
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 25 August 2022
Sec. Brain Imaging Methods
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.994458
This article is part of the Research Topic Women in Brain Imaging Methods 2022 View all 4 articles
 Anna K. Bonkhoff1
Anna K. Bonkhoff1 Teresa Ullberg2,3
Teresa Ullberg2,3 Martin Bretzner1,4
Martin Bretzner1,4 Sungmin Hong1
Sungmin Hong1 Markus D. Schirmer1
Markus D. Schirmer1 Robert W. Regenhardt1
Robert W. Regenhardt1 Kathleen L. Donahue1
Kathleen L. Donahue1 Marco J. Nardin1
Marco J. Nardin1 Adrian V. Dalca5,6
Adrian V. Dalca5,6 Anne-Katrin Giese7
Anne-Katrin Giese7 Mark R. Etherton1
Mark R. Etherton1 Brandon L. Hancock6
Brandon L. Hancock6 Steven J. T. Mocking6
Steven J. T. Mocking6 Elissa C. McIntosh8
Elissa C. McIntosh8 John Attia9,10
John Attia9,10 John W. Cole11
John W. Cole11 Amanda Donatti12
Amanda Donatti12 Christoph J. Griessenauer13,14
Christoph J. Griessenauer13,14 Laura Heitsch15,16
Laura Heitsch15,16 Lukas Holmegaard17,18
Lukas Holmegaard17,18 Katarina Jood17,18
Katarina Jood17,18 Jordi Jimenez-Conde19,20
Jordi Jimenez-Conde19,20 Steven J. Kittner11
Steven J. Kittner11 Robin Lemmens21,22
Robin Lemmens21,22 Christopher R. Levi23,24
Christopher R. Levi23,24 Caitrin W. McDonough25
Caitrin W. McDonough25 James F. Meschia26
James F. Meschia26 Chia-Ling Phuah16
Chia-Ling Phuah16 Stefan Ropele27
Stefan Ropele27 Jonathan Rosand1,6,28
Jonathan Rosand1,6,28 Jaume Roquer19,20
Jaume Roquer19,20 Tatjana Rundek10
Tatjana Rundek10 Ralph L. Sacco10
Ralph L. Sacco10 Reinhold Schmidt27
Reinhold Schmidt27 Pankaj Sharma29
Pankaj Sharma29 Agnieszka Slowik30
Agnieszka Slowik30 Alessandro Sousa12
Alessandro Sousa12 Tara M. Stanne31
Tara M. Stanne31 Daniel Strbian32
Daniel Strbian32 Turgut Tatlisumak17,18
Turgut Tatlisumak17,18 Vincent Thijs33,34
Vincent Thijs33,34 Achala Vagal35
Achala Vagal35 Daniel Woo36
Daniel Woo36 Ramin Zand37
Ramin Zand37 Patrick F. McArdle38
Patrick F. McArdle38 Bradford B. Worrall39,40
Bradford B. Worrall39,40 Christina Jern31,41
Christina Jern31,41 Arne G. Lindgren42,43
Arne G. Lindgren42,43 Jane Maguire44
Jane Maguire44 Ona Wu6
Ona Wu6 Petrea Frid43
Petrea Frid43 Natalia S. Rost1
Natalia S. Rost1 Johan Wasselius2,3* on behalf of the MRI-GENIE GISCOME Investigators, the International Stroke Genetics Consortium
Johan Wasselius2,3* on behalf of the MRI-GENIE GISCOME Investigators, the International Stroke Genetics ConsortiumBackground purpose: A substantial number of patients with acute ischemic stroke (AIS) experience multiple acute lesions (MAL). We here aimed to scrutinize MAL in a large radiologically deep-phenotyped cohort.
Materials and methods: Analyses relied upon imaging and clinical data from the international MRI-GENIE study. Imaging data comprised both Fluid-attenuated inversion recovery (FLAIR) for white matter hyperintensity (WMH) burden estimation and diffusion-weighted imaging (DWI) sequences for the assessment of acute stroke lesions. The initial step featured the systematic evaluation of occurrences of MAL within one and several vascular supply territories. Associations between MAL and important imaging and clinical characteristics were subsequently determined. The interaction effect between single and multiple lesion status and lesion volume was estimated by means of Bayesian hierarchical regression modeling for both stroke severity and functional outcome.
Results: We analyzed 2,466 patients (age = 63.4 ± 14.8, 39% women), 49.7% of which presented with a single lesion. Another 37.4% experienced MAL in a single vascular territory, while 12.9% featured lesions in multiple vascular territories. Within most territories, MAL occurred as frequently as single lesions (ratio ∼1:1). Only the brainstem region comprised fewer patients with MAL (ratio 1:4). Patients with MAL presented with a significantly higher lesion volume and acute NIHSS (7.7 vs. 1.7 ml and 4 vs. 3, pFDR < 0.001). In contrast, patients with a single lesion were characterized by a significantly higher WMH burden (6.1 vs. 5.3 ml, pFDR = 0.048). Functional outcome did not differ significantly between patients with single versus multiple lesions. Bayesian analyses suggested that the association between lesion volume and stroke severity between single and multiple lesions was the same in case of anterior circulation stroke. In case of posterior circulation stroke, lesion volume was linked to a higher NIHSS only among those with MAL.
Conclusion: Multiple lesions, especially those within one vascular territory, occurred more frequently than previously reported. Overall, multiple lesions were distinctly linked to a higher acute stroke severity, a higher total DWI lesion volume and a lower WMH lesion volume. In posterior circulation stroke, lesion volume was linked to a higher stroke severity in multiple lesions only.
The stroke field has seen substantial advancements in recent years. This progress was particularly due to robust clinical trials that have impacted treatment pathways (Puy and Cordonnier, 2022). Studies designed to improve our understanding of which lesion metrics are important for stroke outcomes can contribute to such progress in complementary ways, and support novel therapeutical approaches.
Neuroimaging is a core element of modern acute stroke management (Lövblad et al., 2015; Regenhardt et al., 2022b). It has been unparalleled in its capacity to derive detailed lesion characteristics. Especially after the introduction of elaborate imaging modalities, such as diffusion-weighted imaging (DWI), it became possible to reliably disentangle the acute versus chronic, lacunar versus non-lacunar, and single versus multiple nature of ischemic lesions. While all these lesion characteristics deserve further exploration, this present work investigates single versus multiple lesions.
An early computed tomography (CT)-based study estimated the frequency of multiple lesions to be ∼2% (Bogousslavsky, 1991). Later magnetic resonance imaging (MRI)-focused re-evaluations, however, suggested a frequency in the range of 10–30% (Ay et al., 1999; Baird et al., 2000b; Roh et al., 2000; Wen et al., 2004; Caso et al., 2005; Cho et al., 2007; Mustanoja et al., 2013; Depuydt et al., 2014; Novotny et al., 2017). These more recent estimates indicate that multiple lesions are a common and clinically relevant phenomenon. Concurrently, accumulating evidence suggests associations between the presence of multiple lesions and specific stroke etiologies, such as large-artery atherosclerosis (Roh et al., 2000; Wen et al., 2004) and cardioembolism (Novotny et al., 2017). In addition, multiple stroke lesions have also been linked to a higher initial stroke severity (Novotny et al., 2019), higher risk of death (Wen et al., 2004) and stroke recurrence (Wang et al., 2019).
Lesion volume has long been known to explain substantial variability in acute and chronic stroke outcomes (Löuvbld et al., 1997; Baird et al., 2000a; Thijs et al., 2000; Vogt et al., 2012; Regenhardt et al., 2022a). This explanatory capacity rendered lesion volume a clinically important prognostic marker. Conceivably, a higher lesion volume in the case of multiple lesions could readily explain the association with less favorable outcomes. However, the links between single and multiple lesion status, lesion volume and stroke outcomes have not been assessed thoroughly. This omission may largely be due to the unavailability of individual lesion volume information in many large stroke databases. Altogether, the in-depth investigation of lesion volume interaction effects with other lesion characteristics may be particularly important: More and more studies indicate that the association between lesion volume and outcomes is more complex than initially believed. For example, the links between lesion volume and long-term functional outcomes were shown to differ for small and large lesions (Ospel et al., 2021). Further work indicated that only a fraction of the endovascular treatment benefit could be traced back to the reduction in lesion volume (Al-Ajlan et al., 2016).
