- or West-European descent, and 44 (49%) were male. None of the m-TD mothers, or their children, presented a diagnosis of ASD, hyperactivity or learning disabilities.
A pool of 30 m-ASD plasma samples was used to screen for novel antibodies using serological antigen selection (SAS) (Table 1). A pool of plasma samples from 20-mTD, of which the youngest child was age- and gender-matched [mean (SD) age 4.3 (1.3) years, 16 (80%) were male] were used in the counterselection of the SAS procedure.
This study was conducted in accordance with the Helsinki Declaration. All m-ASD and m-TD study subjects provided written informed consent, and this study was approved by the local ethics committees of Jessa Hospital and Hasselt University.
Commercially obtained poly A+ RNA isolated from the brains of 59 spontaneously aborted male and female Caucasian fetuses, of 20−33 weeks of gestation (Clontech/Takara, Saint-Germain-en-Laye, France) was used to construct a human fetal brain cDNA phage display library according to the previously described procedure (Vandormael et al., 2017). This phage display library, expressing human fetal brain antigens, but also non-physiological peptides, was used to screen for antibody reactivity in 30 pooled m-ASD plasma samples (Table 1) using SAS, using the previously described procedure, with minor modifications (Somers et al., 2005, 2009; Quaden et al., 2020). In brief, plasma antibodies from the m-ASD SAS pool (n = 30) with reactivity against phage or bacterial proteins were first removed by pre-absorbing plasma to cyanogen-bromide sepharose beads (Merck, United States) coupled with phage and bacterial protein extracts. Next, in a first positive selection round of the SAS procedure, phage particles from the human fetal brain cDNA phage display library were pre-incubated with the m-ASD SAS pool in phosphate buffered saline (PBS) pH 7.4 with 2% (w/v) skimmed milk powder (MPBS), and incubated for 2 h rotating at room temperature (RT). A MaxiSorp Immuno tube (Thermo Fisher Scientific) was coated with 10 μg/ml of polyclonal rabbit anti-human immunoglobulin (Ig) G antibody (DAKO, United States) overnight at 4°C. After washing twice with PBS with 0.1% Tween20 (0.1% PBS-T) and twice with PBS, the tube was blocked with 2% MPBS for 2 h at RT, and washed again with PBS-T and PBS. The positive pre-incubation mixture was transferred to the coated Immuno tube, incubated 30 mins rotating, and 2 h while standing at RT, and washed 10 times with PBS-T and 10 times with PBS. The bound phage-antibody complexes were eluted with 100mM triethylamine, neutralized with 1M Tris-HCl pH7.4, and amplified by infecting TG1 bacteria and plating on 2xTY plates with 100 μg/ml ampicillin and 2% glucose (2xTYAG). The output of this first SAS round was used as input for three additional positive selection SAS rounds. After elution and neutralization of the output of the fourth and final positive selection round, phage particles were precipitated and used immediately (no amplification) for a round of counterselection using a pool of 20 m-TD plasma samples. Here, phage particles were pre-incubated with the m-TD plasma pool, and phage particles not bound by a rabbit anti-human IgG-coated Immuno tube were amplified by infecting TG1 bacteria and plating on 2xTYAG plates. After this final SAS round, colonies were randomly picked and stored in liquid 2xTYAG medium with 10% glycerol at −80°C. The DNA sequence of the M13 geneVI-cDNA fusion was characterized by Sanger sequencing, and the amino acid sequence of the corresponding displayed antigen was determined using DNAnalyzer software (Quaden et al., 2020). Amino acid homology between the antigen sequences and human proteins was determined using the RefSeq Select proteins database on NCBI and the blastp algorithm, sorted by E-value.
Antibody reactivity in pooled or individual plasma samples against antigens displayed on the surface of purified phage clones was measured using phage ELISA, as described previously (Quaden et al., 2020). In brief, half area 96 well Microlon high-binding microplates (Greiner, Belgium) were coated overnight at 4°C with 2 μg/ml anti-M13 mouse monoclonal antibody (clone MM05T, Sino Biological, China) diluted in coating buffer (0.1 M sodium carbonate bicarbonate buffer, pH9.6). After washing [3 times using phosphate buffered saline (PBS) pH7.4 with 0.1% Tween-20, 1 time using PBS, shaking at RT], plates were blocked in PBS with 2% (w/v) skimmed milk powder (MPBS) for 2 h, shaking at 37°C. After washing, diluted antigen-expressing phage particles (7.0 × 1011 cfu/ml in 2% MPBS) were added, and incubated for 1 h standing at 37°C, followed by 30 mins shaking at RT. After washing, plates were incubated with diluted serum samples (1/100 in 2% MPBS) for 1 h standing at 37°C, and 30 mins shaking at RT. After washing, plates were incubated with cross-adsorbed goat anti-human IgG-Fc, conjugated with horseradish peroxidase (Bethyl, United States) diluted 1/10,000 in 2% MPBS, for 1 h shaking at RT. Finally, after washing, plates were colored with 3,3’,5,5’-tetramethylbenzidine for 10 mins in the dark, stopped using 1.8 M H2SO4, and absorbance was read at 450nm.
For each sample, the antibody reactivity is expressed as the ratio of the average optical density (OD) signal of the antigen-expressing phage clone, over the average OD of an empty phage, not expressing any antigen. All samples were tested in duplicate, and experiments were performed independently at least twice, with a coefficient of variation of the ratio < 20% between experiments. For each antigen, a cut-off for positivity was calculated as the mean antibody reactivity plus 5 times standard deviation of the non-reactive m-TD and m-ASD samples. This non-reactive sample group was determined using change-point analysis using the R package “change-point” selecting the Pruned Exact Linear Time (PELT) algorithm (Killick et al., 2012; Lardeux et al., 2016). Antibody reactivity against a panel of antigens included the combined antibody-positivity for at least one of the antigens included in the panel.
During competition ELISA, plasma samples were pre-incubated with increasing concentrations of synthetic peptide (0−30 μg/ml, >85% purity, GL Biochem, China) corresponding to the peptide sequences of UH-ASD.1, UH-ASD.8, or UH-ASD.12, or using recombinant His-tagged human RPL23 (0−30 μg/ml, Proteintech, United Kingdom) for UH-ASD.5, before being added to the respective antigen-expressing phage and to empty phage in a regular phage ELISA, as described in Quaden et al. (2020). Results are expressed as the ratio of antigen-expressing phage OD over empty phage OD. For UH-ASD.1, the antigenic peptide sequence following the cloning adaptor was used (UH-ASD.1: GKIRQPIGLF), while UH-ASD.8 was tested as three partially overlapping peptides, UH-ASD.8.1 (ASVPEYTGPRLYKEPSAKSNKFIIHNALS), UH-ASD.8.2 (SNKFIIHNALSHCCLAGKVNEPQKNRIL), and UH-ASD.8.3 (VNEPQKNRILEEIEKSKANHFLILFRDS). UH-ASD.12 was elongated with 5 N-terminal amino acids originating from the translated cloning adaptor (UH-ASD.12.1 SRPRDAATTF).
Post-test probability was calculated from pre-test odds and the positive likelihood ratio (LR+) of the applied test. Pre-test probability for full siblings was derived by multiplying the ASD prevalence of the general population (1.85%) (Maenner et al., 2020) with the relative risk for ASD in full siblings (9.3) (Hansen et al., 2019). Pre-test probability for preterm births was obtained from ASD prevalence in preterm infants (Agrawal et al., 2018). The LR+ for the M-CHAT was obtained from the pooled sensitivity (83%) and specificity (53%) of 13 studies (Yuen et al., 2018). The LR+ for the (early) SCQ at 36 months with cut-off 15, cut-off 11 for ASD with phrase speech, and cut-off 11 without phrase speech, was obtained from the sensitivity (respectively 20%, 34%, and 69%) and specificity (respectively 99%, 89%, and 89%) of a large sample of the Norwegian Mother and Child Cohort Study (Suren et al., 2019). The LR+ for the ADOS was obtained from the pooled sensitivity (94%) and specificity (80%) of 12 studies (Randall et al., 2018).