We here build upon a large, uniquely well-phenotyped imaging dataset of patients with acute ischemic stroke (AIS) originating from the international, multi-site MRI–Genetics Interface Exploration (MRI-GENIE) study (Giese et al., 2017) to investigate three main research aims: First, we focused on phenotyping multiple versus single ischemic lesions in radiological terms. We here primarily computed the occurrence of multiple versus single ischemic lesions while taking information on the individual vascular territory into account. In the following, we evaluated whether there were any differences in sociodemographic and clinical characteristics of patients presenting with either multiple or single acute ischemic lesions. In final analyses, we assessed the interaction effects of multiple versus single lesions and lesion volume on acute stroke severity and chronic functional outcomes. With respect to these final analyses, we aimed to evaluate the hypothesis that lesion volume relates to stroke outcomes in varying ways depending on multiple versus single lesion status. Altogether, the unique availability of (i) a large dataset of multimodal clinical MRI scans and clinical information, (ii) extensive automated segmentations of acute infarct lesions and white matter hyperintensity (WMH) lesions in combination with (iii) comprehensive manual scan evaluations by neuroradiologist experts (Drake et al., 2020) allowed us to perform analyses with an unprecedented level of detail.
The present study relied on data of patients with AIS gathered for the international MRI–Genetics Interface Exploration (MRI-GENIE) study (Giese et al., 2017), which, itself, was based upon the Stroke Genetics Network (SiGN) collaboration (Meschia et al., 2013). In brief, this study’s primary aim was to facilitate the genetic analysis of acute and chronic cerebrovascular neuroimaging phenotypes with an emphasis on creating a large database of acute and well-characterized MRI scans. While MRI-GENIE recruited 3,301 patients overall, we here focused on patients with complete radiological reports and discernible acute infarct diffusion-weighted imaging (DWI) lesions (n = 2,468). Patients gave written informed consent in accordance with the Declaration of Helsinki. The study protocol was approved by Massachusetts General Hospital’s Institutional Review Board (Protocol #: 2001P001186 and 03P000836).
MRI–Genetics Interface Exploration patients underwent acute MRI examinations. Most examinations occurred within the first 48 h of hospital admission and featured DWI, as well as FLAIR sequences. In view of the multi-site character of the study, a variety of imaging parameters have been employed. A comprehensive overview is given in the Supplementary material. Two board-certified neuroradiologists (JW and MD) manually reviewed all individual scans. They captured detailed information on lesion location (e.g., side, vascular territory) and further lesion characteristics (e.g., cortical/subcortical, single/multiple, lacunar/non-lacunar lesions for supratentorial strokes). An ischemic injury was classified as “multiple ischemic lesions stroke” if there were either multiple lesions within any of the predefined anatomical areas, or if there were distinct lesions in more than one of the predefined anatomical areas. Vascular territories encompassed the anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA) and vertebrobasilar territory (cerebellum and brainstem), each coded separately for the left and right hemispheres. Importantly, lesions did not have to occur in multiple vascular territories to be considered as multiple lesions. Therefore, we also assigned the multiple status within one vascular territory. It must be noted that borders are not distinct between vascular territories and substantial variation exists between individuals. Hence, the association of lesions to vascular territories is somewhat subjective for such border-zone areas. An exhaustive description of the structured reporting tool used can be found in Drake et al. (2020). Furthermore, DWI lesion volume, as well as WMH burden were estimated from automatically generated segmentations of DWI-defined stroke lesions (Wu et al., 2019) and FLAIR-defined WM lesions, respectively (Schirmer et al., 2019).
Sociodemographic and clinical data comprised information on age, sex, stroke severity, stroke etiology, and comorbidities/cardiovascular risk factors. Stroke etiology was captured via the causative classification of stroke system (CCS) (Arsava et al., 2010), stroke severity was measured via the National Institutes of Health Stroke Scale during the acute hospital stay (NIHSS, 0–42, 0: no measured deficits, 42: maximum stroke severity). Functional outcomes were captured via the modified Rankin Scale (mRS, 0: no symptoms at all, 6: death) at 3–6 months. Comorbidities included hypertension, coronary artery disease, diabetes mellitus, atrial fibrillation, history of smoking and prior stroke.
Our primary focus was the characterization of multiple ischemic lesions, i.e., their frequency of occurrence, spatial predilection, and associations to clinical factors and stroke outcomes. Multiple lesions were defined as >1 ischemic lesion, which could be located either within one vascular territory or multiple vascular territories. Patients were categorized according to the number of lesions and the number of involved vascular territories: Single lesion in a single vascular territory, multiple lesions in a single vascular territory, two single lesions in two vascular territories, multiple lesions in two vascular territories, and so forth. Subsequently, we compared the frequency of single versus multiple lesions within each vascular territory. These descriptive statistics are reported as means and associated standard deviations (SDs). Associations of single versus multiple lesion status with clinical characteristics were evaluated via two-sample t-tests or Fisher’s exact tests as appropriate. The level of significance was set to p < 0.05 after correction for multiple comparisons.
In more granular analyses, we scrutinized the links between lesion volume and single versus multiple lesion status (as the exposures of interest) and stroke severity and unfavorable functional outcome (mRS > 2) (as the outcomes of interest). These analyses were motivated by the hypothesis that patients with multiple stroke lesions would experience more severe strokes despite similar lesion volumes. Initially, we performed analyses for all stroke patients with available stroke severity and functional outcome data. Subsequently, we stratified for anterior versus posterior strokes. We excluded patients with lacunar stroke in the analysis of anterior circulation stroke patients, given that they, per definition, exclude multiple lesions. Lacunar stroke was defined as a single subcortical supratentorial lesion smaller than 1.5 cm. The same analysis without the exclusion of patients with lacunar stroke is included in our Supplementary material. We explained acute stroke severity by means of Bayesian hierarchical linear regression. Similarly, we employed Bayesian hierarchical logistic regression to explain 3–6-months unfavorable functional outcomes. The choice of these Bayesian models was motivated by the ease with which interaction effects can be evaluated: The hierarchical structure of the Bayesian models allowed us to estimate the effect of lesion volume on stroke outcomes separately for multiple and single ischemic lesions. However, since both of these estimates were obtained in the same model, we could directly compare these estimates and quantify their difference. The usage of Bayesian hierarchical models was furthermore supported by their successful application in several of our previous stroke outcome studies (Bonkhoff et al., 2020, 2021a,2022b). Log-transformed total DWI lesion volume represented the input to explain stroke severity, i.e., the acute NIHSS score, or unfavorable functional outcomes, i.e., mRS > 2, as the output. Single versus multiple lesion status was integrated via the hierarchical structure of our model. In this way, we obtained an estimate of the association of DWI lesion volume with stroke severity separately for those patients with a single versus those with multiple lesions (c.f., Supplementary material for model specifications). As in previous work (Bonkhoff et al., 2021b,2022a), we determined whether group estimates substantially differed via checking the overlap with zero for the difference distributions of posteriors (single – multiple lesions).
Data can be made available to researchers for the purpose of reproducing the here reported results, pending the permission for data sharing by Massachusetts General Hospital’s institutional review board. Analyses were implemented in Python 3.7, hierarchical models relied on pymc3 (Salvatier et al., 2016).
We included a total of 2,466 MRI-GENIE patients with available MRI examinations and visible acute DWI stroke lesions in this study [mean age (standard deviation (SD)]: 63.4 (14.8), 39.0% women, Table 1).