All statistical analyses were performed using SAS JMP Pro version 14, and a P < 0.05 was considered statistically significant. The presence of antibodies against individual or panels of antigens, was compared using Fisher’s exact test between m-ASD and m-TD groups.
To analyze correlations between anti-UH-ASD seropositivity in m-ASD samples, and corresponding clinical and demographic characteristics in mothers and their children with ASD, the Fisher’s exact test was used for categorical variables, the Student’s t-test was used for variables with parametric distribution, and the Wilcoxon/Kruskal Wallis tests was used for variables with non-parametric distribution.
To identify novel IgG isotype m-ASD autoantibodies with reactivity toward human fetal brain antigens, an in vitro representation of the proteins expressed in the developing human fetal brain was created. To this end, the mRNA expressed in human fetal brains from weeks 20 till 33 of gestation was converted to cDNA, and randomly fused to the filamentous phage gene VI, resulting in a cDNA phage display library consisting of 3.95 × 106 recombinant clones. Sequencing analysis showed that the cDNA coding sequences of approximately 14%, or 5.5 × 105 clones of this phage display library were fused in frame with phage gene VI, which therefore correctly express (fragments of) known human proteins. The remaining 86% of this library consists of out-of-frame cDNA fusions or fusion to non-coding cDNA sequences, which result in the expression of non-physiological peptides.
This novel fetal brain cDNA phage display library was used to screen for antibody reactivity in pooled m-ASD plasma samples from the Simons Simplex Collection (SSC) of the Simons Foundation Autism Research Initiative (SFARI) (Table 1) using SAS (Somers et al., 2005; Vandormael et al., 2017). These m-ASD samples had been obtained at ASD diagnosis of the child, at which time the child was between 3 and 7 years, and had no younger siblings. Sequencing analysis of a selection of isolated phage clones identified 149 novel candidate antigenic targets of m-ASD antibodies. Antibody reactivity against each of these individual antigens was measured by phage ELISA using pools of m-ASD (5 pools of 10 samples each) and m-TD controls (2 pools of 10 samples each), resulting in the selection of 33 antigens (called UH-ASD.1 till UH-ASD.33, Supplementary Table 1) with increased antibody reactivity in m-ASD pools (results not shown). A preliminary screening in a subpopulation of individual samples resulted in the selection of 6 antigens that were further analyzed (results not shown).
DNA sequencing of the fusion between filamentous phage gene VI and the human fetal brain cDNA insert, allowed to determine the amino acid (aa) sequence of the antigens expressed on the phage surface (Table 2). The 6 selected antigens, called UH-ASD.1, UH-ASD.5, UH-ASD.7, UH-ASD.8, UH-ASD.12, and UH-ASD.19, were composed of protein sequences between 5 and 134 aa in length (Table 2). Of these, UH-ASD.1, UH-ASD.12, and UH-ASD.19 are non-physiological peptides resulting from the fusion to, and translation of non-coding DNA sequences. However, these antigens show partial homology with several human proteins (Table 2). On the other hand, UH-ASD.5, UH-ASD.7 and UH-ASD.8 correctly express fragments of known human proteins. The sequence of the UH-ASD.5 antigen corresponds almost entirely to the full-length sequence of the 60S ribosomal protein L23 (RPL23) of 140 aa, lacking only the 6 N-terminal aa. The UH-ASD.7 antigen consists of the last 12 C-terminal aa of the 335 aa glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Finally, UH-ASD.8 contains an internal 64 aa part (aa 1129-1192) of calmodulin-regulated spectrin-associated protein family member 3 (CAMSAP3). This sequence comprises 57 aa of the so-called CKK domain, a C-terminal domain common to CAMSAP family proteins, which is required for binding to microtubules (Baines et al., 2009).
Antibody reactivity against each of the 6 novel UH-ASD antigens was determined in individual plasma samples of 238 m-ASD and 90 m-TD using phage ELISA. Antibody reactivity against these individual antigens was present in 2.5% (6/238) to 19.3% (46/238) of m-ASD, and in 0% (0/90) to 14.4% (13/90) of m-TD samples (Table 3). The UH-ASD.7 (GAPDH) antigen showed the highest antibody reactivity in both the m-ASD and the m-TD groups. For each of the 6 UH-ASD antigens, antibody reactivity was higher in the m-ASD than in the m-TD group, albeit not significant. In order to be able to discriminate m-ASD from m-TD samples, the 4 antigens with the highest positive likelihood ratio (LR+), UH-ASD.1, UH-ASD.5 (RPL23), UH-ASD.8 (CAMSAP3), and UH-ASD.12, were combined into a panel. Combined antibody reactivity against at least one of the 4 UH-ASD antigens of this panel was significantly higher in m-ASD (16%) compared to m-TD (4.4%) samples (p = 0.005). Similarly, a second panel of antigenic targets was composed of the 3 autoantigens UH-ASD.5 (RPL23), UH-ASD.7 (GAPDH), and UH-ASD.8 (CAMSAP3), which showed more autoantibody reactivity in m-ASD samples (25.2%) compared to m-TD controls (15.6%), but could not significantly discriminate between these two groups (p = 0.075).
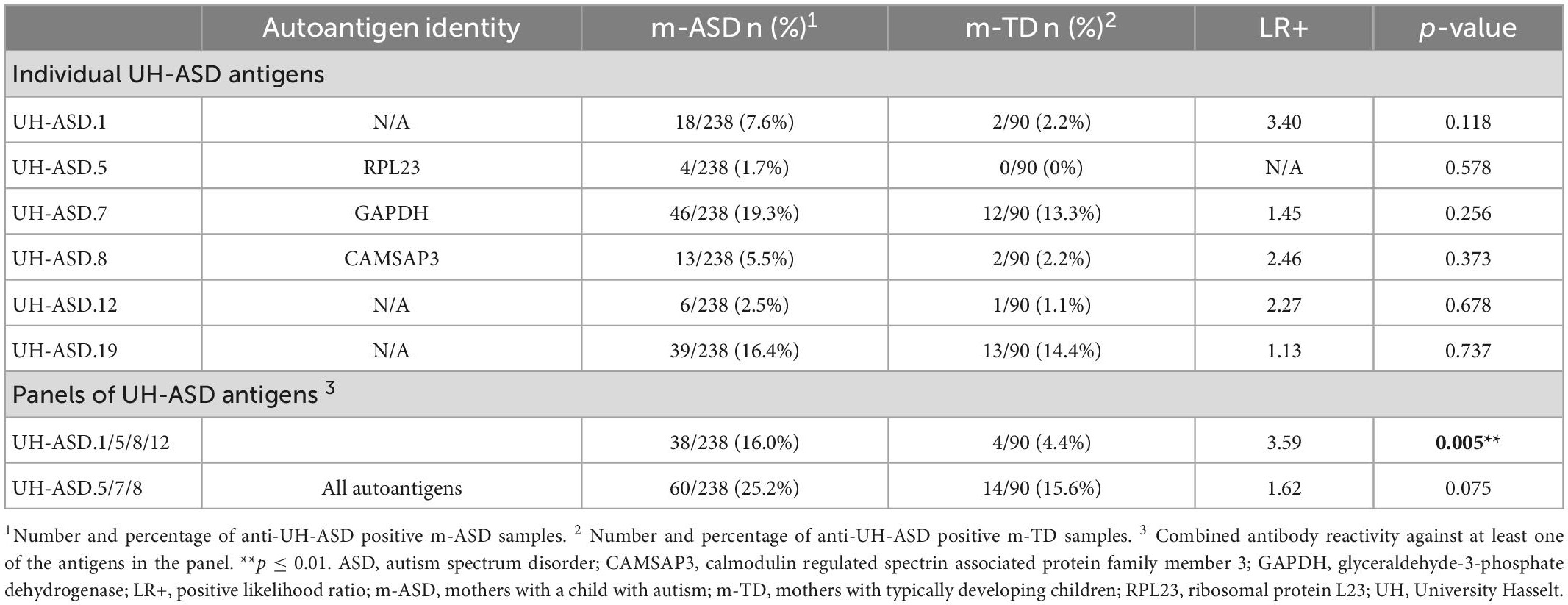
Table 3. Antibody reactivity to individual and combinations of UH-ASD antigens in m-ASD and m-TD samples.
Determination of antibody reactivity against our UH-ASD antigens has been performed using phage ELISA, where each respective antigen was displayed at the surface of phage particles. In order to confirm that the m-ASD antibodies specifically bind to the displayed antigen, a competition ELISA using synthetic peptide or recombinant protein was performed for the antigens of the panel composed of UH-ASD.1, UH-ASD.5 (RLP23), UH-ASD.8 (CAMSAP3), and UH-ASD.12. Clear competition could be seen using synthetic peptides for the short UH-ASD.1 and UH-ASD.12 antigens, which were formed by the expression of non-coding sequences (Figures 1A, B). Antibody specificity to the 1129 to 1192 aa fragment of CAMSAP3, expressed by UH-ASD.8, was tested using three overlapping synthetic peptides (UH-ASD.8.1, 8.2, and 8.3). Since the UH-ASD.8.1 peptide showed evident competition, while the partially overlapping peptide UH-ASD.8.2 did not, antibody reactivity could be narrowed down to a region between aa 1129 and 1146 of CAMSAP3 (Figure 1C). On the other hand, competition using recombinant full length RPL23 protein could not lower binding to UH-ASD.5 (RLP23) under the tested conditions (data not shown).
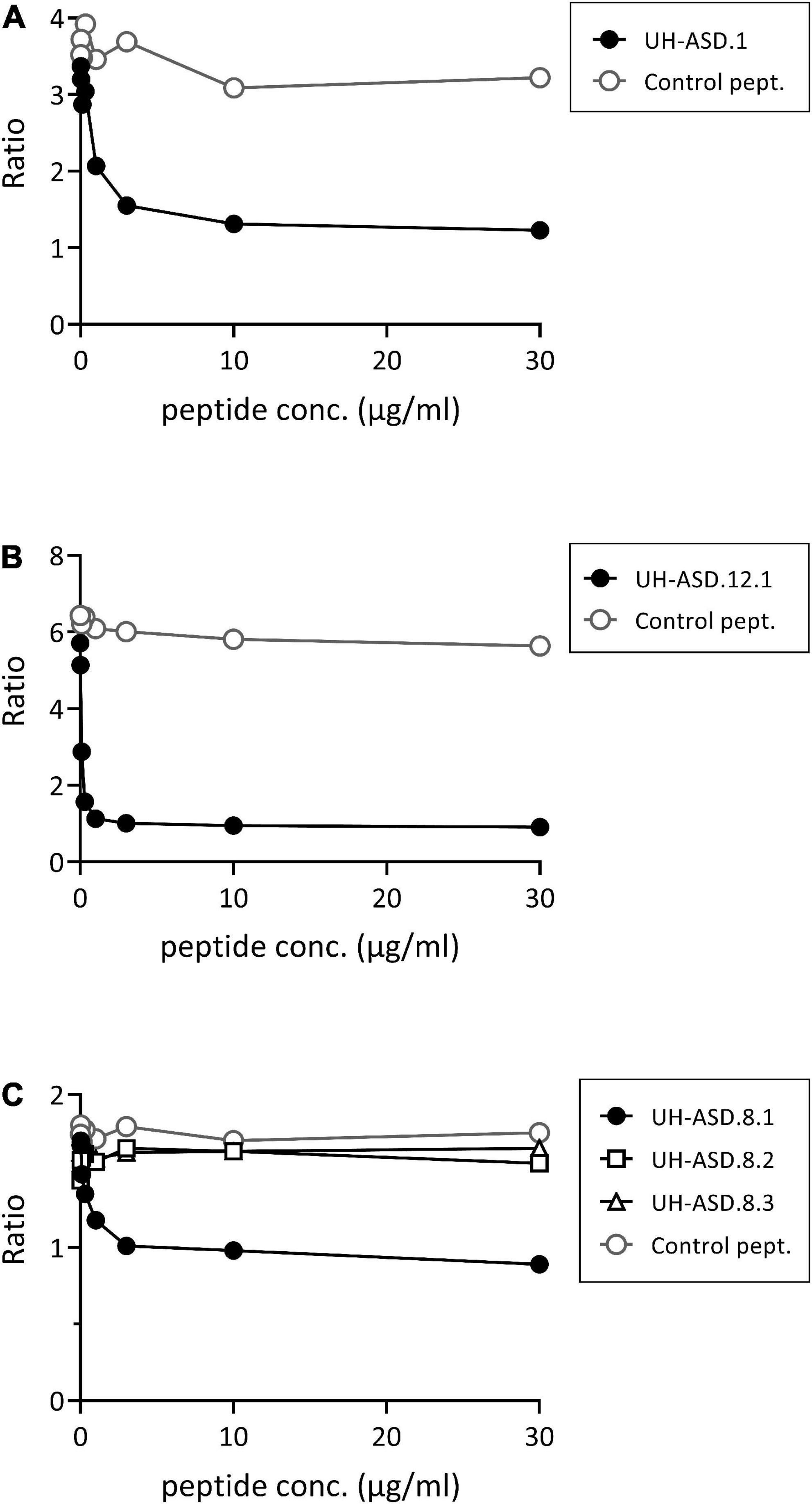
Figure 1. Competition for binding phage-displayed antigens using synthetic peptides. In a phage ELISA, an antibody-positive plasma sample was pre-incubated in solution with increasing amounts of synthetic peptide, corresponding to the respective displayed antigens of UH-ASD.1 (A), UH-ASD.12.1 (B), or three partially overlapping peptides for UH-ASD.8 (8.1, 8.2, and 8.3) (C). As a control the samples were pre-incubated with similar amounts of a control peptide. Antibody reactivity is expressed as the ratio of OD specific phage/OD empty phage. ASD, autism spectrum disorder; UH, University Hasselt.
Demographic and clinical parameters of mothers, and of their child with ASD, were compared in m-ASD samples that were seropositive or seronegative for antibodies against the panel of UH-ASD.1/5/8/12 antigens (Table 4). The maternal parameters that were investigated, i.e., maternal age at birth and maternal autoimmune disease, were not significantly different in m-ASD samples that were antibody positive or negative for this antigenic panel. Likewise, presence or absence of maternal antibody reactivity against the UH-ASD.1/5/8/12 antigens, was not correlated with any of the parameters of the child with ASD, including gender, gestational age, age at diagnosis, Calibrated Severity Score (CSS), Aberrant Behavior Checklist (ABC), IQ and Verbal IQ, regression, presence of other developmental disorders, and febrile and non-febrile seizures.