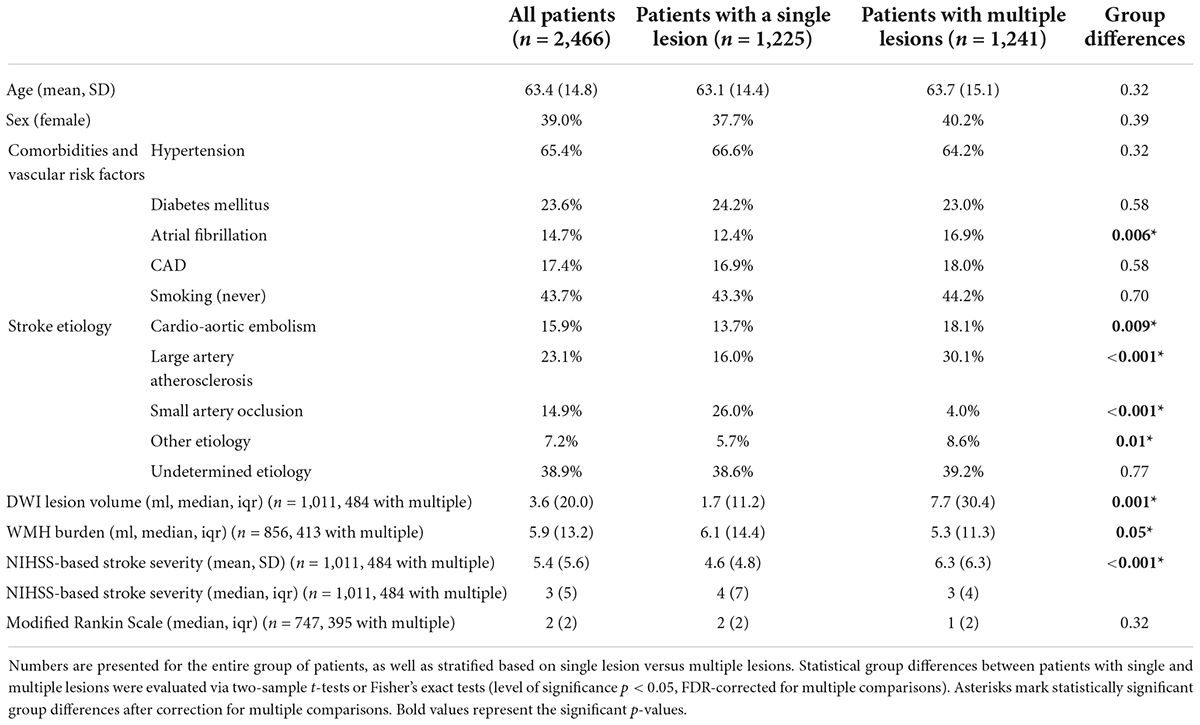
Table 1. Clinical characteristics of included MRI–Genetics Interface Exploration (MRI-GENIE) patients with acute ischemic stroke (AIS).
Patients with a single ischemic lesion in a single vascular territory constituted 49.7% (1,225/2,466), while 50.3% experienced multiple ischemic lesions (1,241/2,466). Most of these patients with multiple lesions had all their lesions within one vascular territory (37.4%, 922/2,466). Further multiple lesion constellations were comparably less frequent: 4.1% (102/2,466) patients with a single lesion in a first and multiple lesions in a second vascular territory, 3.2% with two single lesions in two vascular territories. Figure 1 presents a visual overview of these lesion and territory constellations.
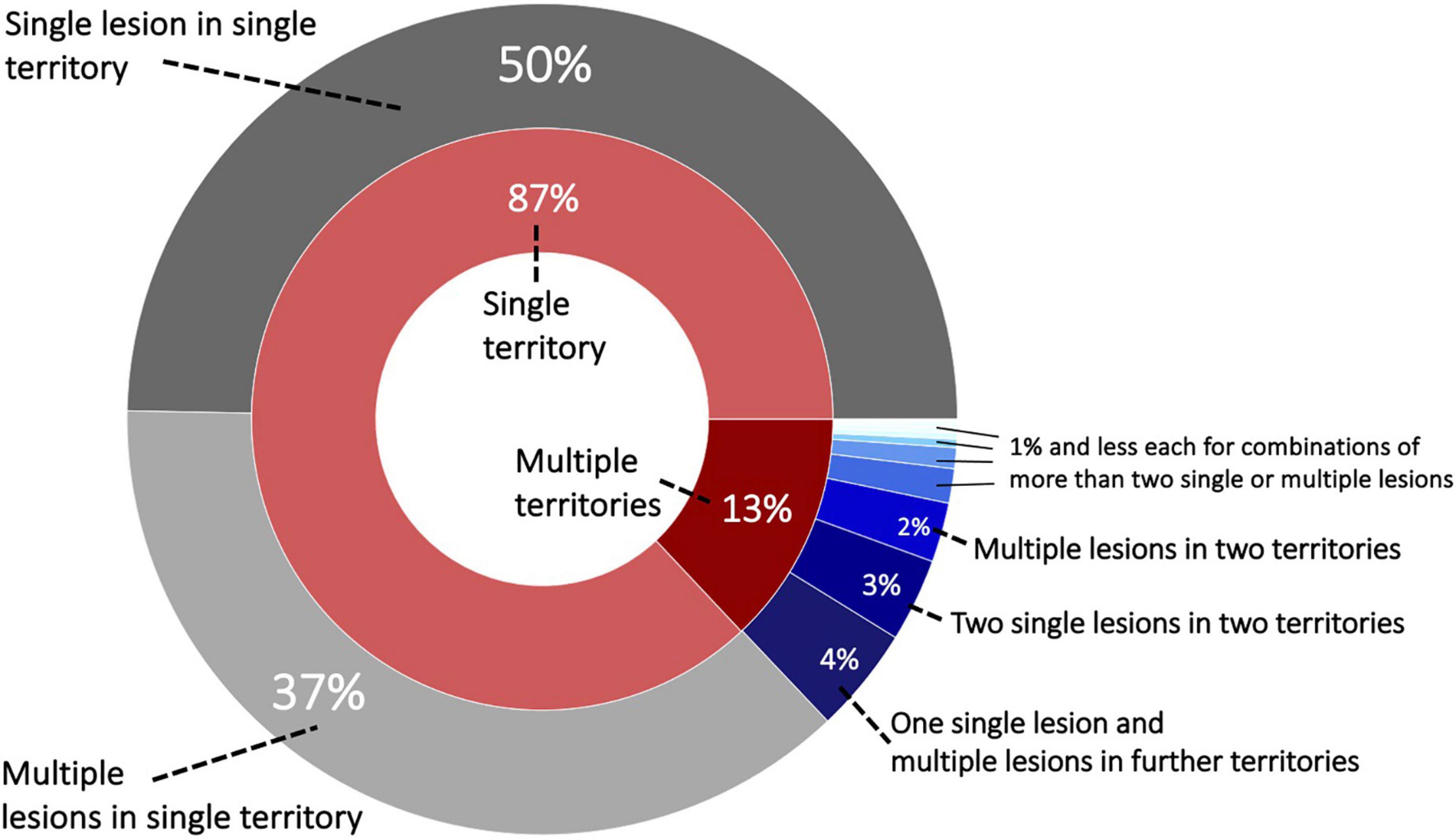
Figure 1. Sunburst plot of lesion and territory constellations. Most lesions, i.e., 87%, occurred within one vascular territory and the majority out of these were characterized as a single lesion [nine vascular territories: anterior cerebral artery (ACA left/right), middle cerebral artery (MCA left/right), posterior cerebral artery (PCA left/right) and vertebrobasilar territory (cerebellum left/right and brainstem)].
A total of 196 patients (8.0%) presented with bilateral stroke (ACA, MCA, PCA, and cerebellar strokes, excluding the brainstem). Furthermore, 138 strokes (5.6%) occurred in both supra- and infratentorial territories and 46 (1.9%) in both the anterior and posterior circulation.
When evaluating each vascular territory separately, the frequency of multiple versus single lesions remained largely the same, i.e., ∼50% (from 43% for right ACA to 55% for right cerebellum, Table 2). The brainstem represented a noteworthy exception: Single lesions within the brainstem were four times more likely than multiple lesions (81 vs 19%).
There were no significant differences in age or sex between patients with a single lesion and those with multiple lesions [mean (SD) age 63.1 (14.8) years, 37.7% women vs. 63.7 (15.1) years, 40.2% women]. Similarly, patients with single vs. multiple lesions did not differ significantly in the frequencies of the comorbidities hypertension (66.6 vs. 64.2%), diabetes mellitus (24.2 vs. 23.0%) and coronary artery disease [16.9 vs. 18.0%, all p-values > 0.05 after False Discovery Rate (FDR)-correction for multiple comparisons]. However, significantly more patients with multiple lesions had a diagnosis of atrial fibrillation (12.4 vs. 16.9%, pFDR-value = 0.005). Furthermore, patients with multiple lesions were more likely to be non-smokers (43.3 vs. 50.0%, pFDR-value = 0.002). With respect to stroke etiology, stroke patients with multiple lesions were significantly more likely to be diagnosed with cardioembolic and large artery occlusion strokes (cardioembolic: 13.7 vs. 18.1%, pFDR-value = 0.008; LAO: 16.0 vs. 30.1%, pFDR-value < 0.001), as well as strokes of the category “other etiology” (5.7 vs. 8.6%, pFDR-value = 0.01). In contrast, patients with single lesions more frequently experienced small artery occlusion strokes (26.0 vs. 4.0%, pFDR-value < 0.001).