Finally, we explored whether testing for antibodies against the UH-ASD.1/5/8/12 antigens could provide added value during the diagnostic process. We selected a number of diagnostic trajectories involving three mains steps, starting from at-risk groups, followed by the application of a screening instrument, and finally a diagnostic instrument (Figure 2). In these trajectories, the effect of additional testing for anti-UH-ASD.1/5/8/12 antibodies during the screening step, was calculated. In each consecutive step, the pre- or post-test probability for actually having ASD was calculated in case the tests gave a positive result (Figure 2). To this end, we used reported prevalences and positive likelihood ratios for siblings of a child with ASD (Hansen et al., 2019; Maenner et al., 2020), or children with preterm birth (Agrawal et al., 2018) as risk groups, for the M-CHAT (Yuen et al., 2018), or the early SCQ (Yuen et al., 2018; Suren et al., 2019) as screening instruments, and finally, for the ADOS (Randall et al., 2018) as a diagnostic instrument. When testing for anti-UH-ASD.1/5/8/12 antibodies would be added in combination with the M-CHAT screening, the post-test probability after a positive ADOS could be increased from 62 to 87% for siblings, and from 38 to 68% for preterm births (Figure 2). Screening siblings and children with preterm birth using the regular SCQ cut-off of 15, already gave sufficiently high post-test probabilities after ADOS, of approximately 90% or higher, even in the absence of additional antibody testing (not shown). On the other hand, the lower SCQ cut-off of 11 is frequently used to increase the sensitivity in younger children (Barnard-Brak et al., 2016; Moody et al., 2017), but because of lower specificity, this also decreases the probability for correct diagnosis after ADOS (Figure 2). Using this lower SCQ cut-off, additional testing for anti-UH-ASD.1/5/8/12 antibodies again increased the post-test probability after a positive ADOS, from 75 to 92% for siblings, and from 52 to 80% for preterm births, in case phrase speech was present in the SCQ. In the absence of phrase speech during the SCQ, testing for our antibody panel would only cause a meaningful increase in post-test probability for the preterm births, increasing from 69 to 89%.
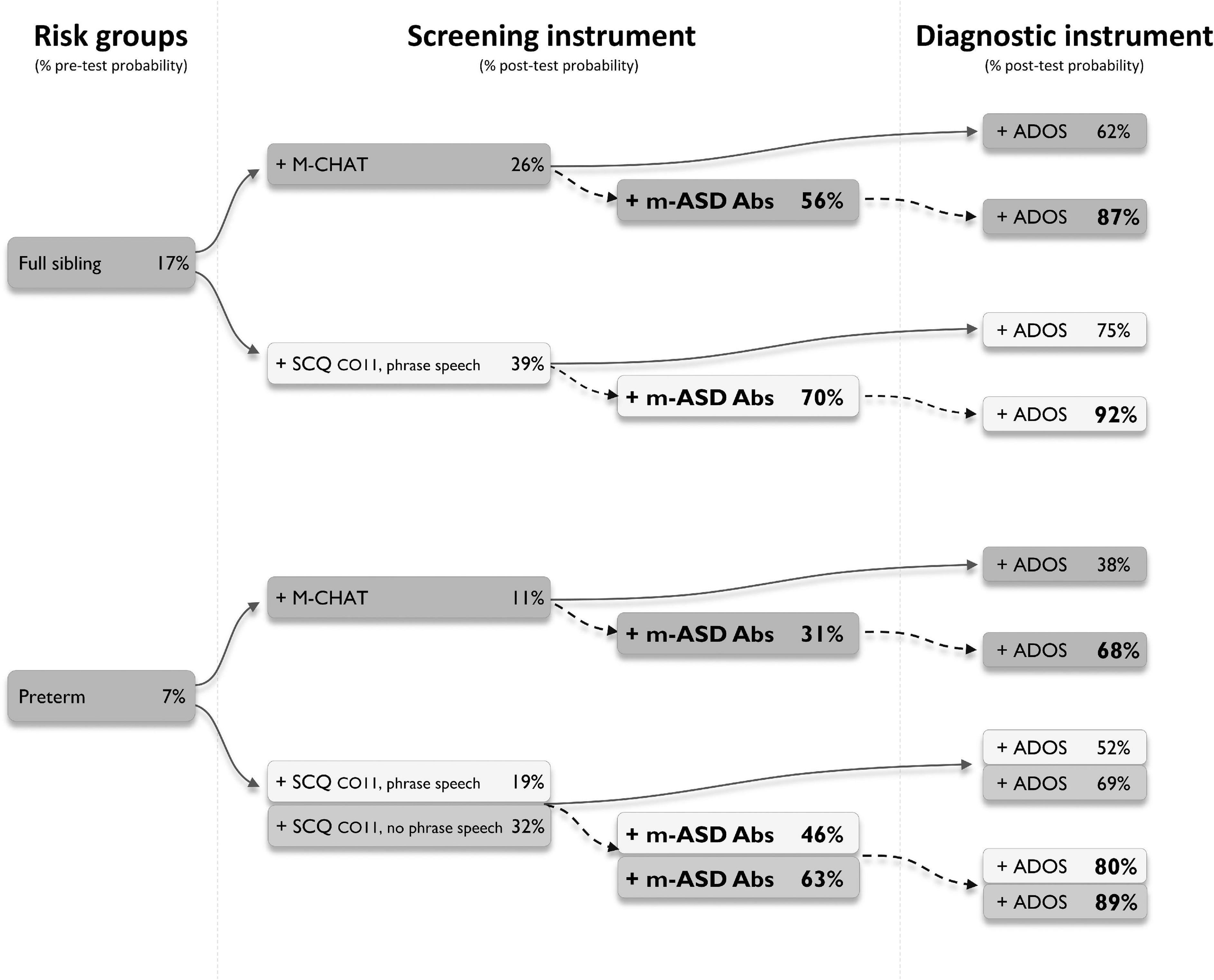
Figure 2. Simulated diagnostic trajectories with and without additional m-ASD antibody testing. Different diagnostic trajectories were studied, and the stepwise increase in post-test probability for having autism spectrum disorder (ASD) was calculated, when a person would be part of an ASD risk group (full ASD sibling or preterm), would be positive for one of the indicated screening instruments (M-CHAT or SCQ), and would be positive for the ADOS as a diagnostic instrument (solid arrows). For each trajectory, the added value of testing for anti-UH-ASD.1/5/8/12 antibodies was calculated (dashed arrows). Pre-test probability for full siblings was obtained from Killick et al. (2012), Maenner et al. (2020), for preterm births from Lardeux et al. (2016). Positive likelihood ratios to calculate post-test probabilities for M-CHAT were obtained from Hansen et al. (2019), for early SCQ from Agrawal et al. (2018), and for ADOS from Randall et al. (2018). ADOS, Autism Diagnostic Observational Schedule; CO, cut-off; M-CHAT, Modified Checklist for Autism in Toddlers; m-ASD Abs, maternal autism spectrum disorder antibodies; SCQ, Social Communication Questionnaire.
In the current study, we have identified novel m-ASD antibodies against 6 antigens originating from a human fetal brain library, and validated their reactivity in mothers of a child with autism and mothers of typically developing children. Three of these antigens, UH-ASD.5, UH-ASD.7, and UH-ASD.8, correspond to parts of proteins expressed in the human fetal brain, respectively RPL23, GAPDH, and CAMSAP3. This is the first time that these three proteins have been identified as targets of maternal autoantibodies in ASD. Furthermore, antibody reactivity against a panel of 4 UH-ASD antigens could increase the probability of a correct early diagnosis in certain risk populations, when applied in conjunction with early screening instruments.
We have used the SAS technology to screen for novel m-ASD autoantibodies. A custom-made cDNA phage display library was generated from mRNA expressed in human fetal brains, which thus forms an in vitro representation of the antigens expressed during human fetal brain development. The use of the SAS technology has proven to be a successful method for identifying novel antibody biomarkers in other diseases such as rheumatoid arthritis, axial spondylarthritis, multiple sclerosis and spinal cord injury (Somers et al., 2008, 2011; Palmers et al., 2016; Quaden et al., 2020). This is the first time that human fetal brain antigens have been used in a comprehensive screening for targets of m-ASD antibodies, as previous studies have screened starting from protein extracts of Rhesus macaque fetal brain (Braunschweig et al., 2013), or used a candidate-based approach (Brimberg et al., 2016). With approximately 5.5 × 105 protein coding genes cloned in the correct reading frame, our phage display library has an average 29-fold coverage of the number of protein coding genes in the human genome (Ezkurdia et al., 2014). However, because of the starting material used to create this library, it will be highly enriched for genes which are expressed in the human brain during weeks 20 to 33 of fetal development. During this period, several processes of brain development have been linked to ASD, such as neurogenesis, neuronal migration, synaptogenesis and gliogenesis, and the beginning of myelination (reviewed by Estes and McAllister, 2016).