We also had access to information on lesion volume and acute stroke severity for a subset of 1,011 patients (n = 484 patients with multiple lesions, 47.9%). Patients with multiple lesions presented both with a higher stroke severity [NIHSS 4.6 (4.8) vs. 6.3 (6.3), pFDR-value < 0.001], as well as a higher lesion volume [median (IQR) 1.7 (11.2) ml vs. 7.7 (30.4) ml, pFDR-value = 0.001]. Despite this higher lesion volume in the case of multiple lesions, lesion distributions themselves were qualitatively similar. In case of both multiple and single lesions, there was a predilection for subcortical infarcts in vicinity to the lateral ventricles (Figure 2). On the other hand, patients with single lesions were characterized by a significantly larger WMH burden [median (IQR) 6.1 (14.4) ml vs. 5.3 (11.3) ml, pFDR-value = 0.048]. Patients with single lesions and patients with multiple lesions did not significantly differ in their post-stroke functional outcome [median (IQR) mRS 2 (2) vs. 1 (2), pFDR-value = 0.29, information available for 747 patients (n = 395 patients with multiple lesions, 52.9%)].
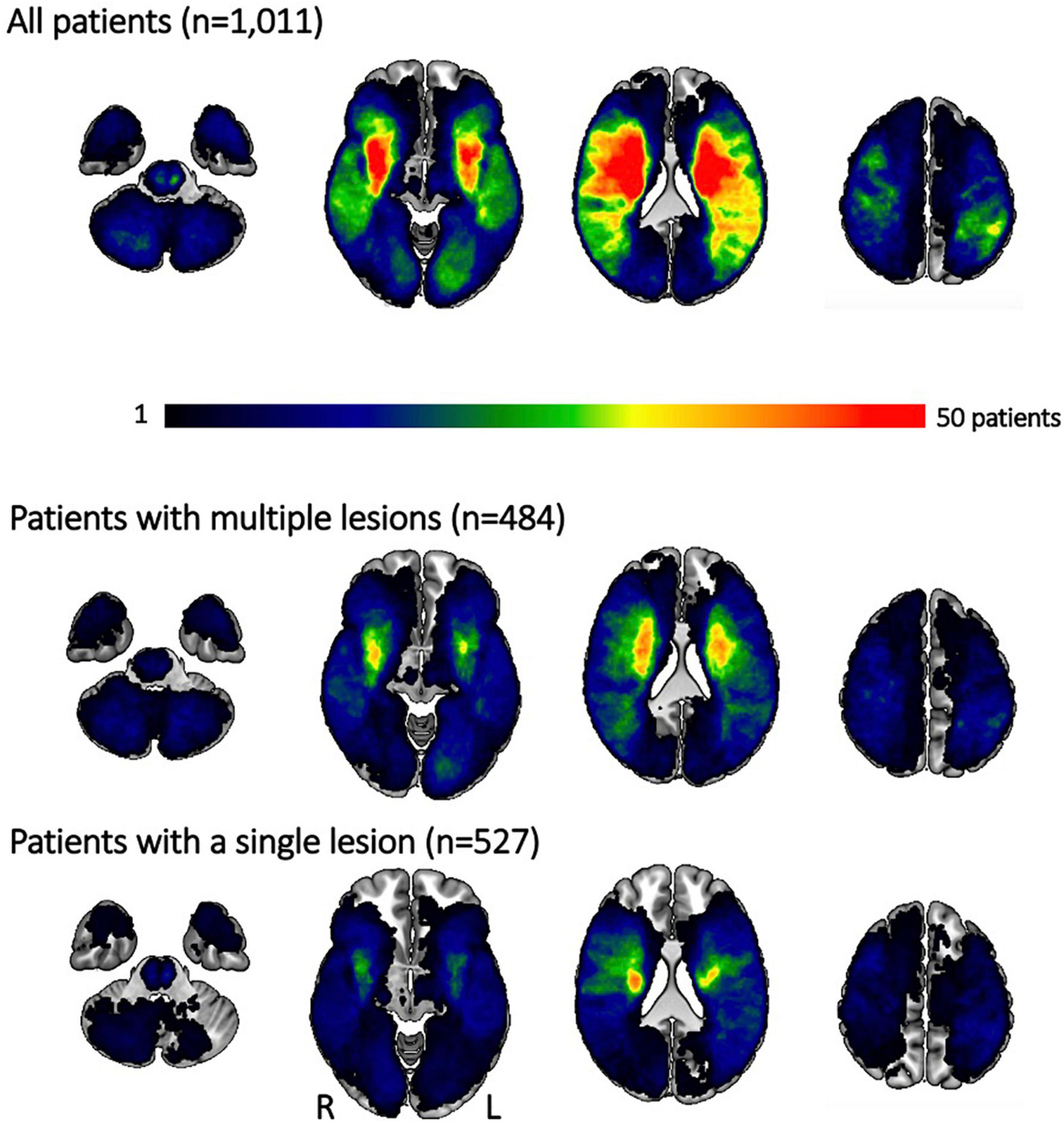
Figure 2. Lesion overlap for all patients and separately for those experiencing multiple and single lesions. Most lesions occurred subcortically, predominantly affecting the white matter in the vicinity of lateral ventricles. Patients with multiple lesions had larger stroke lesions on average, resulting in more extensive regions of substantial overlap. Qualitatively, the distribution of lesions was, however, comparable between patients with multiple and single lesions. Please note that the size of the original cohort was decreased due to lesion segmentation and stroke severity availability.
These analyses were conducted within the subsample of 1,011 patients with available lesion volume and stroke severity data. Lesion volume was positively linked to stroke severity across all patients: These effects of lesion volumes were comparable between patients with single and multiple lesions (posterior distribution for single lesions: mean: 1.15, 90% highest probability density interval (HDPI): 1.01–1.28, posterior distribution for multiple lesions: mean: 1.21, 90% HPDI: 1.08–1.33; difference of posterior distributions: mean: −0.06, 90% HPDI: −0.11 to 0.003, hence overlapping with zero, Figure 3, upper row). Findings remained the same, when considering only those patients with anterior circulation strokes and excluding patients with lacunar lesions: Once again, the effect of lesion volume on stroke severity was comparable for patients with a single and with multiple lesions (posterior distribution for single lesions: mean: 1.56, 90% HPDI: 1.39–1.84, posterior distribution for multiple lesions: mean: 1.57, 90% HPDI: 1.4–1.76; difference of posterior distributions: mean: −0.012, 90% HDPI: −0.091 to 0.064; Figure 3, middle row, c.f., Supplementary material for results without exclusion of lacunar stroke patients). In the case of posterior circulation stroke, lesion volume had varying effects on stroke severity depending on the single versus multiple lesion status (posterior distribution for single lesions: mean: 0.208, 90% HPDI: −0.0698 to 0.477, posterior distribution for multiple lesions: mean: 0.39, 90% HPDI: 0.127–0.619; difference of posterior distributions: mean: −0.182, 90% HPDI: −0.314 to −0.0689, not overlapping with zero; Figure 3, bottom row). Therefore, lesion volume had a more prominent role in stroke severity in the sample of patients with multiple lesions in the posterior circulation.
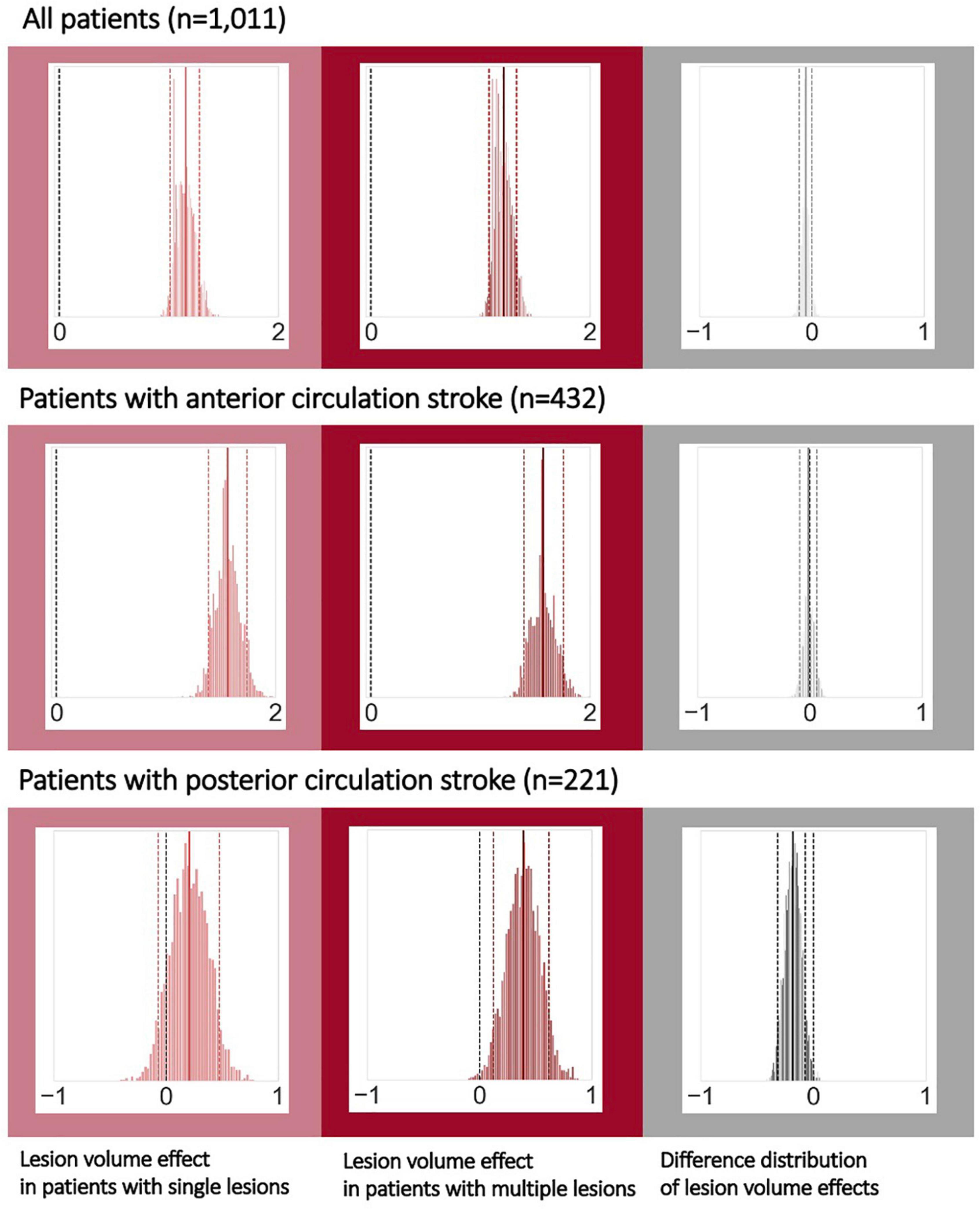
Figure 3. Bayesian hierarchical modeling: Lesion volume effects on stroke severity depending on the multiple versus single lesion status. The left column presents the lesion volume effects on stroke severity in the case of single lesions, while the middle column presents the lesion volume effects in the case of multiple lesions and the right column represents their difference. A total of 292 patients with non-lacunar, anterior circulation strokes had multiple lesions (hence 140 with single lesions). For posterior circulation stroke, 117 patients had multiple, and 104 patients had single lesions. Effects can be considered substantial if the zero is not included in the 90% highest probability density interval (HPDI, indicated by the dashed lines). Therefore, when analyzing all patients (upper row) or all patients with non-lacunar, anterior circulation strokes, lesion volume was noticeably linked to stroke severity – stroke severity was higher, the higher the lesion volume, independent of single or multiple lesions. However, for posterior circulation strokes, lesion volume had a substantial effect on stroke severity only in case of multiple lesions, yet not in case of single lesions. These varying links between lesion volume and stroke severity for single and multiple lesions were underscored by a difference distribution not overlapping with zero (right column, bottom row).
A total of 747 patients had available information on 3-month functional outcomes. When analyzing the entirety of patients, we once again ascertained positive links between lesion volume and unfavorable functional outcomes. These effects did not differ between the groups of patients with single and multiple lesions (difference of posterior distributions: mean: 0.002, 90% HPDI: −0.025 to 0.034). Results were qualitatively similar for patients with anterior circulation stroke and non-lacunar lesions. In case of posterior circulation stroke, lesion volume was not markedly associated with unfavorable functional outcomes, neither for single, nor for multiple lesions.
We leveraged a large, exceptionally well-characterized sample of 2,466 patients with AIS to investigate the intricacies of multiple ischemic lesions. We found that multiple lesions were frequent and occurred in 50% of all patients. Most of the multiple lesions occurred within one specific vascular territory. Only a total of 13% of all patients had lesions in multiple vascular territories. Combined lesions in both anterior and posterior circulation brain region were rare (∼2%). This low estimate contrasted with an overall higher rate of bilateral stroke lesions (∼8%). Our data corroborate previously reported higher rates of atrial fibrillation, cardioembolic and large artery occlusion etiologies (Roh et al., 2000; Cho et al., 2007; Depuydt et al., 2014) in patients with MAL. Similarly, stroke severity (Novotny et al., 2019) was higher in patients with MAL. Furthermore, we could gain novel insights into the links between single versus multiple lesions and the volumes of DWI and WMH lesions. We found that WMH lesion volume was significantly higher in patients with single lesions. This observation can potentially be explained by the higher prevalence of small vessel etiology in single lesion stroke. However, it has to be noted that the absolute difference was only 0.8 ml. In contrast, DWI lesion volume was significantly higher in those patients experiencing multiple lesions. What is more, for posterior circulation lesions, our findings suggest that lesion volume is linked to a higher stroke severity in the case of only multiple, but not single lesions.
Our finding that 50% of patients experience multiple lesions is in stark contrast with reports of multiple lesions in ∼2% in the earliest studies (Bogousslavsky, 1991) and even with those ones of up to ∼30% in more recent studies (Roh et al., 2000). These vastly varying numbers may arise due to both differences in the scanner technical capabilities at the time of study execution, as well as the definitions of multiple lesions. Plausibly, the sensitivity of lesion detection has substantially increased over the years. On the one hand, there was large-scale transitions from CT to MRI scans. On the other hand, MRI field strengths progressed from 1T and 1.5T to 3T MRI in many places. A thinner slice acquisition may increase the sensitivity even further (Erdur et al., 2019). We here only investigated MRI scans which are more sensitive than early CT scans. However, our multi-center imaging data was widely heterogeneous: Most scans were acquired at a field strength of 1.5T, some at 3T, and slice thicknesses varied from 2 to 7 mm.
The increase in the percentage of patients with multiple lesions in our study may also stem from varying definitions of multiple lesions. Our definition of “multiple” relied on whether there were several topographically discrete, isolated, unconnected lesions, independent of the actual vascular territory. With that, our approach differs from many previous studies. These studies rather considered lesions to be multiple only, if they occurred in several vascular territories. If we had applied this stricter criterion of multiple vascular territories in our study, we would have observed multiple lesions in only ∼13% of the cases. Hence, this estimate would have been substantially lower than the one of ∼50%, if vascular territories are not taken into account. Importantly, the exact definitions of vascular territories differed in previous studies as well: Some authors employed broad categories of hemisphere-specific anterior, middle, and posterior cerebral artery strokes (ACA, MCA, and PCA, respectively) (Erdur et al., 2019). In contrast, other authors differentiated between more subtle territories. Novotny et al. (2017), for example, additionally incorporated individual leptomeningeal branches of the ACA and MCA, the anterior choroidal artery (AchA) and numerous perforating branches of arteries in the posterior circulation. Baird and colleagues, on the other hand, modified criteria introduced by Bogousslavsky et al. (1996). They discriminated between the anterior cerebral artery, middle cerebral artery (lenticulostriate, superior or inferior division), penetrating artery in the deep basal ganglia or white matter, anterior choroidal artery, and watershed strokes (Baird et al., 2000b). The extent of territories likely has an appreciable effect on the frequency of single versus multiple lesions. Conceivably, increasing the territory size leads to the count of fewer multiple lesions.
There may not be an objectively superior way to define multiple lesions. Practically, it may be safer to assume that lesions are truly distinct and unconnected, when occurring in separate vascular territories (Baird et al., 2000b). However, false positives may have been more of a concern, when working with low resolution (CT) images, but less so nowadays. False positives here refer to situations in which lesions are presumed to be multiple, while they are not. Altogether, it may therefore be of importance to be aware of employed MAL definitions when evaluating and comparing the results of different studies. In this study, we decided to employ a non-territory-based definition of multiple lesions to ascertain an upper limit for the occurrence of multiple lesions. We hence defined associations to clinical characteristics in this context, as discussed in the following.
The in-depth investigations of multiple lesions are of particular clinical value if they augment our insights on potential stroke etiologies and outcomes. This new knowledge could then be instrumentalized to optimize preventative regimens and acute treatments. The high frequency of multiple lesions of 50% further underscores their relevance in general: Many patients could potentially benefit from any optimization.