Our SAS screening resulted in the identification of 149 novel candidate fetal brain antigens. Initial testing for antibody reactivity in m-ASD and m-TD samples resulted in six antigens, UH-ASD.1, UH-ASD.5 (RLP23), UH-ASD.7 (GAPDH), UH-ASD.8 (CAMSAP3), UH-ASD.12, and UH-ASD.19 with increased antibody reactivity in m-ASD samples. Further validation in a large number of m-ASD and m-TD plasma samples showed that antibody reactivity against at least one of the 4 antigens with the highest LR+, UH-ASD.1, UH-ASD.5, UH-ASD.8, and UH-ASD.12, is significantly more present in m-ASD than in m-TD, with a sensitivity of 16.0% and a specificity of 95.6%. Presence of these antibodies did not correlate with the clinical characteristics that were investigated, neither of the mothers, nor from their children with ASD. Consequently, these antibodies could not identify subpopulations of ASD individuals based on phenotype, probably due to their limited presence. Of note, mothers that were positive for these antibodies did not show increased prevalence of a number of autoimmune diseases that are characterized by the presence of autoantibodies, such as rheumatoid arthritis and systemic lupus erythematosus. Therefore, the antibodies with self-reactivity identified in this study do not seem to originate from intrinsic autoimmune processes or disorders in the mother, although maternal autoimmunity has been implicated with increased risk for development of autism in the child (Chen et al., 2016; Wojcik et al., 2017; Spann et al., 2019).
Early identification of children with ASD is critical for early intervention, which has been shown to increase the chance of a better outcome and reduce the long-term healthcare cost (Peters-Scheffer et al., 2012; Estes et al., 2015; Landa, 2018). In recent years, general developmental screening, but also screening using ASD-specific tools, such as M-CHAT and SCQ, have been introduced in primary care routines as a first-line tool for identifying children at risk (Robins et al., 2001; Eaves et al., 2006; Hyman et al., 2020). However, the performance of these screening tools is not always sufficient to identify ASD individuals, especially in young children. When applied in a universal setting, these screening instruments have the limitation of low probability of correctly identifying children with ASD, because of the relatively low ASD prevalence in the general population (Yuen et al., 2018; Suren et al., 2019). Even focusing on the at-risk populations of siblings, and children with preterm births, leaves much room for improvement, depending on the screening instrument used. In this study, we determined the added value of our newly identified antibody biomarkers in calculating the post-test probability for correct diagnosis in a number of different scenarios, combining at-risk populations, the M-CHAT and early SCQ screening tools, and the ADOS for diagnosis. Adding antibody testing to the M-CHAT screening instrument greatly increased the confidence in the final diagnosis. Testing positive for anti-UH-ASD.1/5/8/12 antibodies could also clearly increase the post-test probability when combined with screening using SCQ obtained at 36 months, especially when the lower cut-off value of 11 was used, which allows a much higher sensitivity in younger children (Barnard-Brak et al., 2016; Moody et al., 2017). In children who show early phrase speech, which represent about 76% of the children with ASD (Suren et al., 2019), this benefit of additional antibody testing showed the largest added value. A major advantage of these antibody biomarkers is that they can be easily measured in the maternal blood at early time points during the child’s development. One of the limitations is that the m-ASD sample would only be positive in about 16% of cases using these specific biomarkers. Application of a panel of additional early biomarkers, which can be other similar maternal serological markers, but also EEG, eye tracking, genetic or metabolic markers, could increase the number of children with ASD that are correctly recognized in an early phase (Vanaken et al., 2021).
Functional studies in animal models have shown that maternal autoantibodies which target human fetal brain proteins can enter fetal brain tissue in utero, induce modifications in the developing brain, and trigger ASD-like behaviors in the offspring (Garty et al., 1994; Zimmerman et al., 2007; Croen et al., 2008; Martin et al., 2008; Singer et al., 2008, 2009; Goines et al., 2011; Palmeira et al., 2012; Braunschweig et al., 2013; Brimberg et al., 2013; Jones et al., 2020). Studying such autoantibodies and their target antigens can therefore provide a better understanding of the underlying causes of disease. In the future, such antibodies might be used to better inform patients, they might allow physicians to make targeted therapy choices, and can also provide possible novel points of entry for pharmaceutical companies, to tackle the underlying disease process at the molecular level. In this study, we found 3 novel m-ASD autoantibodies, directed respectively against RPL23, the C-terminus of GAPDH, and part of the tubulin-binding domain (CKK domain) of CAMSAP3. RPL23 is a structural protein of the large ribosomal subunit (60S), and has not yet been associated with ASD. However, many components of the translation machinery have been described as autism risk genes (reviewed in Chen et al., 2019), and a strong signature or translational downregulation has been described in male fetal brains after maternal immune activation (Kalish et al., 2021). The dehydrogenase GAPDH is a glycolytic enzyme which catalyzes the oxidation of glyceraldehyde-3-phosphate into 1,3-bisphosphoglycerate. Anti-GAPDH autoantibodies are not specific for m-ASD, and have been described in autoimmune diseases such as systemic lupus erythematosus (SLE), and have been related to cognitive dysfunction in these patients (Delunardo et al., 2016). Anti-GAPDH antibodies have also been detected in patients with schizophrenia and major depression, and administration of these immunoglobulins to mice leads to cognitive and behavioral alterations (Delunardo et al., 2016). Remarkably, autoantibodies against other glycolytic enzymes, i.e., lactate dehydrogenase (LDH) A and B, and neuron-specific enolase, have been described in m-ASD samples (Braunschweig et al., 2013; Ramirez-Celis et al., 2020). These autoantibodies might even actively contribute to the development of autistic behaviors, as female mice which have been immunized against 4 antigens, including LDH-A and LDH-B, produce offspring with altered development and social interactions (Jones et al., 2020). Finally, CAMSAP3 is an essential protein in the organization of non-centrosomal microtubules, stabilizing their minus ends through the CKK domain (Baines et al., 2009). Through its regulation of these microtubules, CAMSAP3 is a key player in cell polarity in specific cell types such as neurons and epithelial cells (Toya et al., 2016; Pongrakhananon et al., 2018; Chen et al., 2020). CAMSAP3 is a leading player in axon development, and its loss in neurons leads to formation of multiple axons (Pongrakhananon et al., 2018). Alterations in the morphology of neurons, changes in polarity, and consequently, modifications in the connectivity, have been described both in individuals with ASD and in models of ASD, which makes CAMSAP3 an interesting candidate target in the study of the etiology of the disease (Donovan and Basson, 2017). Of note, RPL23, GAPDH, and CAMSAP3 are intracellular proteins, and whether or how autoantibodies targeting them would affect brain development, would need to be further explored. It has been shown that autoantibodies targeting the intracellular proteins LDH-A and LDH-B, STIP1 and CRMP1 are able to induce autism-related behavioral changes in a mouse model, however, but how these antibodies target their antigens is also still unknown (Jones et al., 2020). In addition, RPL23, GAPDH, and CAMSAP3, and most of the described m-ASD antigens described by others, are not uniquely expressed in the fetal brain, and are also found postnatally, and in other tissues.
For the remaining three anti-UH-ASD antibodies, the exact in vivo antigens are currently not known, as they are reactive against peptides generated from the translation of non-coding sequences (UH-ASD.1, UH-ASD.12, and UH-ASD.19). These peptides probably form mimotopes, which are sequences that mimic the epitopes the antibodies were initially formed against. Nevertheless, some of these mimotope antigens show aa homology with proteins implicated in ASD, such as Teashirt homolog 3, whose gene deletion causes a syndrome with high autism prevalence in affected patients (Caubit et al., 2016), and ASD-like behavioral deficits in a knockout mouse model (Chabbert et al., 2019). Still, whether anti-UH-ASD antibodies are actually directed against these, or other human proteins, remains to be investigated.