Despite differences in MAL frequency, we here observed constellations of stroke etiologies in patients with single vs. multiple lesions similar to those described in previous studies (Roh et al., 2000; Cho et al., 2007; Depuydt et al., 2014). Cardioembolic and large artery occlusion etiologies were significantly more common in patients with multiple lesions. Patients with single lesions experienced significantly more small artery occlusion etiology strokes. In line with this pattern of associated etiologies, we could also ascertain a significantly higher WMH lesion load in patients with only single lesions and a higher frequency of atrial fibrillation in patients with multiple lesions. However, one important observation is that these differences in stroke etiology were not absolute. Every stroke etiology occurred in both single and multiple lesion stroke. For example, ∼14% of patients with a single lesion were still categorized as having had a cardioembolic stroke. In contrast, 4% of patients with multiple lesions were assigned small artery occlusion etiology. This latter finding may be well in line with prior work that described multiple lesions occurring in lacunar stroke (Caso et al., 2005). Importantly, the authors of this referenced study confirmed small artery disease as the underlying etiology in ∼57% of patients with multiple lacunar lesions. In the remaining 43% of patients with multiple lacunar lesions, etiologies other than small artery disease were determined after closer investigation.
Given these previous insights, it may thus be worth reevaluating more atypical constellations of stroke etiology and multiple vs. single lesion, such as small artery occlusion etiology in patients with multiple lesions. An enhanced understanding of causes of multiple and single lesions could then have appreciable therapeutic consequences for secondary prevention.
Previous studies focused on MAL reported varying findings with respect to the acute stroke severity. Some authors observed non-significant differences in NIHSS scores (Wen et al., 2004; Depuydt et al., 2014), while others described higher NIHSS scores, along with higher acute mRS scores in patients with multiple lesions (Novotny et al., 2019). We here ascertained further evidence for a significantly higher acute stroke severity in patients with multiple lesions. Due to the availability of information on individual stroke volumes, we could enrich our investigations by additionally scrutinizing interaction effects of single versus multiple lesion status and lesion volume on outcomes. Of note, we determined similar links between lesion volume and stroke severity in case of anterior circulation stroke for both patients with single, as well as multiple lesions. This finding suggests that a higher stroke severity in patients with MAL may be primarily due to a correspondingly higher lesion volume. However, the nature of these links changed when focusing on patients with posterior circulation strokes. We here saw a more pronounced increase in stroke severity in case of multiple discrete lesions. In other words, the same lesion volume was linked to a higher stroke severity in case of multiple, as compared to single lesions. The brainstem, representing one of the main regions affected by a stroke in the posterior circulation, hosts numerous relevant nerve nuclei and the cortical spinal tract in close spatial proximity. Multiple smaller lesions may inevitably affect more of these centers than a larger, but single lesion could. This circumstance could eventually result in the proportionally higher stroke severity in multiple lesion stroke. Intriguingly, we observed a predominance of single lesions in the brainstem region that stood in stark contrast to all other vascular territories: While multiple lesions usually occurred in approximately half of all cases, they constituted only 19% of brainstem lesions. Future studies are warranted to explore potential explanatory mechanisms and study the effect this difference may exhibit on the effect of lesion volume on stroke outcomes.
All in all, the nature of associations differed depending on the single versus multiple lesion status and affected vascular territory. Therefore, these findings strongly support the notion that links between lesion volume and stroke outcomes need to be dissected with the greatest care possible. Future research could take our approach to the next level and go beyond only differentiating between anterior versus posterior circulation stroke. Exemplarily, it could be promising to test for varying effects of multiple and single lesions for individual brain regions and white matter tracts, as defined by common brain atlases [e.g., the Harvard Oxford atlas (Desikan et al., 2006)].
Further, we did not note any significant group differences with respect to more long-term, 3–6-months functional outcome. These functional outcomes were measured on the modified Rankin Scale that represents a global assessment of the symptomatic consequences of stroke (0: no symptoms to 6: death) (Van Swieten et al., 1988; Erler et al., 2022). The scale is most frequently employed as a primary endpoint in acute stroke treatment trials (National Institute of Neurological Disorders Stroke rt-PA Stroke Study Group, 1995; Berkhemer et al., 2015; Braun et al., 2021). Nonetheless, it captures stroke sequelae in a rather coarse-grained way. Given this limitation, it is difficult to elucidate whether the observed initial differences in stroke severity were only short-lasting and not present anymore at 3 months (e.g., due to a proportionally greater recovery) or were long-lasting, but too subtle to be detected. When compared to single lesions, multiple ischemic lesions were previously linked to a higher rate of death and stroke recurrence (Wen et al., 2004), suggesting some prolonged effect. Eventually, future investigations are needed to confirm that links between single and multiple lesions and long-term functional outcome do also not arise in case of larger sample sizes. Additionally, it would be insightful to examine links to more sophisticated scales, such as the Fugl-Meyer score (Fugl-Meyer et al., 1975) for motor functions or the mini-mental state exam (Folstein et al., 1975) for cognitive functions.
Our large sample size, as well as detailed central reads of individual scans by expert neuroradiologists are two essential strengths of this study. The multi-center character may also suggest a good generalization to stroke populations at large. However, there are multiple important limitations. It should be noted that severe stroke patients may have been underrecruited in the original studies (c.f., our median NIHSS of 3). Additionally, information on functional outcome was only available in a subset of our patient sample. Further, the focus on MRI scans may have introduced a selection bias, given that patients with CT scans were not considered. Another limitation is that we did not have readily available information on acute stroke treatment. Therefore, associations to the frequency and efficacy of thrombolysis and thrombectomy, as well as their influence on the final lesion volume remain to be estimated in future work. Similarly, it would have been interesting to follow up on previous work that highlighted links between multiple lesions and hyperviscosity (Roh et al., 2000), or investigated symptomatic presentations suggesting multiple lesions (Depuydt et al., 2014). However, we did not have access to these clinical details. Lastly, we here focused on estimating effects for total DWI and WMH lesions volumes and compared stroke lesion distributions only qualitatively (Figure 2). Future work could go one step further even and evaluate lesion distributions and location in quantitative ways and additionally employ techniques such as radiomics (Bretzner et al., 2021; Regenhardt et al., 2021) or image contrast enhancement (Versaci et al., 2015) to augment the imaging-based lesion information.
We here assessed a large and radiologically uniquely deep-phenotyped cohort of patients with acute ischemic stroke and present evidence that multiple ischemic lesions occur more frequently than previously reported. Multiple ischemic lesions were detected in almost half of all cases. In analyses leveraging information on lesion volume, we uncovered distinct interaction effects with multiple lesions and vascular territories: The link between lesion volume and stroke severity was the same for both single and multiple lesions in the case of anterior circulation strokes. However, in the case of posterior circulation stroke, lesion volume was linked to a higher stroke severity in multiple lesion stroke compared to single lesion stroke. However, this association did not carry through to ∼3-to-6-months functional outcome.
The datasets presented in this article are not readily available because data can be made available to researchers for the purpose of reproducing the here reported results, pending the permission for data sharing by Massachusetts General Hospital’s institutional review board. Requests to access the datasets should be directed to AB, YWJvbmtob2ZmQG1naC5oYXJ2YXJkLmVkdQ==.
The studies involving human participants were reviewed and approved by Massachusetts General Hospital’s Institutional Review Board (Protocol #: 2001P001186 and 2003P000836). The patients/participants provided their written informed consent to participate in this study.
AB, TU, NR, and JW conceived and designed the study, led data interpretation, and prepared the manuscript. AB led data analysis. TU, MB, SH, MS, RR, MB, and OW also contributed to data analysis. KD, MN, ADa, A-KG, ME, BH, SJTM, EM, JA, JC, ADo, CG, LHe, LHo, KJ, JJ-C, SK, RL, CL, CM, JMe, C-LP, SR, JaR, JoR, TR, RSa, RSc, PS, ASl, ASo, TS, DS, TT, VT, AV, JW, DW, RZ, PM, BW, CJ, AL, JMa, OW, and PF contributed to data acquisition, management, and preprocessing. All authors contributed to results interpretation and final manuscript preparation.
The MRI-GENIE study was funded by NIH NINDS (R01NS086905). AB was supported by a Massachusetts General Hospital Executive Committee on Research (MGH ECOR) Fund for Medical Discovery (FMD) Clinical Research Fellowship Award. MB was supported by the Société Française de Neuroradiologie, Société Française de Radiologie and Fondation ISITE-ULNE. AV was in part supported by National Institutes of Health and National Institute of Neurological Disorders and Stroke (NIH-NINDS, R01 NS103824, RF1 NS117643, R01 NS100417, U01NS100699, and U01NS110772). CJ was supported by the Swedish Research Council (2018-02543 and 2021-01114), the Swedish state under the agreement between the Swedish government and the county councils, the “Avtal om Läkarutbildning och Medicinsk Forskning” (ALF) agreement (ALFGBG-720081); the Swedish Heart and Lung Foundation (20190203). AL was supported by the Swedish Research Council (2019-01757), The Swedish Government (under the “Avtal om Läkarutbildning och Medicinsk Forskning, ALF”), The Swedish Heart and Lung Foundation, Region Skåne, Lund University, Skåne University Hospital, Sparbanksstiftelsen Färs och Frosta, Fremasons Lodge of Instruction Eos in Lund and National Institutes of Health (NIH, 1R01NS114045-01). NR was in part supported by National Institutes of Health and National Institute of Neurological Disorders and Stroke (NIH-NINDS, R01NS082285, R01NS086905, and U19NS115388). JW received funding from the Crafoord Foundation (#20180610 and #20200548), the Swedish state under the agreement between the Swedish government and the county councils, the “Avtal om Läkarutbildning och Medicinsk Forskning” (ALF) agreement (YF-aALF-43435), and the Skåne University Hospital Research Funds (#96437 and 96438). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
We are grateful to our colleagues at the J. Philip Kistler Stroke Research Center for valuable support and discussions. Furthermore, we are grateful to our research participants without whom this work would not have been possible.
ME had received personal fees for consulting from Astra Zeneca and WorldCare Clinical Group. CG had received consulting honoraria from Microvention and Strykere and research funding from Medtronic and Penumbra. AV had received research funding from Cerenovus. AL had received personal fees from Bayer, Astra Zeneca, BMS Pfizer, and Portola. NR had received compensation as scientific advisory consultant from Omniox, Sanofi Genzyme and AbbVie Inc. TU received personal fees for consulting from AstraZeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.994458/full#supplementary-material
Al-Ajlan, F. S., Goyal, M., Demchuk, A. M., Minhas, P., Sabiq, F., Assis, Z., et al. (2016). Intra-arterial therapy and post-treatment infarct volumes: insights from the ESCAPE randomized controlled trial. Stroke 47, 777–781. doi: 10.1161/STROKEAHA.115.012424
Arsava, E. M., Ballabio, E., Benner, T., Cole, J. W., Delgado-Martinez, M. P., Dichgans, M., et al. (2010). The causative classification of stroke system: an international reliability and optimization study. Neurology 75, 1277–1284. doi: 10.1212/WNL.0b013e3181f612ce
Ay, H., Oliveira-Filho, J., Buonanno, F. S., Ezzeddine, M., Schaefer, P. W., Rordorf, G., et al. (1999). Diffusion-weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke 30, 2644–2650. doi: 10.1161/01.str.30.12.2644
Baird, A. E., Lövblad, K.-O., Dashe, J. F., Connor, A., Burzynski, C., Schlaug, G., et al. (2000a). Clinical correlations of diffusion and perfusion lesion volumes in acute ischemic stroke. CED 10, 441–448. doi: 10.1159/000016105
Baird, A. E., Lövblad, K. O., Schlaug, G., Edelman, R. R., and Warach, S. (2000b). Multiple acute stroke syndrome: marker of embolic disease? Neurology 54, 674–674. doi: 10.1212/WNL.54.3.674
Berkhemer, O. A., Fransen, P. S., Beumer, D., van den Berg, L. A., Lingsma, H. F., Yoo, A. J., et al. (2015). A randomized trial of intraarterial treatment for acute ischemic stroke. New England J. Med. 372, 11–20. doi: 10.1056/NEJMoa1411587
Bogousslavsky, J. (1991). Double infarction in one cerebral hemisphere. Ann. Neurol. 30, 12–18. doi: 10.1002/ana.410300104
Bogousslavsky, J., Bernasconi, A., and Kumral, E. (1996). Acute multiple infarction involving the anterior circulation. Arch. Neurol. 53, 50–57. doi: 10.1001/archneur.1996.00550010068017
Bonkhoff, A. K., Bretzner, M., Hong, S., Schirmer, M. D., Cohen, A., Regenhardt, R. W., et al. (2022a). Sex-specific lesion pattern of functional outcomes after stroke. Brain Commun. 4:fcac020.
Bonkhoff, A. K., Hong, S., Bretzner, M., Schirmer, M. D., Regenhardt, R. W., Arsava, E. M., et al. (2022b). Association of stroke lesion pattern and white matter hyperintensity burden with stroke severity and outcome. Neurology 95, e79–e88.
Bonkhoff, A. K., Hope, T., Bzdok, D., Guggisberg, A. G., Hawe, R. L., Dukelow, S. P., et al. (2020). Bringing proportional recovery into proportion: bayesian modelling of post-stroke motor impairment. Brain 143, 2189–2206. doi: 10.1093/brain/awaa146
Bonkhoff, A. K., Lim, J.-S., Bae, H.-J., Weaver, N. A., Kuijf, H. J., Biesbroek, J. M., et al. (2021a). Generative lesion pattern decomposition of cognitive impairment after stroke. Brain Commun. 3:fcab110. doi: 10.1093/braincomms/fcab110
Bonkhoff, A. K., Schirmer, M. D., Bretzner, M., Hong, S., Regenhardt, R. W., Brudfors, M., et al. (2021b). Outcome after acute ischemic stroke is linked to sex-specific lesion patterns. Nat. Commun. 12:3289. doi: 10.1038/s41467-021-23492-3
Braun, R. G., Heitsch, L., Cole, J. W., Lindgren, A., de Havenon, A., Dude, J. A., et al. (2021). What the modified rankin isn’t ranking: domain-specific outcomes for stroke clinical trials. Neurology 97, 367–377. doi: 10.1212/WNL.0000000000012231
Bretzner, M., Bonkhoff, A. K., Schirmer, M. D., Hong, S., Dalca, A. V., Donahue, K. L., et al. (2021). MRI radiomic signature of white matter hyperintensities is associated with clinical phenotypes. Front. Neurosci. 15:691244. doi: 10.3389/fnins.2021.691244
Caso, V., Budak, K., Georgiadis, D., Schuknecht, B., and Baumgartner, R. W. (2005). Clinical significance of detection of multiple acute brain infarcts on diffusion weighted magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 76, 514–518. doi: 10.1136/jnnp.2004.046383
Cho, A.-H., Kim, J. S., Jeon, S.-B., Kwon, S. U., Lee, D. H., and Kang, D.-W. (2007). Mechanism of multiple infarcts in multiple cerebral circulations on diffusion-weighted imaging. J. Neurol. 254, 924–930. doi: 10.1007/s00415-006-0397-3
Depuydt, S., Sarov, M., Vandendries, C., Guedj, T., Cauquil, C., Assayag, P., et al. (2014). Significance of acute multiple infarcts in multiple cerebral circulations on initial diffusion weighted imaging in stroke patients. J. Neurol. Sci. 337, 151–155. doi: 10.1016/j.jns.2013.11.039
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Drake, M., Frid, P., Hansen, B. M., Wu, O., Giese, A.-K., Schirmer, M. D., et al. (2020). Diffusion-Weighted imaging, MR angiography, and baseline data in a systematic multicenter analysis of 3,301 MRI scans of ischemic stroke patients—neuroradiological review within the MRI-GENIE study. Front. Neurol. 11:577. doi: 10.3389/fneur.2020.00577
Erdur, H., Milles, L. S., Scheitz, J. F., Villringer, K., Haeusler, K. G., Endres, M., et al. (2019). Clinical significance of acute and chronic ischaemic lesions in multiple cerebral vascular territories. Eur. Radiol. 29, 1338–1347. doi: 10.1007/s00330-018-5684-8
Erler, K. S., Wu, R., DiCarlo, J. A., Petrilli, M. F., Gochyyev, P., Hochberg, L. R., et al. (2022). Association of modified rankin scale with recovery phenotypes in patients with upper extremity weakness after stroke. Neurology 98, e1877–e1885. doi: 10.1212/WNL.0000000000200154
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Fugl-Meyer, A. R., Jääskö, L., Leyman, I., Olsson, S., and Steglind, S. (1975). The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabilitation Med. 7, 13–31.