The results of this study should be interpreted while considering some limitations. Our novel fetal brain cDNA phage display library probably cannot account for autism-specific (neo)antigens that would arise from specific mutations or altered gene splicing, or for genes specifically expressed before, or after 20 to 33 weeks of gestation. Moreover, because of our cloning method, expression of human proteins is biased toward C-terminal fragments. In addition, bacterial expression of our cDNA phage display library gives constraints with respect to protein folding, post-translational modification and the expression of membrane proteins.
Secondly, although phage particles of the UH-ASD.5 antigen express 134 of the 140 amino acids of human RPL23, competition experiments could not definitely confirm that anti-UH-ASD.5 antibody reactivity was directed against the RPL23 protein. This might be caused by differences in folding between RPL23, expressed on the phage surface and recombinant RPL23, or anti-UH-ASD.5 antibody reactivity might be directed against an epitope formed by the fusion site between the adaptor used for cloning, and the beginning of the RPL23 sequence. Therefore, as our described reactivity values of the antibody panel rely upon UH-ASD.5, further determination of the exact epitope is required before use in clinical settings.
Finally, as we used maternal plasma samples taken at the time of diagnosis of the child, we need to assume that the presence of antibodies at the time of sampling is representative for the situation during the pregnancy, 3−7 years earlier. In most persons, the presence and levels of protective antibodies against pathogens, or of disease-associated autoantibodies, are stable over a period of several years to decades (Amanna et al., 2007; Gkrouzman et al., 2021). However, the antibody reactivities described in the current study should be confirmed in maternal plasma samples obtained during pregnancies of children which went on to develop autism later, such as from the Finnish or Norwegian birth cohorts (Lampi et al., 2011; Magnus et al., 2016), which are coupled to clinical data involving long-term follow up of child development. Finally, in the future, the specificity of our UH-ASD antibodies should be studied in maternal samples coupled to children who do not have ASD, but show conditions that are often ASD co-morbidities such as ADHD, epilepsy or mental impairment.
Maternal antibodies against a combination of 4 novel human fetal brain antigens are increased in our cohort of mothers of children with autism. These antibodies can provide a novel tool to support the early recognition of children with ASD, providing added value when used in combination with early screening tools in at-risk populations. Moreover, the discovery of autoantibodies directed against the fetal brain antigens RPL23, GAPDH, and CAMSAP3, could provide further understanding the role of maternal autoantibodies in ASD pathophysiology.
The datasets presented in this article are not readily available because data are available upon reasonable request. The authors commit to making the relevant anonymized reactivity and patient data available for a specified purpose approved by the institution and the principal investigator of the study, and with a signed data access agreement. Requests to access the datasets should be directed to VS, dmVlcmxlLnNvbWVyc0B1aGFzc2VsdC5iZQ==.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee UHasselt. The patients/participants provided their written informed consent to participate in this study.
PV, BB, and VS designed the study. RM-C and PV acquired the experimental data and drafted the manuscript. RM-C, JL, and PV performed the data analysis. BB, VS, and JL revised the manuscript critically for the important intellectual content. All authors were involved in interpretation of data and approved the final draft for publication.
This work was supported by the Research Foundation - Flanders [SB-FWO, 1S54717 (RM-C) and G098618N (PV)] and a Simons Foundation Autism Research Initiative - Explorer Award (SFARI, 534849).
The authors thank Dr. Marc Raes (Jessa Hospital) for help with m-TD recruitment, Kim Ulenaers, Veronique Pousset, and Anne Bogaers (Hasselt University, Biomedical Research Institute) for assistance with the sample collection, and Igna Rutten and Josianne Bleus (Hasselt University, Biomedical Research Institute) for their excellent technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2023.1067833/full#supplementary-material
Agrawal, S., Rao, S., Bulsara, M., and Patole, S. (2018). Prevalence of autism spectrum disorder in preterm infants: A Meta-analysis. Pediatrics 142:e20180134. doi: 10.1542/peds.2018-0134
Amanna, I., Carlson, N., and Slifka, M. (2007). Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915. doi: 10.1056/NEJMoa066092
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders, Fifth Edition (DSM-V), 5 th Edn. Washington, DC: American Psychiatric Association. doi: 10.1176/appi.books.9780890425596
Bagnall-Moreau, C., Huerta, P., Comoletti, D., La-Bella, A., Berlin, R., Zhao, C., et al. (2020). In utero exposure to endogenous maternal polyclonal anti-Caspr2 antibody leads to behavioral abnormalities resembling autism spectrum disorder in male mice. Sci. Rep. 10:14446. doi: 10.1038/s41598-020-71201-9
Baines, A., Bignone, P., King, M., Maggs, A., Bennett, P., Pinder, J., et al. (2009). The CKK domain (DUF1781) binds microtubules and defines the CAMSAP/ssp4 family of animal proteins. Mol. Biol. Evol. 26, 2005–2014. doi: 10.1093/molbev/msp115
Baird, G., Charman, T., Baron-Cohen, S., Cox, A., Swettenham, J., Wheelwright, S., et al. (2000). A screening instrument for autism at 18 months of age: A 6-year follow-up study. J. Am. Acad. Child Adolesc. Psychiatry 39, 694–702. doi: 10.1097/00004583-200006000-00007
Barnard-Brak, L., Brewer, A., Chesnut, S., Richman, D., and Schaeffer, A. (2016). The sensitivity and specificity of the social communication questionnaire for autism spectrum with respect to age. Autism Res. 9, 838–845. doi: 10.1002/aur.1584
Braunschweig, D., Krakowiak, P., Duncanson, P., Boyce, R., Hansen, R., Ashwood, P., et al. (2013). Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl. Psychiatry 3:e277. doi: 10.1038/tp.2013.50
Brimberg, L., Mader, S., Jeganathan, V., Berlin, R., Coleman, T., Gregersen, P., et al. (2016). Caspr2-reactive antibody cloned from a mother of an ASD child mediates an ASD-like phenotype in mice. Mol. Psychiatry 21, 1663–1671. doi: 10.1038/mp.2016.165
Brimberg, L., Sadiq, A., Gregersen, P., and Diamond, B. (2013). Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol. Psychiatry 18, 1171–1177. doi: 10.1038/mp.2013.101
Caubit, X., Gubellini, P., Andrieux, J., Roubertoux, P., Metwaly, M., Jacq, B., et al. (2016). TSHZ3 deletion causes an autism syndrome and defects in cortical projection neurons. Nat. Genet. 48, 1359–1369. doi: 10.1038/ng.3681