Giese, A.-K., Schirmer, M. D., Donahue, K. L., Cloonan, L., Irie, R., Winzeck, S., et al. (2017). Design and rationale for examining neuroimaging genetics in ischemic stroke: the MRI-GENIE study. Neurol. Genet. 3:e180. doi: 10.1212/NXG.0000000000000180
Löuvbld, K.-O., Baird, A. E., Schlaug, G., Benfield, A., Siewert, B., Voetsch, B., et al. (1997). Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann. Neurol. 42, 164–170. doi: 10.1002/ana.410420206
Lövblad, K.-O., Altrichter, S., Pereira, V. M., Vargas, M., Gonzalez, A. M., Haller, S., et al. (2015). Imaging of acute stroke: CT and/or MRI. J. Neuroradiol. 42, 55–64. doi: 10.1016/j.neurad.2014.10.005
Meschia, J. F., Arnett, D. K., Ay, H., Brown, R. D. Jr., Benavente, O. R., et al. (2013). Stroke Genetics Network (SiGN) study: design and rationale for a genome-wide association study of ischemic stroke subtypes. Stroke 44, 2694–2702. doi: 10.1161/STROKEAHA.113.001857
Mustanoja, S., Putaala, J., Haapaniemi, E., Strbian, D., Kaste, M., and Tatlisumak, T. (2013). Multiple brain infarcts in young adults: clues for etiologic diagnosis and prognostic impact. Eur. J. Neurol. 20, 216–222. doi: 10.1111/j.1468-1331.2012.03872.x
National Institute of Neurological Disorders, and Stroke rt-Pa Stroke Study Group (1995). Tissue plasminogen activator for acute ischemic stroke. New England J. Med. 333, 1581–1588. doi: 10.1056/NEJM199512143332401
Novotny, V., Khanevski, A. N., Bjerkreim, A. T., Kvistad, C. E., Fromm, A., Waje-Andreassen, U., et al. (2019). Short-term outcome and in-hospital complications after acute cerebral infarcts in multiple arterial territories. Stroke 50, 3625–3627. doi: 10.1161/STROKEAHA.119.027049
Novotny, V., Thomassen, L., Waje-Andreassen, U., and Naess, H. (2017). Acute cerebral infarcts in multiple arterial territories associated with cardioembolism. Acta Neurol. Scand. 135, 346–351. doi: 10.1111/ane.12606
Ospel, J. M., Mayank, A., Hill, M. D., Menon, B. K., Demchuk, A., McTaggart, R., et al. (2021). Strength of association between infarct volume and clinical outcome depends on the magnitude of infarct size: results from the ESCAPE-NA1 trial. AJNR Am. J. Neuroradiol. 42, 1375–1379. doi: 10.3174/ajnr.A7183
Puy, L., and Cordonnier, C. (2022). Stroke research in 2021: insights into the reorganisation of stroke care. Lancet Neurol. 21, 2–3. doi: 10.1016/S1474-4422(21)00410-5
Regenhardt, R. W., Bonkhoff, A. K., Bretzner, M., Etherton, M. R., Das, A. S., Hong, S., et al. (2022a). Association of infarct topography and outcome after endovascular thrombectomy in patients with acute ischemic stroke. Neurology 98, e1094–e1103. doi: 10.1212/WNL.0000000000200034
Regenhardt, R. W., Nolan, N. M., Rosenthal, J. A., McIntyre, J. A., Bretzner, M., Bonkhoff, A. K., et al. (2022b). Understanding delays in MRI-based selection of large vessel occlusion stroke patients for endovascular thrombectomy. Clin. Neuroradiol. Online ahead of print. doi: 10.1007/s00062-022-01165-y
Regenhardt, R. W., Bretzner, M., Zanon Zotin, M. C., Bonkhoff, A. K., Etherton, M. R., Hong, S., et al. (2021). Radiomic signature of DWI-FLAIR mismatch in large vessel occlusion stroke. J. Neuroimaging 32, 63–67. doi: 10.1111/jon.12928
Roh, J.-K., Kang, D.-W., Lee, S.-H., Yoon, B.-W., and Chang, K.-H. (2000). Significance of acute multiple brain infarction on diffusion-weighted imaging. Stroke 31, 688–694. doi: 10.1161/01.STR.31.3.688
Salvatier, J., Wiecki, T. V., and Fonnesbeck, C. (2016). Probabilistic programming in Python using PyMC3. PeerJ Computer Sci. 2:e55. doi: 10.7717/peerj-cs.55
Schirmer, M. D., Dalca, A. V., Sridharan, R., Giese, A.-K., Donahue, K. L., Nardin, M. J., et al. (2019). White matter hyperintensity quantification in large-scale clinical acute ischemic stroke cohorts–The MRI-GENIE study. NeuroImage: Clin. 23:101884. doi: 10.1016/j.nicl.2019.101884
Thijs, V. N., Lansberg, M. G., Beaulieu, C., Marks, M. P., Moseley, M. E., and Albers, G. W. (2000). Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? a multivariable analysis. Stroke 31, 2597–2602. doi: 10.1161/01.STR.31.11.2597
Van Swieten, J. C., Koudstaal, P. J., Visser, M. C., Schouten, H. J., and Van Gijn, J. (1988). Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19, 604–607. doi: 10.1161/01.STR.19.5.604
Versaci, M., Calcagno, S., and Morabito, F. C. (2015). “Image contrast enhancement by distances among points in fuzzy hyper-cubes,” in Proceedings of the International Conference on Computer Analysis of Images and Patterns, (Berlin: Springer). doi: 10.1007/978-3-319-23117-4_43
Vogt, G., Laage, R., Shuaib, A., and Schneider, A. (2012). Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: an analysis of the virtual international stroke trials archive (VISTA) database. Stroke 43, 1266–1272. doi: 10.1161/STROKEAHA.111.646570
Wang, G., Jing, J., Pan, Y., Meng, X., Zhao, X., Liu, L., et al. (2019). Does all single infarction have lower risk of stroke recurrence than multiple infarctions in minor stroke? BMC Neurol. 19:7. doi: 10.1186/s12883-018-1215-0
Wen, H. M., Lam, W. W. M., Rainer, T., Fan, Y. H., Leung, T. W. H., Chan, Y. L., et al. (2004). Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology 63, 1317–1319. doi: 10.1212/01.WNL.0000140490.22251.B6
Keywords: magnetic resonance imaging, acute ischemic stroke, lesion volume, multiple acute ischemic lesions, quantitative imaging, Bayesian hierarchical regression
Citation: Bonkhoff AK, Ullberg T, Bretzner M, Hong S, Schirmer MD, Regenhardt RW, Donahue KL, Nardin MJ, Dalca AV, Giese A-K, Etherton MR, Hancock BL, Mocking SJT, McIntosh EC, Attia J, Cole JW, Donatti A, Griessenauer CJ, Heitsch L, Holmegaard L, Jood K, Jimenez-Conde J, Kittner SJ, Lemmens R, Levi CR, McDonough CW, Meschia JF, Phuah C-L, Ropele S, Rosand J, Roquer J, Rundek T, Sacco RL, Schmidt R, Sharma P, Slowik A, Sousa A, Stanne TM, Strbian D, Tatlisumak T, Thijs V, Vagal A, Woo D, Zand R, McArdle PF, Worrall BB, Jern C, Lindgren AG, Maguire J, Wu O, Frid P, Rost NS and Wasselius J (2022) Deep profiling of multiple ischemic lesions in a large, multi-center cohort: Frequency, spatial distribution, and associations to clinical characteristics. Front. Neurosci. 16:994458. doi: 10.3389/fnins.2022.994458
Received: 14 July 2022; Accepted: 02 August 2022;
Published: 25 August 2022.
Edited by:
Mario Sansone, University of Naples Federico II, ItalyReviewed by:
Mario Versaci, Mediterranea University of Reggio Calabria, ItalyCopyright © 2022 Bonkhoff, Ullberg, Bretzner, Hong, Schirmer, Regenhardt, Donahue, Nardin, Dalca, Giese, Etherton, Hancock, Mocking, McIntosh, Attia, Cole, Donatti, Griessenauer, Heitsch, Holmegaard, Jood, Jimenez-Conde, Kittner, Lemmens, Levi, McDonough, Meschia, Phuah, Ropele, Rosand, Roquer, Rundek, Sacco, Schmidt, Sharma, Slowik, Sousa, Stanne, Strbian, Tatlisumak, Thijs, Vagal, Woo, Zand, McArdle, Worrall, Jern, Lindgren, Maguire, Wu, Frid, Rost and Wasselius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johan Wasselius, am9oYW4ud2Fzc2VsaXVzQG1lZC5sdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.