Chabbert, D., Caubit, X., Roubertoux, P., Carlier, M., Habermann, B., Jacq, B., et al. (2019). Postnatal Tshz3 deletion drives altered corticostriatal function and autism spectrum disorder-like behavior. Biol. Psychiatry 86, 274–285. doi: 10.1016/j.biopsych.2019.03.974
Chen, S., Zhong, X., Jiang, L., Zheng, X., Xiong, Y., Ma, S., et al. (2016). Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behav. Brain Res. 296, 61–69. doi: 10.1016/j.bbr.2015.08.035
Chen, Y., Chang, Y., and Huang, Y. (2019). Dysregulated translation in neurodevelopmental disorders: An overview of autism-risk genes involved in translation. Dev. Neurobiol. 79, 60–74. doi: 10.1002/dneu.22653
Chen, Y., Zheng, J., Li, X., Zhu, L., Shao, Z., Yan, X., et al. (2020). Wdr47 controls neuronal polarization through the camsap family microtubule minus-end-binding proteins. Cell Rep. 31:107526. doi: 10.1016/j.celrep.2020.107526
Choi, G., Yim, Y., Wong, H., Kim, S., Kim, H., Kim, S., et al. (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939. doi: 10.1126/science.aad0314
Croen, L., Braunschweig, D., Haapanen, L., Yoshida, C., Fireman, B., Grether, J., et al. (2008). Maternal mid-pregnancy autoantibodies to fetal brain protein: The early markers for autism study. Biol. Psychiatry 64, 583–588. doi: 10.1016/j.biopsych.2008.05.006
de la Torre-Ubieta, L., Won, H., Stein, J., and Geschwind, D. (2016). Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 22, 345–361. doi: 10.1038/nm.4071
Delobel-Ayoub, M., Saemundsen, E., Gissler, M., Ego, A., Moilanen, I., Ebeling, H., et al. (2020). Prevalence of autism spectrum disorder in 7-9-year-old children in Denmark, Finland, France and Iceland: A population-based registries approach within the ASDEU project. J. Autism Dev. Disord. 50, 949–959. doi: 10.1007/s10803-019-04328-y
Delunardo, F., Soldati, D., Bellisario, V., Berry, A., Camerini, S., Crescenzi, M., et al. (2016). Anti-GAPDH autoantibodies as a pathogenic determinant and potential biomarker of neuropsychiatric diseases. Arthritis Rheumatol. 68, 2708–2716. doi: 10.1002/art.39750
Donovan, A., and Basson, M. (2017). The neuroanatomy of autism - a developmental perspective. J. Anat. 230, 4–15. doi: 10.1111/joa.12542
Eaves, L., Wingert, H., Ho, H., and Mickelson, E. (2006). Screening for autism spectrum disorders with the social communication questionnaire. J. Dev. Behav. Pediatr. 27(2 Suppl.), S95–S103. doi: 10.1097/00004703-200604002-00007
Estes, A., Munson, J., Rogers, S., Greenson, J., Winter, J., and Dawson, G. (2015). Long-term outcomes of early intervention in 6-year-old children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 54, 580–587. doi: 10.1016/j.jaac.2015.04.005
Estes, M., and McAllister, A. (2016). Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777. doi: 10.1126/science.aag3194
Ezkurdia, I., Juan, D., Rodriguez, J., Frankish, A., Diekhans, M., Harrow, J., et al. (2014). Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum. Mol. Genet. 23, 5866–5878. doi: 10.1093/hmg/ddu309
Fischbach, G., and Lord, C. (2010). The Simons Simplex Collection: A resource for identification of autism genetic risk factors. Neuron 68, 192–195. doi: 10.1016/j.neuron.2010.10.006
Frye, R., Vassall, S., Kaur, G., Lewis, C., Karim, M., and Rossignol, D. (2019). Emerging biomarkers in autism spectrum disorder: A systematic review. Ann. Transl. Med. 7:792. doi: 10.21037/atm.2019.11.53
Garty, B., Ludomirsky, A., Danon, Y., Peter, J., and Douglas, S. (1994). Placental transfer of immunoglobulin G subclasses. Clin. Diagn. Lab. Immunol. 1, 667–669. doi: 10.1128/cdli.1.6.667-669.1994
Gkrouzman, E., Sevim, E., Finik, J., Andrade, D., Pengo, V., Sciascia, S., et al. (2021). Antiphospholipid antibody profile stability over time: Prospective results from the APS ACTION clinical database and repository. J. Rheumatol. 48, 541–547. doi: 10.3899/jrheum.200513
Goines, P., Haapanen, L., Boyce, R., Duncanson, P., Braunschweig, D., Delwiche, L., et al. (2011). Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav. Immun. 25, 514–523. doi: 10.1016/j.bbi.2010.11.017
Gotham, K., Pickles, A., and Lord, C. (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J. Autism Dev. Disord. 39, 693–705. doi: 10.1007/s10803-008-0674-3
Han, V., Patel, S., Jones, H., and Dale, R. (2021). Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579. doi: 10.1038/s41582-021-00530-8
Hansen, S., Schendel, D., Francis, R., Windham, G., Bresnahan, M., Levine, S., et al. (2019). Recurrence risk of autism in siblings and cousins: A multinational, population-based study. J. Am. Acad. Child Adolesc. Psychiatry 58, 866–875. doi: 10.1016/j.jaac.2018.11.017
Hyman, S., Levy, S., Myers, S., and Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics (2020). Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics 145:e20193447. doi: 10.1542/9781610024716-part01-ch002
Jones, K., Pride, M., Edmiston, E., Yang, M., Silverman, J., Crawley, J., et al. (2020). Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Mol. Psychiatry 25, 2994–3009. doi: 10.1038/s41380-018-0126-1
Kalish, B., Kim, E., Finander, B., Duffy, E., Kim, H., Gilman, C., et al. (2021). Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat. Neurosci. 24, 204–213. doi: 10.1038/s41593-020-00762-9
Killick, R., Fearnhead, P., and Eckley, I. (2012). Optimal detection of changepoints with a linear computational cost. J. Am. Stat. Assoc. 107, 1590–1598. doi: 10.1080/01621459.2012.737745
Kim, J., Mohanty, S., Ganesan, L., Hua, K., Jarjoura, D., Hayton, W., et al. (2009). FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J. Immunol. 182, 2583–2589. doi: 10.4049/jimmunol.0803247
Kowal, C., Athanassiou, A., Chen, H., and Diamond, B. (2015). Maternal antibodies and developing blood-brain barrier. Immunol. Res. 63, 18–25. doi: 10.1007/s12026-015-8714-5
Lampi, K., Banerjee, P., Gissler, M., Hinkka-Yli-Salomäki, S., Huttunen, J., Kulmala, U., et al. (2011). Finnish prenatal study of autism and autism spectrum disorders (FIPS-A): Overview and design. J. Autism Dev. Disord. 41, 1090–1096. doi: 10.1007/s10803-010-1132-6
Landa, R. (2018). Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int. Rev. Psychiatry 30, 25–39. doi: 10.1080/09540261.2018.1432574
Lardeux, F., Torrico, G., and Aliaga, C. (2016). Calculation of the ELISA’s cut-off based on the change-point analysis method for detection of Trypanosoma cruzi infection in Bolivian dogs in the absence of controls. Mem. Inst. Oswaldo Cruz 111, 501–504. doi: 10.1590/0074-02760160119
Linsen, L., Vanhees, K., Vanoppen, E., Ulenaers, K., Driessens, S., Penders, J., et al. (2019). Raising to the challenge: Building a federated biobank to accelerate translational research-The University Biobank Limburg. Front. Med. 6:224. doi: 10.3389/fmed.2019.00224
Lipkin, P., Macias, M., and Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics (2020). Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics 145:e20193449.
Lord, C., Cook, E., Leventhal, B., and Amaral, D. (2000). Autism spectrum disorders. Neuron 28, 355–363. doi: 10.1016/S0896-6273(00)00115-X
Lord, C., Rutter, M., and Le Couteur, A. (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24, 659–685. doi: 10.1007/BF02172145
Maenner, M., Shaw, K., Baio, J., Washington, A., Patrick, M., DiRienzo, M., et al. (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 69, 1–12. doi: 10.15585/mmwr.ss6904a1
Magnus, P., Birke, C., Vejrup, K., Haugan, A., Alsaker, E., Daltveit, A., et al. (2016). Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol. 45, 382–388. doi: 10.1093/ije/dyw029
Martin, L., Ashwood, P., Braunschweig, D., Cabanlit, M., Van de Water, J., and Amaral, D. (2008). Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav. Immun. 22, 806–816. doi: 10.1016/j.bbi.2007.12.007
Mazon-Cabrera, R., Vandormael, P., and Somers, V. (2019). Antigenic targets of patient and maternal autoantibodies in autism spectrum disorder. Front. Immunol. 10:1474. doi: 10.3389/fimmu.2019.01474
Modabbernia, A., Velthorst, E., and Reichenberg, A. (2017). Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 8:13. doi: 10.1186/s13229-017-0121-4
Moody, E., Reyes, N., Ledbetter, C., Wiggins, L., DiGuiseppi, C., Alexander, A., et al. (2017). Screening for Autism with the SRS and SCQ: Variations across Demographic, developmental and behavioral factors in preschool children. J. Autism Dev. Disord. 47, 3550–3561. doi: 10.1007/s10803-017-3255-5
Palmeira, P., Quinello, C., Silveira-Lessa, A., Zago, C., and Carneiro-Sampaio, M. (2012). IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012:985646. doi: 10.1155/2012/985646
Palmers, I., Ydens, E., Put, E., Depreitere, B., Bongers-Janssen, H., Pickkers, P., et al. (2016). Antibody profiling identifies novel antigenic targets in spinal cord injury patients. J. Neuroinflammation 13:243. doi: 10.1186/s12974-016-0713-5
Peters-Scheffer, N., Didden, R., Korzilius, H., and Matson, J. (2012). Cost comparison of early intensive behavioral intervention and treatment as usual for children with autism spectrum disorder in The Netherlands. Res. Dev. Disabil. 33, 1763–1772. doi: 10.1016/j.ridd.2012.04.006
Pongrakhananon, V., Saito, H., Hiver, S., Abe, T., Shioi, G., Meng, W., et al. (2018). CAMSAP3 maintains neuronal polarity through regulation of microtubule stability. Proc. Natl. Acad. Sci. U.S.A. 115, 9750–9755. doi: 10.1073/pnas.1803875115
Quaden, D., Vandormael, P., Ruytinx, P., Geusens, P., Corten, K., Vanhoof, J., et al. (2020). Antibodies against three novel peptides in early axial spondyloarthritis patients from two independent cohorts. Arthritis Rheumatol. 72, 2094–2105. doi: 10.1002/art.41427
Ramaekers, V., Quadros, E., and Sequeira, J. (2013). Role of folate receptor autoantibodies in infantile autism. Mol. Psychiatry 18, 270–271. doi: 10.1038/mp.2012.22
Ramirez-Celis, A., Edmiston, E., Schauer, J., Vu, T., and Van de Water, J. (2020). Peptides of neuron specific enolase as potential ASD biomarkers: From discovery to epitope mapping. Brain Behav. Immun. 84, 200–208. doi: 10.1016/j.bbi.2019.12.002
Randall, M., Egberts, K., Samtani, A., Scholten, R., Hooft, L., Livingstone, N., et al. (2018). Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst. Rev. 7:CD009044. doi: 10.1002/14651858.CD009044.pub2
Robins, D., Fein, D., Barton, M., and Green, J. (2001). The Modified Checklist for Autism in Toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. J. Autism Dev. Disord. 31, 131–144. doi: 10.1023/A:1010738829569
Singer, H., Morris, C., Gause, C., Gillin, P., Crawford, S., and Zimmerman, A. (2008). Antibodies against fetal brain in sera of mothers with autistic children. J. Neuroimmunol. 194, 165–172. doi: 10.1016/j.jneuroim.2007.11.004
Singer, H., Morris, C., Gause, C., Pollard, M., Zimmerman, A., and Pletnikov, M. (2009). Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J. Neuroimmunol. 211, 39–48. doi: 10.1016/j.jneuroim.2009.03.011
Smith, S., Li, J., Garbett, K., Mirnics, K., and Patterson, P. (2007). Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007
Somers, K., Geusens, P., Elewaut, D., De Keyser, F., Rummens, J., Coenen, M., et al. (2011). Novel autoantibody markers for early and seronegative rheumatoid arthritis. J. Autoimmun. 36, 33–46. doi: 10.1016/j.jaut.2010.10.003
Somers, K., Stinissen, P., and Somers, V. (2009). Optimization of high-throughput autoantibody profiling for the discovery of novel antigenic targets in rheumatoid arthritis. Ann. N. Y. Acad. Sci. 1173, 92–102. doi: 10.1111/j.1749-6632.2009.04654.x
Somers, V., Govarts, C., Hellings, N., Hupperts, R., and Stinissen, P. (2005). Profiling the autoantibody repertoire by serological antigen selection. J. Autoimmun. 25, 223–228. doi: 10.1016/j.jaut.2005.09.023
Somers, V., Govarts, C., Somers, K., Hupperts, R., Medaer, R., and Stinissen, P. (2008). Autoantibody profiling in multiple sclerosis reveals novel antigenic candidates. J. Immunol. 180, 3957–3963. doi: 10.4049/jimmunol.180.6.3957
Spann, M., Timonen-Soivio, L., Suominen, A., Cheslack-Postava, K., McKeague, I., Sourander, A., et al. (2019). Proband and familial autoimmune diseases are associated with proband diagnosis of autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 58, 496–505. doi: 10.1016/j.jaac.2018.09.444
Suren, P., Saasen-Havdahl, A., Bresnahan, M., Hirtz, D., Hornig, M., Lord, C., et al. (2019). Sensitivity and specificity of early screening for autism. BJPsych Open 5:e41. doi: 10.1192/bjo.2019.34
Toya, M., Kobayashi, S., Kawasaki, M., Shioi, G., Kaneko, M., Ishiuchi, T., et al. (2016). CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 113, 332–337. doi: 10.1073/pnas.1520638113
Vanaken, G., Noens, I., Roeyers, H., van Esch, L., Warreyn, P., Steyaert, J., et al. (2021). Ethics of returning children’s individual research findings: From principles to practice. Eur. Child Adolesc. Psychiatry 30, 1163–1171. doi: 10.1007/s00787-020-01606-4
Vandormael, P., Verschueren, P., De Winter, L., and Somers, V. (2017). cDNA phage display for the discovery of theranostic autoantibodies in rheumatoid arthritis. Immunol. Res. 65, 307–325. doi: 10.1007/s12026-016-8839-1
Wojcik, S., Bernatsky, S., Platt, R., Pineau, C., Clarke, A., Fombonne, E., et al. (2017). Risk of autism spectrum disorders in children born to mothers with rheumatoid arthritis: A systematic literature review. Arthritis Care Res. 69, 1926–1931. doi: 10.1002/acr.23235
Yuen, T., Penner, M., Carter, M., Szatmari, P., and Ungar, W. (2018). Assessing the accuracy of the Modified Checklist for Autism in Toddlers: A systematic review and meta-analysis. Dev. Med. Child Neurol. 60, 1093–1100. doi: 10.1111/dmcn.13964
Zimmerman, A., Connors, S., Matteson, K., Lee, L., Singer, H., Castaneda, J., et al. (2007). Maternal antibrain antibodies in autism. Brain Behav. Immun. 21, 351–357. doi: 10.1016/j.bbi.2006.08.005
Keywords: autoantibodies, autism spectrum disorder, risk factor, pregnancy, biomarker
Citation: Mazón-Cabrera R, Liesenborgs J, Brône B, Vandormael P and Somers V (2023) Novel maternal autoantibodies in autism spectrum disorder: Implications for screening and diagnosis. Front. Neurosci. 17:1067833. doi: 10.3389/fnins.2023.1067833
Received: 12 October 2022; Accepted: 09 January 2023;
Published: 02 February 2023.
Edited by:
Jordi Julvez, Pere Virgili Health Research Institute (IISPV), SpainReviewed by:
Edward Quadros, SUNY Downstate Health Sciences University, United StatesCopyright © 2023 Mazón-Cabrera, Liesenborgs, Brône, Vandormael and Somers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Veerle Somers,  dmVlcmxlLnNvbWVyc0B1aGFzc2VsdC5iZQ==
dmVlcmxlLnNvbWVyc0B1aGFzc2VsdC5iZQ==
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.